Abstract
Our ability to predict how species will respond to human-induced rapid environmental change (HIREC) may depend upon our understanding of transgenerational plasticity (TGP), which occurs when environments experienced by previous generations influence phenotypes of subsequent generations. TGP evolved to help organisms cope with environmental stressors when parental environments are highly predictive of offspring environments. HIREC can alter conditions that favored TGP in historical environments by reducing parents’ ability to detect environmental conditions, disrupting previous correlations between parental and offspring environments, and interfering with the transmission of parental cues to offspring. Because of the propensity to produce errors in these processes, TGP will likely generate negative fitness outcomes in response to HIREC, though beneficial fitness outcomes may occur in some cases.
Considering Transgenerational Plasticity in the Context of Human-Induced Rapid Environmental Change
Humans are profoundly affecting the global abundance and distribution of organisms by facilitating habitat loss and fragmentation [1], introducing exotic species [2], overharvesting wild populations [3], increasing pollutant exposure [4], and altering the global climate [5]. While some species (e.g., invasive species, commensal pests) have been successful [6] under human-induced rapid environmental change (HIREC) (see Glossary) [7], other species exhibit maladaptive responses that contribute to declines and even increased extinction risk [8]. This may be because organisms lack the ability to effectively detect or respond to novel environments [9] or because cue-response systems that were beneficial in past conditions become detrimental under HIREC [10,11]. To date, the vast majority of theoretical and empirical work on plastic responses to HIREC has focused on within-generational plasticity (WGP) (e.g., [12,13]). However, organisms can also convey environmental information across generations via transgenerational plasticity (TGP), which evolved as another mechanism to help organisms cope with changing environments [14]. HIREC is expected to alter the historical conditions under which TGP evolved by changing how well environmental cues indicate environmental conditions (cue reliability), the magnitude or rate of environmental variation across time or space (environmental variability), or the ways in which current environmental conditions predict future conditions (e.g., temporal or spatial autocorrelation). While recent reviews have explored related topics on TGP and climate change (e.g., [15]), and models predict that variation in trait transmission from parents to offspring can affect species persistence in human-modified landscapes [16], we lack a conceptual framework that formulates general hypotheses about how TGP will affect organismal fitness in response to a broad range of potential forms of HIREC.
TGP occurs when the environment experienced by one generation influences behavioral, physiological, morphological, or life-history traits in future generations, sometimes in ways that increase fitness (reviewed in [17–19]). TGP can occur via either the mother or the father (i.e., maternal or paternal effects) and persist for multiple generations (e.g., grandparental effects), although here we concentrate on the relationship between parents and their offspring. TGP can operate in response to short-lived or long-lasting experiences that occur at any point in a parent’s lifetime [20] (Box 1). TGP can operate via mechanisms such as epigenetic marks in gametes, sperm miRNAs, hormones in ovo or in utero, microbiota, parental care, or parental habitat selection or niche construction [21–24], and be transmitted to offspring at different stages of offspring development (Box 2). Here, we focus on TGP that has evolved primarily to transmit information to offspring rather than state-based TGP, in which parental state (e.g., injury) alters offspring phenotypes. While some state-based TGP (e.g., body condition) also provides information to offspring about their potential environment, HIREC is expected to specifically alter the conditions under which information-based TGP is likely to evolve; namely, environments with strong temporal autocorrelation in which parental environments reliably predict offspring environments [25–28]. TGP may not evolve in systems that lack this predictability [29,30].
Box 1. The Importance of the Timing and Duration of Parent’s Exposure to HIREC.
We generally hypothesize that HIREC that occurs early in the parent’s lifetime may have outsized impacts on parental fitness, and therefore offspring fitness, compared with HIREC experienced later in life [67]. This could be because: (i) plasticity is often highest early in development [43]; (ii) certain TGP mechanisms (e.g., methylation, histone modifications) can only be established early in development [68]; or (iii) changes in parental state induced in early development can compound across parents’ lifetimes. In cichlids, for example, maternal food availability early, but not late, in life impacted maternal investment in offspring [69]. Further, HIREC encountered by parents early in life can alter key life history decisions (e.g., dispersal or habitat choice) that may have stronger effects on offspring than conditions encountered after those life history choices have been made. Finally, parents may be more likely to transmit accurate information about environmental shifts if HIREC occurs before parents’ sensitive windows have closed because parents are sampling and integrating information about the new environmental conditions during those critical periods.
HIREC that occurs just before parents reproduce is also expected to affect offspring via TGP. First, if a high degree of temporal autocorrelation existed historically (e.g., snow thaw is followed by increased food availability), past selection should have favored parents that transmit cues received close to reproduction to their offspring; this sensitivity may be retained in response to HIREC [70]. Second, HIREC that occurs close to reproduction may trigger certain state-based TGP mechanisms, such as cellular damage or physiological or neurogenomic changes, that alter offspring phenotypes [20]. This has been shown in male flour beetles: fathers that experienced a brief heat wave immediately before reproduction produced less fit offspring, likely because of reductions in sperm viability [71].
When HIREC occurs repeatedly and/or continuously (e.g., warming) rather than a single event (e.g., hurricane), earlier parental exposure is likely correlated with longer exposure time. Longer exposure time should reduce parental uncertainty about the environment [72] by better allowing parents to assess changes in temporal autocorrelation or variability or to assess if novel conditions are relevant or dangerous to offspring. Repeated exposure to HIREC may potentially reduce the negative impacts of HIREC on offspring; for example, in sea urchins, short-term parental exposure to low pH induces fitness costs in offspring, but this is not true when parents are exposed to low pH earlier and for longer time periods [73].
Box 2. Common TGP Mechanisms, Their Timing of Delivery from Parents to Offspring, and Their Potential to Affect Offspring Phenotypes.
