Abstract
Aims
Many patients are unable to achieve guideline-recommended LDL cholesterol (LDL-C) targets, despite taking maximally tolerated lipid-lowering therapy. Bempedoic acid, a competitive inhibitor of ATP citrate lyase, significantly lowers LDL-C with or without background statin therapy in diverse populations. Because pharmacodynamic interaction between statins and bempedoic acid is complex, a dose–response model was developed to predict LDL-C pharmacodynamics following administration of statins combined with bempedoic acid.
Methods and results
Bempedoic acid and statin dosing and LDL-C data were pooled from 14 phase 1–3 clinical studies. Dose–response models were developed for bempedoic acid monotherapy and bempedoic acid–statin combinations using previously published statin parameters. Simulations were performed using these models to predict change in LDL-C levels following treatment with bempedoic acid combined with clinically relevant doses of atorvastatin, rosuvastatin, simvastatin, and pravastatin. Dose–response models predicted that combining bempedoic acid with the lowest statin dose of commonly used statins would achieve a similar degree of LDL-C lowering as quadrupling that statin dose; for example, the predicted LDL-C lowering was 54% with atorvastatin 80 mg compared with 54% with atorvastatin 20 mg + bempedoic acid 180 mg, and 42% with simvastatin 40 mg compared with 46% with simvastatin 10 mg + bempedoic acid 180 mg.
Conclusion
These findings suggest bempedoic acid combined with lower statin doses offers similar LDL-C lowering compared with statin monotherapy at higher doses, potentially sparing patients requiring additional lipid-lowering therapies from the adverse events associated with higher statin doses.
Keywords: Dose response, Drug interaction, Hypercholesterolaemia, LDL cholesterol, Statin
Introduction
LDL cholesterol (LDL-C) plays a key role in the development of atherosclerotic plaques and, subsequently, cardiovascular events. Lowering LDL-C levels reduces the risk of atherosclerotic cardiovascular disease proportionally to the absolute reduction in LDL-C.1,2 Statins remain the cornerstone of lipid-lowering therapy.3,4 Statins competitively inhibit 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase, the rate-limiting enzyme for de novo cholesterol synthesis, resulting in up-regulation of hepatic LDL receptors and a reduction in circulating LDL-C.5
Many patients with hypercholesterolaemia remain above guideline-recommended LDL-C thresholds despite treatment with maximally tolerated statin doses with or without the addition of non-statin agents (e.g. ezetimibe) and thus remain at elevated risk for cardiovascular disease.6 Adverse effects (primarily muscle symptoms) can limit the maximally tolerated statin dose to low-dose therapy, or may make patients not adhere to their treatment or stop their statin therapy completely. Therefore, there is a high unmet need for additional non-statin therapies to help patients achieve lipid-lowering goals.
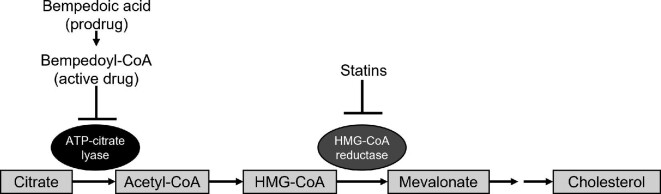
Bempedoic acid, an oral, once-daily medication that lowers LDL-C in patients with hypercholesterolaemia, is approved for use in the United States and Europe with varying indications.7 Bempedoic acid is a competitive inhibitor of ATP citrate lyase, an enzyme two steps upstream of HMG-CoA reductase (the target of statins), and lowers LDL-C by decreasing cholesterol synthesis and up-regulating LDL receptors, thus impacting LDL metabolism through this well-established pathway (Figure 1). Results from phase 3 trials of bempedoic acid in patients receiving maximally tolerated statins—including patients who were unable to tolerate the lowest approved statin doses due to adverse events—consistently demonstrated clinically meaningful reductions in LDL-C as compared with placebo.8–12 Additionally, the degree of LDL-C lowering with bempedoic acid was greater in the pool of statin-intolerant patients receiving low dose, very low dose, or no background statin therapy (82% of whom were not taking statins) compared with the pool of patients who were receiving higher doses of background statins (91% of whom were taking moderate- or high-intensity statins; placebo-corrected least squares mean % change from baseline of −24.5% vs. −17.8%, respectively).12 Consistent with the model predictions, in the subset of patients who took no background statin, the placebo-corrected least squares mean LDL-C reduction from baseline was slightly greater (−27.2%) when compared with the higher dose statin or the entire lower dose statin pools.12
Figure 1.
Mechanism of action of bempedoic acid relative to statins. ATP, adenosine triphosphate; HMG-CoA, 3-hydroxy-3-methylglutaryl coenzyme A.
During phase 3 clinical trials, the addition of bempedoic acid to stable statin therapy was studied; it is important to further evaluate the impact of altering statin doses in combination with bempedoic acid because the pharmacokinetic and pharmacodynamic interaction between statins and bempedoic acid is complex. The objective of this study was to evaluate predictions of LDL-C pharmacodynamics using a dose–response model to assess the potential benefits and risks of adjustments in the statin dose when administered in combination with bempedoic acid.
Methods
Data
Data on bempedoic acid dosing and LDL-C concentrations were pooled from 14 clinical studies (1 phase 1,13 4 phase 2,14–17 and 3 phase 3 studies8–10 with published results and 2 phase 1 and 4 phase 2 studies with data on file) conducted as part of the clinical development of bempedoic acid. Study populations included healthy volunteers (one study) and patients with dyslipidaemia, hypercholesterolaemia, heterozygous familial hypercholesterolaemia, or type 2 diabetes mellitus who received placebo monotherapy, bempedoic acid monotherapy, statin monotherapy, or bempedoic acid in combination with statins. Patients receiving concomitant ezetimibe therapy were excluded from this analysis. Patients receiving stable background therapy with proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors with placebo or bempedoic acid from a single study were included in the bempedoic acid monotherapy analysis. A sensitivity analysis was performed to assess the impact of baseline PCSK9 treatment on bempedoic acid monotherapy model parameters.
