Abstract
Background
In the Netherlands, the bivalent human papillomavirus (HPV) vaccine has been offered to preadolescent girls via the National Immunization Program in a 2-dose schedule since 2014. The current study estimates vaccine effectiveness (VE) against HPV infections up to 4 years postvaccination among girls eligible for routine 2-dose immunization.
Methods
A cohort study (HAVANA2) was used in which participants annually filled out an online questionnaire and provided a vaginal self-sample for determination of HPV by the SPF10-LiPA25 assay, able to detect 25 HPV types. VE against incident type-specific infections and pooled outcomes was estimated by a Cox proportional hazards model with shared frailty between the HPV types.
Results
In total, 2027 girls were included in the study, 1098 (54.2%) of whom were vaccinated with 2 doses. Highest incidence rate was 5.0/1000 person-years (HPV-51) among vaccinated participants and 9.1/1000 person-years (HPV-74) among unvaccinated participants. Adjusted pooled VE was 84.0% (95% confidence interval [CI], 27.0%–96.5%) against incident HPV-16/18 infections and 86.5% (95% CI, 39.5%–97.08%) against cross-protective types HPV-31/33/45.
Conclusions
Four years postvaccination, 2 doses of bivalent HPV vaccine were effective in the prevention of incident HPV-16/18 infections and provided cross-protection to HPV-31/33/45. Our VE estimates rival those from 3-dose schedules, indicating comparable protection by 2-dose schedules.
Keywords: human papillomavirus, vaccination, immunization schedule, observational study, reduced dosing
Persistent infections with human papillomavirus (HPV) are associated with development of clinical disease, including anogenital or oropharyngeal cancers (in case of high-risk HPV type infections) and anogenital or laryngeal warts (in case of low-risk HPV type infections) [1, 2]. From 2006 onward, 3 vaccines targeting different combinations of HPV types have been registered, which were initially licensed and offered according to a 3-dose (3D) schedule (recommended schedule: 0, 1, and 6 months). The European Medicines Agency licensed a 2-dose (2D) schedule (0 and 6 months) for all available HPV vaccines in 2014 for vaccine recipients aged 9–14 years [3]. Immunobridging studies demonstrated comparable immunogenicity between 9- to 14-year-old 2D-vaccinated and 15- to 26-year-old 3D-vaccinated individuals. As efficacy of vaccination was already shown among 3D vaccine recipients, comparable efficacy was expected after 2 doses in case of noninferior immunogenicity [4, 5]. When comparing girls vaccinated at similarly young age, antibody levels against HPV vaccine types following 2 doses are within acceptable ranges compared to 3 doses [6–8], although noninferiority of HPV-18 antibodies is still inconclusive [9, 10].
Ultimately, noninferiority of reduced-dosing schedules needs to be assessed on vaccine efficacy and effectiveness outcomes. However, assessment of protection against virological and clinical outcomes following a 2D schedule requires long follow-up and results are still limited, especially for the bivalent vaccine (2vHPV). Originally, HPV vaccine trials in young women used cervical intraepithelial neoplasia grade 2 (CIN2+) or higher as outcome. Since the introduction of HPV vaccination, HPV infections are endorsed as intermediate endpoint for monitoring vaccine effectiveness (VE) [11]. Only a few studies have shown protection against HPV infections or CIN2+ after 2 doses, including randomized controlled trials (RCTs), a cross-sectional study, and a linkage study [12–14]. Other observational data also indicated protection from reduced dosing schedules (including both 1- and 2-dose vaccination) [15–17]. However, the vast majority of these studies were not conducted in the context of a recommended 2D schedule, so information was retrieved from incompletely vaccinated individuals (ie, those who did not complete 3D vaccination series) [13, 18]. Therefore, numbers may be small, age at vaccination may be higher, and the interval between first and second vaccine can be shorter than recommended, possibly leading to lower antibody levels or waning of antibodies [19]. Together, this might affect VE estimates. As preadolescents who were vaccinated according to prescribed 2D regimens do not yet attend cervical cancer screening programs, monitoring of HPV infections is necessary to assess VE of 2D vaccination in a population-based setting.
The current study aims to estimate VE of 2vHPV vaccination against incident genital HPV infections after a 2-dose recommended schedule from a Dutch longitudinal cohort study. In the Netherlands, 2vHPV vaccination targeting high-risk types HPV-16 and HPV-18 was included in the National Immunization Program (NIP) beginning in 2010, initially as a girls-only vaccine in a 3D schedule [20]. The 3D schedule was replaced by a 2D schedule in 2014, starting with girls born in 2001 (eligible for vaccination in the year they turn 13). Vaccine uptake has been suboptimal in the Netherlands, ranging between 46% and 61% [21], which facilitates comparisons between vaccinated and unvaccinated individuals from the same birth cohorts. We report data up to 4 years postvaccination among routinely vaccinated Dutch girls from the first birth cohort eligible for the 2D schedule.
METHODS
Study Design
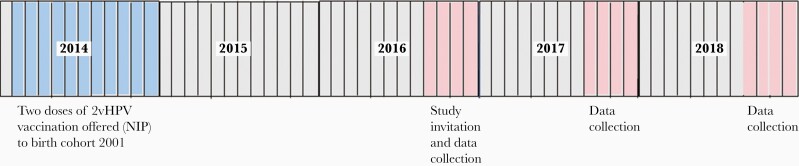
In 2016, letters of invitation were sent to 11 770 vaccinated and 27 491 unvaccinated girls from birth cohort 2001. A longitudinal cohort study was initiated: HAVANA2 (HPV Amongst Vaccinated and Nonvaccinated Adolescents After 2 Doses). Girls and their parents signed an informed consent before inclusion in the study (n = 2476 correct informed consents, response rate 6.3%). Vaccination status of participants was acquired through the national vaccination registry, Praeventis [22]. Every year, participants filled out a web-based questionnaire and collected a vaginal self-sample (Viba-Brush, Rovers Medical Devices, Oss, the Netherlands). We report data from 2016, 2017, and 2018 (Figure 1), that is, up to 4 years postvaccination. This study adhered to the tenets of the Declaration of Helsinki and was approved by the Medical Ethics Committee of the VU University Medical Center (2009/022).
