Abstract
Bones are the third most common location for solid tumor metastasis affecting up to 10% of patients with solid tumors. When the spine is involved, thoracic and lumbar vertebrae are frequently affected. Access to spinal lesions can be through minimally invasive surgery (MIS) or traditional open surgery (OS). This study aims to determine which method provides an advantage. Following the PRISMA (Preferred Inventory for Systematic Reviews and Meta-Analysis) guidelines, a systematic review was conducted to identify studies that compare MIS with OS in patients with spinal metastatic disease. Data were analyzed using Review Manager ver. 5.3 (RevMan; Cochrane, London, UK). Ten studies were included. Operative time was similar among groups at −35.23 minutes (95% confidence interval [CI], −73.36 to 2.91 minutes; p=0.07). Intraoperative bleeding was lower in MIS at −562.59 mL (95% CI, −776.97 to −348.20 mL; p<0.00001). OS procedures had higher odds of requiring blood transfusions at 0.26 (95% CI, 0.15 to 0.45; p<0.00001). Both approaches instrumented similar numbers of levels at −0.05 levels (95% CI, −0.75 to 0.66 levels; p=0.89). We observed a decreased need for postoperative bed rest at −1.60 days (95% CI, −2.46 to −0.74 days; p=0.0003), a shorter length of stay at −3.08 days (95% CI, −4.50 to −1.66 days; p=0.001), and decreased odds of complications at 0.60 (95% CI, 0.37 to 0.96; p=0.03) in the MIS group. Both approaches revealed similar reintervention rates at 0.65 (95% CI, 0.15 to 2.84; p=0.57), effective rates of reducing metastasis-related pain at −0.74 (95% CI, −2.41 to 0.94; p=0.39), and comparable scores of the Tokuhashi scale at −0.52 (95% CI, −2.08 to 1.05; p=0.41), Frankel scale at 1.00 (95% CI, 0.60 to 1.68; p=1.0), and American Spinal Injury Association Scale at 0.53 (95% CI, 0.21 to 1.37; p=0.19). MIS appears to provide advantages over OS. Larger and prospective studies should fully detail the role of MIS as a treatment for spine metastasis.
Keywords: Metastasis, Spine, Surgery, Cancer surgery, Minimally invasive surgical procedures
Introduction
Bones are the third most common location for solid tumor metastasis, affecting up to 10% of patients with solid tumors [1,2]. These tumors most commonly arise from the breast, prostate, and lungs, and their presence indicates advanced-stage disease [3–6]. Thoracic and lumbar vertebrae are most commonly affected when the spine is involved [7]. Although many patients remain asymptomatic, those who develop symptoms tend to have a poor quality of life (QoL) mainly due to neurological pain or dysfunction. The underlying reasons for neoplasias’ predilection for bone, and more specifically the spine, have yet to be elucidated.
Increased survival in cancer patients has contributed to an increase in both the incidence and prevalence of spinal metastatic disease. Therefore, its presence remains an important clinical challenge for physicians, chiefly due to the considerable impact on patient morbidity and QoL. This burden extends to resource consumption for healthcare systems, as it is associated with a remarkable increase in hospital resource expenditure and requires multiple outpatient visits [8,9].
The optimal treatment of spinal metastatic disease is individualized for each patient, involving a multidisciplinary collaboration among health care providers [10]. Over the years, several treatment options have appeared, such as surgery, pharmacotherapy, and radiation [10]. Surgery remains the best treatment option for pain and neurological symptoms caused by spinal instability [11]. Surgical interventions usually rely on resection and stabilization, both of which have immediate and lasting effects on pain and neurological function [12,13]. Access to spinal lesions through surgical procedures can be done through minimally invasive surgery (MIS) or traditional open surgery (OS). Recently, MIS has gained in popularity; evidence has shown that it constitutes a safe and effective technique as measured by variables such as blood loss, operative time, postoperative drainage before discharge, and mean hospital stay [14,15]. As with any surgery, MIS and OS carry an unavoidable risk for complications in patients already strained by their disease. Surgery should be an option only in cases of final-stage cancer, when life expectancy is >8–12 weeks and when postoperative benefits outweigh the inherent risks [16].
Therefore, in this systematic review and meta-analysis of current published data comparing MIS and OS, we aim to elucidate the ideal surgical management of spinal metastatic disease with the latest available evidence.
Materials and Methods
1. Literature search strategy
In November 2020, following the PRISMA (Preferred Inventory for Systematic Reviews and Meta-Analysis) guidelines, we performed a systematic search in the PubMed, Web of Science, Scopus, and Google Scholar databases, identifying studies comparing the surgical management of spinal metastatic disease through either MIS or OS [17]. The search terms we used in the article titles and abstracts were “spinal metastasis,” “spine metastasis,” “surgical,” “surgery,” “treatment,” “minimally invasive,” “MIS,” and “open surgery.” The MeSH terms we included were “humans,” “minimally invasive surgical procedures,” “spinal neoplasms/secondary,” “spinal neoplasms/surgery,” “treatment outcome,” “operative time,” “pain measurement,” “postoperative complications,” “quality of life,” and “treatment outcome.” Fig. 1 presents the workflow of the data recollection. We also screened related articles for possible inclusion to broaden the search.
Fig. 1.
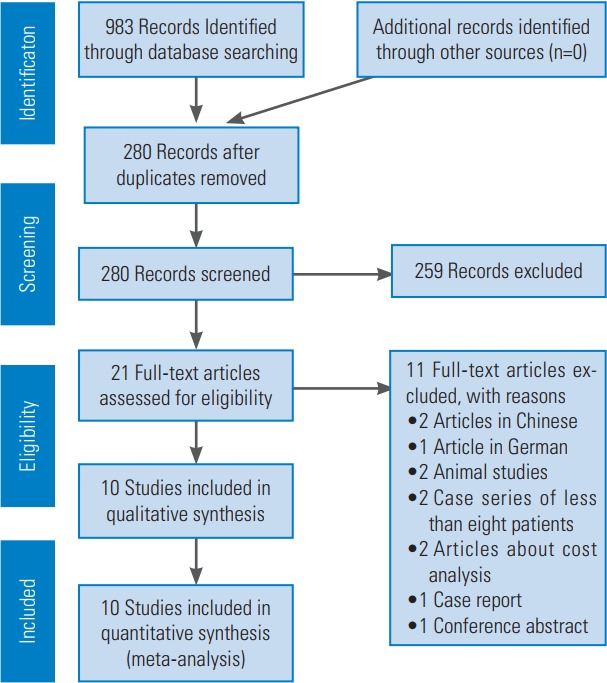
PRISMA (Preferred Inventory for Systematic Reviews and Meta-Analysis) flowchart of search strategy and included studies.