Determination of pre-statin baseline
Patients enrolled into bempedoic acid and statin combination treatment arms were already receiving stable statin therapy at study entry; therefore, their baseline LDL-C concentrations reflect the LDL-C concentration following stable statin therapy. Conversely, baseline LDL-C concentrations in any studies in healthy volunteers or patients receiving bempedoic acid monotherapy represent their baseline prior to any lipid-lowering drugs. To pool these data for analysis, imputations of individual patient baseline LDL-C concentrations measured at study entry were estimated to the ‘pre-statin’ baseline for patients who were receiving stable statin doses prior to combination with bempedoic acid in these trials (see Supplementary material online, Detailed Methods).
Development of dose–response model
Overall approach
Indirect-effect dose–response models were developed independently from subject-level data for bempedoic acid monotherapy and for bempedoic acid administered in combination with atorvastatin, rosuvastatin, simvastatin, or pravastatin.
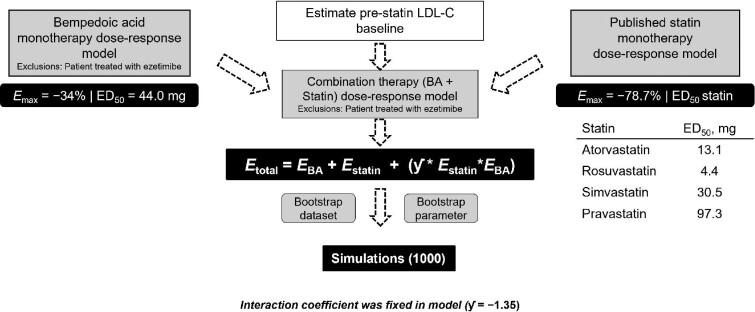
Following identification of the bempedoic acid monotherapy dose–response model, individual bempedoic acid–statin combination dose–response models were developed; model parameters for bempedoic acid response were obtained from the bempedoic acid monotherapy model and those for statin dose response were obtained from a published analysis using trial-level data.18 The overall approach is depicted in Figure 2. The first‐order conditional estimation with interaction method in NONMEM® (version 7; ICON Development Solutions, Ellicott City, MD, USA) was used for model development. The first-order rate constant describing elimination of LDL-C in the indirect-effect model was fixed to a value consistent with the literature.19–21 Post-processing of the results was performed using R (version 3.5, The R Foundation for Statistical Computing, Indianapolis, IN, USA).
Figure 2.
Model-based analysis workflow: bempedoic acid plus statin combination therapy. BA, bempedoic acid; ED50, median effective dose; Emax, maximum effective dose; EBA, efficacy of bempedoic acid; Estatin, efficacy of statin; Etotal, total efficacy; LDL-C, LDL cholesterol.
Model evaluation
Model fit of the observed data was assessed visually for each model by overlaying the typical model-predicted LDL-C % change from baseline vs. dose over the corresponding observed mean [95% confidence interval (CI)] values.
Model-based predictions
Simulations were performed for each of the bempedoic acid–statin combination therapy models to predict LDL-C change from baseline at week 12. Combined bempedoic acid and statin model predictions were further evaluated using posterior predictive checks with parametric bootstrapping of model parameters and non-parametric bootstrapping of the observed datasets used to incorporate uncertainty. The simulated individual predicted change from baseline in LDL-C at week 12 was summarized for statin monotherapy and for bempedoic acid–statin combination therapy for each individual statin and dose combination. The additional LDL-C lowering when bempedoic acid was added to stable background statin therapy at specific doses (ΔLDL-C) was manually computed using the following formula:
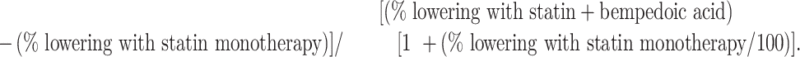 |
Simulations were also performed to predict attainment of absolute LDL-C goals among patients treated with various bempedoic acid–statin combinations. In these simulations, a parametric bootstrap of bempedoic acid–statin combination model parameters was performed. To facilitate comparison across the background statin agents, the population baseline LDL-C and corresponding inter-individual variability parameters from the atorvastatin–bempedoic acid combination model were used for all combinations. This model represents the highest baseline LDL-C condition estimated across background statin therapy. One patient was simulated for each unique dosing condition with each set of parameters resulting in a total of 1000 simulated patients per dose level. Summary statistics of individual predicted LDL-C were tabulated across the 1000 simulated patients in each dosing condition at treatment week 12. Additionally, the proportion of simulated patients achieving clinically relevant LDL-C targets of <100 mg/dL (2.6 mmol/L) and <70 mg/dL (1.8 mmol/L) was determined for each dosing condition and plotted to provide a visual assessment of target attainment.
Results
Model parameters
Statins
The model parameters for each statin are presented (Figure 2). The maximal effect as proportional change from baseline (Emax) and Hill coefficient did not differ among statins, but each statin had a unique dose needed to achieve 50% of maximal effect (ED50). All statins shared a similar shape of the dose–response relationship, with a similar maximal effect of 79% reduction in LDL-C over placebo.18
Bempedoic acid
A simple Emax model best described the dose–response relationship for bempedoic acid. A sigmoidal Emax model was tested; however, the Hill coefficient was not precisely estimated with the 95% CI including the null value of 1 at which the sigmoidal Emax model reduces to a simple Emax relationship. The maximal reduction in LDL-C with bempedoic acid alone was 34% with an ED50 estimated to be 44 mg. All model parameters are shown in Table 1. In a sensitivity analysis, exclusion of patients with concomitant PCSK9 inhibitor use did not result in a meaningful difference in model parameters; therefore, patients with concomitant PCSK9 inhibitor use were included in the assessment of bempedoic acid monotherapy (data not shown).
