Abstract
Methods
Eight databases, PubMed, CINAHL, Web of Science, Embase, PsycINFO, Cochrane Library, Popline, and Maternity and Infant Care, were searched, covering the period of January 2000 to January 2022. Studies that had examined the association between SBI and any form of child mortality were included. The findings of the included studies were summarized through fixed-effects or random-effects meta-analysis and the model was selected based on the heterogeneity index.
Results
A total of 51 studies were included. Of them, 19 were conducted in Ethiopia, 10 in Nigeria and 7 in Bangladesh. Significant higher likelihoods of stillbirth (odds ratio (OR) = 2.11; 95% confidence interval (CI) = 1.32-3.38), early neonatal mortality (OR = 1.58; 95% CI = 1.04-2.41), perinatal mortality (OR = 1.71; 95% CI = 1.32-2.21), neonatal mortality (OR = 1.85; 95% CI = 1.68-2.04), post-neonatal mortality (OR = 3.01; 95% CI = 1.43-6.33), infant mortality (OR = 1.92; 95% CI = 1.77-2.07), child mortality (OR = 1.67; 95% CI = 1.27-2.19) and under-five mortality (OR = 1.95; 95% CI = 1.56-2.44) were found among babies born in short birth intervals than those who born in normal intervals.
Conclusions
SBI significantly increases the risk of child mortality in LMICs. Programmes to reduce pregnancies in short intervals need to be expanded and strengthened. Reproductive health interventions aimed at reducing child mortality should include proper counselling on family planning, distribution of appropriate contraceptives and increased awareness of the adverse effects of SBI on maternal and child health.
Currently, the global neonatal mortality rate is 17 per 1000 live births and under-five mortality (U5M) is 37 per 1000 live births [1,2]. These rates declined substantially in the Millennium Development Goal period, 2000-2015 [3]. The Sustainable Development Goal targets to reduce neonatal and U5M to less than 12 and 25 per 1000 live births, respectively [3]. A majority of these deaths occur in low- and lower-middle-income countries (LMICs), where the neonatal mortality and U5M rates are around 10 and 15 times higher than in high-income countries [4,5]. The deaths rates are even higher among the children of their first month of life. Over 46% of the 5.2 million U5M recorded in 2019 occurred in the first month of life, around 33% occurred on the first day of birth and close to 75% within the first week of birth [3]. The most common causes of these deaths are complications during pregnancy as well as adverse birth outcomes including premature birth, low birth weight and birth defects [6]. Significant progress can be made in reducing these deaths by offering appropriate maternal health care services.
The World Health Organization (WHO) recommends an optimal time-space between two successive births, 24 months for two subsequent conceptions and 33 months for one conception to the next live birth. Shorter than these recommended durations are identified as short birth intervals (SBIs). Between 11% and 66% of all live births in LMICs occur in short intervals and contribute to several adverse pregnancy and birth outcomes, including maternal and under-five mortality [7-11]. Possible pathways of such contributions are both direct and indirect. For instance, mothers giving birth in SBIs are usually undernourished and anemic, which, in turn, contribute to increased risks of pregnancy complications and maternal mortality. SBIs increase the risks of a range of adverse outcomes of child health, including preterm birth, under-nutrition and low-birth-weight [6,12], and these outcomes subsequently contribute to U5M in several LMICs [13,14].
Relevant studies exploring these associations were conducted mostly in African contexts, although the prevalence of SBI is higher in Asian countries (33%) than in African countries (20%) [7]. Moreover, maternal health care services use, which can significantly reduce adverse pregnancy and birth outcomes, are substantially different in LMICs, with higher rates in Asian countries and lower in African countries [15,16]. Thus, the observed associations in African countries may not be generalisable in other LMICs. Moreover, studies conducted in the context of African countries with regard to SBI examined mainly the relationship with U5M [10,17,18] but rarely with other forms of child mortality, such as neonatal mortality and infant mortality. The relationships of SBIs are not uniform across these various forms of child mortality. For instance, the negative effect of SBI was found stronger for neonatal mortality than infant mortality [19]. Having a detailed picture of the relationships between SBIs and various forms of child mortality such as stillbirth, neonatal mortality, post-natal mortality, infant mortality in the context of LMICs generated from a systematic review may help overcome some of these limitations, offer an overall overview. Such a study would inform with detailed risks of SBIs on the child mortality across the respective durations of each of these health outcomes and assist in evidence-based policy and programmes. However, systematic reviews that have been conducted so far examined the effects of SBI on low-birth-weight, preterm births and stillbirths [6,20-23]. Also, the individual studies around the effects of SBI on different forms of child mortality are scarce in the context of LMICs and available studies are country-specific [24]. Consequently, the summary effects of SBI on several form of child mortality in LMICs are still unknown. We therefore conducted this study to determine the effects of SBI on various forms of child mortality in LMICs.
METHODS
We conducted a systematic review and meta-analysis and reported the findings following the Preferred Reporting Items of Systematic Review and Meta-analysis (PRISMA) consensus statement on the conduct of meta-analysis of observational studies. Relevant and available studies about the relationship between SBIs and various forms of child mortality were included.
Search strategy
A systematic literature search was conducted in January 2022 in the eight databases: PubMed, Cumulative Index to Nursing & Allied Health (CINAHL), Web of Science, Embase, PsycINFO, Cochrane Library, Popline, and Maternity and Infant Care. Studies published since the establishment of the MDGs from January 2000 to January 2022 were included. Searches were conducted on the basis of the individual comprehensive search strategies for each database. We developed search strategies using a combination of free text words, words in title/abstracts and medical subject headings (MeSH) terms of exposure, outcomes and settings. The exposure-related terms were birth intervals or short birth intervals or pregnancy intervals. The outcome-related terms were stillbirths or early neonatal mortality or perinatal mortality or neonatal mortality or post-neonatal mortality or infant mortality or child mortality or under-five mortality. Finally, LMICs’ names were included as the terms related to study settings. They are Afghanistan, Angola, Armenia, Bangladesh, Benin, Bhutan, Bolivia, Burkina Faso, Burundi, Cabo Verde, Cambodia, Cameroon, Central African Republic, Chad, Comoros, Congo, Cote d'Ivoire, Djibouti, Egypt, El Salvador, Eritrea, Ethiopia, Gambia, Georgia, Ghana, Guatemala, Guinea-Bissau, Haiti, Honduras, India, Indonesia, Jordan, Kenya, Kiribati, Korea, Kosovo, Kyrgyz Republic, Laos, Lesotho, Liberia, Madagascar, Malawi, Mali, Mauritania, Micronesia, Moldova, Mongolia, Morocco, Mozambique, Myanmar, Namibia, Nepal, Nicaragua, Niger, Nigeria, Pakistan, Papua New Guinea, Philippine, Rwanda, Sao Tome & Principe, Senegal, Sierra Leone, Solomon Island, Somalia, South Sudan, Sri Lanka, Sudan, Swaziland, Syria, Tajikistan, Tanzania, Timor-Leste, Togo, Tunisia, Uganda, Ukraine, Uzbekistan, Vanuatu, Vietnam, West Bank and Gaza, Yemen, Zambia, Zimbabwe. For this classification, we followed the 2021 World Bank country classification [25]. The term LMICs was also considered together with country names. We combined these terms using the Boolean operators (AND, OR). The Full search strategy and search results for each database are presented in Tables S1 to S8 in the Online Supplementary Document. Further searches for eligible studies were conducted by reviewing selected journals’ websites and reference lists of the selected articles.
