Abstract
Objectives:
On March 2020, the WHO declared coronavirus disease 2019 (COVID-19) pandemic. COVID-19 is associated with various clinical syndromes, with electrolytes imbalances involved. This review aims to quantify the prevalence and outcomes of hyponatremia among COVID-19 patients, as well as to review the underlying pathophysiological mechanisms of hyponatremia among these patients.
Methods:
Using Preferred Reporting Items for Systematic Reviews and Meta Analyses guidelines, we conducted a systematic literature search using the electronic databases of Google Scholar, MEDLINE (PubMed), WHO Virtual Health Library, and ScienceDirect, without limitations regarding gender, geographical area, race or publication date, up until December 13, 2021. Primary outcomes measured were mortality, intensive care unit (ICU) admission, assisted ventilation need, and length of hospital stay (LOS). Secondary outcome was the mechanism underlying hyponatremia among COVID-19 patients.
Results:
From a total of 52 included studies, 23 underwent quantitative analysis. For the primary outcomes; proportions, odds ratios (OR), and standardized mean difference (SMD) were calculated using random effects model. The prevalence of hyponatremia was found to be 25.8%. Hyponatremia was found to be significantly associated with increased odds for mortality (OR = 1.97[95% CI, 1.50–2.59]), ICU admission (OR = 1.91 [95% CI, 1.56–2.35]), assisted ventilation need (OR = 2.04 [95% CI, 1.73–2.38]), and with increased LOS (SMD of 5.74 h [95% CI, 0.092–0.385]). Regarding the mechanisms underlying hyponatremia, syndrome of inappropriate anti-diuretic hormone secretion (SIADH) was most commonly reported, followed by adrenal insufficiency, and finally hypovolemic hyponatremia due to gastrointestinal losses.
Conclusion:
Hyponatremia among COVID-19 patients is generally associated with poor outcomes, with SIADH being the most common underlying mechanism.
Keywords: Adverse outcomes, COVID-19, hyponatremia, prevalence
Introduction
Coronavirus disease 2019 (COVID-19) is an implacable emerging pandemic caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Since its emergence, millions of cases have been detected with unrelenting death tolls.[1] COVID-19 is associated with different complications during and after the infection including acute respiratory distress syndrome, arterial/venous thrombosis, and multiorgan failure, with electrolytes disturbances being one of them.[2-7] In the majority of cases, the electrolyte disorder is caused by multiple factors.[8,9] Such electrolytes disturbances have important implications not only for patient management but also for a better understanding of the pathophysiologic mechanisms underlying COVID-19.
Hyponatremia is considered the most important and most common electrolyte disturbance that presents in clinical practice, with an increased risk of death.[10-12] The most prevalent cause of hyponatremia is the syndrome of inappropriate anti-diuretic hormone secretion (SIADH) contributing to nearly 50% of cases,[11] with an increase in this percentage in specific conditions such as brain and lung pathologies.[13]
Several studies have demonstrated the prevalence of hyponatremia among COVID-19 patients.[14-16] However; in this review, we aimed to demonstrate the pooling prevalence of hyponatremia among patients with COVID-19 infection, with a focus on reflecting the impact of hyponatremia on the severity and outcome of the disease. A recent meta-analysis has found that hyponatremia is associated with poor outcomes in patients with COVID-19.[17] However, this review did not account for each outcome separately, which is what we also targeted in this review. This would give a reflection of each outcome associated with hyponatremia and hence aid in a clear understanding of the disease. In addition, we aimed to provide a narrative review of the different presentations and mechanisms involved in hyponatremia reported in patients with COVID-19 infection, which would help shed light on the unexpected clinical presentation of the disease, and hence allow for better detection and prompt management.
Methods
Design of the study
The methodology of this study was generated from the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement.[18] A pre-published protocol is unavailable for this review.
