Abstract
Introduction:
Older adults with acute myeloid leukemia(AML) often have significant comorbidities. We hypothesized that greater comorbidity burden predicts worse one-month mortality and overall survival(OS) in patients≥60 years with AML
Materials and methods:
We included 50,668 patients≥60 years diagnosed between 2004–2014 from the National Cancer Database; patients were divided into three groups with Charlson comorbidity index(CCI) 0, 1, and≥2. Chi-square tests were used to examine the association between CCI and different variables. We utilized logistic regression and cox proportional hazard models to determine predictors of one-month mortality and OS, respectively.
Results:
Among the entire cohort, 65% had CCI 0, 24% had CCI 1, and 11% had CCI≥2. Thirty-four percent did not receive chemotherapy. Patients with CCI 0 were more likely to receive chemotherapy, especially multiagent chemotherapy and undergo upfront HCT. In multivariate analyses, one-month mortality and OS were significantly worse with CCI 1 or≥2, compared to CCI 0 in the entire cohort as the subgroup of only those patients who received chemotherapy. Younger age, male gender, higher annual income, academic facility, longer travel distance, and acute promyelocytic leukemia were associated with improved OS.
Conclusion:
In one of the largest real-world studies of older adults with AML, we demonstrated that greater comorbidity, measured by higher CCI, independently predicted worse early mortality and OS in older patients with AML. Higher CCI was more common with increasing age and correlated with lower likelihood of receiving chemotherapy and HCT. Whether optimal comorbidity management and supportive care may improve outcomes needs to be studied further.
Microabstract
We hypothesized that higher Charlson comorbidity index(CCI) predicts worse one-month mortality and overall survival(OS) in patients≥60 years with acute myeloid leukemia(AML). In our National Cancer Database study, patients with CCI 0 were more likely to receive chemotherapy and undergo upfront hematopoietic cell transplant. One-month mortality and OS were significantly worse with CCI 1 or ≥2, compared to CCI 0.
Introduction
Older adults ≥ 60 years comprise more than half of total acute myeloid leukemia (AML) cases; the median age at diagnosis of AML is 65–70 years (1, 2). Several studies report worse outcomes and higher mortality in older patients with AML compared to their younger counterparts, which may be associated with both patient- and disease-related factors (2, 3). Outcomes for older patients, in fact, has remained largely unchanged over past few decades (4, 5). Older patients generally have poor performance status, abnormal organ dysfunction, and significant comorbidities (6, 7). Older adults are also reported to have poor disease biology with higher incidence of unfavorable cytogenetics, decreased response to intensive chemotherapy, and multidrug resistance (3, 7–9). Thus, intensive therapy such as chemotherapy and hematopoietic cell transplant (HCT) may not be offered as an option with concerns for higher mortality and morbidity (8, 10, 11).
In clinical practice, different scores are used to assess the risk of treatment-related toxicities and predict outcomes in patients with multiple comorbidities. One of the scores, Charlson comorbidity index (CCI), calculated based on 19 different medical conditions, weighs the comorbidities to measure patients’ burden of disease (12). CCI is known to be of prognostic significance in various underlying diseases, including different malignancies and has been studied and modified extensively since its development. Deyo modification of CCI is commonly used, which consists of 17 variables; 3 variables- leukemia, ly(2)mphoma, and localized solid tumors in original CCI are combined into a single variable- any malignancy other than metastatic solid tumors (supplement table 1) (13). Increase in number of comorbidities increases the CCI score and predicts worse outcomes (14).
We hypothesized that comorbidity burden predicts outcomes in AML. In this context, we analyzed the effect of CCI on one-month mortality and overall survival (OS) in older patients with AML.
Materials and Methods
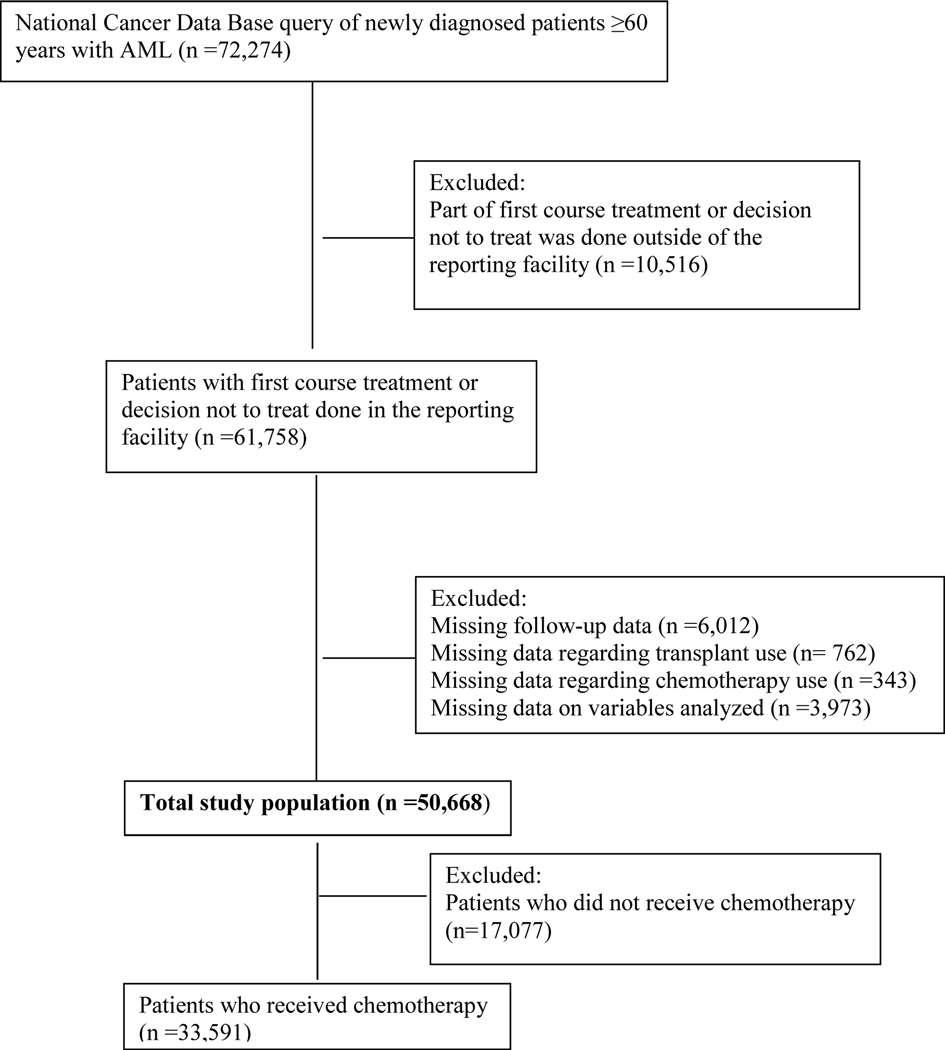
