Review of waterlogging as a yield limiting stress and the benefits and challenges of applying a modern phenotyping approach to improving waterlogging tolerance of major crops.
Keywords: Abiotic stress, breeding, flooding, phenotyping, plant imaging, waterlogging
Abstract
Yield losses to waterlogging are expected to become an increasingly costly and frequent issue in some regions of the world. Despite the extensive work that has been carried out examining the molecular and physiological responses to waterlogging, phenotyping for waterlogging tolerance has proven difficult. This difficulty is largely due to the high variability of waterlogging conditions such as duration, temperature, soil type, and growth stage of the crop. In this review, we highlight use of phenotyping to assess and improve waterlogging tolerance in temperate crop species. We start by outlining the experimental methods that have been utilized to impose waterlogging stress, ranging from highly controlled conditions of hydroponic systems to large-scale screenings in the field. We also describe the phenotyping traits used to assess tolerance ranging from survival rates and visual scoring to precise photosynthetic measurements. Finally, we present an overview of the challenges faced in attempting to improve waterlogging tolerance, the trade-offs associated with phenotyping in controlled conditions, limitations of classic phenotyping methods, and future trends using plant-imaging methods. If effectively utilized to increase crop resilience to changing climates, crop phenotyping has a major role to play in global food security.
Introduction
The Intergovernmental Panel on Climate Change (IPCC) has predicted increased weather extremes for much of the world’s crop-producing arable regions (Masson-Delmotte et al., 2021). The Food and Agriculture Organization of the United Nations (FAO) forecasted that an increase of about 70% in food production by 2050 is required to meet the demand of an increasing population (FAO, 2021). At the intersection of these monumental challenges is improving crop tolerance to a wide range of stresses exacerbated by more volatile climates. Recent scientific developments have made technologies for genetic analysis increasingly accessible. However, a bottleneck has arisen in the collection of quality phenotypic data and big data analysis to advance crop breeding programmes for stress improvement compared with genetic analysis. Traditional phenotyping methods are labour and time intensive and unsuitable for the large-scale screening of germplasm that is required for breeding purposes. High-throughput phenotyping through technological advances is expected to reduce the labour and time required to obtain phenotypic data while, importantly, increasing the scale of genetic screens, improving reliability of data and the volume of genetic resources that can be investigated.
Water stress is set to be one of the most devastating factors for temperate broad-acre crop yields as many regions are set to encounter more frequent droughts while others are set for regular flooding and waterlogging events. Thus, maintenance of yield under such increased weather extremes is a pivotal objective for future breeding programmes. The mechanisms of and responses to waterlogging have been well studied over the years (Kramer, 1951; Mittra and Stickler, 1961; Grable, 1966; Watson et al., 1976; Trought and Drew, 1980; Jackson and Drew, 1984). Applying the most recent technological advances in genotyping and phenotyping is key to progress from the fundamental groundwork of the past as we aim to limit crop losses to waterlogging. Waterlogging causes an average yield decrease of around a third; however, this impact varies greatly between crop species and with other stress conditions (Tian et al., 2021). Yield reductions range greatly between species, from little reduction in adapted crops such as cultivated rice (Oryza sativa L.) to substantial declines in highly sensitive crops such as maize (Zea mays L.) (Tian et al., 2020). Waterlogging tolerance may vary greatly within the same species, with valuable genetic resources found in wild relatives and landraces (Zhang et al., 2017). Waterlogging is a highly variable stress as many compounding factors can affect the plant responses, with the duration of the stress period being the most critical of these (Ren et al., 2016; Tian et al., 2021). Also, the growth stage of the plant during which waterlogging occurs can differently affect the final yield (Cannell et al., 1980; de San Celedonio et al., 2014). Changes in soil redox potential greatly alter soil nutrient composition and can lead to elemental toxicity (e.g. iron) or deficiency (e.g. nitrogen) (Bjerre and Schierup, 1985; Huang et al., 2015). Abiotic stress seldom occurs in isolation and combinations of stress can result in compounding effects on yield (Mittler, 2006). The co-occurrence of salinity and waterlogging stresses is increasing worldwide due to intensive irrigation systems, rise of saline water tables, and sea water intrusion (Zeng et al., 2013). Salinity and waterlogging interact in their effects on plant ion relations, growth, and survival as waterlogging causes oxygen deficiency and energy deficits that impair ion transport processes, which are key salinity tolerance mechanisms, resulting in exacerbated effects compared with salinity alone (as reviewed by Barrett-Lennard, 2003; Bennett et al., 2009; Barrett-Lennard and Shabala, 2013).
According to Sasidharan et al., 2017, flooding refers to excessively wet conditions that can be further summarized: submergence or partial submergence occurs when the entire plant is below the water level or when the entire root system and part of the above ground organs are submerged, respectively, whereas waterlogging occurs when the root zone of the crop has become flooded as soil water content reaches saturation while the above ground plant remains above the water level (Sasidharan et al., 2017). The change in medium from air to water greatly reduces the rate of gas diffusion, which in turn upsets the regular balance of nutrient uptake and gas exchange in the rhizosphere (Armstrong, 1980). A range of responses to flooding stress have evolved in the plant kingdom. Aside from rice as the notable exception, most of the world’s important crops are not well-adapted to aquatic or semi-aquatic agriculture. In this review, we focus on waterlogging as it is the most prevailing stress affecting crops across farmers’ fields.
Feeding 9 billion people by 2050 will require major societal changes and increased efficiency at all stages of food production. To address such notable challenges, it is of the utmost importance to maintain yield under stressful conditions, particularly in light of climate change effects. All available measures will need to be taken, from use of best agronomic practices to reduce the effects of waterlogging (Kaur et al., 2020) to the development of tolerant crops. Research and resources will need to be allocated for the improvement of crop resistance to both biotic and abiotic stresses. Studying crop adaptation to stress requires the examination and phenotyping of germplasm in diverse environments, ranging from pot systems to field studies. In this review we aim to assist students and researchers by outlining different phenotyping methods and the challenges associated with them. Here we provide an overview of the challenges faced in adapting crops to waterlogging and the role of plant phenotyping for a sustainable future.
What are the impacts of waterlogging on plant physiology?
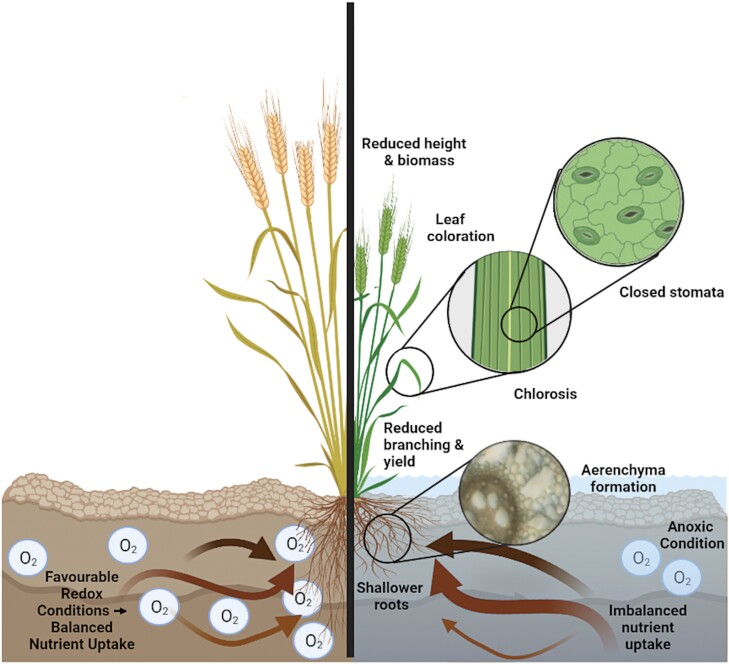
The sessile nature of plants forces them to develop strategies for coping with stresses present in their environment. Waterlogging rapidly reduces oxygen supply to the roots, preventing aerobic respiration and forcing a switch to fermentation for energy (Ashraf, 2012). Fermentation provides only a short-term and less efficient solution to the energy crisis, contributing only a small fraction of the energy produced under control conditions (Gibbs and Greenway, 2003). Under anaerobic fermentation, starch reserves are rapidly depleted and harmful by-products such as alcohols, aldehydes and reactive oxygen species (ROS) are generated (Sairam et al., 2011; Sauter, 2013; Tamang et al., 2014). Such reduced energy production will in turn result in decreased nutrient uptake, growth, and cell maintenance (Herzog et al., 2016). Photosynthetic activity and stomatal conductance decrease (Gomes and Kozlowski, 1980; Malik et al., 2001; Lee et al., 2017; Posso et al., 2018; Zhang et al., 2019) due to reduced chlorophyll degradation, damage of photosystem II, reduced photosynthetic enzyme activity (Amri et al., 2014; Wu et al., 2015; Ploschuk et al., 2018), low nitrogen content (Drew and Sisworo, 1979), and ROS damage (Andrade et al., 2018). ROS are put into action in stress signalling, yet they need to be tightly controlled as their excess can induce oxidative damage to organelles and impact vital cell structures (Ahanger et al., 2018). In addition, when photosynthetic activity is halted, excess light is absorbed leading to accumulation of ROS (Carvalho et al., 2015; Anee et al., 2019). During waterlogging there is an overall shift by the plant from energy use for growth to use for survival, leading to reductions in growth, height, and yield (Collaku and Harrison, 2002; Tian et al., 2020; Tian et al., 2021), which may ultimately lead to death (Smethurst et al., 2005; Tamang et al., 2014). Taken together, waterlogging impacts plant physiology at different levels ranging from photosynthetic effects to lower yield (Fig. 1).
Fig. 1.
Physiology of waterlogging in a plant. On the left, a barley plant under non-waterlogging conditions in well oxygenated soil. On the right, a barley plant under waterlogging stress in anoxic soil, showing a list of common physiological responses to waterlogging. Barley is used as a hypothetical example, yet these physiological responses are common to other crops experiencing waterlogging. Elements of the figure were created using Biorender.com.
Several factors influence the severity of waterlogging stress including temperature (Trought and Drew, 1982; Lin et al., 2016; Chen et al., 2017), the rhizosphere microbiome (Lin and Sauter, 2018), growth stage (Ren et al., 2016; Wang et al., 2017; Tian et al., 2021), plant species/accession (Perata et al., 1992; Arguello et al., 2016; Tian et al., 2021), stress duration (Masoni et al., 2016; Ren et al., 2016), and soil texture and chemical composition (Boem et al., 1996; Jiménez et al., 2015). At higher temperature plants are more susceptible to waterlogging, as temperature stress on its own causes hormonal imbalance, and a reduction in photosynthetic rate and carbohydrates (Lin et al., 2016; Chen et al., 2017). Waterlogging stress is exacerbated by high temperatures possibly due to an increased oxygen depletion and consumption (Trought and Drew, 1982). Waterlogging impacts plants differently according to their growth stage. For example, in wheat, waterlogging at tillering stage leads to a reduction in spike and grain numbers, while waterlogging at booting stages reduces grain weight (Wu et al., 2015). Waterlogging duration is a crucial determinant of stress severity; for example, maize grain weight reduction after 6 d of waterlogging was about twice as much as after 3 d (Masoni et al., 2016; Ren et al., 2016).
