Abstract
Background
This phase 1 placebo-controlled study assessed the safety and immunogenicity of 2-dose regimens of Ad26.ZEBOV (adenovirus serotype 26 [Ad26]) and MVA-BN-Filo (modified vaccinia Ankara [MVA]) vaccines with booster vaccination at day 360.
Methods
Healthy US adults (N = 164) randomized into 10 groups received saline placebo or standard or high doses of Ad26 or MVA in 2-dose regimens at 7-, 14-, 28-, or 56-day intervals; 8 groups received booster Ad26 or MVA vaccinations on day 360. Participants reported solicited and unsolicited reactogenicity; we measured immunoglobulin G binding, neutralizing antibodies and cellular immune responses to Ebola virus glycoprotein.
Results
All regimens were well tolerated with no serious vaccine-related adverse events. Heterologous (Ad26,MVA [dose 1, dose 2] or MVA,Ad26) and homologous (Ad26,Ad26) regimens induced humoral and cellular immune responses 21 days after dose 2; responses were higher after heterologous regimens. Booster vaccination elicited anamnestic responses in all participants.
Conclusions
Both heterologous and homologous Ad26,MVA Ebola vaccine regimens are well tolerated in healthy adults, regardless of interval or dose level. Heterologous 2-dose Ad26,MVA regimens containing an Ebola virus insert induce strong, durable humoral and cellular immune responses. Immunological memory was rapidly recalled by booster vaccination, suggesting that Ad26 booster doses could be considered for individuals at risk of Ebola infection, who previously received the 2-dose regimen.
Keywords: Ebola vaccine, heterologous, homologous, 2-dose, Ad26.ZEBOV, MVA-BN-Filo, safety, immunogenicity
Safety and immunogenicity of 2-dose Ad26.ZEBOV and MVA-BN-Filo vaccine regimens were evaluated in healthy US adults (phase 1 study). Regimens were well tolerated and immunogenic, with higher antibody responses afterose 2 for heterologous regimens with increased dosing intervals.
First described over 40 years ago [1], recurrent outbreaks of Ebola virus (EBOV) disease (EVD) have occurred with increasing frequency in some African countries [2, 3]. After the major epidemic in 2014–2016 in Sierra Leone, Guinea, and Liberia, further outbreaks have occurred in the Democratic Republic of the Congo (DRC); the 2018–2020 outbreak was the second largest ever, with 3481 cases and 2299 fatalities [4, 5]. During the 2014–2016 outbreak, several vaccine candidates underwent accelerated development, each targeting the glycoprotein (GP) of Zaire ebolavirus (ZEBOV) [6, 7]. Among these, a single-dose, recombinant vesicular stomatitis virus–based vaccine (rVSV-ZEBOV-GP) was efficacious when used in a ring-vaccination strategy in both the 2014–2016 outbreak in Guinea and the 2018–2020 outbreak in DRC [8, 9]. This reactive use vaccine was granted conditional approval for use in adults in Europe and in the United States [10, 11].
Furthermore, Janssen developed a heterologous 2-dose vaccination strategy consisting of a monovalent, recombinant, replication-incompetent adenovirus serotype 26 (Ad26) viral vector encoding ZEBOV GP (Ad26.ZEBOV) with a multivalent, recombinant nonreplicating modified vaccinia Ankara (MVA) viral vector encoding the GPs from Zaire and Sudan ebolaviruses, and Marburg virus, and Taï Forest ebolavirus nucleoprotein (MVA-BN-Filo) [12]. Heterologous 2-dose schedules using adenovirus- and MVA-vectored vaccines are potent inducers of humoral and cellular immune responses and are well tolerated in clinical trials against malaria [13‒15], human immunodeficiency virus (HIV) [16], and hepatitis C [17]. Furthermore, heterologous 2-dose regimens using Ad26,Ad35 [18, 19] or Ad26,MVA [20] gave protection against EBOV challenge in nonhuman primates.
Phase 1 studies conducted in the United Kingdom [12] and in African countries [21, 22] showed that this heterologous 2-dose vaccination strategy was well tolerated and highly immunogenic in healthy adults. After analysis of these and other studies, this regimen received approval for marketing under exceptional circumstances for prophylactic use in adults and children ≥1 year old in the European Union [23]. The Rwandan Food and Drug Administration has also granted exceptional approval under emergency conditions to conduct a mass vaccination campaign in areas that border DRC. In this US-based phase 1 study, we report the safety and immunogenicity of a variety of heterologous and homologous 2-dose vaccination regimens based on different dosages, sequences, and intervals of Ad26 and MVA vaccines.
METHODS
Participants and Study Design
This phase 1, randomized, placebo-controlled, observer-blind, single-center trial was conducted in Rockville, Maryland, United States, according to the Declaration of Helsinki and good clinical practice guidelines. The protocol was approved by the National Research Ethics Service (no 14/SC/1408) and registered on ClinicalTrials.gov (NCT02325050). Participants provided written informed consent before enrollment.
After a 28-day screening period, healthy adults aged 18–50 years who were willing to use approved contraception throughout the study, and to provide written informed consent, were enrolled. Exclusion criteria included any prior immunization with an Ebola candidate vaccine or any MVA- or Ad26-vectored candidate vaccine; previous diagnosis of EVD; exposure to EVD, including travel to West Africa in the preceding 12 months; or known allergies to any vaccine components.
The study was performed in 3 parts with 10 groups (Table 1). In part 1, participants randomly allocated to 6 groups (groups 1–6) were further randomized 5:1 (groups 1–4) or 9:1 (groups 5–6, after enrollment of 3 open-label sentinel participants) to receive either active vaccine or placebo. A first dose of vaccine or placebo was administered on day 1, followed by the second dose 14, 28, or 56 days later, with follow-up visits at 7 and 21 days after dose 2, and at 180, 240, and 360 days after dose 1. In part 1, standard doses of Ad26.ZEBOV (Ad26) and MVA-BN-Filo (MVA) were used. In part 2, group 7 received standard-dose Ad26 or placebo followed by high-dose MVA (hdMVA) or placebo 14 days later, and group 8 received high-dose Ad26 (hdAd26) or placebo followed by hdMVA 28 days later. Participants in groups 9 and 10 received standard doses of MVA or placebo followed by standard doses of Ad26 or placebo 7 and 14 days later, respectively. In parts 1 and 2, an open-label booster vaccination with either Ad26 (groups 1–5 and 7), hdAd26 (group 8), or MVA (group 6) was administered on day 360 to those who completed the primary vaccination with the active vaccine.
Table 1.
