Abstract
To review the recent progress of machine learning for the early diagnosis of depression (major depressive disorder). The source of data was 32 original studies in the Web of Science. The search terms were “depression” (title) and “random forest” (abstract). The eligibility criteria were the dependent variable of depression, the interventions of machine learning (the decision tree, the naïve Bayesian, the random forest, the support vector machine and/or the artificial neural network), the outcomes of accuracy and/or the area under the receiver operating characteristic curve (AUC) for the early diagnosis of depression, the publication year of 2000 or later, the publication language of English and the publication journal of SCIE/SSCI. Different machine learning methods would be appropriate for different types of data for the early diagnosis of depression, e.g., logistic regression, the random forest, the support vector machine and/or the artificial neural network in the case of numeric data, the random forest in the case of genomic data. Their performance measures reported varied within 60.1–100.0 for accuracy and 64.0–96.0 for the AUC. Machine learning provides an effective, non-invasive decision support system for early diagnosis of depression.
Keywords: Depression, Early diagnosis, Machine learning
INTRODUCTION
Major depressive disorder (depression hereafter) is a leading cause of disease burden in the world, affecting 300 million people on the globe [1-6]. Depression, defined as “a mood disorder that causes a persistent feeling of sadness and loss of interest,” [1] is a diagnostic category of mental disorder together with anxiety disorder [2]. Its global incidence registered a rapid growth of 47.86% from 172 million to 258 million during 1990 to 2017 [3]. It ranked third in the world for 2017 and second in Korea for 2010 in terms of years lost to disability and disability-adjusted life years, respectively [4,5]. It is considered to have a wide range of determinants including demographic factors (age, sex), socioeconomic status (education, employment, income), neighborhood conditions (crowding, housing, pollution, violence) and health-related factors (drinking, exercise, smoking, diseases, genetics) [6,7]. Recently, on the other hand, the terms “deep learning,” “machine learning” and “artificial intelligence” have attracted great attention all over the globe. For instance, their Google trends recorded ten-fold expansions from 10 to 100 during 2013–2018. Artificial intelligence can be defined as “the capability of a machine to imitate intelligent human behavior” (the Merriam-Webster dictionary). The definition of machine learning can be a division of artificial intelligence to “extract knowledge from large amounts of data.” [8]
Six common machine learning algorithms are the decision tree, the naïve Bayesian predictor, the random forest, the support vector machine, the artificial neural network, and the deep neural network (deep learning). A decision tree has three components: an intermediate node (a test on an independent variable), a branch (an outcome of the test) and a terminal node (a value of the dependent variable). A naïve Bayesian predictor makes an early diagnosis based on Bayes’ theorem, which states that the probability of the dependent variable given certain values of independent variables comes from the probabilities of the independent variables given a certain value of the dependent variable. A random forest is a collection of many decision trees, which make majority votes on the dependent variable (“bootstrap aggregation”). Let us take a random forest with 1,000 decision trees as an example. Let us assume that original data includes 10,000 participants. Then, the training and test of this random forest takes two steps. Firstly, new data with 10,000 participants is created based on random sampling with replacement, and a decision tree is created based on this new data. Here, some participants in the original data would be excluded from the new data and these leftovers are called out-of-bag data. This process is repeated 1,000 times, i.e., 1,000 new data are created, 1,000 decision trees are created and 1,000 out-of-bag data are created. Secondly, the 1,000 decision trees make predictions on the dependent variable of every participant in the out-of-bag data, their majority vote is taken as their final prediction on this participant, and the out-of-bag error is calculated as the proportion of wrong votes on all participants in the out-of-bag data.
A support vector machine originates a line or space called a “hyperplane” (a collection of “support vectors”). The hyperplane divides data with the greatest distance between different sub-groups [8]. An artificial neural network is a network of “neurons”, i.e., information units combined through weights. Usually, the artificial neural network has one input layer, one, two or three intermediate layers and one output layer. Neurons in a previous layer connect with “weights” in the next layer and these weights represent the strengths of connections between neurons in a previous layer and their next-layer counterparts. This process starts from the input layer, continues through intermediate layers and ends in the output layer (feedforward operation). Then, learning happens: these weights are accommodated based on how much they contributed to the loss, a difference between the actual and predicted final outputs. This process starts from the output layer, continues through intermediate layers and ends in the input layer (backpropagation operation). The two operations are replicated until a certain expectation is met regarding the accurate diagnosis of the dependent variable. Finally, a deep neural network is an artificial neural network with a large number of intermediate layers, e.g., 5, 10 or even 1,000. The deep neural network is called “deep learning” given that learning “deepens” through numerous intermediate layers [9].
Traditional research considers a limited scope of predictors for the early diagnosis of disease, whereas adopting logistic regression with an unrealistic assumption of ceteris paribus, i.e., “all the other variables staying constant.” In this context, emerging literature uses artificial intelligence for the early diagnosis of disease, e.g., arrhythmia [9], birth outcome [10-15], cancer [16-19], comorbidity [20-22], menopause [23] and temporomandibular disease [24,25]. It does not require unrealistic assumptions of “all the other variables staying constant” while managing to analyze which predictors are more important for the early diagnosis of the dependent variable. The purpose of this study is to review the recent progress of machine learning for the early diagnosis of depression.
