Abstract
Purpose:
The use of concurrent doublet chemotherapy with radiation for locoregionally advanced cervical cancer (LACC) is limited by gastrointestinal and hematologic toxicity. By reducing radiation dose to bowel and bone marrow, image guided intensity modulated radiation therapy (IG-IMRT) may improve chemotherapy tolerance. The goal of this study was to determine whether IG-IMRT could lead to improved tolerance to concurrent cisplatin and gemcitabine for LACC.
Methods and Materials:
We conducted an open-label, nonrandomized, prospective phase 1 dose escalation trial at a tertiary academic cancer center (ClinicalTrials.gov identifier: NCT01554410). We enrolled patients with stage IB-IVA cervical cancer, with either an intact cervix or posthysterectomy with residual/recurrent pelvic or paraortic nodal involvement, undergoing radical pelvic or extended field chemoradiation therapy. Treatment consisted of chemoradiation with IG-IMRT (45–47.6 Gy, 25–28 fractions to the pelvis ± paraortic nodes with simultaneous nodal boost to 53.2–59.4 Gy, 28 fractions) plus 5 cycles of concurrent weekly cisplatin 40 mg/m2 with escalating doses of gemcitabine (50, 75, 100, or 125 mg/m2). Cohorts were separated preregistration according to whether the patient received pelvic or extended field IG-IMRT and whether gemcitabine followed (CG) or preceded (GC) cisplatin delivery. Dose-limiting toxicity (DLT) events were monitored up to 30 days after chemoradiation therapy. The primary endpoint was maximum tolerated dose (MTD) resulting in DLT probability ≤20%.
Results:
Between February 2011 and June 2019, 35 patients were registered. Overall, 7 patients (20.0%) experienced DLTs. For the pelvic field cohort, the estimated MTD was 100 mg/m2 with GC sequencing, which is higher than the previously reported MTD for this regimen. The extended field cohort was closed after 2 of 3 patients experienced a DLT at the first dose level.
Conclusions:
IG-IMRT can permit higher doses of concurrent gemcitabine with cisplatin and pelvic radiation for LACC. However, acute toxicity remains a factor with this regimen, depending on radiation volume and chemotherapy sequencing.
Introduction
Multiple randomized trials have established concurrent cisplatin with radiation therapy as the standard of care for locoregionally advanced cervical cancer (LACC).1–5 However, rates of treatment failure remain high, with 5-year disease-free survival of approximately 65%, indicating a need for improved treatment. Ribonucleotide reductase inhibitors, such as gemcitabine, have been studied as a strategy to improve outcomes for LACC. Randomized trials of standard chemoradiation plus concurrent gemcitabine, with or without adjuvant chemotherapy, have found improved response and overall survival.6,7 However, high rates of acute gastrointestinal and hematologic toxicity have prevented wider adoption of the cisplatin/gemcitabine doublet. Furthermore, multi-institutional phase 1 trials have found that concurrent gemcitabine given at doses used in the randomized trials (125 mg/m2 weekly) were infeasible secondary to toxicity.8,9
The sequence of delivery of gemcitabine and cisplatin has been postulated as a factor affecting its tolerance, due to the timing of the inhibition of nuclear excision repair with gemcitabine in relation to platinum adduct formation.9,10 In randomized trials reporting improved outcomes with gemcitabine, cisplatin was delivered first, followed by gemcitabine. In contrast, in trials indicating lower tolerance to gemcitabine, gemcitabine was delivered first, followed by cisplatin. However, other studies have indicated that toxicity is greater when cisplatin precedes gemcitabine.11
Another factor hypothesized to affect chemotherapy tolerance is normal tissue radiation dose and volume. For example, it has long been known that extended radiation fields including paraortic nodal regions are more toxic than pelvic-only fields.12 More recently, modern radiation techniques, such as image guided intensity modulated radiation therapy (IG-IMRT), have been shown to improve targeting accuracy and reduce gastrointestinal and hematologic toxicity by limiting dose to bowel and bone marrow, respectively.13,14 Such reductions could permit improved chemotherapy tolerance, particularly in the context of highly toxic regimens. However, the cost, complexity, and limited availability of technology have slowed the wider adoption of IG-IMRT into routine practice.15–17 In this study, we sought to test the hypothesis that treatment of LACC with IG-IMRT permits improved tolerance to the concurrent gemcitabine-cisplatin doublet and to assess the extent to which radiation volume (ie, extended field vs pelvic only field) and chemotherapy sequencing affected tolerance of this regimen.
Methods and Materials
Study design, setting, and participants
This was a single-center, open-label, dose-escalation, phase 1 trial conducted from February 2011 through June 2019. Eligible patients were aged ≥ 18 years and had pathologically confirmed, International Federation of Gynecology and Obstetrics 2009 stage IB-IVA squamous cell carcinoma or adenocarcinoma of the cervix, with either an intact cervix or posthysterectomy with residual gross pelvic disease, or biopsy-proven paraortic nodal involvement (either resected or unresected). Patients with recurrent disease confined to the pelvis after hysterectomy alone were eligible. Pretreatment diagnostic evaluation included a history/physical examination, cervical biopsy, chest x-ray or chest computed tomography (CT), and pelvic CT or magnetic resonance imaging in all patients. 18F-fluorodeoxyglucose positron emission tomography/CT (PET/CT), cystoscopy, and sigmoidoscopy were used if indicated. All patients had a Karnofsky performance status ≥60, absolute neutrophil count ≥ 1500/μL, platelet count ≥ 100,000/μL, hemoglobin count ≥ 8.0 gm/dL, serum creatinine ≤1.5 mg/dL, and aminotransferases <1.5 times the upper limit of normal for age.
Patients were ineligible if they had prior invasive malignancy (except nonmelanomatous skin cancer) with active disease within the past 3 years; were pregnant/lactating; received growth factors or erythropoietic drugs within 7 days of registration; or had active uncontrolled infection, paraortic nodal disease only, distant metastases, chemo-therapy within 3 years of registration, prior radiation therapy to the pelvis or abdomen, or a history of hematologic disease.
The study was approved by the University of California San Diego Institutional Review Board and registered at ClinicalTrials.gov (NCT01554410). All patients provided written informed consent.
Study procedures
Chemotherapy was administered intravenously once weekly starting on day 1 of external beam radiation therapy. Chemotherapy consisted of 5 cycles of cisplatin 40 mg/m2 (70 mg maximum) with concurrent gemcitabine in escalating dose cohorts of 50, 75, 100, or 125 mg/m2. Patients were assigned to separate cohorts before registration according to whether they would receive pelvic or extended-field radiation and whether cisplatin would precede (CG) or follow (GC) gemcitabine in sequencing. The dosing scheme began with CG sequencing, with escalating gemcitabine doses from 50 to 125 mg/m2 in 25 mg/m2 increments, followed by a change to GC sequencing at the highest tolerated dose level, with a de-escalation of 1 to 2 dose levels depending on observed toxicity (Fig. 1).
