Abstract
Background
Maintenance treatment with long‐acting beta2‐agonists and inhaled corticosteroids (LABA/ICS) can relieve asthma symptoms and reduce the frequency of exacerbations, but there are limited treatment options for people who do not gain control on combination LABA/ICS. Long‐acting muscarinic antagonists (LAMA) are a class of inhaled drug which have been effective for people with chronic obstructive pulmonary disease and are now becoming available for people with asthma to take alongside their LABA/ICS inhaler.
Objectives
To assess the effects of adding a long‐acting muscarinic antagonist (LAMA) to combination long‐acting beta2‐agonists (LABA) and inhaled corticosteroids (ICS) in adults whose asthma is not well controlled by LABA/ICS.
Search methods
We identified trials from the Cochrane Airways Review Group Specialised Register (CAGR) up to January 2016. We also searched ClinicalTrials.gov, the WHO trials portal, and reference lists of other reviews, and we contacted trial authors for additional information.
Selection criteria
We included parallel randomised controlled trials (RCTs) of at least 12 weeks' duration. Studies met the inclusion criteria if they compared LAMA as an add‐on to LABA/ICS versus LABA/ICS alone for adults with asthma. We included studies reported as full text, those published as abstract only, and unpublished data. Primary outcomes were exacerbations requiring oral corticosteroids (OCS), validated measures of asthma control, and serious adverse events (including mortality).
Data collection and analysis
Two review authors screened searches and independently extracted details on risk of bias and numerical data. We analysed dichotomous data as odds ratios (ORs) and continuous data as mean differences (MD) using a random‐effects model. We rated all outcomes using GRADE.
Main results
We found four double‐blind, double‐dummy trials comparing LAMA to placebo, including 1197 people with asthma taking combination LABA/ICS. One of the trials was designed to study glycopyrronium bromide but was withdrawn prior to enrolment, and the other three all studied tiotropium bromide (mostly 5 µg once daily via Respimat) over 48 to 52 weeks. People in the trials had a mean forced expiratory volume in one second (FEV1) of 55% of their predicted value, indicating severe asthma.
People randomised to take tiotropium add‐on had fewer exacerbations requiring oral corticosteroids than those continuing to take LABA/ICS alone, but the confidence intervals did not rule out no difference (OR 0.76, 95% CI 0.57 to 1.02; moderate quality evidence). Over 48 weeks, 328 out of 1000 people taking their usual LABA/ICS would have to take oral corticosteroids for an exacerbation compared with 271 if they took tiotropium as well (95% CI 218 to 333 per 1000). Analyses comparing the number of exacerbations per patient in each group (rate ratio) and the time until first exacerbation (hazard ratio) were in keeping with the main result. Quality of life, as measured by the Asthma Quality of Life Questionnaire (AQLQ) was no better for those taking tiotropium add‐on than for those taking LABA/ICS alone when considered in light of the 0.5 minimal clinically important difference on the scale (MD 0.09, 95% CI − 0.03 to 0.20), and evidence for whether tiotropium increased or decreased serious adverse events in this population was inconsistent (OR 0.60, 95% CI 0.24 to 1.47; I2 = 76%).
Within the secondary outcomes, exacerbations requiring hospital admission were too rare to tell whether tiotropium was beneficial over LABA/ICS alone. There was high quality evidence showing benefits to lung function (trough FEV1 and forced vital capacity (FVC)) and potentially small benefits to asthma control. People taking tiotropium add‐on were less likely to experience non‐serious adverse events.
Authors' conclusions
Tiotropium add‐on may have additional benefits over LABA/ICS alone in reducing the need for rescue oral steroids in people with severe asthma. The effect was imprecise, and there was no evidence for other LAMA preparations. Possible benefits on quality of life were negligible, and evidence for the effect on serious adverse events was inconsistent. There are likely to be small added benefits for tiotropium Respimat 5 µg daily on lung function and asthma control over LABA/ICS alone and fewer non‐serious adverse events. The benefit of tiotropium add‐on on the frequency of hospital admission is still unknown, despite year‐long trials.
Ongoing and future trials should clearly describe participants' background medications to help clinicians judge how the findings relate to stepwise care. If studies test LAMAs other than tiotropium Respimat for asthma, they should be at least six months long and use accepted and validated outcomes to allow comparisons of the safety and effectiveness between different preparations.
Keywords: Adult; Aged; Humans; Middle Aged; Administration, Inhalation; Adrenal Cortex Hormones; Adrenal Cortex Hormones/therapeutic use; Adrenergic beta‐2 Receptor Agonists; Adrenergic beta‐2 Receptor Agonists/therapeutic use; Asthma; Asthma/drug therapy; Disease Progression; Drug Therapy, Combination; Drug Therapy, Combination/methods; Muscarinic Antagonists; Muscarinic Antagonists/adverse effects; Muscarinic Antagonists/therapeutic use; Quality of Life; Randomized Controlled Trials as Topic; Tiotropium Bromide; Tiotropium Bromide/adverse effects; Tiotropium Bromide/therapeutic use
Plain language summary
Does adding tiotropium, a long‐acting muscarinic antagonist (LAMA), to combination therapy (LABA/ICS) help to control asthma?
Adding the LAMA tiotropium Respimat inhaler to combination LABA/ICS inhaler may reduce the need for rescue oral steroids. A noticeable benefit on quality of life is unlikely, and we couldn't tell if it reduced hospital admissions, but adding tiotropium has some benefit on lung function, asthma control, and non‐serious side effects.
More detail about the studies and results:
Taking a daily inhaler containing a long‐acting beta2‐agonist and an inhaled corticosteroid (LABA/ICS) can improve symptoms and reduce the likelihood of asthma attacks. If this doesn't help, another type of inhaled drug called a long‐acting muscarinic antagonist (LAMA), which has been effective for people with other breathing conditions, is now available for people with asthma to take as well as their LABA/ICS inhaler.
We wanted to find out whether adding a LAMA to LABA/ICS is better than continuing LABA/ICS alone for adults with asthma.
We found four relevant studies, but one was withdrawn before anyone was signed up. The other three compared a LAMA called tiotropium Respimat to placebo for around a year, with participants in both groups continuing to take their usual LABA/ICS inhaler. People generally had quite poor lung function when they entered the studies, suggesting their asthma was not well controlled ‐ in respiratory medicine, this is known as 'severe asthma'.
Over 48 weeks, 328 out of 1000 people taking their usual LABA/ICS had to take a course of oral steroids compared with 271 if they took tiotropium as well. However, uncertainty in the results meant that rather than there being 271 people taking oral steroids, there could be anywhere from 218 to 333 people per 1000 who would have to take oral steroids, so we couldn't be sure of the benefit. Quality of life scores were not that different between those who took tiotropium and those who didn't. The studies showed different results for whether people taking tiotropium were more likely to suffer a serious side effect, but fewer people had non‐serious side effects if they took tiotropium.
We couldn't tell whether taking tiotropium on top of LABA/ICS reduced the number of people who had to go to hospital for an asthma attack because it didn't happen often enough for us to have confidence in the result. There was high quality evidence that showed benefits to lung function and probably small benefits on measures of asthma control.
Summary of findings
Summary of findings for the main comparison. Summary of findings table 1.
| LAMA versus placebo in adults with asthma taking background LABA/ICS | ||||||
|
Patient or population: adults with asthma
Setting: outpatient
Intervention: LAMA + background LABA/ICS
Comparison: LABA/ICS alone The studies randomised participants to LAMA or placebo and required participants to be taking background LABA/ICS. The durations shown are the weighted means of the studies included in each analysis. | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with LABA/ICS | Risk with LAMA + LABA/ICS | |||||
|
Exacerbations requiring oral corticosteroids 48 weeks |
328 per 1000 | 271 per 1000 (218 to 333) | OR 0.76 (0.57 to 1.02) | 907 (2 RCTs) | ⊕⊕⊕⊝ Moderate1 | Analyses comparing the number of exacerbations per patient in each group (rate ratio) and the time until first exacerbation (hazard ratio) were in keeping with the main result |
|
Quality of life (AQLQ) 7‐point scale from 1 to 7 Higher scores are better 48 weeks |
The mean AQLQ was 5.03 | The mean AQLQ score in the LAMA group was 0.09 better (0.03 better to 0.20 worse) |
— | 907 (2 RCTs) | ⊕⊕⊕⊕ High2 | No benefit of LAMA add‐on over LABA/ICS alone. The MCID for the AQLQ is 0.5. |
|
Serious adverse events 49 weeks |
96 per 1000 | 60 per 1000 (25 to 134) | OR 0.60 (0.24 to 1.47) | 1197 (3 RCTs) | ⊕⊕⊝⊝ Low3, 4 | Evidence does not suggest LAMA increases adverse events. Post hoc sensitivity analysis removing Ohta 2014 gave a more precise estimate but did not change the conclusions. |
|
Exacerbations requiring hospital admission 49 weeks |
43 per 1000 | 30 per 1000 (15 to 59) | OR 0.68 (0.34 to 1.38) | 1191 (3 RCTs) | ⊕⊕⊝⊝ Low5, 6 | Too few events to detect whether there is a benefit of LAMA add‐on. |
|
Lung function (change in trough FEV1 L) 49 weeks |
The mean change in trough FEV1 was 0.08 L | The mean change in trough FEV1 (L) in the intervention group was 0.07 higher (0.03 higher to 0.11 higher) |
— | 1191 (3 RCTs) | ⊕⊕⊕⊕ High7 | Some benefit of LAMA add‐on over LABA/ICS alone |
|
Asthma control (ACQ) 7‐point scale from 0 to 6 Lower scores are better 48 weeks |
The mean asthma control (ACQ) was 2.13 | The mean asthma control (ACQ) in the intervention group was 0.13 better (0.23 better to 0.02 better) |
— | 907 (2 RCTs) | ⊕⊕⊕⊕ High | Scores with LAMA add‐on were better than LABA/ICS alone, but the difference was not clinically significant (MCID = 0.5) |
|
Any adverse events 49 weeks |
813 per 1000 | 753 per 1000 (693 to 803) | OR 0.70 (0.52 to 0.94) | 1197 (3 RCTs) | ⊕⊕⊕⊕ High7 | The listed events were reported in at least 2% of patients who underwent randomisation |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). For the continuous outcomes, we calculated a weighted mean of the scores in the control groups. ACQ: Asthma Control Questionnaire; AQLQ: Asthma Quality of Life Questionnaire; CI: confidence interval; FEV1 : forced expiratory volume in one second; LABA/ICS: combined long‐acting beta2‐agonist and inhaled corticosteroid; LAMA: long‐acting muscarinic antagonist; MCID: minimal clinically important difference; OR: odds ratio; RCT: randomised controlled trial. | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1 Less than 300 events in the analysis. The confidence intervals included an important benefit of LAMA add‐on and no difference (− 1 imprecision). 2 Both confidence intervals were within the 0.5 minimal clinically important difference for the scale. The effect suggests no important difference between the treatments (no downgrade). 3 I2 = 76%, P = 0.02. Ohta 2014 showed significantly fewer serious adverse events on LAMA while the other two trials did not show a difference against LABA/ICS alone (− 1 inconsistency). 4 The confidence intervals included appreciable harm on either treatment, largely because Ohta 2014 had a much larger effect in favour of LAMA. Ohta 2014 was different from the other two studies because it had two dose groups that were combined in the analysis. In addition, the study included participants who were taking either ICS alone as background treatment or LABA/ICS. As such, some results are from participants who do not meet all inclusion criteria for this review. A post hoc sensitivity analysis removing this study made the effect much more precise (− 1 indirectness, no downgrade for imprecision). 5Ohta 2014 showed a much larger effect in favour of LAMA add‐on than the other two studies, which may be due to the indirectness of the population (− 1 indirectness). However, the Ohta 2014 effect was based on far fewer events so carried less weight and the confidence intervals included the effects of the other two studies (no downgrade for inconsistency). 6Removing Ohta 2014 in a post hoc sensitivity analysis on the basis of an indirect population did not significantly improve the precision of the estimate (− 1 imprecision). 7The study with an indirect population contributed to this outcome but its results were not inconsistent with the other studies (no downgrade for indirectness).
Background
Description of the condition
Asthma is a "common chronic non‐communicable disease that affects as many as 334 million people of all ages in all parts of the world" (Global Asthma Report 2011). It is the 14th most important disorder in terms of the extent and duration of disability, not only because of recurring physical symptoms like wheezing, shortness of breath, chest tightness, and cough, but also because of associated psychological and social effects (GINA 2014; Global Asthma Report 2011). Symptoms are caused by chronic inflammation of the airways, which are hyperresponsive to various risk factors (e.g. allergens, tobacco, infection), leading to narrowing of the airways and mucus production (GINA 2014). Much of the burden is felt in low‐ and middle‐income countries, where treatment costs lead to uncontrolled symptoms and exacerbations, but studies report avoidable morbidity and mortality worldwide as the result of inappropriate or insufficient management of the disease (Global Asthma Report 2011; NRAD 2014).
Treatment recommended by internationally recognised guidelines follows a stepwise approach to maintain symptom control, prevent exacerbations and minimise drug costs and side effects (e.g. BTS/SIGN 2014; GINA 2014). Regular clinic visits, self monitoring and an asthma action plan are important if patients are to receive treatment consistent with their level of asthma control, which is commonly assessed by frequency and severity of symptoms, limitation of daily activities, rescue inhaler use, and lung function (GINA 2014; NRAD 2014).
Description of the intervention
Many people with asthma take daily controller medication to "prevent symptoms, improve lung function, and prevent attacks" (GINA 2014), and as an as‐needed reliever inhaler for quick relief of symptoms. Inhaled corticosteroids (ICS) are an effective controller medication for asthma and are the preferred initial controller choice when people require regular daily therapy (Adams 2008a; Adams 2008b; GINA 2014). If low‐dose inhaled corticosteroids are ineffective, they can be combined with a long‐acting beta2‐agonist (LABA) in stepwise management (Ducharme 2008; Ducharme 2010). Limited step‐up options are available for patients who continue to have frequent symptoms and exacerbations while taking combination LABA/ICS, but data are emerging to support the use of long‐acting muscarinic antagonist (LAMA) add‐on therapy for this group of patients (Lipworth 2014), and the licence for one LAMA has recently been extended for this indication (eMC 2014). The licence extension applies only to tiotropium delivered via the Respimat device ‐ not to tiotropium via the HandiHaler device nor to other available LAMAs such as aclidinium and glycopyrronium. LAMAs are not yet available in a single inhaler with LABA/ICS, so patients taking these three types of medications have to take LABA/ICS in a single inhaler and LAMA in another. Twice daily preparations of combination LABA/ICS are common (salmeterol/fluticasone propionate or formoterol/budesonide), but once‐daily preparations are emerging (vilanterol/fluticasone furoate), and LAMA are taken once daily.
How the intervention might work
LAMAs ease muscle contraction and mucus secretion by blocking acetylcholine receptors on airway smooth muscle, glands, and nerves (Moulton 2011). Used as triple therapy (i.e. LAMA/LABA/ICS), studies have also suggested that combining a LABA and a LAMA may lead to additional bronchodilation through the interaction of their different mechanisms, although this theory requires further study (Kerstjens 2012). For patients with poorly controlled asthma, treatment guidelines recommend that the ICS component within the LABA/ICS combination be increased rather than adding on other therapies, as there is limited evidence of benefit from the addition of other therapeutic classes such as leukotriene antagonists and methylxanthines (GINA 2014). The addition of a LAMA for added long‐acting bronchodilation may provide an alternative option, allowing doses of steroids to be minimised to reduce the risk of side effects (Fardon 2007). Inhaled corticosteroids have been associated with dose‐related systemic side effects such as hypothalamo‐pituitary adrenal (HPA) axis suppression, reduction in bone density, cataracts, and skin bruising (Lipworth 1999; Pandya 2014). LAMAs are associated with their own side effects, which include dry mouth, metallic taste, mydriasis, and urinary retention (Therapeutic Choices 2014).
Why it is important to do this review
Now that one preparation of LAMA has been licensed for asthma (eMC 2014), it is important for researchers to critically assess the evidence base for its use in the clinical scenario for which it is indicated. Limited treatment options are available for patients whose asthma does not respond well to LABA/ICS, so there is a need to fully assess the efficacy and safety of potential therapies to improve the quality of life of this group of patients.
Objectives
To assess the effects of adding a long‐acting muscarinic antagonist (LAMA) to combination long‐acting beta2‐agonists (LABA) and inhaled corticosteroids (ICS) in adults whose asthma is not well controlled by LABA/ICS.