Parents convey information to their offspring through a variety of potential mechanisms (Figure I). The effects of TGP on offspring phenotypes will also depend on the point in development when offspring receive parental cues: (i) fertilization (formed in parents prior to offspring fertilization); (ii) during development or gestation; and (iii) post-emergence during the parental care period. Pre-fertilization mechanisms apply to most sexually reproducing organisms, developmental/gestational mechanisms only apply to organisms where parents have some influence over the environment of the developing offspring (e.g., live bearers, nesting birds), and post-emergence mechanisms only apply to organisms that provide parental care. In some species, early cues transmitted via one mechanism (e.g., gametes) can be confirmed or refuted by cues received later in development (e.g., parental care [74]) or from the offspring’s own experience (WGP [75]). If HIREC causes parents to misidentify environmental cues, TGP can set offspring on incorrect developmental trajectories that are not easily readjusted by WGP or later parental cues. A striking example involves parental choice of offspring habitat that offspring cannot leave until they mature (e.g., oviposition site choice by amphibians or aquatic insects).
We predict that parental cues received earliest in development will have the greatest influence on offspring fitness (positive or negative). First, the earlier a phenotype begins to develop, the greater the change in affected traits and the harder it is to reverse those changes later in life (epiphenotype problem [76]). Second, earlier cues are more likely to be received before potential sensitive windows of development have closed, increasing the likelihood that affected traits are still plastic. Third, parental cues received early in development may be difficult to alter via WGP; in contrast, during the post-emergence parental care period, offspring can simultaneously integrate TGP and WGP. Life history will also alter offspring’s ability to update parental cues with WGP; offspring that develop outside of parents’ bodies can begin sampling their own environment earlier in development.
We also predict that early cues may be least predictive of offspring environments because of the time lag between when parental cues are delivered to offspring and when offspring will encounter the predicted environment. Further, some pre-fertilization mechanisms of TGP are a result of parents’ early life decisions or experiences (e.g., parental state, habitat choice) that may no longer be reflective of the current environment; this is particularly likely to occur for long-lived organisms relative to short-lived organisms.
Figure I. Potential Mechanisms of Transgenerational Plasticity.
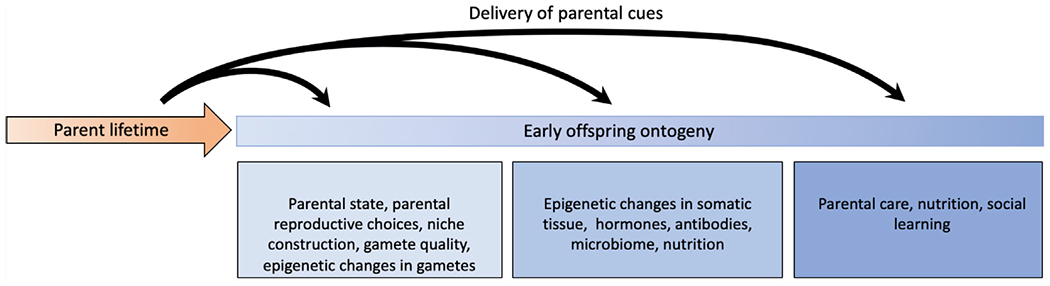
Transgenerational plasticity can operate through a variety of mechanisms listed above that affect offspring at various stages of ontogeny, including at fertilization, during development/gestation, and after emergence. While some mechanisms are specifically linked to one stage of offspring development (e.g., epigenetic changes in gametes conveyed at fertilization), other mechanisms (e.g., parental state, niche construction) are relevant for offspring at multiple points in development. Regardless of when parental cues are delivered to offspring, many transgenerational plasticity mechanisms have lifelong effects on offspring.
TGP differs from WGP in several key aspects that may influence how organisms respond to HIREC. First, with TGP, parents must have the sensory/cognitive ability to correctly detect and identify environmental conditions, possess a mechanism to transmit this information to offspring (e.g., methylation), and offspring must possess a mechanism to integrate these parental cues during development (e.g., epigenetic marks escaping erasure at fertilization [17]; see [31] for a recent review of TGP mechanisms). In contrast, with WGP, individuals detect environmental conditions and integrate that information into estimates of their environment, which then triggers biochemical, hormonal, or neurological responses that elicit phenotypic change. The multiple steps of information transfer between generations increase the scope for error with TGP relative to WGP. Further, because information obtained from parents is likely to be less current than information obtained from an individual’s own experience, there is greater potential for phenotypic/environmental mismatches with TGP [25,26]. Importantly, TGP does not necessarily require active detection or cognition on the part of parents; TGP might occur because offspring phenotypes are highly correlated with the phenotypes of past generations (e.g., egg size [32]; cascading maternal effects [28,33]). In these cases, HIREC may produce phenotypic/environmental mismatches by altering the selection regime favoring such parent–offspring correlations.
If parents can transmit reliable information to offspring about their potential environment, TGP may have benefits beyond what offspring can achieve with WGP alone [28,30,34], particularly when: (i) it is risky for offspring to sample their environment; (ii) offspring are unable to sample their future environments (e.g., organisms that undergo ontogenetic niche shifts); (iii) it takes a substantial amount of time for offspring to generate a plastic response via WGP alone; or (iv) selective pressures are highest early in life. These scenarios might favor parental priming via TGP [17] and can enhance offspring’s sensitivity to relevant environmental conditions [35,36]. For example, in Caenorhabditis elegans, learned avoidance behavior of pathogens in the parental generation can be transmitted for up to four generations via epigenetic changes in sensory neurons and small RNA pathways [37]. This priming can be especially important in response to HIREC, where many of the selective pressures are amplified (e.g., increased drought), such that even a small advantage via TGP is important for survival. Finally, TGP offers greater possibilities for diversified bet hedging (DBH) if, for example, parental experiences do not reliably predict future conditions. With bet hedging, parents increase the phenotypic variance of their offspring, thus potentially increasing geometric mean offspring fitness and population persistence under HIREC [38] (Box 3).
Box 3. Bet Hedging as Another Means for TGP: Will It Fare Better in Response to HIREC?