Search strategy and inclusion criteria
We conducted a systematic literature search using the electronic databases of Google Scholar, MEDLINE (PubMed), WHO Virtual Health Library, and ScienceDirect without limitations regarding gender, geographical area, race, or publication date up until December 13, 2021. The search terms used were “COVID-19,” “COVID 19,” “2019-nCoV,” “2019 nCoV,” “COVID-19,” “ COVID-19,” “2019 Novel Coronavirus,” “COVID-19,” “ COVID-19,” “SARS Coronavirus* 2,” “SARS-CoV-2,” “SARS CoV 2,” “2019 Novel Coronavirus*,” “Novel Coronavirus, 2019,” “Wuhan Seafood Market Pneumonia Virus,” “SARS-CoV-2 Virus*,” “SARS CoV 2 Virus*,” “2019-nCoV,” “Virus, COVID-19,” “Wuhan Coronavirus*,” “Coronavirus*, Wuhan,” “SARS Coronavirus* 2,” “Coronavirus* 2, SARS,” “SARS-CoV-2,” “ Hyponatremia*,” “low sodium,” “decrease sodium,” “low Na,” “decrease Na,” “reduce sodium,” and “reduce Na’ and Dysnatremia.” The retrieved results were transferred into Rayyan software for references management, where initial titles/abstracts screening was done and duplicates were removed.[19] The target population was the COVID-19 patients with hyponatremia and the criteria for articles to be included were cross-sectional, cohort, and case–control studies that assessed the prevalence of hyponatremia and/or association between hyponatremia and any poor outcome in COVID-19 patients in comparison to normonatremic patients, and case report that discussed the causes of hyponatremia in COVID-19 patients. We excluded editorials, conference abstracts, reviews, non-English papers, and studies with insufficient data. The titles and abstracts of the retrieved articles were screened, after which the full-text of studies classed as relevant were reviewed for potential inclusion. All steps were executed by at least two independent reviewers, and any conflict between the reviewers was further brought for group discussion to resolve it.
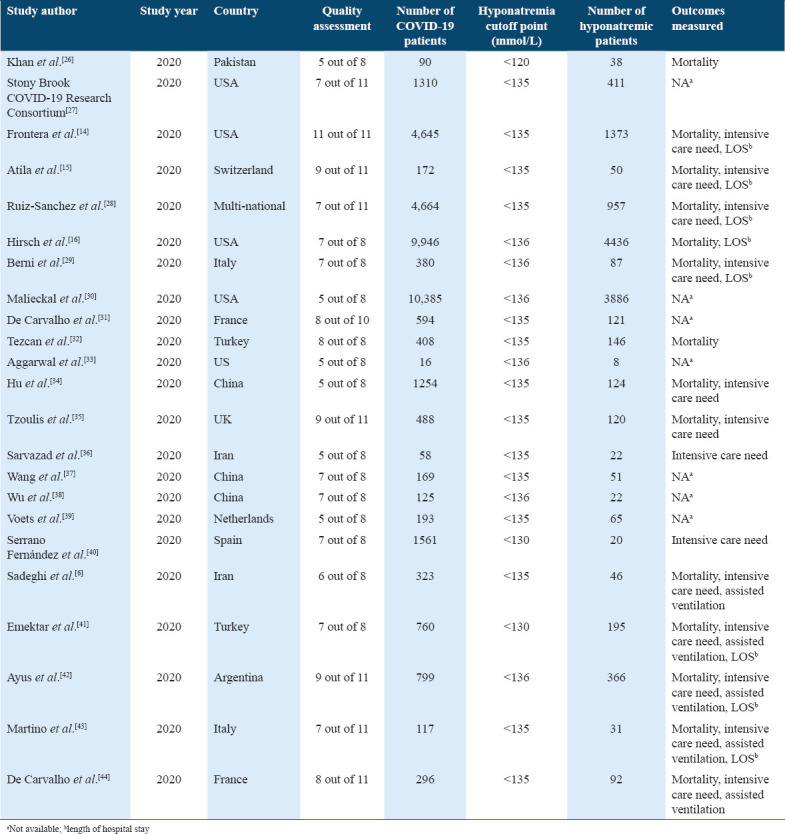
Quality assessment
Quality assessment of the included studies was done using the Joanna Briggs Institute (JBI) critical appraisal checklists by two independent reviewers to assess the methodological quality of studies and the possibility of bias in study design, data analysis, and conduct. The quality assessment tool consisted of 11, eight, and 10 questions for cohort, cross-sectional, and case–control studies, respectively. Using Yes, No, Unclear, and Not applicable reply to each question, values are assigned representing the extent to which they met the criteria. The mean score of two authors was taken for final decision and studies with a score yes, greater than or equal to six out of 11, five out of eight, or six out of 10 were included in the study.[20]
Exposure and outcomes
The exposure of our study was hyponatremia, defined as sodium measurement lower than 136 mEq/L (mmol/L).[21] The data of interest have been extracted by six independent reviewers in data extraction sheets. Any conflict between the reviewers was resolved by discussion and agreement. The baseline characteristics were extracted from each study in Excel sheets, which included: Authors, year of study, study region, hyponatremia cutoff point, number of COVID-19 patients diagnosed with reverse transcriptase-polymerase chain reaction testing, number of normonatremic and hyponatremic COVID-19 patients, age and gender of both groups, the cause of hyponatremia if identified, and comorbidities in hyponatremic and normonatremic groups. Furthermore, poor outcomes data were extracted (number of deaths, length of hospital stay [LOS], number of patients that needed intensive care unit [ICU] admission, and assisted ventilation; including, invasive and non-invasive ventilation).