The first effects of waterlogging are experienced in the rhizosphere as microorganisms compete with roots for limited oxygen (Lin and Sauter, 2018). Furthermore, waterlogging affects availability of nutrients in soil (Sharma et al., 2018), causing an imbalance in nutrient uptake of plants and leading to both shortages and toxic build-ups of different plant nutrients (Boem et al., 1996; Jiménez et al., 2015). The nutrient uptake in waterlogged soils is affected by changes in the chemical reduction of some nutrients (notably nitrate, ferric, and manganese ions) due to the anaerobic respiration by soil bacteria (Ponnamperuma, 1972), limited root surface, as well as reduced proton motive force, less negative membrane potential and reduced metabolic control of xylem loading. Waterlogging substantially reduces plant concentrations of nitrogen, phosphorus, potassium, magnesium, copper, zinc, as well as toxicity of iron and manganese (Steffens et al., 2005; Tong et al., 2021). In particular, nitrogen and phosphorus shortages will reduce plant growth and consequently biomass (Zambrosi et al., 2014), causing leaf chlorosis due to the remobilization of nitrogen to new leaves (Drew and Sisworo, 1977). Amelioration of such nutritional effects has been achieved after nitrogen application (Zhou et al., 1997) and phosphorus fertilizer (Pang et al., 2007; Ylivainio et al., 2018).
How do plants respond to waterlogging?
The low O2 status of the rhizosphere under waterlogged conditions prevents root respiration, which in turn impacts oxygen-requiring metabolic processes, causing changes in plant-growth and nutritional status and leading to cell damage and even death (Drew and Sisworo, 1979; Trought and Drew, 1980). Thus, rapid sensing and signalling of stress are vital to allow for adaptation and damage control. Roots are the first organ to sense waterlogging, and hence play a key role in the waterlogging stress response. Once a plant detects waterlogging, its priority is to reinstate the oxygen supply to the roots, which can be achieved by altering its root morphology and anatomy (Pedersen et al., 2021). Under waterlogging conditions and due to lack of oxygen, older roots die, but some species can produce new roots closer to the surface—adventitious roots—which act as an acclimation mechanism to low O2 status, facilitating gas exchange (Sauter, 2013; Pedersen et al., 2021). In some species such as rice, some roots can even grow from the shoot area to further reduce the gas diffusion distance (Sauter, 2013; Steffens and Rasmussen, 2016).
The most emblematic physiological response to waterlogging is the development of aerenchyma to enhance the internal supply of O2 along the roots (Justin and Armstrong, 1987; Jackson and Armstrong, 1999). Aerenchyma consists of porous spaces in roots that improve gas diffusion and facilitate oxygen transport from above-ground structures to the roots (Colmer, 2003; Loreti et al., 2016). Aerenchyma can form in primary and secondary tissue (Yamauchi et al., 2018). Primary aerenchyma can be lysigenous or schizogenous, where lysigenous aerenchyma is formed by the death and lysis of cortical cells in roots and schizogenous aerenchyma is formed by separation of adjacent cells through differentiation division and/or expansion of cortical cells (with no cell death) (Yamauchi et al., 2018). Secondary aerenchyma develops from phellogen, forming spongy tissue filled with air spaces outside of stem, hypocotyl, and roots (Yamauchi et al., 2018). Rice is an exception because aerenchyma is formed constitutively (i.e. prior to waterlogging stress), promoting cell survival and enabling a faster induction of further aerenchyma formation. Root cortical aerenchyma formation is another morphological adaptation of plants to waterlogging. For example, barley genotypes with higher root cortical aerenchyma produced significantly higher yield under waterlogging (Manik et al., 2022a). In addition, relatively consistent correlation has been reported between adventitious root development and root cortical aerenchyma formation in barley (Manik et al., 2022b). In addition to aerenchyma, radial oxygen loss from the roots can be reduced through the formation of a barrier that prevents oxygen leakage into surrounding soil and enhances O2 diffusion to root tips (Abiko et al., 2012; Watanabe et al., 2017; Pedersen et al., 2021). Some crops such as maize and wheat cannot form a radial oxygen loss barrier (Abiko et al., 2012), yet they have developed other structural changes to cope with radial oxygen loss, namely increased cortex-to-stele ratio and smaller surface area to volume, with both strategies promoting a diffusion of O2 along the roots to overcome the root energy crisis (Armstrong and Beckett, 1987; Pedersen et al., 2021).
Waterlogging tolerance mechanisms, including signalling pathways, genes and quantitative trait loci associated with tolerance-related traits, have been thoroughly reviewed (Bailey-Serres and Voesenek, 2010; Mancuso and Shabala, 2010; Fukao et al., 2019; Khan et al., 2020; Jia et al., 2021; Tong et al., 2021). Moreover, the crucial role of transcriptional and translational regulations of specific genes in plant adaptation to waterlogging has been reported (Licausi and Perata, 2009). Here we provide a simplified summary of the molecular mechanisms underlying plants’ response to waterlogging. Inducible aerenchyma formation is dependent on ethylene and ROS signalling pathways through the induction of cell death and the development of above-root primordia during adventitious root formation (Jackson and Armstrong, 1999; Mergemann and Sauter, 2000; Steffens and Sauter, 2009; Yamauchi et al., 2018). Waterlogging prevents gases from leaving the roots through the soil, leading to ethylene build-up in the roots (Voesenek and Sasidharan, 2013), a reduction in root growth (Loreti et al., 2016), promotion of auxin biosynthesis (Qi et al., 2019), cell elongation (Schopfer, 2001), and root gravitropism (Joo et al., 2001). Due to the importance of ethylene and ROS in response to waterlogging, several genes that are involved in ROS production have been identified, including RBOH, (Sagi and Fluhr, 2006), as well as ethylene response factors (ERFs).
Ethylene response factor group VII (ERFVII) is known to respond to low O2 availability through the mediation of the N-degron rule pathway (formerly known as the N-end rule pathway) of targeted proteolysis (for a complete review of the N-degron pathway see Holdsworth et al., 2020). ERFVII transcription factors act as oxygen sensors through the oxidation of the tertiary destabilizing residue cysteine (Gibbs et al., 2011; Licausi et al., 2011). Substrates containing destabilizing residues of the N-degron pathway mediate proteasomal degradation of proteins via specific E3 ligases (Garzon et al., 2007; Holdsworth et al., 2020). The ERFVII are highly conserved in flowering plants (Nakano et al., 2006) and have been reported in Arabidopsis, rice, and poplar, demonstrating the crucial role of this transcription factor family in response to waterlogging (Loreti et al., 2005; Lasanthi-Kudahettige et al., 2007; Kreuzwieser et al., 2009). The most well-known ERFVII genes include those of rice responses to rapid or deep-water flooding, namely submergence 1 (Sub1A) and Snorkel Skl1 and Skl2, respectively (Khan et al., 2020). For example, manipulation of ERFs has been shown to improve waterlogging tolerance through the reduced expression of barley N-recognin E3 ligase (HvPRT6 gene) (Mendiondo et al., 2016).
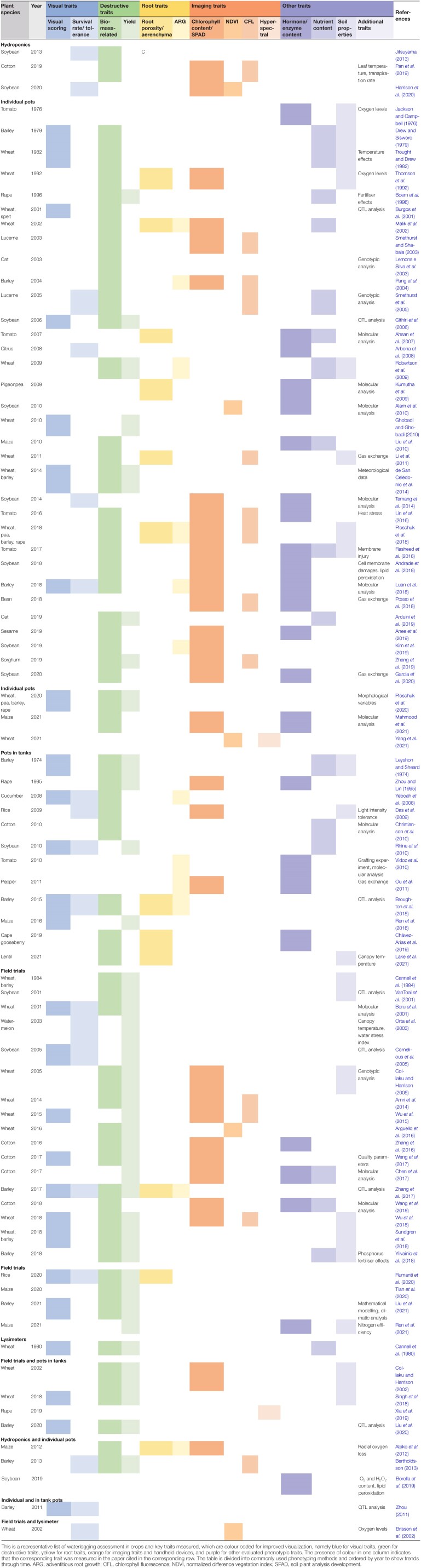
Our understanding of waterlogging sensing, signalling, and response has been greatly expanded in recent years through exemplary molecular lab work (for reviews see Bailey-Serres and Voesenek, 2010; Zhou et al., 2020). In contrast to drought, waterlogging stress per se can be more complex and varied. In fact, there are numerous developmental responses to waterlogging that differ between roots and shoots as well as between species. Crop species will respond differently to waterlogging, and some responses can be considered to be adaptations (e.g. aerenchyma formation) while others can be interpreted as an injury (e.g. chlorosis) (as reviewed by Parent et al., 2008; Zhou, 2010). As a result of such complexity, standardization of waterlogging protocols has proved to be difficult. Instead, a plethora of approaches exist in the literature (e.g. pots in tanks or field trials) across a range of crop species, where numerous traits such as biomass, root porosity, photosynthetic parameters, and chlorophyll content have been assessed (Table 1).
Table 1.
Compilation of crop phenotyping approaches to waterlogging tolerance found in literature
Phenotyping in controlled conditions is typically a straightforward approach to assess crop performance under abiotic stress, including waterlogging, as it simplifies and reduces other confounding effects. Breakthroughs in elucidating the intricacies of waterlogging stress response greatly aid our understanding, but there is a gap between advances in the laboratory and the stress tolerance of crops in the field. Phenotyping for waterlogging in controlled conditions only simulates to some degree what happens in field conditions, due to the intricacies of field trials. Field-based work for waterlogging is easier said than done, due to several challenges ranging from levelling the soil to availability of water sources. Nevertheless, studies have been carried out to bridge the gap between controlled and field conditions (Collaku and Harrison, 2002; Setter et al., 2009; Zou et al., 2014), showing that some traits were significantly correlated between the two settings. We believe that advances in genetic analysis paired with plant phenotyping are pivotal to bridging this gap towards a more food secure future.
What are the advantages and challenges when phenotyping for waterlogging?
Due to the variability of waterlogging stress and its compounding factors in terms of plant response, there are no standardized phenotyping protocols for screening waterlogging stress. Phenotyping for waterlogging has been achieved using different methods (e.g. pots in tanks or field assays) across a range of crop species while assessing a plethora of traits such as biomass or chlorophyll content (Table 1). Further experimental variation is observed for stress duration, growth stage during stress imposition, and the inclusion of recovery periods (Striker, 2008, 2012; Tian et al., 2021).
