Abstract
Introduction:
Blood levels of uremic toxin, asymmetric dimethylarginine (ADMA), are strongly associated with mortality in sepsis, renal failure, and cardiovascular and renal disease patients.
Methods:
An extracorporeal approach to reduce pathological ADMA was developed. The dimethylarginine dimethylaminohydrolase (DDAH) was immobilized on agarose beads to prepare a cartridge. The efficacy of cartridge for ADMA lowering in blood was investigated.
Results:
The DDAH-beads and cartridge reduced ADMA from solution or plasma. The magnitude of ADMA removal was dependent on the quantity of DDAH linked to the beads and the flow rate. When tested in association with plasmapheresis, the DDAH-cartridge was highly effective in ADMA removal from the blood and improved the arginine/ADMA ratio in a pig model.
Conclusion:
A new, safe and effective extracorporeal approach to lower ADMA was develop which may have application in improving outcome in patients with vascular complications and risk of mortality associated with high ADMA.
Keywords: ADMA, Uremic Toxin, Extracorporeal Device
Introduction
The uremic toxin, asymmetric dimethylarginine (ADMA) induces multiple cellular responses with deleterious consequences [1]. High serum ADMA concentration is present in sepsis patients [2–9] which correlate with disease severity (higher in septic shock) and mortality [2, 5, 6, 8]. ADMA is a strong predictor of 30-day mortality in sepsis patients [6, 8]. In patients admitted to ICU, high serum ADMA is associated with higher mortality [10–12]. Elevated ADMA level is independently associated with higher risk of all-cause mortality in end stage renal disease [13, 14] and renal transplant recipients [15]. More recently, it was found that high serum ADMA predicted hospital mortality in COVID-19 patients [16]. ADMA levels are also strongly associated with mortality in patients with cardiovascular disease [17, 18], heart failure [19, 20] and chronic kidney disease [21].
ADMA causes vascular dysfunction by reducing nitric oxide production [22, 23]. In addition, ADMA induces oxidative stress, mitochondrial dysfunction [24–26] and other direct effects on cellular functions [27, 28]. The pathological ADMA can induce vasoconstriction [29], platelet aggregation [30, 31], immune cell adhesion [32], vascular leak [33, 34], red cell deformity [35, 36] and generation of reactive oxygen species (ROS) [37].
The deleterious effects of ADMA on vascular functions or endotoxemia have been illustrated in preclinical models in which ADMA is increased by DDAH-gene deletion [29]. Alternatively, transgenic expression or adenoviral DDAH-1 gene delivery in rodents reduced ADMA, improved vascular function and reduced disease severity [38–40], reduced lung injury and improved outcome in LPS induced organ damage [34]. In models of ischemia-reperfusion, reduction of ADMA significantly reduced injury to heart, kidney, and liver [38, 40]. Further, pharmacological treatment with DDAH reduced ADMA and protected kidney from ischemia-reperfusion injury [41]. These multiple lines of evidence support the pathological role of ADMA, and potential therapeutic benefit of lowering ADMA. At the present time, ADMA lowering therapies are not available. We have developed a highly safe extracorporeal approach to selectively lower ADMA in plasma.
Materials and Methods
Generation and Characterization of Recombinant DDAH
Human and Pseudomonas Aeruginosa-DDAH (PA-DDAH) genes were cloned expressed in E. coli and purified using a Ni-sepharose column as previously described [41]. Purity was determined using SDS gel-electrophoresis. DDAH activity was determined by a colorimetric assay [42].
ADMA Measurement
ADMA in plasma was analyzed by HPLC method [43, 44]. Samples prepared by solid-phase extraction using an Oasis MCX Cartridge (Waters, USA) were derivatized with ortho-phthaldialdehyde (Sigma-Aldrich, USA). The analytes were heated at 30 °C for 1 min and injected into the HPLC Chromolith performance RP-18e column. Chromatography was performed with mobile phase A (25 mM potassium phosphate, pH 6.5) and B (methanol/THF) (97/3 by volume) at a flow rate of 2 mL/min and detected by fluorescence detector.
Generation of Immobilized DDAH for Extracorporeal Device.
PA-DDAH was dialyzed against 0.1 M phosphate, pH 8.0 and incubated with agarose-NHS (Fisher Scientific, USA) for 2 h at room temperature. The agarose bead conjugated PA-DDAH were packed into a cartridge, washed with 0.1 M phosphate pH 8.0 and stored at 4 °C. The cartridge is designated as therapeutic extracorporeal device (TED).
Ex-vivo ADMA Lowering in Blood
The Prismaflex System (Baxter, USA) was set up in total plasma exchange (TPE) mode with the TPE 2000 filter. For the shorter duration ex-vivo study with blood, the plasmapheresis configuration was modified to incorporate the TED into the effluent line and allowed return of plasma to the blood using a 4-way stopcock. The prescription was programmed as plasma exchange with the effluent rate matching the replacement rate. Removal of ADMA was tested using 0.55 L citrated blood spiked with 2 μM ADMA while stirring. A double lumen 14 × 24 cm hemodialysis catheter (SLX, Medcomp, USA) was inserted into the blood to enable access and return. Blood flow and plasma loss were 100 mL/min and 600 mL/h respectively, resulting in a filtration fraction of 12–13%. Blood and plasma samples pre- and post- TED were collected at 0, 15, 30, and 60 min and analyzed for ADMA.
Plasmapheresis Set Up for Porcine Model
For longer duration studies with the pigs, Prismaflex System was modified as shown in Figure 1. During the initial period of TPE, a colloid solution was used as replacement (Vetstarch, Zoetis, USA) until enough plasma was accumulated in the effluent bag. The effluent was then used as replacement solution. Pigs (60–70 lb) were premedicated (i.m.) with Telazol and Xylazin, masked down with isoflurane, intubated and maintained with isoflurane. An ear vein catheter was placed for intravenous fluids (Lactated Ringer’s 5 mL/kg/h) and an arterial line was placed to measure arterial blood pressure. A 14 Fr × 30 cm hemodialysis catheter (SLX, Medcomp, USA) was inserted into the right external jugular vein by modified Seldinger technique. Baseline vital parameters were assessed during TPE. Heparin was used as anticoagulant. Heparin bolus (75–100 U/kg) was administered intravenously 15 min prior to the start of the extracorporeal therapy followed by an intravenous infusion using an integrated syringe pump at rate of 50–100 U/kg/h. Clotting times were monitored via activated clotting time using the ACT Plus Automated Coagulation Timer System (Medtronic, USA). Clotting times were measured prior to administering heparin and then every 30–60 min or as clinically indicated with a goal of keeping ACT at 1.6 – 2× baseline. The average baseline ACT was 82 ± 14 seconds. The average ACT after heparin bolus (75–100 U/kg) was 148 ± 23 seconds or 1.8× average baseline ACT. Heparin infusion was started at an average of 72 ± 21 U/kg/h and the average rate at the end of the experiment was 88 ± 26 U/kg/h. There was no evidence of clotting complications. The pigs were connected to the Prismaflex and TPE without TED for 15–30 min with blood flow of 100 mL/min and effluent/replacement flow of 1000 mL/h until an effluent volume of about 200–250 mL was reached. The TED was then introduced and replacement solution was changed from the colloid solution to the plasma that had been harvested in previous cycle. The effluent/replacement flow was increased to 1000 mL/h. Whole blood and plasma samples pre and post TED were collected at 0, 60, 120, 180 and 240 min. The ADMA in pig blood was at normal levels without administration of exogenous ADMA.
Fig. 1.