Study selection
Two authors (MZI and MAB) independently reviewed all articles based on the inclusion and exclusion criteria presented in Table 1. They first conducted title and abstract screening. Articles selected in this stage were considered for full-text review. Disagreements were resolved through discussion and involving the senior author (MNK) if deemed necessary. We used online platforms, including COVIDENCE, EndNote X9 and Zoom online meetings to complete this review.
Table 1.
Inclusion and exclusion criteria considered in this study
| Topics | Criteria for inclusion | Criteria for exclusion |
|---|---|---|
| Study type |
Peer-reviewed |
Non-peer reviewed |
| Language |
In English only |
Other than English language |
| Exposure |
Short birth interval |
|
| Outcome |
Any form of child mortality including stillbirths, neonatal mortality, perinatal mortality and under-5 mortality (U5M) |
Other than the child mortality |
| Country type |
Low and lower-middle income countries |
Higher-middle- and high-income countries |
| Participant | Women with pregnancy outcomes | Women with or without pregnancy outcome and have any suppressible conditions – eg, HIV/AIDS or other STI infections, having chronic non-communicable diseases |
Data extraction
Prior to tabulating the final data, a data extraction template was designed, trialled, and modified following the “Strengthening the Reporting of Observational Studies in Epidemiology” guidelines [26]. Two authors (MZI and MAB) independently extracted the relevant data, including authors’ names, design of the study, study sample size, study setting, and the category(ies) of child mortality. Other information collected were effects size (eg, odds ratio (OR)) and underlying data used to calculate it and whether it was adjusted or unadjusted for confounders. Similar to the previous step, disagreements between the data collectors were resolved through discussion and involving the senior author (MNK) when deemed necessary.
Quality assessment of included studies
We used the modified Newcastle-Ottawa Scale (NOS) for the quality assessment of the included studies [27]. The items included in the scale were different for the cross-sectional, case-control and cohort studies. Two authors (MZI and MAB) checked included articles and gave 1 point for each item if the study met the relevant item condition. Aggregated scores were then used to measure overall study quality as good (score 8 to 9), moderate (score 5 to 7) and low (score <5) [28,29].
Exposure variable
Our exposure of interest was SBI, classified dichotomously as Yes and No. Some articles included in this review used slightly different intervals to define SBI. However, for the quantitative synthesis, we followed the WHO classification of SBI [30]. The articles that did not follow the WHO classification were synthesized narratively.
Outcome variables
The following seven forms of child mortality were our outcome variables: stillbirth (a baby dies after 28 weeks of pregnancy, but before or during birth), early neonatal mortality (death between 0 and 7 completed days of birth), perinatal death (death within 28 weeks of gestation to one week of live birth), neonatal death (deaths among live births during the first 28 completed days of life), post-neonatal mortality (death after 7 days to 28 completed days of birth), infant death (death before completing the first year of age), child mortality (death between the first and fifth birthday) and U5M (death after birth, but before reaching the age of five years) [31-36].
Statistical analysis
We used extracted ORs as the basis of analysis. If ORs were unavailable in the paper, we first calculated unadjusted ORs. For the studies conducted in multiple countries and that reported multiple ORs, we computed pooled ORs first. We used the fixed-effects and random-effects models to calculate the summary estimate for the overall effects of SBI on stillbirth, early neonatal mortality, perinatal mortality, neonatal mortality, post-neonatal mortality, infant mortality, child mortality and U5M. We did not reclassify these mortality measures. For instance, both neonatal mortality and post-neonatal mortality are parts of infant mortality and they could be pooled together to get the summary estimate for infant mortality. The model was selected based on the heterogeneity assessment. An I2 statistic with a p-value (<0.05) was estimated for each meta-analysis to describe the extent of heterogeneity. When the test for heterogeneity was moderate (50%-74%) or high (75%-100%), the pooled estimates of ORs were computed by using the random-effects model [37]. We also explored the source of heterogeneity using subgroup analysis and meta-regression once moderate or higher heterogeneity was identified. For this, pre-specified sub-groups were considered including the study sample size, confounding adjustment, study design, and study settings. Publication bias was explored by visual inspection of Funnel Plot asymmetry and Egger’s regression test [38]. The trim-and-fill method was used when evidence of publication bias was found, and to estimate and adjust for potentially missing studies, the effect size was recalculated accordingly [39]. Statistical software, STATA version 15.1 (Stata.corp), was used for all analyses.
RESULTS
Search results
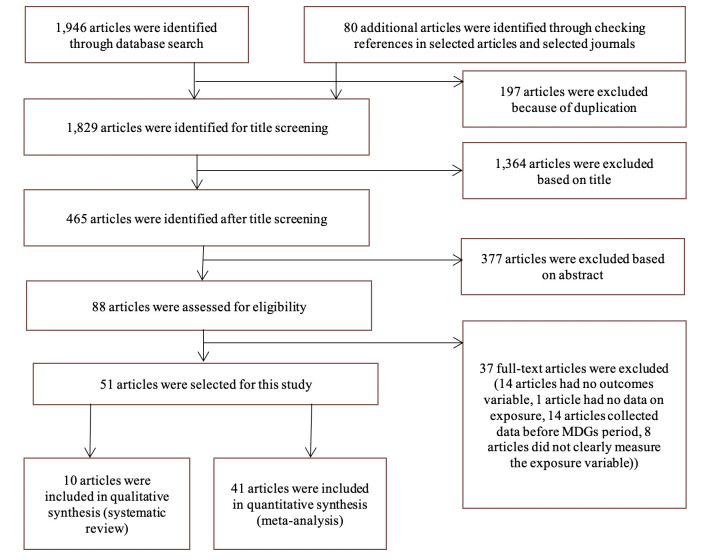
We found 1946 studies in eight databases and additional 80 studies through hand searching and reference checking. Together, there were 2026 studies, of which 197 studies were duplicates and thereby excluded. Of the remaining 1829 studies, 1741 studies were excluded through title and abstract screening. Full-text reviews were conducted for the remaining 88 articles and 37 articles were excluded based on full-text review. We excluded those 37 studies because they did not report any mortality outcome (n = 14), or collected data before the starting point of MDGs (ie, prior to January 2000) (n = 14), or did not clearly report the measurement criteria (n = 8) or did not have any data on exposure variable (n = 1). A total of 51 studies were finally included in this study, 41 included in the quantitative synthesis and the remaining 10 studies included in the narrative synthesis (Figure 1). They were moderate to good in quality (see Tables S10-S12 in the Online Supplementary Document).
Figure 1.
Schematic presentation of the studies included and excluded in the systematic review.
Study characteristics
Of the 51 studies included, a majority were conducted in Ethiopia (n = 19) [40-58], Nigeria (n = 10) [17,59-67] and Bangladesh (n = 7) [68-74] (Table S9 in the Online Supplementary Document). Four studies were conducted in multiple countries [17,56,57,70]. Forty-five of the included studies were of cross-sectional design. More than two-thirds (n = 36) of the included studies used national-level data and fourteen studies reported adjusted ORs.
