Abstract
Background and Objective
An ever-growing body of evidence supports the impact of cytokine modulation on the patient’s phenotypic drug response. The aim of this systematic review was to analyze the clinical studies that assessed the pharmacokinetics of victim drugs of this drug–disease interaction in the presence of different scenarios of cytokine modulation in comparison with baseline conditions.
Methods
We conducted a systematic review by searching the PubMed-MEDLINE database from inception until February 2022 to retrieve prospective and/or retrospective observational studies, population pharmacokinetic studies, phase I studies, and/or case series/reports that investigated the impact of cytokine modulation on the pharmacokinetic behavior of victim drugs. Only studies providing quantitative pharmacokinetic data of victim drugs by comparing normal status versus clinical conditions with documented cytokine modulation or by assessing the influence of anti-inflammatory biological agents on metabolism and/or transport of victim drugs were included.
Results
Overall, 26 studies were included. Rheumatoid arthritis (6/26; 23.1%) and sepsis (5/26; 19.2%) were the two most frequently investigated pro-inflammatory clinical scenarios. The victim drug most frequently assessed was midazolam (14/26; 53.8%; as a probe for cytochrome P450 [CYP] 3A4). Cytokine modulation showed a moderate inhibitory effect on CYP3A4-mediated metabolism (area under the concentration–time curve increase and/or clearance decrease between 1.98-fold and 2.59-fold) and a weak-to-moderate inhibitory effect on CYP1A2, CYP2C9, and CYP2C19-mediated metabolism (in the area under the concentration–time curve increase or clearance decrease between 1.29-fold and 1.97-fold). Anti-interleukin-6 agents showed remarkable activity in counteracting downregulation of CYP3A4-mediated activity (increase in the area under the concentration–time curve between 1.75-fold and 2.56-fold).
Conclusions
Cytokine modulation may cause moderate or weak-to-moderate downregulation of metabolism/transport of victim drugs, and this may theoretically have relevant clinical consequences.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40262-022-01173-8.
Key Points
| Inflammation represents an under-appreciated variable that may significantly impact the patient’s phenotypic drug response. The impact of inflammation-induced downregulation of CYP450 (CYP)-mediated drug metabolism and or of drug transporter-mediated uptake was assessed for a limited number of victim drugs. |
| Pro-inflammatory cytokines showed a moderate inhibitory effect on CYP3A4, and a weak-to-moderate inhibitory effect on CYP2C9-mediated, CYP2C19-mediated, and CYP1A2-mediated drug metabolism. A positive relationship between the magnitude of the pro-inflammatory cytokine levels over time and the degree of inhibition of CYP-mediated metabolism or transporter-mediated uptake was documented. |
| Administration of anti-interleukin-6 biological agents showed remarkable reverting activity towards the downregulation of different CYP isoenzymes (especially of CYP3A4) in patients with chronic inflammatory conditions. |
| A ‘patient-centered’ strategy based not only on the pharmacokinetic features of the victim drugs, but also on the patient’s underlying conditions (i.e., specific inflammatory conditions, magnitude of inflammatory biomarkers over time, eventual administration of anti-inflammatory biological agents) should be carefully implemented for pursuing appropriate decision making on dose adjustment. |
Introduction
Cytokine release syndrome (CRS) and a cytokine storm are two life-threatening systemic inflammatory syndromes characterized by immune-cell hyperactivation and by elevated levels of circulating cytokines, which can be triggered by different causes [1]. Although CRS has recently gained the spotlight thanks to severe forms of coronavirus disease 2019, several other clinical conditions may be responsible. Among these, sepsis/septic shock, polytrauma, acute pancreatitis, massive burns, chimeric antigen receptor T-cell therapy, autoimmune disorders, hematological malignancies, bone marrow transplantation engraftment, and immunotherapy are some of the most relevant [1]. Commonly, CRS may result in immune dysregulation responsible for constitutional symptoms, systemic inflammation, multiorgan dysfunction, and multiorgan failure leading potentially to death [1]. Cytokine release syndrome may have a variable time onset, severity, and duration depending on the underlying causes and the administered treatments [2]. Regardless of which the initial trigger is, late-stage clinical manifestations are usually very similar.
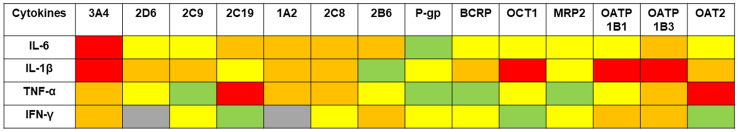
Notably, systemic inflammation and cytokine modulation represent an under-appreciated variable that may significantly impact the patient’s phenotypic drug response [3–5]. Cytokine modulation may promote an increase in pro-inflammatory cytokine production and release (i.e., interleukin (IL)-6, IL-1, tumor necrosis factor-α [TNF-α], or interferon-γ [IFN-γ]), which may, on the one hand, trigger acute inflammation and, on the other hand, maintain chronic inflammatory responses [6]. Preclinical evidence demonstrated that pro-inflammatory cytokines may significantly downregulate the expression and the activity of different isoforms of cytochrome P450 (CYP450) [Fig. 1], such as CYP3A4, CYP1A2, and CYP2C19, and also those of some drug transporters [7]. Drugs that behave as substrates of these CYP isoenzymes and/or these transporters may become victims of clinically relevant drug–disease interactions triggered by cytokine modulation, and consequently undergo major modifications of their pharmacokinetics.
Fig. 1.
Impact of pro-inflammatory cytokines on different cytochrome P450 (CYP) isoenzymes and transporters retrieved from preclinical studies (adapted with permission from [7]). Red box: reduction in activity more than five-fold; orange box: reduction in activity 2-fold to 5-fold; yellow box: reduction in activity 1.25-fold to 2-fold; green box: no significant reduction in activity; gray box: no data. BCRP breast cancer resistance protein, IFN interferon, IL interleukin, MRP2 multidrug resistance-associated protein 2, OATP organic anion transporting polypeptide, OAT2 organic anion transporter 2, OCT1 organic cation transporter 1, P-gp P-glycoprotein, TNF tumor necrosis factor
Several clinical studies carried out both in the adult and in the pediatric settings documented that systemic inflammation may affect the metabolism of several victim drugs. Psychotropic drugs, sedative agents, immunosuppressants, antifungal agents, and antiviral agents were the most investigated victim drugs [3, 8–11]. Additionally, it has also been postulated that the downregulation of CYP activity caused by systemic inflammation could be reverted by some anti-inflammatory biological agents to baseline pre-inflammatory levels [11, 12].
The aim of this systematic review was to analyze the clinical studies that assessed the pharmacokinetics of victim drugs of this drug–disease interaction in the presence of different scenarios of cytokine modulation in comparison with baseline conditions. Whenever feasible, practical considerations for the management of victim drugs in these clinical scenarios have been provided.
Methods
This is a systematic review that investigated the available evidence of the influence that CRS and/or increases in pro-inflammatory biomarker levels may have on the pharmacokinetic (PK) behavior of victim drugs under different drug–disease scenarios. The systematic review was conducted according to the Preferred Reporting Items for Systematic Review and Meta-Analyses (PRISMA) guidelines [13]. A PICOS framework was developed in order to design the literature search according to the following criteria: participants: adult and children subjects who received victim drug probes for different CYP isoenzymes and/or transporters; intervention: acute or chronic inflammatory conditions; comparator: baseline clinical conditions before the onset of inflammation or after the administration of biological agents inducing or suppressing inflammation; outcome: variations in PK parameters of selected victim drugs.
A literature search was conducted on PubMed-MEDLINE (search performed on 10 February, 2022) in order to retrieve all the prospective or retrospective observational studies, the population PK studies, the phase I studies, and/or the case series/reports that investigated this drug–disease interaction. The following search string was specifically created: (“cytochrome” OR “cytochrome p450” OR “transporter”) AND (“cytokine storm” OR “cytokine” OR “interleukin” OR “interleukin-6”).
Inclusion criteria were: (1) clinical studies that assessed quantitative data of the main PK parameters (i.e., peak concentration [Cmax], average or trough steady-state concentrations [Cavg,ss or Cmin,ss] according to continuous or intermittent infusion, clearance [CL] or apparent oral clearance [CL/F], area under the concentration–time curve [AUC], or terminal half-life [t1/2]) of victim drugs comparatively during normal status versus clinical conditions with documented cytokine modulation or increased levels of pro-inflammatory biomarkers and (2) clinical studies that assessed the influence of anti-inflammatory biological agents on the metabolism of victim drugs. Exclusion criteria were: (1) clinical studies that did not assess quantitative data of the main PK parameters of victim drugs comparatively during normal status versus clinical conditions with cytokine modulation and/or those that assessed only trough concentrations (Cmin) and/or normalized Cmin; (2) pre-clinical studies in the same settings; and (3) conference abstracts and/or studies published in languages other than English.
For each included study, the following data were retrieved: first author, study year, study design and population, number of included patients, victim drug (with dosage) probes for different CYP isoenzymes and/or transporters, inflammatory condition, pro-inflammatory biomarker levels, proposed or documented underlying mechanism for drug–disease interaction, PK parameters (i.e., Cmax, Css, CL, AUC, and t1/2), and pharmacodynamic effect. Studies were categorized into three different subgroups according to study design: (1) studies assessing the influence of cytokine modulation during acute or chronic inflammatory conditions on the pharmacokinetics of drugs that behave as substrates of CYP; (2) studies assessing the influence of anti-inflammatory biological agents during acute or chronic inflammatory conditions on the pharmacokinetics of drugs that behave as substrates of CYP; and (3) studies assessing the influence of cytokine modulation during acute or chronic inflammatory conditions on the pharmacokinetics of drugs that behave as substrates of transporters.
The influence of cytokine modulation on the pharmacokinetics of the victim drug was quantified by calculating the percentage changes of mean or median AUC, Cmax, or CL values observed during acute or chronic inflammatory conditions compared with those observed under a baseline status. Strong, moderate, or weak effects of the drug–disease interaction were defined as the occurrence of a more than five-fold increase in the plasma AUC values or a ≥ 80% decrease in oral CL, of a two-fold to five-fold increase in the plasma AUC values or a 50–80% decrease in oral CL, and of a 1.25-fold to 2-fold increase in the plasma AUC values or a ≤ 50% decrease in oral CL, respectively [14].
Results
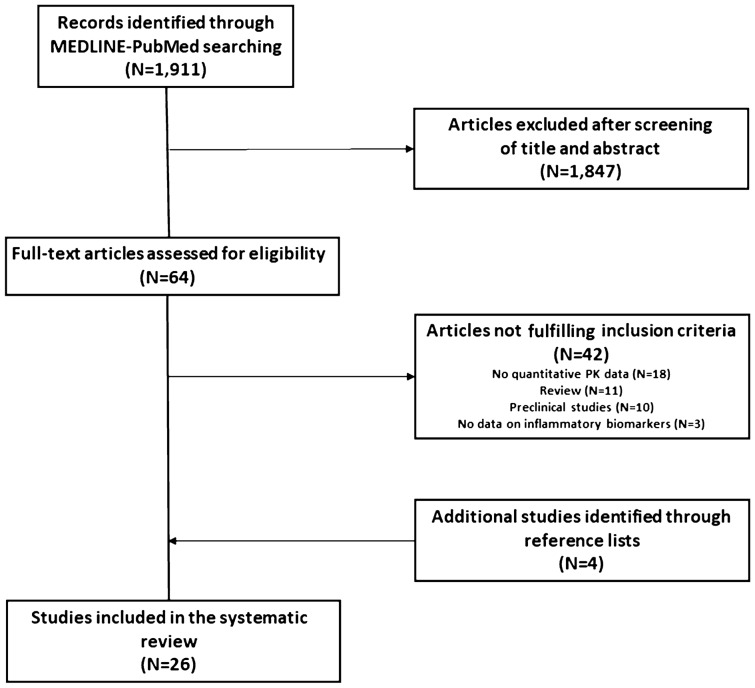
The search strategy identified 1911 articles of potential interest. After initial screening of titles and abstracts, 1847 studies were excluded. Overall, 64 full-text articles were assessed for eligibility, and 42 of these were excluded according to the following criteria: lack of quantitative PK data (18 studies), reviews (11 studies), preclinical studies (ten studies), and a lack of data on inflammatory biomarkers (three studies). After manual screening of reference lists of included studies, four more studies were identified, thus the final review included 26 original studies assessing the PK behavior of victim drugs [15–40] (Fig. 2).