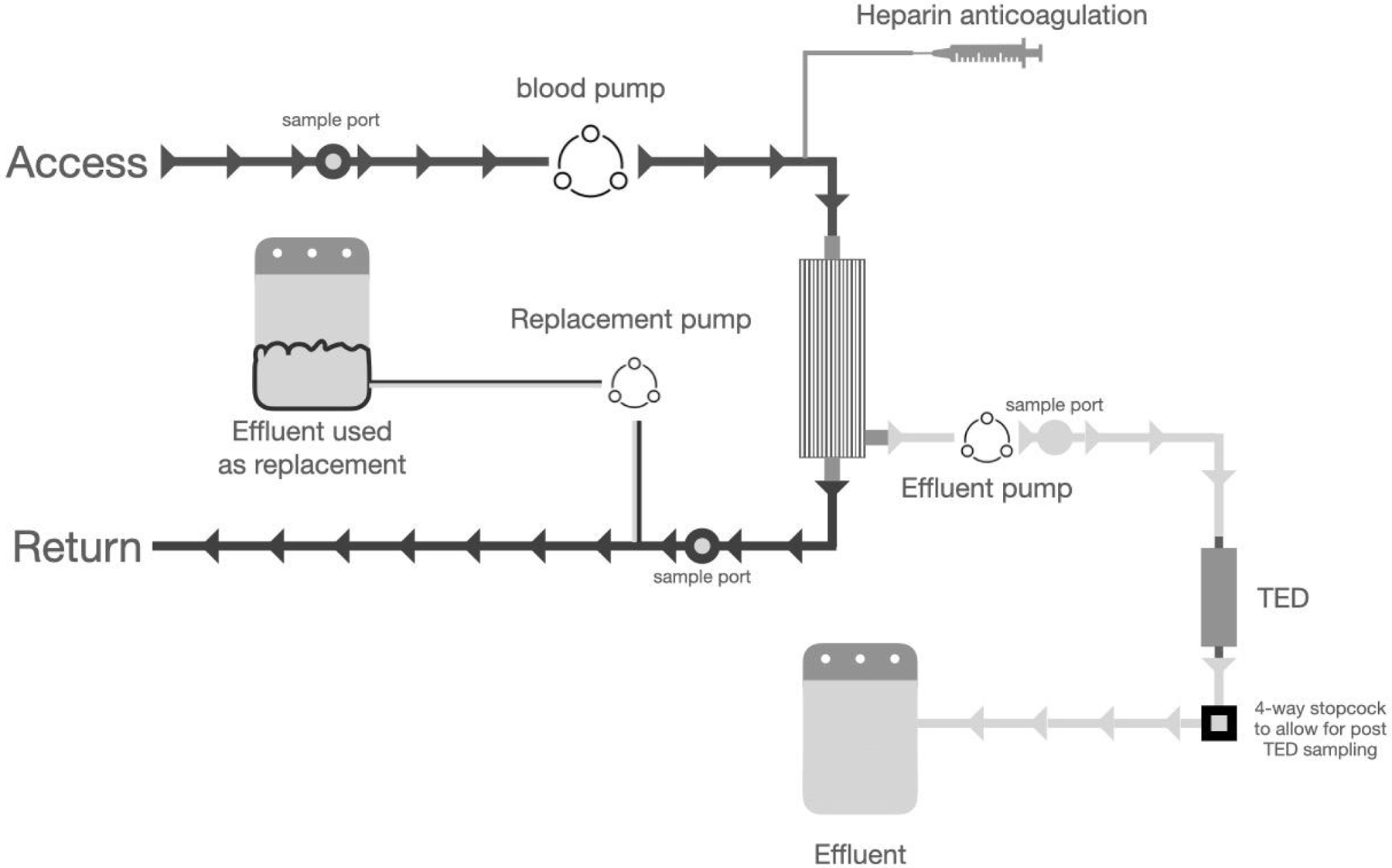
In order to successfully insert a device in the Baxter Prismaflex TPE 2000 System, it was configured as shown here. TED was connected distal to the membrane filter. The prescription was programmed for plasma exchange. During the initial phase, a colloid solution is used as replacement solution. Once enough plasma is available in the effluent bag, it was transferred to a sterile bag and used as replacement solution. This set up avoided the flow error messages from the plasmapheresis system which could lead to early termination of the study.
Statistical Analysis
Statistical significance was evaluated by student t test or one-way analysis of variance (ANOVA), followed by Dunnett’s test for multiple comparison between the experimental groups. All data presented as mean ± SD, P value of < 0.05 were considered statistically significant. *P < 0.05, **P < 0.01, ***P < 0.001.
Results
Preparation of Recombinant DDAH
In order to develop an extracorporeal device, recombinant human and PA-DDAH were prepared for immobilization on agarose beads. As shown in Figure 2a, purified enzymes were homogenous with >90% purity. Figure 2b shows that PA-DDAH was 6–10 folds more active as compared to the human DDAH-1. In addition, as compared to the human DDAH-1, PA-DDAH exhibited higher level of expression in E. coli and greater stability during the purification which offered significant advantages for product manufacturing. Based on the superior activity, protein expression and stability, we have used recombinant PA-DDAH, for further studies on immobilization and device construction.
Fig. 2.
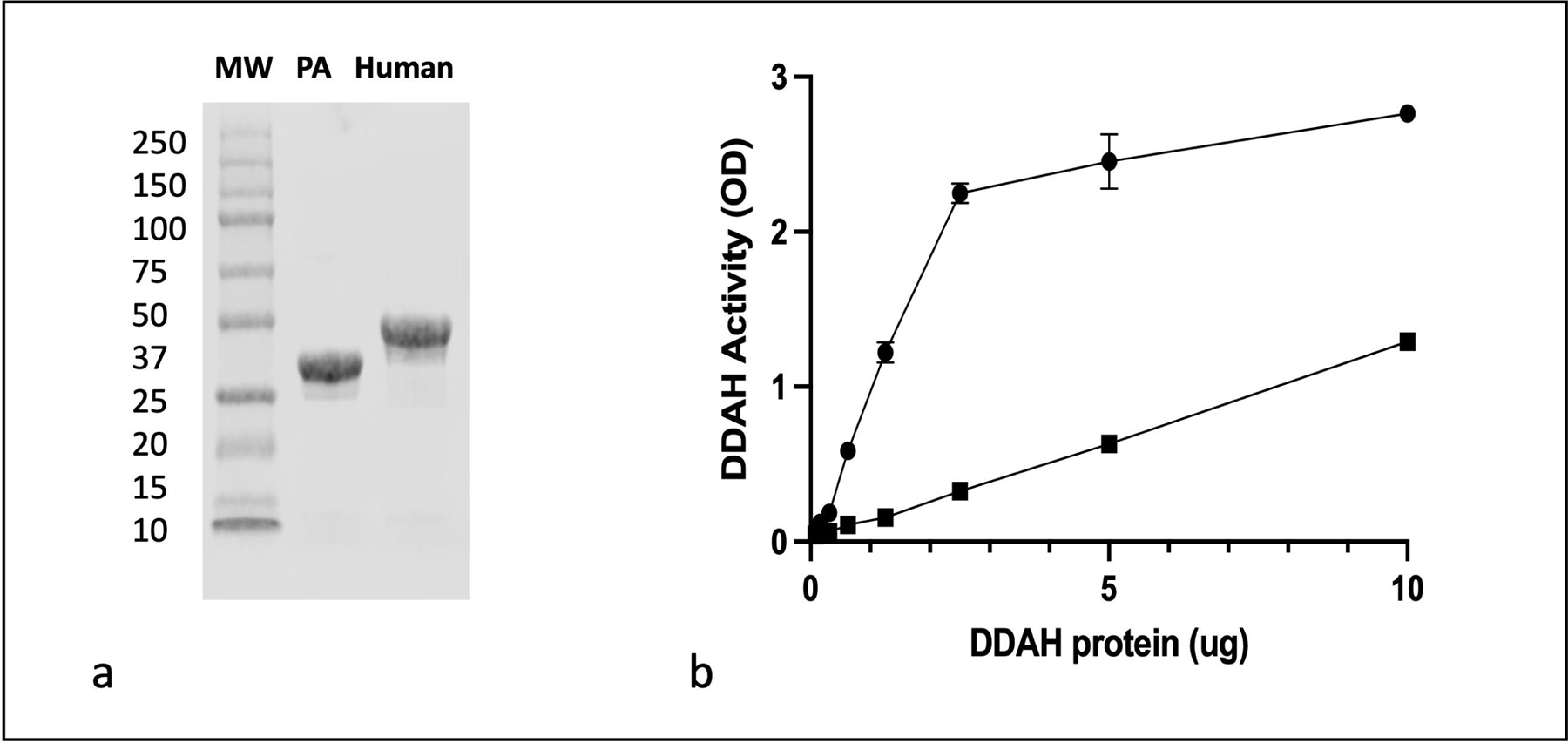
Human DDAH-1 and PA-DDAH were cloned and expressed in E.coli. (a) Shows the purity of recombinant PA (lane 2), recombinant human DDAH-1 (lane 3) and molecular weight markers (MW, lane 1) as determined by SDS gel electrophoresis. (b) Shows enzymatic activity of the recombinant PA (●) and human (■) DDAH as measured by generation of citrulline from ADMA which is measured by previously described colorimetric assay (n=3). All data are given as mean ± SD.
