Abstract
Background
The Coronavirus 19 pandemic has raised new relevant questions regarding the management of patients with multiple sclerosis (pwMS) treated with different immunosuppressive and immunomodulant drugs. In most COVID-19 outcomes analyses, due to the small available sample size, patients treated with cladribine were grouped with patients treated with other treatments.
Methods
Three major databases (PubMed, Scopus and Web of Science) and the most recent MS congress libraries were searched for extracting original articles on COVID-19 and multiple sclerosis. The key inclusion criteria were the presence of data on pwMS treated with cladribine and with documented positivity for COVID-19. The quality of the included studies was evaluated using a modified version of the Dutch Cochrane center critical review checklist proposed by MOOSE. A common-effect meta-analysis was used for estimating the pooled proportion of patients with severe events (hospitalizations, pneumonia, ICU admissions and deaths) and heterogeneity was assessed by the I2 statistic.
Results
13 articles were included in the analysis and the median quality of the articles reached a level of 4. The selected studies included 5138 patients with COVID-19, of whom 107 (2.1%) were treated with cladribine. Pooled estimates of hospitalization and death were 9.36% and 0% for patients treated with cladribine, 14.98% and 2.66% for pwMS under other treatments.
Conclusion
These results indicate that pwMS treated with cladribine are not at a greater risk of developing a severe form of COVID-19.
Registration
The protocol was registered with the International Prospective Register of Systematic Reviews (PROSPERO: CRD42022329464),
Keywords: Multiple sclerosis, COVID-19 severity, Cladribine, Disease modifying treatments
1. Introduction
The coronavirus disease 2019 (COVID-19), due to its significant and unpredictable long-lasting impact on public health, has raised relevant questions regarding the infection risk in vulnerable patients and the management of subjects treated with immunotherapies (Iaffaldano et al., 2022). In fact, immunosuppressive conditions, whether it is induced by immunological disorders or by medications, may alter immunocompetence modifying the ability to mount an effective immune response to infection and/or vaccination (Agrati et al., 2021).
Multiple sclerosis (MS), a chronic demyelinating disorder of the central nervous system (CNS), involves immune response abnormalities and is often treated with disease modifying therapies (DMTs), causing different degrees of systemic immunosuppression. For these reasons, patients with MS (pwMS) might be exposed to an increased risk of COVID-19 severe complications (Capasso et al., 2020; Sharifian-Dorche et al., 2021) and so they are considered as fragile subjects. Taking into account the infection contagiousness and the hospital promiscuity during the first wave of COVID-19 pandemic, also the disease management, from therapeutic strategies to scheduling routine clinical follow-up and rehabilitation plans, has undergone to a considerable change (Sharifian-Dorche et al., 2021).
At the beginning of COVID-19 spread, different empirical recommendations were developed by scientific societies for assisting the neurologists in the management of pwMS and indicating either to delay or to continue treatments, depending on their mechanisms of action, the impact, and the effects duration on the immune-system (Laroni et al., 2021). These national and international guidelines were initially more precautionary, subsequently the first evidence became available allowing a better evaluation of the DMTs safety profile and an update of the different scientific societies’ positions.
So far, different evidence has been published regarding the evaluation of the impact of DMTs on the severity of COVID-19 in pwMS. They are largely reassuring but they have also suggested that MS therapies may be related to a different severity of COVID-19 infection (Laroni et al., 2021; Sormani et al., 2021; Salter et al., 2021; Simpson-Yap et al., 2021) and have consistently correlated risk factors as male sex, an older age, the presence of comorbidities, and higher disability to a more severe disease outcome (Sormani et al., 2021; Salter et al., 2021; Louapre et al., 2020).
Cladribine tablets, counted among immune cell depleting medications, is a purine chlorinated analog of deoxyadenosine that acts through the transient depletion of lymphocytes followed by immune reconstitution phase. As a semi-selective therapy, its effects are more pronounced on the adaptive immune response, impacting most specific B subsets than T cells, while its impact on the innate immune system is limited (Amor et al., 2020; Zheng et al., 2020).
In most COVID-19 outcomes analysis, due to the small available sample size, patients treated with cladribine were grouped with patients treated with other treatments, often in the “other DMT” group (Sormani et al., 2021; Salter et al., 2021; Louapre et al.; 2020; Dalla Costa et al., 2020; Mantero et al., 2020), and therefore it is not possible to derive results specifically related to patients treated with cladribine. .
We conducted here a meta-analysis for estimating the severity of COVID-19 events, evaluated as mortality and hospitalization rate, among patients treated with cladribine. The methodology is the same used in another review referring to anti-CD20 drugs category (Schiavetti et al., 2022).
2. Methods
2.1. Article selection
A comprehensive online search of the literature to detect the most relevant studies published from 2020 to March 2022 was performed in Scopus, Web of Science, PubMed and among the abstracts presented at the 2020 and 2021 ECTRIMS meeting and the most relevant congresses held before March 2022.
