Abstract
Capsicum spp. fruits (CFs) are a basic ingredient in the diet and have been used as active ingredients in the pharmaceutical, cosmetic, and food products, due to their antioxidant, anti-inflammatory, antiseptic, and antimicrobial properties. The antimicrobial activity is the most studied property due to its effectiveness against pathogenic species, however, few studies have focused on the mechanisms of action involved. Therefore, this review discusses the effects generated by the CFs compounds on the viability and metabolism of microorganisms, highlighting the mechanisms by which these compounds exert their antimicrobial effects. The information provided shows that CFs are mainly source of capsaicinoids and phenolic compounds responsible for the inhibition of bacteria, yeasts, and fungi, through an increase in the permeabilization of the membrane and cell wall. Also, these compounds show an antiviral effect associated with the inactivation of virus binding proteins, preventing their replication and infection. Despite this, there is still a lack of information about the mechanisms that regulate the interactions between CFs compounds and food-important-microorganisms. Therefore, future research should focus on new antimicrobial compounds from CFs for their subsequent use against novel infectious agents, mainly virus of importance in health such as SARS-CoV-2.
Keywords: Capsaicin, Hydroxycinnamic acid, Lactic acid bacteria, Yeast, Virus
Introduction
The genus Capsicum belongs to the Solanaceae family and includes approximately 35 species differentiated by the size of the plant and its fruits (Olatunji and Afolayan 2019). Capsicum spp., commonly named chili pepper or ají, has been present since ancient times in many cultures. Despite their popularity, only five species (Capsicum baccatum, Capsicum chinense, Capsicum frutescens, Capsicum pubescens, and Capsicum annuum), have been domesticated and cultivated; being C. annuum the one with highest consumption and the most economically important for food industry (Rudrapal and Sarwa 2020).
Capsicum spp. fruits (CFs) are the second most consumed vegetables worldwide, whose gastronomic importance lies in their versatility to be consumed either fresh, dried, fermented, or as a spice. For the Latin American countries, the CFs are a basic ingredient in their daily diet (Melgar-Lalanne et al. 2017). Additionally, industries such as pharmaceutical and cosmetic have included bioactive compounds from CFs in their products. For example, capsaicin, a compound responsible for the pungency of CFs, is used as additive in topic products (gel, patches, and creams) to relieve muscle and joint pain, due to its anti-inflammatory activity. Also, this compound is used in hair treatments against alopecia, as well as raw material for obtaining colorants and resins for industrial purposes. Nevertheless, additional properties have been reported not only for capsaicinoids but also for other compounds present in CFs, such as peptides and phenolics compounds. These properties include weight loss, body thermoregulation, antioxidant, and antimicrobial properties (Adaszek et al. 2019; Baenas et al. 2019). The latter has been widely studied and has gained interest due to the growing reports where CFs are used as natural antimicrobials against pathogenic microorganisms, however, there are few studies about the mechanisms triggered by the interaction between CFs compounds and microorganisms. Therefore, the present review aims to discuss the antimicrobial effects of CFs, emphasizing the mechanisms of action of their bioactive compounds on the metabolism of bacteria, fungi, and viruses, as well as addressing the applications of CFs as functional ingredients within the food industry.
Applications of Capsicum fruits in food industry products
Consumers are currently showing greater interest in healthier and more innovative foods, whose ingredients promote well-being in general, including disease prevention (Guiné et al. 2020). Regarding these demands, the food industry has opted for the use of compounds of plant origin, which, in addition to serving as additives to generate color, aroma, and flavor, present bioactivities that can result in the generation of new functional foods with positive effects on human health. In this sense, the CFs have been an important source of compounds such as capsaicinoids, carotenoids, polyphenols, tannins, flavonoids, and vitamin C and A, used in the food industry either as preservatives, additives, or technological ingredients with antioxidant, anti-inflammatory, and antimicrobial activities (Baenas et al. 2019).
Capsaicin, the most known and used bioactive compound extracted from CFs, is commercially available as a liquid oleoresin, which contains, in addition to capsaicinoids, other natural antioxidants. Example of this is an oleoresin obtained from the habanero pepper (C. chinense), used by the food industry to grant sensory and functional attributes to newly formulated products in the market where it is added. Also, this oleoresin has been used as a coloring and flavoring agent in a wide number of processed food products that include cheeses, meats, mortadella, sausages, soups, sauces, snacks, condiments, sweets, and even alcoholic beverages (Domínguez-Cañedo et al. 2015).
On the other hand, Capsicum flours have been recently used as complement for fruit and vegetable flours intended for breading or seasoning different foods, providing them with flavor and color, as well as improve their stability and antioxidant capacity (de Sá Mendes et al. 2019). For example, Kaur et al. (2020), have reported that the substitution of 10% (w/w) of conventional wheat flour for powdered red pepper during the baking process, improves the nutritional value, bioactive properties, and appearance characteristics of the bakery products. Similarly, other types of substitutions have allowed the obtention of different presentations such as pizza doughs, croutons, rolls, and tortillas (Maldonado et al. 2018). Thus, Capsicum flour can be considered a potential ingredient with functional properties given by the type of bioactive compounds it contains, which increase both the sensory and nutritional value of the final product (de Sá Mendes et al. 2020).
Additionally, bioactive compounds from CFs such as carotenoids and capsaicinoids have also been extracted and used as additives in the food industry, mainly to generate color and pungency in food products, respectively. Carotenoids of CFs include mainly capsanthin and capsoroubin, as well as β-carotene, β-cryptoxanthin, lutein, zeaxanthin, antheraxanthin, and violaxanthin, which are recognized for acting as antioxidant, anticancer, anti-inflammatory, and antiallergic agents. In the food industry, carotenoids are intended to be used as natural colorants, to avoid the incidence of digestive problems, allergies, insomnia, hyperactivity, and cancer in humans, related to the use of synthetic colorants (Baenas et al. 2019).
Moreover, capsaicin (8-methyl-N-vanillyl-trans-6-nonenamide) and dihydrocapsaicin (N-(4-hydroxy-3-methoxybenzyl)-8-methylnonenamide) are used as ingredients in the food industry to give flavor and the CFs characteristic aroma. The meat industry is the one that makes use of these capsaicinoids the most, due to both their ability to confer the characteristic sensory profile related to the spice foods and their antimicrobial properties reported against Gram-negatives and Gram-positives bacteria, which justifies the wide use of these bioactive compounds in the manufacture of meat pastes such as sausages and chorizo (Alsebaeai et al. 2020).
