Abstract
Achieving malaria elimination requires a better understanding of the transmissibility of human infections in different transmission settings. This study aimed to characterize the human infectious reservoir in a high endemicity setting in eastern Uganda, using gametocyte quantification and mosquito feeding assays. In asymptomatic infections, gametocyte densities were positively associated with the proportion of infected mosquitoes (β = 1.60; 95% CI, 1.32–1.92; P < .0001). Combining transmissibility and abundance in the population, symptomatic and asymptomatic infections were estimated to contribute to 5.3% and 94.7% of the infectious reservoir, respectively. School-aged children (5–15 years old) contributed to 50.4% of transmission events and were important drivers of malaria transmission.
Keywords: malaria transmission, Plasmodium falciparum, gametocytes, mosquito feeding assays, Uganda
In an area of intense malaria transmission in Uganda, school-aged children (5–15 years old) and asymptomatic infections were identified as major contributors to the malaria human infectious reservoir and could be considered as important targets for malaria control interventions.
The declining global burden of malaria has resulted in a renewed interest in malaria elimination, which will depend on effective deployment of transmission-reducing interventions and a good understanding of the infectious reservoir [1]. Human to mosquito transmission of Plasmodium parasites is initiated when a malaria vector takes a human blood meal containing male and female gametocytes, the transmissible parasite stages [2]. Mosquito feeding assays (MFAs) that allow malaria vectors to feed on an individual’s venous blood are the only tools currently available for quantification of onward transmission [3]. Epidemiological assessments using MFAs to characterize the human infectious reservoir are sparse and report conflicting findings on the contribution of symptomatic, asymptomatic, and submicroscopic infections to malaria transmission [4, 5]. The importance of submicroscopic infections in transmission was underscored by a recent longitudinal cohort study conducted in a low-transmission setting in Uganda, where submicroscopic infections were responsible for 15% of mosquito infections and asymptomatic infections among school-aged children were highly important for transmission [6]. In high-transmission settings, the average age at which infections are acquired is lower [7], asymptomatically infected individuals may harbor higher parasite densities [8], and clinical malaria episodes may occur in the presence of ongoing infections. These differences make it unclear whether patterns in transmission potential and the relative contributions of different populations to the infectious reservoir are similar between high- and low-transmission settings. Here, we examined gametocyte density and infectivity among symptomatic and asymptomatic malaria infections to assess the human infectious reservoir in an area of intense malaria transmission in Uganda.
METHODS
Study Site
Our study was conducted in an area that spans the border between Busia-Tororo Districts in Eastern Uganda and included Buteba Parish (Busia District) and Kayoro and Osukuru Parishes (Tororo District). Tororo is under intensive vector control with long-lasting insecticidal nets (LLINs) and indoor-residual spraying while Busia district receives only LLINs and is characterized by intense P. falciparum malaria transmission (Supplementary Table 1) [9].
Procedures
An all-age cohort consisting of 80 households with a maximum of 9 residents, including at least 2 children younger than 10 years, was followed from August 2020 to June 2021. Household and participant selection is described in detail elsewhere [10]. Following baseline sampling, participants entered a cohort with scheduled visits every 4 weeks and passive case detection at a study clinic. At each visit, a standard clinical evaluation was done and venous blood samples were collected. Participants with a parasite-positive thick blood smear and subjective fever or a tympanic temperature above 38.0°C were classified as symptomatic and treated accordingly [10]. Symptomatic episodes were considered unique when occurring at least 4 weeks apart. Asymptomatic infections were defined as the detection of parasites in the absence of symptoms and were not treated, in accordance with local guidelines.
The presence and density of P. falciparum parasites was assessed by var gene acidic terminal sequence (varATS) quantitative polymerase chain reaction (qPCR), with a lower limit of detection of 0.05 parasites/µL using 200µL whole-blood samples [6]. Of all qPCR-positive samples (n = 1912), a subset was used for gametocyte quantification, including all qPCR-positive symptomatic follow-up visit samples (n = 244), the first asymptomatic qPCR-positive follow-up visit sample for each cohort participant (n = 306), and all samples included in direct membrane feeding assays (n = 182). With 7 extraction failures, this resulted in a total of 732 gametocyte measurements. Gametocytes were quantified from 100 µL whole blood in RNA preservative (RNAprotect Cell Reagent; Qiagen) by quantitative reverse transcriptase PCR (qRT-PCR) targeting male (PfMGET) and female (CCp4) mRNA transcripts after automated extraction (Total Nucleic Acid Isolation Kit-High Performance; Roche Applied Science) with a lower limit of detection of 0.01 gametocytes/µL [11]. (Primer and probe sequences are described in Supplementary Table 2.) Gametocyte densities were adjusted for background transcripts in asexual parasites [11].
