Abstract
Objective:
To assess whether neonatal morbidities evident by the time of hospital discharge are associated with subsequent CP or death.
Study Design:
This is a secondary analysis of data from a multi-center placebo-controlled trial of magnesium sulfate for the prevention of CP. The association between pre-specified intermediate neonatal outcomes (n=11) and demographic and clinical factors (n=10) evident by the time of discharge among surviving infants (n=1889) and the primary outcome of death or moderate/severe CP at age 2 (n=73) was estimated, and a prediction model was created.
Results:
Gestational age in weeks at delivery (OR 0.74, 95% CI 0.67–0.83), grade III or IV intraventricular hemorrhage (IVH) (OR 5.3, CI 2.1–13.1), periventricular leukomalacia (PVL) (OR 46.4, CI 20.6–104.6), and male gender (OR 2.5, CI 1.4–4.5) were associated with death or moderate/severe CP by age 2. Outcomes not significantly associated with the primary outcome included respiratory distress syndrome, bronchopulmonary dysplasia, seizure, necrotizing enterocolitis, neonatal hypotension, 5-minute Apgar score, sepsis, and retinopathy of prematurity. Using all patients, the ROC curve for the final prediction model had an area under the curve of 0.84 (CI 0.78–0.89). Using these data, the risk of death or developing CP by age 2 can be calculated for individual surviving infants.
Conclusion:
IVH and PVL were the only neonatal complications evident at discharge that contributed to an individual infant’s risk of the long-term outcomes of death or CP by age 2. A model that includes these morbidities, gestational age at delivery, and gender is predictive of subsequent neurologic sequelae.
Keywords: cerebral palsy, magnesium sulfate, premature birth
INTRODUCTION
Preterm birth remains a major cause of perinatal morbidity and mortality, with long term neurologic disability being a potential consequence. Rates of long term handicap approach 25% for preterm survivors born before 25 weeks’ gestation.1 Factors known at or before delivery that have been associated with rate of impairment include birth weight, gender, twin gestation, and maternal exposure to antenatal corticosteroids.2,3 Other clinical findings that have also been associated with increased risk of long term neurologic handicap include intrapartum chorioamnionitis, intraventricular hemorrhage and periventricular leukomalacia, with evidence of cerebral palsy often being present by age 2. Other neonatal findings are also thought to influence the rate of major morbidity, such as respiratory distress syndrome and sepsis, but insufficient data exist to predict rates of long-term neurologic handicap for individual infants based on clinical findings. The purpose of this investigation is to evaluate (among infants born prematurely who survive to hospital discharge) which neonatal outcomes contribute to the risk of death or a diagnosis of moderate or severe cerebral palsy by age 2. Further, we aimed to construct and validate a model for predicting the risk of developing this outcome.
STUDY DESIGN
This is a secondary analysis of a trial of antenatal magnesium sulfate for the prevention of cerebral palsy conducted by the Eunice Kennedy Shriver National Institute of Child Health and Human Development Maternal Fetal Medicine Units Network.4 This randomized, double-masked multi-center clinical trial enrolled patients at 24 0/7 −31 6/7 weeks’ gestation who were at high risk for preterm delivery because of premature rupture of membranes, advanced premature labor with cervical dilation of 4–8 cm, or indicated preterm delivery anticipated within 2–24 hours. Exclusion criteria included major fetal anomalies and maternal hypertension or preeclampsia, as these patients routinely received magnesium sulfate for seizure prophylaxis. After randomization, patients received either a loading dose of 6 grams of intravenous magnesium sulfate, followed by continuous infusion of 2 grams per hour, or a matching placebo. Detailed maternal and neonatal data were collected by trained, certified research nurses. Neonatal cranial ultrasound films were obtained and read centrally by three independent pediatric radiologists blinded to clinical information, for a diagnosis of intraventricular hemorrhage (IVH) or periventricular leukomalacia (PVL). For deliveries before 32 weeks’ gestation, neonatal cranial ultrasounds were performed by 3 days of age, at 21–28 days of age, and either at hospital discharge or 36–40 weeks post-menstrual age, and read centrally by three independent pediatric radiologists. Infants were followed through two years of age corrected for prematurity with evaluation of neuromotor function by study certified pediatricians or pediatric neurologists. Cerebral palsy at or beyond 2 years of age was diagnosed based on delay of gross motor developmental milestones, and abnormalities of muscle tone, reflexes, and movement, and was considered moderate or severe if the child was unable to walk independently or was unable to grasp and release an object. The primary outcome for this trial was a composite of stillbirth or infant death by 1 year of age or moderate or severe cerebral palsy at or beyond age 2, and was ascertained in 96% of the children. Additional details of this study have been published elsewhere.4 The primary study was approved by the Institutional Review Board at all participating centers, and all patients gave informed consent.