Statistical analysis
We calculated the prevalence of hyponatremia and the risk of mortality, need for intensive care and assisted ventilation, and LOS among hyponatremic COVID-19 patients through DerSimonian-Laird random effect model estimates for meta-analysis of proportion, odds ratios (OR), and standardized mean difference (SMD).[22] Heterogeneity among the studies was assessed using the I2 test, which describes the variability of the effect size across the studies between the two groups. Prediction intervals (PI) for the true effects were calculated.
Publication bias was assessed by the visual examination of the funnel plot, Egger’s regression intercept, and Begg and Mazumdar rank correlation.[23] When asymmetry of funnel plot was perceived, Duvall and Tweedie’s trim-and-fill analysis was used to add the imputed studies, and the adjusted value was calculated.[24] All analyses were carried out using Comprehensive Meta-analysis version 3.3.070 (CMA 3) software.[25]
Results
Quantitative analysis
Studies characteristics and demographic data of the included patients
Our search retrieved records for 721 published articles. We also hand-searched the bibliography of included articles and relevant reviews but included no additional studies. The records screened after the removal of duplicates were 535 articles. We excluded 424 records in the title/abstracts screening phase after applying the exclusion criteria. Then, full texts of 111 studies were assessed for eligibility and quality. Overall, 52 studies conducted from 2020 to 2021 were included in the review, with a total of 23 of the studies[6,14-16,26-44] including 38,753 patients that met the eligibility criteria were included in the quantitative analysis. The majority of studies included carried low-moderate risk of bias according to JBI critical appraisal checklists. The studies selection process and the baseline characteristics of the included studies are summarized in Figure 1 and Table 1. Data for the age of the participants were obtainable from 11 studies[6,14-16,28,29,34,41-44] and the mean age of the hyponatremic and normonatremic groups was 63.50±1.9 years and 61.20±1.11, respectively. The SMD between the two groups was 0.113 years (P = 0.076).
Figure 1.
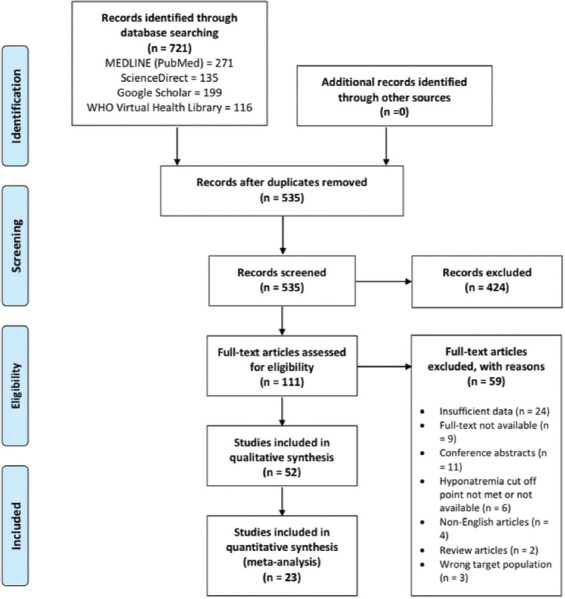
Flowchart for the study selection process
Table 1.