Figure 1.
Study design and first years of follow-up. Abbreviations: 2vHPV, bivalent human papillomavirus vaccine; NIP, National Immunization Program.
Laboratory Analyses
Self-collected vaginal samples were stored in 1 mL buffered saline at −20°C. DNA was isolated from 200 µL of suspension using the MagNA Pure DNA and viral NA small volume Kit (Roche, Mannheim, Germany). DNA was eluted in 100 µL of elution buffer, of which 10 µL was used for amplification of HPV DNA. Amplification was performed using the broad-spectrum SPF10 primer cocktail. Amplified HPV DNA was detected with a DNA enzyme-linked immunoassay (HPV-DEIA, Labo Biomedical Products, Rijswijk, the Netherlands). HPV-DEIA–positive amplicons were subsequently analyzed in a reverse line blot assay (HPV-LiPA25, Labo Biomedical Products, Rijswijk, the Netherlands). The reverse line blot assay is able to detect 25 HPV genotypes, including high-risk types 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, and 59. Additionally, it can detect 13 low-risk types: 6, 11, 34, 40, 42, 43, 44, 53, 54, 66, 70, and 74. HPV types 68, 73, and 97 are also detected, but since no distinction between these types can be made, they are all classified as HPV-68.
Statistical Analyses
For inclusion in the analyses, participants needed to hand in a vaginal self-sample in the first study round and be either unvaccinated or vaccinated according to 2D schedule before study start. Unvaccinated participants who decided to initiate HPV vaccination during the study were included in the unvaccinated group until the year they started vaccination and were censored thereafter. For participants with missing follow-up data in the questionnaires, data from previous years were used for analyses if possible (last observation carried forward). Participants were censored for the remainder of the follow-up period if they did not contribute a self-sample to a year.
To explore possible associations with HPV vaccine uptake, sociodemographic characteristics among vaccinated and unvaccinated girls were described per study year. Differences in characteristics between vaccinated and unvaccinated girls over time were analyzed in a generalized estimating equation (GEE) binomial model with logit link and an exchangeable correlation structure. Each characteristic was modeled as a function of vaccination status, study round, and their interaction, to assess potential trend differences over time. Additionally, we examined which characteristics were associated with HPV infection (irrespective type or persistence) using a time-dependent GEE with a Poisson distribution and a log-link. Characteristics significantly associated with HPV in univariable models (P < .05) were included in a multivariable model to identify characteristics independently associated with HPV. These were considered as covariates to adjust for in VE analyses. An additional category was included for missing observations per characteristic. For the final models we included age, ethnicity, ever had sexual intercourse, and ever used contraception to adjust the estimates. The other characteristics that were associated with HPV were all related to sexual behavior and were not included in the model as this resulted in nonconvergence (to many variables in model, data not shown).
Type-specific HPV prevalence was determined per year. Incidence was defined as being positive for a specific HPV type, preceded by a negative sample in the previous year (except for infections in the first year). Persistence was defined as being HPV positive for the same HPV type in (at least) 2 consecutive years. Type-specific incidence and persistence rates were calculated as the number of infections divided by the person-years at risk (Poisson approach). Infections (events) were counted at the year in which they were detected. Person-years were counted as the time between vaccination or vaccine eligibility for unvaccinated participants (set at 30 June 2014, halfway during the year girls were eligible) and the end of follow-up or the time of an event, whichever came first. This reflects the time girls were at risk for developing an incident or persistent infection. The maximum number of person-years per participant was 4 (2014–2018).
VE against incident HPV infections was estimated for all HPV types available in the HPV-LiPA25 using a Cox proportional hazards model with shared frailty between HPV types. VE was calculated as 1 – hazard ratio × 100%. For HPV types with zero infection events among vaccinated girls (ie, VE = 100%), approximate lower bounds of the 95% confidence intervals (CIs) were obtained by the Peto estimator for the hazard ratio, based on the log-rank statistic [23]. For other types, event-specific hazards were adjusted using time-dependent sociodemographic characteristics as previously identified. The frailty term in the Cox model denotes a random effect on the individual level, representing residual heterogeneity in HPV infection risk irrespective of type. VE against all HPV types was estimated by 1 multivariate model, with covariate effects estimated simultaneously for all types. As pooled outcomes, we considered vaccine types (HPV-16/18), cross-protective types (HPV-31/33/45), high-risk types (HPV-16/18/31/33/45/52/58), low-risk types associated with genital warts (HPV-6/11), and a combination of vaccine and cross-protective types (HPV-16/18/31/33/45). Pooled outcomes were estimated as weighted averages of types included in a particular combination to obtain more precise estimates compared to type-specific VE or a priori specification of combined outcomes. All statistical analyses were performed using SAS software version 9.4 (SAS Institute, Cary, North Carolina).
RESULTS
A total of 2027 girls handed in a vaginal self-sample in the first study year, 1098 (54.2%) of whom were vaccinated against HPV according to a 2D schedule at age 12 (in the year they turned 13, according to the NIP). The number of girls participating amounted to 1666 in the third year due to loss to follow-up (Table 1). At study start, the mean age was 15 years. In the first part of Table 1, the entire study population is described regarding sociodemographic characteristics. Sexual activity increased from 12% to 43% over the first 3 years. In general, vaccinated and unvaccinated girls were comparable regarding sociodemographic characteristics, except for contraception use: Vaccinated participants were more likely to ever have used contraception (odds ratio [OR], 1.18 [95% CI, 1.01–1.37]). Table 1 also shows sexual behavior characteristics among the sexually active participants only. No differences between vaccinated and unvaccinated participants were seen in sociodemographic or sexual characteristics over time.
Table 1.