We utilized the National Cancer Data Base (NCDB) Participant User File (PUF) to identify patients aged ≥60 years, who were diagnosed between 2004 and 2014. NCDB, a joint program of the Commission on Cancer of the American College of Surgeons and the American Cancer Society, captures approximately 70% of new diagnoses of cancer in the United States (15). Adult patients with AML were captured using International Classification of Diseases for Oncology, Version 3, codes 9840–9861, 9865–9874, and 9891–9931. The CONSORT diagram (Figure 1) illustrates the cohort selection. We excluded patients who received part of first course treatment or decided not to treat outside of the reporting facility as well as the patients with missing data (n=10,516). For a subgroup analysis of patients who received chemotherapy, we excluded the patients who did not receive chemotherapy (n=17,077). Deyo modification of CCI was used to divide patients into 3 groups with CCI scores of 0, 1, and ≥2. In NCDB database, the calculation of the Charlson-Deyo score excludes any comorbidity code identified as malignant neoplasms from the total score, since all patients have a diagnosis of cancer. Other variables analyzed in our study included age, sex, race, education, income, insurance, facility location (urban/rural), facility type, receipt of chemotherapy, receipt of hematopoietic cell transplant (HCT), distance traveled to the treatment facility, and histology. The educational and income status are not patient-level data; they are an estimated average of the population residing in the zip code of the patient, as determined by census data of the year 2012. Further information on classification of other variables in NCDB database is detailed in our prior publication (16). Our study, with deidentified data, was not considered a human subject research by the institutional review board at the University of Nebraska Medical Center.
Figure 1.
CONSORT diagram for cohort selection. AML indicates acute myeloid leukemia
Statistical analysis
Chi-square test was used to determine the relationship between one-month mortality and each of the categorical potential predictors. Variables with p-values <0.20 from the univariate analyses were used in a multiple logistic regression model to predict one-month mortality, and backward selection was used to determine the final model. In the final model, each of the variables was presented as odds ratios (OR) and 95% confidence interval (CI). For OS, we performed univariate survival analyses and generated Kaplan-Meier curves. Within each variable, the KM curves for each stratum were compared using the log rank test. The p-values from the log rank test of the survival analysis for each variable were presented. The variables with p-values <0.20 were included in a multivariate cox-proportional model and the model was reduced using backward elimination where 0.05 was the criteria for a term to remain in the model. A subgroup analysis using the same methods was performed among patients who received chemotherapy. PC SAS version 9.4 was used for all summaries and analyses.
Results
A total of 50,668 patients were included in the analysis: 35% were 60–69 years of age, 44% were female, 81% were white, 64% received chemotherapy, and 4% received HCT. Among the entire study population, 65% had CCI 0, 24% had CCI 1, and 11% had CCI ≥2 (Table 1). Patients with CCI 0, compared to those with CCI 1 or ≥2 were slightly younger, had higher annual income and private insurance, received treatment at academic center, and travelled longer distance for treatment. Patients with CCI 0 were more likely to receive chemotherapy, especially multiagent chemotherapy (42% vs 39% vs 28%) and slightly more likely to undergo upfront HCT (4% vs 3% vs 1%).
Table 1:
Patient characteristics
| Variable | CCI 0 N=32,983 (%) | CCI 1 N=12,018 (%) | CC score ≥2 N=5,667 (%) | P-value |
|---|---|---|---|---|
| Age | <0.001 | |||
| 60–69 | 11848 (36%) | 4011 (33%) | 1681 (30%) | |
| 70–79 | 12087 (37%) | 4510 (38%) | 2197 (39%) | |
| 80–89 | 7863 (24%) | 3022 (25%) | 1553 (27%) | |
| ≥90 | 1185 (4%) | 475 (4%) | 236 (4%) | |
|
| ||||
| Sex | 0.01 | |||
| Male | 18185 (55%) | 6762 (56%) | 3220 (57%) | |
| Female | 14798 (45%) | 5256 (44%) | 2447 (43%) | |
|
| ||||
| Race | <0.001 | |||
| White | 29448 (89%) | 10600 (88%) | 4990 (88%) | |
| African American | 2111 (6%) | 955 (8%) | 466 (8%) | |