Fig. 2.
Study selection process. PK pharmacokinetic
Among these 26 studies, 15 assessed the influence of cytokine modulation on the pharmacokinetics of drugs that behave as substrates of the CYP system during acute or chronic inflammatory conditions [15–29]; nine assessed the impact of anti-inflammatory biological agents in counteracting the inhibitory effect of pro-inflammatory cytokines on the pharmacokinetics of drugs that behave as substrates of CYP during acute or chronic inflammatory conditions [30–38]; two assessed the impact of the influence of cytokine modulation on the pharmacokinetics of drugs that behave as substrates of transporters during acute or chronic inflammatory conditions [39, 40]. Details of clinical characteristics and PK parameters retrieved in these three subgroups of studies are provided in Tables 1, 2, and 3 and Table 1 of the Electronic Supplementary Material (ESM), respectively.
Table 1.
Studies of acute or chronic inflammatory conditions assessing the effects of cytokine modulation on the pharmacokinetics of CYP substrates
| Study and year of publication | Study population and design (N of included patients) | Drug and dosage | Inflammatory conditions and biomarker levels | Mechanism | Pharmacokinetic effect | Pharmacodynamic effect | Clinical relevance |
|---|---|---|---|---|---|---|---|
| Chen et al., 1994 [15] | Patients undergoing an allogenic bone marrow transplantation (6) | Cyclosporine (probe for 3A4) |
Transplant engraftment Assessment of serum IL-6, TNF-α, CRP, and α1-glycoprotein acid Mean IL-6 serum levels (day 1 post-transplant vs engraftment) 10.7 ± 3.9 units/mL vs 212.2 ± 56.8 units/mL Mean CRP serum levels (day 1 post-transplant vs engraftment) 20.3 ± 2.3 mg/L vs 179.2 ± 12.5 mg/L |
Downregulation of CYP metabolism mediated by increase in IL-6 levels | A 3.6-fold increase was found in mean cyclosporine Css at transplant engraftment compared with day 2 after transplant | Not assessed | A correlation between the times of the peaks in IL-6 and cyclosporine levels (p = 0.03) was found |
| Shedlofsky et al., 1994 [16] | Single-sequence phase I study: healthy male volunteers (12) | Antipyrine 250 mg (probe for several CYP isoenzymes), hexobarbital 500 mg (probe for 1A2), theophylline 150 mg (probe for 1A2) before and after a single dose of LPS (day 1) or two doses of LPS (days 1–2) |
Sepsis Assessment of IL-6, TNF-α, CRP, and α1-glycoprotein acid Mean serum TNF-α (baseline vs day 1): 4 ± 1 pg/mL vs. 53 ± 34 pg/mL Mean serum IL-6 (baseline vs day 1) 3 ± 1 pg/mL vs 31 ± 36 pg/mL Mean serum CRP (baseline vs day 1): 0 ± 0 mg/dL vs 2.7 ± 0.5 mg/dL |
Downregulation of CYP metabolism mediated by increase in pro-inflammatory cytokines levels |
Antipyrine CL (baseline vs day 1): 2.33 ± 0.43 L/h vs 2.06 ± 0.40 L/h (12% decrease) Hexobarbital CL (baseline vs day 1): 19.5 ± 5.8 L/h vs 17.9 ± 4.2 L/h (8% decrease) Theophylline CL (baseline vs day 1) 3.30 ± 0.33 L/h vs 3.08 ± 0.60 L/h (7% decrease) Antipyrine CL (baseline vs day 2) 35% decrease (95% CI 18–48) Hexobarbital CL (baseline vs day 2) 27% decrease (95% CI 14–34) Theophylline CL (baseline vs day 2): 22% decrease (95% CI 12–32) |
Not assessed | LPS-induced inflammation was associated with significant downregulation of CYP-mediated drug metabolism, occurring several h after initiation of the inflammatory response. The extent of downregulation correlated with the intensity of inflammatory response (increase in IL-6 and TNF-α levels) |
| Shedlofsky et al., 1997 [17] | Single-sequence phase I study: healthy female volunteers (7) | Antipyrine 250 mg (probe for several CYP isoenzymes), hexobarbital 500 mg (probe for 1A2), theophylline 150 mg (probe for 1A2) before and after a single dose of LPS (day 1) or two doses of LPS (days 1–2) |
Sepsis Assessment of IL-6, TNF-α, CRP, and α1-glycoprotein acid |
Downregulation of CYP metabolism mediated by increase in pro-inflammatory cytokine levels |
Mean CL decrease for antipyrine: 31% (95% CI 21–41) Mean CL decrease for hexobarbital: 20% (95% CI 10–31) Mean CL decrease for theophylline: 20% (95% CI 10–30) |
Not assessed | No sex difference in downregulation of CYP-mediated drug metabolism caused by LPS-induced inflammation |
| Gorski et al., 2000 [18] | Double-blind cross-over phase I PK study: healthy volunteers (12) | Tolbutamide (probe for 2C9), caffeine (probe for 1A2), dextromethorphan (probe for 2D6 and 3A4), midazolam (probe for 3A4) | Administration of IL-10 8 mcg/kg/day for 6 days | Downregulation of CYP metabolism mediated by increase in IL-10 levels |
Mean caffeine CL (placebo vs IL-10 group): 5.2 ± 1.6 vs 5.5 ± 1.9 Mean unbound tolbutamide CL (placebo v. IL-10 group): 22.7 ± 9.5 vs 23.2 ± 11.4 Mean urinary dextromethorphan/dextrorphan metabolic ratio (placebo vs IL-10 group): 0.020 ± 0.028 vs 0.020 ± 0.049 Mean midazolam CL (placebo vs IL-10 group): 29.2 ± 6.5 vs 25.4 ± 5.7 |
Not assessed | Administration of IL-10 did not alter CYP1A2, CYP2C9, and CYP2D6 activities. Conversely, CYP3A-mediated biotransformation was reduced by administration of IL-10, although to a modest extent with no clinically significant effect |
| Carcillo et al., 2003 [19] | Case-control PK study: consecutive children with sepsis (51) vs critically ill children without sepsis (6) | Antipyrine 18 mg/kg (probe for 1A2, 2B6, 2C8, 2C9, 2C18, 3A4) |
Sepsis Assessment of serum IL-6 levels |
Downregulation of CYP metabolism mediated by increase in IL-6 levels |
Mean t1/2 10.4 ± 3.6 h (control) vs 30.9 ± 27.4 h (sepsis) Mean CL 0.74 ± 0.31 mL/min/kg (control) vs 0.38 ± 0.28 mL/min/kg (sepsis) |
Not assessed | Significant correlation between antipyrine elimination half-life and serum IL-6 levels |
| Moises et al., 2008 [20] | Case-control phase I study: pregnant women without documented diseases (10) vs pregnant women with gestational diabetes (6) | Lidocaine 200 mg (probe for 3A4 and partially for 1A2) | Gestational diabetes | Downregulation of CYP3A4 metabolism mediated by increase in pro-inflammatory cytokine levels |
Median (IQR) AUC0–∞ values (control group vs gestational diabetes group): 256.01 (238.87–308.07) mcg/mL/min vs. 455.95 (333.48–661.13) mcg/mL/min Median (IQR) CL values (control group vs gestational diabetes group): 781.43 (650.67–839.08) mL/min vs. 438.86 (321.89–605.85) mL/min |
Not assessed | The apparent clearance of lidocaine was reduced in diabetic patients compared with healthy women, suggesting that gestational diabetes could inhibit the CYP1A2/CYP3A4 isoforms responsible for the metabolism of this drug and its metabolite |
| Veringa et al., 2016 [24] | Prospective observational study: hospitalized patients treated with voriconazole (34) | Voriconazole (probe for 2C9/3A4) 4 mg/kg twice daily |
Hematological malignancies, solid organ transplantation, or chronic pulmonary diseases in patients requiring antifungal prophylaxis or treatment Assessment of CRP |
Downregulation of CYP metabolism mediated by increase in pro-inflammatory cytokine levels | Voriconazole CL decreased by 0.99299N, and voriconazole Cmin increased by 1.005321 N, where N is the difference in CRP units (mg/L) | Not assessed | The metabolism of voriconazole is decreased during inflammation due to CYP inhibition with a potential increase in serum levels and the need for dosage adjustment |
| Vet et al., 2016 [25] | Population PK study: critically ill children (83) | Midazolam (probe for 3A4) 100 mcg/kg LD + 100 mcg/kg/h CI |
Sepsis/inflammation in critically ill patients Assessment of CRP, IL-6, TNF-α, IL-1a, IL-1b, IL-2, IL-4, IL-10, IFN-γ, IL-8, MCP-1, MIP-1a, MIP-1b, FGF-β, GCS-F, GMCS-F |
Downregulation of CYP3A4 mediated by increase in pro-inflammatory biomarker levels |
Midazolam CL decreased by 65.4% when serum CRP levels increased from 10 to 300 mg/L Serum CRP and IL-6 levels are covariate impacting on midazolam CL |
Not assessed | Inflammation strongly reduces midazolam CL due to downregulation of 3A4, possibly leading to midazolam over-exposure and associated toxicity |
| Gravel et al., 2019 [26] | Case-control PK study: healthy volunteers (35) vs patients with type 2 diabetes (38) |
CYP probe drug cocktail: caffeine (1A2), bupropion (2B6), tolbutamide (2C9), omeprazole (2C19), dextromethorphan (2D6), midazolam (3A4), or chlorzoxazone alone (2E1) |
Type 2 diabetes Assessment of serum IFN-γ, TNF-α, IL-1, IL-6 levels |
Activity of 2B6 ↓ 45% in T2D Activity of 2C19 ↓ 46% in T2D Activity of 3A4 ↓ 38% in T2D Activity of 1A2 ↑ 23% in T2D No difference in activity for 2C9, 2D6, and 2E1 |
