Abstract
Background
Genicular radiofrequency ablation is an established therapy for chronic knee pain. An analysis comparing different probe sizes and technologies has not yet been undertaken for this indication. This large retrospective, comparison study from a single-center comprehensive pain management practice aims to do that.
Methods
Outcomes of 170 patients who underwent traditional radiofrequency ablation (tRFA) for chronic knee pain were compared to 170 consecutive patients who received cooled radiofrequency ablation (CRFA) with similar (p=0.5) pre-procedural pain scores.
Results
The VAS pain score at the first post-procedure visit at 4–6 weeks decreased to 5.07±2.8 cm for tRFA and to 4.26 ± 3.2 cm for CRFA (p<0.001 for both from baseline). The difference was profound and significantly better in the favor of CRFA (p<0.001) as the duration of reduction of pain scores by greater than 50% was 2.6 months for tRFA and 11.1 months for CRFA. There were only 15 patients (8.8%) who continued to receive >50% of pain relief in tRFA at 12 months, as opposed to 78 (46%) at 12 months for CRFA. We compared the initial outcomes and long-term pain relief. Long-term outcomes were better for the bigger lesion size treatment group patients.
Conclusion
We conclude that the duration and intensity of pain relief were of a greater magnitude after the larger diameter probe cooled RFA.
Keywords: radiofrequency ablation, chronic knee pain, knee osteoarthritis, pain management
Introduction
Osteoarthritis (OA) is the most common cause of the chronic knee pain producing lifetime prevalence of almost 45%.1 Treatment options include non-surgical interventions like intra-articular steroid injections, hyaluronic acid, intra-articular platelet-rich plasma therapy and total knee arthroplasty. Radiofrequency ablation (RFA) has emerged as another therapeutic option for the refractory chronic knee pain due to various pathologies. RFA, introduced in the 1970s, has been used to treat various chronic pain conditions like trigeminal neuralgia, lumbar zygapophyseal joint mediated pain, and recently chronic knee pain.2 RF systems deliver targeted thermal energy to the nearby neural tissues to cause thermal coagulation and localized neuronal destruction. Radiofrequency (RF) causes the ions (Na+, K+ and Cl−) inside the tissue to oscillate, resulting in friction from the ions’ movement to generate heat.2 Conventional or traditional RF (tRFA) protocols typically involve heating up the targeted local tissue to temperatures ranging from 80°C to 90°C for about 90 seconds.2 tRF lesions are elliptical and follow the shape of the electrode active tip. Although further increases in temperature and/or time of RFA may lead to tissue charring at the electrode interface, size of the lesion production can be altered to a certain degree by adjusting temperature and/or denervation time.3
Cooled radiofrequency ablation (CRFA) involves circulating cool water through the probe tip to maintain the lower temperatures at the tissue tip interface. The circulated water carries the heat away from the tissue–tip interface, which reduces desiccation and charring of tissues in contact with the probe. While the tip of the probe temperature is managed at 60°C, studies have demonstrated that the tissue temperature reaches about 80°C. CRFA delivers spherical lesions which are larger than lesions produced by conventional RF, thus increasing the likelihood of ablating targeted neural tissue.4 Furthermore, cooled RF probes can deliver a significantly more energy to surrounding neural tissues, which has been hypothesized to result in more durable clinical outcomes when compared to the small gauge conventional electrodes.5
It seems that the proper RF technique and the lesion size are but two important factors affecting long-term outcomes when utilizing RFA to treat chronic knee pain.6–8 Outcomes of tRFA were variable in success rate and provided interval time of pain relief. Most studies averaged 6-months of an effective analgesia when tRFA is used.11–18
Evidence on long-term effectiveness of CRFA first emerge in case series reports19,20 followed by prospective, multicenter, randomized trial comparing the effectiveness of CRFA to intraarticular corticosteroid (IAS) injections in the management of chronic knee pain from osteoarthritis in 151 subjects. At 6 months, 74.1% of study subjects had more than 50% of pain relief after CRFA. The CRFA group also had more favorable outcomes when it comes to Oxford knee score, global perceived effect, and mean change in non-opioid medication use.21 This study was continued prospectively through 12, 18 and 24 months.22,23 At 12 months, 65% of the original CRFA study subjects reported a pain reduction of >50% from baseline. The 12-month results noted above were found consistent in another multicenter RCT which suggests a similar degree and longevity of the pain relief and consequent improvements in functional capacity.24
In order to examine if the real-world data closely match the results from two previously published RCTs, Kapural et al25 retrospectively studied 183 patients who had undergone CRFA after successful genicular blocks. Sixty-five percent of the patients obtained greater than 50% pain relief and 77% had a two or more-point decrease in the Visual Analog Scale (VAS). The mean duration of the greater than 50% pain relief was about 12.5 months.
To date, a head-to-head comparison of the two technologies outcomes was not reported. Our large retrospective study compares the outcomes of two large consecutive patient cohorts who received either a tRFA or a CRFA of genicular nerves for chronic knee pain within the same interventional pain practice.
