Abstract
Multi-drug resistant (MDR) Shigella flexneri 2a, one of the leading bacterial agents of diarrhoeal mortality, has posed challenges in treatment strategies. The present study was conducted to identify potential therapeutic biomarkers using gene interaction network (GIN) in order to understand the cellular and molecular level interactions of both antimicrobial resistance (AMR) and virulence genes through topological and clustering metrics. Statistically significant differential gene expression (DGE), structural chemistry and dynamics were incorporated to elucidate biomarker for sustainable therapeutic regimen against MDR S. flexneri. Functional enrichments and topological metrics revealed evgS, ybjZ, tolC, gyrA, parC and their direct interactors to be associated with diverse AMR mechanisms. Histidine kinase EvgS was considered as the hub protein due to its highest prevalence in the molecular interactome profiles of both the AMR (71.6%) and virulence (45.8%) clusters interconnecting several genes concerning two-component system (TCS). DGE profiles of ΔPhoPQ (deleted regulatory PhoP and sensor PhoQ) led to the upregulation of TCS comprising EvgSA thereby validating EvgS as a promising therapeutic biomarker. Druggability and structural stability of EvgS was assessed through thermal shifts, backbone stability and coarse dynamics refinement. Structure–function relationship was established revealing the C-terminal extracellular domain as the drug-binding site which was further validated through molecular dynamics simulation. Structure elucidation of identified biomarker followed by secondary and tertiary structural validation would prove pivotal for future therapeutic interventions against subverting both AMR and virulence posed by this strain.
Supplementary Information
The online version contains supplementary material available at 10.1007/s13205-022-03325-w.
Keywords: Differential gene expression, Druggability, Gene interaction network, Molecular Dynamics Simulation, Protein Modelling, Shigella flexneri 2a str. 301
Introduction
Shigella, a facultative anaerobic Gram-negative enteropathogen, causes mild to severe watery diarrhoea in humans. They are the main causative agents of paediatric acute gastrointestinal infections leading to dysentery. It leads to immense morbidity and mortality to infants below 5 years of age, with an infective dose of a mere 10–100 bacterium count (Sethuvel et al. 2017b; Anandan et al. 2017). The disease transmission occurs through oral–faecal route in humans, which serve as the sole hosts for this bacterium. Shigella spp. are divided into several serogroups and serotypes depending on the variation in the lipopolysaccharide structure of O-antigen (Khaghani et al. 2014). Amongst four major serogroups of Shigella, S. flexneri is dominant in developing countries (Mao et al. 2013). In India, several studies reported S. flexneri 2a as the most virulent serotype affecting children (Anandan et al. 2017). The antimicrobial resistance (AMR) traits in Shigella spp. have been recorded since the 1940s, accounting for Trimethoprim-sulfamethoxazole, Tetracycline and Chloramphenicol resistance followed by Ampicillin, Cotrimoxazole and Nalidixic acid resistance by early 1990s. Administration of Fluoroquinolones was thereby routinely performed till the end of the past decade when S. flexneri 2a developed resistance against Norfloxacin, Ciprofloxacin and Ofloxacin (Sur et al. 2004; Livio et al. 2014). The World Health Organization ranked Fluoroquinolone resistant Shigella spp. as a medium priority enteric pathogen (www.who.int). Presently, there are no vaccines available for treating shigellosis. As serotype-specific immunity governs in humans, it is a cumbersome task to develop vaccines for each serotype, sub-serotypes and non-serotypeable Shigella spp. (Sethuvel et al. 2017b, a).
Several virulence and AMR genes in Shigella spp. conferring resistance to different antibiotics have been identified till date. Though extensive researches on genomics and proteomics have been carried out, we still lack effective treatment strategies to combat the multi-drug resistance (MDR) patterns. This prompted us to perform gene interaction network (GIN) analysis of its AMR and virulent genes (VGs) to reveal the molecular-level cross-talk among them. GIN analysis has gained mammoth importance and is extensively studied by researchers around the World to understand the complex mechanisms and functioning of genes/proteins of an organism (Martínez-Vázquez et al. 2012; Miryala et al. 2018). Our research group has extensively analysed various pathogens of clinical importance using GIN approach to unveil potential alternative drug-targets as a therapeutic measure (Debroy et al. 2020; Naha et al. 2020, 2021; Miryala et al. 2021a, b; Shankar et al. 2021; Vasudevan et al. 2021; Miryala and Ramaiah 2022). Based on several reports of virulence and pathogenesis, we have selected S. flexneri 2a str. 301, as the strain of interest (Zhu et al. 2018). The present study aimed to explore alternate therapeutic targets/biomarkers to be targeted against both AMR and virulence for curbing down the pathogenesis of the strain.
Materials and methods
Retrieval of AMR and VGs
The VGs and AMR specific to the strain were retrieved from several comprehensive and manually curated public repositories like PATRIC (https://www.patricbrc.org/), CARD (https://card.mcmaster.ca/), Victors (http://www.phidias.us/victors/), VFDB (http://www.mgc.ac.cn/VFs/main.htm), and NDARO (https://www.ncbi.nlm.nih.gov/pathogens/antimicrobial-resistance/).
Gene interactome, functional enrichments and network construction
The interaction profiles each of AMR and VGs were independently curated from the STRING v11.0 (https://string-db.org/) and DAVID (https://david.ncifcrf.gov/) databases. Both experimentally validated and theoretically predicted interactions (P-value < 0.05) were considered with a confidence scores (CS) ranging from 0 to 1. Functional enrichment analysis (FEA) were performed to retrieve gene ontology (GO) terms, domain information and pathway enrichments from Pfam, InterPro, SMART and KEGG databases (Szklarczyk et al. 2017). The GIN networks were finally constructed and visualised in Cytoscape v3.8.0 (https://cytoscape.org/) (Smoot et al. 2011). The clustering analysis was performed using MCODE that assigned each cluster with a score based on density, cluster size and connectivity of the nodes. Further, topological parameters were calculated and represented graphically using NetworkAnalyzer and CytoHubba, respectively (Bader and Hogue 2003). Further, the common set of genes from both AMR and virulence network were screened out and the interactome was constructed to understand the molecular-level cross-talks linking both the networks. The experimentally evident interactions (CS > 0.9) were considered to decipher the key biological pathway/mechanism bridging both the systems. The gene(s) involved in vital functional enrichments and attained better topological metrics, in both AMR and VG network, was considered as the hub-gene. The role of hub-gene in controlling both the networks was further assessed upon studying suitable DGE profiles specific to the strain.
DGE analysis
Based on functional enrichments, topological metrics and literary evidence, the interactome profiles of gene(s) responsible for linking both AMR and virulent pathways were better understood by studying the DGE profiles. DGE of Shigella flexneri 2a str. 301 was analysed with the microarray expression profiles [GSE107365] from NCBI-GEO database (https://www.ncbi.nlm.nih.gov/geo/). The experimental design was cross-referenced from the cited literature in which the regulation functions of ΔPhoPQ in S. flexneri were investigated through in-vitro procedures (Lin et al. 2018). Microarray analysis and normalization were performed using kit-based standardized protocols while subsequent validations of the differential gene expressions were carried out using qRT-PCR. The wild and mutant samples were grown to mid-log (6 h) and early-stationary (10 h) phases adhering to the standard protocols as followed throughout the in-vitro experimental work. The corresponding microarray dataset encompassing samples at 6 h and 10 h, was thereafter opted in the current study for analyzing the DGE profiles. The DGE were computed using GEO2R to record the up-regulation and down-regulation pattern of various genes. DGE analysis was performed based on statistically significant P-values (< 0.05) and log Fold Change (logFC) values ranging from − 0.5 to + 0.5 (Miryala et al. 2019). The differential regulation pattern of several genes would justify the effective role of the hub-gene in controlling Shigella pathogenesis.
Protein target modelling and refinement
The structure of the expressed hub-gene was either screened from RCSB-PDB (https://www.rcsb.org/) or else modeled to build the complete 3D structure as described previously (Basu et al. 2021). The structure was refined to reduce the unstable bond torsions, poor rotamers and outliers through the GalaxyRefine (http://galaxy.seoklab.org/refine/) server for structure enhancement by furnishing and reorienting the side-chains (Heo et al. 2013). Energy minimisation was performed in-vacou with GROMOS96-43B1 force-field with 2000 steps each for steepest-descents and conjugate-gradients using the Swiss-PDB Viewer v4.1.0 (Kaplan and Littlejohn 2001). The secondary structures were predicted from the SOPMA (https://npsa-prabi.ibcp.fr/cgi-bin/npsa_automat.pl?page=%20npsa_sopma.html) and were further validated using the PSIPRED (http://bioinf.cs.ucl.ac.uk/psipred/) server (Geourjon and Deléage 1995; McGuffin et al. 2000). The tertiary structure was analysed from the ProSA-Web (https://prosa.services.came.sbg.ac.at/prosa.php) (Wiederstein and Sippl 2007) and the SAVES6.0 (https://saves.mbi.ucla.edu/) servers. The protein/domain structures were visualised in UCSF-Chimera v1.9 (Pettersen et al. 2004).