Fig. 1.
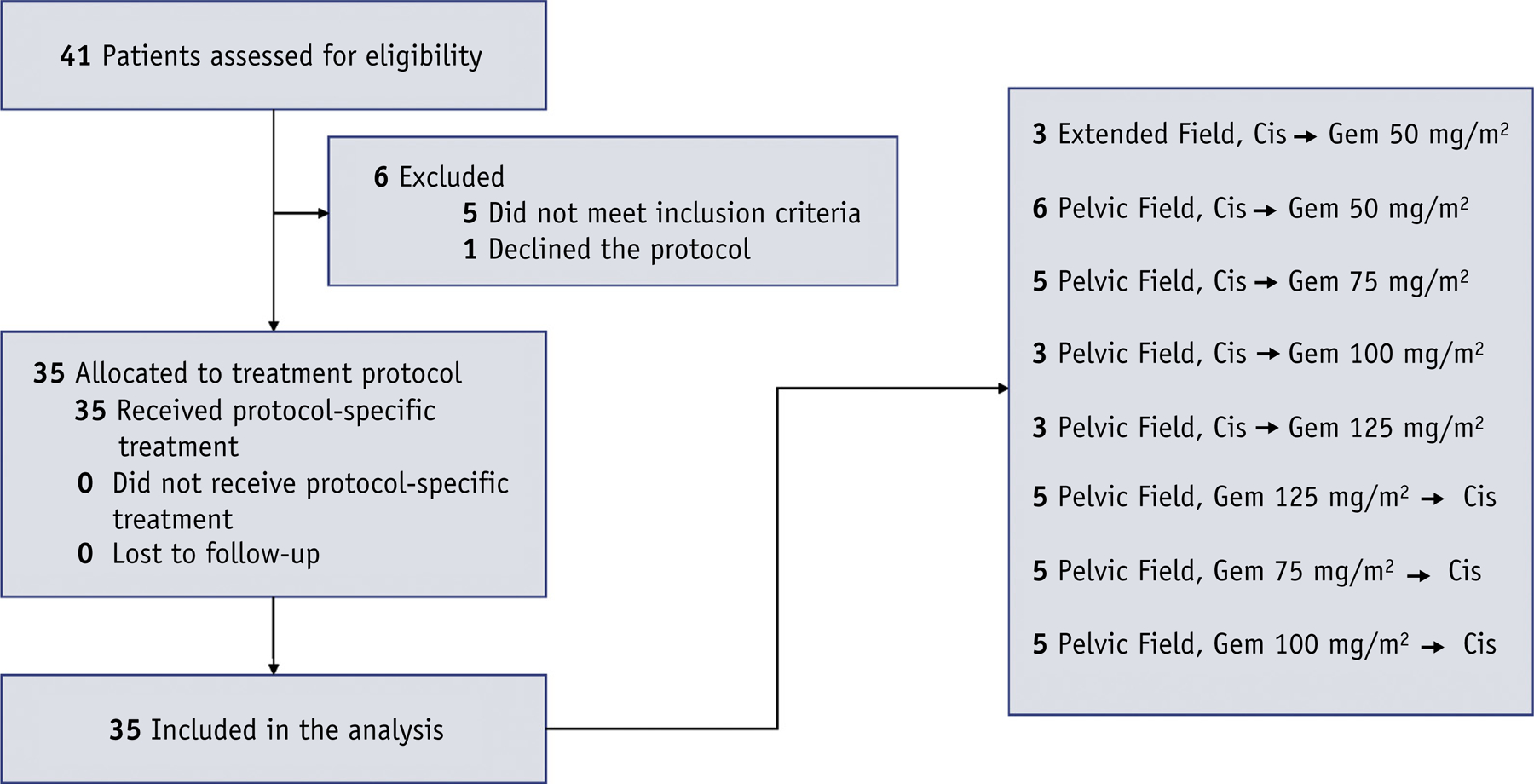
CONSORT diagram and cohort distribution.
Radiation therapy consisted of IG-IMRT to the whole pelvis, 45.0 to 47.6 Gy in 25 to 28 daily fractions, 5 fractions per week. For patients with no involved lymph nodes, 45.0 Gy in 25 fractions was used; otherwise, 47.6 Gy in 28 fractions was used. Patients with grossly involved lymph nodes (determined by abnormal metabolic activity on PET/CT) received a simultaneous integrated boost to 54.0 to 59.4 Gy in 28 fractions, with the final dose determined by bowel tolerance (D0.03cc <62.1 Gy EQD2). No size cutoff was specified for the nodal boost.
Clinical target volumes (CTVs) included the primary tumor and gross nodes, cervix, uterus (as applicable), parametria, upper vagina, and common, external, internal, and presacral lymph nodes. For patients in the extended field cohort, paraortic nodal fields extended to the superior border of L1. The CTV did not include the bladder or rectum. Planning target volumes were 0.5 cm around lymph node regions and nodal boosts (as applicable), 1.0 cm around the parametria and vagina, and 1.5 cm around the primary tumor, cervix, and uterus, or the vaginal cuff for postoperative patients. Use of an internal target volume obtained by fusing the CTV from full and empty bladder scans and then adding a 0.7 cm planning margin was optional at the discretion of the treating radiation oncologist.
Key normal tissue requirements for IG-IMRT planning were bowel volume receiving >45 Gy (V45) ≤250 cm3, bone marrow volume receiving >10 Gy (V10) <90%, and bone marrow volume receiving >20 Gy (V20) <75%. Bowel was defined as the outermost extent of the bowel loops beginning from the axial slice 1 cm above the superior border of the planning target volume and extending to the most inferior extent in the pelvis, excluding the distal descending colon and sigmoid colon. Bone marrow was defined as the outer contour of the os coxae, sacrum, acetabulae, L4-L5 (plus L1-L3 for extended field patients), and proximal femora extending to the inferior border of the ischial tuberosities. Active bone marrow was defined as the subset of the bone marrow with standardized uptake value greater than the mean standardized uptake value for the bone marrow. Active bone marrow sparing was not an initial requirement of the protocol and was implemented in later patients who underwent pretreatment PET/CT, based on supporting data.18
An optional parametrial boost (4–10 Gy in 2–5 fractions) was delivered according to the discretion of the treating physician and was not delivered if a simultaneous integrated boost plan was used. Radiation was delivered using 6 or 15 MV photon volumetric modulated arc therapy with image guidance using daily kV-kV and cone beam CT; note that this differs from Radiation Therapy Oncology Group 1203, which did not require cone beam CT with each fraction in the postoperative setting.13 Patients with intact cervix received volume-directed high-dose-rate brachy-therapy consisting of 24 to 30 Gy in 3 to 5 fractions to the high-risk clinical target volume. Patients who were post-hysterectomy with gross disease received 21 to 25 Gy in 3 to 5 fractions using intracavitary or interstitial brachytherapy; gross disease not amenable to brachytherapy was treated with stereotactic body radiation therapy (25–26 Gy in 5 fractions). Chemotherapy was not delivered on the same day as brachytherapy.
