Abstract
To more fully explore the role of unsaturated fatty acids in high-pressure, low-temperature growth, the fabF gene from the psychrotolerant, piezophilic deep-sea bacterium Photobacterium profundum strain SS9 was characterized and its role and regulation were examined. An SS9 strain harboring a disruption in the fabF gene (strain EA40) displayed growth impairment at elevated hydrostatic pressure concomitant with diminished cis-vaccenic acid (18:1) production. However, growth ability at elevated pressure could be restored to wild-type levels by the addition of exogenous 18:1 to the growth medium. Transcript analysis did not indicate that the SS9 fabF gene is transcriptionally regulated, suggesting that the elevated 18:1 levels produced in response to pressure increase result from posttranscriptional changes. Unlike many pressure-adapted bacterial species such as SS9, the mesophile Escherichia coli did not regulate its fatty acid composition in an adaptive manner in response to changes in hydrostatic pressure. Moreover, an E. coli fabF strain was as susceptible to elevated pressure as wild-type cells. It is proposed that the SS9 fabF product, β-ketoacyl–acyl carrier protein synthase II has evolved novel pressure-responsive characteristics which facilitate SS9 growth at high pressure.
Increased hydrostatic pressure and reduced temperature elicit similar physical effects on the phase and fluidity properties of membrane lipids. As growth temperature is lowered or growth pressure is elevated, biological membranes undergo a reversible change from a fluid disordered state to a nonfluid ordered state (22, 29). Such changes would seem particularly problematic for life in deep ocean environments. Many poikilothermic organisms respond to decreased temperature and/or increased hydrostatic pressure by altering their membrane lipid composition, apparently to tailor the membrane with physical properties suited to prevailing environmental conditions. Such changes may include increases in fatty acyl chain unsaturation, decreases in mean chain length, increased methyl branching, cis/trans isomerization of unsaturated fatty acid double bonds, increases in the ratio of anteiso branching relative to iso branching, acyl chain shuffling between the phospholipid sn-1 and sn-2 positions, or phospholipid headgroup composition changes (22, 25, 36, 39). Among these changes, the most common change observed among deep-sea bacteria involves the incorporation into membrane phospholipids of increased proportions of unsaturated fatty acids (UFAs) (1, 11, 12, 47). UFAs adopt a more expanded conformation, pack less compactly, and possess lower melting temperatures than their saturated counterparts, allowing for their less orderly alignment within membrane phospholipids (22). This response presumably functions to offset the membrane gelling effects of increased pressure or decreased temperature, thereby maintaining biological membranes in a fluidity or phase optimized for growth. In addition to producing increased amounts of monounsaturated fatty acids (MUFAs) such as palmitoleic acid (16:1n-9) and cis-vaccenic acid (18:1n-11), many deep-sea bacteria also produce substantial quantities of omega-3 polyunsaturated fatty acids (PUFAs) at high pressure (1, 12, 47, 48).
The way in which a bacterial species modulates its membrane fatty acid unsaturation depends on its method of UFA synthesis and involves either an aerobic or anaerobic mechanism. In gram-positive bacteria and cyanobacteria, a double bond is introduced into a preexisting fatty acid chain by means of an oxygen-dependent desaturase system (13, 44). In contrast, gram-negative bacteria employ an anaerobic pathway, whereby at a discrete point in the elongation cycle of fatty acid biosynthesis a cis double bond is introduced (38). Some bacteria utilize both the anaerobic and aerobic desaturation pathways (34).
In Escherichia coli, where the mechanisms of anaerobic UFA synthesis have been well characterized, the response to temperature downshift entails the restructuring of membrane fatty acid composition by increasing the amount of 18:1 and decreasing the amount of palmitic acid (16:0) incorporated into membrane phospholipids (33). This regulation is an intrinsic property of the fatty acid biosynthetic enzyme β-ketoacyl-ACP (acyl carrier protein) synthase II (KAS II), product of the fabF gene (10, 14, 17). E. coli KAS II is one of three isozymes that catalyze the elongation of fatty acyl chains. Specifically, KAS II catalyzes the elongation of palmitoleoyl-ACP (16:1) to cis-vaccenoyl-ACP (18:1) in UFA synthesis. Neither mRNA nor protein synthesis is required for increased 18:1 production at reduced temperature, indicating that thermal modulation of fatty acid production is controlled at the level of KAS II activity (17). Indeed, the elongation activity of KAS II is temperature dependent, exhibiting decreased Km for palmitoleoyl-ACP and increased relative Vmax at reduced temperatures (16, 18). E. coli fabF mutants possess a deficiency in 18:1 synthesis as well as a loss of 18:1 thermal regulation (19).
Because of the critical role of fabF (KAS II) in thermal modulation of UFA production in E. coli, we predicted that a similar role exists for KAS II in the deep-sea bacterium Photobacterium profundum strain SS9 and furthermore that SS9 KAS II is required for 18:1 piezoregulation and piezoadaptation. Such properties could distinguish the SS9 KAS II enzyme from its homologue in bacteria which have not evolved adaptations for substantially elevated pressures. Consistent with this hypothesis, recent studies in our lab using the fatty acid biosynthesis inhibitor cerulenin and mutants altered in the abundance of various UFAs indicated that MUFAs but not PUFAs are required for high-pressure and low-temperature adaptation in P. profundum strain SS9 (1). Here we report the cloning of the SS9 fabF gene, the engineering of an SS9 mutant harboring a disruption in fabF, and the growth characteristics and fatty acid analysis of this mutant. In addition, an E. coli fabF mutant and parental strain were compared with respect to fatty acid composition and growth ability as a function of pressure and temperature. Our results indicate that SS9 fabF has evolved novel characteristics critical to pressure sensing and high-pressure adaptation.
MATERIALS AND METHODS
Strains and growth conditions.
All bacterial strains and plasmids used in this study are listed in Table 1. P. profundum strains were routinely cultured at 15°C, 1 atm (=0.101 MPa) in 2216 marine medium (28 g/liter; Difco Laboratories, Detroit, Mich.). All temperature experiments (15 and 4°C) were conducted aerobically in 2216 marine medium for P. profundum strains unless otherwise indicated. E. coli strains SJ16 and MR86 were graciously provided by John E. Cronan, Jr. E. coli strains were routinely cultured in Luria-Bertani (LB) media (30). For solid media, agar (Difco Laboratories) was added at 17 g/liter. The antibiotics kanamycin (50 μg/ml for E. coli; 200 μg/ml for P. profundum strains), streptomycin (50 μg/ml for E. coli; 150 μg/ml for P. profundum strains), rifampin (100 μg/ml), and tetracycline (12 μg/ml) were added to the media when required. All antibiotics were obtained from Sigma Chemical Co. (St. Louis, Mo.). Exogenous supplementation of marine media with fatty acids (i.e., Na+ salts) is not possible due to insolubility problems resulting from the presence of a high concentration of divalent cations. Tween compounds, however, are highly soluble in marine media and have been used for exogenous supplementations (1). Oleic acid (18:1) in the form of Tween 80 (polyoxyethylenesorbitan monooleate; Sigma) was added at a final concentration of 0.025% (vol/vol).
TABLE 1.