METHODS
Figure 1 shows the flow diagram of this study. Thirty two original studies were selected for review out of 120 original studies in the Web of Science with the search terms “depression” (title) and “random forest” (abstract). The eligibility criteria of this review were: 1) the intervention(s) of the decision tree, the naïve Bayesian predictor, the random forest, the support vector machine and/or the artificial neural network; 2) the outcome(s) of accuracy and/or the area under the receiver operating characteristic curve (AUC) for the early diagnosis of depression; 3) the publication year of 2000 or later; 4) the publication language of English; 5) the publication journal of Science Citation Index Expanded and/or Social Science Citation Index; and 6) depression being the dependent variable. The following summary measures were adopted: machine learning methods, sample size, data type, performance measures, and important predictors. Here, accuracy can be defined as the proportion of correct predictions over all observations, while the AUC can be defined as the area under the plot of the true positive rate (sensitivity) against the false positive rate (1- specificity) at various threshold settings.
Figure 1.
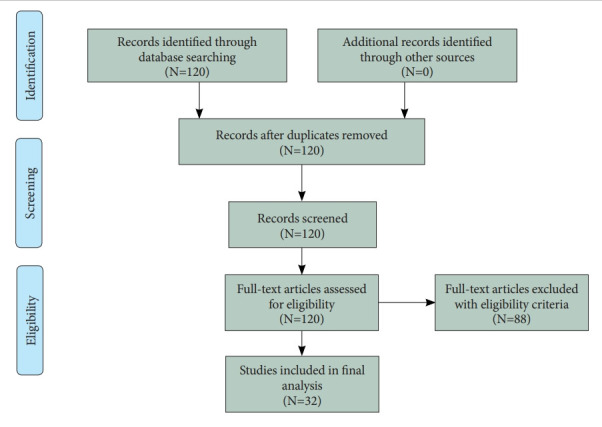
Flow diagram.
RESULTS
Review summary
The summary of review is shown in Tables 1 and 2 [26-57]. The tables have five summary measures, i.e., machine learning methods, sample size, data type, performance measures, important predictors, and whether the variable importance of the random forest is reported (VI-Yes 1). Based on the results of this review, different machine learning methods would be appropriate (i.e., would show the best performance measures) for different types of data for the early diagnosis of depression: 1) logistic regression, the random forest, the support vector machine and/or the artificial neural network in the case of numeric data; 2) the random forest in the case of genomic data; 3) the random forest and/or the support vector machine in the case of radiomic data; and 4) the random forest in the case of social-network-service data. Their performance measures reported varied within 60.1–100.0 for accuracy, 68.8–95.0 for sensitivity, 76.0–94.0 for specificity, and 64.0–96.0 for the AUC (Table 1). According to the findings of this review, indeed, the following predictors would be important variables for the early diagnosis of depression: comorbid psychopathology, symptom-related disability, treatment credibility, access to therapists, time spent using certain internet-intervention modules; pain-fatigue (symptom intensity scale), comorbidity; 30 microbial markers (gut microbiota); psychological elasticity, income level; upper body movements-postures; brain connectivity within posterior cingulate cortex, within insula, between posterior cingulate cortex and insula/hippocampus-amygdala, between insula and precuneus, between superior parietal lobule and medial prefrontal cortex; single-nucleotide polymorphisms (rs12248560, rs878567, rs17710780); cingulate isthmus asymmetry, pallidal asymmetry, ratio of the paracentral to precentral cortical thickness, ratio of lateral occipital to pericalcarine cortical thickness; self-assessed cardiac-related fear, sex, number of words to answer the first homework assignment for internet-delivered psychotherapy (Table 2). However, machine learning is a datadriven method and more study is to be done with more external data for greater external validity.
Table 1.
Summary of review: methods, sample size, data type and performance measures
| ID | Methods | Sample size | Data type | Performance measures |
|---|---|---|---|---|
| 26 | Semisupervised RF | 115 | Numeric | RMSE 4.50 |
| 27 | RF | 1,549 | Numeric | Accuracy Validation 94.2 Test 93.3 |
| 28 | LR DT NB RF SVM ANN | 28,755 | Numeric | AUC RF 88.4 SVM 86.4 |
| 29 | AR EN-RF | 283 | Numeric | R2 EN-RF 0.25 AR 0.17 |
| 30 | LR RF | 22,131 | Numeric | Only Coefficient P-Values Reported |
| 31 | RF | 97 | Genomic | AUC 81.0 |
| 32 | Expert vs. RF (Feature Selection); SVM vs. RF (Classification) | 508 | Numeric | RF-RF AUC 78.0 Sensitivity 69.0 |
| 33 | RF | 126 | Genomic | Accuracy 87.3 |
| 34 | RF | 3,669 | Numeric | Accuracy 91.6 |
| 35 | Lasso RF SVM | 120 | Radiomic | Accuracy 95.0 90.0 100.0 |
| 36 | 10 Models | 620 | Numeric | RF Accuracy 89.0/91.0 Internal/External |
| 37 | 1 RF vs. 2 RFs | 135 | SNS | Early Risk Detection Error: 2 RFs 10.0% Lower vs. 1 RF |
| 38 | RF | 201 | Radiomic | Accuracy 82.4 |
| 39 | RF | 412 | Numeric | Accuracy 76.8/81.1 Imbalanced/Balanced Data |
| 40 | LR DT NB RF SVM ANN | 43 | EEG | Accuracy 90.24–97.56 |
| 41 | LR RF | 439 | Numeric | Only Coefficient P-Values Reported |
| 42 | LR RF ANN | 637 | Numeric | AUC 87.0–91.0 |
| 43 | LR RF | 150 | Genomic | Only Coefficient P-Values Reported |
| 44 | LR RF | 4,270 | Numeric | AUC 76.0-79.0 |
| 45 | RF | 111 | Numeric | Sensitivity 69.7, Specificity 76.8 |
| 46 | RF | 5,895 | Numeric | Sensitivity 86.7, Specificity 91.9 |
| 47 | EN (Feature Selection); RF (Classification) | 41 | Radiomic | Accuracy 85.4 |
| 48 | RF | 42 | Radiomic | Accuracy 71.0–78.0 |
| 49 | RF | 656 | Numeric | Sensitivity 95.0, Specificity 87.0 |
| 50 | LR RF SVM | 238 | Numeric | LR AUC 93.8 |