2. Study inclusion
Included studies were either retrospective or prospective and provided clear statistical comparisons of MIS versus OS, reporting at least one of the following outcomes: operative time or bleeding, cases requiring transfusions, number of instrumented levels, postoperative change in pain or length of bed rest, hospital length of stay (LOS), complications rates, necessity for surgical reintervention, and American Spinal Injury Association (ASIA), Frankel, and Tokuhashi scores.
The Tokuhashi scoring system, which was designed to yield an estimated patient survival time on the basis of preoperative evaluation, considers six items: general condition, number of extraspinal or spinal metastases, primary cancer site, state of paralysis (as graded by Frankel classification), and metastases to visceral organs [18]. The Frankel classification is a grading system that includes five groups (A–E) based on the severity of the neurological deficit, with A (a total absence of motor and sensory function below the level of lesion) and E (a normal motor and sensory function), possibly with abnormal reflexes present [19]. The ASIA score, also based on the degree of neurological impairment, similarly includes five groups (A–E), with A (no motor or sensory function is preserved in the sacral segments S4–S5) and E (total neurological normality) [20]. Restrictions on studies included conference abstracts, case reports, series of <8 patients, animal studies, cadaveric models, and studies not in English or Spanish, with no restriction on publication year.
3. Data screening and extraction
Two reviewers independently screened the articles for inclusion, with articles matching inclusion criteria retrieved for further data extraction. Primary extracted data included those variables previously mentioned in the inclusion criteria. Any discrepancies were solved by a third reviewer and two senior neurosurgeons with >10 years of experience in the treatment of complex neurosurgical and spinal pathology.
4. Quality assessment
Each reviewer independently graded the studies using the Newcastle-Ottawa Scale [21].
5. Statistical analysis
Statistical analysis was performed using Review Manager ver. 5.3 (RevMan; Cochrane, London, UK). Heterogeneity was measured using I2 (%), to which studies obtaining values of >50% were considered heterogeneous and analyzed through random-effects models. Studies with values of <50% were considered homogeneous and were analyzed through fixed-effects models. Continuous variables were analyzed using standardized mean differences with a 95% confidence interval (CI) and dichotomous variables were analyzed using odds ratios with a 95% CI as well. Those p-values <0.05 were considered significant. Hazard ratios were estimated from Kaplan-Meier curves using Tierney’s method [22].
If the included studies reported variables of interest in median and range or median and interquartile range, mean and standard deviation (SD) were estimated using a methodology of Wan et al. [23]. For studies that included means but not SDs and that had enough data (e.g., p-value, group sizes), we used Cochrane’s Handbook for Systematic Reviews of Interventions (ver. 6.1; Cochrane) to estimate SDs with the t-value [24]. We calculated the estimations on the impact of the intervention as defined by changes to means and SDs as follows:
Results
1. Overall
A total of 10 studies met the inclusion criteria totaling 577 patients, of which 271 underwent MIS and 305 underwent OS. Breast and lung primary malignancies were by far the most common origin of metastasis (123 patients with breast cancer, 114 with lung cancer), followed by genitourinary and prostate cancers (50 patients with genitourinary cancer, 43 with prostate cancer). Table 1 summarizes the analyzed variables. Common indications for the surgical treatment of spinal metastasis were generally palliation of compressive symptoms, acute neurological deficit, intractable pain, and spinal instability. Table 2 specifies the studies’ characteristics such as study design, spinal anatomical location of metastasis, population, indications, type of procedure, mean age, and type of primary cancer [15,25–33].
Table 1.
Summary of analysis results
| Variable | No. of studies | Population | MD or OD or HR (95% CI) | p-value | Heterogeneity | ||
|---|---|---|---|---|---|---|---|
| MIS | OS | I2 % | p-value | ||||
| Operative time | 10 | 271 | 305 | −35.23 (−73.36 to 2.91) | 0.07 | 96 | <0.00001 |
| Operative bleeding | 10 | 271 | 305 | −562.59 (−776.97 to −348.20) | <0.00001 | 94 | <0.0001 |
| Instrumented levels | 5 | 125 | 120 | −0.05 (−0.75 to 0.66) | 0.89 | 72 | 0.006 |
| Transfusions | 5 | 119 | 122 | 0.26a) (0.15 to 0.45) | <0.00001 | 50 | 0.09 |
| Postoperative bed rest | 2 | 48 | 44 | −1.60 (−2.46 to −0.74) | 0.0003 | 0 | 0.90 |
| Length of stay | 6 | 170 | 220 | −3.08 (−4.50 to −1.66) | 0.001 | 89 | <0.00001 |
| Complications | 9 | 244 | 287 | 0.60a) (0.37 to 0.96) | 0.03 | 6 | 0.38 |
| Reinterventions | 3 | 66 | 73 | 0.65a) (0.15 to 2.84) | 0.57 | 0 | 0.93 |
| Change in pain | 4 | 102 | 93 | −0.74 (−2.41 to 0.94) | 0.39 | 88 | <0.0001 |
| ECOG grading | 2 | 57 | 48 | −0.25 (−0.68 to 0.18) | 0.26 | 0 | 0.50 |
| Tokuhashi | 5 | 128 | 118 | −0.52 (−2.08 to 1.05) | 0.41 | 85 | <0.0001 |
| Frankel | 3 | 104 | 160 | 1.00 (0.60 to 1.68) | 1.0 | 0 | 0.40 |
| ASIA Scale | 2 | 37 | 40 | 0.53 (0.21 to 1.37) | 0.19 | 36 | 0.21 |
| Survival | 2 | 61 | 43 | 0.81b) (0.56 to 1.16) | 0.25 | 0 | 0.37 |
MIS, minimally invasive surgery; OS, open surgery; MD, mean difference; OD, odds ratio; HR, hazard ratio; CI, confidence interval; ECOG, Eastern Cooperative Oncology Group; ASIA, American Spinal Injury Association.
Indicates OR.
Indicates HR.
Table 2.