Table 1.
Bempedoic acid monotherapy dose–response model
| Parameter | Estimate | SE | 95% CI |
|---|---|---|---|
| Baseline LDL-C, mg/dL | 147.1 | 1.1 | (144.9, 149.3) |
| k out, h–1 | 0.01 | FIX | — |
| E max-BA | −0.34 | 0.01 | (−0.36, −0.32) |
| ED50-BA, mg | 44.0 | 3.8 | (36.6, 51.3) |
| HillBA | 1 (FIX) | — | — |
| Residual error | |||
| Proportional, % | 8.4 | — | (7.5, 9.3) |
| Additive, mg/dL | 11.4 | 0.6 | (10.3, 12.5) |
| Inter-individual variability, % | |||
| Baseline LDL-C | 23.0 | — | (21.9, 24.1) |
| Emax-BA | 36.2 | — | (32.6, 39.4) |
BA, bempedoic acid; CI, confidence interval; ED50-BA, dose of bempedoic acid needed to achieve 50% of maximal effect; Emax-BA, maximal bempedoic acid effect as proportional change from baseline; FIX, model parameter fixed to estimated value without error; HillBA, Hill coefficient for bempedoic acid; kout, first-order rate constant for elimination of LDL cholesterol; LDL-C, LDL cholesterol; SE, standard error.
Bempedoic acid–statin interaction
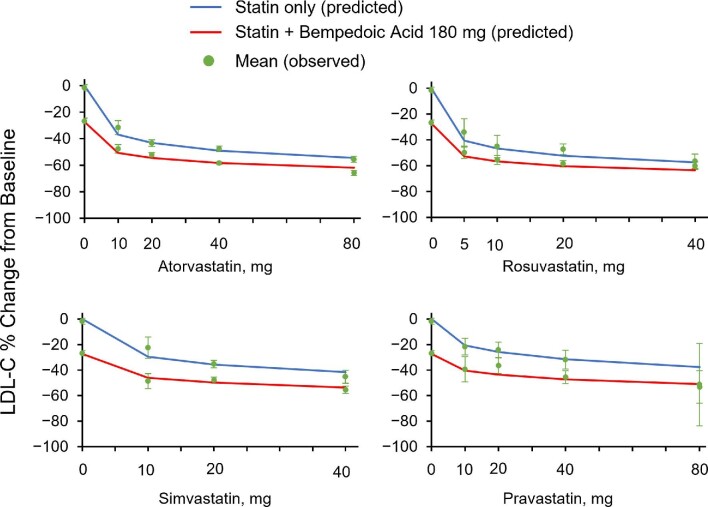
Combination models of bempedoic acid with individual statins were developed to predict LDL-C change from baseline using parameters from the bempedoic acid model described earlier and from the published statin dose–response model. The previously published dose–response model parameters for individual statins consisted of a common Emax (79% reduction from baseline) and unique ED50 values for each statin.18 Pre-treatment baseline LDL-C values were estimated using these individual statin dose–response model parameters for patients who were treated with maximally tolerated statins before enrolment. Parameters for the combined model with each statin are shown in Table 2. Based on sensitivity analysis, the interaction parameter γ was fixed to a common value across the individual statins to describe the effect of combination with bempedoic acid. Figure 3 shows the impact of bempedoic acid 180 mg on the statin dose–response relationship for each statin. In all cases, the model-predicted % LDL-C changes from baseline were within the 95% CI of the corresponding observed mean values, indicating that the models adequately described the observed data. Lowering of LDL-C, in terms of percentage change from baseline, was less than additive with combination therapy. However, the addition of bempedoic acid still provided benefit over individual statin therapy alone.
Table 2.
Bempedoic acid–statin combination model
| Parameter | Atorvastatin | Simvastatin | Rosuvastatin | Pravastatin |
|---|---|---|---|---|
| Baseline LDL-C, mg/dL | 177.9 (176.0, 179.7) | 160.4 (158.2, 162.5) | 171.9 (169.4, 174.6) | 156.7 (154.5, 158.8) |
| k out, h–1 | 0.01 (FIX) | 0.01 (FIX) | 0.01 (FIX) | 0.01 (FIX) |
| E max-statin | −0.787 (FIX) | −0.787 (FIX) | −0.787 (FIX) | −0.787 (FIX) |
| ED50-statin, mg | 13.1 (FIX) | 30.5 (FIX) | 4.4 (FIX) | 97.3 (FIX) |
| Hillstatin | 0.451 (FIX) | 0.451 (FIX) | 0.451 (FIX) | 0.451 (FIX) |
| E max-BA | −0.34 (FIX) | −0.34 (FIX) | −0.34 (FIX) | −0.34 (FIX) |
| ED50-BA, mg | 43.96 (FIX) | 43.96 (FIX) | 43.96 (FIX) | 43.96 (FIX) |
| HillBA | 1 (FIX) | 1 (FIX) | 1 (FIX) | 1 (FIX) |
| γ statin-BA | −1.35 (FIX) | −1.35 (FIX) | −1.35 (FIX) | −1.35 FIX |
| Residual error | ||||
| Proportional, % | 16.1 (15.7, 16.5) | 13.5 (12.8, 14.3) | 15.1 (14.4, 15.7) | 10.0 (8.9, 11.0) |
| Additive, mg/dL | 8.8 (9.5, 8.1) | 13.3 (14.3, 12.3) | 11.7 (10.6, 12.8) | 17.9 (18.9, 16.9) |
| Inter-individual variability, % | ||||
| Baseline LDL-C | 27.1 (26.3, 27.8) | 25.5 (24.5, 26.5) | 30.4 (29.2, 31.4) | 24.2 (23.1, 25.2) |
Values in parentheses are 95% CI.