Based on the above, recent research has focused on the use of extracts from Capsicum annuum var. annuum, C. chinense, and Capsicum annuum var. acuminatum L., as possible substitutes for artificial preservatives applicated against the microbiological deterioration generated by bacteria (e.g., Escherichia coli and Lactobacillus casei) and fungi (e.g., Penicillium spp.) species, extending the shelf life (Sánchez-López et al. 2019). Despite these results, there are few studies focused on revealing the interaction mechanisms present between the bioactive compounds from CFs and the different microbial species. Thus, in the following sections, the effects of Capsicum compounds on different microbial species and the mechanisms of action reported so far will be addressed.
Effects of Capsicum compounds on bacteria, fungi, and virus
Capsicum bioactive compounds such as peptides, phenolics, and capsaicinoids, can exert beneficial effects on human health, including antimicrobial properties, which are of great interest due to the controversial results observed in different studies, since these antimicrobial compounds do not seem to exert the same activity in all microbial genera and species, it being understood that the effects not only depend on the concentration and nature of compound, but also on the strain in question (Table 1). According to this, in the following sections, the effects of Capsicum bioactive compounds on different microbial species will be described.
Table 1.
Antimicrobial activities of bioactive compounds from Capsicum spp. fruits
| Activity | Capsicum specie | Compound | Microorganism | Mechanism of action | References |
|---|---|---|---|---|---|
| Antibacterial | Capsicum sp. | Capsaicin | S. pyogenes | Reduction of hemolytic activity, cell invasion and biofilm production | Rossi et al. (2020) |
| Capsicum sp. | Capsaicin |
E. coli ATCC 25,922 S. aureus ATCC 25,923 Proteus mirabilis ATCC 14,153 Proteus vulgaris ATCC 13,315 P. aeruginosa ATCC 27,853 Enterobacter aerogenes ATCC 13,048 Bacillus thuringiensis S. Typhimurium SL 1344 Streptococcus mutans ATCC 25,175 |
Disruption of the bacteria cell wall | Akyuz et al. (2018) | |
| Capsicum annuum | Capsianosides | L. monocytogenes | Calcium chelating capacity | Bacon et al. (2016) | |
| Antifungal | Capsicum annuum L | Peptides | Candida species | Permeabilization of plasma membrane and oxidative stress | Taveira et al. (2017) |
| Antiviral | Capsicum baccatum var. pendulum (Willd.) Eshbaugh | Trypsin inhibitor | PepYMV* | Binding and blocking of the active site and suppression of enzymatic activity | Moulin et al. (2014) |
| Capsicum sp. | Capsaicin | Lassa virus | Blocking the pH-dependent viral fusion of the surface glycoprotein SSP-GP2TM* | Tang et al. (2020) | |
| Capsicum sp. | Capsaicin | SARS-CoV-2* | Binding to viral 3C-like protease, promoting structural changes | Gonzalez-Paz et al. (2020); Jo et al. (2020) |
*PepYMV: pepper yellow mosaic virus, SSP-GP2™ stable signal peptide GP2 transmembrane region, SARS-CoV-2 severe acute respiratory syndrome coronavirus-2
Peptides
Antimicrobial peptides (AMPs) are low molecular weight (less than 10 kDa) peptides with amphipathic character and a net positive charge at physiological pH. AMPs isolated from Capsicum seeds, plants, and fresh fruits show different effects on microbial species (bacteria, fungi, enveloped viruses, and parasites) important for human health (da Silva et al. 2020). For example, a thionin-like peptide from C. annuum (CaThi), demonstrated potent antimicrobial capacity against E. coli ATCC 25,922, Pseudomonas aeruginosa, and Saccharomyces cerevisiae 1038 (Afroz et al. 2020; Taveira et al. 2017). Likewise, da Silva et al. (2020), identified and characterized two defensins CaDef2.1 and CaDef2.2 from ripened C. annuum L. fruits with antimicrobial activity against opportunistic yeasts of medical importance (Candida albicans CE022, Candida tropicalis CE017 and Candida parapsilosis CE002), and pathogenic bacteria (Mycobacterium tuberculosis strains ATCC 27,294 and Beijing M299). The effectiveness of the peptides was related to a membrane permeabilization capacity, leading to apoptosis and a consequent loss of cell viability. These results agree with those observed by Aguieiras et al. (2021), who exposed the same strains of C. albicans, C. tropicalis as well as Candida buinensis 3982, to AMPs from C. chinense, being C. albicans the most sensitive yeast to a fraction from AMPs identified as CcDef3 (Capsicum chinense Defensin 3). Similarly, the CaThi peptide also showed a synergic effect when it was mixed with fluconazole against six Candida strains of clinical importance (Taveira et al. 2017). These results contribute to future developments on new therapeutic substances against pathogenic species, with a more efficient response at lower concentrations of the antimicrobial agents.
Similarly, the seeds from CFs have been considered as a source of AMPs, for example, peptide enriched fractions (5 to 8.5 kDa) obtained from C. chinense Jacq. seeds have shown antifungal activity against C. albicans, Pichia membranifaciens, S. cerevisiae, Kluyveromyces marxiannus, and promoted structural elongation in C. tropicalis cells (Brito-Argáez et al. 2016). In all the cases described, the antifungal effect was related to the increase in the permeability of the cell membrane; although this has not been the only mechanism reported by which the AMPs from Capsicum exert their antimicrobial activity. A peptide extracted from C. baccatum, identified as CSP37, has been effective to control the proliferation of Staphylococcus epidermidis, a pathogenic microorganism associated with infections related to medical devices, through anti-adhesive properties when was used as surface coatings (Von Borowski et al. 2020).
Capsaicinoids
The principal characteristic of CFs is their pungency, generated by the presence of capsaicinoids. Commonly, in CFs, the capsaicin, dihydrocapsaicin, nordihydrocapsaicin, homocapsaicin, and homodihydrocapsaicin are the majority capsaicinoids; which are defined as acid amides of vanillylamine with C9-C12 branched fatty acid chain, differing only in the saturation of the acyl group (Uarrota et al. 2021). Cultivars of C. chinense are recognized as the most pungent due to their high capsaicin concentration. The studies about the antimicrobial effects of pungent CFs are based on the capsaicin concentration, however, the results obtained from these studies are ambiguous and strain dependent. As reported by Peredo-Lovillo et al. (2019), the addition of an aqueous extract of orange habanero pepper (C. chinense Jacq.), with a concentration of 8.71 mg/g dry weight (d.w.) of capsaicin and 4.72 mg/g d.w. of dihydrocapsaicin added at 50% (v/v), to a culture broth, generated a bacteriostatic effect on the two lactic acid bacteria (LAB) Leuconostoc citreum and Lactobacillus casei Shirota after 24 and 12 h of exposure, respectively; while an inhibitory effect on Staphylococcus aureus ATCC 25,923 was shown by this same extract concentration after 4 h of exposure. The authors observed that, in addition to the inhibitory effect, the incorporation of the extract induced an increase in the cell size of both LAB, but only L. citreum showed an improvement in its mucin-binding capacity, a potentially probiotic property not observed in control cells. Similarly, Mokhtar et al. (2017), evaluated the inhibitory effect of capsaicinoids extracted from green bell pepper (C. annuum L. var. biskra) against 13 pathogenic bacteria and three beneficial strains, observing that S. aureus ATCC 6538 and Listeria monocytogenes ATCC 7644 were more sensitive to capsaicin and dihydrocapsaicin; whilst Lactobacillus acidophilus CECT 4529, Lactobacillus plantarum CECT 748 and Bifidobacterium animalis subsp. animalis Bb12 did not show inhibition after capsaicinoids exposition. These effects agree with those previously observed by Mokhtar et al. (2016), who reported a tolerance of Lactobacillus rhamnosus LbRE-LSAS and Bifidobacterium longum ATCC 15,707 to a capsaicinoids-extract from Algerian C. annuum L.; while the pathogenic bacteria L. monocytogenes ATCC 1392, Enterococcus hirae ATCC 1054, S. aureus ATCC 49,444 were inhibited by the extract, except for a wild strain of Klebsiella pneumoniae.