Generation of Matrix-Immobilized PA-DDAH
The cross-linking of PA-DDAH to beads was investigated using the amino groups of the protein and the beads containing N-hydroxysuccinamide as the linker. Figure 3a shows a concentration-dependent conjugation of DDAH to the beads. The stoichiometry of conjugation was dependent on the DDAH concentration and therefore beads containing varying levels of DDAH can be generated. Figure 3b shows that beads-immobilized protein produced fully active DDAH. Figure 3c shows that the bead conjugated DDAH was stable for more than 28 days at 4 °C. These results demonstrated the generation of a highly active and stable preparation of immobilized DDAH that was used for the fabrication of a therapeutic device.
Fig. 3.
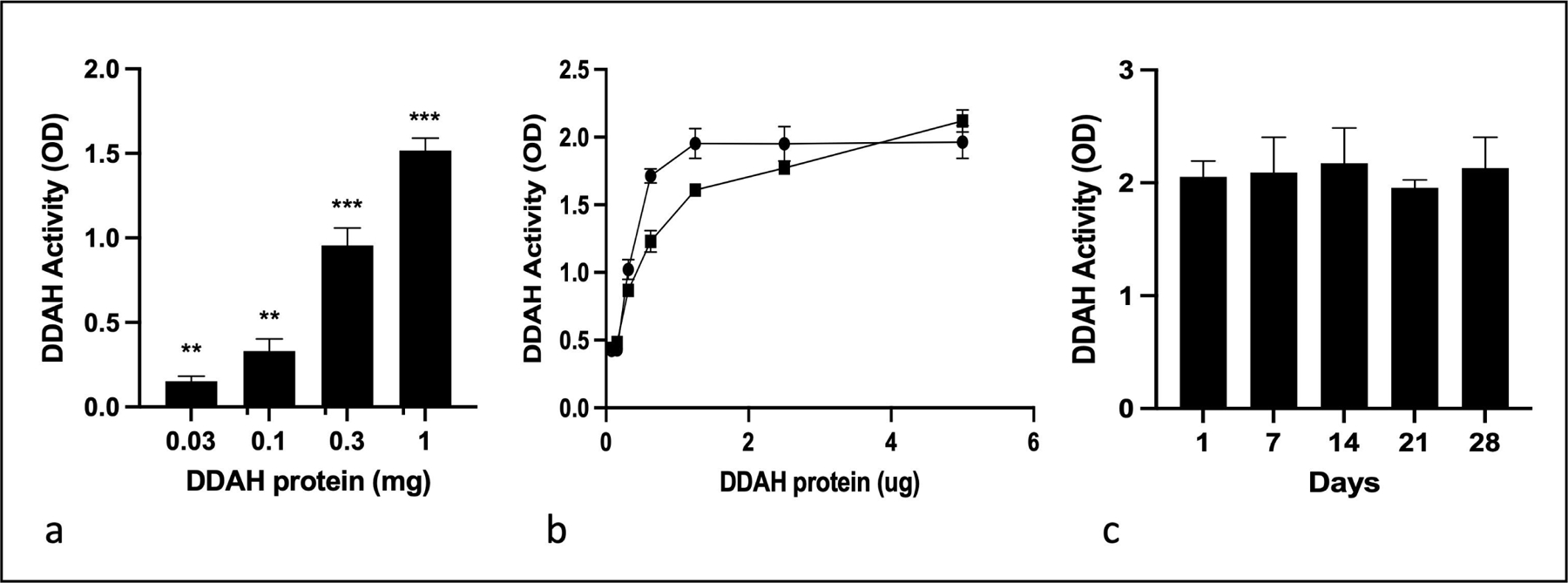
Generation and characterization of bead-immobilized PA-DDAH. PA-DDAH was dialyzed against 0.1 M phosphate, pH 8. Indicated concentrations in 500 μL buffer were incubated with 20 mg of agarose-NHS beads for 2 h at room temperature, while mixing. The conjugated beads were washed and assayed for DDAH activity. (a) Concentration-dependence of DDAH conjugation to the agarose beads (n=3). (b) Shows that the activity of the enzyme was fully retained after conjugation, activity prior to conjugation and (●) and after conjugated (■) (n=3). Stability of the immobilized PA-DDAH was determined after storage of the beads for up to one month at 4 ˚C (n=3). All data are given as mean ± SD. *P < 0.05, **P < 0.01, ***P < 0.001.
ADMA Lowering in Plasma by Immobilized PA-DDAH
In order to determine if bead conjugated PA-DDAH can reduce ADMA in plasma, we added different concentrations of beads to plasma and measured ADMA. Figure 4 shows a dose (4a) and time (4b) -dependent lowering of ADMA in plasma. 100% of ADMA was reduced when excess beads were added. Thus, immobilized PA-DDAH was highly effective in lowering ADMA from plasma. The dose and time dependence show that a device to lower desired ADMA concentration can be constructed by adjusting the volume of the beads and the duration of exposure to the beads.
Fig. 4.
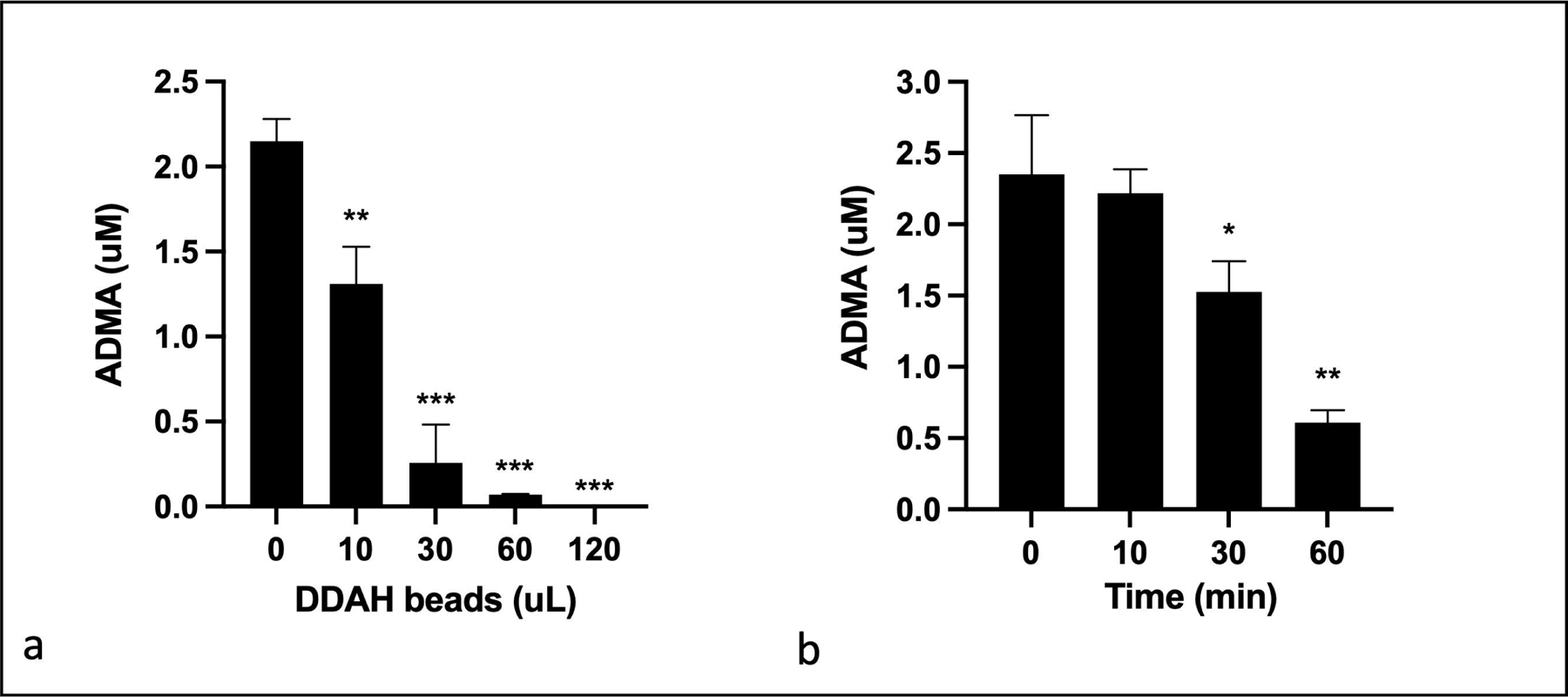
ADMA lowering in plasma by PA-DDAH-beads. (a) Different amounts of PA-DDAH-beads were incubated with pig plasma containing 2 μM ADMA for 30 min at room temperature. The mixture was then lightly centrifuged to separate beads from the plasma. ADMA in the plasma was determined using HPLC method (n=3). (b) 10 μL of PA-DDAH-beads were incubated with pig plasma for various times at room temperature and ADMA was determined using HPLC method (n=3). All data are given as mean ± SD. *P < 0.05, **P < 0.01, ***P < 0.001.