| 51 | Lasso RF SVM | 62 | Radiomic | Lasso SVM Accuracy 90.0 |
| 52 | RF SVM | 126 | Numeric | Accuracy RF 60.1 SVM 59.1 |
| 53 | LR RF SVM GB | 170 | Numeric | GB Accuracy 76.9 |
| 54 | LR RF GB | 573,634 | Numeric | Accuracy LR 71.7 RF 72.0 GB 72.2; AUC LR 71.7 RF 72.0 GB 72.2 |
| 55 | LR DT RF GB | 47 | Numeric | LR Accuracy 91.0 AUC 96.0 Sensitivity 92.9 Specificity 94.0 |
| 56 | RF | 84,317 | Numeric | AUC 78.9 Sensitivity 68.8–83.9 Specificity 76.0–92.2 |
| 57 | RF | 90 | Numeric | AUC 64.0 |
Different machine learning methods would be appropriate (i.e., would show the best performance measures) for different types of data for the early diagnosis of depression: 1) logistic regression, the random forest, the support vector machine and/or the artificial neural network in the case of numeric data; 2) the random forest in the case of genomic data; 3) the random forest and/or the support vector machine in the case of radiomic data; and 4) the random forest in the case of social-network-service data. Their performance measures reported varied within 60.1–100.0 for accuracy, 68.8–95.0 for sensitivity, 76.0–94.0 for specificity, and 64.0–96.0 for the AUC. ANN, artificial neural network; AR, augoregressive; AUC, area under the receiver operating characteristic curve; DT, decision tree; EEG, electroencephalogram; EN, elastic net; GB, gradient boosting; LR, logistic regression; NB, naïve bayes; RF, random forest; RMSE, root mean squared error; SNS, social network service; SNP, single nucleotide polymorphism; SVM, support vector machine
Table 2.
Summary of review: important predictors and whether variable importance (VI) is reported
| ID | Important predictors | VI-yes | Participants/class/predictors |
|---|---|---|---|
| 26 | Cognitive-behavioral features | Participants: 35 labeled 80 unlabeled | |
| 27 | Patient health questionnaire-9 items | Participants: university students | |
| 28 | Demographic, health-behavioral factors | Participants: pregnancy risk assessment monitoring system enrollee | |
| 29 | Comorbid psychopathology, symptom-related disability, treatment credibility, access to therapists, time spent using certain internetintervention (deprexis) modules | 1 | |
| 30 | Pain-fatigue (symptom intensity scale), comorbidity | 1 | Participants: rheumatoid arthritis patients |
| 31 | 30 Microbial markers (gut microbiota) | 1 | Predictors: 16s-ribosomal rna gene sequences |
| 32 | Psychological elasticity, depression during the third trimester, income level | 1 | Participants: women with delivery |
| 33 | Blood-derived methylome and transcriptome features | ||
| 34 | Upper body movements-postures | 1 | Participants: university students |
| 35 | 19 Features of brain connectivity | Participants: parkinson’s disease patients | |
| 36 | Demographic, health-behavioral factors | Participants: 510/110 elders for internal/external validation | |
| 37 | SNS-derived behavioral patterns | ||
| 38 | Brain connectivity within posterior cingulate cortex, within insula, between posterior cingulate cortex and insula/hippocampus-amygdala, between insula and precuneus, between superior parietal lobule and medial prefrontal cortex | 1 | Participants: 156 advanced parkinson’s disease patients and 45 normal controls (predictors: 42 brain connectivity networks) |
| 39 | Fewer contacts, fewer calls, more messages | ||
| 40 | Higuchi’s fractal dimension, sample entropy | ||
| 41 | Fluoxetine more important than cognitive-behavioural therapy, two combined more important than one | ||
| 42 | Patient-reported immune-mediated inflammatory disease measures | ||
| 43 | SNPs rs12248560, rs878567, rs17710780 | 1 | Participants: 150 depression patients on 6-month regular therapy from the psycolaus cohort (predictors: 44 snps in existing literature) |
| 44 | Psychosometric properties in general health questionnaire | ||
| 45 | Six cognitive-behavioral tasks | Class: anxiety, depression or mixed vs. Healthy | |
| 46 | Motor activity recorded in a wearable device | ||
| 47 | Prefrontal cortical activation during working memory task anticipation | Class: unipolar vs. Bipolar depression | |
| 48 | Cingulate isthmus asymmetry, pallidal asymmetry, ratio of the paracentral to precentral cortical thickness, ratio of lateral occipital to pericalcarine cortical thickness | 1 | Class: depression relapse after electroconvulsive therapy |
| 49 | 4–6 Computerized-adaptive-diagnostic-test measures | ||
| 50 | Sex, age, medical insurance, marital status, education level, household income, pathological stage, psychosocial measures (social skills rating system, pittsburgh sleep quality index, european organization for research and treatment of cancer quality of life questionnaire [QLQ-C30]) | Participants: non-hodgkin’s lymphoma patients with chemotherapy | |
| 51 | Left precuneus, left precentral gyrus, left inferior frontal cortex (pars triangularis), left cerebellum | ||
| 52 | 120 Behavioral patterns based on smartphone censors including app adherence | ||
| 53 | Whole body kinematic cues | ||
| 54 | Age, race | Participants: women with delivery | |
| 55 | Physical activity and light exposure measured by a wearable device, sleep efficiency measured in a survey | ||
| 56 | Demographic, health-behavioral factors | ||