Relationship between short birth interval on child mortality
We found significantly higher likelihoods of stillbirth, early neonatal mortality, perinatal mortality, neonatal mortality, post-neonatal mortality, infant mortality, child mortality and U5M among children of mothers having SBI than their counterpart mothers having optimal birth intervals (Table 2).
Table 2.
Summary measures of the relationships between short birth interval and various forms of child mortality, publication bias, and Trim and Fill estimates for low- and lower-middle-income countries, January 2000 to January 2022
| Characteristics |
Number of studies |
Summary estimates * |
Egger bias test P-value |
Trim and Fill estimates † |
||
|---|---|---|---|---|---|---|
|
OR (95%, CI)
|
Heterogeneity index
|
Number of missing studies
|
OR (95%, CI)
|
|||
|
Adverse outcomes
| ||||||
| Stillbirth |
4 |
2.11 (1.32-3.38) |
76.0% |
0.651 |
0 |
2.11 (1.32-3.38) |
| Early neonatal mortality |
1 |
1.58 (1.04-2.41) |
0.0% |
0.422 |
0 |
1.58 (1.04-2.41) |
| Perinatal mortality |
4 |
1.71 (1.32-2.21) |
76.5% |
0.090 |
0 |
1.71 (1.32-2.21) |
| Neonatal mortality |
17 |
1.85 (1.68-2.04) |
72.6% |
<0.01 |
3 |
1.76 (1.60-1.95) |
| Post-neonatal mortality |
1 |
3.01 (1.43-6.33) |
0.00% |
0.428 |
0 |
3.01 (1.43-6.33) |
| Infant mortality |
12 |
1.92 (1.77-2.07) |
51.4% |
<0.01 |
2 |
1.88 (1.73-2.04) |
| Child mortality |
9 |
1.67 (1.27-2.19) |
89.3% |
<0.01 |
2 |
1.44 (1.09-1.90) |
| Under-five mortality | 9 | 1.95 (1.56-2.44) | 90.3% | <0.01 | 1 | 1.89 (1.52-2.34) |
OR – odds ratio, CI – confidence interval
Note: Non-short birth interval was considered the reference category.
*Summary estimates were based on random-effects methods except for the early neonatal mortality and post-neonatal mortality which were based on the fixed-effects model.
†The trim-and-fill method simulates studies that are likely to be missing from the literature due to publication or other forms of bias. The trim-and-fill OR estimates what the pooled OR would be if these missing studies were included in the analysis.
We found evidence of moderate to higher heterogeneity for the likelihoods of stillbirths, perinatal mortality, neonatal mortality, infant mortality, child mortality and U5M (Table 2). Stratified analysis was conducted to explore the source of heterogeneity across included characteristics, including sample size, confounding factors, study design and study setting. Results are presented in Table 3. We found different likelihoods of stillbirths, perinatal mortality, neonatal mortality, infant mortality, child mortality and U5M across sample size, confounding factors, study design and study setting.
Table 3.
Stratified analysis of pooled OR of six forms of child mortality across selected characteristics
| Characteristics |
Stillbirths |
Perinatal mortality |
Neonatal mortality |
Infant mortality |
Child mortality |
Under-five mortality |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Pooled OR (95%, CI)
|
Heterogeneity, P value
|
Meta-regression, P value
|
Pooled OR (95%, CI)
|
Heterogeneity, P value
|
Meta-regression, P value
|
Pooled OR (95%, CI)
|
Heterogeneity, P value
|
Meta-regression, P value
|
Pooled OR (95%, CI)
|
Heterogeneity, P value
|
Meta-regression, P value
|
Pooled OR (95%, CI)
|
Heterogeneity, P value
|
Meta-regression, P value
|
Pooled OR (95%, CI)
|
Heterogeneity, P value
|
Meta-regression, P value
|
|
|
Sample size
| ||||||||||||||||||
| ≤21 816 |
2.20 (1.13-4.29) |
<0.05 |
0.845 |
1.84 (1.02-3.34) |
<0.05 |
0.781 |
1.69 (1.40-2.04) |
<0.01 |
<0.05 |
1.74 (1.42-2.14) |
0.227 |
0.253 |
1.02 (0.42-2.51) |
<0.01 |
0.033 |
2.02 (1.09-3.75) |
<0.01 |
0.912 |
| >21 816 |
1.93 (1.20-3.10)* |
NA |
|
1.66 (1.15-2.39) |
<0.01 |
|
1.97 (1.88-2.07) |
<0.01 |
|
1.95 (1.81-2.11) |
<0.05 |
|
1.91 (1.49-2.45) |
<0.01 |
|
1.93 (1.63-2.28) |
<0.01 |
|
|
Confounders
| ||||||||||||||||||
| Adjusted |
2.61 (1.91-3.57) |
0.295 |
<0.01 |
2.58 (1.61-4.13)* |
NA |
0.154 |
1.74 (1.49-2.03) |
<0.01 |
0.463 |
2.12 (1.57-2.88) |
0.131 |
0.636 |
1.42 (1.15-1.75)* |
NA |
0.686 |
1.80 (1.65-1.97) |
0.474 |
0.830 |
| Unadjusted |
1.27 (0.89-1.82)* |
NA |
|
1.58 (1.21-2.06) |
<0.01 |
|
1.90 (1.66-2.17) |
<0.01 |
|
1.90 (1.75-2.07) |
<0.05 |
|
1.72 (1.24-2.38) |
<0.01 |
|
2.01 (1.36-2.97) |
<0.01 |
|
|
Study design
| ||||||||||||||||||
| Prospective cohort |
0 |
|
|
2.58 (1.61-4.13)* |
NA |
|
1.10 (0.80-1.51)* |
NA |
|
0 |
|
|
0 |
|
|
0 |
|
|
| Cross-sectional |
1.96 (1.13-3.39) |
<0.05 |
0.522 |
1.58 (1.21-2.06) |
<0.01 |
0.154 |
1.90 (1.73-2.08) |
<0.01 |
<0.05 |
1.90 (1.77-2.05) |
<0.05 |
0.076 |
1.67 (1.27-2.19) |
<0.01 |
<0.01 |
1.94 (1.53-2.39) |
<0.01 |
0.869 |
| Case-control |
2.99 (1.35-6.62)* |
NA |
|
0 |
|
|
0 |
|
|
3.57 (1.82-7.01)* |
NA |
|
0 |
|
|
2.08 (1.22-3.57)* |
NA |
|
|
Study setting
| ||||||||||||||||||
| National |
1.93 (1.20-3.10)* |
NA |
0.845 |
1.99 (1.68-2.35)* |
NA |
0.418 |
1.91 (1.73-2.10) |
<0.01 |
0.082 |
1.94 (1.82-2.07) |
0.118 |
0.354 |
1.72 (1.24-2.38) |
<0.01 |
0.686 |
1.87 (1.46-2.39) |
<0.01 |
0.383 |
| Regional | 2.20 (1.13-4.29) | <0.05 | 1.59 (1.18-2.15)* | <0.01 | 1.56 (1.10-2.20) | <0.01 | 2.17 (0.92-5.11) | <0.05 | 1.42 (1.15-1.75)* | NA | 2.46 (1.69-3.59) | <0.01 | ||||||
OR – odds ratio, CI – confidence interval, NA – not applicable
*Odds ratio for single study.
