Abstract
Mounting evidence highlights the pivotal role of enteric microbes as a dynamic interface with the host. Indeed, the gut microbiota, located in the lumen of the gastrointestinal (GI) tract, influence many essential physiological processes that are evident in both healthy and pathological states. A key signaling molecule throughout the body is serotonin (5-hydroxytryptamine; 5-HT), which acts in the GI tract to regulate numerous gut functions including intestinal motility and secretion. The gut microbiota can modulate host 5-HT systems both directly and indirectly. Direct actions of gut microbes, evidenced by studies using germ-free animals or antibiotic administration, alter the expression of key 5-HT-related genes to promote 5-HT biosynthesis. Indirectly, the gut microbiota produce numerous microbial metabolites, whose actions can influence host serotonergic systems in a variety of ways. This review summarizes the current knowledge regarding mechanisms by which gut bacteria act to regulate host 5-HT and 5-HT-mediated gut functions, as well as implications for 5-HT in the microbiota-gut-brain axis.
Keywords: tryptophan, gut-brain axis, bacteria, tryptophan synthase, enteric nervous system, microbial metabolites
Despite decades of research, we are only beginning to fully appreciate the immense complexity in which microbes colonize the gut and interact dynamically with the host in healthy and pathological conditions. Located in the lumen of the gastrointestinal (GI) tract on the order of tens of trillions of microorganisms, the gut microbiota demonstrate remarkable capacity to influence nearly every physiological system in the body1,2.
Research into the ecology of the microbiome demonstrates that bacteria colonization is diverse and varies along the GI tract3. Notably, the microbial communities become increasingly heterogeneous progressing from the oral to distal ends of the gut due to the diverse microhabitats associated with each region of the GI tract. For example, fewer bacteria are present in the small intestine of the gut where transit time is short and pH is low, in comparison to the colon, which harbors the highest numbers and biodiversity of bacteria and is the primary site for bacterial fermentation.
Pivotal early insights into host-microbiota interactions stem largely from germ-free (GF) and fecal microbiota transplantation (FMT) studies2. GF studies exploit the postnatal timing of microbiota colonization of the GI tract, such that rodents born and raised in a sterile environment develop without a microbiome. Studies employing this strategy demonstrate that GF mice exhibit abnormal physiology, such as slowed colonic motility4 and total intestinal transit time5. Notably, colonization with either normal mouse-derived or human-derived microbiota can restore these GF-induced deficits in motility6.
FMT is the process by which stool harvested from a donor is transplanted into the intestines of a recipient in order to alter the microbiota composition7. Preclinical FMT studies demonstrate remarkable transfers of microbiota-driven disease symptomologies in both rodent models and humans, including transfers of slow transit constipation8, rapid transit diarrhea9, and intestinal barrier dysfunction9. While GF and FMT approaches certainly have clinically translatable limitations, they nevertheless reliably demonstrate that healthy gut function is microbiota-dependent.
Serotonin in the gut
Serotonin (5-hydroxytryptamine; 5-HT) is a prominent signaling molecule throughout the body. The major source of 5-HT is from the enterochromaffin (EC) cells in the epithelium of the GI tract10. EC cells are a subset of enteroendocrine cells that synthesize, store, and release 5-HT in a regulated manner10. EC cells synthesize 5-HT from tryptophan (Trp) using the rate-limiting enzyme tryptophan hydroxylase 1 (Tph1), and since Tph1 is not saturated under baseline Trp concentrations, it is likely that elevated Trp could increase metabolic 5-HT output11. Myenteric neurons also express 5-HT, though at much lower levels than in the mucosa.
Termination of 5-HT signaling involves a serotonin-selective reuptake transporter (SERT) to remove 5-HT from the interstitial space. 5-HT is then degraded intracellularly by monoamine oxidase A (MAO-A) to form 5-hydroxyindoleacetic acid (5-HIAA). SERT is expressed by all mucosal epithelial cells in the intestines and functions as an important regulator of interstitial 5-HT availability12.
In the gut, 5-HT released from EC cells in response to luminal chemical and mechanical stimuli functions as a critical activator of many GI reflexes by signaling through a variety of receptors located on intrinsic and extrinsic afferent nerve fibers10. Mucosal 5-HT can initiate the peristaltic reflex, a component of propulsive motility10. In the intestinal epithelium, 5-HT stimulates secretory responses acting primarily on the 5-HT3 receptor (5-HT3R) and the 5-HT4R, with evidence for paracrine-mediated secretion through the 5-HT2R as well12. 5-HT release and activation of 5-HT3R located on vagal afferent neurons can result in various reflex responses including pancreatic secretion, gallbladder contraction, and inhibition of gastric emptying when released as part of the normal digestive response, as well as nausea and vomiting when released at higher concentrations in pathological conditions.
Numerous approaches to treat clinical GI disorders target the enteric serotonergic system with the goal of promoting healthy gut function. For example, in patients with irritable bowel syndrome, 5-HT3R antagonists have been used to treat diarrhea13, and 5-HT4R agonists are used to treat constipation14,15. Taken together, 5-HT is a critical mediator of many important gut functions and consequently is an important target in the context of GI dysfunction.
Direct Mechanisms
5-HT’s prominent role in the regulation of GI function is well documented15. However, the contribution of gut microbes in impacting host gut-derived 5-HT signaling is a burgeoning field that may offer key insights into the link between the microbiota and GI function.
Early studies examining the role of the microbiota in gut-derived 5-HT regulation demonstrated that GF mice have significantly lower serum 5-HT levels16,17, decreased colonic Tph1 mRNA expression17, and increased colonic SERT mRNA expression17 as compared to control mice.
More recently, two landmark studies provided further strength toward the notion that the microbiota can regulate gut-derived 5-HT levels5,18 (FIG. 1A). A study by Kashyap and colleagues examined the impact of the gut microbiota on host colonic 5-HT production18. The authors utilized three groups of mice with differing microbiotas: GF mice, GF mice colonized with human gut microbiota (HM), and conventionally raised (CR) mice with normal mouse microbiomes. HM and CR mice exhibit significantly higher colonic Tph1 mRNA and protein levels, as well as higher colonic 5-HT concentrations, compared to GF mice. Notably, there is no difference in EC cell density in the proximal colon between the groups, suggesting a microbiome-induced effect of increased Tph1 transcription.
Figure 1.
