Abstract
At present, lactic acid bacteria (LAB) fermentation is commonly considered as an effective strategy to remarkably drive the improvement of flavor and nutritional value, and extend shelf-life of fermented foods. In this study, the by-product of tea manufacture, including broken tea segments and tea stalk, was used to produce fermented tea beverages. In addition, the residual components of matrices and bacterial metabolites were measured, as well as the sensory quality of the beverage was evaluated. Subsequently, the determination of monosaccharides, volatile aroma profile, free amino acids, biogenic amines and organic acids, and several functional substances involving γ-aminobutyric acid (GABA), polyphenols, caffeine and L-theanine were carried out. The results revealed that glucose, fructose, mannose and xylose are principal carbon source of Lactobacillus plantarum RLL68 during the fermentation; moreover, the abundance of aromatic substances is varied dramatically and the characteristic flavors of the beverages, particularly fermentation for 48 h and 72 h, are imparted with sweet and fruity odor on the basis of initial nutty and floral odor; Meanwhile, the organoleptic qualities of fermented beverages is also enhanced. Furthermore, the levels of organic acids and GABA are elevated, while the bitter amino acids, as well as some bioactive substances including tea polyphenols and L-theanine are declined; Besides, the caffeine level almost remains constant, and quite low levels of various biogenic amines are also observed. The results of this study will provide the theoretical basis to steer and control the flavor and quality of the fermented tea beverages in the future.
Keywords: By-products of black tea manufacture, Fermentation, Lactobacillus plantarum RLL68, Characteristic metabolites, Quality control
Graphical abstract
Highlights
-
•
The dynamic variation of characteristic metabolites of the beverage was elucidated.
-
•
The characteristic flavors changed from nutty and floral to sweet and fruity.
-
•
L. plantarum fermentation bring both beneficial and adverse impacts.
1. Introduction
In recent years, owing to the major drawbacks involving allergy to dairy products, lactose intolerance, and elevated serum cholesterol implicated to the intake of fermented dairy and meat products for a large percentage of consumers, the fermented foods derived from the vegetative matrices including fruit, vegetable and cereal are attracted more attentions (Rodriguez et al., 2021). As one important food microbiology, lactic acid bacteria (LAB), has been applied in food fermentation for thousand years. The LABs, in particular, involving Lactobacillus plantarum, Lactobacillus casei, Lactobacillus rhamnosus, Lactobacillus delbrueckii, Leuconostoc mesenterica, Streptococcus thermophilus, etc., are granted with GRAS (Generally regard as safety) status and dominate the fermentation microbiota in majority of fermented foods (Dan et al., 2019; Di Cagno et al., 2017; Dongmo et al., 2017; Gupta and Abu-Ghannam, 2012). In addition, the foods are imparted with pleasant and distinct flavor, as well as a lots of functional substances including short chain fatty acids, extracellular polysaccharides, vitamins and bioactive peptides are also produced after being fermented by LAB (Gharehyakheh, 2021; Liu et al., 2011; McFeeters, 2004; Mugampoza et al., 2019). Moreover, the reduction of undesirable flavors in the foods, for instance, the elimination of beany flavor in soymilk were observed (Blagden and Gilliland, 2005); besides, some detrimental components including nitrite N-nitrosodimethylamine, and biogenic amines in fermented dry sausage, kimchi and sauerkraut, are also declined through being fermented with LABs (Chen et al., 2016; Kim et al., 2017; Rabie et al., 2011). Furthermore, the LABs including Pediococcus pentosaceus and Lactobacillus namurensis can reduce the levels of biogenic amines and cholesterol of fermented pork sausage and simultaneously produce γ-aminobutyric acid (Kantachote et al., 2016).
At present, the LAB-fermented products derived from fruits, vegetables, grains and tea have gradually become the popular beverages and foodstuff due to their appetizing and nutritious properties (Johanningsmeier and McFeeters, 2013; Mozzi et al., 2013; Vasilean et al., 2021; Zhao and Shah, 2014). Notably, the appetizing flavour of fermented food is resulted from the accumulation of substantial volatile aroma compounds and tasty substances, and majority of them are attributed to the primary and secondary metabolites of LABs (Di Cagno et al., 2017). In essence, the microbial enzymes of LABs exert action on fermentable carbohydrate, nitrogenous compounds and other food matrix, while converting the substrates into organic acids, amino acids, bioactive compounds and volatile components during the fermentation (Dan et al., 2019; Di Renzo et al., 2018; Smid and Kleerebezem, 2014). Naturally, the chemical composition of both food matrix and the metabolites responsible for the flavor in the fermentation niches are also undergone a dynamic variation process.
It is well-known that the black tea is one of the most pleasant and popular beverages, and accounts for about 78% of the total tea production in the world, due to its distinct flavor and healthy effects (Kraujalyte et al., 2016). Presently, China is the largest contributor to black tea production (Yun et al., 2021), meanwhile, plenty of by-products involving broken tea segments and tea stems are also generated in the process of black tea production every year. To reduce the waste of the by-products, it is one of the desirable strategies to produce fermented black tea beverages. In this study, a bacteriocin-producing Lactobacillus plantarum isolated from Chinese sauerkraut, was utilized to produce fermented black tea beverage, and the dynamics of core bacterial metabolites and the components of matrices were investigated. The results of this study can provide the theoretical basis for developing fermented tea beverage, as well as steering and controlling the quality of beverage.
2. Materials and methods
2.1. Preparation of fermented black tea beverage
Lactobacillus plantarum RLL 68 (Deposited in China General Microbiology Culture Collection Center) was cultivated in liquid MRS medium to exponential phase, the bacterial cells were collected through being centrifugated at 4 °C, 8000 g for 5 min and then re-suspended in fresh sterilized water and diluted to an optical density (at 600 nm, OD600) of 0.6. In addition, 10 g broken black tea segments was soaked in 2 L fresh boiling water for 5 min, the supernatant was harvested by filtering with gauze, added with 10 g glucose and sterilized at 115 °C for 15 min to get black tea soup. Finally, the addition of 1% bacterial suspension mentioned above into the black tea soup was conducted and cultivated at 37 °C for static fermentation.
2.2. Determination of viable count and pH
The aliquots of the fermented tea beverage were harvested at the indicated times including 0 h, 24 h, 48 h, 72 h and 96 h during fermentation, then serially diluted with saline (0.85%), and were plated in triplicate on MRS agar, and the number of Colony forming units (CFUs) were counted after incubation for 18 h at 37 °C. Additionally, the supernatant of black tea soup was obtained by centrifuging at 8000 g for 5 min, and the pH values were determined using pH meter (Shanghai Sanxin instrumentation, INC., Shanghai, China).
2.3. Measurement of monosaccharide
Monosaccharide composition analysis of fermented black tea beverage was performed following the method (Shi et al., 2017) described previously with slight modification. Briefly, the aliquots obtained at different time intervals were centrifugated at 15000 g for 10 min, and the supernatants were collected after being filtered with 0.22 μm microporous filter membrane; and next, the supernatants were transferred to a 50 mL volumetric flask and diluted 5 or 10 times using ultrapure water. Finally, the composition of monosaccharide in the fermented black tea beverage was determined using a high-performance anion exchange chromatographic (HPAEC) system coupled with a pulse amperometric detector (PAD) (Dionex-5500, Dionex Corporation, Sunnyvale, California, USA). NaOH solution (100 mM) was used as isocratic eluent and a Dionex Carbo-Pac PA20 analytical column (3 mm × 150 mm) was used and maintained at 30 °C. Meanwhile, the injection volume was 10 μL, and the flowrate of mobile phase was 0.5 mL/min.