Sociodemographic Characteristics by Study Year Among Vaccinated and Unvaccinated Participants
| Characteristic | Study Year 1 (2016) | Study Year 2 (2017) | Study Year 3 (2018) | Associated With Vaccination Status | Associated With HPV | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Unvaccinated (n = 929) | Vaccinated (n = 1098) | Unvaccinated (n = 802) | Vaccinated (n = 944) | Unvaccinated (n = 767) | Vaccinated (n = 899) | |||||||||
| No. | (%) | No. | (%) | No. | (%) | No. | (%) | No. | (%) | No. | (%) | P Valuea | P Value | |
| Age, y, mean (range) | 14.97 (14–15) | 14.96 (14–15) | 15.98 (15–16) | 15.97 (15–16) | 16.97 (16–17) | 16.96 (16–17) | <.0001 | |||||||
| Dutch ethnicity | .7328 | .0468 | ||||||||||||
| Yes | 769 | (82.8) | 926 | (84.3) | 671 | (83.7) | 772 | (81.8) | 651 | (84.9) | 748 | (83.2) | ||
| No | 102 | (11. 0) | 120 | (10.9) | 75 | (9.4) | 97 | (10.3) | 79 | (10.3) | 85 | (9.5) | ||
| Degree of urbanization | .4166 | .1892 | ||||||||||||
| Low | 422 | (45.4) | 476 | (43.4) | 367 | (45.8) | 405 | (42.9) | 359 | (46.8) | 400 | (44.5) | ||
| High | 443 | (47.7) | 558 | (50.8) | 379 | (47.3) | 462 | (48.9) | 371 | (48.4) | 433 | (48.2) | ||
| Highest educational level | .4803 | .1249 | ||||||||||||
| Low | 365 | (39.3) | 437 | (39.8) | 283 | (35.3) | 328 | (34.7) | 275 | (35.9) | 316 | (35.2) | ||
| High | 506 | (54.5) | 609 | (55.5) | 463 | (57.7) | 541 | (57.3) | 450 | (58.7) | 510 | (56.7) | ||
| Ever used contraception | .03 | .0261 | ||||||||||||
| No | 625 | (67.3) | 734 | (66.8) | 416 | (51.9) | 420 | (44.5) | 282 | (36.8) | 275 | (30.6) | ||
| Yes | 246 | (26.5) | 312 | (28.4) | 330 | (41.1) | 449 | (47.6) | 448 | (58.4) | 558 | (62.1) | ||
| Smoking past year | .3544 | .4011 | ||||||||||||
| No | 745 | (80.2) | 910 | (82.9) | 585 | (72.9) | 696 | (73.7) | 523 | (68.2) | 603 | (67.1) | ||
| Yes | 126 | (13.6) | 136 | (12.4) | 161 | (20.1) | 173 | (18.3) | 207 | (27.0) | 230 | (25.6) | ||
| Sexual orientation | .6632 | .5703 | ||||||||||||
| Heterosexual | 822 | (88.5) | 984 | (89.6) | 693 | (86.4) | 808 | (85.6) | 672 | (87.6) | 764 | (85.0) | ||
| Other | 47 | (5.1) | 58 | (5.3) | 50 | (6.2) | 61 | (6.5) | 58 | (7.6) | 67 | (7.5) | ||
| Ever had sexual intercourse | .6947 | .0195 | ||||||||||||
| No | 745 | (80.2) | 921 | (83.9) | 545 | (68.0) | 614 | (65.0) | 400 | (52.2) | 451 | (50.2) | ||
| Yes | 126 | (13.6) | 123 | (11.2) | 201 | (25.1) | 255 | (27.0) | 330 | (43.0) | 382 | (42.5) | ||
| Among sexually active participants only | ||||||||||||||
| Ever used condoms | .6577 | .9772 | ||||||||||||
| No | 14 | (11.1) | 14 | (11.4) | 20 | (10.0) | 18 | (7.1) | 35 | (10.6) | 40 | (10.5) | ||
| Yes | 112 | (88.9) | 109 | (88.6) | 181 | (90.0) | 237 | (92.9) | 295 | (89.4) | 342 | (89.5) | ||
| Age at first sexual intercourse | .5910 | .0270 | ||||||||||||
| <15 y | 68 | (54.0) | 70 | (56.9) | 55 | (27.4) | 62 | (24.3) | 50 | (15.2) | 57 | (14.9) | ||
| ≥15 y | 56 | (44.4) | 51 | (41.5) | 142 | (70.6) | 193 | (75.7) | 277 | (83.9) | 325 | (85.1) | ||
| No. of sex partners in past year | .6992 | .3574 | ||||||||||||
| 1–2 | 113 | (89.7) | 115 | (93.5) | 174 | (86.6) | 221 | (86.7) | 268 | (81.2) | 316 | (82.7) | ||
| ≥3 | 7 | (5.6) | 6 | (4.9) | 15 | (7.5) | 21 | (8.2) | 35 | (10.6) | 45 | (11.8) | ||
| No. of sex partners in lifetime | .7329 | <.0001 | ||||||||||||
| 1–2 | 111 | (88.1) | 108 | (87.8) | 159 | (79.1) | 209 | (82.0) | 250 | (75.8) | 284 | (74.3) | ||
| ≥3 | 15 | (11.9) | 14 | (11.4) | 38 | (18.9) | 45 | (17.6) | 77 | (23.3) | 96 | (25.1) | ||
| Has current sex partner | .9926 | .0238 | ||||||||||||
| No | 44 | (34.9) | 48 | (39.0) | 75 | (37.3) | 80 | (31.4) | 109 | (33.0) | 130 | (34.0) | ||
| Yes | 81 | (64.3) | 75 | (61.0) | 126 | (62.7) | 175 | (68.6) | 220 | (66.7) | 251 | (65.7) | ||
| Condom use with partner | .6802 | .0011 | ||||||||||||
| Never | 12 | (9.5) | 12 | (9.8) | 23 | (11.4) | 30 | (11.8) | 59 | (17.9) | 67 | (17.5) | ||
| Always or sometimes | 64 | (50.8) | 60 | (48.8) | 93 | (46.3) | 135 | (52.9) | 143 | (43.3) | 161 | (42.1) | ||
| Diagnosed with STI past year | .3394 | .0092 | ||||||||||||
| No | 125 | (99.2) | 121 | (98.4) | 196 | (97.5) | 253 | (99.2) | 321 | (97.3) | 373 | (97.6) | ||
| Yes | 1 | (0.8) | 2 | (1.6) | 5 | (2.5) | 2 | (0.8) | 9 | (2.7) | 9 | (2.4) |
Abbreviations: HPV, human papillomavirus; STI, sexually transmitted infection.
a P values for main effect of vaccination status are reported (no time interaction).