When future environmental conditions are unpredictable, it may be adaptive for parents to increase phenotypic variation among offspring of the same genotype, that is, express diversified bet hedging (DBH) [77,78], to ensure that at least a portion of offspring will have phenotypes that match the current environment [27,79]. DBH is only a form of TGP if a parent’s experience alters the amount of phenotypic variation in offspring. There is growing evidence that DBH occurs in both plants [80] and animals [38]; for example, three-spine stickleback mothers increase intraclutch egg size variability when temperatures are highly variable [81].
DBH may outperform other forms of TGP in response to HIREC. Because DBH alters the variance in offspring phenotypes rather than the mean offspring phenotype [77,78], DBH may be less sensitive to reductions in autocorrelation or increases in environmental variability caused by HIREC because parents using DBH do not have to predict the precise environmental conditions that their offspring will encounter. How well populations respond to HIREC may therefore depend more on variation in offspring phenotypes than on mean phenotypes [15], particularly when uncertainty is high (e.g., parents have low information about the potential mean of offsprings’ environment), directional plasticity is ineffective because offspring must make decisions before reliable information is available, or when uncertainty in past environments has favored DBH. For example, if DBH was effective in historical environments because of existing variation in the relationship between environmental conditions (e.g., how well temperature and photoperiod correlate), we expect that it will be a key component of organisms’ response to HIREC.
DBH will only be adaptive in response to HIREC if parents adjust offspring traits in ways that affect offspring fitness. For example, increasing variance in egg size will only be adaptive if egg size is linked to differences in offspring fitness in the altered environment. Since DBH does not alter the mean of offspring phenotypes, it is, on its own, unlikely to allow populations to adaptively respond to sustained, directional environmental change. DBH is also unlikely to help populations persist under HIREC unless past and future environmental conditions favor DBH [82], but the existence of DBH might reduce the negative effects of HIREC within a population and provide time for other types of responses (e.g., plasticity and selection) to arise.
How HIREC Alters Environments in Ways That May Influence the Benefits of TGP
TGP is likely to be beneficial if: (i) parents can detect and identify current environmental conditions, (ii) parental environments accurately predict offspring environments, and (iii) parents can accurately transmit information to offspring so that it can be integrated into offspring phenotypes [17]. Here, we outline a framework that highlights how HIREC is likely to produce errors in one or more of these processes if HIREC produces a mismatch between current environmental conditions and historical conditions that made TGP adaptive in the past (Figure 1). Namely, if HIREC produces conditions that increase temporal/spatial environmental variability relative to historic environments, decrease temporal/spatial autocorrelation, or creates conditions where previously reliable environmental cues become unreliable or in which environmental variability and autocorrelation are unknown, there is high potential that TGP may produce detrimental phenotypes in offspring. If, however, the conditions that favored the evolution of TGP are maintained after HIREC, TGP has the potential to substantially enhance offspring adaptive responses to HIREC. While offspring are not necessarily passive recipients of parental information (Box 2), we focus on parents because they directly experience environmental conditions produced by human activities.
Figure 1. Overview of the Key Determinants of the Transgenerational Consequences of Human-Induced Rapid Environmental Change (HIREC) Using a Hypothetical Example.
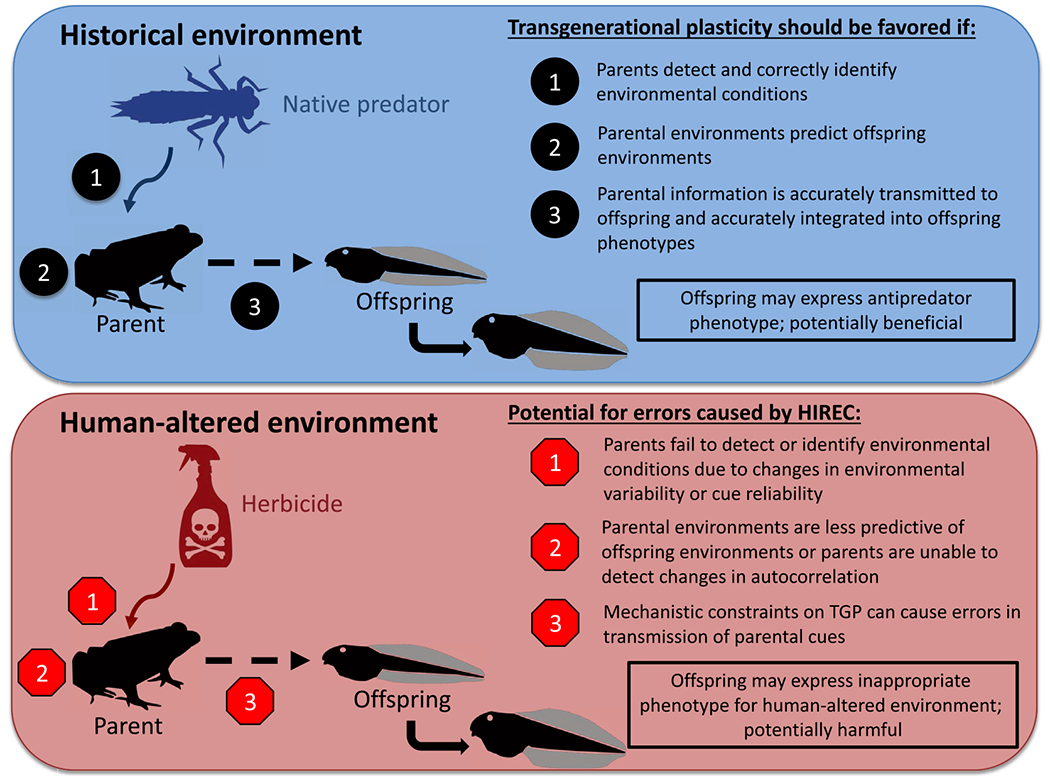
Transgenerational plasticity (TGP) is a process by which offspring phenotypes are altered by environments experienced by previous generations (e.g., parents). Parental experiences can be conveyed to offspring through a variety of potential mechanisms, but TGP involves three general processes (top panel). In historical environments, TGP is more likely to be favored when these processes occur with minimal error. This is likely if: (i) parents possess the sensory/biochemical systems to accurately detect and identify environmental conditions and cue reliability is high, (ii) temporal/spatial environmental variability is low or similar to historic conditions and/or temporal/spatial autocorrelation is high, and (iii) parents can accurately transmit information about their environment to offspring and offspring can accurately integrate that information into their phenotype. Human-altered environments (bottom panel) increase the potential for errors in each of these processes. This may be due to the introduction of novel environmental conditions (which may reduce cue reliability), increases in environmental variability, or decreases in environmental autocorrelation relative to historic environments. HIREC may also increase the likelihood of mismatches between offspring phenotypes and human-altered environments and lead to detrimental effects of TGP. Visual example in both panels adapted from [66], who found that direct exposure to predator risk cues from dragonfly larvae cause tadpole prey to develop an antipredator phenotype (deeper tails) and that exposure to herbicides can elicit this same phenotypic response. We extend this WGP example to suggest possible errors in the process of TGP. Images by M. Bensky.