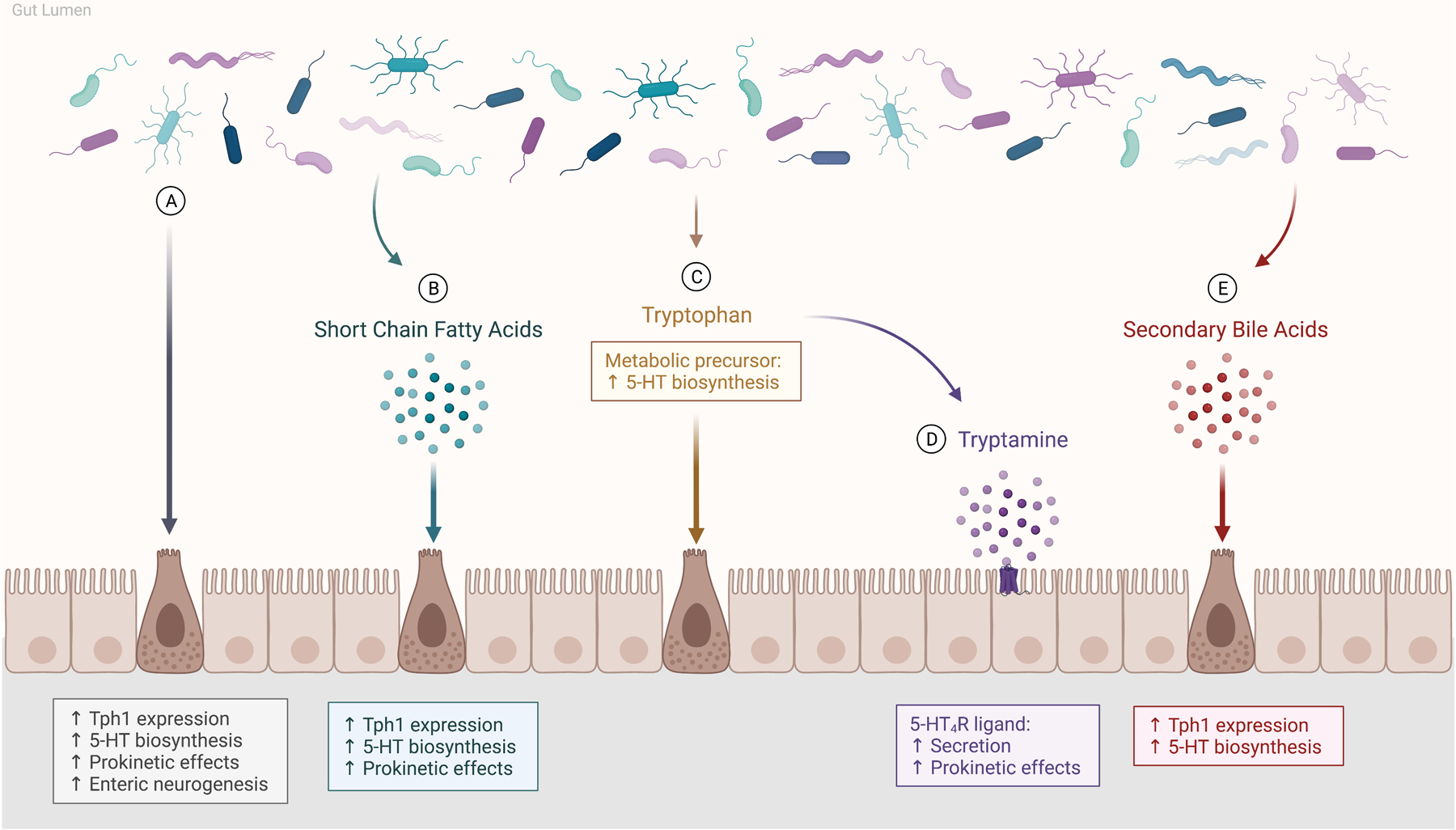
The gut microbiota mediate host serotonin (5-HT) through direct and indirect means. (A) Gut bacteria act directly on enterochromaffin (EC) cells to increase colonic tryptophan hydroxylase 1 (Tph1) expression and promote 5-HT synthesis. (B-E) Enteric bacteria alter host 5-HT indirectly through microbial metabolites, including short chain fatty acids, tryptophan, tryptamine, and secondary bile acids. (B) Short chain fatty acids are dietary-derived metabolites produced by bacterial fermentation that stimulate 5-HT synthesis and release by EC cells, and induce prokinetic effects on intestinal motility. (C) Tryptophan is an essential amino acid and its metabolism is under control of the gut microbiota. It functions as the metabolic precursor to 5-HT as well as tryptamine. (D) Tryptamine acts as a ligand for the 5-HT4 receptor (5-HT4R) to stimulate secretion by intestinal epithelial cells, thereby inducing prokinetic actions. (E) Secondary bile acids, formed by the gut microbiota from primary bile acids, promote Tph1 expression and stimulate 5-HT synthesis. Created with BioRender.com.
Along similar lines, Hsiao and colleagues demonstrated that the gut microbiota promotes host 5-HT biosynthesis5. Consistent with the findings described above, adult GF mice exhibit decreased colonic 5-HT levels as well as decreased colonic mRNA expression of Tph1, while also exhibiting significantly higher concentrations of Trp in feces. Colonization of GF mice with spore-forming bacteria from healthy mice restores colonic 5-HT levels and elevates colonic Tph1 expression. Notably, the restorative increases in 5-HT are blocked by the administration of the Tph inhibitor para-chlorophenylalanine (PCPA), suggesting that host Tph activity is necessary for promoting 5-HT biosynthesis.
Taken together, it is now generally accepted that the gut microbiota plays a key role in regulating the serotonergic system of the host primarily through altering the expression of 5-HT-related genes. Specifically, intestinal microbiota influence Tph1 transcription, thereby promoting 5-HT biosynthesis. While the microbiota have also been shown to influence SERT expression5,17, this likely represents an indirect, compensatory effect to deficient 5-HT biosynthesis, rather than a direct effect by the microbiota. Inhibition of Tph via PCPA administration modulates SERT expression5, suggesting that changes in SERT expression occur independently of the intestinal microbiota. Furthermore, the strong positive correlations between colonic mRNA expressions of SERT and other key 5-HT-related genes such as 5-HT4R does not depend on microbiota colonization status19, providing further support that SERT is not directly mediated by the gut microbiota. Additionally, GF mice do not exhibit differences in mRNA expression of enzymes that release, package, or break down 5-HT5. Therefore, it is likely that the microbiota influence enteric 5-HT biosynthesis primarily through the elevation of Tph1 expression.
Gut microbiota may also alter expression levels of a key 5-HT receptor, the 5-HT3R, known to be involved in intestinal secretion and peristalsis. Indeed, Bhattarai et al. found a microbiota-mediated modulation of host colonic secretion through altered epithelial 5-HT3R expression20. GF mice display elevated 5-HT3R expression levels compared to HM mice, which correspond with increased colonic secretory responses. Notably, there was no microbiota-mediated effects on 5-HT4R expression, or on 5-HT4R-dependent colonic secretory responses.
Further support for the notion that the microbiota influence the enteric serotonergic system comes from antibiotic studies. Administration of broad-spectrum antibiotics can deplete luminal bacteria in an inducible and temporally controlled manner. Unlike GF studies, in which microbiota alterations are persistent throughout development, antibiotics provide an alternative strategy to alter microbiota composition following normal development, and to assess microbiota-dependent physiological processes and behavior.