Methods
Criteria for considering studies for this review
Types of studies
We included parallel and cross‐over randomised controlled trials (RCTs) of at least 12 weeks' duration. We included studies reported as full text, those published as abstract only and unpublished data.
We did not exclude studies on the basis of blinding.
Types of participants
We included studies in adults (aged 18 years or older) with asthma who were taking LABA/ICS combination therapy. We excluded trials that included participants with other chronic respiratory comorbidities (e.g. chronic obstructive pulmonary disease, bronchiectasis).
If studies included adults and adolescents or children younger than age 12 and data were not reported separately, we included them if the mean age in both groups was over 18 years.
Types of interventions
We included trials assessing a LAMA add‐on to any dose of LABA/ICS combination therapy versus the same dose of LABA/ICS alone. We included studies comparing LAMA with placebo if they required participants to be taking LABA/ICS combination therapy for inclusion in the trial, and if the dose taken was equivalent in intervention and comparison groups.
We included studies involving the addition of the following LAMA at any dose.
Tiotropium (Spiriva Handihaler or Respimat).
Aclidinium bromide (Eklira Genuair).
Glycopyrronium bromide (Seebri Breezhaler).
We included studies that allowed participants to continue using additional short‐ or long‐acting medications (e.g. salbutamol, terbutaline and ipratropium, leukotriene receptor antagonists), provided they were not part of the randomised treatment.
Types of outcome measures
Primary outcomes
Exacerbations requiring oral corticosteroids
Quality of life (measured on a validated asthma scale, e.g. Asthma Quality of Life Questionnaire, AQLQ)
Serious adverse events (all causes)
Secondary outcomes
Exacerbations requiring hospital admission
Lung function (preferably trough forced expiratory volume in one second, or FEV1)
Asthma control (measured on a validated scale, e.g. Asthma Control Questionnaire (ACQ), Asthma Control Test)
Any adverse events
Reporting in the trial of one of more of the outcomes listed here was not an inclusion criterion for the review.
If trials reported exacerbations as a composite of more than one definition (e.g. patients with one or more exacerbations requiring hospitalisation or visit to the emergency department), we analysed them separately.
Search methods for identification of studies
Electronic searches
We identified trials from the Cochrane Airways Group Specialised Register (CAGR), which is maintained by the Trials Search Co‐ordinator for the Group. The register contains trial reports identified through systematic searches of bibliographic databases, including the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, EMBASE, the Cumulative Index to Nursing and Allied Health Literature (CINAHL), the Allied and Complementary Medicine Database (AMED) and PsycINFO. The CAGR also includes records identified by handsearching respiratory journals and meeting abstracts (please see Appendix 1 for further details). We searched all records in the CAGR using the search strategy presented in Appendix 2.
We also conducted a search of www.ClinicalTrials.gov and the World Health Organization (WHO) trials portal (www.who.int/ictrp/en/) using search terms adapted from the strategy in Appendix 2. We searched all databases from their inception to January 2016 and we imposed no restriction on language of publication.
Searching other resources
We checked the reference lists of all primary studies and review articles for additional references. We searched relevant manufacturers' websites for trial information.
We searched for errata or retractions published in full text on PubMed (www.ncbi.nlm.nih.gov/pubmed) for included studies in August 2015.
Data collection and analysis
Selection of studies
Two review authors (KMK and KD) independently screened titles and abstracts for inclusion of all potential studies identified as a result of the search and coded them as 'retrieve' (eligible or potentially eligible/unclear) or 'do not retrieve'. We retrieved the full‐text study reports/publications; two review authors (KMK and KD) independently screened the full text, identified studies for inclusion and identified and recorded reasons for exclusion of ineligible studies. We resolved disagreements through discussion. We identified and excluded duplicates and collated multiple reports of the same study, so that each study rather than each report was the unit of interest in the review. We recorded the selection process in sufficient detail to complete a Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) flow diagram and a 'Characteristics of excluded studies' table (Moher 2009).
Data extraction and management
We used a data collection form that had been piloted on at least one study in the review to document study characteristics and outcome data. One review author (KMK) extracted the following study characteristics from included studies, and a second review author (KD) spot‐checked them for accuracy against the trial reports.
Methods: study design, total duration of study, details of any 'run‐in' period, number of study centres and locations, study setting, withdrawals, dates of study.
Participants: N, mean age, age range, sex, severity of condition, diagnostic criteria, baseline lung function, smoking history, inclusion criteria, exclusion criteria.
Interventions: intervention, comparison, concomitant medications, excluded medications.
Outcomes: primary and secondary outcomes specified and collected, time points reported.
Notes: funding for trial, notable conflicts of interest of trial authors.
Two review authors (KMK and KD) independently extracted outcome data from included studies. We noted in the 'Characteristics of included studies' table if outcome data were not reported in a useable way. We resolved disagreements by consensus. One review author (KMK) transferred data into the Review Manager file (RevMan 2014). We double‐checked that data had been entered correctly by comparing data presented in the systematic review versus information provided in the study reports.
Assessment of risk of bias in included studies
Two review authors (KMK and KD) independently assessed risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), and we resolved disagreements by discussion. We assessed risk of bias according to the following domains.
Random sequence generation.
Allocation concealment.
Blinding of participants and personnel.
Blinding of outcome assessment.
Incomplete outcome data.
Selective outcome reporting.
Other bias.
We graded each potential source of bias as high, low, or unclear and provided a quote from the study report together with a justification for our judgement in the 'Risk of bias' table. We summarised risk of bias judgements across different studies for each of the domains listed. We considered blinding separately for different key outcomes when necessary (e.g. for unblinded outcome assessment, risk of bias for all‐cause mortality may be very different than for a patient‐reported pain scale). When information on risk of bias related to unpublished data or correspondence with a trialist, we noted this in the 'Risk of bias' table.
When considering treatment effects, we took into account risk of bias for studies that contributed to each outcome separately.
Assessment of bias in conducting the systematic review
We conducted the review according to our published protocol and reported deviations from it in the 'Differences between protocol and review' section of the systematic review (Kew 2015).
Measures of treatment effect
We analysed dichotomous data as odds ratios (OR), and continuous data as mean differences (MD) or standardised mean differences (SMD). We entered data presented as a scale with a consistent direction of effect. We narratively described skewed data reported as medians and interquartile ranges. We analysed data from cross‐over trials using generic inverse variance (GIV), only if double‐counting of participants had been accounted for. If trials presented both raw data and adjusted analyses (e.g. accounting for baseline differences), we used the latter.
We undertook meta‐analyses only when meaningful (i.e. if treatments, participants and underlying clinical questions were similar enough for pooling to make sense).
When a single trial reported multiple trial arms, we included only the relevant arms. If two comparisons (e.g. drug A vs placebo and drug B vs placebo) were combined in the same meta‐analysis, we halved the control group to avoid double‐counting.
If both change from baseline and endpoint scores were available for continuous data, we used change from baseline unless most studies reported endpoint scores. If a study reported outcomes at multiple time points, we used the end‐of‐study measurement.
When both an analysis including only participants who completed the trial and an analysis that imputed data for participants who were randomly assigned but did not provide endpoint data (e.g. last observation carried forward) were available, we used the latter.
For dichotomous outcomes, we assumed equivalence of treatments only if the OR estimate and its 95% confidence interval fell between the predefined arbitrary limits of 0.9 and 1.1.
Unit of analysis issues
For dichotomous outcomes, we used participants rather than events as the unit of analysis (i.e. number of adults admitted to hospital rather than number of admissions per adult). However, if exacerbations were reported as rate ratios, we analysed them on this basis. For cross‐over trials, we included data only if we could analyse them appropriately using generic inverse variance to control for intercorrelation of matched pairs.
Dealing with missing data
We contacted investigators or study sponsors to verify key study characteristics and obtained missing numerical outcome data when possible (e.g. when a study was reported as abstract only). When this was not possible and the missing data were thought to introduce serious bias, we performed a sensitivity analysis to explore the impact of including such studies in the overall assessment of results.
Assessment of heterogeneity
We used the I2 statistic to measure heterogeneity among the trials in each analysis. If we identified substantial heterogeneity, we reported this and explored possible causes by prespecified subgroup analysis.
Assessment of reporting biases
If we were able to pool more than 10 trials, we created and examined a funnel plot to explore possible small study and publication biases.
Data synthesis
We used a random‐effects model and performed a sensitivity analysis with a fixed‐effect model if the I2 value was greater than 30%.
Subgroup analysis and investigation of heterogeneity
We planned to carry out the following subgroup analyses for the primary outcomes, using the formal test for subgroup differences in Review Manager 5 (RevMan 2014).
Duration of therapy (≤ 6 months vs > 6 months).
Dose and type of LABA/ICS (e.g. formoterol/budesonide 9/320 vs salmeterol/fluticasone 50/250 µg).
Dose and type of LAMA (e.g. tiotropium HandiHaler 18 µg vs tiotropium Respimat 5 µg).
Sensitivity analysis
We planned to carry out sensitivity analyses for the primary outcomes by excluding the following.
Studies at high risk of bias for blinding of participants and personnel.
Unpublished data (i.e. no peer‐reviewed full paper available).
Cross‐over trials.
'Summary of findings' table
We created a 'Summary of findings' table using the seven prespecified outcomes from our protocol (Kew 2015). We used the five Grades of Recommendation, Assessment, Development and Evaluation (GRADE) considerations (study limitations, consistency of effect, imprecision, indirectness and publication bias) to assess the quality of the body of evidence as it related to studies that contributed data to the meta‐analyses for prespecified outcomes. We used methods and recommendations as described in Section 8.5 and Chapter 12 of the Cochrane Handbook for Systematic Reviews of Interventions and used GRADEpro software (Higgins 2011). We justified all decisions to downgrade or upgrade the quality of studies by using footnotes, and we made comments when necessary to aid readers' understanding of the review.
Results
Description of studies
Results of the search
We screened the titles and abstracts of 84 records identified in the main electronic search conducted in January 2016. We also looked for trials in a similar, less specific search in October 2014 (71 records) and from the WHO trials portal (45 records), the EU clinical trials register (28 records), ClinicalTrials.gov (27 records), and the Novartis trial registry (7 records). We attempted searches of Boehringer Ingelheim and AstraZeneca, which also make LAMA products, but these websites linked directly to the registries we had already searched. We removed 83 duplicate records and screened the titles and abstracts of the remaining 179 records. Both authors agreed to exclude 105 records after viewing titles and abstracts, and we reviewed full‐text articles for the other 74 records. At this stage, we excluded 36 with reasons, collating them into 28 excluded studies (see Excluded studies) plus one ongoing study. Thirty‐six records relating to four unique studies met the inclusion criteria, with many of these listed under both Kerstjens 2012a and Kerstjens 2012b, as the reports described both trials. We present the study flow in Figure 1.
1.
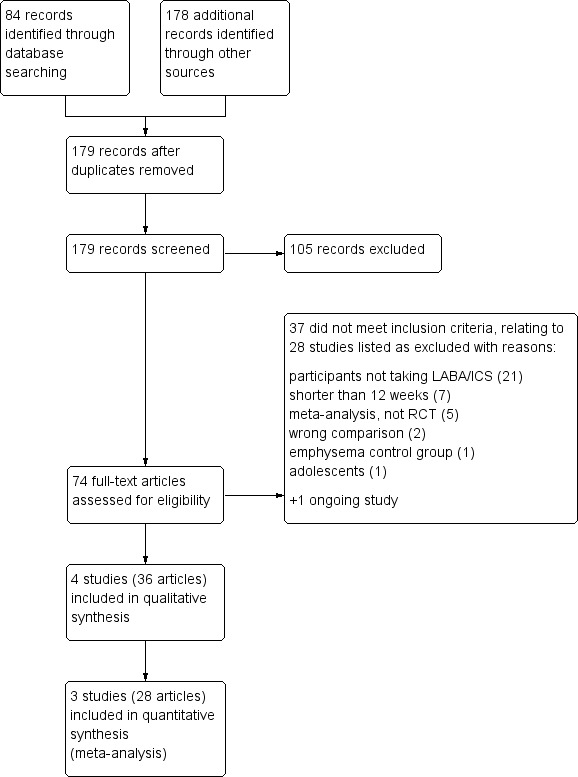
Study flow diagram.
Included studies
Four studies met the inclusion criteria, one of which was withdrawn prior to enrolment (NCT02127697). The other three studies were all multicentre, parallel, double‐blind, double‐dummy randomised controlled trials sponsored by Boehringer‐Ingelheim. Two were 48‐week twin trials conducted at over 70 study centres in multiple countries (the twin trials were registered separately as Kerstjens 2012a and Kerstjens 2012b, but other reports of the studies treated the trials as twins) and one was a 52‐week study conducted across 55 sites in Japan (Ohta 2014). Kerstjens 2012a and Kerstjens 2012b randomised patients to one of two groups, tiotropium Respimat at a dose of 5 µg once daily or placebo. Ohta 2014 was a three‐arm study randomising people to receive one of two doses of tiotropium Respimat, 2.5 µg or 5 µg daily, or placebo. The total number of participants randomised to the three completed studies was 1197. Summary characteristics of the included studies are shown in Table 2.
1. Summary of included studies.
| Study ID | Country | Total N | Weeks | Design | LABA/ICS background | LAMA add‐on | Age (years) | % FEV1 |
| Kerstjens 2012a | International | 459 | 48 | P, R, DB/DD | Stable high dose LABA/ICS | Tiotropium (Respimat) 5 µg | 53.4 | 54.6 |
| Kerstjens 2012b | International | 453 | 48 | P, R, DB/DD | Stable high dose LABA/ICS | Tiotropium (Respimat) 5 µg | 52.5 | 55.0 |
| Ohta 2014 | Japan | 285 | 52 | P, R, DB/DD | Medium ICS +/‐ LABA | Tiotropium (Respimat) 2.5/5 µg | 44.5 | NR |
| NCT02127697 | International | Withdrawn | 52 | P, R, DB/DD | Any stable dose LABA/ICS | Glycopyrronium | NA | NA |
DB/DD: double‐blind, double‐dummy; % FEV1 : forced expiratory volume in 1 second, percentage of the predicted normal value; LABA/ICS: inhaled corticosteroids/long‐acting‐beta2‐agonist combination; NA: not applicable;NR: not reported; P: parallel; R: randomised
The three completed studies had similar designs and recruited similar cohorts of patients. Inclusion criteria that were common across the trials were that patients were aged between 18 and 75 years, diagnosed with asthma before age 40 as confirmed at screening with a range of similar lung function requirements, and had a score of at least 1.5 on the ACQ to confirm that it was symptomatic. All studies excluded patients with chronic obstructive pulmonary disorder (COPD) and other unstable medical illnesses as well as patients who were current smokers or had a pack‐year history of more than 10 years. Stipulations regarding concomitant drug use were comparable, requiring that treatment with other asthma drugs had stopped at least four weeks before enrolment.
The twin trials were more stringent with criteria relating to the duration and severity of asthma, requiring participants to have at least a five‐year history of asthma, at least one exacerbation needing treatment with systemic glucocorticoids in the previous year, and stable high doses of LABA/ICS. Ohta 2014 required only a 12‐week history of symptomatic asthma, and crucially that participants could be taking stable medium doses of ICS, "alone or in a fixed combination with a LABA, for at least four weeks". This meant that a subset of participants in the latter study were only taking ICS and did not meet the criteria for this review, but we chose to include the study because baseline data showed that 56.8 percent were taking a LABA. We did not anticipate this possibility and so assessed its impact with sensitivity analyses and downgraded the quality in the GRADE assessment for indirectness of the study population.
Excluded studies
Of the 38 articles we excluded after viewing full texts, NCT01696214 was listed as an ongoing study, and 37 records related to 28 excluded studies that did not meet the inclusion criteria. The most common reason for exclusion was that participants were excluded if they were taking regular LABA/ICS; these studies assessed tiotropium for people with less severe asthma who were currently taking ICS monotherapy. We excluded 7 articles because they described studies shorter than 12 weeks, 5 because they were reports of meta‐analyses rather than RCT reports, 2 because they compared the wrong treatments, 1 because the control group had emphysema, and 1 because the study recruited adolescents under 18 years.
Risk of bias in included studies
We rated the three completed studies contributing data to the meta‐analyses as having low risk of bias across domains (see Figure 2). We judged the remaining study to be at unclear or high risk of bias across the domains, mainly because it was withdrawn without explanation before enrolling participants.