Baseline LDL values are from studies of patients with hypercholesterolaemia who were treated with maximally tolerated statins. Patients treated with ezetimibe were excluded from the analysis.
BA, bempedoic acid; ED50-BA, dose of bempedoic acid needed to achieve 50% of maximal effect; ED50-statin, dose of statin needed to achieve 50% of maximal effect; Emax-BA, maximal bempedoic acid effect as proportional change from baseline; Emax-statin, maximal statin effect as proportional change from baseline; FIX, model parameter fixed to estimated value without error; HillBA, Hill coefficient for bempedoic acid; Hillstatin, Hill coefficient for statins; kout, first-order rate constant for elimination of LDL cholesterol; γstatin-BA, interaction coefficient between statins and bempedoic acid; LDL-C, LDL cholesterol.
Figure 3.
Combination model fit. The observed mean change (red circles) at week 12 is shown with a 95% confidence interval. Solid lines are model-predicted mean change at week 12 for statin monotherapy (blue) and bempedoic acid–statin combinations (red). LDL-C, LDL cholesterol.
Model-based predictions of LDL-C change from baseline
Model-predicted LDL-C lowering with bempedoic acid 180 mg as monotherapy was −27% (90% CI: −29%, −26%). As shown in Table 3, bempedoic acid–statin combination at 25% of the maximum statin dose was predicted to reduce LDL-C levels to a similar or greater extent than maximal dose statin monotherapy (atorvastatin, 54% vs. 54%; simvastatin, 46% vs. 42%; rosuvastatin, 57% vs. 57%; and pravastatin, 44% vs. 38%). At 50% of the maximum statin dose, the bempedoic acid–statin combination provided a reduction in LDL-C levels that was greater than that obtained with maximal dose statin therapy alone (atorvastatin, 58% vs. 54%; simvastatin, 50% vs. 42%; rosuvastatin, 60% vs. 57%; and pravastatin, 47% vs. 38%). ΔLDL-C, which represents the additional LDL-C lowering attributed to bempedoic acid when added to stable background statin therapy at the indicated doses, aligns with data on the LDL-C lowering observed in phase 3 studies where bempedoic acid was added to maximally tolerated background statin therapy.
Table 3.
Predicted % LDL-C lowering from baseline to week 12 by statins alone and in combination with bempedoic acid
| Mean (90% CI) LDL-C % change from baselinea | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Atorvastatin | Simvastatin | Rosuvastatin | Pravastatin | |||||||||
| Statin dose (mg) | Alone | + BA 180 mg | ΔLDL-Cb | Alone | + BA 180 mg | ΔLDL-Cb | Alone | + BA 180 mg | ΔLDL-Cb | Alone | + BA 180 mg | ΔLDL-Cb |
| 0 | — | −27 (−29, −26) | — | — | −27 (−29, −26) | — | — | −27 (−29, −26) | — | — | −27 (−29, −26) | — |
| 10 | −37 (−40, −30) | −51 (−55, −46) | −22 | −30 (−36, −23) | −46 (−50, −42) | −23 | −46 (−55, −39) | −57 (−62, −52) | −20 | −21 (−26, −16) | −41 (−45, −37) | −25 |
| 20 | −43 (−51, −36) | −54 (−60, −50) | −19 | −36 (−43, −29) | −50 (−54, −45) | −22 | −52 (−61, −44) | −60 (−66, −55) | −17 | −26 (−32, −20) | −44 (−48, −39) | −24 |
| 40 | −49 (−57, −41) | −58 (−63, −53) | −18 | −42 (−50, −34) | −54 (−59, −49) | −21 | −57 (−66, −49) | −63 (−69, −58) | −14 | −32 (−38, −25) | −47 (−52, −43) | −22 |
| 80 | −54 (−63, −46) | −62 (−67, −56) | −17 | — | — | — | — | — | — | −38 (−45, −31) | −51 (−56, −46) | −21 |
BA, bempedoic acid; LDL-C, LDL cholesterol.
Data represent 1000 simulations using bootstrapped data and parameter estimates.
ΔLDL-C represents the % reduction in LDL-C due to bempedoic acid when added to stable background therapy at the designated statin dose.
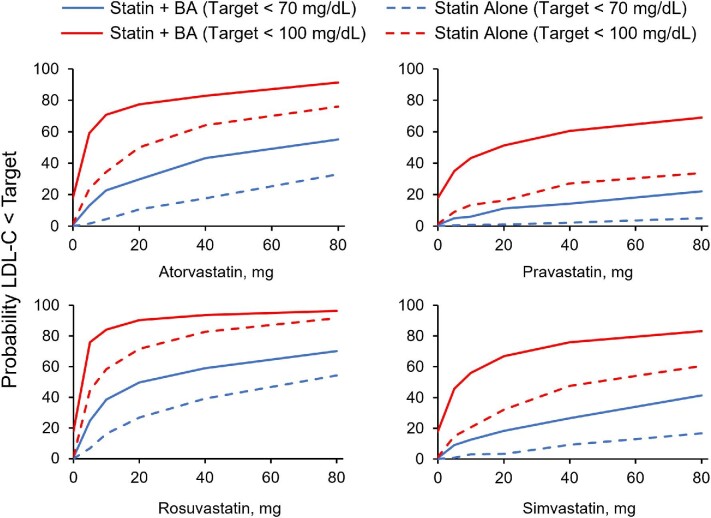
In terms of predicted absolute LDL-C levels, the model-predicted mean LDL-C baseline with no lipid-lowering therapy was 183.1 mg/dL (4.7 mmol/L). Starting from this common baseline value, the model-predicted mean steady-state LDL-C with bempedoic acid 180 mg alone was estimated to be 132.7 mg/dL (3.4 mmol/L; Table 4). With the combination of bempedoic acid and individual statins at 50% of the maximum statin dose, the bempedoic acid–statin combination achieved lower absolute LDL-C levels than did the maximal dose statin administered as monotherapy [e.g. atorvastatin, 77.5 vs. 84.2 mg/dL (2.0 mmol/L vs. 2.2 mmol/L)]. Based on predicted absolute values, the predicted probabilities of achieving LDL-C <100 mg/dL (2.6 mmol/L) and <70 mg/dL (1.8 mmol/L) are depicted in Figure 4. The addition of bempedoic acid to statins markedly increased the probability of achieving both LDL-C thresholds.