Additionally, pure capsaicin has also been tested, showing antimicrobial activity against pathogenic bacteria such as Helicobacter pylori NCTC 11,916 with a minimum inhibitory concentration (MIC) of 0.0625 mg/mL (Tayseer et al. 2020). Conversely, this compound has different effects on the viability and metabolism of beneficial bacteria strains. For instance, Sharma et al. (2013), observed that the addition of pure capsaicin during the curd formation of milk inoculated with L. acidophilus ATCC 435, increased both the D-glucose consumption and L-lactate production of the bacteria, but did not alter its viable cells count throughout the curdling process. This effect was also observed in milk added with fresh red pepper, allowing to correlate the bacterial metabolic increase with the concentration of natural capsaicin in milk. Furthermore, capsaicinoids have been used to reduce the virulence of pathogenic bacteria. Erfanimanesh et al. (2019), employed pure capsaicin to reduce the expression of genes related to cholera and zonula occludens toxin synthesis, in three strains of V. cholera (O1 serotype Ogawa, ATCC 14,035, and O1 serotype Inaba PTCC 1611). Another study showed a reduction of the virulence parameters such as the hemolytic activity, cell invasion and biofilm production of Streptococcus pyogenes exposed to sublethal capsaicin concentration (Rossi et al. 2020).
In fungi, pure capsaicin addition inhibits the growth of Aspergillus section Nigri strains (ATHUM 6997, 6998, 6999, 7000), as well as the biosynthesis of ochratoxin A production. So, capsaicin emerges as a natural preservative for foods and crops that have ochratoxin A and Aspergillus section Nigri contamination problems (Kollia et al. 2019).
In addition to the previous antimicrobial effects, recent studies report antiviral effects of capsaicin. Tang et al. (2020), showed that pure capsaicin acts as an inhibitor of Lassa virus (LASV) entry in different cell lines. LASV is an arenavirus that causes a severe and fatal viral hemorrhagic fever in humans. Likewise, an in silico study showed that capsaicin molecule is capable of strongly binding to viral 3C-like protease, involved in the propagation process of severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), promoting structural changes and thus, causing the inactivation of this protease by enzyme folding. This finding suggests that capsaicin could be a candidate as a model drug for a novel treatment against SARS-CoV-2 (Gonzalez-Paz et al. 2020; Jo et al. 2020). Thus, capsaicin can be considered as a potential antiviral compound.
Capsianosides and capsinoids
Capsianosides are acyclic diterpene glycosides isolated from C. annuum plants, which can also influence the metabolism of microorganisms. Regarding this, Bacon et al. (2016), obtained eluted fractions from an extract of C. annuum var. annuum (Jalapeño cv. “Del Mar”), where one of them contained a capsianoside with inhibitory effect against L. monocytogenes, but did not affect the growth of Salmonella enterica Baildon and E. coli O157:H7 incubated in the same growth conditions.
On the other hand, capsinoids are present in sweet CFs sharing a similar structure with the capsaicinoids. Capsinoids structure is made up of an ester of an aliphatic hydroxyl group in vanillyl alcohol with a fatty acid, being capsiate and dihydrocapsiate the major compounds of this chemical classification (Uarrota et al. 2021). A recent study carried out by Prasch et al. (2019), reported a higher antibacterial activity of capsinoids against Mycobacterium smegmatis, compared to that of capsaicinoids. These results highlight the importance of focusing new research on those minor compounds that are not recognized for their functionality and technological importance.
Phenolic compounds
As mentioned above, pungency is the principal characteristic of CFs, related to the content of capsaicinoids present in the fruits. However, the capsaicinoids are not the only bioactive phytochemicals present in CFs, other compounds such as phenolics are also found in these fruits and some of them have shown antimicrobial activity, antibiotic synergism, and bacterial virulence removal (Ordaz-Trinidad et al. 2018).
In CFs, the phenolic compounds are mostly hydroxycinnamic or cinnamic acids, derived from phenolic acids, which have been related to the reduction in the risk of cardiovascular diseases, due to their antioxidant activity exerted once they are consumed (Cortés-Rodríguez et al. 2019). In addition to their antioxidant activity, phenolic compounds and their derivatives have shown antibacterial effects against foodborne pathogenic bacteria. Martínez-Arámburu et al. (2015), used sodium ferulate and sodium caffeate, previously identified in CFs extracts, as antibacterial agents against S. enterica ser. Typhimurium and L. monocytogenes. Sodium ferulate generated a bacteriostatic effect on S. enterica ser. Typhimurium at concentrations of 0.3 and 0.6%, and in L. monocytogenes at 0.6%. Meanwhile, same effect was shown by sodium caffeate in both pathogenic bacteria but at a concentration of 0.15%. Likewise, the effect of hydroxycinnamic and cinnamic acids has been tested on the growth of non-pathogenic bacteria such as LAB. Cortés-Rodríguez et al. (2019), compared the tolerance of four strains of LAB (Lactobacillus delbruekii subsp. bulgaricus ATCC 11,842, Streptococcus thermophilus ATCC 19,987, L. plantarum 299v and L. casei Shirota) and L. monocytogenes ATCC 19,115, exposed to mixtures of sodium cinnamate, coumarate, caffeate and ferulate at similar concentrations to those naturally present in fresh CFs. The authors reported that the mixtures of hydroxycinnamic and cinnamic acids showed antilisterial activity, while LAB strains had higher survivability to the effect of the mixtures. These findings were similar to those reported by Mokhtar et al. (2015), who observed that a C. annuum L. extract rich in caffeic acid did not show antibacterial activity against the beneficial bacteria L. rhamnosus LbRE-LSAS and B. longum ATCC 15,707; however, the pathogenic bacteria L. monocytogenes ATCC 1392, Enterococcus hirae ATCC 10,541, K. pneumoniae (wild type) and S. aureus ATCC 49,444 were sensitive to the phenolic extract and its other polyphenolic constituents (coumarin, quercetin, and kaempferol). Likewise, chrysoeriol, a flavonoid with antioxidant and anti-inflammatory activities isolated from C. frutescens, showed antibacterial activity against Gram-positive (Enterococcus faecalis, Bacillus subtilis, and S. aureus) and Gram-negative (P. aeruginosa, K. pneumoniae, and E. coli) bacteria. This flavonoid was more efficient to inhibit E. coli at a MIC of 0.06 µg/mL (Nascimento et al. 2014).