2.
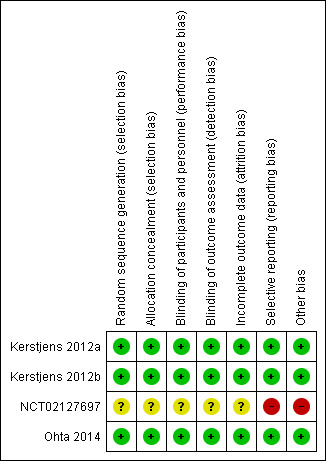
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
The three completed studies were all at low risk of selection bias. They were all published in peer‐reviewed journals and adequately described methods of random sequence generation using computerised random number generators, and allocation concealment using web‐ or phone‐based automated allocation systems. We were unable to make a judgement about the withdrawn study for selection bias as it did not enrol any participants.
Blinding
All three studies contributing to the meta‐analyses were double‐blind until after database lock by means of matching placebo inhalers. As such, all trials were at low risk of performance and detection bias. As with selection bias, we were unable to make a judgement about the study that was withdrawn.
Incomplete outcome data
The three completed studies all had dropout below 15% across the included arms. They all used the intention‐to‐treat population for the analyses, which included all patients who received at least one dose of the study medication ‒ the vast majority of randomised participants in all three studies. As with the other domains, we were unable to make a judgement about the study that was withdrawn.
Selective reporting
All of the studies had registered protocols so it was possible to compare the prospective list of outcomes with the reported data. The three completed studies reported all outcomes in full and so we rated them as having low risk of bias. We rated the withdrawn study as at high risk of bias because, although no participants were enrolled, there was no publicly available information about the reasons why the study did not go ahead, and the information about the study was not sufficient to assess bias thoroughly.
Other potential sources of bias
We did not observe any other sources of bias in three studies, but we note the fact that NCT02127697 was withdrawn as a high risk of bias, as it was not clear why this occurred.
Effects of interventions
See: Table 1
We present evidence for the primary and secondary outcomes with an assessment of the quality of evidence in Table 1. While the review aimed to assess evidence for any LAMA preparation, the results are currently for tiotropium Respimat only.
Primary outcomes
Exacerbations requiring oral corticosteroids
Fewer people taking tiotropium add‐on had exacerbations that needed treatment with oral corticosteroids, but the confidence intervals (CIs) for the effect estimate included no difference (OR 0.76, 95% CI 0.57 to 1.02; participants = 907; studies = 2; I2 = 1%; Analysis 1.1). Over 48 weeks, 328 out of 1000 people taking their usual LABA/ICS would have to take oral corticosteroids for an exacerbation compared with 271 if they also took tiotropium, but the confidence intervals ranged from 218 to 333 per 1000. This imprecision is partly explained by there only being two studies in the analysis, which observed fewer than 300 events between them, and we downgraded the evidence to moderate quality for this reason.
1.1. Analysis.
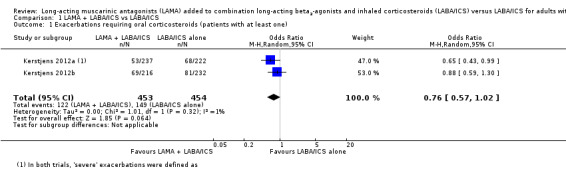
Comparison 1 LAMA + LABA/ICS vs LABA/ICS, Outcome 1 Exacerbations requiring oral corticosteroids (patients with at least one).
We also looked at data for the number of exacerbations per patient, which accounted for people who had multiple exacerbations during the study period. The rate ratio for this outcome was in favour of adding tiotropium but the confidence intervals included a possible benefit of LABA/ICS alone (rate ratio 0.79, 95% CI 0.53 to 1.17; participants = 907; studies = 2). An analysis of time to the first exacerbation also favoured tiotropium add‐on, but again the confidence intervals did not rule out no effect (hazard ratio 0.80, 95% CI 0.63 to 1.01; participants = 907; studies = 2).
Quality of life
Two studies reporting scores from the Asthma Quality of Life Questionnaire (AQLQ) did not show a benefit of tiotropium over LABA/ICS alone (MD 0.09, 95% CI − 0.03 to 0.20; participants = 907; studies = 2; I2 = 0%; Analysis 1.4). The effect estimate favoured tiotropium add‐on, but the confidence intervals included a benefit of LABA/ICS alone, and they were both well within the minimal clinically important difference for the scale (0.5).
1.4. Analysis.
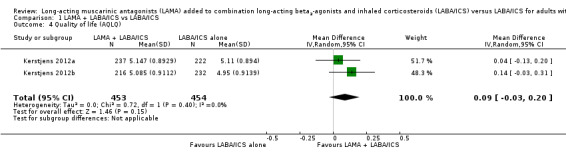
Comparison 1 LAMA + LABA/ICS vs LABA/ICS, Outcome 4 Quality of life (AQLQ).
Serious adverse events (all causes)
The effect estimate suggested fewer serious adverse events when people took tiotropium, but the difference against LABA/ICS alone was not statistically significant, and there was a large degree of inconsistency between individual studies (OR 0.60, 95% CI 0.24 to 1.47; participants = 1197; studies = 3; I2 = 76%; Analysis 1.5). Pooling all three studies, the confidence intervals included appreciable harm on either treatment, largely because Ohta 2014 had a much larger effect in favour of tiotropium. Ohta 2014 was different from the other two studies because it combined two dose groups in the analysis. In addition, the study included participants who were taking either ICS alone as background treatment or LABA/ICS. Consequently, some results are from participants who do not meet all inclusion criteria for this review. A post hoc sensitivity analysis removing this study made the effect much more precise but did not change the interpretation that there was not a clear difference. We downgraded for inconsistency and indirectness but not for imprecision, and so rated it as low quality.
1.5. Analysis.
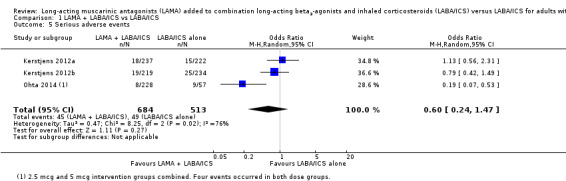
Comparison 1 LAMA + LABA/ICS vs LABA/ICS, Outcome 5 Serious adverse events.
Secondary outcomes
Exacerbations requiring hospital admission
Tiotropium add‐on did not reduce the number of people needing to go to hospital for an exacerbation of their asthma (OR 0.68, 95% CI 0.34 to 1.38; participants = 1191; studies = 3; I2 = 11%; Analysis 1.6). While there were slightly more hospital visits in those not taking tiotropium, the difference between groups was not statistically significant, and the confidence intervals were wide. Ohta 2014 showed a much larger effect in favour of tiotropium add‐on than the other two studies, which may be due to the indirectness of the population, as described above. However, the effect in this study carried less weight because it was based on very few events, and the confidence intervals included the effects of the other two studies. Removing the study in a post hoc sensitivity analysis on the basis of an indirect population did not significantly improve the precision of the estimate. We downgraded the evidence for indirectness and imprecision, rating it as low quality.
1.6. Analysis.
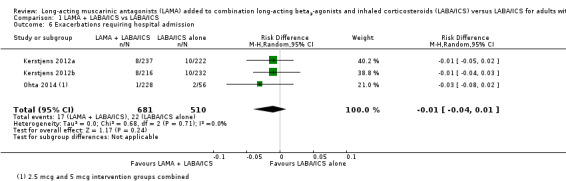
Comparison 1 LAMA + LABA/ICS vs LABA/ICS, Outcome 6 Exacerbations requiring hospital admission.
Lung function
Change in lung function, as measured by trough FEV1, was 0.07 L better in those taking tiotropium in addition to LABA/ICS (MD 0.07, 95% CI 0.03 to 0.11; participants = 1191; studies = 3; I2 = 0%; Analysis 1.7). The study with a partly indirect population contributed to this outcome, but its results were not inconsistent with the other studies, so we did not downgrade the evidence for indirectness, rating it as high quality. We also analysed a second lung function measure that was reported in three studies, and the results were consistent with a modest benefit of tiotropium add‐on over LABA/ICS alone (trough FVC: MD 0.07, 95% CI 0.02 to 0.13; participants = 1191; studies = 3; I2 = 0%; Analysis 1.8).
1.7. Analysis.
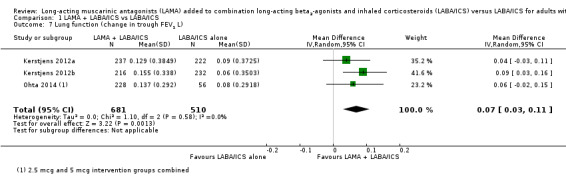
Comparison 1 LAMA + LABA/ICS vs LABA/ICS, Outcome 7 Lung function (change in trough FEV1 L).
1.8. Analysis.
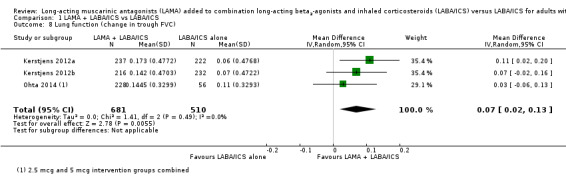
Comparison 1 LAMA + LABA/ICS vs LABA/ICS, Outcome 8 Lung function (change in trough FVC).
Asthma control
Scores on the Asthma Control Questionnaire (ACQ) were slightly better with tiotropium add‐on compared with LABA/ICS alone, but the difference was not clinically significant (MD − 0.13, 95% CI − 0.23 to − 0.02; participants = 907; studies = 2; I2 = 0%; Analysis 1.9). We did not downgrade the evidence and rated it as high quality.
1.9. Analysis.
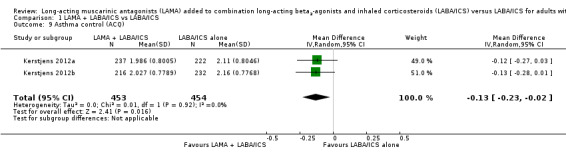
Comparison 1 LAMA + LABA/ICS vs LABA/ICS, Outcome 9 Asthma control (ACQ).
We also found data for the number of people meeting the criteria for 'response' on the ACQ (an improvement in the total score of at least 0.5 points). The twin trials and one other study reported this outcome as a pooled result; it favoured of tiotropium add‐on, but the confidence intervals did not exclude the possibility that people on LABA/ICS alone did better (OR 1.42, 95% CI 0.88 to 2.29; participants = 1192; studies = 2; I2 = 51%; Analysis 1.10).
1.10. Analysis.
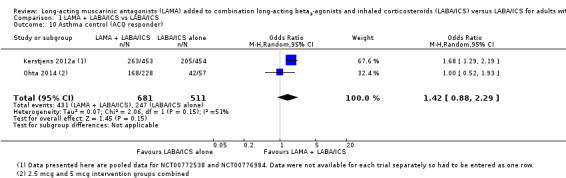
Comparison 1 LAMA + LABA/ICS vs LABA/ICS, Outcome 10 Asthma control (ACQ responder).
Any adverse events
People taking tiotropium add‐on were less likely to have adverse events than those taking LABA/ICS alone (OR 0.70, 95% CI 0.52 to 0.94; participants = 1197; studies = 3; I2 = 0%; Analysis 1.11). The study with an indirect population contributed to this outcome, but its results were not inconsistent with the other studies, so we did not downgrade the evidence for indirectness, rating it as high quality.
1.11. Analysis.
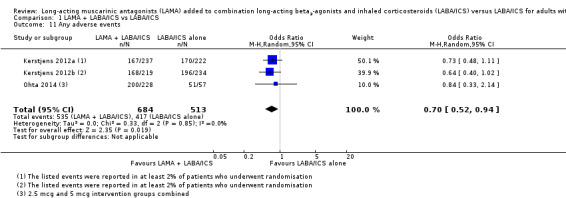
Comparison 1 LAMA + LABA/ICS vs LABA/ICS, Outcome 11 Any adverse events.
Subgroup analysis and investigation of heterogeneity
Duration of therapy (≤ 6 months vs > 6 months)
All of the studies lasted longer than six months, so it was not possible to explore a possible effect of study duration through a subgroup analysis. However, at times studies reported some outcomes at midpoint (either 24 or 26 weeks) and endpoint (48 or 52 weeks). Within the primary outcomes, this was only true for the AQLQ, and results were not different at the midpoint than at the primary endpoint analysis (MD 0.11, 95% CI − 0.03 to 0.24 at 24 weeks; MD 0.09, 95% CI − 0.03 to 0.20 at 48‐week endpoint; Analysis 1.12).
1.12. Analysis.
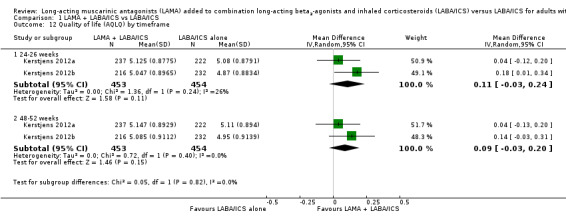
Comparison 1 LAMA + LABA/ICS vs LABA/ICS, Outcome 12 Quality of life (AQLQ) by timeframe.
Dose and type of LABA/ICS (e.g. formoterol/budesonide 9/320 vs salmeterol/fluticasone 50/250 µg)
The included studies required participants to be taking LABA/ICS but did not include a particular combination product as part of the randomised treatment (i.e. participants continued with whatever they were taking prior to the trial). The twin trials required participants to be taking stable high doses of LABA/ICS, and Ohta 2014 required participants to be taking stable medium doses of ICS, "alone or in a fixed combination with a LABA, for at least four weeks". As such, we could not make a clear comparison across studies on the basis of different types or doses of background treatment.
Dose and type of LAMA (e.g. tiotropium HandiHaler 18 µg vs tiotropium Respimat 5 µg)
The twin trials both compared tiotropium Respimat 5 µg with placebo, and only these two studies appeared in the analyses for exacerbations requiring oral steroids and quality of life. In the third primary outcome, serious adverse events, the effect in the third study, which included an additional group receiving 2.5 µg Ohta 2014, was much more in favour of LAMA add‐on, but this could not be explained by dose (4/114 events in each groups). Within‐study analyses in Ohta 2014 showed that "adjusted mean trough FEV1 and trough PEFR [peak expiratory flow rate] responses were significantly higher with tiotropium 5 μg (but not 2.5 μg) versus placebo" at week 52, but differences between the two doses were not statistically significant for other outcomes.
Sensitivity analyses
Studies at high risk of bias for blinding of participants and personnel
All of the studies were at low risk of bias for these domains, so it was not necessary to conduct this planned sensitivity analysis.
Unpublished data (i.e. no peer‐reviewed full paper available)
Athough there was not always a peer‐reviewed publication available, all of the studies were registered, and all of the data included in the meta‐analysis were freely available on ClinicalTrials.gov. As such, it was not necessary to conduct this sensitivity analysis.
Cross‐over trials
No cross‐over trials met the inclusion criteria, so it was not necessary to conduct this planned sensitivity analysis.
Discussion
Summary of main results
We found four double‐blind, double‐dummy trials that compared LAMA to placebo for people with asthma who were already taking combination LABA/ICS. One of the trials was designed to study glycopyrronium bromide but was withdrawn prior to enrolment, and the three others studied tiotropium bromide over 48 to 52 weeks. People in the trials generally had quite poor lung function, with FEV1 of around 55% of their predicted value.
People randomised to take a LAMA add‐on had fewer exacerbations requiring oral corticosteroids than those continuing to take LABA/ICS alone, although the confidence intervals included no difference (OR 0.76, 95% CI 0.57 to 1.02), so we considered the evidence to be moderate quality. Over 48 weeks, 328 out of 1000 people taking their usual LABA/ICS would have to take oral corticosteroids for an exacerbation compared with 271 if they took a LAMA as well (95% CI 218 to 333 per 1000). Analyses comparing the number of exacerbations per patient in each group (rate ratio) and the time until first exacerbation (hazard ratio) were in keeping with the main result. Quality of life (AQLQ) was no better for those taking LAMA add‐on than those taking LABA/ICS alone when considered in light of the 0.5 minimal clinically important difference on the scale (MD 0.09, 95% CI − 0.03 to 0.20), and evidence for whether LAMA increased or decreased serious adverse events in this population was inconsistent (OR 0.60, 95% CI 0.24 to 1.47; I2 = 76%).