2.4. Characterization of volatile profile
The volatile profile of the fermented black tea beverage was measured following the method described previously (Xu et al., 2020) with slight modification. Briefly, 5 mL of samples were obtained at different time intervals during fermentation, and placed into 12 mL headspace vials, 1 g NaCl and 10 μL of 2-octanol (10 mg/mL, internal standard) were added. The vials were placed in a thermostatic block (40 °C) with a stirrer for 20 min at 600 rpm, and the fiber of PDMS solid-phase microextraction head (Supelco, Bellefonte, PA, USA) was inserted and maintained in the sample headspace for 30 min, and then it was removed and immediately inserted into the GC/MS system (Agilent Technologies, Santa Clara, USA) for analysis. In addition, the operating conditions were as follows: DB-WAX capillary column (Agilent Technologies, 30 m × 0.25 mm ID, film thickness 0.25 μm); the injection port temperature is 250 °C, and the initial temperature of column temperature box is 40 °C, and hold this temperature for 8 min; then the temperature was raised to 150 °C at the rate of 4 °C/min, and then raised the temperature to 250 °C at the rate of 20 °C/min for 5 min. The carrier gas is helium with purity of 99.999% and the flow rate was 1 mL/min; the ion source temperature was 230 °C, and ionization mode of EI was used with ionization energy of 70 EV and interface temperature of 250 °C, the four-stage rod temperature was set as 150 °C, meanwhile, the SCAN mode was adopted and the ion fragment scanning range is set as 30–500 m/Z with solvent delay time of 2.5 min. Finally, the matching degree ≥85 was chosen. Data were expressed like relative peak area respect to internal standard. Moreover, the semi-quantitative analysis of the flavor compounds was conducted following the formula:
2.5. Determination of free amino acids
The 800 μL supernatants of liquid samples were mixed with 200 μL 10% Sulfosalicylic acid solution after being filtered with 0.45 μm microporous filter membrane, and been standing at 4 °C for 12 h; And then the supernatants were obtained by centrifugating at 15000 g for 20 min. Additionally, the supernatants were analyzed using A300 Automatic amino acid analyzer (Membrapure GmbH, Germany), after being diluted 3 times with diluent buffer (Membrapure GmbH, Germany) and filtered with 0.22 μm microporous filter membrane.
2.6. Measurement of organic acids
The composition of organic acids was determined according to the method described previously (Ozcelik et al., 2016). Briefly, the samples were analyzed using LC-20 A high performance liquid chromatography (Shimadzu, Japan) after being centrifuged at 15000 g for 5 min and filtered with 0.22 μm microporous filter membrane. The high-performance liquid chromatography (HPLC) analysis conditions were showed here: the mobile phase is sodium dihydrogen phosphate solution (0.01 M, pH 2.7); The column was reversed-phase C18 column (4.6 × 200 mm, 5 μm, Agilent Technologies Inc., USA), with the column temperature of 27 °C; and the detection wavelength was set as 210 nm using UV detector; The injection volume is 20 μL and the flow rate was 0.8 mL/min.
2.7. Measurement of biogenic amines
The biogenic amines contents in the fermented black tea beverage were measured according to a previously described method (Pineda et al., 2012) with slight modification. The samples were centrifugated at 15000 g for 10 min, and the supernatants were collected. Then, 100 μL supernatant above was taken into a 1.5 mL Ep tube and mixed with 400 μL Na2CO3–NaHCO3 buffer (0.1 M, pH 10.0), 300 μL acetone and 200 μL Dns-Cl solution (10 mg/mL). And the dansylation was conducted through vortex mixing for 30 s and then being incubated at 60 °C for 60 min. Finally, the samples were analyzed using a LC-20 A high performance liquid chromatography (Shimadzu, Japan), after the solutions being filtered with 0.22 μm filter membrane; An Agilent ZORBAX SB-C18 (4.6 um × 250 mm, 5 um) was used and the column temperature was set as 40 °C. Moreover, a binary mobile phase composed of water (A) and acetonitrile (B) were used with a flow rate of 0.6 mL/min. The following gradient program was performed: 0–6 min, 35%∼55% B; 6–16 min, 55%∼60% B; 16–24 min, 60%∼90% B; 24–35 min, 90%e∼0% B (isocratic step), and 35–40 min, 90%∼35% B. Detection was performed with the injection volume of 20 μL in the UV-range at 254 nm.
2.8. Sensory evaluation
The sensory evaluation of fermented black tea beverage for aroma, transparency, color, sweetness, bitterness, sourness and overall acceptability was conducted following the method described previously (Yyh et al., 2021). Briefly, the samples acquired during the fermentation were provided to a panel of 24 trained panelists, half male and half female, to evaluate the sensory quality of the samples; additionally, a 10-point hedonic scale ranging from 1 to 10 (1, dislike extremely to 10, like extremely) was used, as well as the total score was weighted and added according to each index mentioned above.
2.9. Determination of bioactive components
2.9.1. γ-Aminobutyric acid
The content of γ-Aminobutyric acid (GABA) in fermented black tea beverage was measured following the previously described method (Gharehyakheh, 2021). The supernatants of samples were collected after centrifugation, and passed the 0.22 μm filter membrane. Then 20 μL liquid samples were taken and mixed well with 100 μL boric acid buffer (0.4 M, pH 10.2) and 20 μL o-phthaldialdehyde (OPA) derivatizing agent (dissolving 10 mg OPA and 20 μL 2-mercaptoethanol in 2.5 mL acetonitrile), and react for 5 min under the light proof and room temperature condition. The conditions for HPLC analysis are following: mobile phase of A is chromatography grade Acetonitrile (Merck KGaA, Germany), and the solution B is CH3COONa (0.02 M, pH 7.3) + 200 μL Triethylamine. A reverse inverted C18 column (5 μm, 4.6 × 250 mm) was used and the column temperature was remained at 40 °C. Additionally, the injection volume of 20 μL and detection wavelength of 338 nm were taken. Moreover, a gradient program of HPLC analysis was used and showed following: at 0~20 min, 100% solution A and flow rate of 1.0 mL/min; at 20~24 min, 92% solution A, 1.0 mL/min; at 24~24.5 min, 60% solution A, and 1.5 mL/min; at 26.5~30 min, 0% solution A, 1.0 mL/min.
2.9.2. L-theanine
The L-theanine in the sample was determined according to the method described by Chen et al. (Cheng et al., 2019). Briefly, the supernatants of the samples were obtained through centrifugating. And the final measurement samples were acquired by passing the 0.22 μm filter membrane. Additionally, the conditions for HPLC analysis are described here: mobile phases solution A is Ultra-pure water (filtering through a 0.45 μm membrane and ultrasonic treatment for 20 min) and solution B is chromatography grade Acetonitrile (Merck KGaA, Germany). The injection volume was 20 μL, detection wavelength was 210 nm, and An Agilent inverted C18 column (5 μm, 4.6 × 250 mm), at 27 ± 0.5 °C, was used to conduct chromatographic separation with a flow rate of 0.8 mL/min. The gradient program was performed as 100% solution A at 0~10 min, 20% solution A at 12~20 min, 100% solution A at 22~40 min. L-theanine standards was purchased from Solarbio Science & Tecnology Co., Ltd (Beijing, China).