The search strategy consisted of a combination of terms and keywords as follows: for PubMed Database: (MS) OR (Multiple Sclerosis) AND (COVID-19 OR Coronavirus OR SARS-CoV-2 OR Covid) AND (cladribine); for Scopus Database: (TITLE-ABS-KEY (coronavirus OR covid OR COVID-19 OR sars-cov-2) AND TITLE-ABS-KEY (cladribine) AND TITLE-ABS-KEY (ms OR multiple AND sclerosis)); for Web of Science: MS or multiple sclerosis (All Fields) and cladribine (All Fields) and Covid or coronavirus or COVID-19 or SARS-CoV-2 (All Fields).
The systematic review included articles reporting data on COVID-19 course for pwMS treated with cladribine. However, studies were excluded if they were out of topic, systematic reviews and duplicated manuscripts among the sources, with overlapping patients.
Eligibility assessment of the first screening was performed independently by two reviewers (AA and IS), based on title and abstract. The second examination was done on full text with the inclusion and exclusion criteria reported above by both reviewers (AA and IS), independently. Disagreements between the reviewers were resolved by double-checking and discussion to achieve a consensus.
The following data were extracted from the identified studies: authors, title, country, sample size, number of males/females, mean age with range, number of patients with progressive MS, number of patients with relapsing MS, median last EDSS, mean MS duration with range, most recent MS treatments, number of hospitalizations (overall and for pwMS treated with Cladribine), number of patients admitted to the ICU, number of patients with pneumonia, number of deaths (overall and for pwMS treated with Cladribine). In case of missing data, an email was sent to the corresponding authors asking to complete the lacking information.
The review protocol was registered with PROSPERO (registration number: CRD42022329464).
2.2. Quality assessment
The quality of selected articles was evaluated by two authors according to a modified version of the Dutch Cochrane center critical review checklist proposed by MOOSE (Stroup, 2000). The key MOOSE domains include: (I) Clear definition of study population; (II) Clear definition of outcomes and outcomes assessment; (III) Independent assessment of outcome parameters; (IV) Sufficient follow-up; (V) No selective loss during follow-up; and (VI) Important confounders and prognostic factors identified. Each domain, based on published data, could be completed, and rated as follows: yes (1 point), no/unclear (0 points). However, since two domains (IV and V) were considered irrelevant for the purpose of this analysis, only four (I, II, III, VI) were combined in an overall reporting quality score (ranging from 0 to 4 points). A study was defined of highest quality if all criteria were rated as “yes” because free from intra-study bias.
2.3. Outcomes
The outcomes selected for the evaluation of Covid-severity among all pwMS with confirmed positivity were: the number of hospitalization, the presence of pneumonia, the number of ICU admissions and cases of deaths. For the subgroup of patients in treatment with cladribine, only number of hospitalization and deaths were considered as clinical outcomes.
2.4. Statistical analysis
A pooled estimate of the proportion of hospitalizations, and deaths among pwMS was obtained by a common-effect meta-analysis with logit transformation. The I2 statistic (the ratio of between-study variance to the observed variance) was computed for quantifying heterogeneity.
Case reports were excluded from the meta-analysis to avoid overestimating the rate of severe outcomes (case reports usually refer to severe cases). Meta-analysis was performed using R software, version 4.1.3 (packages “metafor” and “meta”).
3. Results
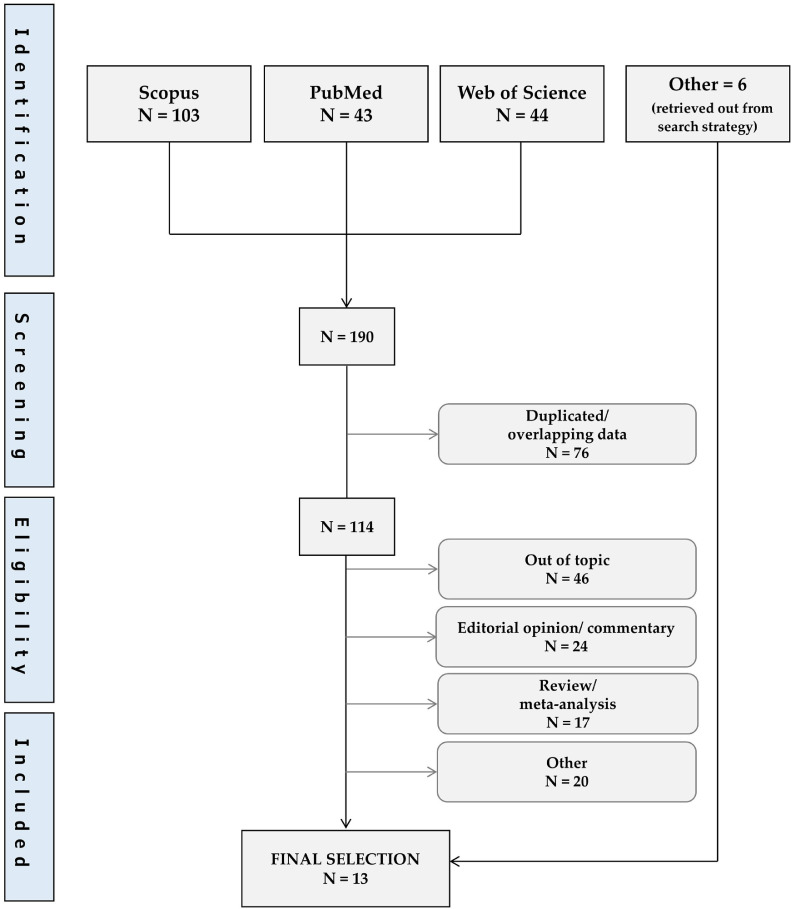
The first literature search revealed a total of 190 publications, 76 were excluded for potentially duplicated or overlapping data. Of the remaining 114, 7 were selected for the final analysis, along with 6 retrieved out from search strategy (Fig. 1 ). References of 13 selected manuscripts (Sormani et al., 2021; Salter et al., 2021; Louapre et al., 2020; Hervas-Garcia et al., 2020; Czarnowska et al., 2021; Arrambide et al., 2021; Spelman et al., 2022; Drulovic et al., 2021; Fragoso et al., 2021; Bsteh et al., 2021; Pérez et al., 2021; Clinical Outcomes, 2022) are shown in Table 1 .
