Abstract
Background
This study investigated whether the association between family history of breast cancer in first-degree relatives and breast cancer risk varies by breast density.
Methods
Women aged 40 years and older who underwent screening between 2009 and 2010 were followed up until 2020. Family history was assessed using a self-reported questionnaire. Using Breast Imaging Reporting and Data System (BI-RADS), breast density was categorized into dense breast (heterogeneously or extremely dense) and non-dense breast (almost entirely fatty or scattered areas of fibro-glandular). Cox regression model was used to assess the association between family history and breast cancer risk.
Results
Of the 4,835,507 women, 79,153 (1.6%) reported having a family history of breast cancer and 77,238 women developed breast cancer. Family history led to an increase in the 5-year cumulative incidence in women with dense- and non-dense breasts. Results from the regression model with and without adjustment for breast density yielded similar HRs in all age groups, suggesting that breast density did not modify the association between family history and breast cancer. After adjusting for breast density and other factors, family history of breast cancer was associated with an increased risk of breast cancer in all three age groups (age 40–49 years: aHR 1.96, 95% confidence interval [CI] 1.85–2.08; age 50–64 years: aHR 1.70, 95% CI 1.58–1.82, and age ≥65 years: aHR 1.95, 95% CI 1.78–2.14).
Conclusion
Family history of breast cancer and breast density are independently associated with breast cancer. Both factors should be carefully considered in future risk prediction models of breast cancer.
Keywords: Breast cancer risk, Family history of breast cancer, Breast density
Highlights
-
•
This cohort study found that first-degree family history was associated with a 1.7 to 2-fold increased breast cancer risk after adjustment for breast density.
-
•
Further, breast density did not modify the association between family history and breast cancer and thus is independently associated with breast cancer.
-
•
Both family history of breast cancer and breast density should be considered in future breast cancer risk prediction models.
1. Introduction
A family history of breast cancer is one of the most well-known risk factors for breast cancer. Women with a family history of breast cancer in their first-degree relatives have an approximately two-fold higher risk than women without a family history, and the risk varies depending on the number of family members with breast cancer and age at breast cancer diagnosis of family members [1]. Breast cancer-susceptible genes and a shared environment are suggested to contribute to the familial clustering of breast cancer [[1], [2], [3]]. Mutations in rare and moderate to high-penetrance genes such as BRCA1, BRCA2, PTEN, and TP53 account for less than 25% of familial risk [4], and common, low-risk single nucleotide polymorphisms identified through genome-wide association studies, known as polygenic factors, account for 18% of the familial risk [5,6].
Mammographic breast density, which is a strong risk factor for breast cancer associated with a 4- to 6-fold increased risk, is affected by both genetics and environmental factors [7], and familial correlations of breast density have been identified between twins and family members [[8], [9], [10]]. As some of the breast cancer risk factors showed familial clustering or association, several studies focused on the assumption that they could modify the effect of each other on the risk of breast cancer. Breast density and its change-associated breast cancer risk were higher in women with a family history of breast cancer [11], suggesting an interaction between family history and breast density [12]. In addition, in older women, the breast cancer risk associated with first-degree family history differed according to breast density and age group [13]. Other studies found no interaction between breast density and family history of breast cancer on breast cancer risk [14,15].
As the combination of mammographic density and family history of breast cancer with previously developed models would improve the risk classification [16], identification of the combined effect of family history and breast density would be useful in mammographic screening settings where breast density is measured. However, these associations have yet to be fully examined in Asian women. In this study, we aimed to determine whether a family history of breast cancer in first-degree relatives and the type of first-degree relatives is associated with an increased risk of breast cancer, and to identify whether the association varies by mammographic breast density.
2. Materials and methods
2.1. Study settings and study population
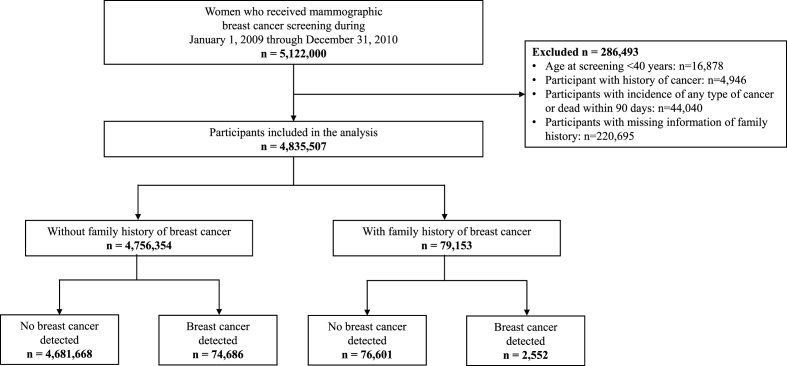
This cohort study used data from the National Health Insurance Service (NHIS) - National Health Information Database (NHID) in Korea. The NHIS is a mandatory health insurance system for the entire Korean population. The NHIS-NHID database contains information on demographics, healthcare utilization, deaths, and national health screening results for the Korean population [17,18]. As part of national health screening programs, biennial mammographic breast cancer screening is offered to women aged 40 years or older. This study population included women who underwent mammographic breast cancer screening between January 1, 2009, and December 31, 2010. All breast cancer screening participants were followed until the date of breast cancer diagnosis, date of death, or December 31, 2020, whichever came first. If women underwent mammographic screening more than once between 2009 and 2010, we used data from the first screening. We excluded participants whose age at screening was ˂ 40 years, who had a history of cancer before the date of mammographic screening, and those with unknown information on family history of breast cancer. In addition, women who died or developed any type of cancer within 90 days after the date of breast cancer screening were excluded to avoid the possibility of detecting cancer cases through screening. Among the 5,122,000 women who underwent mammography screening between 2009 and 2010, after applying the above-mentioned exclusion criteria, the final study sample included 4,835,507 individuals. Among them, 79,153 women had a family history of breast cancer (Fig. 1).
Fig. 1.
Flow diagram of the selection of eligible population.
This study was approved by the Institutional Review Board of Hanyang University College of Medicine (approval no. HYUIRB-202106-003-1). Based on the Institutional Review Board approval, the NHIS granted permission to utilize the NHIS-NHID database. The requirement for informed consent was waived because all screened populations agreed to transfer their screening results to NHIS-NHID, and the NHIS database was constructed after the anonymization of individual identities.