Many laboratory-based screenings for waterlogging focus specifically on anoxia to elucidate the mechanisms of hypoxia response. Much work undertaken in model species such as Arabidopsis has been used to expand our knowledge of waterlogging stress. Highly controlled conditions and growth media allow for the isolation of anoxia stress by removing compounding factors found in the field or pots. Laboratory-based methods such as hydroponics, agar, and starch have been used to simulate the hypoxic conditions of waterlogging (Hunt et al., 2002; Bertholdsson, 2013; Miricescu et al., 2021). The use of such methods with a tighter control over the growth media, i.e. hydroponics or individual pots, improves reproducibility and allows for easier harvesting of roots. However, such methods remove the important interaction of soil type and soil toxicity persisting after treatment (Pang et al., 2004; Khabaz-Saberi et al., 2006). In general, laboratory-based phenotyping uses highly controlled environments, either glasshouses or fully controlled growth chambers. Glasshouse experiments offer more controlled conditions at a smaller scale while maintaining fairly similar environmental conditions to the region in which they are located (light intensity, photoperiod, and temperature). Moreover, other abiotic and biotic stresses can be better controlled within glasshouse experiments while providing easily obtained environmental data. Glasshouses can offer varying levels of environmental control ranging from basic slatted-side glasshouses that only provide wind protection (Pang et al., 2004) to highly controlled conditions including use of supplemental lighting, temperature control, and automated watering (Luan et al., 2018). Glasshouse trials also reduce the risk of disease, which may influence phenotypic results. Growth chambers offer further control of environmental factors such as light and temperature at a, once again, minimized scale.
Several waterlogging experiments for physiological and genetic purposes, use individual pots or pots placed in containers, including concrete or plastic tanks (Table 1). Potted experiments allow greater control over soil composition and root architecture (Negrão and Julkowska, 2020). Although potted experiments present a vastly different root environment from field conditions, there are benefits to individual samples (Ploschuk et al., 2018). Roots are more accessible for analysis within potted experiments allowing for better investigation of morphological changes such as aerenchyma or radial oxygen loss barrier formation in response to waterlogging (Rasheed et al., 2018). Allowing for destructive and non-destructive root phenotyping is a key advantage of using pots over field experiments as damage during harvest is reduced, allowing for detailed investigation of root morphology under waterlogging stress (Cannarozzi et al., 2018). In general, waterlogging stress is achieved by reaching ~110–120% field capacity. We recommend estimating the field capacity of the soil in question using a target weight that is maintained throughout the course of the experiment. Duration of stress and recovery period should be optimized based on phenotyping objectives, crop species, growth stage, and experimental setup (Striker, 2008).
The controlled conditions of a pot in a glasshouse or growth chamber provide high resolution of individual sample data at a lower throughput. On the other hand, phenotyping in the field can provide lower resolution data, yet field trials represent crop performance at a more agronomically relevant scale as yield can be assessed (Negrão and Julkowska, 2020). Waterlogging screening in pots containing soil substrate offers conditions closer to the ‘natural’ field environment compared with the very tightly controlled environment found in hydroponics or other substrates (e.g. perlite). The key issue to consider with glasshouse trials is whether the results obtained strongly mirror those of the field. There are many environmental elements in the field that will affect results and that need to be considered when comparing results from the glasshouse. The disparity between glasshouse and field trials is also present when conducting phenotyping.
Screening for waterlogging tolerance in the field comes with a flood of challenges as waterlogging is a highly variable stress. Temperature, pH, and nutrient and mineral quantities will change based on the water source and/or the duration of the waterlogging event. Waterlogging events caused by rainfall are sporadic in field conditions due to differences in soil elevation, compaction, and nutrient heterogeneity, which can introduce significant noise into the data. Careful selection of field site, experimental design, and replication is critical to assess differences between experimental units (i.e. comparing cultivar tolerance). Phenotyping in field conditions for waterlogging tolerance will also need to take such variability into consideration. Ideally, one would prefer to use a naturally waterlogging prone site for repeated trials because artificially waterlogging a field site will require substantial resources as water must be supplied continuously to maintain the flooded conditions. Although field research such as that of Borrego-Benjumea et al. (2021) utilizes such sites, the availability of naturally waterlogging prone environments for agronomic, physiological, or genetic experiments is limited and so waterlogging must be artificially simulated. Creating homogeneous and reproducible field conditions for waterlogging is an arduous endeavour with many factors that must be considered such as laser levelling field sites in some cases. Firstly, very few locations will have precipitation reliable enough to guarantee waterlogged conditions and so a water source must be available on site. Secondly, the water source selected is very important as agricultural run-off and mineral contents can add extra factors to the waterlogging stress. In fact, irrigating with enough water to reach field capacity can result in the rapid accumulation of any chemical constituents present in the water source. Thirdly, the duration of stress in field trials is a decision that should not be taken lightly because waterlogging duration is a key factor in determining the damage sustained by the crop. Moreover, the duration of the stress will impact the cost of the experiment as well as the farm environment. The cost of sourcing and pumping water to the experimental site may limit not only the duration of the waterlogging treatment but also the scale of the screening. Finally, waterlogging for the duration of the crop growing season is only possible where water usage and resource availability are less restricted. Waterlogging duration usually ranges from 8 to 28 d for crop species experiments (Striker, 2008). The interaction of waterlogging treatment duration and crop growth stage at stress imposition can have varied effects on final crop yield (Cannell et al., 1980; Belford et al., 1985; de San Celedonio et al., 2014; Tian et al., 2021).
We emphasize the need to perform a pilot or optimization trial to test the soil percolation, water flow rates required, duration of stress, etc. Also, it is important to note that during the stress imposition period, one must monitor the levels of water content in the soil, which can be achieved gravimetrically (i.e. removing soil core samples and obtaining wet and dry weights) or with water and oxygen probe sensors. This is helpful for determining the overall stress resistance among genetic resources, as well as ensuring trackability of changes occurring in different years of field testing. After the considerable effort of experimental setup and the continual waterlogging of the field site has been completed, it is important to ensure that meaningful data are carefully logged, obtained, and analysed.
Classically phenotypic scoring in glasshouse environments (e.g. visual stress scoring) or field environments (e.g. flowering time) has been undertaken by trained labourers; however, this process is tedious and subjective to the assessment of those undertaking the scoring (Negrão and Julkowska, 2020). Furthermore, morphological traits such as height, growth stage, and chlorosis can be recorded, yet such recording is laborious and time-consuming and depends on the scale of the screening and size of the experiment. Technological advances now allow for high-throughput collection of data via imaging sensors mounted on phenotyping platforms for relatively low cost. High-throughput phenotyping utilizes a range of sensors that can be mounted on ground, aerial, or even orbiting platforms. Imaging sensors provide objective data that can be used to track and assess the plant response to stress non-invasively over time, thus offering an unprecedented amount of quantitative data (Negrão and Julkowska, 2020).
Can image-based methods be used to phenotype for waterlogging stress?
Phenotyping using imaging sensors and platforms continues to advance at an ever-increasing rate, meaning that the bottleneck of phenotypic data collection is eased, and new challenges come to the forefront (Roitsch et al., 2019). Increased spectra, scale of experiments and frequency of imaging all result in higher accuracy and consequently vast amounts of data. It is important that data is utilized to the fullest, ideally by maintenance of open-source databases employing FAIR (findable, available, identifiable, reusable) principles. As mentioned before, standardization of protocols and the reporting of in-depth environmental data and metadata are key to promote the usability of datasets for years to come. Here we introduce image-based phenotyping, highlighting its associated challenges and its promise in assessing waterlogging tolerance.
Imaging sensors can be classified based on the portion of the electromagnetic spectrum they cover. Researchers use visible imaging sensors that cover a wavelength range of 400–700 nm to capture the information lost due to the human eye’s limitations. More commonly known as red, green, blue (RGB) sensors, these visible imaging sensors are cheap to manufacture and it requires little experience to interpret the collected data. Imaging sensors that go beyond the visible wavelength are considered spectral imaging sensors, being categorized by the number of bands and how narrow each band is in a spectral image. If a spectral image has between three and 10 wide bands, it is generally referred to as a multispectral image. However, if the image instead consists of hundreds of bands, then it is referred to as a hyperspectral cube. Spectral imaging sensors are expensive and require extensive knowledge to interpret the results. However, due to its broad application and great potential for stress determination, spectral imaging has been extensively used in crop phenotyping (Beisel et al., 2018; Bruning et al., 2020).
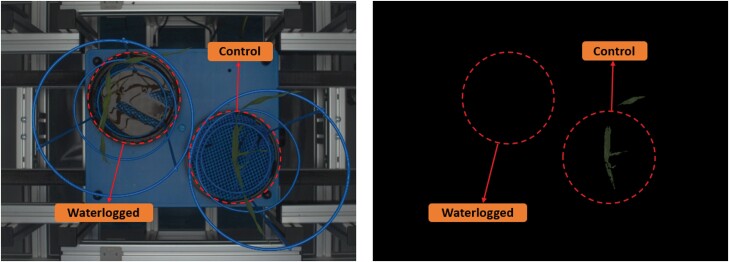
In controlled environments, i.e. glasshouses and growth chambers, imaging sensors can range from affordable low-cost Raspberry Pi cameras to expensive custom-built imaging suites (López-López et al., 2016; Tovar et al., 2018). The use of low-cost equipment was demonstrated by Xia et al. (2019), where two imaging sensors, namely RGB and hyperspectral, were used to detect waterlogging stress in oilseed rape. In this study, the authors combined hyperspectral images, ranging from 400 to 1000 nm and targeting 240 channels, with quadratic discriminant analysis, enabling the detection of waterlogging stress in oilseed rape using only six optimal wavelength channels with almost 95% accuracy (Xia et al., 2019). Regardless of cost, imaging sensors still encounter challenges that hinder the quality of the images produced. For example, low-cost devices such as the Raspberry Pi can struggle to handle the impact of image noise. Image noise can be defined as a visual distortion, including variation in brightness, grainy structure, or fluctuations of colours, which can be resolved by an imaging expert. On the other hand, custom-built imaging suites eliminate the influence of noise that may leak onto an image but are expensive and require trained personnel. Image noise can also refer to undesirable objects (e.g. pots, frames, and machinery) that obstruct the image’s main feature— the plant. To remove image noise, a technique called ‘chroma key’ is used to split an image into sub-groups—image segmentation (Agata et al., 2007). In plants, the standard colour blue is used to assist the segmentation process. Segmentation is not easy and is frequently hindered due to challenges relating to the acquisition of each image. Imaging waterlogged plants offers an extra challenge to segmentation as water in the pot reflects the lights of the imaging sensor, reducing the effectiveness of the image segmentation method (Fig. 2). Another challenge to image segmentation is unwanted algal growth. The use of gravel or plastic pellets in the pots to reduce water reflection can help in overcoming the segmentation challenge of waterlogged plants. Furthermore, multidisciplinary research teams comprising computer scientists and computer vision experts are likely to play a substantial role in the improvement of the segmentation process.
Fig. 2.
Segmentation of barley plants imaged using a photon system instruments (PSI) imaging suite. (A) Two barley plants (one waterlogged and one control) imaged with a RGB sensor, Sony IMX253LQR-C with a resolution of 4112 × 3006 pixels. (B) Image after application of a colour segmentation method to remove the unwanted noise of the image.
The use of artificial intelligence (AI) has increased in popularity in plant stress phenotyping using imaging technologies. One sub-field of AI is machine learning (ML), which is commonly defined as the development of a computer system that can learn from a dataset without following explicit instructions (Mitchell, 1997). ML has considerably changed how the process of image segmentation and stress classification can be studied. The typical use of ML is split based on the learning process into supervised or unsupervised. Supervised learning (e.g. support vector machines, neural networks) requires an annotated dataset (images labelled with stress vs. non-stress) to train itself on the classification method. In contrast, unsupervised learning does not require an annotated dataset to learn, but instead uses alternative approaches to analyse data (e.g. clustering). Taken together, supervised learning methods have been shown to produce impressive results in plant stress phenotyping (see review by Singh et al., 2016). This has been demonstrated by Kaneda et al. (2017) where both environmental data and RGB images of tomato plants exposed to drought stress were analysed using ML. The authors used a combination of a sliding-window-based support vector regression and a convolutional neural network (both supervised methods) for the accurate prediction of stress traits targeting image data of leaves based on plant wilting motion, demonstrating the advantage of ML with low-cost sensors using low number of images (Kaneda et al., 2017). Unsupervised methods have been advocated for both plant disease classification and segmentation, yet these methods struggle to obtain similar accuracies generated using supervised learning. A new promising computer science trend argues for self-supervised learning (SSL), which is the process of training an algorithm using an unlabelled dataset. This was demonstrated by Nagasubramanian et al. (2021, Preprint) where four different types of SSL models were trained and tested on two different plant stress datasets, comprising of biotic and abiotic foliar stresses in soybean plants. Excitingly, SSL significantly improved the data curation process and annotation efficiency for image-based plant stress classification compared with commonly used supervised learning methods.