Fig. 1.
Flow diagram for study selection.
Table 1.
List of included studies.
| Nr | First author | Title | Journal | Year |
|---|---|---|---|---|
| 01 | Louapre C. | Clinical Characteristics and Outcomes in Patients with Coronavirus Disease 2019 and Multiple Sclerosis | JAMA Neurol. | Jul,2020 |
|
02 |
Oreja-Guevara C. | COVID-19 in cladribine-treated patients with Multiple Sclerosis | 8th Joint ACTRIMS-ECTRIMS Meeting | Sep,2020 |
| 03 | Hervás-García J. V. | Seroprevalence of SARS-CoV-2 in multiple sclerosis patients under immunomodulatory treatment in lleida (study emCOVID-19) | Mult Scler. | Dec,2020 |
| 04 | Salter A. | Outcomes and Risk Factors Associated With SARS-CoV-2 Infection in a North American Registry of Patients with Multiple Sclerosis | JAMA Neurol. | Mar,2021 |
| 05 | Czarnowska A. | Clinical course and outcome of SARS-CoV-2 infection in multiple sclerosis patients treated with disease-modifying therapies - the Polish experience | Neurol Neurochir Pol. | Apr,2021 |
| 06 | Sormani M. P. | Disease-Modifying Therapies and Coronavirus Disease 2019 Severity in Multiple Sclerosis | Annals of Neurology | Jan,2021 |
| 07 | Arrambide G. | SARS-CoV-2 Infection in Multiple Sclerosis: Results of the Spanish Neurology Society Registry | Neurol Neuroimmunol Neuroinflamm. | Jun,2021 |
|
08 |
Spelman T. | Increased rate of hospitalization for COVID-19 among rituximab-treated multiple sclerosis patients: A study of the Swedish multiple sclerosis registry | Mult Scler. | Jul,2021 |
| 09 | Drulovic J. | Humoral response to SARS-CoV-2 COVID-19 vaccines in patients with multiple sclerosis treated with immune reconstitution therapies | Mult Scler Relat Disord. | Jul,2021 |
| 10 | Fragoso Y. D | Coronavirus disease 2019 in Latin American patients with multiple sclerosis | Mult Scler Relat Disord. | Jul,2021 |
| 11 | Bsteh G. | COVID-19 severity and mortality in multiple sclerosis are not associated with immunotherapy: Insights from a nation-wide Austrian registry | Plos one | Jul,2021 |
| 12 | Perez A. C. | COVID-19 severity and outcome in multiple sclerosis: Results of a national, registry-based, matched cohort study | Mult Scler Relat Disord. | Aug,2021 |
| 13 | Yavorskaya V. | Clinical Outcomes in Patients With COVID-19 During Two Phase IV Studies of Cladribine Tablets for Treatment of Multiple Sclerosis: An Update |
AAN 2022 | Apr,2022 |
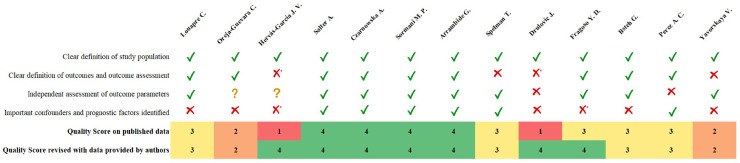
The median quality score of included studies was 3.0 (range 1–4) but considering implemented details provided by authors the value improved to 4.0 (range: 2–4) (Fig. 2 ).
Fig. 2.
Quality score_MOOSE criteria.
These 13 selected studies include 5138 adult pwMS with COVID-19 infections, aged between 17 and 82 years, females for the 72%, with a disease duration ranging from 0 to 40 years and with relapsing remitting form of the disease for about 82%. Out of 5138 pwMS, 107 patients (2.1%) were in treatment with cladribine. The baseline characteristics are reported in Table 2 .