The effect of hydroxycinnamic acids from CFs has been poorly evaluated in fungi species. However, it has been reported that the addition of ferulic acid decreased the production of mycotoxins (enniatins) and subsequently inhibited Fusarium avenaceum (Gautier et al. 2020). This study indicates that the hydroxycinnamic acids present in CFs could have an important effect on the regulation in the metabolism and viability of those fungi contaminating food and crops, therefore it is recommended to expand the studies on this topic.
Moreover, phenolic compounds present in CFs have shown antiviral activity. Extracted phenylpropanoids and flavonoids from jalapeño, serrano, guajillo, ancho, sweet pepper (C. annuum L. var. annuum), and habanero (C. chinense Jacq.), such as gallic, caffeic, p-coumaric, ferulic, chlorogenic, t-cinnamic acids, and luteolin, kaempferol, rutin, and quercetin were analyzed as probable antiviral agents against Herpes simplex virus type 1 (HSV-1) in an in vitro test, using Vero cells (ATCC CCL-81). Sweet pepper extract has shown the highest antiviral activity, followed by the ancho and guajillo extracts. Conversely, habanero extract presented the lowest antiviral activity, mainly due to the low concentration of phenolic compounds in this fruit (Ordaz-Trinidad et al. 2018). Similarly, Hafiz et al. (2017), evaluated the antiviral effect of polyphenols extracted from C. annuum against HSV-1 and Herpes simplex virus type 2 (HSV-2). The authors found an effective antiviral activity at a concentration of 25 μg/mL of C. annuum methanolic extract.
Mechanisms of action of the Capsicum compounds on bacteria, fungi, and virus
As previously described, the Capsicum compounds generate metabolic and structural changes that can culminate in the inhibition or inactivation of microorganisms such as bacteria, fungi, and viruses (Figs. 1 and 2). Thus, in the following section, the mechanisms of action derived from the interactions of the bioactive compounds of CFs and the microorganisms are described.
Fig. 1.
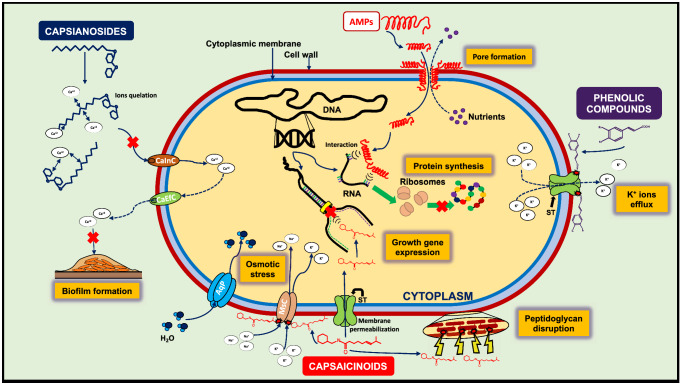
Antibacterial mechanisms of bioactive compounds from Capsicum spp. fruits. Antimicrobial peptides (AMPs) interact with the cell wall components, increasing membrane permeability and pores formation. Subsequently, the AMPs penetrate the bacterial cell and interfere with the RNA transcription affecting the protein synthesis. Similarly, phenolic compounds can adhere to the outer surface of the cell wall, where change the hydrophobicity and the charge of the cell surface, triggering a leakage of K+ ions from the interior of the cell through the solute transporters (ST), and thus, the inhibition of the bacterial cell. Furthermore, capsaicinoids can attach to the cell wall through lipidic interactions, which generate the disruption of the peptidoglycan structure and increase the fluidity of the cell membrane, facilitating the entry of ions (Ca+2 and K+) and capsaicin to the cytoplasm. The entry of solutes into the cell produces an osmotic stress, which, in turn, triggers a higher water uptake facilitating cellular lysis. Also, the capsaicin can interfere with the expression of genes related to the growth and reproduction of the bacteria, which culminates in changes in the growth rate as well as in the inhibition of the microorganism. Finally, the capsianosides are involved in suppressing the ability of bacteria to adhere and aggregate, since they have the capacity to chelate Ca+2 ions from the outside, necessary for the turgor and rigidity of the biofilms synthetized and excreted by the cell. CaInC Calcium influx channel, CEfC Calcium efflux channel, AqP Aqua porins, MsC Mechanosensitive channels
Fig. 2.
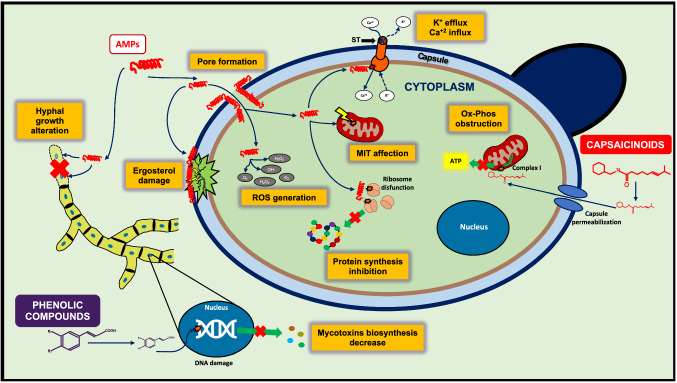
Antifungal mechanisms of bioactive compounds from Capsicum spp. fruits. In yeast, the antimicrobial peptides (AMPs) generate both cell permeabilization (increase the Ca+2 influx and K+ efflux), and pore formation, as well as the ergosterol structure alteration, leading to cellular capsule collapse and then, the cellular inhibition. The AMPs can enter to the cytoplasm where can induce the production of oxygen reactive species (ROS), affecting the cellular redox balance. Furthermore, the AMPs can affect the protein synthesis and mainly the mitochondrial (MIT) function. On the other hand, capsaicinoids disrupt the cell membranes and interrupt the adenosine triphosphate (ATP) production in MIT, the latter due to their bind with the complex I involved in the electron transport chain present (also known as oxidative phosphorylation), as the final part in the respiration process of the cell. In fungi, the AMPs participate as inhibitors of the hyphal growth, whereas the phenolic compounds alter the genetic material (DNA), preventing the expression of the genes responsible for the synthesis of mycotoxins. ST solutes transporters
Bacteria
AMPs from CFs are peptides that act as a form of defense against bacterial attacks (Bacon et al. 2016). Alteration of cell membrane potential and permeability, as well as membrane pores induction and cell aggregation, are the antibacterial mechanisms by which AMPs can penetrate the cell membrane and once inside, may interact with proteins and nucleic acids, establish a potential cell-killing mechanism (Afroz et al. 2020).