Perturbation of the gut microbiota using antibiotic administration alters host 5-HT, 5-HT-mediated gut functions, and enteric nervous system (ENS) neuroanatomy. Mice treated with antibiotics exhibit lower levels of colonic 5-HT and decreased Tph1 expression5,21, which parallel the findings of GF-associated deficits to the gut 5-HT system. Furthermore, these alterations in 5-HT levels result in functional changes to intestinal transit, as antibiotic-treated mice display slower whole gut transit21–24 and slower colonic motility times21,24 compared to controls. In addition, these functional deficits are accompanied by loss of enteric neurons22. Interestingly, antibiotic-induced changes are reversible. Following reconstitution of the microbiota, mice display restored GI motility and enteric neurogenesis22.
The evidence for whether specific commensal bacteria found in the human gut synthesize 5-HT de novo in physiologically relevant concentrations remains unclear. Indeed, certain bacteria have been shown to synthesize 5-HT in vitro25,26. However, this 5-HT synthesis may occur through indirect means and independent of Tph27. Thus far, no human commensal bacteria that synthesize 5-HT have been identified.
Indirect Mechanisms
Insights into host-microbiota dynamics further demonstrate that gut bacteria actively produce microbial metabolites capable of impacting the host. Emerging evidence suggests that these metabolites are key aspects of communication between the microbiota and the host to regulate physiological function in health and disease states. In the context of the enteric 5-HT system, microbial metabolites can stimulate 5-HT biosynthesis and release, leading to alterations in gut functions. This section highlights four key categories of microbial metabolites and summarizes the evidence of how they influence gut-derived 5-HT (Fig. 1 B–E).
Short chain fatty acids
A notable group of microbial metabolites known to influence host physiology are short chain fatty acids (SCFAs). SCFAs are the products of bacterial fermentation of dietary fiber in the colon28. The most abundant SCFAs are acetate, propionate, and butyrate, which are present in an approximate molar ratio of 60:20:20, respectively. These dietary-derived microbial metabolites play a prominent role in the regulation of a variety of host physiological processes29.
SCFAs influence the enteric 5-HT system by stimulating the release of 5-HT by intestinal EC cells, which express SCFA receptors30. An early study by Fukumoto et al. demonstrated that intraluminal perfusion of SCFAs into the proximal colon of rats induced release of 5-HT, and consequently accelerated colonic transit and motility31.
SCFAs can also promote 5-HT biosynthesis. Stimulatory actions of acetate and butyrate on EC cells promote colonic Tph1 expression and 5-HT production in an in vitro human EC cell model18. Furthermore, application of butyrate or propionate on RIN14B chromaffin cell culture leads to an elevation of 5-HT release and increase in Tph1 expression5. In addition to regulating Tph1 expression, SCFAs can also impact expression levels of key 5-HT receptors. Indeed, acetate has been shown to decrease 5-HT3R expression in vitro in GF mouse-derived colonoids20. Taken together, SCFAs are microbial metabolites that play an important role in the stimulation of enteric 5-HT biosynthesis.
Tryptophan
A critical process that is influenced by gut microbes is Trp metabolism32. Trp is an essential amino acid, indicating that it cannot be synthesized de novo and therefore must be supplied through dietary intake. The recommended daily allowance of dietary Trp for adults is 4 mg/kg/day, or roughly 250–450 mg/day. Considering that the average intake for most adults is approximately 800–1000 mg/day33, most individuals achieve this recommended daily intake of dietary Trp readily to meet their protein metabolic demands.
Once ingested, Trp is absorbed nearly completely in the small intestine of the GI tract, specifically within intestinal epithelial cells known as enterocytes34. Two transporters facilitate this absorptive process. On the apical membrane of enterocytes, Trp absorption is mediated by the epithelial amino acid transporter B0AT1 (Slc6a19)34, along with all other neutral amino acids. On the basal membrane of enterocytes, Trp transport occurs via the basolateral aromatic amino acid transporter TAT1 (Slc16a10)35. Internalized Trp is then distributed to tissues through the body via the circulatory system.
Trp functions primarily as a component of protein synthesis. In the GI tract, Trp undergoes three different avenues of metabolism: 5-HT synthesis, kynurenine synthesis, and production of metabolites that act as ligands of the aryl hydrocarbon receptor (AhR). Though it is estimated that the kynurenine synthesis pathway accounts for over 90% of Trp metabolism, the significance of Trp metabolism in the kynurenine and AhR pathways is outside the scope of this manuscript, but this information can be found in recent reviews of the topic36,37.
The gut microbiota plays an active role in Trp availability, although the mechanisms and functional implications of this remain to be elucidated. While the majority of Trp is absorbed in the small intestine, it is possible some Trp can also reach the colon where it is subject to catabolism by the gut bacteria. GF mice exhibit 40% greater levels of plasma Trp than their conventional counterparts38. These increased Trp levels could result from a variety of factors, such as GF-induced deficits in host Tph1 activity5,18, lack of tryptophanase activity by enteric bacteria to metabolize dietary Trp38, or altered metabolism down other pathways. Consistent with GF mice, antibiotic-treated mice also display elevated levels of Trp, in both the colonic mucosa39 and cecal contents40.
Microbially-mediated changes in Trp availability may have an impact on gut 5-HT availability and 5-HT-mediated intestinal functions, though more research is needed to characterize these effects. Some bacteria are known to possess the enzymatic capabilities to metabolize Trp directly using key enzymes in its catabolic and anabolic pathways. Specifically, Trp-catabolizing bacteria produce tryptophanase, the enzyme responsible for metabolizing Trp into indole, pyruvate, and ammonia36. Conversely, Trp-synthesizing bacteria produce tryptophan synthase, an enzyme that catalyzes the biosynthesis of Trp from the metabolite indole36. However, it remains to be determined whether these bacteria are present as human gut commensals.
Tryptamine
Another key metabolite indirectly influenced by gut microbes is tryptamine. Tryptamine is an indoleamine that is formed by the decarboxylation of L-tryptophan. Though it is present at relatively low concentrations in mammalian tissue, it is known to have a physiologically significant role in interacting with the host.
The characterization of tryptamine’s potential functions in the ENS was brought about by an early study by Takaki et al. demonstrating that tryptamine stimulates the release of endogenous 5-HT within the GI systems of guinea pigs41. More recent work indicates that tryptamine acts as a 5-HT4R ligand42. 5-HT4Rs are G-protein-coupled receptors that are expressed in the epithelium of the GI tract, with the greatest level of expression in the distal colon14. Stimulation of 5-HT4Rs produces prokinetic effects15, and 5-HT4R agonists promote colonic propulsive motility14,43.
Tryptamine-induced activation of 5-HT4Rs accelerates gut transit through increased secretion by intestinal epithelial cells42. Tryptamine can also, acting as a 5-HT4R agonist, stimulate mucus release from goblet cells and prevent epithelial barrier disruption44, which has important implications for gut-derived inflammatory conditions such as irritable bowel syndrome.