Table 2.
Baseline characteristics.
| Nr | First Author |
Demography |
MS data |
Disease Modifying Treatments |
||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sample | Females/Males | Age, yrs (range) | Progressive/RR | Last EDSS | MS duration, yrs(range) | DMT unknown | Untreated | Cladribine | Alemtuzumab | Azathioprine | Glatiramer Acetate | Dimethyl fumarate | Fingolimod | Interferon beta | Methotrexate | Mitoxantrone | Natalizumab | Ocrelizumab | Rituximab | Teriflunomide | Ozanimod | Other | ||
| 01 | Louapre C. | 347 | 249/98 | 31.8-57.4 | 65/282 | 2.0 | 3.5-23.5 | 63 | 3 | 1 | 0 | 33 | 35 | 42 | 20 | 1 | 0 | 57 | 38 | 17 | 33 | 0 | 4 | |
| 02 | Oreja-Guevara C. | 14 | 9/5 | 28.1-52.1 | 1/13 | 1.0 | 0.8-18.6 | 0 | 0 | 14 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 03 | Hervás-García J. V. | 19 | 12/7 | 41-67 | 1/18 | 2.0 | 3-24 | 0 | 0 | 1 | 1 | 0 | 0 | 5 | 2 | 3 | 0 | 0 | 2 | 3 | 2 | 0 | 0 | 0 |
| 04 | Salter A. | 1626 | 1202/421 | 34.5-60.9 | 280/1275 | 3.2-23 | 237 | 14 | 9 | 84 | 208 | 106 | 53 | 2 | 0 | 170 | 484 | 77 | 82 | 1 | 41 | |||
| 05 | Czarnowska A. | 396 | 282/114 | 18-68 | 24/372 | 2.0 | 0-33 | 0 | 5 | 1 | 0 | 42 | 164 | 16 | 82 | 0 | 3 | 35 | 20 | 0 | 25 | 3 | 0 | |
| 06 | Sormani M. P. | 844 | 593/251 | 18-82 | 135/676 | 2.0 | 4.7-17.1 | 151 | 11 | 14 | 10 | 70 | 174 | 94 | 73 | 1 | 1 | 85 | 89 | 5 | 64 | 0 | 2 | |
| 07 | Arrambide G. | 326 | 221/105 | 33.3-56.3 | 63/263 | 2.0 | 3-19 | 59 | 6 | 18 | 0 | 13 | 41 | 27 | 36 | 0 | 0 | 26 | 23 | 33 | 37 | 7 | ||
| 08 | Spelman T. | 476 | 340/136 | 19–78 | 67/407 | 2.0 | 0.0–40.7 | 18 | 8 | 5 | 9 | 48 | 20 | 18 | 57 | 7 | 262 | 11 | 13 | |||||
| 09 | Drulovic J. | 18 | 13/5 | 33-51.1 | 0/18 | 3.0 | 6.6-22.2 | 0 | 11(+7)* | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 10 | Fragoso Y. D. | 73 | 50/23 | 17-72 | 4/69 | 2.0 | 0-26 | 0 | 3 | 1 | 1 | 0 | 10 | 15 | 14 | 10 | 0 | 0 | 6 | 5 | 3 | 5 | 0 | 0 |
| 11 | Bsteh G. | 126 | 90/36 | 21-79 | 28/98 | 2.0 | 8 | 37 | 2 | 2 | 1 | 11 | 19 | 16 | 6 | 0 | 0 | 10 | 12 | 2 | 0 | 0 | ||
| 12 | Perez A. C. | 843 | 616/227 | 39.8-70.6 | 511 | 51 | 1 | 1 | 55 | 45 | 14 | 26 | 18 | 72 | 17 | 24 | 1 | 7 | ||||||
| 13 | Yavorskaya V. | 30 | 25/5 | 19-55 | 0 | 0 | 30 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |||
Out of 18 patients in treatment with Cladribine, 11 developed COVID-19 infection. In some manuscripts DMTs and/or MS type are not available for all individuals
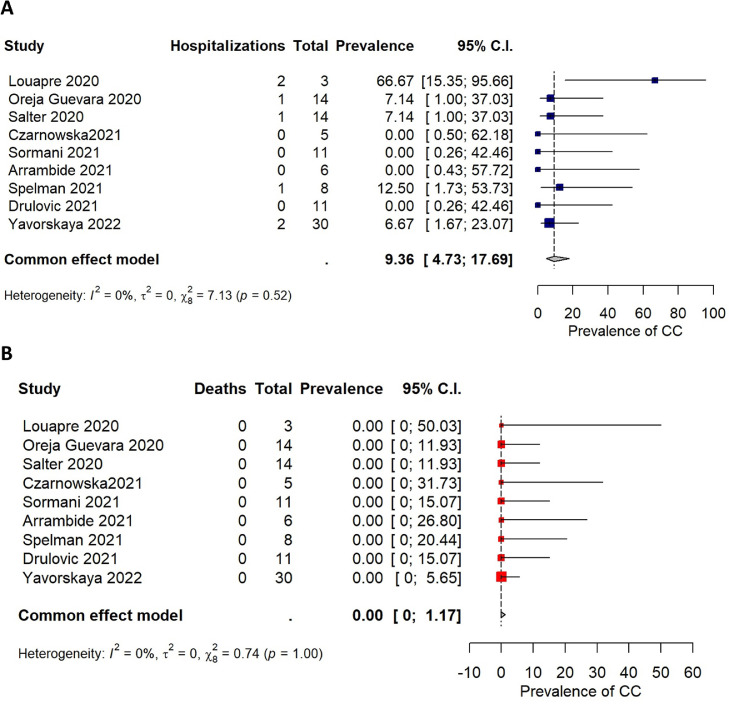
Findings from COVID-19 severity outcomes and results from the meta-analysis are reported in Table 3 . A total of 1029 out of the 5138 included patients were hospitalized (pooled estimate: 14.98%; 95%CI = [10.47%; 20.97%]), and 157 died (pooled estimate: 2.66%; 95%CI = [1.79%; 3.92%]). Among the 107 patients treated with cladribine and reporting COVID-19 severity outcomes, 7 were hospitalized (pooled estimate: 9.36%; 95%CI = [4.73%; 17.69%]) (Fig. 3 A) and no deaths were reported (pooled estimate: 0.00%; 95%CI = [0.00%; 1.17%]) (Fig. 3B).