Endpoints and statistical analysis
The primary aim was to estimate the maximum tolerated dose (MTD) of gemcitabine in combination with IG-IMRT and standard 40 mg/m2 cisplatin. In addition, we sought to gauge the impact of both radiation volume and chemo-therapy sequencing on treatment tolerance, with particular attention to the MTD of gemcitabine associated with GC sequencing. A dose-limiting toxicity (DLT) event was defined as any of the following occurring from protocol registration until 30 days after the completion of all therapy: grade 4 neutropenia lasting >7 days, neutropenic fever, grade 4 thrombocytopenia, symptomatic grade 3 thrombocytopenia, any grade 3 or 4 nonhematologic toxicity (except nausea and/or vomiting lasting <24 hours or diarrhea delaying therapy <6 days), or any treatment-related morbidity causing a delay of therapy for >2 weeks, following the Gynecologic Oncology Group definition.9
Toxicity was assessed using the Common Terminology Criteria for Adverse Events version 4.0. Each patient was monitored before treatment and weekly during treatment through a physical examination with vital signs, complete blood count with differential, comprehensive metabolic panel, and clinical evaluation for toxicity and adverse events. Acute toxicity was defined as occurring between trial registration and up to 30 days after treatment completion, with late toxicity 31 or more days after treatment completion. Secondary endpoints included locoregional failure, disease-free survival, and overall survival. Event times were calculated from enrollment date until first occurrence of the event (or censoring).
The protocol called for dose escalation if 0 of the first 3 patients experienced a DLT, expansion to 6 patients if 1 of the first 3 experienced a DLT, and closure if 2 or more patients at the given dose level experienced a DLT. Escalation was allowed if the observed probability of a DLT at the given dose level did not exceed 20%. To estimate MTD, we followed guidelines for the standard design above.19 In addition, on exploratory analysis we fit a univariate logistic regression with gemcitabine dose as the predictor and DLT as the outcome to estimate the dose giving a 20% probability of a DLT. Log transformation was used to ensure confidence intervals excluded negative values. We also performed a sensitivity analysis to determine whether enrolling a sixth patient to the final cohort (GC, 100 mg/m2) would affect our conclusions. Time to recurrence was described using cumulative incidence functions. Overall and disease-free survival were estimated using the Kaplan-Meier method. Linear mixed-effects models with a random intercept, adjusting for time and baseline value, were used to assess effects of chemotherapy sequencing on longitudinal blood counts. Statistical analyses were performed using R Studio, version 3.6.1 (R Foundation for Statistical Computing). All tests were 2-sided with P < .05 indicating statistical significance.
Results
Patient characteristics
Overall, 41 patients were screened for the trial and 35 were ultimately enrolled into 8 cohorts (Table 1). All patients completed treatment, and the median follow-up for all patients was 47 months (interquartile range, 24–65). The majority of patients were treated with primary chemoradiation and underwent pretreatment PET/CT, with roughly even distribution among stage IB (n = 12 [34%]), stage II (n = 10 [29%]), and stage III-IVA (n = 13 [37%]). Three patients (9%) were treated for residual (n = 1) or recurrent (n = 2) pelvic disease detected at 2, 6, and 7 months posthysterectomy, respectively.
Table 1.
Patient characteristics
| Characteristic | n (%) |
|---|---|
| Age, y | |
| ≤40 | 14 (40) |
| >40 to ≤50 | 6 (17) |
| >50 to ≤60 | 11 (31) |
| >60 | 4 (11) |
| Race/ethnicity | |
| White, non-Hispanic/Latina | 15 (43) |
| Hispanic/Latina | 16 (46) |
| Other | 4 (11) |
| Karnofsky performance status score | |
| 100 | 22 (63) |
| 90 | 5 (14) |
| 80 | 6 (17) |
| 70 | 2 (6) |
| Body mass index, kg/m2 | |
| ≥20 to <25 | 8 (23) |
| ≥25 to <30 | 11 (31) |
| ≥30 to <35 | 10 (29) |
| ≥35 | 6 (17) |
| Smoking status | |
| Current | 9 (26) |
| Former | 8 (23) |
| Never | 18 (51) |
| FIGO stage (2009) | |
| IB | 12 (34) |
| IIA | 2 (6) |
| IIB | 8 (23) |
| IIIA | 1 (3) |
| IIIB | 11 (31) |
| IVA | 1 (3) |
| Tumor histology | |
| Squamous cell carcinoma | 24 (69) |
| Adenocarcinoma | 10 (29) |
| Adenosquamous carcinoma | 1 (3) |
| Tumor grade | |
| Moderately differentiated | 12 (34) |
| Poorly differentiated | 17 (49) |
| Unknown | 6 (17) |
| Pretreatment imaging | |
| PET/CT | 33 (94) |
| MRI | 16 (46) |
| Surgery | |
| None (intact cervix) | 32 (91) |
| Posthysterectomy, residual disease | 1 (3) |
| Posthysterectomy, recurrent disease | 2 (6) |
| No. of cisplatin cycles | |
| ≤3 | 3 (9) |
| 4 | 9 (26) |
| 5 | 22 (63) |
| 6 | 1 (3) |
| No. of gemcitabine cycles | |
| ≤3 | 4 (11) |
| 4 | 8 (23) |
| 5 | 22 (63) |
| 6 | 1 (3) |
| Radiation approach | |
| Teletherapy | |
| Extended (paraortic) field | 3 (9) |
| Inguinal field | 3 (9) |
| Simultaneous integrated boost | 21 (60) |
| Parametrial boost | 8 (23) |
| Stereotactic boost | 3 (9) |
| Brachytherapy | |
| Intracavitary tandem + ovoids/ring | 32 (91) |
| Intracavitary cylinder | 2 (6) |
| Interstitial implant | 4 (11) |
| Treatment duration, d | |
| ≤60 | 30 (86) |
| >60 to ≤70 | 3 (9)* |
| >70 | 2 (6)* |
Abbreviations: FIGO = International Federation of Gynecology and Obstetrics; MRI = magnetic resonance imaging; PET/CT = positron emission tomography/computed tomography.
Of 5 treatment durations >60 d, 4 were due to hospitalization and 1 (63 d) was due to a delay in boost scheduling.
Treatment tolerance
Overall, 7 of 35 patients (20%) experienced a DLT (Table 2). Two of 3 patients enrolled in the first extended field cohort (CG, 50 mg/m2) experienced a DLT, and the trial was discontinued for this arm. Both patients received inguinal radiation. One patient experienced a grade 4 pulmonary embolism. The other experienced a suspected drug-related hypersensitivity reaction and was admitted with epigastric abdominal pain, torso rash, and fever of unknown origin, requiring admission to the intensive care unit and pressor support for systemic inflammatory response syndrome.