Within the secondary outcomes, exacerbations requiring hospital admission were too rare to tell whether LAMA was beneficial over LABA/ICS alone, and we considered the evidence to be low quality. There was high quality evidence showing benefits to lung function (trough FEV1 and FVC) and potentially small benefits to asthma control. People taking a LAMA add‐on were less likely to experience non‐serious adverse events.
Overall completeness and applicability of evidence
We found a limited number of studies that met the inclusion criteria for our review, and there were differences between them that need to be considered when interpreting the evidence. Ohta 2014 enrolled a patient population with less severe asthma, allowing people to participate if they were only on background treatment with stable medium‐dose ICS and if their asthma symptoms had occurred for as little as 12 weeks. This is in contrast to the twin studies, where the patients had to have persistent airway limitation despite a background therapy of high dose inhaled glucocorticoids with LABAs and at least one exacerbation that was treated with systemic glucocorticoids in the previous year (Kerstjens 2012a; Kerstjens 2012b). The difference in asthma severity translates to different points of pharmacotherapy management within the asthma treatment algorithm. When determining the appropriate step to initiate LAMA therapy, it is imperative to know at what stage of asthma drug management provides the most benefit to patients. In addition, all of the trials evaluated the addition of a LAMA versus placebo. There were no trials that were included in the review that directly compared the addition of a LAMA to the addition of another active comparator.
Previous reviews that have evaluated LAMA addition to inhaled corticosteroids have indicated that trials need durations of at least six months in order to identify exacerbations (Anderson 2015). While the trials included in this review were of sufficient duration, from 48 to 52 weeks, exacerbations requiring hospital admission occurred at too low of a rate to assess whether there was a benefit of LAMA add‐on.
The included trials investigated the addition of tiotropium delivered via the Respimat device. The one trial that was withdrawn prior to completion was going to investigate the use of glycopyrronium (NCT02127697). Tiotropium delivered via the Handihaler device and the newer LAMA agents such as umeclidinium and aclidinium that have recently been released have yet to be evaluated in the treatment of asthma. We cannot be certain that the results that we have seen with tiotropium delivered via the Respimat device will be consistently found within this therapeutic class. The studies added tiotropium Respimat to LABA/ICS therapy; however the exact LABA/ICS combination was not specified. The twin trials required participants to be taking stable high doses of LABA/ICS (Kerstjens 2012a; Kerstjens 2012b), and Ohta 2014 required participants to be taking stable medium doses of ICS alone or in a fixed combination with a LABA. As with all study‐based subgroup analyses, comparing the high dose LABA/ICS trials to the medium dose trial would be an observational result that could be confounded by any number of other factors (age, tiotropium dose, adherence, comorbidities, the presence and type of LABA). As such, especially given the small number of trials that are currently available, we did not feel able to draw conclusions regarding the benefits or harms of tiotropium according to the background dose of LABA/ICS.
The evidence that we found did not suggest that LAMAs increased the risk of serious adverse events. However, the trials included a limited number of patients with strict inclusion criteria. Drug companies sponsored all of the included studies. While generally we found them to have low risk of bias, the use of LAMAs outside a strict study environment may lead to either different effects on the measured outcomes or signals for adverse events.
Quality of the evidence
Our confidence in the evidence varied considerably across the primary and secondary outcomes. The most common reason for downgrading evidence quality was imprecision in the estimates, which was partly due to the relatively small number of studies. In addition, for rarer events such as exacerbations requiring hospital admission and serious adverse events, longer studies would be better able to assess any difference between groups more robustly. Only the analyses on quality of life, lung function, asthma control and any adverse events included sufficient people or events to confer confidence in the direction of effect; due to the width of the confidence intervals, we could not conclude that LAMA add‐on was better than LABA/ICS alone for exacerbations requiring oral corticosteroids, serious adverse events or exacerbations requiring hospital admission.
Ohta 2014 introduced indirectness into some of the analyses, which we tested and described with post hoc sensitivity analyses. The study required participants to be taking ICS with or without a LABA, so some participants did not meet the prespecified inclusion criteria for this review and may have had less severe asthma. The results of this study introduced clinical and statistical heterogeneity into the serious adverse events analysis in particular, but also into the analysis of exacerbations requiring hospital admission and ACQ responders. It was the only study to include two doses of tiotropium Respimat, which may also have contributed to differences with the other studies.
Despite these limitations, we did not consider any of the analyses to be compromised by internal risk of bias in the included studies, which were all double‐blind, double‐dummy randomised trials. Nor did we suspect publication bias either within the included studies or due to the absence of other unpublished trials. However, we note that the glycopyrronium trial NCT02127697 was withdrawn without explanation, and that all of the studies were funded by industry, and this represents a potential for bias in the evidence base.
Potential biases in the review process
We closely followed the methods set out in our review protocol (Kew 2015), which was developed in line with Cochrane guidelines. In addition to trial registry searches required by Cochrane, we conducted extensive additional searches of manufacturer databases to identify unpublished studies. Industry‐funded studies conducted since the development of LAMAs should all have been registered and reported on trial registries, but it is possible that other independent studies have been conducted and not yet made available.
It is possible that the decision to include Ohta 2014 in this review introduced bias, although we considered this eventuality throughout the review process and fully addressed it with sensitivity analyses and the respective GRADE ratings for analyses to which the trial contributed.
Agreements and disagreements with other studies or reviews
There have been three other meta‐analyses that have investigated the addition of LAMA for patients with asthma (Lee 2014; Rodrigo 2015; Tian 2014). All three included only trials that evaluated tiotropium. The most recent publication included patients aged 12 years and over who were receiving maintenance therapy with either an inhaled corticosteroid or an inhaled corticosteroid plus a LABA for a minimum duration of four weeks (Rodrigo 2015). Reviewers divided the thirteen studies that met the inclusion criteria into three treatment groups: tiotropium once daily as an add‐on to ICS in patients with mild to moderate asthma, tiotropium once daily added to ICS versus twice daily LABA/ICS in patients with moderate asthma, and tiotropium once daily as add‐on to LABA/ICS versus LABA/ICS in patients with severe asthma. The last treatment group had three studies and was comparable to the focus of our meta‐analysis. However, only one of the included studies also met our inclusion criteria; we had to exclude the other two because they were too short in duration for our review. The review found an improvement in FEV1 with addition of the LAMA. Reviewers defined exacerbations as the number of patients with one or more episodes requiring the use of systemic corticosteroids, and they concluded that the number needed to treat for an additional beneficial outcome (NNTB) was 17 for this outcome, with a difference in occurrence of 18.2% versus 24.0%. This differs from our findings, which did not show a statistically significant difference.
Rodrigo 2015 was the only one of the three publications that separately evaluated the addition of LAMA to LABA/ICS. The remaining two publications included trials with different background therapies, which they analysed together (Lee 2014; Tian 2014). Lee 2014 included a total of five studies, which varied from adding tiotropium to various ICS doses to adding tiotropium to LABA/ICS combination therapy. They found similar improvements in FEV1 in addition to a decrease in the odds of having a severe acute exacerbation of asthma (OR 0.73, 95% CI 0.56 to 0.96). However, their definition of this outcome was an exacerbation that showed a decline in a patient's respiratory symptoms leading to the use of systemic corticosteroids or the increased use of ICS or other asthma medications, and only two of the trials actually evaluated this outcome. Tian 2014 also included a mix of studies that evaluated either ICS alone or combinations of LABA/ICS as background therapy. In addition, one of the included trials evaluated tiotropium in adolescents aged 12 to 17 years old. Despite these differences, their statistical analysis yielded similar results to what we found: asthma exacerbations were less frequent in the tiotropium group (OR 0.70, 95% CI 0.52 to 0.96, P = 0.02). However, they did not provide a definition for this outcome.
None of the above trials found an increase in adverse events with the addition of tiotropium (Lee 2014; Rodrigo 2015; Tian 2014), and this review suggests that tiotropium may lead to fewer non‐serious adverse events than using LABA/ICS alone.
There have been a number of trials and meta‐analyses that have evaluated the use of tiotropium as an add‐on to ICS alone. These trials have found improvements in peak expiratory flow (PEF), FEV1 and more importantly, the occurrence of asthma exacerbations, with the addition of tiotropium (Anderson 2015; Rodrigo 2015).
Authors' conclusions
Implications for practice.
Tiotropium add‐on may have additional benefits over LABA/ICS alone to reduce the need for rescue oral steroids in people with severe asthma.The effect was imprecise, and there was no evidence for other LAMA preparations. Possible benefits on quality of life were negligible, and evidence for the effect on serious adverse events was inconsistent. There are likely to be small added benefits of tiotropium Respimat 5 µg daily on lung function and asthma control over LABA/ICS alone, and fewer non‐serious adverse events. The benefit of tiotropium add‐on on the frequency of hospital admission is not yet known, despite year‐long trials.
Implications for research.
Ongoing and future trials should be clear about the background medications taken by participants to help clinicians judge how the findings relate to stepwise care. If LAMAs other than tiotropium Respimat are tested for asthma, trials should be at least six months long and use accepted and validated outcomes to allow comparisons between the safety and effectiveness of different preparations.
Acknowledgements
We would like to thank Liz Stovold for developing the search strategy and running the electronic database searches and Emma Chen and Vanessa Chu for their translation support.
Chris Cates was the Editor for this review and commented critically on the review.
The Background and Methods sections of this protocol are based on a standard template used by the Cochrane Airways Group.
Appendices
Appendix 1. Sources and search methods for the Cochrane Airways Group Specialised Register (CAGR)
Electronic searches: core databases
| Database | Frequency of search |
| CENTRAL | Monthly |
| MEDLINE (Ovid) | Weekly |
| EMBASE (Ovid) | Weekly |
| PsycINFO (Ovid) | Monthly |
| CINAHL (EBSCO) | Monthly |
| AMED (EBSCO) | Monthly |
Handsearches: core respiratory conference abstracts
| Conference | Years searched |
| American Academy of Allergy, Asthma and Immunology (AAAAI) | 2001 onwards |
| American Thoracic Society (ATS) | 2001 onwards |
| Asia Pacific Society of Respirology (APSR) | 2004 onwards |
| British Thoracic Society Winter Meeting (BTS) | 2000 onwards |
| Chest Meeting | 2003 onwards |
| European Respiratory Society (ERS) | 1992, 1994, 2000 onwards |
| International Primary Care Respiratory Group Congress (IPCRG) | 2002 onwards |
| Thoracic Society of Australia and New Zealand (TSANZ) | 1999 onwards |
MEDLINE search strategy used to identify trials for the CAGR
Asthma search
1. exp Asthma/
2. asthma$.mp.
3. (antiasthma$ or anti‐asthma$).mp.
4. Respiratory Sounds/
5. wheez$.mp.
6. Bronchial Spasm/
7. bronchospas$.mp.
8. (bronch$ adj3 spasm$).mp.
9. bronchoconstrict$.mp.
10. exp Bronchoconstriction/
11. (bronch$ adj3 constrict$).mp.
12. Bronchial Hyperreactivity/
13. Respiratory Hypersensitivity/
14. ((bronchial$ or respiratory or airway$ or lung$) adj3 (hypersensitiv$ or hyperreactiv$ or allerg$ or insufficiency)).mp.
15. ((dust or mite$) adj3 (allerg$ or hypersensitiv$)).mp.
16. or/1‐15
Filter to identify RCTs
1. exp "clinical trial [publication type]"/
2. (randomized or randomised).ab,ti.
3. placebo.ab,ti.
4. dt.fs.
5. randomly.ab,ti.
6. trial.ab,ti.
7. groups.ab,ti.
8. or/1‐7
9. Animals/
10. Humans/
11. 9 not (9 and 10)
12. 8 not 11
The MEDLINE strategy and RCT filter are adapted to identify trials in other electronic databases.
Appendix 2. Search strategy to identify relevant trials from the CAGR
#1 AST:MISC1
#2 MeSH DESCRIPTOR Asthma Explode All
#3 asthma*:ti,ab
#4 #1 or #2 or #3
#5 MeSH DESCRIPTOR Adrenal Cortex Hormones
#6 inhal* NEAR (corticosteroid* or steroid* or glucocorticoid*)
#7 beclomethasone* or beclometasone* OR triamcinolone* OR fluticasone* OR budesonide* OR betamethasone* OR flunisolide* OR ciclesonide* OR mometasone*
#8 ICS:TI,AB
#9 #5 or #6 or #7 or #8
#10 MeSH DESCRIPTOR Adrenergic beta‐Agonists
#11 long* NEAR beta* NEAR agonist*
#12 LABA:TI,AB
#13 *formoterol
#14 salmeterol
#15 vilanterol
#16 #10 or #11 or #12 or #13 or #14 or #15
#17 Muscarinic* NEXT Antagonist*
#18 LAMA:TI,AB
#19 Glycopyrronium*
#20 NVA237
#21 Seebri OR Breezhaler
#22 Aclidinium*
#23 LAS34273
#24 Turdorza or Pressair or Eklira or Genuair
#25 tiotropium*
#26 Spiriva
#27 umeclidinium*
#28 GSK573719
#29 #17 or #18 or #19 or #20 or #21 or #22 or #23 or #24 or #25 or #26 or #27 or #28
#30 #9 AND #16 and #29
#31 triple* NEAR2 therap*
#32 #4 AND (#30 OR #31)
[Note: in search line #1, MISC1 denotes the filed in which the reference has been coded for condition, in this case, asthma]
Data and analyses
Comparison 1. LAMA + LABA/ICS vs LABA/ICS.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Exacerbations requiring oral corticosteroids (patients with at least one) | 2 | 907 | Odds Ratio (M‐H, Random, 95% CI) | 0.76 [0.57, 1.02] |
| 2 Exacerbations requiring oral corticosteroids (number per patient) | 2 | 907 | Rate Ratio (Random, 95% CI) | 0.79 [0.53, 1.17] |
| 3 Time to first exacerbation requiring oral corticosteroids | 2 | 907 | Hazard Ratio (Random, 95% CI) | 0.80 [0.63, 1.01] |
| 4 Quality of life (AQLQ) | 2 | 907 | Mean Difference (IV, Random, 95% CI) | 0.09 [‐0.03, 0.20] |
| 5 Serious adverse events | 3 | 1197 | Odds Ratio (M‐H, Random, 95% CI) | 0.60 [0.24, 1.47] |
| 6 Exacerbations requiring hospital admission | 3 | 1191 | Risk Difference (M‐H, Random, 95% CI) | ‐0.01 [‐0.04, 0.01] |
| 7 Lung function (change in trough FEV1 L) | 3 | 1191 | Mean Difference (IV, Random, 95% CI) | 0.07 [0.03, 0.11] |
| 8 Lung function (change in trough FVC) | 3 | 1191 | Mean Difference (IV, Random, 95% CI) | 0.07 [0.02, 0.13] |
| 9 Asthma control (ACQ) | 2 | 907 | Mean Difference (IV, Random, 95% CI) | ‐0.13 [‐0.23, ‐0.02] |
| 10 Asthma control (ACQ responder) | 2 | 1192 | Odds Ratio (M‐H, Random, 95% CI) | 1.42 [0.88, 2.29] |
| 11 Any adverse events | 3 | 1197 | Odds Ratio (M‐H, Random, 95% CI) | 0.70 [0.52, 0.94] |
| 12 Quality of life (AQLQ) by timeframe | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 12.1 24‐26 weeks | 2 | 907 | Mean Difference (IV, Random, 95% CI) | 0.11 [‐0.03, 0.24] |
| 12.2 48‐52 weeks | 2 | 907 | Mean Difference (IV, Random, 95% CI) | 0.09 [‐0.03, 0.20] |
1.2. Analysis.
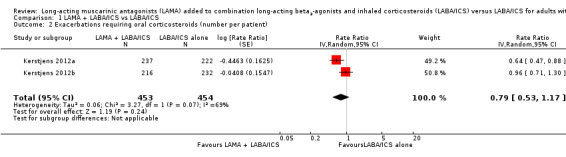
Comparison 1 LAMA + LABA/ICS vs LABA/ICS, Outcome 2 Exacerbations requiring oral corticosteroids (number per patient).