We also found evidence of publication bias for neonatal mortality (Figure S4a in the Online Supplementary Document), infant mortality (Figure S6a in the Online Supplementary Document), child mortality (Figure S7a in the Online Supplementary Document) and U5M (Figure S8a in the Online Supplementary Document). Thus, we conducted the Trim and Fill methods to estimate the number of missing studies. However, after considering the missing studies, the estimated ORs were around similar to the corresponding summary estimates.
Our narrative synthesis also produced a consistent result with the main summary estimates (Table 4). Of the ten studies included, six showed a higher prevalence of U5M among babies born after SBIs. Three studies reported SBI as a significant risk factor for child mortality. SBI was also reported as a significant risk factor of infant mortality in two studies.
Table 4.
Findings from the narrative review of the relationship between short birth interval and six forms of child mortality in low- and lower-middle-income countries, January 2000 to January 2022
| Study | Study design, country | Sample | Results |
|---|---|---|---|
| Ejigu AG et al., 2019 [53] |
Cross-sectional study, Ethiopia |
A total of 418 pregnant women were included during their ANC visits and followed up during pregnancy and after the live births. |
The likelihood of child mortality was 3.6 times (aOR = 3.60; 95% CI = 1.35-9.59) higher among babies born in short intervals than babies born in normal intervals. |
| Hailemichael HT, 2020 [54] |
Case-control study, Ethiopia |
A total of 405 mothers (135 cases and 270 controls) were recruited from public and private hospitals during delivery care services. |
Women who gave birth in SBIs were 5.21 times (aOR = 5.21; 95% CI = 1.89-13.86) more likely to experience child mortality than mothers having normal inter-pregnancy intervals. |
| Asiki G et al., 2016 [75] |
Cohort study, Uganda |
Data of a total of 1830 children were analyzed, extracted from census, vital registrations, pregnancy registrations, and medical survey rounds. |
Around 55% (HR = 0.45; 95% CI = 3%, 79%) lower risk and 26% (HR = 1.26%-95% CI = 0.40-3.97) higher risk of U5M mortality were found among babies born in 1-2 y and >2 y intervals, respectively, compared to <1 y interval. |
| Mekonnen Y et al., 2013 [55] |
Cross-sectional study, Ethiopia |
This study analyzed data of 32 428 children, extracted from the Ethiopian Demography Health Survey. |
This study reported 2.2 times (HR = 2.19; 95% CI = 1.89-2.52) likelihood of infant mortality among babies born in SBI than those born in normal intervals. |
| Kayode GA et al., 2012 [66] |
Cross-sectional study, Nigeria |
This study analyzed data of 28 647 children, extracted from the Nigerian Demography Health Survey. |
Around 51% (OR = 0.49; 95% CI = 0.43-0.56) and 70% (OR = 0.30; 95% CI = 0.26-0.34) lower likelihoods of U5M were found among mothers who gave births in 18-36 mo and >36 mo intervals, respectively, compared to mothers gave birth in <18 mo intervals. |
| Budu et al., 2021 [17] |
Cross- sectional study, eight countries in West Africa |
Total of 52 877 childbearing women’s data were analyzed, extracted from nationally representative Demographic and Health Surveys. |
The likelihood of under-five mortality was 1.82 (95% CI = 1.64-2.00) times higher among SBI-babies compared to the babies born in normal intervals. |
| Biradar R et al., 2019 [67] |
Cross-sectional study, Nigeria |
This study analyzed data of 7468 under-five children, nationally representative Demographic and Health Surveys. |
As compared to the mothers having less than 2 y interval in two most recent live births, mothers having 2-3 y and above 3 y interval in their most recent two live births had 6% (aOR = 0.94; 95%CI = 0.79-1.11) and 23% (aOR = 0.77; 95% CI = 0.65-0.92) lower likelihoods of U5M. |
| Worku MG et al., 2021 [76] |
Cross-sectional study, Ethiopia |
This study analyzed data of 3446 live births, extracted from nationally representative Demographic and Health Surveys. |
The likelihoods of U5M were found 43% (aOR = 0.57; 95%CI = 0.41-0.81) and 65% (aOR = 0.35; 95% CI = 0.22-0.55) lower among mothers having intervals of 2-3 y and above three years in their most recent two live births as compared to the mothers with intervals of less than two years. |
| Tesma GA et al., 2021 [57] |
Cross-sectional study, twelve Est African countries |
This study analyzed data of 138 803 under-five children extracted from the Demographic and Health Survey. |
The likelihood of U5M (aOR = 0.53; 95%CI = 0.50-0.57) declined among mothers having births intervals of 2-4 y as compared to the mothers having birth intervals of less than two years. |
| Woldeamanuel BT et al., 2019 [77] | Cross-sectional study, Ethiopia | This study analyzed data of 10 274 under-five children extracted from the Demographic and Health Survey. | Compared to the mothers having birth intervals of 36 mo and more, the likelihoods of U5M were found higher among mothers having birth intervals of 1-18 mo (OR = 2.16; 95% CI = 1.82-2.57) and 19-36 mo (OR = 1.32; 95% CI = 1.18-1.47). |
ANC – antenatal care, aOR – adjusted odds ratio, HR – hazard ratio, CI – confidence interval, U5M – under 5 mortality, OR – odds ratio, mo -months
DISCUSSION
This study aimed to identify the relationship between SBI and several forms of child mortality in LMICs. The results suggest that the likelihoods of various forms of child mortality were 1.58-3.01 times higher among mothers who gave births in short intervals than their counterparts who gave births in normal intervals. These summary effects were found different across study characteristics, including study design, sample size, confounder adjustment and study settings, however, their directions were similar to the corresponding summary estimates. Evidence of publication bias was observed for neonatal mortality, infant mortality, child mortality and U5M, although their corresponding likelihoods were similar before and after adjusting the hypothetical number of studies due to potential publication bias. Overall, our findings show evidence of the adverse effects of SBI on child mortality in LMICs and highlight the critical importance of appropriate policies and programmes to reduce the prevalence of SBI and its adverse effects on child health thereby.
The duration from perinatal mortality to U5M, the eight forms of mortality we examined in this study, covers a period from conception up to 5 years of birth. Our overall findings suggest that across these various bands of durations, the risks of child mortality are around 2-folds in the context of LMICs, except for post-neonatal mortality, the risk for which was 3-folds but estimated from a single study. A recent and large study with data from 77 countries found that birth intervals are an important factor for perinatal outcomes in low-income countries but are much less consequential in high-income settings [78]. In conjunction with the results of this and other similar studies, our findings suggest that a substantial proportion of these deaths attributable to SBIs in LMICs are avoidable. The literature consistently suggests that women’s education, awareness of the adverse effects of SBI on fetuses, babies and mothers and regular antenatal and postnatal care can substantially reduce both SBIs and adverse health outcomes for children and mothers [79,80]. Indeed, the difference between low- and high-income countries in terms of the effects of SBIs on various forms of child mortality is determined by these social, behavioral and public health aspects, which are avoidable.