Vaccines Received at Each Time Point by Study Groupa
| Study Part 1 | Study Part 2 | Study Part 3 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Group 1 | Group 2 | Group 3 | Group4 | Group 5 | Group 6 | Group 7 | Group 8 | Group 9 | Group 10 | |
| Recipients, no. | ||||||||||
| Active vaccine | 15 | 15 | 15 | 15 | 9 | 9 | 14 | 15 | 15 | 15 |
| Placebo | 3 | 3 | 3 | 3 | 1 | 1 | 3 | 3 | 3 | 3 |
| Time point (day) | ||||||||||
| d 1 | MVA | MVA | MVA | Ad26 | MVA | Ad26 | Ad26 | hdAd26 | MVA | MVA |
| Placebo | Placebo | Placebo | Placebo | Placebo | Placebo | Placebo | Placebo | Placebo | Placebo | |
| d 8 | … | … | … | … | … | … | … | … | Ad26 | … |
| … | … | … | … | … | … | … | … | Placebo | … | |
| d 15 | Ad26 | … | … | … | MVA | Ad26 | hdMVA | … | … | Ad26 |
| Placebo | … | … | … | Placebo | Placebo | Placebo | … | … | Placebo | |
| d 29 | … | Ad26 | … | MVA | … | … | … | hdMVA | … | … |
| … | Placebo | … | Placebo | … | … | … | Placebo | … | … | |
| d57 | … | … | Ad26 | … | … | … | … | … | … | … |
| … | … | Placebo | … | … | … | … | … | … | … | |
| d 360 | Ad26 | Ad26 | Ad26 | Ad26 | Ad26 | MVA | Ad26 | hdAd26 | … | … |
Abbreviations: Ad26, Ad26.ZEBOV; hdAd26, high-dose Ad26; hdMVA, high-dose MVA; MVA, MVA-BN-Filo.
aEach standard dose of Ad26 contained 5 × 1010 viral particles, and hdAd26 had 1 × 1011 viral particles per dose. Each standard dose of MVA contained 1 × 108 50% tissue culture infective dose (TCID50), and each hdMVA dose contained 4.4 × 108 TCID50. The placebo used was 0.9% saline.
Vaccines
All vaccines were supplied as 0.5-mL doses for intramuscular injection in the deltoid. Each standard dose of Ad26 contained 5 × 1010 viral particles, and hdAd26 had 1 × 1011 viral particles per dose. MVA standard doses contained 1 × 108 50% tissue culture infective dose (TCID50), and each hdMVA contained 4.4 × 108 TCID50. The placebo used was 0.9% saline.
Outcomes
The primary objective was to assess the safety of the different vaccine regimens in terms of serious adverse events (SAEs), adverse events (AEs), and solicited local and systemic AEs. Secondary objectives included evaluation of EBOV GP–specific humoral and cellular immune responses.
Safety Assessments
After monitoring for 1 hour for AEs immediately after vaccination, participants recorded solicited local and systemic AEs on diary cards on the day of each study injection and for 7 subsequent days. Unsolicited AEs reported to 28 days after dose 1, 21 days after dose 2, and 21 days after the booster dose (where applicable) were summarized. SAEs were reported throughout the study.
Immunogenicity Assessments
Serum samples to assess immune responses were obtained before dose 1 (baseline), 7 days and 28 days (group 3 only) after dose 1, immediately before and 21 days after dose 2 and then at 180, 240, and 360 days after dose 1 (groups 1–10). Responses to booster vaccination in groups 1–8 were measured 2, 7, 21, 42, 90, 180, 240, and 360 days after the booster. EBOV GP–specific immunoglobulin G (IgG) binding antibodies were measured by means of EBOV GP Filovirus Animal Nonclinical Group enzyme-linked immunosorbent assay (FANG ELISA) at the Battelle Biomedicals Research Center in West Jefferson, Ohio, United States, and expressed as ELISA units (EU) per milliliter, as described elsewhere [12]. Virus neutralizing antibodies were measured using EBOV GP pseudovirions encoding the GP of a Makona variant EBOV isolate from the 2014 outbreak at Monogram Biosciences, expressed as the 50% inhibitory concentration (IC50) titers (value giving 50% inhibition on a standard curve).
Frozen peripheral blood mononuclear cell samples were analyzed using an intracellular cytokine staining assay (expressed as the total cytokine responses percentage of subset; reported as median response), and an enzyme-linked immunospot interferon (IFN) γ assay (expressed as spot-forming units per 106 peripheral blood mononuclear cells; reported as median reportable value) at the HIV Vaccine Trials Network Laboratory in Seattle, Washington, United States, as described elsewhere [12, 24]. Results of IFN-γ enzyme-linked immunospot responses are reported in the Supplementary Materials (Supplementary Results and Supplementary Table 3).
Statistical Analysis
No formal statistical testing of safety or immune response data was planned or performed because of the small sample sizes in each vaccination group. Analysis of safety was based on descriptive summaries of AEs, clinical laboratory values, and vital signs and was performed on the full analysis set. This set consists of all participants who were randomized and received ≥1 dose of study vaccine, regardless of the occurrence of protocol deviations. Descriptive statistics and graphic representations of immunogenicity analyses are presented on all randomized and vaccinated participants with ≥1 evaluable postvaccination immunogenicity sample. Definitions of responders are provided in the Supplementary Materials.
RESULTS
Baseline Characteristics and Participant Disposition
The study ran from January 2015 to May 2017. Of 164 randomized and vaccinated participants, 1 participant in group 7 received hdMVA as dose 1 (instead of Ad26) and was not included in safety or immunogenicity analyses. Four participants did not receive dose 2 (1 withdrew, 1 as lost to follow-up, and 2 were placed on study hold by the sponsor, although both subsequently completed the study) (Figure 1). Baseline demographic characteristics were well balanced across groups (Table 2).
Figure 1.
Study flowcharts showing groups receiving vaccines at 7- and 14-day (top) or 28- and 56-day (bottom) intervals. Unless otherwise indicated, Ad26.ZEBOV (adenovirus serotype 26 [Ad26]) was administered at a dose of 5 × 1010 viral particles and MVA-BN-Filo (modified vaccinia Ankara [MVA]) at a dose of 1 × 108 50% tissue culture infective dose (TCID50). The placebo used was 0.9% saline. Vaccination regimens are shown as “dose 1, dose 2 (booster).” Groups included active and placebo recipients. Groups 1 (MVA,Ad26 [Ad26]) and 10 (MVA,Ad26) are combined; all other groups are shown individually. Abbreviations: hdA626, high-dose Ad26 (1 × 1011 viral particles); hdMVA, high-dose MVA (4.4 × 108 TCID50).
Table 2.