The baseline characteristics of the included studies in quantitative analysis
Data for the sex of participants were obtainable from 11 studies,[6,14-16,28,29,34,37,41-44] and males constituted 67.85% and 55.60% of the hyponatremic and normonatremic groups, respectively. From the total population of the aforementioned 11 studies, 13,300 of the patients (59.90%) were male.
Quality assessment result
The quality score of the included articles ranges from 63% to 100% as per JBI critical appraisal checklists.
Prevalence of hyponatremia, mortality, and need for intensive care
Based on the 23 included studies,[6,14-16,26-44] the overall prevalence of hyponatremia among COVID-19 patients was 25.8% [95% CI, 21.8–30.2%]. Begg’s and Egger’s test P values were 0.31 and 0.01, respectively.
The prevalence of mortality among hyponatremic COVID-19 patients was estimated using meta-analysis of proportions from data available from 14 studies.[6,14-16,26,28,29,32,34,35,41-44] Among 9626 hyponatremic patients, the prevalence of death was 23.7% [95% CI, 20–28.1%]. The proportion of hyponatremic patients who needed intensive care and/or assisted ventilation from 13 studies[6,14,15,26,28,29,32,34-36,41-44] was 41.4% [95% CI, 33.9–49.3%], with 22.8% [95% CI, 17.3–29.4%] needed assisted ventilation,[6,14,15,29,32,34,35,41-44] and 29.5% [95% CI, 22.7–37.3%] were admitted to ICU.[6,15,29,32,36,40-44]
Risk of mortality among hyponatremic COVID-19 patients
Based on the 23 included studies,[6,14-16,26-44] the overall prevalence of hyponatremia among COVID-19 patients was 25.8% [95% CI, 21.8–30.2%] [Figure 2]. The funnel plot for the included studies was asymmetrical on visual examination, with trim-and-fill analysis imputing seven virtual studies to the left [Figure 3]. Begg’s and Egger’s test P values were 0.31 and 0.01, respectively. A total of 14 studies[6,14-16,26,28,29,32,34,35,41-44] with a sum of 24,342 patients had sufficient data for estimating the risk of death among hyponatremic COVID-19 patients compared to normonatremic COVID-19 patients. The hyponatremic group had a higher risk of mortality, with a pooled OR of 1.97 (95% CI, 1.50–2.59) [Figure 4]. The I2 was 88.20% and the PI of the true effect size was 2.15 (95% CI, 0.75–5.15). The funnel plot was asymmetrical, and Duval and Tweedie’s trim-and-fill analysis indicated one virtual study [Figure 5], resulting in an adjusted OR 1.86 (95% CI, 1.42–2.44). However, Egger’s test (P = 0.06) and Begg’s test (P = 0.22) indicated no publication bias. Meta-analysis of adjusted OR for mortality among hyponatremic COVID-19 patients was done. Seven studies[14,15,28,29,32,35,42] provided sufficient data, with estimated adjusted OR of 1.67 (95% CI, 1.24–2.09, P = 0.000, I2 =27.43).
Figure 2.
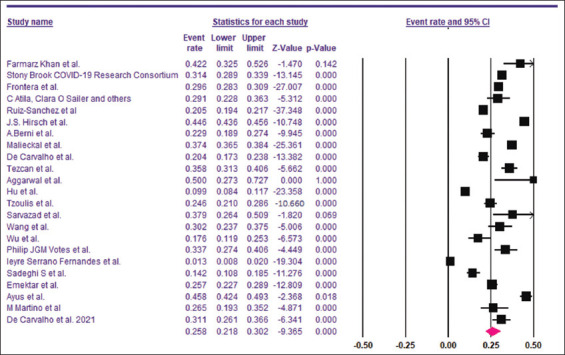
Pooled prevalence of hyponatremia among COVID-19 patients
Figure 3.