Strains and plasmids used in this study
| Strain or plasmid | Description | Reference or source |
|---|---|---|
| Strains | ||
| Photobacterium profundum | ||
| SS9 | Wild type | 11 |
| DB110 | Lac− Rifr; SS9 derivative | 8 |
| EA40 | fabF disruption mutant; DB110 derivative | This study |
| E. coli | ||
| SJ16 | panD2 zad-220::Tn10 | 24 |
| MR86 | fabF::Kmr insertion; SJ16 derivative | 31 |
| MG1655 | λ− | 21 |
| 1100 | λ−glnV44 rfbD1 endA1 spoT1 thi-1 | 15 |
| Plasmids | ||
| pCR2.1 | Vector for cloning PCR products, Kmr | Invitrogen |
| pEA39 | 740-bp internal fragment of SS9 fabF in pCR2.1, Kmr | This study |
| pMUT100 | Mobilizable suicide plasmid, Kmr | 7 |
| pEA40 | 740-bp internal fragment of SS9 fabF in pMUT100, Kmr | This study |
| pEA401 | SS9 genomic library clone containing fabF and flanking DNA, Kmr | This study |
| pKT231 | Broad-host-range vector, Kmr Smr | 3 |
| pEA44 | SS9 fabF in pKT231, Smr | This study |
High-pressure growth studies.
High-pressure cultivation of P. profundum strains for growth studies or fatty acid analysis were conducted as previously described (1). Cultivation of E. coli strains at elevated pressures was similarly performed. Each E. coli culture was grown to stationary phase in LB medium at 1 atm. Stationary-phase cultures were diluted 1/400 into LB medium buffered with HEPES (100 mM, pH 7.5; Sigma) containing 22 mM glucose (Sigma). The diluted culture was used to fill 4.5- or 15-ml polyethylene transfer pipettes (Samco, San Fernando, Calif.). Pipettes were filled completely and then heat sealed with a hand-held heat sealing clamp (Harwil, Oxnard, Calif.). Cells were incubated at 0.1 or 30 MPa (1 or 300 atm, respectively) of hydrostatic pressure at 37°C (unless otherwise stated) in stainless steel pressure vessels equipped with quick-connect fittings for rapid decompression and recompression as described by Yayanos and Van Boxtel (50).
DNA sequencing and analysis.
Double-stranded DNA sequencing reactions were performed using a Taq DyeDeoxy terminator cycle sequencing kit (Applied Biosystems Inc., Foster City, Calif.) and run on an Applied Biosystems 373A DNA sequencer. Global similarity searches were performed using the BLAST network service (2). Multiple alignments were performed using ClustalW (23) in conjunction with GeneDoc software (35).
fabF insertional inactivation mutagenesis.
An internal fragment of the SS9 fabF gene was initially PCR amplified from P. profundum strain DB110 genomic DNA, using primers fabF1 5′-GTGTCCAAGCGTCGTGTAGTTGT-3′ and fabF4 5′-GCGTGTTCGTACTCTTCAAG-3′. These primers were created by analysis of conserved regions from alignment of the E. coli and Vibrio harveyi fabF gene sequences in GenBank. The resultant 740-bp PCR product was cloned into pCR2.1 (Invitrogen, Carlsbad, Calif.), generating pEA39, and sequenced using M13R and T7 primers to confirm the identity of the product. The PCR product was then subcloned into the mobilizable suicide plasmid pMUT100 (Kanr) (7) as an EcoRI fragment yielding pEA40. Bacterial conjugations were used to transfer plasmid pEA40 from E. coli into P. profundum strain DB110 as described by Chi and Bartlett (8). Kanr exconjugants arose from integration of plasmid pMUT100 into the chromosome of P. profundum strain DB110 in a single crossover event giving rise to two deleted copies of the gene, one copy with a 5′ deletion and the other with a 3′ deletion. These experiments yielded P. profundum strain EA40 containing a disruption in the fabF gene and was confirmed by Southern blot analysis (40). Genomic DNA from P. profundum strains EA40 and DB110 was digested with restriction enzymes BglII, HindII, HpaI, or PstI and probed using the fabF internal fragment harbored on pEA39 labeled with [α-32P]dCTP by random priming (Life Technologies, Gaithersburg, Md.).
Isolation of the SS9 fabF gene.
A P. profundum SS9 genomic library (6) was screened using an internal fragment of SS9 fabF in order to identify recombinant library clones harboring the SS9 fabF gene. Colony hybridizations were performed according to standard protocols (40). Plasmid DNA was isolated from positively hybridizing clones and subsequently sequenced. The SS9 fabF gene was PCR amplified from strain DB110 genomic DNA, using primers fabF-F(5′-CTAGTAATGGCTCTTGAAGAAG-3′) and fabF-R(5′-AATTCTTCACGGCAAAATTA-3′). The PCR product was cloned into pCR2.1 and subsequently subcloned into pKT231 (3) as a HindIII-XhoI fragment, yielding pEA44.
Fatty acid analyses.
Extraction and analysis of fatty acid methyl ester preparations via combined gas chromatography-mass spectrometry were performed as previously described (1). Compounds were identified by comparison of retention times with those of known standards (Sigma) as well as sample mass spectra data compared to the Hewlett-Packard G1034C MS ChemStation software NBS75K library containing mass spectra data of 75,000 known compounds. Fatty acids are denoted as number of carbon atoms:number of double bonds. Inner and outer membrane separation and fatty acid analysis on P. profundum strain EA40 grown in the presence of 0.025% Tween 80 at 28 MPa (9°C) were performed in order to show that the 18:1 from Tween 80 was incorporated into membrane phospholipids as previously described (1).
RNA isolation and Northern analyses.
Total RNA was extracted from P. profundum strains grown at various temperatures and pressures using the RNAzol B method (Tel-Test, Inc., Friendswood, Tex.). Equivalent amounts of RNA (10 μg) were electrophoresed through 1.2% formaldehyde agarose, blotted onto a Magnacharge nylon transfer membrane (MSI, Westboro, Mass.), and subjected to Northern analysis using the PCR product contained within pEA39 as the hybridization probe labeled with [α-32P]dCTP by random priming (Life Technologies). Hybridizations were conducted using QuikHyb hybridization solution (Stratagene, La Jolla, Calif.) at a temperature of 64°C.
Nucleotide sequence accession number.
The sequence of P. profundum strain SS9 fabF, along with the partial sequences of acpP and pabC, is deposited in the GenBank database under accession no. AF188707.
RESULTS
Isolation and analysis of the P. profundum strain SS9 fabF gene.
Using PCR primers designed from conserved portions of known fabF sequences (E. coli and V. harveyi), a 740-bp product was amplified from P. profundum strain DB110 genomic DNA and subcloned into pCR2.1 (Invitrogen) to generate pEA39, and its nucleotide sequence was determined. Global similarity searches using gapped-BLAST (2) indicated that the insert DNA present on pEA39 contained an open reading frame with a high degree of similarity to FabF proteins from V. harveyi (E value of 5E-107) and from E. coli (E value of 2E-101). The entire SS9 fabF gene was then isolated following colony blot hybridization of a SS9 genomic library (6), using the insert on pEA39 as a hybridization probe. From all of the three positively hybridizing clones obtained in this way, sequence analysis indicated the presence of fabF DNA. One of the three plasmids chosen for further study, pEA401, contained a 7.5-kb insert. Sequence analysis of pEA401 revealed a gene organization flanking fabF identical to that present in E. coli, V. harveyi, and Pseudomonas aeruginosa (27, 31, 41); in particular it contained the 3′ end of acpP, followed by fabF and pabC. Further sequence downstream of SS9 pabC contained on pEA401 was not obtained.