Summary of included studies
| Author | Design | Location | Cohort | Indications | Procedure | MIS | OS | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
||||||||||
| Cohort | Mean age (yr) | Primary lesion | Cohort | Mean age (yr) | Primary lesion | ||||||
| H_uang et al. [25] (2006) | Retrospective observational | Thoracic | 46 | Intractable back pain and/or neurological deficit | MIS: MASS; OS: traditional OS | 29 | 58 | Breast (6); lung (5); hepatoma (4); others (14) | 17 | 57 | Breast (3); lung (1); hepatoma (2); others (11) |
|
| |||||||||||
| Fang et al. [28] (2012) | Retrospective observational | Thoracic; lumbar | 41 | Acute progressive neurological deficits, intractable pain, impending fracture of the vertebral body | Posterior total en bloc spondylectomy; mini-open corpectomy | 24 | 56.6 | Gastric (1); lung (5); rectal (0); thyroid (4); prostate (3); lymphoma (0); liver (1); colon (0); breast (6); unidentified (4) | 17 | 51 | Gastric (2); lung (5); rectal (1); thyroid (1); prostate (0); lymphoma (1); liver (3); colon (1); breast (2); unidentified (1) |
|
| |||||||||||
| Lau and Chou [29] (2015) | Retrospective observational | Thoracic | 49 | Spinal cord compression | MIS: complete posterior-approach transpedicular corpectomy with expandable cage reconstruction of anterior spinal column; OS: traditional OS | 28 | 59.5 | Lung (3); breast (5); renal/bladder (2); others (14) | 21 | 55.8 | Lung (6); breast (2); renal/bladder (6); others (11) |
|
| |||||||||||
| Miscusi et al. [30] (2015) | MIS group: prospective cohort; OS group: retrospective cohort | Thoracic | 42 | Myelopathy (excluded patients with neurological deficit greater than 24 hr+modified Bauer Score >2) | MIS: minimally invasive laminotomy/laminectomy+percutaneous stabilization; OS: open laminectomy or laminotomy+stabilization | 23 | 58.4 | Lung (7); breast (6); myeloma (4); kidney (1); melanoma (3); others (2) | 19 | 57.8 | Lung (8); breast (6); kidney (2); prostate (2); ovary (1) |
|
| |||||||||||
| Hansen-Algenstaedt et al. [31] (2017) | Prospective observational | Thoracic; lumbar | 60 | Intractable pain and/or neurological deficit | MIS: central small incision with circumferential decompression and percutaneous pedicle screw system; OS: standard open | 30 | 61,8 | Breast (14); prostate (3); lung (5); thyroid (1); others (7) | 30 | 60.2 | Breast (4); prostate (8); lung (3); thyroid (4); others (11) |
|
| |||||||||||
| Kumar et al. [26] (2017) | Prospective observational | Thoracic; lumbar | 45 | Neurologic deficit; spinal instability | MIS: posterior instrumentation; OS: posterior instrumentation | 27 | 62 | Lung (7); breast (3); GI (2); renal (2); prostate (1); others (12) | 18 | 65 | Lung (5); breast (5); GI (1); renal (0); prostate (5); others (2) |
|
| |||||||||||
| Hikata et al. [32] (2017) | Retrospective observational | Thoracic; lumbar | 50 | Neurologic dysfunction; intractable pain | MIS: percutaneous screw and rod placement+neural tissue decompression fixation; OS: open pedicle screw placement and decompression+fixation | 25 | 63.6 | Lung (7); breast (3); prostate 4; kidney (2); lymphoma (2); liver (1); others (6) | 25 | 60.2 | Lung (2); prostate (0); breast (4); lymphoma (1); liver (3); others (15) |
|
| |||||||||||
| Saadeh et al. [33] (2019) | Matched retrospective | Cervical; thoracic; lumbar | 40 | Intractable pain | MIS: hybrid MIS transpedicular; OS: open transpedicular | 20 | 56.4 | Breast (4); lung (4); colon (2); renal (2); squamous (2); others (6) | 20 | 60.3 | Breast (4); lung (4); colon (2); renal (2); squamous (2); others (6) |
|
| |||||||||||
| Morgen et al. [27] (2020) | Non-blinded randomized controlled parallel-group trial | Thoracic; lumbar | 49 | Back pain and/or neurological impairment; MSCC between T5–L3 | MIS: MASS; OS: traditional OS | 23 | 65.9 | Lung (3); breast (9); prostate (4); unidentified (4); renal (2); pancreatic (1); melanoma (0); thyroid (0); lymphoma (3); others (0) | 26 | 67.6 | Lung (6); breast (7); prostate (1); unidentified (1); renal (3); pancreatic (0); melanoma (1); thyroid (1); lymphoma (0); others (3) |
|
| |||||||||||
| Zhu et al. [15] (2021) | Retrospective observational | Thoracic; lumbar | 154 | Progressive paralysis due to spinal cord compression or intolerable back pain as a result of the instability of pathologic fracture | Minimally invasive spine surgery; traditional OS | 49 | 53.84 | Lung (9); breast (12); kidney (7); liver (2); thyroid (3); myeloma (3); colorectal (1); unknown (3); prostate (2); nasopharynx (3); uterus (1); others (3) | 105 | 54.10 | Lung (19); breast (18); kidney (8); liver (12); thyroid (4); myeloma (4); colorectal (4); unknown (9); prostate (4); nasopharynx (5); uterus (2); others (16) |
MIS, minimally invasive surgery; OS, open surgery; MASS, minimal access spinal surgery; GI, gastrointestinal; MSCC, metastatic spinal cord compression.
2. Operative outcomes
Analyzed operative outcomes included intraoperative time and bleeding volume, as well as the necessity for transfusions measured by the number of packed red blood cells transfused and the number of levels that underwent instrumentation. Included studies ranged from 5 to 10.
1) Operative time
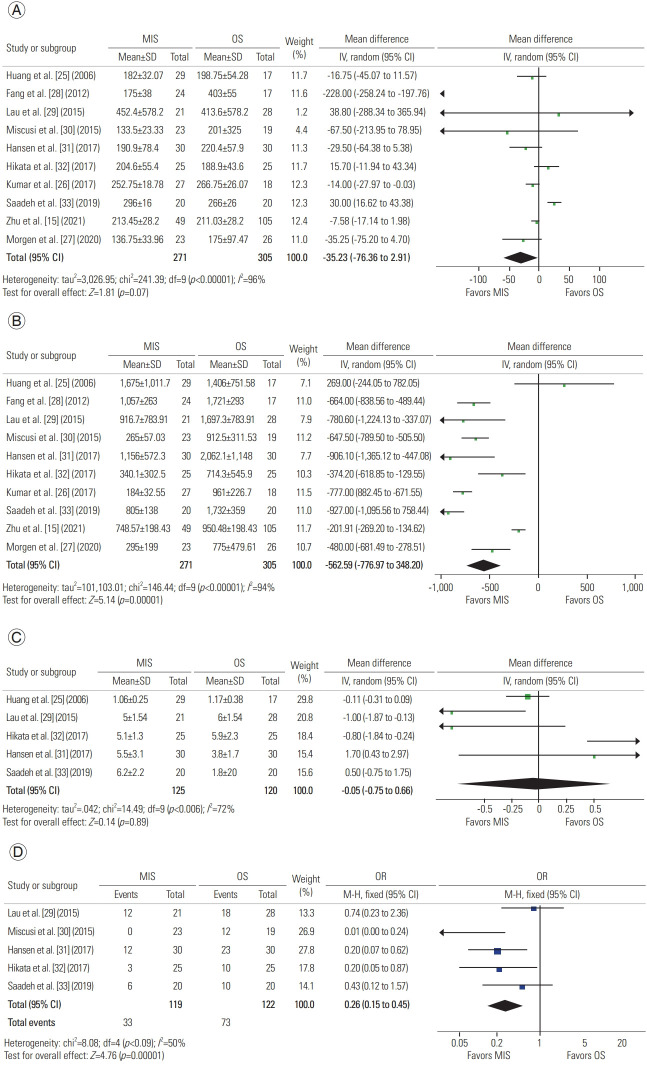
A total of 10 studies described operative time, with 271 patients in the MIS group and 305 in the OS group. A meta-analysis of this data revealed a mean difference of −35.23 minutes (95% CI, −73.36 to 2.91 minutes; p=0.07), suggesting that MIS is similar in operative time to OS (Fig. 2A) [15,25–33].