Can Parents Detect and Identify Environmental Conditions Produced by HIREC?
Parents may be especially likely to detect and correctly identify environmental conditions produced by HIREC when it alters mean conditions by intensifying (e.g., increased temperatures associated with global warming [8]) or weakening (e.g., reduced nutrient limitation via anthropogenic nutrient inputs [39]) the same environmental stressors or conditions that were present in historic conditions. Because there should be minimal effects on cue reliability, environmental variability, or autocorrelation, parents can continue to use historically existing cues to identify such HIREC-induced changes in mean conditions. Parents may be particularly likely to detect mean changes if the absolute change is large (e.g., extreme drought) or quick (e.g., sudden habitat loss) because the cue is strong and detection error is less likely [40].
In contrast, it may be more difficult for parents to detect and correctly identify environmental conditions if HIREC introduces novel conditions that lack historical context (e.g., anthropogenic noise) or increases environmental variability of historically existing conditions. In the case of novel conditions, parents may fail to respond because they lack appropriate cue-response systems; for example, novel olfactory cues emitted by invasive predators may underlie the failure of native prey to respond appropriately to invasive predators [41]. When HIREC increases environmental variability relative to historic environments, parents may fail to detect HIREC-induced environmental conditions or fully incorporate environmental changes into their phenotype because conditions occur outside of sensitive windows when parents are most responsive to environmental stimuli. For example, humans have a sensitive window of development in middle childhood; environmental conditions (e.g., food availability) experienced during this period, but not after, alter the phenotypes of their grandchildren [42]. However, HIREC may lengthen parental exposure to certain environmental conditions (e.g., longer growing season) or lengthen parents’ sensitive windows (by increasing environmental variability [43]) such that relevant environmental conditions still overlap with parents’ sensitive windows.
Increased environmental variability caused by HIREC may also result in disruptions in relationships between historically related conditions (e.g., temperature and day length) or the decoupling of key phenological shifts [44] such that previously reliable environmental cues become unreliable indicators of current conditions. These may be difficult for parents to detect if organisms evolved to rely heavily on one of many correlated environmental cues during a certain time period (e.g., temperature as a proxy for seasonality). This may result in mistimed parental reproduction, which may have strong effects on offspring survival or alter key life history shifts in offspring themselves [45,46]. For example, the decoupling of winter freezing events and warming spring temperatures produced by HIREC caused the timing of winter moth reproduction to be out of sync with peak food offspring availability, which may negatively affect the survival of the newly hatched winter moth caterpillars [44].
Do Parental Environments Accurately Predict Offspring Environments?
Strong temporal and/or spatial autocorrelation between parental and offspring environments is critical for selection to favor TGP in historical environments [30]. The likelihood that parental environments remain predictive of offspring environments after HIREC depends on the extent to which environmental autocorrelation that was present before HIREC is maintained after HIREC. When HIREC increases or decreases the mean of an environmental condition relative to historical conditions without affecting autocorrelation or variability, parental environments should remain similarly predictive of offspring environments compared with historical conditions. Indeed, there are numerous examples of TGP generating seemingly adaptive offspring phenotypes in response to mean environmental changes (e.g., reductions in mean ocean pH in marine fishes [47], reductions in mean salinity due to increased freshwater inputs in a wetland perennial [48]).
If HIREC creates more variable environmental conditions relative to historic conditions or produces novel conditions, temporal and/or spatial autocorrelation between parental and offspring environments may be reduced relative to historic conditions. If parents fail to detect this change in autocorrelation, parents may convey information to offspring that is no longer relevant in human-altered environments. However, repeated exposure to HIREC-induced environmental conditions may allow parents to learn novel patterns of environmental variability. Parents’ ability to repeatedly sample the environment is a potential advantage of TGP compared with WGP, where offspring have a more limited sampling window (Box 1). Importantly, HIREC may also create environments that are more homogeneous in both space and time (e.g., increased spatial and temporal autocorrelation in temperature caused by climate change [49]), thereby increasing the predictive power of parental experiences for offspring.
Finally, the effects of changes in autocorrelation between parental and offspring environments should further depend on organisms’ life history. For example, HIREC may be especially disruptive to short-lived organisms because historically, temporal autocorrelation between parental and offspring environments should be strongest in organisms that have short generation times [30].
Can Parental Information Be Integrated into Offspring Phenotypes?
Even if parents successfully detect and identify environmental conditions produced by HIREC, offspring phenotypes may also be influenced by constraints on existing mechanisms of TGP. HIREC-induced environmental conditions may activate existing TGP pathways that would have produced a response that was appropriate in historical environments, but is inappropriate under HIREC. For example, organisms that evolved to live in high predation environments might have pathways by which stress caused by predation can influence sperm, such as circulating cortisol that binds to receptors in the epididymis and causes the release of extracellular vesicles that transmit small RNAs to sperm [50]. This same pathway may be erroneously activated in response to another HIREC-induced environmental stressor, thereby causing offspring to inappropriately express an antipredator phenotype. For example, insecticides can inappropriately induce an antipredator defense (helmets) in Daphnia [51]; these effects in the parental generation may persist into future generations. In certain cases, however, activation of a general stress pathway by parents may increase offspring fitness by serving as an indicator of poor environmental quality and inducing traits (e.g., dispersal) that can improve offspring fitness across a range of stressful environments. For example, maternal exposure to warmer temperatures increases offspring tolerance of a toxic macroalga in zooplankton [52]. TGP may therefore be adaptive if parental and offspring environments are both stressful, even if they are stressful for different reasons.