Table 3.
COVID-19 severity outcomes.
|
OVERALL |
CLADRIBINE |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Nr | Author | N | Hospitalization | Pneumonia | ICU admission | Death | N | Hospitalization | Death |
| 01 | Louapre C. | 347 | 73 (21.0%) | - | 4 (1.2%) | 12 (3.5%) | 3 | 2 (66.7%) | 0 (0.0%) |
| 02 | Oreja-Guevara C. | 14 | 1 (7.1%) | 1 (7.1%) | 0 (0.0%) | 0 (0.0%) | 14 | 1 (7.1%) | 0 (0.0%) |
| 03 | Hervás-García J. V. | 19 | 1 (5.3%) | 1 (5.3%) | 0 (0.0%) | 0 (0.0%) | 1^ | 0 (0.0%) | 0 (0.0%) |
| 04 | Salter A. | 1626 | 320 (19.7%) | 112 (6.9%) | 104 (6.4%) | 54 (3.3%) | 14 | 1 (7.1%) | 0 (0.0%) |
| 05 | Czarnowska A. | 396 | 27 (6.8%) | 1 (0.3%) | - | 1 (0.3%) | 5 | 0 (0.0%) | 0 (0.0%) |
| 06 | Sormani M. P. | 844 | 96 (11.4%) | 99 (11.7%) | 38 (4.5%) | 13 (1.5%) | 11 | 0 (0.0%) | 0 (0.0%) |
| 07 | Arrambide G. | 326 | 69 (21.2%) | - | 7 (2.1%) | 7 (2.1%) | 6 | 0 (0.0%) | 0 (0.0%) |
| 08 | Spelman T. | 476 | 73 (15.3%) | - | 19 (4.0%) | 8 (1.7%) | 8 | 1 (12.5%) | 0 (0.0%) |
|
09 |
Drulovic J. | 18 | 0 (0.0%) | 5 (27.8%) | 0 (0.0%) | 0 (0.0%) | 11 | 0 (0.0%) | 0 (0.0%) |
| 10 | Fragoso Y. D. | 73 | 15 (20.5%) | 20 (27.4%) | 6 (8.2%) | 2 (2.7%) | 1^ | 0 (0.0%) | 0 (0.0%) |
| 11 | Bsteh G. | 126 | 12 (9.5%) | - | - | 4 (3.2%) | 2^ | 0 (0.0%) | 0 (0.0%) |
| 12 | Perez A. C. | 843 | 340 (40.3%) | - | 87 (10.3%) | 56 (6.6%) | 1^ | 0 (0.0%) | 0 (0.0%) |
| 13 | Yavorskaya V. | 30 | 2 (6.7%) | - | - | 0 (0.0%) | 30 | 2 (6.7%) | 0 (0.0%) |
| POOLED ESTIMATE | |||||||||
| Proportion [95% IC] | 14.98 [10.47 – 20.97] | 8.40 [2.90 – 21.92] | 4.44 [2.76 – 7.06] | 2.66 [1.79 – 3.92] | 9.36 [4.73 – 17.69] | 0.00 [0.00 – 1.17] | |||
| I^2 [95% IC] | 95.48 [89–23 – 98.70] | 97.50 [91.71 – 99.64] | 88.61 [62.82 – 96.45] | 71.23 [30.81 – 90.50] | 0.00 [0.00 – 72.22] | 0.00 | |||
Case reports excluded from the meta-analysis
Fig. 3.
Forest plots cladribine.