Plasma C4h caffeine ↑ 23% in T2D Urine Ae0–8h bupropion ↓ 45% in T2D Plasma AUC0–24 tolbutamide ↑ 26% in T2D Plasma AUC0–8 omeprazole ↑ 46% in T2D Plasma AUC0–8 dextromethorphan No difference in T2D Urine Ae0–8 h chlorzoxazone ↓ 9% in T2D Plasma AUC0–24 midazolam ↑ 38% in T2D |
Not assessed | IFN-γ and TNF-α serum levels are independent variables associated with intersubject variability in CYP1A2, 2C19, and 2B6 activity in patients with T2D compared with healthy volunteers |
| Trousil et al., 2019 [27] | Case-control PK study: healthy volunteers (21) vs patients with stage III/IV epithelial ovarian cancer (22) | Caffeine 100 mg (probe for 1A2), chlorzoxazone 250 mg (2E1), dextromethorphan 30 mg (2D6), omeprazole 40 mg (2C19/3A4) |
Advanced-stage ovarian cancer Assessment of CRP, IL-6, IL-8, TNF-α, IL-1β Median inflammatory biomarker levels (volunteers vs patients): CRP: 17 v. 182 mg/L IL-6: 12.89 vs 37.33 pg/mL IL-8: 25.62 vs 71.61 pg/mL TNF-α: 27.71 vs 45.42 pg/mL |
Downregulation/upregulation of CYP metabolism mediated by increase in pro-inflammatory cytokine levels |
3.28-fold increase in chlorzoxazone CL in patients with cancer vs healthy volunteers 42% decrease in omeprazole CL in patients with cancer vs healthy volunteers Significant association between pro-inflammatory cytokines/CRP and increased 2E1 activity or 3A4 decreased activity |
Not assessed | The presence of tumor-associated inflammation differentially affected CYP4 isoenzyme activity, leading to increase metabolism for 2E1 substrates and decrease metabolism for 3A4 substrates |
| Cojutti et al., 2020 [28] | Population PK study in patients with COVID-19 (30) vs patients with HIV (25) | Darunavir (3A4) |
COVID-19 Assessment of serum IL-6 levels Median (IQR) serum IL-6 levels (patients with COVID-19 vs patients with HIV): 31 (10–114.75) pg/mL vs 2 (2–2.75) pg/mL |
Downregulation of CYP3A4 activity mediated by increase in IL-6 levels |
Mean darunavir CL/F 4.1 mL/h (patients with COVID-19) vs 10.3 mL/h (patients with HIV; p < 0.001) Median darunavir AUC 161,387.0 (patients with COVID-19) vs 75,727.0 ng·h/mL (patients with HIV; p < 0.001) |
Not assessed |
IL-6 was the only clinical variable highly significantly associated with darunavir CL/F in a multivariate regression analysis (p < 0.001). CART analysis found that an IL-6 level of 18 pg/mL may adequately split the SARS-CoV-2 population in patients with low vs high darunavir CL/F |
| Lenoir et al., 2020 [29] | Prospective open-label observational study: patients undergoing elective hip surgery (30) | Caffeine 50 mg (probe for 1A2), bupropion 20 mg (2B6), flurbiprofen 10 mg (2C9), omeprazole 10 mg (2C19), dextromethorphan 10 mg (2D6), and midazolam 1 mg (3A4), measured before (day 0) and after surgery (day 1 and 3) and at discharge |
Elective hip surgery Assessment of IL-6, CRP, TNF-α, IL-1β, and IFN-γ |
Downregulation of CYP activity mediated by increase in pro-inflammatory cytokine levels |
MR 1A2: ↓ 53.2% (day 1 post-surgery) MR 2C19: ↓ 57.5% (day 3 post-surgery) MR 3A4: ↓ 61.3% (day 3 post-surgery) MR 2B6: ↑ 120.1% (day 1 post-surgery) MR 2C9: ↑ 79.1% (day 1 post-surgery) MR 2D6: ↓ 50.0% (no statistical significance) |
Not assessed |
Surgery and acute inflammation have a major impact on the activity of six major CYPs in an isoform-specific manner of different magnitude and velocity |
Ae amount excretion, AUC area under the concentration–time curve, C concentration, CI continuous infusion, Cmax peak concentration, CL clearance, COVID-19 coronavirus disease 2019, CRP C-reactive protein, Css steady-state concentration, CYP cytochrome P450, FGF fibroblast growth factor, GCS-F granulocyte colony-stimulating factor, GMCS-F granulocyte-macrophage colony-stimulating factor, HIV human immunodeficiency virus, IFN interferon, IL interleukin, IQR interquartile range, LD loading dose, LPS lipopolysaccharide, MCP monocyte chemotactic protein, MIP macrophage inflammatory protein, MR metabolic ratio, PK pharmacokinetic, SARS-CoV-2 severe acute respiratory syndrome coronavirus 2, T2D type 2 diabetes, TNF tumor necrosis factor, t1/2 half-life, ↑ increased, ↓ decreased
Table 2.
Physiologically based pharmacokinetic models investigating the effects of cytokine modulation on the pharmacokinetics of CYP substrates
| Study, year of publication | Study population and design (N of included patients) | Drug and dosage | Inflammatory conditions and biomarker levels | Mechanism | Pharmacokinetic effect | Pharmacodynamic effect | Clinical relevance |
|---|---|---|---|---|---|---|---|
| Machavaram et al., 2013 [21] | Physiologically based pharmacokinetic model: patients with rheumatoid arthritis (12) or requiring a bone marrow transplant (5) | Simvastatin 40 mg (probe for 3A4) and cyclosporine 1.5 mg/kg (3A4) |
Rheumatoid arthritis or bone marrow transplant Assessment of serum IL-6 levels |
Downregulation of CYP3A4 metabolism mediated by increase in IL-6 levels |
Elevated simvastatin AUC in virtual patients with rheumatoid arthritis, following 100 pg/mL of IL-6 was comparable to observed clinical data (59% vs 58%) In virtual patients requiring a bone marrow transplant, 500 pg/ml of IL-6 resulted in increase in cyclosporine AUC that was in good agreement with the observed data (45% vs 39%) |
Not assessed | The application of physiologically based PK models is suitable for the prediction of drug–disease interactions via suppression of CYP by elevated levels of IL-6 in patients with rheumatoid arthritis or requiring a bone marrow transplant |
| Xu et al., 2015 [23] | Physiologically based pharmacokinetic model: virtual patients with non-Hodgkin lymphoma receiving blinatumomab | CYP probe drug cocktail: simvastatin (3A4), midazolam (3A4), theophylline (1A2), caffeine (1A2), warfarin (2C9) |
Non-Hodgkin lymphoma Assessment of IL-6 and IL-10 serum levels |
Downregulation of CYP metabolism mediated by transient increase in pro-inflammatory cytokines due to blinatumomab administration |
Maximal suppression of transient IL-6 on CYP isoenzymes: 3A4: 28%, occurred at 48 h, lasted for 7 days 1A2: 9%, occurred at 48 h, lasted for 7 days 2C9: 17%, occurred at 70 h, lasted for 9 days Mean AUC (95% CI) ratio (transient IL-6 increase vs baseline) Simvastatin 1.9 (1.8–2.0) Midazolam 1.7 (1.6–1.8) Theophylline 1.1 (1.0–1.1) Caffeine 1.2 (1.1–1.3) Warfarin 1.2 (1.0–1.4) Mean Cmax (95% CI) ratio (transient IL-6 increase vs baseline) Simvastatin 1.7 (1.6–1.8) Midazolam 1.2 (1.1–1.3) Theophylline 1.0 (1.0–1.0) Caffeine 1.0 (1.0–1.1) Warfarin 1.0 (1.0–1.0) |
Not assessed | The magnitude of the suppressive effect of transient cytokine elevation on hepatic CYP enzyme activities is <30% for up to a week. Changes in exposures to substrates of CYP3A4, CYP1A2, and CYP2C9 are expected to be lower than two-fold and the magnitude of CYP suppression is highly dependent on the duration of cytokine elevation |
| Machavaram et al., 2019 [22] | Physiologically based pharmacokinetic model: virtual patients with neuromyelitis optica or neuromyelitis optica spectrum disorders in North European Caucasian, Japanese, or Chinese populations | CYP probe drug cocktail: caffeine 100 mg (1A2), warfarin 5 mg (2C9), omeprazole 20 mg (2C19), dextromethorphan 30 mg (2D6), midazolam 0.03 mg/kg (3A4), or simvastatin 40 mg (3A4) |
Neuromyelitis optica or neuromyelitis optica spectrum disorders Assessment of IL-6 serum levels according to a previous study performed in patients with rheumatoid arthritis |
Downregulation of CYP metabolism mediated by increase in IL-6 levels |
Mean fold change in AUC (steady-state IL-6 levels from 10 to 100 pg/mL): Simvastatin AUC ↑ 2.36-fold Midazolam AUC ↑ 2.08-fold Omeprazole AUC ↑ 1.97-fold Dextromethorphan AUC ↑ 1.37-fold Warfarin AUC ↑ 1.29-fold Caffeine AUC ↑ 1.07-fold |
Not assessed |
Increasing levels of IL-6 led to predicted increases in exposure to the CYP probe substrates tested, with the exception of caffeine (CYP1A2), being the CYP3A4 substrates the most sensitive to IL-6-mediated suppression There were no notable ethnic differences between the North European Caucasian, Japanese, and Chinese populations in the sensitivity of the change in pharmacokinetics of CYP probe substrates following IL-6-mediated suppression |
AUC area under concentration–time curve, C concentration, CI confidence interval, Cmax peak concentration, CYP cytochrome P450, IL interleukin, PK pharmacokinetic, ↑ increased
Table 3.