The gut microbiota can play a role in tryptamine production. For example, GF mice exhibit significantly lower levels of tryptamine in feces compared to HM mice45. Furthermore, specific microbes present in the human intestinal microbiota possess the enzymatic capacity to decarboxylase Trp to produce tryptamine27. It remains an open question whether, and to what degree, microbiota-mediated metabolism of Trp into tryptamine influences host physiology and 5-HT-related gut functions. Nonetheless, bacterially-mediated metabolism of Trp into tryptamine represents an exciting avenue for future investigation in the context of potential therapeutic applications.
Bile acids / secondary bile acids
Bile acids (BAs) are water-soluble, cholesterol-derived surfactants that are critical for digestive and absorptive processes. The effects of BAs in the lumen of the intestine have been shown to be region-specific. Colonic EC cells express the G protein-coupled bile acid receptor 1 (GPBAR1; also known as TGR5), for which BAs are endogenous ligands46. Notably, activation of GPBAR1 by intestinal BAs promotes 5-HT release and mediates prokinetic actions47,48.
The gut bacteria play an active role in biotransforming primary BAs in the colon through deconjugation and dehydroxylation to produce the secondary BAs, deoxycholic acid (DCA) and lithocholic acid (LCA). The regulation of secondary BA metabolism by the gut microbiota is further supported by GF studies in which GF mice exhibit nonexistent levels of secondary BAs in intestinal tissue compared to conventional mice49,50. Similarly, antibiotic-treated mice display decreased levels of secondary BAs21.
Secondary BAs are key microbial metabolites that can impact the enteric serotonergic system. DCA, induced by spore-forming microbes, has been shown to elevate 5-HT levels and increase Tph1 expression, both in vitro in RIN14B chromaffin cells and in vivo in mice5. Furthermore, reductions in relative abundances of bile-metabolizing bacteria is associated with impaired Trp metabolism, leading to reduced 5-HT bioavailability and delayed intestinal motility51. Taken together, it is evident that microbially-regulated alterations in secondary BAs can influence 5-HT bioavailability and consequently induce changes in gut function.
Beneficial bacteria applications
A transient approach to manipulate the gut microbiota is through oral administration of isolated bacterial strains such as those included in non-colonizing “probiotic” formulations. Numerous studies report beneficial effects on host physiological processes of orally-delivered bacteria in both animal models and humans. In the context of GI function, for example, administration of certain bacteria can prevent stress-induced intestinal barrier dysfunction52,53 and colonic dysfunction54, as well as reduce whole gut transit time55–57.
Despite evident impacts of bacteria administration on many functions, the underlying mechanisms of action of such bacteria are not well elucidated. Along with the need for more mechanistic-based investigations, it is becoming clear that there is a need for a deeper understanding of bacterial species and strain-specific effects. Certainly these investigations are underway and will yield stimulating findings in the coming years.
A developing avenue of exploration and potential therapeutic exploitation is the use of specific microbial strains that can alter the production and regulation of key bioactive metabolites. Indeed, certain strains have been shown to change the bioavailability of enteric 5-HT. A study by Nzakizwanayo et al. (2015) demonstrated that Escherichia coli Nissle 1917, a bacteria found in “probiotics” currently available on the market, enhances 5-HT bioavailability in a dose-dependent manner in an ex-vivo model58. Furthermore, this strain increases intracellular 5-hydroxytryptophan (5-HTP) levels and decreases 5-HIAA levels, supporting a hypothesis that Escherichia coli Nissle 1917 alters 5-HT through synthesis and clearance, respectively.
Spore-forming bacteria can promote 5-HT biosynthesis through the elevation of host colonic Tph1 expression5. Furthermore, monoassociation of GF mice with the strain Clostridium ramosum increases host 5-HT bioavailability59. Expression levels of ileal and colonic Tph1 and MAO-A are significantly greater in Clostridium ramosum monocolonized mice compared to GF controls. In addition, in vitro stimulation with Clostridium ramosum provokes an increased 5-HT release in RIN14B chromaffin cells as well as organoids from murine small intestine and colon.
Commensal lactic acid bacteria represent another class of “beneficial” bacteria known to exert effects on the enteric serotonergic system. For example, studies report that increased intestinal SERT concentrations are associated with Bifidobacterium (B.) dentium60, B. longum61, Lacticaseibacillus (formerly classified as Lactobacillus) rhamnosus62,63, Lactobacillus acidophilus61, and Limosilactobacillus (formerly classified as Lactobacillus) reuteri64. Additionally, monoassociation with the strain B. dentium promotes the secretion of microbial metabolites, including the SCFA acetate, that act to modulate the host serotonergic system60. Specifically, mice monoassociated with B. dentium displayed increased intestinal 5-HT levels and upregulated colonic 5-HT4R and SERT mRNA expression.
Taken together, mounting evidence suggests that the administration of beneficial isolated bacteria can alter host gut-derived 5-HT and influence 5-HT-mediated gut functions in a transient, inducible, and reversible way. Further resolution of the underlying mechanisms of actions of specific bacteria will move the field forward to encourage investigations into potential therapeutic applications.
Implications for 5-HT in the microbiota-gut-brain axis
Overview of the microbiota-gut-brain axis
The gut-brain axis refers to the bidirectional line of communication between the gut and the brain through neural, immune, and endocrine means. Dating back to the foundational theoretical work of William James and Carl Lange in the 1800s, a wealth of research has since demonstrated top-down contributions of psychological stress and emotions on GI function1. Recently, a growing body of research on the microbiome highlights its remarkable capacity for bottom-up modulation, leading many in the field to expand the concept into the “microbiome-gut-brain axis”.
The brain communicates with the GI tract in a top-down fashion primarily through parallel efferent pathways originating in the hypothalamus, amygdala, and cortical regions. Such pathways include the sympathetic and parasympathetic components of the autonomic nervous system, the hypothalamic-pituitary-adrenal (HPA) axis, and descending monoaminergic pathways. Sympathetic effects on the gut are inhibitory and act to slow intestinal transit and secretion1. Parasympathetic effects, on the other hand, act in an excitatory manner to stimulate motility, secretion, and release of key signaling molecules such as serotonin, gastrin, and somatostatin1. The HPA axis coordinates adaptive stress responses and regulates numerous homeostatic systems, including digestion and metabolism65.
The gut communicates with the brain primarily through neural and endocrine means. The vagus nerve acts as a bidirectional conduit for information passing between the GI tract and the brain1,66. Moreover, gut peptides and neuropeptides, released in response to luminal stimuli, can act in a paracrine manner to activate receptors located on adjacent vagal afferents. These peptides can also act in an endocrine manner when released into the circulation to signal to various brain regions, particularly circumventricular organs such as the hypothalamus and area postrema66.