The underlying reasons for relatively high child mortality among babies born in SBIs touch several aspects, including maternal reproductive health conditions, mother-child behavior, and mothers’ socio-demographic attributes [19,81]. Following a live birth, women need time to return to the normal stage from maternal nutritional depletion and may compromise the ability to support fetal growth, which could result in fetal malnutrition and increased risk of infection and death during childhood. Also, SBI can cause cervical insufficiency, incomplete healing of uterine scars, abnormal remodelling of endometrial blood vessels, ruptured membrane, and anemia [10,12,22]. All these may ultimately contribute to the adverse outcomes in the subsequent pregnancy. Other issues such as competition between short-spaced siblings for parental time, material resources and inadequate parental attention have also been identified as possible causes of child mortality among babies born in SBIs [6,82]. These socio-behavioral issues make infants susceptible to infectious diseases (eg, diarrhea, acute respiratory infection) and child nutritional disorders (stunting, wasting, and underweight) [83-85], which, in LMICs, are major causes of child mortality [86].
Another leading cause of the relatively high prevalence of SBI in LMICs is unintended conceptions. The prevalence of unintended pregnancies is higher among the disadvantaged women than their advantaged counterparts, mainly due to infrequent use of modern contraception [87], ineffective contraceptives use [88], inadequate use of emergency contraception [89], or combinations of these three. Many of these women suffer from depression due to unintended pregnancies, and they have limited access to antenatal health care, delivery health care and postnatal health care. Moreover, disadvantaged women, in general, and women having SBI, in particular, tend to depend on their prior pregnancy experiences [90]. In addition, they are more likely to face cultural restrictions in utilizing maternal health care services and have usually lower knowledge about the adverse child health outcomes [5]. Negative effects of unintended conception are continued following the live birth and affect preventative and curative care of the newborn, including exclusive breastfeeding and newborn care [91]. The adverse effects may exacerbate further since mothers giving birth in SBIs may not be able to ensure adequate care for two infants of almost the same age; and ultimately, all these, together, can increase the risk of child mortality.
A previous systematic review conducted based on the studies published in the Ethiopian context reported a higher likelihood of infant mortality among babies born in SBIs (OR = 2.03; 95% CI = 1.52-2.70) [24] than the estimate we found (OR = 1.88; 95% CI = 1.71, 2.08). This difference could be due to the sample variation since that study summarized papers only in the Ethiopian context, where a higher parity and lower use of maternal health care services are more prevalent than many other LMICs [15,16]. Since our summary estimates for the effects of SBI on U5M, neonatal mortality and stillbirths are the first in the context of LMICs, we are unable to compare them.
This study has several strengths and some limitations. As far we know, this is the first study in LMICs that summarized the effects of SBI on various forms of child mortality. All studies conducted in LMICs since the year 2000, when the MDGs were established aiming to reduce maternal and child mortality, were included in this review. Moreover, we used comprehensive search techniques for data extraction and selection of eligible studies and followed the “Strengthening the Reporting of Observational Studies in Epidemiology” and PRISMA guidelines for reporting study findings. In addition, we considered all forms of child mortality and analyzed them separately following the relevant international classifications. However, the summary estimates presented in this study were mainly based on cross-sectional studies. We did not search unpublished papers and gray literature, some of which could contribute to publication bias, although it is likely to be insignificant as Egger’s test and funnel plots suggest. The slight asymmetry that we observed may arise due to the inclusion of a small number of studies on particular forms of mortality, substantial between-study heterogeneity and/or similar studies size. However, we found a similar trend in results of summarized ORs when we captured publication bias by using the trim-and-fill method, thus suggesting that our findings are reliable.
CONCLUSIONS
This study found significantly higher likelihoods of several forms of child mortality among babies born in short intervals than their counterparts born in normal intervals. These indicate SBI is an important public health challenge in LMICs for achieving a significant reduction in child mortality in the current round of the world development goals, the SDGs, to be achieved by 2030. Policies and programs need to be strengthened to improve reproductive and maternal health care services, reduce the occurrences of short interval births and ensure health care services in every pregnancy. Initiatives are also needed to increase awareness about the adverse effect of short interval birth.
Additional material
Acknowledgments
We acknowledge the support of the Population Science and Human Resource Development Department of the University of Rajshahi, Bangladesh where this research was conducted.
Footnotes
Funding: The authors did not receive any specific funding for this study.
Authorship contributions: MZI, MMR, and MNK developed the study concept. MZI and MAB reviewed the articles independently, extracted data, and assessed study quality. MNK and MZI conducted the formal analysis and MZI drafted the manuscript. MMI and MNK critically reviewed and edited all previous versions of the manuscript. All authors approved the final version of this manuscript.
Disclosure of interest: The authors completed the ICMJE Disclosure of Interest Form (available upon request from the corresponding author) and disclose no relevant interests.
REFERENCES
- 1.Belizán JM, McClure EM, Goudar SS, Pasha O, Esamai F, Patel A, et al. Neonatal death in Low-Middle Income Countries: A Global Network Study. Am J Perinatol. 2012;29:649-56. 10.1055/s-0032-1314885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Child mortality and causes of death. Available: https://www.who.int/data/gho/data/themes/topics/topic-details/GHO/child-mortality-and-causes-of-death. Accessed: 27 May 2022.
- 3.Goal 3 | Department of Economic and Social Affairs. Available: https://sdgs.un.org/goals/goal3. Accessed: 27 May 2022.
- 4.Belizán JM, McClure EM, Goudar SS, Pasha O, Esamai F, Patel A, et al. Neonatal death in Low-Middle Income Countries: A Global Network Study. Am J Perinatol. 2012;29:649-56. 10.1055/s-0032-1314885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.O’Hare B, Makuta I, Chiwaula L, Bar-Zeev N.Income and child mortality in developing countries: a systematic review and meta-analysis. J R Soc Med. 2013;106:408-14. 10.1177/0141076813489680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Conde-Agudelo A, Rosas-Bermudez A, Castaño F, Norton MH.Effects of birth spacing on maternal, perinatal, infant, and child health: a systematic review of causal mechanisms. Stud Fam Plann. 2012;43:93-114. 10.1111/j.1728-4465.2012.00308.x [DOI] [PubMed] [Google Scholar]
- 7.Aleni M, Mbalinda SN, Muhindo R.Birth Intervals and Associated Factors among Women Attending Young Child Clinic in Yumbe Hospital, Uganda. Int J Reprod Med. 2020;2020:1326596. 10.1155/2020/1326596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Determinants of interpregnancy interval among parturient in Port Harcourt, Nigeria. Available: https://www.smjonline.org/article.asp?issn=1118-8561;year=2016;volume=19;issue=4;spage=180;epage=184;aulast=Bassey. Accessed: 27 May 2022.