Preparation of Extracorporeal Device
TED was prepared by packing the desired volume of the PA-DDAH-beads into a cartridge. ADMA lowering in vitro was studied using various cartridge sizes and flow rates. Figure 5 shows that 100% ADMA from the solution was removed at a flow rate of 0.3 mL/min (5b). Increasing the flow rate to 1.0 mL/min which reduced ADMA exposure time with the DDAH-beads, resulted in removal of ~50% ADMA (5c). Likewise, the degree of ADMA removal can be adjusted by changing the amount of DDAH present on the beads and the size of the cartridge. These studies showed that, ADMA lowering can be controlled by the volume of the beads in a cartridge, DDAH concentration on the beads and the flow rate.
Fig. 5.
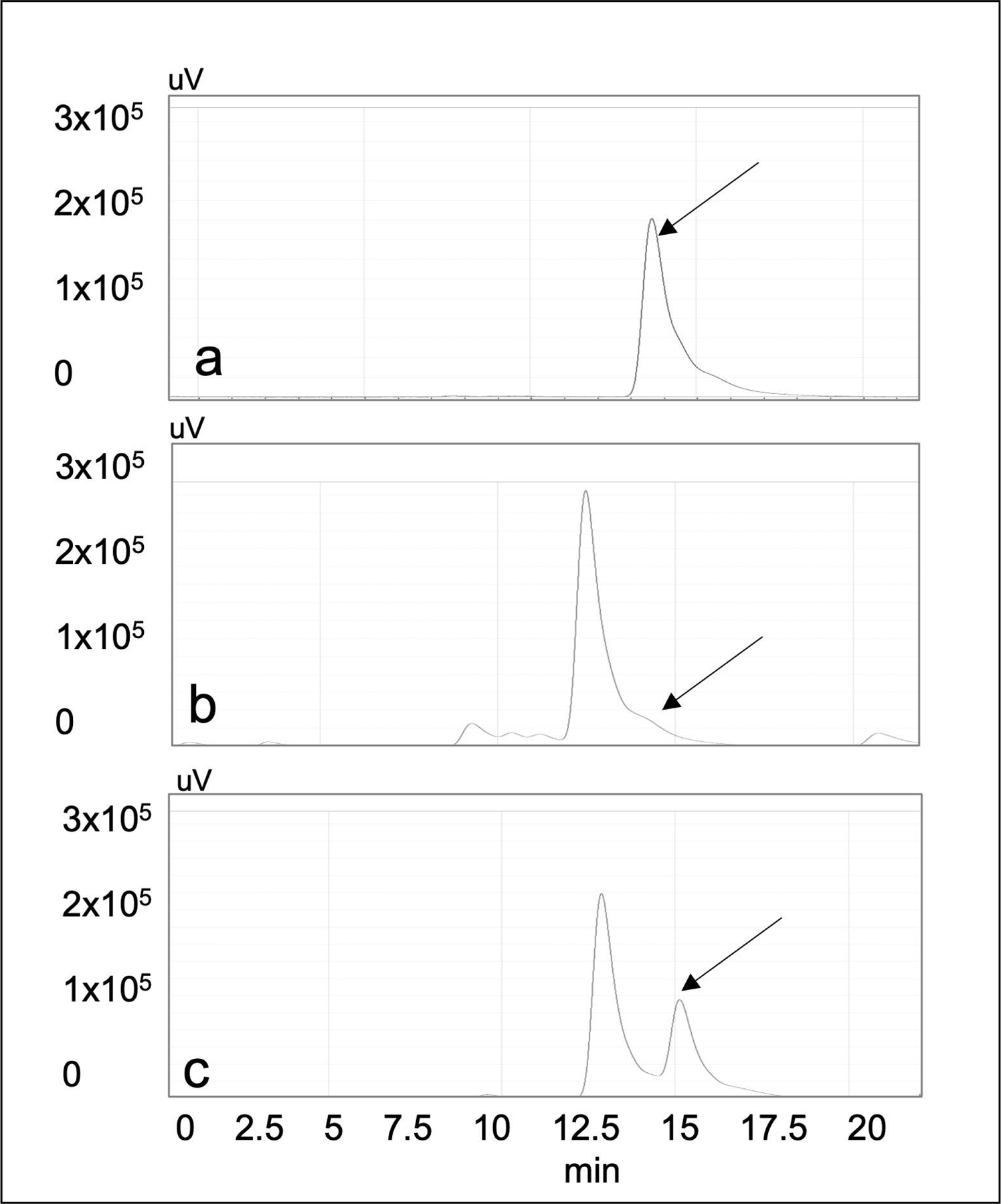
Removal of ADMA by a cartridge containing PA-DDAH beads. The HPLC traces show elution time of ADMA and citrulline generated as results of ADMA hydrolysis. (a) ADMA peak eluted from the HPLC chromatography of the starting solution (arrow). (b) HPLC chromatography of ADMA solution after passing through 1 mL DDAH-cartridge at 0.3 mL/min showing complete conversion of ADMA to citrulline, (c) HPLC chromatography of solution after passing through 1 mL DDAH-cartridge at 1.0 mL/min showing partial conversion of ADMA to citrulline. The x-axis is the elution time from the HPLC column and y-axis is the fluorescence signal indicating ADMA or citrulline levels.
Ex-Vivo ADMA Lowering in Blood
ADMA lowering in blood was tested by using TED in association with plasmapheresis. The TED was incorporated into the effluent line of the extracorporeal circuit distal to the apheresis filter. The citrated pig blood from the 0.55 L flask was processed with a blood flow rate of 100 mL/min. Plasma flow rate through the TED was 600 mL/h. Figure 6a shows the ADMA concentration in plasma before entering and immediately after eluting from the TED. At each time point, >90% of ADMA was removed from plasma. Based on the ADMA concentration in the blood at pre- and post- plasmapheresis, plasmapheresis alone did not reduce ADMA. Figure 6b shows that ADMA concentration in the blood was reduced over time leading to a 50% reduction after 60 min of plasmapheresis. These results demonstrated that a prototype TED inserted in the plasmapheresis system effectively lowered ADMA from blood.