Serotonin in the brain
In the central nervous system (CNS), the primary sites of 5-HT synthesis and storage are clusters of neurons in midbrain and pontine regions, with the raphe nuclei accounting for the majority of serotonergic cell bodies and projection fibers67. The rate limiting enzyme of neuronal 5-HT synthesis is the isoform tryptophan hydroxylase 2 (Tph2)68. Notably, gut 5-HT and CNS 5-HT are considered separate pools, since 5-HT does not cross the blood-brain barrier (BBB).
5-HT activates a variety of receptors located pre- and post-synaptically, with the 5-HT1, 5-HT2, and 5-HT4 families being most common in the CNS. The diverse array of receptor subtypes offers an expansive range of 5-HT-mediated actions in the brain69. In the CNS, 5-HT availability, in addition to serotonergic neurons expressing SERT, is also controlled by presynaptic autoreceptors that act as negative feedback mechanisms to inhibit further 5-HT release into the synaptic cleft70. While 5-HT’s functions in the gut are fairly well characterized, its actions in the brain, though extensively studied, remain more elusive. Serotonin has been implicated in a myriad of functions, including but not limited to mood71, anxiety72, food consumption73, reward74, and sleep75,76.
Converging lines of evidence indicate that dysfunctions in microbiota-gut-brain communication can have salient pathophysiological consequences, and at least some of these appear to involve 5-HT. Indeed, it is estimated that nearly 60% of individuals diagnosed with anxiety or depression also report symptoms of intestinal dysfunction77. Clinically, pharmacological treatments for anxiety and depression target the 5-HT system and act to increase 5-HT levels by a variety of mechanisms, including selectively inhibiting or impairing 5-HT reuptake, inhibiting 5-HT metabolism, or increasing 5-HT release67. 5-HT’s enigmatic role in mood is of particular relevance to gut-brain communication given the high comorbidities of anxiety and depressive symptoms and GI dysfunction. However, such treatments pose distinct disadvantages. Notably, the delayed onset of action of selective serotonin reuptake inhibitors (SSRIs) as well as mixed efficacies67 suggest alternative or adjuvant treatment options are worth pursuing. The microbiome is one such promising target.
Tryptophan as an potential microbiota-gut-brain target
Given that 5-HT does not cross the BBB, changes in circulating 5-HT levels are unlikely to influence 5-HT signaling in the CNS. However, intervention in microbiota-gut-brain 5-HT signaling may be possible through Trp acting as a precursor-mediated signal. Given that Trp metabolism is decisively regulated by the gut microbiota, it is possible that Trp is a critical point of intersection within the microbiota-gut-brain axis. Early studies demonstrate that the relationship between brain Trp concentrations and 5-HT synthesis is directly proportional, such that an injection of Trp produces a rapid elevation of brain Trp concentrations, and consequently a rapid rise in 5-HT synthesis78,79.
Subsequent work to elucidate this relationship reveals that the ability of Trp to influence brain 5-HT synthesis depends principally on three factors. The first factor is the competitive nature by which Trp crosses the BBB. Upon absorption in the gut, circulating Trp must compete with other large neutral amino acids (LNAAs) to enter the brain through the amino acid transporter. The bioavailability of Trp to cross the BBB therefore depends on the sum of the competing amino acids, often expressed as the “tryptophan ratio”33,80. Altering the tryptophan ratio by either raising plasma Trp levels or lowering concentrations of other LNAAs can thus maximize the amount of Trp available for brain 5-HT synthesis.
The competitive transport mechanism for Trp is particularly relevant during food consumption. Studies in rats suggest the composition of a meal can indirectly determine brain Trp uptake and subsequent 5-HT synthesis due to the meal’s effects on the Trp ratio. A protein-rich meal, for instance, raises both plasma Trp and other LNAA levels to a similar degree, thus the net effect on brain Trp and 5-HT is negligible81. On the other hand, a carbohydrate-rich meal increases plasma Trp concentrations and decreases concentrations of the other LNAAs through insulin activation, which therefore favors Trp transport into the brain to increase precursor availability and 5-HT synthesis33,82.
The second main factor depends on the enzyme kinetics of Tph2, which like Tph1, is only partially saturated under normal conditions. Thus, in theory, raising or lowering brain Trp concentrations could respectively increase or decrease the rate of brain 5-HT synthesis78,83. Further investigation is warranted to determine the characteristics by which this occurs.
Lastly, in order to influence CNS 5-HT-mediated behaviors, changes in Trp levels must ultimately affect neuronal 5-HT release, following which 5-HT can interact with various receptors to influence behavior. In support of this concept, Sharp et al. used in vivo microdialysis in rats to demonstrate that pre-treatment with Trp significantly enhanced 5-HT release upon electrical stimulation of serotonergic dorsal raphe nucleus neurons84.
Though it is thought that less than 5% of dietary Trp is used for 5-HT synthesis throughout the body, the serotonergic impacts on the CNS are demonstrated in experiments involving manipulations of dietary Trp availability. Indeed, acute Trp depletion via consumption of a Trp-free diet produces striking reductions in brain Trp and 5-HT levels in both animal models and humans85–88. Conversely, Trp loading via ingestion of pure Trp, α-lactalbumin, or carbohydrates induces robust increases in brain 5-HT levels80,89. Furthermore, such modulation of 5-HT levels may have impacts at the behavioral level, as several rodent studies report Trp manipulations impact anxiety- and depressive-like behavior85,90.
Certain gut bacteria may also have the ability to alter Trp levels as well. A novel, microbiome-targeted strategy to manipulate Trp availability and subsequent 5-HT synthesis could exploit certain bacteria’s biochemical capacity to manipulate Trp. By possessing key enzymes in Trp catabolism or anabolism, such bacteria may be able to alter availability of the amino acid for absorption. Oral administration of these bacteria may impact microbiota-gut-brain signaling by altering Trp levels, and ultimately modulate 5-HT-mediated physiological and behavioral effects. Further investigation is necessary to better understand how to utilize orally administered bacteria as a potential therapeutic interface between microbiota-gut-brain communication and function.
Conclusion
The dynamic interactions between the host and the gut microbiota underlie many physiological processes. It is evident that a deeper understanding of how gut microbes act to mediate the enteric serotonergic system will drive the field forward towards clinical applications for the targeted use of bacteria to enhance intestinal function and behavior.
Acknowledgments
The authors wish to thank Ms. Molly Hurd for proofing the manuscript.
Funding information
Preparation of this article was supported by a grant from the National Institutes of Health (NOA R21AT011203)
Footnotes
Conflict of interest statement No conflicts to report.