Methods
Outcome data are on 340 patients treated from January 2013 to December of 2019 who underwent radiofrequency denervation of a painful knee using either a tRFA or CRFA system at our Institute during their clinical management of chronic knee pain from osteoarthritis. Outcomes of 170 consecutive patients who underwent conventional RF with complete records and at least 12 months’ follow-up were compared to 170 consecutive patients who received cooled radiofrequency ablation in this retrospective study. There were 23 incomplete records in the tRFA group and 16 in the CRFA group (therefore, the total charts reviewed were 193 for tRFA and 186 for CRFA). Following the Forsyth Medical Center IRB (Winston-Salem, NC; No18-916) study approval, data were collected from our electronic medical records. Patient identifiers during data collection were removed and not used again. The nature of this retrospective study did not require further reclaim of patient’s identifiers. As such, Western IRB waived the need for obtaining informed consent from the patients.
Data collected included patients identifier, date of birth/age, sex, BMI, baseline numeric pain score, geniculate block (one or two diagnostic blocks and numeric pain score after the block/blocks that provided greater pain relief), daily total use of opioid medications at baseline (morphine equivalents in mg), daily total use of opioid medications approximately 6 months after the tRFA or CRFA of genicular nerves (oral morphine equivalents (OME) in mg), other sources of chronic pain, number of other chronic pain sources, assessment of opioid dependency/abuse susceptibility (based on opioid risk assessments during follow-ups), number of repeated genicular denervations, numeric pain scores after the repeated procedure. An assessment of the degree of osteoarthritis based on MRI/X-Ray imaging in four stages (Kellgren–Lawrence grade) as previously reported20,21 was completed prior to RFA, all patients included underwent one or two diagnostic blocks at the three nerve targets noted below under fluoroscopic guidance. Typically, if a patient received two blocks, the time interval between the two procedures was 2–3 weeks. The minimum requirements to progress to RFA required at least 50% relief. Universally, 0.5% bupivacaine was used at 1–1.5 cc per site. RF ablation was performed also under fluoroscopic guidance, in the supine position, under intravenous sedation and the targeted knee slightly bent. The electrodes were inserted into three previously described locations of superomedial, superolateral, and inferomedial genicular nerves.8,17,18,20 Electrode adjustment was performed to optimize sensory stimulation at 50 Hz, with concordant pain or pressure sensation at <0.5 V and 1 mL of 2% lidocaine then injected via the electrode to reduce procedure-related pain. tRFA was completed using a variety of electrode gauges ranging from 16- to 22-gauge, 100 mm radiofrequency needles with 10 mm active tips, while the cooled RF system consisted of 17-gauge, 75 mm cooled electrodes with 4 mm active tips (Coolief, Avanos Medical, Alpharetta, Georgia, USA). For conventional RF, the temperature was set at 80°C for 90s, whereas the cooled RF probe held a temperature of 60°C with a 150 s lesion time. At the completion of tRFA procedure, the knee was injected with 1–2 cc of 0.25% bupivacaine and 10 mg of triamcinolone, while at the completion of CRFA 1–2 cc of 0.5% bupivacaine only.
Patient populations were similar in terms of OA grade (Kellgren–Lawrence) at baseline. Grade was assessed in 123 patients in the tRFA group and 141 in the CRFA group. There were 72 patients’ images graded 1–2, and 51 grade 3–4 in the tRFA cohort, while there were 79 patients with grade 1–2 and 62 with grade 3–4 in the CRFA group. We were unable to assess the rest of the patients (47 in the tRFA group and 29 in the cRFA group). Follow-up x-rays were not obtained.
Data were detailed using descriptive statistics for continuous variables. We used Student t-test and, when needed, Mann–Whitney rank sum test to determine whether the VAS (Visual Analog Scale) pain scores, opioid usage attained following RF denervation of the affected knee using either tRFA or CRFA were significantly different. Similar analyses were carried out to determine changes in the numeric pain scores between the two RF treatments. We calculated the percentage of patients who maintained pain relief following either RF ablation. For multiple group comparisons, Kruskal–Wallis test was used first. All analyses were completed using the Sigma Plot Version 14 software for Windows (SYSTAT Software, San Jose, CA). A p value <0.05 was considered to be statistically significant.