Direct membrane feeding assays were performed on selected individuals (1) when diagnosed with symptomatic malaria or (2) at the scheduled visit following detection of parasites above a qPCR threshold of 100 parasites/µL. This inclusion criteria was implemented to avoid many uninformative feeds, as parasite densities below this threshold are unlikely to result in mosquito infection [6]. Feeding procedures are described in detail elsewhere [6] and involved locally reared Anopheles gambiae ss mosquitoes being offered a heparin blood sample through glass feeders. Ten days postfeeding, fully fed mosquitoes were dissected in 1% mercurochrome and oocysts were detected at 40× magnification by 2 independent microscopists (J.O. and E.M.).
Statistical Analyses
Total parasite and gametocyte densities were log transformed and modelled using a Gaussian distribution with clinical status and age group as covariates. The association between gametocyte density and total parasite density was determined by mixed effects linear regression models for symptomatic and asymptomatic infections separately, whilst including participant-specific random effects to adjust for multiple observations from the same individual. The association between proportion of infected mosquitoes and gametocyte density was determined using generalized linear models assuming a binomial distribution with a log-link for symptomatic and asymptomatic infections separately. Parasite-positive visits were used to estimate the contribution of symptomatic and asymptomatic infections to the infectious reservoir. The contribution of different age groups (younger than 5 years, 5–15 years, 16 years and older) to the infectious reservoir was estimated using all available visits (including parasite-negative observations), as previously performed and detailed in the Supplementary Methods [6]. All statistical analyses were performed in R (version 3.1.12). Full details of statistical analyses are shown in the Supplementary material.
Ethical Approval
Written informed consent was received from all eligible study participants. The Uganda National Council for Science and Technology, Makerere University School of Medicine, the University of California San Francisco, and the London School of Hygiene and Tropical Medicine provided ethical approval for the study.
RESULTS
Between August 2020 and June 2021, 501 residents from 80 households were enrolled and followed up. As a consequence of enrolment purposefully targeting the cross-border area, 20 households were located in Buteba, 40 in Kayoro, and 20 in Osukuru. At enrolment, 37.4% (192/513) of participants were aged younger than 5 years, 29.4% (151/513) were 5–15 years, and 33.1% (170/513) were 16 years or older.