References
- 1.Mayer EA. Gut feelings: the emerging biology of gut–brain communication. Nature Reviews Neuroscience. 2011;12:453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cryan JF, O’Riordan KJ, Cowan CSM, et al. The Microbiota-Gut-Brain Axis. Physiol Rev. 2019;99(4):1877–2013. [DOI] [PubMed] [Google Scholar]
- 3.Hillman ET, Lu H, Yao T, Nakatsu CH. Microbial Ecology along the Gastrointestinal Tract. Microbes Environ. 2017;32(4):300–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vincent AD, Wang X-Y, Parsons SP, Khan WI, Huizinga JD. Abnormal absorptive colonic motor activity in germ-free mice is rectified by butyrate, an effect possibly mediated by mucosal serotonin. American Journal of Physiology-Gastrointestinal and Liver Physiology. 2018;315(5):G896–G907. [DOI] [PubMed] [Google Scholar]
- 5.Yano JM, Yu K, Donaldson GP, et al. Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis. Cell. 2015;161(2):264–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kashyap PC, Marcobal A, Ursell LK, et al. Complex interactions among diet, gastrointestinal transit, and gut microbiota in humanized mice. Gastroenterology. 2013;144(5):967–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gupta S, Allen-Vercoe E, Petrof EO. Fecal microbiota transplantation: in perspective. Therapeutic advances in gastroenterology. 2016;9(2):229–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ge X, Zhao W, Ding C, et al. Potential role of fecal microbiota from patients with slow transit constipation in the regulation of gastrointestinal motility. Scientific reports. 2017;7(1):441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De Palma G, Lynch MD, Lu J, et al. Transplantation of fecal microbiota from patients with irritable bowel syndrome alters gut function and behavior in recipient mice. Sci Transl Med. 2017;9(379). [DOI] [PubMed] [Google Scholar]
- 10.Mawe GM, Hoffman JM. Serotonin signalling in the gut—functions, dysfunctions and therapeutic targets. Nature Reviews Gastroenterology &Amp; Hepatology. 2013;10:473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.O’Mahony SM, Clarke G, Borre YE, Dinan TG, Cryan JF. Serotonin, tryptophan metabolism and the brain-gut-microbiome axis. Behav Brain Res. 2015;277:32–48. [DOI] [PubMed] [Google Scholar]
- 12.Gershon MD, Tack J. The serotonin signaling system: from basic understanding to drug development for functional GI disorders. Gastroenterology. 2007;132(1):397–414. [DOI] [PubMed] [Google Scholar]
- 13.Andresen V, Montori VM, Keller J, West CP, Layer P, Camilleri M. Effects of 5-hydroxytryptamine (serotonin) type 3 antagonists on symptom relief and constipation in nonconstipated irritable bowel syndrome: a systematic review and meta-analysis of randomized controlled trials. Clin Gastroenterol Hepatol. 2008;6(5):545–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hoffman JM, Tyler K, MacEachern SJ, et al. Activation of colonic mucosal 5-HT(4) receptors accelerates propulsive motility and inhibits visceral hypersensitivity. Gastroenterology. 2012;142(4):844–854.e844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mawe GM, Hoffman JM. Serotonin signalling in the gut--functions, dysfunctions and therapeutic targets. Nat Rev Gastroenterol Hepatol. 2013;10(8):473–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wikoff WR, Anfora AT, Liu J, et al. Metabolomics analysis reveals large effects of gut microflora on mammalian blood metabolites. Proc Natl Acad Sci U S A. 2009;106(10):3698–3703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sjögren K, Engdahl C, Henning P, et al. The gut microbiota regulates bone mass in mice. J Bone Miner Res. 2012;27(6):1357–1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reigstad CS, Salmonson CE, Rainey JF 3rd, et al. Gut microbes promote colonic serotonin production through an effect of short-chain fatty acids on enterochromaffin cells. FASEB J. 2015;29(4):1395–1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reigstad CS, Linden DR, Szurszewski JH, Sonnenburg JL, Farrugia G, Kashyap PC. Correlated gene expression encoding serotonin (5-HT) receptor 4 and 5-HT transporter in proximal colonic segments of mice across different colonization states and sexes. Neurogastroenterol Motil. 2016;28(9):1443–1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bhattarai Y, Schmidt BA, Linden DR, et al. Human-derived gut microbiota modulates colonic secretion in mice by regulating 5-HT(3) receptor expression via acetate production. American journal of physiology Gastrointestinal and liver physiology. 2017;313(1):G80–G87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ge X, Ding C, Zhao W, et al. Antibiotics-induced depletion of mice microbiota induces changes in host serotonin biosynthesis and intestinal motility. J Transl Med. 2017;15(1):13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vicentini FA, Keenan CM, Wallace LE, et al. Intestinal microbiota shapes gut physiology and regulates enteric neurons and glia. Microbiome. 2021;9(1):210–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Caputi V, Marsilio I, Filpa V, et al. Antibiotic-induced dysbiosis of the microbiota impairs gut neuromuscular function in juvenile mice. Br J Pharmacol. 2017;174(20):3623–3639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grasa L, Abecia L, Forcén R, et al. Antibiotic-Induced Depletion of Murine Microbiota Induces Mild Inflammation and Changes in Toll-Like Receptor Patterns and Intestinal Motility. Microbial Ecology. 2015;70(3):835–848. [DOI] [PubMed] [Google Scholar]
- 25.Shishov VA, Kirovskaya TA, Kudrin VS, Oleskin AV. Amine neuromediators, their precursors, and oxidation products in the culture of Escherichia coli K-12. Applied Biochemistry and Microbiology. 2009;45(5):494–497. [PubMed] [Google Scholar]
- 26.Roshchina VV. Evolutionary Considerations of Neurotransmitters in Microbial, Plant, and Animal Cells. In: Lyte M, Freestone PPE, eds. Microbial Endocrinology: Interkingdom Signaling in Infectious Disease and Health. New York, NY: Springer New York; 2010:17–52. [Google Scholar]
- 27.Williams BB, Van Benschoten AH, Cimermancic P, et al. Discovery and characterization of gut microbiota decarboxylases that can produce the neurotransmitter tryptamine. Cell host & microbe. 2014;16(4):495–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miller TL, Wolin MJ. Pathways of acetate, propionate, and butyrate formation by the human fecal microbial flora. Appl Environ Microbiol. 1996;62(5):1589–1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tan J, McKenzie C, Potamitis M, Thorburn AN, Mackay CR, Macia L. The role of short-chain fatty acids in health and disease. Adv Immunol. 2014;121:91–119. [DOI] [PubMed] [Google Scholar]