2.2. Exposure
Information on family history was collected by a self-administered survey at the time of breast cancer screening. The questionnaire surveyed information on first-degree family history of six cancer types, including breast, cervical, gastric, colorectal, liver, and lung cancer [19]. For each cancer site, participants were asked whether they had a family history of a particular cancer in parents, siblings (brother/sister), and children (daughter/son). In this study, we focused on information on family history of breast cancer among mothers and sisters.
Mammography breast density was classified using the fourth edition of Breast Imaging Reporting and Data System (BI-RADS) density categories [20]. BI-RADS category A indicates almost entirely fat (parenchyma <25%), category B indicates scattered fibroglandular density (parenchyma is 25–50%), category C is heterogeneously dense (parenchyma is 51–75%), and category D is extremely dense (parenchyma >75%). Breast density was further categorized into two groups: the non-dense breast group (almost entirely fat and scattered fibroglandular) and the dense breast group (heterogeneously dense and extremely dense).
2.3. Outcomes of interest
The main outcome of this study was incidence of breast cancer. Breast cancer cases were defined by health care utilization data with disease codes of the International Classification of Diseases code of invasive breast cancer (C50.0–C50.9) and ductal carcinoma in situ (DCIS) (D05.0–D05.9), in combination with the catastrophic illness code [21].
2.4. Measures of covariates
The following factors were considered as covariates: age (continuous variable), age at menarche (categorical variables: <15 years, 15–16 years, >16 years, and unknown), number of children (categorical variables: nulliparous, 1 child, ≥2 children, and unknown), breastfeeding history (categorical variables: never, ever, and unknown), oral contraceptive use (categorical variables: never, ever, and unknown), hormone replacement therapy (categorical variables: never, ever, and unknown), smoking history (categorical variables: never, ever, and unknown), and number of days of alcohol consumption per week (categorical variables: none, 1 day per week, ≥2 per week, and unknown), which were measured using a self-administered questionnaire at the same time as breast cancer screening. In addition, body mass index (BMI) (continuous variable), which was calculated using measured height and weight values by trained medical staff during the health examination, was included. Missing values of covariates were treated as dummy categories in the analysis.
2.5. Statistical analysis
Descriptive statistics of baseline characteristics of total participants by the presence of family history of breast cancer in first-degree relatives were presented. In the analysis, participants were categorized into three age groups: 40–49 years, 50–64 years, and ≥65 years. The five-year cumulative incidence was calculated using non-parametric methods to account for the competing risk, stratified by breast density classification, age group, and family history at baseline. Gray's test was used to statistically compare the cumulative incidence functions [22]. Cox proportional hazard regression analysis was conducted to calculate the hazard ratio (HR) and 95% confidence intervals (CIs) of the association between the presence of family history of breast cancer in first-degree relatives and breast cancer risk adjusted for the above-mentioned covariates, with or without adjustment for breast density. In addition, the association between the presence of a family history of breast cancer in first-degree relatives and breast cancer risk was assessed, stratified by breast density (non-dense and dense).
Two sets of sensitivity analyses were conducted. The first analysis was conducted after excluding DCIS cases (including only invasive breast cancer cases). Given that the association between BMI and breast cancer development has opposite directions in pre- and postmenopausal women, we conducted a sensitivity analysis with the addition of the interaction between BMI and menopausal status in the regression models. The results of these sensitivity analyses are presented in the Supplemental Tables.
All reported P-values were two-sided with a type I error (α < 0.05) and considered statistically significant. Statistical analyses were performed using SAS statistical software (version 9.4, SAS Institute, Cary, NC, USA).
3. Results
3.1. Characteristics of the study population
Of the 4,835,507 participants included in the final analysis, 79,153 (1.6%) reported having a family history of breast cancer. During a mean (standard deviation) follow-up of 10.4 (1.7) years (median, 10.8 years), 74,686 and 2552 breast cancer cases were diagnosed among those with and without a family history of breast cancer, respectively (Fig. 1). The distribution of covariates in the study cohort and across the strata of first-degree family history are presented in Table 1. Among women without a family history of breast cancer, 45.1% were women aged 50–64 years, 42.1% had dense breasts, and 42.2% had premenopausal status. In women with any family history of breast cancer, the proportion of the younger age group was higher, with 37.6% of women aged 40–49 years and 48.9% aged 50–64 years. The proportion of women with dense breasts was likewise greater in those with a family history of breast cancer (51.8%). Notably, in participants whose first-degree family history involved the mother, the proportion of dense breasts was 59.4%.
Table 1.
Characteristics of participants by family history of breast cancer in the cohort.