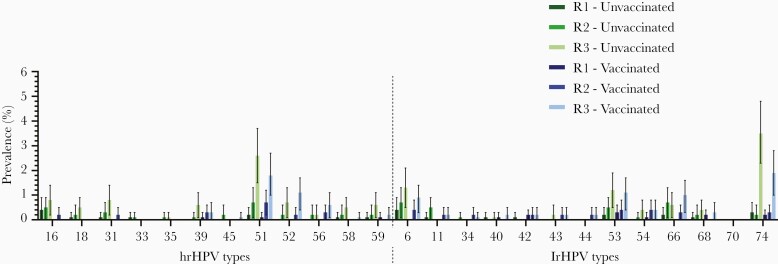
The prevalence of type-specific HPV infections was low in the first study year among both vaccinated and unvaccinated girls (Figure 2). Prevalence of any HPV type infection (both low risk and high risk) increased from 1.7% in year 1 to 11.0% in year 3 for unvaccinated participants and from 1.1% to 8.0% for vaccinated participants, respectively. HPV-51 was the most prevalent high-risk type, while HPV-74 was the most prevalent low-risk type, irrespective of vaccination status. Type-specific incidence rates ranged from 0.0 to 9.1 per 1000 person-years (HPV-74) among unvaccinated and from 0.0 to 5.0 per 1000 person-years (HPV-51) for vaccinated girls. Due to the low number of persistent infections, persistence rates could only be calculated for a limited number of HPV types. Highest persistence rates were 1.2 per 1000 person-years (HPV-16) among unvaccinated participants and 0.8 per 1000 person-years (HPV-51) among vaccinated participants (Table 2).
Figure 2.
Type-specific prevalence with 95% confidence intervals of high-risk and low-risk human papillomavirus (hrHPV and lrHPV, respectively) types among vaccinated and unvaccinated participants per study year.
Table 2.
Type-Specific Incidence and Persistence Rates per 1000 Person-Years Among Vaccinated and Unvaccinated Participants
| HPV Type | Incidence Rates per 1000 PY (95% CI) |
Persistence Rates per 1000 PY (95% CI) |
||
|---|---|---|---|---|
| Unvaccinated | Vaccinated | Unvaccinated | Vaccinated | |
| High-risk types | ||||
| 16 | 1.8 (1.5–2.1) | 0.5 (.3–.7) | 1.2 (.5–3.2) | 0.0 |
| 18 | 1.5 (1.3–1.8) | 0.0 (.0–.3) | 0.0 | 0.0 |
| 31 | 2.7 (2.4–3.1) | 0.5 (.3–.7) | 0.3 (.0–2.2) | 0.0 |
| 33 | 0.6 (.4–.8) | 0.0 (.0–.3) | 0.0 | 0.0 |
| 35 | 0.6 (.4–.8) | 0.0 (.0–.3) | 0.0 | 0.0 |
| 39 | 1.8 (1.5–2.1) | 1.8 (1.5–2.0) | 0.0 | 0.0 |
| 45 | 0.6 (.4–.8) | 0.0 (.0–.3) | 0.0 | 0.0 |
| 51 | 6.7 (6.1–7.3) | 5.0 (4.6–5.5) | 0.6 (.2–2.4) | 0.8 (.2–2.3) |
| 52 | 2.1 (1.8–2.5) | 2.3 (2.0–2.6) | 0.0 | 0.3 (.0–1.8) |
| 56 | 0.9 (.7–1.1) | 2.0 (1.7–2.3) | 0.3 (.0–2.2) | 0.3 (.0–1.8) |
| 58 | 1.2 (.9–1.5) | 0.3 (.1–.4) | 0.0 | 0.0 |
| 59 | 2.1 (1.8–2.5) | 0.8 (.6–1.0) | 0.3 (.0–2.2) | 0.0 |
| Low-risk types | ||||
| 6 | 4.9 (4.4–5.4) | 2.3 (2.0–2.6) | 0.9 (.3–2.8) | 0.0 |
| 11 | 1.2 (.9–1.5) | 0.5 (.4–.7) | 0.3 (.0–2.2) | 0.3 (.0–1.8) |
| 34 | 0.0 (.0–.3) | 0.8 (.6–1.0) | 0.0 | 0.0 |
| 40 | 0.3 (.2–.5) | 0.8 (.6–1.0) | 0.0 | 0.0 |
| 42 | 0.3 (.2–.5) | 1.0 (.8–1.2) | 0.0 | 0.5 (.1–2.0) |
| 43 | 0.6 (.4–.8) | 0.8 (.6–1.0) | 0.0 | 0.0 |
| 44 | 0.0 (.0–.3) | 1.0 (.8–1.2) | 0.0 | 0.0 |
| 53 | 3.3 (2.9–3.8) | 3.5 (3.2–3.9) | 0.6 (.2–2.4) | 0.5 (.1–2.0) |
| 54 | 1.2 (.9–1.5) | 1.8 (1.5–2.0) | 0.0 | 0.5 (.1–2.0) |
| 66 | 3.0 (2.7–3.4) | 2.0 (1.8–2.3) | 0.0 | 0.3 (.0–1.8) |
| 68 | 1.2 (.9–1.5) | 1.0 (.8–1.2) | 0.0 | 0.0 |
| 70 | 0.0 (.0–.3) | 0.0 (.0–.3) | 0.0 | 0.0 |
| 74 | 9.1 (8.5–9.8) | 4.8 (4.4–5.2) | 0.0 | 0.0 |
The 95% confidence intervals for zero observations were calculated based on the rule of 3.