The SS9 fabF gene was found to display a high degree of similarity and identity to both the E. coli and V. harveyi fabF genes at both nucleotide and deduced amino acid sequence levels. At the DNA level, the SS9 fabF sequence was 73 and 69% identical to fabF sequences of V. harveyi and E. coli, respectively. In addition, possible Rho-independent terminator sequences are present within the SS9 acpP-fabF intergenic region (ending 70 bp upstream of the fabF GTG start) and the fabF-pabC intergenic region (ending 67 bp upstream of the pabC ATG start). These structures are similar in location to putative terminators identified upstream of fabF in V. harveyi, P. aeruginosa, and E. coli and downstream of fabF in V. harveyi and P. aeruginosa. The predicted amino acid sequence of SS9 KAS II (FabF) was 79 (90) and 76% (88%) identical (similar) to KAS II sequences of V. harveyi and E. coli, respectively. No dramatic differences in pI value or amino acid composition were observed between the KAS II enzymes analyzed.
fabF transcript analysis.
To identify and determine the sizes of SS9 fabF transcripts, Northern blotting was performed (Fig. 1). Northern blot analysis of strain DB110 revealed two fabF probe-specific transcripts, one major transcript at approximately 1.9 kb and one minor transcript of approximately 1.5 kb. Zhang and Cronan (52) examined the expression of the E. coli fabF gene and identified two transcripts identical in size to those produced by SS9. Their analysis revealed that the 1.5-kb transcript was a fabF-specific mRNA, whereas the 1.9-kb transcript was most likely the product of cotranscription of fabF and the upstream acpP gene. In SS9, neither of these transcripts exhibited differential abundance at decreased temperature or elevated pressure.
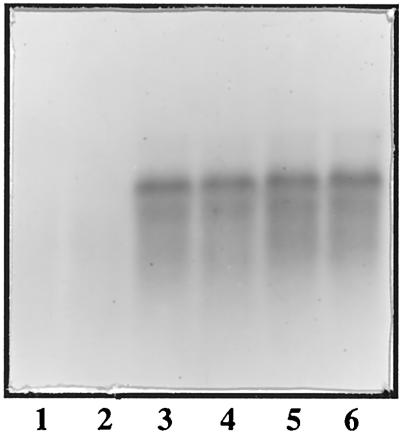
FIG. 1.
Northern analysis of fabF expression in P. profundum strains EA40 (fabF) and DB110 (wild type). Cells were grown to late exponential phase for total RNA isolation and probed using internal fragment of SS9 fabF as described in Materials and Methods. Lanes: 1, EA40 at 4°C; 2, EA40 at 15°C; 3, DB110 at 4°C; 4, DB110 at 15°C; 5, DB110 at 28 MPa (9°C); 6, DB110 at 0.1 MPa (9°C).
Isolation of an SS9 fabF mutant.
To explore the in vivo regulation and function of KAS II in SS9, a fabF mutant was constructed. The isolation of an SS9 fabF mutant followed the introduction of an internal fragment of the SS9 fabF gene, harbored on pEA40, into strain DB110 by conjugal transfer. The ColE1 replicon on this plasmid is mobilizable but replication impaired in SS9 and thus serves as a suicide plasmid allowing selection of plasmid integrants into targeted regions of the chromosome, i.e., those cloned on the plasmid. Kanr exconjugants were screened initially by examining their fatty acid profiles at 4°C compared to 15°C. One exconjugant displaying greatly reduced levels of 18:1 at 4°C was designated EA40 and saved for further study. The creation of a fabF insertion mutation in EA40 was verified by both Southern and Northern blotting. Southern analysis revealed the replacement of specific restriction endonuclease fragments in strain DB110 by DNA approximately 6.3 kb larger in the case of strain EA40 (data not shown). In addition, no fabF-specific transcripts were detected in total RNA extracted from mutant EA40 grown under various temperature conditions (Fig. 1), providing additional verification of the insertional inactivation of fabF in this strain.
Characterization of the fabF mutant as a function of temperature.
The percentages and types of fatty acids produced by strains DB110 and EA40 under different growth conditions are listed in Table 2. When examined at a temperature of 15°C, mutant EA40 exhibited greatly diminished 18:1 levels relative to the parental strain DB110 (4 versus 9.9%, respectively). Moreover, when examined at the reduced temperature of 4°C, mutant EA40 displayed further reductions in 18:1 content, contrary to that observed in strain DB110. At 4°C strain, DB110 produced approximately 10.3% 18:1, whereas the fabF mutant produced only 0.7% 18:1.
TABLE 2.
Fatty acid composition of P. profundum strains as a function of varying temperature and pressurea
| Fatty acid species | Mean wt% fatty acid species ± SD
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Wild type
|
fabF
|
|||||||
| Temp (°C)
|
Pressure (MPa)
|
Temp (°C)
|
Pressure (MPa)
|
|||||
| 15 | 4 | 0.1 MPa | 28 | 15 | 4 | 0.1 MPa | 28 | |
| 12:0 | 1.8 ± 0.9 | 2.7 ± 1.0 | 1.5 ± 0.1 | 2.2 ± 0.6 | 3.2 ± 0.5 | 4.3 ± 1.4 | 4.0 ± 1.3 | 3.4 ± 0.6 |
| 14:0 | 4.9 ± 1.4 | 3.3 ± 0.8 | 5.0 ± 0.3 | 1.6 ± 0.7 | 13.2 ± 1.7 | 18.6 ± 3.2 | 19.6 ± 1.3 | 14.0 ± 1.8 |
| 14:1 | 1.2 ± 0.8 | 2.5 ± 0.5 | 0.8 ± 0.1 | 0.7 ± 0.1 | 4.6 ± 1.1 | 6.3 ± 0.8 | 4.8 ± 0.9 | 3.1 ± 0.8 |
| Iso-16:0 | 9.8 ± 3.1 | 0.2 ± 0.1 | 1.2 ± 1.3 | |||||
| 16:0 | 21.7 ± 2.8 | 20.2 ± 3.8 | 21.4 ± 1.5 | 17.5 ± 3.2 | 19.2 ± 2.6 | 10.5 ± 2.2 | 19.7 ± 1.3 | 16.2 ± 1.2 |
| 16:1 | 41.3 ± 8.3 | 48.8 ± 6.8 | 64.1 ± 1.5 | 48.7 ± 9.6 | 41.5 ± 4.8 | 39.3 ± 6.8 | 31.4 ± 1.1 | 44.9 ± 0.7 |
| 12-OH | 3.0 ± 1.2 | 3.4 ± 1.3 | 0.6 ± 0.1 | 2.0 ± 0.8 | 3.8 ± 1.0 | 4.1 ± 1.2 | 3.4 ± 1.2 | 5.2 ± 0.1 |
| 18:0 | 1.1 ± 0.4 | 0.2 ± 0.1 | 0.2 ± 0.0 | 0.1 ± 0.1 | 1.9 ± 0.4 | 0.1 ± 0.0 | 0.7 ± 0.1 | 0.4 ± 0.1 |
| 18:1 | 9.9 ± 2.6 | 10.3 ± 1.7 | 3.6 ± 0.1 | 16.2 ± 1.6 | 4.0 ± 0.8 | 0.7 ± 0.1 | 1.1 ± 0.3 | 1.1 ± 0.3 |
| 20:5 | 5.3 ± 1.1 | 8.4 ± 1.1 | 2.7 ± 0.1 | 11.0 ± 0.8 | 7.1 ± 1.7 | 15.9 ± 2.1 | 15.1 ± 3.8 | 11.4 ± 1.5 |
| UFA/SFA ratiob | 1.95 | 2.65 | 2.53 | 3.57 | 1.53 | 1.91 | 1.20 | 1.77 |
Data represent values derived from triplicate samples harvested in late-exponential-phase growth; 15 and 4°C cultures were grown aerobically at 0.1 MPa; 0.1- and 28-MPa cultures were grown at 9°C in 2216 marine medium containing 22 mM glucose buffered with 100 mM HEPES.