Studies assessing the influence of anti-inflammatory biological agents administered during acute or chronic inflammatory conditions on the pharmacokinetics of drugs that behave as substrates of CYP
| Study and year of publication | Study population and design (N of included patients) | Drug and dosage | Inflammatory conditions and biomarker levels | Mechanism | Pharmacokinetic effect | Pharmacodynamic effect | Clinical relevance |
|---|---|---|---|---|---|---|---|
| Schmitt et al., 2011 [30] | Multicenter, open-label, randomized, single-sequence disease–drug interaction study: patients with rheumatoid arthritis (12) | Simvastatin 40 mg administered as a single dose on days 1, 15, and 43 (probe for 3A4), and a single infusion of tocilizumab 10 mg/kg on day 8 |
Rheumatoid arthritis and assessment of IL-6 and CRP Mean IL-6 serum levels (baseline vs day 15): 50 pg/mL vs 256 pg/mL Mean CRP serum levels (baseline vs day 15): 5 mg/dL vs 0.3 mg/dL |
Reversal of CYP downregulation after tocilizumab administration due to IL-6 inhibition |
Mean effect ratio (day 15/day 1): Simvastatin AUC0–∞: 43% (90% CI 34–55) Simvastatin Cmax: 43% (90% CI 33–55) Mean effect ratio (day 43/day 1): Simvastatin AUC0–∞: 61% (90% CI 47–78) Simvastatin Cmax: 60% (90% CI 46–76) |
Not assessed |
Tocilizumab approximately halves the simvastatin AUC by almost doubling simvastatin clearance (a CYP3A4-dependent process) as compared with baseline values in patients with rheumatoid arthritis. This finding could be clinically remarkable also for other drugs metabolized by CYP3A4 |
| Zhuang et al., 2015 [31] | Open-label, multicenter, phase I study: patients with rheumatoid arthritis (12) | Midazolam 0.03 mg/kg (probe for 3A4), warfarin 10 mg (2C9), omeprazole 20 mg (2C19), caffeine 100 mg (1A2) 1 week before, and 1,3, and 6 week after a single dose of 300 mg sirukumab |
Rheumatoid arthritis treated with sirukumab Assessment of serum CRP levels (baseline vs day 14/28/49, after sirukumab administration at day 8): 25.3 mg/L vs 1.8/0.5/0.7 mg/L |
Reversal of CYP downregulation after sirukumab administration due to IL-6 inhibition |
Geometric mean Cmax ratio (1/3/6 weeks after sirukumab administration vs baseline): Midazolam 0.77 (0.56–1.05); 0.69 (0.50–0.94); 0.66 (0.48–0.90) Omeprazole 0.61 (0.42–0.89); 0.62 (0.43–0.91); 0.70 (0.48–1.02) Warfarin 1.00 (0.89–1.15); 1.00 (0.87–1.15); 0.99 (0.86–1.14) Caffeine 1.04 (0.75–1.43); 1.10 (0.80–1.52); 1.05 (0.76–1.45) Geometric mean AUC ratio (1/3/6 weeks after sirukumab administration vs baseline): Midazolam 0.70 (0.51–0.96); 0.65 (0.47–0.89); 0.67 (0.49–0.92) Omeprazole 0.55 (0.32–0.96); 0.59 (0.34–1.02); 0.63 (0.36–1.09) Warfarin 0.82 (0.73–0.92); 0.82 (0.73–0.92); 0.81 (0.72–0.91) Caffeine 1.20 (0.75–1.91); 1.34 (0.84–2.15); 1.28 (0.80–2.04) |
Not assessed |
Sirukumab may reverse IL-6-mediated suppression of CYP3A, CYP2C9, and CYP2C19 activities in patients with active inflammation associated with rheumatoid arthritis |
| Tran et al., 2016 [32] | Two-sequences phase I study: patients with multiple sclerosis (20) | Caffeine 200 mg (probe for 1A2), warfarin 10 mg (2C9), omeprazole 40 mg (2C19), dextromethorphan 30 mg (2D6), midazolam 5 mg (3A4) before and after administration of daclizumab 150 mg every 4 weeks | Multiple sclerosis | Potential reversal downregulation of CYP mediated by IL-2 blockade |
Geometric mean AUC ratio (probe substrate with daclizumab/probe substrate alone): Caffeine 1.03 (90% CI 0.93–1.14) Midazolam 1.01 (90% CI 0.89–1.15) Warfarin 1.00 (90% CI 0.95–1.06) Omeprazole 1.00 (90% CI 0.88–1.13) Dextromethorphan 1.01 (90% CI 0.76–1.34) |
Not assessed | In patients with multiple sclerosis, daclizumab has no effect on CYP activity |
| Jiang et al., 2016 [33] | Physiologically based pharmacokinetic model: patients with rheumatoid arthritis (12) | Midazolam 0.03 mg/kg (probe for 3A4), warfarin 10 mg (2C9), caffeine 100 mg (1A2), and omeprazole 20 mg (2C19) administered one week before (day 1) and three weeks after (day 29) sirukumab 300 mg single dose | Rheumatoid arthritis and assessment of IL-6 | Reversal of CYP downregulation after sirukumab administration due to IL-6 inhibition |
Geometric mean AUC ratio (day 29/day 1): Midazolam 0.65 (90% CI 0.47–0.89) Omeprazole 0.59 (90% CI 0.34–1.02) Warfarin 0.82 (90% CI 0.73–0.92) Caffeine 1.34 (90% CI 0.84–2.15) |
Not assessed | Sirukumab may reverse CYP downregulation affecting CYP3A4, CYP2C9, and CYP2C19, resulting in a decrease of substrate exposure (range 20–40%) compared with inflammatory conditions |
| Lee et al., 2017 [34] | Open-label, single-sequence, nonrandomized, phase I study: patients with rheumatoid arthritis (19) | Simvastatin 40 mg (probe for 3A4) administered one day before and seven days after sarilumab 200 mg |
Rheumatoid arthritis and assessment of IL-6 and CRP Mean IL-6 serum levels (baseline vs day 8): 47.5 pg/mL vs 219.9 pg/mL Mean CRP serum levels (baseline vs day 8): 22.1 mg/L vs 1.9 mg/L |
Reversal of CYP3A4 downregulation after sarilumab administration due to IL-6 inhibition |
Mean effect ratio (day 8/baseline): Simvastatin AUC0–∞: 54.7% (90% CI 47.2–63.3) Simvastatin Cmax: 54.1% (90% CI 42.2–69.4) |
Not assessed |
Sarilumab treatment resulted in a reduction in exposure of simvastatin, consistent with reversal of IL-6- mediated CYP3A4 suppression in patients with active rheumatoid arthritis |
| Davis et al., 2018 [35] | Open-label, multicenter study, single-sequence phase I PK study: patients with moderate-to-severe atopic dermatitis (14) | Midazolam 2 mg (probe for 3A4), omeprazole 20 mg (2C19), warfarin 10 mg (2C9), caffeine 100 mg (1A2), and metoprolol 100 mg (2D6) administered before (day 1) and after dupilumab 300 mg/week (day 36) |
Moderate-to-severe atopic dermatitis and assessment of CCL-17 and LDH Mean CCL-17 serum levels (baseline vs day 35): 8060 ± 17,200 pg/mL vs 667 ± 402 pg/mL |
Potential downregulation of CYP isoenzymes due to increased IL-4/IL-13 levels and reversal of activity after dupilumab administration |
Geometric mean AUC ratio (day 36/day 1): Midazolam 0.98 (90% CI 0.87–1.09) Omeprazole 1.00 (90% CI 0.88–1.12) Warfarin 0.90 (90% CI 0.83–0.98) Caffeine 1.12 (90% CI 0.87–1.45) Metoprolol 1.29 (90% CI 1.10–1.51) |
Not assessed |
No drug–disease interaction was identified in patients with type 2 inflammation. Blockade of IL-4/IL-13 signaling in patients with type 2 inflammation does not appear to significantly affect CYP enzyme activities; the use of dupilumab in patients with atopic dermatitis is unlikely to influence the pharmacokinetics of CYP substrates |
| Khalilieh et al., 2018 [36] | Open-label, fixed-sequence, two-period trial: patients with moderate-to-severe psoriasis (20) | Midazolam 2 mg (probe for 3A4), warfarin 10 mg (2C9), caffeine 200 mg (1A2), omeprazole 40 mg (2C19), and dextromethorphan 30 mg (2D6) administered at day 1 (period 1). In period 2, probe cocktail was administered on day 57 after tildrakizumab 200 mg on day 1 and 29 | Moderate-to-severe psoriasis and assessment of CRP and IL-6 | Potential reversal of CYP downregulation after tildrakizumab administration due to IL-23 inhibition |
Geometric mean AUC ratio (probe + tildrakizumab vs probe alone) Midazolam 1.11 (90% CI 0.94–1.32) Omeprazole 0.96 (90% CI 0.77–1.19) Warfarin 1.07 (90% CI 0.98–1.17) Dextromethorphan 1.20 (90% CI 1.00–1.45) Caffeine 1.14 (90% CI 1.01–1.28) Geometric mean Cmax ratio (probe + tildrakizumab vs probe alone) Midazolam 1.06 (90% CI 0.86–1.29) Omeprazole 0.99 (90% CI 0.85–1.15) Warfarin 0.99 (90% CI 0.95–1.03) Dextromethorphan 1.17 (90% CI 0.96–1.43) Caffeine 0.96 (90% CI 0.88–1.05) |
Not assessed |
Tildrakizumab had no clinically relevant effect on the pharmacokinetics of any of the probe substrates tested, possibly related to the lower degree of inflammation in psoriasis compared with other pro-inflammatory diseases. There were no clinically relevant changes in IL-6 or CRP before and after tildrakizumab administration |
| Bruin et al., 2019 [37] | Open-label, multicenter study, single-sequence phase I PK study: patients with moderate-to-severe plaque psoriasis (24) | Midazolam 5 mg (3A4) administered before (day 0) and after secukinumab 300 mg/week (day 8 and 36) | Moderate-to-severe psoriasis and assessment of CRP, TNF-α, IL-6, and BD-2 | Potential reversal of CYP3A4 downregulation after secukinumab administration due to IL-17A inhibition |
Midazolam AUC0–∞ day8/baseline 0.99 (90% CI 0.88–1.12) Midazolam AUC0–∞ day36/baseline 0.97 (90% CI 0.85–1.09) |
Not assessed |
Secukinumab can be used in the treatment of psoriasis without significant PK interactions with drugs metabolized by CYP3A4. Because of only slightly increased cytokine levels, such as IL-17A and IL-6, in patients with psoriasis, a reduction in cytokine levels to levels observed in healthy subjects will have no impact on any of the CYP activities |
| Zhu et al., 2020 [38] | Open-label, multicenter, phase I study: patients with moderate-to-severe psoriasis (14) | Midazolam (probe for 3A4), warfarin (2C9), omeprazole (2C19), dextromethorphan (2D6), and caffeine (1A2) before (day 1) and after (day 15 and 36) guselkumab 200 mg administration | Moderate-to-severe psoriasis | Potential reversal of CYP downregulation after guselkumab administration due to IL-23 inhibition |
Geometric mean Cmax ratio (15/36 day vs day 1): Midazolam 1.11 (0.75–1.65); 1.14 (0.77–1.69) Warfarin 1.07 (0.90–1.27); 0.90 (0.74–1.11) Omeprazole 0.96 (0.72–1.28); 0.96 (0.67–1.36) Dextromethorphan 1.06 (0.46–2.43); 1.33 (0.55–3.18) Caffeine 1.07 (0.94–1.22); 1.06 (0.89–1.26) Geometric mean AUC ratio (15/36 day vs day 1): Midazolam 1.01 (0.70–1.45); 1.04 (0.75–1.44) Warfarin 1.12 (0.90–1.40); 1.05 (0.82–1.36) Omeprazole 0.96 (0.61–1.52); 1.19 (0.75–1.90) Dextromethorphan 1.13 (0.56–2.26); 1.24 (0.46– 3.31) Caffeine 1.00 (0.77–1.31); 1.02 (0.77–1.35) |
Not assessed |
No significant drug interactions between guselkumab and substrates of various CYP enzymes are reported. Dose adjustment for concomitant CYP substrates in patients treated with guselkumab does not seem to be necessary |
Ae amount excretion, AUC area under the concentration–time curve, C concentration, CCL-17 chemokine 17, CI confidence interval, Cmax peak concentrations, CRP C-reactive protein, CYP cytochrome P450, IL interleukin, LDH lactate dehydrogenase, PK pharmacokinetic, T2D type 2 diabetes, TNF-α tumor necrosis factor-α
The total number of subjects in the included studies was 596 [479 (80.4%) were patients and 117 (19.6%) were healthy volunteers]. Rheumatoid arthritis (6/26; 23.1%) and sepsis (5/26; 19.2%) were the two most frequent pro-inflammatory clinical scenarios investigated. The most frequently assessed victim drugs of drug–disease interactions during cytokine modulation and/or pro-inflammatory clinical conditions were midazolam (14/26; 53.8%; as a probe for CYP3A4), omeprazole (10/26; 38.5%; as a probe for 2C19), warfarin (8/26; 30.8%; as a probe for 2C9), and statins (7/26; of which, five with simvastatin and one each with atorvastatin and fluvastatin; 26.9%; as a probe for CYP3A4 and organic anion transporting polypeptide [OATP] 1B1). No study investigated any type of pharmacodynamic consequence due to PK variations.
Studies Assessing the Influence of Cytokine Modulation on the Pharmacokinetics of Drugs that Behave as Substrates of the CYP System During Acute or Chronic Inflammatory Conditions
Among the 12 clinical PK studies retrieved (Table 1) [15–20, 24–29], the most frequently investigated scenarios were sepsis and/or infections. Overall, in all of these studies, a weak-to-moderate inhibitory effect of pro-inflammatory cytokines on the CYP-mediated metabolism of victim drugs was found.
In a population PK study performed in 83 critically ill pediatric patients with sepsis, Vet et al. [25] found that both IL-6 levels and serum C-reactive protein (CRP) were significant covariates in decreasing midazolam CL. Interestingly, a simulated CRP increase from 10 to 300 mg/L, by causing a 65.4% decrease of midazolam CL, resulted in a moderate inhibition of the CYP3A4-mediated metabolism.