Abbreviations: CI, confidence interval; HPV, human papillomavirus; PY, person-years.
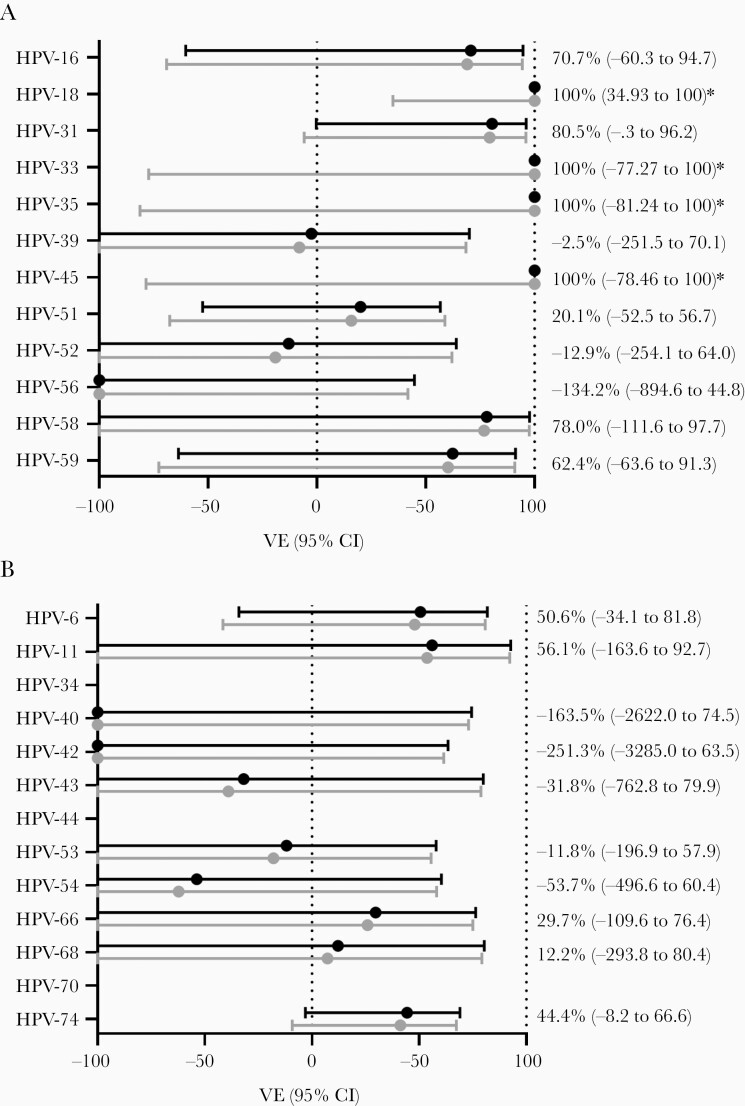
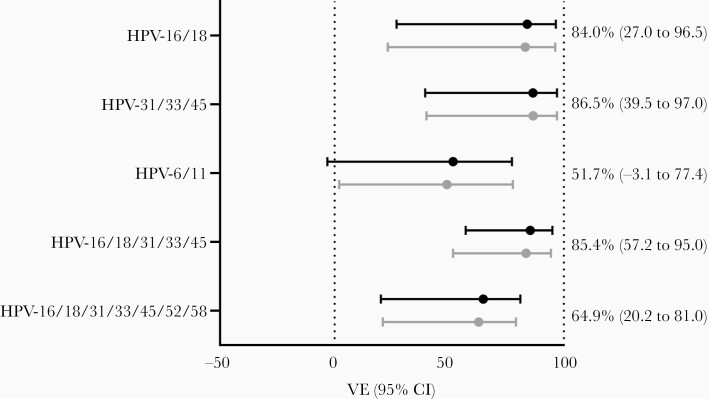
VE against incident infections was calculated for all high-risk types (Figure 3A), all low-risk types (Figure 3B) and for pooled outcomes (Figure 4). Type-specific VE estimates against HPV-18, -33, -35, and -45 were all 100%, as no infections among vaccinated participants were detected, but only VE against HPV-18 was statistically significant in unadjusted analyses (P = .0148, log-rank test). For other types, estimates were adjusted for age, ethnicity, ever had sexual intercourse, and ever used contraception. In the pooled outcomes analyses, adjusted VE against vaccine types HPV-16/18 was 84.0% (95% CI, 27.0%–96.5%). The VE against cross-protective types HPV-31/33/45 was 86.5% (95% CI, 39.5%–97.08%). Moreover, the VE against incident infections with high-risk types HPV-16/18/31/33/45/52/58 was 64.9% (95% CI, 20.2%–81.0%), while VE against low-risk types 6 and 11 was 51.7% (95% CI, –3.1% to 77.4%). The complete models including estimates for covariates are included in Supplementary Data 1.
Figure 3.
Type-specific vaccine effectiveness (VE) estimates against incident human papillomavirus (HPV) infections with 95% confidence intervals (CIs). Crude (gray dots) and adjusted estimates (black dots) are shown for high-risk (A) and low-risk (B) HPV types. VE was adjusted for age, ethnicity, ever had sexual intercourse, and ever used contraception. *For HPV types with no infections among vaccinated participants, confidence estimates could only be included for the crude estimates (using the Peto estimator for the hazard ratio, based on the log-rank statistic).
Figure 4.
Vaccine effectiveness (VE) estimates for pooled outcomes against incident human papillomavirus (HPV) infections with 95% confidence intervals (CIs) for crude (gray dots) and adjusted estimates (black dots). VE was adjusted for age, ethnicity, ever had sexual intercourse, and ever used contraception.