The cohort for this analysis included infants who were discharged alive from the hospital nursery. The association between neonatal outcomes evident by the time of hospital discharge and the primary outcome of death or moderate/severe cerebral palsy by age 2 was analyzed. For univariate analysis, the Wilcoxon rank sum test was used for continuous variables and the chi square test or Fisher exact test for categorical variables. The original data set was divided into a training set and a test set using a computer based simple random sampling method. The training set was used to identify predictive factors for the outcomes of interest and build predictive models based on logistic regression. A backward proceeding stepwise logistic regression model was used. Variables in the model were pre-specified intermediate neonatal outcomes that included chest compressions at birth, Apgar score at 5 minutes <4, respiratory distress syndrome (RDS), bronchopulmonary dysplasia (BPD), severe intraventricular hemorrhage (IVH, grades III or IV), periventricular leukomalacia (PVL), seizure, necrotizing enterocolitis (NEC), sepsis, retinopathy of prematurity (ROP), and neonatal hypotension (treated with vasopressor agents and/or volume expansion). Demographic and clinical variables included in the analysis were maternal age, smoking status during current pregnancy, education level, maternal race/ethnicity (African-American versus white/Hispanic versus not Hispanic), treatment group assignment, gestational age at delivery, mode of delivery, twin gestation, male gender, and clinically-diagnosed chorioamnionitis. Since few infants were found to be small for gestational age, and almost all infants were exposed in utero to corticosteroids, these two variables were not included in the analyses. Also, because gestational age at delivery and birth weight were highly correlated, only gestational age at delivery was included. In the case of twin gestations, one twin from each pair was randomly selected.
After applying the regression model to the test set, the predicted probabilities were compared to the observed rates. Predicted and empirical probabilities were plotted and smoothly connected to form a curve. The corresponding 95% confidence intervals for the curve were calculated from the normal approximation. The model was considered validated if the confidence intervals contained the 45° straight line. The final predictive model utilized all data and included the variables found to be significant from the training set model.
A nominal two-sided P value less than 0.05 was considered to indicate statistical significance. No adjustments were made for multiple comparisons. Statistical analyses were conducted with SAS software (SAS Institute, Cary, NC).
RESULTS
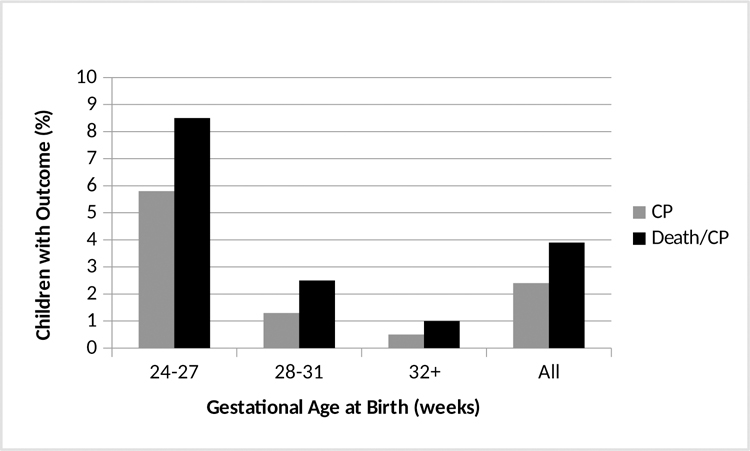
There were 1889 infants who survived until discharge from the nursery for whom there was follow up information regarding death or major morbidity by the age of 2. They were born at a mean (± standard deviation) gestational age of 29.8 ± 2.9 weeks and birth weight of 1439 ± 533 grams. A total of 73 (3.9%) developed the primary outcome. Of these, 28 died and 45 were diagnosed with moderate or severe cerebral palsy by age 2. The outcomes were inversely related to gestational age (Figure 1). Comparisons of pregnancy characteristics and neonatal complications for those neonates who ultimately did and did not develop the primary outcome are shown in Table 1. All neonatal outcomes evaluated in this analysis were significantly more common among those infants who developed the primary outcome in the univariate analysis.