Early studies carried out in healthy volunteers showed that administration of one or two doses 24 h apart of lipopolysaccharide, by promoting increases in pro-inflammatory cytokines, caused a weak inhibitory effect of CYP3A4-mediated and CYP1A2-mediated metabolism of antipyrine (decrease in mean CL of 35% and 31%), theophylline (decrease in mean CL of 22% and 20%), and hexobarbital (decrease in mean CL of 27% and 20%), with no sex difference [16, 17].
A case-control study tested the potential influence of tumor-associated inflammation by comparing the PK behavior of probe drugs of CYP1A2 (caffeine), CYP2C19 (omeprazole), CYP2D6 (dextromethorphan), CYP3A4 (omeprazole), and CYP2E1 (chlorzoxazone) activity between 21 healthy volunteers and 22 patients with advanced epithelial ovarian cancer [27]. The findings supported that also in this setting pro-inflammatory cytokines may promote a downregulation of CYP2C19-mediated and CYP3A4-mediated metabolism of omeprazole (42% decrease in CL). Conversely, it is worth noting that tumor-associated inflammation resulted in an upregulation of CYP2E1 (3.28-fold increase in chlorzoxazone CL).
A prospective observational study assessed the potential influence of severity of inflammation on voriconazole metabolism in 34 hospitalized patients, mainly affected by hematological malignancies or receiving a solid organ transplantation [24]. After correction for other factors that could have influenced voriconazole metabolism (age, sex, voriconazole dose and route of administration, liver enzymes, and use of interacting co-medications), the authors showed that CRP levels were significantly associated with decreased voriconazole metabolism during inflammation, resulting in higher voriconazole trough concentrations (p < 0.001), and in both a lower voriconazole metabolic ratio (p < 0.001) and lower CL (p < 0.001). Interestingly, a simulated scenario of an absolute CRP increase at 200 mg/L resulted in a moderate inhibition of CYP3A4-mediated voriconazole metabolism in all of the different CYP2C19 phenotypes (voriconazole CL decrease of 70%, 80%, and 90% for ultra-rapid, extensive, and intermediate metabolizers, respectively).
In a comparative population PK study of darunavir in 30 patients with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) versus 25 patients with human immunodeficiency virus, Cojutti et al. found that IL-6 levels were a significant covariate of darunavir oral CL only among patients with SARS-CoV-2 [28]. In patients with SARS-CoV-2, median darunavir oral CL was 60.2% lower (4.1 L/h vs 10.3 L/h; p < 0.001) and median drug exposure was 2.1-fold higher (AUC of 161,387.0 vs 75,727.0 ng·h/mL; p < 0.001) than in patients with human immunodeficiency virus, resulting in a moderate inhibition of the CYP3A4-mediated metabolism. Notably, a classification and regression tree analysis found that an IL-6 level of 18 pg/mL may adequately split the SARS-CoV-2 population in patients with low versus high darunavir CL (2.78 L/h vs 7.24 L/h; p < 0.0001).
Additionally, we retrieved also three retrospective physiologically based PK models that investigated the impact of cytokine modulation on the CYP-mediated metabolism of some victim drugs (Table 2) [21–23]. In a physiologically based PK model carried out among patients with neuromyelitis optica spectrum disorders, a simulated ten-fold increase of steady-state IL-6 levels compared with baseline (from 10 to 100 pg/mL) resulted in moderate inhibition of CYP3A4-mediated metabolism of simvastatin and midazolam (mean AUC increase of 2.36-fold and 2.08-fold, respectively) [22]. In the same study, the simulated ten-fold IL-6 increase caused a weak-to-moderate inhibition of the CYP2C19-mediated metabolism of omeprazole (mean AUC increase of 1.97-fold), and a weak inhibition of both the CYP2C9-mediated metabolism of warfarin and the CYP2D6-mediated metabolism of dextromethorphan (mean increase AUC of 1.29-fold and 1.37-fold, respectively).
Likewise, in another physiologically based PK model carried out among patients with non-Hodgkin lymphoma, a transient raise of pro-inflammatory cytokine levels promoted by blinatumomab administration was responsible for a weak inhibition of CYP3A4-mediated metabolism (maximum effect 28% at 48 h) of both simvastatin (mean AUC increase of 1.9-fold) and midazolam (mean AUC increase of 1.7-fold) [23]. Conversely, in the same study, the inhibitory effect was much lower on CYP1A2-mediated metabolism (maximum effect 9% at 48 h) of theophylline (mean AUC increase of 1.2-fold) and on CYP2C19-mediated metabolism (maximum effect 17% at 70 h) of warfarin (mean AUC increase of 1.2-fold) [23].
Finally, in a physiologically based PK model including 12 patients with rheumatoid arthritis (RA) and five bone marrow transplant recipients, Machavaram et al. [21] showed that a virtual serum IL-6 level of 100 pg/mL caused a weak inhibition of CYP3A4-mediated metabolism of both simvastatin (mean AUC increase of 1.59-fold) and cyclosporine (mean AUC increase of 1.45-fold).
In summary, pro-inflammatory cytokines, and especially IL-6, were shown to exert a moderate inhibition of CYP3A4-mediated metabolism towards different victim drugs during acute or chronic inflammatory conditions. A less pronounced inhibition was observed against CYP2C9-mediated, CYP2C19-mediated, and CYP1A2-mediated metabolism. Although no study documented the pharmacodynamic consequences of this, it may be hypothesized that patients who are treated with victim drugs of CYP3A4-mediated metabolism during inflammatory conditions might experience over-exposure and the potential emergence of adverse events. Conversely, the upregulation of CYP2E1 observed in the presence of tumor-associated inflammation may promote faster detoxification and could be explained by the well-known prominent role that this isoenzyme may have in the metabolism of toxins and procarcinogens.
Studies Assessing the Influence of Anti-Inflammatory Biological Agents in Counteracting the Inhibitory Effect of Pro-Inflammatory Cytokines on the Pharmacokinetics of Drugs That Behave as Substrates of CYP During Acute or Chronic Inflammatory Conditions
Among the nine retrieved studies (Table 3) [30–38], the most frequently investigated scenario was that of the impact of anti-IL-6 agents in counteracting the downregulation of CYP activity in patients affected by RA. The influence of the anti-IL-6 agent tocilizumab on the PK behavior of the CYP3A4 victim drug simvastatin was assessed in a multicenter, randomized, single-sequence study enrolling 12 patients with RA [30]. At 1 week after tocilizumab administration, patients showed a significant decrease in CRP levels (0.3 mg/dL vs 5.0 mg/dL) and an increase in IL-6 levels (256 pg/mL vs 50 pg/mL) compared with baseline. At 1 week and 5 weeks after tocilizumab administration, mean simvastatin AUC was significantly decreased by 43% (90% CI 34–55) and 61% (90% CI 47–78) compared with baseline values, respectively, thus resulting in a moderate counteracting effect.
In another open-label, single-sequence, non-randomized phase I study, the impact of a different anti-IL-6 agent, namely sarilumab, on the PK behavior of simvastatin was assessed in 19 patients affected by RA [34]. Even in this study, at 1 week after sarilumab administration, patients showed a significant decrease in CRP levels (1.9 mg/dL vs 22.1 mg/dL) and an increase in IL-6 levels (219.9 pg/mL vs 47.5 pg/mL) compared with baseline. Mean simvastatin AUC calculated at 1 week after sarilumab administration was 54.7% lower than baseline values, resulting in a moderate counteracting effect.
A multicenter phase I study tested in 12 patients with RA the impact of the anti-IL-6 agent sirukumab on the PK behavior of probe drugs of CYP3A4 (midazolam), CYP2C9 (warfarin), CYP2C19 (omeprazole), and CYP1A2 (caffeine) [31]. A significant decrease in CRP levels at 1, 3, and 6 weeks after sirukumab administration was observed compared with baseline conditions (1.8/0.5/0.7 mg/L vs 25.3 mg/L). At 1, 3, and 6 weeks after sirukumab administration, a decrease in mean AUC compared with baseline conditions was found for midazolam, omeprazole, and warfarin, with ranges of 30–35%, 37–45%, and 17–18%, respectively. Conversely, no significant variation in mean AUC between pre-sirukumab and post-sirukumab administration was reported for caffeine.
Likewise, Jiang et al. [33] found in a physiologically based PK model including 12 patients with RA that at 3 weeks after sirukumab administration, the mean AUC of midazolam, omeprazole, and warfarin was decreased by 35%, 41%, and 18%, respectively, compared with baseline conditions. Conversely, no significant variation in mean AUC between pre-sirukumab and post-sirukumab administration was reported for caffeine.
Conversely, no significant variation in mean AUC and Cmax of caffeine, warfarin, omeprazole, dextromethorphan, and midazolam was found between pre-administration and post-administration of an anti-IL-2 agent (daclizumab) [32] and an anti-IL-23 agent (tildrakizumab or guselkumab) [36, 38]. Similarly, no significant difference in mean AUC of caffeine, warfarin, omeprazole, metoprolol, and midazolam was reported between pre-administration and post-administration of an anti-IL-4/IL-13 agent (dupilumab) [35]. Likewise, no variation in mean midazolam AUC compared with baseline conditions was observed between pre-administration and post-administration of an anti-IL-17 agent, secukinumab, among 24 patients affected by moderate-to-severe plaque psoriasis [37].
In summary, these findings showed that anti-IL-6 agents could have clinical relevance in counteracting the downregulation of CY3A4, CYP2C19, and CYP2C9 isoenzyme activity caused by chronic inflammatory conditions. The increased CL of victim drugs of CYP3A4-mediated, CYP2C9-mediated, or CYP2C19-mediated metabolism that may occur during treatment with anti-IL6 agents could lead to remarkable drug under-exposure and a lack of efficacy, thus potentially requiring dosing increases. Conversely, none of the anti-inflammatory biological agents that may block pro-inflammatory pathways other than IL-6 was able to reverse downregulation of different CYP isoenzymes (namely 1A2, 2C9, 2C19, 2D6, and 3A4) in the tested clinical scenarios, namely multiple sclerosis, atopic dermatitis, and psoriasis.
Studies Assessing the Influence of Cytokine Modulation on the Pharmacokinetic of Drugs That Behave as Substrates of Transporters During Acute or Chronic Inflammatory Conditions
Overall, only two studies were retrieved (Table 1 of the ESM) [39, 40], and both investigated the inhibitory effect that pro-inflammatory cytokines may have on the OAPT1B1-mediated transport of statins during chronic inflammatory conditions. The first was a case-control PK study that tested the potential influence of a chronic inflammation state on the PK behavior of fluvastatin by comparing the profile of this probe drug of the hepatic sinusoidal OATP1B1 activity between 15 patients with RA and ten healthy volunteers [39]. Patients with RA had significantly higher median IL-6 levels (11.2 pg/mL vs 4.94 pg/mL; p < 0.05) and TNF-α levels (39.9 pg/mL vs 21.1 pg/mL; p < 0.05) compared with healthy subjects. A 2.59-fold increase of fluvastatin AUC with a 2.52-fold decrease in fluvastatin CL were found in patients with RA compared with healthy volunteers.