Fig. 2.
Forest plot of meta-analysis of the following variables: (A) operative time, (B) operative bleeding, (C) instrumented levels, and (D) transfusions. MIS, minimally invasive surgery; OS, open surgery; SD, standard deviation; IV, independent variable; CI, confidence interval; df, degrees of freedom; OR, odds ratio.
2) Operative bleeding
A total of 10 studies described operative bleeding, with 271 patients in the MIS group and 305 in the OS group. A meta-analysis of this data revealed a mean difference of −562.59 mL (95% CI, −776.97 to −348.20 mL; p<0.00001), concluding that MIS procedures result in less intraoperative bleeding than do OS procedures (Fig. 2B) [15,25–33].
3) Instrumented levels
A total of five studies described the number of instrumented levels, with 125 patients in the MIS group and 120 in the OS group. A meta-analysis of this data revealed a mean difference of −0.05 levels (95% CI, −0.75 to 0.66 levels; p=0.89), suggesting that both approaches are adequate for instrumenting various levels (Fig. 2C) [25,29,31–33].
4) Transfusions
A total of five studies described transfusions, with 119 patients in the MIS group and 122 in the OS group. A meta-analysis of this data revealed an odds ratio of −0.26 (95% CI, 0.15 to 0.45; p<0.00001). As suggested by increased intraoperative bleeding volumes, OS procedures have higher odds of requiring transfusions (Fig. 2C) [29–33].
3. Postoperative outcomes
Postoperative outcomes of interest included postoperative bed rest time, hospital LOS, and complications and reintervention rates, as well as postoperative changes in pain scores. Included studies ranged from 2 to 9.
1) Postoperative bed rest
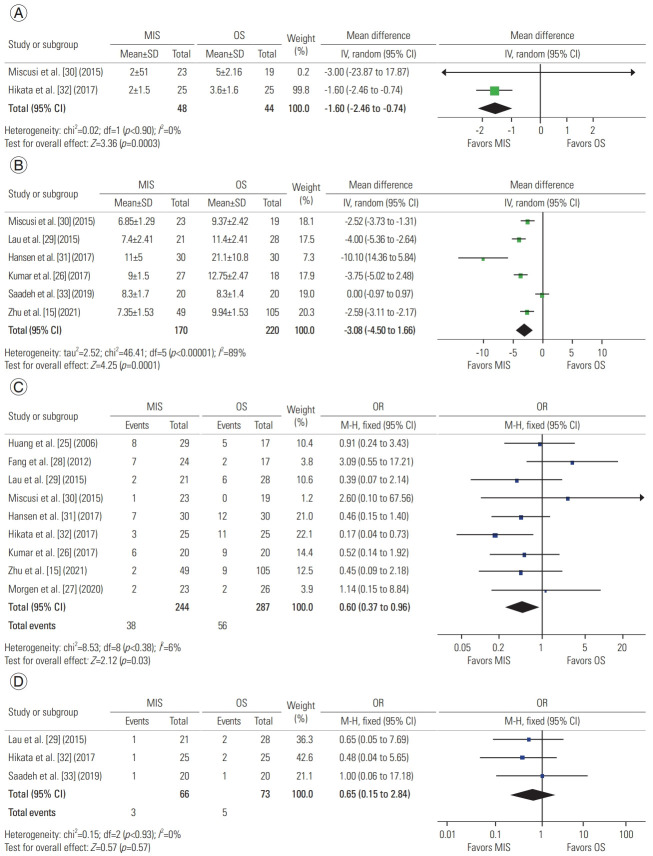
A total of two studies described postoperative bed rest, with 48 patients in the MIS group and 44 in the OS group. A meta-analysis of this data revealed a mean difference of −1.60 days (95% CI, −2.46 to −0.74 days; p=0.0003), concluding a decreased need for bed rest in MIS procedures (Fig. 3A) [30,32].
Fig. 3.
Forest plot of meta-analysis of the following variables: (A) postoperative bed rest, (B) length of stay, (C) complications, and (D) reinterventions. MIS, minimally invasive surgery; OS, open surgery; SD, standard deviation; IV, independent variable; CI, confidence interval; df, degrees of freedom; OR, odds ratio.
2) Length of stay
A total of six studies described postoperative LOS, with 170 patients in the MIS group and 220 in the OS group. A meta-analysis of this data revealed a mean difference of −3.08 days (95% CI, −4.50 to −1.66 days; p=0.001), suggesting that MIS procedures are associated with a shorter LOS (Fig. 3B) [15,26,29–31,33].
3) Complications
A total of nine studies described complications, with 244 patients in the MIS group and 287 in the OS group. A meta-analysis of this data revealed an odds ratio of 0.60 (95% CI, 0.37 to 0.96; p=0.03), suggesting decreased odds of complications in MIS as compared to OS (Fig. 3C) [15,25–32].
4) Reinterventions
A total of three studies described reintervention, with 66 patients in the MIS group and 73 in the OS group. A meta-analysis of this data revealed an odds ratio of 0.65 (95% CI, 0.15 to 2.84; p=0.57), suggesting that both approaches undergo similar reintervention rates (Fig. 3D) [29,32,33].