Fig. 6.
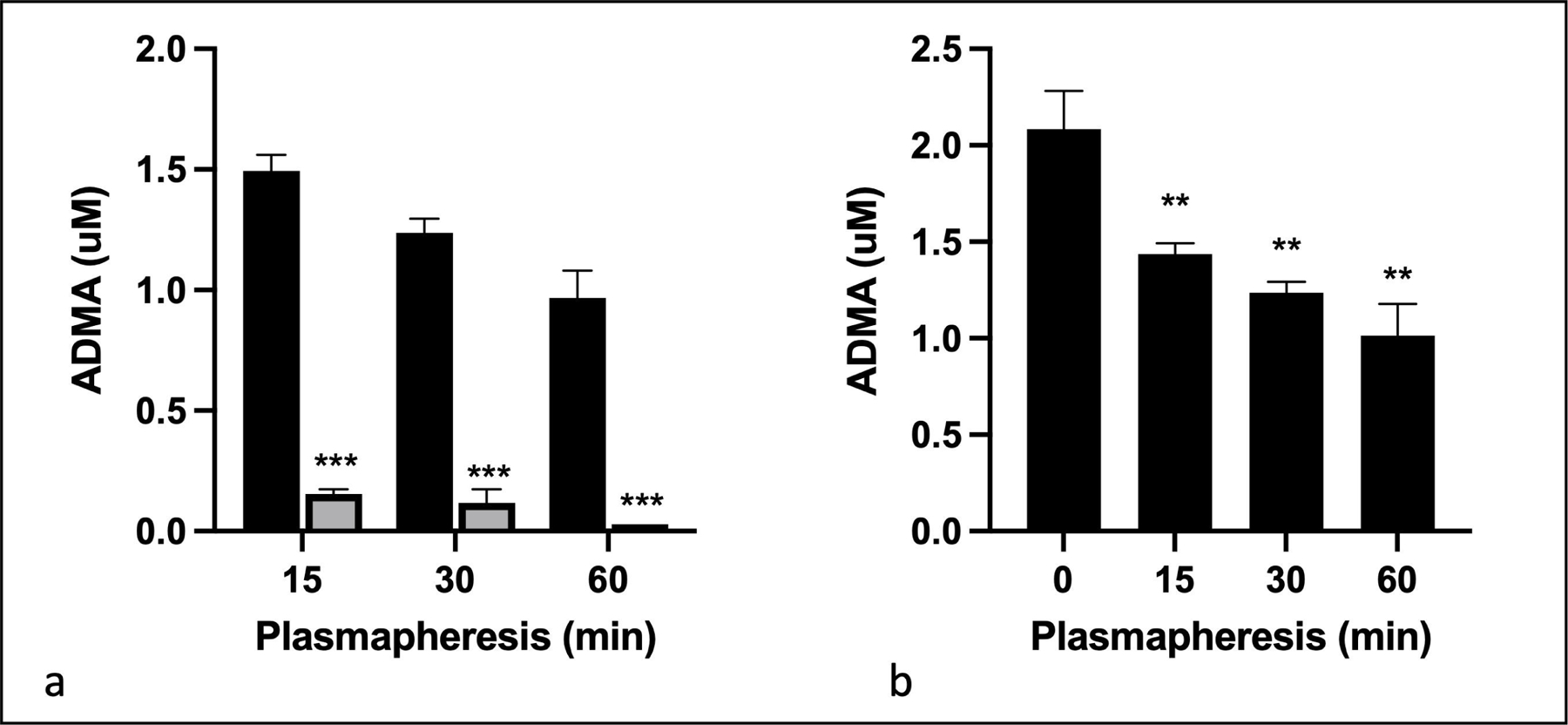
Removal of ADMA from blood in association with plasmapheresis. Pig blood (0.55 L) was subjected to filtration plasma separation using Baxter Prismaflex at a blood flow rate of 100 mL/min. The TED was prepared by packing 7 mL of PA-DDAH beads into the cartridge. Plasma was routed through the TED at a flow rate of 600 mL/h. (a) shows ADMA concentration in plasma samples entering the TED at the indicated period after plasmapheresis (black bars) and eluting from the TED (gray bars). ADMA concentration was determined in triplicate using HPLC method. (b) ADMA in pig blood samples collected after the indicated time of plasmapheresis and TED was analyzed at each time point in triplicate using HPLC method. All data are given as mean ± SD. *P < 0.05, **P < 0.01, ***P < 0.001.
Reduction of ADMA and Improvement of Arginine/ADMA in a Porcine Model
Following the demonstration of ex-vivo ADMA lowering, we tested the ability of the TED to lower ADMA in live animals. Blood from the anesthetized pigs was subjected to plasmapheresis and plasma samples from pre- and post- TED and whole blood from the animals were collected. The specificity of ADMA lowering was determined by testing the effect of TED on the plasma concentration of ADMA, symmetric dimethylarginine (SDMA) and arginine. As shown in Figure 7, TED reduced ADMA without affecting SDMA or arginine, demonstrating that ADMA lowering was specific. Measurements of ADMA, SDMA and arginine in the blood of live animals showed changes in all analytes after 4 h of plasmapheresis. These broader changes in blood chemistry are likely due to the anesthesia and the stressful experimental conditions. Under such conditions the data are commonly normalized to SDMA which is not affected by the treatment [45]. Figure 8 shows that TED produced a time dependent decrease in ADMA/SDMA ratio. Likewise, the arginine/ADMA which is an important measure of the effect on endothelial function was increased. These data showed that subjection of live pigs to TED reduced ADMA and increased the arginine/ADMA ratio.
Fig. 7.
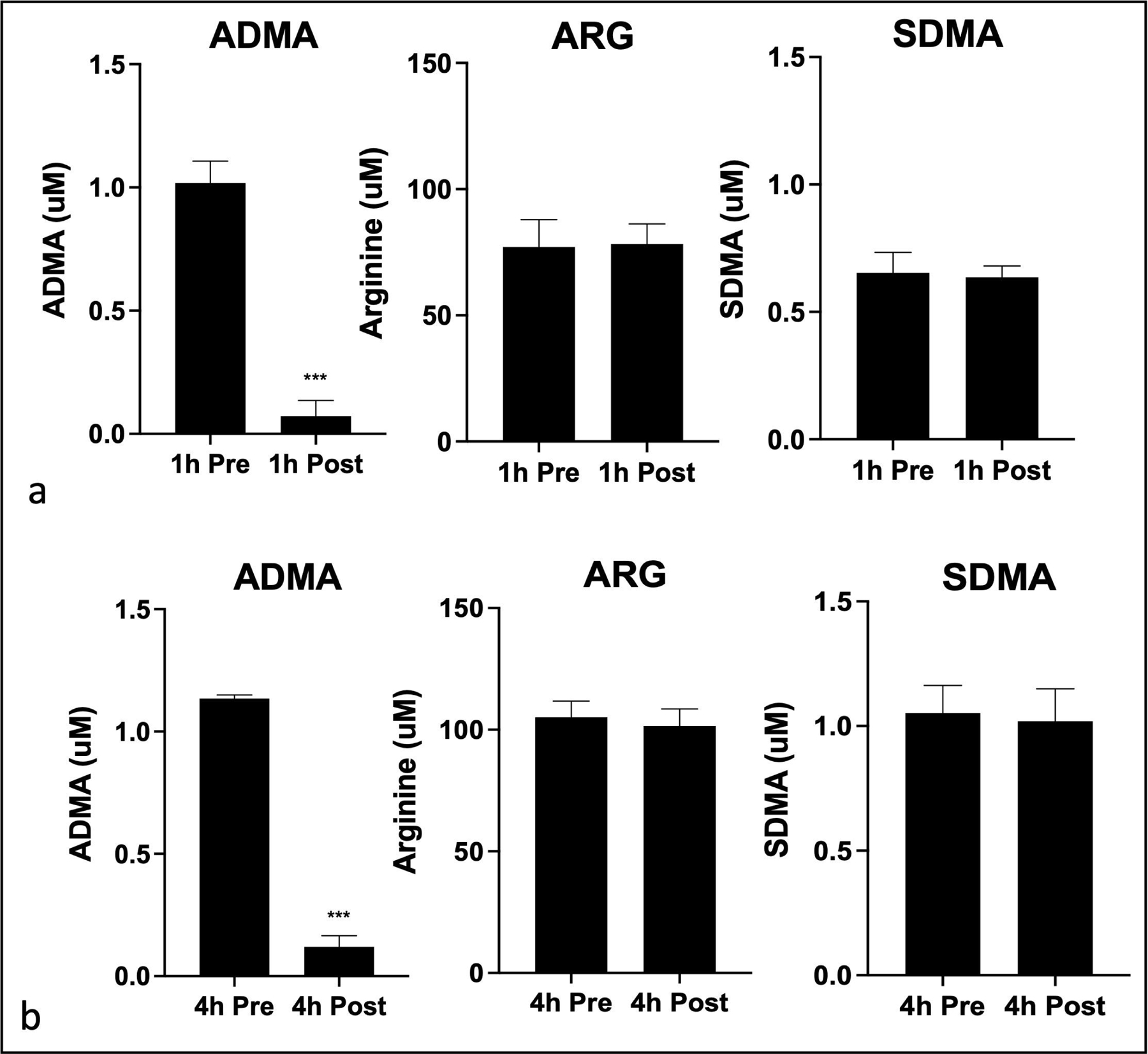
Specificity ADMA lowering in pigs by TED in association with plasmapheresis. The TED was prepared with 15 mL of PA-DDAH beads in a cartridge and incorporated in the plasmapheresis system as in Figure 1. Plasma samples were collected and analyzed for ADMA, SDMA and arginine. Shown here are concentration is pre- and post- TED samples collected at 1 h (a) and 4 h (b) (n=3). All data are shown as mean ± SD. *P < 0.05, **P < 0.01, ***P < 0.001.