| Other | 1424 (4%) | 463 (4%) | 211 (4$) | |
|
| ||||
| No high school diploma * | <0.001 | |||
| ≥21% | 4748 (14%) | 1941 (16%) | 974 (17%) | |
| 13%−20.9% | 8230 (25%) | 3097 (26%) | 1563 (28%) | |
| 7%−12.9% | 11380 (35%) | 4174 (35%) | 1959 (35%) | |
| < 7% | 8625 (26%) | 2806 (23%) | 1171 (21%) | |
|
| ||||
| Annual household income * | <0.001 | |||
| <$38,000 | 5183 (16%) | 2215 (18%) | 1125 (20%) | |
| $38000-$47999 | 7895 (24%) | 2962 (25%) | 1493 (26%) | |
| $48000-$62999 | 9010 (27%) | 3269 (27%) | 1514 (27%) | |
| ≥$63,000 | 10895 (33%) | 3572 (30%) | 1535 (27%) | |
|
| ||||
| Location type | 0.02 | |||
| Urban | 32331 (98%) | 11730 (98%) | 5548 (98%) | |
| Rural | 652 (2%) | 288 (2%) | 119 (2%) | |
|
| ||||
| Facility type | <0.001 | |||
| Academic | 15342 (47%) | 5294 (44%) | 2253 (40%) | |
| Non-academic | 17641 (53%) | 6724 (56%) | 3414 (60%) | |
|
| ||||
| Insurance type | <0.001 | |||
| Medicare | 24133 (73%) | 9252 (77%) | 4545 (80%) | |
| Private | 7223 (22%) | 2179 (18%) | 841 (15%) | |
| None/Medicaid/Other | 1627 (5%) | 587 (5%) | 281 (5%) | |
|
| ||||
| Chemotherapy | <0.001 | |||
| No chemotherapy | 10255 (31%) | 4274 (36%) | 2548 (45%) | |
| Single agent | 7993 (24%) | 2889 (24%) | 1414 (25%) | |
| Multi agent | 13956 (42%) | 4636 (39%) | 1605 (28%) | |
| Unknown | 779 (2%) | 219 (2%) | 100 (2%) | |
|
| ||||
| Received HCT | <0.001 | |||
| Yes | 1426 (4%) | 303 (3%) | 54 (1%) | |
| No | 31557 (96%) | 11715 (97%) | 5613 (99%) | |
|
| ||||
| Distance traveled (miles) † | <0.001 | |||
| 0–4.9 | 8731 (26%) | 3360 (28%) | 1709 (30%) | |
| 5–10.9 | 7555 (23%) | 2773 (23%) | 1323 (23%) | |
| 11–30.9 | 8222 (25%) | 2984 (25%) | 1369 (24%) | |
| ≥31 | 8475 (26%) | 2901 (24%) | 1266 (22%) | |
|
| ||||
| Histology | <0.001 | |||
| APL | 1337 (4%) | 622 (5%) | 358 (6%) | |
| Core binding factor AML | 699 (2%) | 253 (2%) | 133 (2%) | |
| Therapy-related AML/AML | 3941 (12%) | 1223 (10%) | 570 (10%) | |
| MRC | ||||
| All others | 27006 (82%) | 9920 (83%) | 4606 (81%) | |
based on aggregate census data from the patient’s zip code
distance between the center of the patients’ zip code of residence and the treatment facility
AML- Acute myeloid leukemia; APL- Acute promyelocytic leukemia; CCI- Charlson comorbidity index; CI- Confidence interval; HCT- Hematopoietic cell transplant; MRC- Myelodysplasia related changes
One-month mortality was 24%, 34% and 45% for patients with CCI 0, 1, and ≥2 respectively (p<0.001). In a univariate analysis, lower CCI (24% vs 34% vs 44% for CCI 0, 1, and ≥2, respectively, p<0.001) was associated with lower one-month mortality (supplement table 2). In a multivariate analysis, one-month mortality was significantly worse in patients with CCI 1 (OR 1.5, 95% CI 1.4–1.6) or ≥2 (OR 2.3, 95% CI 2.2–2.5), in comparison to those with CCI 0 (Table 2). Other independent factors with improved one-month mortality included younger age, male gender, higher annual income, academic facility, availability of private insurance, longer distance traveled, and acute promyelocytic leukemia.
Table 2:
Multivariate logistic regression for one-month mortality
| Variable | Odds ratio | 95% CI | p-value |
|---|---|---|---|
| Charlson comorbidity index | |||
| 0 | 1 | ||
| 1 | 1.55 | 1.47–1.62 | <0.001 |
| ≥2 | 2.35 | 2.21–2.5 | <0.001 |
|
| |||
| Age | |||
| 60–69 | 1 | ||
| 70–79 | 1.62 | 1.53–1.71 | <0.001 |
| 80–89 | 2.72 | 2.57–2.89 | <0.001 |
| ≥90 | 4.41 | 3.97–4.89 | <0.001 |
|
| |||
| Sex | |||
| Female | 1 | ||
| Male | 0.9 | 0.91–0.98 | 0.01 |
|
| |||
| Annual houelhold income * | |||
| ≥$63,000 | 1 | ||
| $48000–62999 | 1.09 | 1.03–1.51 | 0.001 |
| $38000–47999 | 1.14 | 1.08–1.21 | <0.001 |
| <$38,000 | 1.21 | 1.14–1.29 | <0.001 |
|
| |||
| Location type | |||
| Urban | 1 | ||
| Rural | 1.16 | 1.01–1.34 | 0.02 |
|
| |||
| Facility type | |||
| Academic | 1 | ||
| Non-academic | 1.52 | 1.45–1.58 | <0.001 |
|
| |||
| Insurance type | |||
| Private | 1 | ||
| Medicare | 1.2 | 1.13–1.27 | <0.001 |
| None/All | 1.25 | 1.12–1.39 | <0.001 |
|
| |||
| Distance traveled (miles) † | |||
| 0–4.9 | 1 | ||
| 5–10.9 | 0.9 | 0.85–0.95 | 0.0003 |
| 11–30.9 | 0.86 | 0.82–0.91 | <0.001 |
| ≥31 | 0.82 | 0.77–0.87 | <0.001 |
|
| |||
| Histology | |||
| APL | 1 | ||
| Core binding factor AML | 0.56 | 0.47–0.67 | <0.001 |