4. Discussion
The hospitalization rate of the overall MS population, as reported in the studies included in this analysis, was between 0% and 40% and the death rate was between 0% and 7%. Actually, the range mentioned above would include much closer values (respectively 0% - 21% and 0% - 4%) if the incidence reported from Perez et al. is not considered (hospitalization rate 340/843, 40%; death rate 56/843, 7%). In this study, that is a data collection using an administrative claims-based method (Pérez et al., 2021) several limitations were reported, including the risk of possible misclassification, underreporting and/or inconsistent ICD coding practices being.
The pooled estimate of the hospitalization rate was 14.98% (high heterogeneity: 95.4%), lower than the rate reported in the systematic review on COVID-19 in pwMS from Barzegar et al. (2021) (20.7%). The collection of data from different countries, often referred to different periods of pandemic and based on different study designs, could explain these results. However, the hospitalization rate of the general population is ranging from 2.9 to 30% of all COVID-19 cases (Coronavirus Disease, 2020) and that of pwMS falls in this interval. The demographic characteristics of pwMS are generally younger with more females than the general population, which should automatically put this cohort of patients at lower risks of hospitalization (Barzegar et al., 2021).
In this analysis only 157 out of 5138 (3.1%) patients presented a fatal event, resulting in a pooled estimate of 2.66 (95%CI: 1.79-3.92), with a high heterogeneity among studies (I2= 71.2%), that is a number in line with previous studies (Barzegar et al., 2021). No deaths (out of 107 subjects) were observed in the group of patients treated with cladribine and even if the size of this group is small, this observation indicates that there is no evidence of an increased risk of severe COVID-19 in pwMS receiving cladribine.
Declaration of Competing Interest
This scientific collaborative research project research thesis has been funded by Merck Serono S.p.A., Rome, Italy, an affiliate of Merck KGaA (CrossRef Funder ID: 10.13039/100009945). AA is an employee of Merck Serono S.p.A., Rome, Italy, an affiliate of Merck KGaA, Darmstadt, Germany.
Acknowledgments
We really thank authors which have provided some additional data of their samples or have confirmed some unclear measures.
References
- Iaffaldano P., Lucisano G., Manni A., Paolicelli D., Patti F., Capobianco M., Brescia Morra V., Sola P., Pesci I., Lus G., De Luca G., Lugaresi A., Cavalla P., Montepietra S., Maniscalco G.T., Granella F., Ragonese P., Vianello M., Brambilla L., Trojano M. Risk of getting COVID-19 in people with multiple sclerosis: a case-control study. Neurol. Neuroimmunol. Neuroinflamm. 2022;9(2):e1141. doi: 10.1212/NXI.0000000000001141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agrati C., Di Cosimo S., Fenoglio D., Apolone G., Ciceri F., Ciliberto G., Baldanti F., Costantini M., Giannarelli D., Ippolito G., Locatelli F., Mantovani A., Morrone A., Tagliavini F., Uccelli A., Zinzani P.L., Silvestris N., Rescigno M. COVID-19 vaccination in fragile patients: current evidence and an harmonized transdisease trial. Front. Immunol. 2021;12 doi: 10.3389/fimmu.2021.704110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capasso N., Palladino R., Montella E., Pennino F., Lanzillo R., Carotenuto A., Petracca M., Iodice R., Iovino A., Aruta F., Pastore V., Buonomo A.R., Zappulo E., Gentile I., Triassi M., Brescia Morra V., Moccia M. Prevalence of SARS-CoV-2 antibodies in multiple sclerosis: the hidden part of the iceberg. J. Clin. Med. 2020;9(12):4066. doi: 10.3390/jcm9124066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharifian-Dorche M., Sahraian M.A., Fadda G., Osherov M., Sharifian-Dorche A., Karaminia M., Saveriano A.W., La Piana R., Antel J.P., Giacomini P.S. COVID-19 and disease-modifying therapies in patients with demyelinating diseases of the central nervous system: a systematic review. Mult. Scler. Relat. Disord. 2021;50 doi: 10.1016/j.msard.2021.102800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laroni A., Schiavetti I., Sormani M.P., Uccelli A. COVID-19 in patients with multiple sclerosis undergoing disease-modifying treatments. Mult. Scler. 2021;27(14):2126–2136. doi: 10.1177/1352458520971817. [DOI] [PubMed] [Google Scholar]
