Abstract
During persistent pain, the dorsal spinal cord responds to painful inputs from the site of injury, but the molecular modulatory processes have not been comprehensively examined. Using transcriptomics and multiplex in situ hybridization, we identified the most highly regulated receptors and signaling molecules in rat dorsal spinal cord in peripheral inflammatory and post-surgical incisional pain models. We examined a time course of the response including acute (2 hrs) and longer term (2 day) time points after peripheral injury representing the early onset and instantiation of hyperalgesic processes. From this analysis, we identify a key population of superficial dorsal spinal cord neurons marked by somatotopic upregulation of the opioid neuropeptide precursor prodynorphin, and two receptors: the neurokinin 1 receptor, and anaplastic lymphoma kinase. These alterations occur specifically in the glutamatergic subpopulation of superficial dynorphinergic neurons. In addition to specific neuronal gene regulation, both models showed induction of broad transcriptional signatures for tissue remodeling, synaptic rearrangement, and immune signaling defined by complement and interferon induction. These signatures were predominantly induced ipsilateral to tissue injury, implying linkage to primary afferent drive. We present a comprehensive set of gene regulatory events across two models that can be targeted for the development of non-opioid analgesics.
Keywords: RNA-Seq, Persistent pain, Transcriptomics, Opioids, Spinal cord
Introduction
The underlying neurobiological and behavioral modifications needed to promote healing of an organism provide an empirical framework for understanding normal and pathological pain states. At a minimum, there is a dualistic modulation of pain sensitivity to promote resumption of physical activity yet prevent tissue re-injury. Throughout the post-injury persistent pain state, opponent neural processes participate in modulation of a sensory set-point to balance acute and tonic incoming nociceptive information to control resolution of the hyperalgesic state as the organism heals. In terms of neural mechanisms, we hypothesize the opponent process consists of a suppression of tonic nociceptive input from the injured region and a concomitant accentuation of potentially injurious stimuli. Behavioral and electrophysiological research shows that responses to acute potentially damaging stimuli are enhanced. 45, 48, 129 Both of the opposing processes have adaptive significance, allowing activities of daily living to resume while simultaneously protecting the organism from further damage. Algesic mediators released peripherally during injury states tonically stimulate nociceptive primary afferent endings. To counterbalance this, the central apparatus adapts to desensitize spinal circuits to persistent “low level” inputs while maintaining sensitization of more intense insults. These plastic events must be established rapidly to serve a protective function and promote productive behavior yet must be reversible so that the neurobehavioral adaptations resolve with the injury.
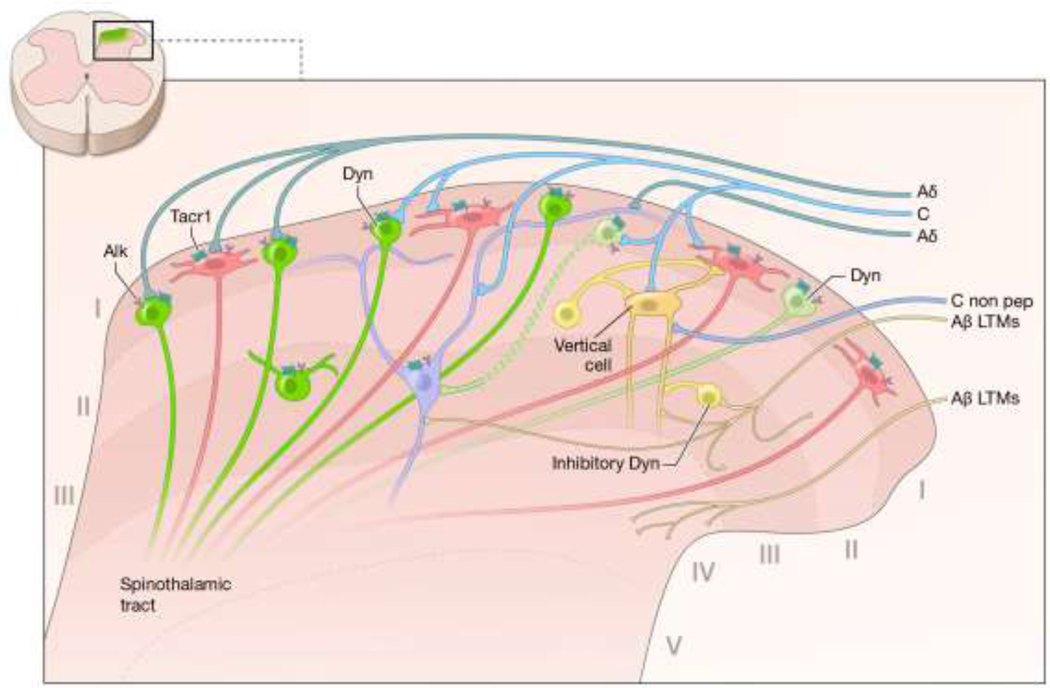
In pathological pain states in humans, the hyperalgesic process becomes maladaptive indicating failure and/or exacerbation of one or more steps in the normally protective process. Throughout the hyperalgesic process, gene induction is initiated to enact and support long-term changes in excitability and signaling characteristics of the nociceptive circuit. Many of the alterations occur in the superficial laminae of the dorsal spinal cord, 48 where sensory inputs are received, integrated, and projected rostrally to multiple regions of the brain. Investigations of the processes involved in this plasticity have revealed roles for NMDA receptors, 109 inhibitory synapses, non-neural cells,54 and a variety of other mechanisms which could potentially be manipulated to produce analgesia. 111 Elements induced in response to persistent inflammatory nociceptive input encompass gene induction,116 peptide biosynthesis93 and physiological alterations79, 143, 144 and continue to be studied. Investigating the intricacies of the neural circuits, and the molecular identities of the cells involved in these processes is particularly relevant to understanding the dynamic states induced by persistent or neuropathic pain models.37, 108 We describe two distinct gene regulatory programs that occur in the dorsal spinal cord receiving input from the damaged hindlimb in rat models of either hind paw inflammation or surgical incision. The first of these programs is a unilateral neuronal and microglial gene regulatory response. The second program is a bilateral generalized immune response characterized by innate immune activation signatures.
The present report demonstrates prominent transcriptional regulation of a glutamatergic-dynorphinergic neuronal population in dorsal spinal cord during acute phase hyperalgesia. The induction of the neuropeptide precursor prodynorphin is a known indicator of spinal neural responses to peripheral injury.21, 48, 57, 93, 129 However the role of these dynorphinergic cells, and consequently the analgesic utility of the spinal kappa opioid receptor system is an ongoing question.9, 38, 147 We aim to further investigate some of the dynorphinergic mechanism by assessing the whole tissue transcriptome at the time of peak prodynorphin mRNA induction (48 hrs).48, 129 We characterize the most prominent signaling molecules and receptors that accompany this induction. For several genes, this induction colocalized to the same neuronal subpopulation. Further, using comprehensive transcriptional profiling of both the ipsilateral and contralateral dorsal horn over the first two days of the hyperalgesic process, it is possible to deconvolute bilateral immune processes related to immune defensive priming from unilateral neural and microglial processes related to the hyperalgesic state. The results identify key regulatory receptors that represent new avenues for the study of pain, and the development of potential analgesic agents.
Methods
Overall structure of the study, selection of hyperalgesic models, and selection of timepoints.
The present study uses the inflammatory agent carrageenan to cause a sterile abscess in the hind paw, which strongly stimulates the immune system. This sulfated polysaccharide has been shown to activate B-cells, and has also been used as an adjuvant.25, 35, 146 Intraplantar injection of carrageenan causes profound hyperalgesia, especially in response to thermal stimuli.25, 48, 49 Alongside this model, we also examined transcriptional changes in response to hind paw surgical incision.116 In both models, peripheral tissue damage drives pro-inflammatory signaling cascades and DRG afferent activation, leading to central sensitization and behavioral changes characterized by hyperresponsiveness and guarding behavior to protect the injured hind limb from further injury. We examined two timepoints during this response based on previous studies delineating the greatest transcriptional responses. First, we examined rapid induction of immediate early genes at 2 hrs. Second, we examined the later phase (48 hrs) of spinal cord gene expression, which includes target genes activated by transcription factor binding initiated at the 2 hr timepoint.49, 99 The overall model of how these events develop over the hyperalgesic time course has been described extensively.129 Transcriptional activation of these target genes is maximal at around 48 hrs for several target genes, and this timepoint may represent a transcriptional response aimed at driving the spinal cord to a new set point. Subsequently, after the spinal circuitry has reached homeostasis, transcriptional changes are less pronounced, and may be involved in maintenance rather than establishment of this phenomenon.48, 129 Therefore, the present study examines the molecular transcriptomic and anatomical pathways and correlates of the initiation events in response to peripheral injury. This set of investigations is important to determine the precise drivers of the hyperalgesic remodeling occurring in response to tissue damage. Further, the receptors and signaling molecules engaged in the establishment of hyperalgesia may hold important clues for the development of anti-hyperalgesic and analgesic drug development. These foundational studies may also provide insight into the instantiation of chronic pain conditions as these early events may drive subsequent long-term pathological alterations.
Animal care and rat nociceptive behavioral testing
All animal work was performed under an approved animal care and use committee protocol at the Clinical Center, National Institutes of Health. For all experiments, rats were housed on a 12 hr light-dark cycle and provided food and water ad libitum. All procedures were conducted under a protocol approved by the Animal Care and Use Committee of the NIH Clinical Center. Within each experiment, animals were born on approximately the same date and were obtained at the same time. In all behavioral paradigms in which there were multiple groups (i.e. drug-treated vs. control) injections were performed by a separate investigator and the experimenter testing the animals was blinded to treatment group.
For behavioral testing in Figure 1, adult male Sprague-Dawley rats (220–350g, Envigo) were used. Hyperalgesia was induced by injection of 6mg (4%) of type IV λ-carrageenan (C-3889, Sigma-Aldrich) into the plantar surface of the hind paw (N=6 animals). The resulting edema was measured with a digital caliper (Figure 1A)129 For nociceptive testing, rats were placed on an elevated glass platform, habituated for approximately 10 minutes, and stimulated using a radiant thermal heat source (Figure 1B; Plantar Test, Ugo Basille, Monvalle, Italy).128 Subsequent to radiant thermal testing, animals were placed on an elevated wire mesh grid, allowed to habituate again, and mechanical sensitivity was assayed using Von Frey filaments (Figure 1C) following the up-down method.116 After Von Frey filament testing, a pinprick stimulus was delivered to examine guarding behavior.4, 5 In the uninflamed state, this produces a rapid Aδ-fiber mediated withdrawal and/or orientation to the stimulus.5 In the inflamed state, however, animals respond to the pinprick stimulation by guarding the stimulated paw, the duration of which was scored with a stopwatch (Figure 1D).
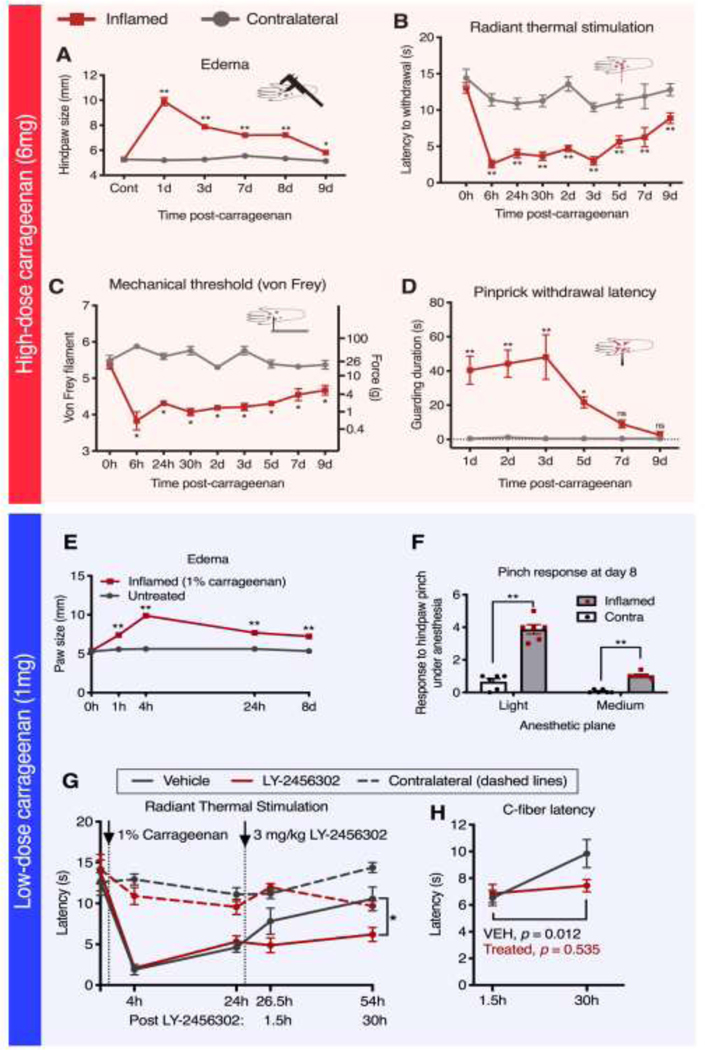
Figure 1. Paw width and behavioral response after intraplantar injection with high and low dose carrageenan.
A, B, C, D. Animals were injected with high-dose (150 μl, 4%, 6mg) carrageenan and then tested for peripheral edema and behavioral changes. A. Injection of high-dose carrageenan caused maximal edema at 1 day, and was mostly resolved by 9 days. B. Radiant thermal stimulation was performed to assess thermal hyperalgesia for high-dose carrageenan, showing maximal sensitivity at 6 hrs. This effect begins to resolve at 3 days and does not full resolve by 9 days. C. Mechanical allodynia was assessed for high-dose carrageenan by von Frey filament testing, showing increased mechanical sensitivity at every timepoint tested. D. For high-dose carrageenan, pinprick stimulation causes guarding behavior in inflamed but not uninflamed animals. The duration of the guarding response was assessed in inflamed animals. The guarding response was significantly prolonged in animals between 1–5 days after administration of the inflammatory agent. E, F, G, H. Another cohort of animals were injected with a low-dose (150 μl, 1%, 1 mg) of carrageenan, and also tested for changes in behavior. E. Injection of low-dose carrageenan resulted in significant but less severe inflammation (relative to high-dose). Peak measurements were observed at 4 hours, largely resolving over 24–48 hrs but lasting at least 8 days. F. Persistent hyperalgesia 8 days after low-dose carrageenan was observed in response to stronger mechanical stimulation (i.e. pinch) in the inflamed hind paw of anesthetized rats. G. I.P. injection of a kappa-opioid receptor antagonist (3mg/kg LY-2456302) subsequent to low-dose carrageenan injection resulted in prolonged unilateral thermal hyperalgesia at 30 hours following drug administration (54 hours following inflammation). Specifically, animals injected with drug exhibited significantly shorter response latencies to radiant thermal stimulation on the inflamed paw than animals injected with vehicle. H. Similarly, C-fiber selective thermal stimulation (with a 1000 mA laser at 13 cm distance from paw) of the inflamed hind paw showed recovery in the vehicle group which did not occur with drug treatment. A, B, D, E, G, H. Significance testing was performed using two-way ANOVA followed by Holm-Šidák corrections. C. Significance testing was performed using repeated Mann-Whitney U-tests followed by Holm- Šidák corrections (N=6); *, p < 0.05; **, p < 0.01; Error bars represent SEM.
A separate cohort of animals was characterized for responses to a lower dose of carrageenan. The 1% dose of carrageenan produces a less severe inflammation (relative to 6mg) that largely resolves over 24–48 hrs, allowing more sensitivity for modulation by pharmacological interventions. Edema measurements for the 1% dose were taken as described above (Figure 1E). The data in Figure 1E are shown based on multiple experiments with different endpoints and include different numbers of animals at each timepoint (0 hr, N=10; 1 hr, N=4; 4 hrs, N=16; 24 hrs, N=12; 8 d, N=5). This is important because the maximal inflammation with 6 mg (4%) carrageenan leads to a level of hypersensitivity that reduces assay sensitivity in pharmacological studies (note the short withdrawal latencies at the 6hr timepoint, Figure 1B).47, 48, 129 The medium (1%) dose of carrageenan also provides the potential to observe either an increase or a decrease in hyperalgesia. Behavioral responses to mechanical pinch delivered to ipsilateral and contralateral paws were characterized at day 8 after inflammation with 1% carrageenan (N=6 rats), a timepoint at which awake behaving responses have largely resolved. Rats were immobilized with a restraint dose of ∼1% isoflurane through a nose cone114 and toothed forceps were used to test their nociceptive responsiveness under either light or medium isoflurane anesthetic gas.51 These stronger stimuli under light anesthesia were capable of unmasking hyperalgesia long after the resolution of standard awake behaving responses. The light and medium doses corresponded to approximately 1% or 1.2% isoflurane respectively, delivered by nose cone (Figure 1F), and were adapted from a previous study.114 The pinch was delivered using approximately 1kg of instantaneous force as measured by a pressure application monitor (38500, Ugo Basille) with the forceps positioned such that one toothed end was on the dorsum and one toothed end was situated in the mid-plantar region (Figure 1F). Scoring was tabulated by adding one point for each hind paw paddle-like movement in response to a single pinch, with some animals paddling 4–5 times in response to a single pinch under light anesthesia. This response was not entirely stereotyped and sometimes resulted in a longer duration of guarding-like behavior where the paw was retracted for several seconds, which occurred in place of paddling. Each 2 seconds of this guarding response was counted as a single point to tabulate the response score. However, the majority of strong responses were observed as multiple paddles, and both behaviors (paddling and increased guarding-like durations) were only observed on the inflamed paw. For Figure 1A, B, D and E significance testing was performed using two-way ANOVA followed by Holm-Šidák corrections. In Figure 1C, significance testing was performed using repeated Mann-Whitney U-tests followed by Holm-Šidák corrections; *, p < 0.05; **, p < 0.01; Error bars represent SEM.
Administration of the kappa opioid antagonist LY-2456302
The selective kappa opioid antagonist,121 LY-2456302 was obtained from the NIDA Drug Supply Program, and dissolved in sterile 0.9% saline solution containing 0.1% tween 20, 0.05% ascorbate, 10% N-Methyl-2-pyrrolidone, 5% ethanol, and 5% Cremophor EL. This solution, without the addition of drug compound, was used as the vehicle control. This compound was administered at 3 mg/kg by intraperitoneal injection. A group of 18 male adult rats was habituated to testing until baseline behavioral values were stable (3 test sessions over 3 days). Additional details of these experiments (Figure 1G, H) are found in the supplementary methods.
Overall experimental design and methodology of the three core transcriptomic experiments in the present report
The current report examines the effects of unilateral hind paw inflammation or incision on the dorsal horn transcriptome. Four independently-powered sequencing experiments were analyzed in the present study, of which three were performed as part of this study, and one (surgical incision) is reanalyzed from a previous study.116 For the experiment referred to as “Inflammation 1”, male animals were examined at 0 hrs, 2 hrs and 48 hrs, for the ipsilateral and contralateral dorsal spinal quadrants. In this experiment we looked for induction at 2 hrs and 48 hrs vs. naïve controls, and also examined the minor impact on the contralateral side as an additional control. In Inflammation 2, we expand the most impactful time point (48 hrs ipsilateral) into male and female animals, comparing each to their respective naïve control within sexes. Note that two of the experiments are considered together (the independently analyzed and powered male vs. female experiments in Inflammation 2) as these samples were processed at the same time, as described in the supplementary methods. Within each separate experiment, tissue samples were collected by the same individual using identical methodologies as described elsewhere and performed at the same time. Between experiments, the same equipment and protocol were used with minor variations. In all cases, sequencing and library preparation was performed at the National Institutes of Health Intramural Sequencing Center as described previously (with minor variations).115, 127, 128, 130 Detailed methodology for each of the experiments is found in the supplementary materials.
Alignment and quantification of RNA-Seq datasets
Alignment, quantification, and quality control were performed using the MAGIC pipeline (version magic.2017_06_19) and a genomic target based on Rn6 annotations. Quantification and normalization of gene counts were performed as described previously.73, 159 Gene quantification is reported as significant fragments per kilobase per million aligned reads (sFPKM), a normalized expression metric that adjusts for library size, gene length, insert size of the library, and level of genomic contamination. Sequencing data examined in the present manuscript are available for download through the Sequence Read Archive under the BioProject PRJNA593520: Transcriptomic analyses of dorsal spinal cord after peripheral inflammation. Data from the surgical incision are available under BioProject PRJNA412076: Transcriptomic analyses of dorsal spinal cord after surgical incision.116 Analysis of dog and human DRG transcriptomics was also mined from our previously published datasets (Supplementary Table 1).50, 130
Statistical analyses and determination of differentially expressed genes (DEGs) from RNA-Seq
Differential genes were determined using DESeq2 (v1.26.0)78 in R (v3.6.1) using identical parameters for alignment, quantification and statistical analyses for each of the experiments. Briefly, for each dataset, genes were filtered by demanding a counts per million of at least 0.25 in at least 3 biological replicates before statistics were calculated. The remainder of the DESeq2 pipeline was run using standard procedures, with an adjusted p-value of 0.01 after Benjamini-Hochberg (B-H) correction. This is the standard adjustment for this method.
Expression ratios were used as a representation of gene changes in the dataset. Throughout the manuscript the following formula is used:
In this calculation, 0.1 was added to both the numerator and the denominator to reduce the impact of dividing by very small values as when genes with a low basal expression are strongly induced. This is also a consideration for genes contributed by recruited leukocytes that are not present in basal conditions.
Scatter plots of expression ratio vs. sFPKM for the 2 hr vs. control comparison in the Inflammation 1 dataset.
Average ipsilateral sFPKM values and expression ratios (2 hrs/control) were calculated between the control and ipsilateral 2 hrs timepoints of the Inflammation 1 dataset. Significant (p < 0.01) genes with an expression ratio >2 at 2 hrs in the Inflammation 1 dataset were selected and graphed against their corresponding average sFPKM values (Figure 2A). A selection of the time course data (control, 2 hrs, 48 hrs) for 10 of the most highly induced genes at 2 hrs are presented individually (Figure 2B). These 10 genes were selected because they were the top 10 induced genes by significance.