In field environments, unmanned aerial vehicles (UAVs), wheel-mounted sensors, and satellite imagery are the most popular choices for imaging (Li et al., 2014; Sankaran et al., 2019). The use of ML has been applied to analyse the large influx of data generated from field-based imaging with UAVs. For example, Zhou et al. (2021) proposed the use of ML algorithms to quantify the effects of flooding stress on soybeans. In this study, soybean field plots were imaged with multispectral and thermal sensors mounted on a UAV, and supervised learning (feed forward neural network) was used to predict the flooding injury score of each plot imaged (Zhou et al., 2021). Results from this study indicate the effectiveness of ML in estimating the flooding injury score for soybean, demonstrating how field imaging phenotyping and ML could be used to assess waterlogging response in the future. Nevertheless, researchers have also found some success using low-cost handheld imaging devices that do not require expert training, the challenge here being the scale of the research experiment (Rodriguez-Moreno et al., 2017). Handheld imaging devices are limited in both the quality and quantity of the data obtained and can only be efficient for small-scale experiments. Spectral-based imaging sensors present several challenges in the field, including sensor calibration, increased sensor weight, and additional software requirements to analyse spectral images and take into account changes in light due to clouds, sun movement, and shadows. Field image-based phenotyping is subject to a series of challenges due to the influence of external factors. The first challenge is collecting ground-truth data before and during the experiment. Ground-truthing is the acquisition of a value that has been directly observed/measured, and proven true (e.g. flowering time or yield), and is essential to prove or refute the hypothesis of an experiment or statistical model using an imaging method. Thus, the ground-truthing challenge relates to data collection (scoring and harvesting) and the effect of environmental factors on the process (weather data). The second, and most obvious challenge, is data processing. The standard approach to analyse UAV imagery involves transforming many geotagged images into a single georeferenced orthomosaic, providing a bird’s-eye view experiment by stitching many small images together. The stitching process is facilitated by pre-flight geocoordinate collection and flight planning followed by post-flight processing to build an orthomosaic. The orthomosaic is then used for subsequent analysis such as the calculation of vegetation indices (Roberts et al., 2018). In waterlogging field experiments, irrigation equipment such as pipes will act as unwanted objects that will affect further downstream analysis (e.g. commonly used machine learning algorithms). Soil colour changes between waterlogged and control plots will also need to be accounted for during data processing. Due to the nature of saturated soils, aerial imaging is preferred over wheel-mounted platforms to phenotype for waterlogging stress. Also, increased soil evaporation in waterlogged field plots may also complicate thermal imaging analysis due to the introduction of ‘background’ noise.
As previously discussed, the root system plays a critical role in waterlogging responses. Indeed, the plasticity of roots enables plants to change their root system architecture in response to dynamic environmental conditions (Lobet et al., 2019). Root system architecture is defined as the geometric arrangement of structural root features in the three-dimensional soil space (Meister et al., 2014). Due to the difficulties in phenotyping below the soil level, roots are generally less analysed than aboveground organs. To date, classical two-dimensional (2D) techniques such as agar plates or rhizotrons have been used widely to understand root development (e.g. Nagel et al., 2012). Recently, germination/growth pouches have been utilized to study root traits in plants (Acharya et al., 2017; Huang et al., 2020). However, the plant root is a three-dimensional (3D) structure and the results from 2D techniques are often difficult to extrapolate to field conditions (Topp et al., 2013). Currently three tomographic techniques, X-ray computed tomography (CT), magnetic resonance imaging, and positron emission tomography, are applied for 3D phenotyping of roots in soil (see recent reviews by Atkinson et al., 2019; Wasson et al., 2020). Recently, an X-ray CT method was reported to visualize aerenchyma formation in barley roots after 9 d post-waterlogging in 2D and 3D without the requirement for chemical fixation (Kehoe et al., 2022). The X-ray CT method is less destructive, and minimal preparation or fixation time is required. The application of X-ray CT methods can be modified to different plant species for root phenotyping under waterlogging, hence opening new avenues for promising studies.
Several methods have been developed to phenotype different root traits such as crown roots, root surface area (Koyama et al., 2021), or complete root specimens in a soil core (Kücke et al., 1995) and monolith large boxes (Teramoto et al., 2020). The use of ML in root phenotyping has also been demonstrated by putting into action a convolutional neural network- supervised learning method in a field trench (Teramoto and Uga, 2020). Despite the importance of root system architecture in response to waterlogging, there is not a standard root phenotyping methodology to study different aspects of root system architecture under waterlogged conditions. Hence, the imaging research area has much to offer to the research community, and we look forward to seeing several of these root phenotyping methods being optimized for waterlogging studies in crops.
Image-based methods have generated large volumes of data with researchers struggling to handle ‘big data’ and produce the tools needed to mitigate the risks presented by this new data influx. Additionally, generated data (whether image or textual data) must be adequately stored and maintained to remain accessible many years after the experiment is conducted. Thus, data must be based on a data management plan and deposited through an open-source digital repository, with proper security measures to certify that it is protected from malicious intent. The goal of any research experiment that handles data (regardless of size) is to: (i) ensure the data can be found; (ii) make the data openly accessible; (iii) make the data interoperable; and (iv) ensure the data are reusable.
By following the FAIR principles, researchers have found various ways to counter big data challenges (Wilkinson et al., 2016). These principles are typically built into the research plan from the beginning of the experiment, simplifying the handling of the increase in volume, complexity, and creation speed of the data. Having a FAIR dataset opens new possibilities for future analysis, ensuring that waterlogging research is reproducible, expediting the development of stress tolerance crops.
Conclusion
As the effects of the climate crisis begin to increase worldwide, more and more pressure will be applied to food security. Food producers will face new and frequent challenges as weather extremes become more prevalent. Increased waterlogging events are but one of the yield-reducing challenges that will be faced more frequently and intensely. Our ability to maintain yield in the face of these challenges is vital to prevent food shortages. Currently, developing stress resistant cultivars is a slow and laborious process. Combining improvements in genetic analysis and vast amounts of data that can be quickly generated by image-based methods will facilitate the streamlined development and release of resilient cultivars. Yield loss to waterlogging and all future yield-reducing stresses must be counteracted using all available avenues. A combined research effort from the fields of agriculture, biology, genetics, robotics, computer science, and engineering is required.
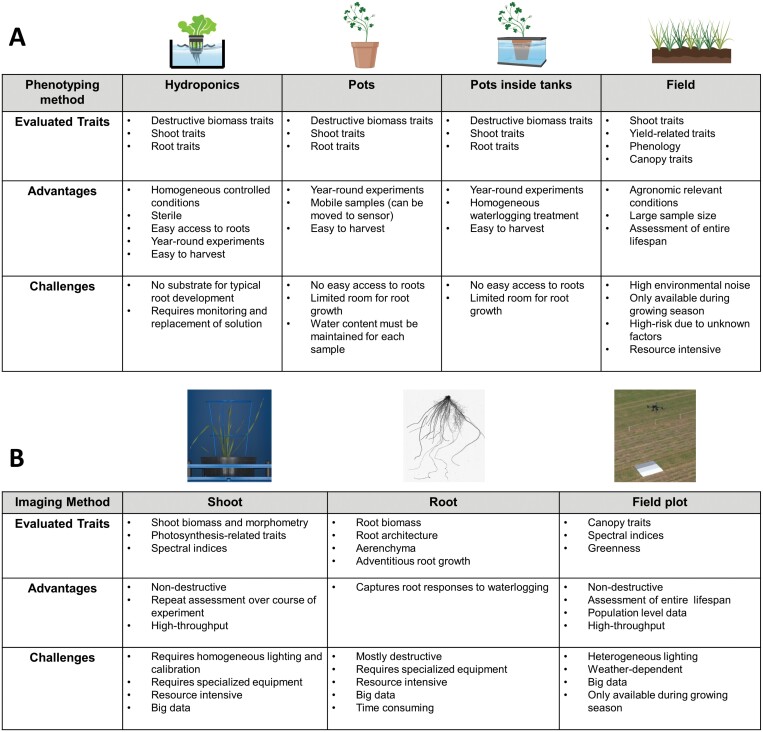
Each waterlogging phenotyping setup (Fig. 3A) and imaging phenotyping method (Fig. 3B) comes with a plethora of advantages and challenges. Thus, the optimal experimental setup should be determined by the research question, target species, available facilities, timeline, and resources (both human and financial). Reproducibility within waterlogging tolerance trials has so far proven elusive (Setter et al., 2009). Combining multiple waterlogging phenotyping methods, for example pots and field, as well as using different imaging sensors will generate extensive datasets, and promises to facilitate and improve the characterization of waterlogging tolerance among crop species. Improved land management and drainage to reduce the prevalence and crop/cultivar selection for waterlogging-tolerant cultivars in high-risk areas will reduce crop losses in the short-term. Unlocking the extensive genetic resources available for all major crops, including landraces and wild relatives, will undoubtedly accelerate waterlogging tolerance. Phenotyping has a large role to play in this progress. From low-throughput image-based systems (e.g. Raspberry Pi) to high-throughput phenotyping in the field (i.e. UAVs), now, like never before, we can monitor the effects of stress on crops non-destructively and through time. Improving tolerance to waterlogging provides unique challenges due to its complex interaction between environment and genotype. Thus, a targeted and proactive phenotyping approach to the challenges ahead will be needed to maintain food security.
Fig. 3.
Summary of waterlogging setup systems and phenotyping imaging methods used to evaluate waterlogging stress. (A) Comparison of different waterlogging methods, traits evaluated, advantages, and challenges associated with hydroponics, waterlogging within individual pots, pots within tanks, and waterlogging in field conditions. (B) Comparison of different imaging targets (single plant shoot, single plant root, and plot level canopy) and associated advantages and challenges of each approach using imaging sensors. Figure adapted from (Negrão and Julkowska, 2020). Elements of the figure were created using Biorender.com.
Acknowledgements
We thank the special issue editors and the three anonymous reviewers for their critical reading and helpful comments that improved this manuscript.
Contributor Information
Patrick Langan, School of Biology and Environmental Science, University College Dublin, Dublin, Ireland.
Villő Bernád, School of Biology and Environmental Science, University College Dublin, Dublin, Ireland.
Jason Walsh, School of Biology and Environmental Science, University College Dublin, Dublin, Ireland; School of Computer Science and UCD Energy Institute, University College Dublin, Dublin, Ireland.
Joey Henchy, School of Biology and Environmental Science, University College Dublin, Dublin, Ireland.
Mortaza Khodaeiaminjan, School of Biology and Environmental Science, University College Dublin, Dublin, Ireland.
Eleni Mangina, School of Computer Science and UCD Energy Institute, University College Dublin, Dublin, Ireland.
Sónia Negrão, School of Biology and Environmental Science, University College Dublin, Dublin, Ireland.
Roland Pieruschka, Forschungszentrum Jülich, Germany.
Author contributions
Conceptualization: PL and SN; data curation: PL, VB, and JH; visualization: VB and JW; writing original draft: all authors; writing, review and editing: PL and SN; funding acquisition: SN.
Conflict of interest
The authors have no conflicts to declare.
Funding
This research was supported by Science Foundation Ireland centre by the SFI President of Ireland Future Research Leaders to SN under Grant No. 18/FRL/6197.