- 9.DaVanzo J, Hale L, Razzaque A, Rahman M.The effects of pregnancy spacing on infant and child mortality in Matlab, Bangladesh: How they vary by the type of pregnancy outcome that began the interval. Population Studies. 2008;62:131-54. 10.1080/00324720802022089 [DOI] [PubMed] [Google Scholar]
- 10.Kozuki N, Walker N.Exploring the association between short/long preceding birth intervals and child mortality: using reference birth interval children of the same mother as comparison. BMC Public Health. 2013;13:S6. 10.1186/1471-2458-13-S3-S6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mahande MJ, Obure J.Effect of interpregnancy interval on adverse pregnancy outcomes in northern Tanzania: a registry-based retrospective cohort study. BMC Pregnancy Childbirth. 2016;16:140. 10.1186/s12884-016-0929-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Conde-Agudelo A, Rosas-Bermúdez A, Kafury-Goeta AC.Effects of birth spacing on maternal health: a systematic review. Am J Obstet Gynecol. 2007;196:297-308. 10.1016/j.ajog.2006.05.055 [DOI] [PubMed] [Google Scholar]
- 13.Murarkar S, Gothankar J, Doke P, Pore P, Lalwani S, Dhumale G, et al. Prevalence and determinants of undernutrition among under-five children residing in urban slums and rural area, Maharashtra, India: a community-based cross-sectional study. BMC Public Health. 2020;20:1559. 10.1186/s12889-020-09642-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Villar J, Restrepo-Méndez MC, McGready R, Barros FC, Victora CG, Munim S, et al. Association Between Preterm-Birth Phenotypes and Differential Morbidity, Growth, and Neurodevelopment at Age 2 Years: Results From the INTERBIO-21st Newborn Study. JAMA Pediatr. 2021;175:483-93. 10.1001/jamapediatrics.2020.6087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yaya S, Ghose B.Global Inequality in Maternal Health Care Service Utilization: Implications for Sustainable Development Goals. Health Equity. 2019;3:145-54. 10.1089/heq.2018.0082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leventhal DGP, Crochemore-Silva I, Vidaletti LP, Armenta-Paulino N, Barros AJD, Victora CG.Delivery channels and socioeconomic inequalities in coverage of reproductive, maternal, newborn, and child health interventions: analysis of 36 cross-sectional surveys in low-income and middle-income countries. Lancet Glob Health. 2021;9:e1101-9. 10.1016/S2214-109X(21)00204-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Budu E, Ahinkorah B, Ameyaw E, Seidu AA, Zegeye B, Yaya S.Does Birth Interval Matter in Under-Five Mortality? Evidence from Demographic and Health Surveys from Eight Countries in West Africa. BioMed Res Int. 2021;2021:5516257. 10.1155/2021/5516257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ezeh OK, Ogbo FA, Odumegwu AO, Oforkansi GH, Abada UD, Goson PC, et al. Under-5 Mortality and Its Associated Factors in Northern Nigeria: Evidence from 22,455 Singleton Live Births (2013-2018). Int J Environ Res Public Health. 2021;18:9899. 10.3390/ijerph18189899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rutstein SO.Effects of preceding birth intervals on neonatal, infant and under-five years mortality and nutritional status in developing countries: evidence from the demographic and health surveys. Int J Gynaecol Obstet. 2005;89:S7-24. 10.1016/j.ijgo.2004.11.012 [DOI] [PubMed] [Google Scholar]
- 20.Conde-Agudelo A, Rosas-Bermúdez A, Kafury-Goeta AC.Birth spacing and risk of adverse perinatal outcomes: a meta-analysis. JAMA. 2006;295:1809-23. 10.1001/jama.295.15.1809 [DOI] [PubMed] [Google Scholar]
- 21.Kozuki N, Lee AC, Silveira MF, Victora CG, Adair L, Humphrey J, et al. The associations of birth intervals with small-for-gestational-age, preterm, and neonatal and infant mortality: a meta-analysis. BMC Public Health. 2013;13:S3. 10.1186/1471-2458-13-S3-S3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mohammed S, Abayneh M, Bekele D, Chan G, Worku A, Girma E. Short birth interval predicts the risk of preterm birth among pregnant women in Sub-Saharan Africa: A systematic review and meta-analysis. In Review; 2020. Available: https://www.researchsquare.com/article/rs-30085/v1. Accessed: 27 May 2022.
- 23.Kangatharan C, Labram S, Bhattacharya S.Interpregnancy interval following miscarriage and adverse pregnancy outcomes: systematic review and meta-analysis. Hum Reprod Update. 2017;23:221-31. [DOI] [PubMed] [Google Scholar]
- 24.Dadi AF.A Systematic Review and Meta-Analysis of the Effect of Short Birth Interval on Infant Mortality in Ethiopia. PLoS One. 2015;10:e0126759. 10.1371/journal.pone.0126759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.New World Bank country classifications by income level: 2021-2022. Available: https://blogs.worldbank.org/opendata/new-world-bank-country-classifications-income-level-2021-2022. Accessed: 27 May 2022.
- 26.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. PLoS Med. 2007;4:e296. 10.1371/journal.pmed.0040296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wells G, Shea B, O’Connell D, Peterson JE, Welch V, Losos M, et al. The Newcastle–Ottawa Scale (NOS) for Assessing the Quality of Non-Randomized Studies in Meta-Analysis. 2000. Available: https://www.ohri.ca//programs/clinical_epidemiology/oxford.asp. Accessed: 27 May 2022.
- 28.Stang A.Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25:603-5. 10.1007/s10654-010-9491-z [DOI] [PubMed] [Google Scholar]
- 29.Sharmin S, Kypri K, Khanam M, Wadolowski M, Bruno R, Mattick RP.Parental Supply of Alcohol in Childhood and Risky Drinking in Adolescence: Systematic Review and Meta-Analysis. Int J Environ Res Public Health. 2017;14:287. 10.3390/ijerph14030287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Report of a WHO technical consultation on birth spacing: Geneva, Switzerland 13-15 June 2005. World Health Organization; 2007. Report No.: WHO/RHR/07.1. Available: https://apps.who.int/iris/handle/10665/69855. Accessed: 27 May 2022.
- 31.Neonatal and perinatal mortality: country, regional and global estimates. 2006; Available: https://apps.who.int/iris/handle/10665/43444. Accessed: 27 May 2022.
- 32.Infant mortality rate (probability of dying between birth and age 1 per 1000 live births). Available: https://www.who.int/data/gho/data/indicators/indicator-details/GHO/infant-mortality-rate-(probability-of-dying-between-birth-and-age-1-per-1000-live-births) . Accessed: 27 May 2022.
- 33.Under-five mortality rate (probability of dying by age 5 per 1000 live births). Available: https://www.who.int/data/gho/data/indicators/indicator-details/GHO/under-five-mortality-rate-(probability-of-dying-by-age-5-per-1000-live-births) . Accessed: 27 May 2022.