UFAs, 14:1, 16:1, 18:1, and 20:5; SFAs, 12:0, 14:0, 16:0, and 18:0.
In concert with the reduction of 18:1, the fabF mutant exhibited elevated levels of the saturated fatty acid 14:0 and the MUFA 14:1 and also upregulated the abundance of the omega-3 PUFA all-cis-5,8,11,14,17-eicosapentaenoic acid (EPA; 20:5). Specifically at 4°C, mutant EA40 also displayed dramatically reduced 16:0 and 16:1 levels relative to parental strain DB110. We have previously noted that chemical mutants of SS9 or drug treatments which reduce the amount of MUFAs produced by SS9 result in increased 14:0 and EPA levels (1). We do not yet understand how it is that a fabF mutation induces such pleiotropic effects on fatty acid composition. As previously reported for P. profundum strain SS9, differences in iso-16:0 content reflect differences in phase of growth at time of harvesting (1). Overall, the ratios of unsaturated to saturated fatty acids (UFA/SFA) of mutant EA40 were comparable to those for strain DB110 under various temperature conditions (Table 2).
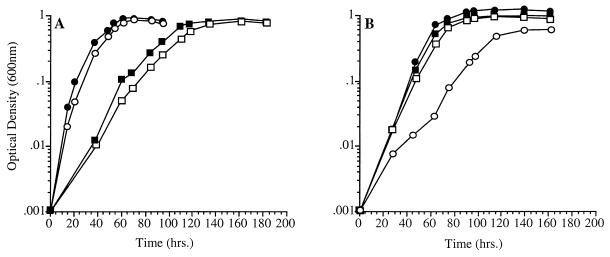
The growth characteristics of the fabF mutant at two temperatures are shown in Fig. 2A. At both 15 and 4°C, mutant EA40 displayed growth rates and yields essentially identical to those for parental strain DB110. These results are consistent with findings in E. coli, where no growth defect at reduced temperatures have been ascribed to fabF strains (43).
FIG. 2.
Growth characteristics of fabF disruption mutant P. profundum strain EA40 and strain DB110 as a function of temperature (A) and pressure (B). (A) ●, DB110 at 15°C; ○, EA40 at 15°C; ■, DB110 at 4°C; □, EA40 at 4°C. (B) ●, DB110 at 28 MPa; ○, EA40 at 28 MPa; ■, DB110 at 0.1 MPa; □, EA40 at 0.1 MPa.
Characterization of the fabF mutant as a function of pressure.
Table 2 displays the fatty acid profiles of mutant EA40 and strain DB110 at 0.1 MPa (9°C) and 28 MPa (9°C). At 0.1 MPa (9°C), mutant EA40 exhibited markedly reduced 18:1 and 16:1 levels and substantially increased 14:0, 14:1, and EPA levels compared to strain DB110. Similarly, at elevated pressure the most dramatic alterations in fatty acid content of mutant EA40 relative to strain DB110 included severe reduction in 18:1 content (1.1 versus 16.2%, respectively) and elevated 14:0 content. These high-pressure EA40 values are similar to those of the mutant grown at 4°C (0.1 MPa). However, at the elevated pressure of 28 MPa (9°C), mutant EA40 did not upregulate EPA production as it did at 4°C. EPA content actually decreased upon a shift in growth pressure from 0.1 MPa (9°C) to 28 MPa (9°C) in mutant EA40 (15.1 versus 11.4%, respectively). This is in contrast to strain DB110, wherein EPA content increased from 2.7 to 11% upon pressurization to 28 MPa. Moreover, the UFA/SFA ratios of mutant EA40 at 28 and 0.1 MPa (1.77 and 1.20, respectively) were substantially lower than those for strain DB110 (3.57 and 2.53, respectively).
The growth characteristics of mutant EA40 at two pressures are shown in Fig. 2B. The effect of fabF disruption on cell growth was detrimental at elevated pressure. At 0.1 MPa (9°C), the mutant and parental strains exhibited essentially identical growth abilities. However, at an elevated pressure of 28 MPa (9°C), mutant EA40 displayed an extended lag phase, decreased growth rate, and reduced overall yield in comparison to strain DB110. These results represent the first identification of a growth phenotype associated with sole disruption or mutation of the fabF gene.
It is conceivable that the difference between temperature and pressure growth and fatty acid regulation observed in strain EA40 is the result of an anaerobic effect (which pressure cultivation necessitates). In other words, high-pressure cultivation conditions alone may be responsible for the observed high-pressure growth phenotype in strain EA40. To address this possibility, strains EA40 and DB110 were grown at 4 and 15°C (0.1 MPa) under conditions identical to those used with pressure cultivation (in heat-sealable bulbs with glucose and HEPES added). These experiments showed no apparent cold sensitivity in strain EA40, suggesting that the high-pressure-sensitive phenotype of EA40 is the result of pressure effects and not the result of cultivation conditions (data not shown).
Nutritional complementation of strain EA40.
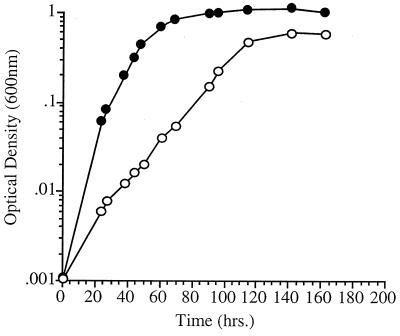
If the basis of the high-pressure growth defect in EA40 stems from its reduced abundance of 18:1, it should be possible to nutritionally complement this defect by providing an exogenous supply of this fatty acid in the growth medium. This approach was previously used with partial success in overcoming the pressure-sensitive and cold-sensitive growth characteristics of a chemical mutagen-derived mutant of SS9 deficient in the production of both 16:1 and 18:1 fatty acids (1). The growth characteristics of mutant EA40 at 28 MPa (9°C) in the presence or absence of exogenous 18:1 in the form of 0.025% Tween 80 are shown in Fig. 3. In the presence of exogenous 18:1, mutant EA40 exhibited completely restored growth characteristics at elevated pressure. Fatty acid analysis of inner and outer membrane and total phospholipid fractions of strain EA40 grown in the presence of Tween 80 at 28 MPa (9°C) revealed incorporation of 18:1 into the membrane phospholipids, suggesting that the exogenous 18:1 supplied is actively utilized for membrane restructuring (data not shown). These results provide further evidence of the need for 18:1 fatty acid for growth at high pressure.
FIG. 3.
Nutritional complementation of P. profundum fabF mutant EA40 at elevated pressure (28 MPa, 9°C). ●, plus 18:1 supplementation; ○, minus 18:1.
E. coli does not regulate fatty acid composition in an adaptive manner in response to pressure changes.
Although the effects of pressure on fatty acid composition in numerous piezophilic and piezotolerant bacteria have been well documented (1, 11, 12, 47), the effect of a pressure increase on the fatty acid composition of a non-pressure-adapted bacterial species has not yet been described. To determine whether the observed effect of elevated pressure on deep-sea bacterial fatty acids is reflective of an adaptive feature specific to microorganisms which have evolved in high-pressure, pressure-variable environments, or if the response simply reflects a similar physical effect of high pressure and low temperature on some aspect of cell structure or physiology (i.e., membrane fluidity), experiments were conducted using E. coli as a representative of a mesophilic bacterial species.