In Gram-positive bacteria, AMPs seem to pass the cell wall with relative ease, while in Gram-negative bacteria, the AMPs can cross the cell wall by a charge-exchange mechanism, where cationic peptides compete with Ca2+ and Mg2+ ions bound to lipopolysaccharide, once they are attached to the outer membrane proteins (Malanovic and Lohner 2016). According to this, it has been proposed membrane models (barrel-stave pore, toroidal pore, and carpet model) associated with cationic AMPs-membrane interactions, membrane disruption, and membrane permeability. By combining both translocation and membrane affinity properties, AMPs can get into bacterial membranes and lead to the disruption of the cell. However, the antibacterial mechanisms of AMPs depend on factors such as peptide structure, the peptide:lipid ratio and the lipid membrane properties (Afroz et al. 2020).
Otherwise, Baenas et al. (2019), reported that capsianosides can inhibit the growth and survival of Gram-positive bacteria due to their capacity of chelating calcium, which is used to provide an adequate ionic environment in cation-dependent membrane transport system and improves the biofilm architecture development. Conversely, the Gram-negative bacteria such as S. enterica and E. coli lack of cell wall requirement of calcium, which could mean the ineffectiveness of capsianosides against the formation of biofilms generated by this type of bacteria (Bacon et al. 2016).
Similarly, the antibacterial effect of capsaicin is related to the disruption of peptidoglycan structure due to lipid-lipid interactions providing liquidity in the cell wall (Akyuz et al. 2018). Also, the antibacterial effect of capsaicin depends on its concentration, and its mechanisms of action involve osmotic stress, destroying cell membrane structures, and inhibiting the expressions of the genes responsible for bacterial cell growth (Adaszek et al. 2019). However, it has been reported that bacteria such as Actinoplanes utahensis NRLL 12,052, Bacillus species isolated from Korean kimchi, and Streptomyces mobaraensis possess capsaicin-degradative enzymes (Adams et al. 2020). The presence of these types of enzymes could be one of the reasons why some bacteria are not inhibited by capsaicin.
Regarding phenolic compounds, it has been reported that ferulic and gallic acids lead to a change in hydrophobicity and a decrease of negative surface charge in cytoplasmic membrane of pathogenic bacteria; causing local rupture and pore formation with leakage of essential intracellular constituents (Bouarab-Chibane et al. 2019). This supposes the formation of pores in the cell membrane, generating structural damage and subsequent inhibition of microorganisms. Meanwhile, LAB use hydroxycinnamic acids as external electron acceptors, allowing cofactor recovery and gaining additional metabolic energy to tolerate the stressful conditions generated by phenolic compounds. This adaptation is one of the mechanisms by which LAB are not inhibited by these acids, which represents an advantage over other undesirable microorganisms (Filannino et al. 2018). Based on the above, the antibacterial effects of the compounds present in CFs are not always inhibitory and appear to be strain dependent.
Fungi
The induction of cell membrane permeabilization is the main antifungal mechanism of Capsicum peptides. However, some studies showed that not only is permeabilization the cause of fungi death, as they may have multiple targets after the interaction with the membrane causing, e.g., reactive oxygen species (ROS) induction, inhibition of protein synthesis and mitochondrial activity, and may trigger signaling cascades that lead to apoptosis of the cell (da Silva et al. 2020).
The permeability of cell membranes treated with AMPs has been well described for peptides such as plant defensins, which can disrupt the ergosterol and change the permeability, resulting in the entry of Ca+2 ions, the efflux of K+ ions, and the collapse of membrane potential. Nevertheless, current studies have suggested other intracellular targets, and the capacity of membrane permeabilization could be a secondary event caused by the endogenous increase in ROS production (da Silva et al. 2020). Peptide-rich leaf and seed extracts of different species of the Capsicum, e.g., C. annuum L. exhibited significant antifungal effect via inhibiting the growth or germination and hyphae formation (Afroz et al. 2020). The alteration of hyphal growth may be due to the inhibition of cell wall biosynthesis, which results from interaction between plant defensins and fungal membrane (da Silva et al. 2020).
In contrast, capsaicinoids can inhibit fungi binding to complex I of the electron transport chain, stopping the oxidative phosphorylation and subsequent ATP production, to finally generate cell inhibition. Nonetheless, it has been reported that pathogenic fungi of wild CFs possess alternative complex I enzymes, which allow the fungi to continue producing energy in the presence of capsaicinoids (Adams et al. 2020). In the case of yeasts, S. cerevisiae lacks complex I, resulting in insensitivity to capsaicin. This deficiency is covered with alternative NADH dehydrogenases, which can transfer electrons from NADH to downstream complexes. Similarly, many fungi have been reported to possess genes for alternative respiratory enzymes, that could be a mechanism by which fungi could tolerate capsaicinoids (Adams et al. 2020). Another strategy to tolerate capsaicinoids is the enzymes that degrade them, which, despite being widely reported in bacteria, have only been found in Aspergillus oryzae (Lee et al. 2015). This indicates that fungi have multiple mechanisms by which tolerate capsaicinoids.
As mentioned above, the hydroxycinnamic acids present in CFs have not been evaluated on fungi. Nonetheless, a study reported by Gautier et al. (2020), has shown that the addition of ferulic acid in growth medium of Fusarium avenaceum decreases the production of enniatins through the downregulation of the esyn1 and kivr genes, which are associated with the mycotoxin biosynthesis.
Virus
There is few information about the antiviral mechanisms of the bioactive compounds from CFs. Despite this, it has been observed that C. baccatum var. pendulum UENF 1624, can generate trypsin inhibitors (TryInh) after being inoculated with the pepper yellow mosaic virus (PepYMV, Potyvirus, Potyvirae). These TryInh, are serine proteinase inhibitors that can inactivate the pathogen-derived proteinase by binding and blocking of the active site, suppressing its enzymatic activity (Moulin et al. 2014).