Assuming that organisms can overcome potential mechanistic constraints, the timing of when parents encounter HIREC may be a key determinant of its effects on offspring phenotypes. Specifically, parental sensitivity to environmental conditions may change across their lifetime, and different TGP mechanisms may be capable of being activated at different parental life stages such that the accuracy and likelihood of parental transmission of information to offspring can change over parents’ lifetime and influence offspring responses to HIREC (Box 1).
Overall Fitness Consequences of HIREC-Induced TGP
HIREC will likely reduce parents’ ability to detect and assess their own environment, alter historical relationships in the degree of autocorrelation between parental and offspring environments, and limit the accuracy of information transmission and reception between parents and offspring. Because errors can occur at each stage, we argue that TGP is especially prone to errors compared with WGP, which may have severe consequences for offspring fitness in human-altered environments.
If parents fail to detect or correctly identify an environmental condition produced by HIREC or if parental environments are no longer predictive of offspring environments, TGP may result in false positives [46], when parents classify a benign condition as stressful (e.g., ecotourism). TGP could also result in false negatives if parents do not recognize the new environmental condition or cue (offspring have a false cue of safety, e.g., novel predator [53]), if HIREC masks stimuli from the environment (e.g., toxins [54]), or if HIREC degrades parents’ ability to personally detect or transmit cues to offspring. For example, zebra finch parents acoustically signal their developing offspring about potential temperature conditions [55] and increased anthropogenic noise (e.g., road noise) may obscure these signals. False negatives and positives may also arise if parents incorrectly ‘teach’ offspring about the value of novel resources or habitats, causing offspring to over- or under-value novel resources or habitats and potentially increase the frequency of ecological/evolutionary traps [56,57]. False negatives and positives may be particularly costly for offspring in the context of TGP because parental information can change offspring phenotypes in potentially irreversible ways, such as inducing dispersal [58] or morphological change (e.g., winged aphid morphs [59]). False negatives and positives may also result in offspring with strong, but incorrect, estimates of the environment that will impede their ability to respond via WGP later in life. For example, if parents signal to offspring that something is dangerous, offspring may avoid that experience and thus never learn that it is harmless.
If conditions that favored TGP before HIREC remain in place after HIREC, parental information may increase the likelihood of offspring success [15,18]. This can arise through a variety of mechanisms, such as reprogramming of offspring stress responses [20], changes in parental care or oviposition site [60], or plasticity in reproductive timing [45]. Adaptive responses to HIREC-induced environmental conditions can also arise if an initial bias is refined through repeated exposure to an evolutionarily novel condition and persist across generations via epigenetic changes (e.g., methylation, chromatin structure) [61]. For example, in rice, direct exposure to heavy metals causes methylation and upregulation of genes involved in the uptake and translocation of heavy metals in the parental generation, a response that is inherited in progeny for two subsequent generations [62]. Similarly, in mice, males that were trained to associate a novel odor with a stressor produce offspring with increased sensitivity to that novel odor, which is mediated via changes in olfactory sensory neurons [63].
Concluding Remarks
HIREC is likely to make TGP maladaptive if it alters one or more of the conditions that made TGP adaptive in historical environments. As environments become more variable and unpredictable, TGP may facilitate species declines, at least until parents can evolve mechanisms to better detect novel environmental conditions or evolve novel TGP pathways to more accurately convey information about novel environmental conditions to offspring (see Outstanding Questions). However, TGP may also allow rapid adaptation of organisms to HIREC and be a key trait in allowing species to become invasive [34]. Fitness consequences of TGP under HIREC might be less severe for certain organisms, such as those that are: (i) less dependent on parental cues (e.g., those that evolved in environments with low temporal autocorrelation) or parental care (e.g., precocial organisms); or (ii) highly social, such that socially learned adaptive responses to novel environmental conditions can easily spread through a population [64]. Our framework highlights critical areas of future research that should be empirically tested to improve our understanding of how TGP will affect organisms in human-altered environments: namely, how changes in environmental variability, autocorrelation, cue reliability, and the introduction of novel conditions affect each of the three key processes of TGP. We also encourage further incorporation of TGP into models of phenotypic plasticity [26–28] to improve estimates of how organisms will respond to environmental change. For example, TGP can be easily integrated into Bayesian updating models via the concept of a ‘prior’, and may help explain variation in the response of seemingly naive individuals to their environment [65]. Finally, given that TGP can extend to multiple generations (grandparents, great-grandparents [17]), assessing the fitness consequences of TGP through multiple generations is a key next step. Overall, we suggest that across all timescales, TGP should be better integrated into theoretical and empirical assessments of how organisms will respond to human-altered environments.
Outstanding Questions.
How will HIREC affect the evolution of TGP as a mechanism for phenotypic change in natural systems? Are there general HIREC-induced conditions under which TGP will be more favored compared with WGP? How quickly can existing TGP mechanisms evolve/adapt to novel HIREC such that TGP can produce beneficial offspring phenotypes?
In what ways does information from parents, personal experience, and alleles interact to inform organisms’ responses to HIREC?
How does an organism’s life-history strategy (e.g., dispersal, long/short lived, altricial/precocial, generalist/specialist) influence the effectiveness of TGP versus WGP in generating adaptive responses to HIREC?
How can individual or social learning as forms of WGP versus TGP affect organisms’ responses to HIREC? Does TGP modify the capacity for learning and thus organisms’ responses to HIREC?