2.9.3. Tea polyphenols
The determination of tea polyphenols content in samples was conducted according to a method described by Jiang etc al (Jiang et al., 2020). Briefly, 1 mL of supernatants mentioned above were put into a 25 mL volumetric flask and 5 mL Folin–Ciocalteu reagent (10%, 10 times of diluted) and 4 mL saturated Na2CO3 were added. Then the volume was made up to 25 mL with distilled water. The absorbance was taken at 765 nm on a GENESYS 10 S spectrophotometer (Thermo Fisher Technology (China) Co., Ltd, Shanghai, China) after incubation of 60 min at room temperature. Total polyphenols were expressed as per cent polyphenols equivalent to gallic acid. Three parallel samples were determined, and the mean value of tea polyphenols content was used as the measured value of the sample.
2.9.4. Caffeine
The Caffeine of the fermented black tea beverages was measured on the basis of the previously described method with slight modification (Wang et al., 2005). Briefly, 1 mL supernatants of the samples were taken and mixed with 0.1 g MgO powder with full vortex, and were centrifugated at 15000 g for 10 min. The supernatants were collected and filtered through a 0.22 μm filter membrane to obtain the final measurement samples. The HPLC analysis was carried out with a LC-20 A HPLC system (Shimadzu, Kyoto, Japan) with an ultraviolet detector at 272 nm. An inverted C18 column (5 μm, 4.6 × 250 mm, Agilent, USA) was used with the temperature of 25 °C. Moreover, the injection volume was 20 μL, and the mobile phase solution used was methanol-water (24:76, v/v) at a flow rate of 1.0 mL/min.
2.10. Statistical analysis
Each sample will be set three parallel tests were conducted. And all the data obtained are the average values of three test measurements. In addition, the Drawing Software Origin 8.0 was used.
3. Results
3.1. The changes of viable counts and pH in fermented black tea beverage
Whether the black tea beverage can be fermented by L. plantarum, it can be reflected with the changes of viable counts and pH of the beverage during fermentation. As showed in Fig. 1, the viable counts of L. plantarum is increased gradually from 105 to 107 cfu/mL, when the black tea beverage was fermented from 0 h to 8 h. While, stepwise decrease of the viable counts was observed with the fermenting time extending, especially from 36 h to 96 h, and the viable counts were declined from 107 to 101 cfu/mL. Moreover, accompanied by the changes of viable counts, the variation trend of pH exhibited descending from 4.7 to 3.8. It is indicated that L. plantarum RLL 68 can tolerate diverse environmental stresses from the niches of black tea beverage, as well as accomplish the growth and fermentation.
Fig. 1.
The changes of L. plantarum viable counts and pH of black tea beverages, when the beverages are fermented for different times.
3.2. Monosaccharide composition analysis
Monosaccharide is the main carbohydrate resource responsible for substance and energy metabolism of L. plantarum during fermentation, in particular, providing the carbon skeleton and energy for cell growth and biosynthesis. As showed in Table 1, five monosaccharides including glucose, fructose, mannose, xylose and galactose were detected. In addition, the content of glucose was declined gradually from 6.46 mg/mL to 5.11 mg/mL and fructose was reduced from 0.41 mg/mL to 0.10 mg/mL, in addition, mannose was decreased from 0.122 mg/mL to 0.010 mg/mL in the beverages during fermentation; moreover, xylose was exhausted from 0.74 mg/mL to 0 mg/mL after being fermented for 24 h. It is implied that glucose, fructose, mannose and xylose are the principal carbohydrate resource during the fermentation in the black tea beverage.
Table 1.
The variation of monosaccharide contents in black tea beverages during fermentation.
| No | Items | Content of different monosaccharide (mg/mL) |
||||
|---|---|---|---|---|---|---|
| 0 h | 24 h | 48 h | 72 h | 96 h | ||
| 1 | Glucose | 6.46 ± 0.42 | 5.74 ± 0.36 | 5.02 ± 0.21* | 4.72 ± 0.19* | 5.11 ± 0.21* |
| 2 | Fructose | 0.41 ± 0.012 | 0.15 ± 0.013** | 0.12 ± 0.012** | 0.08 ± 0.014** | 0.10 ± 0.015** |
| 3 | Mannose | 0.122 ± 0.001 | 0.018 ± 0.002* | 0.008 ± 0.001* | 0.006 ± 0.002* | 0.010 ± 0.002* |
| 4 | Xylose | 0.74 ± 0.012 | ˗ | ˗ | ˗ | ˗ |
| 5 | Galactose | 0.010 ± 0.004 | 0.006 ± 0.001 | 0.006 ± 0.004 | 0.008 ± 0.002 | 0.007 ± 0.002 |
Note: “*“ and “**“ represents P ≤ 0.05 and P ≤ 0.01. “˗“ means that content is below the detection limit.
3.3. Changes of volatile organic compounds (VOCs) of black tea beverage during fermentation
The VOCs profile of the fermented beverage was analyzed using Solid-phase microextraction technique (SPME)-Gas chromatography/Mass spectrometry (GC/MS), and the mass spectrum differences of different groups is observed (Fig. 2A). In addition, a total of 59 VOCs (Table S1), belonging to various chemical classes involving Aldehydes, Olefins, Ketones, Esters, Alcohols, Organic acids, Phenols and other minor compounds were identified in the black tea beverage during fermentation; moreover, the volatile aromatic profile of the black tea beverage is keeping in dynamic variation, when the fermentation is ongoing (Fig. 2B). Additionally, as showed in Fig. 3C, the VOCs of unfermented black tea beverage are mainly composed of trans-beta-Ocimene (significant odor of grass leaves and flower), Phenylethyl Alcohol (odor of natural rose flowers), Nonanal (aroma of rose, citrus and strong oil), Formic acid, octyl eater, Trans-beta-Ionone (violet aroma), Benzaldehyde (bitter almond, cherry and nut aromas), 2,4-dimethyl-Benzaldehyde (almond smell), Furfural (pungent cinnamon and almond aromas), 2-Furanmethanol (almond like aroma) and so on. After being fermented for 24 h, the main composition of the volatile components in the beverage is consisted of 2-Hexen-1-ol/(Z)- (strong odor of immature fruit), Benzene acetaldehyde (sweet aroma of fruit), 3,5-dimethyl-Benzaldehyde (almond flavor), Indole (natural fragrance of flowers and fruits), Phenol, Formic acid octyl ester, beta-Pinene (turpentine aroma), beta-Myrcene (light balm aroma) and alpha-methyl-Benzene methanol (rose and lily fragrance). Moreover, fermentation for 48 h, the key volatile components are consisted of 2-methoxy-3-(2-propenyl)-Phenol, alpha-methyl-Benzene methanol (clove and jasmine aroma), 1-Hexanol (fruits aroma), beta-Ocimene, Acetic acid, Citral (lemon aroma), E1-(2-furanyl)-thanone, Acetophenone (fruits aroma), Methyl salicylate (mint aroma), Phenol, Indole (fruity aroma), 2-Undecanone (odor of rue and peach) and Octyl chloroformate. Furthermore, 72 h later, the volatile profile of the beverage is composed of Geraniol (with an odor of sweet rose), 3,7-dimethyl-1,6-Octadien-3-ol, (E)- 2-Hexen-1-ol (immature fruits aroma), Citral, 1-(2-furanyl)-Ethanone, Acetophenone, Methyl salicylate (strong holly aroma), Acetic acid, Bicyclo [2.2.1]hept-2-ene, 1,7,7-trimethyl-, beta-Pinene, Phenol, beta-Ocimene, Indole, 2-Undecanone, beta-Myrcene, 1-Hexanol, 2-Tridecanone (fruits aroma), Octyl chloroformate, Geranic acid, 2-Hexenoic acid (pineapple, hawthorn and strawberry aroma), Styrene (sweet Aroma) and trans-beta-Ocimene. Finally, as being fermented for 96 h, the volatile components are mainly comprised of (Z)-3-Nonen-1-ol (delicate fragrance), Geranic acid, Eugenol (clove-like aroma), 2,6,6-trimethyl-1-Cyclohexene-1-carboxaldehyde (sweet, grass like aroma and saffron pattern fragrance), D-Limonene,2,6,6-trimethyl-1,3-Cyclohexadiene-1-carboxaldehyde (sweet and grass like aroma), 2-Hexenoic acid, Geranic acid, Octyl chloroformate, 2-Undecanone, 2,4-dimethyl-Benzaldehyde, beta-Ocimene, Phenol, Acetic acid, Acetophenone, 1-(2-furanyl)-Ethanone, Citral and 3,7-dimethyl-1,6-Octadien-3-ol (lily and banksia rose aroma).