On the other hand, a study performed by Tang et al. (2020), showed that pure capsaicin acts as an inhibitor of LASV entry in different cell lines, by blocking the pH-dependent viral fusion of the surface glycoprotein SSP-GP2TM. Similarly, an in silico study showed that capsaicin is capable of strongly binding to viral 3C-like protease, involved in the propagation process of severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), promoting structural changes and thus, causing the inactivation of this protease by enzyme folding (Gonzalez-Paz et al. 2020; Jo et al. 2020). Meanwhile, a previous study has shown that methanolic extracts of C. annuum have antiviral activity against HSV-1 and HSV-2, associated with the ability of the phytochemical compounds of the extract to interfere in the attachment points and penetration of the virus in cells, modifying the viral and cellular receptors, resulting in the prevention of viral infection (Hafiz et al. 2017). Based on the above, it is possible that CFs serve as a natural source of bioactive compounds with antiviral activity, however, there is still not enough evidence to clarify the mechanisms of action of these compounds and their use as functional ingredients in foods.
Perspectives and conclusion
The growing market for functional ingredients arises in response to the global demand for consumption of natural ingredients, which, in addition to preserving the sensory qualities and shelf life of industrialized products, provide greater well-being in people. In this sense, the CFs emerge as a natural ingredients and source of bioactive compounds with antibacterial, antifungal, and antiviral activities. Capsaicinoids and phenolic compounds are the majority bioactive compounds from CFs, and their antimicrobial effects are mainly based on the cell membrane disruption and pore formation in pathogenic bacteria and fungi, however, regarding viruses, studies are scarce, and their mechanisms remain unclear or poor studied.
Therefore, according to the information presented in this review, it is recommended that future research focus on the isolation, identification of new antimicrobial compounds from CFs and their subsequent use against novel infectious agents, whether bacteria, fungi, or viruses such as SARS-CoV-2, which has gained great interest in recent years.
Acknowledgements
None
Abbreviations
- AMPs
Antimicrobial peptides
- AqP
Aquaporine
- ATP
Adenosine triphosphate
- CaEfC
Calcium efflux channel
- CaInC
Calcium influx channel
- CaThi
Thionine-like peptide
- CaDef2.1
Capsicum annuum Defensin 2.1
- CaDef2.2
Capsicum annuum Defensin 2.2
- CcDef3
Capsicum chinense Defensin 3
- CFs
Capsicum Spp. Fruits
- d.w.
Dry weight
- HSV-1
Herpes simlex virus type 1
- HSV-2
Herpes simplex virus type 2
- LAB
Lactic acid bacteria
- LASV
Lassa virus
- MIC
Minimum inhibitory concentration
- MIT
Mitochondria
- MsC
Mechanosensitive channel
- Ox-Phos
Oxidative phospohorylation
- PepYMV
Pepper yellow mosaic virus
- ROS
Reactive oxygen species
- SARS-CoV-2
Severe acute respiratory syndrome coronavirus-2
- ST
Solutes transporter
- SSP-GP2™
Stable signal peptide GP2 transmembrane region
- TryInh
Trypsin inhibitors
Authors’ contribution
HERL Conceptualization, investigation, visualization, writing-original draft, writing-review & Editing; JC Conceptualization, investigation, writing-original draft. LGR Conceptualization, investigation, writing-original draft; CGSC Investigation, writing-original draft; ÁMFC Visualization, investigation; SGP Visualization, investigation; MMGT Visualization, investigation; KOMA Visualization, investigation; APL Project administration, supervision, visualization, writing-review & editing.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
Code availability
not applicable.
Declarations
Conflicts of interest
The authors declare that they have no Conflicts of interest.
Ethics approval
not applicable.
Consent to participate
not applicable.
Consent for publication
not applicable.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Adams CA, Zimmerman K, Fenstermacher K, Thompson MG, Skyrud W, Behie S, Pringle A. Fungal seed pathogen of wild chili peppers possess multiple mechanisms to tolerate capsaicinoids. Appl Environ Microbiol. 2020;86(3):e01697–e1719. doi: 10.1128/aem.01697-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adaszek Ł, Gadomska D, Mazurek Ł, Łyp P, Madany J, Winiarczyk S. Properties of capsaicin and its utility in veterinary and human medicine. Res Vet Sci. 2019;123:14–19. doi: 10.1016/j.rvsc.2018.12.002. [DOI] [PubMed] [Google Scholar]
- Afroz M, Akter S, Ahmed A, Rouf R, Shilpi J, Tiralongo E, Sarker S, Göransson U, Uddin SJ. Ethnobotany and antimicrobial peptides from plants of the solanaceae family: an update and future prospects. Front Pharmacol. 2020;11:565. doi: 10.3389/fphar.2020.00565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguieiras MCL, Resende LM, Souza TAM, Nagano CS, Chaves RP, Taveira GB, Carvalho AO, Rodrigues R, Gomes VM, Mello ÉO. Potent anti-Candida fraction isolated from Capsicum chinense fruits contains an antimicrobial peptide that is similar to plant defensin and is able to inhibit the activity of different α-amylase enzymes. Probiotics Antimicrob Proteins. 2021;13(3):862–872. doi: 10.1007/s12602-020-09739-3. [DOI] [PubMed] [Google Scholar]
- Akyuz L, Kaya M, Mujtaba M, Ilk S, Sargin I, Salaberria AM, Labidi J, Cakmak Y, Islek C. Supplementing capsaicin with chitosan-based films enhanced the anti-quorum sensing, antimicrobial, antioxidant, transparency, elasticity and hydrophobicity. Int J Biol Macromol. 2018;115:438–446. doi: 10.1016/j.ijbiomac.2018.04.040. [DOI] [PubMed] [Google Scholar]
- Alsebaeai M, Chauhan AK, Arvind Yadav P. Consumption of Green Chilli and Its Nutritious Effect on Human Health. In: Mishra P, Mishra RR, Adetunji CO, editors. Innovations in Food Technology. Singapore: Springer; 2020. [Google Scholar]