How do existing relationships between organisms’ use of TGP and WGP explain organisms’ ability to cope with HIREC, particularly in the context of evolutionary match/mismatch?
How does TGP influence differences in personality or cognition of offspring in response to HIREC?
How might nonparental forms of TGP (i.e., information from other conspecifics or heterospecifics in previous generations) affect organismal responses to HIREC?
How does TGP affect organisms’ plasticity in response to multiple stressors?
Highlights.
Human activities are dramatically altering ecological communities. While many organisms are threatened by human-induced rapid environmental change (HIREC), others are thriving. This variability is often attributed to differences in genetic variation and/or within-generational plasticity, but transgenerational plasticity (TGP) may be another key (often overlooked) process that contributes to this variation.
We develop a framework that explores how TGP can affect organisms’ responses to HIREC. We highlight three sequential processes in the detection and transmission of parental cues to offspring that are critical for TGP to be beneficial in a given environment.
Because many hypotheses regarding TGP in human-altered environments have yet to be tested, our framework summarizes potential positive and negative outcomes and outlines key areas for future study.
Acknowledgments
We thank M. Bensky for creating the images for Figure 1 and S. Gignoux-Wolfsohn, R. Fletcher, and three anonymous reviewers for providing helpful comments that improved the manuscript. While writing the manuscript, J.K.H. was supported by a National Institutes of Health NRSA fellowship (award #F32GM121033), S.C.D. was supported by a Smithsonian Institutional Postdoctoral Fellowship, A.M.B. was supported by NSF IOS-1121980 and 191100 and NIH 2R01GM082937-06A1, B.L. was supported by NSF IOS-1557831, and A.S. was supported by NSF IOS-1456724.
Glossary
- Autocorrelation
similarity between environmental conditions in a temporal or spatial series.
- Cue reliability
how well environmental cues reflect environmental conditions.
- Diversified bet hedging (DBH)
when parents increase phenotypic variance in their offspring to lower the variance in genotypic fitness; can be a type of TGP if the parents’ environment modifies offspring phenotypic variation.
- Ecological trap
a type of evolutionary trap; when organisms choose a suboptimal habitat, even though there is a better quality habitat available, because previously reliable environmental cues have become unreliable.
- Environmental mismatch
when the parents’ environment differs from the offspring’s environment; can result in phenotypic mismatches.
- Environmental variability
the extent to which environmental conditions vary from the mean.
- Evolutionary trap
when an environmental change reduces the reliability of previously reliable environmental cues, such that previously adaptive phenotypes become maladaptive.
- Human-induced rapid environmental change (HIREC)
environmental change caused by human activities that is occurring at scales and magnitudes faster and larger than those that organisms have likely experienced in their evolutionary past.
- Phenotypic mismatch
when individuals express a phenotype that is inappropriate for their current environmental conditions.
- Sensitive window
a period of development where an individual’s environment shapes phenotypic development more strongly relative to other life stages.
- Transgenerational plasticity (TGP)
the effect of a previous generation’s environment on the phenotypes of a subsequent generation (also referred to as intergenerational plasticity). TGP can act through maternal or paternal pathways via a variety of mechanisms (see Box 2 for common TGP mechanisms) and can also include grandparental effects, which we do not discuss here
- Within-generational plasticity (WGP)
the effect of an individual’s experience on their phenotype.
References
- 1.Legrand D et al. (2017) Eco-evolutionary dynamics in fragmented landscapes. Ecography 40, 9–25 [Google Scholar]
- 2.Langkilde T et al. (2017) Behavioral adaptations to invasive species: benefits, costs, and mechanisms of change. Adv. Study Behav 49, 199–235 [Google Scholar]
- 3.Kuparinen A and Festa-Bianchet M (2017) Harvest-induced evolution: insights from aquatic and terrestrial systems. Philos. Trans. R. Soc. Lond. B Biol. Sci 372, 20160036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Saaristo M et al. (2018) Direct and indirect effects of chemical contaminants on the behaviour, ecology and evolution of wildlife. Proc. Biol. Sci 285, 20181297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beever EA et al. (2017) Behavioral flexibility as a mechanism for coping with climate change. Front. Ecol. Environ 15, 299–308 [Google Scholar]
- 6.Wolkovich EM et al. (2013) Temperature-dependent shifts in phenology contribute to the success of exotic species with climate change. Am. J. Bot 100, 1407–1421 [DOI] [PubMed] [Google Scholar]
- 7.Sih A et al. (2011) Evolution and behavioural responses to human-induced rapid environmental change. Evol. Appl 4, 367–387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hughes TP et al. (2017) Global warming and recurrent mass bleaching of corals. Nature 543, 373. [DOI] [PubMed] [Google Scholar]
- 9.Bonamour S et al. (2019) Phenotypic plasticity in response to climate change: the importance of cue variation. Philos. Trans. R. Soc. Lond. B Biol. Sci 374, 20180178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Robertson BA et al. (2013) Ecological novelty and the emergence of evolutionary traps. Trends Ecol. Evol 28, 552–560 [DOI] [PubMed] [Google Scholar]
- 11.Parmesan C and Hanley ME (2015) Plants and climate change: complexities and surprises. Ann. Bot 116, 849–864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Davidson AM et al. (2011) Do invasive species show higher phenotypic plasticity than native species and, if so, is it adaptive? A meta-analysis. Ecol. Lett 14, 419–431 [DOI] [PubMed] [Google Scholar]
- 13.Wong B and Candolin U (2015) Behavioral responses to changing environments. Behav. Ecol 26, 665–673 [Google Scholar]
- 14.Jablonka E et al. (1995) The adaptive advantage of phenotypic memory in changing environments. Philos. Trans. R. Soc. Lond. B Biol. Sci 350, 133–141 [DOI] [PubMed] [Google Scholar]