| Characteristics | No. (%) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| All participants |
First-degree family history |
|||||||||
| None |
Any |
Mother only |
Sister only |
|||||||
| (n = 4,835,507) | (n = 4,756,354) | (n = 79,153) | (n = 21,257) | (n = 47,520) | ||||||
| Age (mean/SD) | 55.18 | 10.6 | 55.21 | 10.7 | 53.35 | 9.4 | 49.73 | 8.6 | 53.53 | 8.8 |
| Age group | ||||||||||
| 40-49 | 1,638,491 | 33.9 | 1,608,712 | 33.8 | 29,779 | 37.6 | 11,706 | 55.1 | 16,486 | 34.7 |
| 50-64 | 2,181,452 | 45.1 | 2,142,761 | 45.1 | 38,691 | 48.9 | 8059 | 37.9 | 25,320 | 53.3 |
| ≥65 | 1,015,564 | 21.0 | 1,004,881 | 21.1 | 10,683 | 13.5 | 1492 | 7.0 | 5714 | 12.0 |
| Breast density | ||||||||||
| Non-dense breast | 2,653,446 | 54.9 | 2,616,838 | 55.0 | 36,608 | 46.3 | 8267 | 38.9 | 21,871 | 46.0 |
| Dense breast | 2,037,890 | 42.1 | 1,996,878 | 42.0 | 41,012 | 51.8 | 12,624 | 59.4 | 24,753 | 52.1 |
| Unknown | 144,171 | 3.0 | 142,638 | 3.0 | 1533 | 1.9 | 366 | 1.7 | 896 | 1.9 |
| Age at menopause (years) | ||||||||||
| Premenopausal | 2,040,587 | 42.2 | 2,002,665 | 42.1 | 37,922 | 47.9 | 13,393 | 63.0 | 21,894 | 46.1 |
| <45 | 189,279 | 3.9 | 186,995 | 3.9 | 2284 | 2.9 | 477 | 2.2 | 1311 | 2.8 |
| 45-52 | 1,748,197 | 36.2 | 1,723,088 | 36.2 | 25,109 | 31.7 | 4815 | 22.7 | 15,607 | 32.8 |
| ≥53 | 613,556 | 12.7 | 602,875 | 12.7 | 10,681 | 13.5 | 1836 | 8.6 | 6839 | 14.4 |
| Unknown | 243,888 | 5.0 | 240,731 | 5.1 | 3157 | 4.0 | 736 | 3.5 | 1869 | 3.9 |
| BMI (mean/SD) | 23.87 | 3.2 | 23.87 | 3.2 | 23.69 | 3.1 | 23.47 | 3.2 | 23.7 | 3.1 |
| Age at menarche (years) | ||||||||||
| <15 | 1,084,901 | 22.4 | 1,061,654 | 22.3 | 23,247 | 29.4 | 7934 | 37.3 | 13,521 | 28.5 |
| 15-16 | 1,938,785 | 40.1 | 1,905,907 | 40.1 | 32,878 | 41.5 | 8415 | 39.6 | 20,222 | 42.6 |
| ≥17 | 1,654,640 | 34.2 | 1,633,268 | 34.3 | 21,372 | 27.0 | 4436 | 20.9 | 12,909 | 27.2 |
| Unknown | 157,181 | 3.3 | 155,525 | 3.3 | 1656 | 2.1 | 472 | 2.2 | 868 | 1.8 |
| Parity | ||||||||||
| Nulliparous | 177,052 | 3.7 | 173,218 | 3.6 | 3834 | 4.8 | 1236 | 5.8 | 2220 | 4.7 |
| 1 child | 452,177 | 9.4 | 443,425 | 9.3 | 8752 | 11.1 | 2823 | 13.3 | 5189 | 10.9 |
| ≥2 children | 4,130,944 | 85.4 | 4,065,006 | 85.5 | 65,938 | 83.3 | 17,018 | 80.1 | 39,788 | 83.7 |
| Unknown | 75,334 | 1.6 | 74,705 | 1.6 | 629 | 0.8 | 180 | 0.9 | 323 | 0.7 |
| Breastfeeding | ||||||||||
| Never | 655,715 | 13.6 | 642,173 | 13.5 | 13,542 | 17.1 | 4635 | 21.8 | 7806 | 16.4 |
| Ever | 4,085,871 | 84.5 | 4,021,054 | 84.5 | 64,817 | 81.9 | 16,397 | 77.1 | 39,291 | 82.7 |
| Unknown | 93,921 | 1.9 | 93,127 | 2.0 | 794 | 1.0 | 225 | 1.1 | 423 | 0.9 |
| Oral contraceptive use | ||||||||||
| Never | 3,848,907 | 79.6 | 3,786,648 | 79.6 | 62,259 | 78.7 | 17,085 | 80.4 | 37,464 | 78.8 |
| Ever | 904,602 | 18.7 | 888,474 | 18.7 | 16,128 | 20.4 | 3963 | 18.6 | 9660 | 20.3 |
| Unknown | 81,998 | 1.7 | 81,232 | 1.7 | 766 | 1.0 | 209 | 1.0 | 396 | 0.8 |
| Physical activity | ||||||||||
| No | 1,399,233 | 28.9 | 1,380,235 | 29.0 | 18,998 | 24.0 | 5029 | 23.7 | 11,212 | 23.6 |
| Yes | 3,398,775 | 70.3 | 3,339,185 | 70.2 | 59,590 | 75.3 | 16,097 | 75.7 | 35,974 | 75.7 |
| Unknown | 37,499 | 0.8 | 36,934 | 0.8 | 565 | 0.7 | 131 | 0.6 | 334 | 0.7 |
| Smoking status | ||||||||||
| Never smoked | 4,596,805 | 95.1 | 4,522,102 | 95.1 | 74,703 | 94.4 | 19,850 | 93.4 | 45,020 | 94.7 |
| Ever smoked | 219,547 | 4.5 | 215,420 | 4.5 | 4127 | 5.2 | 1320 | 6.2 | 2330 | 4.9 |
| Unknown | 19,155 | 0.4 | 18,832 | 0.4 | 323 | 0.4 | 87 | 0.4 | 170 | 0.4 |
| Drinking frequency during the last year | ||||||||||
| No | 3,879,292 | 80.2 | 3,817,574 | 80.3 | 61,718 | 78.0 | 15,929 | 74.9 | 37,225 | 78.3 |
| 1 day/week | 556,634 | 11.5 | 546,300 | 11.5 | 10,334 | 13.1 | 3221 | 15.2 | 6087 | 12.8 |
| ≥2 day/week | 362,434 | 7.5 | 355,885 | 7.5 | 6549 | 8.3 | 1978 | 9.3 | 3878 | 8.2 |
| Unknown | 37,147 | 0.8 | 36,595 | 0.8 | 552 | 0.7 | 129 | 0.6 | 330 | 0.7 |
| Hormone replacement therapy after menopause (years) | ||||||||||
| Never use | 2,290,029 | 81.9 | 2,257,564 | 82.0 | 32,465 | 78.7 | 6278 | 79.8 | 20,113 | 78.5 |
| <5 | 339,902 | 12.2 | 333,484 | 12.1 | 6418 | 15.6 | 1137 | 14.5 | 4121 | 16.1 |
| ≥5 | 75,553 | 2.7 | 74,083 | 2.7 | 1470 | 3.6 | 214 | 2.7 | 940 | 3.7 |
| Unknown | 89,436 | 3.2 | 88,558 | 3.2 | 878 | 2.1 | 235 | 3.0 | 452 | 1.8 |
Abbreviations: BMI, body mass index; SD, standard deviation.