DISCUSSION
We studied VE of 2 doses of the HPV-16/18 vaccine in the first birth cohort eligible for reduced-dosing schedule vaccination in the routine vaccination program of the Netherlands. With a 4-year postvaccination follow-up, we demonstrate protection against incident HPV-16/18 infections as well as cross-protection against HPV-31/33/45 infections. To our knowledge, this is the first observational study reporting VE of 2vHPV vaccination against type-specific HPV positivity among routinely 2D vaccinated young women. Our VE estimates compare well to those derived from birth cohorts eligible for the 3D schedule, indicating similar protection of the 2D schedule [24–26].
An important aspect of this study is that the 2 doses of HPV vaccination were routinely offered in the NIP and replaced the initial 3D schedule based on immunological data. In this context, evidence for effectiveness based on virological and clinical outcomes is imperative and should be compared to the effectiveness following 3 doses. Previous research on the 3D schedule can provide various benchmarks, since studies may report effectiveness against incident, prevalent, or persistent infections, with increasing expectation for effectiveness, respectively. Data from Dutch surveillance studies among 3D vaccine-eligible girls from the catch-up campaign (slightly older at vaccination compared to our participants) indicated that VE against incident HPV-16/18 infections was 70% (95% CI, 52%–82%) 4 years postvaccination [24]. For HPV-16/18/31/45, VE was 72% (95% CI, 58%–82%). For persistent HPV-16/18 infections up to 6 years postvaccination from the same study, VE was 97.7% (95% CI, 83.5%–99.7%) [25]. Another Dutch surveillance study among sexual health clinic visitors eligible for 3D vaccination reported a VE of 89.9% (95% CI, 81.7%–94.4%) against prevalent HPV-16/18 infections [26]. Together, these findings align well with the observations from the current 2D study in which VE of 84% against incident HPV-16/18 infections is found. The primary and intermediate endpoints for HPV vaccination as indicated by the World Health Organization include persistent infections [11]. Due to low numbers, VE against persistent infections was not yet included in our current analyses. With prolonged follow-up of the current cohort, these estimates can be reported in the future.
Routinely offered 2vHPV vaccination according to a 3D schedule has been in place outside the Netherlands as well. A Scottish study indicated a VE of 89.1% (95% CI, 85.1%–92.3%) against vaccine-type infections among girls offered vaccination at age 12–13 [13]. For HPV-31/33/45 the VE was 85.1% (95% CI, 77.3%–90.9%). VE declined with increasing age of vaccination. In general, VE observed in the current study following a 2D schedule seems comparable to 3D schedule findings, which is in line with the immunological data and immunobridging studies on the basis of which the 2D schedule was licensed.
Two-dose VE estimates can also be evaluated based on clinical trial data. Even though the trials were not designed or powered to study the 2-dose schedule specifically, they often report their findings from minority groups receiving <3 doses. Our point estimate of 84.0% for HPV-16/18 is in line with those of the 2D-vaccinated, HPV-naive cohorts in the HPV PApilloma TRIal against Cancer In young Adults (PATRICIA) and the Costa Rica Vaccine Trial (CRVT) (81.2%) [18]. As indicated before, women receiving their first and second vaccination without proper time interval might have lower antibody levels following vaccination [5, 19]. Subanalyses in the CRVT data indeed showed higher efficacy among those receiving vaccinations 6 months apart as compared to a shorter interval, also affecting the possibility of cross-protection against HPV-31/33/45 [18]. In the current analyses, all 2D-vaccinated girls received their second dose at >5 months apart from their first dose, likely contributing to our high VE estimates in general. Our findings also agree well with observations from a Dutch serosurveillance study conducted among routinely 2D-vaccinated girls [27]. It was found that seroprevalence was 100% up to 2 years postvaccination for vaccine types with corresponding high avidity levels, likely resulting in solid protection against vaccine types and cross-protective types alike.
Other (high-risk) HPV types against which (cross-protective) effects were observed included HPV-31/33/45 (VE, 86.5%), which is in line with observations from previous research [28], although our estimates were higher. A linkage study between vaccination status and cervical screening from Scotland indicated cross-protection against HPV-31/33/45 of 40.3% among 2D-vaccinated women, but this was among those who did not complete routinely offered 3D vaccination. Regarding type-specific significant VE estimates in general, this was only observed for HPV-18 in unadjusted analyses. This could be due to rather low numbers of type-specific infections and could become measurable with prolonged follow-up time, as observed in 3D schedules and other 2D studies [25, 29].
We found a borderline nonsignificant VE against HPV-6/11 infections (51.7% [95% CI, –3.1% to 77.4%]). These low-risk types are not targeted by HPV-16/18 vaccination. Although 1 of the first RCTs on 2vHPV vaccination [30] also indicated partial protection against HPV-6/11 infections among the HPV-naive cohort, only very few other surveillance studies could replicate such an effect [31]. Some studies have shown a partial protective effect of 2vHPV vaccination against anogenital warts [32], but these were not focused on specific low-risk types. Moreover, based on phylogenetic distance, (cross-) protection against HPV-6 and 11 is not expected [33]. Thus, this finding remains inconclusive and requires further research, for example on the possible biological mechanism underlying cross-protection to low-risk types from the 2vHPV vaccine.
Strengths of the current study include the longitudinal, population-based design. This is the first birth cohort from the Netherlands eligible for 2D vaccination according to protocol, which we were able to follow from a young age. Vaccinated and unvaccinated participants were comparable regarding sociodemographic and sexual characteristics, except for contraception use. This is in line with previous studies, providing no suggestion that HPV vaccination status does affect sexual (risk) behavior [34] and would as such, influence effectiveness of the program or confound VE estimates. Furthermore, our participants were comparable to the general Dutch population regarding sexual behavior, in which the median age for sexual intercourse was 17.5 years for girls in 2017 [35]. However, we also acknowledge some limitations. Due to ethical considerations related to the participants’ age at vaccination, we were unable to start follow-up directly after vaccination and to select HPV-negative girls at time of vaccination. Consequently, we might have missed infections that were acquired and cleared between vaccination and the first measurement, and we attributed infections at year 1 to infection at that moment. Also, the rather young age of participants led to a still limited number of type-specific infections, hence decreasing power in type-specific VE estimates. Last, there was a relatively low response rate regarding participation to this study. This might affect generalizability of results to the general population if specific subgroups were more likely to be included. Although participants were less likely to have a migration background compared to the general population [36], they were comparable regarding sexual behavior. Therefore, we think the bias of our estimates will be limited.