- 15.Donelson JM et al. (2018) Transgenerational plasticity and climate change experiments: where do we go from here? Glob. Chang. Biol 24, 13–34 [DOI] [PubMed] [Google Scholar]
- 16.Fletcher RJ et al. (2012) How the type of anthropogenic change alters the consequences of ecological traps. Proc. Biol. Sci 279, rspb20120139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bell AM and Hellmann JK (2019) An integrative framework for understanding the mechanisms and multigenerational consequences of transgenerational plasticity. Annu. Rev. Ecol. Evol. Syst Published online July 23, 2019. 10.1146/annurev-ecolsys-110218-024613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Herman J and Sultan S (2011) Adaptive transgenerational plasticity in plants: case studies, mechanisms, and implications for natural populations. Front. Plant Sci 2, 102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mousseau TA and Fox CW (1998) Maternal Effects as Adaptations (Oxford University Press; ) [Google Scholar]
- 20.Sheriff MJ et al. (2017) Integrating ecological and evolutionary context in the study of maternal stress. Integr. Comp. Biol 57, 437–449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Groothuis TG et al. (2019) Revisiting mechanisms and functions of prenatal hormone-mediated maternal effects using avian species as a model. Philos. Trans. R. Soc. Lond. B Biol. Sci 374, 20180115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vivas M et al. (2015) Maternal effects on tree phenotypes: considering the microbiome. Trends Plant Sci 20, 541–544 [DOI] [PubMed] [Google Scholar]
- 23.Curley JP and Champagne FA (2016) Influence of maternal care on the developing brain: mechanisms, temporal dynamics and sensitive periods. Front. Neuroendocrinol 40, 52–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McJunkin K (2018) Maternal effects of microRNAs in early embryogenesis. RNA Biol 15, 165–169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Uller T (2008) Developmental plasticity and the evolution of parental effects. Trends Ecol Evol 23, 432–438 [DOI] [PubMed] [Google Scholar]
- 26.Leimar O and McNamara JM (2015) The evolution of transgenerational integration of information in heterogeneous environments. Am Nat 185, E55–E69 [DOI] [PubMed] [Google Scholar]
- 27.McNamara JM et al. (2016) Detection vs. selection: integration of genetic, epigenetic and environmental cues in fluctuating environments. Ecol Lett 19, 1267–1276 [DOI] [PubMed] [Google Scholar]
- 28.Kuijper B and Hoyle RB (2015) When to rely on maternal effects and when on phenotypic plasticity? Evolution 69, 950–968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Uller T et al. (2013) Weak evidence for anticipatory parental effects in plants and animals. J Evol Biol 26, 2161–2170 [DOI] [PubMed] [Google Scholar]
- 30.Burgess SC and Marshall DJ (2014) Adaptive parental effects: the importance of estimating environmental predictability and offspring fitness appropriately. Oikos 123, 769–776 [Google Scholar]
- 31.Bošković A and Rando OJ (2018) Transgenerational epigenetic inheritance. Annu Rev Genet 52, 21–41 [DOI] [PubMed] [Google Scholar]
- 32.Pick JL et al. (2019) The more you get, the more you give: positive cascading effects shape the evolutionary potential of prenatal maternal investment. Evol Lett 3, 412–423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McGlothlin JW and Galloway LF (2014) The contribution of maternal effects to selection response: an empirical test of competing models. Evolution 68, 549–558 [DOI] [PubMed] [Google Scholar]
- 34.Dyer AR et al. (2010) Synthesis: the role of adaptive trans-generational plasticity in biological invasions of plants. Evol Appl 3, 179–192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Holeski LM et al. (2012) Transgenerational defense induction and epigenetic inheritance in plants. Trends Ecol Evol 27, 618–626 [DOI] [PubMed] [Google Scholar]
- 36.Donelan SC and Trussell GC (2018) Parental and embryonic experiences with predation risk affect prey offspring behaviour and performance. Proc Biol. Sci 285, 20180034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moore RS et al. (2019) Piwi/PRG-1 argonaute and TGF-β mediate transgenerational learned pathogenic avoidance. Cell 177, 1827–1841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Crean AJ and Marshall DJ (2009) Coping with environmental uncertainty: dynamic bet hedging as a maternal effect. Philos Trans R Soc Lond B Biol Sci 364, 1087–1096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Snell-Rood E et al. (2015) Life-history evolution in the Anthropocene: effects of increasing nutrients on traits and trade-offs. Evol Appl 8, 635–649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Johnson DD et al. (2013) The evolution of error: error management, cognitive constraints, and adaptive decision-making biases. Trends Ecol Evol 28, 474–481 [DOI] [PubMed] [Google Scholar]
- 41.Carthey AJ et al. (2017) Novel predators emit novel cues: a mechanism for prey naivety towards alien predators. Sci Rep 7, 16377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pembrey M et al. (2014) Human transgenerational responses to early-life experience: potential impact on development, health and biomedical research. J Med Genet 51, 563–572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fawcett TW and Frankenhuis WE (2015) Adaptive explanations for sensitive windows in development. Front Zool 12, S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Visser ME and Holleman LJ (2001) Warmer springs disrupt the synchrony of oak and winter moth phenology. Proc Biol Sci 268, 289–294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Donohue K (2009) Completing the cycle: maternal effects as the missing link in plant life histories. Philos Trans R Soc Lond B Biol Sci 364, 1059–1074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sheriff MJ et al. (2018) Error management theory and the adaptive significance of transgenerational maternal-stress effects on offspring phenotype. Ecol Evol 8, 6473–6482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Munday PL (2014) Transgenerational acclimation of fishes to climate change and ocean acidification. F1000Prime Rep. 6, 99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Van Zandt PA and Mopper S (2004) The effects of maternal salinity and seed environment on germination and growth in Iris hexagona. Evol Ecol Res 6, 813–832 [Google Scholar]