Figure 1.
Percentage of patients with moderate/severe cerebral palsy (CP), or either death or moderate or severe CP among preterm infants who survive the neonatal period, by gestational age at which they delivered.
Table 1.
Pregnancy characteristics and neonatal outcomes of those infants who did or did not develop the primary outcome of death or moderate/severe CP by age 2, univariate analysis
| No primary outcome N=1816 | Primary outcome N=73 | p | |
|---|---|---|---|
| Gestational age at birth (wk) | 29.9 (2.9) | 27.5 (2.5) | < 0.001 |
| Birth Weight (g) | 1455 (553) | 1033 (373) | < 0.001 |
| MgSO4 treatment group | 888 (49) | 27 (37) | 0.046 |
| Cesarean delivery | 684 (38) | 31 (43) | 0.407 |
| 5-minute Apgar <4 | 35 (1.9) | 6 (8) | 0.004 |
| Chest compression | 42 (2.3) | 5 (7) | 0.033 |
| Hypotension | 302 (17) | 28 (38) | < 0.001 |
| RDS | 865 (48) | 57 (78) | < 0.001 |
| BPD | 293 (16) | 35 (48) | < 0.001 |
| Sepsis | 267 (15) | 29 (40) | < 0.001 |
| Seizure | 21 (1.2) | 5 (7) | 0.003 |
| IVH grade III/IV | 28 (1.5) | 12 (16) | < 0.001 |
| PVL | 15 (0.8) | 22 (30) | < 0.001 |
| NEC | 126 (6.9) | 13 (18) | < 0.001 |
| ROP | 407 (22) | 39 (53) | < 0.001 |
Data are presented as mean (standard deviation) or n (%).
In the stepwise logistic regression model, only gestational age at birth, intraventricular hemorrhage grades III or IV, periventricular leukomalacia, and male gender remained independently associated with risk of death or moderate or severe cerebral palsy at age 2 (Table 2). Utilizing the training set analysis, a curve of predicted probabilities and 95% confidence intervals was constructed and plotted on a graph comparing predicted and empirical probabilities. The 45° line on this graph was largely within the confidence intervals, validating the model (figure 2). For the four factors identified as significant plus treatment group, the ROC curve for the final prediction model had an area under the curve (AUC) of 0.84 (CI 0.78–0.89). For the outcome of moderate/severe CP at age 2 alone (n=45), the final model also included neonatal hypotension (OR 3.3, CI 1.5–7.4), and the AUC was 0.91 (CI 0.84–0.97).
Table 2.
Final logistic regression model for death or moderate/severe CP
| Variable | Odds ratio | 95% confidence interval |
|---|---|---|
| Gestational age at birth (wk) | 0.74 | 0.67 – 0.83 |
| Treatment with MgSO4 | 0.69 | 0.40 – 1.19 |
| IVH grade III or IV | 5.29 | 2.14 – 13.09 |
| PVL | 46.43 | 20.61 – 104.6 |
| Male gender | 2.51 | 1.41 – 4.46 |
Covariates included in the initial logistic regression model were the following: chest compressions at birth, Apgar score at 5 minutes <4, RDS, BPD, IVH grades III or IV, PVL, seizure, NEC, sepsis, ROP, neonatal hypotension, maternal age, smoking status, education, maternal race/ethnicity, treatment group assignment, gestational age at delivery, mode of delivery, twin gestation, male gender, and chorioamnionitis.
Figure 2.
Nomogram calibration for the prediction models for A) post-discharge death or moderate/severe CF, and B) moderate/severe CP. The reference which an ideal nomogram would produce is represented by the dotted diagonal line; the solid line represents the actual performance (dashed lines represent the 95% confidence band).
The final models were then used to illustrate predicted probabilities and confidence intervals for cerebral palsy or death in hypothetical patients that were created with selected clinical information (Table 3). The equations describing the models are below the Table.
Table 3.