In conclusion, at 4 years postvaccination, 2 doses of 2vHPV vaccine were effective in the prevention of incident HPV-16/18 infections and additionally provided cross-protection against HPV-31/33/45. This is one of the first population-based observational studies investigating the 2D schedule in a regular immunization program setting, and indicates that protection is comparable to 3D schedules and to observations from RCTs. Our findings are promising regarding future clinical impact of reduced-dosing schedules.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. We gratefully acknowledge the following people for their valuable contributions to either study design, data collection, or statistical analyses: Robine Donken, Najima Lamkaraf, Suzan Leussink, Petra Oomen, Tim Severs, and Nienke Voerman.
Financial support. This work was supported by the Dutch Ministry of Health, Welfare and Sport.
Potential conflicts of interest. All authors: No reported conflicts of interest.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Contributor Information
Joske Hoes, Centre for Infectious Disease Control, National Institute for Public Health and the Environment, Bilthoven, The Netherlands; Department of Epidemiology and Data Science, Amsterdam University Medical Center, location VU University Medical Center, Amsterdam, The Netherlands.
Audrey J King, Centre for Infectious Disease Control, National Institute for Public Health and the Environment, Bilthoven, The Netherlands.
Tessa M Schurink van’t Klooster, Centre for Infectious Disease Control, National Institute for Public Health and the Environment, Bilthoven, The Netherlands; Department of Epidemiology and Data Science, Amsterdam University Medical Center, location VU University Medical Center, Amsterdam, The Netherlands.
Johannes Berkhof, Department of Epidemiology and Data Science, Amsterdam University Medical Center, location VU University Medical Center, Amsterdam, The Netherlands.
Johannes A Bogaards, Department of Epidemiology and Data Science, Amsterdam University Medical Center, location VU University Medical Center, Amsterdam, The Netherlands; Department of Epidemiology and Data Science, Amsterdam University Medical Center, location Academic Medical Center, Amsterdam, The Netherlands.
Hester E de Melker, Centre for Infectious Disease Control, National Institute for Public Health and the Environment, Bilthoven, The Netherlands.
References
- 1. Baseman JG, Koutsky LA. The epidemiology of human papillomavirus infections. J Clin Virol 2005; 32:16–24. [DOI] [PubMed] [Google Scholar]
- 2. Burchell AN, Winer RL, de Sanjosé S, Franco EL. Chapter 6: epidemiology and transmission dynamics of genital HPV infection. Vaccine 2006; 24:S52–61. [DOI] [PubMed] [Google Scholar]
- 3. Committee for Medicinal Products for Human Use . Assessment report for Cervarix. London, UK: European Medicines Agency, 2013. [Google Scholar]
- 4. Pedersen C, Petaja T, Strauss G, et al. ; HPV Vaccine Adolescent Study Investigators Network . Immunization of early adolescent females with human papillomavirus type 16 and 18 L1 virus-like particle vaccine containing AS04 adjuvant. J Adolesc Health 2007; 40:564–71. [DOI] [PubMed] [Google Scholar]
- 5. Romanowski B, Schwarz TF, Ferguson LM, et al. . Immunogenicity and safety of the HPV-16/18 AS04-adjuvanted vaccine administered as a 2-dose schedule compared with the licensed 3-dose schedule: results from a randomized study. Hum Vaccin 2011; 7:1374–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dobson SR, McNeil S, Dionne M, et al. . Immunogenicity of 2 doses of HPV vaccine in younger adolescents vs 3 doses in young women: a randomized clinical trial. JAMA 2013; 309:1793–802. [DOI] [PubMed] [Google Scholar]
- 7. Romanowski B, Schwarz TF, Ferguson LM, et al. . Immune response to the HPV-16/18 AS04-adjuvanted vaccine administered as a 2-dose or 3-dose schedule up to 4 years after vaccination: results from a randomized study. Hum Vaccin Immunother 2014; 10:1155–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Iversen OE, Miranda MJ, Ulied A, et al. . Immunogenicity of the 9-valent HPV vaccine using 2-dose regimens in girls and boys vs a 3-dose regimen in women. JAMA 2016; 316:2411–21. [DOI] [PubMed] [Google Scholar]
- 9. Donken R, Dobson SR, Marty KD, et al. . Immunogenicity of 2 and 3 doses of the quadrivalent HPV vaccine up to 120 months post-vaccination; follow-up of a randomized clinical trial. Clin Infect Dis 2020; 71.4:1022–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Donken R, Knol MJ, Bogaards JA, van der Klis FR, Meijer CJ, de Melker HE. Inconclusive evidence for non-inferior immunogenicity of two-compared with three-dose HPV immunization schedules in preadolescent girls: a systematic review and meta-analysis. J Infect 2015; 71:61–73. [DOI] [PubMed] [Google Scholar]
- 11. International Agency for Research on Cancer HPV Working Group . Primary end-points for prophylactic HPV vaccine trials. Lyon, France: IARC, 2014. [PubMed] [Google Scholar]
- 12. Basu P, Bhatla N, Ngoma T, Sankaranarayanan R. Less than 3 doses of the HPV vaccine—review of efficacy against virological and disease end points. Hum Vaccin Immunother 2016; 12:1394–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kavanagh K, Pollock KG, Cuschieri K, et al. . Changes in the prevalence of human papillomavirus following a national bivalent human papillomavirus vaccination programme in Scotland: a 7-year cross-sectional study. Lancet Infect Dis 2017; 17:1293–302. [DOI] [PubMed] [Google Scholar]