Table 4.
Predicted mean absolute LDL cholesterol lowering from baseline to week 12 with bempedoic acid–statin combination
| Mean LDL-C (mg/dL)a | ||||||||
|---|---|---|---|---|---|---|---|---|
| Atorvastatin | Simvastatin | Rosuvastatin | Pravastatin | |||||
| Statin dose (mg) | Alone | + BA 180 mg | Alone | + BA 180 mg | Alone | + BA 180 mg | Alone | + BA 180 mg |
| 0 | 183.1 | 132.7 | 183.1 | 132.7 | 183.1 | 132.7 | 183.1 | 132.7 |
| 10 | 116.2 | 89.6 | 129.0 | 99.6 | 98.2 | 79.5 | 143.4 | 108.6 |
| 20 | 104.0 | 84.0 | 117.7 | 92.5 | 87.8 | 72.6 | 136.2 | 104.3 |
| 40 | 94.2 | 77.5 | 106.3 | 86.0 | 79.0 | 67.6 | 124.6 | 96.9 |
| 80 | 84.2 | 70.6 | — | — | — | — | 115.9 | 90.3 |
BA, bempedoic acid; LDL-C, LDL cholesterol.
Data represent 1000 simulation subjects using unique parameter estimates.
Figure 4.
Proportion of patients with LDL cholesterol who achieved LDL cholesterol targets of <100 mg/dL (2.6 mmol/L) and <70 mg/dL (1.8 mmol/L) by statin dose as monotherapy or in combination with bempedoic acid. BA, bempedoic acid; LDL-C, LDL cholesterol.
Discussion
Current guidelines recommend the combination of maximally tolerated statins with non-statin agents such as bempedoic acid to intensify LDL-C lowering in patients who cannot achieve desired LDL-C goals with maximally tolerated statin therapy alone.22–23 In addition to greater efficacy with combined statin and non-statin therapy compared with either therapy alone, this combination approach may reduce the risk of cardiovascular events while sparing patients from potential side effects of higher statin doses,22–23 such as skeletal muscle symptoms and adverse glycaemic effects. This study used a combined bempedoic acid–statin dose–response model to predict LDL-C lowering for bempedoic acid 180 mg combined with various statin doses. Our model showed that adding bempedoic acid to statin therapy is at least equivalent to or more effective in lowering LDL-C than an increase in a statin dose after initial statin treatment. Combinations of bempedoic acid plus the lowest dose of each statin were predicted to achieve similar reductions in LDL-C levels as quadrupling the lowest statin dose as monotherapy. The model predictions suggest that bempedoic acid not only provides additional LDL-C lowering when used in combination with maximally tolerated statins, but also may help maintain the overall LDL-C lowering efficacy when adverse muscle effects need to be managed with statin dose reduction.
Because the data were modelled on a population of patients with a variety of clinical characteristics, the findings would be expected to apply to diverse patient populations. A larger proportion of patients (∼10–40% higher, depending on the statin and statin dose) were predicted to achieve guideline-based LDL-C goals with the combination of statin and bempedoic acid at all levels of statin dosing compared with statin monotherapy, which demonstrates the magnitude of benefit likely to be gained from the addition of bempedoic acid to a statin regimen. Triple combination therapy with bempedoic acid, a statin, and ezetimibe may provide further LDL-C lowering. A recent study showed mean LDL-C lowering from baseline with bempedoic acid 180 mg + ezetimibe 10 mg + atorvastatin 20 mg after 6 weeks of treatment was −63.6%.24 In comparison, LDL-C reductions from baseline at week 12 were −36.2% with bempedoic acid 180 mg + ezetimibe 10 mg25 and in our model −54% (90% CI: −60%, −50%) with bempedoic acid 180 mg + atorvastatin 20 mg.
Model predictions of LDL-C lowering achieved with combination therapy appeared to be less than additive based on the value of the interaction coefficient (γ) identified in a sensitivity analysis. This finding is consistent with the site of action of bempedoic acid, which is upstream of HMG-CoA reductase in the cholesterol biosynthetic pathway and may limit the potential for completely additive pharmacodynamic effects of the combination. The narrowing difference in lowering LDL-C with increasing statin dose between statin alone and the bempedoic acid–statin combination may not reflect the pharmacology of the bempedoic acid–statin interaction but the multiplicative, rather than additive, mathematics of combining therapies with differing relative efficacy expressed in proportional terms. For example, if two lipid-lowering therapies both reduce LDL-C by 50% when administered as monotherapy, the effect of their combination is a 75% reduction, not 100% as would be derived by adding the percentages. Nevertheless, bempedoic acid provided a significant additional benefit compared with statins alone. Further, these data indicate that the addition of bempedoic acid to statins markedly increases the probability of achieving LDL-C goals.