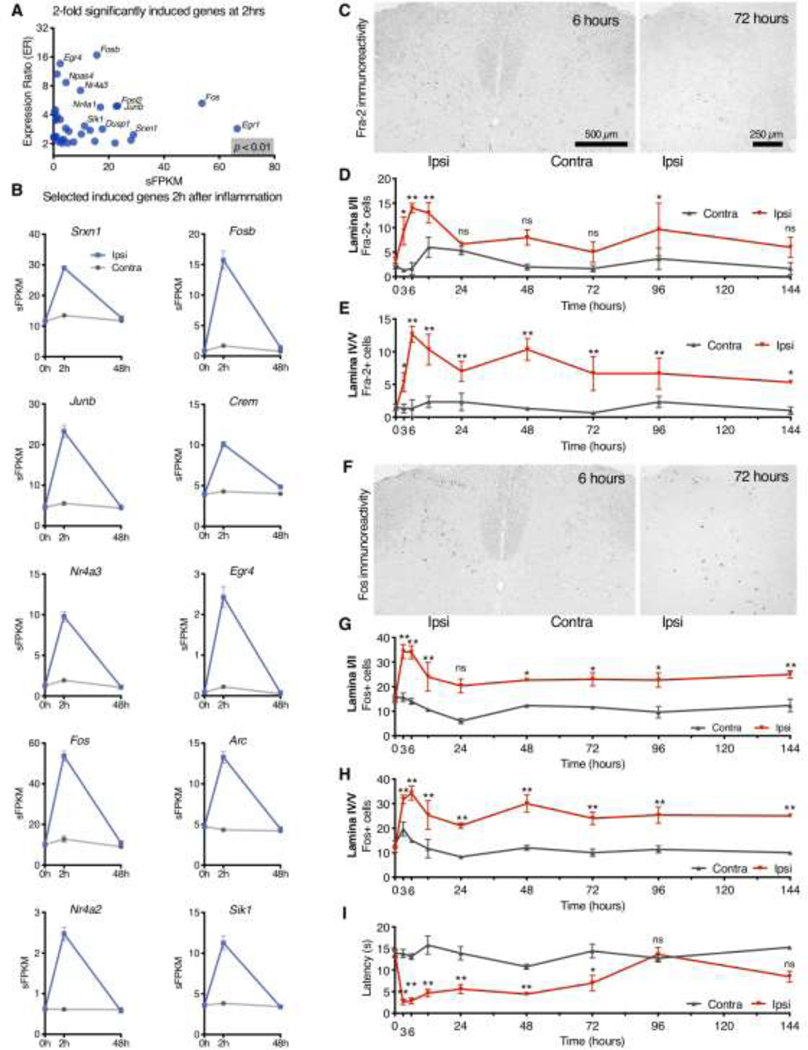
Figure 2. Immediate early gene regulation in response to carrageenan inflammation.
Rats were inflamed unilaterally with carrageenan and RNA-Seq analyses were performed to examine genes regulated at 2 hrs in the ipsilateral dorsal horn. A. Differential genes (p < 0.01) above two-fold induction are plotted for the Inflammation 1 experiment. B. Time course data are shown for the 10 most significantly induced genes after sorting by p-value (naïve control vs. 2 hr ipsilateral), including Fos and Fosl2 (Fra-2). C-H. Sections of the dorsal spinal cord from unilaterally inflamed rats were stained for either Fra-2 or with a panFos antibody at 8 timepoints after injection. C. More Fra-2+ cells were observed on the side of the spinal cord ipsilateral to peripheral inflammation, with peak Fra-2+ cells at 6 hours. Fra-2+ cells were counted at every timepoint in lamina I/II and in lamina IV/V. D. Induction of Fra-2 after inflammation lasted at least 48 hours in lamina I/II E. and at least 144 hrs in lamina IV/V. F. Fos immunoreactivity peaked between 3 and 6 hrs, and was similarly strongly ipsilateral. G, H. The same lamina-specific counting method was used to quantify Fos+ cells, with Fos immunoreactivity, which persisted for at least 144 hrs after inflammation in both laminar regions examined. I. Radiant thermal stimulation was performed at the same time as tissue collection to show hyperalgesia in these animals. D, E, G, H, I: Two-way ANOVA with Holm-Šídák corrections (N=3 rats per timepoint vs. N=6 control rats, 4 sections per animal; see methods). Comparisons in post-hoc testing are for ipsilateral datapoint (timepoint vs. time zero control). Asterisks show significance at *, p < 0.05; **, p < 0.01. Error bars show SEM.
Tissue array preparation and immunohistochemistry
For the experiments in Figure 2C-I, adult female Sprague-Dawley rats (Harlan, 220–280 g) were inflamed by injection of 150 μl 4% carrageenan and used to construct a tissue array for immunohistochemistry (details of construction in Supplementary Figure 2). Animals were deeply anesthetized with a Ketamine/Xylazine mixture and transcardially perfused with normal saline followed by Streck’s Tissue Fixative (STF). Spinal cords were removed, post-fixed in STF overnight and placed in 70% ETOH. In total, 3 tissue arrays were constructed, each containing spinal cords collected from animals at the following timepoints: 3, 6, 12, 24, 48, 72, 96, and 144 hrs after inflammation. Additionally, each array also contained 2 naïve controls per block. Note that this is a grand total of 10 different animals per array (30 different animals total), and that all animals were analyzed (N=3 animals per timepoint, N=6 animals for the control). For each animal, 4 sections were analyzed and averaged (3 tissue arrays × 10 animals per array × 4 counts per animal = 120 counted sections total).
The FOS gene family consists of 4 members: Fos (coding for c-Fos), Fosb (coding for FosB), Fosl1 (coding for Fos-related antigen 1, Fra-1), and Fosl2 (coding for Fos-related antigen 2, Fra-2), all of which encode leucine zipper transcription factors. Like c-Fos, Fra-1 and Fra-2 have been shown to form stable heterodimers with c-Jun, JunB or JunD in vitro, and all of these complexes bind to AP-1 enhancer elements.141 From transcriptional data, it has been observed that the induction of FOS family genes occurs rapidly and resolves by 48 hours. While Fos and Fosb have been examined with respect to hyperalgesia, there have been limited studies on Fra-2 (Fosl2) despite its high degree of induction and similar pattern to Fos and Fosb. These observations expand the combinatorial degrees of freedom for AP-1 transcriptional regulation. Specifically, we examined the time course of the induction of Fos and Fra-2 protein over time using antibody staining.
After deparaffinization, and antigen retrieval, sections were washed 2 times with PBS, and incubated in blocking solution for 2 hrs (10% horse serum and 0.1% Triton X-100 in PBS). Sections were subsequently incubated overnight at 4ºC with a primary global Fos antibody (generated against a conserved region of the Fos family of transcription factors99 and diluted 1:50,000), or a primary FRA-2 antibody (generated against the N-terminal region of the protein135 and diluted 1:7,500). This antibody was a generous gift of Ruben Baler.135 Sections were incubated with a secondary antibody conjugated to a peroxidase labelled-extran polymer (Dako Corporation, Carpinteria, CA) for 30 min at room temperature. Sites of peroxidase activity were visualized using diaminobenzadine (DAB) as a chromagen. Four slides from each block were chosen at random, stained with the Fos or FRA-2 antibodies and staining was quantified by cell counting. Positively staining nuclei in laminae 1, 2, 4, 5, and 6 were counted and recorded using a light microscope (all analyses were performed by one investigator, WTM).
Behavioral data (radiant thermal hyperalgesia testing) from the same female rats as in the IHC studies in Figures 2C-H are shown in Figure 2I and were performed as described in the section on behavioral testing. The N for this time course is 3–6 animals per timepoint, as these animals were also used for tissue array construction as the time course developed. Statistics were performed identically to those described for Figure 2C-H. Notably, the device used was functionally similar to the Ugo Basile device, but was custom made by Fred Brown at the National Institutes of Dental and Craniofacial Research (NIH, Bethesda, MD).36
Overall transcriptomic alterations in the ipsilateral dorsal spinal cord 2 hrs after peripheral inflammation
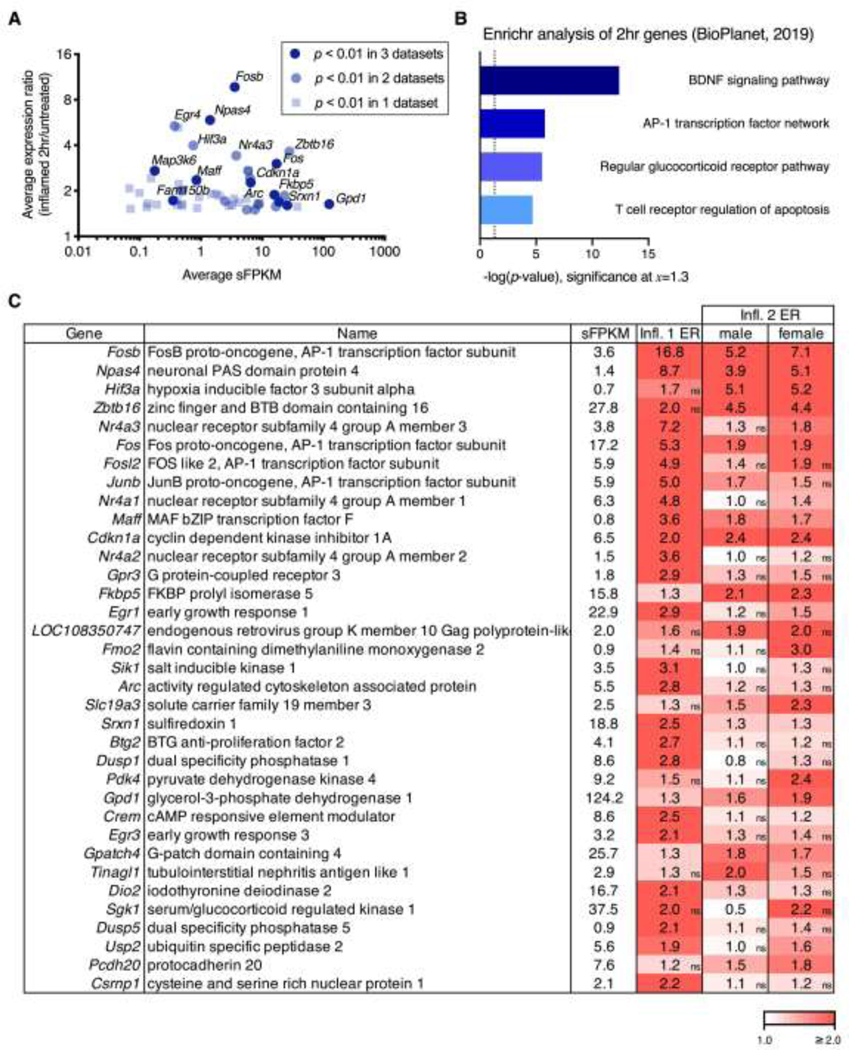
As noted in the description of experimental designs above, the 2 hrs timepoint was collected for 3 inflammation datasets. A first study (Inflammation 1) was done in male rats to examine ipsilateral and contralateral changes, and a second inflammation study (Inflammation 2) was done examining male and female rats at 2 hrs after inflammation. Expression data (sFPKMs) were averaged across these three datasets to compute a grand average sFPKM value. This grand average sFPKM is defined as the average of all individual sample sFPKMs in the Inflammation 1, Inflammation 2: male, or Inflammation 2: female comparisons in either the naïve control or 2 hr ipsilateral groups. Expression ratios (2 hr/controls) were also calculated for each dataset, as well as their grand average across the three datasets. The grand average expression ratio was calculated by averaging the three expression ratios (Inflammation 1, Inflammation 2: male, and Inflammation 2: female) for the 2 hrs comparison. A scatter plot was constructed comparing the grand average expression (sFPKMs, x-axis) to this overall expression ratio difference (y-axis) across the three datasets. For display, only genes with an expression ratio ≥ 50% change (grand average expression ratio ≥1.5 or ≤0.67) that were significant in at least one dataset (p < 0.01) were plotted. Within the plot in Figure 3A, genes were subsequently colored by significance, with genes significant (p < 0.01) in all three datasets in the darkest color. The top 35 genes significant in any dataset were selected based on highest grand average expression ratio (2 hrs/control) after filtering for average sFPKM ≥ 0.5 to remove lowly expressed genes. These genes were run through Enrichr for pathway enrichment analysis, and the -log p-value was plotted for 5 of the top 6 pathways predicted using the NCATS BioPlanet 2019 tools (Figure 3B).11, 43 The value plotted in all graphs showing data from Enrichr are -Log of the adjusted p-value reported by Enrichr following the methods described in Chen, et al 2013.11 Briefly, this is a Fisher’s exact test which estimates the likelihood of a gene list showing a relationship to the given pathway by random chance. The 35 top genes used in the analysis in Figure 3B were tabulated and colored by expression ratio (Figure 3C). Non-significant expression ratios are indicated as “ns” (not significant).
Figure 3. Overall changes at 2 hrs after peripheral carrageenan in the ipsilateral dorsal spinal cord.
For the three datasets in which a 2 hr timepoint was collected (Inflammation 1, Inflammation 2 male, and Inflammation 2 female), sFPKMs from ipsilateral and control (naïve) samples were averaged across all three datasets. The grand average sFPKM was plotted against a grand average of the expression ratio computed by averaging the ER at the 2 hr timepoint across all three datasets. A. The selection criteria for graphing was average expression ratio above 50% change (≥1.5 or ≤0.67). Genes that met this expression ratio were then colored based on how many of the three datasets they were significant in at p < 0.01. B. The top 35 genes significant in any dataset were selected based on highest grand average expression ratio (2 hr:control) after filtering for average sFPKM ≥ 0.5. These genes were run through Enrichr for pathway enrichment analysis, and the -log p-value was plotted, yielding BDNF signaling pathway, and the AP-1 transcription factor network as the top results (lowest p-value). The top 6 predicted pathways are consistent with activation of neuronal immediate early genes such as Fos and Jun which are part of the AP-1 transcription complex, as well as the activation of the Bdnf pathway which is closely coupled to neuroplastic events. C. The 35 top genes used in the Enrichr analysis are tabulated and colored by expression ratio. Non-significant expression ratios are indicated by an “ns” in the bottom right corner of cells that did not meet significance in a given comparison.
Scatter plots of expression ratio vs. sFPKM for 48 hrs timepoint
In order to visualize the overall gene changes for the 48 hrs timepoint in all four of the core datasets, significant genes were selected for each (ipsilateral vs. naïve control, p < 0.01) and plotted with average sFPKM on the x-axis, and expression ratio (48 hrs/control) on the y-axis. Expression ratios were calculated as indicated by the previous formula. In the two datasets where there is a comparison between ipsilateral vs. contralateral (Inflammation 1 and Incision), the ipsilateral data points are shown in red translucent points, whereas the contralateral are shown in smaller black points (Figure 4A, B). In the dataset from Inflammation 2, points were colored according to different criteria. For the males, the significance selection and expression ratios are derived from the male data points, which are shown in red as before (Figure 4C), whereas the corresponding female expression ratios for these genes are shown in black. Conversely, for the female dataset, the significance selection and expression ratios are derived from the female data (Figure 4D), with the corresponding male expression ratios plotted in black. The number of significantly increasing and decreasing DEGs for each dataset is drawn to scale to the right of each scatter plot. Here, the red bar extending above y=1 depicts the number of increasing DEGs whereas the black bar extending below y=1 depicts the decreasing DEGs. The number of DEGs in each category is shown within each bar. Due to the small number of DEGs in the female rats, a more extensive treatment of the methodology for comparing male to female, as well as assessment of various metrics about this dataset are discussed in Supplementary Figure 3.
Figure 4. Differentially expressed genes at 48 hrs after inflammation or incision.
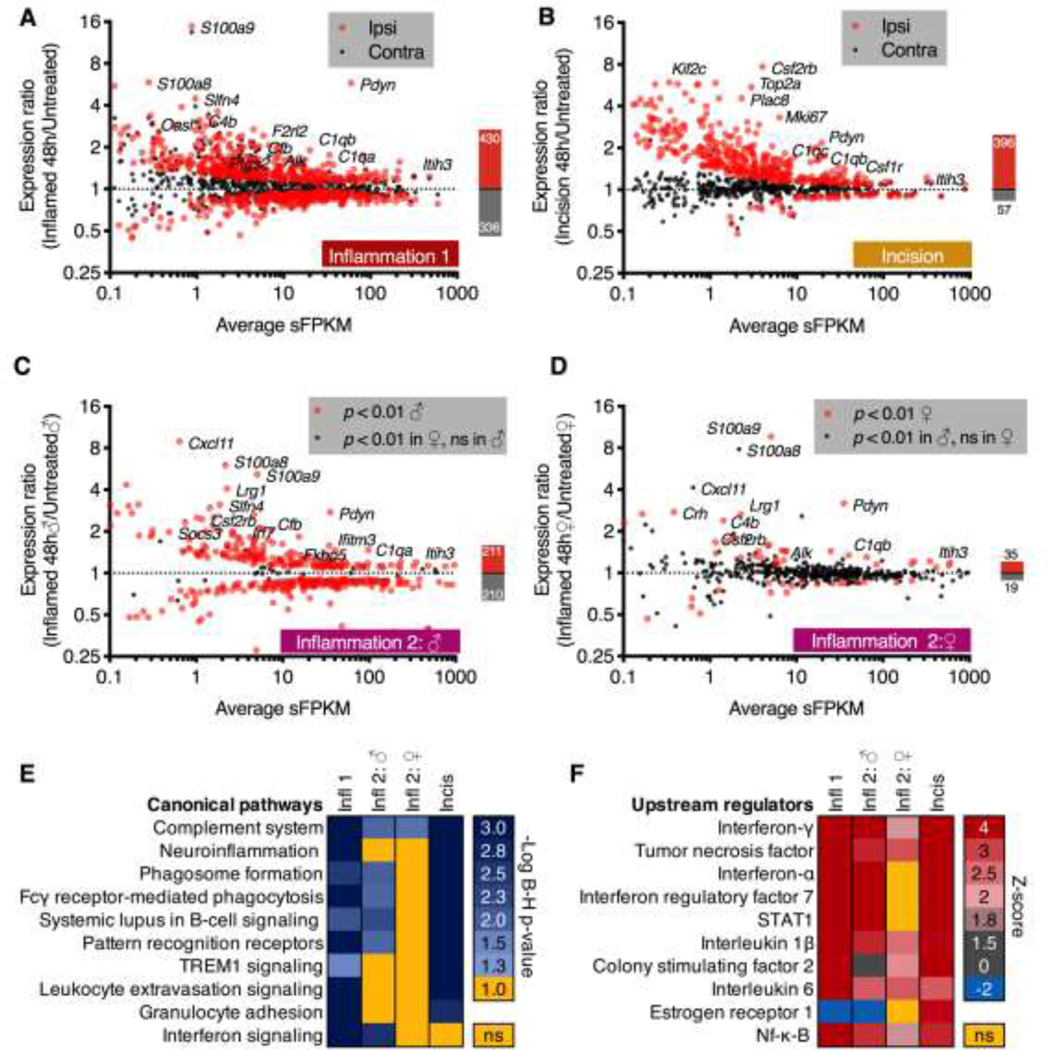
The dorsal spinal cord was dissected from rats with unilateral hind paw carrageenan or surgical incision, which are models of inflammatory and post-operative pain conditions, respectively. For each dataset, DESeq2 was performed on the naïve control vs. 48 hrs comparison (control vs. ipsilateral and control vs. contralateral), and significant (adj. p < 0.01) genes were plotted as average expression in sFPKM vs. expression ratio (48 hrs/untreated). Ipsilateral expression ratios are displayed as red points, and the corresponding contralateral expression ratios for these genes are displayed as black points. These data are shown A. for the Inflammation 1 dataset and B. for the Incision dataset. Notably, the Incision dataset showed a more unilateral induction as indicated by the presence of the majority of black dots (contralateral) around the y=1 (no change) dotted line. C, D. A second inflammation experiment (Inflammation 2) was performed on both male and female animals following similar parameters as in Inflammation 1 (panel A). The contralateral side was not analyzed in these experiments. C. Expression ratios for genes significant in the males are plotted as red points, and genes significant in the female but not male animals are plotted as black points. For the control vs. 48 hr timepoint for male animals a large number of significant genes were observed including many of the most highly significant genes in the original dataset (Pdyn, C1qa, C4b, for example). D. Expression ratios for genes significant in the females are plotted as red points. Genes significant in the males but not the females are plotted as black points. Females in general showed far fewer significant genes, although notably some of the most highly significant genes in the other datasets (Pdyn, C1qa, C1qb) were elevated. These four DEG lists and expression ratios were used to predict canonical pathway and upstream regulator modulation in Ingenuity. E. Canonical pathway activation was assessed in a comparison analysis, and significant pathways were exported into a heatmap. Within the heatmap, cells represent the -Log of the Benjamini-Hochberg corrected p < 0.05 (equal to 1.3). Rows are sorted by level of significance with nonsignificant cells colored in yellow. Most of the non-significant pathways are found in the female dataset. F. Upstream regulators were plotted similarly, with the Z-score of activation used. For these values, the majority of significant Z-scores were positive indicating activation. Note that many of these pathways are related to innate immune activation.
Canonical pathway enrichment and upstream regulator determination using Ingenuity.
Datasets in the current paper were analyzed using Ingenuity Pathway Analysis (Qiagen). For these analyses, expression ratios and p-values (calculated in DESEQ2 as described above) were uploaded to Ingenuity using standard procedures. Subsequently, analyses were performed considering results from all tissue types. A core analysis was performed on each of the four datasets (Inflammation 1, Incision, Inflammation 2 male, and Inflammation 2 female) followed by a comparison analysis. The resulting canonical pathway predictions were ranked by -Log Benjamini-Hochberg (B-H) corrected p-value in each of the four datasets and are plotted as a heatmap. Specifically, the cutoff of adjusted p-value < 0.05 (-Log B-H > 1.3) was used, and values falling below this threshold are indicated in yellow (ns, Figure 4E), whereas a gradient of significant results was plotted in blue. Similarly, the upstream regulators were exported and ranked by Z-score which indicated the direction and magnitude of the predicted regulation (Figure 4F). This Z-score is calculated internally by the IPA software according to their database and algorithms with positive Z-scores indicating activation, and negative Z-scores indicating inhibition. The resulting values were ranked by average Z-score across all conditions, and plotted as a heatmap with the scale corresponding to the magnitude. This scale also contains a gray hue if the Z-score is between 0 and 2, as Z-scores below 2 are thought to be less reliable. For each of these data points, the B-H corrected p-value of the interaction is also calculated, and was significant in all but one cell of the heatmap (indicated by ns, yellow cells).
Prediction of protein-protein interaction networks and gene function analysis for DEGs at the 48 hr timepoint.