3.2. Cumulative incidence of breast cancer by first-degree family history
Women with a first-degree family history of breast cancer had a higher five-year cumulative incidence rate of breast cancer than women without a family history of breast cancer, irrespective of breast density. In addition, the cumulative incidence of breast cancer was higher in the younger age group. (Table 2). The highest 5-year cumulative incidence was observed in women aged 40–49 with dense breasts and family history (20.1, 95% CI = 18.3–22.0) and women aged ≥65 years with non-dense breasts and without family history showed the lowest incidence (3.8, 95% CI = 3.7–3.9). In women with dense breasts without a family history of breast cancer, the 5-year incidence rate per 1000 women was 9.8 (95% CI 9.6–9.9) in the age group of 40–49 years, 9.2 (95% CI 9.0–9.5) in the age group of 50–64 years, and 8.5 (95% CI 8.2–8.9) in the age group of ≥65 years. Higher incidence rate was observed in women with dense breasts with family history in same age groups: 20.1 (95% CI 18.3–22.0), 15.9 (95% CI 13.9–18.1), and 15.4 (95% CI 12.1–19.4), respectively. Among those with a family history in mothers, the highest 5-year cumulative incidence risk per 1000 women was observed in women with dense breasts aged 40–49 years (21.2, 95% CI 18.4–24.4). Similarly, women with a family history in sisters, women with dense breasts and in the age group 40–49 years, had the highest cumulative incidence risk of 19.9 (95% CI 17.6–22.5). Overall, breast density was positively associated with breast cancer risk in all types of family history (any, mother only, and sister only) in all age groups, and the 5-year cumulative risk increased in those with dense breasts.
Table 2.
Frequency and five-year cumulative incidence of breast cancer by family history of breast cancer, breast density, and age groups.
| Age group and breast density | 5-year cumulative incidence, cases per 1000 women |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| None |
Any |
Mother only |
Sister only |
|||||||||
| n | Incidence (95% CI) | n | Incidence (95% CI) | n | Incidence (95% CI) | n | Incidence (95% CI) | |||||
| Age 40–49 | ||||||||||||
| Non-dense | 6672 | 5.8 | (5.6–6.0) | 209 | 13.0 | (10.5–15.8) | 89 | 14.2 | (10.2–19.1) | 107 | 13.0 | (9.8–17.0) |
| Dense | 26,320 | 9.8 | (9.6–9.9) | 1009 | 20.1 | (18.3–22.0) | 410 | 21.2 | (18.4–24.4) | 567 | 19.9 | (17.6–22.5) |
| Age 50–64 | ||||||||||||
| Non-dense | 10,857 | 5.3 | (5.2–5.5) | 331 | 10.7 | (9.1–12.5) | 71 | 11.0 | (7.7–15.2) | 227 | 11.2 | (9.2–13.6) |
| Dense | 13,659 | 9.2 | (9.0–9.5) | 501 | 15.9 | (13.9–18.1) | 102 | 12.8 | (9.3–17.2) | 341 | 16.5 | (14.1–19.3) |
| Age ≥65 | ||||||||||||
| Non-dense | 11,342 | 3.8 | (3.7–3.9) | 320 | 9.7 | (8.2–11.4) | 46 | 8.8 | (5.6–13.2) | 215 | 10.9 | (8.9–13.3) |
| Dense | 3987 | 8.5 | (8.2–8.9) | 144 | 15.4 | (12.1–19.4) | 23 | 14.9 | (7.7–26.4) | 100 | 17.4 | (13.0–22.9) |
Abbreviations: CI, confidence interval; n, number of breast cancer cases.
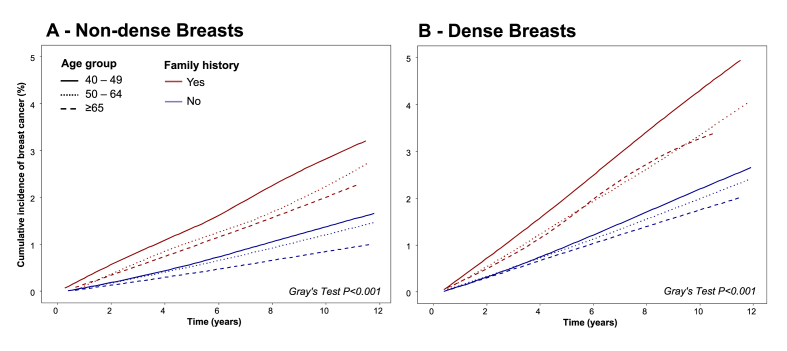
Fig. 2 shows the cumulative incidence function of breast cancer risk during the study period between women with and without a family history of breast cancer stratified by age group and breast density. Among women without dense breasts (Fig. 1A), those with a family history of breast cancer had a higher risk of breast cancer across all age groups than those without a family history. Patterns were similar among women with dense breasts (Fig. 1B); however, the cumulative risks were high overall. In addition, the highest incidence was observed in the youngest age group (aged 40–49), and there was a substantial difference compared with the other two older age groups, especially in women with a family history. In addition, in all age groups, women with dense breasts had a higher incidence rate of breast cancer compared to women with non-dense breasts.
Fig. 2.
Cumulative Incidence of Subsequent Breast Cancer, by First-Degree Family History, Mammographic Breast Density, and Age Group. The red lines represent women with a family history of breast cancer, and the blue lines represent women without a family history of breast cancer. The vertical solid lines represent the age group of 40-49 years, the dotted lines represent the age group of 50-64, and the dashed lines represent the age group of ≥65 years. Gray's tests were used to assess statistical differences between curves with and without family history in each subgroup.
3.3. Hazard ratios associated with breast cancer risk according to family history, breast density, and age group
The associations between family history of breast cancer and breast cancer risk were statistically significant in all age groups as well as both before and after adjusting for breast cancer density (Table 3). After adjusting for breast density, the HRs of family history of breast cancer slightly decreased in all age groups, but remained significant with an adjusted HR of approximately 2. Among women with non-dense breasts, participants with a family history had a 1.85- to 2.34-fold increased risk of future breast cancer, relative to those without family history. Similar findings were observed in women with dense breasts, with an elevated risk in women with a family history compared to those without family history of breast cancer: age group 40–49 aHR, 1.95 (95% CI 1.83–2.07), age group 50–64 aHR, 1.65 (95% CI 1.51–1.80), and age group ≥65 aHR, 1.74 (95% CI 1.47–2.06). Overall, the association between family history and breast cancer risk did not change substantially when adjusted for BI-RADS breast density or stratified by breast density.