References
- Abiko T, Kotula L, Shiono K, Malik AI, Colmer TD, Nakazono M.. 2012. Enhanced formation of aerenchyma and induction of a barrier to radial oxygen loss in adventitious roots of Zea nicaraguensis contribute to its waterlogging tolerance as compared with maize (Zea mays ssp. mays). Plant, Cell & Environment 35, 1618–1630. [DOI] [PubMed] [Google Scholar]
- Acharya BR, Roy Choudhury S, Estelle AB, Vijayakumar A, Zhu C, Hovis L, Pandey S.. 2017. Optimization of phenotyping assays for the model monocot Setaria viridis. Frontiers in Plant Science 8, 2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agata H, Yamashita A, Kaneko T.. 2007. Chroma key using a checker pattern background. IEICE Transactions on Information and Systems 90, 242–249. [Google Scholar]
- Ahanger MA, Gul F, Ahmad P, Akram NA.. 2018. Environmental stresses and metabolomics—deciphering the role of stress responsive metabolites. In: Ahmad P, Ahanger MA, Singh VP, Tripathi DK, Alam P, Alyemeni MN, eds. Plant metabolites and regulation under environmental stress. Academic Press, 53–67. [Google Scholar]
- Ahsan N, Lee DG, Lee SH, Kang KY, Bahk JD, Choi MS, Lee IJ, Renaut J, Lee BH.. 2007. A comparative proteomic analysis of tomato leaves in response to waterlogging stress. Physiologia Plantarum 131, 555–570. [DOI] [PubMed] [Google Scholar]
- Alam I, Lee DG, Kim KH, Park CH, Sharmin SA, Lee H, Oh KW, Yun BW, Lee BH.. 2010. Proteome analysis of soybean roots under waterlogging stress at an early vegetative stage. Journal of Biosciences 35, 49–62. [DOI] [PubMed] [Google Scholar]
- Amri M, El Ouni M, Salem MB.. 2014. Waterlogging affect the development, yield and components, chlorophyll content and chlorophyll fluorescence of six bread wheat genotypes (Triticum aestivumL.). Bulgarian Journal of Agricultural Science 20, 647–657. [Google Scholar]
- Andrade CA, Souza KRD, Santos MO, Silva DM, Alves JD.. 2018. Hydrogen peroxide promotes the tolerance of soybeans to waterlogging. Scientia Horticulturae 232, 40–45. [Google Scholar]
- Anee TI, Nahar K, Rahman A, Mahmud JA, Bhuiyan TF, Alam MU, Fujita M, Hasanuzzaman M.. 2019. Oxidative damage and antioxidant defense in Sesamum indicum after different waterlogging durations. Plants 8, 196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arbona V, Hossain Z, Lopez-Climent MF, Perez-Clemente RM, Gomez-Cadenas A.. 2008. Antioxidant enzymatic activity is linked to waterlogging stress tolerance in citrus. Physiologia Plantarum 132, 452–466. [DOI] [PubMed] [Google Scholar]
- Arduini I, Baldanzi M, Pampana S.. 2019. Reduced growth and nitrogen uptake during waterlogging at tillering permanently affect yield components in late sown oats. Frontiers in Plant Science 10, 1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arguello MN, Mason RE, Roberts TL, Subramanian N, Acuña A, Addison CK, Lozada DN, Miller RG, Gbur E.. 2016. Performance of soft red winter wheat subjected to field soil waterlogging: Grain yield and yield components. Field Crops Research 194, 57–64. [Google Scholar]
- Armstrong W. 1980. Aeration in higher plants. In: Woolhouse HW, ed. Advances in Botanical Research, Vol. 7. Academic Press, 225–332. [Google Scholar]
- Armstrong W, Beckett P.. 1987. Internal aeration and development of stela anoxia in submerged roots: a multishelled mathem mathematical model combining axial diffusion of oxygen in the cortex with radial losses to the stele, the wall layers and the rhizosphere. New Phytologist 105, 221–245. [Google Scholar]
- Ashraf MA. 2012. Waterlogging stress in plants: A review. African Journal of Agricultural Research 7, 1976–1981. [Google Scholar]
- Atkinson JA, Pound MP, Bennett MJ, Wells DM.. 2019. Uncovering the hidden half of plants using new advances in root phenotyping. Current Opinion in Biotechnology 55, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey-Serres J, Voesenek LACJ.. 2010. Life in the balance: a signaling network controlling survival of flooding. Current Opinion in Plant Biology 13, 489–494. [DOI] [PubMed] [Google Scholar]
- Barrett-Lennard E. 2003. The interaction between waterlogging and salinity in higher plants: causes, consequences and implications. Plant and Soil 253, 35–54. [Google Scholar]
- Barrett-Lennard EG, Shabala SN.. 2013. The waterlogging/salinity interaction in higher plants revisited – focusing on the hypoxia-induced disturbance to K+ homeostasis. Functional Plant Biology 40, 872–882. [DOI] [PubMed] [Google Scholar]
- Beisel NS, Callaham JB, Sng NJ, Taylor DJ, Paul A-L, Ferl RJ.. 2018. Utilization of single-image normalized difference vegetation index (SI-NDVI) for early plant stress detection. Applications in Plant Sciences 6, e01186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belford RK, Cannell RQ, Thomson RJ.. 1985. Effects of single and multiple waterloggings on the growth and yield of winter wheat on a clay soil. Journal of the Science of Food and Agriculture 36, 142–156. [Google Scholar]
- Bennett SJ, Barrett-Lennard EG, Colmer TD.. 2009. Salinity and waterlogging as constraints to saltland pasture production: A review. Agriculture, Ecosystems & Environment 129, 349–360. [Google Scholar]
- Bertholdsson N-O. 2013. Screening for barley waterlogging tolerance in nordic barley cultivars (Hordeum vulgare L.) using chlorophyll fluorescence on hydroponically-grown plants. Agronomy 3, 376–390. [Google Scholar]
- Bjerre GK, Schierup H-H.. 1985. Influence of waterlogging on availability and uptake of heavy metals by oat grown in different soils. Plant and Soil 88, 45–56. [Google Scholar]
- Boem FHG, Lavado RS, Porcelli CA.. 1996. Note on the effects of winter and spring waterlogging on growth, chemical composition and yield of rapeseed. Field Crops Research 47, 175–179. [Google Scholar]
- Borella J, Becker R, Lima MC, Colares de Oliveira DS, Braga EJB, Barneche de Oliveira AC, Do Amarante L.. 2019. Nitrogen source influences the antioxidative system of soybean plants under hypoxia and re-oxygenation. Scientia Agricola 76, 51–62. [Google Scholar]
- Borrego-Benjumea A, Carter A, Zhu M, Tucker JR, Zhou M, Badea A.. 2021. Genome-wide association study of waterlogging tolerance in barley (Hordeum vulgare L.) under controlled field conditions. Frontiers in Plant Science 12, 711654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boru G, van Ginkel M, Kronstad WE, Boersma L.. 2001. Expression and inheritance of tolerance to waterlogging stress in wheat. Euphytica 117, 91–98. [Google Scholar]
- Brisson N, Rebière B, Zimmer D, Renault P.. 2002. Response of the root system of a winter wheat crop to waterlogging. Plant and Soil 243, 43–55. [Google Scholar]
- Broughton S, Zhou G, Teakle NL, Matsuda R, Zhou M, O’Leary RA, Colmer TD, Li C.. 2015. Waterlogging tolerance is associated with root porosity in barley (Hordeum vulgare L.). Molecular Breeding 35, 27. [Google Scholar]
- Bruning B, Berger B, Lewis M, Liu H, Garnett T.. 2020. Approaches, applications, and future directions for hyperspectral vegetation studies: An emphasis on yield-limiting factors in wheat. The Plant Phenome Journal 3, e20007. [Google Scholar]
- Burgos MS, Messmer M, Stamp P, Schmid J.. 2001. Flooding tolerance of spelt (Triticum spelta L.) compared to wheat (Triticum aestivum L.): A physiological and genetic approach. Euphytica 122, 287–295. [Google Scholar]
- Cannarozzi G, Weichert A, Schnell M, Ruiz C, Bossard S, Blosch R, Plaza-Wuthrich S, Chanyalew S, Assefa K, Tadele Z.. 2018. Waterlogging affects plant morphology and the expression of key genes in tef (Eragrostis tef). Plant Direct 2, e00056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannell R, Belford R, Gales K, Thomson R, Webster C.. 1984. Effects of waterlogging and drought on winter wheat and winter barley grown on a clay and a sandy loam soil. Plant and Soil 80, 53–66. [Google Scholar]
- Cannell RQ, Belford RK, Gales K, Dennis CW, Prew RD.. 1980. Effects of waterlogging at different stages of development on the growth and yield of winter wheat. Journal of the Science of Food and Agriculture 31, 117–132. [Google Scholar]
- Carvalho LC, Vidigal P, Amâncio S.. 2015. Oxidative stress homeostasis in grapevine (Vitis vinifera L.). Frontiers in Environmental Science 3, 20. [Google Scholar]
- Chávez-Arias CC, Gómez-Caro S, Restrepo-Díaz H.. 2019. Physiological, biochemical and chlorophyll fluorescence parameters of Physalis Peruviana L. seedlings exposed to different short-term waterlogging periods and fusarium wilt infection. Agronomy 9, 213. [Google Scholar]
- Chen Y, Wang H, Hu W, Wang S, Wang Y, Snider JL, Zhou Z.. 2017. Combined elevated temperature and soil waterlogging stresses inhibit cell elongation by altering osmolyte composition of the developing cotton (Gossypium hirsutum L.) fiber. Plant Science 256, 196–207. [DOI] [PubMed] [Google Scholar]
- Christianson JA, Llewellyn DJ, Dennis ES, Wilson IW.. 2010. Global gene expression responses to waterlogging in roots and leaves of cotton (Gossypium hirsutum L.). Plant and Cell Physiology 51, 21–37. [DOI] [PubMed] [Google Scholar]
- Collaku A, Harrison S.. 2002. Losses in wheat due to waterlogging. Crop Science 42, 444–450. [Google Scholar]
- Collaku A, Harrison S.. 2005. Heritability of waterlogging tolerance in wheat. Crop Science 45, 722–727. [Google Scholar]
- Colmer T. 2003. Long-distance transport of gases in plants: a perspective on internal aeration and radial oxygen loss from roots. Plant, Cell & Environment 26, 17–36. [Google Scholar]
- Cornelious B, Chen P, Chen Y, de Leon N, Shannon JG, Wang D.. 2005. Identification of QTLs underlying water-logging tolerance in soybean. Molecular Breeding 16, 103–112. [Google Scholar]
- Das KK, Panda D, Sarkar RK, Reddy JN, Ismail AM.. 2009. Submergence tolerance in relation to variable floodwater conditions in rice. Environmental and Experimental Botany 66, 425–434. [Google Scholar]
- de San Celedonio RP, Abeledo LG, Miralles DJ.. 2014. Identifying the critical period for waterlogging on yield and its components in wheat and barley. Plant and Soil 378, 265–277. [Google Scholar]
- Drew M, Sisworo E.. 1977. Early effects of flooding on nitrogen deficiency and leaf chlorosis in barley. New Phytologist 79, 567–571. [Google Scholar]
- Drew M, Sisworo E.. 1979. The development of waterlogging damage in young barley plants in relation to plant nutrient status and changes in soil properties. New Phytologist 82, 301–314. [Google Scholar]
- FAO. 2021. The state of food security and nutrition in the world 2021. Transforming food systems for food security, improved nutrition and affordable healthy diets for all. Rome: Food and Agriculture Organization of the United Nations. [Google Scholar]