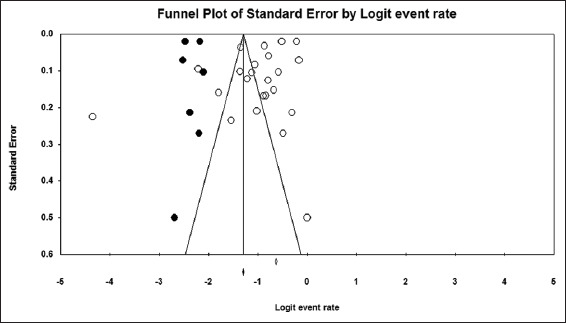
Funnel plot of the studies included in the meta-analysis
Figure 4.
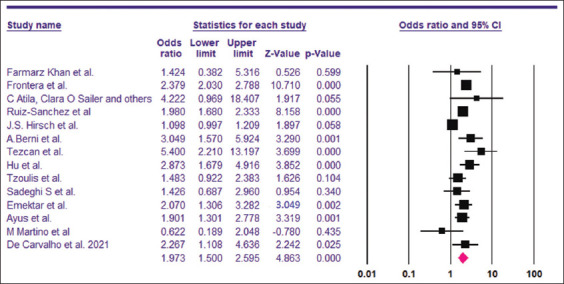
Pooled OR of mortality among hyponatremic COVID-19 patients
Figure 5.
Funnel plot showing no evidence for publication bias among the studies included to calculate pooled OR of mortality among hyponatremic COVID-19 patients
The need for intensive care among hyponatremic COVID-19 patients
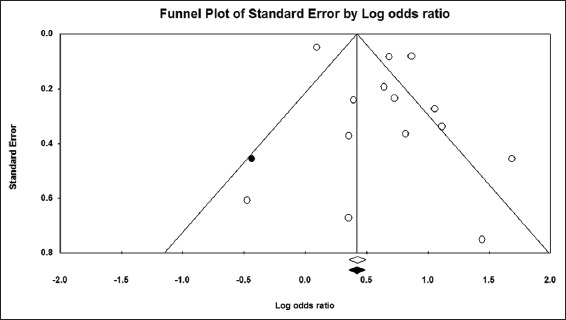
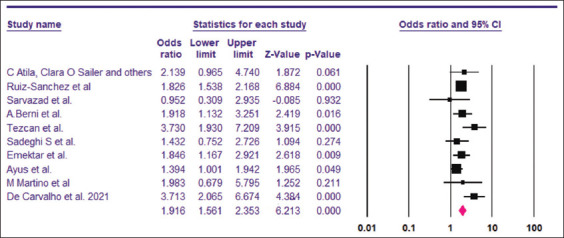
The pooled OR for the need for intensive care among hyponatremic COVID-19 patients was found to be 1.91 (95% CI, 1.56–2.35) [Figure 6]. The I2 was 38.75% and the PI 1.91 (95% CI, 1.16–3.15). A total of 7977 patients were included from 10 studies.[6,15,28,29,32,36,41-44] Visual examination of the funnel plot showed asymmetry. Trim-and-fill analysis imputed one virtual study [Figure 7], with an adjusted OR of 1.96 (95% CI, 1.59–2.41). However, the results of Begg’s test (P = 0.36) and Egger’s test (P = 0.29) indicated no publication bias.
Figure 6.
Pooled OR for the need for intensive care among hyponatremic COVID-19 patients
Figure 7.
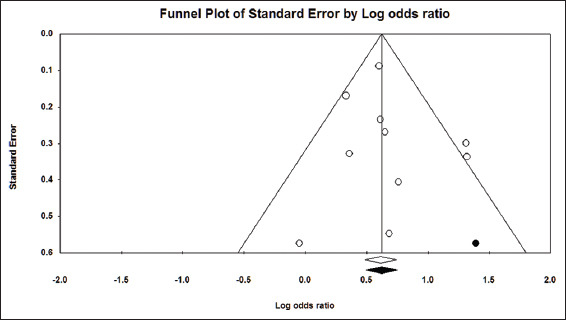
Funnel plot showing no evidence for publication bias among the studies included to calculate pooled OR for the need for intensive care among hyponatremic
Two studies[15,31] provided the adjusted OR for the need of ICU admission. Pooled OR using random effects model was found to be 2.80 (95% CI, 1,82–4.30, P = 0.000, I2 =0).