Study Injections and Baseline Demographic Characteristics in the Full Analysis Set
| Interval | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 7 d | 14 d | 28 d | 56 d | ||||||||||
| Study Injections and Characteristics | Group 9 | Groups 1 and 10a | Group 5 | Group 6 | Group 7 | Placebo | Group 2 | Group 4 | Group 8 | Placebo | Group 3 | ||
| Study injections | |||||||||||||
| Dose 1 | MVA | Placebo | MVA | MVA | Ad26 | Ad26 | Placebo | MVA | Ad26 | hdAd26 | Placebo | MVA | Placebo |
| Dose 2 | Ad26 | Placebo | Ad26 | MVA | Ad26 | hdMVA | Placebo | Ad26 | MVA | hdMVA | Placebo | Ad26 | Placebo |
| Boosterb | None | None | Ad26 | Ad26 | MVA | Ad26 | None | Ad26 | Ad26 | hdAd26 | None | Ad26 | None |
| Participants, no. | 15 | 3 | 30 | 9 | 9 | 14 | 11 | 15 | 15 | 15 | 9 | 15 | 3 |
| Female sex, no. (%) | 6 (40) | 3 (100) | 6 (20) | 3 (33) | 6 (67) | 4 (29) | 7 (64) | 8 (53) | 6 (40) | 8 (53) | 2 (22) | 9 (60) | 2 (67) |
| Age, median (range), y | 28 (21–43) | 26 (25–28) | 26 (19–47) | 34 (20–48) | 39 (20–49) | 36.5 (23–50) | 25 (22–37) | 29 (20–50) | 35 (20–49) | 37 (24–50) | 27 (21–48) | 32 (21–50) | 21 (19–26) |
| Race, no. (%) | |||||||||||||
| White | 6 (40) | 1 (33) | 15 (50) | 7 (78) | 5 (56) | 9 (64) | 6 (55) | 8 (53) | 8 (53) | 9 (60) | 4 (44) | 7 (47) | 2 (67) |
| Black/AA | 7 (47) | 2 (67) | 12 (40) | 2 (22) | 4 (44) | 4 (29) | 5 (46) | 6 (40) | 6 (40) | 4 (27) | 4 (44) | 7 (47) | 1 (33) |
| Asian | 0 | 0 | 3 (10) | 0 | 0 | 0 | 0 | 0 | 0 | 1 (7) | 0 | 0 | 0 |
| Multiple | 2 (13) | 0 | 0 | 0 | 0 | 1 (7) | 0 | 1 (7) | 1 (7) | 0 | 0 | 1 (7) | 0 |
| Other | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (7) | 0 | 0 | 0 |
| BMI, median (range)c | 24.3 (20.1–32.1) | 29.1 (27.5–32.4) | 27.1 (20.5–34.7) | 25.9 (22.3–34.6) | 29.9 (20.6–34.6) | 26.4 (20.6–34.5) | 24.9 (20.6–28.8) | 26.0 (22.5–33.0) | 24.3 (18.6–34.7) | 30.0 (22.4–33.7) | 27.5 (21.7–33.7) | 25.9 (19.7–33.8) | 25.3 (23.4–29.9) |
Abbreviations: Ad26, Ad26.ZEBOV; AA, African/American; BMI, body mass index; hdAd26, high-dose Ad26; hdMVA, high-dose MVA; MVA, MVA-BN-Filo.
aThis column combines groups 1 (MVA,Ad26 [dose 1, dose 2], [Ad26 booster]) and 10 (MVA,Ad26).
bBooster at day 360.
cBMI calculated as weight in kilograms divided by height in meters squared.
Safety
No deaths, SAEs, or AEs leading to discontinuation were reported, and all regimens demonstrated an acceptable safety profile. Solicited local AEs, mainly reports of mild to moderate injection-site pain with some of injection-site warmth and pruritus, occurred after 70.1% of Ad26, 53.9% of MVA, 48.0% of hdAd26, 64.3% of hdMVA, and 19.2% of placebo doses (Table 3). Solicited local AEs were transient, with a maximum duration of 10 days and median time to onset of 1–6 days.
Table 3.
Frequency of Solicited Local and Systemic Adverse Events With Standard and High Doses of Ad26 and MVA Vaccines and Placebo
| Frequency of AE, % of Doses | |||||
|---|---|---|---|---|---|
| Type of Solicited AE | Ad26 (n = 184)a | MVA (n = 115)a | hdAd26 (n = 25)a |
hdMVA (n = 28)a |
Placebo (n = 52)a |
| Local AEsb | 70.1 | 53.9 | 48.0 | 64.3 | 19.2 |
| Pain | 67.9 | 53.0 | 48.0 | 64.3 | 17.3 |
| Pruritus | 4.9 | 4.3 | 0.0 | 0.0 | 3.8 |
| Warmth | 12.5 | 12.2 | 20.0 | 10.7 | 1.9 |
| Systemic AEs | 52.7 | 40.0 | 60.0 | 28.6 | 42.3 |
| Arthralgia | 15.2 | 7.0 | 12.0 | 0.0 | 0.0 |
| Chills | 23.4 | 6.1 | 24.0 | 0.0 | 0.0 |
| Fatigue | 37.0 | 27.0 | 32.0 | 14.3 | 23.1 |
| Headache | 32.6 | 22.6 | 28.0 | 14.3 | 9.6 |
| Myalgia | 23.9 | 13.9 | 28.0 | 10.7 | 5.8 |
| Nausea | 16.8 | 8.7 | 8.0 | 7.1 | 13.5 |
| Pruritus | 3.3 | 2.6 | 0.0 | 0.0 | 3.8 |
| Pyrexia | 12.0 | 2.6 | 16.0 | 3.6 | 0.0 |
| Rash | 2.7 | 0.0 | 0.0 | 0.0 | 3.8 |
| Vomiting | 3.8 | 2.6 | 0.0 | 0.0 | 3.8 |
Abbreviations: Ad26, Ad26.ZEBOV; AE, adverse event; hdAd26, high-dose Ad26; hdMVA, high-dose MVA; MVA, MVA-BN-Filo.
aParenthetical numbers represent number of injections for each vaccine or placebo.
bThere were no reports of injection site erythema, induration, swelling or severe solicited local AEs.
Solicited systemic events were reported after 52.7% of Ad26, 40.0% of MVA, 60.0% of hdAd26, 28.6% of hdMVA, and 42.3% of placebo doses (Table 3), most considered related to the study administrations. The most frequent systemic AEs were headache, fatigue, and myalgia. Severe solicited systemic AEs were reported after 15 of 404 vaccinations (3.7%) (13 Ad26 , 1 MVA, and 1 hdMVA vaccination), and included arthralgia (n = 1), chills (n = 9), fatigue (n = 4), headache (n = 4), myalgia (n = 3), nausea (n = 2), pyrexia (n = 3), and vomiting (n = 1). All solicited systemic AEs were transient. The investigator considered 3 cases of fever >38.9°C to be at least possibly related to study vaccine.