5) Change in pain
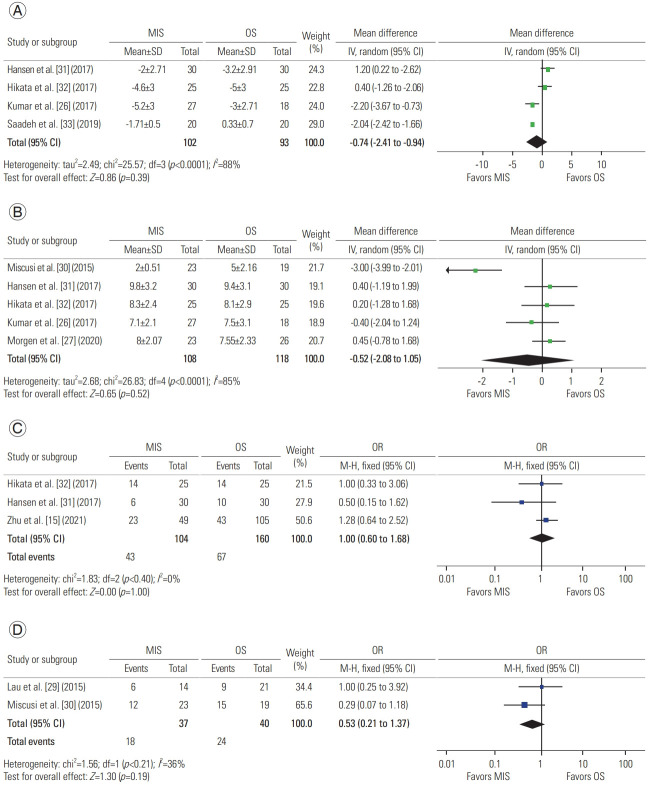
A total of four studies described the change in pain, with 102 patients in the MIS group and 93 in the OS group. A meta-analysis of this data revealed a mean difference of −0.74 in Visual Analog Scale score (95% CI, −2.41 to 0.94; p=0.39), suggesting that both approaches are effective for reducing pain (Fig. 4A) [26,31–33].
Fig. 4.
Forest plot of meta-analysis of the following variables: (A) change in pain, (B) Tokuhashi score, (C) Frankel grade, and (D) American Spinal Injury Association (ASIA) score. MIS, minimally invasive surgery; OS, open surgery; SD, standard deviation; IV, independent variable; CI, confidence interval; df, degrees of freedom; OR, odds ratio.
4. Clinical grading and scoring
Two to five studies described clinical grading scores, including ASIA, Frankel, and Tokuhashi scores.
1) Tokuhashi score
Five studies reported Tokuhashi scores, with 128 patients in the MIS group and 118 in the OS group. When analyzed, the scores revealed a mean difference of −0.52 (95% CI, −2.08 to 1.05; p=0.41), suggesting similar scores for both approaches (Fig. 4B) [26,27,30–32].
2) Frankel grade
Three studies compared Frankel’s grades, displaying before and after intervention values and defining changes in grouping, with 104 patients in the MIS group and 160 in the OS group. Analysis of this data reporting postoperative improvement reveals an odds ratio of 1.00 (95% CI, 0.60 to 1.68; p=1.0), suggesting similar Frankel grades for each procedure (Fig. 4C) [15,31,32].
3) ASIA score
Two studies compared ASIA scores, displaying before and after intervention values and defining changes in ASIA grading, with 37 patients in the MIS group and 40 in the OS group. Analysis of this data reporting postoperative improvement revealed an odds ratio of 0.53 (95% CI, 0.21 to 1.37; p=0.19), suggesting similar neurological outcomes for both approaches (Fig. 4D) [29,30].
4) ECOG performance status
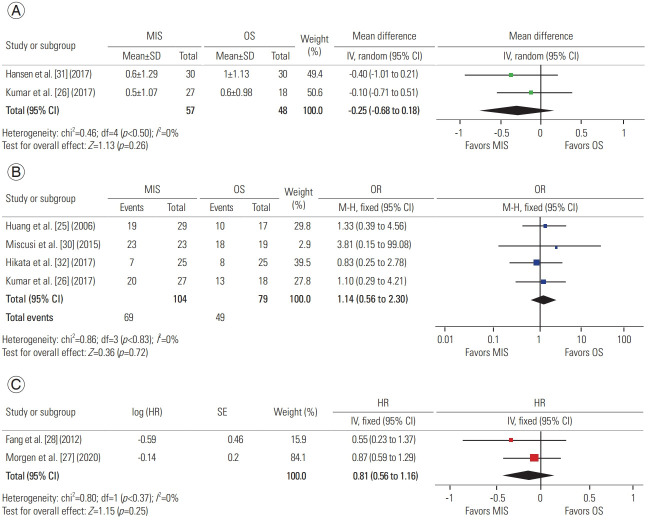
Two studies compared Eastern Cooperative Oncology Group (ECOG) Performance Status, displaying before and after intervention values and defining changes in ECOG grading, with 57 patients in the MIS group and 48 in the OS group. Analysis of this data reporting postoperative improvement revealed a mean difference of −0.25 (95% CI, −0.68 to 0.18; p=0.26), suggesting similar performance status outcomes for both approaches (Fig. 5A) [26,31].
Fig. 5.
Forest plot of meta-analysis of the following variables: (A) Eastern Cooperative Oncology Group (ECOG) Performance Status, (B) survival odds ratio (OR), and (C) survival hazard ratio (HR). MIS, minimally invasive surgery; OS, open surgery; SD, standard deviation; IV, independent variable; CI, confidence interval; df, degrees of freedom; SE, standard error.
5) Survival
Four studies provided the number of patients in each cohort surviving at least 3 months after surgical intervention, with 104 patients in the MIS group and 79 in the OS group. Analysis of this data revealed a survival odds ratio of 1.14 (95% CI, 0.56 to 2.30; p=0.72), suggesting similar survival odds for both approaches (Fig. 5B) [25,26,30,32]. Two studies provided survival curves, and data extraction and analysis revealed a survival hazard ratio of 0.81 (95% CI, 0.56 to 1.16; p=0.25), suggesting similar survival hazard ratios for both approaches (Fig. 5C) [27,28].
Discussion
As our ability to treat cancer increases, further dilemmas arise when facing patients’ complex burdens. Patients’ willingness to undergo aggressive treatment is multifactorial and complex [34–37]. Our therapeutic arsenal must reflect respect for patients’ decision autonomy, presenting the best available therapeutics to match their decisions and preferences. Although cancer is a widely variable spectrum of diseases with an even larger spectrum of presentations, advanced stages of the disease and its related complications present a heavier toll on patient QoL [38].
The presence of spinal metastasis is not only a terminal harbinger but also a heavy burden on patients’ remaining time [10,39], shifting the focus from curative treatment to prolongation and palliation [40]. Although individual overall survival is heavily influenced by both host and tumor biology, 2-year survival is poor in patients with spinal metastasis, with estimated values ranging from 9% for disease arising from lung cancer to 44% for that arising from breast or prostate cancer [39]. Overall, up to 20% of patients with spinal metastatic disease are alive after 2 years [10]. Huang et al. [25] reported similar survival rates for patients in either the MIS or OS group; however, more studies are needed to draw clearer conclusions about which approach has a greater impact on survival. This research represents a challenge because patients usually also undergo other forms of therapy such as radiation or chemotherapy, making controlling for confounding difficult. Our analysis of both surviving patients at the end of the reporting period and survival curve analysis found no differences in overall survival between procedures. Because neither procedure is curative, efforts to measure impact have been focused on QoL outcomes.