| Therapy-related AML/AML MRC | 0.47 | 0.42–0.53 | <0.001 |
| All other | 0.84 | 0.77–0.93 | 0.0006 |
based on aggregate census data from the patient’s zip code
distance from patients’ residence to the treatment facility
AML- Acute myeloid leukemia; APL- Acute promyelocytic leukemia; CI- Confidence interval; MRC- Myelodysplasia related changes
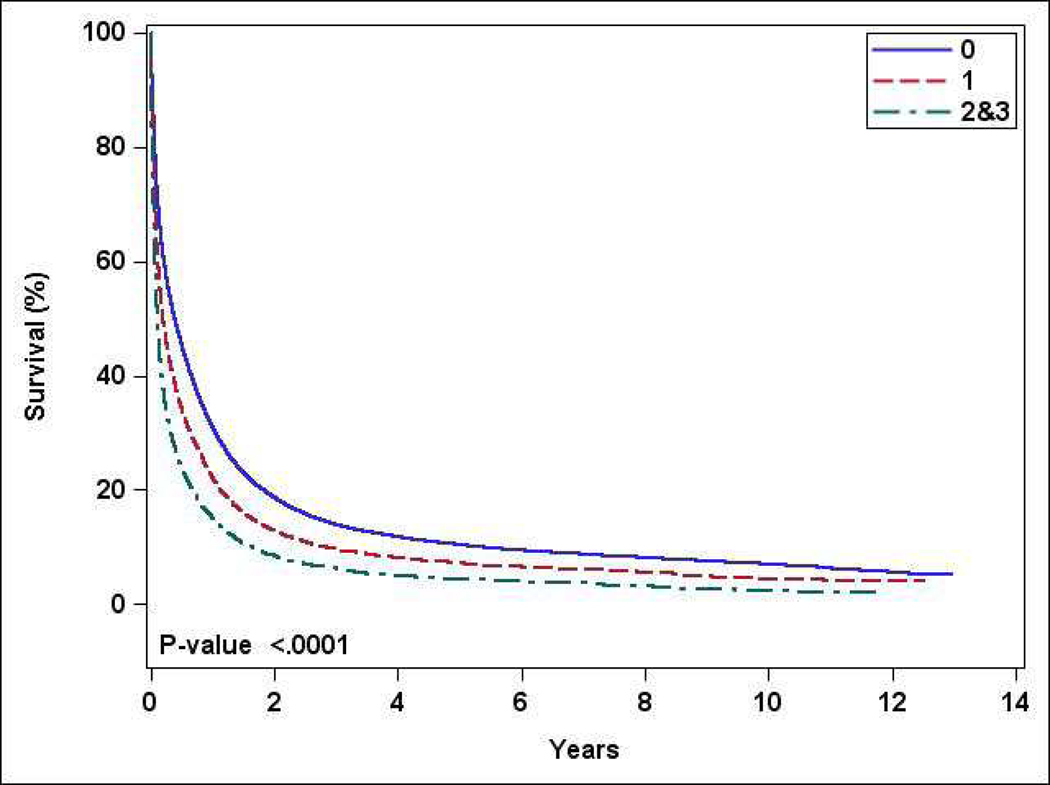
The median one-year OS for the entire study population was 27% (supplement figure 1). The median OS for patients with CCI 0, 1, and ≥2 was 31%, 22%, and 15% respectively (p<0.001) (Figure 2). In a univariate analysis, treatment at academic center, receipt or chemotherapy, and receipt of HCT were associated with higher OS (supplement table 3). In a multivariate analysis, OS was worse with CCI 1 (Hazard ratio [HR] 1.27, 95% CI 1.24–1.31) and ≥2 (HR1.53, 95% CI 1.49–1.58), compared to CCI 0 (Table 3). Age 60–69 years, male gender, higher annual income, academic facility, longer travel distance, and acute promyelocytic leukemia were associated with improved OS.
Figure 2:
Overall survival stratified by Charlson comorbidity index (0 vs 1 vs ≥2)
Table 3:
Cox proportional hazard model for overall survival
| Variable | Hazard ratio | 95% CI | p-value |
|---|---|---|---|
| Charlson comorbidity index | |||
| 0 | 1 | ||
| 1 | 1.27 | 1.24–1.3 | <0.001 |
| ≥2 | 1.53 | 1.49–1.58 | <0.001 |
|
| |||
| Age | |||
| 60–69 | 1 | ||
| 70–79 | 1.24 | 1.21–1.27 | <0.001 |
| 80–89 | 1.46 | 1.41–1.5 | <0.001 |
| ≥90 | 1.73 | 1.65–1.83 | <0.001 |
|
| |||
| Sex | |||
| Female | 1 | ||
| Male | 1.02 | 1.007–1.04 | 0.006 |
|
| |||
| Race | |||
| White | 1 | ||
| African American | 0.99 | 0.95–1.02 | 0.5 |
| Other | 0.92 | 0.88–0.96 | 0.001 |
|
| |||
| Annual household income * | |||
| ≥$63,000 | 1 | ||
| $48000–62999 | 1.04 | 1.01–1.06 | 0.001 |
| $38000–47999 | 1.06 | 1.03–1.09 | <0.001 |
| <$38,000 | 1.11 | 1.07–1.14 | <0.001 |
|
| |||
| Facility type | |||
| Academic | 1 | ||
| Non-academic | 1.07 | 1.05–1.09 | <0.001 |
|
| |||
| Insurance type | |||
| Private | 1 | ||
| Medicare | 1.17 | 1.08–1.14 | <0.001 |
| None/All | 1.05 | 1.01–1.1 | 0.03 |
|
| |||
| Type of chemotherapy | |||
| No chemotherapy | 1 | ||
| Single agent | 0.5 | 0.49–0.52 | <0.001 |
| Multi agent | 0.38 | 0.37–0.39 | <0.001 |
|
| |||
| Receipt of HCT | |||
| Yes | 1 | ||
| No | 2.14 | 2.01–2.28 | <0.001 |
|
| |||
| Distance traveled (miles) † | |||
| 0–4.9 | 1 | ||
| 5–10.9 | 0.97 | 0.94–0.99 | 0.04 |
| 11–30.9 | 0.98 | 0.95–1.006 | 0.13 |
| ≥31 | 1.009 | 0.98=1.03 | 0.5 |
|
| |||
| Histology | |||
| APL | 1 | ||
| Core binding factor AML | 1.67 | 1.53–1.81 | <0.001 |
| Therapy-related AML/AML MRC | 2.05 | 1.93–2.17 | <0.001 |
| All other | 2.4 | 2.27–2.53 | <0.001 |
|
| |||
| Year of diagnosis | |||
| 2010–2014 | 1 | ||
| 2004–2009 | 1.05 | 1.03–1.07 | <0.001 |
based on aggregate census data from the patient’s zip code
distance from patients’ residence to the treatment facility
AML- Acute myeloid leukemia; APL- Acute promyelocytic leukemia; CCI- Charlson comorbidity index; CI- Confidence interval; HCT- Hematopoietic cell transplant; MRC-Myelodysplasia related changes
Subgroup analysis among treated patients
A total of 33,591 patients in our study population received chemotherapy- 45% were 60–69 years of age, 89% were white, 60% received multiagent chemotherapy, and 5% received HCT. Sixty-eight percent had CCI 0, 23% had CCI 1, and 9% had CCI ≥2 (supplement table 4). Patients with CCI 0 were more likely to receive multiagent chemotherapy (61% vs 60% vs 51%) and more likely to undergo upfront HCT (6% vs 4% vs 2%).
One-month mortality was 13%, 19% and 26% for patients with CCI 0, 1, and ≥2 respectively (p<0.001). In a univariate analysis, lower CCI (13% vs 19% vs 26% for CCI 0, 1, and ≥2, respectively, p<0.001) was associated with lower one-month mortality (supplement table 5). Multivariate analysis confirmed significantly worse one-month mortality in patients with CCI 1 (OR 1.5, 95% CI 1.4–1.6) or ≥2 (OR 2.2, 95% CI 2.05–2.5), in comparison to those with CCI 0 (supplement table 6). Younger age, race, higher annual income, academic facility, availability of private insurance, and acute promyelocytic leukemia were associated with improved one-month mortality. Male gender, distance traveled, and facility location (urban/rural) did not influence one-month mortality.