| 57 | Self-assessed cardiac-related fear, sex, number of words to answer the first homework assignment | 1 | Class: adherence to internet-delivered psychotherapy for myocardial infarction patients’ anxiety and depression |
The following predictors would be important variables for the early diagnosis of depression: comorbid psychopathology, symptom-related disability, treatment credibility, access to therapists, time spent using certain internet-intervention modules; pain-fatigue (symptom intensity scale), comorbidity; 30 microbial markers (gut microbiota); psychological elasticity, income level; upper body movements-postures; brain connectivity within posterior cingulate cortex, within insula, between posterior cingulate cortex and insula/hippocampus-amygdala, between insula and precuneus, between superior parietal lobule and medial prefrontal cortex; single-nucleotide polymorphisms (rs12248560, rs878567, rs17710780); cingulate isthmus asymmetry, pallidal asymmetry, ratio of the paracentral to precentral cortical thickness, ratio of lateral occipital to pericalcarine cortical thickness; self-assessed cardiac-related fear, sex, number of words to answer the first homework assignment for internet-delivered psychotherapy. ANN, artificial neural network; AR, augoregressive; AUC, area under the receiver operating characteristic curve; DT, decision tree; EEG, electroencephalogram; EN, elastic net; GB, gradient boosting; LR, logistic regression; NB, naïve bayes; RF, random forest; RMSE, root mean squared error; SNS, social network service; SNP, single nucleotide polymorphism; SVM, support vector machine
Numeric data
The review of major studies with numeric data is presented in this section. The aim of a recent study [29] was to adopt numeric data and machine learning for analyzing the associations of depression with participant characteristics and 8-week internet intervention (Deprexis). The data came from 283 adults in the United States and their demographic, psychopathological, environmental and intervention variables were considered. The R2 performance of an ensemble with the elastic net and the random forest (0.25) were better than the auto-regressive model (0.17) for the prediction of post-treatment depression. Based on random forest VI, important predictors for the early diagnosis of depression were comorbid psychopathology, low symptom-related disability, treatment credibility, lower access to therapists and time spent using certain internet-intervention (Deprexis) modules. The contribution of a single predictor was small but the ensemble model with a rich collection of various predictors showed reasonable performance. Another study [30] employs numeric data and machine learning to identify important predictors for the early diagnosis of rheumatoid arthritis patients’ depression. The source of the data was 22,131 rheumatoid arthritis patients during 1999–2008 and their demographic, socioeconomic and health-related variables were included, especially, regarding pain-fatigue (Symptom Intensity Scale) and comorbidity. Most predictors were statistically significant in logistic regression but two predictors (pain-fatigue and comorbidity) were dominant in terms of random forest VI. This finding highlights the centrality of the two predictors regarding the clinical implication.
The early diagnosis of postpartum depression is a major issue in medicine and a recent study [32] builds two-stage machine learning methods to solve this issue, i.e., feature selection then depression diagnosis. The data came from a cohort of 508 women, the Edinburgh Postnatal Depression Scale within 42 days after delivery was used as the dependent variable, and their demographic, socioeconomic and health-related factors were considered as the independent variables. Four combinations of prediction models were used in this study: expert vs. random forest in feature selection; support vector machine vs. random forest in depression diagnosis. The random forest-random forest combination registered the best performance measures, AUC 78.0 and sensitivity 69.0. Psychological elasticity, depression during the third trimester and the income level ranked the top 3 in terms of random forest VI. The early diagnosis of depression for university students is another great challenge in health and another study [34] employs the random forest and China’s data on upper body movement alone (e.g., head posture, arm/body swing) to achieve the accuracy of 91.6. In a similar vein, low adherence to internet-delivered psychotherapy for myocardial infarction patent’s depression is an important research topic and a recent study [57] uses the random forest to identify important factors for the adherence. The source of the data was 90 myocardial infarction patients participated in Uppsala University Psychosocial Care Programme Heart study in Sweden. Adherence was defined as completing more than two homework assignments during 14-week therapy. The top-3 factors for the adherence were selfassessed cardiac-related fear, sex and the number of words to answer the first homework assignment. Examining a causal mechanism between linguistic factors and internet-delivered psychotherapy is expected to make a great contribution in this direction.