- 34.Lehtonen L, Gimeno A, Parra-Llorca A, Vento M.Early neonatal death: A challenge worldwide. Semin Fetal Neonatal Med. 2017;22:153-60. 10.1016/j.siny.2017.02.006 [DOI] [PubMed] [Google Scholar]
- 35.Tashiro A, Yoshida H, Okamoto E.Infant, neonatal, and postneonatal mortality trends in a disaster region and in Japan, 2002–2012: a multi-attribute compositional study. BMC Public Health. 2019;19:1085. 10.1186/s12889-019-7443-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Afshan K, Narjis G, Qureshi IZ, Cappello M.Social determinants and causes of child mortality in Pakistan: Analysis of national demographic health surveys from 1990 to 2013. J Paediatr Child Health. 2020;56:457-72. 10.1111/jpc.14670 [DOI] [PubMed] [Google Scholar]
- 37.Higgins JPT, Thompson SG, Deeks JJ, Altman DG.Measuring inconsistency in meta-analyses. BMJ. 2003;327:557-60. 10.1136/bmj.327.7414.557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Egger M, Smith GD, Schneider M, Minder C.Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629-34. 10.1136/bmj.315.7109.629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Duval S, Tweedie R.Trim and fill: A simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56:455-63. 10.1111/j.0006-341X.2000.00455.x [DOI] [PubMed] [Google Scholar]
- 40.Mekonnen Dagne H, Takele Melku A, Abdurkadir Abdi A.Determinants of Stillbirth Among Deliveries Attended in Bale Zone Hospitals, Oromia Regional State, Southeast Ethiopia: A Case-Control Study. Int J Womens Health. 2021;13:51-60. 10.2147/IJWH.S276638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lakew D, Tesfaye D, Mekonnen H.Determinants of stillbirth among women deliveries at Amhara region, Ethiopia. BMC Pregnancy Childbirth. 2017;17:375. 10.1186/s12884-017-1573-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fenta SM, Biresaw HB, Fentaw KD.Risk factor of neonatal mortality in Ethiopia: multilevel analysis of 2016 Demographic and Health Survey. Trop Med Health. 2021;49:14. 10.1186/s41182-021-00303-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Andargie G, Berhane Y, Worku A, Kebede Y.Predictors of perinatal mortality in rural population of Northwest Ethiopia: a prospective longitudinal study. BMC Public Health. 2013;13:168. 10.1186/1471-2458-13-168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tesema GA, Seretew WS, Worku MG, Angaw DA.Trends of infant mortality and its determinants in Ethiopia: mixed-effect binary logistic regression and multivariate decomposition analysis. BMC Pregnancy Childbirth. 2021;21:362. 10.1186/s12884-021-03835-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tessema ZT, Tesema GA.Incidence of neonatal mortality and its predictors among live births in Ethiopia: Gompertz gamma shared frailty model. Ital J Pediatr. 2020;46:138. 10.1186/s13052-020-00893-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Basha GW, Woya AA, Tekile AK.Determinants of neonatal mortality in Ethiopia: an analysis of the 2016 Ethiopia Demographic and Health Survey. Afr Health Sci. 2020;20:715-23. 10.4314/ahs.v20i2.23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kidus F, Woldemichael K, Hiko D.Predictors of neonatal mortality in Assosa zone, Western Ethiopia: a matched case control study. BMC Pregnancy Childbirth. 2019;19:108. 10.1186/s12884-019-2243-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tesema GA, Gezie LD, Nigatu SG.Spatial distribution of stillbirth and associated factors in Ethiopia: a spatial and multilevel analysis. BMJ Open. 2020;10:e034562. 10.1136/bmjopen-2019-034562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tesfaye B, Atique S, Elias N, Dibaba L, Shabbir SA, Kebede M.Determinants and development of a web-based child mortality prediction model in resource-limited settings: A data mining approach. Comput Methods Programs Biomed. 2017;140:45-51. 10.1016/j.cmpb.2016.11.013 [DOI] [PubMed] [Google Scholar]
- 50.Shifa GT, Ahmed AA, Yalew AW.Maternal and child characteristics and health practices affecting under-five mortality: A matched case control study in Gamo Gofa Zone, Southern Ethiopia. PLoS One. 2018;13:e0202124. 10.1371/journal.pone.0202124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tesema GA, Worku MG.Individual-and community-level determinants of neonatal mortality in the emerging regions of Ethiopia: a multilevel mixed-effect analysis. BMC Pregnancy Childbirth. 2021;21:12. 10.1186/s12884-020-03506-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shifti DM, Chojenta C, Holliday E, Loxton D.Effects of short birth interval on neonatal, infant and under-five child mortality in Ethiopia: a nationally representative observational study using inverse probability of treatment weighting. BMJ Open. 2021;11:e047892. 10.1136/bmjopen-2020-047892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ejigu AG, Yismaw AE, Limenih MA.The effect of sex of last child on short birth interval practice: the case of northern Ethiopian pregnant women. BMC Res Notes. 2019;12:75. 10.1186/s13104-019-4110-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hailemichael HT, Debelew GT, Alema HB, Weldu MG, Misgina KH.Determinants of adverse birth outcome in Tigrai region, North Ethiopia: Hospital-based case-control study. BMC Pediatr. 2020;20:10. 10.1186/s12887-019-1835-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mekonnen Y, Tensou B, Telake DS, Degefie T, Bekele A.Neonatal mortality in Ethiopia: trends and determinants. BMC Public Health. 2013;13:483. 10.1186/1471-2458-13-483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yaya S, Uthman OA, Ekholuenetale M, Bishwajit G, Adjiwanou V.Effects of birth spacing on adverse childhood health outcomes: evidence from 34 countries in sub-Saharan Africa. J Matern Fetal Neonatal Med. 2020;33:3501-8. 10.1080/14767058.2019.1576623 [DOI] [PubMed] [Google Scholar]
- 57.Tesema GA, Teshale AB, Tessema ZT.Incidence and predictors of under-five mortality in East Africa using multilevel Weibull regression modeling. Arch Public Health. 2021;79:196. 10.1186/s13690-021-00727-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Assefa N, Berhane Y, Worku A, Tsui A.The hazard of pregnancy loss and stillbirth among women in Kersa, East Ethiopia: a follow up study. Sex Reprod Healthc. 2012;3:107-12. 10.1016/j.srhc.2012.06.002 [DOI] [PubMed] [Google Scholar]
- 59.Morakinyo OM, Fagbamigbe AF.Neonatal, infant and under-five mortalities in Nigeria: An examination of trends and drivers (2003-2013). PLoS One. 2017;12:e0182990. 10.1371/journal.pone.0182990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Akinyemi JO, Bamgboye EA, Ayeni O.Trends in neonatal mortality in Nigeria and effects of bio-demographic and maternal characteristics. BMC Pediatr. 2015;15:36. 10.1186/s12887-015-0349-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ezeh OK, Uche-Nwachi EO, Abada UD, Agho KE.Community-and proximate-level factors associated with perinatal mortality in Nigeria: evidence from a nationwide household survey. BMC Public Health. 2019;19:811. 10.1186/s12889-019-7151-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Patel KK, Prasad JB, Biradar RA.Trends in and determinants of neonatal and infant mortality in Nigeria based on Demographic and Health Survey data. J Biosoc Sci. 2021;53:924-34. 10.1017/S0021932020000619 [DOI] [PubMed] [Google Scholar]