Protein druggability prediction and stability analysis upon coarse and molecular dynamics simulations
The functional domains of the expressed hub-gene (drug-target) were curated from Pfam, SMART and InterPro servers (Schultz et al. 2000; Hunter et al. 2009; Finn et al. 2016). The domains were further checked for drug-binding action and active-site conformation using the DoGSiteScorer (http://dogsite.zbh.uni-hamburg.de) server (Volkamer et al. 2012). Further, correlation and stability parameters were assessed upon determining the inherent thermal shifts (normalised B-factor), backbone stability and coarse dynamics refinement. Backbone stability was estimated from the DynaMine (http://dynamine.ibsquare.be/) server (Cilia et al. 2013, 2014) while coarse dynamics was performed with the CABSflex2.0 (http://biocomp.chem.uw.edu.pl/CABSflex2) server (Jamroz et al. 2013) as described previously (Chaudhary et al. 2020; Naha et al. 2021; Shankar et al. 2021; Basu et al. 2021).
Based on critical understanding of the domain harbouring drug-binding site (active-site), structural stability was predicted based on instability and aliphatic indices from the ProtParam (https://web.expasy.org/protparam/) server which was further validated by performing Molecular Dynamics Simulation (MDS). MDS was carried out with GROMACS 2020.2 package in an aqueous environment for a timescale of 50,000 ps. CHARMM36-Feb2021 force-field and simple-point charge water model (TIP3P) was used to build the protein topology. Solvation of the protein was performed after centering the protein inside a cubic box with a uniform dimension of 1.0 nm (Philippi et al. 2019). Neutralisation of the system was achieved upon addition of requisite counter ions (Na+ or Cl−). Energy minimisation was carried out with the steepest-descent method for 50,000 steps and a potential energy gradient threshold of 1000 kJ/mol/nm. Finally, the system was equilibrated under standard NVT (constant Number of particles, Volume, Temperature) and NPT (constant Number of particles, Pressure, Temperature) ensembles for 100 ps. For treating long-range electrostatic interactions, particle-mesh Ewald summation was adopted with an order of 4.0 and Fourier spacing of 0.16 nm. For pressure scaling, Parrinello Rahman proposed barostat coupling ensemble was used by applying motion equations to box vectors followed by MD production to generate the trajectory data (Zeng et al. 2011; Zheng et al. 2011; Jayaraman et al. 2019; Lemkul 2019). The trajectories were visualised using the Grace software (https://plasma-gate.weizmann.ac.il/Grace/).
Results and discussion
Retrieved genes and their interaction profile
A total of 89 AMR genes and 274 VGs were retrieved from a set of 119 AMR and 499 VGs, respectively, after refining and eliminating the redundant entries [Supplementary Information SI 1(a)]. Upon subjecting the refined AMR and VG datasets separately to the STRING database, interaction profiles of 88 AMR genes with 888 interactions and 273 VGs with 3780 interactions were obtained. The total functional partners that accounted for different AMR and VGs were 28 and 103 (highest CS); 32 and 128 (high CS); 58 and 184 (medium CS); 83 and 259 (low CS), respectively, [Supplementary Information SI 1(b-c)].
GIN analysis
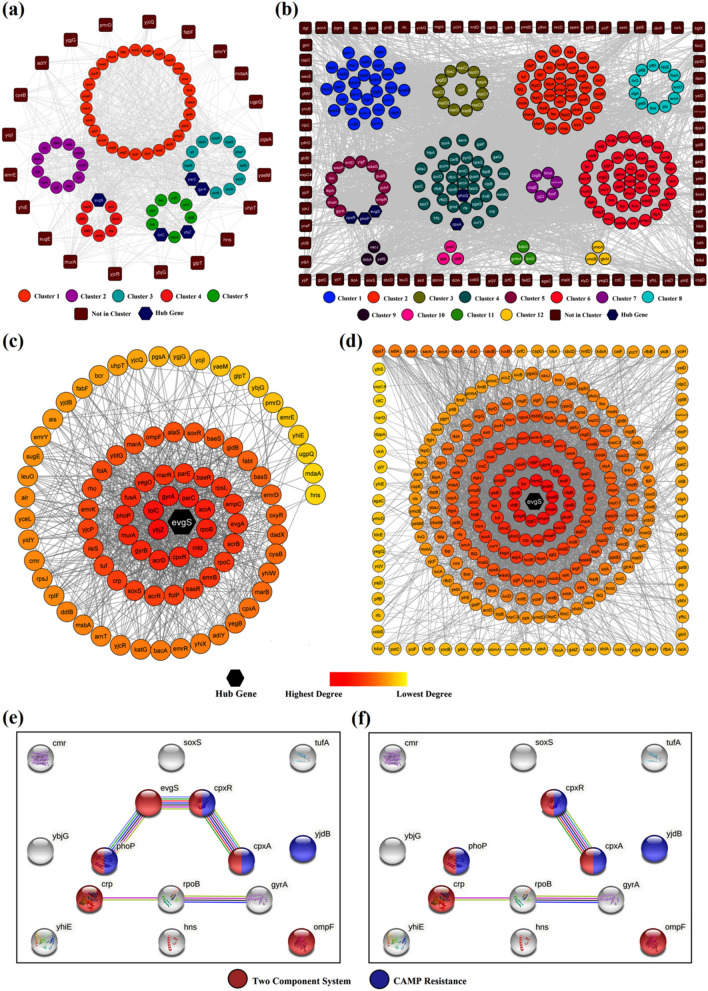
Clustering analysis of both the interaction networks, as illustrated in Fig. 1a, b, resulted in five interconnected AMR clusters (C1–C5) while 12 clusters (C1–C12) were obtained from VGs [Supplementary Information SI 1(d)]. MCODE clustering analysis portrayed all five AMR clusters to be densely interconnected as revealed through highest clustering coefficient of C2 (0.58) followed by C1 (0.5), C3 (0.48), C5 (0.42) and C4 (0.4). The average number of neighbours of C1 (25.73), signified connectivity of ~ 30% with all other nodes of the network [Supplementary Information SI 1(e–f)]. AMR and VGs were sorted based on their interaction degrees to identify the hub-genes bearing the highest number of interactions responsible for molecular cross-talks (Fig. 1c, d). FEA was performed to identify multiple GO terms and pathway enrichments that are listed in Supplementary Information SI 2.
Fig. 1.
GIN Analysis of AMR and VGs: a Clustering analysis of 88 AMR genes. b Clustering analysis of 273 VGs. c AMR genes arranged based on total number of interactions. d VGs arranged based on total number of interactions. e Multiple TCS genes being interlinked in presence of evgS. f Multiple TCS genes being disconnected in absence of evgS
Important GO terms for AMR genes were response to antibiotic (ampC, gyrA,B, ybjZ, yjcP,Q,R, fabI, marA, uppP) [GO:0046677]. arnT was responsible in lipid A biosynthetic process [GO:0009245], and lipopolysaccharide biosynthetic process [GO:0009103] that were involved in cell membrane integrity and functions. The genes alr, bacA, ddlB, murA, uppP and ybjG were responsible for peptidoglycan biosynthetic process [GO:0009252; sfl00550]. A vital resistance mechanism against glycopeptide antibiotic Vancomycin [sfl01502] was evident in the study, which was regulated by alr, dadX, and ddlB genes [sfl00473]. Though vancomycin resistance was seen predominant in Gram-positive bacteria (Naha et al. 2020), Stock and Wiedemann in 1999 reported total glycopeptide resistance for both Vancomycin and Teicoplanin across all Shigella strains (Stock and Wiedemann 1999). Resistance to β-lactams [GO:0017001; sfl01501] was also evident due to decreased permeability of porin channels and over expression of class-C cephalosporinase AmpC in this strain.
Several point mutations in DNA gyrase (GyrA and GyrB) and Topoisomerase IV enzymes (ParC and ParE) resulted in fluoroquinolone resistance that were majorly highlighted via mutation of drug-targets, MDR efflux pumps and decreased permeability of porin channels. Frequent mutations in GyrA contributed towards complete resistance towards fluoroquinolones while mutations in ParC reduced susceptibility towards ciprofloxacin (Poole 2000; Azmi et al. 2014). Simultaneously, mutations in RpoB and RpoC cause several genetic variants imparting Rifampin resistance that arrest transcription by inhibiting DNA-dependent RNA polymerase action [slf03020] (Campbell et al. 2001; Munita and Arias 2016; Jerbi and Springborg 2018).