Spinal metastatic lesions can present in various manners, mainly as pain, fractures, neurological deficits arising from cord compression, and hypercalcemia [41]. Cord compression can be present in up to 14% of patients with spinal metastatic disease and usually results in intractable pain, negatively affecting mobility and continence [42]. Although not therapeutic per se, palliation of pain and related complications can be achieved through surgery, increasing patient QoL [43,44]. Surgery, radiation, or both are still utilized as effective treatment measures [45,46]. Overall, both MIS and OS procedures provide similar pain reduction, with Kumar et al. [26] reporting a shorter time to radiotherapy when patients underwent MIS procedures. Further studies should compare time with additional therapeutic measures and the success of these in each group.
Our analysis found no differences in Tokuhashi scores between either application, suggesting that both can be used regardless of patient prognosis. Additionally, both techniques presented with similar findings when comparing patients with improvements in Frankel and ASIA scores.
It is important to state that, among the variables analyzed within the included studies, authors described only the three previously mentioned scoring methods to evaluate patients. We encourage future studies to include other popular scoring methods such as the Spinal Instability Neoplastic Score and the Neurologic, Oncologic, Mechanical, and Systemic decision framework, to broaden the clinical picture and facilitate decision making. Regarding QoL, the ECOG Performance Status appeared in only two studies. Data analysis revealed no difference among both approaches with respect to preoperative and postoperative changes.
Several studies from various countries, including the United States, Italy, and the United Kingdom, have demonstrated that the reduced operative costs derived from lower LOS and complication rates offset the initial steep cost for implementating MIS [18–20]. These savings can be destined to fund more surgeries on similar patients or toward the overall hospital budget, allowing greater patient access to healthcare. Our study determined similar operative times between the procedures, allowing for both techniques to be viable in settings where a fast operating room turnover is needed. However, the MIS approaches had a reduced intraoperative bleeding volume, which positively affects both the patient and the healthcare setting, as this finding translates into fewer transfusions and postoperative LOS. Additional economical aspects should be analyzed when comparing costs derived from LOS, transfusions, reinterventions, rehabilitation, and pain management, as patients who undergo MIS procedures require significantly shorter postoperative LOS and ambulate earlier. Future studies should integrate cost analysis into these outcomes, as well as describe the outcomes of cases that required conversion from MIS to OS and this conversion’s possible implications for costs and patient morbidity.
Cancer patients are already in a vulnerable state due to their proinflammatory condition blunting the body’s healing capacity, which is compounded by the deleterious effects that radiotherapy and chemotherapy have on wound healing [47,48]. MIS poses a lighter impact on the body’s physiology and thus can provide a quicker recovery route for cancer patients [49,50]. These effects can be complicated further by radiotherapy applications, which also blunt reparatory capacity [51]. These overall vulnerabilities may seem to be reflected in similar rates of reinterventions between the techniques. However, overall complications are present less frequently in MIS interventions, and quicker ambulation suggests a quicker recovery as well. These findings also advocate for MIS application refinement, allowing MIS to match and surpass OS procedures.
Surgical procedures in patients with cancer also present a risk of furthering cancer cell dissemination because of tumor cell shedding during surgery as well as the upregulation of adhesion molecules and inflammatory changes that allow cancer cells to enhance their migration and invasion [52,53]. The potential severity of this issue in patients who already have a metastatic disease with a limited life expectancy is yet to be fully determined. Whether this phenomenon has clinical implications on spinal metastatic surgery techniques and approaches requires further study. Morgen et al. [27] provided a survival analysis and comparison of patients receiving MIS and OS procedures, finding no significant differences between them.
Future studies could improve on the drawn conclusions by including cost analysis, as well as long-term patient survival and time to additional treatment. Our study has limitations derived from the broad generalization of various interventions designated as MIS or OS, and future studies should directly compare equal interventions. Further study limitations arise from the small pool of available studies as well as the small cohort size. Difficulty determining prior applications of chemotherapy and radiotherapy in each study presents a challenge, as the ideal timing and management remain somewhat unclear. This field could benefit from larger randomized prospective studies with additional subgroup analysis of primary neoplasia as well as staging and relevant tumor characteristics. However, time availability in these patients poses a logistical challenge for research, as patient priorities and willingness to participate may change. Methodological limitations exist and are associated with the bulk of included studies being retrospective, as well as limited database inclusion and estimation of values where real values are absent and, importantly, by differences in the overall therapeutic schemes that patients may have received concurrently with surgical interventions such as radiation or chemotherapy. Future studies should also perform subgroup analyses of minor grouping features that could impact outcomes such as primary cancer, staging, and surgical intervention indication.
Conclusions
MIS approaches for the management of spinal metastatic disease are associated with various advantages over OS approaches. MIS procedures match traditional OS procedures in operative time and number of instrumented levels and outperform in associated bleeding and transfusions. In addition, patients can benefit by a shorter time to ambulation and postoperative LOS, with similar complication rates. These improved outcomes are achieved while performing similarly to OS procedures in pain reduction and changes in Frankel and ASIA grading. Regarding survival, both techniques proved to be similar. Information generated by this study can be useful in the decision-making process of multidisciplinary teams when approaching patients with such a complex medical oncology; however, the creation of management guidelines based on current available evidence and future higher quality studies are needed to establish the best treatment scenario.
Footnotes
Conflict of Interest
No potential conflict of interest relevant to this article was reported.