Fig. 8.
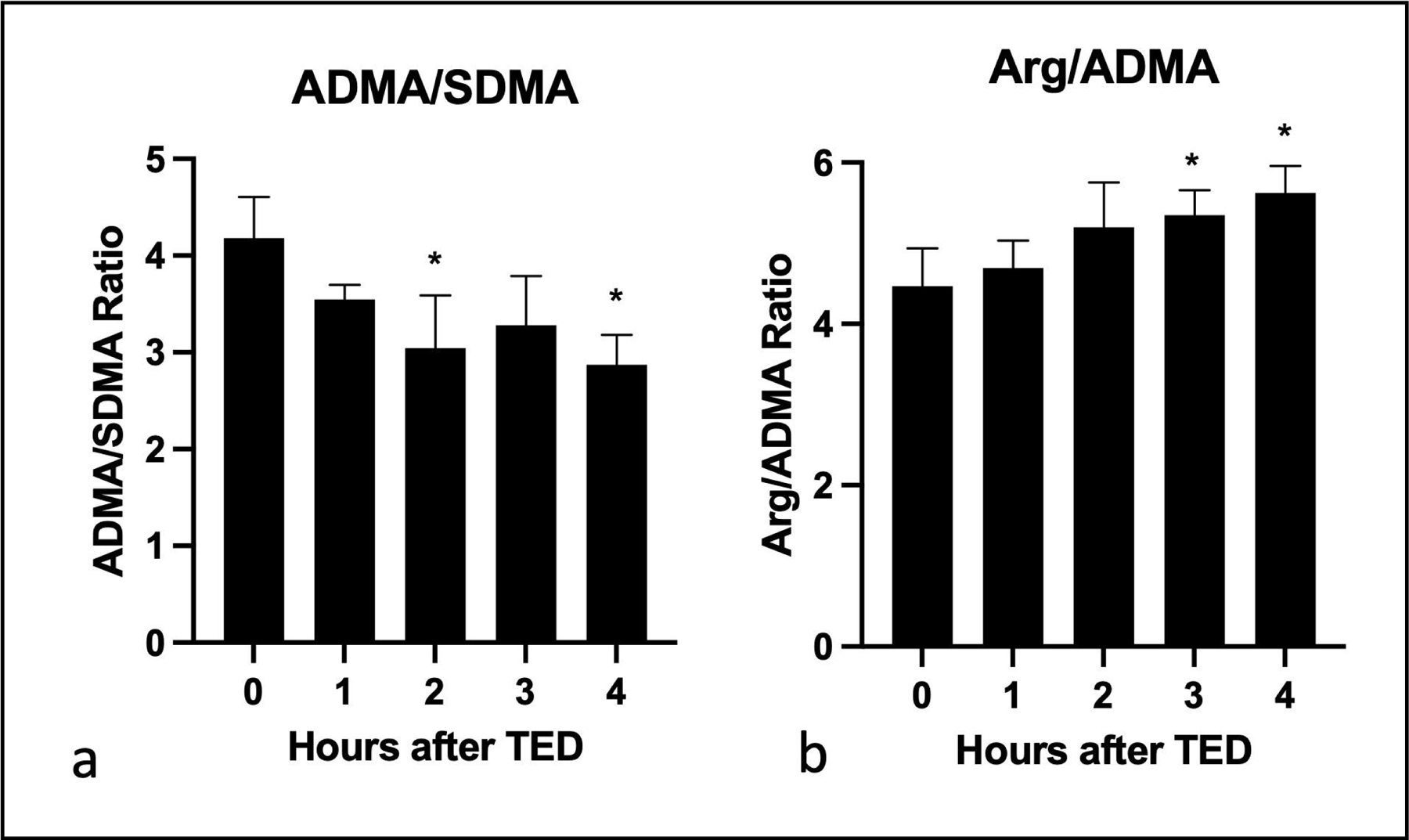
ADMA lowering and improved arginine/ADMA ratios in pigs undergoing plasmapheresis with TED. Pig blood samples were collected hourly during plasmapheresis with TED. ADMA, SDMA and arginine concentration was determined using HPLC method (n=3). ADMA changes normalized to SDMA (a) and arginine (b) are shown here. All data are given as mean ± SD. *P < 0.05, **P < 0.01, ***P < 0.001.
Discussion/Conclusion
In this study, we have developed a novel therapeutic bio-device that effectively and specifically lowered the uremic toxin ADMA from the blood. The TED was prepared by conjugating a uniquely high activity PA-DDAH to beads and optimized for ADMA lowering by adjusting the flow rate or the concentration of conjugated PA-DDAH. The device was tested in ex-vivo studies using pig blood and then in vivo using a porcine model following plasmapheresis. The results show that TED effectively reduced ADMA and improved arginine/ADMA ratio in the pig model. Unlike the non-specific anion exchange columns [46] which reduce ADMA, arginine other critical blood constituents, TED was specific for the removal of ADMA. The arginine/ADMA ratio in an important parameter for reducing the pathological action of ADMA on vascular and mitochondrial function. The selective removal of ADMA without reducing arginine and other essential blood constituents is an important attribute for the efficacy of the device.
The beneficial effects of reducing ADMA have been well documented in preclinical studies in which ADMA was reduced by increasing the expression of DDAH gene [38–40]. These studies have shown that reduction of ADMA improved endothelial function and offered protection from organs damage under stress, ischemia, and cardiovascular and renal disease [38–40, 47, 41]. Although, ADMA lowering by DDAH gene expression in animal studies has provided an important proof of concept, the therapeutic modalities to lower pathological ADMA in humans are not available. Our studies show that ADMA can be safely removed from the blood using TED. The extracorporeal device would be particularly applicable for lowering ADMA in hospitalized patients known the have high ADMA such as those with acute kidney, heart and lung injury [13, 17, 14, 19, 18, 20, 21], sepsis [5, 6, 8], COVID-19 [16] and admitted to the ICU [10–12].