In order to prioritize genes for further analysis from the 48 hr timepoint, two approaches to selection of relevant DEGs for the inflammation dataset were employed. The first (primary) method selects the overlapping DEGs from the first inflammation dataset (Inflammation 1) and either the male or female datasets (Inflammation 2)(Figure 5A). Within this more detailed analysis, we also discuss the rationale for considering DEGs across either dataset in Inflammation 2 (Supplementary Figure 3). This list results in 111 DEGs that were significant in at least two inflammation datasets. A second subset of significant genes was selected by filtering for consistently upregulated genes across all four datasets (described in Supplementary Figure 4).
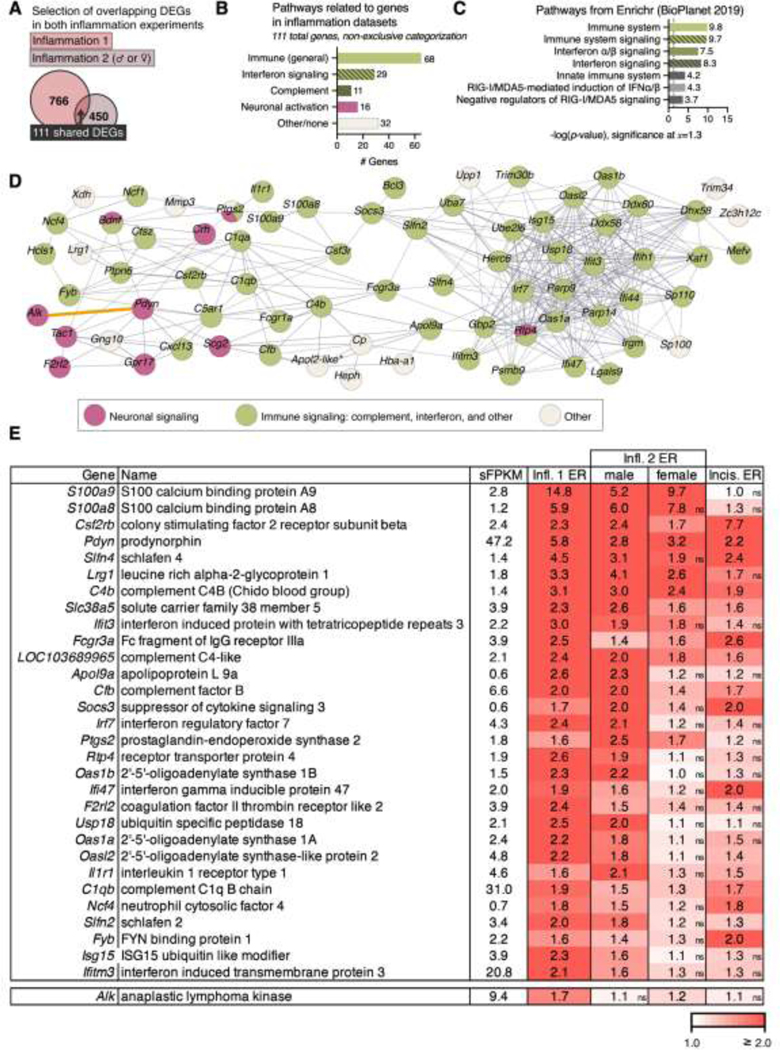
Figure 5. Analysis of genes shared by the inflammation and male vs. female dataset at the 48 hr timepoint.
A. In order to focus on the highest confidence inflammation-induced genes, 111 differentially expressed genes across both datasets were examined. B. These 111 significant genes across the two inflammation experiments were assigned to pathways by performing a targeted literature search. The most predominant signature was related to immune responses, with interferon-related and complement-related genes forming the largest subset of immune genes. C. Pathway analysis was also performed in Enrichr using the BioPlanet database, corroborating the immune nature of the 48 hr inflammation genes. This method also corroborated interferon-related signaling. D. A protein-protein interaction network was created for these 111 shared DEGs using the STRING database and colored according to functional characterization as immune (green), neuronal signaling (pink), or other (gray). For this analysis, disconnected nodes are not shown. Similar to the canonical pathway analysis in Figure 4E, the predominant functional category was immune-related (67/111 genes). Genes were further categorized into general immune, interferon signaling, complement cascade, and neuronal signaling. Among the centrally connected nodes is the interferon regulatory factor 7 (Irf7, middle right), a transcription factor which is induced by IFN-γ and has been implicated in crosstalk with toll-like receptor agonists 62. Notably, while there are relatively few neuronal nodes, they are interconnected (pink circles in panel B). The anaplastic lymphoma kinase (Alk) node was connected to Pdyn manually (orange connector, see results). E. The top thirty genes (after filtration for sFPKM ≥ 0.5) by overall expression ratio in the inflammation datasets are shown. Cells are colored by expression ratio. When gene change was not significant for a particular dataset, it is indicated with an “ns” next to the associated expression ratio. The Alk gene, was also identified within this gene list, and was included as an additional row in this table.
In order to understand the functional significance of these 111 shared DEGs a systematic targeted literature search was performed to identify gene function. The methodology for this literature search is found in the supplementary materials. The results from this literature search are tabulated in Figure 5B, where the immune-related groups are labeled in green, and the neuronal group is labeled in pink. As a complementary approach to the manual literature search, this gene list was also queried using the Enrichr database to predict the functional significance of these genes. Specifically, the NCATS BioPlanet database from 201943 was queried within Enrichr to assign function. The outputs from this query (significance of pathway enrichment relative to “background”) are sorted by -log p-values (Figure 5C).
Genes showing significance in two inflammation datasets were used as an input list for a protein-protein interaction network prediction using STRING.142 This approach allowed all prediction methods at medium confidence (0.4, default) with disconnected nodes (those with no predicted connection to other nodes) hidden (Figure 5D). Connections between nodes represent confidence. Genes were then designated into a functional category, based upon a manual literature review. Subsequently, all nodes were colored according to support for their involvement in the prominent pathways that emerged from this review: immune function, complement signaling, or interferon pathway activation (green), or neuronal function (pink). Those without strong evidence of either designation were left as white, and where attribution to multiple functions was found the symbol was split. The Alk gene was manually connected to Pdyn (orange connector) based on the findings of the current report, as this new connection did not appear in STRING. Based on the observation that several of the highly enriched neuronal genes are neuropeptide precursors, a selection of neuropeptide precursor genes is also plotted in Supplementary Figure 5.
A selection of the top 30 genes for the 48 hr timepoint is shown in tabular form (Figure 5E) along with gene names and expression ratios for each dataset. These top 30 genes were selected by ranking for overall expression ratio at 48 hrs after filtering for genes ≥1 sFPKM. Non-significant changes are indicated in the bottom right corner of each cell (ns). Cells are colored according to expression ratio, saturating at 2.0 (100% increase above baseline).
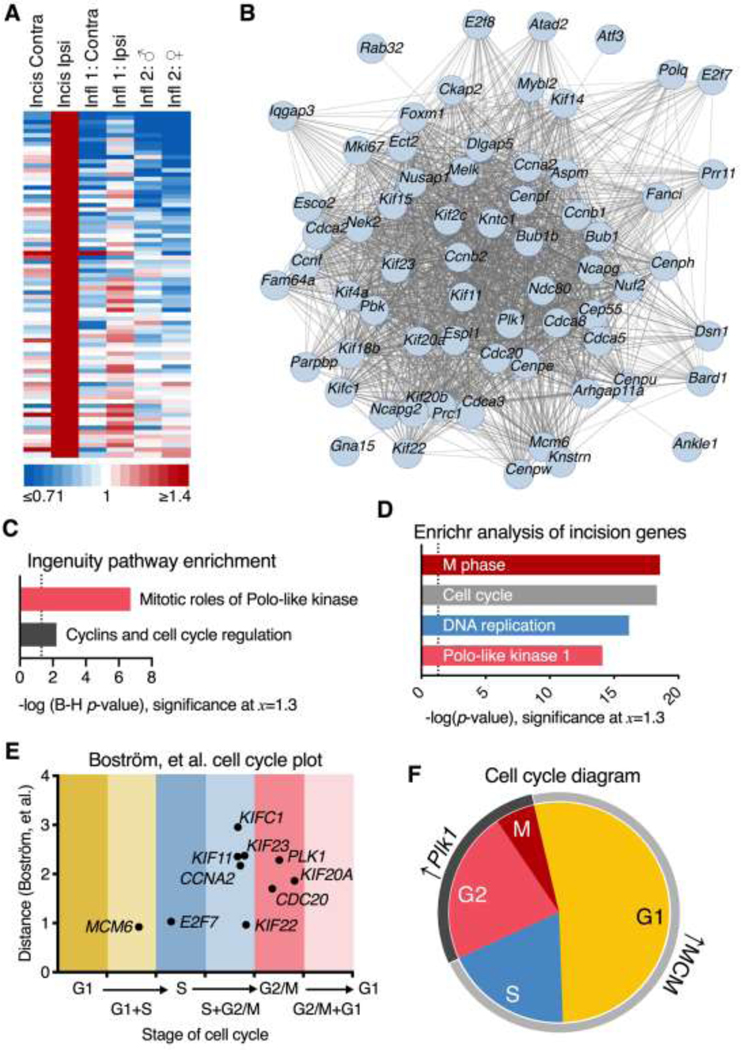
Analysis of incision-enriched genes at the 48 hr timepoint and comparison to a transcriptomic database of cell cycle genes.
Genes enriched in the incision dataset were analyzed similarly to those in Figure 5 and Supplementary Figure 4. The selection for incision-enriched genes was performed by selecting genes with an ER ≥1.4 in the incision dataset, with a maximum ER ≤1.3 in any other dataset. The resulting selection was sorted by the ascending average ER of the three inflammation datasets. This selection was further refined by cutting off at a maximum average ER in the inflammation datasets of ≤1.1. This selection procedure resulted in 79 genes, which are displayed in a heatmap (Figure 6A) showing the degree of enrichment. These genes were input into STRING to generate the plot shown in Figure 6B using the same parameters as in Figure 5D. Ingenuity Pathway Analysis was also used to identify significant pathways regulated in the dataset (Figure 6C) using parameters described in the section on Ingenuity analytic parameters, and plotted as a bar chart showing the -Log B-H corrected p-values of the two highest confidence predicted pathways. Enrichr analysis was performed as in Figure 5C (Figure 6D).
Figure 6. Incision-enriched proliferation signature genes and their relationship to the cell cycle.
A. In order to identify potential unique signatures of the incision response in the dorsal spinal cord, incision enriched genes were identified by filtering for genes that change selectively in this one dataset. These criteria selected for 79 incision-enriched genes. B. STRING analysis was performed on the top incision-enriched genes (panel A), resulting in a densely interconnected single gene network (only connected nodes are shown). C. Canonical pathway analysis (Ingenuity) predicted strong activation of mitotic cascades engaging Polo-like kinase (Plk1) signaling, and activation of a variety of cell cycle control pathways. D. This result was also consistent with Enrichr pathway prediction which specifically identified M phase transition pathways in addition to corroborating Plk1 activation. E. Using an RNA-Seq database 6 of genes enriched at different phases of the cell cycle, each gene was mapped using the methodology described in Boström, et al.6 Briefly, this method estimates the degree of change (y-axis) and the stage of the cell cycle (x-axis) to determine at which point of the cell cycle the gene is overexpressed. F. Notably, Plk1 has been described as important for many points in the cell cycle functionally, but in terms of transcriptional regulation is overexpressed selectively in G2 and M, indicating that the induction of this gene represents cells currently in those phases. Several MCM gene family members were also identified, which were generally enriched in the G1 and G1/S phase of the cell cycle.
Due to the prevalence of cell cycle genes in the incision dataset, further analysis was performed to more precisely identify which phases of the cell cycle were enriched. Using an established RNA-Seq database6 of genes enriched at different phases of the cell cycle, we estimated the enrichment of genes representing each phase of the cell cycle. Individual plots of genes (including Plk1) which drive some of these results are also found in Supplementary Figure 6. Each gene was mapped using the methodology and data from Boström, et al.6, which includes a measure of distance that serves as an estimation factor for degree of enrichment. This variable (distance) is used as the y-axis in Figure 6E, and estimates the degree of transcriptional activation expected for a given x-value. The x-value plotted represents the expected phase of the cell cycle at which such an induction is predicted to occur. A schematic representation was constructed to represent the findings of this analysis (Figure 6F).
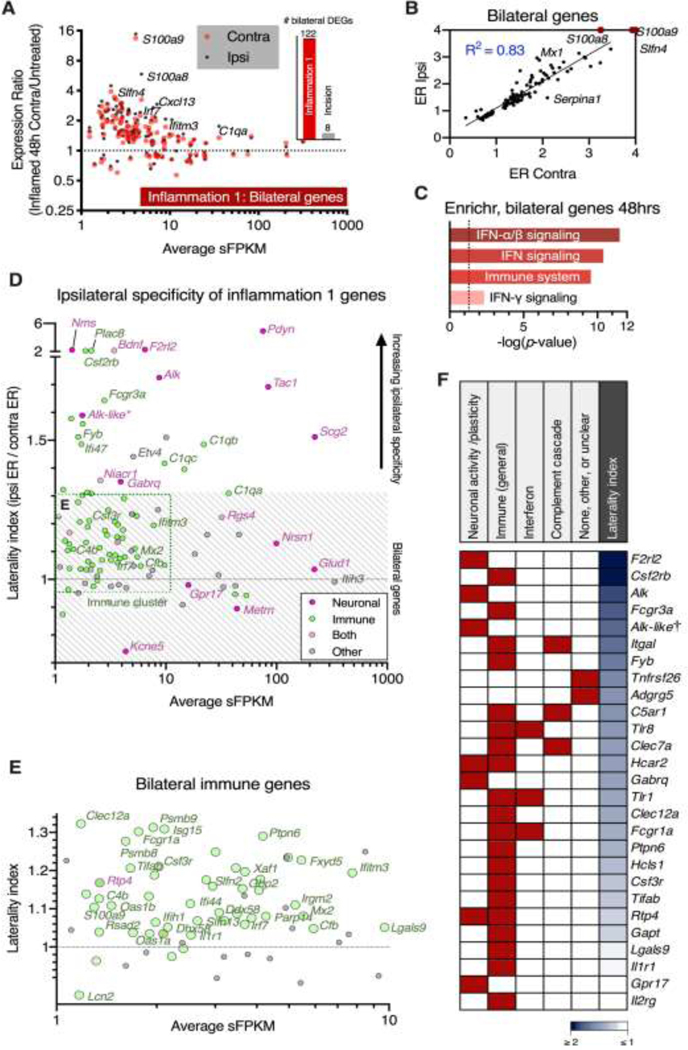
Analysis of bilateral genes in the Inflammation 1 dataset
A scatter plot comparing the average sFPKM and expression ratio on the contralateral side of the inflammation dataset was constructed as described for Figure 4A (Figure 7A). Note that this analysis was also performed for the incision, but resulted in only 8 genes, with very small expression ratios (bar chart in Figure 7A). These 122 DEGs in the Inflammation 1 dataset were classified as bilaterally-induced based on the high degree of correlation between ipsilateral and contralateral for this subset (Figure 7B) calculated as an R2 of the ipsilateral and contralateral expression ratios (linear regression, Prism, GraphPad 8). Gene function analysis was performed using Enrichr with the same parameters and methodology as in Figure 5C (Figure 7C). In order to classify Inflammation 1 genes as ipsilateral vs. bilateral, the ratio of ratios was taken (ipsilateral ER / contralateral ER). The resulting ratio of ratios shows the degree of enrichment on the ipsilateral side, with y = 1 corresponding to perfectly bilateral induction. The genes selected for this analysis were the 151 genes shown in Supplementary Figure 4 and Figure 5 (p < 0.01 in both inflammation experiments and/or correlated across all experiments at the 48 hr timepoint). The range of y-values considered as strongly bilateral was approximately 0.7 ≤ y ≤ 1.3 which represents genes that have similar changes on both ipsilateral and contralateral sides (gray hatching in Figure 7D). A small number of genes were highly ipsilateral (y ≥ 2) shown on the broken axis. Genes on this plot were colored according to the classification in Figure 5 (immune, green; neuronal, pink) with a small number of genes sharing both classifications (green core, pink outline). A cluster of immune genes is highlighted (green dotted outline, bottom left of graph). This selection is enlarged in Figure 7E for better visualization of these bilateral immune genes. Using this classification scheme, including functional analysis from Figure 5 and bilaterality from Figures 7D and 7E, a heatmap was constructed classifying all receptors in this gene list based on these criteria (Figure 7F). Red squares indicate classification in one of the various functional categories. The column of blue squares to the right of the heatmap represents degree of ipsilateral enrichment (dark blue is highly ipsilateral). Genes are sorted by degree of ipsilateral enrichment.
Figure 7. Identification and classification of ipsilateral neuronal, and bilateral immune genes.
Bilateral gene signatures were examined using the significant genes on the contralateral side in the Inflammation 1 and incision experiments at the 48 hr timepoint. A. Genes significant on the contralateral side at 48 hrs in the Inflammation 1 experiment were selected. For each gene, expression ratios (48 hrs vs. naïve control) are shown on the y-axis, and average sFPKM (across control and 48 hrs in Inflammation 1) on the x-axis. Contralateral expression ratios are plotted as red points, and corresponding ipsilateral expression ratios for these genes are plotted as black points. Relative to the ipsilateral side, a small number of genes were significant on the contralateral side at the 48 hr timepoint. This phenomenon was largely specific to the inflammation, as shown in the bar graph comparing the number of significant contralateral genes in the Inflammation 1 (122) and Incision (8) experiments. B. The genes significant on the contralateral side were highly correlated (R2 = 0.83) with the expression ratio (48 hrs/Control) of the same genes on the ipsilateral side, indicating these genes were bilaterally induced. Axes were constrained to a maximum expression ratio of 4 for visibility, with the expression ratios for S100a8, S100a9, and Slfn4 plotted at the axis limit, as these three genes have changes far greater than the others in this plot. C. The gene pathways identified among these bilateral genes were highly immune-related, and encompassed type-1 and type-II interferon signaling (Enrichr, BioPlanet). D. An ipsilateral enrichment index was created by calculating a ratio of ratios (ipsilateral ER/contralateral ER). In this analysis y=1 corresponds to equal induction on both ipsilateral and contralateral sides, whereas higher numbers correspond to a greater degree of ipsilateral induction. Many neuronal genes were found to be highly ipsilateral, including Pdyn, Alk and F2rl2 (see also Figure 5). A cluster of immune-related genes was identified with low expression (≤10 sFPKM) and relatively bilateral induction (gray hatched area 0.7 < y < 1.3 indicates strongly bilateral genes). E. This immune cluster was enlarged, and includes many antiviral innate immune genes including several interferon-induced or interferon-regulatory genes, and antiviral components such as Irf7 and Mx2 (see also Figure 5, Supplementary 4). F. Degree of ipsilateral enrichment was used to rank order receptors, as this criterion supports neuronal involvement, and a relationship to sensory inputs. The top genes by these criteria included F2rl2 and Alk.
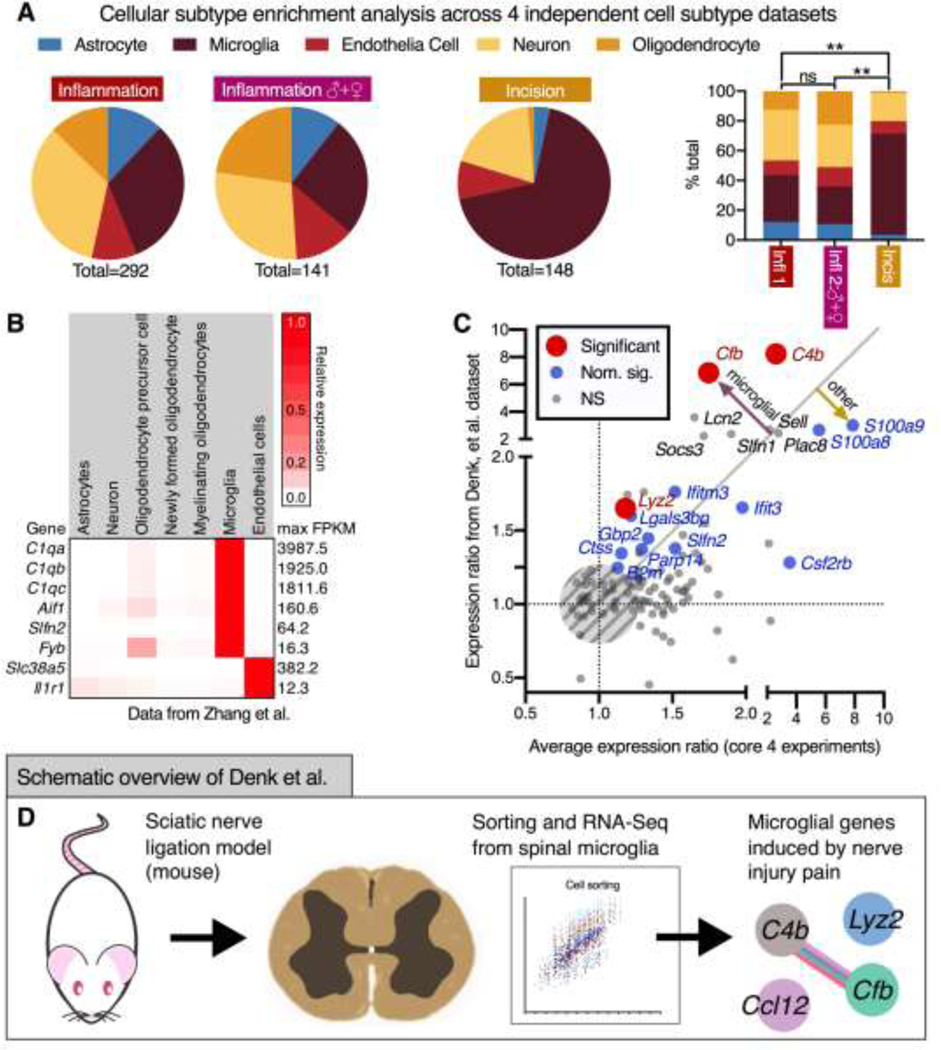
Comparison of the present dataset to single-cell and sorted brain cell populations for estimation of cellular origin
In order to identify the cellular origin of DEGs in the present dataset, four previously published datasets were queried. These datasets use either single-cell sequencing or sorted cell populations to identify enrichment and/or markers across cellular subpopulations. The first dataset was created from purified cell types from the mouse cortex (Zhang, et al.160), measuring basal expression by cell type in the mouse brain, including several broad neuronal categories.160 The second dataset used (Zeisel, et al.158) was a single-cell sequencing experiment from mouse cortex and hippocampus.158 The third dataset (Keren-Shaul, et al.64) was another single-cell sequencing experiment of mouse brain, specifically targeting the characterization of immune populations.64 Finally, the fourth database used single-nucleus RNA-Seq to survey cellular diversity from postmortem human cortex.39 For the Zeisel et al. dataset, enriched genes were supplied in the original publication, and were used directly. These datasets are analyzed in Figures 8A and 8B. Due to the prevalence of microglial and other immune signatures in the present datasets, a comparison was performed to a previously published study using isolated microglia after a sciatic nerve ligation injury.19 The expression ratio from Denk, et al.19 was mined and correlated with the average expression ratio from the four core datasets in the present manuscript. (Figure 8C.) Points were colored according to significance in the Denk, et al dataset. This visualization shows the genes that are changing in both datasets despite substantive differences in experimental design. An overview for the experiment mined for these analyses is depicted diagrammatically in Figure 8D. The detailed methods for these analyses of other datasets are found in the supplementary materials.