Genomic and radionomic data
The review of important studies with genomic and radiomic data is reported in this section. The aim of a recent study [31] is to adopt genomic data and machine learning for analyzing the effect of quetiapine treatment on microbiota and investigating the utility of microbiota as a biomarker for the diagnosis and treatment of bipolar depression (BD). Based on the results of univariate analysis for 16S-ribosomal RNA gene sequences, the composition of gut microbiota is significantly different between BD participants and their normal counterparts. Bacteroidetes and Firmicutes were dominant in BD patients and their normal counterparts, respectively. Quetiapine treatment for BD participants altered the composition of their gut microbiota. According to the findings of the random forest, 30 microbial markers were effective predictors of BD participants compared to their normal counterparts (AUC 81.0). This study concludes that quetiapine treatment would change the composition of gut microbiota and microbial markers would be effective for the diagnosis and treatment of BD. Likewise, another study [43] employed genomic data and the random forest to examine the effects of generic variants on major depressive disorder (depression hereafter) during 6-month regular therapy. The source of the data was 150 depression patients on 6-month regular therapy from the population-based PsyCoLaus cohort in Switzerland. The independent variables were 44 single nucleotide polymorphisms in existing literature. Among the 44 predictors, rs12248560, rs878567, and rs17710780 ranked the top 3 in terms of random forest VI. This study was a rare attempt to demonstrate that combining different types of data would break new ground for this area.
It is noteworthy to address recent innovations based on the combination of radiomic data and machine learning for the early diagnosis of depression. Depression is reported to be under-diagnosed in Parkinson’s disease patients, given that the two diseases are overlapped in their symptoms and it is very challenging to take accurate measures in old patients with Parkinson’s disease. In this context, a recent study [38] uses radiomic data and the random forest to highlight brain connectivity networks as effective predictors of depression among Parkinson’s disease patients. The data came from 156 advanced Parkinson’s disease patients and 45 normal controls. The independent variables were their 42 brain connectivity networks. Among the 42 predictors, the following networks ranked the top 6 in terms of random forest VI: 1) within posterior cingulate cortex; 2) within insula; 3/4) between posterior cingulate cortex and insula/hippocampus-amygdala; 5) between insula and precuneus; and 6) between superior parietal lobule and medial prefrontal cortex. The accuracy of the random forest was 82.4. This study concludes that brain connectivity networks would be useful predictors of depression among Parkinson’s disease patients based on radiomic data and machine learning. In a similar context, the focus of another study [48] was to improve the prediction of depression relapse after 6-month electroconvulsive therapy (which is reported to have a relapse rate higher than 50%). The source of the data was 42 depression patients with 6-month electroconvulsive therapy in the United States. Top predictors in terms of random forest VI were cingulate isthmus asymmetry, pallidal asymmetry, the ratio of the paracentral to precentral cortical thickness and the ratio of lateral occipital to pericalcarine cortical thickness (accuracy 71.0–78.0). Structural imaging features are expected to have great utility for the prediction of the prediction of depression relapse after 6-month electroconvulsive therapy.
DISCUSSION
This study presented one of the most comprehensive reviews regarding the recent progress of machine learning for the early diagnosis of depression. This study reviewed thirty two original studies out of 120 original studies in the Web of Science. Also, this study covered a wide range of summary measures, i.e., machine learning methods, sample size, data type, performance measures, important predictors, and whether the VI of the random forest is reported (VI-Yes 1). Current studies on the early diagnosis of depression based on machine learning has the following limitations. Firstly, many studies adopted cross-sectional data and employing longitudinal data would strengthen the performance of machine learning. Secondly, many studies used data with small sizes in single centers. Using big data (e.g., national health insurance claims data) would make valuable contributions for this area. Thirdly, most studies did not consider possible mediating effects among predictors. Fourthly, some studies reported accuracy or the AUC below 70.0 and these results would not be appropriate as diagnostic tests. Likewise, one study reported the accuracy of 100.0 and there could be overfitting in that study. Fifthly, binary categories (no, yes) are popular now but they can be refined to multiple categories with more clinical insights. Sixthly, combining different types of machine learning approaches for different types of depression data would bring new innovations in many aspects. Seventhly, this study compared the performance measures of the six machine learning methods only for different data types. How other data characteristics affect the performance measures of machine learning approaches would be an important topic for future research. In conclusion, however, this study demonstrates that machine learning provides an effective, non-invasive decision support system for early diagnosis of depression.
Footnotes
Availability of Data and Material
The datasets generated or analyzed during the study are available from the corresponding author on reasonable request.
Conflicts of Interest
The authors have no potential conflicts of interest to disclose.
Author Contributions
Conceptualization: Kwang-Sig Lee, Byung-Joo Ham. Data curation: Kwang-Sig Lee, Byung-Joo Ham. Formal analysis: Kwang-Sig Lee, ByungJoo Ham. Funding acquisition: Byung-Joo Ham. Investigation: Kwang-Sig Lee, Byung-Joo Ham. Methodology: Kwang-Sig Lee, Byung-Joo Ham. Project administration: Kwang-Sig Lee, Byung-Joo Ham. Resources: Byung-Joo Ham. Software: Kwang-Sig Lee, Byung-Joo Ham. Supervision: Kwang-Sig Lee, Byung-Joo Ham. Validation: Kwang-Sig Lee, Byung-Joo Ham. Visualization: Kwang-Sig Lee, Byung-Joo Ham. Writing—original draft: Kwang-Sig Lee, Byung-Joo Ham. Writing—review & editing: KwangSig Lee, Byung-Joo Ham.