- 14. Johnson Jones ML, Gargano JW, Powell M, et al. ; HPV-IMPACT Working Group . Effectiveness of 1, 2, and 3 doses of human papillomavirus vaccine against high-grade cervical lesions positive for human papillomavirus 16 or 18. Am J Epidemiol 2020; 189:265–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Verdoodt F, Dehlendorff C, Kjaer SK. Dose-related effectiveness of quadrivalent human papillomavirus vaccine against cervical intraepithelial neoplasia: a Danish nationwide cohort study. Clin Infect Dis 2020; 70:608–14. [DOI] [PubMed] [Google Scholar]
- 16. Markowitz LE, Naleway AL, Klein NP, et al. . Human papillomavirus vaccine effectiveness against HPV infection: evaluation of one, two, and three doses. J Infect Dis 2020; 221:910–8. [DOI] [PubMed] [Google Scholar]
- 17. Rodriguez AM, Zeybek B, Vaughn M, et al. . Comparison of the long-term impact and clinical outcomes of fewer doses and standard doses of human papillomavirus vaccine in the United States: a database study. Cancer 2020; 126:1656–67. [DOI] [PubMed] [Google Scholar]
- 18. Kreimer AR, Struyf F, Del Rosario-Raymundo MR, et al. . Efficacy of fewer than three doses of an HPV-16/18 AS04-adjuvanted vaccine: combined analysis of data from the Costa Rica vaccine and PATRICIA Trials. Lancet Oncol 2015; 16:775–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. D’Addario M, Redmond S, Scott P, et al. . Two-dose schedules for human papillomavirus vaccine: systematic review and meta-analysis. Vaccine 2017; 35:2892–901. [DOI] [PubMed] [Google Scholar]
- 20. De Melker HE, Conyn-Van Spaendonck MA, Boot HJ, Coutinho RA. Introduction to vaccination against cervical cancer [in Dutch]. Ned Tijdschr Geneeskd 2009; 153:658–61. [PubMed] [Google Scholar]
- 21. van Lier E, Oomen P, Giesbers H, et al. . Vaccination rate and annual report: National Immunization Program Netherlands 2018 [in Dutch]. Bilthoven, the Netherlands: National Institute for Public Health and the Environment, 2019. [Google Scholar]
- 22. van Lier A, Oomen P, de Hoogh P, et al. . Praeventis, the immunisation register of the Netherlands: a tool to evaluate the National Immunisation Programme. Euro Surveill 2012; 17:20153. [DOI] [PubMed] [Google Scholar]
- 23. Lin DY, Dai L, Cheng G, Sailer MO. On confidence intervals for the hazard ratio in randomized clinical trials. Biometrics 2016; 72:1098–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Schurink-van “tKlooster TM, de Melker HE. The national immunisation programme in the Netherlands. Surveillance and developments in 2014–2015. Bilthoven, the Netherlands: National Institute for Public Health and the Environment, 2015. [Google Scholar]
- 25. Donken R, King AJ, Bogaards JA, Woestenberg PJ, Meijer CJLM, de Melker HE. High effectiveness of the bivalent human papillomavirus (HPV) vaccine against incident and persistent HPV infections up to 6 years after vaccination in young Dutch women. J Infect Dis 2018; 217:1579–89. [DOI] [PubMed] [Google Scholar]
- 26. Woestenberg PJ, King AJ, van Benthem BHB, et al. ; Medical Microbiological Laboratories and the Public Health Services . Bivalent vaccine effectiveness against type-specific HPV positivity: evidence for cross-protection against oncogenic types among Dutch STI clinic visitors. J Infect Dis 2018; 217:213–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Schurink-van ‘t Klooster TM, Donken R, Schepp RM, van der Klis FRM, de Melker HE. Persistence of immune response following bivalent HPV vaccination: a follow-up study among girls routinely vaccinated with a two-dose schedule. Vaccine 2018; 36:7580–7. [DOI] [PubMed] [Google Scholar]
- 28. Malagón T, Drolet M, Boily MC, et al. . Cross-protective efficacy of two human papillomavirus vaccines: a systematic review and meta-analysis. Lancet Infect Dis 2012; 12:781–9. [DOI] [PubMed] [Google Scholar]
- 29. Apter D, Wheeler CM, Paavonen J, et al. ; HPV PATRICIA Study Group . Efficacy of human papillomavirus 16 and 18 (HPV-16/18) AS04-adjuvanted vaccine against cervical infection and precancer in young women: final event-driven analysis of the randomized, double-blind PATRICIA trial. Clin Vaccine Immunol 2015; 22:361–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Szarewski A, Skinner SR, Garland SM, et al. . Efficacy of the HPV-16/18 AS04-adjuvanted vaccine against low-risk HPV types (PATRICIA randomized trial): an unexpected observation. J Infect Dis 2013; 208:1391–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Latsuzbaia A, Arbyn M, Tapp J, et al. . Effectiveness of bivalent and quadrivalent human papillomavirus vaccination in Luxembourg. Cancer Epidemiol 2019; 63:101593. [DOI] [PubMed] [Google Scholar]
- 32. Woestenberg PJ, Guevara Morel AE, Bogaards JA, et al. . Partial protective effect of bivalent HPV16/18 vaccination against anogenital warts in a large cohort of Dutch primary care patients. Clin Infect Dis 2020. 2021; 73:291–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bogaards JA, van der Weele P, Woestenberg PJ, van Benthem BHB, King AJ. Bivalent human papillomavirus (HPV) vaccine effectiveness correlates with phylogenetic distance from HPV vaccine types 16 and 18. J Infect Dis 2019; 220:1141–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Donken R, Tami A, Knol MJ, et al. . Changes in (risk) behavior and HPV knowledge among Dutch girls eligible for HPV vaccination: an observational cohort study. BMC Public Health 2018; 18:837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. de Graaf H, van den Borne M, Nikkelen S, Twisk D, Meijer S.. Sex under 25: sexual health of young people in the Netherlands in 2017 [in Dutch]. Utrecht, the Netherlands: Rutgers/Soa AIDS, 2017. [PubMed] [Google Scholar]
- 36. Statistics Netherlands . StatLine. The Netherlands in figures. Available at: https://opendata.cbs.nl/statline/#/CBS/nl/. Accessed July 2020.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.