Table 3 shows the fatty acid profiles of E. coli strains MR86 (fabF::Kmr) and its parental strain SJ16 at 30 MPa (37°C) and 0.1 MPa (37°C) in pressurizable bulb cultures. As described in Materials and Methods, high-pressure cultivation necessitated growth under microaerobic conditions with glucose and HEPES added to the media. In contrast to the fatty acid changes observed in wild-type E. coli strains in response to temperature downshift, wherein increased production of UFAs 18:1 and 16:1 are observed at the expense of 16:0 (33), no changes in fatty acid profile were evident when either strain (SJ16 or MR86) was grown at high pressure (Table 2). The UFA/SFA ratios of strain SJ16 at the two pressures were essentially identical, 0.41 at 30 MPa and 0.38 at 0.1 MPa. Similar results were obtained for fabF strain MR86 at various pressures. At 30 MPa (37°C), strain MR86 experiences a slight reduction in UFA production and an increase in SFA content relative to 0.1 MPa (37°C) cultivation. Specifically, high-pressure incubation resulted in an overall increase in 16:0 in combination with a reduction in 16:1 content. Consequently, substantially reduced UFA/SFA ratios are observed at elevated pressure in this strain (0.16 at 30 MPa, compared to 0.38 at 0.1 MPa). Unlike pressure increase, temperature decrease (from 37°C to 15°C) elicited approximately a twofold increase in UFA/SFA ratios in both strains (approximately 0.6 to 1.2; our unpublished results).
TABLE 3.
Fatty acid composition of E. coli strains as a function of varying pressurea
| Fatty acid species | Mean wt% fatty acid species ± SD
|
|||
|---|---|---|---|---|
| Wild type
|
fabF
|
|||
| 0.1 MPa | 30 MPa | 0.1 MPa | 30 MPa | |
| 12:0 | 4.54 ± 0.3 | 4.89 ± 0.9 | 3.75 ± 0.6 | 3.96 ± 0.2 |
| 14:0 | 10.82 ± 1.7 | 9.81 ± 1.4 | 11.29 ± 0.0 | 11.07 ± 1.8 |
| 16:0 | 47.02 ± 4.5 | 49.22 ± 9.1 | 50.79 ± 6.2 | 62.54 ± 0.1 |
| 16:1 | 14.55 ± 5.3 | 15.24 ± 0.6 | 24.43 ± 9.6 | 12.60 ± 1.9 |
| 18:0 | 1.63 ± 1.0 | 1.74 ± 2.2 | 0.72 ± 0.4 | 1.51 ± 0.8 |
| 18:1 | 11.90 ± 2.1 | 9.70 ± 4.7 | 1.10 ± 0.1 | 0.46 ± 0.2 |
| 14-OH | 9.54 ± 1.1 | 9.40 ± 1.6 | 7.92 ± 2.5 | 7.86 ± 0.2 |
| UFA/SFA ratiob | 0.41 | 0.38 | 0.38 | 0.16 |
Data represents values derived from triplicate samples harvested in mid-exponential-phase growth. Cultures were grown at 37°C in LB medium containing 22 mM glucose buffered with 100 mM HEPES.
UFAs, 16:1 and 18:1; SFAs, 12:0, 14:0, 16:0, and 18:0.
Results comparable to those obtained with SJ16 and MR86 were obtained with other E. coli strains, specifically MG1655 (21) and 1100 (15), which also displayed lower UFA/SFA ratios at 30- than at 0.1-MPa cultivation. Moreover, fatty acid analysis was conducted on all four of the E. coli strains studied at 30 and 0.1 MPa at a temperature of 30°C in addition to 37°C. No dramatic fatty acid content differences were observed in any of the E. coli strains at 30 MPa (30°C) compared to 0.1 MPa (30°C) cultivation (data not shown). The conclusion from these studies is that E. coli does not modulate fatty acid production in an adaptive manner in response to a pressure increase, and therefore the response of many piezophilic bacteria to increase UFA levels with pressure is likely to be an acclimatory feature specific to microorganisms inhabiting high-pressure environments.
Similar to arguments made with P. profundum strain EA40, it is possible that the lack of fatty acid regulation at elevated pressure in E. coli strains is the result of anaerobic rather than pressure effects. When grown at 15 and 37°C (0.1 MPa) in pressurizable bulbs, all E. coli strains (MR86, SJ16, MG1655, and 1100) exhibited approximately twofold increases in UFA/SFA ratios and no apparent decreased temperature sensitivities (data not shown). These results suggest that the lack of an adaptive fatty acid regulatory response of E. coli with exposure to elevated pressure is not a factor of the conditions used for pressure cultivation.
E. coli fabF mutants do not display increased pressure sensitivity.
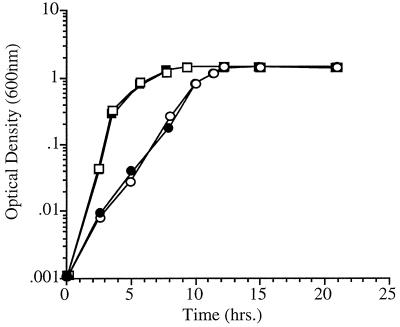
Another contrasting feature between SS9 and E. coli concerns the role of KAS II in growth at elevated pressure. The growth characteristics of E. coli strain SJ16 and its fabF mutant derivative MR86 at various pressures are shown in Fig. 4. Previous reports have indicated that E. coli fabF mutants are not cold sensitive (20). Likewise, at both 0.1 and 30 MPa (37°C), the growth of strain MR86 was identical to that of parental strain SJ16. Finally, no differences in the growth characteristics of the two strains were observed even when pressure cultivation was performed at reduced temperatures (30 and 15°C, 30 MPa; data not shown). Thus, even the combined effects of elevated pressure and reduced temperature did not result in differential growth susceptibility in fabF strain MR86 relative to strain SJ16.
FIG. 4.
Growth characteristics of E. coli fabF disruption mutant MR86 and parental strain SJ16 as a function of pressure. See Materials and Methods for cultivation conditions. Pressure experiments were conducted at a temperature of 37°C. ●, SJ16 at 30 MPa; ○, MR86 at 30 MPa; ■, SJ16 at 0.1 MPa; □, MR86 at 0.1 MPa.
DISCUSSION
The state of the physical environment (i.e., pressure and temperature) influences the physical properties of biological membranes (22). Decreases in temperature or increases in hydrostatic pressure increase the molecular order of the fatty acyl chains and promotes tighter packing of the phospholipids. The biological response to such environmental conditions often entails the retailoring of membrane composition, most notably increases in fatty acid unsaturation (39, 49). This modification is believed to optimize membrane structure and function by offsetting the direct effects imposed by temperature and pressure.
Previous studies employing the deep-sea bacterium P. profundum strain SS9 have revealed direct correlations between UFA production and growth ability at low temperature or elevated pressure (1, 4). In the present study, we have targeted a key enzyme involved in UFA production, the KAS II product of the fabF gene. Results presented here indicate that SS9 KAS II is required for (i) the piezoregulation of cis-vaccenic acid (18:1) and (ii) piezophilic growth. In addition, our data indicate that pressure modulation of fatty acid levels is likely to be an adaptive feature unique to high-pressure-adapted microorganisms.
Since the fabF gene plays an essential role in thermal regulation of fatty acid composition in E. coli, we hypothesized that the SS9 fabF gene may play a similar role in the increased production of 18:1 observed in response to pressure increase. P. profundum strain EA40 containing an insertionally inactivated fabF gene was engineered using a reverse genetics methodology employing the suicide plasmid pMUT100 (7). Based on the fatty acid profile of strain EA40, a dual role for SS9 KAS II has been revealed. In addition to temperature regulation, the lack of 18:1 modulation in response to pressure increase in strain EA40 suggests that SS9 KAS II is responsible for the substantial increase in 18:1 levels observed in response to pressure increase in fabF+ strains (Table 2).