- Sormani M.P., De Rossi N., Schiavetti I., Carmisciano L., Cordioli C., Moiola L., Radaelli M., Immovilli P., Capobianco M., Trojano M., Zaratin P., Tedeschi G., Comi G., Battaglia M.A., Patti F., Salvetti M., the Musc-19 Study Group. Nozzolillo A., Bellacosa A., Mantero V. Disease-modifying therapies and coronavirus disease 2019 severity in multiple sclerosis. Ann. Neurol. 2021;89(4):780–789. doi: 10.1002/ana.26028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salter A., Fox R.J., Newsome S.D., Halper J., Li D.K.B., Kanellis P., Costello K., Bebo B., Rammohan K., Cutter G.R., Cross A.H. Outcomes and risk factors associated with SARS-CoV-2 infection in a north american registry of patients with multiple sclerosis. JAMA Neurol. 2021;78(6):699. doi: 10.1001/jamaneurol.2021.0688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson-Yap S., De Brouwer E., Kalincik T., Rijke N., Hillert J.A., Walton C., Edan G., Moreau Y., Spelman T., Geys L., Parciak T., Gautrais C., Lazovski N., Pirmani A., Ardeshirdavanai A., Forsberg L., Glaser A., McBurney R., Schmidt H., Bergmann A.B., Braune S., Stahmann A., Middleton R., Salter A., Fox R.J., van der Walt A., Butzkueven H., Alroughani R., Ozakbas S., Rojas J.I., van der Mei I., Nag N., Ivanov R., Sciascia do Olival G., Dias A.E., Magyari M., Brum D., Mendes M.F., Alonso R.N., Nicholas R.S., Bauer J., Chertcoff A.S., Zabalza A., Arrambide G., Fidao A., Comi G., Peeters L. Associations of disease-modifying therapies with COVID-19 severity in multiple sclerosis. Neurology. 2021;97(19):e1870–e1885. doi: 10.1212/WNL.0000000000012753. Nov 9Epub 2021 Oct 5. PMID: 34610987; PMCID: PMC8601210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louapre C., Collongues N., Stankoff B., Giannesini C., Papeix C., Bensa C., Deschamps R., Créange A., Wahab A., Pelletier J., Heinzlef O., Labauge P., Guilloton L., Ahle G., Goudot M., Bigaut K., Laplaud D.-A., Vukusic S., Lubetzki C. Clinical characteristics and outcomes in patients with coronavirus disease 2019 and multiple sclerosis. JAMA Neurol. 2020;77(9):1079. doi: 10.1001/jamaneurol.2020.2581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amor S., Baker D., Khoury S.J., Schmierer K., Giovanonni G. SARS-COV -2 and multiple sclerosis: not all immune depleting DMTS are equal or bad. Ann. Neurol. 2020;87(6):794–797. doi: 10.1002/ana.25770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng C., Kar I., Chen C.K., Sau C., Woodson S., Serra A., Abboud H. Multiple sclerosis disease-modifying therapy and the COVID-19 pandemic: implications on the risk of infection and future vaccination. CNS Drugs. 2020;34(9):879–896. doi: 10.1007/s40263-020-00756-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalla Costa G., Leocani L., Montalban X., Guerrero A.I., Sørensen P.S., Magyari M., Dobson R.J.B., Cummins N., Narayan V.A., Hotopf M., Comi G. Real-time assessment of COVID-19 prevalence among multiple sclerosis patients: a multicenter European study. Neurol. Sci. 2020;41(7):1647–1650. doi: 10.1007/s10072-020-04519-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantero V., Abate L., Balgera R., Basilico P., Salmaggi A., Cordano C. Assessing the susceptibility to acute respiratory illness COVID-19-related in a cohort of multiple sclerosis patients. Mult. Scler. Relat. Disord. 2020;46 doi: 10.1016/j.msard.2020.102453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiavetti I., Ponzano M., Signori A., Bovis F., Carmisciano L., Sormani M.P. Severe outcomes of COVID-19 among patients with multiple sclerosis under anti-CD-20 therapies: a systematic review and meta-analysis. Mult. Scler. Relat. Disord. 2022;57 doi: 10.1016/j.msard.2021.103358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroup D.F. Meta-analysis of observational studies in epidemiologya proposal for reporting. JAMA. 2000;283(15):2008. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- Hervas-Garcia J.V., Gil-Sanchez A., Gonzalez-Mingot C., Nogueras L., Quibus L., Sancho A., Quirant B., Peralta S., Solana M.J., Ramo C. Seroprevalence of SARS-CoV-2 in multiple sclerosis patients under immunomodulatory treatment in lleida (study em COVID-19) Mult. Scler. 2020:59. [Google Scholar]