Results
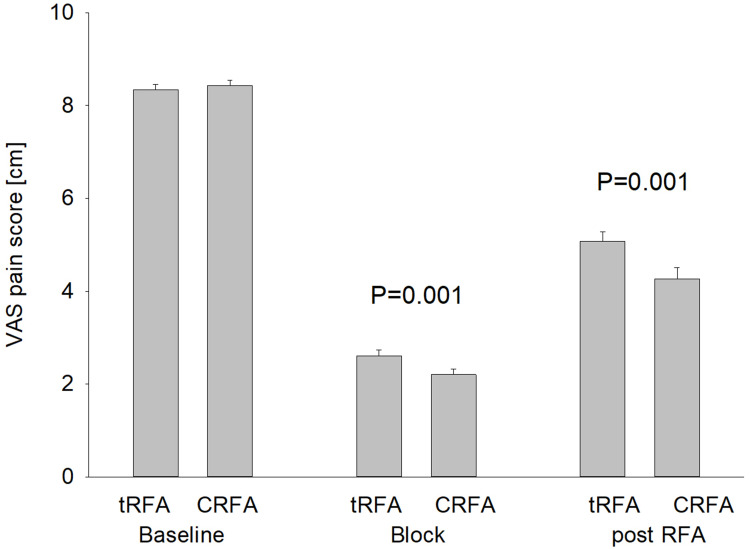
Outcomes of 170 consecutive patients who received tRFA were compared to 170 patients who underwent a CRFA, all from the same group pain practice (see Table 1). There were 111 women and 59 men in the tRFA cohort and 108 women and 62 men in the CRFA cohort. Patients were of comparable age (median age for tRFA group was 63.5 years and for CRFA group 61 years (p=0.297)). Their body mass index (BMI) was 33.9 and 35.9, respectively placing them, on average, into obese population of the patients (p=0.066). Baseline VAS pain scores were comparable and not statistically different, with an average pain score in the tRFA group of 8.3 ± 1.4 cm and in the CRFA group 8.4 ± 1.5 cm. Their average opioid consumption was 48.6 ± 85 mg MSO4 (morphine sulfate) equivalent (median 25) in the tRFA group vs 53 ± 61 mg (median 35) in the CRFA group. Both patient groups maintained, on average, two other chronic pain sources elsewhere in their body (Table 1). We found VAS pain scores after diagnostic blocks comparable at2.6 ± 1.6 for the tRFA group vs 2.2 ± 1.45 for the CRFA group. These VAS score changes were significantly different (p=0.011; Table 1; Figure 1).
Table 1.
Compared of the Two Similar Patient Cohorts Who Were Well Balanced in Most of Baseline Characteristics That Include Age, Number of Males and Females in Each Group, BMI (Body Mass Index), Baseline VAS Pain Scores, VAS Pain Scores After Diagnostic Geniculate Blocks, Daily Opioids Usage at Baseline and Number of Other Chronic Pain Sources per Individual Patient
| Characteristics | tRFA (n=170) | CRFA (n=170) | Statistics |
|---|---|---|---|
| Age (years) | Mean, 63.3 | Mean, 61.77 | P=0.297 |
| Median, 63.5 | Median, 61 | ||
| Sex | Female, n=111 | Female, n=108 | P=0.95 |
| Male, 59 | Male, 62 | ||
| BMI (kg/m2) | 35.9 | 33.9 | P=0.066 |
| Baseline VAS (cm) | Mean, 8.3 | Mean, 8.4 | P=0.505 |
| Median, 8 | Median, 8 | ||
| Diagnostic block VAS (cm) | Mean, 2.6 | Mean, 2.2 | P=0.011 |
| Median, 3 | Median, 2 | ||
| Opioid daily dose (mg) | Mean, 48.6 | Mean, 53 | P=0.05 |
| Median, 25 | Median, 35 | ||
| Other chronic pain sources | Median, 2 | Median, 2 | P=0.624 |
Figure 1.
Initial (4–6 weeks) pain relief after tRFA vs CRFA. Improvements in pain scores after the diagnostic blocks, and then either tRFA or CRFA. Notice a significant improvement in pain scores after the geniculate block, but also following tRFA and CRFA. Improvements after the CRFA were somewhat better than tRFA (p=0.010) when measured 4–6 weeks after the procedure.