The need for assisted ventilation among hyponatremic COVID-19 patients
The pooled OR for the need for assisted ventilation among hyponatremic COVID-19 patients was found to be 2.04 (95% CI, 1.73–2.38) [Figure 8]. The I2 was 18.14% and the PI 2.04 (95% CI, 1,83–3.01). A total of 9642 patients were included from 11 studies.[6,14,15,29,32,34,35,41-44] Visual examination of the funnel plot showed symmetrical distribution of the studies around the mean effect [Figure 9]. This was supported by the results of trim-and-fill analysis, Begg’s test (P = 0.43) and Egger’s test (P = 0.43), indicating no publication bias.
Figure 8.
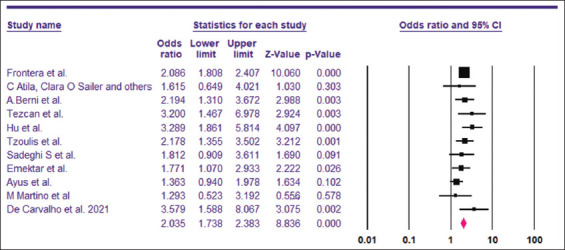
Pooled OR for the need for assisted ventilation among hyponatremic COVID-19 patients
Figure 9.
Funnel plot showing no evidence for publication bias among the studies included to calculate pooled OR for the need for assisted ventilation among hyponatremic COVID-19 patients
Two studies[15,35] provided the adjusted OR for the need of assisted ventilation. Pooled OR using random effects model was found to be 2.49 (95% CI, 1.68–3.68).
LOS in the two groups
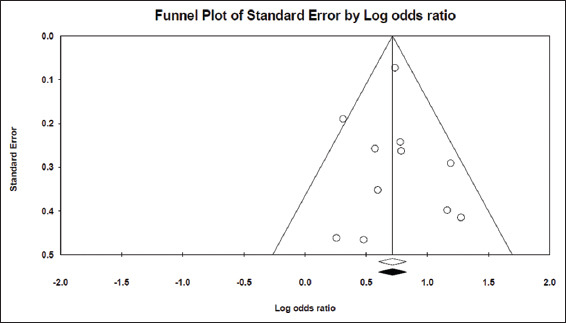
The LOS was compared between hyponatremic and normonatremic COVID-19 patients. Eight studies[14-16,28,29,41-43] including 21,483 patients provided sufficient data for LOS. Hyponatremic COVID-19 patients were found to have higher LOS when compared to normonatremic COVID-19 patients, with SMD of 0.239 days (5.74 h) (95% CI, 0.092–0.385, P = 0.001), as shown in Figure 10. I2 was found to be 93.60%. PI was 0.235 (95% CI, −0.25–0.73). The funnel plot was asymmetrical on visual examination and trim-and-fill analysis indicated three missing studies to the right, with an adjusted SMD of 0.428 (95% CI, 0.197–0.659) days [Figure 11]. Begg’s and Egger’s tests P values were 0.26 and 0.28, respectively, indicating no publication bias.
Figure 10.
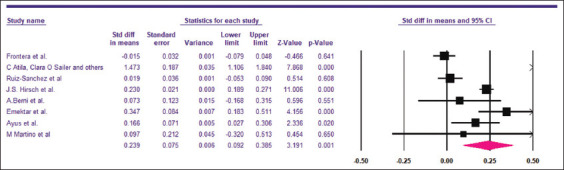
Pooled SMD for the Length of hospital stay among hyponatremic COVID-19 patients
Figure 11.