Fig. 2.
(A) The total ion chromatogram of GC-MS analysis of various fermented black tea beverages; (B) The heat map of volatile flavor compound profiles of samples fermented by L. plantarum RLL 68; Principal component analysis (PCA) of volatile organic compounds in the beverage from different fermentation stages, figure of PCA score scatter plot (D) and hierarchical clustering (E).
Fig. 3.
The variation in contents of free amino acids (A), organic acids (B) and biogenic amines(C) in black tea soup during fermentation.
Meanwhile, principal component analysis of the principal VOCs in the fermented tea beverages were conducted. As showed in Fig. 2 (D, E), all the fermented groups with dramatically different VOCs are clearly divided into three groups; among them, 72 h and 96 h fermentation group are classified as group one, and the principal VOCs of the two groups, consisting of.alpha.-Terpineol, Oxime-, methoxy-phenyl-, 2-Furancarboxaldehyde, 5-methyl-, Ethanone, 1-(2-furanyl)-, Benzyl nitrile, Benzaldehyde, 2,4-dimethyl-,.beta.-Myrcene, Geranic acid, Indole, etc. are observed in first and fourth quadrants; in addition, the unfermented group is classified as group two and the VOCs of this group, including Phenylethyl Alcohol, 3-Hexen-1-ol, (Z)-, Benzeneacetaldehyde, Phenol, 2,4-bis(1,1-dimethylethyl)-, 3-Hexen-1-ol, (Z)-, trans-.beta.-Ionone, Benzenemethanol,.alpha.-methyl-, and 3-Nonen-1-ol, (Z)-, are mainly concentrated in the first and second quadrant; moreover, 24 h and 48 h fermentation group as group three, and the major VOCs composing of Benzyl alcohol, Benzaldehyde, 1-Hexanol, Citral, trans-.beta.-Ocimene, etc. are distributed in third quadrant.
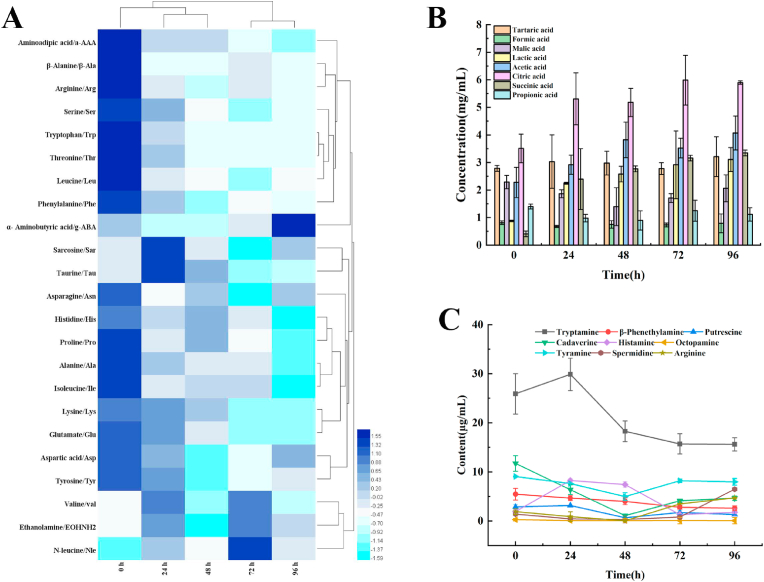
3.4. Free amino acids
The content and composition of free amino acids in black tea beverage is also in dynamic variation during the whole fermentation; moreover, a total of 24 amino acids was identified in the fermented beverage (Table S2). As showed in Fig. 3A, the contents of Alanine, Histidine, Serine, Phenylalanine, Tryptophan, Tyrosine, Leucine, Isoleucine, Arginine, Lysine, Glutamate and Threonine are declined, as well as others almost remain constant with slight fluctuation, when the black tea beverage was fermented at different stages. It is meant that part of amino acids, in the beverage, are consumed during fermentation.
3.5. Organic acids in fermented black tea beverage
The composition and content of organic acids is another one of the essential quality indexes of LAB-fermentation foods. As showed in Fig. 3B, after being fermented, 8 organic acids were identified in the black tea beverage, including tartaric acid, formic acid, malic acid, lactic acid, acetic acid, citric acid, succinic acid and propionic acid. Among them, the levels of lactic acid, acetic acid, citric acid and succinic acid rise during the fermentation, while the contents of both formic acid and malic acid are decreased slightly; Whereas, the contents of tartaric acid and propionic acid are varied irregularly. It is implied that other organic acids except for lactic acid are also produced, when the black tea beverage is fermented by L. plantarum RLL68.
3.6. Biogenic amines
Biogenic amines, as the deamination products of amino acids in foods, are associated to fermentative microbes, which is a vital safety index of fermented foods. As showed in Fig.3 C, 9 biogenic amines including Tryptamine, Phenethylamine, Putrescine, Cadaverine, histamine, Octopamine, Tyramine, Spermidine and Arginine were identified, nevertheless their levels are considerable low. In addition, the content of Tryptamine was being reduced from 25.88 μg/mL to 15.59 μg/mL gradually, during fermentation, while other biogenic amines remain low-levels (below 10.0 μg/mL) with slight fluctuation. It is indicated that low level of biogenic amine is produced and accumulated in the beverage, after fermentation by L. plantarum RLL 68.