1.3. Analysis.
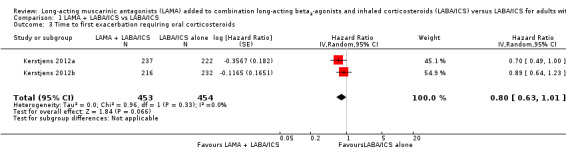
Comparison 1 LAMA + LABA/ICS vs LABA/ICS, Outcome 3 Time to first exacerbation requiring oral corticosteroids.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Kerstjens 2012a.
| Methods |
Study design: 48‐week, parallel, double‐blind RCT Setting: 73 study centres in 14 countries (Australia, Canada, Denmark, Germany, Italy, Japan, Netherlands, Russia, Serbia, South Africa, Turkey, Ukraine, United Kingdom, United States) |
|
| Participants |
Population: 459 people were randomised to receive tiotropium or placebo Baseline characteristics: N randomised: tiotropium 237; placebo 222 N completed: tiotropium 211; placebo 202 Mean age (SD): tiotropium 52.9 (12.4); placebo 53.9 (12.8) % male: tiotropium 38.4; placebo 35.6 % predicted FEV1(SD): tiotropium 54.6 (12.2); placebo 54.6 (12.2) Duration of asthma, years (SD): tiotropium 31 (NR); placebo 28 (NR) Inclusion criteria: informed consent form; male or female patients aged 18‐75 years; ≥ 5‐year history of asthma and diagnosis made before age 40; diagnosis of severe persistent asthma that is symptomatic despite treatment with high, stable doses of ICS and a LABA; history of ≥ 1 asthma exacerbation(s) in the past year; evidence of treated, severe, persistent asthma in post bronchodilatory pulmonary function tests; never‐smokers or ex‐smokers who stopped smoking ≥ 1 year prior to enrolment and who have a smoking history of < 10 pack‐years; able to use the Respimat inhaler correctly; able to perform all trial‐related procedures including technically acceptable pulmonary function tests and use of the electronic diary/peak flow meter Exclusion criteria: significant disease other than asthma; clinically relevant abnormal screening haematology or blood chemistry; recent history (i.e. ≤ 6 months) of myocardial infarction, hospitalisation for cardiac failure during the past year, cardiac arrhythmia requiring treatment within the past year, known active TB, resection, radiation or chemotherapy for malignancy within previous 5 years (treated basal cell carcinoma allowed), lung diseases other than asthma (e.g. COPD), significant alcohol or drug abuse within previous 2 years, thoracotomy with pulmonary resection; current or recent pulmonary rehabilitation program (previous 6 weeks); OCS at stable doses > 5 mg prednisolone equivalent daily or 10 mg every second day; known hypersensitivity to anticholinergic drugs or any components of the tiotropium inhaler; pregnant or nursing women or women of childbearing potential not using a highly effective method of birth control; investigational drug use within 4 weeks or 6 half‐lives (whichever is greater); treated in the previous 4 weeks with tiotropium (Spiriva), beta‐blocker, oral beta‐adrenergic, or other non‐approved 'experimental' drugs for routine asthma therapy that are not recommended by international guidelines; any asthma exacerbation or RTI in the 4 weeks prior to the trial; previously randomised in this trial or in the respective twin trial (Kerstjens 2012b) or currently participating in another trial; known narrow‐angle glaucoma |
|
| Interventions |
Intervention: tiotropium Respimat 5 µg once daily Control: placebo Respimat inhaler taken once daily Background treatment: usual treatment with high, stable doses of inhaled corticosteroids and a long‐acting beta adrenergic agent |
|
| Outcomes |
Primary: peak FEV1 response within 3 h post dosing after 24 weeks, trough FEV1 response after 24 weeks, time to first severe exacerbation during 48 weeks (pooled with twin trial) Secondary: range of lung function measures at 24 and 48 weeks (peak FEV1 0‐3 hours, trough FEV1, peak FVC, trough FVC, FEV1 AUC, FVC AUC, trough morning and evening PEF, PEF variability), all exacerbations and severe exacerbations (time to first, number per patient, and number of patients with at least 1), hospitalisations for exacerbations (time to first, number per patient, and number of patients with at least 1), AQLQ total score, ACQ, symptom‐free days, rescue medication use |
|
| Notes |
Funding: Boehringer Ingelheim with collaboration from Pfizer ID number(s): NCT00772538; PrimoTinA‐asthma 1; 205.416; 2008‐001413‐14 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The randomization schedule was generated by a validated system (PMX CTM, release 3.3.0 HP2, Propack Data) with the use of a pseudo–random number generator and a supplied seed number." |
| Allocation concealment (selection bias) | Low risk | "The randomisation code will be kept by Clinical Trial Support (within Medical Data Services/Biostatistics and Data Management) up to database lock. They will only release it according to this protocol." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Patients, investigators, and everyone involved in the analysis or with an interest in this double‐blind trial (except members of the independent data monitoring committee for the unblinded interim analysis) will remain blinded with regard to the randomised treatment assignments up to database lock unless foreseen otherwise in this protocol." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "Patients, investigators, and everyone involved in the analysis or with an interest in this double‐blind trial (except members of the independent data monitoring committee for the unblinded interim analysis) will remain blinded with regard to the randomised treatment assignments up to database lock unless foreseen otherwise in this protocol." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Patient dropout was 11% and 9% in the treatment and control groups, respectively, and the ITT population used for the analyses included everyone who was randomised and received at least 1 dose of medication (appears to be everyone). |
| Selective reporting (reporting bias) | Low risk | Full trial results according to the published protocol are available on ClinicalTrials.gov and in a published paper with the twin trial. |
| Other bias | Low risk | None noted |
Kerstjens 2012b.
| Methods |
Study design: 48‐week, parallel, double‐blind RCT Setting: 75 study centres in 15 countries (Australia, Canada, Denmark, Germany, Italy, Japan, Netherlands, New Zealand, Russia, Serbia, South Africa, Turkey, Ukraine, United Kingdom, United States) |
|
| Participants |
Population: 453 people were randomised to receive tiotropium or placebo Baseline characteristics: N randomised: tiotropium 219; placebo 234 N completed: tiotropium 198; placebo 203 Mean age (SD): tiotropium 51.4 (12.5); placebo 53.6 (11.7) % male: tiotropium 42.0; placebo 42.3 % predicted FEV1(SD): tiotropium 55.1 (12.8); placebo 55.0 (12.6) Duration of asthma, years (SD): tiotropium 26 (NR); placebo 28 (NR) Inclusion criteria: informed consent form; male or female patients aged 18‐75 years; ≥ 5‐year history of asthma and diagnosis made before age 40; diagnosis of severe persistent asthma that is symptomatic despite treatment with high, stable doses of ICS and a LABA; history of ≥ 1 asthma exacerbation(s) in the past year; evidence of treated, severe, persistent asthma in postbronchodilatory pulmonary function tests; never‐smokers or ex‐smokers who stopped smoking ≥ 1 year prior to enrolment and who have a smoking history of < 10 pack‐years; able to use the Respimat inhaler correctly; able to perform all trial‐related procedures including technically acceptable pulmonary function tests and use of the electronic diary/peak flow meter Exclusion criteria: significant disease other than asthma; clinically relevant abnormal screening haematology or blood chemistry; recent history (i.e. ≤ 6 months) of myocardial infarction, hospitalisation for cardiac failure during the past year, cardiac arrhythmia requiring treatment within the past year, known active TB, resection, radiation or chemotherapy for malignancy within previous 5 years (treated basal cell carcinoma allowed), lung diseases other than asthma (e.g. COPD), significant alcohol or drug abuse within previous 2 years, thoracotomy with pulmonary resection; current or recent pulmonary rehabilitation program (previous 6 weeks); OCS at stable doses > 5 mg prednisolone equivalent daily or 10 mg every second day; known hypersensitivity to anticholinergic drugs or any components of the tiotropium inhaler; pregnant or nursing women or women of childbearing potential not using a highly effective method of birth control; investigational drug use within 4 weeks or 6 half‐lives (whichever is greater); treated in the previous 4 weeks with tiotropium (Spiriva), beta‐blocker, oral beta‐adrenergic, or other non‐approved 'experimental' drugs for routine asthma therapy that are not recommended by international guidelines; any asthma exacerbation or RTI in the 4 weeks prior to the trial; previously randomised in this trial or in the respective twin trial (Kerstjens 2012a) or currently participating in another trial; known narrow‐angle glaucoma. |
|
| Interventions |
Intervention: Tiotropium Respimat 5 µg once daily Control: Placebo Respimat inhaler taken once daily Background treatment: usual treatment with high, stable doses of inhaled corticosteroids and a long‐acting beta adrenergic agent |
|
| Outcomes |
Primary: peak FEV1 response within 3 hours postdosing after 24 weeks, trough FEV1 response after 24 weeks, time to first severe exacerbation during 48 weeks (pooled with twin trial) Secondary: range of lung function measures at 24 and 48 weeks (peak FEV1 0‐3 hours, trough FEV1, peak FVC, trough FVC, FEV1 AUC, FVC AUC, trough morning and evening PEF, PEF variability), all exacerbations and severe exacerbations (time to first, number per patient, and number of patients with at least 1), hospitalisations for exacerbations (time to first, number per patient, and number of patients with at least 1), AQLQ total score, ACQ, symptom‐free days, rescue medication use |
|
| Notes |
Funding: Boehringer Ingelheim with collaboration from Pfizer ID number(s): NCT00776984; PrimoTinA‐asthma 2; 205.417; 2008‐001414‐25 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The randomization schedule was generated by a validated system (PMX CTM, release 3.3.0 HP2, Propack Data) with the use of a pseudo–random number generator and a supplied seed number." |
| Allocation concealment (selection bias) | Low risk | "The randomisation code will be kept by Clinical Trial Support (within Medical Data Services/Biostatistics and Data Management) up to database lock. They will only release it according to this protocol." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Patients, investigators, and everyone involved in the analysis or with an interest in this double‐blind trial (except members of the independent data monitoring committee for the unblinded interim analysis) will remain blinded with regard to the randomised treatment assignments up to database lock unless foreseen otherwise in this protocol" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "Patients, investigators, and everyone involved in the analysis or with an interest in this double‐blind trial (except members of the independent data monitoring committee for the unblinded interim analysis) will remain blinded with regard to the randomised treatment assignments up to database lock unless foreseen otherwise in this protocol" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Patient dropout was 10% and 13% in the treatment and control groups respectively, and the ITT population used for the analyses included everyone who was randomised and received at least 1 dose of medication (448/453 randomised) |
| Selective reporting (reporting bias) | Low risk | Full trial results according to the published protocol are available on clinicaltrials.gov and in a published format with the twin trial |
| Other bias | Low risk | None noted |
NCT02127697.
| Methods |
Study design: 52‐week parallel, double‐blind RCT Setting: 30 countries listed, does not mention the number of participating centres |
|
| Participants |
Population: This trial was withdrawn before any participants were enrolled Inclusion criteria: written informed consent; male and female adult patients aged 18‐75 years; diagnosis of asthma (according to GINA 2012) ≥ 5 years previous to trial, made before the patient was 40; increase in FEV1 of ≥ 12% and ≥ 200 mL within 30 min of 400 µg salbutamol/360 µg albuterol (or equivalent); pre‐bronchodilator FEV1 of ≥ 50 and ≤ 80% predicted normal; treated with a stable dose of a fixed dose inhaled corticosteroid (ICS) and long‐acting beta2‐agonist (LABA) combination for at least 4 weeks prior to screening; total daily dose of ICS of ≥ 800 µg/d of budesonide or equivalent; symptomatic with a mean ACQ‐5 score ≥ 1.5; documented history of ≥ 1 asthma exacerbations in the previous 12 months that required systemic corticosteroids, emergency room visit, hospital treatment or intubation Exclusion criteria: contraindicated for or hypersensitivity to any of the following inhaled drugs, drugs of a similar class, or any component thereof: muscarinic antagonist agents, sympathomimetic amines, lactose or any of the other excipients of the study drug, long‐ and short‐acting beta2‐agonists, corticosteroids; women of child‐bearing potential; resting QTcF ≥ 450 ms (male) or ≥ 460 ms (female); BMI > 40 kg/m2; clinically significant comorbidity; asthma exacerbation that required systemic corticosteroids, emergency room visit, hospital treatment or intubation in the 6 weeks prior to screening; smoked or inhaled tobacco products within 6 months, or a smoking history of > 10 pack years; history of chronic lung diseases other than asthma. Maintenance immunotherapy for allergies must have been so for ≥ 3 months prior to run‐in, and must be expected to remain unchanged throughout the course of the study |
|
| Interventions |
Intervention: glycopyrronium bromide once daily ‐ dose not specified Control: placebo Background treatment: stable dose of a fixed dose inhaled corticosteroid (ICS) and long‐acting beta2‐agonist (LABA) combination for at least 4 weeks prior to screening |
|
| Outcomes |
Primary: trough FEV1 at 26 weeks Secondary: time to first moderate or severe asthma exacerbation (52 weeks), ACQ (26 weeks), ACQ‐5, ACQ‐6 and ACQ‐7 at various time points, AQLQ (12, 26, 52 weeks), SGRQ (12, 26, 52 weeks), variety of lung function measures at day 1 and weeks 4, 26, and 52 (peak FEV, FEV1 standardised AUC, predose FEV1, trough FEV1, morning and evening PEF, FVC), mean daily number of puffs of rescue medication, rate of moderate or severe exacerbation, rate of severe exacerbation, time to first severe exacerbation, time to first mild, moderate or severe exacerbation, rate of mild moderate or severe exacerbation, asthma control diary symptom score |
|
| Notes | WITHDRAWN PRIOR TO ENROLLMENT Funding: Novartis Pharmaceuticals ID number(s): NCT02127697, CNVA237B2301 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Study was withdrawn prior to enrolment |
| Allocation concealment (selection bias) | Unclear risk | No participants enrolled |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No participants enrolled |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No participants enrolled |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No participants enrolled |
| Selective reporting (reporting bias) | High risk | Study was withdrawn prior to enrolment and hence no results are available and minimal methods reported on the trial registration page |
| Other bias | High risk | Not clear why the study was withdrawn |
Ohta 2014.