Table 2.
Detailed description of chemotherapy dosing and adverse events
| Patient | Cohort | Gemcitabine dose, mg/m2 | Sequencing | DLT | Severe adverse events (grade) |
|---|---|---|---|---|---|
| 1 | 1A | 50 | CG | No | Late ureteral stricture (3) |
| 2 | 1A | 50 | CG | No | - |
| 3 | 1A | 50 | CG | Yes | Acute nausea, emesis (3) |
| 4 | 1B | 50 | CG | No | - |
| 5 | 1B | 50 | CG | No | - |
| 7 | 1B | 50 | CG | No | - |
| 6 | EF-1A | 50 | CG | No | - |
| 13 | EF-1A | 50 | CG | Yes | Acute pulmonary embolism (4) |
| 17* | EF-1A | 50 | CG | Yes | Acute drug hypersensitivity (4) |
| 8† | 2A | 75 | CG | No | - |
| 9 | 2A | 75 | CG | Yes | Acute nausea, emesis (3) |
| Late ureteral stricture (3) | |||||
| 10 | 2A | 75 | CG | No | Late radiation cystitis (3) |
| 11 | 2B | 75 | CG | No | - |
| 12 | 2B | 75 | CG | No | - |
| 14‡ | 3A | 100 | CG | No | - |
| 15 | 3A | 100 | CG | No | - |
| 16 | 3A | 100 | CG | No | - |
| 18*,§ | 4A | 125 | CG | No | - |
| 19 | 4A | 125 | CG | No | - |
| 20 | 4A | 125 | CG | No | - |
| 26 | 6A | 75 | GC | No | - |
| 27 | 6A | 75 | GC | No | - |
| 28 | 6A | 75 | GC | No | - |
| 29*,§ | 6B | 75 | GC | No | - |
| 30|| | 6B | 75 | GC | No | - |
| 31¶ | 7A | 100 | GC | No | - |
| 32|| | 7A | 100 | GC | No | - |
| 33|| | 7A | 100 | GC | No | - |
| 34 | 7B | 100 | GC | Yes | Acute nausea, diarrhea (3) |
| 35|| | 7B | 100 | GC | No | - |
| 21 | 5A | 125 | GC | Yes | Acute thrombocytopenia (4) |
| 22 | 5A | 125 | GC | No | - |
| 23 | 5A | 125 | GC | No | - |
| 24 | 5B | 125 | GC | No | - |
| 25 | 5B | 125 | GC | Yes | Acute nausea, diarrhea (3) |
Abbreviations: CG = cisplatin followed by gemcitabine; EF = extended (paraortic) radiation field; GC = gemcitabine followed by cisplatin.
Received stereotactic boost.
Postoperative, residual disease.
Patient assigned to cohort 3A inadvertently, so only 5 patients were enrolled in cohort 2.
Postoperative, recurrent disease.
Received interstitial brachytherapy boost.
Cohort 7 was added after a protocol amendment was approved during the enrollment of cohort 6, so dose escalation proceeded after only 5 patients were enrolled to cohort 6. The trial was closed administratively after 5 patients were enrolled in cohort 7.
In the pelvic field arm, 1 DLT occurred in the first cohort (CG, 50 mg/m2), 1 DLT occurred in the second cohort (CG, 75 mg/m2), 2 DLTs occurred in the fifth cohort (GC, 125 mg/m2), and 1 DLT occurred in the seventh cohort (GC, 100 mg/m2). Four of the DLTs were grade 3 nausea/vomiting and/or diarrhea requiring hospitalization, with symptoms extending beyond 24 hours. The other DLT was a grade 4 thrombocytopenia in a patient receiving GC at 125 mg/m2.
Based on the logistic model results, the overall estimated MTD for gemcitabine delivered with concurrent cisplatin and pelvic IG-IMRT, irrespective of sequencing, was 121 mg/m2 (95% confidence interval [CI], 39–372 mg/m2). The estimated MTD for gemcitabine delivered specifically with GC sequencing was 107 mg/m2 (95% CI, 86–134 mg/m2). On sensitivity analysis, assuming a sixth patient had been enrolled to the GC 100 mg/m2 cohort and experienced a DLT, the estimated MTD was gemcitabine (99 mg/m2; 95% CI, 74–132 mg/m2). Because the primary conclusion would have been unaffected by an additional enrollment, the protocol was closed administratively on July 31, 2019 after evaluation by the review committee, and the MTD was determined to be 100 mg/m2 with GC sequencing.
Adverse events
Details of adverse events are shown in Table 3. There were no grade 5 adverse events. The most common grade 3 to 4 acute toxicities were hematologic (lymphopenia: 32 [91%]; neutropenia: 17 [49%], thrombocytopenia: 8 [23%]; anemia: 7 [20%]) and gastrointestinal (diarrhea: 7 [20%]; nausea: 2 [6%]). Common acute grade 2 nonhematologic and nongastrointestinal events were fatigue (16 [46%]) and dysuria (7 [20%]). Other severe acute adverse events each occurring in 1 patient (3%), regardless of attribution, were grade 3 pyelonephritis, grade 3 thrombus, and grade 2 abscess.
Table 3.