Figure 8. Pathway and cellular subtype marker analysis for inflammation-enriched and shared gene signatures at the 48 hr timepoint.
A. In order to examine cell type enrichment among the differentially expressed genes, four transcriptomic datasets were queried for cell type enrichment. The techniques for comparing these datasets and selection criteria are described in the methods. The result of this analysis was the prevalence of microglial marker genes among the significant genes, particularly in the incision dataset. B. Data from Zhang et al., one of the publicly available datasets used in this cell-marker analysis (which uses RNA-Seq to classify mouse brain cell types) are shown for several strongly enriched representative genes. C. Based on the large number of microglial marker genes, DEGs from the inflammation and incision datasets were compared to another experiment by Denk, et al.19, which examined transcriptional effects of sciatic nerve ligation in sorted mouse microglia. Expression ratios (induced/control) averaged between the four core datasets of the present manuscript were plotted against expression ratios and colored according to significance from the original publication. The gray line (x=y) indicates equal change in both datasets. Notably, two of the most significant genes were the complement genes C4b (Complement component 4B) and Cfb (Complement factor B) indicating that these genes may be contributed by microglial activation. D. The experimental outline of the experiment from which the data were mined for this comparison is shown. Briefly, microglia were purified using a Percoll gradient at day 7 to examine transcriptional consequences in these cells after injury using a nerve ligation model.
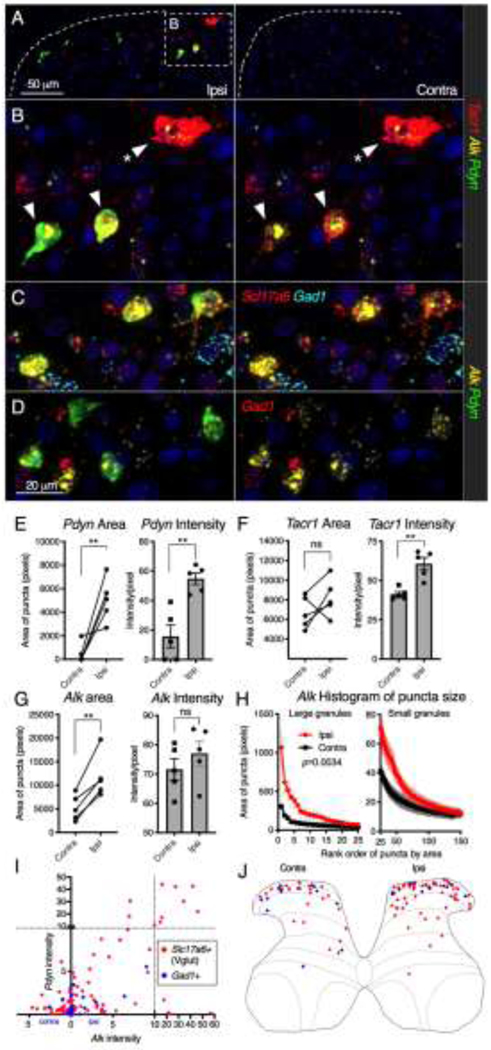
Multiplex fluorescent in situ hybridization
For multiplex fluorescent in situ hybridization, blocks were generated from animals unilaterally inflamed with 150 μl 4% carrageenan as described above. Animals were deeply anesthetized and perfused intracardially at the 48 hr timepoint with cold saline followed by 4% paraformaldehyde. Tissue was dissected and post-fixed in 4% paraformaldehyde overnight. Samples were embedded in paraffin blocks and 6 μm sections were cut and mounted by Histoserv Inc. (Germantown, MD). Paraffin blocks were created such that each block contained at least one naïve control and one inflamed animal so that a control and inflamed group were stained simultaneously with identical parameters. Additionally, since unilateral inflammation was used in these experiments, the contralateral side was available as a within-animal control. Multiplex in situ hybridization was performed using the RNAscope® Multiplex Fluorescent assays v2 and the probes listed in Supplementary Table 1 (Advanced Cell Diagnostics, Newark, CA) with Tyramide Signal Amplification (Opal™ Reagent Systems; Perkin Elmer, Waltham MA) as described previously.129 Quantification was performed according to standard procedures in Fiji (ImageJ version 2.1.0). Detailed methods of these procedures are found in the supplementary materials. Statistics were performed for this analysis by computing the area under the curve for ipsilateral and contralateral data using the trapezoid method followed by a ratio-paired t-test (Prism, GraphPad 8).
Results
Behavioral characterization of the carrageenan inflammation model of hyperalgesia.
Injection of adjuvant agents such as carrageenan, phorbol esters and complete Freund’s adjuvant into the hind paw produces a profound hyperalgesia initiated by a peripheral inflammatory cascade at the site of insult. 25, 48, 120 Unilateral hind paw injection with 150 μl 4% carrageenan caused paw edema within 1 hour, which was maximal at 1 day, and largely resolved by 9 days (Figure 1A). Radiant thermal stimulation measures C-fiber mediated responses to heat, and also shows rapid sensitization (6 hrs), and partial resolution between days 3 and 9 (Figure 1B). Mechanical allodynia was measured using graded von Frey filaments and showed sensitization to low threshold mechanical stimulation at every day tested with a similar time course of onset and resolution as thermal stimulation (Figure 1C). Duration of guarding after a high threshold (pin prick) mechanical stimulus was assessed. While uninflamed animals did not guard in response to this stimulation, exhibiting only rapid withdrawal and stance resumption, guarding behavior was robustly evoked upon stimulation of the inflamed hind paw but not the contralateral un-inflamed paw, and this guarding behavior was significantly elevated over control 1–5 days after inflammation with an apparent maximum guarding duration between 1–3 days (Figure 1E).
Phases of hyperalgesia and the effects of kappa opioid receptor antagonism on hyperalgesia.
Rat hind paws were unilaterally injected with 150uL of 1% carrageenan and nocifensive behavior was characterized as before. Inflammation produces edema lasting at least 8 days, with peak increase in paw width occurring at 4 hrs (Figure 1E). Despite the rapid resolution to an acute mechanical (pinprick) or thermal (laser) stimulus (Supplementary Figure 1), persistent hyperalgesia was still evident in response to stronger mechanical stimulation delivered with toothed forceps under a light or medium restraint dose of anesthesia (approximately 1.0 – 1.2% isoflurane; Figure 1F) at 8 days post-inflammation, suggesting that hypersensitivity is still present and can be unmasked.
Kappa opioid antagonists have been shown to modulate inflammatory hyperalgesia.38 We hypothesized that dorsal horn induction of preprodynorphin (Pdyn) mRNA at 2 days49 regulates hyperalgesia through a kappa-ergic mechanism, and that this kappa-ergic regulation modulates nociceptive sensitivity. The hyperalgesia in the inflamed rats slowly starts to resolve between 4 and 30 hrs after intraplantar carrageenan. Treatment with the kappa-opioid receptor antagonist LY-2456302 (3mg/kg, i.p., see methods) attenuated resolution of the thermal hyperalgesia between 24 and 30 hrs following drug administration (54 hours following carrageenan injection, Figure 1G). Animals injected with LY-2456302 exhibited significantly shorter response latencies (greater hyperalgesia) to radiant thermal stimulation on the inflamed paw than animals injected with vehicle (ipsilateral data points, treated vs. control, p = 0.037, Holm-Šidák multiple comparison’s test, Prism 8). A similar trend in difference between treated and vehicle was obtained using laser C-fiber stimulation of the inflamed hind paw, but was not significant at 30 hrs (Figure 1G, control vs. treated at 30 hrs, p = 0.057, Holm-Šidák multiple comparison’s test). However, there was a significant difference between 1.5 hrs and 30 hrs after injection in the vehicle group (p = 0.0123), but not in the treated group (p = 0.535) supporting the idea that drug treatment attenuated recovery. In both cases the interaction term between drug and time was not significant in the model (p = 0.61 radiant thermal test; p = 0.064 with the laser). In aggregate, these results are consistent with a small effect size using this dose, and these timepoints. No effect of the drug on directly observed spontaneous nocifensive behaviors, A-delta fiber stimulation, or hind paw edema was detected (Supplementary Figure 1).
Early transcriptional events in the ipsilateral dorsal spinal cord in Inflammation 1
The inflammation 1 experiment was analyzed to look at ipsilateral effects of carrageenan in the dorsal spinal quadrant of male rats 2 hrs and 48 hrs after inflammation. RNA-Seq transcriptomic data from Inflammation 1 were analyzed to compare the 2 hr ipsilateral (post-inflammation) timepoint to naïve (uninflamed) controls. In order to prioritize genes for further analysis, significant genes (p < 0.01) with an expression ratio (2 hr/control) greater than 2 (Figure 2A) were selected. This identified 37 genes, of which the 10 most significant genes are plotted individually (Figure 2B). These 10 DEGs are ordered from highest to lowest significance with Fosb (ER=16.8), Early growth response 4 (Egr4, ER=13.7), and Nuclear receptor subfamily 4, group A, member 3 (Nr4a3, ER=7.2) having the highest expression ratios. Neuronal PAS domain protein 4 (Npas4) was also among the highest expression ratio significant genes (ER = 8.7). Note that all of these genes are highly specific for the ipsilateral side, with virtually no induction on the contralateral side.
Among the most significant genes at 2 hrs were many of the well-characterized canonical immediate early genes (IEGs) encoding members of the Activating protein 1 (AP-1) transcription factor complex. The AP-1 complex is a heterodimer including proteins from the c-Fos, c-Jun, and activating transcription factor (ATF) families.59 Specifically, Fos, Fosb, Junb, and Fosl2, as well as Atf3, are significantly upregulated at 2 hrs following carrageenan-induced inflammation. The members of this complex are generally thought to be activity-coupled, and this induction is consistent with alterations in spinal neuronal excitability occurring at this timepoint138 and with the observation of early AP-1 transcription factor induction in pain models in response to nociceptive stimulation.44, 94 Interestingly, with the present cohort, degree of inflammation and analytical pipeline, several canonical AP-1 transcription factor genes were not significantly upregulated at 2 hrs, including Jun (c-Jun), and Jund (JunD), suggesting preferential or accentuated activation of certain members of this family. Notably, while phospho-CREB, which is structurally similar to the AP-1 transcription factor complex has also been shown to be activated in this model, the post-translational modification yielding phospho-CREB is more prominent than changes in CREB protein alone.55, 86 We did not observe changes in Creb1, Creb3, or Creb5 transcript levels, although the elevation in negative regulator cAMP responsive element modulator (Crem) was highly significant (ER = 2.5). Crem is homologous to CREB, binding to cAMP-responsive promoter elements, and to AP-1 sites.80 These transcription factor induction events are involved in regulation of upstream enhancer elements of several opioid peptide precursors such as proenkephalin (Penk) and prodynorphin (Pdyn) in response to painful stimuli20, 85, 94, 137 at approximately 48 hrs, and provide justification for the examination of this timepoint.
Transcription factors that are not a part of the AP-1 pathway are also induced at 2 hrs. Of these, one of the most prominent DEGs was Egr4, (ER=13.7) which encodes a zinc finger transcription factor. The EGR transcription factor family has been studied for its prominent role in long term potentiation and plastic events, although Egr4 is less studied than family members such as Egr1 and Egr3. By comparison the elevation of Egr1 (also known as KROX-24, NGFI-A, and ZIF-268) also was significant with an ER = 2.9. This gene has been well characterized for its role in synaptic plasticity, and has been linked to persistent inflammatory pain.67 Among the most significantly regulated transcription factors was Npas4 which is a key regulator of inhibitory synapse formation.77 Along with phospho-CREB, Npas4 binds to the Bdnf promoter where it activates transcription and is thought to be involved in plastic events.112 Note the induction of Bdnf at the 48 hr timepoint is consistent with the early Npas4 event driving later Bdnf transcription. Nuclear receptor subfamily 4, group A, member 3 (Nr4a3) is also known as NOR-1 and is an immediate early gene in the NR4A family. This family of nuclear receptors is strongly associated with neuroplasticity and long-term potentiation, although Nr4a3 in particular is less well studied than the other paralogs, it also has roles in metabolic control and immune activation.125 Another gene that was strongly induced (and the most highly significant gene in the Inflammation 1 dataset at 2 hrs) was Sulfiredoxin 1 (Srxn1, ER= 2.49), an oxidoreductase involved in cellular response and resistance to oxidative stress. Notably, Srxn1 induction can be driven by AP-1 transcription factor binding in response to synaptic activity as a mechanism to cope with the neuronal activity-associated oxidative stress.103
Immunohistochemical assessment of AP-1 protein family members after peripheral inflammation.
Using immunocytochemistry for protein expression, Fra-2 (Fosl2) and c-Fos exhibited elevation over a prolonged time period and in a lamina-specific fashion (Figure 2C-H). While the increased mRNA levels of these IEG-like genes had resolved by 2 days, the protein levels of Fos and Fra-2 were elevated for substantially longer. A total of 12 sections for each timepoint were counted (Figure 2C-H). Statistics were performed (Figure 2C-H) using a two-way ANOVA (time × side) with testing for control vs. timepoint (3, 6, 12, 24, 48, 72, 96, and 144 hrs) performed using Holm-Šidák’s multiple comparison test in Prism 8 (GraphPad, San Diego, CA). Fra-2 positive nuclear counts showed the largest numbers between 3 and 24 hrs but the elevation was significantly sustained in lamina I/II for as long as 96 hours. In lamina IV/V the Fra-2+ signal was sustained over a longer period, showing significant elevation as late as 144 hrs (6 days). Relative to Fra-2, Fos staining was more consistently elevated with significance reached for both lamina I/II and IV/V for all 6 days. In all four of these two-way ANOVAs (side × time), the side factor and interaction factor were significant (p < 0.01). In all cases but one (Fra-2, lamina I/II), the time factor was also significant (p < 0.01). Comparisons shown in the figure represent statistics calculated between control and the timepoint at which the asterisk is shown (Holm-Šídák’s multiple comparisons test, Prism 8). Similar to the results in Figure 1B, profound thermal hyperalgesia was observed in these animals after carrageenan injection out to about 72 hrs (Figure 2I; Analyzed as with Figure 2C-H, Interaction, time and side, p < 0.001).
Overall changes in the ipsilateral dorsal spinal cord at 2 hrs after peripheral inflammation
Of the 2-fold significantly induced genes shown in Figure 2A, the genes that are significant in one or more 2 hr datasets are explored in greater depth in Figure 3A. By plotting expression (sFPKM) vs. overall expression ratio, genes with above average fold change or expression values are labeled (Figure 3A). This highlights those genes, such as Fosb, which have the highest overall expression ratio (ER = 9.7), and the highest induction in any single dataset (ER = 16.8 in Inflammation 1). The Fosb gene encodes a member of the AP-1 transcription factor subunit that acts in complex with other AP-1 members. Notably, the Fosb paralog Fos is also prominent within this graphical representation (overall ER = 3.0). Many of the members of the AP-1 pathway that were upregulated at 2 hrs in the Inflammation 1 dataset (Figure 2) were increased across all three inflammation datasets (Figure 3A,C). While the prominence of AP-1 family transcription factors was also observed in the analysis in Figure 2, some additional elements are detected in the combined dataset analysis. For example, the basic region and leucine zipper transcription factor MafF (Maff), was more prominent because of its significance in all three datasets in this aggregated analysis (overall ER = 2.4). MafF is a small Maf (musculoaponeurotic fibrosarcoma) lacking the N-terminal transactivation domain,60 and its induction may indicate either activation of transcription (if in complex with other transcription factors), or transcriptional repression, since it can bind but not activate transcription.
Genes significant in at least one of the three inflammation datasets at 2 hrs were filtered to remove lowly expressed genes (see methods) and analyzed further with Enrichr, showing significant enrichment in several pathways related to neuronal activation (Figure 3B). This revealed that the most significant pathway appears to be related to BDNF signaling, which plays a role in activity-coupled plastic events and sensitization to pain at the level of the spinal cord.84 The second most significant pathway was the AP-1 transcription factor pathway, which is discussed extensively above. Notably, Egr1 was identified in the Enrichr analysis as a gene responsible for inclusion in the pathways that encompass Bdnf signaling, AP-1 transcription, and glucocorticoid production.22 The data from the three inflammation experiments are compared in Figure 3C using the top 35 genes across the datasets according to the formula found in the methods (briefly, highest expression ratio for genes significant in at least one dataset). While variability is scattered through all three groups, the gene induction changes in Inflammation 1 had fewer non-significant entries than Inflammation 2, and the females in Inflammation 2 exhibited more non-significant entries than the other two groups. The dose of carrageenan is the same, however, the rats were obtained from two different vendors. At present we do not have a clear explanation for the differences. Nonetheless the aggregate data indicate a rapid and broad induction of gene regulatory processes in the dorsal horn.
Persistent (2 day) signatures of gene regulation in the spinal cord dorsal horn and comparison between carrageenan inflammation and surgical incision.
In each of the four core datasets, the significantly changing genes at the 48 hr timepoint were compared to naïve controls (Figure 4A-D). For the Inflammation 1 experiment, ipsilateral and contralateral gene changes at 48 hrs were also examined (each relative to the naïve control) to identify the highest magnitude of gene changes in this dataset (Figure 4A). The majority of genes displayed upregulation (430 upregulated vs. 336 downregulated). One of the most significantly regulated genes, Pdyn, which codes for preprodynorphin, is an example of a highly ipsilateral gene from this experiment. A comparable analysis was also performed on an earlier dataset (Incision experiment) examining gene changes in the dorsal horn after surgical incision (Figure 4B).116 In contrast to inflammation, incision showed very little contralateral induction (note that the black dots are clustered around x=1). Similar to the inflammation paradigm, Pdyn is among the most highly significant and highly regulated ipsilateral genes in the dataset. However, non-overlapping differences are detected, and genes such as the transcript encoding the canonical proliferation marker Ki67 (Mki67) are prominently increased in the incision (Figure 4B) but not the inflammation. In general, the distribution of data points is similar between these two experiments with the majority of significant genes upregulated in both cases (396 up vs. 57 down in the incision). Notably, while these experiments (inflammation and incision) were performed at two different times, the expression ratios are calculated relative to their respective controls, thus representing the change in expression from their respective baseline levels. For the contralateral side, a large number of trends in gene changes were observed on the contralateral side (black dots), in the inflammation, but not the incision dataset. These changes were generally in the same direction as that on the ipsilateral side inflamed side.
Similar to the analyses in Figure 4A and 4B which compare the ipsilateral induction to that on the contralateral side within each dataset, we also examined the most significant genes in male vs. female rats after hind paw inflammation with carrageenan (Figure 4C, D). The male rats showed vastly more significant genes overall relative to female rats (421 DEGs in the male vs. 54 in the female). Note that in both males and females the Pdyn gene induction is prominent as with the other two experiments. From these scatter plots, two genes encoding proteins in the complement component 1q (C1q) complex C1qa and C1qb are also prominent ipsilaterally-induced genes in all four of the datasets.16 Many of the most significant genes across all datasets are examined further below.
Due to the large difference in number of significant genes between male and female experiments, general characteristics of the datasets were examined (Supplementary Figure 3). Overall, there was a strong correlation between male and female inflammation-induced genes, although often the females showed a lesser magnitude of gene change. Specifically, plots are shown for Meteorin (Metrn), Complement component 5a receptor 1 (C5ar1), Growth arrest and DNA damage inducible gamma (Gadd45g), Ribosomal protein 21 (Rps21), and Sodium/potassium transporting ATPase interacting 4 (Nkain4), which had among the most different inflammation-induced expression ratios by sex (Supplementary Figure 3). However, the overall sex difference among these genes is generally small, and there was little apparent functional relationship between these genes, further reinforcing the idea that the datasets are generally correlated.
Ingenuity canonical pathway and upstream regulator analysis for immune pathway genes altered 48 hrs after inflammation or incision
To examine the biological significance of these gene changes, a comparison analysis was performed in Ingenuity to determine the most significant pathway alterations. This analysis shows the general biological relevance of the gene changes across the datasets by examining canonical pathways and predicted upstream regulators of these gene changes. From these results, complement signaling, and neuroinflammation were identified as the most highly regulated canonical pathways (Figure 4E). Similarly, interferon-γ signaling, was the most highly significant upstream regulator (Figure 4F), with interferon signaling also appearing as a canonical pathway. Due to the few genes changing significantly in the female inflammation dataset, only 1 of the 10 most significant pathways (the complement system) was significant in the female dataset, while the male sets were largely consistent. Of note, the nonsignificant female pathways trend in the same direction as those in the male suggesting similar activation of these pathways in the female rat spinal cord but occurring at a lower magnitude. These results strongly suggest the involvement of multiple immune pathways at the 48 hr timepoint, particularly complement system engagement which was the most highly significant pathway across all four datasets. They also corroborate the strong neuroinflammatory activation that predominates the transcriptional signature at 48 hrs. In addition to neuroinflammatory pathways, the granulocyte adhesion pathway was also activated, as evidenced by the strong elevation in S100a8 and S100a9 transcripts across all datasets, indicating the presence of adherent neutrophils.87 Several of the other canonical pathways identified in Figure 4E are also identified in subsequent analyses, where they are treated in more depth.