Table 3.
Cox regression hazard ratios for effects of breast density and family history of breast cancer on breast cancer by age group in the study cohort.
| Age group and breast density | HR (95% CI) relative to no family history |
|||||
|---|---|---|---|---|---|---|
| Any |
Mother only |
Sister only |
||||
| HR | 95% CI | HR | 95% CI | HR | 95% CI | |
| Age 40–49 years | ||||||
| Model unadjusted for densitya | 1.99 | (1.88–2.11) | 2.03 | (1.86–2.21) | 1.99 | (1.84–2.14) |
| Model adjusted for breast densityb | 1.96 | (1.85–2.08) | 2.01 | (1.84–2.19) | 1.95 | (1.81–2.11) |
| Model stratified by breast densitya | ||||||
| Non-dense breast | 1.97 | (1.72–2.26) | 2.09 | (1.69–2.57) | 1.85 | (1.53–2.24) |
| Dense breast | 1.95 | (1.83–2.07) | 1.97 | (1.78–2.17) | 1.96 | (1.81–2.13) |
| Age 50–64 years | ||||||
| Model unadjusted for densitya | 1.74 | (1.63–1.87) | 1.61 | (1.38–1.86) | 1.79 | (1.65–1.95) |
| Model adjusted for breast densityb | 1.70 | (1.58–1.82) | 1.57 | (1.35–1.82) | 1.74 | (1.60–1.89) |
| Model stratified by breast densitya | ||||||
| Non-dense breast | 1.83 | (1.64–2.04) | 1.75 | (1.39–2.22) | 1.92 | (1.68–2.19) |
| Dense breast | 1.65 | (1.51–1.80) | 1.47 | (1.21–1.79) | 1.67 | (1.50–1.86) |
| Age ≥65 years | ||||||
| Model unadjusted for densitya | 2.02 | (1.84–2.21) | 1.85 | (1.46–2.34) | 2.24 | (2.00–2.50) |
| Model adjusted for breast densityb | 1.95 | (1.78–2.14) | 1.80 | (1.43–2.28) | 2.15 | (1.93–2.41) |
| Model stratified by breast densitya | ||||||
| Non-dense breast | 2.09 | (1.87–2.33) | 1.83 | (1.37–2.45) | 2.34 | (2.04–2.68) |
| Dense breast | 1.74 | (1.47–2.06) | 1.80 | (1.19–2.71) | 1.88 | (1.54–2.29) |
Abbreviations: HR, hazard ratio; CI, confidence interval.
Model was adjusted for age, BMI, smoking, drinking, exercise, age at menarche, age at menopause, childbirth, breastfeeding, hormone replacement therapy, and oral contraceptive use.
Model was adjusted for age, BMI, smoking, drinking, exercise, age at menarche, age at menopause, childbirth, breastfeeding, hormone replacement therapy, oral contraceptive use, and breast density (with 4 categories).
The strength of the association between family history and breast cancer was stronger in women with mothers as first-relative compared to those with sisters as first-relative in the age group 40–49 years but was lower in older age groups. After adjusting for breast density, women aged 40–49 with first-degree family history in mother had a 2.01-fold (95% CI 1.84–2.19) greater risk of breast cancer, and those with family history in sister had 1.95-fold higher risk (95% CI 1.81–2.11) compared to women without family history. Women aged ≥65 years with first-degree family history in mothers had a 1.80-fold (95% CI 1.43–2.28) greater risk of breast cancer, and those with family history in sister had 2.15-fold higher risk (95% CI 1.93–2.41) than women without a family history. Sensitivity analysis including only invasive breast cancer cases (Supplemental Table 1) and considering the interaction between BMI and menopausal status (Supplemental Table 2) yielded results consistent with the main analysis.
4. Discussion
In this cohort study, we evaluated the association between family history of breast cancer in first-degree relatives and the risk of future breast cancer in a population-based cohort, and further assessed whether the association would change when considering BI-RADS breast density together. We observed that women with a family history in any first-relative had an increased risk of breast cancer compared to women without a family history of breast cancer, and the risk was higher in women with dense breasts compared to women with non-dense breasts. Results with or without adjusting for breast density or stratified by breast density yielded similar findings that having a family history was associated with an overall 1.7- to 2-fold elevated risk of breast cancer depending on age group, suggesting an independent association of family history and breast cancer from breast density. Relationship types of family history in first-degree (any, mother, or sister) showed a comparably increased risk of breast cancer, irrespective of breast density. In addition, we found that the highest incidence was observed in the youngest age group (aged 40–49 years), which was consistent with previous statistics in Korean women [23]. In Korean women, the incidence of age-specific breast cancer peaks in the group aged 45–49 years and then decreases as age increases [23], which has different pattern in Western women, whose incidence of age-specific breast cancer increases with age [24]. Thus, the higher incidence in the youngest age group compared with the older age groups in our study reflects the reported trend in statistics in Korean women.
Our findings are consistent with those of previous studies showing that women with dense breasts had a higher risk of breast cancer [[25], [26], [27], [28], [29]], and this risk was elevated if the women additionally had a first-degree family history. The percentage of dense tissue in mammographic screening can explain 14% of the association between family history of breast cancer and breast cancer risk [30], and the combined effect of family history of breast cancer and higher levels of breast density has been shown to be associated with an elevated risk of breast cancer previously [[30], [31], [32]]. Several previous studies have reported findings on the relationship between family history of breast cancer, mammographic breast density, and the risk of breast cancer [[30], [31], [32], [33]]. Crest et al. found that women with first-degree-relative family history had a 1.5-fold increased risk (aOR 1.46, 95% CI 1.05–2.01) of breast cancer development compared to women with no family history [32]. Recent findings from the Breast Cancer Surveillance Consortium Cohort [34] reported an increased risk from 1.4- to 1.5-fold across different age groups after adjusting for breast density. Consistent with our study, the results of the association between family history or breast cancer and breast cancer risk in this study [34] did not substantially change before and after accounting for BI-RADS breast density, suggesting an independent association between each other and breast cancer risk.