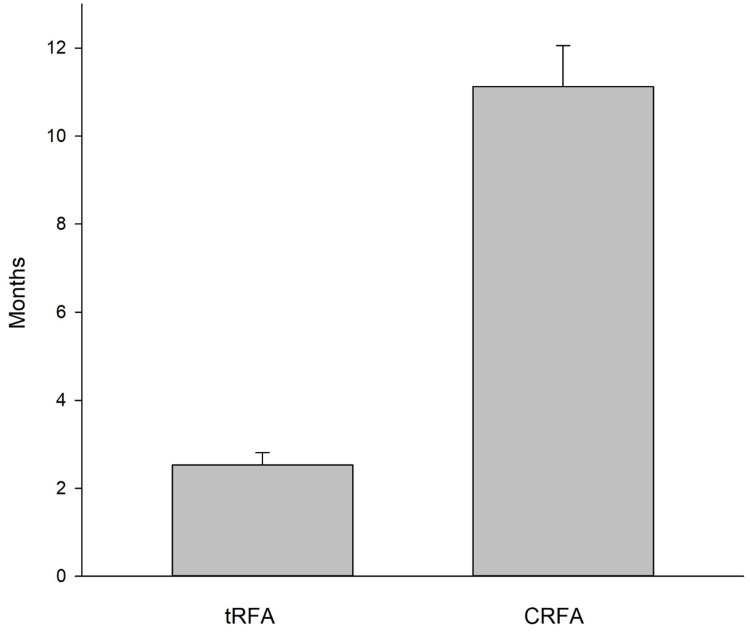
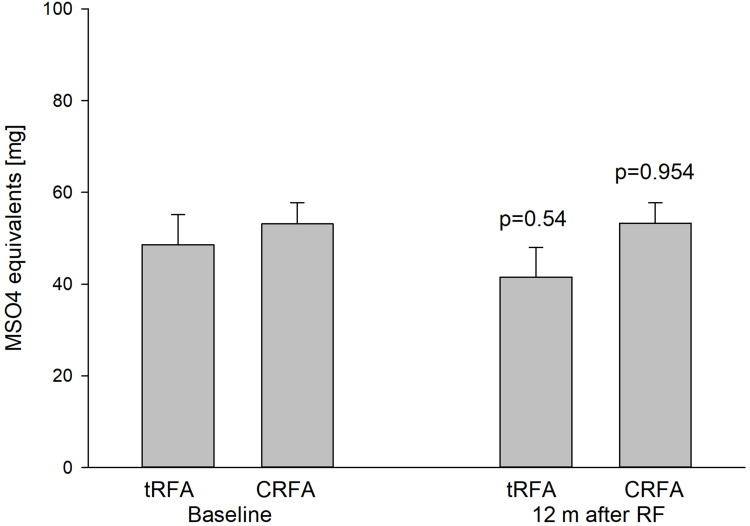
Improvements in VAS pain scores at the first visit (4–6 weeks after the procedure) after either the tRFA or the CRFA were significant. For the tRFA VAS pain scores decreased to 5.07±2.8 cm and for CRFA to 4.26 ± 3.2 cm (p=0.001 for both from baseline). CRFA provided better short-term improvements in the VAS pain scores (p=0.010; Figure 1) than tRFA. The time interval of pain relief (>50% of pain decrease) was an average of only 2.6 months for tRFA and 11.1 months for CRFA group. The difference was profound and significantly better in the favor of CRFA (p=0.001; Figure 2). There were 35 out of 170 patients in the tRFA group who maintained >50% of pain relief for 6 months or longer (or 20.6%) and only 15 out of 170 patients (8.8%) who continue receiving >50% of pain relief at 12 months, as opposed to 107 out of 170 (63%) patients in CRFA cohort who received >50% of pain relief at 6 months and 78 (46%) at 12 months. Opioid usage changed slightly to 41.5±20 mg OME for tRFA and to 53.2 ± 32 mg OME for CRFA (Figure 3). There was no significant change in opioid usage in either patient cohort (p=0.054 and p=0.954, respectively (Table 1; Figure 3).
Figure 2.
Long-term maintenance of pain relief after tRFA vs CRFA. Notice that a significant pain relief was maintained in average after the CRFA (11.1 months, while only 2.6 months for tRFA [p=0.001], n=170 for both).
Figure 3.
Opioid usage before and one year after CRFA vs tRFA. Baseline opioid measurement was taken just before the RFA was completed. There was no significant decrease in opioid usage before or after either tRFA or CRFA (p=0.54 and p=0.954, respectively).
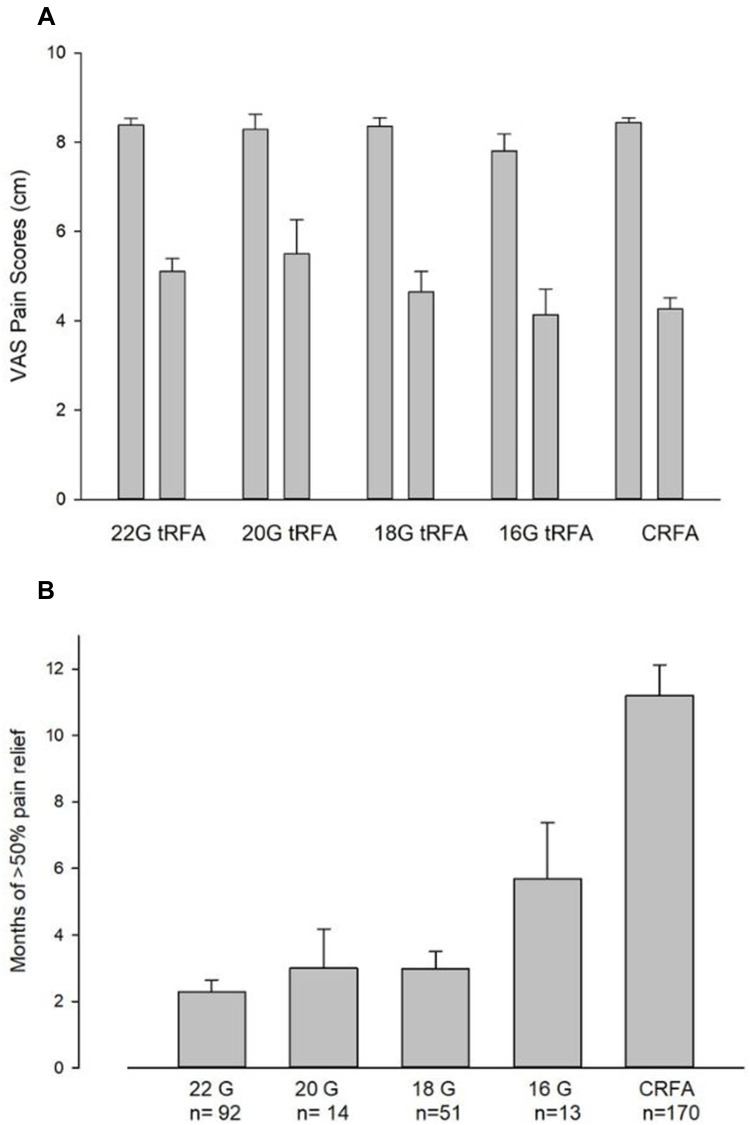
We compared initial outcomes and long-term maintenance of pain relief between sub-cohorts of the patients who received RFA using either 22G (n=92), 20G (n=14). 18G (n=51), 16G (n=13) or CRFA (n=170) (Figure 4). While the initial pain outcomes were similar for any electrode diameter used (Figure 4A), long-term positive outcomes (>50% of pain relief) were prolonged in a bigger lesion size treatment groups, where the largest CRFA also provided longest pain relief (see Figure 4B).