To predict what drives pathway activation, the top ten upstream regulators from this comparison analysis are investigated in Figure 4F. Importantly, 9 of 10 upstream regulators have a positive z-score in the heatmap, indicating activation of these regulators. As a technical note, Z-scores with absolute values below 2 were colored with a gray hue denoting that they may be unreliable as indicators of activation. Two of the ten upstream regulators were interferons γ and α (which had the highest and third highest Z-scores, respectively). This is consistent with the induction of several interferon-induced genes including Interferon regulatory factor 7 (Irf), which is also the 4th highest upstream regulator in this analysis. Another upstream regulator that was identified in this analysis is Colony stimulating factor 2 (Csf2). One of the receptors for Csf2, colony stimulating factor 2 receptor subunit β (Csf2rb) was significantly upregulated, and was the major gene identified in this pathway. Specifically, the Csf2rb gene encodes a shared subunit of receptors for Csf2 (also known as GM-CSF), interleukin 3, and interleukin 5. Colony stimulating factor receptor 3 (Csf3r), another receptor for GM-CSF, is also significantly upregulated in the Inflammation 1 dataset, and in the male dataset of Inflammation 2 (ER = 1.4 in both) corroborating involvement of GM-CSF signaling, which has been linked to microglial proliferation.68
Interleukins 6 and 1β are two highly significant predicted upstream regulators in all four datasets. These two interleukins as well as tumor necrosis factor alpha (2nd highest Z-score, Figure 4F) are pro-inflammatory molecules secreted by several CNS cell types including microglia.133 Moreover, interplay between these molecules has been shown to contribute to hyperalgesic responses in the spinal cord by inducing synaptic and neuronal activity.63 Significant interleukin gene signatures drive the results in Figure 4E and 4F among both sexes. For example, a key significant gene that drives pathway activation in the two male inflammation datasets was induction of the Interleukin 1 receptor type 1 (Il1r1), the primary receptor for interleukin 1β (IL-1β, encoded by Il1b). The Mediterranean fever (Mefv) gene was also induced, which encodes the pyrin protein. Pyrin is a tripartite motif-containing protein (also known as Trim20), which is expressed mainly in neutrophils and macrophages, and modulates production of IL-1β.30 Another tripartite domain containing protein (Trim30) is also elevated, and negatively regulates the NLRP3 inflammasome, the assembly of which leads to release of several pro-inflammatory cytokines including interleukins-1β and 18.42 Moreover, induction of Suppressor of cytokine signaling 3 (Socs3) suggests activation of a negative feedback mechanism for interleukin-6.15 In summary, several of the most significant genes across the datasets are implicated in the positive and negative regulation of interleukin signaling pathways.
Signal transducer and activator of transcription 1 (Stat1) was another predicted upstream regulator according to this analysis and was significantly induced in the Inflammation 1 dataset (ER = 1.2). Of note, signal transducer and activator of transcription 3 (Stat3) is also significantly induced in this dataset (ER = 1.2), and the interaction between Stat1 and Stat3 has been linked to the expression of inflammatory signatures in microglia.113 Moreover, these two STAT proteins are reciprocally regulated, with STAT1 promoting innate immune activation, and STAT3 acting to inhibit inflammation.118
Analysis of transcriptional signatures shared between carrageenan inflammation in male and female animals, and surgical incision.
In order to further examine the biological significance of the genes at the 48 hr timepoint, we selected 111 high confidence genes that were significant in both the Inflammation 1 and Inflammation 2 datasets (Figure 5A). As a complementary approach, 87 genes were selected based on upregulation in all four datasets, and these 87 genes were also examined through a literature search. The precise criteria for the selections are explained in Supplementary Figure 4. Broad functional categories were assigned to genes from both selection criteria based on the targeted literature search. The majority of genes (68/111) in the first selection showed a connection to immune or immune-related function based on published literature (Figure 5B, green). Of these 68 immune genes, two non-exclusive subcategories were observed. These two subcategories of immune function are interferon signaling (29/68) and complement (11/68) pathways, with 3 genes overlapping between interferon and complement (Figure 5B). Similarly, the genes in the selection from Supplementary Figure 4 also showed evidence of immune signaling (65/87), with the major immune pathways being interferon-related (28/87) and/or complement (11/87). Note that between the 111 genes selected based purely on significance, and the 87 selected from correlated upregulation, 47 genes overlapped for a combined total of 151 genes. Due to the functional overlap between the genes in these two groups, results from the full 151 genes are reported together in some parts of the following sections, although the analyses in the main body of the paper are confined to the 111 genes reported in Figure 5.
To confirm the pathway determinations, these 111 genes (Figure 5) were then entered into Enrichr and 7 of the top 10 results are shown (Figure 5C). These top ten pathways were all immune-related, and some were omitted due to similarity. Notably, the appearance of pathways such as interferon-α/β signaling and classical complement activation from Enrichr corroborates our manually-curated literature review. The 111 genes were used to predict protein-protein interactions using the STRING database, showing a high degree of predicted interactions between these genes (represented by the connectedness of nodes). The nodes in the STRING plot were recolored to reflect the broad functional categories (Figure 5D), and show that, in general, the neuronal (pink) nodes are connected at the left side of the plot, whereas the immune signaling genes are connected throughout. These pathways are highly enriched in all four core datasets suggesting that the innate immune response is highly overlapping between the inflammation and incision models. Individual genes in this STRING plot and the above analyses are discussed below.
Among the immune-related genes, a large number correspond to interferon signaling, such as the interferon-induced GTPases Mx1 and Mx2, which are large dynamin-like proteins that self-assemble and bind various viral nucleocapsids as part of the intracellular antiviral defense pathway.34 We also observed induction of interferon-induced protein with tetratricopeptide repeats 3 (Ifit3), which encodes a component of an antiviral complex activated by IFN-α, and IFN-induced transmembrane proteins (Ifitm3), which encodes a transmembrane protein that inhibits replication of influenza and other virus types. Both are engaged to defend against pathogens.23, 156 It is notable that while these genes have been studied in regard to viral defense, they are induced in many contexts in which pathogens are present or the immune system is activated, and presumably there is no viral exposure in our paradigms. The appearance of interferon gamma inducible protein 47 (Ifi47) and Suppressor of cytokine signaling 3 (Socs3) are also notable because of their role in interferon-γ-related pathways.15 Thus, both type I and type II interferon responses in inflammation conditions are apparent. Also of note is the induction of Schlafen gene family members 1, 2, 3, 4, and 13. These proteins contain a unique Schlafen domain and have been implicated in a variety of functions including immune response and cell proliferation. Schlafen family members 2 and 13 (Slfn2, Slfn13) were categorized under the interferon signaling pathway due to their known induction and regulation by IFN-α.61, 82 In the analysis in Supplementary Figure 4, the interferon-α-related Schlafen 1 (Slfn1) was also induced, 61 While Schlafen family members 3 and 4 were identified in the analysis in Supplementary Figure 4, these two family members do not have a known relationship to interferon signaling. Interferon regulatory factor 7 (Irf7) was identified as a highly significant gene across the datasets. Irf7 is a critical regulator of type I (α/β) interferon production and is activated by pathogenic infection in a wide variety of contexts. This activation occurs subsequent to several canonical signaling pathways that are generally activated downstream of pathogen-associated molecular pattern recognition.97 Notably, the Irf7 paralog Interferon regulatory factor 8 (Irf8), a critical factor in the conversion of microglia into a reactive phenotype, was significantly upregulated in Inflammation 1 and Incision datasets,81 and was approaching significance in the Inflammation 2 dataset (p = 0.016 in Inflammation 2: male).
From the complement pathway, C1qa, C1qb, and C1qc were among the strongest upregulated transcripts, each encoding a chain for C1q, a key subcomponent of C1 (component 1), which activates the classical complement cascade.16 C1q is also a pattern recognition receptor which binds to a variety of exogenous and endogenous danger motifs.69 Another component of the classical cascade, C4b (complement component 4b) also features within this subset of genes.66 Interestingly, Cfb (complement factor b) is a component of the alternative cascade, suggesting activation of multiple complement pathways as opposed to simply the classical cascade.100 Moreover, C5ar1, a G-protein coupled receptor for the complement component 5a, was also found to change significantly on the ipsilateral side (Figure 7F).16 In the dorsal horn, C5a has been shown to be released from macrophages134 and is also expressed by microglial cells.52 Additionally, C5ar antagonists have been proposed as treatment for several modalities of pain including neuropathic pain and burn,90 supporting the idea of a functional engagement of this receptor in the hyperalgesic processes.
Another prominent functional category contained genes related to neuronal signaling and neuroplasticity (16/111; note that the immune and neuronal categories are non-exclusive). This included both neuropeptides, whose biosynthesis is generally activity-correlated in peptidergic neurons,129 as well as neuronal signaling receptors. At the lower left side of the STRING plot (Figure 5D), are several of the interconnected genes related to neuronal signaling (pink nodes). These included the neuropeptide precursors for dynorphin (Pdyn), Substance P (Tac1), Corticotropin releasing hormone (Crh), and the peptide-related gene encoding secretogranin 2 (Scg2). Additionally, Proenkephalin (Penk) was significant in the Inflammation 1 dataset (ER = 1.3, p = 1.1×10−8) which is consistent with another recent study identifying modulation of this opioid peptide precursor, and earlier studies.21, 129 In aggregate, the large number of these peptidergic precursor genes suggests activation of several subtypes of peptidergic neurons in the dorsal horn over the preceding 48 hrs. Notably, the induction of both kappa and mu/delta opioid receptor agonist peptide precursors was observed. Within the STRING analysis, the node containing Pdyn was manually connected to anaplastic lymphoma kinase (Alk) as a result of co-localization (see Figure 9). In addition to these signaling proteins, the protease activated receptor 3 (PAR3, encoded by F2rl2) was increased, which has been localized to somatostatin+ neurons in the dorsal spinal cord.10 This family of receptors has been posited to be important regulators of inflammatory pain, and are also expressed in DRG nociceptors (Supplementary Table 2).123 Among the genes that could potentially be involved in neuroplastic regulation, Meteorin was identified, which is thought to be made by astrocyte lineage cells and has been shown to promote axon extension similar to nerve growth factor.98 Notably, Meteorin was among the most differently induced genes by sex (Supplementary Figure 3).
Figure 9. Fluorescent in situ hybridization for inflamed spinal dorsal horn at 48 hrs.
Sections of lumbar spinal cord were taken from unilaterally inflamed animals (48 hrs) and multiplex fluorescent in situ hybridization was performed. A. Triple label for Tacr1 (red), Alk (yellow), and Pdyn (green) with a counterstain (DAPI, blue) revealed a unilateral increase in each of these transcripts in the dorsal spinal cord. B. The densely-positive Pdyn cells in the superficial medial dorsal spinal cord show colocalization of Alk and Tacr1 in a large superficial neuron (arrowhead, asterisk) and colocalization between Alk and Pdyn in two smaller neurons with less Tacr1 reactivity. C. The Alk/Pdyn co-positive subset was further investigated and shown to also co-label with Slc17a6 (Vglut2), a marker of glutamatergic neurons. D. Conversely, hybridization of the section for Gad1 showed that the Alk/Pdyn co-positive subset were Gad1 negative. E. The area and intensity/pixel of Pdyn signal was significantly increased ipsilaterally. F. Intensity/pixel, but not area of the Tacr1 signal was increased. G. Area but not intensity/pixel of Alk was increased. H. Rank order plots of regions using thresholding by area are shown for Alk, revealing that the effect is driven by the appearance of larger agglomerated regions of Alk reactivity. I. Pdyn/Slc17a6 copositive cells (red) and Pdyn/Gad1 copositive cells (blue) were identified in the superficial dorsal quadrants both ipsilateral and contralateral to the site of inflammation in lumbar cords from 3 inflamed animals. Pdyn and Alk signal intensities of identified cells were measured and plotted; contralateral and ipsilateral cells are graphed in the negative and positive x-axis, respectively. A subset of densely Pdyn/Alk/Slc17a6 copositive cells is found in the ipsilateral dorsal quadrant. J. The laminar distribution of Pdyn+/Slc17a6+ (red) and Pdyn+/Gad1+ (blue) cells from inflamed animals are displayed for two of the three animals. The third shows a similar result, and is shown in Supplementary Figure 8. Statistics were performed using paired T-tests; **, p < 0.01; N=5. All significant results were at least p < 0.05 with Mann-Whitney U-test. Error bars represent standard error of the mean.
The brain derived neurotrophic factor gene (Bdnf) was significantly upregulated in two inflammation datasets. Because of its expression in microglia as well as in neurons, Bdnf was assigned to both the “immune” and neuronal categories.14 Consequently, upregulation of Bdnf may result in changes to microglial signaling (considered immune-like in this analysis) and neuronal remodeling. Heterozygous Bdnf knockout rats have been reported to have transcriptomic alterations in interferon pathways similar to those described above,128 including Interferon regulatory factor 7 (Irf7). Changes in interferon genes were also noted in a previous study from our lab on the effects of a heterozygous deletion of Bdnf in the spinal cord and dorsal root ganglion.128 Genes significant in both inflammation and heterozygous Bdnf knockout conditions included some of the most significant genes in the present datasets, including C4b, Crh, and Irf7. This suggests that Bdnf signaling may play a role in bridging the neuronal-immune communication in the spinal cord, and may be part of the interferon-regulatory mechanism.
Many of the most highly significant genes from the inflammation datasets, such as C4b and Cfb are also significant in the incision. This is an indication of the concordance of these datasets. Notably, while the selection criteria (based on significance in the inflammation datasets) does result in several genes in this selection failing to show significance in the incision, none of the genes shown in Figure 5E trend in the opposite direction (downwards). This is generally an indication that there is a high degree of overlap between the incision and the inflammation for the high confidence genes in this analysis. These results viewed in aggregate suggest that a highly immune-like transcriptional signature is activated in the dorsal horn in response to both peripheral inflammation and incision. Note that this change was more ipsilateral in the incision dataset, which had almost no changes on the contralateral side. Nonetheless, complement activation was more prominent ipsilaterally in the inflammation as well, suggesting that this process is coupled to afferent drive.
Analysis of incision-enriched gene signatures.
A subpopulation of genes were identified in the incision-induced population that assembled into a highly interconnected single network related to cell division and progression of the cell cycle (Figure 6A and B). This analysis results from the 79 genes identified in the heatmap shown in Figure 6A, which is based on enrichment in the incision dataset relative to the other experiments (described in detail in the methods). When subjected to functional protein association network analysis, they arranged into a connected node containing 66 of these genes. Pathway analysis showed strong activation corresponding to the mitotic roles of Polo-like kinase (Plk1), as well as one related to cyclins and cell cycle progression (Figure 6C). The large number of genes related to the cell cycle prompted an investigation of transcriptional regulation throughout the cell cycle to identify which phase was most represented. In part, this is because Plk1 is involved in regulation of many stages of the cell cycle.27 Additionally, several cell cycle markers are sometimes activated during events such as neuronal differentiation or dendritic remodeling which involve cell growth. Enrichr gene analysis also supports this finding and specifically implicates mitotic M phase gene regulation (the most significant pathway identified, Figure 6D). Using an RNA-Seq database6 of genes enriched at different phases of the cell cycle, each gene was mapped using the methodology and data presented in a previous study by Boström, et al., examining cell cycle transcriptomics.6 Briefly, this method estimates the degree of change (y-axis) and the stage of the cell cycle (x-axis) to determine at which point of the cell cycle the gene is overexpressed by referencing sequencing captured from cells in different stages from G1 through G2/M. Notably, Plk1 has been described as important for many points in the cell cycle, but is transcriptionally-induced selectively in G2 the portion of interphase immediately preceding mitosis, and M-phase. Several other genes that are considered to have broad roles in mitotic functions were also observed in the Boström dataset to be selectively induced during the G2/M phase (Figure 6E). The overall location of a small subset of representative genes are depicted in their respective phase of the cell cycle. (Figure 6E). These genes include cyclin-dependent kinase 1 (Cdk1),96 which functions in the same pathway as Plk1, as well as cell-division cycle protein 20 (Cdc20), a well-known inhibitor of the anaphase-promoting complex.157 In addition to these genes, we identified a comparatively smaller group of significant genes involved in the G1/S phase of the cell cycle including several minichromosome maintenance complex component (MCM) genes and the transcription factor E2f7. Notably, Mcm5 and Mcm6, which we identify in our study, contain E2F binding sites in their promoter region, and are thought to be primarily induced by E2F transcription factors.101 In aggregate, these findings from STRING, pathway analysis, and the literature show a highly interconnected canonical mitotic activation pathway specific to the incision relative to the inflammation.
Identification of ipsilateral neuronal, and bilateral immune receptors from transcriptomic datasets
Statistical analysis showed a smaller number of significant genes on the contralateral side at 48 hrs than for the ipsilateral side in both the Inflammation 1 and Incision datasets (the two experiments containing contralateral measurements.) In the Inflammation 1 dataset, 122 genes were significant on the contralateral side. The majority of these genes were increasing and appeared to be bilaterally induced (note black dots indicating ipsilateral data in Figure 7A). The incision did not contain reliable contralateral genes (Figure 7A inset bar graph). The ipsilateral and contralateral expression ratios were highly correlated for the contralateral DEGs (p < 0.001, R2 = 0.83) indicating bilateral induction (Figure 7B). Note that S100a8, S100a9 and Slfn4 are plotted at the edge of the graph, as they would be outside the axis limits, which were cut to an expression ratio of 4 (300% increase) to avoid the use of a log scale. For the bilaterally-induced genes, the gene function analysis in Enrichr identified a strong immune signature predominated by both type I and type II interferon signaling (Figure 7C).
Classification of genes as ipsilateral or bilateral was performed by examining the degree of enrichment on the ipsilateral side (Figure 7D). In this analysis, several genes were highly ipsilateral, including F2rl2, Alk, and Pdyn, (discussed in the results for Figure 5). This analysis also identified the tachykinin 1 precursor (Tac1), neuromedin S (Nms), and Bdnf as highly ipsilateral, further emphasizing that the neuronal and particularly peptidergic responses among neurons are highly ipsilateral, similar to what was observed for immediate early genes at the 2 hr timepoint (Figure 2, 3). A dense cluster of immune genes was identified with relatively low expression (sFPKM ≤ 10) and bilateral induction (0.9 ≤ y ≤ 1.3, Figure 7E). This immune cluster includes several of the antiviral immune genes discussed above such as Dhx58, and various interferon-induced and interferon-regulated transcripts (see results for Figure 5). This finding is consistent with the induction of these antiviral and other defensive genes independent of neuronal activation. This is also consistent with the generalized and spatially-delocalized elevation of S100a8 and S100a9 on both the contralateral side, as well as in ventral spinal cord and brain in response to peripheral inflammation87 Indeed, several genes such as Irf7, Isg15, OAS family members are among the most strongly induced genes in several models of viral infection.2, 107 Notably, those genes which are overlapping between the present dataset and viral-induced gene signatures from previous studies are generally widespread, and not limited to the afferent activated nervous tissue (i.e., ipsilateral dorsal horn). Thus, the presence of carrageenan appears to stimulate a body-wide immune response (termed “defensive priming”) as this substance is isolated from algae and is recognized as foreign by pattern recognition receptors. However, among the bilateral genes, there is a general trend towards ipsilateral enrichment as indicated by the majority of the points skewing above y = 1 (Figure 7D). This is suggestive of a concurrent and additive ipsilateral driver of some genes in these innate immune pathways. Potentially, this could be explained by the interferon pathway being shared between defensive priming systems and those induced in microglia and other glia during neuronal-induced hyperalgesic responses.
The overall classification scheme, including functional analysis and laterality, was used to prioritize the receptors identified in this analysis. These receptors were ordered by degree of ipsilateral enrichment to focus on those receptors that were most likely to be involved in neuroplastic events and hyperalgesia. Notably, those towards the bottom of the sort that are highly bilateral might be expected to participate in defensive priming by the innate immune system (Figure 7F). The most strongly ipsilateral receptors were F2rl2, Alk, Csf2rb, and Bcl3. We also detected an uncharacterized gene encoding an Alk-like rat transcript (LOC108351182) as among the most ipsilateral receptor genes. This Alk-like transcript encodes an open reading frame containing a region highly homologous to that of Alk but lacking the C-terminal intracellular kinase domain. Notably, a third gene, Alkal2 (previously known as Fam150b), which encodes a secreted protein thought to be an endogenous ligand of Alk,32 was also significant in the Inflammation 1 dataset. Detailed characterization and plots of the Alk-like transcript and Alkal2 are found in Supplementary Figure 10.
Examination of cell subtype markers for inflammation-enriched and shared gene signatures
Despite the immune signature that emerged from the combined output of several bioinformatic approaches, its specific nature was unclear. For example, while the association with immune, interferon, and complement systems was evident, the specific cell types engaged by these processes were incompletely defined. Several genes in the dataset were suggestive of specific cellular subclasses. The detection of calcium-binding proteins S100a8 and S100a9 after the inflammation is consistent with the adherence and chemotaxis of myeloid cells such as neutrophils,124 which express very high levels of this transcript (∼45% of the neutrophil cytosol is estimated to be composed of these two proteins).153 The low read counts of these transcripts are also consistent with the sparsity of intraluminally-adherent neutrophils from in situ hybridization studies.87 While the S100a8 and 9 genes were present bilaterally, the more unilateral induction of genes such as Fyn binding protein (Fyb) and complement factor 1 chains a, b, and c (C1qa, C1b, and C1qc) suggests the engagement of microglia at the 48 hr timepoint.8, 24, 151 To systematically examine these cell type marker genes, a bioinformatics comparison approach was employed. The list of DEGs from the present experiments was queried against an integrated database that was constructed from 4 transcriptomic studies characterizing nervous system cell types. 39, 64, 158, 160 From this analysis, it became evident that strong markers of microglia were prominent in the overall dataset (Figure 8C), and these comprised the largest proportion of DEGs in the dataset that corresponded to an identifiable cell population. The predominance of microglial marker genes was shared throughout the datasets but was particularly prominent in the incision dataset. This supports the previous evidence of immune-related transcriptional signatures, and possibly identifies microglia as the source for many such changes. To exemplify the cell-subtype specificity for some of the genes from the analysis, data from Zhang et al.160 were also visualized in Figure 8B.