- Czarnowska A., Brola W., Zajkowska O., Rusek S., Adamczyk-Sowa M., Kubicka-Bączyk K., Kalinowska-Łyszczarz A., Kania K., Słowik A., Wnuk M., Marona M., Podlecka-Piętowska A., Nojszewska M., Zakrzewska-Pniewska B., Jasińska E., Gołuch K., Lech B., Noga M., Perenc A., Popiel M., Lasek-Bal A., Puz P., Maciejowska K., Kucharska-Lipowska M., Lipowski M., Kapica-Topczewska K., Chorąży M., Tarasiuk J., Kochanowicz J., Kulikowska J., Wawrzyniak S., Niezgodzińska-Maciejek A., Pokryszko-Dragan A., Gruszka E., Budrewicz S., Białek M., Kurkowska-Jastrzębska I., Kurowska K., Stępień A., Włodek A., Ptasznik V., Pawełczyk M., Sobolewski P., Lejmel H., Strzalińska K., Maciejowski M., Tutaj A., Zwiernik J., Litwin A., Lewańczyk B., Paprocka I., Zwiernik B., Pawlos A., Borysowicz A., Narożnik A., Michałowska A., Nosek K., Fudala M., Milewska-Jędrzejczak M., Kułakowska A., Bartosik-Psujek H. Clinical course and outcome of SARS-CoV-2 infection in multiple sclerosis patients treated with disease-modifying therapies - the Polish experience. Neurol. Neurochir. Pol. 2021;55(2):212–222. doi: 10.5603/PJNNS.a2021.0031. Epub 2021 Apr 15. PMID: 33856686. [DOI] [PubMed] [Google Scholar]
- Arrambide G., Llaneza-González M.Á., Costa-Frossard França L., Meca-Lallana V., Díaz E.F., Moreno-Torres I., García-Domínguez J.M., Ortega-Suero G., Ayuso-Peralta L., Gómez-Moreno M., Sotoca-Fernández J.J., Caminero-Rodríguez A.B., Rodríguez de Antonio L.A., Corujo-Suárez M., Otano-Martínez M.A., Pérez-Miralles F.C., Reyes-Garrido V., Ayuso-Blanco T., Balseiro-Gómez J.J., Muñoz-Pasadas M., Pérez-Molina I., Arnal-García C., Domingo-Santos Á., Guijarro-Castro C., Íñiguez-Martínez C., Castellanos-Pinedo F., Castillo-Triviño T., Cerdán-Santacruz D.M., Pérez-Sempere Á., Torres B.S., Álvarez de Arcaya A., Costa-Arpín E., Durán-Ferreras E., Fragoso-Martínez M., González-Platas M., Landete Pascual L., Millán-Pascual J., Oreja-Guevara C., Meca-Lallana J.E. SARS-CoV-2 infection in multiple sclerosis: results of the spanish neurology society registry. Neurol. Neuroimmunol. Neuroinflamm. 2021;8(5):e1024. doi: 10.1212/NXI.0000000000001024. Jun 24PMID: 34168057; PMCID: PMC8225011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spelman T., Forsberg L., McKay K., Glaser A., Hillert J. Increased rate of hospitalisation for COVID-19 among rituximab-treated multiple sclerosis patients: a study of the Swedish multiple sclerosis registry. Mult. Scler. 2022;28(7):1051–1059. doi: 10.1177/13524585211026272. JunEpub 2021 Jul 2. PMID: 34212816. [DOI] [PubMed] [Google Scholar]
- Drulovic J., Ivanovic J., Martinovic V., Tamas O., Veselinovic N., Cujic D., Gnjatovic M., Mesaros S., Pekmezovic T. Humoral response to SARS-CoV-2 COVID-19 vaccines in patients with multiple sclerosis treated with immune reconstitution therapies. Mult. Scler. Relat. Disord. 2021;54 doi: 10.1016/j.msard.2021.103150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fragoso Y.D., Schiavetti I., Carmisciano L., Ponzano M., Steinberg J., Treviño-Frenk I., Ciampi E., Vecino M.C.A., Correa E.P., Carcamo C., Gomes S., Pimentel M.L.V., Santos G.A.C., Vrech C., Winckler T.C.A., Sormani M.P. Coronavirus disease 2019 in Latin American patients with multiple sclerosis. Mult. Scler. Relat. Disord. 2021;55 doi: 10.1016/j.msard.2021.103173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bsteh G., Assar H., Hegen H., Heschl B., Leutmezer F., Di Pauli F., Gradl C., Traxler G., Zulehner G., Rommer P., Wipfler P., Guger M., Enzinger C., Berger T. AUT-MuSC investigators. COVID-19 severity and mortality in multiple sclerosis are not associated with immunotherapy: insights from a nation-wide Austrian registry. PLoS One. 2021;16(7) doi: 10.1371/journal.pone.0255316. Jul 27PMID: 34314457; PMCID: PMC8315529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez C.A., Zhang G.-Q., Li X., Huang Y., Lincoln J.A., Samudralwar R.D., Gupta R.K., Lindsey J.W. COVID-19 severity and outcome in multiple sclerosis: results of a national, registry-based, matched cohort study. Mult. Scler. Relat. Disord. 2021;55 doi: 10.1016/j.msard.2021.103217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clinical Outcomes Clinical outcomes in patients with COVID-19 during two phase IV studies of cladribine tablets for treatment of multiple sclerosis: an update (P11-4.005) Neurology. 2022;98(18):1358. victoria yavorskaya, radmila karan, laszlo borsi, nektaria alexandri. MaySupplement. [Google Scholar]
- Barzegar M., Mirmosayyeb O., Gajarzadeh M., Afshari-Safavi A., Nehzat N., Vaheb S., Shaygannejad V., Maghzi A.-H. COVID-19 among patients with multiple sclerosis: a systematic review. Neurol. Neuroimmunol. Neuroinflamm. 2021;8(4):e1001. doi: 10.1212/NXI.0000000000001001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coronavirus Disease 2019 (COVID-19): Epidemiology update. Updated: September 20, 2020. Accessed September 21, 2020. health-infobase.canada.ca/COVID-19/epidemiological-summary-COVID-19-cases.htm.