References
- 1.Greenlee RT, Murray T, Bolden S, Wingo PA. Cancer statistics, 2000. CA Cancer J Clin. 2000;50:7–33. doi: 10.3322/canjclin.50.1.7. [DOI] [PubMed] [Google Scholar]
- 2.Hernandez RK, Wade SW, Reich A, Pirolli M, Liede A, Lyman GH. Incidence of bone metastases in patients with solid tumors: analysis of oncology electronic medical records in the United States. BMC Cancer. 2018;18:44. doi: 10.1186/s12885-017-3922-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cetin K, Christiansen CF, Jacobsen JB, Norgaard M, Sorensen HT. Bone metastasis, skeletal-related events, and mortality in lung cancer patients: a Danish population-based cohort study. Lung Cancer. 2014;86:247–54. doi: 10.1016/j.lungcan.2014.08.022. [DOI] [PubMed] [Google Scholar]
- 4.Hagberg KW, Taylor A, Hernandez RK, Jick S. Incidence of bone metastases in breast cancer patients in the United Kingdom: results of a multi-database linkage study using the general practice research database. Cancer Epidemiol. 2013;37:240–6. doi: 10.1016/j.canep.2013.01.006. [DOI] [PubMed] [Google Scholar]
- 5.Major PP, Cook RJ, Lipton A, Smith MR, Terpos E, Coleman RE. Natural history of malignant bone disease in breast cancer and the use of cumulative mean functions to measure skeletal morbidity. BMC Cancer. 2009;9:272. doi: 10.1186/1471-2407-9-272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Norgaard M, Jensen AO, Jacobsen JB, Cetin K, Fryzek JP, Sorensen HT. Skeletal related events, bone metastasis and survival of prostate cancer: a population based cohort study in Denmark (1999 to 2007) J Urol. 2010;184:162–7. doi: 10.1016/j.juro.2010.03.034. [DOI] [PubMed] [Google Scholar]
- 7.Maccauro G, Spinelli MS, Mauro S, Perisano C, Graci C, Rosa MA. Physiopathology of spine metastasis. Int J Surg Oncol. 2011;2011 doi: 10.1155/2011/107969. 107969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pockett RD, Castellano D, McEwan P, Oglesby A, Barber BL, Chung K. The hospital burden of disease associated with bone metastases and skeletal-related events in patients with breast cancer, lung cancer, or prostate cancer in Spain. Eur J Cancer Care (Engl) 2010;19:755–60. doi: 10.1111/j.1365-2354.2009.01135.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Skov Dalgaard K, Gammelager H, Svaerke C, Kurics T, Cetin K, Christiansen CF. Hospital use among patients with lung cancer complicated by bone metastases and skeletal-related events: a population-based cohort study in Denmark. Clin Epidemiol. 2015;7:363–8. doi: 10.2147/CLEP.S78301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Delank KS, Wendtner C, Eich HT, Eysel P. The treatment of spinal metastases. Dtsch Arztebl Int. 2011;108:71–9. doi: 10.3238/arztebl.2011.0071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ciftdemir M, Kaya M, Selcuk E, Yalniz E. Tumors of the spine. World J Orthop. 2016;7:109–16. doi: 10.5312/wjo.v7.i2.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Falicov A, Fisher CG, Sparkes J, Boyd MC, Wing PC, Dvorak MF. Impact of surgical intervention on quality of life in patients with spinal metastases. Spine (Phila Pa 1976) 2006;31:2849–56. doi: 10.1097/01.brs.0000245838.37817.40. [DOI] [PubMed] [Google Scholar]
- 13.Quan GM, Vital JM, Aurouer N, et al. Surgery improves pain, function and quality of life in patients with spinal metastases: a prospective study on 118 patients. Eur Spine J. 2011;20:1970–8. doi: 10.1007/s00586-011-1867-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lu VM, Alvi MA, Goyal A, Kerezoudis P, Bydon M. The potential of minimally invasive surgery to treat metastatic spinal disease versus open surgery: a systematic review and meta-analysis. World Neurosurg. 2018;112:e859–68. doi: 10.1016/j.wneu.2018.01.176. [DOI] [PubMed] [Google Scholar]
- 15.Zhu X, Lu J, Xu H, et al. A comparative study between minimally invasive spine surgery and traditional open surgery for patients with spinal metastasis. Spine (Phila Pa 1976) 2021;46:62–8. doi: 10.1097/BRS.0000000000003690. [DOI] [PubMed] [Google Scholar]
- 16.Igoumenou VG, Mavrogenis AF, Angelini A, et al. Complications of spine surgery for metastasis. Eur J Orthop Surg Traumatol. 2020;30:37–56. doi: 10.1007/s00590-019-02541-0. [DOI] [PubMed] [Google Scholar]
- 17.Moher D, Liberati A, Tetzlaff J, Altman DG PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg. 2010;8:336–41. doi: 10.1016/j.ijsu.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 18.Bae HW, Davis RJ, Hisey MS, Nunley PD, Jackson RJ. Comparing one-level versus two-level cervical TDR and one-level versus two-level ACDF at seven-year follow-up. Spine J. 2016;16:S203–4. [Google Scholar]
- 19.Lucio JC, Vanconia RB, Deluzio KJ, Lehmen JA, Rodgers JA, Rodgers W. Economics of less invasive spinal surgery: an analysis of hospital cost differences between open and minimally invasive instrumented spinal fusion procedures during the perioperative period. Risk Manag Healthc Policy. 2012;5:65–74. doi: 10.2147/RMHP.S30974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vertuani S, Nilsson J, Borgman B, et al. A cost-effectiveness analysis of minimally invasive versus open surgery techniques for lumbar spinal fusion in Italy and the United Kingdom. Value Health. 2015;18:810–6. doi: 10.1016/j.jval.2015.05.002. [DOI] [PubMed] [Google Scholar]
- 21.Wells GA, Shea B, O’Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses [Internet] Ottawa (ON): Ottawa Hospital Research Institute; 2000. [cited 2020 Dec 10]. Available from: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp . [Google Scholar]
- 22.Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials. 2007;8:16. doi: 10.1186/1745-6215-8-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. 2014;14:135. doi: 10.1186/1471-2288-14-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Higgins JP, Li T, Deeks JJ. Chapter 6: choosing effect measures and computing estimates of effect [Internet] London: Cochrane Training; 2020. [cited 2020 Dec 10]. Available from: https://training.cochrane.org/handbook/current/chapter-06 . [Google Scholar]
- 25.Huang TJ, Hsu RW, Li YY, Cheng CC. Minimal access spinal surgery (MASS) in treating thoracic spine metastasis. Spine (Phila Pa 1976) 2006;31:1860–3. doi: 10.1097/01.brs.0000225995.56028.46. [DOI] [PubMed] [Google Scholar]
- 26.Kumar N, Malhotra R, Maharajan K, et al. Metastatic spine tumor surgery: a comparative study of minimally invasive approach using percutaneous pedicle screws fixation versus open approach. Clin Spine Surg. 2017;30:E1015–21. doi: 10.1097/BSD.0000000000000400. [DOI] [PubMed] [Google Scholar]
- 27.Morgen SS, Hansen LV, Karbo T, Svardal-Stelmer R, Gehrchen M, Dahl B. Minimal access vs. open spine surgery in patients with metastatic spinal cord compression: a one-center randomized controlled trial. Anticancer Res. 2020;40:5673–8. doi: 10.21873/anticanres.114581. [DOI] [PubMed] [Google Scholar]