The large number of microglial markers present throughout the datasets prompted further investigation into whether the immune signature could truly be attributed to microglial activation. To that end, data from Denk, et al.19, which examined the transcriptional effects of sciatic nerve ligation (SNL) in isolated mouse microglia, were used to search for microglial genes in our inflammation transcriptomic datasets that are derived from mixed tissue.19 Of particular interest, two of the significant genes from Denk, et al. were also highly significant across all four core datasets: Complement factor B (Cfb), and Complement C4B (C4b). Interestingly, these are representative genes from both the classical (C1qa, C1qb, C1qc, and C4b) and alternative (Cfb) complement cascades. Moreover, various other genes were nominally significant in the SNL-induced model, including Ifit3, and Ifitm3, which imply the involvement of microglia in interferon signaling as well. Notably, while Denk, et al identified the neutrophil-enriched gene S100a8, it was induced more strongly in whole tissue than in purified microglia. This is to be expected given that the major source of this transcript in the spinal cord after peripheral inflammation is the arrival of neutrophils that adhere to the luminal surface of endothelial cells.87 Similarly, the identification of cytokine receptor common subunit beta (Csf2rb) towards the middle right of the graph (Figure 8C) indicates likely enrichment in whole tissue (dorsal spinal cord) relative to microglia despite being present in microglial cells. The complement component 5a receptor 1 (C5ar1) although not significant in the Denk, et al. dataset, also has support in the literature for its expression in microglia.7, 72 Taken together, these analyses are consistent with the idea that some of the most significant gene changes, including that of Cfb and C4b are from microglia. Moreover, they imply that these changes may be consistent with those from other dissimilar pain models, such as the nerve injury model employed by Denk, et al19. Finally, they implicate microglia as at least one contributing source of the immune-like signatures detected in the broader dataset.
Fluorescent multiplex in situ hybridization of neurons expressing prodynorphin and Alk 48 hours after peripheral inflammation
Six μm sections of lumbar spinal cord were taken from unilaterally inflamed animals (48 hr timepoint) for multiplex fluorescent in situ hybridization. Triple labelling for Alk, Tacr1 and Pdyn showed a unilateral increase in each of these transcripts in the medial superficial dorsal spinal cord (lamina I/IIo; Figure 9A,B). The section in Fig 9 is 6 μm and to provide more of a summation image, several multiplex-labeled sections are overlaid after 2D registration and shown in Supplementary Figure 8. The Alk /Pdyn co-positive subset was further investigated by co-labeling with Slc17a6 (Vglut2), a marker of glutamatergic excitatory neurons, and glutamic acid decarboxylase (Gad1), the biosynthetic enzyme for the inhibitory transmitter gamma-aminobutyric acid (GABA; Figure 9C). The upregulated Alk/Pdyn co-positive subset was observed to co-express Vglut2 and, conversely, were largely Gad1 negative (Figure 5D). The area and intensity/pixel of Pdyn signal was quantified, and both were significantly increased ipsilaterally (Figure 9E). Intensity/pixel, but not area of the Tacr1 signal was increased (Figure 9F), suggesting that the same cells stain for Tac1, but the density of staining is larger. Conversely, area but not intensity/pixel of Alk was increased (Figure 9G). For plots in Figure 9, error bars represent standard error of the mean. Statistics (Figure 9E-G) were performed using paired T-tests; **, p < 0.01; N=5. All significant results were at least p < 0.05 with Mann-Whitney U-test. Rank order plots of threshold-selected regions by area (see methods) are shown for Alk, to portray the change in area for this label (Figure 9H). This distribution showed significant (p = 0.0034, ratio paired t-test) separation of the size bins towards the high end of puncta size, indicating sites of large agglomerations Alk+ granules in the ipsilateral side (such as whole cells filled with Alk+ fluorescent grains). The appearance of these larger agglomerations apparently drive the overall change in area. It is also important to note that Alk reactivity is prominent in some cells in the basal state, indicating a reserve of transcript for receptor protein biosynthesis. An analysis was performed for all Pdyn+ cells in N=3 animals (n = 181 cells), where each cell was designated as excitatory or inhibitory based on co-staining for either Vglut2 (Slc17a6) or Gad1, respectively, and the intensity of Pdyn and Alk was measured within each cell (Figure 9I). Among these Pdyn+ cells, Gad1 or Slc17a6 labeled 181 of 181 cells across N=3 animals, with no Pdyn+ cells negative for co-labels. This analysis revealed that a small subset of densely Pdyn/Alk/Slc17a6 co-positive neurons are found in the ipsilateral dorsal quadrant. This subset contains on average ≥10x the signal of Pdyn and Alk as other ipsilateral neurons, and any neuron identified on the contralateral side. The laminar distribution of Pdyn+/Slc17a6+ (red) and Pdyn+/Gad1+ (blue) cells from 2/3 representative inflamed animals are displayed, with the most evident induction occurring in lamina I and II outer (IIo)(Figure 9J). Sections from the third animal, which showed a similar pattern of induction, are shown in Supplemental Figure 8. In contrast to Alk, the Pdyn+ cells were negative for Gpr83, a G-protein coupled receptor 28 also regulated by inflammation (Supplementary Figure 9).
The overall findings of these staining experiments, which show that the Alk receptor is expressed by the dynorphinergic large excitatory neurons in lamina I and IIo were used as justification to test the Alk inhibitors ceritinib and alectinib for analgesic activity. These studies did show anti-hyperalgesic actions but also reduction in paw edema with ceritinib, but not alectinib, suggesting a peripheral anti-inflammatory action may also be contributing to the anti-hyperalgesic actions. The compounds are highly insoluble and have a variety of toxic side effects that may affect behavioral measurements. For these reasons, more research on both antagonists and agonists is needed. (Supplementary Figures 11 and 12).
Discussion
In the present report, we elaborate the regulatory processes that are shared between two commonly used pain models: peripheral carrageenan inflammation and surgical incision. Using transcriptomic profiling and molecular neuroanatomy we investigated gene regulation engaged in these models over the early timepoints. While other studies have examined gene changes in chronic pain models,139 the present study is large, containing several independent sequencing datasets at different time points and incorporating data from both inflammation and incisional models. We further validated that the major changes occurring at the later, 48 hour, time point in the inflammation model were similar in male and female animals. The data further provide discrimination of two major processes: one directed at neurons and upregulation of their intercellular signaling molecules, and a second encompassing innate immune processes including microglial activation. These changes were also investigated with respect to laterality, as the neuronal signatures were highly lateralized and coupled to afferent drive in both models, whereas the inflammatory and immune signatures showed partial bilateral induction in some pathways in the inflammation model. These pathways are explored in the context of nociceptive behaviors and circuit alterations related to hyperalgesia with an emphasis on signaling molecules and receptors useful for future pharmacological investigations.
During persistent pain states, the somatosensory circuit becomes sensitized to protect the injured limb. At nociceptive primary afferent terminals in spinal dorsal horn, the degree of stimulation required to elicit release of substance P and other signals from nociceptors to spinal neurons is rapidly reduced.40 Persistent noxious stimulation is accompanied by rapid post-translational modification of proteins.53, 86, 102, 105 Moreover, increased firing rates and expanded receptive fields of post-synaptic dorsal horn neurons reflect their hyperexcitability to incoming afferent stimulation.45, 74 Simultaneously, gene changes begin in specific subpopulations of second-order spinal neurons,20, 48, 99, 122 which modulate the sensitization of spinal circuitry in response to continued nociceptive inputs. There remains a gap in the fundamental knowledge of the molecular pathways and receptors that are engaged by and modulate this sensitization process. Our experiments investigate these molecular changes related to neuronal plasticity in the early and later phases of incision and inflammatory pain models.
Activation of second-order neurons is accompanied by rapid upregulation of immediate-early genes such as Fos, which couple the neuronal activity to transcriptional alterations. This induction is associated with subsequent plasticity in the form of upregulation of other genes in the later phases of the sensitization process, such as Pdyn, which is strongly induced by 2 days.20, 86, 94 We extend the investigation of the earliest (2 hr) responding gene signatures to identify a broader network of activity-coupled genes (Figure 2). The range of this induction is broader than previously appreciated, including additional members of the AP-1 transcription factor family, for example. While the transcriptional event is rather short lived, protein staining for two members of the AP-1 family revealed sustained retention of both proteins in neuronal nuclei in superficial and deep dorsal horn. The temporal relationships between mRNA and protein are known for some of the defining molecules of this paradigm, and were used in the selection of the time points examined.48, 129 This early induction shapes the transcriptional network regulating downstream responses. The induction of Arc, for instance, has been described in enkephalinergic spinal neurons, where it is thought to participate in antinociception.41 Simultaneously, the basic helix-loop-helix transcription factor Npas4, which is strongly induced after inflammation, regulates activity-dependent inhibitory synapse density in mammalian CNS.77 Importantly, while the 48 hr timepoint represents the maximal induction for genes such as Pdyn, these transient mRNA induction events, are in some cases indicative of sustained long-term protein elevation. On the protein level, some of these changes persist for several days beyond the mRNA induction (Figure 2), and continue to drive longer-term biochemical changes.129 These events promote fine tuning of the spinal responses to peripheral injury, and the combinatorial nature of transcription factor interactions may provide a mechanism for activation of opponent processes that simultaneously drive excitatory and inhibitory regulatory processes. Future studies are necessary to more fully understand the precise induction patterns in specific cell types, but the identification of the strongest induction patterns in the present study forms a scaffold for future investigations.
While the early (2 hr) response was predominated by neuronal activity-coupled transcription, the 48 hr timepoint shows a bifurcated response affecting both neuronal and innate immune pathways. Upstream of both inflammation and incisional gene changes, we identified alterations consistent with interferon-γ (IFN-γ), tumor necrosis factor (TNF) signaling, and complement activation (Figure 4).31, 132 These pathways have been described during microglial activation,104, 145 although most likely additional cells such as astrocytes are also engaged. Complement-dependent microglial signaling participates in synaptic plasticity, which occurs in response to incoming afferent signals.132, 155 Proliferating and/or activated microglial cells mediate complement-dependent synaptic remodeling or synaptic stripping in neuropathic pain and deafferentation models.65 Of note, this complement-dependent synaptic remodeling is often dependent on, and activated by neuronal signaling, or absence thereof, as well as neuronal plasticity events.132, 150 However, this has generally not been extensively described in inflammation and incisional models to date.12 While the profile in both datasets contains a strong non-neural activation signature, the innate immune activation in the incision model also exhibits a unique additional proliferative signature including Plk1, Cdk1, and kinesin family members indicating G2 to M phase transition,6 and commitment to mitosis (Figure 6). This signal is most likely generated by microglial cells, for which proliferation has been described in neuropathic and incisional models.33, 56, 70, 116, 140, 154 Notably, however, additional studies are required to establish the spatial relationship of both the complement activation signature and proliferation events relative to the prodynorphinergic neurons. Specifically, the data may suggest a local proliferation event proximal to the activated dynorphinergic neuron related to tissue remodeling.
Within the spinal cord, at a minimum, there is a bidirectional neuron-immune interaction, where immune-like signals such as chemokines both feed back onto afferent terminals and also modulate post-synaptic spinal circuits. This bidirectional signaling involves interferons and Bdnf (Figure 5).110 While we were not able to detect expression of any interferons, this bilateral signal presumably infiltrates from the circulation. In contrast, Bdnf is induced predominantly ipsilateral to the inflammation, where it is expressed by both microglia and second order spinal neurons (Figure 7).18 This finding is also consistent with other reports of Bdnf induction in pain models.76, 83 In rats with a heterozygous Bdnf deletion, we previously described alterations in dorsal spinal cord in similar immune-like pathways (Supplementary Figure 6).128 Release of Bdnf by both second-order neurons and microglia in the superficial spinal cord modulates excitability of spinal networks, a phenomenon which has been particularly well-described in neuropathic pain models,26 and in learning and memory.106 Notably, this may also be part of the coupling mechanism between neuronal activation, microglia and immune-like signatures, allowing multiple cell types to coordinate. 95, 106, 148
The inflammatory gene signature at 48 hrs included a large group of bilaterally induced innate immune system genes, which include the neutrophil genes S100a8 and S100a9.87 Injection of the foreign sulfated seaweed polysaccharide λ-carrageenan strongly activates the immune system by stimulating the pattern recognition receptor Toll-like receptor 4.146 The recognition of this sulfated polysaccharide is similar to the response observed with injection of highly inflammatory bacterial cell wall components.35, 136 We hypothesize that immune systems throughout the body are stimulated by this xenobiotic agent, including the contralateral spinal cord, to engage in the production of antiviral and antimicrobial gene signatures, and is in part driven by interferon signaling. We refer to this process as “defensive priming” and it is observed after a number of other inflammatory events, including genuine viral infection, and could represent a protective transcription program to limit the spread of invasive pathogens. We initially observed one component of this process, seen as a generalized neutrophil arrest during carrageenan hind paw inflammation,87 but transcriptomic analyses reveals that the process is more complex and likely involves additional cell types besides arresting neutrophils such as astrocytes and endothelial cells.
We extend the analysis of the transcriptomic results to receptor genes regulated during the hyperalgesic process. By filtering for the most ipsilaterally regulated receptor genes, we selected receptors that were enriched in neuronal responses in the spinal cord. This analysis identified several promising candidates for analgesic drug discovery, especially anaplastic lymphoma kinase (Alk), a receptor tyrosine kinase first described as a result of a gene rearrangement in non-Hodgkin’s lymphoma.13 In nervous tissue, ALK is a neuronal receptor, and is expressed in both the spinal cord and peripheral sensory neurons, where it has been identified as a marker for TrkA+ nociceptors. 17 Transfection of wild type ALK in cultured neurons promotes increased neuronal proliferation, whereas ALK inhibition or Alk knockdown blocks these effects.119 These observations support the idea that wildtype ALK promotes neuronal growth. In the context of post-mitotic spinal neurons, ALK most likely participates in neurite outgrowth, synaptic remodeling, or phenotypic switching. The upregulation of this membrane-bound tyrosine kinase may also be indicative of a neuronal growth process coupled to the complement activation observed coincidentally. That is, the complement could be activated alongside these neuronal signals to facilitate rearrangement induced by growth factor and peptidergic signaling cascades, as complement is responsible for synapse elimination involved in information coding in the brain.152 Notably, Alk and Pdyn were among the genes most consistently regulated across models and in males and females. In situ labeling shows strongly Alk+ neurons are also strongly Pdyn+ in ipsilateral dorsal horn after inflammation. Quantitative analysis suggests that Alk/Pdyn co-positive neurons express at least 10x the levels of Pdyn and Alk relative to any contralateral neuron examined. Taken together with the observed, albeit limited, functional data on wildtype Alk in the nervous system,17, 91 our data suggests that the Alk/Pdyn co-positive neurons have changed or are in the process of changing their phenotype, producing vastly more Alk and Pdyn transcript than any positive neuron on the contralateral side. This is consistent with a major shift in the molecular behavior of these neurons, where they transition to production of vastly more prodynorphin precursor and processed peptides, and potentially undergo Alk-mediated neurite outgrowth.91 Our behavioral examinations of Alk inhibitors in animals with unilateral peripheral hind paw inflammation were not fully conclusive. In part, this was due to apparent anti-inflammatory actions of ceritinib, an effect that predisposes behavioral assessments to type I error, and the general toxicity of these molecules (Supplementary Figure 11, 12). Nonetheless, additional pharmacological studies are planned.
The transcriptomic measurements of neuronally-enriched, unilaterally upregulated genes and multilabel in situ hybridization produced a focus on a small, but highly regulated subset of neurons in the superficial layers of the dorsal horn. These neurons are glutamatergic and dynorphinergic, and express high levels of Alk and Tacr1. Additionally, these neurons undergo a transcriptional phenotypic switch that, quantitatively, is the most specific and highest magnitude transcriptional alteration occurring during the hyperalgesic state. Our observations are consistent with a number of other studies reporting excitatory dynorphinergic neurons,131 including the identification of a population of excitatory dynorphinergic neurons that synapses onto Tacr1+ projection neurons in lamina III.3 Furthermore, after peripheral inflammation a subset of dynorphinergic neurons in superficial laminae have been shown to project to the mesencephalic parabrachial area.92, 93 Figure 10 depicts a summary diagram of the up-regulated excitatory dynorphinergic cells, alongside known key relationships between other dorsal spinal cord neurons engaged in persistent pain. By contrast, inhibitory dynorphinergic neurons comprise a substantial proportion of the total dynorphinergic neurons, and are reported to inhibit itch,58 among other functions, indicating a multifunctional role for spinal dynorphin systems. Pharmacological studies find that kappa-opioid receptor signaling can be anti-hyperalgesic.38 However, these data must be taken in context with those of other studies suggesting that dynorphin and kappa-opioid receptor signaling in the spinal cord can enhance neuronal excitability and expand receptive fields,46, 75 possibly in part due to non-opioid actions of dynorphin A1–17 at the NMDA receptor.9 Our findings with a pharmacological antagonist suggest that endogenous dynorphinergic signaling can promote resolution of the hyperalgesic state (Figure 1, lower portion). Because some dynorphinergic neurons project to higher brain centers, specifying the exact location and the potential interaction with the glutamate co-transmitter will require further investigation.
Figure 10. Schematic diagram of cells involved in plastic responses to inflammatory hyperalgesia.
The induction of dynorphin in superficial neurons of the dorsal spinal cord occurs in excitatory projection neurons (green). Activation also occurs in adjacent densely Tacr1+, Dyn- superficial projection neurons (red). Inhibitory dynorphin cells (yellow) do not appear to show strong transcriptional regulation, and only a small percentage of these cells shows an induction of dynorphin. Large lamina III projection neurons (blue perikarya) have been shown previously to receive inputs from dynorphinergic terminals, although it remains unclear if these terminals come from the strongly induced superficial dynorphinergic neurons, or another population. Several components of spinal circuitry were also included from recent reviews88, 108, including the pathway by which non-noxious touch information can be transmitted to nociceptive projection neurons through intermediate pathways and excitatory vertical cells (orange). Notably, the description of Dyn+ neurons as projection neurons is based on nerve tracing experiments showing these projections extending to the mesencephalic parabrachial area.93
While sex was included in the present set of experiments, the analysis of the female rats did not show substantial sex differences in responses to inflammation at the level of the spinal cord (see Supplementary Figure 3). For example, while the female dataset was particularly variable at the 48 hr time point, in general it showed the same biological pathways as those in the males. This could be interpreted either as the same set of pathways with a more variable or lesser magnitude difference, or it could be a technical limitation of the study. This highlights another example where detailed anatomical and functional studies may resolve the underlying nature of the immune-like ipsilateral changes, which presumably are related to afferent drive. Such mechanistic functional studies would be interesting to extend in females given existing literature describing sexual dimorphisms in pain models.89
In addition to probing nociceptive mechanisms in spinal cord, these findings delineate the use of RNA-Seq for target discovery in the pain field. Several of the receptors in Figure 7 may be promising candidates for future analgesic research. This report expands upon our previous research using transcriptomic profiling for target identification in dorsal root ganglion, trigeminal ganglion and in peripheral nerve.29, 50, 73, 127 We have also delineated a strategy for using transcriptomics to improve translation of experimental analgesics,50 and performed detailed investigations of the mechanisms of novel non-opioid methods for pain control in humans.130 Moreover, the added insight gained by consideration of datasets from the two pain models (inflammation and incision), comparison of naïve, ipsi-and contralateral spinal cord and by leveraging outside datasets from published studies allowed for greater inferences to be made about the quantitative nature, cell types involved, relevance, and correlation of neuronal gene changes and broader bodily responses. Additionally, while efforts have been made to ascertain the overall pain gene signatures in the DRG after various pain models using microarray,71 a more concerted effort can be made using RNA-Seq for both the DRG and dorsal spinal cord by analyzing multiple datasets together using the same sequence analysis pipeline.129 Moreover, once neuronal circuits are defined on a molecular basis, the potential for precise functional studies can be enhanced using genetic labeling and/or activation paradigms, and eventually integration of transcriptomics with multi-neuronal ensemble recordings.149 For example, this approach has been taken for identifying the modulatory role of dynorphinergic spinal neurons in itch.58 These considerations underscore the advantages articulated by Akil, et al. that comparison of multiple techniques and experiments can produce a greater level of insight in neuroscience research.1 The large-data approach is characteristic of neurogenetics but is clearly beneficial to understanding pain models and pain mechanisms, and shows promise for realizing new precision pharmacology for pain management. These results and others reinforce the utility behind a growing trend towards integration of massively multiplex techniques such as RNA-Seq, lipidomics, proteomics and intravital imaging to enable functional characterization of these molecularly delineated cell types. This multi-omics approach enables discovery of new mechanisms of pain and pain control, and ultimately new therapeutics.50, 117, 126, 128, 130
Supplementary Material
Perspective:
The deadly impact of the opioid crisis and the need to replace morphine and other opioids in clinical practice is well recognized. Embedded within this research is an overarching goal of obtaining foundational knowledge from transcriptomics to search for non-opioid analgesic targets. Developing such analgesics would address unmet clinical needs.
Highlights.
We present the molecular response in dorsal spinal cord to peripheral inflammation.
Identify ALK as an induced neuronal tyrosine kinase receptor in dynorphinergic neurons.
Distinguish interferon immune responses from afferent driven complement increase.
Identify targetable analgesic signaling molecules induced by peripheral injury.
Articulate application of RNA-Seq to analgesic drug discovery.
Acknowledgements
The authors thank Ms. Natalie Labinger and Mr. Christopher Martin for their assistance with behavioral assessments.