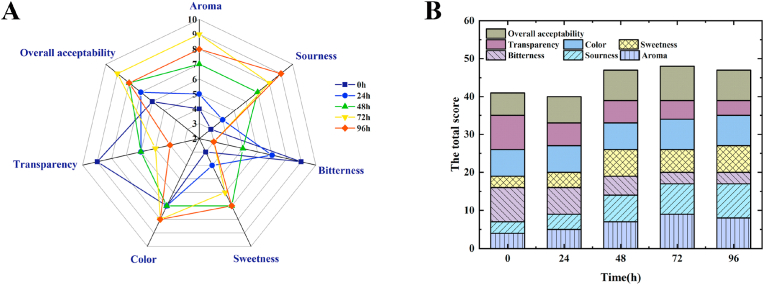
3.7. Sensory evaluation of fermented tea beverage
The sensory evaluations of fermented black tea beverages were conducted, and each sample was scored independently, according to the 10-point hedonic scale for color, shape, flavor, texture, taste, and overall acceptability. The higher the score in the spider diagram, the more intense is the attributor of black tea beverages. As showed in Fig. 4A, unfermented beverage displays a more intense bitterness and transparency, but lower intensity of aroma, sourness, colour and sweetness. While, the sensory evaluation scores of sourness, aroma, sweetness and overall acceptability are increased; meanwhile, the scores of bitterness and transparency reduce, when the beverages were fermented by L. plantarum RLL68. Moreover, the total sensory evaluation scores were obtained, and it is demonstrated that the total score of unfermented tea beverage is similar to 24 h-fermented group; furthermore, the scores of the beverages fermented for both 48 h and 72 h display a rising trend, and then the score of 96 h-fermented group declines slightly (Fig. 4B).
Fig. 4.
(A) Spider web diagram of the sensory evaluation of the black tea beverages fermented for different times and (B) total scores of fermentation beverages sensory evaluation.
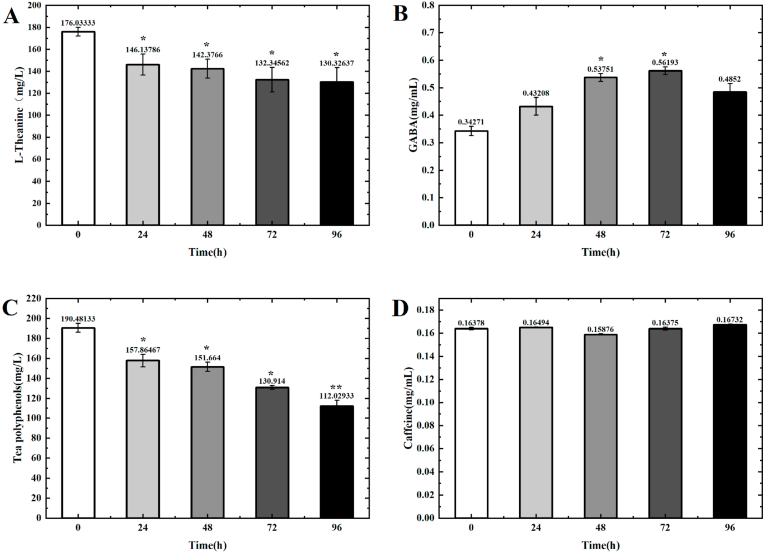
3.8. Bioactive components
It is well-known that many of functionally bioactive components are contained in black tea beverage, thus, the impacts of L. plantarum RLL68 fermentation on the bioactive components was investigated in further. As showed in Fig. 5A, the level of GABA in the beverage increases from 0.34 mg/mL to 0.66 mg/mL, when it is fermented from 0 h to 72 h, and then reduces to 0.58 mg/mL after fermentation for 96 h. In addition, the level of L-Theanine reduces from 176.03 mg/L to 132.35 mg/L (Fig. 5B), meanwhile, the content of tea polyphenols declines from 190.48 mg/L to 112.03 mg/L (Fig. 5C), when the beverage is fermented from 0 h to 96 h. Moreover, the caffeine remains almost constant during the whole fermentation, with a slight variation of 0.158 mg/mL to 0.167 mg/mL (Fig. 5D). Hance, the results indicated that L. plantarum RLL68 fermentation can enhance γ-aminobutyric acid level, and reduce the contents of L-Theanine and tea polyphenols, moreover, displays little impact on the content of caffeine.
Fig. 5.
The contents of bioactive components in black tea soup, including γ- Amino acid butyric acid (A), L-theanine, (B)polyphenols (C) and caffeine (D) after being fermented for different times. “*“ and “**“ represents P ≤ 0.05 and P ≤ 0.01. “˗“ means that content is below the detection limit.
4. Discussion
Lactic acid fermentation is commonly considered as a feasible, valuable and sustainable technology and process to improve the nutritious and sensory properties of fermented foods, and also to extend its shelf-life, due to producing postbiotics, flavor components, bacteriocin, organic acids and hydrogen peroxide (Han et al., 2021; Moradi et al., 2021; Rodriguez et al., 2021). Thus, large amount of LAB fermented foods are emerging under the deriving of market demands (Liu et al., 2011). In addition, elucidation of the metabolic pathways implicated to conversing original substrates to flavor and bioactive components during the LAB fermentation is of considerable current research interests (Ganzle, 2015).
It is well-known that the aroma is one of the crucial factors responsible for the quality of tea or/and tea beverages. To date, around 600 volatile aromatic compounds have been identified in tea leaves or tea beverages (Kraujalyte et al., 2016). Besides that, the compounds closely related to taste in the black tea matrices, including monosaccharide, organic acid, free amino acid, polyphenol, caffeine and other phytochemicals, are also the primary contributors to flavor of tea beverages. Moreover, the compounds mentioned above are also the substrates of L. plantarum RLL68 when the fermentation occurs, and the aromatic and palatable properties of the black tea beverages are enhanced through producing new derived compounds. The odor activity value (OAV) is an important factor to reflect the contribution of individual VOC to the overall aroma of the fermented beverages. It was considered that the VOC with OAV threshold beyond 1 (OAV>1) is defined as significant contributor to the overall aroma of beverage. In this study, the aroma profile of fermented beverages is characterized by the presence of 59 VOCs; and among them, a total of 16 key volatile flavor compounds with OAV>1 in different fermented beverages are found, as showed in Supplemental Table S3. Notably, the unfermented black tea beverage is conferred nutty and floral flavor due to the principal odorants composing of Phenylethyl Alcohol, Geraniol, Benzyl alcohol, Benzaldehyde, Benzeneacetaldehyde and Furfural, etc. In comparison, the fermented beverages are added with sweet and fruity flavor owning to the major aroma contributors composing of 3-Hexen-1-ol/(Z)-, Benzeneacetaldehyde, Indole,.beta.-Myrcene, trans-.beta.-Ocimene, Phenylethyl Alcohol, Geraniol, etc., especially after fermentation for 48 h and 72 h with L. plantarum RLL68. Additionally, several gustatory substances including monosaccharide, amino acids, and tea polyphenols are decreased, meanwhile, the levels of organic acids including lactic acid, acetic acid, citric acid and succinic acid are also elevated during the fermentation. Correspondingly, the organoleptic qualities of fermented beverages are also enhanced, involving in reduction of bitterness, the improvement of sweetness, sourness and overall acceptability. Hance, it is proposed that, when the beverages are fermented by L. plantarum RLL68, the bitter amino acids involving Histidine, Phenylalanine, Tryptophan, Tyrosine, Leucine, Isoleucine, Arginine and Lysine (Wang et al., 2014) as well as tea polyphenols are decreased, which may be the reason responsible for bitterness reduction. In contrast, the total phenolics and flavonoids content are elevated, when the black tea beverage is fermented with a yeast Starmerella davenportii strain Do18 (Tu et al., 2020). The flavonoids and polymeric phenolic compounds of the black tea can be degraded by the enzymes (e.g. β-glucosidase) released from yeast and other microorganisms, and form smaller molecules (e.g. phenolic acids), resulting in increase of total phenols (Marcel Cardoso de Noronha et al.). It is supposed that L. plantarum RLL68 may be deficient of these enzymes, thus, the reduction of tea polyphenols is observed. Besides, the sweet and fruity flavors and organic acids accumulation may be the vital contributors to boost sweetness, sourness and overall acceptability of fermented tea beverage. According to previous study, it was also observed that the ethyl esters with fruity notes arose in the fermented teas using monoculture of Saccharomyces boulardii CNCM I-745 or co-culture of Saccharomyces boulardii CNCM I-745 and Lactiplantibacillus plantarum 299 V (Wang et al., 2022).