In Shigella, a three-component system comprising acrAB-tolC played an active role in resistance nodulation division (RND) efflux pump mechanism [IPR003423; IPR027463]. Genes ybjZ, msbA and yojI contributed towards ATP binding cassette (ABC) efflux pump mechanism [GO:0046677; GO:0043190] imparting resistance against β-lactams [GO:0008800; sfl01501], Tetracyclines [IPR001411; GO:0015238; IPR004638], and acriflavin [IPR001036]. Resistance due to over-expression of AcrAB is controlled by four (AcrR, MarA, SoxS and Rob) regulatory proteins (Zhu et al. 2018). The former three regulators were expressed in the FEA which included bacterial regulatory helix-turn-helix proteins, AraC family [PF00165; PF12833]; and arabinose operon control protein [SM00342]. The genes emrE, sugE [PF00893; IPR000390] responded towards small multidrug resistance (SMR) efflux pumps while emrD, yceL, yegB from (C1); emrB from (C2); bcr, cmr, yidY from (C5); and emrY, glpT, uhpT from (NIC) played vital roles towards major facilitator superfamily (MFS) efflux pump mechanisms [IPR011701; IPR020846; IPR036259; PF07690] (Ghosh et al. 2020). Different resistance mechanisms, along with their contributing AMR genes, are tabulated in Table 1.
Table 1.
MDR mechanisms along with their contributing AMR/VGs and antibiotics to which each mechanism show resistance
| Sl. No | MDR Mechanism | AMR/VGs | Antibiotics |
|---|---|---|---|
| 1 | Decreased permeability of porin channels | acrA, acrB, ampC, ompC, ompF, ompA, ompR, tolC | β-Lactams, fluoroquinolones, tetracyclines |
| 2 | Inactivation of antibiotic molecule | acrA, acrB, ampC, ompF, tolC | Penicillins, cephalosporins |
| 3 | Mutation of target sites | gyrA, gyrB, parC, parE, rpoB, rpoC | Fluoroquinolones, rifampin |
| 4 | Complete replacement or bypass of target site | alr, dadX, ddlB, bacA, murA, ybjG | Vancomycin, teicoplanin |
| 5 | Global cell adaptive response (CAMP resistance) | acrA, acrB, arnT, basR, basS, cpxA, cpxR, dsbA, htrA, marA, phoP, phoQ, pmrD, tolC, yjdB | Daptomycin |
| 6 | Efflux pumps | ||
| a. SMR | emrE, sugE | β-Lactams, fluoroquinolones, chloramphenicol, fusidic acid | |
| b. RND | acrA, acrB, acrR, acrD, emrK, tolC | ||
| c. MFS | bcr, cmr, emrB, emrD, emrY, glpT, uhpT, yceL, yegB, yidY | ||
| d. ABC | msbA, ybjZ, yojI | ||
VG network resulted in a total of 41 VGs contributing significantly towards pathogenesis [GO:0009405]. Other processes include O-antigen biosynthetic process [GO:0009243] by rfbA,B,D and rfe which were significant in giving rise to various serovars in Shigella spp. due to acetylation/glucosylation of the lipopolysaccharide of O-antigen (Morona et al. 1995a, b). Further, Shigella expressed ipgC, lon, yihE and ynaF when subjected to antimicrobial stress [GO:0006950] that facilitates the bacterium in gaining resistance against several efflux resistance mechanisms. Lipopolysaccharide core region biosynthetic process [GO:0009244] by waaP and gmhA; Kdo2-lipid A biosynthetic process [GO:0036104] by msbB and msbB2 are part of Lipid-A biosynthetic mechanisms which are essential for bacterial cell survival and viability. Our results correlates with earlier GIN reports on Escherichia coli which shares resemblance with the Shigella spp. (Miryala and Ramaiah 2019).
Finally, based on direct interactions, clustering and topological metrics from GIN analysis, the chromosomal genes evgS, ybjZ, tolC, gyrA and parC were found to interact with their functional partners via shortest path lengths, high centralities and maximum interactors. These five genes and their interactors were directly associated with various AMR mechanisms. The druggability of TolC, GyrA and ParC are well studied, thus validating the current in-silico approach for evaluating alternate drug-targets as a therapeutic strategy. Irrational usage of antibiotics against these targets resulted in mutation/alteration of these proteins by bacteria resulting AMR (Martínez-Jiménez et al. 2013). The present study thus hypothesised two novel drug-targets EvgS (expressed evgS) and MacB (expressed ybjZ) which are yet to be explored for potential lead molecules. These targets are found only in bacteria and are pivotal for cell survival and signal transduction [PF00512; IPR001789; SM00388]. It was evident that evgS possessed better connectivity and topological scores with the highest number of interactions in both AMR (71.6%) and VG (45.8%) networks. With an intent to obstruct both AMR and virulence network through a single drug-target, common genes from both networks were screened. Based on experimental evidence, the interactome profile of 15 common genes revealed four AMR and VGs (evgS, phoP, cpxR and cpxA) that interacted strongly responding towards two component system (TCS) [sfl02020] and CAMP resistance [sfl01503] mechanisms (Fig. 1e). However, in absence of evgS, the interaction amongst these genes was interrupted indicating the importance of evgS in bridging both AMR and VG networks (Fig. 1f). Therefore, evgS was selected as the hub gene over MacB. Our results were further strengthened by examining DGE profiles to correlate the regulation patterns of various TCS genes alongside several key genes in the strain.
Scrutinising gene expression profiles
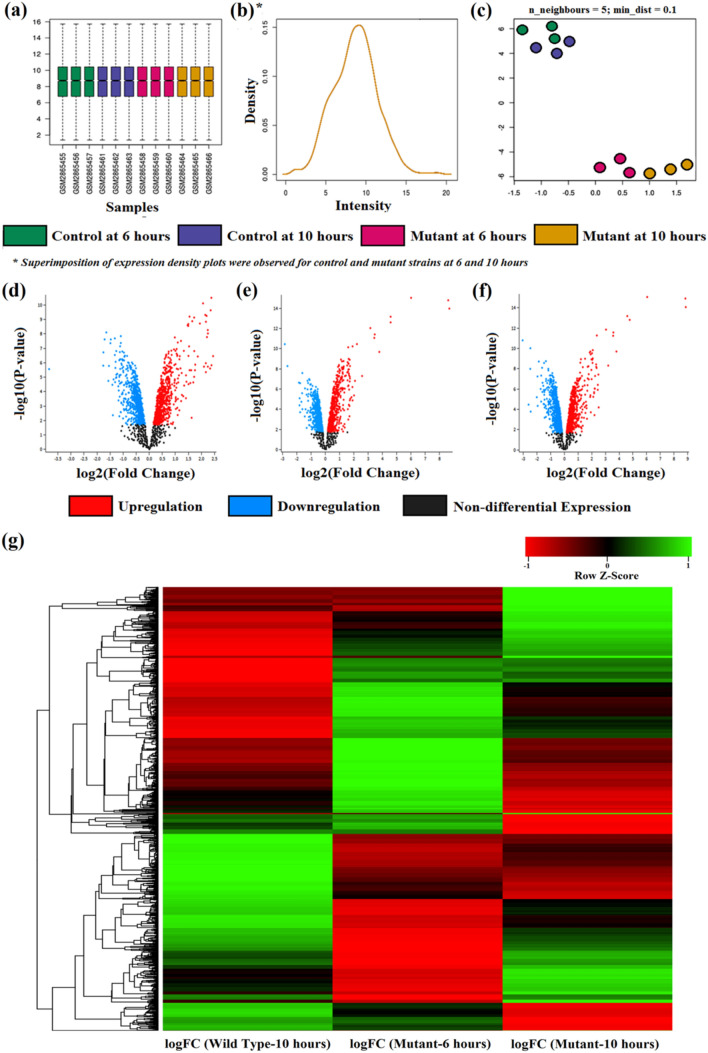
The TCS comprising hybrid sensory histidine kinase (EvgS) phosphorylates response regulator (EvgA) [sfl02020] plays significant roles in phosphorelay signal transduction system [GO:0000160; GO:0000155] and transcriptional regulation [GO:0006355]. Another important TCS comprising PhoP and PhoQ, plays important roles in cellular response upon Mg2+ starvation and acid resistance mechanisms (Utsumi 2017). Literary evidence suggests strong cross-talks between these two TCS systems indicating interdependency on each other (Eguchi et al. 2004). Therefore, the role of EvgS in regulating TCS of S. flexneri was analysed in mutated PhoPQ (ΔPhoPQ) strain upon analysing the microarray profiles of GSE107365. The differential expression of ΔPhoPQ strain was studied at mid-log phase (6 h) and early stationary phase (10 h) for both wild-type (WT) and mutant (MT) strains. Finally, the DGE was performed by comparing each dataset of WT at10h (WT-10), MT at 6 h (MT-6) and 10 h (MT-10) individually with the WT at 6 h (WT-6).