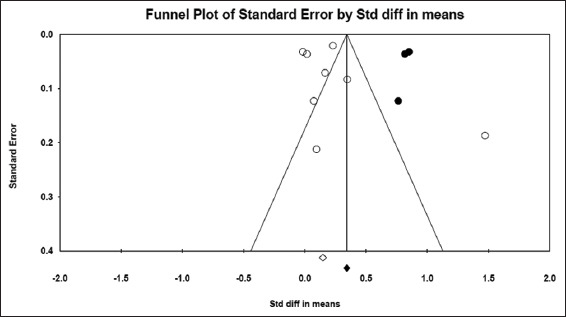
Funnel plot showing no evidence for publication bias among the studies included to calculate pooled SMD for the length of hospital stay among hyponatremic COVID-19 patients
Qualitative results
Managing hyponatremia could be challenging, as the main etiologies that lead to it have entirely distinct management approaches, hence understanding the mechanisms involved in COVID-19-related hyponatremia, are crucial for an appropriate management.[45]
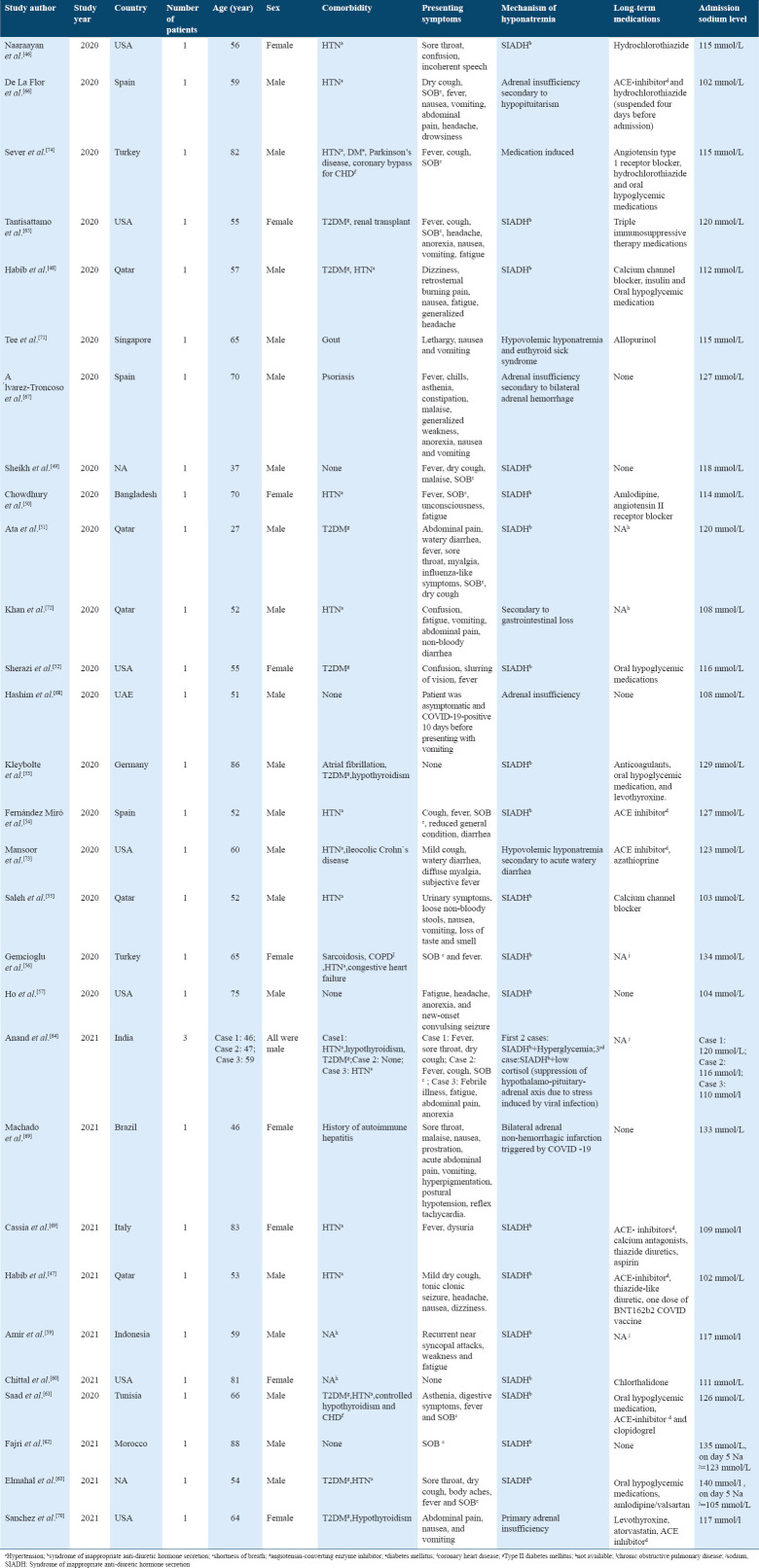
Among the case reports gathered from literature, 29 papers have investigated the mechanism of hyponatremia. The majority of case reports (n = 20) have reported that the SIADH was the primary cause of hyponatremia in their patients.[46-65] Adrenal insufficiency was reported in five case reports.[66-70] While in three cases, hyponatremia was attributed to gastrointestinal losses giving a picture of hypovolemic hyponatremia.[71-73] Whereas, Sever et al. have reported that the cause of hyponatremia was merely related to the use of angiotensin receptor blocker and thiazide diuretics.[74]
In addition, Frontera et al.[14] examined the mechanisms of hyponatremia in the subgroup of patients with severe hyponatremia (serum sodium ≤120 mmol/L) (n = 36). They found that the most common mechanisms were SIADH secretion (36%) and hypovolemia (36%), followed by tea and toast syndrome (22%), then hypervolemia (6%).
The clinical presentation may also give clue to the etiology of hyponatremia, which will aid in the initial management. The presenting features of patients with COVID-19-related hyponatremia ranged from common COVID-19 respiratory and/or gastrointestinal symptoms, including nausea, vomiting, and non-bloody diarrhea, to CNS symptoms of confusion, headache, incoherent speech, tonic-clonic seizure, and unconsciousness, mostly attributed to the low sodium levels. Interestingly, in one case report by Kleybolte et al.,[53] an 86-year-old gentleman had no presenting symptoms suggestive of either COVID-19 or hyponatremia and was found to have plasma sodium level of 129 mmol/l upon admission. In another case by Fajri et al.,[62] an 88-year-old man was asymptomatic on presentation, with mild hyponatremia of 135 mmol/l, 5 days later, he developed confusion, temporospatial disorientation, headache, and generalized hypotonia, where his sodium levels have dropped to 123 mmol/L. Baseline information extracted from included case reports are summarized in Table 2.