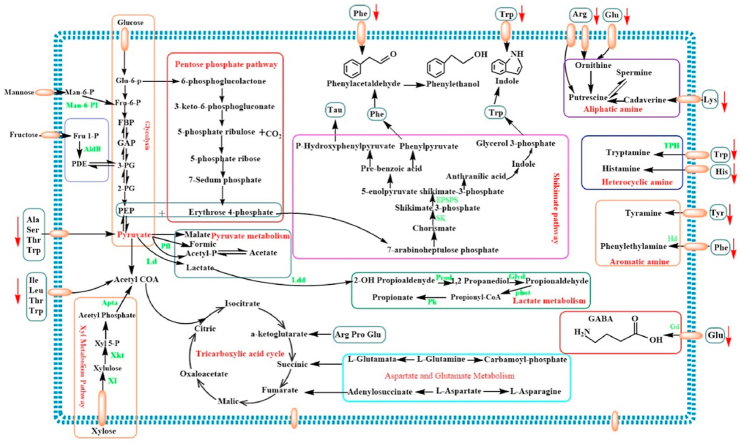
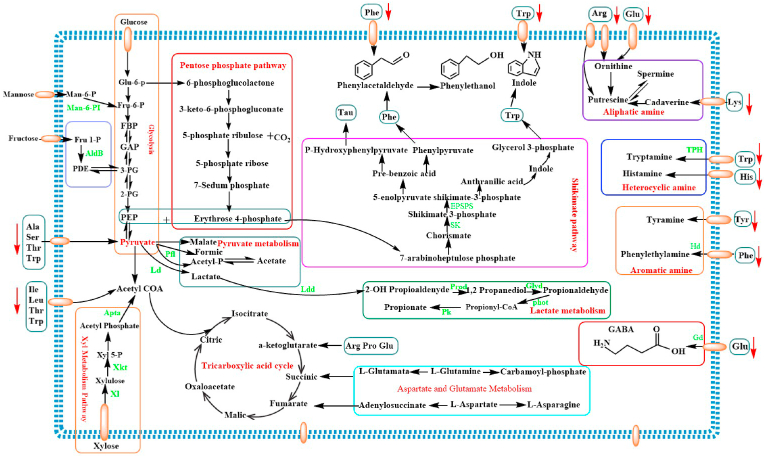
During fermentation by LAB, the production of specific flavor compounds depends closely on the main constituents of food matrices delivering precursors for the conversion to various alcohols, aldehydes, acids, esters and ketones; and the flavor formation is a complex process involving generation of precursor molecules and the conversion of the precursor molecules into the final flavor metabolites (Smid and Kleerebezem, 2014). Just as we known that both the fermentable constituents of the food matrices and the metabolites are undergoing a dynamic variation resulting from the metabolic pathways of LAB or/and the decomposition of original substrates. Moreover, majority of the VOCs formation routes derived from LABs rely on the presence of functional metabolic pathways (Smid and Kleerebezem, 2014); The metabolic pathways involving in biosynthesizing various metabolites responsible for the unique flavor during the L. plantarum RLL 68 fermentation is summarized and demonstrated in Fig. 6, according to the determinations of constituents in black tea beverages. Initially, the glucose, fructose, xylose and mannose, as the vital fermentable monosaccharide, participate in the carbohydrate central metabolic process of L. plantarum. Among them, Glucose can convert to Phosphoenolpyruvate (PEP) and pyruvate through glycolysis pathway or/and Erythrose 4-phosphate by participating in pentose phosphate pathway; additionally, the precursors of aromatic amino acids formation, including Erythrose 4-phosphate and PEP, convert to 7-arabinoheptulose phosphate, and then Trp, Tyr and Phe are formed through the shikimate pathway using 7-arabinoheptulose phosphate as the substrate. Subsequently, the phenylacetaldehyde can be derived from Phe by means of phenylacetaldehyde synthase, and then convert to phenyl ethanol through reduction reaction; Trp, in addition, convert to indole. Moreover, other monosaccharides are generally fermented by L. plantarum RLL 68 through the following pathways: Fructose → Fru-1-P → PDE → 3-PG → PEP → Pyruvate; Xylose→Xyluose→Xyl-5-P→Acetyl Phosphate→ Acetyl CoA, then convert to mevalonate-5-phosphate or citrate; Mannose→Man-6-P→Fru-6-P→Pyruvate. Thereby, the pyruvate is the key branching point of homolactic metabolism of hexoses, and it can be reduced to lactate or convert to Acetyl CoA or citrate. Furthermore, the amino acids are also the potential substrates for pyruvate and lactate formation. For instance, Ala, Ser, Thr and Trp can be deaminated to produce ammonia and pyruvate; besides that, the amino acids of Ile, Leu, Thr and Trp can convert to Acetyl CoA. And then, the pyruvate and Acetyl CoA enter the pyruvate metabolism pathway and tricarboxylic acid cycle to produce the organic acids of lactic acid, propionic acid, malic acid, formic acid, acetic acid and citric acid. Meanwhile, Asp and Glu can convert to a-ketoglutarate, succinic and fumarate through transamination or/and metabolism of aspartate and glutamate. Moreover, Glu also can convert to GABA through decarboxylation of glutamate decarboxylase. Furthermore, some amino acids including Arg, Lys, His, Tyr, Phe and Glu are also converted to biological amines of putrescine, spermine, histamine, tyramine, phenylethylamine through decarboxylation, although the low-level of biogenic amines were detected in black tea beverages fermented using L. plantarum RLL 68. Notably, biogenic amines (BAs) are commonly produced in majority of fermented foods, and is closely associated to the health of human beings, for instance, appropriate BAs can promote growth and metabolism and eliminate free radicals, whereas excessive intake of BAs can lead to toxicity to humans involving headache, hypotension and palpitation (Zhang et al., 2019). According to previous studies, it is considered that the metabolic pathway of by-products of some organisms including Lactobacillus especially, is the main contributor for bioamines biosynthesis (Barbieri et al., 2019). In this study, low levels of bioamines in the fermented beverage may be attribute to the low concentration of amino acids and diverse environmental stresses from the niches of black tea beverage.