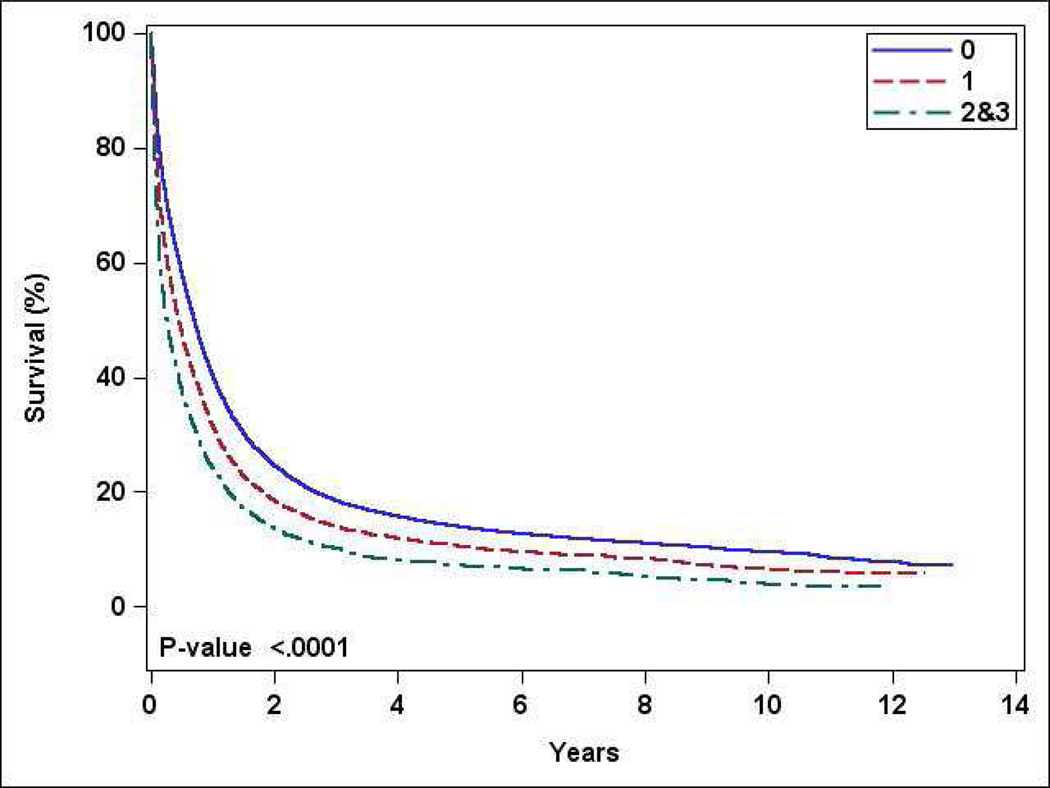
The median one-year OS for patients receiving chemotherapy was 37% (supplement figure 2); median OS for patients with CCI 0, 1, and ≥2 was 40%, 32%, and 24% respectively (p<0.001) (Figure 3). Factors such as treatment at academic center, receipt of multiagent chemotherapy, and receipt of HCT were associated with higher OS in univariate analysis (supplement table 7). In a multivariate analysis, OS was worse with CCI 1 (HR 1.24, 95% CI 1.22–1.28) and ≥2 (HR 1.52, 95% CI 1.46–1.58), compared to CCI 0 (supplement table 8). Other independent factors with improved OS included age 60–69 years, male gender, higher annual income, academic facility, and acute promyelocytic leukemia.
Figure 3:
Overall survival stratified by Charlson comorbidity index (0 vs 1 vs ≥2) for patients who received chemotherapy
Discussion
Outcomes of older adults with AML is generally poor; however, some older adults can achieve remission and attain long-term survival. Predicting chances of survival can help educate patients and families and inform decision-making. While characteristics of AML such as karyotype are frequently used in clinical practice for prognostication, validated measures of comorbidities are less commonly used. CCI is a widely used and validated comorbidity measure that can be easily calculated at the time of diagnosis of AML, thus, providing a tool to further prognosticate patients’ outcomes.
We report the results of one of the largest studies assessing the effect of comorbidity burden on outcomes of older adults with AML. In this real-world analysis, approximately 1/3rd of patients were found to have CCI of 1 or ≥2; older patients, unlike their younger counterparts, were more likely to have CCI 1 or ≥2. A greater comorbidity burden was associated with lower use of chemotherapy or HCT, lower use of multiagent chemotherapy, and independently predicted worse early mortality and OS in older patients with AML, consistent with results of prior studies (11, 17–22). Different reasons for lower use of chemotherapy or HCT in older adults with significant comorbidities include concerns for chemotherapy intolerance and higher risk of early mortality, as well as paucity of data from clinical trials (3, 8, 23).
In our study, 2/3rd of all patients received chemotherapy; prior studies report utilization of chemotherapy in 30–60% of older adults with AML, although specific treatment type or the intent of treatment is not always reported (6, 17–20, 22, 24). Our results indicate that chemotherapy, one of the most important prognostic factors in AML, is less commonly used in patients with higher CCI (11). Thus, to remove the effects of chemotherapy use on outcomes, we performed a subgroup analysis on only those patients who received chemotherapy. Greater comorbidity burden, indeed, had negative effects on early mortality and OS of patients who received chemotherapy, similar to the results for the entire cohort.
Usefulness of CCI as a prognostic tool has been analyzed in a few studies in older adults with AML with variable results (19, 20, 24–26). Our results are supported by two registry-based studies in which higher CCI predicted early death and worse OS, and chemotherapy was less likely to improve OS in patient with CCI ≥2, compared to CCI 0 or 1 (19, 20). A Danish registry database study of AML patients receiving intensive therapy analyzed the association of outcomes in AML with modified CCI and the number of comorbid diseases (24). In contrast to our results, higher modified CCI score or the presence of comorbidities, after adjustment of performance status and other factors, was not predictive of OS in adult AML patients of any age, including those ≥60 years. Similarly, Tawfik et al. analyzed 144 older patients out of total 277 AML patients treated with intensive induction therapy (25). No significant association between CCI and outcomes, including OS, remission or early mortality was reported; although, presence of diabetes and renal disease independently affected 30-day mortality and remission rates, respectively. The studies that did not demonstrate the effect of CCI analyzed impact of comorbidities only among patients receiving intensive induction therapy, which excludes a sizeable population of older adults who are not fit for intensive therapy and are receiving supportive care or less intensive treatment (25–29).