At least 1 unsolicited AE was reported after 47.3% of Ad26, 53.9% of MVA, 36.0% of hdAd26, 39.3% of hdMVA, and 48.1% of placebo doses. Severe unsolicited AEs were uncommon and were considered unrelated or doubtfully related to vaccine, except for 1 case of severe night sweats and decreased appetite. No severe unsolicited AEs were reported with placebo. There were no marked differences in the overall occurrence of unsolicited AEs between different regimens, dosing intervals, doses, or booster vaccinations. There were no noticeable trends in laboratory abnormalities. Any grade 3 abnormalities were usually reported as unsolicited AEs, and none were considered to be related to study vaccinations.
Immunogenicity
All 2-dose heterologous vaccination regimens tested in this study were immunogenic, inducing humoral and cellular immune responses regardless of vaccine sequence and dose levels (Figures 2 and 3 and Table 4). After dose 1, standard-dose Ad26 or hdAd26 vaccination induced higher binding antibody responses than MVA. After dose 2, response levels were boosted for both vaccine sequences regardless of the interval. Homologous regimens were less immunogenic, particularly the MVA regimen.
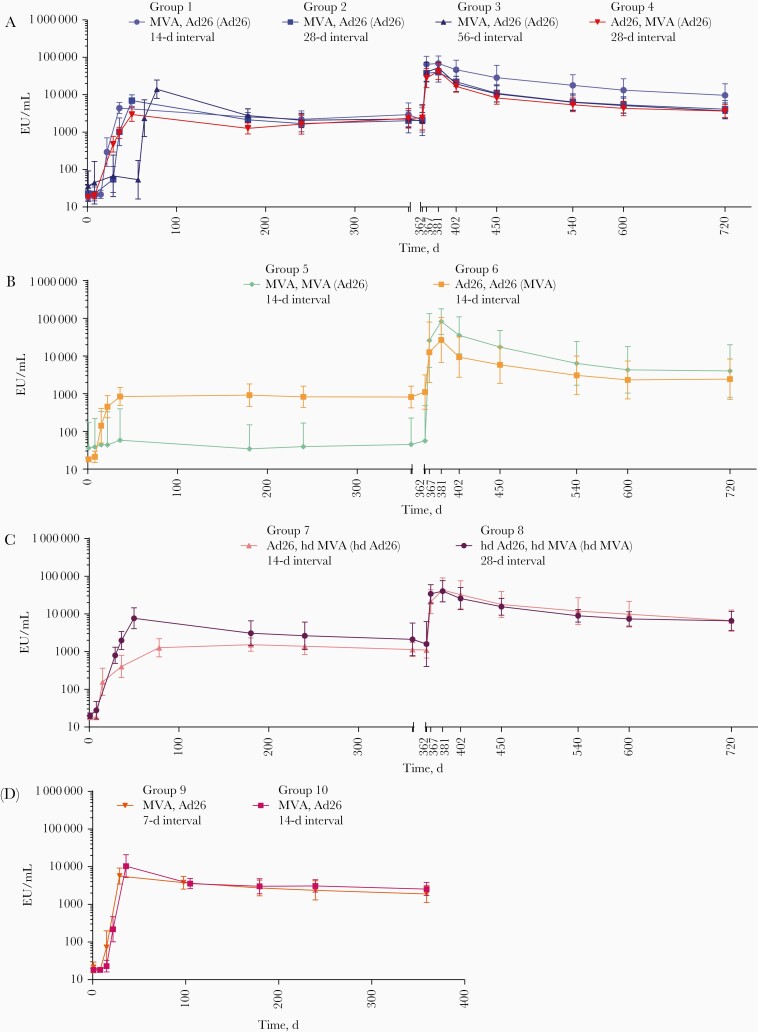
Figure 2.
Anti–Ebola virus glycoprotein immunoglobulin G binding antibody responses after heterologous or homologous 2-dose vaccination with Ad26.ZEBOV (adenovirus serotype 26 [Ad26]) and/or MVA-BN-Filo (modified vaccinia Ankara [MVA]) and booster vaccination at day 360 for groups 1–4 (A), 5 and 6 (B), 7 and 8 (C), and 9 and 10 (no booster vaccinations) (D). Vaccination regimens are shown as “dose 1, dose 2 (booster).” Responses represent geometric mean concentrations with 95% confidence intervals. Abbreviations: EU, enzyme-linked immunosorbent assay units; hdAd26, high-dose Ad26; hdMVA, high-dose MVA.
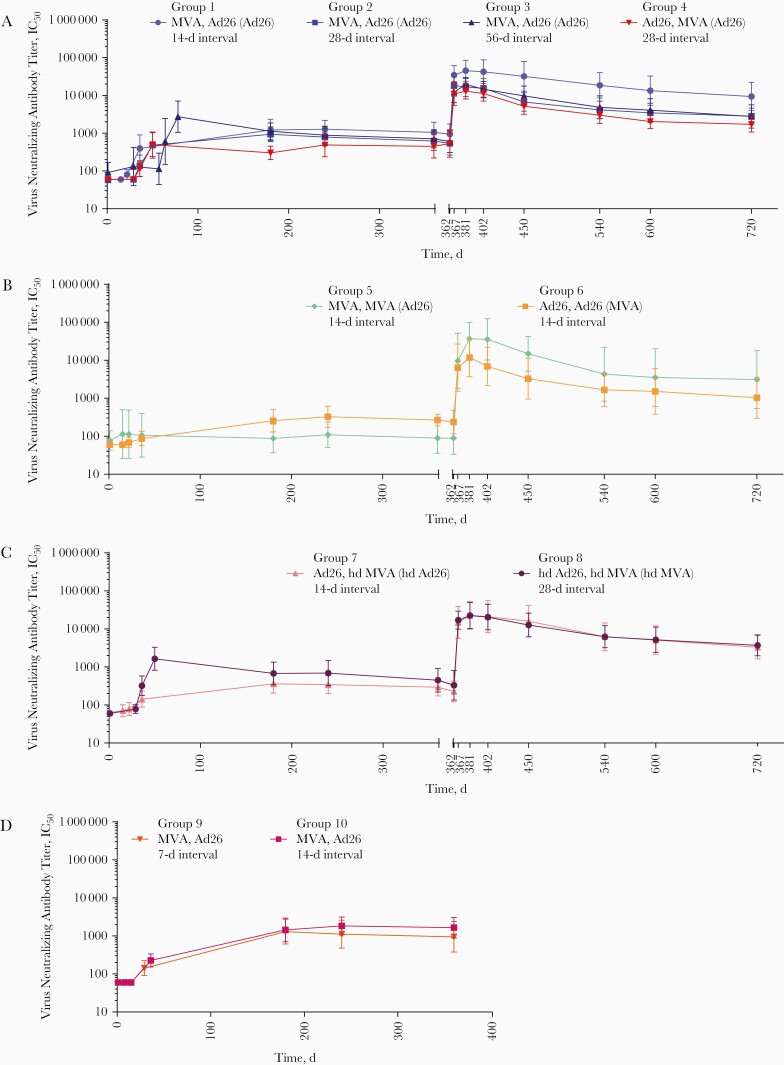
Figure 3.