Studies in E. coli have shown that thermal modulation of 18:1 production does not involve de novo enzyme synthesis (17). Such regulation resides at the level of enzyme activity, where KAS II exhibits increased catalytic efficiency at reduced temperature (16, 18). Northern analyses were performed using P. profundum strain DB110 cultivated under different pressure and temperature conditions in order to determine if the SS9 fabF gene exhibits differential expression. Identical transcripts (sizes and amounts) were detected under all pressure and temperature conditions examined, suggesting that the SS9 fabF gene is not transcriptionally regulated in response to various cultivation parameters (Fig. 1). In light of these results, we hypothesize that SS9 KAS II displays increased catalytic activity at elevated pressure just as E. coli KAS II does at reduced temperature. Given the high level of identity between KAS II of SS9 and that of non-pressure-adapted organisms (E. coli KAS II is 76/88% identical/similar to SS9 KAS II), it will be of interest to analyze the structure and function of SS9 KAS II in comparison to the E. coli enzyme. Of course, the fact that SS9 fabF is not transcriptionally regulated does not preclude the possibility that some posttranscriptional processing event occurs which influences KAS II abundance at elevated pressure, thereby influencing 18:1 synthesis.
In strain DB110, a pressure increase from 0.1 MPa to 28 MPa results in greater than a fourfold increase in 18:1 levels, rising up to 16% of total fatty acids (Table 2). However, under identical conditions, 18:1 comprised only 1.1% of the total fatty acids in mutant EA40. The inability to regulate 18:1 levels at elevated pressure resulted in pronounced high-pressure sensitivity in this strain (Fig. 2). The fact that supplementation of this mutant with exogenous 18:1 resulted in restoration of wild-type-like growth rates and yields at elevated pressure (Fig. 3) suggests that the 18:1 defect is responsible for its high-pressure-sensitive growth phenotype. These results represent the first reported growth alteration observed in any bacterial strain in which fabF is the only lesion in lipid synthesis. It should be noted, however, that E. coli strains harboring a mutation in fabF as well as an additional temperature-sensitive mutation in fabB (KAS I) are incapable of producing any long-chain fatty acids at the nonpermissive temperature (19).
In contrast to high-pressure conditions, strain EA40 exhibited no apparent growth sensitivity at reduced temperature despite producing only trace levels of 18:1 at 4°C. Contrary to pressure increase, temperature decrease did not elicit substantially elevated 18:1 production in wild-type SS9 strains (Table 2). Hence, some aspect of high-pressure growth ability appears reliant upon 18:1 production which is not apparent at low temperature. The fact that EPA levels increase significantly in response to temperature downshift in mutant EA40 but not with pressure increase is puzzling. It would appear SS9 is capable of compensating for decreased 18:1 levels at 4°C by increased EPA production but not at increased pressure. It could be either the change in global membrane fluidity (or membrane phase state) or that within the local environment of a key membrane protein that accounts for the pressure sensitivity of 18:1-deficient strains. Membrane proteins which are known or implicated as important for piezophilic growth include CydD (required for the assembly of the cytochrome bd respiratory complex [26]), RseC (an inner membrane protein of unknown function [5, 9]), and the ToxR transcription factor (which regulates the differential expression of outer membrane protein encoding genes as a function of pressure [45]). It is possible that membrane perturbation resulting from altered 18:1 synthesis contributes to the decreased activity of some key membrane component and consequently pressure sensitivity.
Attempts to complement mutant EA40 by the introduction of the wild-type SS9 fabF gene into this strain were unsuccessful. Despite the creation of numerous SS9 fabF constructs, expression problems prevented complementation of either the 18:1 defect or the high-pressure-sensitive phenotype of mutant EA40 by fabF containing plasmids (our unpublished results). One possibility is that all of our plasmid constructs lacked a fabF promoter. It could be that the two fabF transcripts observed in SS9 by Northern blotting result from a single promoter upstream of acpP and that the fabF-specific message arises from a posttranscriptional processing event. However, even SS9 fabF containing plasmids which placed fabF transcription under the control of the Kanr promoter on pKT231 (3) still failed to transcribe fabF, even when a potential Rho-independent terminator sequence was removed from fabF upstream DNA. These results suggest that some mechanism for tight control of fabF transcription may exist in SS9. Coordinating fabF expression with that of other fab cluster genes is critical to viability in E. coli. Excess fabF transcription leads to the cessation of fatty acid synthesis as a result of blockage of fatty acyl chain elongation (42). Because of these FabF toxicity effects, E. coli fabF mutants have yet to be genetically complemented.
Fatty acid compositional adjustment is a near-ubiquitous response to temperature change among bacteria (39). However, unlike temperature, pressure variation is a seldom encountered environmental parameter outside of the deep sea or deep subsurface. Previous studies which have documented pressure regulation of fatty acids in deep-sea bacteria have inferred that such changes reflect acclimation to pressure change (1, 11, 12, 47). However, another possibility is that because high pressure exerts a physical change on membrane structure similar to that of a drop in temperature (30), most microbes would perceive high pressure as low temperature and respond accordingly to restore membrane fluidity or phase. Our results with E. coli indicate that at least for this organism, the latter possibility is not the case even though this mesophile is quite piezotolerant, capable of growing at pressures up to 50 MPa (54).
Fatty acid profiling of E. coli was performed at various pressures to determine whether fatty acid composition in this mesophile is responsive to pressure change as it is to temperature change. Because various E. coli strains were discovered to exhibit similar fatty acid compositions at low and high pressures, the results indicated that the capacity for thermal regulation of UFAs in bacteria does not necessarily predispose microorganisms to respond in a similar fashion to pressure changes, despite the fact that both parameters can be manipulated to produce similar effects on membrane structure. These results are in accordance with observations made using cultures of the mesophilic protozoan Tetrahymena pyriformis NT-1 (28). As with E. coli, exposure of T. pyriformis to 26 MPa did not result in changes in fatty acid composition or fluidity of microsomal membranes. Of course, the possibility exists that not all surface-living bacteria would respond as E. coli does to elevated pressure. For example, bacteria which possess membrane-localized desaturases may increase UFA production at elevated pressure due to activation of such enzymes in response to membrane fluidity changes.
While investigating pressure effects on the fatty acids produced by E. coli, we also examined the effect of loss of KAS II on E. coli growth at elevated pressure (Fig. 4). Previous reports have described the growth characteristics of E. coli at elevated hydrostatic pressure (6, 53, 54). Upon pressurization, E. coli experiences retardation in growth and reproduction owing in part to inhibition in macromolecular synthesis and cell division (51, 55). The response of E. coli to elevated hydrostatic pressure results in a unique stress response which results in induction of numerous heat shock and cold shock proteins as well as many proteins which appear solely in response to high pressure (46). Despite numerous studies having investigated high-pressure effects on specific functions in E. coli, it is still unclear as to the key pressure point(s) which limits its growth ability at elevated pressure (32, 37). Due to the physical effects elevated pressure is known to exert on biological membranes (22), it was hypothesized that the state of the membrane could represent such a pressure point. Since an E. coli fabF mutant did not exhibit increased pressure sensitivity, something other than membrane phospholipid structure most likely limits E. coli growth at high pressure. Possible limiting factors include aspects of chromosome partitioning (6), macromolecular synthesis (51), cytochrome function (26), or proton translocation and ATP production (32).