The extracorporeal device represents a safe and effective treatment to lower pathological ADMA. ADMA is lowered without drug exposure to the patient. Direct administration of PA-DDAH could pose a risk of immunogenicity. The treatment can be tailored to individual patient as a personalized therapy by calibrating the duration of the procedure and the plasma flow rate. Furthermore, unlike a drug molecule which can last for a long time in the body and potentially produce adverse effects, ADMA lowering by TED can be terminated at will. Further development and clinical testing of the TED could offer a potential therapy for patients with high ADMA and risk of vascular and hemodynamic complications.
A limitation of the current device is its use for clinical studies in the current format. The agarose-based device is designed for preclinical proof of concept studies in conjunction with plasmapheresis and not with dialysis. Future studies will develop polysulfone or cellulase triacetate based polymer that are FDA approved for use in dialysis and will produce device for clinical testing for both in the setting of plasmapheresis and dialysis.
Acknowledgement
We want to thank the Purdue Pre-Clinical Trials Office for their support of this study.
Funding Sources
This study was supported in part with grant from the Indiana Clinical and Translational Sciences Institute Grant Number UL1TR001108 from the National Institutes of Health, National Center for Advancing Translational Sciences, Additional funding was provided through the MTP and AIM grant from IU Health and Indiana-CTSI. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Conflict of Interest Statement
The authors have no conflicts of interest to disclose
Statement of Ethics
This study was approved by the Purdue University Institutional Animal Care and Use Committee (# 2010002077).
Data Availability Statement
All data generated or analyzed during this study are included in this article. Further enquiries can be directed to the corresponding author.
References
- 1.Tain YL, Hsu CN.Toxic Dimethylarginines: Asymmetric Dimethylarginine (ADMA) and Symmet ric`Dimethylarginine (SDMA). Toxins (Basel). 2017. Mar 6;9(3). [Google Scholar]
- 2.Mortensen KM, Itenov TS, Haase N, Müller RB, Ostrowski SR, Johansson PI, et al. High Levels of Methylarginines Were Associated With Increased Mortality in Patients With Severe Sepsis. Shock. 2016. Oct;46(4):365–72. [DOI] [PubMed] [Google Scholar]
- 3.Németh B, Kiss I, Péter I, Ajtay Z, Németh Á, Márk L, et al. Monitoring of L-Arginine and Endogenous Dimethylarginines in Survivor Septic Patients - A Pilot Study. In Vivo. 2016. 09-10;30(5):663–9. [PubMed] [Google Scholar]
- 4.Tesfai A, MacCallum N, Kirkby NS, Gashaw H, Gray N, Want E, et al. Metabolomic profiling of amines in sepsis predicts changes in NOS canonical pathways. PLOS ONE. 2017;12(8):e0183025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Winkler MS, Kluge S, Holzmann M, Moritz E, Robbe L, Bauer A, et al. Markers of nitric oxide are associated with sepsis severity: an observational study. Crit Care. 2017. Jul 15;21(1):189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Coskun CN, Usanmaz SE, Savci V, Demirel-Yilmaz E. Time-Dependent Production of Endothelium-Related Biomarkers is Affected Differently in Hemorrhagic and Septic Shocks. Inflammation. 2018. Feb;41(1):33–41. [DOI] [PubMed] [Google Scholar]
- 7.Hansen MB, Rasmussen LS, Garred P, Pilely K, Wahl AM, Perner A, et al. Associations of Plasma Nitrite, L-Arginine and Asymmetric Dimethylarginine With Morbidity and Mortality in Patients With Necrotizing Soft Tissue Infections. Shock. 2018. Jun;49(6):667–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Winkler MS, Nierhaus A, Rösler G, Lezius S, Harlandt O, Schwedhelm E, et al. Symmetrical (SDMA) and asymmetrical dimethylarginine (ADMA) in sepsis: high plasma levels as combined risk markers for sepsis survival. Crit Care. 2018. Sep 19;22(1):216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van Wijk XMR, Yun C, Lynch KL. Evaluation of Biomarkers in Sepsis: High Dimethylarginine (ADMA and SDMA) Concentrations Are Associated with Mortality. J Appl Lab Med. 2021. Apr 29;6(3):592–605. [DOI] [PubMed] [Google Scholar]
- 10.Koch A, Weiskirchen R, Kunze J, Dückers H, Bruensing J, Buendgens L, et al. Elevated asymmetric dimethylarginine levels predict short- and long-term mortality risk in critically ill patients. J Crit Care. 2013. Dec;28(6):947–53. [DOI] [PubMed] [Google Scholar]
- 11.Brinkmann SJ, de Boer MC, Buijs N, van Leeuwen PA. Asymmetric dimethylarginine and critical illness. Curr Opin Clin Nutr Metab Care. 2014. Jan;17(1):90–7. [DOI] [PubMed] [Google Scholar]
- 12.Ghashut RA, Blackwell S, Ryan S, Willox L, McMillan DC, Kinsella J, et al. Assessment of asymmetrical dimethylarginine metabolism in patients with critical illness. Eur J Clin Invest. 2017. Apr;47(4):279–88. [DOI] [PubMed] [Google Scholar]
- 13.Aucella F, Maas R, Vigilante M, Tripepi G, Schwedhelm E, Margaglione M, et al. Methylarginines and mortality in patients with end stage renal disease: a prospective cohort study. Atherosclerosis. 2009. Dec;207(2):541–5. [DOI] [PubMed] [Google Scholar]
- 14.Shafi T, Hostetter TH, Meyer TW, Hwang S, Hai X, Melamed ML, et al. Serum Asymmetric and Symmetric Dimethylarginine and Morbidity and Mortality in Hemodialysis Patients. Am J Kidney Dis. 2017. Jul;70(1):48–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Frenay AR, van den Berg E, de Borst MH, Beckmann B, Tsikas D, Feelisch M, et al. Plasma ADMA associates with all-cause mortality in renal transplant recipients. Amino Acids. 2015. Sep;47(9):1941–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hannemann J, Balfanz P, Schwedhelm E, Hartmann B, Ule J, Müller-Wieland D, et al. Elevated serum SDMA and ADMA at hospital admission predict in-hospital mortality of COVID-19 patients. Scientific Reports. 2021. 2021/05/10;11(1):9895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schlesinger S, Sonntag SR, Lieb W, Maas R. Asymmetric and Symmetric Dimethylarginine as Risk Markers for Total Mortality and Cardiovascular Outcomes: A Systematic Review and Meta-Analysis of Prospective Studies. PLoS One. 2016;11(11):e0165811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu X, Xu X, Shang R, Chen Y. Asymmetric dimethylarginine (ADMA) as an important risk factor for the increased cardiovascular diseases and heart failure in chronic kidney disease. Nitric Oxide. 2018. Aug 1;78:113–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wirth J, Atzler D, di Giuseppe R, Cordts K, Menzel J, Böger RH, et al. Higher serum asymmetric dimethylarginine is related to higher risk of heart failure in the EPIC-Potsdam study. Amino Acids. 2017. Jan;49(1):173–82. [DOI] [PubMed] [Google Scholar]
- 20.Potočnjak I, Radulović B, Degoricija V, Trbušić M, Pregartner G, Berghold A, et al. Serum concentrations of asymmetric and symmetric dimethylarginine are associated with mortality in acute heart failure patients. Int J Cardiol. 2018. Jun 15;261:109–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang H, Xiang S, Dai Z, Fan Y. Asymmetric dimethylarginine level as biomarkers of cardiovascular or all-cause mortality in patients with chronic kidney disease: a meta-analysis. Biomarkers. 2021. Jul 27:1–7. [DOI] [PubMed] [Google Scholar]