The qualitative assessments of the expression data through the boxplot analysis revealed a uniform median-centred value which is an indicative attribute describing the nature of samples taken into consideration (Fig. 2a). The normalised samples depicted in the boxplot allowed cross comparison across the four different study groups viz., WT-6, WT-10, MT-6 and MT-10. Microarray data are prone to technical variations rather than biological variations; hence normalisation of samples is rather obligatory (Leung and Cavalieri 2003). However, uniformity of the samples in the study groups depicted that the sample is normalised and does not require further post processing like force normalisation procedures. The expression density plots further validated the boxplot as all the four profiles were found to be superimposed indicating the data to be normalised and cross-comparable for further analysis (Fig. 2b). The discrete clustering in Uniform Manifold Approximation and Projection curve revealed notable differential expression patterns of MTs from WTs datasets under ΔPhoPQ criterion (Fig. 2c). The DGE profile of WT-10 (Fig. 2d), MT-6 (Fig. 2e) and MT-10 (Fig. 2f) versus WT-6 were projected through the volcano-plots. These volcano-plots represented the upregulation (red coloured dots) and downregulation (blue coloured dots) profiles of WT and MTs upon considering statistically significant log10P values (x-axis) versus the magnitude of expression expressed as logFC values. WT-10 profile highlighted 2168 upregulated genes and 2015 genes were downregulated. However, both the volcano plot concerning the MT profiles (MT-6 and MT-10) appeared similar as MT-6 and MT-10 profiles depicted 2385 and 2189 genes as upregulated while 1799 and 1995 genes were found down-regulated, respectively [Supplementary Information SI 3(a–d)]. For a better interpretation of the DGE patterns of the studied genes, a hierarchical-clustering heatmap was constructed as displayed in Fig. 2g. The DGE pattern from the heatmap further validated the results of the volcano plots by giving a detailed outlook of the differential regulation pattern of the two mutant states (at 6 h and 10 h respectively) when compared with WT at 6 h individually. The heatmap encompassed the logFC values, where the genes with non-differential expression are displayed as black coloured bands while the upregulated and downregulated genes are displayed as green and red coloured bands, respectively.
Fig. 2.
Differential Expression of Genes: a Boxplot analysis. b Expression density plots. c Uniform Manifold Approximation and Projection (UMAP) curve. d Volcano Plot of WT-10 vs. WT-6. e Volcano Plot of MT-6 vs. WT-6. f Volcano Plot of MT-10 vs. WT-6. g Hierarchical clustering heatmap of logFC values depicting gene regulation profiles of WT-10, MT-6 and MT-10 vs. WT-6 respectively
VGs are part of bacterial operon systems solely responsible for host–pathogen interactions. The transcriptional activator virB,F and outer membrane protein IcsA autotransporter gene (virG or icsA) were highly down-regulated in the study. The virF activates virB, which in turn stimulates the expression of structural genes (Sakai et al. 1986; Tobe et al. 1993). The chromosomally encoded DNA-binding protein H-NS plays significant roles in thermal control and brings a negative regulation in the expression of several VFs (Tobe et al. 1991; Morona et al. 1995a, b; Tobe 2008). Experimental evidences portrayed that ΔH-NS strains resulted in increased expression of mxiC and icsB under varied different thermo-osmolarity fluctuations, while the WT restricted their expression levels (Hromockyj et al. 1992; Porter and Dorman 1994). In the current study, expression of hns under ΔPhoPQ influence showed notable up-regulation in both mid-log and stationary phases when compared with the control. As a result, significant decline in invasion, spread and secretory VGs (ipaB,D [sfl05100], mxiA,C,D,E,H,I,J,M, ipaH_1,4) were noted signifying hindrance in shigellosis [sfl05131]. The differential expression of key VGs is graphically represented in Supplementary Information SI 3(e). A total of 12 AMR and seven VGs contributed to Daptomycin resistance via global cell adaptive response against Cationic Anti-Microbial Polypeptides (CAMP) molecules [sfl01503] (Munita and Arias 2016). The transcriptional regulator MxiE in conjugation with Osp proteins induces negative regulation in humans by dodging the innate defence system against the bacterium entry. MxiE suppresses the expression of CAMP molecules thereby preventing bacterial cell lysis (Hase et al. 2002; Sperandio et al. 2008). However, upon PhoPQ suppression significant down-regulation of MxiE and Osp proteins can be achieved which can curb down both CAMP and Daptomycin resistance.
Apart from the invasion genes ipaA,C [sfl05100], other invasions (ipaB,D) and secretory VGs (spaL,N,Q,R,S) were found suppressed in the current study. Mxi-Spa secretion system harbours IpaA,B,C,D, which are expressed upon host-cell contact. The effectors (OspF,G) target several mitogen-activated protein kinases (MAPK) and NF-κB signalling pathways in humans (Arbibe et al. 2007). Transcriptional regulator mxiE and ospB,C2,D1,D3,F,G genes were highly down-regulated signifying suppression in pathogenesis [GO:0009405] under ΔPhoPQ conditions. The expression levels of TCS containing cpxAR was found to be downregulated while evgS and evgA were found upregulated in the log phase of the mutants that normalised as the bacterium approached stationary phase (10 h). Though several reports have established the PhoP as an effective drug-target, resistance against polymyxin B and antimicrobial peptides have already been encountered in Salmonella enterica (Shi et al. 2004). Therefore, there is a need of alternate therapeutic approach to counteract both AMR and virulence mechanisms.
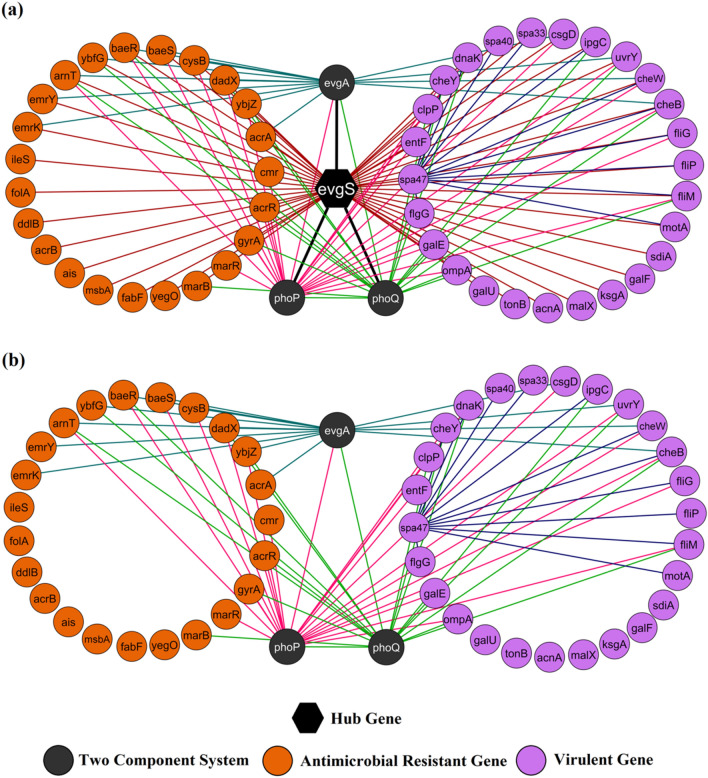
Thus, GIN and DGE analyses have highlighted the role of evgS in both AMR and virulence network. Since the functional interactors of evgS were directly associated with several resistance and virulence mechanisms, interaction profile of evgS with its 52 functional partners (23 AMR genes, 26 VGs, 4 TCS genes) were further analysed. A total of 119 interactions were noted with clustering coefficient and average neighbours being 0.576 and 4.5, respectively. Histidine kinase evgS displayed dense interconnections with the highest connectivity of 98.07% followed by PhoP and PhoQ with 47.17% and 37.73% connectivity, respectively (Fig. 3a). However, in absence of evgS, a notable reduction in interactions (42.85%), network density (~ 24%) and average neighbours (18.14%) were observed indicating sparse interrelationships with most of its direct interactors (Fig. 3b). The current study portrays the diverse molecular cross-talks of evgS with its direct interactors and phoP,Q connecting both AMR and VG network. The study speculates the hindrance in phoP,Q activity upon targeting evgS which can subsequently result in downregulation several VGs as evident from the DGE analysis (Sen et al. 2017). Therefore, EvgS (expressed evgS) can be hypothesised as a potential biomarker from its expression profiles in both WT and MT strains whose druggability needs to be established upon structural analysis and molecular dynamics.
Fig. 3.