Adverse events*
| Adverse event | Grade 0 | Grade 1 | Grade 2 | Grade 3 | Grade 4 |
|---|---|---|---|---|---|
| Acute† | |||||
| Leukopenia | 1 (3) | 3 (9) | 9 (26) | 18 (51) | 4 (11) |
| Neutropenia | 6 (17) | 0 (0) | 12 (34) | 16 (46) | 1 (3) |
| Lymphopenia | 0 (0) | 0 (0) | 3 (9) | 24 (69) | 8 (23) |
| Anemia | 2 (6) | 3 (9) | 23 (66) | 7 (20) | 0 (0) |
| Thrombocytopenia | 1 (3) | 16 (46) | 10 (29) | 7 (20) | 1 (3) |
| Fatigue | 0 (0) | 18 (51) | 16 (46) | 1 (3) | 0 (0) |
| Fever | 32 (91) | 2 (6) | 1 (3) | 0 (0) | 0 (0) |
| Sweating/chills | 31 (89) | 4 (11) | 0 (0) | 0 (0) | 0 (0) |
| Diarrhea | 2 (6) | 13 (37) | 13 (37) | 7 (20) | 0 (0) |
| Nausea | 2 (6) | 19 (54) | 12 (34) | 2 (6) | 0 (0) |
| Vomiting/emesis | 18 (51) | 11 (31) | 6 (17) | 0 (0) | 0 (0) |
| Low appetite/weight loss | 6 (17) | 25 (71) | 4 (11) | 0 (0) | 0 (0) |
| Constipation/bloating | 13 (37) | 20 (57) | 2 (6) | 0 (0) | 0 (0) |
| Abdominal/pelvic pain | 6 (17) | 21 (60) | 7 (20) | 1 (3) | 0 (0) |
| Rectal bleeding | 27 (77) | 7 (20) | 0 (0) | 1 (3) | 0 (0) |
| Rectal pain | 20 (57) | 14 (40) | 1 (3) | 0 (0) | 0 (0) |
| Vaginal bleeding/discharge | 13 (37) | 19 (54) | 3 (9) | 0 (0) | 0 (0) |
| Dysuria | 12 (34) | 16 (46) | 7 (20) | 0 (0) | 0 (0) |
| Frequency/urgency | 8 (23) | 23 (66) | 4 (11) | 0 (0) | 0 (0) |
| Incontinence | 20 (57) | 12 (34) | 3 (9) | 0 (0) | 0 (0) |
| Hematuria | 24 (69) | 10 (29) | 1 (3) | 0 (0) | 0 (0) |
| Dyspnea/cough | 30 (86) | 4 (11) | 0 (0) | 1 (3) | 0 (0) |
| Tinnitus | 31 (89) | 4 (11) | 0 (0) | 0 (0) | 0 (0) |
| Neuropathy | 31 (89) | 4 (11) | 0 (0) | 0 (0) | 0 (0) |
| Weakness/dizziness | 28 (80) | 5 (14) | 2 (6) | 0 (0) | 0 (0) |
| Dermatitis/rash | 17 (49) | 16 (46) | 2 (6) | 0 (0) | 0 (0) |
| Late† | |||||
| Rectal bleeding | - | - | 4(11) | 0 (0) | 0 (0) |
| Fistula | - | - | 2 (6) | 0 (0) | 0 (0) |
| Ureteral stricture | - | - | 0 (0) | 2 (6) | 0 (0) |
| Soft tissue necrosis | - | - | 1(3) | 0 (0) | 0 (0) |
| Pelvic fracture | - | - | 0 (0) | 0 (0) | |
| Cystitis | - | - | 1 (3) | 0 (0) |
Other acute grade 1 events (number of patients): headache (4), itching/dryness (3), anxiety (2), insomnia (2), urinary retention (2), dyspareunia (1), dysphagia (1), alopecia (1).
Scored with the Common Terminology Criteria for Adverse Events, version 4.0, highest grade recorded, regardless of attribution. Data are presented as number (percentage) of patients unless otherwise indicated.
Acute period is from trial registration until 30 d after treatment completion. Late period is 31 or more d after treatment completion.
The most common late high-grade toxicity was grade 2 radiation proctitis, which 4 patients (11%) experienced at a median time of 15 months from study registration. Two patients developed vesicovaginal fistulae and 2 patients developed ureteral strictures. One patient experienced soft tissue radiation necrosis of the vaginal vault treated with hyperbaric oxygen. None of the patients who underwent either interstitial or stereotactic boost developed any high-grade late toxicities.
Compliance
The median total treatment duration was 52 days (range, 41–90). Thirty patients (86%) had treatment completed in fewer than 60 days, and 31 patients (89%) received 4 or more cycles of gemcitabine (Table 1). One patient was given a sixth cycle of cisplatin/gemcitabine (CG, 125 mg/m2) at the discretion of the treating oncologist. One patient received 5 cycles of adjuvant carboplatin and paclitaxel after completing protocol therapy (GC, 75 mg/m2) at an outside institution. Two patients had cisplatin and gemcitabine given in the incorrect sequence for 1 of the delivered cycles. The sixth patient assigned to cohort CG 75 mg/m2 was inadvertently administered 100 mg/m2 and remained at this dose level; because no DLT was observed, the trial proceeded without replacement of a patient at the lower dose cohort.
IG-IMRT plans achieved highly optimized dosimetry to both bowel (mean V45, 192 cm3; standard deviation [SD], 89.5 cm3) and bone marrow (mean [SD] V10, V20, and mean dose, 82.2% [5.9%], 61.0% [9.0%], and 25.7 (2.9) Gy), respectively. Twenty-two plans (63%) were further optimized to spare metabolically active bone marrow identified using PET/CT, with corresponding mean (SD) V10, V20, and mean dose, 84.5% (8.7%), 63.0% (12.7%), and 27.8 (4.8) Gy, respectively. Protocol target constraints, maxima for bowel, bladder, and rectum, and bone marrow V20 were met in all 35 patients. Bowel V45 exceeded 250 cm3 in 4 patients and bone marrow V10 exceeded 90% in 3 patients.
Outcomes
With 28 patients (80%) having at least 24 months of follow-up, 8 patients (23%) experienced treatment failure, and 5 patients (14%) died of progression, with no intercurrent deaths. The 2-year and 3-year overall survival estimates were 86.2% (95% CI, 74.4%-99.8%) and 82.2% (69.1%-97.8%), respectively (Fig. 2). The 2-year and 3-year disease-free survival were 80.0% (95% CI, 66.8%-95.8%) and 76.1% (61.9%-93.4%), respectively (Fig. 2). The 2-year and 3-year cumulative incidence of locoregional failure was 7.1% (95% CI, 2.2%-12.1%) and 11.8% (5.2%-18.4%), respectively (Fig. 2). Two patients developed lung metastasis and received additional systemic therapy and are alive with 24 and 43 months of follow-up. One patient experienced a late, out-of-field suburethral recurrence (65 months) and underwent salvage radiation therapy. One patient (3%) developed a second primary malignancy (small cell lung cancer).
Fig. 2.
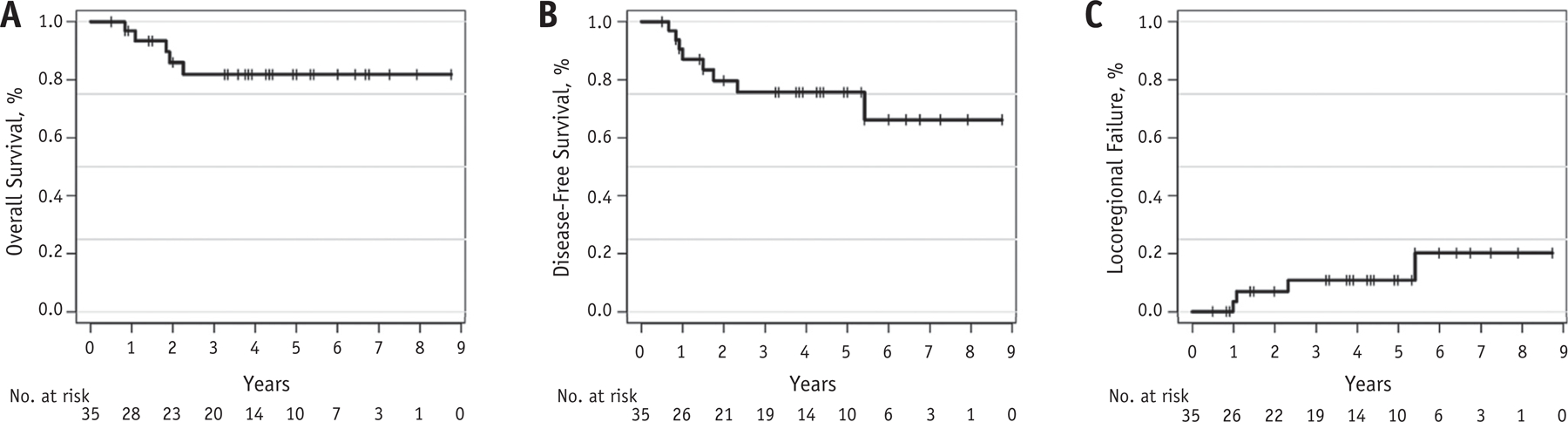
Outcomes for patients with cervical cancer treated with image guided intensity modulated radiation therapy with concurrent cisplatin and gemcitabine. (A) Overall survival. (B) Disease-free survival. (C) Locoregional failure.