Virus neutralizing antibody titer results, determined with luciferase-based virus neutralization assay, after heterologous or homologous 2-dose vaccination with Ad26.ZEBOV (adenovirus serotype 26 [Ad26]) and/or MVA-BN-Filo (modified vaccinia Ankara [MVA]) and booster vaccination at day 360 for groups 1–4 (A), 5 and 6 (B), 7 and 8 (C), and 9 and 10 (no booster vaccinations) (D). Vaccination regimens are shown as “dose 1, dose 2 (booster).” Abbreviations: hdAd26, high-dose Ad26; hdMVA, high-dose MVA; IC50, 50% inhibitory concentration.
Table 4.
Humoral Immune Responder Rates by Study Group
| Interval | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 7 d | 14 d | 28 d | 56 d | |||||||
| Injections or Antibody Response | Group 9 (n = 12)a | Group 1 (n = 15) | Group 5 (n = 9) | Group 6 (n = 9) | Group 7 (n = 13) | Group 10 (n = 15) | Group 2 (n = 15) | Group 4 (n = 15) | Group 8 (n = 15) | Group 3 (n = 15) |
| Study injection | ||||||||||
| Dose 1 | MVA | MVA | MVA | Ad26 | Ad26 | MVA | MVA | Ad26 | hdAd26 | MVA |
| Dose 2 | Ad26 | Ad26 | MVA | Ad26 | hdMVA | Ad26 | Ad26 | MVA | hdMVA | Ad26 |
| Boosterb | None | Ad26 | Ad26 | MVA | Ad26 | None | Ad26 | Ad26 | hdAd26 | Ad26 |
| EBOV binding antibody responders, %c | ||||||||||
| Before dose 2 | 0 | 13 | 11 | 89 | 85 | 14 | 47 | 100 | 100 | 13 |
| After dose 2d | 100 | 100 | 33 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
| d 180 | 100 | 100 | 0 | 100 | 100 | 100 | 93 | 100 | 100 | 93 |
| d 240 | 100 | 100 | 11 | 100 | 100 | 100 | 93 | 100 | 100 | 93 |
| d 360 | 100 | 100 | 11 | 100 | 100 | 100 | 93 | 100 | 100 | 87 |
| d 367 | NA | 100 | 100 | 100 | 100 | NA | 100 | 100 | 100 | 100 |
| d 381 | NA | 100 | 100 | 100 | 100 | NA | 100 | 100 | 100 | 100 |
| d 720 | NA | 100 | 100 | 100 | 100 | NA | 91 | 100 | 100 | 92 |
| EBOV neutralizing antibody responders, %c | ||||||||||
| Before dose 2 | 0 | 0 | 11 | 0 | 8 | 0 | 0 | 0 | 27 | 7 |
| After dose 2d | 67 | 80 | 11 | 33 | 62 | 87 | 80 | 93 | 100 | 100 |
| d 180 | 100 | 100 | 0 | 88 | 92 | 100 | 100 | 93 | 93 | 100 |
| d 240 | 100 | 100 | 22 | 88 | 92 | 100 | 100 | 100 | 93 | 93 |
| d 360 | 92 | 100 | 11 | 100 | 83 | 100 | 100 | 80 | 86 | 87 |
| d 367 | NA | 100 | 100 | 100 | 100 | NA | 100 | 100 | 100 | 100 |
| d 381 | NA | 100 | 100 | 100 | 100 | NA | 100 | 100 | 100 | 100 |
| d 720 | NA | 100 | 100 | 100 | 100 | NA | 100 | 100 | 100 | 92 |
Abbreviations: Ad26, Ad26.ZEBOV; EBOV, Ebola virus; hdAd26, high-dose Ad26; hdMVA, high-dose MVA; MVA, MVA-BN-Filo; NA, not applicable.
aParenthetical numbers represent numbers of participants with data at baseline and 21 days after dose 2.
bBooster at day 360.
cFor both binding and neutralizing antibodies, a participant was a responder if a sample was negative at baseline and positive after baseline, or if a sample was positive at baseline with a >3-fold increase after baseline (see Supplementary Material).
dAt 21 days after dose 2.
EBOV GP–Specific IgG Binding Antibody Responses
First vaccination with either Ad26 or MVA induced EBOV GP–specific IgG binding antibody responses, with higher responder rates after Ad26 vaccination than MVA (Table 4). At day 15, 84.6%–88.9% of the participants had a response after Ad26 vaccination (groups 6 and 7), compared with 11.1%–13.3% after MVA (groups 1 and 5); at day 29 all participants had responded to either Ad26 or hdAd26 (groups 4 and 8), while only 26.7%–46.7% of participants had responded to MVA (groups 2 and 3) (Supplementary Table 1).
Heterologous Vaccination Regimens (Groups 1–4 and 7–10)
Binding antibody responder rates increased after the second heterologous vaccination, with all participants across all groups having EBOV-binding IgG antibodies 21 days after dose 2 (Table 4). At this time point, the highest geometric mean concentration (GMC; 14 048 EU/mL) was obtained after MVA,Ad26 in a 56-day interval (group 3), and GMCs of 2976–7706 EU/mL were achieved in a 28-day interval irrespective of the vaccination sequence (groups 2, 4, and 8) (Figure 2 and Supplementary Table 1). Binding antibody responses persisted in all participants (100%) up to day 360, except for MVA,Ad26 in a 28-day interval (93%; group 2) and MVA,Ad26 in a 56-day interval (87%; group 3) (Table 4). At day 360, the GMCs persisted at similar levels (1138–2940 EU/mL) irrespective of the vaccination interval (Figure 2; Supplementary Table 1).