- Bacon K, Boyer R, Denbow C, O’Keefe S, Neilson A, Williams R. Antibacterial activity of jalapeño pepper (Capsicum annuum var. annuum) extract fractions against select foodborne pathogens. Food Sci Nutr. 2016;5(3):730–738. doi: 10.1002/fsn3.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baenas N, Belović M, Ilic N, Moreno DA, García-Viguera C. Industrial use of pepper (Capsicum annuum L.) derived products: technological benefits and biological advantages. Food Chem. 2019;274:872–885. doi: 10.1016/j.foodchem.2018.09.047. [DOI] [PubMed] [Google Scholar]
- Bouarab-Chibane L, Forquet V, Lantéri P, Clément Y, Léonard-Akkari L, Oulahal N, Degraeve P, Bordes C. Antibacterial properties of polyphenols: characterization and QSAR (quantitative structure-activity relationship) models. Front Microbiol. 2019;10:829. doi: 10.3389/fmicb.2019.00829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brito-Argáez L, Tamayo-Sansores JA, Madera-Piña D, García-Villalobos FJ, Mooc-Puc RE, Kú-González Á, Villanueva MA, Islas-Flores I. Biochemical characterization and immunolocalization studies of a Capsicum chinense Jacq. protein fraction containing DING proteins and anti-microbial activity. Plant Physiol Biochem. 2016;109:502–514. doi: 10.1016/j.plaphy.2016.10.031. [DOI] [PubMed] [Google Scholar]
- Cortés-Rodríguez V, Dorantes-Alvarez L, Hernández-Sánchez H, Paniagua-Castro N, Aparicio-Ozores G, López-Villegas EO, Perea-Flores MJ. Effect of sodium cinnamate, coumarate, caffeate and ferulate mixtures on the viability, morphometric and ultrastructure of lactic-acid bacteria and Listeria monocytogenes. LWT-Food Sci Technol. 2019;112:108240. doi: 10.1016/j.lwt.2019.06.007. [DOI] [Google Scholar]
- da Silva RG, Taveira GB, de Azevedo dos Santos L, Dias Calixto S, Lopes T, Lassounskaia E, Frazão M, Teixeira-Ferreira A, Perales J, Rodrigues R, de Oliveira A, Moreira V. Identification and characterization of two defensins from Capsicum annuum fruits that exhibit antimicrobial activity. Probiotic Antimicrob Proteins. 2020;12(3):1253–1265. doi: 10.1007/s12602-020-09647-6. [DOI] [PubMed] [Google Scholar]
- de Sá MN, de Andrade GÉ. The role of bioactive components found in peppers. Trends Food Sci Technol. 2020;99:229–243. doi: 10.1016/j.tifs.2020.02.032. [DOI] [Google Scholar]
- de Sá MN, Santos M, Santos M, Cameron L, Ferreira M, de Andrade GÉ. Characterization of pepper (Capsicum baccatum)-a potential functional ingredient. LWT-Food Sci Technol. 2019;112:108209. doi: 10.1016/j.lwt.2019.05.107. [DOI] [Google Scholar]
- Domínguez-Cañedo IL, Beristain-Guevara CI, Díaz-Sobac R, Vázquez-Luna A. Degradation of carotenoids and capsaicin in the molecular inclusion complex of oleoresin from habanero pepper (Capsicum chinense) with β-cyclodextrin. CYTA-J Food. 2015;13(1):151–158. doi: 10.1080/19476337.2014.926459. [DOI] [Google Scholar]
- Erfanimanesh S, Eslami G, Taherpour A, Hashemi A. Capsaicin inhibitory effects on Vibrio cholerae toxin genes expression. Avicenna J Phytomed. 2019;9(3):187–194. [PMC free article] [PubMed] [Google Scholar]
- Filannino P, Di Cagno R, Gobbetti M. Metabolic and functional paths of lactic acid bacteria in plant foods: get out of the labyrinth. Curr Opin Biotechnol. 2018;49:64–72. doi: 10.1016/j.copbio.2017.07.016. [DOI] [PubMed] [Google Scholar]
- Gautier C, Pinson-Gadais L, Verdal-Bonnin MN, Ducos C, Tremblay J, Chéreau S, Atanasova V, Richard-Forget F. Investigating the efficiency of hydroxycinnamic acids to inhibit the production of enniatins by Fusarium avenaceum and modulate the expression of enniatins biosynthetic genes. Toxins. 2020;12(12):735. doi: 10.3390/toxins12120735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Paz LA, Lossada CA, Moncayo LS, Romero F, Paz JL, Vera-Villalobos J, Pérez AE, San-Blas E, Alvarado YJ (2020). Theoretical molecular docking study of the structural disruption of the viral 3CL-protease of COVID19 induced by binding of capsaicin piperine and curcumin part 1: a comparative study with chloroquine and hydrochloroquine two antimalaric drugs. Res Sq. 10.21203/rs.3.rs-21206/v1
- Guiné RP, Florença SG, Barroca MJ, Anjos O. The link between the consumer and the innovations in food product development. Foods. 2020;9(9):1317. doi: 10.3390/foods9091317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafiz TA, Mubaraki M, Dkhil M, Al-Quraishy S. Antiviral activities of Capsicum annuum methanolic extract against herpes simplex virus 1 and 2. Pak J Zool. 2017;49(1):251. doi: 10.17582/journal.pjz/2017.49.1.267.272. [DOI] [Google Scholar]
- Jo S, Kim S, Shin DH, Kim MS. Inhibition of SARS-CoV 3CL protease by flavonoids. J Enzyme Inhib Med Chem. 2020;35(1):145–151. doi: 10.1080/14756366.2019.1690480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur R, Kaur K, Wagh RV, Kaur A, Aggarwal P. Red bell pepper (Capsicum annuum L.): optimization of drying conditions and preparation of functional bread. J Food Sci. 2020;85(8):2340–2349. doi: 10.1111/1750-3841.15317. [DOI] [PubMed] [Google Scholar]
- Kollia E, Proestos C, Zoumpoulakis P, Markaki P. Capsaicin, an inhibitor of ochratoxin a production by aspergillus section nigri strain in grapes (Vitis vinifera L.) Food Addit Contam: Part A. 2019;36(11):1709–1721. doi: 10.1080/19440049.2019.1652771. [DOI] [PubMed] [Google Scholar]
- Lee M, Cho JY, Lee YG, Lee HJ, Lim SI, Park SL, Moon JH. Bioconversion of capsaicin by Aspergillus oryzae. J Agric Food Chem. 2015;63(26):6102–6108. doi: 10.1021/acs.jafc.5b01730. [DOI] [PubMed] [Google Scholar]
- Malanovic N, Lohner K. Antimicrobial peptides targeting gram-positive bacteria. Pharmaceuticals. 2016;9(3):59. doi: 10.3390/ph9030059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldonado MB, Santillan AM, González MC, Velázquez TH, Benitez MB, Romero GF, Azócar ASM. Use of bell pepper flour (Capsicum annuum) in the production of artisan bread. Nexo. 2018;31(2):127–143. doi: 10.5377/nexo.v31i2.6836. [DOI] [Google Scholar]