Hypothetical cases and model derived probabilities of death or mod/severe CP based on clinical information that would be available at hospital discharge
| Case scenarios | 1 | 2 | 3 | 4 | 5 | 6 |
|---|---|---|---|---|---|---|
| Gestational age at birth (wk) | 25 | 26 | 26 | 28 | 30 | 30 |
| MgSO4 treatment group | Y | N | N | N | Y | Y |
| IVH Grade III/IV | N | N | Y | Y | N | N |
| PVL | N | Y | N | Y | Y | N |
| Male | N | Y | Y | N | Y | Y |
| Neonatal hypotension | N | N | Y | N | N | N |
| Probability of death or moderate/severe CP* | 4 [2,7] | 82 [65, 92] | 34 [17, 56] | 84 [63, 94] | 49 [28, 70] | 2 [1,3] |
| Probability of moderate/severe CP* | 1 [0.3, 3] | 77 [53, 91] | 32 [14, 58] | 81 [51, 94] | 35 [16, 60] | 0.5 [0.2,1] |
In percent [95% confidence interval]
Probability of death or moderate/severe CP (the values to enter into the formula for categorical markers are 0 for absence and 1 for presence) = 1−{1/(1+exp(4.4730 + [−0.3665 × MgSO4 treatment group] + [−0.2968 × gestational age at birth] + [1.6660 × IVH Grade 3–4] + [3.8380 × PVL] + [0.9182 × male]))}.
Probability of moderate/severe CP =1−{1/(1+exp(3.5226 + [−0.5992 × MgSO4 treatment group] + [−0.3020 × gestational age at birth] + [1.6069 × IVH Grade 3–4] + [4.7433 × PVL] + [0.7797 × male] + [1.1885 × neonatal hypotension]))}.
DISCUSSION
When counseling patients faced with an imminent preterm birth, clinical factors known at that time are important to consider in any estimate of survival or prognosis for long term morbidity. Birth weight and gestational age are recognized as major factors for this prognosis, but other factors ranging from antenatal steroid administration to gender have been found to be important as well. It is reasonable to assume that the long-term prognosis will also be influenced by issues not known until after delivery, but few data exist to allow inclusion of post-natal findings in long term prognostication. In this study we evaluated a range of neonatal findings and complications in prematurely born infants who survive to hospital discharge, for their contribution to the occurrence of death or moderate or severe cerebral palsy by age two. In univariable analysis, every factor examined was associated with the primary outcome. However, in multivariable analysis, only intraventricular hemorrhage (IVH) grades III or IV and periventricular leukomalacia (PVL) are independently associated with this significant morbidity and mortality, while other morbidities including respiratory distress syndrome, bronchopulmonary dysplasia, necrotizing enterocolitis, and retinopathy of prematurity are not statistically significant. Using only IVH and PVL, along with gestational age and newborn gender, more than 80% of the variance in long term survival and motor handicap can be explained. When evaluating only infants surviving to their follow up evaluation, neonatal hypotension is also significant in the model, and over 90% of the variance in motor handicap is explained.
In the primary study, chorioamnionitis was defined as a clinical diagnosis of chorioamnionitis and a body temperature of 37.8° C or greater and no other defined infection. Numerous publications have shown an association between perinatal infection and long-term neurologic injury.5,6 This association is strongest when pregnancies end prematurely. It has been demonstrated in animals that introducing infection into the uterus can lead to white matter injury in the offspring, and motor abnormalities.7,8 In this context, the absence of chorioamnionitis as a significant factor in the multivariable model is surprising. It is possible that the link between perinatal infection and cerebral palsy is mediated through white matter injury recognizable by imaging; in the absence of IVH and PVL, the association between infection and subsequent development of CP is no longer present.
Tyson et al1 similarly used clinical information in an effort to predict mortality or neurodevelopmental impairment by the second year of age. In their analysis limited to infants born between 22 and 25 weeks’ gestation and examining only factors present at or before delivery, they found that gestational age, birth weight, gender, exposure to antenatal corticosteroids, and singleton pregnancy were significant contributors to this outcome. Our analysis differs in that it included infants born between 24–31 weeks’ gestation who survived to hospital discharge, and included short term complications noted before hospital discharge. Also, respiratory distress syndrome, NEC, and ROP, short term outcomes that are frequently included with IVH and PVL in composite outcomes in clinical studies, were not associated with the long-term outcomes examined. The lack of significance of these short-term outcomes is possibly because they have no effect on long term neurologic outcome independent of IVH and PVL in this particular population. PVL is the strongest predictor in the model, but factors that lead to PVL should not be discounted in their importance. Further, other factors in the development of CP might not have been included in this analysis. Although the clinical trial did note a benefit of treatment with magnesium sulfate on the primary outcome, the beneficial effect did not achieve statistical significance in this subset of patients in the logistic regression. This may have been related to the smaller sample size utilized for this analysis. Nonetheless, because treatment with magnesium sulfate was shown to be beneficial in the clinical trial, it was decided to include treatment group in the calculations of area under the curve.