| Methods |
Study design: 52‐week parallel, double‐blind RCT Setting: 55 Boehringer Ingelheim investigational sites in Japan |
|
| Participants |
Population: 285 people were randomised to receive tiotropium 2.5 µg, tiotropium 5 µg, or placebo (57) Baseline characteristics: N randomised: tiotropium low 114; tiotropium high 114; placebo 57 N completed: tiotropium low 106; tiotropium high 106; placebo 52 Mean age (SD): tiotropium low 44.7 (12.1); tiotropium high 42.6 (12.8); placebo 47.8 (13.0) % male: tiotropium low 36.8; tiotropium high 42.1; placebo 33.3 % predicted FEV1(SD): NR Duration of asthma, years (SD): NR Inclusion criteria: informed consent; male or female outpatients aged 18‐75 years; ≥ 12‐week history of asthma at enrolment, diagnosed before 40 years, and confirmed with bronchodilator reversibility (15‐30 min after 400 µg salbutamol) resulting in a FEV1 increase of at least 12% and at least 200 mL; on maintenance treatment with a medium, stable dose of ICS (alone or in a fixed combination with a LABA) for at least 4 weeks prior to visit 1; ACQ ≥ 1.5 at screening; pre‐bronchodilator FEV1 60%‐90% of predicted normal at visit 1; never‐smokers or ex‐smokers for ≥ 1 year and a smoking history of < 10 pack‐years; able to use the Respimat inhaler correctly; able to perform all trial‐related procedures Exclusion criteria: lung or additional significant disease other than asthma; recent history (≤ 6 months) of myocardial infarction; hospitalised for cardiac failure within 1 year; any unstable or life‐threatening cardiac arrhythmia or cardiac arrhythmia requiring intervention or a change in drug therapy within 1 year; known active TB; malignancy or treated for malignancy with resection, radiation therapy or chemotherapy within 5 years (treated basal cell carcinoma allowed); undergone thoracotomy with pulmonary resection; significant alcohol or drug abuse within 2 years; known hypersensitivity to anticholinergic drugs, benzalkonium chloride (BAC), ethylenediaminetetraacetic acid (EDTA), or any other components of the study medication delivery systems; pregnant or nursing women; women of childbearing potential not using a highly effective method of birth control; taken an investigational drug, beta‐blocker, tiotropium (Spiriva), oral beta‐adrenergic, systemic corticosteroids, or other non‐approved/not guideline recommended 'experimental' drugs for asthma within 4 weeks prior to visit 1; topical cardioselective beta‐blocker eye medications for non‐narrow angle glaucoma are allowed; anti‐IgE antibodies, e.g. omalizumab (Xolair), within 6 months prior to visit 1 or during the screening period; any asthma exacerbation or any respiratory tract infection in the four weeks prior to visit 1 or during the screening period; currently participating in another trial; narrow‐angle glaucoma or micturition disorder due to prostatic hyperplasia; below 80% eDiary completion compliance on visit 2 |
|
| Interventions |
Intervention 1: Ttiotropium Respimat 2.5 µg once daily (low group) Intervention 2: tiotropium Respimat 5 µg once daily (high group) Control: placebo Respimat inhaler taken once daily Background treatment: maintenance treatment with a medium, stable dose of inhaled corticosteroids with or without a long‐acting beta2‐agonist. Continuation with pre‐study maintenance therapy and rescue salbutamol was permitted. |
|
| Outcomes |
Primary: number of patients with drug‐related adverse events Secondary: change in trough FEV1, trough FVC, trough PEF from baseline to week 52, change in weekly mean morning and evening PEF and PEF variability, weekly mean number of puffs of rescue medication use per day (change from baseline), weekly mean score of asthma symptoms in the morning and during the day (5‐point verbal rating scale, with 1 representing no impairment) |
|
| Notes |
Funding: Boehringer Ingelheim with collaboration from Pfizer ID number(s): NCT01340209; 205.464 Patients were allowed to continue taking maintenance medication, including LABA, but we were unable to confirm how many did so. For this reason, the study was removed in a sensitivity analysis from the primary outcomes. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Eligible patients were randomised in blocks, 2:2:1" with a "pseudo‐random number generator" |
| Allocation concealment (selection bias) | Low risk | "Randomisation was achieved via a third‐party phone‐ or web‐based system involving a validated pseudo‐random number generator and a supplied seed number, using a block size of 5" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "In order to maintain the blind, patients in the placebo group also used the Respimat SoftMist inhaler, and the placebo inhalation solution was identical in appearance to the tiotropium inhalation solution. Blinding was maintained until after database lock." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | As above, all parties were blind until after database lock. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Total dropout was less than 10% in all groups. "Full analysis set: all patients of the treated set for which baseline and at least 1 post‐baseline efficacy measurement were available". This was used for efficacy measures, and included at least 85% of the randomised population. |
| Selective reporting (reporting bias) | Low risk | Data for all pre‐specified outcomes were published or available in full on ClinicalTrials.gov. |
| Other bias | Low risk | None noted |
ACQ: Asthma Control Questionnaire; AQLQ: Asthma Quality of Life Questionnaire; AUC:area under the curve; COPD: chronic obstructive pulmonary disease; FEV1 : forced expiratory volume in one second; FVC: forced vital capacity; ICS: inhaled corticosteroids; ITT: intention‐to‐treat; LABA: long‐acting beta2‐agonists; NR: not reported;OCS: oral corticosteroids; PEF: peak expiratory flow; RTI: respiratory tract infection; RCT: randomised controlled trial; SD: standard deviation; SGRQ: St George's Respiratory Questionnaire; TB: tuberculosis.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| 2009‐018006‐21 | Participants not taking LABA/ICS combination and too short (4‐week cross‐over) |
| 2010‐018471‐26 | Participants not taking LABA/ICS combination and too short (4‐week cross‐over) |
| Bateman 2011 | Participants not taking LABA/ICS combination |
| Beeh 2013 | Participants not taking LABA/ICS combination, and too short |
| Dusser 2014 | Not an RCT ‐ meta‐analysis |
| Fardon 2007 | Participants not taking LABA/ICS combination, and too short |
| FitzGerald 2014 | Not an RCT ‐ meta‐analysis |
| Haggart 2004 | Participants not taking LABA/ICS combination |
| Haughney 2014 | Not an RCT ‐ meta‐analysis |
| Jiang 2006 | Wrong intervention ‐ triple therapy of traditional Chinese medicine |
| Kerstjens 2011 | Too short, 8 weeks |
| Kerstjens 2015 | Participants not taking LABA/ICS combination |
| Lee 2014 | Participants not taking LABA/ICS combination |
| Lommatzsch 2014 | Wrong design ‐ cross‐over with 4‐week phases |
| Murphy 2014 | Not an RCT ‐ meta‐analysis |
| NCT01573624 | LAMA/ICS against LABA, not in combination as triple therapy |
| NCT02039011 | Too short ‐ 2‐4 weeks |
| Paggiaro 2013 | Participants not taking LABA/ICS combination |
| Peters 2010 | Participants not taking LABA/ICS combination |
| Price 2014 | Not an RCT ‐ meta‐analysis |
| Rajanandh 2014 | Participants not taking LABA/ICS combination |
| Rajanandh 2015 | Participants not taking LABA/ICS combination |
| Rodrigo 2015 | Not an RCT ‐ meta‐analysis |
| Salvi 2009 | Participants not taking LABA/ICS combination and too short |
| Timmer 2014 | Too short |
| Vandewalker 2015 | Adolescent study |
| Vogelberg 2014 | Too short and study of adolescents not adults |
| Yoshida 2013 | Comparison group with comorbid emphysema |
Characteristics of ongoing studies [ordered by study ID]
NCT01696214.
| Trial name or title | A pilot study to determine the feasibility and utility of implementing of the full scale TOM trial (SAPS) |
| Methods | Allocation: randomised Endpoint classification: efficacy study Intervention model: factorial assignment Masking: double‐blind (subject, investigator) Primary purpose: treatment |
| Participants | Estimated enrolment: 20 Inclusion criteria: Males and females, aged 18‐50; smoke ≥ 5 cigarettes per day for at least 5 years; positive urine cotinine test; physician diagnosed asthma; symptomatic, as evidenced by use of SABA ≥ 2 times per week for relief of asthma symptoms, or 1 or more nocturnal awakenings per week for asthma symptoms; pre‐BD FEV1 ≥ 40% predicted; asthma diagnosis confirmed by either albuterol reversibility of FEV1 by 12% or more, or 20% fall in FEV1 at 8 mg or less of methacholine. If over age 45, a DLCO greater than 80% predicted; females of childbearing potential: not pregnant, not lactating and agree to practice an adequate birth control method (abstinence, combination barrier and spermicide, or hormonal) for the duration of the study Exclusion criteria: Diagnosis of COPD or emphysema; other major chronic illnesses in the opinion of the investigator that might interfere with the study, including but not limited to uncontrolled diabetes, uncontrolled HIV infection or other immune system disorder, hyperthyroidism, seizure disorders, renal failure, liver disease, non‐skin cancer, unstable psychiatric illness; recent active substance abuse (in past 6 months); lung disease other than asthma including COPD, bronchiectasis, sarcoidosis, or other significant lung disease; unstable cardiac disease (decompensated CHF, unstable angina, recent MI, atrial fibrillation, supraventricular or ventricular tachycardia, congenital heart disease, or severe uncontrolled hypertension); high risk of near fatal or fatal asthma as defined by the following: 1‐3 ICU admission of asthma in the past year, more than 2 hospitalisations for asthma in the previous year, more than 3 ED visits for asthma in the previous year, intubation or ICU admission for asthma in the past 2 years, use of more than 2 canisters of inhaled SABAs in past month; acute asthma exacerbation in the past 4 weeks (treatment with systemic corticosteroids) |
| Interventions | For this review, the comparison between group 1 and group 4 meets the inclusion criteria:
|
| Outcomes | Primary outcome: Asthma Control Test Secondary outcomes: Asthma Symptom Utility Index (AUSI), FEV1, FVC, FEV1/FVC ratio |
| Starting date | First received: June 25, 2012 Last updated: June 11, 2013 Last verified: June 2013 Study start date: October 2012 Estimated study completion date: September 2016 Estimated primary completion date: September 2014 (final data collection date for primary outcome measure) |
| Contact information | Airway Researchj & Clinical Trials Center San Diego, California, United States, 92103 Contact: Paul Ferguson pferguson@ucsd.edu Principal investigator: Joe Ramsdell, MD |
| Notes | ID number(s): ARCTC‐09 and IR34HL109482‐01A1 |
BD: bronchodilator; COPD: chronic obstructive pulmonary disorder; CHF: congestive heart failure; DLCO: diffusing capacity of the lungs for carbon monoxide; ED: emergency department: FEV1 : forced expiratory volume in one second; FVC: forced vital capacity; ICU: intensive care unit; LTRA: leukotriene receptor antagonist; MI: myocardial infarction; SABA: short‐acting beta2‐agonists.
Differences between protocol and review
We did not anticipate that a study would include only a subset of participants that met the inclusion criteria relating to background medication, and so we did not outline methods to guide how to deal with Ohta 2014. We chose to include the study and describe sensitivity analyses without it where there was heterogeneity in the analyses. We also factored in the partly indirect population in the GRADE ratings for outcomes to which the study contributed data.
We were unable to conduct appropriate subgroup analyses to investigate the potential effect of duration of therapy, dose and type of LABA/ICS and dose and type of LAMA due to the small number of studies included. Where possible, we conducted subgroup analyses by looking at dose groups within multi‐arm studies and splitting the placebo group accordingly.
We were also unable to test the robustness of the analyses by performing sensitivity analyses on the basis of risk of performance bias, although this was because we rated all of the included studies as having a low risk for this domain. We did not include any unpublished data in the analyses and found no cross‐over studies, so these sensitivity analyses were also not possible.
Contributions of authors
Kayleigh Kew (KK): Background and Methods text, sifting the search, duplicate data extraction and risk of bias judgements, data analysis, results text and parts of the discussion.
Karen Dahri (KD): Background and Methods contributions, sifting the search, duplicate data extraction and risk of bias judgements, data interpretation and parts of the discussion.
Sources of support
Internal sources
-
Kayleigh M. Kew, UK.
Supported by St George's, University of London
External sources
-
National Institute for Health Research, UK.
This project was supported by the National Institute for Health Research, via Cochrane Infrastructure, Cochrane Programme Grant or Cochrane Incentive funding to the Cochrane Airways Group. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
Declarations of interest
Kayleigh M. Kew: none known.
Karen Dahri: none known.
New
References
References to studies included in this review
Kerstjens 2012a {published and unpublished data}
- Beck E, Kerstjens H, Engel M, Dahl R, Paggiaro P, Vandewalker E, et al. Tiotropium Respimat add‐on therapy to inhaled corticosteroids (ICS) + long‐acting beta2‐agonists (LABAs) in patients with symptomatic severe asthma: efficacy by level of airway obstruction. Allergo Journal 2014;23(6):57. [Google Scholar]
- Bernstein JA, Kerstjens HAM, Moroni‐Zentgraf P, Engel M, Schmidt H, Halpin DMG. Once‐daily tiotropium is well tolerated as add‐on to standard treatment for patients with symptomatic asthma despite receiving inhaled corticosteroids and long‐acting beta2‐agonists. CHEST Annual Conference; 2013 October 26‐31; Chicago. 2013.
- Casale TB, Dahl R, Virchow JC, Engel M, Moroni‐Zentgraf P, Luehmann R, et al. Tiotropium respimat add‐on therapy reduces exacerbation risk in patients with symptomatic moderate to severe asthma, independent of t helper 2 status. American Thoracic Society International Conference; 2015 May 15‐20; Denver. 2015:A4193.
- Corren J, Frew A, Engel M, Schmidt H, Moroni‐Zentgraf P, Kerstjens HAM. Tiotropium as add‐on therapy to ICS+LABA in patients with symptomatic severe asthma: spirometric assessment over 24 hours [Abstract]. American College of Chest Physicians Annual Meeting; 2013 Oct 26‐31; Chicago. 2013; Vol. 144:91A.
- Corren J, Murphy KR, Bensch G, Dahl R, Paggiaro P, Engel M, et al. Once‐daily tiotropium Respimat decreases the risk of exacerbations, independent of baseline characteristics, in patients with symptomatic severe asthma without evidence of chronic obstructive pulmonary disease. Journal of General Internal Medicine 2014;29:S157‐8. [Google Scholar]
- Dahl R, Casale T, Pizzichini E, Vandewalker M, Virchow JC, Engel M, et al. Once‐daily tiotropium Respimat as add‐on to at least medium‐to high‐dose ICS, with or without LABA, improves lung function in patients with symptomatic asthma, independent of allergic status. Thorax 2014;69(Suppl 2):A177‐8. [Google Scholar]
- Dahl R, Doherty DE, Corren J, Karpel J, Kerstjens HAM, Engel M, et al. Once‐daily tiotropium respimat improves lung function in patients with severe symptomatic asthma independent of leukotriene modifier use. The Journal of Allergy and Clinical Immunology 2014;133(2):AB5. [Google Scholar]
- Dahl R, Paggiaro P, Engel M, Sigmund R, Moroni‐Zentgraf P, Bateman E, et al. Once‐daily tiotropium improves lung function and reduces risk of asthma exacerbation/worsening in patients with symptomatic asthma, regardless of allergic status. Allergy 2013;68(s98):40‐1. [Google Scholar]
- Doherty DE, Castro M, Moroni‐Zentgraf P, Engel M, Luehmann R, Buhl R. Tiotropium respimat add‐on therapy improves lung function, as measured by peak expiratory flow, in adult patients across severities of symptomatic asthma. American Thoracic Society International Conference; 2015 May 15‐20; Denver. 2015:A4258.
- Doherty DE, Tashkin DP, Harrison T W, Engel M, Schmidt H, Moroni‐Zentgraf P, et al. Improvements in lung function with tiotropium as add‐on controller therapy to ICS+LABA for patients with symptomatic severe asthma. Chest 2013;144(4):88A. [Google Scholar]
- FitzGerald JM, Engel M, Moroni‐Zentgraf P, Luehmann R, Bleecker ER. Once‐daily tiotropium respimat add‐on therapy improves symptom control across severities of symptomatic asthma, independent of allergic status. American Thoracic Society Internation Conference; 2015 May 15‐20; Denver. 2015:A4263.
- Frith P, Price D, Bateman E, Paggiaro P, Kaplan A, Engel M, et al. Efficacy of once‐daily tiotropium respimat 5 μg from five phase III trials in adults with symptomatic asthma. Respirology 2015;20(Suppl S2):137. [Google Scholar]
- Halpin DMG, Bateman ED, Moroni‐Zentgraf P, Engel M, Schmidt H, Kerstjens HAM. Tiotropium is effective in patients with severe asthma without evidence of chronic obstructive pulmonary disease. Thorax 2013;68(Suppl 3):A152. [Google Scholar]
- Halpin DMG, Bateman ED, Tashkin DP, Engel M, Dahl R, Paggiaro P, et al. Tiotropium decreases the risk of exacerbations in patients with symptomatic asthma regardless of baseline characteristics including markers of allergic status. Thorax 2013;68(Suppl 3):A149. [Google Scholar]
- Hashimoto S, Engel M, Schmidt H, Moroni‐Zentgraf P, Kerstjens HAM. Once‐daily tiotropium as add‐on to ICS + LABA for patients with severe symptomatic asthma: baseline characteristics in Japanese patients. Respirology 2013;18(S2):88. [Google Scholar]
- Hozawa S, Kerstjens HAM, Tashkin DP, Engel M, Ronald D, Pierluigi P, et al. Tiotropium decreases the risk of exacerbations in patients with symptomatic asthma regardless of baseline characteristics. Respirology 2013;18(Suppl 3):A149. [Google Scholar]
- Kerstjens H, Dahl R, Beck E, Vandewalker M, Engel M, Sigmund R, et al. Tiotropium reduces asthma exacerbations in patients with uncontrolled asthma and persistent airflow obstruction despite treatment in accordance with guidelines. Respirology 2012;17(Suppl 2):A483. [Google Scholar]
- Kerstjens HAM, Engel M, Dahl R, Paggiaro P, Beck E, Vandewalker M, et al. Tiotropium Respimat® add‐on therapy to inhaled corticosteroids (ICS) + long‐acting Β2‐agonists (LABAS) in patients with symptomatic severe asthma: efficacy by level of airway obstruction [Abstract]. American Journal of Respiratory and Critical Care Medicine 2014;189:A1312. [Google Scholar]
- Kerstjens HAM, Engel M, Dahl R, Paggiaro P, Beck E, Vandewalker M, et al. Tiotropium in asthma poorly controlled with standard combination therapy. New England Journal of Medicine 2012;367(13):1198‐207. [DOI] [PubMed] [Google Scholar]
- Kerstjens HAM, Engel M, Moroni‐Zentgraf P, Schmidt H, Bateman E. Tiotropium added to inhaled corticosteroids and long‐acting beta(2)‐agonists reduces episodes of asthma worsening, irrespective of selected patient baseline characteristics. Allergy 2013;68(s98):41. [Google Scholar]
- Kerstjens HAM, Paggiaro P, Vandewalker M, Beck E, Engel M, Sigmund R, et al. Tiotropium provides sustained bronchodilation in patients with uncontrolled asthma and persistent airflow obstruction despite treatment in accordance with guidelines [Abstract]. Respirology 2012;17(Suppl 2):6 [221]. [Google Scholar]
- NCT00772538. A Phase III randomised, double‐blind, placebo‐controlled, parallel‐group trial to evaluate efficacy and safety of tiotropium inhalation solution delivered via Respimat ® inhaler (5 μg/day) over 48 weeks as add‐on controller therapy on top of usual care in patients with severe persistent asthma. https://www.clinicaltrialsregister.eu/ctr‐search/trial/2008‐001413‐14/results (accessed 23 June 2015).