Figure 4.
Relationship between RF electrode size and pain relief achieved after RF (A) and longevity of the >50% of pain relief (B). (A) Initial (4–6 weeks) pain relief after tRFA vs CRFA based on probe gauge. Although there was a tendency for the larger diameter electrodes to improve chronic knee pain somewhat better from the baseline, there was no immediate significant difference in improvements of pain scores between patients receiving RFA when using various electrode diameters for ablation. (B) Long-term maintenance of pain relief after tRFA vs CRFA. There was a significant prolongation of >50% of pain relief when the larger diameter of tRFA electrodes and especially CRFA electrodes were used for the knee denervation. Notice that there were much fewer patients in 20 and 16 G electrode groups (n=14 and n=13, respectively).
We could not find a correlation between the increased patient weight (BMI), daily opioid use (in OME), increased age, or degree of knee degeneration with the amount of baseline pain, or pain relief achieved after the procedures in either group were studied.
Discussion
This observational cohort study compared tRFA against CRFA when used for treatment of chronic knee pain in an otherwise very heterogeneous population of the patients.
Our data analysis showed better outcomes (Figures 1and 4A) at the first follow-up visit (4–6 weeks post RF) and much longer time interval of >50% of pain relief when CRFA is used during genicular denervation as opposed to tRFA (Figure 2). A larger, just published study’s26 conclusions are in line with our conclusions, that the usage of CRFA does provide a better long-term outcome. Although they documented only 3 months’ follow-up outcomes comparing CRFA to tRFA when used in a similar patient group, a higher success rate of 67.5% of patients with more than 30% of pain relief at 3 months was achieved when CRFA used vs 54.5% in tRFA group (p=0.049).26
Chen et al’s study also suggested that the clinical outcomes may be significantly better when the larger diameter of tRFA electrodes are used,26 again in line with data that we detailed here (see Figure 4B). Moreover, we found that the long-term positive outcomes (>6 months) depended mainly on the size of the lesion, as CRFA- and larger tRFA-treated patients received a longer time interval of pain relief (Figures 2 and 4B).
A larger volume denervation area likely captures a greater number of articular branches as previously postulated.27 This greater nerve capture could be a direct cause for this longer and more profound pain relief after the CRFA.4,21,22,25
More surprising are the negative outcomes of the denervation procedure when a small diameter tRFA electrode is used. Negative outcomes (zero pain relief) of tRFA using 22 G RF needle after the positive block of geniculate nerves were stunning with 48 out of 92 patients, or 52%, suggesting that targeted nerves may have been captured by blocks, but missed by RF – likely due to highly variable nerve course as noted in Tran et al’s study.7 Other procedural reasons for such an outcome could be the angle of needle placement and consistent concordant responses to sensory stimuli. Patient selection could play a role, but we could not substantiate such a claim from the data collected. In comparison, there were 50 out of 170, or 29% of the patients, who received no pain relief after the CRFA, suggesting that the larger lesion compensated for the nerve course variability.
Our data are consistent with other published data on tRFA and CRFA. We summarized previously published data in Table 2 to show outcomes transparent and simple to compare. For example, if the tRFA study outcomes in Choi et al9 are compared to CRFA data from Davis’ study,21 74.1% of the patients had more than 50% of pain relief in the CRFA group versus 59–65% in the tRFA group. Davis et al went on to report that 65% of the subjects maintained ≥50% relief at 12 months following a single treatment with CRFA.22 Santana-Pineda et al, in a similarly sized randomized control trial (n=188) of tRFA using more precise ultrasound-guided technique,17 showed an effect similar to Choi in that 59% of the patients at 6 months reported ≥50% relief. It should be noted that only 34.4% reported this same analgesic effect at 12 months. Furthermore, the CRFA efficacy at 12 months was confirmed at 65% within a second, multicenter RCT24 and was also found to be comparable to a large real-world data retrospective study and the CRFA arm of this study.25 It has been suggested that the increased energy input by both larger tRFA probes and CRFA could also be playing a role in differences in effect and durability seen between the two technologies, which warrants further exploration.4 While the results from previous clinical studies indicate that the majority of subjects receiving a CRFA report >50% pain relief at the 6- and 12-month time point, the subjects in this retrospective trial were more heterogeneous and reported multiple sources of chronic pain which may have influenced VAS scores at follow-up, thus affecting the percentage of patients meeting this threshold. However, within the real-world population of this retrospective study, the differences in both efficacy and durability between the cooled and standard radiofrequency ablation are significant.