Funding Statement
This study was supported by the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (NRF-2020M3E5D9080792).
REFERENCES
- 1.Mayo Clinic Depression (major depressive disorder) Available at: https://www.mayoclinic.org/diseases-conditions/depression/symptomscauses/syc-20356007. Accessed January 28, 2022.
- 2.World Health Organization . Depression and other common mental disorders: global health estimates. Geneva: World Health Organization; 2017. [Google Scholar]
- 3.Liu Q, He H, Yang J, Feng X, Zhao F, Lyu J. Changes in the global burden of depression from 1990 to 2017: findings from the global burden of disease study. J Psychiatr Res. 2020;126:134–140. doi: 10.1016/j.jpsychires.2019.08.002. [DOI] [PubMed] [Google Scholar]
- 4.Institute for Health Metrics and Evaluation . Findings from the Global Burden of Disease Study 2017. Seattle: Institute for Health Metrics and Evaluation; 2018. [Google Scholar]
- 5.Lee KS, Park JH. Burden of disease in Korea during 2000-10. J Public Health (Oxf) 2014;36:225–234. doi: 10.1093/pubmed/fdt056. [DOI] [PubMed] [Google Scholar]
- 6.Patel V, Chisholm D, Parikh R, Charlson FJ, Degenhardt L, Dua T, et al. Addressing the burden of mental, neurological, and substance use disorders: key messages from Disease Control Priorities, 3rd edition. Lancet. 2016;387:1672–1685. doi: 10.1016/S0140-6736(15)00390-6. [DOI] [PubMed] [Google Scholar]
- 7.Won E, Ham BJ. Imaging genetics studies on monoaminergic genes in major depressive disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2016;64:311–319. doi: 10.1016/j.pnpbp.2015.03.014. [DOI] [PubMed] [Google Scholar]
- 8.Lee KS, Ahn KH. Application of artificial intelligence in early diagnosis of spontaneous preterm labor and birth. Diagnostics (Basel) 2020;10:733. doi: 10.3390/diagnostics10090733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Park J, Kim JK, Jung S, Gil Y, Choi JI, Son HS. ECG-signal multi-classification model based on squeeze-and-excitation residual neural networks. Appl Sci. 2020;10:6495. [Google Scholar]
- 10.Goodwin LK, Iannacchione MA, Hammond WE, Crockett P, Maher S, Schlitz K. Data mining methods find demographic predictors of preterm birth. Nurs Res. 2001;50:340–345. doi: 10.1097/00006199-200111000-00003. [DOI] [PubMed] [Google Scholar]
- 11.Koivu A, Sairanen M. Predicting risk of stillbirth and preterm pregnancies with machine learning. Health Inf Sci Syst. 2020;8:14. doi: 10.1007/s13755-020-00105-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fergus P, Cheung P, Hussain A, Al-Jumeily D, Dobbins C, Iram S. Prediction of preterm deliveries from EHG signals using machine learning. PLoS One. 2013;8:e77154. doi: 10.1371/journal.pone.0077154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gao C, Osmundson S, Velez Edwards DR, Jackson GP, Malin BA, Chen Y. Deep learning predicts extreme preterm birth from electronic health records. J Biomed Inform. 2019;100:103334. doi: 10.1016/j.jbi.2019.103334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grigorescu I, Cordero-Grande L, Edwards AD, Hajnal J, Modat M, Deprez M. Interpretable convolutional neural networks for preterm birth classification. arXiv [Preprint] 2019 doi: 10.48550/arXiv.1910.00071. [Accessed March 1, 2022]. Available at: [DOI] [Google Scholar]
- 15.Lee KS, Kim ES, Kim DY, Song IS, Ahn KH. Association of gastroesophageal reflux disease with preterm birth: machine learning analysis. J Korean Med Sci. 2021;36:e282. doi: 10.3346/jkms.2021.36.e282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhu SL, Dong J, Zhang C, Huang YB, Pan W. Application of machine learning in the diagnosis of gastric cancer based on noninvasive characteristics. PLoS One. 2020;15:e0244869. doi: 10.1371/journal.pone.0244869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cysouw MCF, Jansen BHE, van de Brug T, Oprea-Lager DE, Pfaehler E, de Vries BM, et al. Machine learning-based analysis of [18F]DCFPyL PET radiomics for risk stratification in primary prostate cancer. Eur J Nucl Med Mol Imaging. 2021;48:340–349. doi: 10.1007/s00259-020-04971-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Park YR, Kim YJ, Ju W, Nam K, Kim S, Kim KG. Comparison of machine and deep learning for the classification of cervical cancer based on cervicography images. Sci Rep. 2021;11:16143. doi: 10.1038/s41598-021-95748-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee KS, Jang JY, Yu YD, Heo JS, Han HS, Yoon YS, et al. Usefulness of artificial intelligence for predicting recurrence following surgery for pancreatic cancer: retrospective cohort study. Int J Surg. 2021;93:106050. doi: 10.1016/j.ijsu.2021.106050. [DOI] [PubMed] [Google Scholar]