The second was a case-control PK study that assessed the potential influence of chronic inflammatory status on the PK behavior of probe drugs of both OATP1B1 (atorvastatin) and CYP3A4 (midazolam) by comparing the PK profiles of these two drugs in healthy volunteers (n = 15), and in patients with controlled (n = 13) or uncontrolled (n = 12) systemic lupus erythematosus (SLE) [40]. Patients with uncontrolled SLE had TNF-α levels and monocyte chemoattractant protein-1 levels that were significantly higher compared with healthy volunteers. By comparing patients with uncontrolled SLE to healthy volunteers, a 1.98-fold increase in atorvastatin AUC (60.47 ng·h/mL vs 30.56 ng·h/mL; p < 0.05) due to a 1.98-fold decrease in atorvastatin CL (330.7 L/h vs 654.5 L/h; p<0.05) was found. Similarly, by comparing patients with uncontrolled SLE to those with controlled SLE, a 1.69-fold increase in atorvastatin AUC (60.47 ng·h/mL vs 35.87 ng·h/mL) due to a 1.69-fold decrease in CL (330.7 L/h vs 557.6 L/h) was observed. Conversely, no differences in midazolam AUC and CL were found between the three groups.
In summary, both studies showed that chronic inflammation may promote a weak-to-moderate downregulation of the hepatic sinusoidal OATP1B1 transport of statins. Noteworthy, clinicians should be aware that the approximatively two-fold increase in statin exposure observed under these circumstances might favor statin-related myopathy. Consequently, in patients with chronic inflammation who are treated with statins, close monitoring of serum creatine kinase should be recommended for preventing the occurrence of this adverse event. Additionally, it should not be overlooked that statins may undergo multiple elimination pathways, thus the eventual contribution of some of these (i.e., CYP2C9 for fluvastatin; CYP3A4, breast cancer resistance protein, and/or P-glycoprotein for atorvastatin) to the magnitude of this drug–disease interaction could not be ruled out.
Discussion
The paradigm of personalized medicine aims to provide the right treatment at the right dose for each patient [11]. In this perspective, the role of inflammation as a potential factor impacting on the phenotypic response to a given drug in a given patient is currently usually overlooked. However, from our analysis, it seems that this attitude could lead to undesired unexpected events.
In this systematic review, we summarized the current evidence about clinical studies that assessed the role of pro-inflammatory cytokines as an underlying mechanism of downregulation of CYP-mediated metabolism and of transporter-mediated uptake, and that of anti-inflammatory biological agents in counteracting this effect. From our analysis, some important findings emerged. First, the extent of the inhibitory effects caused by pro-inflammatory cytokines during acute or chronic inflammation on CYP-mediated metabolism and/or transporter-mediated uptake was extremely variable: never strong, neither toward CYP-mediated metabolism nor toward transporter-mediated uptake; moderate toward CYP3A4-mediated metabolism; and weak-to-moderate toward CYP2C9, CYP2C19, or CYP1A2 isoenzymes. Second, several studies showed a positive relationship between the magnitude of pro-inflammatory cytokine levels over time and the extent of the inhibitory effect on CYP-mediated metabolism. Third, anti-IL-6 biological agents showed remarkable activity in counteracting the downregulation of CYP isoenzymes, particularly CYP3A4, during chronic inflammatory conditions. Unfortunately, no study explored the pharmacodynamic consequences of the PK variations of the different victim drugs, thus the clinical implications of these effects could only be supposed.
In regard to the first point, it could be speculated that the increased exposure to statins, cyclosporin, midazolam, and darunavir shown during acute or chronic inflammatory conditions compared with baseline [15, 25, 28, 39, 40] could lead to drug-related adverse events and/or toxicity when these victim drugs of CYP3A4-mediated metabolism and/or of OATP1B1-mediated uptake are administered to patients having high levels of pro-inflammatory cytokines. Additionally, the weak-to-moderate downregulation of the CYP2C9 and CYP2C19 activity caused by pro-inflammatory cytokines has been investigated only for some probe drugs.
However, it may be argued that the clinical impact of the inhibitory effect might be especially relevant for some other narrow therapeutic index victim agents of these isoenzymes, which have not been investigated yet, namely psychotropic drugs, anticoagulants, and/or novel oral anticancer agents [41–43]. Indeed, some studies have just assessed the role of inflammation on the metabolism of agents such as antipsychotics (i.e., clozapine, risperidone, quetiapine) [44, 45], antiepileptic drugs [46], antidepressants (i.e., citalopram, venlafaxine) [47], immunosuppressant drugs (i.e., sirolimus, tacrolimus) [48, 49], theophylline [50], triazoles (i.e., voriconazole, posaconazole, itraconazole) [51–58], and lopinavir [59, 60]. However, none of these adequately assessed the PK behavior and were consistently excluded from our analysis. Additionally, some of these carried a high risk of bias, as they used only dose-normalized serum concentrations as the main parameter for evaluating differences in drug exposure between pro-inflammatory conditions and baseline [3, 8–11].
Some studies assessed the impact of cytokine modulation on CYP-mediated metabolism during acute or chronic inflammatory conditions by means of retrospective, physiologically based PK models [21–23, 33]. This is an emerging and interesting approach for assessing the potential extent of disease–drug interactions [61]. it should be recognized that prospective and widespread studies are currently lacking. Additionally, the reliability of such a methodology in predicting the magnitude of a specific disease (e.g., neuromyelitis optica [22]) on a drug interaction could be affected when the modeled data come from patients with other types of pro-inflammatory diseases.
In regard to the second point, a correlation between the magnitude of serum pro-inflammatory cytokine levels and the inhibitory activity on CPY450-mediated metabolism has been shown for simvastatin, cyclosporin, voriconazole, midazolam, and darunavir [21, 24, 25, 28]. These clinical findings are consistent with those obtained in preclinical studies that assessed the dose–response effect of pro-inflammatory cytokines on the downregulation of CYP isoenzymes [7]. In in vitro studies, the half-maximal inhibitory concentrations of IL-6, IL-1β, and TNF-α against CYP3A4 were generally lower (from two-fold to 15-fold) compared with other isoenzymes such as CYP1A2, CYP2C8, and CYP2C9, with a concentration-dependent effect in the magnitude of both messenger RNA expression and suppression of enzyme activity [62–65]. This suggests that in clinical scenarios characterized by severe inflammatory conditions, the inhibitory effect of pro-inflammatory cytokines may fluctuate in relation to changing levels over time, and that dosing adjustments could become very challenging under this drug–disease interaction, especially for CYP3A4 victim drugs.
In regard to the third point, it was shown that the administration of the anti-IL-6 agents tocilizumab, sarilumab, and sirukumab in patients with different chronic inflammatory conditions was able to revert by approximately 20–45% the downregulation of some CYP isoenzymes, especially CYP3A4 [30, 31, 33, 34]. Accordingly, it could be hypothesized that a similar counteracting effect could occur even if these anti IL-6 agents were administered to patients with acute inflammatory conditions. Scenarios like these may include administration of anti-IL6 agents to patients with severe coronavirus disease 2019 infections, or those with CRS caused by chimeric antigen receptor T-cell therapy or transplant engraftment [66, 67]. If this could be the case, potential under-exposure and a lack of therapeutic efficacy of victim drugs of CYP3A4-mediated metabolism could occur in the absence of adequate dosing adjustments.
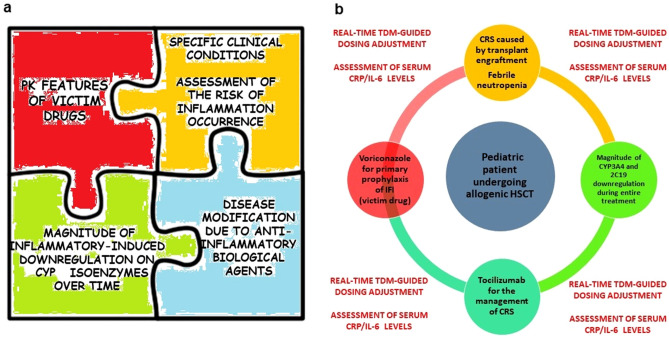
Overall, these considerations should push clinicians to start taking into account the potential role that several aspects of the inflammatory status might have in altering drug exposure to several victim drugs of CYP-mediated metabolism and/or OATP-mediated transport. In order to deal with the best dosing optimization, a ‘patient-centered’ strategy should be necessarily adopted (Fig. 3a). For this purpose, it would be helpful to consider the duration of specific inflammatory condition (short to medium vs long term), the magnitude of the inhibitory effect of pro-inflammatory cytokines over time, and the potential counteracting effect of anti-IL6 agents, as exemplified in Fig. 3b.
Fig. 3.
a Main determinants involved in the impact of systemic inflammation on the patient’s phenotypic response to a specific victim drug. b Illustration of the applicability of the ‘patient-centered’ strategy for the assessment of the impact of inflammation-induced metabolism downregulation in a specific clinical scenario (a pediatric patient undergoing an allogenic hematopoietic stem cell transplant [HSCT] requiring primary prophylaxis for invasive fungal infections [IFI] with voriconazole). In this case, voriconazole as the victim drug exhibits peculiar pharmacokinetic (PK) features (i.e., non-linear kinetic, narrow therapeutic index, extensive metabolism by cytochrome P450 [CYP] 2C19 and CYP3A4 isoenzymes coupled with inhibitory activity on CYP, a high risk of clinically relevant interactions with concomitant drugs, pharmacogenetic variability). These features should be carefully contextualized with the short-term to medium-term risk of cytokine release syndrome (CRS) caused by transplant engraftment or febrile neutropenia, the magnitude of CYP downregulation over time, and the possible administration of tocilizumab for the management of severe CRS. A real-time, therapeutic drug monitoring (TDM)-guided dosing adjustment coupled with intensive monitoring of inflammatory biomarkers could be suggested during the entire treatment with voriconazole. CRP C-reactive protein, IL-6 interleukin-6
A real-time, therapeutic drug monitoring-guided dosing adjustment strategy coupled with intensive monitoring of inflammatory biomarkers (e.g., CRP, IL-6) could provide a valuable support in promptly identifying and managing situations of fluctuations in drug exposure associated with inflammation and/or with the administration of anti-IL6 agents, thus minimizing the risk of adverse events or a lack of efficacy [11]. This strategy could be easily applied to drugs for which therapeutic drug monitoring-based dosing adjustments are widely applied presently, namely azole antifungal agents and/or immunosuppressant agents [68, 69]. However, it should be mentioned that, according to current evidence, no pre-defined dosing adjustments based only on CRP or IL-6 levels may be recommended for these drugs, as the pro-inflammatory status is only one of the determinants that may affect the exposure to these victim drugs.
We acknowledge some limitations of our study. The search was based on a single database, thus the potential exclusion of some other studies could not be ruled out. Translation of the effect of the real findings on the vast majority of victim drugs of CYP-mediated metabolism and transporter-mediated uptake could be only supposed. The lack of a disease–drug interaction found in some specific types of inflammatory conditions (i.e., multiple sclerosis, atopic dermatitis, and psoriasis) could be due to the fact that magnitude of the inflammatory burden may be low and therefore insufficient in downregulating CYP-mediated metabolism of victim agents. Finally, it should be recognized that the potential influence of cytokine modulation on victim drugs of drug transport may only be supposed [70], as clinical evidence on this topic is still limited.
Conclusions
Inflammation had an inhibitory effect that was moderate on CYP3A4-mediated metabolism and weak to moderate on CYP1A2-mediated, CYP2C9-mediated, and CYP2C19-mediated metabolism, with potential clinically relevant implications. Specific inflammatory conditions, magnitude of inhibitory effect over time, and counteracting effects of anti-IL-6 agents may represent key determinants impacting the patient’s phenotypic response to victim drugs. Further studies are warranted to investigate how cytokine modulation may affect the PK behavior of several other drug classes and to explore the pharmacodynamic consequences associated with the inflammatory-induced downregulation of CYP isoenzymes and drug transporters.
Supplementary Information
Below is the link to the electronic supplementary material.
Declarations
Funding
Open access funding provided by Alma Mater Studiorum - Università di Bologna within the CRUI-CARE Agreement.