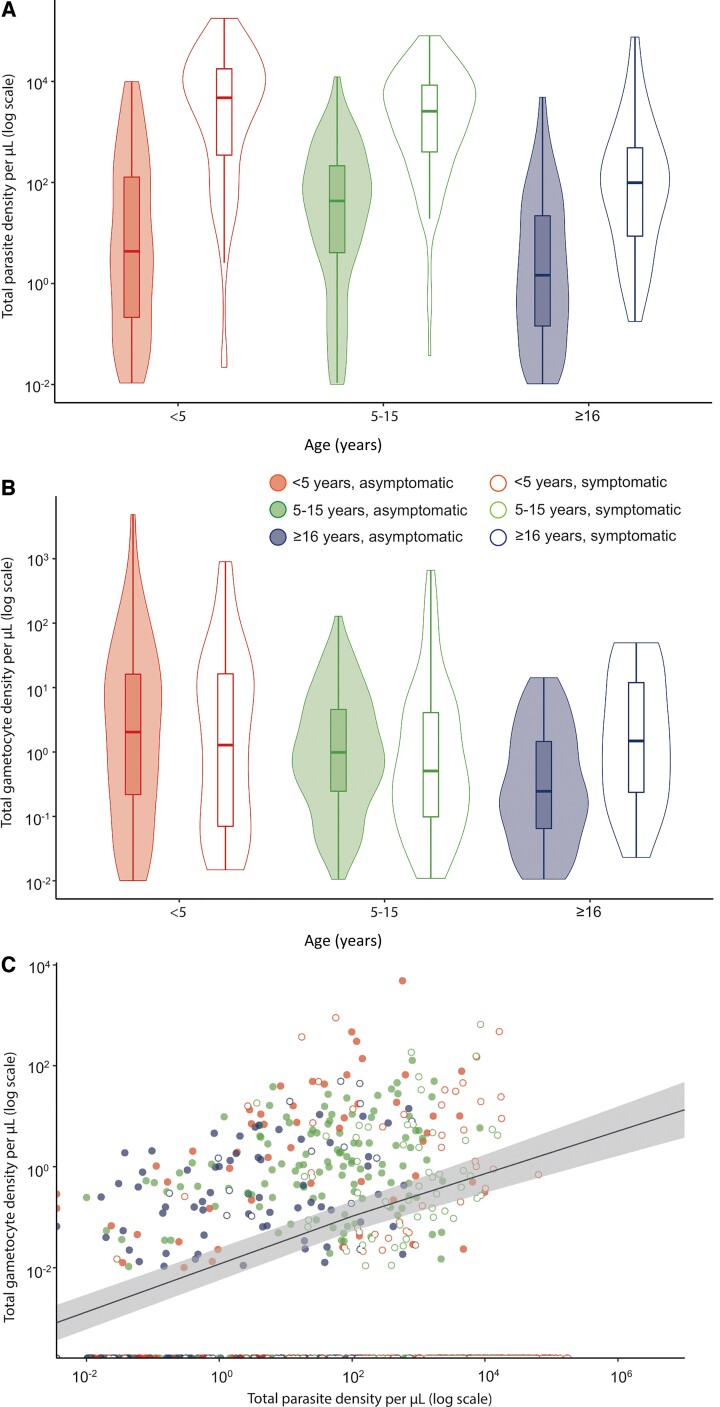
Parasite prevalence by qPCR at enrolment was 46.9% (61/130) in Buteba, 39.0% (99/254) in Kayoro, and 39.3% (46/117) in Osukuru (Supplementary Table 3). When combining these parishes with similar overall levels of parasite carriage and density (Supplementary Figure 1), parasite prevalence at enrolment was 57.0% (85/149) in individuals aged 5–15 years, which was higher than in individuals aged 16 years and older (39.8%, 66/166, P = .003) and individuals younger than 5 years (29.0%, 54/186, P < .0001; Supplementary Table 3). During the study period, 323 episodes of symptomatic malaria were detected. Median parasite density among symptomatic infections was 2222.1 parasites/µL (interquartile range [IQR], 163.4–11 483) compared to 4.22 parasites/µL (IQR, 0.21–83.29) for asymptomatic infections (P < .0001) (Figure 1A and Supplementary Table 4). Gametocyte prevalence by RT-qPCR was lower in symptomatic infections (35.3%, 96/272), compared to asymptomatic infections (55.0%, 231/420, P < .0001; Supplementary Table 5). Median gametocyte density among qRT-PCR–positive samples was highest in asymptomatic individuals younger than 5 years (2.04 gametocytes/µL; IQR, 0.22–16.14) compared to asymptomatic individuals aged 5–15 years (0.99 gametocytes/µL; IQR, 0.25–4.57; P < .007) and asymptomatic individuals aged 16 years and older (0.25 gametocytes/µL; IQR, 0.06–4.57; P < .015; Figure 1B, Supplementary Table 4, and Supplementary Figure 2). Gametocyte density was positively associated with total parasite density in asymptomatic infections (β = .42; 95% confidence interval [CI], .34–.49; P < .0001) and negatively associated in symptomatic infections (β = −.23; 95% CI, –.36 to –.11; P = .0003; Figure 1C).
Figure 1.
Parasite and gametocyte densities and the relationship between them. A, Total parasite density among qPCR-positive symptomatic and asymptomatic infections from different age categories. B, Total gametocyte density among qRT-PCR–positive symptomatic and asymptomatic infections from different age categories. C, Total parasite density in relation to total gametocyte density by qRT-PCR. Closed and open symbols reflect asymptomatic and symptomatic infections, respectively. The best fitted association for asymptomatic infections is represented by the line, and visualizes the association used for imputing gametocyte densities of asymptomatic samples. The shaded area indicates the 95% confidence interval. Abbreviations: qPCR, quantitative polymerase chain reaction; qRT-PCR, quantitative reverse transcriptase polymerase chain reaction.