Other measures of comorbidities are also available. HCT comorbidity index (HCT-CI) has gained popularity in patients undergoing HCT (supplement table 1) (30, 31). The studies that have analyzed HCT-CI as a predictor of outcome in AML patients have reported mixed results (28, 29, 32, 33). For example, a multicenter retrospective study demonstrated that HCT-CI as well as an AML composite model incorporating comorbidity and other AML measures predicted the risk of mortality; however, this study included all adults ≥18 years (34). A single center study of patients ≥ 60 years with newly diagnosed AML and planned intensive chemotherapy did not report any effect of HCT-CI in OS (29). Two studies analyzed effects of both CCI and HCT-CI in AML patients- CCI influenced OS but HCT-CI did not (26, 27). While HCT-CI is valuable to predict outcomes, HCT-CI is not easily computable at the time of diagnosis of AML.
Comorbidities, as an individual disease or as a cumulative burden, can affect outcomes in AML in several ways; the adverse impact may be related to the direct biologic interaction between the comorbidity and the cancer, or the indirect effects of the comorbidity on cancer diagnosis, treatment, and complications (30, 35, 36). Mohammadi et al. reported higher all-cause as well as cancer-specific mortality in AML patients with cerebrovascular, rheumatologic, renal, liver, and psychiatric diseases; highest mortality rate was observed with renal disease (37). Comorbidities may preclude optimal treatment of patients with AML and affect utilization of different drugs; for example, anthracyclines may be contraindicated in patients with severe cardiomyopathy, or total cycles of gemtuzumab ozogamicin may be decreased with hepatic dysfunction (38, 39). Organ dysfunction also results in lower tolerance to treatment-related toxicities and increased risk of life-threatening complications in AML, which may lead to chemotherapy dose-reductions, less intensive therapy, treatment delays, and reduced treatment adherence (40). Drug interactions between comorbidity-specific and cancer-specific treatment may affect management and subsequently, outcomes in AML. However, comorbidities do not always reflect performance status and fitness of the patients, which are important in treatment decisions and overall outcomes in AML (24, 41). Thus, treatment of AML in an older patient with comorbidities should be a collective team effort from oncologists, primary care physicians, geriatricians, pharmacists, nurses, and other ancillary members, with detailed assessment prior to treatment initiation, close supervision during and after the treatment, prompt management of any toxicities or complications, and appropriate supportive measures (29).
Comorbidities, as discussed earlier, have been a significant factor influencing use of intensive chemotherapy which may be associated with superior outcomes compared to lower intensity chemotherapy (11, 24, 25). With significant increase in therapeutic options in last few years, patients can receive effective lower intensity treatments (42). As the data with these newer agents evolves in next few years, it will be interesting to see effects of comorbidities on outcomes.
Our retrospective study has potential limitations. NCDB does not provide data regarding patients’ performance status, detailed molecular and cytogenetic features of AML, and specific chemotherapeutic agents used for treatment, which can affect outcomes. Older patients tend to have high percentage of adverse cytogenetics, adverse mutations, and secondary- or therapy-related disease, which predict lower response rate to frontline therapies (3, 43–46). NCDB does not provide data on response, an important predictor of early mortality and OS (47, 48). In addition, comorbidities alone do not take into consideration patients’ physical fitness. Measures such as geriatric assessment can provide useful information in addition to comorbidity and has been shown to predict OS in AML (29). Our study has several strengths including a large population of unselected older adults with AML treated in real world, inclusion of health system and other factors, and use of CCI, an easily computable measure at the time of diagnosis of AML.
Conclusion
Our real-world study highlights the impact of comorbidity burden in early mortality and OS of older adults with AML. Our results indicate that greater comorbidity is more common with increasing age, correlates with lower likelihood of receiving chemotherapy, and predicts worse outcomes. Trials are warranted to determine the effect of optimized comorbidity management and supportive care to reduce early mortality and improve OS.
Supplementary Material
Clinical practice points.
Older adults often have significant comorbidities. Charlson comorbidity index (CCI), which measures comorbidity burden, is known to be of prognostic significance in various underlying diseases.
We investigated our hypothesis that higher CCI predicts worse one-month mortality and overall survival (OS) in patients ≥60 years with acute myeloid leukemia (AML). In our analysis, patients with CCI 0 were more likely to receive chemotherapy and undergo upfront hematopoietic cell transplant. One-month mortality and OS were significantly worse with CCI 1 or ≥2, compared to CCI 0. Younger age, male gender, higher annual income, academic facility, and longer travel distance were associated with improved OS.
Greater comorbidity burden is associated with worse early mortality and OS in older patients with AML. Whether optimal comorbidity management may improve outcomes needs to be studied further.
Acknowledgements
The National Cancer Data Base (NCDB) is a joint project of the Commission on Cancer of the American College of Surgeons and the American Cancer Society. The data used in the study are derived from a deidentified NCDB file. The American College of Surgeons and the Commission on Cancer have not verified and are not responsible for the analytic or statistical methodology used or the conclusions drawn from these data by the investigators.
Funding
The project described was supported by the National Institute Of General Medical Sciences, 1U54GM115458-01. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
VRB reports receiving consulting fees from Agios, Incyte, Omeros, Takeda, Partnership for health analytic research, LLC and Abbvie, research funding (institutional) from Incyte, Jazz, Tolero Pharmaceuticals, Inc, and National Marrow Donor Program, and drug support for a trial from Oncoceutics. KG reports receiving consulting fees from Pfizer, Novartis, and Shionogi, and has stock in Portola Pharmaceuticals.
Footnotes
Conflict of interest
All other authors declare no conflict of interest.