Longitudinal analysis of acute hematologic toxicity
We did not find evidence that chemotherapy sequencing affected acute hematologic toxicity using linear mixed-effects models (Fig. 3). In addition, no statistically significant differences were observed in ANC (1.03 vs 0.82 k/μL, P = .17), lymphocyte (0.19 vs 0.25 k/μL, P = .08), hemoglobin (9.4 vs 8.4 g/dL, P = .07), or platelet (75 vs 66 k/μL, P = .33) nadirs for CG versus GC sequencing, respectively, among patients receiving ≥75 mg/m2 gemcitabine.
Fig. 3.
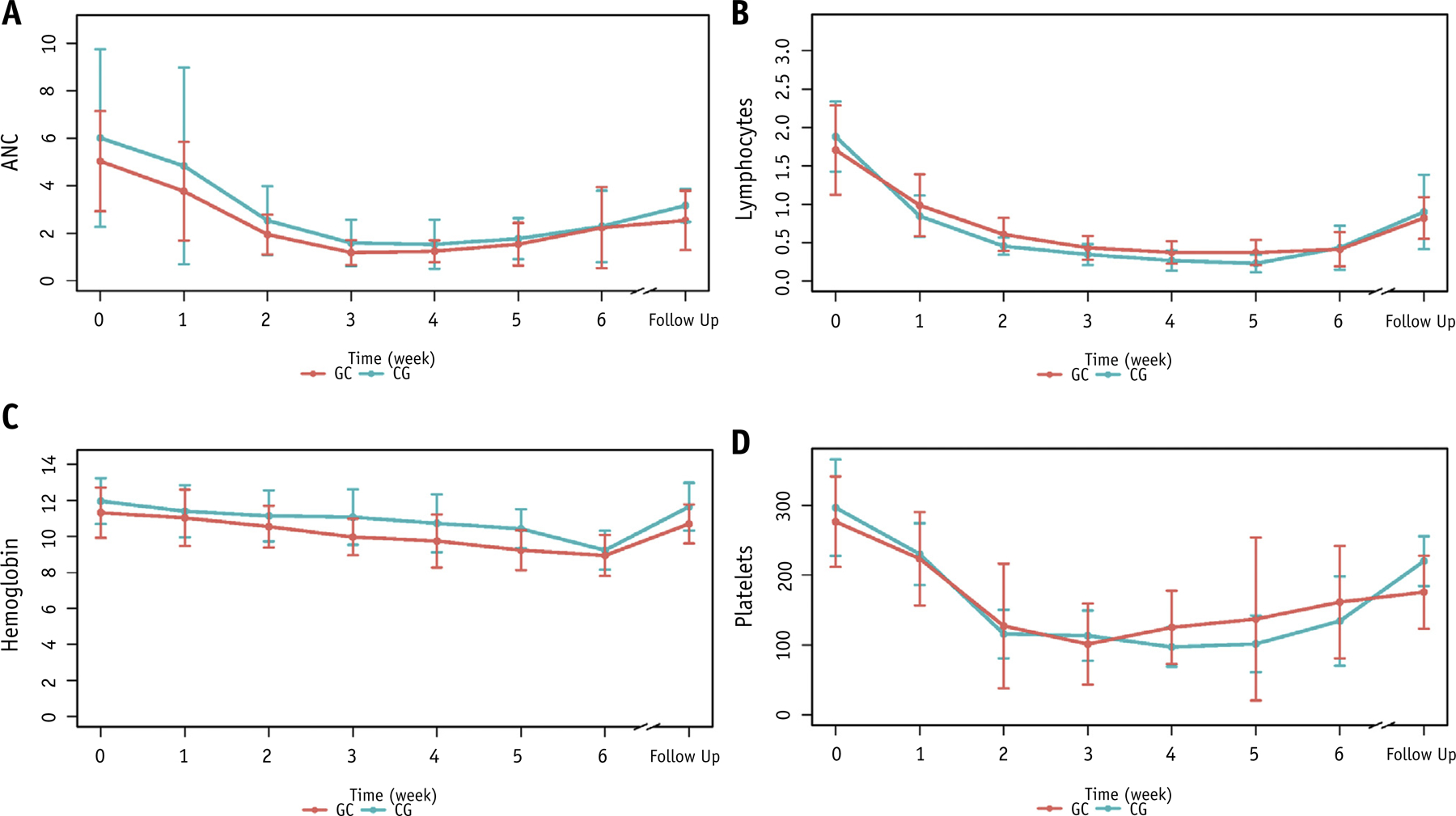
Longitudinal changes in acute blood counts according to chemotherapy sequencing in patients receiving pelvic irradiation with cisplatin 40 mg/m2 weekly and gemcitabine ≥75 mg/m2 weekly. (A) Absolute neutrophil counts, k/μL. (B) lymphocyte counts, k/μL. (C) Hemoglobin levels, g/dL. (D) Platelet counts, k/μL. Follow-up values were obtained 1 to 3 months after completion of therapy. Abbreviations: CG = cisplatin followed by gemcitabine; GC = gemcitabine followed by cisplatin.
Discussion
The premise we tested was that reducing radiation dose to bowel and bone marrow with IG-IMRT could raise the threshold chemotherapy doses for gastrointestinal and hematologic toxicity, respectively, permitting better tolerance to concurrent doublet sensitizing chemotherapy. Previous prospective trials have found that IG-IMRT is effective in reducing acute gastrointestinal and hematologic toxicity,13,14 the principal acute toxicities associated with the concurrent cisplatin/gemcitabine doublet.6 Moreover, previous studies have found high rates of acute toxicity with this regimen using conventional radiation therapy techniques.8,9
We found that with IG-IMRT, gemcitabine doses of 75 to 100 mg/m2 were safe and feasible, with an estimated MTD of 100 mg/m2 with GC sequencing. These doses are higher than found previously using GC sequencing with conventional radiation therapy (50 mg/m2 in 1 study, and infeasible in another),8,9 supporting our primary hypothesis. Overall, 89% of patients received 4 or more cycles of gemcitabine, with lymphopenia being the most common grade 4 hematologic event and 1 instance of grade 4 neutropenia and thrombocytopenia. However, 5 patients encountered high-grade nonhematologic toxicities requiring hospitalization. Moreover, the incidence of acute grade ≥ 3 neutropenia (49%) and diarrhea (20%) remained high (and comparable to the phase 3 trial results from Dueñas-González et al.7—51% and 18%, respectively), despite the ability of IG-IMRT to reduce radiation dose to key organs at risk.