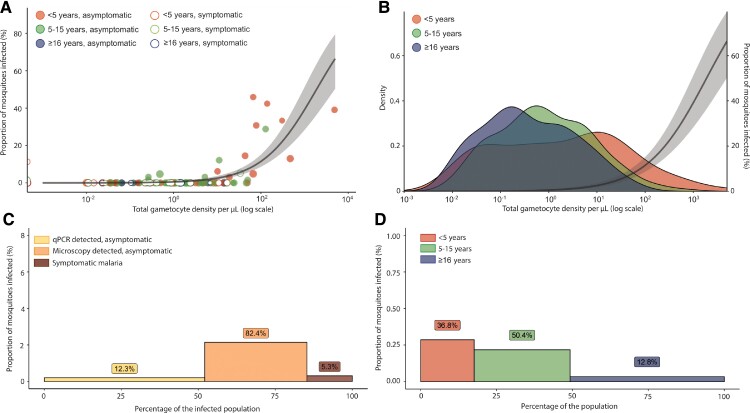
Overall, 182 MFAs were performed with samples from 132 individuals, of which 63 samples were symptomatic and 119 asymptomatic (Supplementary Table 6). Altogether, 11.5% (21/182) of feeding assays resulted in at least 1 infected mosquito and 1.6% (196/12 398) of fed mosquitoes became infected. Gametocyte densities were strongly associated with the proportion of mosquitoes infected upon feeding from asymptomatic samples (β = 1.60; 95% CI, 1.32–1.92; P < .0001), but not from symptomatic samples (β = .18; 95% CI, −.88 to 1.01; P = .68) (Figure 2A and 2B). Among the 4 symptomatic samples that were infectious to mosquitoes, 2 had gametocyte densities below our limit of detection (0.01 gametocyte/µL) and a third sample had detectable gametocyte transcripts, but due to a high total parasite density (36 091 parasites/µL) its gametocyte density was censored as 0 gametocytes/µL [11] (Figure 2A).
Figure 2.
The relationship between gametocyte density and infectivity to mosquitoes and the contribution of different subpopulations to the human infectious reservoir. A, Relationship between total gametocyte density by qRT-PCR and the percentage of infected mosquitoes. The line represents the best-fitted association and the shaded area the 95% CI. B, Gametocyte density among gametocyte-positive individuals from different age categories. The y-axis indicates the density of a gametocyte concentration in the infected population per age category. The best-fitted association between gametocyte density and infectivity to mosquitoes is represented by the line and the shaded area is the 95% CI. C, Contribution of different infection types in the infected population to the infectious reservoir. D, Contribution of different age categories to the human infectious reservoir at a population level. C and D, Contribution to the infectious reservoir for asymptomatic infections was estimated based on imputing gametocyte densities using 1378 asymptomatic samples with known parasite densities, and imputing the proportion of infected mosquitoes using 119asymptomatic samples with mosquito feeding results and known gametocyte densities. Proportion of infected mosquitoes is indicated by the bar heights, the bar widths indicate the proportion of a subpopulation to the (infected) population, and the percentage given above each bar is the estimated proportion of a subpopulation to the infectious reservoir. Abbreviations: CI, confidence interval; qRT-PCR, quantitative reverse transcriptase polymerase chain reaction.
For asymptomatic infections without gametocyte measurements, gametocyte densities were first imputed using the association depicted in Figure 1C. For asymptomatic infections, mosquito infection rates were imputed based on the association with gametocyte densities (Figure 2A), as previously performed [6]. For symptomatic samples, where gametocyte densities were not significantly associated with the proportion of infected mosquitoes, only direct measurements of infectivity were used. We then considered both the infectivity and the relative proportion of different populations in the total population to estimate contributions to the infectious reservoir.
Asymptomatic microscopy-detected infections were estimated to comprise 82.4% of the infectious reservoir, whereas asymptomatic submicroscopic (ie, only detected by qPCR) infections were responsible for 12.3% and symptomatic infections for 5.3% (Figure 2C). Children aged 5–15 years and younger than 5 years were estimated to be responsible for 50.4% and 36.8% of the infectious reservoir, respectively (Figure 2D). The contribution of individuals aged 16 years and older was the lowest (12.8%), despite these individuals comprising 50.8% of the total population.
DISCUSSION
Despite the importance of understanding the human infectious reservoir for malaria control and elimination efforts [1], very few studies have directly quantified this reservoir [4, 6]. The present study aimed to characterize transmissibility in an area of intense perennial transmission in Uganda.