- 20.Lee KS, Park KW. Social determinants of association among diabetes mellitus, visual impairment and hearing loss in a middle-aged or old population: artificial-neural-network analysis of the Korean Longitudinal Study of Aging (2014–2016) Geriatrics (Basel) 2019;4:30. doi: 10.3390/geriatrics4010030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee KS, Park KW. Social determinants of the association among cerebrovascular disease, hearing loss and cognitive impairment in a middleaged or older population: recurrent neural network analysis of the Korean Longitudinal Study of Aging (2014-2016) Geriatr Gerontol Int. 2019;19:711–716. doi: 10.1111/ggi.13716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee KS, Park KW. Artificial intelligence approaches to social determinants of cognitive impairment and its associated conditions. Dement Neurocogn Disord. 2020;19:114–123. doi: 10.12779/dnd.2020.19.3.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ryu KJ, Yi KW, Kim YJ, Shin JH, Hur JY, Kim T, et al. Machine learning approaches to identify factors associated with women’s vasomotor symptoms using general hospital data. J Korean Med Sci. 2021;36:e122. doi: 10.3346/jkms.2021.36.e122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee KS, Kwak HJ, Oh JM, Jha N, Kim YJ, Kim W, et al. Automated detection of TMJ osteoarthritis based on artificial intelligence. J Dent Res. 2020;99:1363–1367. doi: 10.1177/0022034520936950. [DOI] [PubMed] [Google Scholar]
- 25.Lee KS, Jha N, Kim YJ. Risk factor assessments of temporomandibular disorders via machine learning. Sci Rep. 2021;11:19802. doi: 10.1038/s41598-021-98837-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lu S, Shi X, Li M, Jiao J, Feng L, Wang G. Semi-supervised random forest regression model based on co-training and grouping with information entropy for evaluation of depression symptoms severity. Math Biosci Eng. 2021;18:4586–4602. doi: 10.3934/mbe.2021233. [DOI] [PubMed] [Google Scholar]
- 27.Sawangarreerak S, Thanathamathee P. Random forest with sampling techniques for handling imbalanced prediction of university student depression. Information. 2020;11:519. [Google Scholar]
- 28.Shin D, Lee KJ, Adeluwa T, Hur J. Machine learning-based predictive modeling of postpartum depression. J Clin Med. 2020;9:2899. doi: 10.3390/jcm9092899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pearson R, Pisner D, Meyer B, Shumake J, Beevers CG. A machine learning ensemble to predict treatment outcomes following an Internet intervention for depression. Psychol Med. 2019;49:2330–2341. doi: 10.1017/S003329171800315X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wolfe F, Michaud K. Predicting depression in rheumatoid arthritis: the signal importance of pain extent and fatigue, and comorbidity. Arthritis Rheum. 2009;61:667–673. doi: 10.1002/art.24428. [DOI] [PubMed] [Google Scholar]
- 31.Hu S, Li A, Huang T, Lai J, Li J, Sublette ME, et al. Gut microbiota changes in patients with bipolar depression. Adv Sci (Weinh) 2019;6:1900752. doi: 10.1002/advs.201900752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang W, Liu H, Silenzio VMB, Qiu P, Gong W. Machine learning models for the prediction of postpartum depression: application and comparison based on a cohort study. JMIR Med Inform. 2020;8:e15516. doi: 10.2196/15516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bhak Y, Jeong HO, Cho YS, Jeon S, Cho J, Gim JA, et al. Depression and suicide risk prediction models using blood-derived multi-omics data. Transl Psychiatry. 2019;9:262. doi: 10.1038/s41398-019-0595-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fang J, Wang T, Li C, Hu X, Ngai E, Seet BC, et al. Depression prevalence in postgraduate students and its association with gait abnormality. IEEE Access. 2019;7:174425–174437. [Google Scholar]
- 35.Zhang X, Cao X, Xue C, Zheng J, Zhang S, Huang Q, et al. Aberrant functional connectivity and activity in Parkinson’s disease and comorbidity with depression based on radiomic analysis. Brain Behav. 2021;11:e02103. doi: 10.1002/brb3.2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sau A, Bhakta I. Predicting anxiety and depression in elderly patients using machine learning technology. Healthc Technol Lett. 2017;4:238–243. [Google Scholar]
- 37.Cacheda F, Fernandez D, Novoa FJ, Carneiro V. Early detection of depression: social network analysis and random forest techniques. J Med Internet Res. 2019;21:e12554. doi: 10.2196/12554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lin H, Cai X, Zhang D, Liu J, Na P, Li W. Functional connectivity markers of depression in advanced Parkinson’s disease. Neuroimage Clin. 2020;25:102130. doi: 10.1016/j.nicl.2019.102130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Razavi R, Gharipour A, Gharipour M. Depression screening using mobile phone usage metadata: a machine learning approach. J Am Med Inform Assoc. 2020;27:522–530. doi: 10.1093/jamia/ocz221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Čukić M, Stokić M, Simić S, Pokrajac D. The successful discrimination of depression from EEG could be attributed to proper feature extraction and not to a particular classification method. Cogn Neurodyn. 2020;14:443–455. doi: 10.1007/s11571-020-09581-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Foster S, Mohler-Kuo M, Tay L, Hothorn T, Seibold H. Estimating patient-specific treatment advantages in the ‘Treatment for Adolescents with Depression Study’. J Psychiatr Res. 2019;112:61–70. doi: 10.1016/j.jpsychires.2019.02.021. [DOI] [PubMed] [Google Scholar]