Interaction profiles of evgS and its functional partners connecting AMR and VG networks: a evgS displaying maximum number of direct interactions in both AMR and virulence networks. b Sparse interactions amongst functional partners from AMR and virulence networks in absence of evgS
Structural analysis and validation of EvgS as potential drug-target
Unavailability of the complete 3D conformer of EvgS prompted us for in-silico structure characterization. Subsequent validations were crucial to minimize the minimal local and gross errors in protein folding and conformations. BLASTp analysis of EvgS revealed high sequence similarity (99.92%) with acid-sensing system histidine kinase EvgS of multiple species of Enterobacteriaceae family (WP_011069458.1). Therefore, in the present study, in-silico structural validations have been carried out to infer the druggability and stability of EvgS. BLASTp search against RCSB-PDB highlighted two crystallised structures of extracellular domain (4Q0C) and signal transduction domain (6QRJ). However, sequence similarity as per local alignment (https://www.ebi.ac.uk/Tools/psa/emboss_water/) was too low, being 45.2% (residue: 13–577) and 38.2% (residue: 716–1072), respectively. Global alignment (https://www.ebi.ac.uk/Tools/psa/emboss_needle/) of EvgS sequence from UniProt (A0A0H2VW50) revealed 100% identity with S. flexneri 2a (Accession: WP_011110608.1), thus validating the sequence before structural analysis. Extended dual-step modeling as previously described (Basu et al. 2021) was adopted encompassing homology, threading, iterative and molecular dynamics constraints using Swiss-Model (https://swissmodel.expasy.org/), I-Tasser (https://zhanggroup.org/I-TASSER/), Phyre2 (http://www.sbg.bio.ic.ac.uk/phyre2/) and MODELLER 10.0 platforms, thus generating structures comparable to NMR spectroscopy (Schwede 2003; Adzhubei et al. 2013; Kelley et al. 2015; Yang and Zhang 2015; Webb and Sali 2016). The modeled structure was further refined, resulting in 98.6% residues in Ramachandran favoured regions, with low RMSD (0.28 Å) and poor rotamers (0.8). The modeled structure thus portrayed improved conformational integrity with nominal erroneous zones in the protein. The validated structure of EvgS was submitted to Protein Model Database (PMDB; http://srv00.recas.ba.infn.it/PMDB/) having PMDB-ID PM0084192 for easy accessibility to other users for further experimentation analysis (Fig. 4a).
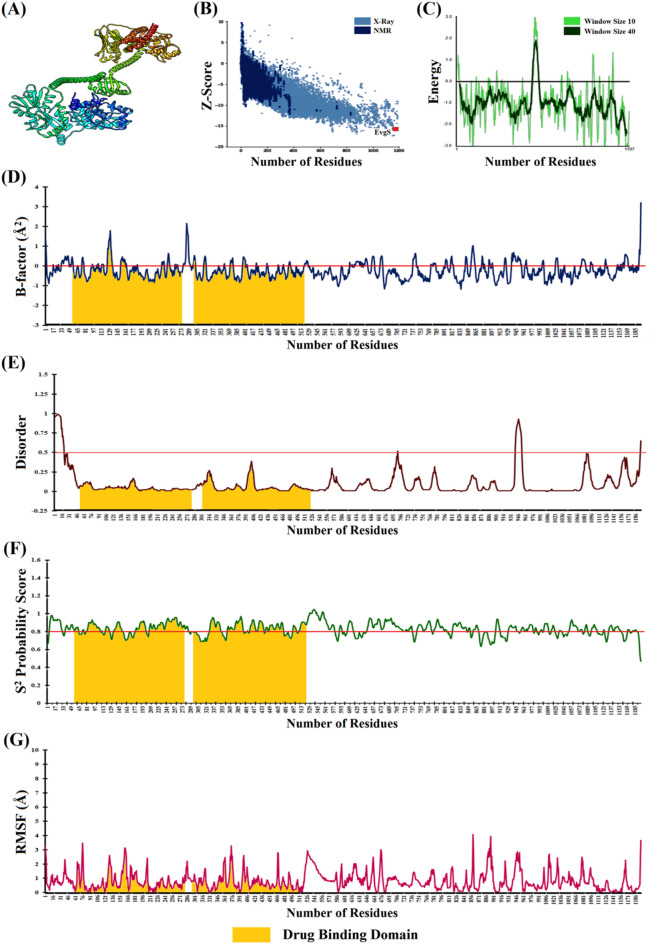
Fig. 4.
Structural Analysis of EvgS: a Optimised modelled structure of EvgS. b Global model quality of EvgS. c Local model quality of EvgS. d Atomic-level fluctuations (Normalised B-factor) in EvgS. e Extent of disorderness in EvgS. f Backbone stability curve of EvgS. g Residue-level fluctuation profile of EvgS
Secondary structural analysis revealed 38.42% of EvgS being exposed and 61.57% was buried. EvgS primarily composed of α-helix (48.37%), followed by extended-strand (14.87%), coils (32.58%) and β-sheets (4.18%). The Global Z-score from ProSA-web indicated the global model quality upon estimating the deviation in energy distribution of the stable modeled structure with random structural conformers. The Z-scores of EvgS (-15.64) implied that the tertiary structure was well poised amongst the experimentally determined proteins indicating minimal global errors in the protein structure (Fig. 4b). The local energetics revealed the local quality of the protein upon tagging energy functions with each amino acid residues. The energy plot of overall EvgS and its functional domains laid well below the threshold cut-off (0.0) signifying proper folding with marginal erroneous conformations (Fig. 4c). The inherent thermal mobility estimated from B-factor curve revealed minimal atomic-level fluctuations of EvgS (average ~ -0.23Å2) due to positional disorders and thermal motions thereby establishing its thermostability profiles (Parthasarathy and Murthy 2000) (Fig. 4d). The native disorders and dynamicity in the functional domains of EvgS were further estimated through the DISOPRED3 server. The PSI-BLAST profile indicated that the probability of disorderness of the entire EvgS (average ~ 0.09) was below the threshold cut-off of 0.5 indicating minimal positional disorders in the functional domains (Fig. 4e). The backbone dynamicity of EvgS elucidated from the N–H S2 bond-order restraints revealed a rigid backbone conformation (average S2 = 0.83). The reduced flexibility of the evolutionarily conserved EvgS highlighted its uniform functional attributes and its potency in formation of macromolecular scaffolds related to TCS (Fig. 4f). The rigid dynamicity profile was well complemented from the minimal residue-level fluctuations (average RMSF = 0.81 Å) upon coarse-grained dynamics indicating the stability of the entire protein (Fig. 4g).
Structure–Function relationship of EvgS
The functionality of several EvgS domains was studied, and their dynamicity was characterised by the atomic and residue-level fluctuations. The N-terminal of EvgS comprised of a signal peptide domain (residue: 1–25), followed by the extracellular domain (residue: 26–535), transmembrane helix (residue: 536–557) and the His-kinase C-terminal cytoplasmic domain (residue: 558–1197) which was involved in signal transduction [PF00512; IPR001789; SM00388]. The extracellular domain played effective roles as the substrate and solute/drug binding site (residue: 57–279 and 303–522) [IPR001638; PF00497; SM00062]. The active-site pocket (area: ~ 700Å2; volume: 868.18Å3; depth: 21.61 Å) at residue 307–487 revealed a high drug-binding score (0.84). Therefore, the study hypothesised the extracellular domain as an effective drug-binding site, which can be explored for designing novel leads for effective inhibition of the protein. The backbone flexibility curve displayed relative rigidity for both solute binding domains (average S2: 0.84 and 0.83, respectively) defining their potency as scaffolds for macromolecular complex formation pertaining to TCS. Simultaneously, coarse dynamics simulations unveiled low residue-level fluctuations (RMSF: 0.75 Å and 0.72 Å, respectively) for both the drug-binding domains. The uniform functionality of the evolutionarily conserved EvgS (sequence similarity: 99.92%) across the Enterobacteriaceae family [WP_011069458.1] comprehended the less dynamic (rigid) nature of the interactive domains. Histidine kinase EvgS displayed minimal gross and local folding errors while establishing stable backbone and coarse dynamics profiles. Thereafter to investigate its stability in dynamic conditions, the modeled EvgS was subjected to MDS analysis.