Conflicts of interest/competing interests
Milo Gatti reports grants from Angelini. Federico Pea participated in a speaker bureau for Angelini, Basilea Pharmaceutica, Gilead, Hikma, Merck Sharp & Dohme, Nordic Pharma, Pfizer, and Sanofi Aventis, and in an advisory board for Angelini, Basilea Pharmaceutica, Correvio, Gilead, Hikma, Merck Sharp & Dohme, Nordic Pharma, Novartis, Pfizer, Shionogi, and Thermo-Fisher.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and material
Not applicable.
Code availability
Not applicable.
Authors’ contributions
MG and FP made substantial contributions to the conception of the manuscript. MG was involved in drafting the manuscript. FP made substantial contributions in revising the manuscript critically for important intellectual content. All authors approved the final version of the manuscript.
References
- 1.Fajgenbaum DC, June CH. Cytokine storm. N Engl J Med. 2020;383:2255–2273. doi: 10.1056/NEJMra2026131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee DW, Santomasso BD, Locke FL, Ghobadi A, Turtle CJ, Brudno JN, et al. ASTCT consensus grading for cytokine release syndrome and neurologic toxicity associated with immune effector cells. Biol Blood Marrow Transplant. 2019;25:625–638. doi: 10.1016/j.bbmt.2018.12.758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.White CM. Inflammation suppresses patients’ ability to metabolize cytochrome P450 substrate drugs. Ann Pharmacother. 2022;56:809–819. doi: 10.1177/10600280211047864. [DOI] [PubMed] [Google Scholar]
- 4.Coutant DE, Hall SD. Disease–drug interactions in inflammatory states via effects on CYP-mediated drug clearance. J Clin Pharmacol. 2018;58:849–863. doi: 10.1002/jcph.1093. [DOI] [PubMed] [Google Scholar]
- 5.Schmith VD, Foss JF. Inflammation: planning for a source of pharmacokinetic/pharmacodynamic variability in translational studies. Clin Pharmacol Ther. 2010;87:488–491. doi: 10.1038/clpt.2009.258. [DOI] [PubMed] [Google Scholar]
- 6.Nathan C. Points of control in inflammation. Nature. 2002;420:846–852. doi: 10.1038/nature01320. [DOI] [PubMed] [Google Scholar]
- 7.Dunvald A-CD, Järvinen E, Mortensen C, Stage TB. Clinical and molecular perspectives on inflammation-mediated regulation of drug metabolism and transport. Clin Pharmacol Ther. 2022;112:277–290. doi: 10.1002/cpt.2432. [DOI] [PubMed] [Google Scholar]
- 8.Vet NJ, de Hoog M, Tibboel D, de Wildt SN. The effect of inflammation on drug metabolism: a focus on pediatrics. Drug Discov Today. 2011;16:435–442. doi: 10.1016/j.drudis.2011.02.014. [DOI] [PubMed] [Google Scholar]
- 9.Lenoir C, Rollason V, Desmeules JA, Samer CF. Influence of inflammation on cytochromes P450 activity in adults: a systematic review of the literature. Front Pharmacol. 2021;12:733935. doi: 10.3389/fphar.2021.733935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lenoir C, Rodieux F, Desmeules JA, Rollason V, Samer CF. Impact of inflammation on cytochromes P450 activity in pediatrics: a systematic review. Clin Pharmacokinet. 2021;60:1537–1555. doi: 10.1007/s40262-021-01064-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stanke-Labesque F, Gautier-Veyret E, Chhun S, Guilhaumou R, French Society of Pharmacology and Therapeutics Inflammation is a major regulator of drug metabolizing enzymes and transporters: consequences for the personalization of drug treatment. Pharmacol Ther. 2020;215:107627. doi: 10.1016/j.pharmthera.2020.107627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.White CM, Sicignano DJ, Smith K. Impact of interferons and biological drug inhibitors of IL-2 and IL-6 on small-molecule drug metabolism through the cytochrome P450 system. Ann Pharmacother. 2022;56:170–180. doi: 10.1177/10600280211022281. [DOI] [PubMed] [Google Scholar]
- 13.Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.European Medicines Agency (EMA). Guideline on the investigation of drug interactions. 2015. https://www.ema.europa.eu/en/investigation-drug-interactions. Accessed 1 May 2022.
- 15.Chen YL, Le Vraux V, Leneveu A, Dreyfus F, Stheneur A, Florentin I, et al. Acute-phase response, interleukin-6, and alteration of cyclosporine pharmacokinetics. Clin Pharmacol Ther. 1994;55:649–660. doi: 10.1038/clpt.1994.82. [DOI] [PubMed] [Google Scholar]
- 16.Shedlofsky SI, Israel BC, McClain CJ, Hill DB, Blouin RA. Endotoxin administration to humans inhibits hepatic cytochrome P450-mediated drug metabolism. J Clin Investig. 1994;94:2209–2214. doi: 10.1172/JCI117582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shedlofsky SI, Israel BC, Tosheva R, Blouin RA. Endotoxin depresses hepatic cytochrome P450-mediated drug metabolism in women. Br J Clin Pharmacol. 1997;43:627–632. doi: 10.1046/j.1365-2125.1997.00603.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gorski JC, Hall SD, Becker P, Affrime MB, Cutler DL, Haehner-Daniels B. In vivo effects of interleukin-10 on human cytochrome P450 activity. Clin Pharmacol Ther. 2000;67:32–43. doi: 10.1067/mcp.2000.103860. [DOI] [PubMed] [Google Scholar]
- 19.Carcillo JA, Doughty L, Kofos D, Frye RF, Kaplan SS, Sasser H, et al. Cytochrome P450 mediated-drug metabolism is reduced in children with sepsis-induced multiple organ failure. Intensive Care Med. 2003;29:980–984. doi: 10.1007/s00134-003-1758-3. [DOI] [PubMed] [Google Scholar]
- 20.Moisés ECD, de Duarte LB, de Cavalli RC, Marques MP, Lanchote VL, Duarte G, et al. Pharmacokinetics of lidocaine and its metabolite in peridural anesthesia administered to pregnant women with gestational diabetes mellitus. Eur J Clin Pharmacol. 2008;64:1189–1196. doi: 10.1007/s00228-008-0544-0. [DOI] [PubMed] [Google Scholar]
- 21.Machavaram KK, Almond LM, Rostami-Hodjegan A, Gardner I, Jamei M, Tay S, et al. A physiologically based pharmacokinetic modeling approach to predict disease-drug interactions: suppression of CYP3A by IL-6. Clin Pharmacol Ther. 2013;94:260–268. doi: 10.1038/clpt.2013.79. [DOI] [PubMed] [Google Scholar]
- 22.Machavaram KK, Endo-Tsukude C, Terao K, Gill KL, Hatley OJ, Gardner I, et al. Simulating the impact of elevated levels of interleukin-6 on the pharmacokinetics of various CYP450 substrates in patients with neuromyelitis optica or neuromyelitis optica spectrum disorders in different ethnic populations. AAPS J. 2019;21:42. doi: 10.1208/s12248-019-0309-y. [DOI] [PubMed] [Google Scholar]
- 23.Xu Y, Hijazi Y, Wolf A, Wu B, Sun Y-N, Zhu M. Physiologically based pharmacokinetic model to assess the influence of blinatumomab-mediated cytokine elevations on cytochrome P450 enzyme activity. CPT Pharmacometr Syst Pharmacol. 2015;4:507–515. doi: 10.1002/psp4.12003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Veringa A, Ter Avest M, Span LFR, van den Heuvel ER, Touw DJ, Zijlstra JG, et al. Voriconazole metabolism is influenced by severe inflammation: a prospective study. J Antimicrob Chemother. 2017;72:261–267. doi: 10.1093/jac/dkw349. [DOI] [PubMed] [Google Scholar]
- 25.Vet NJ, Brussee JM, de Hoog M, Mooij MG, Verlaat CWM, Jerchel IS, et al. Inflammation and organ failure severely affect midazolam clearance in critically ill children. Am J Respir Crit Care Med. 2016;194:58–66. doi: 10.1164/rccm.201510-2114OC. [DOI] [PubMed] [Google Scholar]
- 26.Gravel S, Chiasson J-L, Turgeon J, Grangeon A, Michaud V. Modulation of CYP450 activities in patients with type 2 diabetes. Clin Pharmacol Ther. 2019;106:1280–1289. doi: 10.1002/cpt.1496. [DOI] [PubMed] [Google Scholar]
- 27.Trousil S, Lee P, Edwards RJ, Maslen L, Lozan-Kuehne JP, Ramaswami R, et al. Altered cytochrome 2E1 and 3A P450-dependent drug metabolism in advanced ovarian cancer correlates to tumour-associated inflammation. Br J Pharmacol. 2019;176:3712–3722. doi: 10.1111/bph.14776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cojutti PG, Londero A, Della Siega P, Givone F, Fabris M, Biasizzo J, et al. Comparative population pharmacokinetics of darunavir in SARS-CoV-2 patients vs. HIV patients: the role of interleukin-6. Clin Pharmacokinet. 2020;59:1251–1260. doi: 10.1007/s40262-020-00933-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lenoir C, Daali Y, Rollason V, Curtin F, Gloor Y, Bosilkovska M, et al. Impact of acute inflammation on cytochromes P450 activity assessed by the Geneva cocktail. Clin Pharmacol Ther. 2021;109:1668–1676. doi: 10.1002/cpt.2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schmitt C, Kuhn B, Zhang X, Kivitz AJ, Grange S. Disease–drug–drug interaction involving tocilizumab and simvastatin in patients with rheumatoid arthritis. Clin Pharmacol Ther. 2011;89:735–740. doi: 10.1038/clpt.2011.35. [DOI] [PubMed] [Google Scholar]
- 31.Zhuang Y, de Vries DE, Xu Z, Marciniak SJ, Chen D, Leon F, et al. Evaluation of disease-mediated therapeutic protein-drug interactions between an anti-interleukin-6 monoclonal antibody (sirukumab) and cytochrome P450 activities in a phase 1 study in patients with rheumatoid arthritis using a cocktail approach. J Clin Pharmacol. 2015;55:1386–1394. doi: 10.1002/jcph.561. [DOI] [PubMed] [Google Scholar]
- 32.Tran JQ, Othman AA, Wolstencroft P, Elkins J. Therapeutic protein–drug interaction assessment for daclizumab high-yield process in patients with multiple sclerosis using a cocktail approach. Br J Clin Pharmacol. 2016;82:160–167. doi: 10.1111/bcp.12936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jiang X, Zhuang Y, Xu Z, Wang W, Zhou H. Development of a physiologically based pharmacokinetic model to predict disease-mediated therapeutic protein–drug interactions: modulation of multiple cytochrome P450 enzymes by interleukin-6. AAPS J. 2016;18:767–776. doi: 10.1208/s12248-016-9890-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee EB, Daskalakis N, Xu C, Paccaly A, Miller B, Fleischmann R, et al. Disease–drug interaction of sarilumab and simvastatin in patients with rheumatoid arthritis. Clin Pharmacokinet. 2017;56:607–615. doi: 10.1007/s40262-016-0462-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Davis JD, Bansal A, Hassman D, Akinlade B, Li M, Li Z, et al. Evaluation of potential disease-mediated drug–drug interaction in patients with moderate-to-severe atopic dermatitis receiving dupilumab. Clin Pharmacol Ther. 2018;104:1146–1154. doi: 10.1002/cpt.1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Khalilieh S, Hussain A, Montgomery D, Levine V, Shaw PM, Bodrug I, et al. Effect of tildrakizumab (MK-3222), a high affinity, selective anti-IL23p19 monoclonal antibody, on cytochrome P450 metabolism in subjects with moderate to severe psoriasis. Br J Clin Pharmacol. 2018;84:2292–2302. doi: 10.1111/bcp.13670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bruin G, Hasselberg A, Koroleva I, Milojevic J, Calonder C, Soon R, et al. Secukinumab treatment does not alter the pharmacokinetics of the cytochrome P450 3A4 substrate midazolam in patients with moderate to severe psoriasis. Clin Pharmacol Ther. 2019;106:1380–1388. doi: 10.1002/cpt.1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhu Y, Xu Y, Zhuang Y, Piantone A, Shu C, Chen D, et al. Evaluating potential disease-mediated protein-drug interactions in patients with moderate-to-severe plaque psoriasis receiving subcutaneous guselkumab. Clin Transl Sci. 2020;13:1217–1226. doi: 10.1111/cts.12807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Caris JA, de Benzi JRL, de Souza FFL, de Oliveira RDR, Donadi EA, Lanchote VL. Rheumatoid arthritis downregulates the drug transporter OATP1B1: fluvastatin as a probe. Eur J Pharm Sci. 2020;146:105264. doi: 10.1016/j.ejps.2020.105264. [DOI] [PubMed] [Google Scholar]