All participants who received the booster (groups 1–8) responded within 7 days to a heterologous booster vaccination given at day 360, which induced an anamnestic response in all heterologous vaccination groups with 12.4–38.6-fold increases in GMCs (21 283–65 123 EU/mL) (Supplementary Table 1). Responses persisted in all participants until day 720, except for the MVA,Ad26 28-day interval (91%; group 2) and MVA,Ad26 56-day interval (92%; group 3) participants. At day 720, the GMCs persisted at a level ranging between 3653 and 9668 EU/mL, which represents a 1.6–6.4-fold increase compared with day 360 (Supplementary Table 1).
Homologous Vaccination Regimens (Groups 5 and 6)
At 21 days after dose 2, responses were observed in all participants receiving the Ad26,Ad26 vaccine regimen in a 14-day interval (GMC, 848 EU/mL; group 6), whereas only 3 (33.3%) participants responded after receiving MVA,MVA in a 14-day interval (GMC, 59 EU/mL; group 5). At day 360, responses were maintained in all Ad26,Ad26-vaccinated participants (GMC, 822 EU/mL; group 6), but only in 1 (11.1%) MVA,MVA-vaccinated participant (GMC, 45 EU/mL; group 5).
A heterologous booster vaccination given at day 360 induced a 13.9–269.8-fold increase in GMCs (12 614–26 020 EU/mL) within 7 days, with 100% responders across both groups (Supplementary Table 1). The responses persisted in all participants until day 720 at a level 2.7–41.7-fold higher compared with day 360 (2449–4021 EU/mL).
Neutralizing Antibody Responses
The first vaccination induced very little neutralizing antibody responses, with the highest responder rate observed after hdAd26 vaccination (27%; group 8).
Heterologous Vaccination Regimens (Groups 1–4 and 7–10)
At 21 days after dose 2, the highest responder rate (100%) was observed in the MVA,Ad26 56-day interval (group 3) and hdAd26,hdMVA (group 8) participants (Table 4). At this time point, geometric mean titers (GMTs) ranged between 142 and 2727 IC50, with the highest response detected in MVA,Ad26 56-day interval (group 3) vaccinated participants (Figure 3). At day 360, responses persisted in all of MVA,Ad26 14-day (groups 1 and 10) and MVA,Ad26 28-day participants (group 2), while lower persistence rates (80%–87%) were observed in the other groups. Similar GMTs were observed across all groups at day 360 (293–1649 IC50).
Within 7 days after the Ad26 booster, all participants who received the booster (groups 1–8) displayed a strong increase in neutralizing antibody response (18.6–46.7-fold increase compared with day 360), with a GMT range of 10 951–34 488 IC50. At day 720, the responses persisted in all participants. except for those vaccinated with MVA,Ad26 (Ad26 booster) with a 56-day interval (92%; group 3), with GMTs ranging from 1716 to 9386 IC50 (3.1–10.6-fold increase compared with day 360).
Homologous Vaccination Regimens (Groups 5 and 6)
At 21 days after dose 2, 1 participant in group 5 (MVA,MVA) (11%) and 3 participants in group 6 (Ad26,Ad26) (33%) had a neutralizing antibody response (GMTs below the lower limit of quantitation [LLOQ]). At day 360, neutralizing antibodies were observed in all Ad26,Ad26 recipients (GMT, 267 IC50; group 6) but in only participant 1 (11%) in the MVA,MVA group (GMTs below LLOQ; group 5).
Seven days after heterologous booster vaccination (day 367), an anamnestic response was observed in all participants of both groups. GMTs ranged from 6377–9634 IC50, with an approximate 22.9–52.9-fold increase compared with the prebooster time point. At day 720, the responses persisted in all participants of both groups, with an approximate 3.7–17.2-fold increase in GMT compared with day 360 (1031–3126 IC50).
CD4+ T-Cell Responses
Heterologous Vaccination Regimens (Groups 1–4 and 7–10)
All heterologous regimens induced CD4+ T-cell responses (Supplementary Figure 1). Twenty-one days after dose 2, the highest responder rate was observed for the Ad26,hdMVA 14-day interval group 7 (92%) and the lowest for the MVA,Ad26 56-day interval group 3 (14%), with median response levels ranging from 0.04% to 0.21%. At day 360, 0% (group 3; MVA,Ad26, 56-day interval) to 45% (group 1; MVA,Ad26, 14-day interval) of participants showed a persisting CD4+ T-cell response (median, below LLOQ to 0.06%).
Booster vaccination elicited increases in responder rates within all groups at day 367 (30%–88.9% of participants) compared, with day 360 with median levels ranging between 0.05% and 0.19%. At day 720, responses were observed in 0% to 36.4% of participants across all groups (median, below LLOQ to 0.05%).
Homologous Vaccination Regimens (Groups 5 and 6)
At 21 days after dose 2, 11.1% (Ad26,Ad26; group 6) to 33.3% (MVA,MVA; group 5) of participants had a CD4+ T-cell response (median, below LLOQ). In both homologous regimens, the response was no longer observed at day 360 (Supplementary Figure 1).
The heterologous booster vaccination elicited an increase in responders at day 367 (20% of participants in group 5 [MVA,MVA]; 66.7% of participants in group 6 [Ad26,Ad26]), with median values below the LLOQ (MVA,MVA) and 0.16% (Ad26,Ad26). At day 720, a CD4+ T-cell response was observed in only 1 MVA,MVA participant (14.3%; below LLOQ).
CD8+ T-Cell Responses
Heterologous Vaccination Regimens (Groups 1–4 and 7–10)
All heterologous regimens induced CD8+ T-cell responses (Supplementary Figure 2). At 21 days after dose 2, the highest responder rate was observed for the Ad26,hdMVA 14-day interval group 7 (85%) while the lowest responder rate was observed for the MVA,Ad26 56-day interval group 3 (33.3%). Median CD8+ T-cell responses varied from below the LLOQ to 0.34% across groups. At day 360, responses were observed in all heterologous groups (40%–72.7%), with median values ranging from below the LLOQ to 0.15%.
Within 7 days after booster dose, increases in CD8+ T-cell responses were observed (45.5%–72.7%), with median values varying from 0.05% to 0.46%. At day 720, responses persisted in all regimens (41.7%–72.7%) and median CD8+ T-cell responses ranged from below the LLOQ to 0.24%.
Homologous Vaccination Regimens (Groups 5 and 6)
In the homologous vaccination regimens, only Ad26,Ad26 (group 6) induced a CD8+ T-cell response in 55.6% of participants at 21 days after dose 2 (median, 0.17%), while only 1 participant (11.1%) responded after MVA,MVA (group 5) (median, below LLOQ) (Supplementary Figure 2). At day 360, responses were still observed in both groups (22.2%–50%) with a median value ranging between below LLOQ and 0.22%. Compared with day 360, the booster vaccination did not induce notable response changes in the homologous regimens.