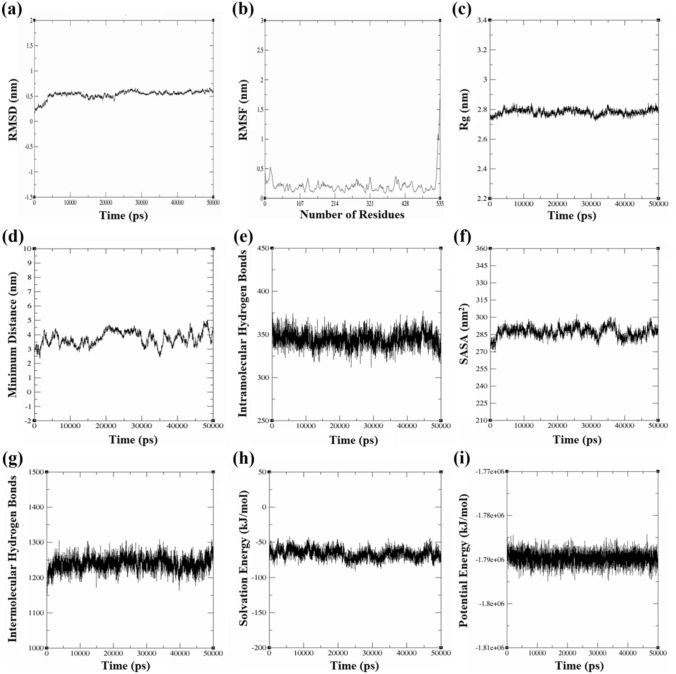
The instability and aliphatic indices of the extracellular drug-binding region (residue: 1–535) was 38.27 (< 40.0) and 95.87, respectively, that implied thermal stability and well-packed protein core (Ikai 1980). Upon MDS analysis, the RMSD trajectory attained equilibrium after initial 5000 ps at 0.53 ± 0.07 nm which was maintained throughout indicating minimal mean deviations concerning protein backbone (Fig. 5a). The RMSF values signified low residual level fluctuations (~ 0.21 ± 0.1 nm) in the drug-binding domain (Fig. 5b). Compactness in the folding patterns of secondary structure into tertiary conformations was established by the steady radius of gyration (Rg) trajectory (~ 2.78 ± 0.01 nm) further strengthening the ProtParam findings (Fig. 5c) (Azam et al. 2012). The conformational coherence between proximal backbone residues was measured to be 3.74 ± 0.4 nm (> 2.0 nm), indicating negligible steric hindrance and stereochemically favourable tertiary structure (Fig. 5d). About 350 intramolecular hydrogen bonds were formed amongst the protein residues that were maintained throughout the timeframe (Fig. 5e). SASA trajectory (287.21 ± 4.5nm2) and persistent intermolecular hydrogen bonds (~ 1240) between domain and solvent molecules signified a uniform layer of solvation (Fig. 5f, g). The free energy of solvation (-66.31 ± 7.2 kJ/mol) plot further confirmed the uniform solvated system (Fig. 5h). The potential energy fluctuations revealed the domain as energetically favourable with mean stability at -1.8e + 06 kJ/mol (Fig. 5i) (Hu et al. 2011; Jayaraman et al. 2019; Basu et al. 2021; Miryala et al. 2021b; Kushwaha et al. 2021). The MDS analysis thus validated the overall stability profile of the extracellular drug-binding domain of EvgS. Further, this stability profile would serve as the standard for evaluating the relative deviations and dynamicity of the EvgS-inhibitor complexes in future studies.
Fig. 5.
MDS analysis of extracellular drug binding domain of EvgS: a RMSD curve. b Residue level RMSF plot. c Rg trajectory. d Minimum distance amongst backbone residues. e Number of intramolecular (protein–protein) hydrogen bonds. f SASA profile. g Number of intermolecular (protein-solvent) hydrogen bonds. h Free energy of solvation. i Potential energy curve
Bacterial TCS proteins being crucial for cell survival mechanisms serves as good therapeutic targets as certain TCS are responsible for regulating the expression of AMR determinants. Moreover, TCS proteins are specific to bacterial origin and studies have reported distinct difference in their phosphorylation patterns from higher eukaryotes in signal transduction mechanisms. Therefore, they can be considered as potent drug-targets due to high structural resemblances of the catalytic domain of histidine kinases across multiple TCS in the same bacterium and also within the same Enterobacteriaceae family (Macielag and Goldschmidt 2000). A few reports suggest the inhibitory action of Imidazolium analogues and salts against structurally similar histidine kinases (EvgS, PhoQ, BvgS and EnvZ) in vancomycin-resistant Enterococcus, penicillin-resistant Streptococcus pneumoniae and oxacillin-resistant Staphylococcus aureus (Roychoudhury et al. 1993; Worthington et al. 2013). However, the stability profile of these salts with TCS protein complexes and the inhibitory action of Imidazolium derivatives against the histidine kinase EvgS particularly in Shigella is not deciphered to the best of our knowledge. Therefore, the study further contemplates the designing a novel TCS inhibitor that can effectively control the rapid upsurge in AMR and virulence posed by the strain.
Conclusion
The present study established EvgS as an alternate drug-target to circumvent both AMR and virulence displayed by the pathogenic S. flexneri 2a str. 301. GIN comprising both AMR and virulence genes highlighted evgS as crucial hub-gene with favourable topological scores and functional enrichments. Interactome profile of evgS and other common genes from AMR and VG network revealed the association with the three TCS pathways in the bacteria which could be intervened upon targeting evgS. It was further confirmed that absence of evgS led to a substantial loss of interactions in the overall AMR and virulence networks. Structural analysis further validated the druggability and conformational stability of the drug-binding domain of EvgS for designing potential inhibitors against it. This strategy can result in a conjoined hindrance to the prevailing virulence and AMR that can be subsequently validated by experimental procedures.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The Authors would like to acknowledge the Indian Council of Medical Research (ICMR), Govt. of India, (IRIS-ID: 2019-0810) for the genesis of the study. The authors gratefully acknowledge the management of Vellore Institute of Technology, Vellore for the necessary facilities to carry out this research work. AN sincerely thank ICMR for his research fellowship. The authors sincerely thank Prof. (Dr.) Anand Anbarasu, VIT-Vellore, for his invaluable intellectual inputs and immense support throughout the research work.
Author contribution
SR: Project supervision; Funding acquisition; Methodology; Draft review. AN: Data curation; in-silico experimentation and analysis; Writing- original draft.
Funding
This research was funded by the Indian Council of Medical Research (ICMR), Govt. of India, through the research grant IRIS-ID: 2020–0690.
Declarations
Conflict of interest
The authors have no competing financial or non-financial interests to declare that are relevant to the content of this article.
Contributor Information
Aniket Naha, Email: aniket.naha@vit.ac.in.
Sudha Ramaiah, Email: sudhaanand@vit.ac.in.
References
- Adzhubei AA, Sternberg MJE, Makarov AA. Polyproline-II helix in proteins: structure and function. J Mol Biol. 2013;425:2100–2132. doi: 10.1016/j.jmb.2013.03.018. [DOI] [PubMed] [Google Scholar]
- Anandan S, Muthuirulandi Sethuvel DP, Gajendiren R, et al. Molecular characterization of antimicrobial resistance in clinical Shigella isolates during 2014 and 2015: trends in South India. Germs. 2017;7:115–122. doi: 10.18683/germs.2017.1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arbibe L, Kim DW, Batsche E, et al. An injected bacterial effector targets chromatin access for transcription factor NF-κB to alter transcription of host genes involved in immune responses. Nat Immunol. 2007;8:47–56. doi: 10.1038/ni1423. [DOI] [PubMed] [Google Scholar]
- Azam SS, Uddin R, Wadood A. Structure and dynamics of alpha-glucosidase through molecular dynamics simulation studies. J Mol Liq. 2012;174:58–62. doi: 10.1016/j.molliq.2012.07.003. [DOI] [Google Scholar]
- Azmi IJ, Khajanchi BK, Akter F, et al. Fluoroquinolone resistance mechanisms of Shigella flexneri isolated in Bangladesh. PLoS ONE. 2014 doi: 10.1371/journal.pone.0102533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bader GD, Hogue CW. An automated method for finding molecular complexes in large protein interaction networks. BMC Bioinform. 2003;4:2. doi: 10.1093/nar/29.1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu S, Naha A, Veeraraghavan B, et al. In silico structure evaluation of BAG3 and elucidating its association with bacterial infections through protein-protein and host-pathogen interaction analysis. J Cell Biochem. 2021 doi: 10.1002/jcb.29953. [DOI] [PubMed] [Google Scholar]
- Campbell EA, Korzheva N, Mustaev A, et al. Structural mechanism for rifampicin inhibition of bacterial RNA polymerase. Cell. 2001;104:901–912. doi: 10.1016/S0092-8674(01)00286-0. [DOI] [PubMed] [Google Scholar]
- Chaudhary MK, Srivastava A, Singh KK, et al. Computational evaluation on molecular stability, reactivity, and drug potential of frovatriptan from DFT and molecular docking approach. Comput Theor CHem. 2020;1191:113031. doi: 10.1016/j.comptc.2020.113031. [DOI] [Google Scholar]
- Cilia E, Pancsa R, Tompa P, et al. From protein sequence to dynamics and disorder with DynaMine. Nat Commun. 2013;4:2741. doi: 10.1038/ncomms3741. [DOI] [PubMed] [Google Scholar]
- Cilia E, Pancsa R, Tompa P, et al. The DynaMine webserver: predicting protein dynamics from sequence. Nucleic Acids Res. 2014;42:264–270. doi: 10.1093/nar/gku270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debroy R, Miryala SK, Naha A, et al. Gene interaction network studies to decipher the multi-drug resistance mechanism in Salmonella enterica serovar Typhi CT18 reveal potential drug targets. MiCrob Pathog. 2020;142:104096. doi: 10.1016/j.micpath.2020.104096. [DOI] [PubMed] [Google Scholar]