In general, a family history of breast cancer increases the risk of early onset breast cancer in combination with inherited genetic risk in the family, compared with late-onset breast cancer [35]. The association between family history of breast cancer and breast cancer risk decreased slightly with age [36]. Findings from the Breast Cancer Surveillance Consortium Cohort likewise found that first-degree family history was associated with an increased risk of breast cancer [33], with a 1.5-fold increased risk in women aged 65–74 years (95% CI, 1.35–1.61) and a 1.4-fold increased risk in women aged 75 years and older (95% CI, 1.28–1.62) [33]. We found that a family history of breast cancer was associated with an increased risk of breast cancer not only in the young age group but also in those aged 60 years and older with comparable strength of the association. Consistently, the Iowa Women's Health Study Family [37] found that elderly women with first-degree history of breast cancer had a 1.5-fold elevated risk (aHR 1.54, 95% CI 1.24–1.93) of breast cancer compared to women with no family history. These results were consistent, with a relatively smaller effect, with our findings that a family history of breast cancer might affect the risk of breast cancer even in older age groups.
The prevalence of women reporting a first-degree family history of breast cancer has increased from 11% to 16% over the last three decades in the United States [36]. The current guideline from the American Cancer Society recommends that women who have a first-degree relative with a BRCA1 or BRCA2 gene mutation without genetic testing should undergo an annual breast magnetic resonance imaging scan in addition to normal mammographic screening [38,39]. In our study, 1.6% of women who underwent screening reported having a family history of breast cancer, and currently no specific recommendation for screening strategy is available for Korean women with a family history of breast cancer. Despite the relatively small proportion, their doubled increase in breast cancer indicates that they could benefit from a tailored screening strategy to detect early breast cancer.
A history of breast cancer in both first- and second-degree relatives results in a substantially increased risk of breast cancer compared to when there is a history of breast cancer in first-degree relatives alone [34]. However, information on family history of breast cancer in this study was available only for first-degree relatives; thus, we were unable to assess the associations with a more extended family history and could not consider family structure. In addition, a family history of breast cancer was collected as self-reported information at the time of screening. Although a family history of breast cancer in first-degree relatives is usually accurately reported [40,41], we cannot fully eliminate the possibility of recall bias. This study used BI-RADS breast density measurements, which were reported by radiologists at multiple screening centers in Korea, which often leads to moderate inter-observer agreement [42]. However, in Korea, a mammography education program to standardize the performance of radiologists is available, which may increase the reproducibility of the interpretation [43]. Inter-radiologist variability was assessed in randomly selected films from the Korean National Breast Cancer Screening Program, reporting an inter-radiologist variability of 0.83, indicating a very high agreement [44].
This study has some limitations. First, our database did not contain information regarding the clinical characteristics of breast cancer, such as tumor characteristics. Therefore, we were unable to assess how the association of family history of breast cancer and breast density differs by clinical characteristics such as hormone receptor status. Second, the BI-RADS breast density measured in our database includes measurements from both film and digital mammography. Considering that more than 90% of the mammography procedures were film mammography [45], the breast density measured in our study could be less affected by screening modalities. However, breast density could vary depending on the radiologist's ability and thus is subject to inter- and intra-observer agreement.
This study had several strengths. The study included more than four million women from a population-based breast cancer screening, which broadly represents the demographic composition of women in Korea and other East Asian populations. Furthermore, the study had a retrospective cohort design, with a large number of breast cancer cases, and complete breast cancer ascertainment from the health insurance database with cancer code for reimbursement, covering the whole population. Our results also accounted for other confounding factors including adiposity, age at menarche, age at menopause, menopausal status, postmenopausal hormone therapy, and other health behaviors. Hence, this comprehensive national cohort study elucidates the association between first-degree family history of breast cancer, breast density, and breast cancer risk. Findings from this study provide new evidence for this relationship and help reinforce existing interventions to prevent or detect breast cancer in women with a family history of breast cancer and dense breasts, who have a higher risk.
Funding statement
This work was supported by a National Research Foundation of Korea grant funded by the Korean government (MSIT) (grant no. 2021R1A2C1011958), and was partly supported by Institute of Information & communications Technology Planning & Evaluation (IITP) grant funded by the Korea government(MSIT) (grant no. 2020-0-01373, Artificial Intelligence Graduate School Program (Hanyang University)) and the research fund of Hanyang University (HY-202100000670036).
Author contributions statement
Thi Xuan Mai Tran: Conceptualization, data curation, formal analysis, methodology, software, visualization, validation, writing original draft, writing-review and editing. Soyeoun Kim: Conceptualization, formal analysis, methodology, project administration, writing-review and editing. Huiyeon Song: Data curation, project administration, writing-review and editing. Boyoung Park: Conceptualization, funding acquisition, supervision, methodology, software, validation, writing-review and editing.
Data availability statement
The data that support the findings of this study are available on the website of the National Health Insurance Sharing Service (https://nhiss.nhis.or.kr/). We accessed the database after submitting the study protocol, the IRB approval document, and the reviewed request form by the committee. Further information is available from the corresponding author upon request.
Ethics statement
This study was approved by the Institutional Review Board of Hanyang University College of Medicine (approval no. HYUIRB-202106-003-1).