Our analysis also allows for the use of information present by hospital discharge to calculate an individual child’s long-term prognosis with considerable accuracy. Using a limited number of clinical factors and neonatal outcomes, parents can be given an individualized prognosis that has been validated. As seen in Table 3 using hypothetical patient data, different combinations of clinical factors lead to a wide range of prognoses. It should be noted that this model was derived from a population born predominantly after preterm premature rupture of membranes, which may limit its generalizability. Other possible limitations include neonatal intervention differences influenced by early gestational age, center to center differences, inclusion criteria for the parent study, and differences in post-discharge care. Further, other factors which are known to be associated with risk of neurologic injury, such as genetic factors and fetal academia are not considered in this analysis. However, these factors are thought to be not as strong as prematurity in the pathway to neurologic injury, which is supported by the high percentage of variation in rates of disability or death predicted by the model.
In summary, this analysis better defines clinical factors and neonatal complications that predict survival and neurodevelopmental function by age 2. A validated model for prediction of individual long-term outcomes is also presented in Table 3. These findings will allow for improved counseling of parents at the time of neonatal discharge, and have the potential to alter the way composite outcomes are used in clinical studies. If the findings that only PVL and IVH are important independent predictors of long-term infant health and development are corroborated in future studies, consideration should be given to limiting composite outcomes in future obstetric and pediatric research to these two clinical findings. Further, research focused on reducing the incidence of PVL and IVH is warranted.
Key Points.
Risk factors for death and long-term neurologic handicap have been identified, but the contribution of factors identified in the neonatal period is poorly defined. Counseling regarding longer term outcomes in individual cases would benefit from a validated calculator.
Only intraventricular hemorrhage and periventricular leukomalacia are significant predictors of death or moderate/severe cerebral palsy for preterm infants who survive to hospital discharge.
Equations are provided which allow calculation of long-term risk based on information available early in the neonatal course.
ACKNOWLEDGEMENTS
The authors thank the following subcommittee members who participated in protocol development and coordination between clinical research centers (Allison T. Northen, M.S.N, R.N.), protocol/data management and statistical analysis (Steven Weiner, M.S. and Elizabeth Thom, Ph.D.), and protocol development and oversight (Deborah G. Hirtz, M.D., Karin Nelson, M.D., and Catherine Y. Spong, M.D.).
The parent trial for this study was registered on ClinicalTrials.gov website with number NCT00014989.
FUNDING
The project described was supported by grants from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) [HD27869, HD34208, HD34116, HD40544, HD27915, HD34136, HD21414, HD27917, HD27860, HD40560, HD40545, HD40485, HD40500, HD27905, HD27861, HD34122, HD40512, HD53907, HD34210, HD21410, HD36801, HD19897], MO1-RR-000080, and by the National Institute of Neurological Disorders and Stroke (NINDS). Comments and views of the authors do not necessarily represent views of the NICHD, NINDS.