- Eguchi Y, Okada T, Minagawa S, et al. Signal transduction cascade between EvgA/EvgS and PhoP/PhoQ two-component systems of Escherichia coli. J Bacteriol. 2004;186:3006–3014. doi: 10.1128/JB.186.10.3006-3014.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finn RD, Coggill P, Eberhardt RY, et al. The Pfam protein families database: towards a more sustainable future. Nucleic Acids Res. 2016;44:D279–D285. doi: 10.1093/nar/gkv1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geourjon C, Deléage G. Sopma: significant improvements in protein secondary structure prediction by consensus prediction from multiple alignments. Bioinformatics. 1995;11:681–684. doi: 10.1093/bioinformatics/11.6.681. [DOI] [PubMed] [Google Scholar]
- Ghosh A, Roymahapatra G, Paul D, Mandal SM. Theoretical analysis of bacterial efflux pumps inhibitors: strategies in-search of competent molecules and develop next. Comput Biol Chem. 2020;87:107275. doi: 10.1016/j.compbiolchem.2020.107275. [DOI] [PubMed] [Google Scholar]
- Hase K, Eckmann L, Leopard JD, et al. Cell differentiation is a key determinant of cathelicidin LL-37/human cationic antimicrobial protein 18 expression by human colon epithelium. Infect Immun. 2002;70:953–963. doi: 10.1128/IAI.70.2.953-963.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heo L, Park H, Seok C. GalaxyRefine: protein structure refinement driven by side-chain repacking. Nucleic Acids Res. 2013;41:384–388. doi: 10.1093/nar/gkt458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hromockyj AE, Tucker SC, Maurelli AT. Temperature regulation of Shigella virulence: identification of the repressor gene virR, an analogue of hns, and partial complementation by tyrosyl transfer RNA (tRNA 1 Tyr ) Mol Microbiol. 1992;6:2113–2124. doi: 10.1111/j.1365-2958.1992.tb01385.x. [DOI] [PubMed] [Google Scholar]
- Hu S-Q, Guo A-L, Yan Y-G, et al. Computer simulation of diffusion of corrosive particle in corrosion inhibitor membrane. Comput Theor Chem. 2011;964:176–181. doi: 10.1016/j.comptc.2010.12.019. [DOI] [Google Scholar]
- Hunter S, Apweiler R, Attwood TK, et al. InterPro: the integrative protein signature database. Nucleic Acids Res. 2009;37:D211–D215. doi: 10.1093/nar/gkn785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikai A. Thermostability and aliphatic index of globular proteins. J Biochem. 1980;88:1895–1898. doi: 10.1093/oxfordjournals.jbchem.a133168. [DOI] [PubMed] [Google Scholar]
- Jamroz M, Kolinski A, Kmiecik S. CABS-flex: server for fast simulation of protein structure fluctuations. Nucleic Acids Res. 2013;41:427–431. doi: 10.1093/nar/gkt332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayaraman M, Rajendra SK, Ramadas K. Structural insight into conformational dynamics of non-active site mutations in KasA: a Mycobacterium tuberculosis target protein. Gene. 2019;720:144082. doi: 10.1016/j.gene.2019.144082. [DOI] [PubMed] [Google Scholar]
- Jerbi J, Springborg M. Reactivity descriptors for DNA bases and the methylation of cytosine. Int J Quantum Chem. 2018 doi: 10.1002/qua.25538. [DOI] [Google Scholar]
- Kaplan W, Littlejohn TG. Swiss-PDB viewer (deep view) Brief BIoinform. 2001;2:195–197. doi: 10.1093/bib/2.2.195. [DOI] [PubMed] [Google Scholar]
- Kelley LA, Mezulis S, Yates CM, et al. The Phyre2 web portal for protein modeling, prediction and analysis. Nat Protoc. 2015;10:845–858. doi: 10.1038/nprot.2015.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khaghani S, Shamsizadeh A, Nikfar R, Hesami A. Shigella flexneri: a three-year antimicrobial resistance monitoring of isolates in a Children Hospital, Ahvaz. Iran Iran J Microbiol. 2014;6:225–229. [PMC free article] [PubMed] [Google Scholar]
- Kushwaha PP, Singh AK, Bansal T, et al. Identification of natural inhibitors against SARS-CoV-2 drugable targets using molecular docking, molecular dynamics simulation, and MM-PBSA approach. Front Cell Infect Microbiol. 2021 doi: 10.3389/fcimb.2021.730288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemkul J. From proteins to perturbed hamiltonians: a suite of tutorials for the GROMACS-2018 molecular simulation package [Article v1.0] Living J Comput Mol Sci. 2019;1:53. doi: 10.33011/livecoms.1.1.5068. [DOI] [Google Scholar]
- Leung YF, Cavalieri D. Fundamentals of cDNA microarray data analysis. Trends Genet. 2003;19:649–659. doi: 10.1016/j.tig.2003.09.015. [DOI] [PubMed] [Google Scholar]
- Lin Z, Cai X, Chen M, et al. Virulence and stress responses of Shigella flexneri regulated by PhoP/PhoQ. Front Microbiol. 2018 doi: 10.3389/fmicb.2017.02689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livio S, Strockbine NA, Panchalingam S, et al. Shigella isolates from the global enteric multicenter study inform vaccine development. Clin Infect Dis. 2014;59:933–941. doi: 10.1093/cid/ciu468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macielag MJ, Goldschmidt R. Inhibitors of bacterial two-component signalling systems. Expert Opin Investig Drugs. 2000;9:2351–2369. doi: 10.1517/13543784.9.10.2351. [DOI] [PubMed] [Google Scholar]
- Mao Y, Cui E, Bao C, et al. Changing trends and serotype distribution of Shigella species in Beijing from 1994 to 2010. Gut Pathog. 2013;5:12. doi: 10.1016/S0966-842X(99)01646-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez-Jiménez F, Papadatos G, Yang L, et al. Target prediction for an open access set of compounds active against Mycobacterium tuberculosis. PLoS Comput Biol. 2013;9:e1003253. doi: 10.1371/journal.pcbi.1003253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez-Vázquez MA, Vázquez-Elizondo G, González-González JA, et al. Effect of antispasmodic agents, alone or in combination, in the treatment of Irritable Bowel Syndrome: systematic review and meta-analysis. Rev Gastroenterol México. 2012;77:82–90. doi: 10.1016/j.rgmx.2012.04.002. [DOI] [PubMed] [Google Scholar]
- McGuffin LJ, Bryson K, Jones DT. The PSIPRED protein structure prediction server. Bioinformatics. 2000;16:404–405. doi: 10.1093/bioinformatics/16.4.404. [DOI] [PubMed] [Google Scholar]
- Miryala SK, Ramaiah S. Exploring the multi-drug resistance in Escherichia coli O157:H7 by gene interaction network: a systems biology approach. Genomics. 2019;111:958–965. doi: 10.1016/j.ygeno.2018.06.002. [DOI] [PubMed] [Google Scholar]
- Miryala SK, Ramaiah S. Cellular and molecular level host-pathogen interactions in Francisella tularensis: a microbial gene network study. Comput Biol Chem. 2022 doi: 10.1016/j.compbiolchem.2021.107601. [DOI] [PubMed] [Google Scholar]
- Miryala SK, Anbarasu A, Ramaiah S. Discerning molecular interactions: a comprehensive review on biomolecular interaction databases and network analysis tools. Gene. 2018;642:84–94. doi: 10.1016/j.gene.2017.11.028. [DOI] [PubMed] [Google Scholar]
- Miryala SK, Anbarasu A, Ramaiah S. Impact of bedaquiline and capreomycin on the gene expression patterns of multidrug-resistant Mycobacterium tuberculosis H37Rv strain and understanding the molecular mechanism of antibiotic resistance. J Cell Biochem. 2019 doi: 10.1002/jcb.28711. [DOI] [PubMed] [Google Scholar]
- Miryala SK, Anbarasu A, Ramaiah S. Gene interaction network to unravel the role of gut bacterial species in cardiovascular diseases: E. coli O157:H7 host-bacterial interaction study. Comput Biol Med. 2021 doi: 10.1016/j.compbiomed.2021.104417. [DOI] [PubMed] [Google Scholar]
- Miryala SK, Basu S, Naha A, et al. Identification of bioactive natural compounds as efficient inhibitors against Mycobacterium tuberculosis protein-targets: a molecular docking and molecular dynamics simulation study. J Mol Liq. 2021 doi: 10.1016/j.molliq.2021.117340. [DOI] [Google Scholar]
- Morona R, van den Bosch LUISA, Manning PA. Molecular, genetic, and topological characterization of O-antigen chain length regulation in Shigella flexneri. J Bacteriol. 1995;177:1059–1068. doi: 10.1128/jb.177.4.1059-1068.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morona R, Macpherson DF, Van Den Bosch L, et al. Lipopolysaccharide with an altered O-antigen produced in Escherichia coli K-12 harbouring mutated, cloned Shigella flexneri rfb genes. Mol Microbiol. 1995;18:209–223. doi: 10.1111/j.1365-2958.1995.mmi_18020209.x. [DOI] [PubMed] [Google Scholar]