- 63.Adedini SA, Odimegwu C, Imasiku ENS, Ononokpono DN, Ibisomi L.REGIONAL VARIATIONS IN INFANT AND CHILD MORTALITY IN NIGERIA: A MULTILEVEL ANALYSIS. J Biosoc Sci. 2015;47:165-87. 10.1017/S0021932013000734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ezeh OK, Agho KE, Dibley MJ, Hall J, Page AN.Determinants of neonatal mortality in Nigeria: evidence from the 2008 demographic and health survey. BMC Public Health. 2014;14:521. 10.1186/1471-2458-14-521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fagbamigbe A, Nnanatu C.Modelling the Spatial Distribution and the Factors Associated with Under-Five Mortality in Nigeria. Spat Demogr. 2021. 10.1007/s40980-021-00078-7 [DOI] [Google Scholar]
- 66.Kayode GA, Adekanmbi VT, Uthman OA.Risk factors and a predictive model for under-five mortality in Nigeria: evidence from Nigeria demographic and health survey. BMC Pregnancy Childbirth. 2012;12:10. 10.1186/1471-2393-12-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Biradar R, Patel KK, Prasad JB.Effect of birth interval and wealth on under-5 child mortality in Nigeria. Clin Epidemiol Glob Health. 2019;7:234-8. 10.1016/j.cegh.2018.07.006 [DOI] [Google Scholar]
- 68.Akter S, Rahman JAMS, Rahman MM, Abedin S.The influence of birth spacing on child survival in Bangladesh: a life table approach. World Health Popul. 2010;12:42-56. [PubMed] [Google Scholar]
- 69.de Jonge HC, Azad K, Seward N, Kuddus A, Shaha S, Beard J, et al. Determinants and consequences of short birth interval in rural Bangladesh: a cross-sectional study. BMC Pregnancy Childbirth. 2014;14:427. 10.1186/s12884-014-0427-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Houweling TAJ, van Klaveren D, Das S, Azad K, Tripathy P, Manandhar D, et al. A prediction model for neonatal mortality in low- and middle-income countries: an analysis of data from population surveillance sites in India, Nepal and Bangladesh. Int J Epidemiol. 2019;48:186-98. 10.1093/ije/dyy194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hossain MM, Mani KKC, Islam MR.Prevalence and Determinants of the Gender Differentials Risk Factors of Child Deaths in Bangladesh: Evidence from the Bangladesh Demographic and Health Survey, 2011. PLoS Negl Trop Dis. 2015;9:e0003616. 10.1371/journal.pntd.0003616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Khan MA, Khan N, Rahman O, Mustagir G, Hossain K, Islam R, et al. Trends and projections of under-5 mortality in Bangladesh including the effects of maternal high-risk fertility behaviours and use of healthcare services. PLoS One. 2021;16:e0246210. 10.1371/journal.pone.0246210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Haq I, Alam M, Islam A, Rahman M, Latif A, Methun MIH, et al. Influence of sociodemographic factors on child mortality in Bangladesh: a multivariate analysis. J Public Health (Berl). 2020;30:1079-86. [Google Scholar]
- 74.Al Kibria GM, Khanam R, Mitra DK, Mahmud A, Begum N, Moin SMI, et al. Rates and determinants of neonatal mortality in two rural sub-districts of Sylhet, Bangladesh. PLoS One. 2018;13:e0206795. 10.1371/journal.pone.0206795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Asiki G, Newton R, Marions L, Seeley J, Kamali A, Smedman L.The impact of maternal factors on mortality rates among children under the age of five years in a rural Ugandan population between 2002 and 2012. Acta Paediatr. 2016;105:191-9. 10.1111/apa.13252 [DOI] [PubMed] [Google Scholar]
- 76.Worku MG, Teshale A, Tesema G.Determinants of under-five mortality in the high mortality regions of Ethiopia: mixed-effect logistic regression analysis. Arch Public Health. 2021;79:55. 10.1186/s13690-021-00578-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Woldeamanuel BT.Socioeconomic, Demographic, and Environmental Determinants of Under-5 Mortality in Ethiopia: Evidence from Ethiopian Demographic and Health Survey, 2016. Child Dev Res. 2019;2019:e1073782. 10.1155/2019/1073782 [DOI] [Google Scholar]
- 78.Molitoris J, Barclay K, Kolk M.When and Where Birth Spacing Matters for Child Survival: An International Comparison Using the DHS. Demography. 2019;56:1349-70. 10.1007/s13524-019-00798-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Abate MG, Angaw DA, Shaweno T.Proximate determinants of infant mortality in Ethiopia, 2016 Ethiopian demographic and health surveys: results from a survival analysis. Arch Public Health. 2020;78:4. 10.1186/s13690-019-0387-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wolde HF, Gonete KA, Akalu TY, Baraki AG, Lakew AM.Factors affecting neonatal mortality in the general population: evidence from the 2016 Ethiopian Demographic and Health Survey (EDHS)—multilevel analysis. BMC Res Notes. 2019;12:610. 10.1186/s13104-019-4668-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gribble JN, Murray NJ, Menotti EP.Reconsidering childhood undernutrition: can birth spacing make a difference? An analysis of the 2002-2003 El Salvador National Family Health Survey. Matern Child Nutr. 2009;5:49-63. 10.1111/j.1740-8709.2008.00158.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.DaVanzo J, Razzaque A, Rahman M, Hale L, Ahmed K, Khan M, et al. The Effects of Birth Spacing on Infant and Child Mortality, Pregnancy Outcomes, and Maternal Morbidity and Mortality in Matlab, Bangladesh. RAND Corporation Publications Department, Working Papers. 2004 Jan 1
- 83.Amugsi DA, Aborigo RA, Oduro AR, Asoala V, Awine T, Amenga-Etego L.Socio-demographic and environmental determinants of infectious disease morbidity in children under 5 years in Ghana. Glob Health Action. 2015;8. . 10.3402/gha.v8.29349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hasan MM, Richardson A.How sustainable household environment and knowledge of healthy practices relate to childhood morbidity in South Asia: analysis of survey data from Bangladesh, Nepal and Pakistan. BMJ Open. 2017;7:e015019. 10.1136/bmjopen-2016-015019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Li Z, Kim R, Vollmer S, Subramanian SV.Factors Associated With Child Stunting, Wasting, and Underweight in 35 Low- and Middle-Income Countries. JAMA Netw Open. 2020;3:e203386. 10.1001/jamanetworkopen.2020.3386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Walson JL, Berkley JA.The impact of malnutrition on childhood infections. Curr Opin Infect Dis. 2018;31:231-6. 10.1097/QCO.0000000000000448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Finer LB, Zolna MR.Declines in Unintended Pregnancy in the United States, 2008–2011. N Engl J Med. 2016;374:843-52. 10.1056/NEJMsa1506575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Finer LB, Henshaw SK.Disparities in rates of unintended pregnancy in the United States, 1994 and 2001. Perspect Sex Reprod Health. 2006;38:90-6. 10.1363/3809006 [DOI] [PubMed] [Google Scholar]
- 89.Trussell J, Koenig J, Ellertson C, Stewart F.Preventing unintended pregnancy: the cost-effectiveness of three methods of emergency contraception. Am J Public Health. 1997;87:932-7. 10.2105/AJPH.87.6.932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Khan MN, Harris ML, Loxton D.Does unintended pregnancy have an impact on skilled delivery care use in Bangladesh? A nationally representative cross-sectional study using Demography and Health Survey data. J Biosoc Sci. 2021;53:773-89. 10.1017/S0021932020000528 [DOI] [PubMed] [Google Scholar]
- 91.Gipson JD, Koenig MA, Hindin MJ.The effects of unintended pregnancy on infant, child, and parental health: a review of the literature. Stud Fam Plann. 2008;39:18-38. 10.1111/j.1728-4465.2008.00148.x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.