One of the mechanisms implicated in muscle-related effects of statins is increased systemic exposure to statins, leading to increased uptake into skeletal muscle.26 Reducing the statin dose by 50% in the presence of bempedoic acid is predicted to reduce the systemic exposure of statin overall by about 25% because of the pharmacokinetic interaction with bempedoic acid, and, by extension, is also likely to lower the risk of myotoxicity. Although bempedoic acid acts on the same cholesterol biosynthesis pathway as statins, bempedoic acid is not activated in skeletal muscle and its addition to statin treatment is, therefore, unlikely to exacerbate statin-related muscle symptoms.27 Among patients in phase 3 studies receiving maximally tolerated statin therapy, myalgia was reported by 2.9% of patients receiving bempedoic acid vs. 3.1% of patients receiving placebo, and muscle weakness was reported by 0.4% of patients receiving either add-on bempedoic acid or placebo.8
The current study has some limitations. The model-based predictions from this study need to be validated further in clinical studies where the statin dose is reduced. The model was derived from non-uniform patient populations and may differ according to the type of dyslipidaemia diagnosed. Uncertainty added into estimating individual baseline pre-statin LDL-C assumes a population distribution of statin responses. However, the use of data from patients in clinical trials who have not achieved their goals in lowering LDL-C while taking a statin could have resulted in a selection bias towards those patients taking statins who responded poorly. Therefore, the estimated pre-statin baseline may have been different. Because of lack of appropriate studies to accurately characterize the interaction between statins and bempedoic acid, it was difficult to estimate the interaction coefficient. A sensitivity analysis was therefore used to fix an interaction coefficient in the model that best described LDL-C lowering by bempedoic acid in the presence/absence of each of the statins. If we could estimate the interaction coefficient and associated confidence interval, we could have made a definitive interpretation of the bempedoic acid–statin interaction. Also, other non-statin therapies were not considered in this model. Effects of covariates (gender, age, presence or absence of familial hypercholesterolaemia) were not evaluated in the present analysis. The data included in the model were from 12-week treatment, so that the combined model was unable to predict longer-term LDL-C lowering with bempedoic acid and reduced doses of statins. Furthermore, the data for bempedoic acid and statin combinations are almost entirely modelled from patients who received a bempedoic acid dose of 180 mg; therefore, there may be greater uncertainty in the predicted effect of bempedoic acid–statin combinations at other bempedoic acid doses.
Conclusions
Dose–response models predicted meaningful reductions in LDL-C when bempedoic acid was combined with several statin dose intensities; therefore, the combination of bempedoic acid with lower dose statins provides therapeutic options for patients who cannot tolerate high-intensity statins or achieve LDL-C thresholds with maximally tolerated statin therapy alone.
Supplementary Material
Acknowledgements
All authors had access to the data and participated in the development, review, critique, and approval of the manuscript throughout the editorial process, and approved the final manuscript draft submitted for publication. All authors agree to be accountable for all aspects of the work, ensuring the accuracy and integrity of the publication. All named authors meet the ICMJE criteria for authorship and have given their approval for this version to be published. Medical writing support, funded by Esperion Therapeutics, Inc., was provided by Meher M. Dustoor, PhD; Kelly M. Cameron, PhD, CMPP; and Callie A.S. Corsa, PhD, of JB Ashtin, who developed the first draft based on an author-approved outline and assisted in implementing author revisions.
Author contributions: S.B.J., R.L.C., S.C., M.G.E., and A.L.C. contributed to the study concept and design. M.G.E. contributed to data acquisition. S.B.J. and R.L.C. contributed to statistical analysis. S.B.J., R.L.C., S.C., M.K., W.J.S., M.G.E., B.M.A., P.H.R.B., G.F.W., and A.L.C. contributed to data interpretation.
Contributor Information
Satyawan B Jadhav, Ann Arbor Pharmacometrics Group, 900 Victors Way #328, Ann Arbor, MI 48108, USA.
Ryan L Crass, Ann Arbor Pharmacometrics Group, 900 Victors Way #328, Ann Arbor, MI 48108, USA.
Sunny Chapel, Ann Arbor Pharmacometrics Group, 900 Victors Way #328, Ann Arbor, MI 48108, USA.
Michael Kerschnitzki, Daiichi Sankyo Europe GmbH, Zielstattstraße 48, 81379 Munich, Germany.
William J Sasiela, Esperion Therapeutics, Inc., 3891 Ranchero Dr, Ann Arbor, MI 48108, USA.
Maurice G Emery, Esperion Therapeutics, Inc., 3891 Ranchero Dr, Ann Arbor, MI 48108, USA.
Benny M Amore, Esperion Therapeutics, Inc., 3891 Ranchero Dr, Ann Arbor, MI 48108, USA.
P Hugh R Barrett, Faculty of Medicine and Health, University of New England, Armidale, NSW 2351, Australia.
Gerald F Watts, School of Medicine, University of Western Australia, Medical Research Foundation Building, Rear 50 Murray Street, Perth, WA 6001, Australia.
Alberico L Catapano, Department of Pharmacological and Biomolecular Sciences, University of Milan and IRCCS Multimedica, Via Balzaretti 9, 20133 Milan, Italy.
Funding
Esperion Therapeutics, Inc.