- 30.Lund ML, Egerod KL, Engelstoft MS, et al. Enterochromaffin 5-HT cells - A major target for GLP-1 and gut microbial metabolites. Mol Metab. 2018;11:70–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fukumoto S, Tatewaki M Fau - Yamada T, Yamada T Fau - Fujimiya M, et al. Short-chain fatty acids stimulate colonic transit via intraluminal 5-HT release in rats. (0363–6119 (Print)). [DOI] [PubMed]
- 32.Bosi A, Banfi D, Bistoletti M, Giaroni C, Baj A. Tryptophan Metabolites Along the Microbiota-Gut-Brain Axis: An Interkingdom Communication System Influencing the Gut in Health and Disease. International Journal of Tryptophan Research. 2020;13:1178646920928984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Richard DM, Dawes MA, Mathias CW, Acheson A, Hill-Kapturczak N, Dougherty DM. L-Tryptophan: Basic Metabolic Functions, Behavioral Research and Therapeutic Indications. Int J Tryptophan Res. 2009;2:45–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Keszthelyi D, Troost FJ, Masclee AA. Understanding the role of tryptophan and serotonin metabolism in gastrointestinal function. Neurogastroenterol Motil. 2009;21(12):1239–1249. [DOI] [PubMed] [Google Scholar]
- 35.Mariotta L, Ramadan T, Singer D, et al. T-type amino acid transporter TAT1 (Slc16a10) is essential for extracellular aromatic amino acid homeostasis control. J Physiol. 2012;590(24):6413–6424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Agus A, Planchais J, Sokol H. Gut Microbiota Regulation of Tryptophan Metabolism in Health and Disease. Cell Host Microbe. 2018;23(6):716–724. [DOI] [PubMed] [Google Scholar]
- 37.Gao J, Xu K, Liu H, et al. Impact of the Gut Microbiota on Intestinal Immunity Mediated by Tryptophan Metabolism. Front Cell Infect Microbiol. 2018;8:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wikoff WR, Anfora AT, Liu J, et al. Metabolomics analysis reveals large effects of gut microflora on mammalian blood metabolites. Proceedings of the National Academy of Sciences. 2009;106(10):3698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hung LY, Parathan P, Boonma P, et al. Antibiotic exposure postweaning disrupts the neurochemistry and function of enteric neurons mediating colonic motor activity. American journal of physiology Gastrointestinal and liver physiology. 2020;318(6):G1042–G1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ogawa Y, Miyoshi C, Obana N, et al. Gut microbiota depletion by chronic antibiotic treatment alters the sleep/wake architecture and sleep EEG power spectra in mice. Scientific Reports. 2020;10(1):19554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Takaki M, Mawe GM, Barasch JM, Gershon MD, Gershon MD. Physiological responses of guinea-pig myenteric neurons secondary to the release of endogenous serotonin by tryptamine. Neuroscience. 1985;16(1):223–240. [DOI] [PubMed] [Google Scholar]
- 42.Bhattarai Y, Williams BB, Battaglioli EJ, et al. Gut Microbiota-Produced Tryptamine Activates an Epithelial G-Protein-Coupled Receptor to Increase Colonic Secretion. Cell host & microbe. 2018;23(6):775–785.e775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Konen JR, Haag MM, Guseva D, et al. Prokinetic actions of luminally acting 5-HT4 receptor agonists. Neurogastroenterology & Motility. 2021;33(4):e14026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bhattarai Y, Jie S, Linden DR, et al. Bacterially Derived Tryptamine Increases Mucus Release by Activating a Host Receptor in a Mouse Model of Inflammatory Bowel Disease. iScience. 2020;23(12):101798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Marcobal A, Kashyap PC, Nelson TA, et al. A metabolomic view of how the human gut microbiota impacts the host metabolome using humanized and gnotobiotic mice. ISME J. 2013;7(10):1933–1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schaap FG, Trauner M, Jansen PLM. Bile acid receptors as targets for drug development. Nature Reviews Gastroenterology & Hepatology. 2014;11(1):55–67. [DOI] [PubMed] [Google Scholar]
- 47.Alemi F, Poole DP, Chiu J, et al. The Receptor TGR5 Mediates the Prokinetic Actions of Intestinal Bile Acids and Is Required for Normal Defecation in Mice. Gastroenterology. 2013;144(1):145–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li N, Koester ST, Lachance DM, Dutta M, Cui JY, Dey N. Microbiome-encoded bile acid metabolism modulates colonic transit times. iScience. 2021;24(6):102508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sayin Sama I, Wahlström A, Felin J, et al. Gut Microbiota Regulates Bile Acid Metabolism by Reducing the Levels of Tauro-beta-muricholic Acid, a Naturally Occurring FXR Antagonist. Cell Metabolism. 2013;17(2):225–235. [DOI] [PubMed] [Google Scholar]
- 50.Selwyn FP, Csanaky IL, Zhang Y, Klaassen CD. Importance of Large Intestine in Regulating Bile Acids and Glucagon-Like Peptide-1 in Germ-Free Mice. Drug Metab Dispos. 2015;43(10):1544–1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Golubeva AV, Joyce SA, Moloney G, et al. Microbiota-related Changes in Bile Acid & Tryptophan Metabolism are Associated with Gastrointestinal Dysfunction in a Mouse Model of Autism. EBioMedicine. 2017;24:166–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zareie M, Johnson-Henry K, Jury J, et al. Probiotics prevent bacterial translocation and improve intestinal barrier function in rats following chronic psychological stress. Gut. 2006;55(11):1553–1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fukui H, Oshima T, Tanaka Y, et al. Effect of probiotic Bifidobacterium bifidum G9–1 on the relationship between gut microbiota profile and stress sensitivity in maternally separated rats. Scientific reports. 2018;8(1):12384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gareau MG, Jury J, MacQueen G, Sherman PM, Perdue MH. Probiotic treatment of rat pups normalises corticosterone release and ameliorates colonic dysfunction induced by maternal separation. Gut. 2007;56(11):1522–1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dimidi E, Christodoulides S, Fragkos KC, Scott SM, Whelan K. The effect of probiotics on functional constipation in adults: a systematic review and meta-analysis of randomized controlled trials. Am J Clin Nutr. 2014;100(4):1075–1084. [DOI] [PubMed] [Google Scholar]
- 56.Marteau P, Cuillerier E, Meance S, et al. Bifidobacterium animalis strain DN-173 010 shortens the colonic transit time in healthy women: a double-blind, randomized, controlled study. Aliment Pharmacol Ther. 2002;16(3):587–593. [DOI] [PubMed] [Google Scholar]
- 57.Krammer HJ, von Seggern H, Schaumburg J, Neumer F. Effect of Lactobacillus casei Shirota on colonic transit time in patients with chronic constipation. coloproctology. 2011;33(2):109–113. [Google Scholar]