- Fukao T, Barrera-Figueroa BE, Juntawong P, Peña-Castro JM.. 2019. Submergence and waterlogging stress in plants: A review highlighting research opportunities and understudied aspects. Frontiers in Plant Science 10, 340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia N, da-Silva CJ, Cocco KLT, Pomagualli D, de Oliveira FK, da Silva JVL, Barneche de Oliveira AC, Do Amarante L.. 2020. Waterlogging tolerance of five soybean genotypes through different physiological and biochemical mechanisms. Environmental and Experimental Botany 172, 103975. [Google Scholar]
- Garzon M, Eifler K, Faust A, Scheel H, Hofmann K, Koncz C, Yephremov A, Bachmair A.. 2007. PRT6/At5g02310 encodes an Arabidopsis ubiquitin ligase of the N-end rule pathway with arginine specificity and is not the CER3 locus. FEBS Letters 581, 3189–3196. [DOI] [PubMed] [Google Scholar]
- Ghobadi M, Ghobadi M.. 2010. Effect of anoxia on root growth and grain yield of wheat cultivars. International Journal of Agricultural and Biosystems Engineering 4, 729–732. [Google Scholar]
- Gibbs DJ, Lee SC, Isa NM, et al. 2011. Homeostatic response to hypoxia is regulated by the N-end rule pathway in plants. Nature 479, 415–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs J, Greenway H.. 2003. Mechanisms of anoxia tolerance in plants. I. Growth, survival and anaerobic catabolism. Functional Plant Biology 30, 1–47. [DOI] [PubMed] [Google Scholar]
- Githiri SM, Watanabe S, Harada K, Takahashi R.. 2006. QTL analysis of flooding tolerance in soybean at an early vegetative growth stage. Plant Breeding 125, 613–618. [Google Scholar]
- Gomes ARS, Kozlowski TT.. 1980. Growth responses and adaptations of Fraxinus pennsylvanica seedlings to flooding. Plant Physiology 66, 267–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grable AR. 1966. Soil aeration and plant growth. Advances in Agronomy 18, 57–106. [Google Scholar]
- Harrison D, De Oliveira MR, Wu C, et al. 2020. Developing a high-throughput method to screen soybean germplasm for hypoxia tolerance in hydroponic systems. Crop Science 62, 592–609. [Google Scholar]
- Herzog M, Striker GG, Colmer TD, Pedersen O.. 2016. Mechanisms of waterlogging tolerance in wheat – a review of root and shoot physiology. Plant, Cell & Environment 39, 1068–1086. [DOI] [PubMed] [Google Scholar]
- Holdsworth MJ, Vicente J, Sharma G, Abbas M, Zubrycka A.. 2020. The plant N-degron pathways of ubiquitin-mediated proteolysis. Journal of Integrative Plant Biology 62, 70–89. [DOI] [PubMed] [Google Scholar]
- Huang CT, Klos KE, Huang YF.. 2020. Genome-wide association study reveals the genetic architecture of seed vigor in oats. G3: Genes, Genomes, Genetics 10, 4489–4503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang X, Shabala S, Shabala L, Rengel Z, Wu X, Zhang G, Zhou M.. 2015. Linking waterlogging tolerance with Mn2+ toxicity: a case study for barley. Plant Biology 17, 26–33. [DOI] [PubMed] [Google Scholar]
- Hunt PW, Klok EJ, Trevaskis B, Watts RA, Ellis MH, Peacock WJ, Dennis ES.. 2002. Increased level of hemoglobin 1 enhances survival of hypoxic stress and promotes early growth in Arabidopsis thaliana. Proceedings of the National Academy of Sciences, USA 99, 17197–17202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson MB, Armstrong W.. 1999. Formation of aerenchyma and the processes of plant ventilation in relation to soil flooding and submergence. Plant Biology 1, 274–287. [Google Scholar]
- Jackson MB, Campbell DJ.. 1976. Waterlogging and petiole epinasty in tomato: the role of ethylene and low oxygen. New Phytologist 76, 21–29. [Google Scholar]
- Jackson MB, Drew MC.. 1984. Effects of flooding on growth and metabolism of herbaceous plants. In: Kozlowski TT, ed. Flooding and plant growth. San Diego: Academic Press, 47–128. [Google Scholar]
- Jia W, Ma M, Chen J, Wu S.. 2021. Plant morphological, physiological and anatomical adaption to flooding stress and the underlying molecular mechanisms. International Journal of Molecular Sciences 22, 1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiménez JC, Cardoso JA, Arango-Londoño D, Fischer G, Rao I.. 2015. Influence of soil fertility on waterlogging tolerance of two Brachiaria grasses. Agronomía Colombiana 33, 20–28. [Google Scholar]
- Jitsuyama Y. 2013. Responses of Japanese soybeans to hypoxic condition at rhizosphere were different depending upon cultivars and ambient temperatures. American Journal of Plant Sciences 4, 1297–1308. [Google Scholar]
- Joo JH, Bae YS, Lee JS.. 2001. Role of auxin-induced reactive oxygen species in root gravitropism. Plant Physiology 126, 1055–1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Justin SHFW, Armstrong W.. 1987. The anatomical characteristics of roots and plant response to soil flooding. New Phytologist 106, 465–495. [Google Scholar]
- Kaneda Y, Shibata S, Mineno H.. 2017. Multi-modal sliding window-based support vector regression for predicting plant water stress. Knowledge-Based Systems 134, 135–148. [Google Scholar]
- Kaur G, Singh G, Motavalli PP, Nelson KA, Orlowski JM, Golden BR.. 2020. Impacts and management strategies for crop production in waterlogged or flooded soils: A review. Agronomy Journal 112, 1475–1501. [Google Scholar]
- Kehoe S, Byrne T, Spink J, Barth S, Ng CKY, Tracy S.. 2022. A novel 3D X-ray computed tomography (CT) method for spatio-temporal evaluation of waterlogging-induced aerenchyma formation in barley. The Plant Phenome Journal 5, e20035. [Google Scholar]
- Khabaz-Saberi H, Setter TL, Waters I.. 2006. Waterlogging induces high to toxic concentrations of iron, aluminum, and manganese in wheat varieties on acidic soil. Journal of Plant Nutrition 29, 899–911. [Google Scholar]
- Khan MIR, Trivellini A, Chhillar H, Chopra P, Ferrante A, Khan NA, Ismail AM.. 2020. The significance and functions of ethylene in flooding stress tolerance in plants. Environmental and Experimental Botany 179, 104188. [Google Scholar]
- Kim KH, Cho MJ, Kim J-M, Lee T, Heo JH, Jeong JY, Lee J, Moon J-K, Kang S.. 2019. Growth response and developing simple test method for waterlogging stress tolerance in soybean. Journal of Crop Science and Biotechnology 22, 371–378. [Google Scholar]
- Koyama T, Murakami S, Karasawa T, Ejiri M, Shiono K.. 2021. Complete root specimen of plants grown in soil-filled root box: sampling, measuring, and staining method. Plant Methods 17, 97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer PJ. 1951. Causes of injury to plants resulting from flooding of the soil. Plant Physiology 26, 722–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreuzwieser J, Hauberg J, Howell KA, Carroll A, Rennenberg H, Millar AH, Whelan J.. 2009. Differential response of gray poplar leaves and roots underpins stress adaptation during hypoxia. Plant Physiology 149, 461–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kücke M, Schmid H, Spiess A.. 1995. A comparison of four methods for measuring roots of field crops in three contrasting soils. Plant and Soil 172, 63–71. [Google Scholar]
- Kumutha D, Ezhilmathi K, Sairam RK, Srivastava GC, Deshmukh PS, Meena RC.. 2009. Waterlogging induced oxidative stress and antioxidant activity in pigeonpea genotypes. Biologia Plantarum 53, 75–84. [Google Scholar]
- Lake L, Izzat N, Kong T, Sadras VO.. 2021. High-throughput phenotyping of plant growth rate to screen for waterlogging tolerance in lentil. Journal of Agronomy and Crop Science 207, 995–1005. [Google Scholar]
- Lasanthi-Kudahettige R, Magneschi L, Loreti E, Gonzali S, Licausi F, Novi G, Beretta O, Vitulli F, Alpi A, Perata P.. 2007. Transcript profiling of the anoxic rice coleoptile. Plant Physiology 144, 218–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HJ, Park ST, Kim SK, Choi CS, Lee SG.. 2017. The effects of high air temperature and waterlogging on the growth and physiological responses of hot pepper. Horticultural Science & Technology 35, 69–78. [Google Scholar]
- Lemons e Silva CF, de Mattos LAT, de Oliveira AC, de Carvalho FIF, de Freitas FA, e Silva SDA.. 2003. Flooding tolerance in oats. Journal of New Seeds 5, 29–42. [Google Scholar]
- Leyshon A, Sheard R.. 1974. Influence of short-term flooding on the growth and plant nutrient composition of barley. Canadian Journal of Soil Science 54, 463–473. [Google Scholar]
- Li C, Jiang D, Wollenweber B, Li Y, Dai T, Cao W.. 2011. Waterlogging pretreatment during vegetative growth improves tolerance to waterlogging after anthesis in wheat. Plant Science 180, 672–678. [DOI] [PubMed] [Google Scholar]
- Li L, Zhang Q, Huang D.. 2014. A review of imaging techniques for plant phenotyping. Sensors 14, 20078–20111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Licausi F, Kosmacz M, Weits DA, Giuntoli B, Giorgi FM, Voesenek LA, Perata P, van Dongen JT.. 2011. Oxygen sensing in plants is mediated by an N-end rule pathway for protein destabilization. Nature 479, 419–422. [DOI] [PubMed] [Google Scholar]
- Licausi F, Perata P.. 2009. Low oxygen signaling and tolerance in plants. Advances in Botanical Research 50, 139–198. [Google Scholar]
- Lin C, Sauter M.. 2018. Control of adventitious root architecture in rice by darkness, light, and gravity. Plant Physiology 176, 1352–1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin H-H, Lin K-H, Syu J-Y, Tang S-Y, Lo H-F.. 2016. Physiological and proteomic analysis in two wild tomato lines under waterlogging and high temperature stress. Journal of Plant Biochemistry and Biotechnology 25, 87–96. [Google Scholar]
- Liu K, Harrison MT, Archontoulis SV, et al. 2021. Climate change shifts forward flowering and reduces crop waterlogging stress. Environmental Research Letters 16, 094017. [Google Scholar]
- Liu K, Harrison MT, Ibrahim A, Manik SMN, Johnson P, Tian X, Meinke H, Zhou M.. 2020. Genetic factors increasing barley grain yields under soil waterlogging. Food and Energy Security 9, e238. [Google Scholar]
- Liu Y-Z, Tang B, Zheng Y-L, Ma K-J, Xu S-Z, Qiu F-Z.. 2010. Screening methods for waterlogging tolerance at maize (Zea mays L.) seedling stage. Agricultural Sciences in China 9, 362–369. [Google Scholar]
- Lobet G, Paez-Garcia A, Schneider H, Junker A, Atkinson JA, Tracy S.. 2019. Demystifying roots: A need for clarification and extended concepts in root phenotyping. Plant Science 282, 11–13. [DOI] [PubMed] [Google Scholar]
- López-López M, Calderón R, González-Dugo V, Zarco-Tejada P, Fereres E.. 2016. Early detection and quantification of almond red leaf blotch using high-resolution hyperspectral and thermal imagery. Remote Sensing 8, 276. [Google Scholar]
- Loreti E, Poggi A, Novi G, Alpi A, Perata P.. 2005. A genome-wide analysis of the effects of sucrose on gene expression in Arabidopsis seedlings under anoxia. Plant Physiology 137, 1130–1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loreti E, van Veen H, Perata P.. 2016. Plant responses to flooding stress. Current Opinion in Plant Biology 33, 64–71. [DOI] [PubMed] [Google Scholar]
- Luan H, Guo B, Pan Y, Lv C, Shen H, Xu R.. 2018. Morpho-anatomical and physiological responses to waterlogging stress in different barley (Hordeum vulgare L.) genotypes. Plant Growth Regulation 85, 399–409. [Google Scholar]