Declaration of competing interest
The authors declare no potential conflicts of interest.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.breast.2022.08.008.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Familial breast cancer: collaborative reanalysis of individual data from 52 epidemiological studies including 58 209 women with breast cancer and 101 986 women without the disease. Lancet. 2001;358:1389–1399. doi: 10.1016/S0140-6736(01)06524-2. [DOI] [PubMed] [Google Scholar]
- 2.Pharoah P.D.P., Antoniou A.C., Easton D.F., Ponder B.A.J. Polygenes, risk prediction, and targeted prevention of breast cancer. N Engl J Med. 2008;358:2796–2803. doi: 10.1056/NEJMsa0708739. [DOI] [PubMed] [Google Scholar]
- 3.Wu H.-C., Do C., Andrulis I.L., John E.M., Daly M.B., Buys S.S., et al. Breast cancer family history and allele-specific DNA methylation in the legacy girls study. Epigenetics. 2018;13:240–250. doi: 10.1080/15592294.2018.1435243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bahcall O. Common variation and heritability estimates for breast, ovarian and prostate cancers. Nat Genet. 2013;10:304. [Google Scholar]
- 5.Michailidou K., Lindström S., Dennis J., Beesley J., Hui S., Kar S., et al. Association analysis identifies 65 new breast cancer risk loci. Nature. 2017;551:92–94. doi: 10.1038/nature24284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yanes T., Young M.-A., Meiser B., James P.A. Clinical applications of polygenic breast cancer risk: a critical review and perspectives of an emerging field. Breast Cancer Res. 2020;22:1–10. doi: 10.1186/s13058-020-01260-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tran T.X.M., Moon S.G., Kim S., Park B. Association of the interaction between mammographic breast density, body mass index, and menopausal status with breast cancer risk among Korean women. JAMA Netw Open. 2021;4 doi: 10.1001/jamanetworkopen.2021.39161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ursin G., Lillie E.O., Lee E., Cockburn M., Schork N.J., Cozen W., et al. The relative importance of genetics and environment on mammographic density. Cancer Epidemiology and Prevention Biomarkers. 2009;18:102–112. doi: 10.1158/1055-9965.EPI-07-2857. [DOI] [PubMed] [Google Scholar]
- 9.Sung J., Song Y.-M., Stone J., Lee K., Jeong J-i, Kim S.-S. Genetic influences on mammographic density in Korean twin and family: the Healthy Twin study. Breast Cancer Res Treat. 2010;124:467–474. doi: 10.1007/s10549-010-0852-9. [DOI] [PubMed] [Google Scholar]
- 10.Sieh W., Rothstein J.H., Klein R.J., Alexeeff S.E., Sakoda L.C., Jorgenson E., et al. Identification of 31 loci for mammographic density phenotypes and their associations with breast cancer risk. Nat Commun. 2020;11:1–11. doi: 10.1038/s41467-020-18883-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim S., Park B. Association between changes in mammographic density category and the risk of breast cancer: a nationwide cohort study in East-Asian women. Int J Cancer. 2021;148:2674–2684. doi: 10.1002/ijc.33455. [DOI] [PubMed] [Google Scholar]
- 12.Maskarinec G., Nakamura K.L., Woolcott C.G., Conroy S.M., Byrne C., Nagata C., et al. Mammographic density and breast cancer risk by family history in women of white and Asian ancestry. Cancer Causes Control. 2015;26:621–626. doi: 10.1007/s10552-015-0551-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Braithwaite D., Miglioretti D.L., Zhu W., Demb J., Trentham-Dietz A., Sprague B., et al. Family history and breast cancer risk among older women in the breast cancer surveillance consortium cohort. JAMA Intern Med. 2018;178:494–501. doi: 10.1001/jamainternmed.2017.8642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Martin L.J., Melnichouk O., Guo H., Chiarelli A.M., Hislop T.G., Yaffe M.J., et al. Family history, mammographic density, and risk of breast cancer. Cancer Epidemiology and Prevention Biomarkers. 2010;19:456–463. doi: 10.1158/1055-9965.EPI-09-0881. [DOI] [PubMed] [Google Scholar]
- 15.Lim S.-E., Ahn H., Lee E.S., Kong S.-Y., Jung S.-Y., Lee S., et al. Interaction effect between breast density and reproductive factors on breast cancer risk in Korean population. Journal of cancer prevention. 2019;24:26. doi: 10.15430/JCP.2019.24.1.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Louro J., Román M., Posso M., Vázquez I., Saladié F., Rodriguez-Arana A., et al. Developing and validating an individualized breast cancer risk prediction model for women attending breast cancer screening. PLoS One. 2021;16 doi: 10.1371/journal.pone.0248930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bahk J., Kim Y.Y., Kang H.Y., Lee J., Kim I., Lee J., et al. Using the national health information database of the national health insurance Service in Korea for monitoring mortality and life expectancy at national and local levels. J Kor Med Sci. 2017;32:1764–1770. doi: 10.3346/jkms.2017.32.11.1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim S., Tran T.X.M., Song H., Ryu S., Chang Y., Park B. Mammographic breast density, benign breast disease, and subsequent breast cancer risk in 3.9 million Korean women. Radiology. 2022 doi: 10.1148/radiol.212727. [DOI] [PubMed] [Google Scholar]
- 19.Hong S., Lee Y.Y., Lee J., Kim Y., Choi K.S., Jun J.K., et al. Trends in cancer screening rates among Korean men and women: results of the Korean national cancer screening survey, 2004–2018. Cancer Res Treat. 2021;53:330–338. doi: 10.4143/crt.2020.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Burnside E.S., Sickles E.A., Bassett L.W., Rubin D.L., Lee C.H., Ikeda D.M., et al. The ACR BI-RADS® experience: learning from history. J Am Coll Radiol. 2009;6:851–860. doi: 10.1016/j.jacr.2009.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim S., Kwon S. Impact of the policy of expanding benefit coverage for cancer patients on catastrophic health expenditure across different income groups in South Korea. Soc Sci Med. 2015;138:241–247. doi: 10.1016/j.socscimed.2015.06.012. [DOI] [PubMed] [Google Scholar]
- 22.Gray R.J. A class of K-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat. 1988:1141–1154. [Google Scholar]
- 23.Hong S., Won Y.J., Lee J.J., Jung K.W., Kong H.J., Im J.S., et al. Cancer statistics in Korea: incidence, mortality, survival, and prevalence in 2018. Cancer Res Treat. 2021;53:301–315. doi: 10.4143/crt.2021.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Saika K., Machii R. Age-specific breast cancer incidence rate in the world. Jpn J Clin Oncol. 2020;50:1481–1482. doi: 10.1093/jjco/hyaa226. [DOI] [PubMed] [Google Scholar]