- NCT00772538. Evaluation of tiotropium 5 µg/day delivered via the Respimat® inhaler over 48 weeks in patients with severe persistent asthma on top of usual care (study I). https://clinicaltrials.gov/ct2/show/NCT00772538 (accessed 22 June 2015).
- Price D, Engel M, Moroni‐Zentgraf P, Schmidt H, Dahl R, Paggiaro P, et al. Once‐daily tiotropium respimat add‐on to ICS + LABA improves symptom control and reduces exacerbations in patients with symptomatic asthma. Thorax 2014;69(Suppl 2):A49‐50. [Google Scholar]
- Tashkin D, Moroni‐Zentgraf P, Engel M, Schmidt H, Kerstjens H. Once‐daily tiotropium respimat add‐on to ICS+LABA reduces risk of severe exacerbation and asthma worsening in patients with asthma, independent of degree of airflow obstruction. Chest 2014;146(4):18A. [Google Scholar]
- Tashkin DP, Moroni‐Zentgraf P, Engel M, Schmidt H, Kerstjens HAM. Once‐daily tiotropium reduces risk of exacerbations and asthma worsening in patients with symptomatic asthma despite treatment with inhaled corticosteroids and long‐acting beta2‐agonists. Chest 2013;144(4):90A. [Google Scholar]
- Vandewalker ML, Engel M, Schmidt H, Siebold W, Moroni‐Zentgraf P, Kerstjens H, et al. Efficacy of tiotropium in patients with asthma in relation to allergic status [Abstract]. The Journal of Allergy and Clinical Immunology 2013;131(2):AB1. [Google Scholar]
- Vandewalker ML, Karpel J, Bensch G, Ford L, Engel M, Sigmund R, et al. Once‐daily tiotropium Respimat add‐on to maintenance therapy improves lung function in patients with moderate or severe symptomatic asthma, independent of T helper 2 inflammatory status. Annals of Allergy, Asthma & Immunology 2014;113(5):A105‐6. [Google Scholar]
Kerstjens 2012b {published and unpublished data}
- 2008‐001414‐25. A Phase III randomised, double‐blind, placebo‐controlled, parallel‐group trial to evaluate efficacy and safety of tiotropium inhalation solution delivered via Respimat ® inhaler (5 μg/day) over 48 weeks as add‐on controller therapy on top of usual care in patients with severe persistent asthma. https://www.clinicaltrialsregister.eu/ctr‐search/trial/2008‐001414‐25/results (accessed 23 June 2015).
- Beck E, Kerstjens H, Engel M, Dahl R, Paggiaro P, Vandewalker E, et al. Tiotropium Respimat add‐on therapy to inhaled corticosteroids (ICS) + long‐acting beta2‐agonists (LABAs) in patients with symptomatic severe asthma: efficacy by level of airway obstruction. Allergo Journal 2014;23(6):57. [Google Scholar]
- Bernstein JA, Kerstjens HAM, Moroni‐Zentgraf P, Engel M, Schmidt H, Halpin DMG. Once‐daily tiotropium is well tolerated as add‐on to standard treatment for patients with symptomatic asthma despite receiving inhaled corticosteroids and long‐acting beta2‐agonists. CHEST Annual Conference; 2013 October 26‐31. 2013.
- Casale TB, Dahl R, Virchow JC, Engel M, Moroni‐Zentgraf P, Luehmann R, et al. Tiotropium respimat add‐on therapy reduces exacerbation risk in patients with symptomatic moderate to severe asthma, independent of t helper 2 status. American Thoracic Society International Conference; 2015 May 15‐20; Denver. 2015:A4193.
- Corren J, Frew A, Engel M, Schmidt H, Moroni‐Zentgraf P, Kerstjens HAM. Tiotropium as add‐on therapy to ICS+LABA in patients with symptomatic severe asthma: spirometric assessment over 24 hours [Abstract]. American College of Chest Physicians Annual Meeting; 2013 Oct 26‐31; Chicago, Illinois. 2013; Vol. 144:91A.
- Corren J, Murphy KR, Bensch G, Dahl R, Paggiaro P, Engel M, et al. Once‐daily tiotropium respimat decreases the risk of exacerbations, independent of baseline characteristics, in patients with symptomatic severe asthma without evidence of chronic obstructive pulmonary disease. Journal of General Internal Medicine 2014;29:S157‐8. [Google Scholar]
- Dahl R, Casale T, Pizzichini E, Vandewalker M, Virchow JC, Engel M, et al. Once‐daily tiotropium Respimat as add‐on to at least medium‐to high‐dose ICS, with or without LABA, improves lung function in patients with symptomatic asthma, independent of allergic status. Thorax 2014;69(Suppl 2):A177‐8. [Google Scholar]
- Dahl R, Doherty DE, Corren J, Karpel J, Kerstjens HAM, Engel M, et al. Once‐daily tiotropium respimat improves lung function in patients with severe symptomatic asthma independent of leukotriene modifier use. The Journal of Allergy and Clinical Immunology 2014;133(2):AB5. [Google Scholar]
- Dahl R, Paggiaro P, Engel M, Sigmund R, Moroni‐Zentgraf P, Bateman E, et al. Once‐daily tiotropium improves lung function and reduces risk of asthma exacerbation/worsening in patients with symptomatic asthma, regardless of allergic status. Allergy 2013;68(s98):40‐1. [Google Scholar]
- Doherty DE, Castro M, Moroni‐Zentgraf P, Engel M, Luehmann R, Buhl R. Tiotropium respimat add‐on therapy improves lung function, as measured by peak expiratory flow, in adult patients across severities of symptomatic asthma. American Thoracic Society International Conference; 2015 May 15‐20; Denver. 2015:A4258.
- Doherty DE, Tashkin DP, Harrison T W, Engel M, Schmidt H, Moroni‐Zentgraf P, et al. Improvements in lung function with tiotropium as add‐on controller therapy to ICS+LABA for patients with symptomatic severe asthma. Chest 2013;144(4):88A. [Google Scholar]
- FitzGerald JM, Engel M, Moroni‐Zentgraf P, Luehmann R, Bleecker ER. Once‐daily tiotropium respimat add‐on therapy improves symptom control across severities of symptomatic asthma, independent of allergic status. American Thoracic Society Internation Conference; 2015 May 15‐20; Denver. 2015:A4263.
- Frith P, Price D, Bateman E, Paggiaro P, Kaplan A, Engel M, et al. Efficacy of once‐daily tiotropium Respimat 5 μg from five phase III trials in adults with symptomatic asthma. Respirology 2015;20(Suppl S2):137. [Google Scholar]
- Halpin DMG, Bateman ED, Moroni‐Zentgraf P, Engel M, Schmidt H, Kerstjens HAM. Tiotropium is effective in patients with severe asthma without evidence of chronic obstructive pulmonary disease. Thorax 2013;68(Suppl 3):A152. [Google Scholar]
- Halpin DMG, Bateman ED, Tashkin DP, Engel M, Dahl R, Paggiaro P, et al. Tiotropium decreases the risk of exacerbations in patients with symptomatic asthma regardless of baseline characteristics including markers of allergic status. Thorax 2013;68(Suppl 3):A149. [Google Scholar]
- Hashimoto S, Engel M, Schmidt H, Moroni‐Zentgraf P, Kerstjens HAM. Once‐daily tiotropium as add‐on to ICS + LABA for patients with severe symptomatic asthma: Baseline characteristics in Japanese patients. Respirology 2013;18(S2):88. [Google Scholar]
- Hozawa S, Kerstjens HAM, Tashkin DP, Engel M, Ronald D, Pierluigi P, et al. Tiotropium decreases the risk of exacerbations in patients with symptomatic asthma regardless of baseline characteristics. Respirology 2013;18(Suppl 3):175. [Google Scholar]
- Kerstjens H, Dahl R, Beck E, Vandewalker M, Engel M, Sigmund R, et al. Tiotropium reduces asthma exacerbations in patients with uncontrolled asthma and persistent airflow obstruction despite treatment in accordance with guidelines. Respirology 2012;17(Suppl 2):16. [Google Scholar]
- Kerstjens HAM, Engel M, Dahl R, Paggiaro P, Beck E, Vandewalker M, et al. Tiotropium Respimat® add‐on therapy to inhaled corticosteroids (ICS) + long‐acting Β2‐agonists (LABAS) in patients with symptomatic severe asthma: efficacy by level of airway obstruction [Abstract]. American Journal of Respiratory and Critical Care Medicine 2014;189:A1312. [Google Scholar]
- Kerstjens HAM, Engel M, Dahl R, Paggiaro P, Beck E, Vandewalker M, et al. Tiotropium in asthma poorly controlled with standard combination therapy. New England Journal of Medicine 2012;367(13):1198‐207. [DOI] [PubMed] [Google Scholar]
- Kerstjens HAM, Engel M, Dahl R, Paggiaro P, Beck E, Vandewalker M, et al. Tiotropium respimat add‐on therapy to inhaled corticosteroids (ICS) + long‐acting beta2‐agonists (LABAS) in patients with symptomatic severe asthma: Efficacy by level of airway obstruction. American Thoracic Society International Conference; 2014 May 16‐21; San Diego. 2014:A1312.
- Kerstjens HAM, Engel M, Moroni‐Zentgraf P, Schmidt H, Bateman E. Tiotropium added to inhaled corticosteroids and long‐acting beta(2)‐agonists reduces episodes of asthma worsening, irrespective of selected patient baseline characteristics. Allergy 2013;68(s98):41. [Google Scholar]
- Kerstjens HAM, Paggiaro P, Vandewalker M, Beck E, Engel M, Sigmund R, et al. Tiotropium provides sustained bronchodilation in patients with uncontrolled asthma and persistent airflow obstruction despite treatment in accordance with guidelines [Abstract]. Respirology 2012;17(Suppl 2):6 [221]. [Google Scholar]
- NCT00776984. Evaluation of tiotropium 5 µg/day delivered via the Respimat® inhaler over 48 weeks in patients with severe persistent asthma on top of usual care (study II). https://clinicaltrials.gov/ct2/show/NCT00776984 (accessed 22 June 2015).
- Price D, Engel M, Moroni‐Zentgraf P, Schmidt H, Dahl R, Paggiaro P, et al. Once‐daily tiotropium Respimat add‐on to ICS + LABA improves symptom control and reduces exacerbations in patients with symptomatic asthma. Thorax 2014;69(Suppl 2):A49‐50. [Google Scholar]
- Tashkin D, Moroni‐Zentgraf P, Engel M, Schmidt H, Kerstjens H. Once‐daily tiotropium Respimat add‐on to ICS+LABA reduces risk of severe exacerbation and asthma worsening in patients with asthma, independent of degree of airflow obstruction. Chest 2014;146(4):18A. [Google Scholar]
- Tashkin DP, Moroni‐Zentgraf P, Engel M, Schmidt H, Kerstjens HAM. Once‐daily tiotropium reduces risk of exacerbations and asthma worsening in patients with symptomatic asthma despite treatment with inhaled corticosteroids and long‐acting beta2‐agonists. Chest 2013;144(4):90A. [Google Scholar]
- Vandewalker ML, Engel M, Schmidt H, Siebold W, Moroni‐Zentgraf P, Kerstjens H, et al. Efficacy of tiotropium in patients with asthma in relation to allergic status [Abstract]. The Journal of Allergy and Clinical Immunology 2013;131(2):AB1. [Google Scholar]
- Vandewalker ML, Karpel J, Bensch G, Ford L, Engel M, Sigmund R, et al. Once‐daily tiotropium Respimat add‐on to maintenance therapy improves lung function in patients with moderate or severe symptomatic asthma, independent of T helper 2 inflammatory status. Annals of Allergy, Asthma and Immunology 2014;113(5):A105‐6. [Google Scholar]
NCT02127697 {published and unpublished data}
- NCT02127697. Study of efficacy and safety of NVA237 in patients with poorly controlled asthma. https://clinicaltrials.gov/ct2/show/NCT02127697 (accessed 22 June 2015).
Ohta 2014 {published and unpublished data}
- Ichinose M, Ohta K, Tohda Y, Engel M, Moroni‐Zentgraf P, Kunimitsu S, et al. Once‐daily tiotropium respimat is well tolerated and efficacious over 52 weeks in Japanese patients with symptomatic asthma receiving inhaled corticosteroids (ICS) + long‐acting B2‐agonist (LABA): A randomized, double‐blind, placebo‐controlled study. Respirology 2014;19(Suppl 3):65. [Google Scholar]
- NCT01340209. Evaluation of tiotropium 2.5 and 5 µg once daily delivered via the Respimat inhaler compared to placebo in patients with moderate to severe persistent asthma. https://clinicaltrials.gov/ct2/show/NCT01340209 (accessed 22 June 2015).
- Ohta K, Ichinose M, Tohda Y, Engel M, Moroni‐Zentgraf P, Kunimitsu S, et al. Long‐term once‐daily tiotropium Respimat is well tolerated and maintains efficacy over 52 weeks in patients with symptomatic asthma in Japan: A randomised, placebo‐controlled study. PLOS ONE 2015;10(4):e0124109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohta K, Ichinose M, Tohda Y, Engel M, Moroni‐Zentgraf P, Kunimitsu S, et al. Once‐daily tiotropium respimat® is well tolerated and efficacious over 52 weeks in Japanese patients with symptomatic asthma receiving inhaled corticosteroids (ICS ± long‐acting Β2‐agonist (LABA): a randomized, double‐blind, placebo‐controlled study [Abstract]. American Journal of Respiratory and Critical Care Medicine 2014;189:A1311. [Google Scholar]
References to studies excluded from this review
2009‐018006‐21 {unpublished data only}
- 2009‐018006‐21. A phase II, randomised, double‐ blind, placebo controlled, cross‐over efficacy and safety comparison of tiotropium 5 μg administered once daily (in the evening) and tiotropium 2.5 μg administered twice daily delivered by the Respimat® inhaler for four weeks versus placebo in patients with moderate persistent asthma. https://www.clinicaltrialsregister.eu/ctr‐search/trial/2009‐018006‐21/results (accessed 23 June 2015).
2010‐018471‐26 {unpublished data only}
- 2010‐018471‐26. A phase II randomised, double‐blind, placebo controlled, cross‐over efficacy and safety comparison of three doses of tiotropium inhalation solution delivered via Respimat® inhaler (1.25, 2.5 and 5.0 μg once daily) versus placebo in patients with moderate persistent asthma. https://www.clinicaltrialsregister.eu/ctr‐search/trial/2010‐018471‐26/results (accessed 23 June 2015).
Bateman 2011 {published data only}
- Bateman ED, Kornmann O, Schmidt P, Pivovarova A, Engel M, Fabbri LM. Tiotropium is noninferior to salmeterol in maintaining improved lung function in B16‐Arg/Arg patients with asthma. The Journal of Allergy and Clinical Immunology 2011;128(2):315‐22. [DOI] [PubMed] [Google Scholar]
Beeh 2013 {published data only}
- Beeh KM, Ablinger O, Moroni‐Zentgraf P, Hollaenderova Z, Pivovarova A, Engel M, et al. Once‐daily add‐on tiotropium: a dose‐finding trial in adult patients with moderate persistent asthma. Allergy 2013;68(s97):376‐7. [Google Scholar]
- Beeh KM, Moroni‐Zentgraf P, Ablinger O, Hollaenderova Z, Unseld A, Engel M, et al. Tiotropium Respimat in asthma: a double‐blind, randomised, dose‐ranging study in adult patients with moderate asthma. Respiratory Research 2014;15:61. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dusser 2014 {published data only}
- Dusser D, Buhl R, Castro M, Kerstjens H, Paggiaro P, Engel M, et al. Once‐daily tiotropium respimat add‐on to at least ICS in adult patients with symptomatic asthma: Pooled safety analysis. European Respiratory Journal 2014;44(Suppl 58):P905. [Google Scholar]
Fardon 2007 {published data only}
- Fardon T, Haggart K, Lee D K C, Lipworth B J. A proof of concept study to evaluate stepping down the dose of fluticasone in combination with salmeterol and tiotropium in severe persistent asthma. Respiratory Medicine 2007;101(6):1218‐28. [DOI] [PubMed] [Google Scholar]
FitzGerald 2014 {published data only}
- FitzGerald J M, Kerstjens H, Paggiaro P, Ohta K, Ichinose M, Moroni‐Zentgraf P, et al. Once‐daily tiotropium respimat add‐on to ICS+/‐LABA improves control across asthma severities. European Respiratory Journal 2014;44(Suppl 58):1894. [Google Scholar]
Haggart 2004 {published data only}
- Haggart K, Fardon TC, Lee DKC, Lipworth BJ. Stepping down inhaled steroids in severe asthma with long acting bronchodilators: utilising effort dependent and independent measures of pulmonary function [Abstract]. British Thoracic Society Winter Meeting; 2004 Dec 1‐3; London, United Kingdom. 2004; Vol. 59:ii72.