- 28.Fang T, Dong J, Zhou X, McGuire RA, Jr, Li X. Comparison of mini-open anterior corpectomy and posterior total en bloc spondylectomy for solitary metastases of the thoracolumbar spine. J Neurosurg Spine. 2012;17:271–9. doi: 10.3171/2012.7.SPINE111086. [DOI] [PubMed] [Google Scholar]
- 29.Lau D, Chou D. Posterior thoracic corpectomy with cage reconstruction for metastatic spinal tumors: comparing the mini-open approach to the open approach. J Neurosurg Spine. 2015;23:217–27. doi: 10.3171/2014.12.SPINE14543. [DOI] [PubMed] [Google Scholar]
- 30.Miscusi M, Polli FM, Forcato S, et al. Comparison of minimally invasive surgery with standard open surgery for vertebral thoracic metastases causing acute myelopathy in patients with short- or mid-term life expectancy: surgical technique and early clinical results. J Neurosurg Spine. 2015;22:518–25. doi: 10.3171/2014.10.SPINE131201. [DOI] [PubMed] [Google Scholar]
- 31.Hansen-Algenstaedt N, Kwan MK, Algenstaedt P, et al. Comparison between minimally invasive surgery and conventional open surgery for patients with spinal metastasis: a prospective propensity score-matched study. Spine (Phila Pa 1976) 2017;42:789–97. doi: 10.1097/BRS.0000000000001893. [DOI] [PubMed] [Google Scholar]
- 32.Hikata T, Isogai N, Shiono Y, et al. A retrospective cohort study comparing the safety and efficacy of minimally invasive versus open surgical techniques in the treatment of spinal metastases. Clin Spine Surg. 2017;30:E1082–7. doi: 10.1097/BSD.0000000000000460. [DOI] [PubMed] [Google Scholar]
- 33.Saadeh YS, Elswick CM, Fateh JA, et al. Analysis of outcomes between traditional open versus mini-open approach in surgical treatment of spinal metastasis. World Neurosurg. 2019;130:e467–74. doi: 10.1016/j.wneu.2019.06.121. [DOI] [PubMed] [Google Scholar]
- 34.Jenkins V, Catt S, Banerjee S, et al. Patients’ and oncologists’ views on the treatment and care of advanced ovarian cancer in the U.K.: results from the ADVOCATE study. Br J Cancer. 2013;108:2264–71. doi: 10.1038/bjc.2013.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Meropol NJ, Egleston BL, Buzaglo JS, et al. Cancer patient preferences for quality and length of life. Cancer. 2008;113:3459–66. doi: 10.1002/cncr.23968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shrestha A, Martin C, Burton M, Walters S, Collins K, Wyld L. Quality of life versus length of life considerations in cancer patients: a systematic literature review. Psychooncology. 2019;28:1367–80. doi: 10.1002/pon.5054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Silvestri G, Pritchard R, Welch HG. Preferences for chemotherapy in patients with advanced non-small cell lung cancer: descriptive study based on scripted interviews. BMJ. 1998;317:771–5. doi: 10.1136/bmj.317.7161.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nayak MG, George A, Vidyasagar MS, et al. Quality of life among cancer patients. Indian J Palliat Care. 2017;23:445–50. doi: 10.4103/IJPC.IJPC_82_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ulmar B, Huch K, Kocak T, et al. The prognostic influence of primary tumour and region of the affected spinal segment in 217 surgical patients with spinal metastases of different entities. Z Orthop Ihre Grenzgeb. 2007;145:31–8. doi: 10.1055/s-2007-960506. [DOI] [PubMed] [Google Scholar]
- 40.McArthur HL, Hudis CA. Has first-line therapy had an impact on general outcome in metastatic breast cancer? Future Oncol. 2007;3:411–8. doi: 10.2217/14796694.3.4.411. [DOI] [PubMed] [Google Scholar]
- 41.Nater A, Martin AR, Sahgal A, Choi D, Fehlings MG. Symptomatic spinal metastasis: a systematic literature review of the preoperative prognostic factors for survival, neurological, functional and quality of life in surgically treated patients and methodological recommendations for prognostic studies. PLoS One. 2017;12:e0171507. doi: 10.1371/journal.pone.0171507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Patchell RA, Tibbs PA, Regine WF, et al. Direct decompressive surgical resection in the treatment of spinal cord compression caused by metastatic cancer: a randomised trial. Lancet. 2005;366:643–8. doi: 10.1016/S0140-6736(05)66954-1. [DOI] [PubMed] [Google Scholar]
- 43.Ibrahim A, Crockard A, Antonietti P, et al. Does spinal surgery improve the quality of life for those with extradural (spinal) osseous metastases?: an international multicenter prospective observational study of 223 patients. Invited submission from the Joint Section Meeting on Disorders of the Spine and Peripheral Nerves, March 2007. J Neurosurg Spine. 2008;8:271–8. doi: 10.3171/SPI/2008/8/3/271. [DOI] [PubMed] [Google Scholar]
- 44.Tang Y, Qu J, Wu J, et al. Effect of surgery on quality of life of patients with spinal metastasis from non-small-cell lung cancer. J Bone Joint Surg Am. 2016;98:396–402. doi: 10.2106/JBJS.O.00629. [DOI] [PubMed] [Google Scholar]
- 45.Klimo P, Jr, Thompson CJ, Kestle JR, Schmidt MH. A meta-analysis of surgery versus conventional radiotherapy for the treatment of metastatic spinal epidural disease. Neuro Oncol. 2005;7:64–76. doi: 10.1215/S1152851704000262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lee RS, Batke J, Weir L, Dea N, Fisher CG. Timing of surgery and radiotherapy in the management of metastatic spine disease: expert opinion. J Spine Surg. 2018;4:368–73. doi: 10.21037/jss.2018.05.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cauley CE, Panizales MT, Reznor G, et al. Outcomes after emergency abdominal surgery in patients with advanced cancer: opportunities to reduce complications and improve palliative care. J Trauma Acute Care Surg. 2015;79:399–406. doi: 10.1097/TA.0000000000000764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Payne WG, Naidu DK, Wheeler CK, et al. Wound healing in patients with cancer. Eplasty. 2008;8:e9. [PMC free article] [PubMed] [Google Scholar]
- 49.Liu CA, Huang KH, Chen MH, et al. Comparison of the surgical outcomes of minimally invasive and open surgery for octogenarian and older compared to younger gastric cancer patients: a retrospective cohort study. BMC Surg. 2017;17:68. doi: 10.1186/s12893-017-0265-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Parameswaran R, Titcomb DR, Blencowe NS, et al. Assessment and comparison of recovery after open and minimally invasive esophagectomy for cancer: an exploratory study in two centers. Ann Surg Oncol. 2013;20:1970–7. doi: 10.1245/s10434-012-2848-7. [DOI] [PubMed] [Google Scholar]
- 51.Haubner F, Ohmann E, Pohl F, Strutz J, Gassner HG. Wound healing after radiation therapy: review of the literature. Radiat Oncol. 2012;7:162. doi: 10.1186/1748-717X-7-162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Krall JA, Reinhardt F, Mercury OA, et al. The systemic response to surgery triggers the outgrowth of distant immune-controlled tumors in mouse models of dormancy. Sci Transl Med. 2018;10 doi: 10.1126/scitranslmed.aan3464. eaan3464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tohme S, Simmons RL, Tsung A. Surgery for cancer: a trigger for metastases. Cancer Res. 2017;77:1548–52. doi: 10.1158/0008-5472.CAN-16-1536. [DOI] [PMC free article] [PubMed] [Google Scholar]