- Martínez-Arámburu D, González-Quijano GK, Dorantes-Alvarez L, Aparicio-Ozores G, López-Villegas EO. Changes in microstructure of salmonella typhimurium and Listeria monocytogenes exposed to hydroxycinnamic salts. Rev Mex Ing Quím. 2015;14(2):347–354. [Google Scholar]
- Melgar-Lalanne G, Hernández-Álvarez AJ, Jiménez-Fernández M, Azuara E. Oleoresins from Capsicum spp.: Extraction methods and bioactivity. Food Bioproc Technol. 2017;10(1):51–76. doi: 10.1007/s11947-016-1793-z. [DOI] [Google Scholar]
- Mokhtar M, Ginestra G, Youcefi F, Filocamo A, Bisignano C, Riazi A. Antimicrobial activity of selected polyphenols and capsaicinoids identified in pepper (Capsicum annuum L.) and their possible mode of interaction. Curr Microbiol. 2017;74(11):1253–1260. doi: 10.1007/s00284-017-1310-2. [DOI] [PubMed] [Google Scholar]
- Mokhtar M, Russo M, Cacciola F, Donato P, Giuffrida D, Riazi A, Farnetti S, Dugo P, Mondello L. Capsaicinoids and carotenoids in Capsicum annuum L.: optimization of the extract method, analytical characterization, and evaluation of its biological properties. Food Anal Methods. 2016;9(5):1381–1390. doi: 10.1007/s12161-015-0311-7. [DOI] [Google Scholar]
- Mokhtar M, Soukup J, Donato P, Cacciola F, Dugo P, Riazi A, Jandera P, Mondello L. Determination of the polyphenolic content of a Capsicum annuum L. extract by liquid chromatography coupled to photodiode array and mass spectrometry detection and evaluation of its biological activity. J Sep Sci. 2015;38(2):171–178. doi: 10.1002/jssc.201400993. [DOI] [PubMed] [Google Scholar]
- Moulin M, Rodrigues R, Ribeiro S, Gonçalves L, Bento C, Sudré C, Vasconcelos IM, Gomes VM. Trypsin inhibitors from Capsicum baccatum var. pendulum leaves involved in pepper yellow mosaic virus resistance. Genet Mol Res. 2014;13(4):9229–9243. doi: 10.4238/2014.November.7.10. [DOI] [PubMed] [Google Scholar]
- Nascimento PLA, Nascimento TCES, Ramos NSM, Silva GR, Galindo JE, Falcão REA, Moreira KA, Porto ALF, Silva TMS. Quantification, antioxidant and antimicrobial activity of phenolics isolated from different extracts of Capsicum frutescens (Pimenta Malagueta) Molecules. 2014;19(4):5434–5447. doi: 10.3390/molecules19045434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olatunji TL, Afolayan AJ. Evaluation of genetic relationship among varieties of Capsicum annuum L. and Capsicum frutescens L. in West Africa using ISSR markers. Heliyon. 2019;5(5):e01700. doi: 10.1016/j.heliyon.2019.e01700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ordaz-Trinidad N, Dorantes-Álvarez L, Salas-Benito J, Barrón-Romero BL, Salas-Benito M, De Nova-Ocampo M. Cytotoxicity and antiviral activity of pepper extracts (Capsicum spp.) Polibotánica. 2018;46:273–285. doi: 10.18387/polibotanica.46.18. [DOI] [Google Scholar]
- Peredo-Lovillo A, Dorantes-Alvarez L, Hernández-Sánchez H, Ribas-Aparicio RM, Cauich-Sánchez PI, Aparicio-Ozores G. Functional properties and microstructure of Leuconostoc citreum and Lactobacillus casei Shirota exposed to habanero pepper extract and inhibition of Staphylococcus aureus. Rev Mex Ing Quím. 2019;18(1):115–129. doi: 10.24275/uam/izt/dcbi/revmexingquim/2019v18n1/Peredo. [DOI] [Google Scholar]
- Prasch S, Duran AG, Chinchilla N, Molinillo JMG, Macías FA, Bucar F. Resistance modulatory and efflux-inhibitory activities of capsaicinoids and capsinoids. Bioorg Chem. 2019;82:378–384. doi: 10.1016/j.-bioorg.2018.10.062. [DOI] [PubMed] [Google Scholar]
- Rossi B, Toschi A, Piva A, Grilli E. Single components of botanicals and nature-identical compounds as a non-antibiotic strategy to ameliorate health status and improve performance in poultry and pigs. Nutr Res Rev. 2020;33(2):218–234. doi: 10.1017/S0954422420000013. [DOI] [PubMed] [Google Scholar]
- Rudrapal M, Sarwa KK (2020). Capsicum: chemistry and medicinal properties of indigenous Indian varieties. In: Capsicum. IntechOpen. 10.5772/intechopen.92241
- Sánchez-López PJ, Rodríguez-Flores F, González-Cortés N, Luna-Jiménez AL, Jiménez-Vera R. Antimicrobial effect of jalapeño chili juice (Capsicum annuum var. Annuum) in sopero cheese. Eur Sci J. 2019 doi: 10.19044/esj.2019.v15n33p238. [DOI] [Google Scholar]
- Sharma S, Jain S, Nair GN, Ramachandran S. Capsicum annuum enhances L-lactate production by Lactobacillus acidophilus: implications in curd formation. J Dairy Sci. 2013;96(7):4142–4148. doi: 10.3168/jds.2012-6243. [DOI] [PubMed] [Google Scholar]
- Tang K, Zhang X, Guo Y. Identification of the dietary supplement capsaicin as an inhibitor of Lassa virus entry. Acta Pharm Sin B. 2020;10(5):789–798. doi: 10.1016/j.apsb.2020.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taveira GB, Mello É, Carvalho AO, Regente M, Pinedo M, de La Canal L, Rodrigues R, Gomes VM. Antimicrobial activity and mechanism of action of a thionin-like peptide from Capsicum annuum fruits and combinatorial treatment with fluconazole against Fusarium solani. Biopolymers. 2017;108(3):e23008. doi: 10.1002/bip.23008. [DOI] [PubMed] [Google Scholar]
- Tayseer I, Aburjai T, Abu-Qatouseh L, Al-Karabieh N, Ahmed W, Al-Samydai A. In vitro anti-Helicobacter pylori activity of capsaicin. J Pure Appl Microbiol. 2020;14(1):279–286. doi: 10.22207/JPAM.14.1.29. [DOI] [Google Scholar]
- Uarrota VG, Maraschin M, de Barrios ÂFM, Pedreschi R. Factors affecting the capsaicinoid profile of hot peppers and biological activity of their non-pungent analogs (Capsinoids) present in sweet peppers. Crit Rev Food Sci Nutr. 2021;61(4):1–7. doi: 10.1080/10408398.2020.1743642. [DOI] [PubMed] [Google Scholar]
- Von Borowski R, Barros MP, da Silva DB, Lopes NP, Zimmer KR, Staats CC, Bernardes de Oliveira C, Giudice E, Macedo AJ, Baggio SC, Gnoatto SCB. Red pepper peptide coatings control Staphylococcus epidermidis adhesion and biofilm formation. Int J Pharm. 2020;574:118872. doi: 10.1016/j.ijpharm.2019. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
not applicable.