Conflict of interest
S.B.J. was part of Ann Arbor Pharmacometrics Group when this work was completed, which has a consulting agreement with Esperion Therapeutics, Inc. R.L.C. and S.C. are part of Ann Arbor Pharmaceuticals Group, which has a consulting agreement with Esperion Therapeutics, Inc. M.K. is an employee of Daiichi Sankyo Europe, GmbH, which has a corporate agreement with Esperion Therapeutics, Inc. W.J.S., M.G.E., and B.M.A. are current (B.M.A.) or former (W.J.S. and M.G.E.) employees of Esperion Therapeutics, Inc., and may hold stock or stock options. P.H.R.B. has received funding/grant support from Amgen, Sanofi, Esperion, and AstraZeneca. G.F.W. has received funding/grant support from Sanofi, Sanofi Regeneron, Amgen, Arrowhead, and Novartis, and honorarium for consultancy from Amgen, Sanofi, Esperion, Kowa, Novartis, Regeneron, AstraZeneca, and Arrowhead. A.L.C. has received research grant(s)/support from Sanofi, Sanofi Regeneron, Amgen, Mylan, and Menarini, and has served as a consultant for or received honoraria from Akcea, Amgen, Sanofi, Esperion, Kowa, Novartis, Ionis Pharmaceuticals, Medco, Mylan, Menarini, MSD, Recordati, Regeneron, and Daiichi Sankyo, and he acknowledges support for his work from the Ministry of Health–Ricerca Corrente–IRCCS MultiMedica.
Data availability statement
To protect patient privacy, the data, analytical methods, and study materials will not be made available to other researchers for purposes of reproducing the results or replicating the procedures.
References
- 1. Baigent C, Blackwell L, Emberson J, Holland LE, Reith C, Bhala N, Peto R, Barnes EH, Keech A, Simes J, Collins R. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet 2010;376:1670–1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ference BA, Ginsberg HN, Graham I, Ray KK, Packard CJ, Bruckert E, Hegele RA, Krauss RM, Raal FJ, Schunkert H, Watts GF, Borén J, Fazio S, Horton JD, Masana L, Nicholls SJ, Nordestgaard BG, van de Sluis B, Taskinen MR, Tokgözoglu L, Landmesser U, Laufs U, Wiklund O, Stock JK, Chapman MJ, Catapano AL. Low-density lipoproteins cause atherosclerotic cardiovascular disease: pathophysiological, genetic, and therapeutic insights: a consensus statement from the European Atherosclerosis Society Consensus Panel. Eur Heart J 2017;38:2459–2472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Grundy SM, Stone NJ, Bailey AL, Beam C, Birtcher KK, Blumenthal RS, Braun LT, de Ferranti S, Faiella-Tommasino J, Forman DE, Goldberg R, Heidenreich PA, Hlatky MA, Jones DW, Lloyd-Jones D, Lopez-Pajares N, Ndumele CE, Orringer CE, Peralta CA, Saseen JJ, Smith SC Jr, Sperling L, Virani SS, Yeboah J. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol 2019;73:e285–e350. [DOI] [PubMed] [Google Scholar]
- 4. Mach F, Baigent C, Catapano AL, Koskinas KC, Casula M, Badimon L, Chapman MJ, De Backer GG, Delgado V, Ference BA, Graham IM, Halliday A, Landmesser U, Mihaylova B, Pedersen TR, Riccardi G, Richter DJ, Sabatine MS, Taskinen M-R, Tokgozoglu L, Wiklund O, ESC Scientific Document Group . 2019 ESC/EAS guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Atherosclerosis 2019;290:140–205. [DOI] [PubMed] [Google Scholar]
- 5. Brown MS, Dana SE, Goldstein JL. Regulation of 3-hydroxy-3-methylglutaryl coenzyme A reductase activity in human fibroblasts by lipoproteins. Proc Natl Acad Sci USA 1973;70:2162–2166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Boekholdt SM, Hovingh GK, Mora S, Arsenault BJ, Amarenco P, Pedersen TR, LaRosa JC, Waters DD, DeMicco DA, Simes RJ, Keech AC, Colquhoun D, Hitman GA, Betteridge DJ, Clearfield MB, Downs JR, Colhoun HM, Gotto AM Jr, Ridker PM, Grundy SM, Kastelein JJ. Very low levels of atherogenic lipoproteins and the risk for cardiovascular events: a meta-analysis of statin trials. J Am Coll Cardiol 2014;64:485–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. NEXLETOL (bempedoic acid) tablets for oral use. Prescribing information. Esperion Therapeutics, Ann Arbor, MI, 2020. [Google Scholar]
- 8. Goldberg AC, Leiter LA, Stroes ESG, Baum SJ, Hanselman JC, Bloedon LT, Lalwani ND, Patel PM, Zhao X, Duell PB. Effect of bempedoic acid vs placebo added to maximally tolerated statins on low-density lipoprotein cholesterol in patients at high risk for cardiovascular disease: the CLEAR Wisdom randomized clinical trial. JAMA 2019;322:1780–1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Laufs U, Banach M, Mancini GBJ, Gaudet D, Bloedon LT, Sterling LR, Kelly S, Stroes ESG. Efficacy and safety of bempedoic acid in patients with hypercholesterolemia and statin intolerance. J Am Heart Assoc 2019;8:e011662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ray KK, Bays HE, Catapano AL, Lalwani ND, Bloedon LT, Sterling LR, Robinson PL, Ballantyne CM. Safety and efficacy of bempedoic acid to reduce LDL cholesterol. N Engl J Med 2019;380:1022–1032. [DOI] [PubMed] [Google Scholar]
- 11. Ballantyne CM, Banach M, Mancini GBJ, Lepor NE, Hanselman JC, Zhao X, Leiter LA. Efficacy and safety of bempedoic acid added to ezetimibe in statin-intolerant patients with hypercholesterolemia: a randomized, placebo-controlled study. Atherosclerosis 2018;277:195–203. [DOI] [PubMed] [Google Scholar]