Fig. 6.
The metabolic pathways of different metabolites in black tea beverages fermented by L. plantarum. Man-6-PI: Mannose-6-P Isomerase, AldB: Aldolase B, Pfl: Pyruvate formate-lyase, Ld: Lactic dehydrogenase, Ldd: Lactaldehyde dehydrogenase, SK: shikimate kinase, EPSPS: synthetase, Prod: propanediol dehydrogenase, Pd: pyruvate dehydrogenase, phot: phosphotransacylase, Pk: propionate kinase, Xi: Xylose isomers, Xkt: Xylose ketose thritol, Apta: Acetyl phosphate transfer alcohol, Gd: glutamic acid decarboxylase, Hd: histidine decarboxylase, TPH: tryptophan hydroxylase.
5. Conclusion
In this study, the analysis of the dynamic variation of the characteristic metabolites, during black tea beverage fermentation by Lactobacillus plantarum RLL68, was conducted. It was concluded that L. plantarum fermentation derives both beneficial and adverse impacts on the metabolic products and the quality of black tea beverages, including the improvement of characteristic flavor and sensory acceptability in further, and reduction of total nutrients and some bioactive components. It is suggested that the result of this study can provide the theoretical basis to steer and control the characteristic metabolites formation and quality of the fermented tea beverages during LAB fermentation.
CRediT authorship contribution statement
Ruili Li: Methodology, Investigation, Writing – original draft, Writing – review & editing, Validation. Weibo Luo: Methodology, Investigation, Writing – original draft, Writing – review & editing, Validation. Yifeng Liu: Investigation, Writing – original draft. Chi Chen: Data curation, Software, Visualization. Shunxian Chen: Investigation, Conceptualization. Jie Yang: and. Peifen Wu: Investigation, Writing – original draft. Xucong Lv: Software, Supervision, Conceptualization. Zhibin Liu: Supervision, Conceptualization. Li Ni: Supervision, Validation, Project administration. Jinzhi Han: Writing – original draft, Writing – review & editing, Supervision, Project administration.
Declaration of competing interest
The authors declare that they have no conflict of interest. Informed consent was obtained from all individual participants included in the study.
Acknowledgments
This study is supported by the Fujian Provincial Natural Science Foundation (No. 2020J01492), and the Open Project Program of the Key Laboratory of Brewing Molecular Engineering of China Light Industry.
Handling editor: Dr. Siyun Wang
Footnotes
Supplementary data to this article can be found online at doi:10.1016/j.crfs.2022.07.014.
Appendix A. Supplementary data
References
- Barbieri F., Montanari C., Gardini F., Tabanelli G. Biogenic amine production by lactic acid Bacteria: a review. Foods. 2019;8(1):17. doi: 10.3390/foods8010017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blagden T.D., Gilliland S.E. Reduction of levels of volatile components associated with the "Beany" flavor in soymilk by Lactobacilli and Streptococci. J. Food Sci. 2005;70(3):186–189. [Google Scholar]
- Chen X., Li J.P., Zhou T., Li J.C., Yang J.N., Chen W.H., Xiong Y.L.L. Two efficient nitrite-reducing Lactobacillus strains isolated from traditional fermented pork (Nanx Wudl) as competitive starter cultures for Chinese fermented dry sausage. Meat Sci. 2016;121:302–309. doi: 10.1016/j.meatsci.2016.06.007. [DOI] [PubMed] [Google Scholar]
- Cheng S., Fu X., Liao Y., Xu X., Zeng L., Tang J., Li J., Lai J., Yang Z. Differential accumulation of specialized metabolite L-theanine in green and albino-induced yellow tea (Camellia sinensis) leaves. Food Chem. 2019;276:93–100. doi: 10.1016/j.foodchem.2018.10.010. [DOI] [PubMed] [Google Scholar]
- Dan T., Chen H.Y., Li T., Tian J.L., Ren W.Y., Zhang H.P., Sun T.S. Influence of Lactobacillus plantarum P-8 on fermented milk flavor and storage stability. Front. Microbiol. 2019;9:1–14. doi: 10.3389/fmicb.2018.03133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Cagno R., Filannino P., Gobbetti M. Lactic acid fermentation drives the optimal volatile flavor-aroma profile of pomegranate juice. Int. J. Food Microbiol. 2017;248:56–62. doi: 10.1016/j.ijfoodmicro.2017.02.014. [DOI] [PubMed] [Google Scholar]
- Di Renzo T., Reale A., Boscaino F., Messia M.C. Flavoring production in kamut (R), quinoa and wheat doughs fermented by Lactobacillus paracasei, Lactobacillus plantarum, and Lactobacillus brevis: a SPME-GC/MS study. Front. Microbiol. 2018;9:429–432. doi: 10.3389/fmicb.2018.00429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dongmo S.N., Sacher B., Kollmannsberger H., Becker T. Key volatile aroma compounds of lactic acid fermented malt based beverages - impact of lactic acid bacteria strains. Food Chem. 2017;229:565–573. doi: 10.1016/j.foodchem.2017.02.091. [DOI] [PubMed] [Google Scholar]
- Ganzle M.G. Lactic metabolism revisited: metabolism of lactic acid bacteria in food fermentations and food spoilage. Curr. Opin. Food Sci. 2015;2:106–117. [Google Scholar]
- Gharehyakheh S. Gamma aminobutyric acid (GABA) production using Lactobacillus sp. Makhdzir Naser-1 (GQ451633) in the cherry-kefir beverage. J. Food Process. Preserv. 2021;45(6):1–12. [Google Scholar]
- Gupta S., Abu-Ghannam N. Probiotic fermentation of plant Based products: possibilities and opportunities. Crit Rev Food Sci. 2012;52(1–3):183–199. doi: 10.1080/10408398.2010.499779. [DOI] [PubMed] [Google Scholar]
- Han J., Meng X., Shen H., Luo W., Yao S., Yang J., Zhu Q., Tian Y., Wang S. Purification, molecular characterization of Lactocin 63 produced by Lactobacillus coryniformis FZU63 and its antimicrobial mode of action against Shewanella putrefaciens. Appl. Microbiol. Biotechnol. 2021;105(18):6921–6930. doi: 10.1007/s00253-021-11503-8. [DOI] [PubMed] [Google Scholar]
- Jiang H., Xu W., Chen Q. Determination of tea polyphenols in green tea by homemade color sensitive sensor combined with multivariate analysis. Food Chem. 2020;319 doi: 10.1016/j.foodchem.2020.126584. [DOI] [PubMed] [Google Scholar]
- Johanningsmeier S.D., McFeeters R.F. Metabolism of lactic acid in fermented cucumbers by Lactobacillus buchneri and related species, potential spoilage organisms in reduced salt fermentations. Food Microbiol. 2013;35(2):129–135. doi: 10.1016/j.fm.2013.03.004. [DOI] [PubMed] [Google Scholar]