DISCLOSURES:
This work was supported by the intramural research programs of the Clinical Center, the National Institute of Neurological Disorders and Stroke and the National Institutes of Nursing Research, National Institutes of Health. This work was also supported by a funds from the National Center for Complementary and Integrative Health (1ZIAAT000017–03), and the Office of Behavioral and Social Sciences Research. TG was supported by the Japan Society for the Promotion of Science Overseas Research Fellowship. Funding for two research fellows (JJK and FAV), was made possible through the NIH Medical Research Scholars Program, a public-private partnership supported jointly by the NIH and contributions to the Foundation for the NIH from the Doris Duke Charitable Foundation (DDCF Grant #2014194), the American Association for Dental Research, the Colgate-Palmolive Company, Genentech, Elsevier, and other private donors.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Akil H, Martone ME, Van Essen DC. Challenges and opportunities in mining neuroscience data. Science. 331:708–712, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barnes BJ, Richards J, Mancl M, Hanash S, Beretta L, Pitha PM. Global and distinct targets of IRF-5 and IRF-7 during innate response to viral infection. J Biol Chem. 279:45194–45207, 2004 [DOI] [PubMed] [Google Scholar]
- 3.Baseer N, Polgar E, Watanabe M, Furuta T, Kaneko T, Todd AJ. Projection neurons in lamina III of the rat spinal cord are selectively innervated by local dynorphin-containing excitatory neurons. J Neurosci. 32:11854–11863, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Benoliel R, Eliav E, Iadarola MJ. Neuropeptide Y in trigeminal ganglion following chronic constriction injury of the rat infraorbital nerve: is there correlation to somatosensory parameters? Pain. 91:111–121, 2001 [DOI] [PubMed] [Google Scholar]
- 5.Blivis D, Haspel G, Mannes PZ, O’Donovan MJ, Iadarola MJ. Identification of a novel spinal nociceptive-motor gate control for A-delta pain stimuli in rats. Elife. 6, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bostrom J, Sramkova Z, Salasova A, Johard H, Mahdessian D, Fedr R, Marks C, Medalova J, Soucek K, Lundberg E, Linnarsson S, Bryja V, Sekyrova P, Altun M, Andang M. Comparative cell cycle transcriptomics reveals synchronization of developmental transcription factor networks in cancer cells. PLoS One. 12:e0188772, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brennan FH, Gordon R, Lao HW, Biggins PJ, Taylor SM, Franklin RJ, Woodruff TM, Ruitenberg MJ. The Complement Receptor C5aR Controls Acute Inflammation and Astrogliosis following Spinal Cord Injury. J Neurosci. 35:6517–6531, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Butovsky O, Jedrychowski MP, Moore CS, Cialic R, Lanser AJ, Gabriely G, Koeglsperger T, Dake B, Wu PM, Doykan CE, Fanek Z, Liu L, Chen Z, Rothstein JD, Ransohoff RM, Gygi SP, Antel JP, Weiner HL. Identification of a unique TGF-beta-dependent molecular and functional signature in microglia. Nat Neurosci. 17:131–143, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Caudle RM, Mannes AJ. Dynorphin: friend or foe? Pain. 87:235–239, 2000 [DOI] [PubMed] [Google Scholar]
- 10.Chamessian A, Young M, Qadri Y, Berta T, Ji RR, Van de Ven T. Transcriptional Profiling of Somatostatin Interneurons in the Spinal Dorsal Horn. Sci Rep. 8:6809, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen EY, Tan CM, Kou Y, Duan Q, Wang Z, Meirelles GV, Clark NR, Ma’ayan A. Enrichr: interactive and collaborative HTML5 gene list enrichment analysis tool. BMC Bioinformatics. 14:128, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen G, Zhang YQ, Qadri YJ, Serhan CN, Ji RR. Microglia in Pain: Detrimental and Protective Roles in Pathogenesis and Resolution of Pain. Neuron. 100:1292–1311, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chiarle R, Voena C, Ambrogio C, Piva R, Inghirami G. The anaplastic lymphoma kinase in the pathogenesis of cancer. Nat Rev Cancer. 8:1123, 2008 [DOI] [PubMed] [Google Scholar]
- 14.Coull JA, Beggs S, Boudreau D, Boivin D, Tsuda M, Inoue K, Gravel C, Salter MW, De Koninck Y. BDNF from microglia causes the shift in neuronal anion gradient underlying neuropathic pain. Nature. 438:10171021, 2005 [DOI] [PubMed] [Google Scholar]
- 15.Croker BA, Krebs DL, Zhang JG, Wormald S, Willson TA, Stanley EG, Robb L, Greenhalgh CJ, Forster I, Clausen BE, Nicola NA, Metcalf D, Hilton DJ, Roberts AW, Alexander WS. SOCS3 negatively regulates IL-6 signaling in vivo. Nat Immunol. 4:540–545, 2003 [DOI] [PubMed] [Google Scholar]
- 16.Degn SE, Jensenius JC, Thiel S. Disease-causing mutations in genes of the complement system. Am J Hum Genet. 88:689–705, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Degoutin J, Brunet-de Carvalho N, Cifuentes-Diaz C, Vigny M. ALK (Anaplastic Lymphoma Kinase) expression in DRG neurons and its involvement in neuron-Schwann cells interaction. Eur J Neurosci. 29:275–286, 2009 [DOI] [PubMed] [Google Scholar]
- 18.Dembo T, Braz JM, Hamel KA, Kuhn JA, Basbaum AI. Primary AfferentDerived BDNF Contributes Minimally to the Processing of Pain and Itch. eNeuro. 5, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Denk F, Crow M, Didangelos A, Lopes DM, McMahon SB. Persistent Alterations in Microglial Enhancers in a Model of Chronic Pain. Cell Rep. 15:1771–1781, 2016 [DOI] [PubMed] [Google Scholar]
- 20.Draisci G, Iadarola MJ. Temporal analysis of increases in c-fos, preprodynorphin and preproenkephalin mRNAs in rat spinal cord. Brain Res Mol Brain Res. 6:31–37, 1989 [DOI] [PubMed] [Google Scholar]
- 21.Draisci G, Kajander KC, Dubner R, Bennett GJ, Iadarola MJ. Up-regulation of opioid gene expression in spinal cord evoked by experimental nerve injuries and inflammation. Brain Res. 560:186–192, 1991 [DOI] [PubMed] [Google Scholar]
- 22.Duclot F, Kabbaj M. The Role of Early Growth Response 1 (EGR1) in Brain Plasticity and Neuropsychiatric Disorders. Front Behav Neurosci. 11:35, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Everitt AR, Clare S, Pertel T, John SP, Wash RS, Smith SE, Chin CR, Feeley EM, Sims JS, Adams DJ, Wise HM, Kane L, Goulding D, Digard P, Anttila V, Baillie JK, Walsh TS, Hume DA, Palotie A, Xue Y, Colonna V, Tyler-Smith C, Dunning J, Gordon SB, Gen II, Investigators M, Smyth RL, Openshaw PJ, Dougan G, Brass AL, Kellam P. IFITM3 restricts the morbidity and mortality associated with influenza. Nature. 484:519–523, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Farber K, Cheung G, Mitchell D, Wallis R, Weihe E, Schwaeble W, Kettenmann H. C1q, the recognition subcomponent of the classical pathway of complement, drives microglial activation. J Neurosci Res. 87:644–652, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fehrenbacher JC, Vasko MR, Duarte DB. Models of inflammation: Carrageenan- or complete Freund’s Adjuvant (CFA)-induced edema and hypersensitivity in the rat. Curr Protoc Pharmacol. Chapter 5:Unit5 4, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ferrini F, De Koninck Y. Microglia control neuronal network excitability via BDNF signalling. Neural Plast. 2013:429815, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Golsteyn RM, Mundt KE, Fry AM, Nigg EA. Cell cycle regulation of the activity and subcellular localization of Plk1, a human protein kinase implicated in mitotic spindle function. J Cell Biol. 129:1617–1628, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gomes I, Bobeck EN, Margolis EB, Gupta A, Sierra S, Fakira AK, Fujita W, Muller TD, Muller A, Tschop MH, Kleinau G, Fricker LD, Devi LA. Identification of GPR83 as the receptor for the neuroendocrine peptide PEN. Sci Signal. 9:ra43, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Goswami SC, Mishra SK, Maric D, Kaszas K, Gonnella GL, Clokie SJ, Kominsky HD, Gross JR, Keller JM, Mannes AJ, Hoon MA, Iadarola MJ. Molecular signatures of mouse TRPV1-lineage neurons revealed by RNA-Seq transcriptome analysis. J Pain. 15:1338–1359, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Grandemange S, Aksentijevich I, Jeru I, Gul A, Touitou I. The regulation of MEFV expression and its role in health and familial Mediterranean fever. Genes Immun. 12:497–503, 2011 [DOI] [PubMed] [Google Scholar]
- 31.Griffin RS, Costigan M, Brenner GJ, Ma CH, Scholz J, Moss A, Allchorne AJ, Stahl GL, Woolf CJ. Complement induction in spinal cord microglia results in anaphylatoxin C5a-mediated pain hypersensitivity. J Neurosci. 27:8699–8708, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Guan J, Umapathy G, Yamazaki Y, Wolfstetter G, Mendoza P, Pfeifer K, Mohammed A, Hugosson F, Zhang H, Hsu AW, Halenbeck R, Hallberg B, Palmer RH. FAM150A and FAM150B are activating ligands for anaplastic lymphoma kinase. Elife. 4:e09811, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Guan Z, Kuhn JA, Wang X, Colquitt B, Solorzano C, Vaman S, Guan AK, Evans-Reinsch Z, Braz J, Devor M, Abboud-Werner SL, Lanier LL, Lomvardas S, Basbaum AI. Injured sensory neuron-derived CSF1 induces microglial proliferation and DAP12-dependent pain. Nat Neurosci. 19:94–101, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Haller O, Kochs G. Interferon-induced mx proteins: dynamin-like GTPases with antiviral activity. Traffic. 3:710–717, 2002 [DOI] [PubMed] [Google Scholar]
- 35.Hanazawa S, Ishikawa T, Yamaura K. Comparison of the adjuvant effect on antibody response of three types of carrageenan and the cellular events in the induction of the effect. Int J Immunopharmacol. 4:521–527, 1982 [DOI] [PubMed] [Google Scholar]
- 36.Hargreaves K, Dubner R, Brown F, Flores C, Joris J. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain. 32:77–88, 1988 [DOI] [PubMed] [Google Scholar]
- 37.Haring M, Zeisel A, Hochgerner H, Rinwa P, Jakobsson JET, Lonnerberg P, La Manno G, Sharma N, Borgius L, Kiehn O, Lagerstrom MC, Linnarsson S, Ernfors P. Neuronal atlas of the dorsal horn defines its architecture and links sensory input to transcriptional cell types. Nat Neurosci. 21:869–880, 2018 [DOI] [PubMed] [Google Scholar]
- 38.Ho J, Mannes AJ, Dubner R, Caudle RM. Putative kappa-2 opioid agonists are antihyperalgesic in a rat model of inflammation. J Pharmacol Exp Ther. 281:136–141, 1997 [PubMed] [Google Scholar]
- 39.Hodge RD, Bakken TE, Miller JA, Smith KA, Barkan ER, Graybuck LT, Close JL, Long B, Johansen N, Penn O, Yao Z, Eggermont J, Hollt T, Levi BP, Shehata SI, Aevermann B, Beller A, Bertagnolli D, Brouner K, Casper T, Cobbs C, Dalley R, Dee N, Ding SL, Ellenbogen RG, Fong O, Garren E, Goldy J, Gwinn RP, Hirschstein D, Keene CD, Keshk M, Ko AL, Lathia K, Mahfouz A, Maltzer Z, McGraw M, Nguyen TN, Nyhus J, Ojemann JG, Oldre A, Parry S, Reynolds S, Rimorin C, Shapovalova NV, Somasundaram S, Szafer A, Thomsen ER, Tieu M, Quon G, Scheuermann RH, Yuste R, Sunkin SM, Lelieveldt B, Feng D, Ng L, Bernard A, Hawrylycz M, Phillips JW, Tasic B, Zeng H, Jones AR, Koch C, Lein ES. Conserved cell types with divergent features in human versus mouse cortex. Nature. 573:61–68, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Honor P, Menning PM, Rogers SD, Nichols ML, Basbaum AI, Besson JM, Mantyh PW. Spinal substance P receptor expression and internalization in acute, short-term, and long-term inflammatory pain states. J Neurosci. 19:7670–7678, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hossaini M, Jongen JL, Biesheuvel K, Kuhl D, Holstege JC. Nociceptive stimulation induces expression of Arc/Arg3.1 in the spinal cord with a preference for neurons containing enkephalin. Mol Pain. 6:43, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hu Y, Mao K, Zeng Y, Chen S, Tao Z, Yang C, Sun S, Wu X, Meng G, Sun B. Tripartite-motif protein 30 negatively regulates NLRP3 inflammasome activation by modulating reactive oxygen species production. J Immunol. 185:7699–7705, 2010 [DOI] [PubMed] [Google Scholar]
- 43.Huang R, Grishagin I, Wang Y, Zhao T, Greene J, Obenauer JC, Ngan D, Nguyen DT, Guha R, Jadhav A, Southall N, Simeonov A, Austin CP. The NCATS BioPlanet - An Integrated Platform for Exploring the Universe of Cellular Signaling Pathways for Toxicology, Systems Biology, and Chemical Genomics. Front Pharmacol. 10:445, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hunt SP, Pini A, Evan G. Induction of c-fos-like protein in spinal cord neurons following sensory stimulation. Nature. 328:632–634, 1987 [DOI] [PubMed] [Google Scholar]
- 45.Hylden JL, Nahin RL, Traub RJ, Dubner R. Expansion of receptive fields of spinal lamina I projection neurons in rats with unilateral adjuvant-induced inflammation: the contribution of dorsal horn mechanisms. Pain. 37:229–243, 1989 [DOI] [PubMed] [Google Scholar]
- 46.Hylden JL, Nahin RL, Traub RJ, Dubner R. Effects of spinal kappa-opioid receptor agonists on the responsiveness of nociceptive superficial dorsal horn neurons. Pain. 44:187–193, 1991 [DOI] [PubMed] [Google Scholar]
- 47.Hylden JL, Thomas DA, Iadarola MJ, Nahin RL, Dubner R. Spinal opioid analgesic effects are enhanced in a model of unilateral inflammation/hyperalgesia: possible involvement of noradrenergic mechanisms. Eur J Pharmacol. 194:135–143, 1991 [DOI] [PubMed] [Google Scholar]
- 48.Iadarola MJ, Brady LS, Draisci G, Dubner R. Enhancement of dynorphin gene expression in spinal cord following experimental inflammation: stimulus specificity, behavioral parameters and opioid receptor binding. Pain. 35:313–326, 1988 [DOI] [PubMed] [Google Scholar]
- 49.Iadarola MJ, Douglass J, Civelli O, Naranjo JR. Differential activation of spinal cord dynorphin and enkephalin neurons during hyperalgesia: evidence using cDNA hybridization. Brain Res. 455:205–212, 1988 [DOI] [PubMed] [Google Scholar]
- 50.Iadarola MJ, Sapio MR, Raithel SJ, Mannes AJ, Brown DC. Long-term pain relief in canine osteoarthritis by a single intra-articular injection of resiniferatoxin, a potent TRPV1 agonist. Pain. 159:2105–2114, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Iadarola MJ, Sapio MR, Wang X, Carrero H, Virata-Theimer ML, Sarnovsky R, Mannes AJ, FitzGerald DJ. Analgesia by Deletion of Spinal Neurokinin 1 Receptor Expressing Neurons Using a Bioengineered Substance P-Pseudomonas Exotoxin Conjugate. Mol Pain. 13:1744806917727657, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jeong H, Na YJ, Lee K, Kim YH, Lee Y, Kang M, Jiang BC, Yeom YI, Wu LJ, Gao YJ, Kim J, Oh SB. High-resolution transcriptome analysis reveals neuropathic pain gene-expression signatures in spinal microglia after nerve injury. Pain. 157:964–976, 2016 [DOI] [PubMed] [Google Scholar]
- 53.Ji RR, Baba H, Brenner GJ, Woolf CJ. Nociceptive-specific activation of ERK in spinal neurons contributes to pain hypersensitivity. Nat Neurosci. 2:1114–1119, 1999 [DOI] [PubMed] [Google Scholar]
- 54.Ji RR, Chamessian A, Zhang YQ. Pain regulation by non-neuronal cells and inflammation. Science. 354:572–577, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ji RR, Rupp F. Phosphorylation of transcription factor CREB in rat spinal cord after formalin-induced hyperalgesia: relationship to c-fos induction. J Neurosci. 17:1776–1785, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jin SX, Zhuang ZY, Woolf CJ, Ji RR. p38 mitogen-activated protein kinase is activated after a spinal nerve ligation in spinal cord microglia and dorsal root ganglion neurons and contributes to the generation of neuropathic pain. J Neurosci. 23:4017–4022, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kajander KC, Sahara Y, Iadarola MJ, Bennett GJ. Dynorphin increases in the dorsal spinal cord in rats with a painful peripheral neuropathy. Peptides. 11:719–728, 1990 [DOI] [PubMed] [Google Scholar]
- 58.Kardon AP, Polgar E, Hachisuka J, Snyder LM, Cameron D, Savage S, Cai X, Karnup S, Fan CR, Hemenway GM, Bernard CS, Schwartz ES, Nagase H, Schwarzer C, Watanabe M, Furuta T, Kaneko T, Koerber HR, Todd AJ, Ross SE. Dynorphin acts as a neuromodulator to inhibit itch in the dorsal horn of the spinal cord. Neuron. 82:573–586, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Karin M, Liu Z, Zandi E. AP-1 function and regulation. Curr Opin Cell Biol. 9:240–246, 1997 [DOI] [PubMed] [Google Scholar]
- 60.Kataoka K, Igarashi K, Itoh K, Fujiwara KT, Noda M, Yamamoto M, Nishizawa M. Small Maf proteins heterodimerize with Fos and may act as competitive repressors of the NF-E2 transcription factor. Mol Cell Biol. 15:2180–2190, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Katsoulidis E, Carayol N, Woodard J, Konieczna I, Majchrzak-Kita B, Jordan A, Sassano A, Eklund EA, Fish EN, Platanias LC. Role of Schlafen 2 (SLFN2) in the generation of interferon alpha-induced growth inhibitory responses. J Biol Chem. 284:25051–25064, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kawai T, Sato S, Ishii KJ, Coban C, Hemmi H, Yamamoto M, Terai K, Matsuda M, Inoue J, Uematsu S, Takeuchi O, Akira S. Interferon-alpha induction through Toll-like receptors involves a direct interaction of IRF7 with MyD88 and TRAF6. Nat Immunol. 5:1061–1068, 2004 [DOI] [PubMed] [Google Scholar]
- 63.Kawasaki Y, Zhang L, Cheng JK, Ji RR. Cytokine mechanisms of central sensitization: distinct and overlapping role of interleukin-1beta, interleukin6, and tumor necrosis factor-alpha in regulating synaptic and neuronal activity in the superficial spinal cord. J Neurosci. 28:5189–5194, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Keren-Shaul H, Spinrad A, Weiner A, Matcovitch-Natan O, Dvir-Szternfeld R, Ulland TK, David E, Baruch K, Lara-Astaiso D, Toth B, Itzkovitz S, Colonna M, Schwartz M, Amit I. A Unique Microglia Type Associated with Restricting Development of Alzheimer’s Disease. Cell. 169:1276–1290 e1217, 2017 [DOI] [PubMed] [Google Scholar]
- 65.Kettenmann H, Kirchhoff F, Verkhratsky A. Microglia: new roles for the synaptic stripper. Neuron. 77:10–18, 2013 [DOI] [PubMed] [Google Scholar]
- 66.Kidmose RT, Laursen NS, Dobo J, Kjaer TR, Sirotkina S, Yatime L, Sottrup-Jensen L, Thiel S, Gal P, Andersen GR. Structural basis for activation of the complement system by component C4 cleavage. Proc Natl Acad Sci U S A. 109:15425–15430, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ko SW, Vadakkan KI, Ao H, Gallitano-Mendel A, Wei F, Milbrandt J, Zhuo M. Selective contribution of Egr1 (zif/268) to persistent inflammatory pain. J Pain. 6:12–20, 2005 [DOI] [PubMed] [Google Scholar]
- 68.Koguchi K, Nakatsuji Y, Okuno T, Sawada M, Sakoda S. Microglial cell cycle-associated proteins control microglial proliferation in vivo and in vitro and are regulated by GM-CSF and density-dependent inhibition. J Neurosci Res. 74:898–905, 2003 [DOI] [PubMed] [Google Scholar]
- 69.Kohl J The role of complement in danger sensing and transmission. Immunol Res. 34:157–176, 2006 [DOI] [PubMed] [Google Scholar]
- 70.LaCroix-Fralish ML, Austin JS, Zheng FY, Levitin DJ, Mogil JS. Patterns of pain: meta-analysis of microarray studies of pain. Pain. 152:1888–1898, 2011 [DOI] [PubMed] [Google Scholar]
- 71.LaCroix-Fralish ML, Austin JS, Zheng FY, Levitin DJ, Mogil JS. Patterns of pain: meta-analysis of microarray studies of pain. Pain. 152:1888–1898, 2011 [DOI] [PubMed] [Google Scholar]
- 72.Lacy M, Jones J, Whittemore SR, Haviland DL, Wetsel RA, Barnum SR. Expression of the receptors for the C5a anaphylatoxin, interleukin-8 and FMLP by human astrocytes and microglia. J Neuroimmunol. 61:71–78, 1995 [DOI] [PubMed] [Google Scholar]
- 73.LaPaglia DM, Sapio MR, Burbelo PD, Thierry-Mieg J, Thierry-Mieg D, Raithel SJ, Ramsden CE, Iadarola MJ, Mannes AJ. RNA-Seq investigations of human post-mortem trigeminal ganglia. Cephalalgia. 38:912–932, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Latremoliere A, Woolf CJ. Central sensitization: a generator of pain hypersensitivity by central neural plasticity. J Pain. 10:895–926, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Laughlin TM, Larson AA, Wilcox GL. Mechanisms of induction of persistent nociception by dynorphin. J Pharmacol Exp Ther. 299:6–11, 2001 [PubMed] [Google Scholar]
- 76.Li CQ, Xu JM, Liu D, Zhang JY, Dai RP. Brain derived neurotrophic factor (BDNF) contributes to the pain hypersensitivity following surgical incision in the rats. Mol Pain. 4:27, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lin Y, Bloodgood BL, Hauser JL, Lapan AD, Koon AC, Kim TK, Hu LS, Malik AN, Greenberg ME. Activity-dependent regulation of inhibitory synapse development by Npas4. Nature. 455:1198–1204, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 15:550, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lu Y, Perl ER. A specific inhibitory pathway between substantia gelatinosa neurons receiving direct C-fiber input. J Neurosci. 23:8752–8758, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Masquilier D, Sassone-Corsi P. Transcriptional cross-talk: nuclear factors CREM and CREB bind to AP-1 sites and inhibit activation by Jun. J Biol Chem. 267:22460–22466, 1992 [PubMed] [Google Scholar]