We also found that tolerance of this regimen was affected by both radiation volume and chemotherapy sequencing. This conclusion is based on the following observations: (1) the extended field cohort closed at the lowest dose level (50 mg/m2) with CG sequencing; (2) with pelvic radiation and CG sequencing, gemcitabine doses up to 125 mg/m2 were well tolerated, consistent with other studies; (3) with pelvic radiation and GC sequencing, gemcitabine doses of 75 to 100 mg/m2 but not 125 mg/m2 were feasible. Note, however, this study did not have sufficient statistical power to compare effects of volume and sequencing using a regression model.
Our results indicate that the extent of acute toxicity reduction with IG-IMRT, while meaningful, is constrained in the setting of intensive chemotherapy. Pelvic radiation causes acute and chronic bone marrow injury and invokes a subacute compensatory hematopoietic response in unirradiated marrow, which is suppressed by intensive chemotherapy.20 Thus, we still encountered significant reductions in peripheral cell counts despite implementing bone marrow sparing. Moreover, most of the DLTs were due to gastrointestinal toxicity (particularly emesis and diarrhea). Toxicity with CG sequencing did not seem to follow a dose-dependent pattern, with all 4 DLTs with CG sequencing occurring at lower dose levels. Previous studies have noted that downregulation of cytidine deaminase, a key enzyme mediating gemcitabine degradation, has been correlated with increased toxicity.21,22 In this study, we did not test for cytidine deaminase enzymatic activity, which may have contributed to varying gemcitabine tolerance.
Gemcitabine is a potent radiosensitizer with high activity in cervical cancer and other diseases. A large phase 3 trial previously found that concurrent and adjuvant CG improved progression-free survival and overall survival compared with standard cisplatin and conventional radiation therapy.7 However, lack of confirmatory phase 3 trials and concerns about acute toxicity have mitigated enthusiasm for this approach. Moreover, a recent phase 2 randomized trial in 107 patients found inferior survival and higher toxicity with the addition of neoadjuvant cisplatin and gemcitabine to standard chemoradiation.23 Nonetheless, interest in testing chemoradiation with concurrent ribonucleotide reductase inhibitors continues, including ongoing NRG trials in vulvar and cervical cancer.24,25 Notably, a recent study in 81 patients found that IMRT and cisplatin (30 mg/m2) followed by gemcitabine (125 mg/m2) was well tolerated, with low rates of acute toxicity and favorable long-term outcomes.26 Our long-term out-comes, albeit in a small sample, also indicate this strategy is worthy of continued investigation in LACC, particularly in light of its cost-effectiveness relative to alternative drugs.
Although IG-IMRT can reduce toxicity in LACC,14,27–30 uncertainty regarding its clinical benefits has slowed routine adoption of this technology. This study was unique in using chemotherapy dose escalation to test a primary radiation question. Our sample also had relatively long follow-up to evaluate long-term toxicity and outcomes and notably included a large proportion (46%) self-identifying as Hispanic/Latina, an underrepresented minority in clinical trials.
One problem we encountered is the definition of a DLT used in this and other trials includes grade 3 gastrointestinal toxicity lasting >24 hours, which was the cause of the majority of our DLT events, potentially overestimating clinically meaningful toxicity. We also encountered several administrative challenges, particularly DLTs in lower-dose cohorts requiring several expansions. A phase 1 design with better operating characteristics may have led to a shorter study duration. The single institution design may affect generalizability to multicenter trials; however, IG-IMRT is being widely used in a multi-institutional phase 3 trial in LACC, suggesting wider adoption of this technology is feasible.25 Comparisons of results from trials conducted in different eras should also be interpreted cautiously, given difficulties in parsing subgroup effects in small studies.
Conclusions
This study provides evidence that IG-IMRT can permit better tolerance of intensive concurrent chemotherapy for LACC. In particular, we found weekly gemcitabine at 100 mg/m2 was feasible and well tolerated when delivered concurrently with and preceding cisplatin, in contrast to prior publications. However, acute toxicity remains a factor with this regimen, depending on radiation volume and chemotherapy sequencing.
Acknowledgments
This work was supported by American Cancer Society (IRG-70–002 to L.M.) and the National Institutes of Health (UL1TR001442 to R.X.). Study data were collected and managed using REDCap electronic data capture tools hosted at University of California San Diego.
Footnotes
Disclosures: none.
Data Sharing Statement: All data generated and analyzed during this study are included in this published article (and its supplementary information files).
References
- 1.Keys HM, Bundy BN, Stehman FB, et al. Cisplatin, radiation, and adjuvant hysterectomy compared with radiation and adjuvant hysterectomy for bulky stage IB cervical carcinoma. N Engl J Med 1999;340:1154–1161. [DOI] [PubMed] [Google Scholar]
- 2.Morris M, Eifel PJ, Lu J, et al. Pelvic radiation with concurrent chemotherapy compared with pelvic and para-aortic radiation for high-risk cervical cancer. N Engl J Med 1999;340:1137–1143. [DOI] [PubMed] [Google Scholar]
- 3.Eifel PJ, Winter K, Morris M, et al. Pelvic irradiation with concurrent chemotherapy versus pelvic and para-aortic irradiation for high-risk cervical cancer: An update of radiation therapy oncology group trial (RTOG) 90–01. J Clin Oncol 2004;22:872–880. [DOI] [PubMed] [Google Scholar]
- 4.Rose PG, Bundy BN, Watkins EB, et al. Concurrent cisplatin-based radiotherapy and chemotherapy for locally advanced cervical cancer. N Engl J Med 1999;340:1144–1153. [DOI] [PubMed] [Google Scholar]
- 5.Green JA, Kirwan JM, Tierney JF, et al. Survival and recurrence after concomitant chemotherapy and radiotherapy for cancer of the uterine cervix: A systematic review and meta-analysis. Lancet 2001;358:781–786. [DOI] [PubMed] [Google Scholar]