- 40.Cestari RN, de Oliveira RDR, de Souza FFL, Pippa LF, Nardotto GHB, Rocha A, et al. Systemic lupus erythematosus activity affects the sinusoidal uptake transporter OATP1B1 evaluated by the pharmacokinetics of atorvastatin. Clin Transl Sci. 2020;13:1227–1235. doi: 10.1111/cts.12808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gatti M, Raschi E, Poluzzi E, Martignani C, Salvagni S, Ardizzoni A, et al. The complex management of atrial fibrillation and cancer in the COVID-19 era: drug interactions, thromboembolic risk, and proarrhythmia. Curr Heart Fail Rep. 2020;17:365–383. doi: 10.1007/s11897-020-00485-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gatti M, De Ponti F, Pea F. Clinically significant drug interactions between psychotropic agents and repurposed COVID-19 therapies. CNS Drugs. 2021;35:345–384. doi: 10.1007/s40263-021-00811-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stipp MC, Acco A. Involvement of cytochrome P450 enzymes in inflammation and cancer: a review. Cancer Chemother Pharmacol. 2021;87:295–309. doi: 10.1007/s00280-020-04181-2. [DOI] [PubMed] [Google Scholar]
- 44.Hefner G, Shams MEE, Unterecker S, Falter T, Hiemke C. Inflammation and psychotropic drugs: the relationship between C-reactive protein and antipsychotic drug levels. Psychopharmacology. 2016;233:1695–1705. doi: 10.1007/s00213-015-3976-0. [DOI] [PubMed] [Google Scholar]
- 45.Ruan C-J, Zang Y-N, Cheng Y-H, Wang C-Y, de Leon J. Around 3% of 1,300 levels were elevated during infections in a retrospective review of 131 Beijing hospital in-patients with more than 24,000 days of clozapine treatment. Psychother Psychosom. 2020;89:255–257. doi: 10.1159/000506355. [DOI] [PubMed] [Google Scholar]
- 46.Yamamoto Y, Takahashi Y, Horino A, Usui N, Nishida T, Imai K, et al. Influence of inflammation on the pharmacokinetics of perampanel. Ther Drug Monit. 2018;40:725–729. doi: 10.1097/FTD.0000000000000556. [DOI] [PubMed] [Google Scholar]
- 47.Hefner G, Shams MEE, Unterecker S, Falter T, Hiemke C. Retrospective pilot study for analysis of antidepressant serum concentrations of citalopram and venlafaxine during inflammation. Pharmacopsychiatry. 2015;48:215–218. doi: 10.1055/s-0035-1559666. [DOI] [PubMed] [Google Scholar]
- 48.Mizuno T, O’Brien MM, Vinks AA. Significant effect of infection and food intake on sirolimus pharmacokinetics and exposure in pediatric patients with acute lymphoblastic leukemia. Eur J Pharm Sci. 2019;128:209–214. doi: 10.1016/j.ejps.2018.12.004. [DOI] [PubMed] [Google Scholar]
- 49.Lemahieu W, Maes B, Verbeke K, Rutgeerts P, Geboes K, Vanrenterghem Y. Cytochrome P450 3A4 and P-glycoprotein activity and assimilation of tacrolimus in transplant patients with persistent diarrhea. Am J Transplant. 2005;5:1383–1391. doi: 10.1111/j.1600-6143.2005.00844.x. [DOI] [PubMed] [Google Scholar]
- 50.Yamaguchi A, Tateishi T, Okano Y, Matuda T, Akimoto Y, Miyoshi T, et al. Higher incidence of elevated body temperature or increased C-reactive protein level in asthmatic children showing transient reduction of theophylline metabolism. J Clin Pharmacol. 2000;40:284–289. doi: 10.1177/00912700022008955. [DOI] [PubMed] [Google Scholar]
- 51.Naito T, Yamada T, Mino Y, Kawakami J. Impact of inflammation and concomitant glucocorticoid administration on plasma concentration of triazole antifungals in immunocompromised patients. Clin Chim Acta. 2015;441:127–132. doi: 10.1016/j.cca.2014.12.024. [DOI] [PubMed] [Google Scholar]
- 52.Märtson A-G, Veringa A, Bakker M, van den Heuvel ER, Touw DJ, van der Werf TS, et al. Posaconazole trough concentrations are not influenced by inflammation: a prospective study. Int J Antimicrob Agents. 2019;53:325–329. doi: 10.1016/j.ijantimicag.2019.01.006. [DOI] [PubMed] [Google Scholar]
- 53.Gautier-Veyret E, Thiebaut-Bertrand A, Roustit M, Bolcato L, Depeisses J, Schacherer M, et al. Optimization of voriconazole therapy for treatment of invasive aspergillosis: pharmacogenomics and inflammatory status need to be evaluated. Br J Clin Pharmacol. 2021;87:2534–2541. doi: 10.1111/bcp.14661. [DOI] [PubMed] [Google Scholar]
- 54.EncaladaVentura MA, Span LFR, van den Heuvel ER, Groothuis GMM, Alffenaar J-WC. Influence of inflammation on voriconazole metabolism. Antimicrob Agents Chemother. 2015;59:2942–2943. doi: 10.1128/AAC.04789-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Encalada Ventura MA, van Wanrooy MJP, Span LFR, Rodgers MGG, van den Heuvel ER, Uges DRA, et al. Longitudinal analysis of the effect of inflammation on voriconazole trough concentrations. Antimicrob Agents Chemother. 2016;60:2727–2731. doi: 10.1128/AAC.02830-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dote S, Sawai M, Nozaki A, Naruhashi K, Kobayashi Y, Nakanishi H. A retrospective analysis of patient-specific factors on voriconazole clearance. J Pharm Health Care Sci. 2016;2:10. doi: 10.1186/s40780-016-0044-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Niioka T, Fujishima N, Abumiya M, Yamashita T, Ubukawa K, Nara M, et al. Relationship between the CYP2C19 phenotype using the voriconazole-to-voriconazole N-oxide plasma concentration ratio and demographic and clinical characteristics of Japanese patients with different CYP2C19 genotypes. Ther Drug Monit. 2017;39:514–521. doi: 10.1097/FTD.0000000000000441. [DOI] [PubMed] [Google Scholar]
- 58.van Wanrooy MJP, Span LFR, Rodgers MGG, van den Heuvel ER, Uges DRA, van der Werf TS, et al. Inflammation is associated with voriconazole trough concentrations. Antimicrob Agents Chemother. 2014;58:7098–7101. doi: 10.1128/AAC.03820-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gregoire M, Le Turnier P, Gaborit BJ, Veyrac G, Lecomte R, Boutoille D, et al. Lopinavir pharmacokinetics in COVID-19 patients. J Antimicrob Chemother. 2020;75:2702–2704. doi: 10.1093/jac/dkaa195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schoergenhofer C, Jilma B, Stimpfl T, Karolyi M, Zoufaly A. Pharmacokinetics of lopinavir and ritonavir in patients hospitalized with coronavirus disease 2019 (COVID-19) Ann Intern Med. 2020;173:670–672. doi: 10.7326/M20-1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.US Food and Drug Administration (US FDA). Drug-drug interaction assessment for therapeutic proteins guidance for industry. Draft guidance for industry. 2020. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/drug-drug-interaction-assessment-therapeutic-proteins-guidance-industry. Accessed 1 May 2022.
- 62.Dickmann LJ, Patel SK, Rock DA, Wienkers LC, Slatter JG. Effects of interleukin-6 (IL-6) and an anti-IL-6 monoclonal antibody on drug-metabolizing enzymes in human hepatocyte culture. Drug Metab Dispos. 2011;39:1415–1422. doi: 10.1124/dmd.111.038679. [DOI] [PubMed] [Google Scholar]
- 63.Dickmann LJ, Patel SK, Wienkers LC, Slatter JG. Effects of interleukin 1β (IL-1β) and IL-1β/interleukin 6 (IL-6) combinations on drug metabolizing enzymes in human hepatocyte culture. Curr Drug Metab. 2012;13:930–937. doi: 10.2174/138920012802138642. [DOI] [PubMed] [Google Scholar]
- 64.Rubin K, Janefeldt A, Andersson L, Berke Z, Grime K, Andersson TB. HepaRG cells as human-relevant in vitro model to study the effects of inflammatory stimuli on cytochrome P450 isoenzymes. Drug Metab Dispos. 2015;43:119–125. doi: 10.1124/dmd.114.059246. [DOI] [PubMed] [Google Scholar]
- 65.Nguyen TV, Ukairo O, Khetani SR, McVay M, Kanchagar C, Seghezzi W, et al. Establishment of a hepatocyte-kupffer cell coculture model for assessment of proinflammatory cytokine effects on metabolizing enzymes and drug transporters. Drug Metab Dispos. 2015;43:774–785. doi: 10.1124/dmd.114.061317. [DOI] [PubMed] [Google Scholar]
- 66.Snow TAC, Saleem N, Ambler G, Nastouli E, Singer M, Arulkumaran N. Tocilizumab in COVID-19: a meta-analysis, trial sequential analysis, and meta-regression of randomized-controlled trials. Intensive Care Med. 2021;47:641–652. doi: 10.1007/s00134-021-06416-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kotch C, Barrett D, Teachey DT. Tocilizumab for the treatment of chimeric antigen receptor T cell-induced cytokine release syndrome. Expert Rev Clin Immunol. 2019;15:813–822. doi: 10.1080/1744666X.2019.1629904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bury D, Tissing WJE, Muilwijk EW, Wolfs TFW, Brüggemann RJ. Clinical pharmacokinetics of triazoles in pediatric patients. Clin Pharmacokinet. 2021;60:1103–1147. doi: 10.1007/s40262-021-00994-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.McCune JS, Bemer MJ. Pharmacokinetics, pharmacodynamics and pharmacogenomics of immunosuppressants in allogeneic haematopoietic cell transplantation: part I. Clin Pharmacokinet. 2016;55:525–550. doi: 10.1007/s40262-015-0339-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Evers R, Piquette-Miller M, Polli JW, Russel FGM, Sprowl JA, Tohyama K, et al. Disease-associated changes in drug transporters may impact the pharmacokinetics and/or toxicity of drugs: a white paper from the International Transporter Consortium. Clin Pharmacol Ther. 2018;104:900–915. doi: 10.1002/cpt.1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.