Among asymptomatic infections, parasite density was positively associated with gametocyte density [3, 6]. As observed before, this association was negative in the symptomatic population [12] where recent infections may be associated with high parasite densities but low or undetectable gametocytes due to their maturation period [13]. The proportion of mosquitoes infected upon feeding was strongly associated with gametocyte density in asymptomatic, but not in symptomatic, parasite carriers. Whereas gametocyte infectivity may be lower during symptoms [6, 13, 14], it is also possible that this lack of positive association is explained by 3 infectious individuals without detectable gametocytes. Given these uncertain associations among symptomatic individuals, we used direct measurements of infectivity for this population while missing observations were imputed for asymptomatic parasite carriers [6].
When considering both the transmissibility and relative frequency in our study cohort, asymptomatic infections were estimated to be responsible for 94.7% of the infectious reservoir, whereas symptomatic infections only comprised 5.3%. Because participants in our study had access to prompt treatment, the frequency and duration of symptomatic infections in a nonresearch settings may differ, potentially influencing their contribution to the infectious reservoir. The close monitoring of our population allowed us to confirm that only a minority of asymptomatic infections progressed to symptomatic episodes (Supplementary Table 7). Previous studies in low-endemic settings in Ethiopia [5] and Uganda [6] also observed a large contribution of asymptomatic malaria infections to the human infectious reservoir. In our setting, submicroscopic infections were responsible for 12.3% of the mosquitoes infected, which is similar to the contribution of submicroscopic infections (15%) in a low-endemic setting [6], despite previous suggestions that submicroscopic infections would contribute less to transmission in high-endemic settings [7]. We further estimated that school-aged children contribute highly to the infectious reservoir (50.4%), followed by children younger than 5 years (36.8%). Compared to a previous study in a neighboring low-endemic area in Tororo, we observed higher contributions of the youngest age group and of symptomatic infections to the infectious reservoir. Clinical cases may carry gametocytes from a longer-term infection whilst a recent superinfection resulted in symptoms. Despite these subtle differences, our findings are in broad agreement with previous studies highlighting the important contribution of school-aged children to the malaria infectious reservoir [6, 15]. A limitation of our study is that we did not quantify (age-dependent) mosquito exposure; this is a crucial component of onward transmission potential and requires dedicated studies.
In conclusion, the present study highlights the importance of asymptomatic infections in malaria transmission. We further identified school-aged children as the most important drivers of transmission in this setting. These findings support the hypotheses that both asymptomatic infections and school-aged children form important contributors to the malaria infectious reservoir, which could be targeted by malaria control interventions.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online (http://jid.oxfordjournals.org/). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Acknowledgments . We thank the study team and the Infectious Diseases Research Collaboration for administrative and technical support. We are grateful to the study participants who participated in this study and their families.
Financial support . This work was supported by the National Institutes of Health (grant numbers AI089674 International Centers of Excellence in Malaria Research Program and AI075045); the Bill and Melinda Gates Foundation (grant number INDIE OPP1173572); and the European Research Council (grant number ERC-CoG 864180 QUANTUM fellowship to T. B.). M. D. C. is supported by the Fogarty International Center (grant numbers P0529898 and TW009343), and a Centennial Travel Award from the American Society of Tropical Medicine and Hygiene. The ClinEpiDB platform is supported by the Bill and Melinda Gates Foundation (grant number OPP1169785). Funding to pay the Open Access publication charges for this article was provided by the Bill & Melinda Gates Foundation.
Potential conflicts of interest . All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Supplementary Material
Contributor Information
John Rek, Infectious Diseases Research Collaboration, Kampala, Uganda.
Sara Lynn Blanken, Department of Medical Microbiology, Radboud University Nijmegen Medical Centre, Nijmegen, The Netherlands.
Joseph Okoth, Infectious Diseases Research Collaboration, Kampala, Uganda.
Daniel Ayo, Infectious Diseases Research Collaboration, Kampala, Uganda.
Ismail Onyige, Infectious Diseases Research Collaboration, Kampala, Uganda.
Eric Musasizi, Infectious Diseases Research Collaboration, Kampala, Uganda.
Jordache Ramjith, Department for Health Evidence, Radboud University Medical Center, Nijmegen, The Netherlands.
Chiara Andolina, Department of Medical Microbiology, Radboud University Nijmegen Medical Centre, Nijmegen, The Netherlands.
Kjerstin Lanke, Department of Medical Microbiology, Radboud University Nijmegen Medical Centre, Nijmegen, The Netherlands.
Emmanuel Arinaitwe, Infectious Diseases Research Collaboration, Kampala, Uganda.
Peter Olwoch, Infectious Diseases Research Collaboration, Kampala, Uganda.