Haughney 2014 {published data only}
- Haughney J, Vandewalker M, Meltzer E, Paggiaro P, Engel M, Unseld A, et al. Once‐daily tiotropium Respimat: safety and tolerability results from five phase iii trials in adults with symptomatic asthma. Thorax 2014;69(Suppl 2):A178‐9. [Google Scholar]
Jiang 2006 {published data only}
- Jiang XP, Tan HY. Clinical observation for triple therapy in the treatment of 30 patients with bronchial asthma. Hunan Zhongyi Zazhi [Hunan Journal of Traditional Chinese Medicine] 2006;22:17‐8. [Google Scholar]
Kerstjens 2011 {published data only}
- Kerstjens HA, Disse B, Schröder‐Babo W, Bantje T A, Gahlemann M, Sigmund R, et al. Tiotropium improves lung function in patients with severe uncontrolled asthma: a randomized controlled trial. The Journal of Allergy and Clinical Immunology 2011;128(2):308‐14. [DOI] [PubMed] [Google Scholar]
Kerstjens 2015 {published data only}
- Casale T, Bleecker E, Meltzer E, Pizzichini E, Schmidt O, Bateman E, et al. Phase III trials to investigate tiotropium as add‐on therapy to inhaled corticosteroids for patients with symptomatic asthma: Trial design and planned statistical analyses. Allergy 2013;68(s97):377. [Google Scholar]
- Casale T, Carr WW, Greos L, Engel M, Bour LJ, Moroni‐Zentgraf P, et al. 24‐hour lung function response to tiotropium Respimatadd‐on to maintenance therapy in symptomatic patients with moderate persistent asthma. Annals of Allergy, Asthma & Immunology 2014;113(5):A108. [Google Scholar]
- Casale TB, Bateman ED, Dahl R, Pizzichini E, Vandewalker ML, Virchow JC, et al. Tiotropium respimat add‐on therapy reduces airflow obstruction in patients with symptomatic moderate asthma, independent of Th2 inflammatory status. The Journal of Allergy and Clinical Immunology 2014;133(2):AB5. [Google Scholar]
- Kerstjens HA, Casale TB, Bleecker ER, Meltzer EO, Pizzichini E, Schmidt O, et al. Tiotropium or salmeterol as add‐on therapy to inhaled corticosteroids for patients with moderate symptomatic asthma: two replicate, double‐blind, placebo‐controlled, parallel‐group, active‐comparator, randomised trials. The Lancet Respiratory Medicine 2015;3(5):367‐76. [DOI] [PubMed] [Google Scholar]
- Kerstjens HAM, Bleecker E, Meltzer E, Casale T, Pizzichini E, Schmidt O, et al. Tiotropium as add‐on to inhaled corticosteroids significantly improves asthma control as reflected by the ACQ responder rate. European Respiratory Society Annual Congress; 2013 7‐11 Sept; Barcelona. 2013.
Lee 2014 {published data only}
- Lee LA, Yang S, Kerwin E, Trivedi R, Edwards LD, Pascoe S. The effect of fluticasone furoate/umeclidinium in adult patients with asthma: a randomized, dose‐ranging study. Respiratory Medicine 2015;109(1):54‐62. [DOI] [PubMed] [Google Scholar]
Lommatzsch 2014 {published data only}
- Lommatzsch M, Moroni‐Zentgraf P, Cornelissen P, Unseld A, Pizzichini E, Timmer W. Tiotropium Respimat in asthma: evaluation of dosing regimens. Allergo Journal 2014;23(6):57. [Google Scholar]
Murphy 2014 {published data only}
- Murphy K, Bensch G, Berger WE, Engel M, Schmidt H, Moroni‐Zentgraf P, et al. Once‐daily tiotropium Respimat add‐on to inhaled corticosteroids + long‐acting beta2‐agonists improves lung function and asthma control and reduces risk of asthma worsening in patients with moderate or severe asthma. Annals of Allergy, Asthma & Immunology 2014;113(5):A107. [Google Scholar]
NCT01573624 {published data only}
- NCT01573624. A multi‐center, randomized, double‐blind, dose‐ranging study to evaluate GSK573719 in combination with fluticasone furoate, fluticasone furoate alone, and an active control of fluticasone furoate/vilanterol combination in subjects with asthma. http://clinicaltrials.gov/show/NCT01573624 (accessed 22 June 2015).
NCT02039011 {published data only}
- NCT02039011. Proof of concept study to evaluate single and chronic dosing effects of ultra‐long acting bronchodilator therapy on mannitol challenge in asthmatic patients taking inhaled corticosteroids ‐ MAN02 Ultra‐long Acting Bronchodilator Therapy in Asthmatics. http://apps.who.int/trialsearch/Trial2.aspx?TrialID=EUCTR2013‐001953‐28‐GB (accessed 23 June 2015).
Paggiaro 2013 {published data only}
- Paggiaro P, Engel M, Tudoric N, Forstner B, Radeczky E, Zubek V, et al. Phase III trial of tiotropium as add‐on therapy to low‐dose inhaled corticosteroids for patients with symptomatic mild persistent asthma: design and planned analyses [Abstract]. European Respiratory Journal 2013;42(Suppl 57):877s [P4133]. [Google Scholar]
Peters 2010 {published data only}
- Lane M. Tiotropium bromide in asthma patients: an alternative to inhaled long‐acting beta‐agonists?. Journal of the Royal College of Physicians of Edinburgh 2010;40(4):321‐2. [DOI] [PubMed] [Google Scholar]
- Peters S P, Kunselman S J, Icitovic N, Moore W C, Pascual R, Ameredes B T, et al. Tiotropium bromide step‐up therapy for adults with uncontrolled asthma. New England Journal of Medicine 2010;363(18):1715‐26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters SP, Bleecker ER, Kunselman SJ, Icitovic N, Moore WC, Pascual R, et al. Predictors of response to tiotropium versus salmeterol in asthmatic adults. The Journal of Allergy and Clinical Immunology 2013;132(5):1068‐74.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Price 2014 {published data only}
- Price D, Bateman ED, Paggiaro P, Kaplan A, Engel M, Schmidt H, et al. Efficacy of once‐daily tiotropium Respimat 5 mug from five phase III trials in adults with symptomatic asthma. Thorax 2014;69(Suppl 2):A50. [Google Scholar]
Rajanandh 2014 {published data only}
- Rajanandh MG, Nageswari A D, Ilango K. Pulmonary function assessment in mild to moderate persistent asthma patients receiving montelukast, doxofylline, and tiotropium with budesonide: a randomized controlled study. Clinical Therapeutics 2014;36(4):526‐33. [DOI] [PubMed] [Google Scholar]
Rajanandh 2015 {published data only}
- Rajanandh MG, Nageswari AD, Ilango K. Assessment of montelukast, doxofylline, and tiotropium with budesonide for the treatment of asthma: which is the best among the second‐line treatment? A randomized trial. Clinical Therapeutics 2015;37(2):418‐26. [DOI] [PubMed] [Google Scholar]
- Rajanandh MG, Nageswari AD, Ilango K. Assessment of various second‐line medications in addition to inhaled corticosteroid in asthma patients: a randomized controlled trial. Clinical and Experimental Pharmacology & Physiology 2014;41(7):509‐13. [DOI] [PubMed] [Google Scholar]
Rodrigo 2015 {published data only}
- Rodrigo GJ, Castro‐Rodriguez JA. What is the role of tiotropium in asthma?: a systematic review with meta‐analysis [Review]. Chest 2015;147(2):388‐96. [DOI] [PubMed] [Google Scholar]
Salvi 2009 {published data only}
- Salvi S, Bhosle K, Brashier B, Limaye S, Mandrekar S, Gaikwad S, et al. Eight weeks of treatment with tiotropium plus fluticasone (t + f ) produces an equally effective therapeutic response as salmeterol plus fluticasone (s + f) in subjects with moderate‐to‐severe asthma [Abstract]. European Respiratory Journal 2009;34(Suppl 53):E276. [Google Scholar]
Timmer 2014 {published data only}
- Timmer W, Moroni‐Zentgraf P, Cornelissen P, Unseld A, Pizzichini E, Buhl R. Once‐daily tiotropium Respimat 5 mug is an efficacious 24‐h bronchodilator in adults with symptomatic asthma. Respiratory Medicine 2015;109(3):329‐38. [DOI] [PubMed] [Google Scholar]
Vandewalker 2015 {published data only}
- Vandewalker M, Harper III T, Moroni‐Zentgraf P, Engel M, Luhmann R, Bernstein JA. Once‐daily tiotropium Respimat add‐on therapy improves lung function in adolescent patients with moderate symptomatic asthma, independent of t helper 2 inflammatory status. Annals of Allergy, Asthma & Immunology 2015;115(5 Suppl 1):A54‐5. [Google Scholar]
Vogelberg 2014 {published data only}
- Vogelberg C, Engel M, Moroni‐Zentgraf P, Leonaviciute‐Klimantaviciene M, Sigmund R, Downie J, et al. Once‐daily tiotropium Respimat add‐on to medium‐dose ics is an efficacious 24‐hour bronchodilator in adolescent patients with symptomatic asthma. Chest 2014;146(4):698A. [Google Scholar]
- Vogelberg C, Leonaviciute‐Klimantaviciene M, Vevere V, Vandewalker M, Engel M, Sigmund R, et al. Dose‐ranging study of tiotropium as treatment for moderate persistent asthma in adolescents. Allergo Journal 2013;22:389. [Google Scholar]
Yoshida 2013 {published data only}
- Yoshida M, Nakano T, Fukuyama S, Matsumoto T, Eguchi M, Moriwaki A, et al. Effects of tiotropium on lung function in severe asthmatics with or without emphysematous changes. Pulmonary Pharmacology and Therapeutics 2013;26(2):159‐66. [DOI] [PubMed] [Google Scholar]
References to ongoing studies
NCT01696214 {published data only}
- NCT01696214. A pilot study to determine the feasibility and utility of implementing of the full scale TOM trial (SAPS). https://clinicaltrials.gov/ct2/show/NCT01696214 (accessed 22 June 2015).
Additional references
Adams 2008a
- Adams NP, Bestall JB, Malouf R, Lasserson TJ, Jones PW. Beclomethasone versus placebo for chronic asthma. Cochrane Database of Systematic Reviews 2008, Issue 4. [DOI: 10.1002/14651858.CD002738.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Adams 2008b
- Adams NP, Bestall JC, Lasserson TJ, Jones P, Cates CJ. Fluticasone versus placebo for chronic asthma in adults and children. Cochrane Database of Systematic Reviews 2008, Issue 4. [DOI: 10.1002/14651858.CD003135.pub4] [DOI] [Google Scholar]
Anderson 2015
- Anderson DE, Kew KM, Boyter AC. Long‐acting muscarinic antagonists (LAMA) added to inhaled corticosteroids (ICS) versus the same dose of ICS alone for adults with asthma. Cochrane Database of Systematic Reviews 2015, Issue 8. [DOI: 10.1002/14651858.CD011397.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
BTS/SIGN 2014
- British Thoracic Society/Scottish Intercollegiate Guidelines Network. British guideline on the management of asthma, October 2014. https://www.brit‐thoracic.org.uk/guidelines‐and‐quality‐standards/asthma‐guideline/ (accessed 16 March 2015).
Ducharme 2008
- Ducharme FM, Ni Chroinin M, Greenstone I, Lasserson TJ. Addition of long‐acting beta2‐agonists to inhaled corticosteroids versus same dose inhaled corticosteroids for chronic asthma in adults and children. Cochrane Database of Systematic Reviews 2008, Issue 5. [DOI: 10.1002/14651858.CD005535.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ducharme 2010
- Ducharme FM, Ni Choinin M, Greenstone I, Lasserson TJ. Addition of long‐acting beta2‐agonists to inhaled steroids versus higher dose inhaled steroids in adults and children with persistent asthma. Cochrane Database of Systematic Reviews 2010, Issue 4. [DOI: 10.1002/14651858.CD005533.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
eMC 2014
- Electronic Medicines Compendium. License extension 19th September 2014: Spiriva Respimat 2.5 micrograms solution for inhalation. https://www.medicines.org.uk/emc/history/20134 (accessed 16 March 2015).
GINA 2014
- Global Initiative for Asthma. Global strategy for asthma management and prevention. http://www.ginasthma.org/ (accessed 16 March 2015).
Global Asthma Report 2011
- International Union Against Tuberculosis and Lung Disease. The Global Asthma Report 2011. http://theunion.org/what‐we‐do/publications/technical/global‐asthma‐report (accessed 16 March 2015).
GRADEpro [Computer program]
- Brozek J, Oxman A, Schünemann H. GRADEpro. Version 3.2 for Windows. Hamilton, Ontario: McMaster University, 2008.
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1 [updated March 2011]. The Cochrane Collaboration, 2011. www.cochrane‐handbook.org.
Kerstjens 2012
- Kerstjens HAM, Engel M, Bateman ED. Tiotropium in asthma [correspondence]. New England Journal of Medicine 2012;367:2552‐3. [DOI] [PubMed] [Google Scholar]
Lipworth 1999
- Lipworth BJ. Systemic adverse effects of inhaled corticosteroid therapy. Archives of Internal Medicine 1999;159(9):941‐55. [DOI] [PubMed] [Google Scholar]
Lipworth 2014
- Lipworth BJ. Emerging role of long acting muscarinic antagonists for asthma. British Journal of Clinical Pharmacology 2014;77(1):55‐62. [DOI] [PMC free article] [PubMed] [Google Scholar]
Moher 2009
- Moher D, Liberati A, Tetzlaff J, Altman D. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. PLoS Medicine 2009;6(7):e1000097. [DOI: 10.1371/journal.pmed.1000097] [DOI] [PMC free article] [PubMed] [Google Scholar]
Moulton 2011
- Moulton BC, Fryer AD. Muscarinic receptor antagonists, from folklore to pharmacology: finding drugs that actually work in asthma and COPD. British Journal of Clinical Pharmacology 2011;163(1):44‐52. [DOI] [PMC free article] [PubMed] [Google Scholar]
NRAD 2014
- Royal College of Physicians. Why asthma still kills: the national review of asthma deaths. www.rcplondon.ac.uk/sites/default/files/why‐asthma‐still‐kills‐full‐report.pdf (accessed 27 March 2015).
Pandya 2014
- Pandya D, Puttanna A, Balagopal V. Systemic effects of inhaled corticosteroids: an overview. The Open Respiratory Medicine Journal 2014;8(Suppl 1):59‐65. [DOI] [PMC free article] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Therapeutic Choices 2014
- McIvor RA. Respiratory disorders: chronic obstructive pulmonary disease. In: Gray J editor(s). Therapeutic Choices. 7th Edition. Ottawa: Canadian Pharmaceutical Association, 2014. [Google Scholar]
Tian 2014
- Tian J, Chen J, Chen R, Chen X. Tiotropium versus placebo for inadequately controlled asthma: a meta‐analysis. Respiratory Care 2014;59(5):654‐66. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Kew 2015
- Kew KM, Dahri K. Long‐acting muscarinic antagonists (LAMA) added to combination long‐acting beta2‐agonists and inhaled corticosteroids (LABA/ICS) versus LABA/ICS for adults with asthma. Cochrane Database of Systematic Reviews 2015, Issue 5. [DOI: 10.1002/14651858.CD011721] [DOI] [PMC free article] [PubMed] [Google Scholar]