Table 2.
Summary of the Studies Using Various Sizes of tRF and CRF Electrodes
| Study | No of Patients | Baseline NRS | NRS After RFA | Time Interval of >50% of Pain Relief | Notes |
|---|---|---|---|---|---|
| Choi et al 20119 | 36–19 tRFA | N/A | N/A | 12 weeks | Limited to 12 weeks |
| Ikeuchi et al 201110 | 35–18 tRFA | 6 | 2 | 10% with >50% pain relief at 6 months | 22G-5mm; Sensory stimulation |
| Kirdemir et al 201713 | 49 tRFA | 8.9 | 3.93 | 12 weeks | 22G-10mm; Sensory stimulation |
| Sari et al 201811 | 37 tRFA | N/A | N/A | N/A | Significant reduction in VAS pain at 1 and 3 months noted. |
| Iannaccone et al12 | 20 tRFA to 6m | >7 | 3.7 | <50% with >50% pain relief at 3 months | 22 G-10 mm Sensory stimulation |
| Santana Pineda et al 201718 | 25 tRFA | 8.5 | 2, 5 at 1 m 5.8 at 12 m | 32% with >50% pain relief at 12 months | 23G 5 mm active tip; ultrasound-guided |
| Santana Pineda et al 202117 | 93 tRFA | 8.36 | 6.59 at 12m | 59% with >50% pain relief at 3 months 34.4% with >50% pain relief at 12 months | 23G 5 mm active tip; ultrasound-guided pulsed RF shown less effective/ ineffective |
| This study tRFA | 170 | 8.3 | 5.07 | 20.6% with >50% pain relief at 6 months 8.8% with >50% of pain relief at 12 months |
22 G worst results |
| Chen Y et al 202126 | CRFA 151 tRFA 103 | 6.6–7.3 | See next column | CRFA –63% with >30%; tRFA – 35% with >30% at 3 months | CRFA – 67.5%; p=0.049tRFA − 54.5% at 3 months18-gauge or larger – 66.1% smaller – 50.0%; p=0.01 |
| Davis et al 201821,22 | 151- CRFA | 7.3 | 3.1 | 65% with >50% pain relief at 12 months | CRFA; optional sensory stimulation |
| Chen A et al24 | 175–88 CRFA | 6.9 | 2.7 | 71% with >50% pain relief at 6 months; 65% at 12 months | Randomized prospective trial comparing CRFA to hyaluronic acid injection |
| McCormick et al19 | 33 CRFA | 8 | Not reported | 35% Success of procedures at 6 months | CRFA; no sensory stimulation. Success defined as combined outcome of >50% reduction in NRS score, reduction of 3.4 or more points in MQSIII score, and PGIC score consistent with “very much improved/improved” |
| Kapural et al25 | 183 CRFA | 8.5 | 4.2 | 12.5 months average, 65% with >50% pain relief | CRFA; Sensory stimulation |
| This study CRFA | 170 CRFA | 8.4 ± 1.5 cm | 4.26 ± 3.2 cm | 11.1 months average 46% with >50% pain relief at 12 months | CRFA; Sensory stimulation |
We could not find a correlation between the increased patient weight (BMI), daily opioid use (in OME), increased age, or degree of knee degeneration with the amount of baseline pain, or pain relief achieved after the procedures in either group studied. This is consistent with our previously published large CRFA cohort.25 Although there was a recent study suggesting a correlation of negative outcomes to opioid usage, we were unable to detect such a correlation. There were only 67 out of 170 patients in the tRFA (39%) and 85 out of 170 (50%) patients in the CRFA cohorts who were using more than 30 mg OME of opioids daily.
Also, when it comes to opioid reduction after either RF treatment, there was no intended effort to wean the patients off opioids in this diverse patient group who had on average two other chronic pain sources, and with fewer patients taking any opioids, we could not show a significant reduction in opioid usage.