Katharine A Collins, Department of Medical Microbiology, Radboud University Nijmegen Medical Centre, Nijmegen, The Netherlands.
Moses R Kamya, Department of Medicine, Makerere University College of Health Sciences, Kampala, Uganda.
Grant Dorsey, Department of Medicine, San Francisco General Hospital, University of California, San Francisco, USA.
Chris Drakeley, Department of Infection Biology, London School of Hygiene and Tropical Medicine, London, United Kingdom.
Sarah G Staedke, Department of Clinical Research, London School of Hygiene and Tropical Medicine, London, United Kingdom.
Teun Bousema, Department of Medical Microbiology, Radboud University Nijmegen Medical Centre, Nijmegen, The Netherlands; Department of Infection Biology, London School of Hygiene and Tropical Medicine, London, United Kingdom.
Melissa D Conrad, Department of Medicine, San Francisco General Hospital, University of California, San Francisco, USA.
References
- 1. Stone W, Goncalves BP, Bousema T, Drakeley C. Assessing the infectious reservoir of falciparum malaria: past and future. Trends Parasitol 2015; 31:287–96. [DOI] [PubMed] [Google Scholar]
- 2. Bradley J, Stone W, Da DF, et al. . Predicting the likelihood and intensity of mosquito infection from sex specific Plasmodium falciparum gametocyte density. Elife 2018; 7:e34463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Goncalves BP, Kapulu MC, Sawa P, et al. . Examining the human infectious reservoir for Plasmodium falciparum malaria in areas of different transmission intensity. Nat Commun 2017; 8:1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Vantaux A, Samreth R, Piv E, et al. . Contribution to malaria transmission of symptomatic and asymptomatic parasite carriers in Cambodia. J Infect Dis 2018; 217:1561–68. [DOI] [PubMed] [Google Scholar]
- 5. Tadesse FG, Slater HC, Chali W, et al. . The relative contribution of symptomatic and asymptomatic Plasmodium vivax and Plasmodium falciparum infections to the infectious reservoir in a low-endemic setting in Ethiopia. Clin Infect Dis 2018; 66:1883–91. [DOI] [PubMed] [Google Scholar]
- 6. Andolina C, Rek JC, Briggs J, et al. . Sources of persistent malaria transmission in a setting with effective malaria control in eastern Uganda: a longitudinal, observational cohort study. Lancet Infect Dis 2021; 21:1568–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Whittaker C, Slater H, Nash R, et al. . Global patterns of submicroscopic Plasmodium falciparum malaria infection: insights from a systemic review and meta-analysis of population surveys. Lancet Microbe 2021; 2:e366–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Slater HC, Ross A, Felger I, et al. . The temporal dynamics and infectiousness of subpatent Plasmodium falciparum infections in relation to parasite density. Nat Commun 2019; 10:1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tumwebaze PK, Katairo T, Okitwi M, et al. . Drug susceptibility of Plasmodium falciparum in eastern Uganda: a longitudinal phenotypic and genotypic study. Lancet Microbe 2021; 2:e441–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Nankabirwa JI, Arinaitwe ERek J, et al. . Malaria transmission, infection, and disease following sustained indoor residual spraying of insecticide in Tororo, Uganda. Am J Trop Med Hyg 2020; 103:1525–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Meerstein-Kessel L, Andolina C, Carrio E, et al. . A multiplex assay for the sensitive detection and quantification of male and female Plasmodium falciparum gametocytes. Malaria J 2018; 17:441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. WWARN Gametocyte Study Group . Gametocyte carriage in uncomplicated Plasmodium falciparum malaria following treatment with artemisinin combination therapy: a systemic review and meta-analysis of individual patient data. BMC Med 2016; 14:79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Barry A, Bradley J, Stone W, et al. . Higher gametocyte production and mosquito infectivity in chronic compared to incident Plasmodium falciparum infections. Nat Commun 2021; 12:2443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ahmad A, Soumare HM, Camara MM, et al. . Infectivity of patent Plasmodium falciparum gametocyte carriers to mosquitoes: establishing capacity to investigate the infectious reservoir of malaria in a low-transmission setting in the Gambia. Trans R Soc Trop Med Hyg 2021; 115:1462–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Coalson JE, Cohee LM, Buchwald AG, et al. . Simulation models predict that school-age children are responsible for most human-to-mosquito Plasmodium falciparum transmission in southern Malawi. Malaria J 2018; 17:147. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.