- 22.Leiper J, Nandi M. The therapeutic potential of targeting endogenous inhibitors of nitric oxide synthesis. Nat Rev Drug Discov. 2011. Apr;10(4):277–91. [DOI] [PubMed] [Google Scholar]
- 23.Schwedhelm E, Boger RH. The role of asymmetric and symmetric dimethylarginines in renal disease. Nat Rev Nephrol. 2011. May;7(5):275–85. [DOI] [PubMed] [Google Scholar]
- 24.Sud N, Wells SM, Sharma S, Wiseman DA, Wilham J, Black SM. Asymmetric dimethylarginine inhibits HSP90 activity in pulmonary arterial endothelial cells: role of mitochondrial dysfunction. Am J Physiol Cell Physiol. 2008. Jun;294(6):C1407–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen N, Leng YP, Xu WJ, Luo JD, Chen MS, Xiong Y. Contribution of endogenous inhibitor of nitric oxide synthase to hepatic mitochondrial dysfunction in streptozotocin-induced diabetic rats. Cell Physiol Biochem. 2011;27(3–4):341–52. [DOI] [PubMed] [Google Scholar]
- 26.Xiong Y, He YL, Li XM, Nie F, Zhou XK. Endogenous asymmetric dimethylarginine accumulation precipitates the cardiac and mitochondrial dysfunctions in type 1 diabetic rats. Eur J Pharmacol. 2021. Jul 5;902:174081. [DOI] [PubMed] [Google Scholar]
- 27.Hasegawa K, Wakino S, Tanaka T, Kimoto M, Tatematsu S, Kanda T, et al. Dimethylarginine Dimethylaminohydrolase 2 Increases Vascular Endothelial Growth Factor Expression Through Sp1 Transcription Factor in Endothelial Cells. Arteriosclerosis, Thrombosis, and Vascular Biology. 2006. 2006/07/01;26(7):1488–94. [DOI] [PubMed] [Google Scholar]
- 28.Jiang D-J, Jia S-J, Dai Z, Li Y-J. Asymmetric dimethylarginine induces apoptosis via p38 MAPK/caspase-3-dependent signaling pathway in endothelial cells. Journal of Molecular and Cellular Cardiology. 2006. 2006/04/01/;40(4):529–39. [DOI] [PubMed] [Google Scholar]
- 29.Leiper J, Nandi M, Torondel B, Murray-Rust J, Malaki M, O’Hara B, et al. Disruption of methylarginine metabolism impairs vascular homeostasis. Nature Medicine. 2007. 2007/02/01;13(2):198–203. [DOI] [PubMed] [Google Scholar]
- 30.Li D, Guo R, Chen QQ, Hu CP, Chen X. Increased plasma level of asymmetric dimethylarginine in hypertensive rats facilitates platelet aggregation: role of plasma tissue factor. Can J Physiol Pharmacol. 2011. Mar;89(3):151–8. [DOI] [PubMed] [Google Scholar]
- 31.Gawryś J, Wiśniewski J, Szahidewicz-Krupska E, Gajecki D, Leśniewska J, Majda F, et al. Increased Intraplatelet ADMA Level May Promote Platelet Activation in Diabetes Mellitus. Oxid Med Cell Longev. 2020;2020:6938629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mangoni AA, Tommasi S, Sotgia S, Zinellu A, Paliogiannis P, Piga M, et al. Asymmetric Dimethylarginine: a Key Player in the Pathophysiology of Endothelial Dysfunction, Vascular Inflammation and Atherosclerosis in Rheumatoid Arthritis? Curr Pharm Des. 2021;27(18):2131–40. [DOI] [PubMed] [Google Scholar]
- 33.Wojciak-Stothard B, Torondel B, Zhao L, Renné T, Leiper JM. Modulation of Rac1 activity by ADMA/DDAH regulates pulmonary endothelial barrier function. Molecular biology of the cell. 2009;20(1):33–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Aggarwal S, Gross CM, Kumar S, Dimitropoulou C, Sharma S, Gorshkov BA, et al. Dimethylarginine Dimethylaminohydrolase II Overexpression Attenuates LPS-Mediated Lung Leak in Acute Lung Injury. American Journal of Respiratory Cell and Molecular Biology. 2013. 2014/03/01;50(3):614–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tsuda K, Nishio I. An association between plasma asymmetric dimethylarginine and membrane fluidity of erythrocytes in hypertensive and normotensive men: an electron paramagnetic resonance investigation. Am J Hypertens. 2005. Sep;18(9 Pt 1):1243–8. [DOI] [PubMed] [Google Scholar]
- 36.Kuwai T, Hayashi J. Nitric oxide pathway activation and impaired red blood cell deformability with hypercholesterolemia. J Atheroscler Thromb. 2006. Dec;13(6):286–94. [DOI] [PubMed] [Google Scholar]
- 37.Druhan LJ, Forbes SP, Pope AJ, Chen C-A, Zweier JL, Cardounel AJ. Regulation of eNOS-Derived Superoxide by Endogenous Methylarginines. Biochemistry. 2008. 2008/07/01;47(27):7256–63. [DOI] [PubMed] [Google Scholar]
- 38.Stuhlinger MC, Conci E, Haubner BJ, Stocker EM, Schwaighofer J, Cooke JP, et al. Asymmetric Dimethyl L-Arginine (ADMA) is a critical regulator of myocardial reperfusion injury. Cardiovascular Research. 2007. Jul 15;75(2):417–25. [DOI] [PubMed] [Google Scholar]
- 39.Jacobi J, Maas R, Cardounel AJ, Arend M, Pope AJ, Cordasic N, et al. Dimethylarginine dimethylaminohydrolase overexpression ameliorates atherosclerosis in apolipoprotein E-deficient mice by lowering asymmetric dimethylarginine. The American journal of pathology. 2010;176(5):2559–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nakayama Y, Ueda S, Yamagishi S, Obara N, Taguchi K, Ando R, et al. Asymmetric dimethylarginine accumulates in the kidney during ischemia/reperfusion injury. Kidney Int. 2014. Mar;85(3):570–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lee Y, Mehrotra P, Basile D, Ullah M, Singh A, Skill N, et al. Specific Lowering of Asymmetric Dimethylarginine by Pharmacological Dimethylarginine Dimethylaminohydrolase Improves Endothelial Function, Reduces Blood Pressure and Ischemia-Reperfusion Injury. J Pharmacol Exp Ther. 2021. Feb;376(2):181–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Knipp M, Vasak M. A colorimetric 96-well microtiter plate assay for the determination of enzymatically formed citrulline. Anal Biochem. 2000. Nov 15;286(2):257–64. [DOI] [PubMed] [Google Scholar]
- 43.de Jong S, Teerlink T. Analysis of asymmetric dimethylarginine in plasma by HPLC using a monolithic column. Analytical Biochemistry. 2006. 2006/06/15/;353(2):287–89. [DOI] [PubMed] [Google Scholar]
- 44.Teerlink T HPLC analysis of ADMA and other methylated l-arginine analogs in biological fluids. Journal of Chromatography B. 2007. 2007/05/15/;851(1):21–29. [DOI] [PubMed] [Google Scholar]
- 45.Rowland MR, Ragina NP, Sarkar J, Uyehara CF, Senagore AJ. Is arginine/asymetric dimethylarginine ratio depletion an indicator of insufficient resuscitation in a porcine model of hemorrhage-reperfusion? Surgery. 2014. Oct;156(4):861–8. [DOI] [PubMed] [Google Scholar]
- 46.Rifai K, Bode-Boeger SM, Martens-Lobenhoffer J, Ernst T, Kretschmer U, Hafer C, et al. Removal of asymmetric dimethylarginine during artificial liver support using fractionated plasma separation and adsorption. Scand J Gastroenterol. 2010. Sep;45(9):1110–5. [DOI] [PubMed] [Google Scholar]
- 47.Lambden S, Kelly P, Ahmetaj-Shala B, Wang Z, Lee B, Nandi M, et al. Dimethylarginine Dimethylaminohydrolase 2 Regulates Nitric Oxide Synthesis and Hemodynamics and Determines Outcome in Polymicrobial Sepsis. Arteriosclerosis, Thrombosis, and Vascular Biology. 2015. 2015/06/01;35(6):1382–92. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this article. Further enquiries can be directed to the corresponding author.