Polyfunctionality of CD4+ and CD8+ T-Cell Responses
In all regimens, CD4+ and CD8+ T-cell responses were mostly polyfunctional, with the majority of the T cells producing 2 or 3 of the investigated cytokines (IFN-γ, interleukin 2 [IL-2], and/or tumor necrosis factor [TNF] α). The most frequent polyfunctional T-cell subsets were IFN-γ +/IL-2+/TNF-α +, IFN-γ −/IL-2+/TNF-α +, and IFN-γ +/IL-2−/TNF-α + among CD4+ T-cell responders and IFN-γ +/IL-2+/TNF-α + and IFN-γ +/IL-2−/TNF-α + among CD8+ T-cell responders.
DISCUSSION
In parts of Africa, Ebola outbreaks are unpredictable and inevitable [3, 5], and multiple approaches are necessary for effective disease control. Adenoviral-vectored Ebola vaccines have been shown to be potent inducers of T- and B-cell responses and to protect against EBOV challenge in a nonhuman primate model [18, 19, 25]. Furthermore, the heterologous 2-dose Ad26,MVA vaccination strategy has been demonstrated to be safe and immunogenic, conferring long-lasting immune responses [12, 21, 22].
In this phase 1 study, we demonstrated that both heterologous and homologous Ad26,MVA Ebola vaccine regimens are well tolerated in healthy adults, regardless of interval (7–56 days), vaccination order, or dose levels. Safety and tolerability data for the 2-dose strategy were comparable with other phase 1 study findings [12, 21, 22]. There were no vaccine-related SAEs and no apparent differences in AE profile after either dose, or between different dose intervals.
All heterologous vaccine regimens induced antibody responses, similar to those previously observed in other phase 1 studies [12, 21, 22]. A clear trend to higher responses with increasing intervals was seen between the 2 doses, irrespective of order of administration. The highest humoral responses were observed 21 days after Ad26,MVA and MVA,Ad26 vaccine regimens administered at 28- and 56-day intervals, respectively. However, in heterologous regimens, antibody levels were generally similar in long-term follow-up regardless of sequence, interval, or use of high-dose vaccines. Homologous 2-dose regimens, particularly the MVA regimen, were less immunogenic.
All heterologous and homologous 2-dose regimens studied induced humoral immune memory, as demonstrated by rapid increases in binding and neutralizing antibody levels within 7 days after a booster vaccination. One year after the booster, binding and neutralizing antibody responses persisted at levels higher than those observed 1 year after initial vaccination (day 360).
The Ad26,hdMVA in a 14-day interval regimen did not induce higher antibody responses than the standard-dose Ad26,MVA in a 28-day interval regimen, suggesting that the higher dose did not compensate for the shorter interval. Although hdAd26,hdMVA tended to induce higher humoral responses than standard-dose Ad26,MVA with a 28-day interval, the difference did not persist until day 360.
Heterologous regimens induced polyfunctional CD4+ and CD8+ T-cell responses in a substantial percentage of participants. However, unlike the humoral responses, these cellular immune responses tended to be higher with MVA,Ad26 at shorter intervals. Cellular responses were much smaller and less persistent in the homologous regimen groups.
Our data show that heterologous Ad26,MVA vaccination regimens produce robust and durable humoral and cellular immune responses against EBOV GP. Homologous regimens resulted in lower humoral responses and little, if any, cellular immune responses. Increasing the dose of either regimen component resulted in small incremental increases in responses, but this was limited to the immediate postvaccination assessments, and the differences did not persist over time.
The main limitations of this study are small numbers of participants and a population not representative of those most at risk of Ebola infection. Both limitations will be addressed by the ongoing clinical development program, which includes larger phase 2 and 3 studies (NCT02416453, NCT02564523, NCT02598388, NCT02509494, NCT02543567, and NCT02543268) in Europe, the United States, and West and East sub-Saharan Africa to evaluate Ad26,MVA regimens with 28-, 56- or 84-day intervals.
In conclusion, 2-dose heterologous regimens with standard doses of Ad26 and MVA provide persistent humoral and cellular immune responses, as well as immune memory to EBOV GP. The heterologous 2-dose Ad26,MVA vaccination strategy is undergoing further clinical assessment in phase 2 and 3 trials encompassing different populations including children, older adults, and people living with HIV infection.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. We thank all the volunteers who took part in the study and the ethical bodies and regulatory authorities of the participating country for their review and approval of the study. Editorial support was funded by Janssen Vaccines & Prevention. We thank National Institute of Allergy and Infectious Disease (NIAID), National Institutes of Health (NIH) for their support (study funded under the Contract HHSN272200800056C), and the team at the Division of Microbiology and Infectious Diseases (DMID/NIAID/NIH) for their collaboration. Medical writing support was provided by Kaedy Bryson of Zoetic Science and Keith Veitch (keithveitch communications), funded by Janssen Pharmaceuticals, and by Marialuisa Quadri (Janssen Vaccines & Prevention). We thank Yvonne Salzgeber (Janssen Vaccines AG) and Marialuisa Quadri (Janssen Vaccines & Prevention) for publication coordination.
The data sharing policy of Janssen, Pharmaceutical Companies of Johnson & Johnson, is available at https://www.janssen.com/clinical-trials/transparency. Requests for access to the study data can be submitted through the Yale Open Data Access (YODA) project site (http://yoda.yale.edu).
Author contributions. All authors had full access to the data. N. G. had final responsibility for the decision to submit the manuscript for publication.
Financial support. The work was supported by NIAID, NIH (contract HHSN272200800056C) and Janssen Vaccines & Prevention B.V.
Potential conflicts of interest. The study sponsor, Jansssen Vaccines & Prevention B.V., was involved in the design and conduct of the trial, and in data collection and analysis. N. G., V. B., K. L., C. R., A. G., B. C., and M. D. were all full-time employees of Janssen at the time of the study. S. B. reports grants from Janssen, Pharmaceutical Companies of Johnson & Johnson, during the conduct of the study, paid to his institution. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Contributor Information
Neil Goldstein, Janssen Infectious Diseases and Vaccines, Leiden, the Netherlands.
Viki Bockstal, Janssen Infectious Diseases and Vaccines, Leiden, the Netherlands.
Stephan Bart, Optimal Research LLC, Rockville, Maryland, USA.
Kerstin Luhn, Janssen Infectious Diseases and Vaccines, Leiden, the Netherlands.
Cynthia Robinson, Janssen Infectious Diseases and Vaccines, Leiden, the Netherlands.
Auguste Gaddah, Janssen Infectious Diseases and Vaccines, Beerse, Belgium.