- 6.Dueñas-Gonzalez A, Cetina-Perez L, Lopez-Graniel C, et al. Pathologic response and toxicity assessment of chemoradiotherapy with cisplatin versus cisplatin plus gemcitabine in cervical cancer. Int J Radiat Oncol Biol Phys 2005;61:817–823. [DOI] [PubMed] [Google Scholar]
- 7.Dueñas-González A, Zarbá JJ, Patel F, et al. Phase III, open-label, randomized study comparing concurrent gemcitabine plus cisplatin and radiation followed by adjuvant gemcitabine and cisplatin versus concurrent cisplatin and radiation in patients with stage IIB to IVA carcinoma of the cervix. J Clin Oncol 2011;29:1678–1685. [DOI] [PubMed] [Google Scholar]
- 8.Swisher EM, Swensen RE, Greer B, et al. Weekly gemcitabine and cisplatin in combination with pelvic radiation in the primary therapy of cervical cancer: A phase I trial of the Puget Sound Oncology Consortium. Gynecol Oncol 2006;101:429–435. [DOI] [PubMed] [Google Scholar]
- 9.Rose PG, Degeest K, McMeekin S, et al. A phase I study of gemcitabine followed by cisplatin concurrent with whole pelvic radiation therapy in locally advanced cervical cancer: A Gynecologic Oncology Group study. Gynecol Oncol 2007;107:274–279. [DOI] [PubMed] [Google Scholar]
- 10.Moufarij MA, Phillips DR, Cullinane C. Gemcitabine potentiates cytotoxicity and inhibits repair of cisplatin-DNA damage in ovarian cancer cell lines. Mol Pharmacol 2003;63:862–869. [DOI] [PubMed] [Google Scholar]
- 11.Kroep JR, Peters GJ, van Moorsel CJ, et al. Gemcitabine-cisplatin: A schedule finding study. Ann Oncol 1999;10:1503–1510. [DOI] [PubMed] [Google Scholar]
- 12.Rotman M, Pajak TF, Choi K, et al. Prophylactic extended-field irradiation of para-aortic lymph nodes in stages IIB and bulky IB and IIA cervical carcinomas. Ten-year treatment results of RTOG 79–20. JAMA 1995;274:387–393. [PubMed] [Google Scholar]
- 13.Klopp AH, Yeung AR, Deshmukh S, et al. Patient-reported toxicity during pelvic intensity-modulated radiation therapy: NRG Oncology-RTOG 1203. J Clin Oncol 2018;36:2538–2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mell LK, Sirák I, Wei L, et al. Bone marrow-sparing intensity modulated radiation therapy with concurrent cisplatin for stage IB-IVA cervical cancer: an international multicenter phase II clinical trial (INTERTECC-2). Int J Radiat Oncol Biol Phys 2017;97:536–545. [DOI] [PubMed] [Google Scholar]
- 15.Lesnock JL, Farris C, Beriwal S, et al. Upfront treatment of locally advanced cervical cancer with intensity modulated radiation therapy compared to four-field radiation therapy: A cost-effectiveness analysis. Gynecol Oncol 2013;129:574–579. [DOI] [PubMed] [Google Scholar]
- 16.Chen LA, Kim J, Boucher K, et al. Toxicity and cost-effectiveness analysis of intensity modulated radiation therapy versus 3-dimensional conformal radiation therapy for postoperative treatment of gynecologic cancers. Gynecol Oncol 2015;136:521–528. [DOI] [PubMed] [Google Scholar]
- 17.Arul Ponni TR, Avinash HU, Nirmala S, et al. Optimal technique of radiotherapy for carcinoma cervix in developing countries: Dosimetric and logistic comparison. J Cancer Res Ther 2018;14:1207–1213. [DOI] [PubMed] [Google Scholar]
- 18.Rose BS, Liang Y, Lau SK, et al. Correlation between radiation dose to 18F-FDG-PET defined active bone marrow subregions and acute hematologic toxicity in cervical cancer patients treated with chemo-radiotherapy. Int J Radiat Oncol Biol Phys 2012;83:1185–1191. [DOI] [PubMed] [Google Scholar]
- 19.Rubinstein LV, Simon RM. Phase I clinical trial design. In: Budman DR, Calvert AH, Rowinsky EK, editors. Handbook of Anticancer Drug Development Philadelphia, PA: Lippincott Williams & Wilkins; 2003. p. 297. [Google Scholar]
- 20.Noticewala SS, Li N, Williamson CW, et al. Longitudinal changes in active bone marrow for cervical cancer patients treated with concurrent chemoradiation therapy. Int J Radiat Oncol Biol Phys 2017;97:797–805. [DOI] [PubMed] [Google Scholar]
- 21.Sugiyama E, Kaniwa N, Kim SR, et al. Pharmacokinetics of gemcitabine in Japanese cancer patients: The impact of a cytidine deaminase polymorphism. J Clin Oncol 2007;25:32–42. [DOI] [PubMed] [Google Scholar]
- 22.Ding X, Chen W, Fan H, et al. Cytidine deaminase polymorphism predicts toxicity of gemcitabine-based chemotherapy. Gene 2015;559: 31–37. [DOI] [PubMed] [Google Scholar]
- 23.da Costa SCS, Bonadio RC, Gabrielli FCG, et al. Neoadjuvant chemotherapy with cisplatin and gemcitabine followed by chemo-radiation versus chemoradiation for locally advanced cervical cancer: A randomized phase II trial. J Clin Oncol 2019;37:3124–3131. [DOI] [PubMed] [Google Scholar]
- 24.U.S. National Library of Medicine. Radiation therapy, gemcitabine hydrochloride, and cisplatin in treating patients with locally advanced squamous cell carcinoma of the vulva Available at: https://clinicaltrials.gov/ct2/show/NCT01595061. Accessed November 10, 2019.
- 25.U.S. National Library of Medicine. Radiation Therapy and Cisplatin With or Without Triapine in Treating Patients With Newly Diagnosed Stage IB2, II, or IIIB-IVA Cervical Cancer or Stage II-IVA Vaginal Cancer Available at: https://clinicaltrials.gov/ct2/show/NCT02466971. Accessed November 10, 2019.
- 26.Drokow EK, Zi L, Qian H, et al. Tolerability, efficacy and feasibility of concurrent gemcitabine and cisplatin (CGP) combined with intensity modulated radiotherapy for loco-regionally advanced carcinoma of the cervix. J Cancer 2020;11:2632–2638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Simpson DR, Song WY, Moiseenko V, et al. Normal tissue complication probability analysis of acute gastrointestinal toxicity in cervical cancer patients undergoing intensity modulated radiation therapy and concurrent cisplatin. Int J Radiat Oncol Biol Phys 2012; 83:e81–e86. [DOI] [PubMed] [Google Scholar]
- 28.Vitzthum LK, Park H, K Zakeri K, et al. Risk of pelvic fracture with radiation therapy in older patients. Int J Radiat Oncol Biol Phys 2020; 106:485–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Klopp AH, Moughan J, Portelance L, et al. Hematologic toxicity in RTOG 0418: A phase 2 study of postoperative IMRT for gynecologic cancer. Int J Radiat Oncol Biol Phys 2013;86:83–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sapienza LG, Salcedo MP, Ning MS, et al. Pelvic insufficiency fractures after external beam radiation therapy for gynecologic cancers: A meta-analysis and meta-regression of 3929 patients. Int J Radiat Oncol Biol Phys 2020;106:475–484. [DOI] [PubMed] [Google Scholar]