- 42.Tennenhouse LG, Marrie RA, Bernstein CN, Lix LM, CIHR Team in Defining the Burden and Managing the Effects of Psychiatric Comorbidity in Chronic Immunoinflammatory Disease Machine-learning models for depression and anxiety in individuals with immune-mediated inflammatory disease. J Psychosom Res. 2020;134:110126. doi: 10.1016/j.jpsychores.2020.110126. [DOI] [PubMed] [Google Scholar]
- 43.Kanders SH, Pisanu C, Bandstein M, Jonsson J, Castelao E, Pistis G, et al. A pharmacogenetic risk score for the evaluation of major depression severity under treatment with antidepressants. Drug Dev Res. 2020;81:102–113. doi: 10.1002/ddr.21609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Elovanio M, Hakulinen C, Pulkki-Råback L, Aalto AM, Virtanen M, Partonen T, et al. General Health Questionnaire (GHQ-12), Beck Depression Inventory (BDI-6), and Mental Health Index (MHI-5): psychometric and predictive properties in a Finnish population-based sample. Psychiatry Res. 2020;289:112973. doi: 10.1016/j.psychres.2020.112973. [DOI] [PubMed] [Google Scholar]
- 45.Richter T, Fishbain B, Fruchter E, Richter-Levin G, Okon-Singer H. Machine learning-based diagnosis support system for differentiating between clinical anxiety and depression disorders. J Psychiatr Res. 2021;141:199–205. doi: 10.1016/j.jpsychires.2021.06.044. [DOI] [PubMed] [Google Scholar]
- 46.Zanella-Calzada LA, Galván-Tejada CE, Chávez-Lamas NM, Gracia-Cortés MDC, Magallanes-Quintanar R, Celaya-Padilla JM, et al. Feature extraction in motor activity signal: towards a depression episodes detection in unipolar and bipolar patients. Diagnostics (Basel) 2019;9:8. doi: 10.3390/diagnostics9010008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Manelis A, Iyengar S, Swartz HA, Phillips ML. Prefrontal cortical activation during working memory task anticipation contributes to discrimination between bipolar and unipolar depression. Neuropsychopharmacology. 2020;45:956–963. doi: 10.1038/s41386-020-0638-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wade BSC, Sui J, Hellemann G, Leaver AM, Espinoza RT, Woods RP, et al. Inter and intra-hemispheric structural imaging markers predict depression relapse after electroconvulsive therapy: a multisite study. Transl Psychiatry. 2017;7:1270. doi: 10.1038/s41398-017-0020-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gibbons RD, Hooker G, Finkelman MD, Weiss DJ, Pilkonis PA, Frank E, et al. The computerized adaptive diagnostic test for major depressive disorder (CAD-MDD): a screening tool for depression. J Clin Psychiatry. 2013;74:669–674. doi: 10.4088/JCP.12m08338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hu C, Li Q, Shou J, Zhang FX, Li X, Wu M, et al. Constructing a predictive model of depression in chemotherapy patients with non-Hodgkin’s lymphoma to improve medical staffs’ psychiatric care. Biomed Res Int. 2021;2021:9201235. doi: 10.1155/2021/9201235. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 51.Shimizu Y, Yoshimoto J, Toki S, Takamura M, Yoshimura S, Okamoto Y, et al. Toward probabilistic diagnosis and understanding of depression based on functional MRI data analysis with logistic group LASSO. PLoS One. 2015;10:e0123524. doi: 10.1371/journal.pone.0123524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wahle F, Kowatsch T, Fleisch E, Rufer M, Weidt S. Mobile sensing and support for people with depression: a pilot trial in the wild. JMIR Mhealth Uhealth. 2016;4:e111. doi: 10.2196/mhealth.5960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Li W, Wang Q, Liu X, Yu Y. Simple action for depression detection: using kinect-recorded human kinematic skeletal data. BMC Psychiatry. 2021;21:205. doi: 10.1186/s12888-021-03184-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Park Y, Hu J, Singh M, Sylla I, Dankwa-Mullan I, Koski E, et al. Comparison of methods to reduce bias from clinical prediction models of postpartum depression. JAMA Netw Open. 2021;4:e213909. doi: 10.1001/jamanetworkopen.2021.3909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kim H, Lee S, Lee S, Hong S, Kang H, Kim N. Depression prediction by using ecological momentary assessment, actiwatch data, and machine learning: observational study on older adults living alone. JMIR Mhealth Uhealth. 2019;7:e14149. doi: 10.2196/14149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kasthurirathne SN, Biondich PG, Grannis SJ, Purkayastha S, Vest JR, Jones JF. Identification of patients in need of advanced care for depression using data extracted from a statewide health information exchange: a machine learning approach. J Med Internet Res. 2019;21:e13809. doi: 10.2196/13809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wallert J, Gustafson E, Held C, Madison G, Norlund F, von Essen L, et al. Predicting adherence to internet-delivered psychotherapy for symptoms of depression and anxiety after myocardial infarction: machine learning insights from the U-CARE heart randomized controlled trial. J Med Internet Res. 2018;20:e10754. doi: 10.2196/10754. [DOI] [PMC free article] [PubMed] [Google Scholar]