- 25.Boyd N.F., Guo H., Martin L.J., Sun L., Stone J., Fishell E., et al. Mammographic density and the risk and detection of breast cancer. N Engl J Med. 2007;356:227–236. doi: 10.1056/NEJMoa062790. [DOI] [PubMed] [Google Scholar]
- 26.Yaghjyan L., Colditz G.A., Rosner B., Tamimi R.M. Mammographic breast density and breast cancer risk: interactions of percent density, absolute dense, and non-dense areas with breast cancer risk factors. Breast Cancer Res Treat. 2015;150:181–189. doi: 10.1007/s10549-015-3286-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pettersson A., Graff R.E., Ursin G., Santos Silva I.D., McCormack V., Baglietto L., et al. Mammographic density phenotypes and risk of breast cancer: a meta-analysis. J Natl Cancer Inst. 2014;106 doi: 10.1093/jnci/dju078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Park B., Cho H.M., Lee E.H., Song S., Suh M., Choi K.S., et al. Does breast density measured through population-based screening independently increase breast cancer risk in Asian females? Clin Epidemiol. 2018;10:61–70. doi: 10.2147/CLEP.S144918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim S., Park B. Association between changes in mammographic density category and the risk of breast cancer: a nationwide cohort study in East-Asian women. Int J Cancer. 2021;148:2674–2684. doi: 10.1002/ijc.33455. [DOI] [PubMed] [Google Scholar]
- 30.Martin L.J., Melnichouk O., Guo H., Chiarelli A.M., Hislop T.G., Yaffe M.J., et al. Family history, mammographic density, and risk of breast cancer. Cancer Epidemiol Biomarkers Prev. 2010;19:456–463. doi: 10.1158/1055-9965.EPI-09-0881. [DOI] [PubMed] [Google Scholar]
- 31.Maskarinec G., Nakamura K.L., Woolcott C.G., Conroy S.M., Byrne C., Nagata C., et al. Mammographic density and breast cancer risk by family history in women of white and Asian ancestry. Cancer Causes Control. 2015;26:621–626. doi: 10.1007/s10552-015-0551-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Crest A.B., Aiello E.J., Anderson M.L., Buist D.S. Varying levels of family history of breast cancer in relation to mammographic breast density (United States) Cancer Causes Control. 2006;17:843–850. doi: 10.1007/s10552-006-0026-6. [DOI] [PubMed] [Google Scholar]
- 33.Braithwaite D., Miglioretti D.L., Zhu W., Demb J., Trentham-Dietz A., Sprague B., et al. Family history and breast cancer risk among older women in the breast cancer surveillance consortium cohort. JAMA Intern Med. 2018;178:494–501. doi: 10.1001/jamainternmed.2017.8642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ahern T.P., Sprague B.L., Bissell M.C.S., Miglioretti D.L., Buist D.S.M., Braithwaite D., et al. Family history of breast cancer, breast density, and breast cancer risk in a U.S. Breast cancer screening population. Cancer Epidemiol Biomarkers Prev. 2017;26:938–944. doi: 10.1158/1055-9965.EPI-16-0801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tilanus-Linthorst M.M., Bartels C.C., Obdeijn A.I., Oudkerk M. Earlier detection of breast cancer by surveillance of women at familial risk. Eur J Cancer. 2000;36:514–519. doi: 10.1016/s0959-8049(99)00337-8. [DOI] [PubMed] [Google Scholar]
- 36.Shiyanbola O.O., Arao R.F., Miglioretti D.L., Sprague B.L., Hampton J.M., Stout N.K., et al. Emerging trends in family history of breast cancer and associated risk. Cancer Epidemiol Biomarkers Prev. 2017;26:1753–1760. doi: 10.1158/1055-9965.EPI-17-0531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sweeney C., Blair C.K., Anderson K.E., Lazovich D., Folsom A.R. Risk factors for breast cancer in elderly women. Am J Epidemiol. 2004;160:868–875. doi: 10.1093/aje/kwh276. [DOI] [PubMed] [Google Scholar]
- 38.Saslow D., Boetes C., Burke W., Harms S., Leach M.O., Lehman C.D., et al. American cancer society guidelines for breast screening with MRI as an adjunct to mammography. CA A Cancer J Clin. 2007;57:75–89. doi: 10.3322/canjclin.57.2.75. [DOI] [PubMed] [Google Scholar]
- 39.Oeffinger K.C., Fontham E.T.H., Etzioni R., Herzig A., Michaelson J.S., Shih Y.-C.T., et al. Breast cancer screening for women at average risk: 2015 guideline update from the American cancer society. JAMA. 2015;314:1599–1614. doi: 10.1001/jama.2015.12783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.John E.M., Canchola A.J., Sangaramoorthy M., Koo J., Whittemore A.S., West D.W. Race/ethnicity and accuracy of self-reported female first-degree family history of breast and other cancers in the northern California breast cancer family registry. Cancer Epidemiol Biomarkers Prev. 2019;28:1792–1801. doi: 10.1158/1055-9965.EPI-19-0444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sijmons R.H., Boonstra A.E., Reefhuis J., Hordijk-Hos J.M., de Walle H.E., Oosterwijk J.C., et al. Accuracy of family history of cancer: clinical genetic implications. Eur J Hum Genet. 2000;8:181–186. doi: 10.1038/sj.ejhg.5200441. [DOI] [PubMed] [Google Scholar]
- 42.Melnikow J., Fenton J.J., Whitlock E.P., Miglioretti D.L., Weyrich M.S., Thompson J.H., et al. Supplemental screening for breast cancer in women with dense breasts: a systematic review for the U.S. Preventive services task force. Ann Intern Med. 2016;164:268–278. doi: 10.7326/M15-1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lee E.H., Jun J.K., Jung S.E., Kim Y.M., Choi N. The efficacy of mammography boot camp to improve the performance of radiologists. Korean J Radiol. 2014;15:578–585. doi: 10.3348/kjr.2014.15.5.578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jo H.-M., Song S., Lee E.H., Ko K., Kang B.J., Cha J.H., et al. Interpretive volume and inter-radiologist agreement on assessing breast density. J Korean Soc Breast Screening. 2018;15:15–22. [Google Scholar]
- 45.Song S.Y., Hong S., Jun J.K. Digital mammography as a screening tool in Korea. Journal of the Korean Society of Radiology. 2021:82. doi: 10.3348/jksr.2021.0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available on the website of the National Health Insurance Sharing Service (https://nhiss.nhis.or.kr/). We accessed the database after submitting the study protocol, the IRB approval document, and the reviewed request form by the committee. Further information is available from the corresponding author upon request.