- Mahmood U, Hussain S, Hussain S, Ali B, Ashraf U, Zamir S, Al-Robai SA, Alzahrani FO, Hano C, El-Esawi MA.. 2021. Morpho-physio-biochemical and molecular responses of maize hybrids to salinity and waterlogging during stress and recovery phase. Plants 10, 1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malik AI, Colmer TD, Lambers H, Schortemeyer M.. 2001. Changes in physiological and morphological traits of roots and shoots of wheat in response to different depths of waterlogging. Functional Plant Biology 28, 1121–1131. [Google Scholar]
- Malik AI, Colmer TD, Lambers H, Setter TL, Schortemeyer M.. 2002. Short-term waterlogging has long-term effects on the growth and physiology of wheat. New Phytologist 153, 225–236. [Google Scholar]
- Mancuso S, Shabala S. (eds). 2010. Waterlogging signalling and tolerance in plants. Berlin, Heidelberg: Springer. [Google Scholar]
- Manik SMN, Quamruzzaman M, Livermore M, Zhao C, Johnson P, Hunt I, Shabala S, Zhou M.. 2022a. Impacts of barley root cortical aerenchyma on growth, physiology, yield components, and grain quality under field waterlogging conditions. Field Crops Research 279, 108461. [Google Scholar]
- Manik SMN, Quamruzzaman M, Zhao C, Johnson P, Hunt I, Shabala S, Zhou M.. 2022b. Genome-wide association study reveals marker trait associations (MTA) for waterlogging-triggered adventitious roots and aerenchyma formation in barley. International Journal of Molecular Sciences 23, 3341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masoni A, Pampana S, Arduini I.. 2016. Barley response to waterlogging duration at tillering. Crop Science 56, 2722–2730. [Google Scholar]
- Masson-Delmotte V, Zhai P, Pirani A, et al. (eds). 2021. IPCC 2021: Climate change 2021: The physical science basis. Contribution of Working Group I to the sixth assessment report of the intergovernmental panel on climate change. Cambridge: Cambridge University Press. [Google Scholar]
- Meister R, Rajani MS, Ruzicka D, Schachtman DP.. 2014. Challenges of modifying root traits in crops for agriculture. Trends in Plant Science 19, 779–788. [DOI] [PubMed] [Google Scholar]
- Mendiondo GM, Gibbs DJ, Szurman-Zubrzycka M, et al. 2016. Enhanced waterlogging tolerance in barley by manipulation of expression of the N-end rule pathway E3 ligase PROTEOLYSIS6. Plant Biotechnology Journal 14, 40–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mergemann H, Sauter M.. 2000. Ethylene induces epidermal cell death at the site of adventitious root emergence in rice. Plant Physiology 124, 609–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miricescu A, Byrne T, Doorly CM, Ng CKY, Barth S, Graciet E.. 2021. Experimental comparison of two methods to study barley responses to partial submergence. Plant Methods 17, 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell TM. 1997. Machine learning. New York: McGraw-Hill. [Google Scholar]
- Mittler R. 2006. Abiotic stress, the field environment and stress combination. Trends in Plant Science 11, 15–19. [DOI] [PubMed] [Google Scholar]
- Mittra MK, Stickler FC.. 1961. Excess water effects on different crops. Transactions of the Kansas Academy of Science 64, 275–286. [Google Scholar]
- Nagasubramanian K, Singh A, Singh A, Sarkar S, Ganapathysubramanian B.. 2021. Plant phenotyping with limited annotation: doing more with less. Earth and Space Science Open Archive, doi: 10.1002/essoar.10508864.1 [Preprint]. [DOI] [Google Scholar]
- Nagel KA, Putz A, Gilmer F, et al. 2012. GROWSCREEN-Rhizo is a novel phenotyping robot enabling simultaneous measurements of root and shoot growth for plants grown in soil-filled rhizotrons. Functional Plant Biology 39, 891–904. [DOI] [PubMed] [Google Scholar]
- Nakano T, Suzuki K, Fujimura T, Shinshi H.. 2006. Genome-wide analysis of the ERF gene family in Arabidopsis and rice. Plant Physiology 140, 411–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negrão S, Julkowska MM.. 2020. Plant phenotyping. eLS, DOI: 10.1002/9780470015902.a0028894. [Google Scholar]
- Orta AH, Erdem Y, Erdem T.. 2003. Crop water stress index for watermelon. Scientia Horticulturae 98, 121–130. [Google Scholar]
- Ou LJ, Dai XZ, Zhang ZQ, Zou XX.. 2011. Responses of pepper to waterlogging stress. Photosynthetica 49, 339. [Google Scholar]
- Pan R, Jiang W, Wang Q, Xu L, Shabala S, Zhang WY.. 2019. Differential response of growth and photosynthesis in diverse cotton genotypes under hypoxia stress. Photosynthetica 57, 772–779. [Google Scholar]
- Pang J, Ross J, Zhou M, Mendham N, Shabala S.. 2007. Amelioration of detrimental effects of waterlogging by foliar nutrient sprays in barley. Functional Plant Biology 34, 221–227. [DOI] [PubMed] [Google Scholar]
- Pang J, Zhou M, Mendham N, Shabala S.. 2004. Growth and physiological responses of six barley genotypes to waterlogging and subsequent recovery. Australian Journal of Agricultural Research 55, 895–906. [Google Scholar]
- Parent C, Capelli N, Berger A, Crèvecoeur M, Dat J.. 2008. An overview of plant responses to soil waterlogging. Plant stress 2, 20–27. [Google Scholar]
- Pedersen O, Sauter M, Colmer TD, Nakazono M.. 2021. Regulation of root adaptive anatomical and morphological traits during low soil oxygen. New Phytologist 229, 42–49. [DOI] [PubMed] [Google Scholar]
- Perata P, Pozueta-Romero J, Akazawa T, Yamaguchi J.. 1992. Effect of anoxia on starch breakdown in rice and wheat seeds. Planta 188, 611–618. [DOI] [PubMed] [Google Scholar]
- Ploschuk RA, Miralles DJ, Colmer TD, Ploschuk EL, Striker GG.. 2018. Waterlogging of winter crops at early and late stages: Impacts on leaf physiology, growth and yield. Frontiers in Plant Science 9, 1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ploschuk RA, Miralles DJ, Colmer TD, Striker GG.. 2020. Waterlogging differentially affects yield and its components in wheat, barley, rapeseed and field pea depending on the timing of occurrence. Journal of Agronomy and Crop Science 206, 363–375. [Google Scholar]
- Ponnamperuma FN. 1972. The chemistry of submerged soils. Advances in Agronomy 24, 29–96. [Google Scholar]
- Posso DA, Borella J, Reissig GN, Bacarin MA.. 2018. Root flooding-induced changes in the dynamic dissipation of the photosynthetic energy of common bean plants. Acta Physiologiae Plantarum 40, 212. [Google Scholar]
- Qi X, Li Q, Ma X, et al. 2019. Waterlogging-induced adventitious root formation in cucumber is regulated by ethylene and auxin through reactive oxygen species signalling. Plant, Cell & Environment 42, 1458–1470. [DOI] [PubMed] [Google Scholar]
- Rasheed R, Iqbal M, Ashraf MA, Hussain I, Shafiq F, Yousaf A, Zaheer A.. 2018. Glycine betaine counteracts the inhibitory effects of waterlogging on growth, photosynthetic pigments, oxidative defence system, nutrient composition, and fruit quality in tomato. The Journal of Horticultural Science and Biotechnology 93, 385–391. [Google Scholar]
- Ren B, Hu J, Liu P, Zhao B, Zhang J.. 2021. Responses of nitrogen efficiency and antioxidant system of summer maize to waterlogging stress under different tillage. PeerJ 9, e11834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren B, Zhang J, Dong S, Liu P, Zhao B.. 2016. Effects of duration of waterlogging at different growth stages on grain growth of summer maize (Zea mays L.) under field conditions. Journal of Agronomy and Crop Science 202, 564–575. [Google Scholar]
- Rhine MD, Stevens G, Shannon G, Wrather A, Sleper D.. 2010. Yield and nutritional responses to waterlogging of soybean cultivars. Irrigation Science 28, 135–142. [Google Scholar]
- Roberts DA, Roth KL, Wetherley EB, Meerdink SK, Perroy RL.. 2018. Hyperspectral vegetation indices. In: Thenkabail PS, Lyon JG, Huete A, eds. Hyperspectral indices and image classifications for agriculture and vegetation. Boca Raton, FL: CRC Press, 3–26. [Google Scholar]
- Robertson D, Zhang H, Palta JA, Colmer T, Turner NC.. 2009. Waterlogging affects the growth, development of tillers, and yield of wheat through a severe, but transient, N deficiency. Crop and Pasture Science 60, 578–586. [Google Scholar]
- Rodriguez-Moreno F, Kren J, Zemek F, Novak J, Lukas V, Pikl M.. 2017. Advantage of multispectral imaging with sub-centimeter resolution in precision agriculture: generalization of training for supervised classification. Precision Agriculture 18, 615–634. [Google Scholar]
- Roitsch T, Cabrera-Bosquet L, Fournier A, Ghamkhar K, Jimenez-Berni J, Pinto F, Ober ES.. 2019. Review: New sensors and data-driven approaches—A path to next generation phenomics. Plant Science 282, 2–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rumanti I, Sitaresmi T, Nugraha Y.. 2020. Rice tolerance variation to long-term stagnant flooding and germination ability under an-aerobic environment. IOP Conference Series: Earth and Environmental Science 423, 012048. [Google Scholar]
- Sagi M, Fluhr R.. 2006. Production of reactive oxygen species by plant NADPH oxidases. Plant Physiology 141, 336–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sairam RK, Dharmar K, Lekshmy S, Chinnusamy V.. 2011. Expression of antioxidant defense genes in mung bean (Vigna radiata L.) roots under water-logging is associated with hypoxia tolerance. Acta Physiologiae Plantarum 33, 735–744. [Google Scholar]
- Sankaran S, Quirós JJ, Miklas PN.. 2019. Unmanned aerial system and satellite-based high resolution imagery for high-throughput phenotyping in dry bean. Computers and Electronics in Agriculture 165, 104965. [Google Scholar]
- Sasidharan R, Bailey-Serres J, Ashikari M, Atwell BJ, Colmer TD, Fagerstedt K, Fukao T, Geigenberger P, Hebelstrup KH, Hill RD.. 2017. Community recommendations on terminology and procedures used in flooding and low oxygen stress research. New Phytologist 214, 1403–1407. [DOI] [PubMed] [Google Scholar]
- Sauter M. 2013. Root responses to flooding. Currrent Opinion in Plant Biology 16, 282–286. [DOI] [PubMed] [Google Scholar]
- Schopfer P. 2001. Hydroxyl radical-induced cell-wall loosening in vitro and in vivo: implications for the control of elongation growth. The Plant Journal 28, 679–688. [DOI] [PubMed] [Google Scholar]
- Setter TL, Waters I, Sharma SK, et al. 2009. Review of wheat improvement for waterlogging tolerance in Australia and India: the importance of anaerobiosis and element toxicities associated with different soils. Annals of Botany 103, 221–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma SK, Kulshreshtha N, Kumar A, Yaduvanshi NPS, Singh M, Prasad KRK, Basak N.. 2018. Waterlogging effects on elemental composition of wheat genotypes in sodic soils. Journal of Plant Nutrition 41, 1252–1262. [Google Scholar]
- Singh A, Ganapathysubramanian B, Singh AK, Sarkar S.. 2016. Machine learning for high-throughput stress phenotyping in plants. Trends in Plant Science 21, 110–124. [DOI] [PubMed] [Google Scholar]