- 81.Masuda T, Tsuda M, Yoshinaga R, Tozaki-Saitoh H, Ozato K, Tamura T, Inoue K. IRF8 is a critical transcription factor for transforming microglia into a reactive phenotype. Cell Rep. 1:334–340, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mavrommatis E, Fish EN, Platanias LC. The schlafen family of proteins and their regulation by interferons. J Interferon Cytokine Res. 33:206–210, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Merighi A, Salio C, Ghirri A, Lossi L, Ferrini F, Betelli C, Bardoni R. BDNF as a pain modulator. Progress in Neurobiology. 85:297–317, 2008 [DOI] [PubMed] [Google Scholar]
- 84.Merighi A, Salio C, Ghirri A, Lossi L, Ferrini F, Betelli C, Bardoni R. BDNF as a pain modulator. Prog Neurobiol. 85:297–317, 2008 [DOI] [PubMed] [Google Scholar]
- 85.Messersmith DJ, Gu J, Dubner R, Douglass J, Iadarola MJ. Basal and inducible transcriptional activity of an upstream AP-1/CRE element (DYNCRE3) in the prodynorphin promoter. Mol Cell Neurosci. 5:238–245, 1994 [DOI] [PubMed] [Google Scholar]
- 86.Messersmith DJ, Kim DJ, Iadarola MJ. Transcription factor regulation of prodynorphin gene expression following rat hindpaw inflammation. Brain Res Mol Brain Res. 53:260–269, 1998 [DOI] [PubMed] [Google Scholar]
- 87.Mitchell K, Yang HY, Tessier PA, Muhly WT, Swaim WD, Szalayova I, Keller JM, Mezey E, Iadarola MJ. Localization of S100A8 and S100A9 expressing neutrophils to spinal cord during peripheral tissue inflammation. Pain. 134:216–231, 2008 [DOI] [PubMed] [Google Scholar]
- 88.Moehring F, Halder P, Seal RP, Stucky CL. Uncovering the Cells and Circuits of Touch in Normal and Pathological Settings. Neuron. 100:349360, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Mogil JS. Qualitative sex differences in pain processing: emerging evidence of a biased literature. Nat Rev Neurosci. 21:353–365, 2020 [DOI] [PubMed] [Google Scholar]
- 90.Morgan M, Deuis JR, Woodruff TM, Lewis RJ, Vetter I. Role of complement anaphylatoxin receptors in a mouse model of acute burninduced pain. Mol Immunol. 94:68–74, 2018 [DOI] [PubMed] [Google Scholar]
- 91.Motegi A, Fujimoto J, Kotani M, Sakuraba H, Yamamoto T. ALK receptor tyrosine kinase promotes cell growth and neurite outgrowth. J Cell Sci. 117:3319–3329, 2004 [DOI] [PubMed] [Google Scholar]
- 92.Nahin RL, Hylden JL, Humphrey E. Demonstration of dynorphin A 1–8 immunoreactive axons contacting spinal cord projection neurons in a rat model of peripheral inflammation and hyperalgesia. Pain. 51:135–143, 1992 [DOI] [PubMed] [Google Scholar]
- 93.Nahin RL, Hylden JL, Iadarola MJ, Dubner R. Peripheral inflammation is associated with increased dynorphin immunoreactivity in both projection and local circuit neurons in the superficial dorsal horn of the rat lumbar spinal cord. Neurosci Lett. 96:247–252, 1989 [DOI] [PubMed] [Google Scholar]
- 94.Naranjo JR, Mellstrom B, Achaval M, Sassone-Corsi P. Molecular pathways of pain: Fos/Jun-mediated activation of a noncanonical AP-1 site in the prodynorphin gene. Neuron. 6:607–617, 1991 [DOI] [PubMed] [Google Scholar]
- 95.Nguyen PT, Dorman LC, Pan S, Vainchtein ID, Han RT, Nakao-Inoue H, Taloma SE, Barron JJ, Molofsky AB, Kheirbek MA, Molofsky AV. Microglial Remodeling of the Extracellular Matrix Promotes Synapse Plasticity. Cell. 182:388–403 e315, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Nigg EA. Polo-like kinases: positive regulators of cell division from start to finish. Curr Opin Cell Biol. 10:776–783, 1998 [DOI] [PubMed] [Google Scholar]
- 97.Ning S, Pagano JS, Barber GN. IRF7: activation, regulation, modification and function. Genes Immun. 12:399–414, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Nishino J, Yamashita K, Hashiguchi H, Fujii H, Shimazaki T, Hamada H. Meteorin: a secreted protein that regulates glial cell differentiation and promotes axonal extension. EMBO J. 23:1998–2008, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Noguchi K, Kowalski K, Traub R, Solodkin A, Iadarola MJ, Ruda MA. Dynorphin expression and Fos-like immunoreactivity following inflammation induced hyperalgesia are colocalized in spinal cord neurons. Brain Res Mol Brain Res. 10:227–233, 1991 [DOI] [PubMed] [Google Scholar]
- 100.Noris M, Remuzzi G. Overview of complement activation and regulation. Semin Nephrol. 33:479–492, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ohtani K, Iwanaga R, Nakamura M, Ikeda M, Yabuta N, Tsuruga H, Nojima H. Cell growth-regulated expression of mammalian MCM5 and MCM6 genes mediated by the transcription factor E2F. Oncogene. 18:2299–2309, 1999 [DOI] [PubMed] [Google Scholar]
- 102.Olah Z, Karai L, Iadarola MJ. Protein kinase C(alpha) is required for vanilloid receptor 1 activation. Evidence for multiple signaling pathways. J Biol Chem. 277:35752–35759, 2002 [DOI] [PubMed] [Google Scholar]
- 103.Papadia S, Soriano FX, Leveille F, Martel MA, Dakin KA, Hansen HH, Kaindl A, Sifringer M, Fowler J, Stefovska V, McKenzie G, Craigon M, Corriveau R, Ghazal P, Horsburgh K, Yankner BA, Wyllie DJ, Ikonomidou C, Hardingham GE. Synaptic NMDA receptor activity boosts intrinsic antioxidant defenses. Nat Neurosci. 11:476–487, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Papageorgiou IE, Lewen A, Galow LV, Cesetti T, Scheffel J, Regen T, Hanisch UK, Kann O. TLR4-activated microglia require IFN-gamma to induce severe neuronal dysfunction and death in situ. Proc Natl Acad Sci. 113:212–217, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Pareek TK, Keller J, Kesavapany S, Pant HC, Iadarola MJ, Brady RO, Kulkarni AB. Cyclin-dependent kinase 5 activity regulates pain signaling. Proc Natl Acad Sci. 103:791–796, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Parkhurst CN, Yang G, Ninan I, Savas JN, Yates JR, 3rd, Lafaille JJ, Hempstead BL, Littman DR, Gan WB. Microglia promote learningdependent synapse formation through brain-derived neurotrophic factor. Cell. 155:1596–1609, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Paulus C, Sollars PJ, Pickard GE, Enquist LW. Transcriptome signature of virulent and attenuated pseudorabies virus-infected rodent brain. J Virol. 80:1773–1786, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Peirs C, Seal RP. Neural circuits for pain: Recent advances and current views. Science. 354:578–584, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Petrenko AB, Yamakura T, Baba H, Shimoji K. The role of N-methyl-D-aspartate (NMDA) receptors in pain: a review. Anesth Analg. 97:11081116, 2003 [DOI] [PubMed] [Google Scholar]
- 110.Pinho-Ribeiro FA, Verri WA Jr, .Chiu IM Nociceptor Sensory NeuronImmune Interactions in Pain and Inflammation. Trends Immunol. 38:5–19, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Price TJ, Gold MS. From Mechanism to Cure: Renewing the Goal to Eliminate the Disease of Pain. Pain Med. 19:1525–1549, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Pruunsild P, Sepp M, Orav E, Koppel I, Timmusk T. Identification of ciselements and transcription factors regulating neuronal activity-dependent transcription of human BDNF gene. J Neurosci. 31:3295–3308, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Przanowski P, Dabrowski M, Ellert-Miklaszewska A, Kloss M, Mieczkowski J, Kaza B, Ronowicz A, Hu F, Piotrowski A, Kettenmann H, Komorowski J, Kaminska B. The signal transducers Stat1 and Stat3 and their novel target Jmjd3 drive the expression of inflammatory genes in microglia. J Mol Med (Berl). 92:239–254, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Raithel SJ, Sapio MR, Iadarola MJ, Mannes AJ. Thermal A-delta Nociceptors, Identified by Transcriptomics, Express Higher Levels of Anesthesia-Sensitive Receptors Than Thermal C-Fibers and Are More Suppressible by Low-Dose Isoflurane. Anesth Analg. 127:263–266, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Raithel SJ, Sapio MR, LaPaglia DM, Iadarola MJ, Mannes AJ. Transcriptional Changes in Dorsal Spinal Cord Persist after Surgical Incision Despite Preemptive Analgesia with Peripheral Resiniferatoxin. Anesthesiology. 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Raithel SJ, Sapio MR, LaPaglia DM, Iadarola MJ, Mannes AJ. Transcriptional Changes in Dorsal Spinal Cord Persist after Surgical Incision Despite Preemptive Analgesia with Peripheral Resiniferatoxin. Anesthesiology. 128:620–635, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ramsden CE, Domenichiello AF, Yuan ZX, Sapio MR, Keyes GS, Mishra SK, Gross JR, Majchrzak-Hong S, Zamora D, Horowitz MS, Davis JM, Sorokin AV, Dey A, LaPaglia DM, Wheeler JJ, Vasko MR, Mehta NN, Mannes AJ, Iadarola MJ. A systems approach for discovering linoleic acid derivatives that potentially mediate pain and itch. Sci Signal. 10, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Regis G, Pensa S, Boselli D, Novelli F, Poli V. Ups and downs: the STAT1:STAT3 seesaw of Interferon and gp130 receptor signalling. Semin Cell Dev Biol. 19:351–359, 2008 [DOI] [PubMed] [Google Scholar]
- 119.Reiff T, Huber L, Kramer M, Delattre O, Janoueix-Lerosey I, Rohrer H. Midkine and Alk signaling in sympathetic neuron proliferation and neuroblastoma predisposition. Development. 138:4699–4708, 2011 [DOI] [PubMed] [Google Scholar]
- 120.Ren K, Dubner R. Inflammatory Models of Pain and Hyperalgesia. ILAR J. 40:111–118, 1999 [DOI] [PubMed] [Google Scholar]
- 121.Rorick-Kehn LM, Witkin JM, Statnick MA, Eberle EL, McKinzie JH, Kahl SD, Forster BM, Wong CJ, Li X, Crile RS, Shaw DB, Sahr AE, Adams BL, Quimby SJ, Diaz N, Jimenez A, Pedregal C, Mitch CH, Knopp KL, Anderson WH, Cramer JW, McKinzie DL. LY2456302 is a novel, potent, orally-bioavailable small molecule kappa-selective antagonist with activity in animal models predictive of efficacy in mood and addictive disorders. Neuropharmacology. 77:131–144, 2014 [DOI] [PubMed] [Google Scholar]
- 122.Ruda MA, Iadarola MJ, Cohen LV, Young WS, 3rd. In situ hybridization histochemistry and immunocytochemistry reveal an increase in spinal dynorphin biosynthesis in a rat model of peripheral inflammation and hyperalgesia. Proc Natl Acad Sci. 85:622–626, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Russell FA, McDougall JJ. Proteinase activated receptor (PAR) involvement in mediating arthritis pain and inflammation. Inflamm Res. 58:119–126, 2009 [DOI] [PubMed] [Google Scholar]
- 124.Ryckman C, Vandal K, Rouleau P, Talbot M, Tessier PA. Proinflammatory activities of S100: proteins S100A8, S100A9, and S100A8/A9 induce neutrophil chemotaxis and adhesion. J Immunol. 170:3233–3242, 2003 [DOI] [PubMed] [Google Scholar]
- 125.Safe S, Jin UH, Morpurgo B, Abudayyeh A, Singh M, Tjalkens RB. Nuclear receptor 4A (NR4A) family - orphans no more. J Steroid Biochem Mol Biol. 157:48–60, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Sapio MR, Fricker LD. Carboxypeptidases in disease: insights from peptidomic studies. Proteomics Clin Appl. 8:327–337, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Sapio MR, Goswami SC, Gross JR, Mannes AJ, Iadarola MJ. Transcriptomic analyses of genes and tissues in inherited sensory neuropathies. Exp Neurol. 283:375–395, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Sapio MR, Iadarola MJ, LaPaglia DM, Lehky T, Thurm AE, Danley KM, Fuhr SR, Lee MD, Huey AE, Sharp SJ, Tsao JW, Yanovski JA, Mannes AJ, Han JC. Haploinsufficiency of the brain-derived neurotrophic factor gene is associated with reduced pain sensitivity. Pain. 160:1070–1081, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Sapio MR, Iadarola MJ, Loydpierson AJ, Kim JJ, Thierry-Mieg D, Thierry-Mieg J, Maric D, Mannes AJ. Dynorphin and Enkephalin Opioid Peptides and Transcripts in Spinal Cord and Dorsal Root Ganglion During Peripheral Inflammatory Hyperalgesia and Allodynia. J Pain. 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Sapio MR, Neubert JK, LaPaglia DM, Maric D, Keller JM, Raithel SJ, Rohrs EL, Anderson EM, Butman JA, Caudle RM, Brown DC, Heiss JD, Mannes AJ, Iadarola MJ. Pain control through selective chemo-axotomy of centrally projecting TRPV1+ sensory neurons. J Clin Invest. 128:1657–1670, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Sardella TC, Polgar E, Garzillo F, Furuta T, Kaneko T, Watanabe M, Todd AJ. Dynorphin is expressed primarily by GABAergic neurons that contain galanin in the rat dorsal horn. Mol Pain. 7:76, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Schafer DP, Lehrman EK, Kautzman AG, Koyama R, Mardinly AR, Yamasaki R, Ransohoff RM, Greenberg ME, Barres BA, Stevens B. Microglia sculpt postnatal neural circuits in an activity and complement-dependent manner. Neuron. 74:691–705, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Sebire G, Emilie D, Wallon C, Hery C, Devergne O, Delfraissy JF, Galanaud P, Tardieu M. In vitro production of IL-6, IL-1 beta, and tumor necrosis factor-alpha by human embryonic microglial and neural cells. J Immunol. 150:1517–1523, 1993 [PubMed] [Google Scholar]
- 134.Shutov LP, Warwick CA, Shi X, Gnanasekaran A, Shepherd AJ, Mohapatra DP, Woodruff TM, Clark JD, Usachev YM. The Complement System Component C5a Produces Thermal Hyperalgesia via Macrophage-to-Nociceptor Signaling That Requires NGF and TRPV1. J Neurosci. 36:5055–5070, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Smith M, Burke Z, Humphries A, Wells T, Klein D, Carter D, Baler R. Tissue-specific transgenic knockdown of Fos-related antigen 2 (Fra-2) expression mediated by dominant negative Fra-2. Mol Cell Biol. 21:3704–3713, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Song J, Bishop BL, Li G, Duncan MJ, Abraham SN. TLR4-initiated and cAMP-mediated abrogation of bacterial invasion of the bladder. Cell Host Microbe. 1:287–298, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Sonnenberg JL, Rauscher FJ 3rd, Morgan JI, Curran T Regulation of proenkephalin by Fos and Jun. Science. 246:1622–1625, 1989 [DOI] [PubMed] [Google Scholar]
- 138.Stanfa LC, Sullivan AF, Dickenson AH. Alterations in neuronal excitability and the potency of spinal mu, delta and kappa opioids after carrageenan-induced inflammation. Pain. 50:345–354, 1992 [DOI] [PubMed] [Google Scholar]
- 139.Starobova H, S WAH , Lewis RJ, Vetter I Transcriptomics in pain research: insights from new and old technologies. Mol Omics. 14:389–404, 2018 [DOI] [PubMed] [Google Scholar]
- 140.Suter MR, Berta T, Gao YJ, Decosterd I, Ji RR. Large A-fiber activity is required for microglial proliferation and p38 MAPK activation in the spinal cord: different effects of resiniferatoxin and bupivacaine on spinal microglial changes after spared nerve injury. Mol Pain. 5:53, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Suzuki T, Okuno H, Yoshida T, Endo T, Nishina H, Iba H. Difference in transcriptional regulatory function between c-Fos and Fra-2. Nucleic Acids Res. 19:5537–5542, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Szklarczyk D, Franceschini A, Wyder S, Forslund K, Heller D, Huerta-Cepas J, Simonovic M, Roth A, Santos A, Tsafou KP, Kuhn M, Bork P, Jensen LJ, von Mering C. STRING v10: protein-protein interaction networks, integrated over the tree of life. Nucleic Acids Res. 43:D447–452, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Takazawa T, Choudhury P, Tong CK, Conway CM, Scherrer G, Flood PD, Mukai J, MacDermott AB. Inhibition Mediated by Glycinergic and GABAergic Receptors on Excitatory Neurons in Mouse Superficial Dorsal Horn Is Location-Specific but Modified by Inflammation. J Neurosci. 37:2336–2348, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Takazawa T, MacDermott AB. Glycinergic and GABAergic tonic inhibition fine tune inhibitory control in regionally distinct subpopulations of dorsal horn neurons. J Physiol. 588:2571–2587, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Tsuda M, Masuda T, Kitano J, Shimoyama H, Tozaki-Saitoh H, Inoue K. IFN-gamma receptor signaling mediates spinal microglia activation driving neuropathic pain. Proc Natl Acad Sci. 106:8032–8037, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Tsuji RF, Hoshino K, Noro Y, Tsuji NM, Kurokawa T, Masuda T, Akira S, Nowak B. Suppression of allergic reaction by lambda-carrageenan: toll-like receptor 4/MyD88-dependent and -independent modulation of immunity. Clin Exp Allergy. 33:249–258, 2003 [DOI] [PubMed] [Google Scholar]
- 147.Vanderah TW, Ossipov MH, Lai J, Malan TP Jr, Porreca F Mechanisms of opioid-induced pain and antinociceptive tolerance: descending facilitation and spinal dynorphin. Pain. 92:5–9, 2001 [DOI] [PubMed] [Google Scholar]
- 148.Vasek MJ, Garber C, Dorsey D, Durrant DM, Bollman B, Soung A, Yu J, Perez-Torres C, Frouin A, Wilton DK, Funk K, DeMasters BK, Jiang X, Bowen JR, Mennerick S, Robinson JK, Garbow JR, Tyler KL, Suthar MS, Schmidt RE, Stevens B, Klein RS. A complement-microglial axis drives synapse loss during virus-induced memory impairment. Nature. 534:538543, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.von Buchholtz LJ, Ghitani N, Lam RM, Licholai JA, Chesler AT, Ryba NJP. Decoding Cellular Mechanisms for Mechanosensory Discrimination. Neuron. 109:285–298 e285, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Vukojicic A, Delestree N, Fletcher EV, Pagiazitis JG, Sankaranarayanan S, Yednock TA, Barres BA, Mentis GZ. The Classical Complement Pathway Mediates Microglia-Dependent Remodeling of Spinal Motor Circuits during Development and in SMA. Cell Rep. 29:3087–3100 e3087, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Walker DG, Link J, Lue LF, Dalsing-Hernandez JE, Boyes BE. Gene expression changes by amyloid beta peptide-stimulated human postmortem brain microglia identify activation of multiple inflammatory processes. J Leukoc Biol. 79:596–610, 2006 [DOI] [PubMed] [Google Scholar]
- 152.Wang C, Yue H, Hu Z, Shen Y, Ma J, Li J, Wang XD, Wang L, Sun B, Shi P, Wang L, Gu Y. Microglia mediate forgetting via complement-dependent synaptic elimination. Science. 367:688–694, 2020 [DOI] [PubMed] [Google Scholar]
- 153.Wang S, Song R, Wang Z, Jing Z, Wang S, Ma J. S100A8/A9 in Inflammation. Frontiers in Immunology. 9, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Wen YR, Suter MR, Ji RR, Yeh GC, Wu YS, Wang KC, Kohno T, Sun WZ, Wang CC. Activation of p38 mitogen-activated protein kinase in spinal microglia contributes to incision-induced mechanical allodynia. Anesthesiology. 110:155–165, 2009 [DOI] [PubMed] [Google Scholar]
- 155.Wu Y, Dissing-Olesen L, MacVicar BA, Stevens B. Microglia: Dynamic Mediators of Synapse Development and Plasticity. Trends Immunol. 36:605–613, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Xiao S, Li D, Zhu HQ, Song MG, Pan XR, Jia PM, Peng LL, Dou AX, Chen GQ, Chen SJ, Chen Z, Tong JH. RIG-G as a key mediator of the antiproliferative activity of interferon-related pathways through enhancing p21 and p27 proteins. Proc Natl Acad Sci U S A. 103:16448–16453, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Yu H Regulation of APC-Cdc20 by the spindle checkpoint. Curr Opin Cell Biol. 14:706–714, 2002 [DOI] [PubMed] [Google Scholar]
- 158.Zeisel A, Munoz-Manchado AB, Codeluppi S, Lonnerberg P, La Manno G, Jureus A, Marques S, Munguba H, He L, Betsholtz C, Rolny C, Castelo-Branco G, Hjerling-Leffler J, Linnarsson S. Brain structure. Cell types in the mouse cortex and hippocampus revealed by single-cell RNA-seq. Science. 347:1138–1142, 2015 [DOI] [PubMed] [Google Scholar]
- 159.Zhang W, Yu Y, Hertwig F, Thierry-Mieg J, Zhang W, Thierry-Mieg D, Wang J, Furlanello C, Devanarayan V, Cheng J, Deng Y, Hero B, Hong H, Jia M, Li L, Lin SM, Nikolsky Y, Oberthuer A, Qing T, Su Z, Volland R, Wang C, Wang MD, Ai J, Albanese D, Asgharzadeh S, Avigad S, Bao W, Bessarabova M, Brilliant MH, Brors B, Chierici M, Chu TM, Zhang J, Grundy RG, He MM, Hebbring S, Kaufman HL, Lababidi S, Lancashire LJ, Li Y, Lu XX, Luo H, Ma X, Ning B, Noguera R, Peifer M, Phan JH, Roels F, Rosswog C, Shao S, Shen J, Theissen J, Tonini GP, Vandesompele J, Wu PY, Xiao W, Xu J, Xu W, Xuan J, Yang Y, Ye Z, Dong Z, Zhang KK, Yin Y, Zhao C, Zheng Y, Wolfinger RD, Shi T, Malkas LH, Berthold F, Wang J, Tong W, Shi L, Peng Z, Fischer M. Comparison of RNA-seq and microarray-based models for clinical endpoint prediction. Genome Biol. 16:133, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Zhang Y, Chen K, Sloan SA, Bennett ML, Scholze AR, O’Keeffe S, Phatnani HP, Guarnieri P, Caneda C, Ruderisch N, Deng S, Liddelow SA, Zhang C, Daneman R, Maniatis T, Barres BA, Wu JQ. An RNAsequencing transcriptome and splicing database of glia, neurons, and vascular cells of the cerebral cortex. J Neurosci. 34:11929–11947, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.