Disclosure
An abstract of this study was accepted for online publication by 2020 American Society of Clinical Oncology Annual Meeting committee.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- 1.Alves AdSBM, Bataglia FB, Conterno LdO, Segato R, Payão SLM. Epidemiological and cytogenetic profiles of patients with hematological malignancies and their relationship with aging. Hematology, Transfusion and Cell Therapy. 2018;40(3):200–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Peyrade F, Gastaud L, Ré D, Pacquelet-Cheli S, Thyss A. Treatment decisions for elderly patients with haematological malignancies: a dilemma. The Lancet Oncology. 2012;13(8):e344e52. [DOI] [PubMed] [Google Scholar]
- 3.Appelbaum FR, Gundacker H, Head DR, Slovak ML, Willman CL, Godwin JE, et al. Age and acute myeloid leukemia. Blood. 2006;107(9):3481–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Luke C, Nguyen A-M, To B, Seshadri R, Hughes T, Bardy P, et al. Myeloid leukaemia treatment and survival-the South Australian experience, 1977 to 2002. Asian Pacific Journal of Cancer Prevention. 2006;7(2):227. [PubMed] [Google Scholar]
- 5.Xie Y, Davies SM, Xiang Y, Robison LL, Ross JA. Trends in leukemia incidence and survival in the United States (1973–1998). Cancer: Interdisciplinary International Journal of the American Cancer Society. 2003;97(9):2229–35. [DOI] [PubMed] [Google Scholar]
- 6.Alibhai SMH, Leach M, Minden MD, Brandwein J. Outcomes and quality of care in acute myeloid leukemia over 40 years. Cancer. 2009;115(13):2903–11. [DOI] [PubMed] [Google Scholar]
- 7.Pant M, Bhatt VR. Early mortality and survival in older adults with acute myeloid leukemia. Int J Hematol Oncol. 2017;6(3):61–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kantarjian H, O’Brien S, Cortes J, Giles F, Faderl S, Jabbour E, et al. Results of intensive chemotherapy in 998 patients age 65 years or older with acute myeloid leukemia or high-risk myelodysplastic syndrome. Cancer. 2006;106(5):1090–8. [DOI] [PubMed] [Google Scholar]
- 9.Leith CP, Kopecky KJ, Godwin J, McConnell T, Slovak ML, Chen I-M, et al. Acute Myeloid Leukemia in the Elderly: Assessment of Multidrug Resistance (MDR1) and Cytogenetics Distinguishes Biologic Subgroups With Remarkably Distinct Responses to Standard Chemotherapy. A Southwest Oncology Group Study. Blood. 1997;89(9):3323–9. [PubMed] [Google Scholar]
- 10.Bhatt VR, Chen B, Gyawali B, Lee SJ. Socioeconomic and health system factors associated with lower utilization of hematopoietic cell transplantation in older patients with acute myeloid leukemia. Bone marrow transplantation. 2018;53(10):1288–94. [DOI] [PubMed] [Google Scholar]
- 11.Bhatt VR, Shostrom V, Gundabolu K, Armitage JO. Utilization of initial chemotherapy for newly diagnosed acute myeloid leukemia in the United States. Blood advances. 2018;2(11):1277–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. Journal of chronic diseases. 1987;40(5):373–83. [DOI] [PubMed] [Google Scholar]
- 13.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9CM administrative databases. Journal of clinical epidemiology. 1992;45(6):613–9. [DOI] [PubMed] [Google Scholar]
- 14.Hall WH, Ramachandran R, Narayan S, Jani AB, Vijayakumar S. An electronic application for rapidly calculating Charlson comorbidity score. BMC cancer. 2004;4(1):94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.About the National Cancer Database 2017. [Available from: https://www.facs.org/quality-programs/cancer/ncdb/about.
- 16.Bhatt VR, Shostrom V, Giri S, Gundabolu K, Monirul Islam KM, Appelbaum FR, et al. Early mortality and overall survival of acute myeloid leukemia based on facility type. American journal of hematology. 2017;92(8):764–71. [DOI] [PubMed] [Google Scholar]
- 17.Lang K, Earle CC, Foster T, Dixon D, Van Gool R, Menzin J. Trends in the treatment of acute myeloid leukaemia in the elderly. Drugs & aging. 2005;22(11):943–55. [DOI] [PubMed] [Google Scholar]
- 18.Menzin J, Lang K, Earle CC, Kerney D, Mallick R. The outcomes and costs of acute myeloid leukemia among the elderly. Archives of internal medicine. 2002;162(14):1597–603. [DOI] [PubMed] [Google Scholar]
- 19.Meyers J, Yu Y, Kaye JA, Davis KL. Medicare fee-for-service enrollees with primary acute myeloid leukemia: an analysis of treatment patterns, survival, and healthcare resource utilization and costs. Applied health economics and health policy. 2013;11(3):275–86. [DOI] [PubMed] [Google Scholar]
- 20.Oran B, Weisdorf DJ. Survival for older patients with acute myeloid leukemia: a population-based study. Haematologica. 2012;97(12):1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Juliusson G, Antunovic P, Derolf Å, Lehmann S, Möllgård L, Stockelberg D, et al. Age and acute myeloid leukemia: real world data on decision to treat and outcomes from the Swedish Acute Leukemia Registry. Blood, The Journal of the American Society of Hematology. 2009;113(18):4179–87. [DOI] [PubMed] [Google Scholar]
- 22.Medeiros BC, Satram-Hoang S, Hurst D, Hoang KQ, Momin F, Reyes C. Big data analysis of treatment patterns and outcomes among elderly acute myeloid leukemia patients in the United States. Ann Hematol. 2015;94(7):1127–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gupta V, Chun K, Yi Q-L, Minden M, Schuh A, Wells R, et al. Disease biology rather than age is the most important determinant of survival of patients ≥ 60 years with acute myeloid leukemia treated with uniform intensive therapy. Cancer. 2005;103(10):2082–90. [DOI] [PubMed] [Google Scholar]
- 24.Østgård LSG, Nørgaard JM, Sengeløv H, Severinsen M, Friis LS, Marcher CW, et al. Comorbidity and performance status in acute myeloid leukemia patients: a nation-wide population-based cohort study. Leukemia. 2015;29(3):548–55. [DOI] [PubMed] [Google Scholar]