- Munita JM, Arias CA. Mechanisms of antibiotic resistance. Microbiol Spectr. 2016 doi: 10.1128/microbiolspec.VMBF-0016-2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naha A, Kumar Miryala S, Debroy R, et al. Elucidating the multi-drug resistance mechanism of Enterococcus faecalis V583: a gene interaction network analysis. Gene. 2020;748:144704. doi: 10.1016/J.GENE.2020.144704. [DOI] [PubMed] [Google Scholar]
- Naha A, Vijayakumar S, Lal B, Shankar BA. Genome sequencing and molecular characterisation of XDR Acinetobacter baumannii reveal complexities in resistance: novel combination of Sulbactam-Durlobactam holds promise for therapeutic intervention. J Cell Biochem. 2021 doi: 10.1002/jcb.30156. [DOI] [PubMed] [Google Scholar]
- Parthasarathy S, Murthy MRN. Protein thermal stability: insights from atomic displacement parameters (B values) Protein Eng. 2000;13:9–13. doi: 10.1093/protein/13.1.9. [DOI] [PubMed] [Google Scholar]
- Pettersen EF, Goddard TD, Huang CC, et al. UCSF Chimera-A visualization system for exploratory research and analysis. J Comput Chem. 2004;25:1605–1612. doi: 10.1002/jcc.20084. [DOI] [PubMed] [Google Scholar]
- Philippi F, Rauber D, Springborg M, Hempelmann R. Density functional theory descriptors for ionic liquids and the charge-transfer interpretation of the Haven ratio. J Phys Chem A. 2019;123:851–861. doi: 10.1021/acs.jpca.8b10827. [DOI] [PubMed] [Google Scholar]
- Poole K. Efflux-mediated resistance to fluoroquinolones in gram-negative bacteria. Antimicrob Agents Chemother. 2000;44:2233–2241. doi: 10.1128/AAC.44.9.2233-2241.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter ME, Dorman CJ. A role for H-NS in the thermo-osmotic regulation of virulence gene expression in Shigella flexneri. J Bacteriol. 1994;176:4187–4191. doi: 10.1128/jb.176.13.4187-4191.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roychoudhury S, Zielinski NA, Ninfa AJ, et al. Inhibitors of two-component signal transduction systems: inhibition of alginate gene activation in Pseudomonas aeruginosa. Proc Natl Acad Sci. 1993;90:965–969. doi: 10.1073/pnas.90.3.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai T, Sasakawa C, Makino S, Yoshikawa M. DNA sequence and product analysis of the virF locus responsible for congo red binding and cell invasion in Shigella flexneri 2a. Infect Immun. 1986;54:395–402. doi: 10.1128/iai.54.2.395-402.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz J, Copley RR, Doerks T, et al. SMART: a web-based tool for the study of genetically mobile domains. Nucleic Acids Res. 2000;28:231–234. doi: 10.1093/nar/28.1.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwede T. SWISS-MODEL: an automated protein homology-modeling server. Nucleic Acids Res. 2003;31:3381–3385. doi: 10.1093/nar/gkg520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen H, Aggarwal N, Ishionwu C, et al. Structural and functional analysis of the Escherichia coli acid-sensing histidine kinase EvgS. J Bacteriol. 2017;199:e00310-17. doi: 10.1128/JB.00310-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sethuvel DPM, Devanga Ragupathi NK, Anandan S, et al. Molecular diagnosis of non-serotypeable Shigella spp.: problems and prospects. J Med Microbiol. 2017;66:255–257. doi: 10.1099/jmm.0.000438. [DOI] [PubMed] [Google Scholar]
- Sethuvel DPM, Devanga Ragupathi NK, Anandan S, Veeraraghavan B. Update on: Shigella new serogroups/serotypes and their antimicrobial resistance. Lett Appl Microbiol. 2017;64:8–18. doi: 10.1111/lam.12690. [DOI] [PubMed] [Google Scholar]
- Shankar C, Basu S, Lal B, et al. Aerobactin, seems to be a promising marker compared to unstable RmpA2 for the identification of hypervirulent carbapenem-resistant Klebsiella pneumoniae: in-silico and in-vitro evidence. Front Cell Infect Microbiol. 2021 doi: 10.3389/fcimb.2021.709681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y, Cromie MJ, Hsu F-F, et al. PhoP-regulated Salmonella resistance to the antimicrobial peptides magainin 2 and polymyxin B. Mol Microbiol. 2004;53:229–241. doi: 10.1111/j.1365-2958.2004.04107.x. [DOI] [PubMed] [Google Scholar]
- Smoot ME, Ono K, Ruscheinski J, et al. Cytoscape 2.8: new features for data integration and network visualization. Bioinformatics. 2011;27:431–432. doi: 10.1093/bioinformatics/btq675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperandio B, Regnault B, Guo J, et al. Virulent Shigella flexneri subverts the host innate immune response through manipulation of antimicrobial peptide gene expression. J Exp Med. 2008;205:1121–1132. doi: 10.1084/jem.20071698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stock I, Wiedemann B. Natural antibiotic susceptibility of Escherichia coli, Shigella, E. vulneris, and E. hermannii strains. Diagn Microbiol Infect Dis. 1999;33:187–199. doi: 10.1016/S0732-8893(98)00146-1. [DOI] [PubMed] [Google Scholar]
- Sur D, Ramamurthy T, Deen J, Bhattacharya SK. Shigellosis: challenges & management issues. Indian J Med Res. 2004;120:45–54. [PubMed] [Google Scholar]
- Szklarczyk D, Morris JH, Cook H, et al. The STRING database in 2017: quality-controlled protein–protein association networks, made broadly accessible. Nucleic Acids Res. 2017;45:D362–D368. doi: 10.1093/nar/gkw937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobe T. The roles of two-component systems in virulence of pathogenic Escherichia coli and Shigella spp. Adv Exp Med Biol. 2008;631:189–199. doi: 10.1007/978-0-387-78885-2_13. [DOI] [PubMed] [Google Scholar]
- Tobe T, Nagai S, Okada N, et al. Temperature-regulated expression of invasion genes in Shigella flexneri is controlled through the transcriptional activation of the virB gene on the large plasmid. Mol Microbiol. 1991;5:887–893. doi: 10.1111/j.1365-2958.1991.tb00762.x. [DOI] [PubMed] [Google Scholar]
- Tobe T, Yoshikawa M, Mizuno T, Sasakawa C. Transcriptional control of the invasion regulatory gene virB of Shigella flexneri: activation by virF and repression by H-NS. J Bacteriol. 1993;175:6142–6149. doi: 10.1128/jb.175.19.6142-6149.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Utsumi R. Bacterial signal transduction networks via connectors and development of the inhibitors as alternative antibiotics. Biosci Biotechnol Biochem. 2017;81:1663–1669. doi: 10.1080/09168451.2017.1350565. [DOI] [PubMed] [Google Scholar]
- Vasudevan K, Basu S, Arumugam A, et al. Identification of potential carboxylic acid-containing drug candidate to design novel competitive NDM inhibitors: an in-silico approach comprising combined virtual screening and molecular dynamics simulation. Res Prepr. 2021 doi: 10.21203/rs.3.rs-784343/v1. [DOI] [Google Scholar]
- Volkamer A, Kuhn D, Rippmann F, Rarey M. Dogsitescorer: a web server for automatic binding site prediction, analysis and druggability assessment. Bioinformatics. 2012;28:2074–2075. doi: 10.1093/bioinformatics/bts310. [DOI] [PubMed] [Google Scholar]
- Webb B, Sali A. Comparative protein structure modeling using modeller. Curr Protoc Bioinform. 2016;54:5–6. doi: 10.1002/cpbi.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiederstein M, Sippl MJ. ProSA-web: Interactive web service for the recognition of errors in three-dimensional structures of proteins. Nucleic Acids Res. 2007;35:407–410. doi: 10.1093/nar/gkm290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worthington RJ, Blackledge MS, Melander C. Small-molecule inhibition of bacterial two-component systems to combat antibiotic resistance and virulence. Future Med Chem. 2013;5:1265–1284. doi: 10.4155/fmc.13.58. [DOI] [PubMed] [Google Scholar]
- Yang J, Zhang Y. I-TASSER server: new development for protein structure and function predictions. Nucleic Acids Res. 2015;43:W174–W181. doi: 10.1093/nar/gkv342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng J, Zhang J, Gong X. Molecular dynamics simulation of interaction between benzotriazoles and cuprous oxide crystal. Comput Theor Chem. 2011;963:110–114. doi: 10.1016/j.comptc.2010.10.006. [DOI] [Google Scholar]
- Zheng H, Wu F, Wang B, Wu Y. Molecular dynamics simulation on the interfacial features of phenol extraction by TBP/dodecane in water. Comput Theor Chem. 2011;970:66–72. doi: 10.1016/j.comptc.2011.05.028. [DOI] [Google Scholar]
- Zhu Z, Zhou X, Li B, et al. Genomic analysis and resistance mechanisms in Shigella flexneri 2a strain 301. Microb Drug Resist. 2018;24:323–336. doi: 10.1089/mdr.2016.0173. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.