- Singh G, Setter TL, Singh MK, Kulshreshtha N, Singh BN, Stefanova K, Tyagi BS, Singh JB, Kherawat BS, Barrett-Lennard EG.. 2018. Number of tillers in wheat is an easily measurable index of genotype tolerance to saline waterlogged soils: evidence from 10 large-scale field trials in India. Crop and Pasture Science 69, 561–573. [Google Scholar]
- Smethurst CF, Garnett T, Shabala S.. 2005. Nutritional and chlorophyll fluorescence responses of lucerne (Medicago sativa) to waterlogging and subsequent recovery. Plant and Soil 270, 31–45. [Google Scholar]
- Smethurst CF, Shabala S.. 2003. Screening methods for waterlogging tolerance in lucerne: comparative analysis of waterlogging effects on chlorophyll fluorescence, photosynthesis, biomass and chlorophyll content. Functional Plant Biology 30, 335–343. [DOI] [PubMed] [Google Scholar]
- Steffens B, Rasmussen A.. 2016. The physiology of adventitious roots. Plant Physiology 170, 603–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffens B, Sauter M.. 2009. Epidermal cell death in rice is confined to cells with a distinct molecular identity and is mediated by ethylene and H2O2 through an autoamplified signal pathway. The Plant Cell 21, 184–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffens D, Hutsch B, Eschholz T, Losak T, Schubert S.. 2005. Water logging may inhibit plant growth primarily by nutrient deficiency rather than nutrient toxicity. Plant Soil and Environment 51, 545–552. [Google Scholar]
- Striker GG. 2008. Visiting the methodological aspects of flooding experiments: Quantitative evidence from agricultural and ecophysiological studies. Journal of Agronomy and Crop Science 194, 249–255. [Google Scholar]
- Striker GG. 2012. Time is on our side: the importance of considering a recovery period when assessing flooding tolerance in plants. Ecological Research 27, 983–987. [Google Scholar]
- Sundgren T, Uhlen A, Waalen W, Lillemo M.. 2018. Field screening of waterlogging tolerance in spring wheat and spring barley. Agronomy 8, 38. [Google Scholar]
- Tamang BG, Magliozzi JO, Maroof MA, Fukao T.. 2014. Physiological and transcriptomic characterization of submergence and reoxygenation responses in soybean seedlings. Plant, Cell & Environment 37, 2350–2365. [DOI] [PubMed] [Google Scholar]
- Teramoto S, Takayasu S, Kitomi Y, Arai-Sanoh Y, Tanabata T, Uga Y.. 2020. High-throughput three-dimensional visualization of root system architecture of rice using X-ray computed tomography. Plant Methods 16, 66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teramoto S, Uga Y.. 2020. A deep learning-based phenotypic analysis of rice root distribution from field images. Plant Phenomics 2020, 3194308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson C, Colmer T, Watkin E, Greenway H.. 1992. Tolerance of wheat (Triticum aestivum cvs Gamenya and Kite) and triticale (Triticosecale cv. Muir) to waterlogging. New Phytologist 120, 335–344. [Google Scholar]
- Tian L, Bi W, Ren X, Li W, Sun L, Li J.. 2020. Flooding has more adverse effects on the stem structure and yield of spring maize (Zea mays L.) than waterlogging in Northeast China. European Journal of Agronomy 117, 126054. [Google Scholar]
- Tian L, Zhang Y, Chen P, Zhang F, Li J, Yan F, Dong Y, Feng B.. 2021. How does the waterlogging regime affect crop yield? A global meta-analysis. Frontiers in Plant Science 12, 634898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong C, Hill CB, Zhou G, Zhang X-Q, Jia Y, Li C.. 2021. Opportunities for improving waterlogging tolerance in cereal crops—physiological traits and genetic mechanisms. Plants 10, 1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topp CN, Iyer-Pascuzzi AS, Anderson JT, et al. 2013. 3D phenotyping and quantitative trait locus mapping identify core regions of the rice genome controlling root architecture. Proceedings of the National Academy of Sciences, USA 110, E1695–E1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tovar JC, Hoyer JS, Lin A, et al. 2018. Raspberry Pi-powered imaging for plant phenotyping. Applications in Plant Sciences 6, e1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trought M, Drew M.. 1982. Effects of waterlogging on young wheat plants (Triticum aestivum L.) and on soil solutes at different soil temperatures. Plant and Soil 69, 311–326. [Google Scholar]
- Trought MCT, Drew MC.. 1980. The development of waterlogging damage in wheat seedlings (Triticum aestivum L.). Plant and Soil 54, 77–94. [Google Scholar]
- VanToai TT, St. Martin SK, Chase K, Boru G, Schnipke V, Schmitthenner AF, Lark KG.. 2001. Identification of a QTL associated with tolerance of soybean to soil waterlogging. Crop Science 41, 1247–1252. [Google Scholar]
- Vidoz ML, Loreti E, Mensuali A, Alpi A, Perata P.. 2010. Hormonal interplay during adventitious root formation in flooded tomato plants. The Plant Journal 63, 551–562. [DOI] [PubMed] [Google Scholar]
- Voesenek LACJ, Sasidharan R.. 2013. Ethylene – and oxygen signalling – drive plant survival during flooding. Plant Biology 15, 426–435. [DOI] [PubMed] [Google Scholar]
- Wang H, Chen Y, Xu B, et al. 2018. Long-term exposure to slightly elevated air temperature alleviates the negative impacts of short term waterlogging stress by altering nitrogen metabolism in cotton leaves. Plant Physiology and Biochemistry 123, 242–251. [DOI] [PubMed] [Google Scholar]
- Wang X, Deng Z, Zhang W, Meng Z, Chang X, Lv M.. 2017. Effect of waterlogging duration at different growth stages on the growth, yield and quality of cotton. PLoS One 12, e0169029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasson AP, Nagel KA, Tracy S, Watt M.. 2020. Beyond digging: noninvasive root and rhizosphere phenotyping. Trends in Plant Science 25, 119–120. [DOI] [PubMed] [Google Scholar]
- Watanabe K, Takahashi H, Sato S, Nishiuchi S, Omori F, Malik AI, Colmer TD, Mano Y, Nakazono M.. 2017. A major locus involved in the formation of the radial oxygen loss barrier in adventitious roots of teosinte Zea nicaraguensis is located on the short-arm of chromosome 3. Plant, Cell & Environment 40, 304–316. [DOI] [PubMed] [Google Scholar]
- Watson E, Lapins P, Barron R.. 1976. Effect of waterlogging on the growth, grain and straw yield of wheat, barley and oats. Australian Journal of Experimental Agriculture 16, 114–122. [Google Scholar]
- Wilkinson MD, Dumontier M, Aalbersberg IJ, et al. 2016. The FAIR guiding principles for scientific data management and stewardship. Scientific Data 3, 160018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, Tang Y, Li C, McHugh AD, Li Z, Wu C.. 2018. Individual and combined effects of soil waterlogging and compaction on physiological characteristics of wheat in southwestern China. Field Crops Research 215, 163–172. [Google Scholar]
- Wu X, Tang Y, Li C, Wu C, Huang G.. 2015. Chlorophyll fluorescence and yield responses of winter wheat to waterlogging at different growth stages. Plant Production Science 18, 284–294. [Google Scholar]
- Xia JA, Cao H, Yang Y, Zhang W, Wan Q, Xu L, Ge D, Zhang W, Ke Y, Huang B.. 2019. Detection of waterlogging stress based on hyperspectral images of oilseed rape leaves (Brassica napus L.). Computers and Electronics in Agriculture 159, 59–68. [Google Scholar]
- Yamauchi T, Colmer TD, Pedersen O, Nakazono M.. 2018. Regulation of root traits for internal aeration and tolerance to soil waterlogging-flooding stress. Plant Physiology 176, 1118–1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang F, Liu T, Wang Q-y, Du M-z, Yang T-l, Liu D-z, Li S-j, Liu S-p.. 2021. Rapid determination of leaf water content for monitoring waterlogging in winter wheat based on hyperspectral parameters. Journal of Integrative Agriculture 20, 2613–2626. [Google Scholar]
- Yeboah MA, Xuehao C, Guohua L, Minghong G, Chenwu X.. 2008. Inheritance of waterlogging tolerance in cucumber (Cucumis sativus L.). Euphytica 162, 145–154. [Google Scholar]
- Ylivainio K, Jauhiainen L, Uusitalo R, Turtola E.. 2018. Waterlogging severely retards P use efficiency of spring barley (Hordeum vulgare). Journal of Agronomy and Crop Science 204, 74–85. [Google Scholar]
- Zambrosi FCB, Ribeiro RV, Marchiori PER, Cantarella H, Landell MGA.. 2014. Sugarcane performance under phosphorus deficiency: physiological responses and genotypic variation. Plant and Soil 386, 273–283. [Google Scholar]
- Zeng F, Shabala L, Zhou M, Zhang G, Shabala S.. 2013. Barley responses to combined waterlogging and salinity stress: separating effects of oxygen deprivation and elemental toxicity. Frontiers in Plant Science 4, 313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F, Zhu K, Wang YQ, Zhang ZP, Lu F, Yu HQ, Zou JQ.. 2019. Changes in photosynthetic and chlorophyll fluorescence characteristics of sorghum under drought and waterlogging stress. Photosynthetica 57, 1156–1164. [Google Scholar]
- Zhang X, Fan Y, Shabala S, Koutoulis A, Shabala L, Johnson P, Hu H, Zhou M.. 2017. A new major-effect QTL for waterlogging tolerance in wild barley (H. spontaneum). Theoretical and Applied Genetics 130, 1559–1568. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Chen Y, Lu H, Kong X, Dai J, Li Z, Dong H.. 2016. Growth, lint yield and changes in physiological attributes of cotton under temporal waterlogging. Field Crops Research 194, 83–93. [Google Scholar]
- Zhou J, Mou H, Zhou J, Ali ML, Ye H, Chen P, Nguyen HT.. 2021. Qualification of soybean responses to flooding stress using UAV-based imagery and deep learning. Plant Phenomics 2021, 9892570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou M. 2010. Improvement of plant waterlogging tolerance. In: Mancuso S, Shabala S (eds). Waterlogging signalling and tolerance in plants. Berlin, Heidelberg: Springer, 267–285. [Google Scholar]
- Zhou M. 2011. Accurate phenotyping reveals better QTL for waterlogging tolerance in barley. Plant Breeding 130, 203–208. [Google Scholar]
- Zhou W, Chen F, Meng Y, Chandrasekaran U, Luo X, Yang W, Shu K.. 2020. Plant waterlogging/flooding stress responses: From seed germination to maturation. Plant Physiology and Biochemistry 148, 228–236. [DOI] [PubMed] [Google Scholar]
- Zhou W, Lin X.. 1995. Effects of waterlogging at different growth stages on physiological characteristics and seed yield of winter rape (Brassica napus L.). Field Crops Research 44, 103–110. [Google Scholar]
- Zhou W, Zhao D, Lin X.. 1997. Effects of waterlogging on nitrogen accumulation and alleviation of waterlogging damage by application of nitrogen fertilizer and mixtalol in winter rape (Brassica napus L.). Journal of Plant Growth Regulation 16, 47–53. [Google Scholar]
- Zou X, Hu C, Zeng L, Cheng Y, Xu M, Zhang X.. 2014. A comparison of screening methods to identify waterlogging tolerance in the field in Brassica napus L. during plant ontogeny. PLoS One 9, e89731. [DOI] [PMC free article] [PubMed] [Google Scholar]