Benoit Callendret, Janssen Infectious Diseases and Vaccines, Leiden, the Netherlands.
Macaya Douoguih, Janssen Infectious Diseases and Vaccines, Leiden, the Netherlands.
References
- 1. Rosello A, Mossoko M, Flasche S, et al. . Ebola virus disease in the Democratic Republic of the Congo, 1976–2014. Elife 2015; 4:e09015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Malvy D, McElroy AK, de Clerck H, Günther S, van Griensven J. Ebola virus disease. Lancet 2019; 393:936–48. [DOI] [PubMed] [Google Scholar]
- 3. Centers for Disease Control and Prevention. Ebola virus disease distribution map: cases of Ebola virus disease in Africa since 1976. https://www.cdc.gov/vhf/ebola/history/distribution-map.html/. Accessed 10 August 2020.
- 4. Burki T. Ebola in the Democratic Republic of the Congo: 1 year on. Lancet Infect Dis 2019; 19:813–4. [DOI] [PubMed] [Google Scholar]
- 5. World Health Organization . Ebola in the Democratic Republic of the Congo—health emergency update. https://www.who.int/emergencies/diseases/ebola/drc-2019. Accessed 10 August 2020.
- 6. WHO Ebola Response Team. After Ebola in West Africa—unpredictable risks, preventable epidemics. N Engl J Med 2016; 375:587–96. [DOI] [PubMed] [Google Scholar]
- 7. Lambe T, Bowyer G, Ewer KJ. A review of phase I trials of Ebola virus vaccines: what can we learn from the race to develop novel vaccines? Philos Trans R Soc Lond B Biol Sci 2017; 372:20160295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Henao-Restrepo AM, Camacho A, Longini IM, et al. . Efficacy and effectiveness of an rVSV-vectored vaccine in preventing Ebola virus disease: final results from the Guinea ring vaccination, open-label, cluster-randomised trial (Ebola Ça Suffit!). Lancet 2017; 389 :505–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. World Health Organization . Preliminary results on the efficacy of rVSV-ZEBOV-GP Ebola vaccine using the ring vaccination strategy in the control of an Ebola outbreak in the Democratic Republic of the Congo: an example of integration of research into epidemic response. https://www.who.int/csr/resources/publications/ebola/ebola-ring-vaccination-results-12-april-2019.pdf. Accessed 24 June 2020.
- 10. European Commission . Vaccine against Ebola: Commission grants first-ever market authorisation. https://ec.europa.eu/commission/presscorner/detail/en/ip_19_6246. Accessed 10 March 2020.
- 11. Food and Drug Administration . First FDA-approved vaccine for the prevention of Ebola virus disease, marking a critical milestone in public health preparedness and response. https://www.fda.gov/news-events/press-announcements/first-fda-approved-vaccine-prevention-ebola-virus-disease-marking-critical-milestone-public-health. Accessed 10 March 2020.
- 12. Milligan ID, Gibani MM, Sewell R, et al. . Safety and immunogenicity of novel adenovirus type 26- and modified vaccinia Ankara-vectored Ebola vaccines: a randomized clinical trial. JAMA 2016; 315:1610–23. [DOI] [PubMed] [Google Scholar]
- 13. Reyes-Sandoval A, Berthoud T, Alder N, et al. . Prime-boost immunization with adenoviral and modified vaccinia virus Ankara vectors enhances the durability and polyfunctionality of protective malaria CD8+ T-cell responses. Infect Immun 2010; 78:145–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sheehy SH, Duncan CJ, Elias SC, et al. . Phase Ia clinical evaluation of the safety and immunogenicity of the Plasmodium falciparum blood-stage antigen AMA1 in ChAd63 and MVA vaccine vectors. PLoS One 2012; 7:e31208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. O’Hara GA, Duncan CJ, Ewer KJ, et al. . Clinical assessment of a recombinant simian adenovirus ChAd63: a potent new vaccine vector. J Infect Dis 2012; 205:772–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Barouch DH, Tomaka FL, Wegmann F, et al. . Evaluation of a mosaic HIV-1 vaccine in a multicentre, randomised, double-blind, placebo-controlled, phase 1/2a clinical trial (APPROACH) and in rhesus monkeys (NHP 13-19). Lancet 2018; 392:232–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Swadling L, Capone S, Antrobus RD, et al. . A human vaccine strategy based on chimpanzee adenoviral and MVA vectors that primes, boosts, and sustains functional HCV-specific T cell memory. Sci Transl Med 2014; 6:261ra153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Geisbert TW, Bailey M, Hensley L, et al. . Recombinant adenovirus serotype 26 (Ad26) and Ad35 vaccine vectors bypass immunity to Ad5 and protect nonhuman primates against ebolavirus challenge. J Virol 2011; 85:4222–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zahn R, Gillisen G, Roos A, et al. . Ad35 and ad26 vaccine vectors induce potent and cross-reactive antibody and T-cell responses to multiple filovirus species. PLoS One 2012; 7:e44115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Van Hoof J. 2015 Janssen Ebola vaccine: emergency track program. Review of Ebola vaccines in phase 1 clinical evaluation. Presented at World Health Organization Meeting, 8 January 2015. http://www.who.int/mediacentre/events/2015/S2.1_Janssen_ZEBOV_vaccine-Revised.pdf. Accessed 10 March 2020. [Google Scholar]
- 21. Mutua G, Anzala O, Luhn K, et al. . Safety and immunogenicity of a 2-dose heterologous vaccine regimen with Ad26.ZEBOV and MVA-BN-Filo Ebola vaccines: 12-month data from a phase 1 randomized clinical trial in Nairobi, Kenya. J Infect Dis 2019; 220:57–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Anywaine Z, Whitworth H, Kaleebu P, et al. . Safety and immunogenicity of a 2-dose heterologous vaccination regimen with Ad26.ZEBOV and MVA-BN-Filo Ebola vaccines: 12-month data from a phase 1 randomized clinical trial in Uganda and Tanzania. J Infect Dis 2019; 220:46–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. European Commission . Vaccine against Ebola: Commission grants new market authorizations. https://ec.europa.eu/commission/presscorner/detail/en/IP_20_1248. Accessed on 13 July 2020.
- 24. Frahm N, DeCamp AC, Friedrich DP, et al. . Human adenovirus-specific T cells modulate HIV-specific T cell responses to an Ad5-vectored HIV-1 vaccine. J Clin Invest 2012; 122:359–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sullivan NJ, Hensley L, Asiedu C, et al. . CD8+ cellular immunity mediates rAd5 vaccine protection against Ebola virus infection of nonhuman primates. Nat Med 2011; 17:1128–31. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.