This retrospective study of the large number of subjects is consistent with previously published studies.17,18,22,25 The CRFA creates a larger lesion compared to conventional RFA.4 In addition, the larger volume lesions can be created without any regard to the angle of the needle in relation to the course of the nerve. It has been clearly demonstrated that the genicular nerves have significant variability in relation to the osseous structures.6,7 By creating larger, angle-independent lesions, the likelihood of ablating the target nerves increases, which could explain the success of CRFA when compared to conventional RFA.5,28,29
There are limitations to this study which may influence outcomes reported. Those include procedural variations (like usage of triamcinolone after the completion of tRFA), missing data, lack of functional capacity assessment and non-blinded nature of the study. Like with any other retrospective study, we tried to set such a deficiency by having a large consecutive cohort of patients studied. Also, it is not clear if this comparison may reach any significance if multiple mono or bipolar lesions are provided as suggested recently.30 The CRFA group also exhibited statistically better improvements in the pain scores that followed diagnostic blocks (Table 1). It is not clear if such an assessment can influence long-term improvements or not. However, the requirement for a successful block was >50% of the pain relief which was reached in every patient in both patient cohorts in order to qualify for RFA.
Disclosure
Dr Leonardo Kapural reports grants, personal fees from Avanos, grants, personal fees from Medtronic, during the conduct of the study. The authors report no other conflicts of interest in this work.
References
- 1.Murphy L, Schwartz TA, Helmick CG, et al. Lifetime risk of symptomatic knee osteoarthritis. Arthritis Rheum. 2008;59(9):1207–1213. doi: 10.1002/art.24021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kapural L, Mekhail N. Radiofrequency ablation for chronic pain control. Curr Pain Headache Rep. 2001;5:517–525. doi: 10.1007/s11916-001-0069-z [DOI] [PubMed] [Google Scholar]
- 3.Haines DE, Verow AF. Observations on electrode-tissue interface temperature and effect on electrical impedance during radiofrequency ablation of ventricular myocardium. Circulation. 1990;82:1034–1038. doi: 10.1161/01.CIR.82.3.1034 [DOI] [PubMed] [Google Scholar]
- 4.Cedeño DL, Vallejo A, Kelley CA, et al. Comparisons of lesion volumes and shapes produced by a radiofrequency system with a cooled, a protruding, or a monopolar probe. Pain Physician. 2017;20(6):E915–E922. doi: 10.36076/ppj.20.5.E915 [DOI] [PubMed] [Google Scholar]
- 5.Zachariah C, Mayeux J, Alas G, et al. Physiological and functional responses of water-cooled versus traditional radiofrequency ablation of peripheral nerves in rats. Reg Anesth Pain Med. 2020;45(10):792–798. doi: 10.1136/rapm-2020-101361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Franco Carlo D, Buvanendran A, Petersohn JD, et al. Innervation of the anterior capsule of the human knee: implications for radiofrequency ablation. Reg Anesth Pain Med. 2015;40(4):363–368. doi: 10.1097/AAP.0000000000000269 [DOI] [PubMed] [Google Scholar]
- 7.Tran J, Pang PWH, Lam K, et al. Anatomical study of the innervation of anterior knee joint capsule: implications for image-guided interventions. Reg Anesth Pain Med. 2018;43:407–414. doi: 10.1097/AAP.0000000000000778 [DOI] [PubMed] [Google Scholar]
- 8.Kapural L. Bigger is better: or is it? Reg Anesth Pain Med. 2018;43:339–340. doi: 10.1097/AAP.0000000000000788 [DOI] [PubMed] [Google Scholar]
- 9.Choi WJ, Hwang SJ, Song JG, et al. Radiofrequency treatment relieves chronic knee osteoarthritis pain: a double-blind randomized controlled trial. Pain. 2011;152:481–487. doi: 10.1016/j.pain.2010.09.029 [DOI] [PubMed] [Google Scholar]
- 10.Ikeuchi M, Ushida T, Izumi M, Tani T. Percutaneous radiofrequency treatment for refractory anteromedial pain of osteoarthritic knees. Pain Med. 2011;12:546–551. doi: 10.1111/j.1526-4637.2011.01086.x [DOI] [PubMed] [Google Scholar]
- 11.Sarı S, Aydın ON, Turan Y, et al. Which one is more effective for the clinical treatment of chronic pain in knee osteoarthritis: radiofrequency neurotomy of the genicular nerves or intra-articular injection? Int J Rheum Dis. 2018;2018(21):1772–1778. [DOI] [PubMed] [Google Scholar]
- 12.Iannaccone F, Dixon S, Kaufman A. A review of long-term pain relief after genicular nerve radiofrequency ablation in chronic knee osteoarthritis. Pain Physician. 2017;20:E437–E444. doi: 10.36076/ppj.2017.E444 [DOI] [PubMed] [Google Scholar]
- 13.Kirdemir P, Çatav S, Alkaya Solmaz F. The genicular nerve: radiofrequency lesion application for chronic knee pain. Turkish J Med Sci. 2017;47:268–272. doi: 10.3906/sag-1601-171 [DOI] [PubMed] [Google Scholar]