- 12. Banach M, Duell PB, Gotto AM Jr, Laufs U, Leiter LA, Mancini GBJ, Ray KK, Flaim J, Ye Z, Catapano AL. Association of bempedoic acid administration with atherogenic lipid levels in phase 3 randomized clinical tials of patients with hypercholesterolemia. JAMA Cardiol 2020;5:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ballantyne CM, Davidson MH, MacDougall DE, Bays HE, Dicarlo LA, Rosenberg NL, Margulies J, Newton RS. Efficacy and safety of a novel dual modulator of adenosine triphosphate-citrate lyase and adenosine monophosphate-activated protein kinase in patients with hypercholesterolemia: results of a multicenter, randomized, double-blind, placebo-controlled, parallel-group trial. J Am Coll Cardiol 2013;62:1154–1162. [DOI] [PubMed] [Google Scholar]
- 14. Thompson PD, Rubino J, Janik MJ, MacDougall DE, McBride SJ, Margulies JR, Newton RS. Use of ETC-1002 to treat hypercholesterolemia in patients with statin intolerance. J Clin Lipidol 2015;9:295–304. [DOI] [PubMed] [Google Scholar]
- 15. Thompson PD, MacDougall DE, Newton RS, Margulies JR, Hanselman JC, Orloff DG, McKenney JM, Ballantyne CM. Treatment with ETC-1002 alone and in combination with ezetimibe lowers LDL cholesterol in hypercholesterolemic patients with or without statin intolerance. J Clin Lipidol 2016;10:556–567. [DOI] [PubMed] [Google Scholar]
- 16. Ballantyne CM, McKenney JM, MacDougall DE, Margulies JR, Robinson PL, Hanselman JC, Lalwani ND. Effect of ETC-1002 on serum low-density lipoprotein cholesterol in hypercholesterolemic patients receiving statin therapy. Am J Cardiol 2016;117:1928–1933. [DOI] [PubMed] [Google Scholar]
- 17. Emery MG, Hanselman JH, MacDougall D, Amore BM, SW J., McGonigal J. Effect of bempedoic acid on the pharmacokinetics and bempedoic acid (BA) effect on metformin (MET) pharmacokinetics (PK) and pharmacodynamics (PD) in patients with type 2 diabetes (T2D): in vitro–in vivo correlation. J Clin Pharmacol Ther 2020;107:S43. [Google Scholar]
- 18. Mandema JW, Hermann D, Wang W, Sheiner T, Milad M, Bakker-Arkema R, Hartman D. Model-based development of gemcabene, a new lipid-altering agent. AAPS J 2005;7:E513–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kesaniemi YA, Grundy SM. Significance of low density lipoprotein production in the regulations of plasma cholesterol level in man. J Clin Invest 1982;70:13–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kim J, Ahn BJ, Chae HS, Han S, Doh K, Choi J, Jun YK, Lee YW, Yim DS. A population pharmacokinetic–pharmacodynamic model for simvastatin that predicts low-density lipoprotein-cholesterol reduction in patients with primary hyperlipidaemia. Basic Clin Pharmacol Toxicol 2011;109:156–163. [DOI] [PubMed] [Google Scholar]
- 21. Wright DF, Pavan Kumar VV, Al-Sallami HS, Duffull SB. The influence of dosing time, variable compliance and circadian low-density lipoprotein production on the effect of simvastatin: simulations from a pharmacokinetic–pharmacodynamic model. Basic Clin Pharmacol Toxicol 2011;109:494–498. [DOI] [PubMed] [Google Scholar]
- 22. Averna M, Banach M, Bruckert E, Drexel H, Farnier M, Gaita D, Magni P, März W, Masana L, Mello ESA, Reiner Z, Ros E, Vrablik M, Zambon A, Zamorano JL, Stock JK, Tokgözoğlu LS, Catapano AL. Practical guidance for combination lipid-modifying therapy in high- and very-high-risk patients: a statement from a European Atherosclerosis Society Task Force. Atherosclerosis 2021;325:99–109. [DOI] [PubMed] [Google Scholar]
- 23. Banach M, Penson PE, Vrablik M, Bunc M, Dyrbus K, Fedacko J, Gaita D, Gierlotka M, Jarai Z, Magda SL, Margetic E, Margoczy R, Durak-Nalbantic A, Ostadal P, Pella D, Trbusic M, Udroiu CA, Vlachopoulos C, Vulic D, Fras Z, Dudek D, Reiner Ž, for the ACS EuroPath Central & South European Countries Project . Optimal use of lipid-lowering therapy after acute coronary syndromes: a position paper endorsed by the International Lipid Expert Panel (ILEP). Pharmacol Res 2021;166:105499. [DOI] [PubMed] [Google Scholar]
- 24. Rubino J, MacDougall DE, Sterling LR, Hanselman JC, Nicholls SJ. Combination of bempedoic acid, ezetimibe, and atorvastatin in patients with hypercholesterolemia: a randomized clinical trial. Atherosclerosis 2021;320:122–128. [DOI] [PubMed] [Google Scholar]
- 25. Ballantyne CM, Laufs U, Ray KK, Leiter LA, Bays HE, Goldberg AC, Stroes ES, MacDougall D, Zhao X, Catapano AL. Bempedoic acid plus ezetimibe fixed-dose combination in patients with hypercholesterolemia and high CVD risk treated with maximally tolerated statin therapy. Eur J Prev Cardiol 2019;27:593–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Turner RM, Pirmohamed M. Statin-related myotoxicity: a comprehensive review of pharmacokinetic, pharmacogenomic and muscle components. J Clin Med 2019;9:1–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Pinkosky SL, Newton RS, Day EA, Ford RJ, Lhotak S, Austin RC, Birch CM, Smith BK, Filippov S, Groot PHE, Steinberg GR, Lalwani ND. Liver-specific ATP-citrate lyase inhibition by bempedoic acid decreases LDL-C and attenuates atherosclerosis. Nat Commun 2016;7:13457. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
To protect patient privacy, the data, analytical methods, and study materials will not be made available to other researchers for purposes of reproducing the results or replicating the procedures.