- Kantachote D., Ratanaburee A., Sukhoom A., Sumpradit T., Asavaroungpipop N. Use of gamma-aminobutyric acid producing lactic acid bacteria as starters to reduce biogenic amines and cholesterol in Thai fermented pork sausage (Nham) and their distribution during fermentation. LWT--Food Sci. Technol. 2016;70:171–177. [Google Scholar]
- Kim S.H., Kang K.H., Kim S.H., Lee S., Lee S.H., Ha E.S., Sung N.J., Kim J.G., Chung M.J. Lactic acid bacteria directly degrade N-nitrosodimethylamine and increase the nitrite-scavenging ability in kimchi. Food Control. 2017;71:101–109. [Google Scholar]
- Kraujalyte V., Pelvan E., Alasalvar C. Volatile compounds and sensory characteristics of various instant teas produced from black tea. Food Chem. 2016;194:864–872. doi: 10.1016/j.foodchem.2015.08.051. [DOI] [PubMed] [Google Scholar]
- Liu S.N., Han Y., Zhou Z.J. Lactic acid bacteria in traditional fermented Chinese foods. Food Res. Int. 2011;44(3):643–651. [Google Scholar]
- Marcel C.N., Rodrigo R.C., Carolina T.S., D’A M.A.V.C., Luciana A., Vinícius G.M., Jose I.R.J., Monique R.E., Luiz C.C., Mariana S.L.F., Frederico A.R.B. Black tea kombucha: physicochemical, microbiological and comprehensive phenolic profile changes during fermentation, and antimalarial activity. Food Chem. 2022;384 doi: 10.1016/j.foodchem.2022.132515. [DOI] [PubMed] [Google Scholar]
- McFeeters R.F. Fermentation microorganisms and flavor changes in fermented foods. J. Food Sci. 2004;69(1):35–37. [Google Scholar]
- Moradi M., Molaei R., Guimaraes J.T. A review on preparation and chemical analysis of postbiotics from lactic acid bacteria. Enzyme microb tech. 2021;143 doi: 10.1016/j.enzmictec.2020.109722. [DOI] [PubMed] [Google Scholar]
- Mozzi F., Ortiz M.E., Bleckwedel J., De Vuyst L., Pescuma M. Metabolomics as a tool for the comprehensive understanding of fermented and functional foods with lactic acid bacteria. Food Res. Int. 2013;54(1):1152–1161. [Google Scholar]
- Mugampoza D., Gkatzionis K., Linforth R.S.T., Dodd C.E.R. Acid production, growth kinetics and aroma profiles of Lactobacillus flora from Stilton cheese. Food Chem. 2019;287:222–231. doi: 10.1016/j.foodchem.2019.02.082. [DOI] [PubMed] [Google Scholar]
- Ozcelik S., Kuley E., Ozogul F. Formation of lactic, acetic, succinic, propionic, formic and butyric acid by lactic acid bacteria. Lwt-Food Sci Tech. 2016;73:536–542. [Google Scholar]
- Pineda A., Carrasco J., Pena-Farfal C., Henriquez-Aedo K., Aranda M. Preliminary evaluation of biogenic amines content in Chilean young varietal wines by HPLC. Food Control. 2012;23(1):251–257. [Google Scholar]
- Rabie M.A., Siliha H., el-Saidy S., el-Badawy A.A., Malcata F.X. Reduced biogenic amine contents in sauerkraut via addition of selected lactic acid bacteria. Food Chem. 2011;129(4):1778–1782. [Google Scholar]
- Rodriguez L.G.R., Gasga V.M.Z., Pescuma M., Van Nieuwenhove C., Mozzi F., Burgos J.A.S. Fruits and fruit by-products as sources of bioactive compounds. Benefits and trends of lactic acid fermentation in the development of novel fruit-based functional beverages. Food Res. Int. 2021;140 doi: 10.1016/j.foodres.2020.109854. [DOI] [PubMed] [Google Scholar]
- Shi X.D., Nie S.P., Yin J.Y., Que Z.Q., Zhang L.J., Huang X.J. Polysaccharide from leaf skin of Aloe barbadensis Miller: Part I. Extraction, fractionation, physicochemical properties and structural characterization. Food Hydrocolloids. 2017;73:176–183. [Google Scholar]
- Smid E.J., Kleerebezem M. Production of aroma compounds in lactic fermentations. Doyle M.P., Klaenhammer T.R., editors. Annu. Rev. Food Sci. Technol. 2014;5:313–326. doi: 10.1146/annurev-food-030713-092339. [DOI] [PubMed] [Google Scholar]
- Tu C., Hu W., Tang S., Meng L., Dong M. Isolation and identification of Starmerella davenportii strain Do18 and its application in black tea beverage fermentation. Food Sci. Hum. Wellness. 2020;9(4):355–362. [Google Scholar]
- Vasilean I., Aprodu I., Garnai M., Munteanu V., Patrascu L. Preliminary investigations into the use of amylases and lactic acid Bacteria to obtain fermented vegetable products. Foods. 2021;10(7) doi: 10.3390/foods10071530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P., Mao J., Meng X., Li X., Liu Y., Feng H. Changes in flavour characteristics and bacterial diversity during traditional fermentation of Chinese rice wines from Shaoxing region. Food Control. 2014;44:58–63. [Google Scholar]
- Wang R., Sun J., Lassabliere B., Yu B., Liu S.Q. Green tea fermentation with Saccharomyces boulardii CNCM I-745 and Lactiplantibacillus plantarum 299V. LWT--Food Sci. Technol. 2022:157. doi: 10.1016/j.crfs.2022.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X.G., Hu S.X., Wan X.C., Pan C.Y. Effect of microbial fermentation on caffeine content of tea leaves. J. Agric. Food Chem. 2005;53(18):7238–7242. doi: 10.1021/jf050495h. [DOI] [PubMed] [Google Scholar]
- Xu X., Zhou S.D., McClements D.J., Huang L., Meng L., Xia X.D., Dong M.S. Multistarter fermentation of glutinous rice with Fu brick tea: effects on microbial, chemical, and volatile compositions. Food Chem. 2020;309:785–790. doi: 10.1016/j.foodchem.2019.125790. [DOI] [PubMed] [Google Scholar]
- Yun J., Cui C.J., Zhang S.H., Zhu J.J., Peng C.Y., Cai H.M., Yang X.G., Hou R.Y. Use of headspace GC/MS combined with chemometric analysis to identify the geographic origins of black tea. Food Chem. 2021;360:130–135. doi: 10.1016/j.foodchem.2021.130033. [DOI] [PubMed] [Google Scholar]
- Yyh A., Xzja B., Jjy A., Yhc A., Dml A., Mhl A. Effect of different lactic acid bacteria on nitrite degradation, volatile profiles, and sensory quality in Chinese traditional paocai. LWT--Food Sci. Technol. 2021;147:597–602. [Google Scholar]
- Zhao D.Y., Shah N.P. Effect of tea extract on lactic acid bacterial growth, their cell surface characteristics and isoflavone bioconversion during soymilk fermentation. Food Res. Int. 2014;62:877–885. [Google Scholar]
- Zhang Y.J., Zhang Y., Zhou Y., Li G.H., Yang W.Z., et al. A review of pretreatment and analytical methods of biogenic amines in food and biological samples since 2010. J. Chromatogr. A. 2019;1605 doi: 10.1016/j.chroma.2019.07.015. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.