- 49.Di Cecco GJ and Gouhier TC (2018) Increased spatial and temporal autocorrelation of temperature under climate change. Sci Rep 8, 14850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chen Q et al. (2016) Epigenetic inheritance of acquired traits through sperm RNAs and sperm RNA modifications. Nat Rev Genet 17, 733–743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hanazato T (1991) Pesticides as chemical agents inducing helmet formation in Daphnia ambigua. Freshwat Biol 26, 419–424 [Google Scholar]
- 52.Lyu K et al. (2017) Cladoceran offspring tolerance to toxic Microcystis is promoted by maternal warming. Environ Pollut 227, 451–459 [DOI] [PubMed] [Google Scholar]
- 53.Guiden PW et al. (2019) Predator–prey interactions in the Anthropocene: reconciling multiple aspects of novelty. Trends Ecol Evol 34, 616–627 [DOI] [PubMed] [Google Scholar]
- 54.Polo-Cavia N et al. (2016) Low levels of chemical anthropogenic pollution may threaten amphibians by impairing predator recognition. Aquat Toxicol 172, 30–35 [DOI] [PubMed] [Google Scholar]
- 55.Mariette MM and Buchanan KL (2016) Prenatal acoustic communication programs offspring for high posthatching temperatures in a songbird. Science 353, 812–814 [DOI] [PubMed] [Google Scholar]
- 56.Gilroy JJ and Sutherland WJ (2007) Beyond ecological traps: perceptual errors and undervalued resources. Trends Ecol Evol 22, 351–356 [DOI] [PubMed] [Google Scholar]
- 57.Schuler MS and Orrock JL (2012) The maladaptive significance of maternal effects for plants in anthropogenically modified environments. Evol Ecol 26, 475–481 [Google Scholar]
- 58.Larios E and Venable DL (2015) Maternal adjustment of offspring provisioning and the consequences for dispersal. Ecology 96, 2771–2780 [DOI] [PubMed] [Google Scholar]
- 59.Sentis A et al. (2018) Evolution without standing genetic variation: change in transgenerational plastic response under persistent predation pressure. Heredity 121, 266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Injaian AS et al. (2018) Effects of experimental anthropogenic noise on avian settlement patterns and reproductive success. Behav Ecol 29, 1181–1189 [Google Scholar]
- 61.Robinson GE and Barron AB (2017) Epigenetics and the evolution of instincts. Science 356, 26–27 [DOI] [PubMed] [Google Scholar]
- 62.Cong W et al. (2019) Transgenerational memory of gene expression changes induced by heavy metal stress in rice (Oryza sativa L.). BMC Plant Biol 19, 282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dias BG and Ressler KJ (2014) Parental olfactory experience influences behavior and neural structure in subsequent generations. Nat Neurosci 17, 89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Barrett B et al. (2019) Counter-culture: does social learning help or hinder adaptive response to human-induced rapid environmental change? Front Ecol Evol 7, 183 [Google Scholar]
- 65.Stamps JA and Frankenhuis WE (2016) Bayesian models of development. Trends Ecol Evol 31, 260–268 [DOI] [PubMed] [Google Scholar]
- 66.Relyea RA (2012) New effects of roundup on amphibians: predators reduce herbicide mortality; herbicides induce antipredator morphology. Ecol Appl 22, 634–647 [DOI] [PubMed] [Google Scholar]
- 67.Jobson MA et al. (2015) Transgenerational effects of early life starvation on growth, reproduction, and stress resistance in Caenorhabditis elegans. Genetics 201, 201–212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nagy C and Turecki G (2012) Sensitive periods in epigenetics: bringing us closer to complex behavioral phenotypes. Epigenomics 4, 445–457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Taborsky B (2006) Mothers determine offspring size in response to own juvenile growth conditions. Biol Lett 2, 225–228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ezard TH et al. (2014) The fitness costs of adaptation via phenotypic plasticity and maternal effects. Funct Ecol 28, 693–701 [Google Scholar]
- 71.Sales K et al. (2018) Experimental heatwaves compromise sperm function and cause transgenerational damage in a model insect. Nat Commun 9, 4771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Fischer B et al. (2013) The evolution of age-dependent plasticity. Am Nat 183, 108–125 [DOI] [PubMed] [Google Scholar]
- 73.Suckling CC et al. (2015) Adult acclimation to combined temperature and pH stressors significantly enhances reproductive outcomes compared to short-term exposures. J Anim Ecol 84, 773–784 [DOI] [PubMed] [Google Scholar]
- 74.Francis D et al. (1999) Nongenomic transmission across generations of maternal behavior and stress responses in the rat. Science 286, 1155–1158 [DOI] [PubMed] [Google Scholar]
- 75.Falcão-Tebas F et al. (2019) Four weeks of exercise early in life reprograms adult skeletal muscle insulin resistance caused by a paternal high-fat diet. J Physiol 597, 121–136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Frankenhuis WE and Panchanathan K (2011) Balancing sampling and specialization: an adaptationist model of incremental development. Proc Biol Sci 278, 3558–3565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Starrfelt J and Kokko H (2012) Bet-hedging—a triple trade-off between means, variances and correlations. Biol Rev 87, 742–755 [DOI] [PubMed] [Google Scholar]
- 78.Seger J and Brockmann H (1987) What is bet-hedging? In Oxford Surveys in Evolutionary Biology, 4th edn, P.H. Harvey and I. Partridge, eds. (Oxford University Press; ), pp. 182–211 [Google Scholar]
- 79.Donaldson-Matasci MC et al. (2013) When unreliable cues are good enough. Am Nat 182, 313–327 [DOI] [PubMed] [Google Scholar]
- 80.Sadeh A et al. (2009) Plastic bet-hedging in an amphicarpic annual: an integrated strategy under variable conditions. Evol Ecol 23, 373–388 [Google Scholar]
- 81.Shama LN (2015) Bet hedging in a warming ocean: predictability of maternal environment shapes offspring size variation in marine sticklebacks. Global Change Biol 21, 4387–4400 [DOI] [PubMed] [Google Scholar]
- 82.Botero CA et al. (2015) Evolutionary tipping points in the capacity to adapt to environmental change. Proc Natl Acad Sci USA 112, 184–189 [DOI] [PMC free article] [PubMed] [Google Scholar]