Footnotes
Conflict Of Interest
None declared
In addition to the authors, other members of the Eunice Kennedy Shriver National Institute of Child Health and Human Development Maternal–Fetal Medicine Units Network are as follows:
Northwestern University, Chicago, IL – G. Mallett, M. Ramos-Brinson., P. Simon
University of Alabama at Birmingham, Birmingham, AL – J.C. Hauth, A. Northen, T. Hill-Webb, S. Tate, K. Nelson, F. Biasini
University of Utah, Salt Lake City, UT – K. Anderson, M. Jensen, L. Williams, L. Fullmer (LDS Hospital), A. Guzman (McKay-Dee Hospital)
University of Texas Southwestern Medical Center, Dallas, TX – M.L. Sherman, J. Dax, L. Faye-Randall, C. Melton, E. Flores
Case Western Reserve University-MetroHealth Medical Center, Cleveland, OH – M. Collin, G. VanBuren, C. Milluzzi, M. Fundzak, C. Santori
The Ohio State University, Columbus, OH – J. Iams, F. Johnson, M.B. Landon, C. Latimer, V. Curry, S. Meadows
Thomas Jefferson University, Philadelphia, PA – A. Sciscione, M.M. DiVito, M. Talucci, S. Desai, D. Paul
University of Tennessee, Memphis, TN – B.M. Sibai, R. Ramsey, W. Mabie, L. Kao, M. Cassie Wayne State University, Detroit, MI – G.S. Norman, D. Driscoll, B. Steffy, M.P. Dombrowski Wake Forest University Health Sciences, Winston-Salem, NC – P.J. Meis, M. Swain, K. Klinepeter, M. O’Shea, L. Steele
University of North Carolina at Chapel Hill, Chapel Hill, NC – K.J. Moise, Jr., S. Brody, J. Bernhardt, K. Dorman
University of Texas Health Science Center at Houston-Children’s Memorial Hermann Hospital, Houston, TX – L.C. Gilstrap, III, M.C Day, E. Gildersleve, F. Ortiz, M. Kerr Columbia University, New York, NY – V. Pemberton, L. Paley, C. Paley, S. Bousleiman, V. Carmona
Brown University, Providence, RI – M. Carpenter, J. Tillinghast, D. Allard, B. Vohr, L. Noel, K. McCarten
University of Cincinnati, Cincinnati, OH – M. Miodovnik, N. Elder, W. Girdler, T. Gratton
University of Chicago, Chicago, IL – A.H. Moawad, M. Lindheimer, P. Jones
University of Miami, Miami, FL – F. Doyle, C. Alfonso, M. Scott, R. Washington
University of Texas Medical Branch, Galveston, TX – G. Hankins, T. Wen, L.A. Goodrum, G.R. Saade, G.L. Olson, H.M. Harirah, E. Martin
University of Texas at San Antonio, San Antonio, TX – E. Xenakis, D. Conway, M. Berkus
University of Pittsburgh, Pittsburgh, PA – T. Kamon, M. Cotroneo, C. Milford
The George Washington University Biostatistics Center – E.Thom, S.J. Weiner, B. Jones-Binns, M. Cooney, M. Fischer, S. McLaughlin, K. Brunette, E. Fricks, T. Williams
National Institute of Neurological Disorders and Stroke, Bethesda, MD – D. Hirtz, K.B. Nelson Eunice Kennedy Shriver National Institute of Child Health and Human Development, Bethesda, MD – C. Spong, S. Tolivaisa, D. McNellis, C. Catz, K. Howell
MFMU Network Steering Committee Chair (University of Pittsburgh, Pittsburgh, PA) –– J. Roberts
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Tyson JE, Parikh NA, Langer J, Green C, Higgins RD. Intensive care for extreme prematurity—moving beyond gestational age. N Engl J Med 2008; 358:1672–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wood NS, Costeloe K, Gibson AT, Hennessy EM, Marlow N, Wilkinson AR. The EPICure study: associations and antecedents of neurological and developmental disability at 30 months of age following extremely preterm birth. Arch Dis Child Fetal Neonatal Ed 205; 90:F134–F140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vohr BR, Wright LL, Poole KP, McDonald SA. Neurodevelopmental outcomes of extremely low birth weight infants <32 weeks’ gestation between 1993 and 1998. Pediatrics 2005; 116:635–43. [DOI] [PubMed] [Google Scholar]
- 4.Rouse DJ, Hirtz DG, Thom E, et al. A randomized, controlled trial of magnesium sulfate for the prevention of cerebral palsy. N Engl J Med 2008; 359:895–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yoon BH, Romero R, Park JS, et al. Fetal exposure to an intra-amniotic inflammation and the development of cerebral palsy at the age of three years. Am J Obstet Gynecol 2000; 182:675–81. [DOI] [PubMed] [Google Scholar]
- 6.Wu YW, Colford JM. Chorioamnionitis as a risk factor for cerebral palsy: a meta-analysis. JAMA 2000; 284:1417–24. [DOI] [PubMed] [Google Scholar]
- 7.Yoon BH, Kim CJ, Romero R, et al. Experimentally induced intrauterine infection causes fetal brain white matter lesions in rabbits. Am J Obstet Gynecol 1997; 177:797–802. [DOI] [PubMed] [Google Scholar]
- 8.Patrick LA, Gaudet LM, Farley AE, Rossiter JP, Tomallty LL, Smith GN. Development of a guinea pig model of chorioamnionitis and fetal brain injury. Am J Obstet Gynecol 2004; 191:1205–11. [DOI] [PubMed] [Google Scholar]