ACKNOWLEDGMENT
This work was supported by grant MCB96-30546 from the National Science Foundation to D.H.B.
REFERENCES
- 1.Allen E E, Facciotti D, Bartlett D H. Monounsaturated but not polyunsaturated fatty acids are required for growth of the deep-sea bacterium Photobacterium profundum strain SS9 at low temperature and high pressure. Appl Environ Microbiol. 1999;65:1710–1720. doi: 10.1128/aem.65.4.1710-1720.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Altschul S F, Madden T L, Schaffer A A, Zhang J, Zhang Z, Miller W, Lipman D J. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bagdasarian M, Lurz R, Ruckert B, Franklin F C, Bagdasarian M M, Frey J, Timmis K N. Specific-purpose plasmid cloning vectors. II. Broad host range, high copy number, RSF1010-derived vectors, and a host-vector system for gene cloning in Pseudomonas. Gene. 1981;16:237–247. doi: 10.1016/0378-1119(81)90080-9. [DOI] [PubMed] [Google Scholar]
- 4.Bartlett D H, Allen E E. Role of unsaturated fatty acids in growth at high pressure and low temperature in the deep-sea bacterium Photobacterium species strain SS9. In: Bennett P B, Demchenko I, Marquis R E, editors. High pressure biology and medicine. Rochester, N.Y: University of Rochester Press; 1998. pp. 32–37. [Google Scholar]
- 5.Bartlett D H, Chi E, Welch T J. High pressure sensing and adaptation in the deep-sea bacterium Photobacterium species strain SS9. In: Hayashi R, Balny C, editors. High pressure bioscience and biotechnology. Amsterdam, The Netherlands: Elsevier Science B.V.; 1996. pp. 29–36. [Google Scholar]
- 6.Bidle K A, Bartlett D H. RecD function is required for high-pressure growth in a deep-sea bacterium. J Bacteriol. 1999;181:2330–2337. doi: 10.1128/jb.181.8.2330-2337.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brahamsha B. A genetic manipulation system for oceanic cyanobacteria of the genus Synechococcus. Appl Environ Microbiol. 1996;62:1747–1751. doi: 10.1128/aem.62.5.1747-1751.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chi E, Bartlett D H. Use of a reporter gene to follow high-pressure signal transduction in the deep-sea bacterium Photobacterium sp. strain SS9. J Bacteriol. 1993;175:7533–7540. doi: 10.1128/jb.175.23.7533-7540.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chi E, Bartlett D H. An rpoE-like locus controls outer membrane protein synthesis and growth at cold temperatures and high pressures in the deep-sea bacterium Photobacterium sp. strain SS9. Mol Microbiol. 1995;17:713–726. doi: 10.1111/j.1365-2958.1995.mmi_17040713.x. [DOI] [PubMed] [Google Scholar]
- 10.Cronan J E, Jr, Rock C O. Biosynthesis of membrane lipids. In: Neidhardt F C, Curtiss III R, Ingraham J L, Lin E C C, Low K B, Magasanik B, Reznikoff W S, Riley M, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella typhimurium: cellular and molecular biology. 2nd ed. Washington, D.C.: American Society for Microbiology; 1996. pp. 612–636. [Google Scholar]
- 11.DeLong E F, Yayanos A A. Adaptation of the membrane lipids of a deep-sea bacterium to changes in hydrostatic pressure. Science. 1985;228:1101–1103. doi: 10.1126/science.3992247. [DOI] [PubMed] [Google Scholar]
- 12.DeLong E F, Yayanos A A. Biochemical function and ecological significance of novel bacterial lipids in deep-sea procaryotes. Appl Environ Microbiol. 1986;51:730–737. doi: 10.1128/aem.51.4.730-737.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.de Mendoza D, Farias R N. Effect of fatty acid supplementation on membrane fluidity in microorganisms. In: Aloia R C, Curtain C C, Gordon L M, editors. Physiological regulation of membrane fluidity. New York, N.Y: Alan R. Liss, Inc.; 1988. pp. 119–149. [Google Scholar]
- 14.de Mendoza D, Ulrich A K, Cronan J E., Jr Thermal regulation of membrane fluidity in Escherichia coli: effects of overproduction of β-ketoacyl-acyl carrier protein synthase I. J Biol Chem. 1983;258:2098–2101. [PubMed] [Google Scholar]
- 15.Durwald H, Hoffmann-Berling H. Endonuclease I-deficient and ribonuclease I-deficient Escherichia coli mutants. J Mol Biol. 1968;34:331–346. doi: 10.1016/0022-2836(68)90257-x. [DOI] [PubMed] [Google Scholar]
- 16.Edwards P, Nelson J S, Metz J G, Dehesh K. Cloning of the fabF gene in an expression vector and in vitro characterization of recombinant fabF and fabB encoded enzymes from Escherichia coli. FEBS Lett. 1997;402:62–66. doi: 10.1016/s0014-5793(96)01437-8. [DOI] [PubMed] [Google Scholar]
- 17.Garwin J L, Cronan J E., Jr Thermal modulation of fatty acid synthesis in Escherichia coli does not involve de novo enzyme synthesis. J Bacteriol. 1980;141:1457–1459. doi: 10.1128/jb.141.3.1457-1459.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Garwin J L, Klages A L, Cronan J J E. Structural, enzymatic, and genetic studies of β-ketoacyl-acyl carrier protein synthases I and II of Escherichia coli. J Biol Chem. 1980;255:11949–11956. [PubMed] [Google Scholar]
- 19.Garwin J L, Klages A L, Cronan J E., Jr β-Ketoacyl-acyl carrier protein synthase II of Escherichia coli. J Biol Chem. 1980;255:3263–3265. [PubMed] [Google Scholar]
- 20.Gelmann E P, Cronan J E., Jr Mutant of Escherichia coli deficient in the synthesis of cis-vaccenic acid. J Bacteriol. 1972;112:381–387. doi: 10.1128/jb.112.1.381-387.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guyer M S. Identification of a sex-factor-affinity site in E. coli as γδ. Cold Spring Harbor Symp Quant Biol. 1981;45:135–140. doi: 10.1101/sqb.1981.045.01.022. [DOI] [PubMed] [Google Scholar]
- 22.Hazel J R, Williams E E. The role of alterations in membrane lipid composition in enabling physiological adaptation of organisms to their physical environment. Prog Lipid Res. 1990;29:167–227. doi: 10.1016/0163-7827(90)90002-3. [DOI] [PubMed] [Google Scholar]
- 23.Higgins D G, Sharp P M. CLUSTAL: a package for performing multiple sequence alignment on a microcomputer. Gene. 1988;73:237–244. doi: 10.1016/0378-1119(88)90330-7. [DOI] [PubMed] [Google Scholar]
- 24.Jackowski S, Rock C O. Turnover of the 4′-phosphopantetheine prosthetic group of acyl carrier protein. J Biol Chem. 1984;259:1891–1895. [PubMed] [Google Scholar]
- 25.Kamimura K, Fuse H, Takimura O, Yamaoka Y. Effects of growth pressure and temperature on fatty acid composition of a barotolerant deep-sea bacterium. Appl Environ Microbiol. 1993;59:924–926. doi: 10.1128/aem.59.3.924-926.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kato C, Tamegai H, Ikegami A, Usami R, Horikoshi K. Open reading frame 3 of the barotolerant bacterium strain DSS12 is complementary with cydD in Escherichia coli: cydD functions are required for cell stability. J Biochem. 1996;120:301–305. doi: 10.1093/oxfordjournals.jbchem.a021413. [DOI] [PubMed] [Google Scholar]