- 14.Protzman NM, Gyi J, Malhotra AD, Kooch JE. Examining the feasibility of radiofrequency treatment for chronic knee pain after total knee arthroplasty. PMR. 2014;6:373–376. doi: 10.1016/j.pmrj.2013.10.003 [DOI] [PubMed] [Google Scholar]
- 15.Gupta A, Huettner DP, Dukewich M. Comparative effectiveness review of cooled versus pulsed radiofrequency ablation for the treatment of knee osteoarthritis: a systematic review. Pain Physician. 2017;20(3):155–171. doi: 10.36076/ppj.2017.171 [DOI] [PubMed] [Google Scholar]
- 16.Ahmed A, Arora D. Ultrasound-guided radiofrequency ablation of genicular nerves of knee for relief of intractable pain from knee osteoarthritis: a case series. Br J Pain. 2018;12:145–154. doi: 10.1177/2049463717730433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Santana Pineda MM, Vanlinthout LE, Santana-Ramírez S, et al. A randomized controlled trial to compare analgesia and functional improvement after continuous neuroablative and pulsed neuromodulative radiofrequency treatment of the genicular nerves in patients with knee osteoarthritis up to one year after the intervention. Pain Med. 2021;22(3):637–652. doi: 10.1093/pm/pnaa309 [DOI] [PubMed] [Google Scholar]
- 18.Santana Pineda MM, Vanlinthout LE, Moreno Martín A, et al. Analgesic effect and functional improvement caused by radiofrequency treatment of genicular nerves in patients with advanced osteoarthritis of the knee until 1 year following treatment. Reg Anesth Pain Med. 2017;42:62–68. doi: 10.1097/AAP.0000000000000510 [DOI] [PubMed] [Google Scholar]
- 19.McCormick ZL, Korn M, Reddy R, et al. Cooled radiofrequency ablation of geniculate nerves for chronic pain due to knee osteoarthritis: a six months outcomes. Pain Medicine. 2017;18:1631–1641. doi: 10.1093/pm/pnx069 [DOI] [PubMed] [Google Scholar]
- 20.Bellini M, Barbieri M. Cooled radiofrequency system relieves chronic knee osteoarthritis pain: the first case-series. Anaesthesiol Intensive Ther. 2015;47:30–33. doi: 10.5603/AIT.2015.0003 [DOI] [PubMed] [Google Scholar]
- 21.Davis T, Loudermilk E, DePalma M, et al. Prospective, multi-center, randomized, cross-over clinical trial comparing the safety and effectiveness of cooled radiofrequency ablation to corticosteroid injection in the management of knee pain from osteoarthritis. Reg Anesth Pain Med. 2018;43:84–91. doi: 10.1097/AAP.0000000000000690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Davis T, Loudermilk E, DePalma M, et al. 12-Month analgesia, and rescue, by cooled radiofrequency ablation treatment of osteoarthritic knee pain: results from prospective, multicenter, randomized, cross-over trial. Reg Anesth Pain Med. 2019;44:499–506. doi: 10.1136/rapm-2018-100051 [DOI] [PubMed] [Google Scholar]
- 23.Hunter C, Davis T, Loudermilk E, et al. Cooled radiofrequency ablation treatment of the genicular nerves in the treatment of osteoarthritic knee pain: 18- and 24-month results. Pain Pract. 2020;20(3):238–246. doi: 10.1111/papr.12844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen AF, Khalouf F, Zora K, et al. Cooled radiofrequency ablation provides extended clinical utility in the management of knee osteoarthritis: 12-month results from a prospective, multi-center, randomized, cross-over trial comparing cooled radiofrequency ablation to a single hyaluronic acid injection. BMC Musculoskelet Disord. 2020;21(1):363. doi: 10.1186/s12891-020-03380-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kapural L, Lee N, Neal K, Long-Term Retrospective BM. Assessment of clinical efficacy of radiofrequency ablation of the knee using a cooled radiofrequency system. Pain Physician. 2019;22(5):489–494. doi: 10.36076/ppj/2019.22.489 [DOI] [PubMed] [Google Scholar]
- 26.Chen Y, Vu TH, Chinchilli VM, et al. Clinical and technical factors associated with knee radiofrequency ablation outcomes: a multicenter analysis. Reg Anesth Pain Med. 2021;46(4):298–30427. doi: 10.1136/rapm-2020-102017 [DOI] [PubMed] [Google Scholar]
- 27.Tran J, Peng P, Agur A. Evaluation of nerve capture using classical landmarks for genicular nerve radiofrequency ablation: 3D cadaveric study. Reg Anesth Pain Med. 2020;45:898–906. doi: 10.1136/rapm-2020-101894 [DOI] [PubMed] [Google Scholar]
- 28.Cosman ER, Dolensky JR, Hoffman RA. Factors that affect radiofrequency heat lesion size. Pain Med. 2014;15:2020–2036. doi: 10.1111/pme.12566 [DOI] [PubMed] [Google Scholar]
- 29.Singh S, Melnick R. Domain heterogeneity in radiofrequency therapies for pain relief: a computational study with coupled models. Bioengineering. 2020;7(2):35. doi: 10.3390/bioengineering7020035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Conger A, Cushman DM, Walker K, et al. A novel technical protocol for improved capture of the genicular nerves by radiofrequency ablation. Pain Med. 2019;20(11):2208–2212. doi: 10.1093/pm/pnz124 [DOI] [PubMed] [Google Scholar]