- 25.Tawfik B, Pardee TS, Isom S, Sliesoraitis S, Winter A, Lawrence J, et al. Comorbidity, age, and mortality among adults treated intensively for acute myeloid leukemia (AML). Journal of geriatric oncology. 2016;7(1):24–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xhaard A, Porcher R, Chien JW, de Latour RP, Robin M, Ribaud P, et al. Impact of comorbidity indexes on non-relapse mortality. Leukemia. 2008;22(11):2062–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Breccia M, Frustaci AM, Cannella L, Stefanizzi C, Latagliata R, Cartoni C, et al. Comorbidities and FLT3-ITD abnormalities as independent prognostic indicators of survival in Elderly acute myeloid leukaemia patients. Hematological Oncology. 2009;27(3):148–53. [DOI] [PubMed] [Google Scholar]
- 28.Harb AJ, Tan W, Wilding GE, Ford L, Sait SNJ, Block AW, et al. Treating octogenarian and nonagenarian acute myeloid leukemia patients—Predictive prognostic models. Cancer. 2009;115(11):2472–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Klepin HD, Geiger AM, Tooze JA, Kritchevsky SB, Williamson JD, Pardee TS, et al. Geriatric assessment predicts survival for older adults receiving induction chemotherapy for acute myelogenous leukemia. Blood. 2013;121(21):4287–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sorror ML, Maris MB, Storb R, Baron F, Sandmaier BM, Maloney DG, et al. Hematopoietic cell transplantation (HCT)-specific comorbidity index: a new tool for risk assessment before allogeneic HCT. Blood. 2005;106(8):2912–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sorror ML, Maris MB, Storer B, Sandmaier BM, Diaconescu R, Flowers C, et al. Comparing morbidity and mortality of HLA-matched unrelated donor hematopoietic cell transplantation after nonmyeloablative and myeloablative conditioning: influence of pretransplantation comorbidities. Blood. 2004;104(4):961–8. [DOI] [PubMed] [Google Scholar]
- 32.Etienne A, Esterni B, Charbonnier A, Mozziconacci M-J, Arnoulet C, Coso D, et al. Comorbidity is an independent predictor of complete remission in elderly patients receiving induction chemotherapy for acute myeloid leukemia. Cancer. 2007;109(7):1376–83. [DOI] [PubMed] [Google Scholar]
- 33.Giles FJ, Borthakur G, Ravandi F, Faderl S, Verstovsek S, Thomas D, et al. The haematopoietic cell transplantation comorbidity index score is predictive of early death and survival in patients over 60 years of age receiving induction therapy for acute myeloid leukaemia. British Journal of Haematology. 2007;136(4):624–7. [DOI] [PubMed] [Google Scholar]
- 34.Sorror ML, Storer BE, Fathi AT, Gerds AT, Medeiros BC, Shami P, et al. Development and Validation of a Novel Acute Myeloid Leukemia–Composite Model to Estimate Risks of Mortality. JAMA Oncology. 2017;3(12):1675–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Extermann M. Measurement and impact of comorbidity in older cancer patients. Critical reviews in oncology/hematology. 2000;35(3):181–200. [DOI] [PubMed] [Google Scholar]
- 36.Yancik R, Ganz PA, Varricchio CG, Conley B. Perspectives on comorbidity and cancer in older patients: approaches to expand the knowledge base. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2001;19(4):1147–51. [DOI] [PubMed] [Google Scholar]
- 37.Mohammadi M, Cao Y, Glimelius I, Bottai M, Eloranta S, Smedby KE. The impact of comorbid disease history on all-cause and cancer-specific mortality in myeloid leukemia and myeloma – a Swedish population-based study. BMC Cancer. 2015;15(1):850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Doxorubicin prescribing information [Doxorubicin prescribing information]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/062921s022lbl.pdf.
- 39.Gentuzumab ozogamicin prescribing inofrmation [Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/761060lbl.pdf.
- 40.Pinto A, Zagonel V, Ferrara F. Acute myeloid leukemia in the elderly: biology and therapeutic strategies. Crit Rev Oncol Hematol. 2001;39(3):275–87. [DOI] [PubMed] [Google Scholar]
- 41.Extermann M, Overcash J, Lyman GH, Parr J, Balducci L. Comorbidity and functional status are independent in older cancer patients. Journal of Clinical Oncology. 1998;16(4):15827. [DOI] [PubMed] [Google Scholar]
- 42.Bhatt VR. Personalizing therapy for older adults with acute myeloid leukemia: Role of geriatric assessment and genetic profiling. Cancer treatment reviews. 2019;75:52–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Grimwade D, Walker H, Harrison G, Oliver F, Chatters S, Harrison CJ, et al. The predictive value of hierarchical cytogenetic classification in older adults with acute myeloid leukemia (AML): analysis of 1065 patients entered into the United Kingdom Medical Research Council AML11 trial. Blood. 2001;98(5):1312–20. [DOI] [PubMed] [Google Scholar]
- 44.Farag SS, Archer KJ, Mrózek K, Ruppert AS, Carroll AJ, Vardiman JW, et al. Pretreatment cytogenetics add to other prognostic factors predicting complete remission and long-term outcome in patients 60 years of age or older with acute myeloid leukemia: results from Cancer and Leukemia Group B 8461. Blood. 2006;108(1):63–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Takeyama K, Seto M, Uike N, Hamajima N, Ino T, Mikuni C, et al. Therapy-related leukemia and myelodysplastic syndrome: a large-scale Japanese study of clinical and cytogenetic features as well as prognostic factors. International journal of hematology. 2000;71(2):144–52. [PubMed] [Google Scholar]
- 46.Kayser S, Döhner K, Krauter J, Köhne CH, Horst HA, Held G, et al. The impact of therapy-related acute myeloid leukemia (AML) on outcome in 2853 adult patients with newly diagnosed AML. Blood. 2011;117(7):2137–45. [DOI] [PubMed] [Google Scholar]
- 47.Foran JM, Sun Z, Claxton DF, Lazarus HM, Thomas ML, Melnick A, et al. Importance of Achieving Complete Remission (CR) after Intensive Therapy for Acute Myeloid Leukemia (AML) in Older Adults Age ≥60 Years: Analysis of Risk Factors for Early Mortality and Re-Induction, and Impact of Quality of Response on Overall Survival (OS) in the ECOG-ACRIN E2906 Randomized Trial. Blood. 2016;128(22):339-. [Google Scholar]
- 48.Löwenberg B, Ossenkoppele GJ, van Putten W, Schouten HC, Graux C, Ferrant A, et al. High-dose daunorubicin in older patients with acute myeloid leukemia. The New England journal of medicine. 2009;361(13):1235–48. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.