- 27.Kutchma A J, Hoang T T, Schwizer H P. Characterization of a Pseudomonas aeruginosa fatty acid biosynthetic gene cluster: purification of acyl carrier protein (ACP) and malonyl-coenzyme A:ACP transacylase (FabD) J Bacteriol. 1999;181:5498–5504. doi: 10.1128/jb.181.17.5498-5504.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Macdonald A G. Homeoviscous theory under pressure. I. The fatty acid composition of Tetrahymena pyriformis NT-1 grown at high pressure. Biochim Biophys Acta. 1984;775:141–149. [Google Scholar]
- 29.Macdonald A G. The role of membrane fluidity in complex processes under high pressure. In: Marquis R E, Zimmerman A M, Jannasch H W, editors. Current perspectives in high pressure biology. London, England: Academic Press; 1987. pp. 207–223. [Google Scholar]
- 30.Macdonald A G, Cossins A R. The theory of homeoviscous adaptation of membranes applied to deep-sea animals. Symp Soc Exp Biol. 1985;39:301–322. [PubMed] [Google Scholar]
- 31.Magnuson K, Carey M R, Cronan J E., Jr The putative fabJ gene of Escherichia coli fatty acid synthesis is the fabF gene. J Bacteriol. 1995;177:3593–3595. doi: 10.1128/jb.177.12.3593-3595.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Marquis R E, Bender G R. Barophysiology of prokaryotes and proton-translocating ATPase. In: Jannasch H W, Marquis R E, Zimmerman A M, editors. Current perspectives in high-pressure biology. London, England: Academic Press Ltd.; 1987. pp. 65–73. [Google Scholar]
- 33.Marr A G, Ingraham J L. Effect of temperature on the composition of fatty acids in Escherichia coli. J Bacteriol. 1962;84:1260–1267. doi: 10.1128/jb.84.6.1260-1267.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Morita N, Gotoh M, Okajima N, Okuyama H, Hayashi H, Higashi S, Murata N. Both the anaerobic pathway and aerobic desaturation are involved in the synthesis of unsaturated fatty acids in Vibrio sp. strain ABE-1. FEBS Lett. 1992;297:9–12. doi: 10.1016/0014-5793(92)80316-9. [DOI] [PubMed] [Google Scholar]
- 35.Nicholas K B, Nicholas H B J. GeneDoc. Pittsburgh, Pa: Pittsburgh Supercomputing Center; 1997. [Google Scholar]
- 36.Okuyama H, Okajima N, Sasaki S, Higashi S, Murata N. The cis/trans isomerization of the double bond of a fatty acid as a strategy for adaptation to changes in ambient temperature in the psychrophilic bacterium, Vibrio sp. strain ABE-1. Biochim Biophys Acta. 1991;1084:13–20. doi: 10.1016/0005-2760(91)90049-n. [DOI] [PubMed] [Google Scholar]
- 37.Pope D H, Berger L R. Inhibition of metabolism by hydrostatic pressure: what limits microbial growth? Arch Mikrobiol. 1973;93:367–370. doi: 10.1007/BF00427933. [DOI] [PubMed] [Google Scholar]
- 38.Rock C O, Cronan J E., Jr Escherichia coli as a model for the regulation of dissociable (type II) fatty acid biosynthesis. Biochim Biophys Acta. 1996;1302:1–16. doi: 10.1016/0005-2760(96)00056-2. [DOI] [PubMed] [Google Scholar]
- 39.Russell N J, Hamamoto T. Psychrophiles. In: Horikoshi K, Grant W D, editors. Extremophiles: microbial life in extreme environments. New York, N.Y: Wiley-Liss, Inc.; 1998. pp. 25–45. [Google Scholar]
- 40.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- 41.Shen Z, Byers D M. Isolation of Vibrio harveyi acyl carrier protein and the fabG, acpP, and fabF genes involved in fatty acid biosynthesis. J Bacteriol. 1996;178:571–573. doi: 10.1128/jb.178.2.571-573.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Subrahmanyam S, Cronan J E., Jr Overproduction of a functional fatty acid biosynthesis enzyme blocks fatty acid synthesis in Escherichia coli. J Bacteriol. 1998;180:4596–4602. doi: 10.1128/jb.180.17.4596-4602.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ulrich A L, de Mendoza D, Garwin J L, Cronan J E., Jr Genetic and biochemical analyses of Escherichia coli mutants altered in temperature-dependent regulation of membrane lipid composition. J Bacteriol. 1983;154:221–230. doi: 10.1128/jb.154.1.221-230.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wada H, Gombos Z, Murata N. Enhancement of chilling tolerance of a cyanobacterium by genetic manipulation of fatty acid desaturation. Nature. 1990;347:200–203. doi: 10.1038/347200a0. [DOI] [PubMed] [Google Scholar]
- 45.Welch T J, Bartlett D H. Identification of a regulatory protein required for pressure-responsive gene expression in the deep-sea bacterium Photobacterium species strain SS9. Mol Microbiol. 1998;27:977–985. doi: 10.1046/j.1365-2958.1998.00742.x. [DOI] [PubMed] [Google Scholar]
- 46.Welch T J, Farewell A, Neidhardt F C, Bartlett D H. Stress response of Escherichia coli to elevated hydrostatic pressure. J Bacteriol. 1993;175:7170–7177. doi: 10.1128/jb.175.22.7170-7177.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wirsen C O, Jannasch H W, Wakeham S G, Canuel E A. Membrane lipids of a psychrophilic and barophilic deep-sea bacterium. Curr Microbiol. 1987;14:319–322. [Google Scholar]
- 48.Yano Y, Nakayama A, Yoshida K. Distribution of polyunsaturated fatty acids in bacteria present in intestines of deep-sea fish and shallow-sea poikilothermic animals. Appl Environ Microbiol. 1997;63:2572–2577. doi: 10.1128/aem.63.7.2572-2577.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yayanos A A. Emipirical and theoretical aspects of life at high pressure in the deep sea. In: Horikoshi K, Grant W D, editors. Extremophiles: microbial life in extreme environments. New York, N.Y: Wiley-Liss, Inc.; 1998. pp. 47–92. [Google Scholar]
- 50.Yayanos A A, Van Boxtel R. Coupling device for quick high pressure connection to 1000 MPa. Rev Sci Instrum. 1982;53:704–705. [Google Scholar]
- 51.Yayanos A A, Pollard E C. A study of the effects of hydrostatic pressure on macromolecular synthesis in Escherichia coli. Biophys J. 1969;9:1464–1482. doi: 10.1016/S0006-3495(69)86466-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang Y, Cronan J E., Jr Polar allele duplication for transcriptional analysis of consecutive essential genes: application to a cluster of Escherichia coli fatty acid biosynthetic genes. J Bacteriol. 1996;178:3614–3620. doi: 10.1128/jb.178.12.3614-3620.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.ZoBell C E. Pressure effects on morphology and life processes of bacteria. London, England: Academic Press; 1970. [Google Scholar]
- 54.ZoBell C E, Cobet A B. Growth, reproduction, and death rates of Escherichia coli at increased hydrostatic pressure. J Bacteriol. 1962;84:1228–1236. doi: 10.1128/jb.84.6.1228-1236.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.ZoBell C E, Cobet A B. Filament formation by Escherichia coli at increased hydrostatic pressures. J Bacteriol. 1963;87:710–719. doi: 10.1128/jb.87.3.710-719.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]