- 58.Nzakizwanayo J, Dedi C, Standen G, Macfarlane WM, Patel BA, Jones BV. Escherichia coli Nissle 1917 enhances bioavailability of serotonin in gut tissues through modulation of synthesis and clearance. Scientific Reports. 2015;5(1):17324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mandić AD, Woting A, Jaenicke T, et al. Clostridium ramosum regulates enterochromaffin cell development and serotonin release. Scientific Reports. 2019;9(1):1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Engevik MA, Luck B, Visuthranukul C, et al. Human-Derived Bifidobacterium dentium Modulates the Mammalian Serotonergic System and Gut-Brain Axis. Cell Mol Gastroenterol Hepatol. 2021;11(1):221–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cao YN, Feng LJ, Wang BM, et al. Lactobacillus acidophilus and Bifidobacterium longum supernatants upregulate the serotonin transporter expression in intestinal epithelial cells. Saudi J Gastroenterol. 2018;24(1):59–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cao Y-N, Feng L-J, Liu Y-Y, et al. Effect of Lactobacillus rhamnosus GG supernatant on serotonin transporter expression in rats with post-infectious irritable bowel syndrome. World J Gastroenterol. 2018;24(3):338–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wang YM, Ge XZ, Wang WQ, et al. Lactobacillus rhamnosus GG supernatant upregulates serotonin transporter expression in intestinal epithelial cells and mice intestinal tissues. Neurogastroenterol Motil. 2015;27(9):1239–1248. [DOI] [PubMed] [Google Scholar]
- 64.Engevik M, Ruan W, Visuthranukul C, et al. Limosilactobacillus reuteri ATCC 6475 metabolites upregulate the serotonin transporter in the intestinal epithelium. Beneficial Microbes. 2021;12(6):583–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Farzi A, Frohlich EE, Holzer P. Gut Microbiota and the Neuroendocrine System. Neurotherapeutics : the journal of the American Society for Experimental NeuroTherapeutics. 2018;15(1):5–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Martin CR, Osadchiy V, Kalani A, Mayer EA. The Brain-Gut-Microbiome Axis. Cellular and Molecular Gastroenterology and Hepatology. 2018;6(2):133–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mohammad-Zadeh LF, Moses L, Gwaltney-Brant SM. Serotonin: a review. Journal of Veterinary Pharmacology and Therapeutics. 2008;31(3):187–199. [DOI] [PubMed] [Google Scholar]
- 68.Walther DJ, Peter J-U, Bashammakh S, et al. Synthesis of Serotonin by a Second Tryptophan Hydroxylase Isoform. Science. 2003;299(5603):76. [DOI] [PubMed] [Google Scholar]
- 69.Uphouse L Multiple Serotonin Receptors: Too Many, Not Enough, or Just the Right Number? Neuroscience & Biobehavioral Reviews. 1997;21(5):679–698. [DOI] [PubMed] [Google Scholar]
- 70.Cerrito F, Raiteri M. Serotonin release is modulated by presynaptic autoreceptors. European journal of pharmacology. 1979;57(4):427–430. [DOI] [PubMed] [Google Scholar]
- 71.Kandel ER, Schwartz JH, Jessell TM, Siegelbaum SA, Hudspeth AJ. Principles of Neural Science, Fifth Edition. McGraw-Hill Education; 2012. [Google Scholar]
- 72.Lowry CA, Johnson PL, Hay-Schmidt A, Mikkelsen J, Shekhar A. Modulation of anxiety circuits by serotonergic systems. Stress (Amsterdam, Netherlands). 2005;8(4):233–246. [DOI] [PubMed] [Google Scholar]
- 73.Simansky KJ. Serotonergic control of the organization of feeding and satiety. Behav Brain Res. 1996;73(1–2):37–42. [DOI] [PubMed] [Google Scholar]
- 74.Li Y, Zhong W, Wang D, et al. Serotonin neurons in the dorsal raphe nucleus encode reward signals. Nature Communications. 2016;7:10503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Muraki Y, Yamanaka A, Tsujino N, Kilduff TS, Goto K, Sakurai T. Serotonergic regulation of the orexin/hypocretin neurons through the 5-HT1A receptor. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2004;24(32):7159–7166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chowdhury S, Yamanaka A. Optogenetic activation of serotonergic terminals facilitates GABAergic inhibitory input to orexin/hypocretin neurons. Scientific reports. 2016;6:36039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Liu L, Zhu G. Gut-Brain Axis and Mood Disorder. Frontiers in psychiatry. 2018;9:223–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Carlsson A, Lindqvist M. Dependence of 5-HT and catecholamine synthesis on concentrations of precursor amino-acids in rat brain. Naunyn-Schmiedeberg’s Archives of Pharmacology. 1978;303(2):157–164. [DOI] [PubMed] [Google Scholar]
- 79.Moir AT, Eccleston D. The effects of precursor loading in the cerebral metabolism of 5-hydroxyindoles. Journal of neurochemistry. 1968;15(10):1093–1108. [DOI] [PubMed] [Google Scholar]
- 80.Fernstrom JD, Wurtman RJ. Brain Serotonin Content: Physiological Regulation by Plasma Neutral Amino Acids. Science. 1972;178(4059):414. [DOI] [PubMed] [Google Scholar]
- 81.Fernstrom JD, Fernstrom MH, Grubb PE, Volk EA. Absence of chronic effects of dietary protein content on brain tryptophan concentrations in rats. J Nutr. 1985;115(10):1337–1344. [DOI] [PubMed] [Google Scholar]
- 82.Caballero B, Finer N, Wurtman RJ. Plasma amino acids and insulin levels in obesity: response to carbohydrate intake and tryptophan supplements. Metabolism. 1988;37(7):672–676. [DOI] [PubMed] [Google Scholar]
- 83.Fernstrom JD. Large neutral amino acids: dietary effects on brain neurochemistry and function. Amino acids. 2013;45(3):419–430. [DOI] [PubMed] [Google Scholar]
- 84.Sharp T, Bramwell SR, Grahame-Smith DG. Effect of acute administration of L-tryptophan on the release of 5-HT in rat hippocampus in relation to serotoninergic neuronal activity: An invivo microdialysis study. Life Sciences. 1992;50(17):1215–1223. [DOI] [PubMed] [Google Scholar]
- 85.Biskup CS, Sánchez CL, Arrant A, Van Swearingen AED, Kuhn C, Zepf FD. Effects of Acute Tryptophan Depletion on Brain Serotonin Function and Concentrations of Dopamine and Norepinephrine in C57BL/6J and BALB/cJ Mice. PLOS ONE. 2012;7(5):e35916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Fadda F Tryptophan-Free Diets: A Physiological Tool to Study Brain Serotonin Function. Physiology. 2000;15(5):260–264. [DOI] [PubMed] [Google Scholar]
- 87.Gessa GL, Biggio G, Fadda F, Corsini GU, Tagliamonte A. Effect of the oral administration of tryptophan-free amino acid mixtures on serum tryptophan, brain tryptophan and serotonin metabolism. Journal of neurochemistry. 1974;22(5):869–870. [DOI] [PubMed] [Google Scholar]
- 88.Nishizawa S, Benkelfat C, Young SN, et al. Differences between males and females in rates of serotonin synthesis in human brain. Proceedings of the National Academy of Sciences. 1997;94(10):5308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Silber BY, Schmitt JAJ. Effects of tryptophan loading on human cognition, mood, and sleep. Neuroscience & Biobehavioral Reviews. 2010;34(3):387–407. [DOI] [PubMed] [Google Scholar]
- 90.Jans LA, Korte-Bouws GA, Korte SM, Blokland A. The effects of acute tryptophan depletion on affective behaviour and cognition in Brown Norway and Sprague Dawley rats. J Psychopharmacol. 2010;24(4):605–614. [DOI] [PubMed] [Google Scholar]