Table 2.
Baseline information extracted from the included case reports
Discussion
The pooled prevalence of hyponatremia among COVID-19 patients from 23 studies included in this systematic review and meta-analysis was found to be 25.8%. A recent systematic review and meta-analysis found similar results, with an incidence of 24%.[17] In comparison, the prevalence of hyponatremia among SARS-CoV-1 pneumonia was estimated at 60%.[43] Several studies have also described the prevalence of hyponatremia among community acquired pneumonia (CAP) patients, with it ranging between 22% and 31.8%.[75-79]
Over two-thirds of hyponatremic patients included in this meta-analysis were male. This might be due to the fact that nearly 60% of COVID-19 patients included were male, but this result also supports the findings of Frontera et al. study, indicating that the severity of hyponatremia increases with age, male gender, and Asian race.[14] Another plausible reason for this result is the relatively lower susceptibility of females to viral infections including COVID-19, as investigated by some studies, where differences in innate immune response, steroid hormones, and the presence of two X chromosomes play a role. There are regulatory genes involved in immune response located on the X chromosome, leading to lower viral load levels and less inflammation in women compared to men. Females have also higher CD4+ T cells and produce higher levels of antibodies. Interestingly, after a viral infection, interleukin-6 (IL-6) production has been observed to be lower in females than in males. This inflammatory cytokine is known to play a role in the development of COVID-19-related hyponatremia as will be discussed further in this review.[80,81] With regard to steroid hormones, few studies have reported that lower levels of testosterone found in men, due to old age, comorbidities, or other factors, were found to be associated with severe SARS-COV-2 infection necessitating ICU admission and leading to higher rates of death.[82,83]
Among hyponatremic patients, the mortality rate in our study was 23.7%. The risk of mortality among hyponatremic patients is almost twice that of normonatremic patients (OR = 1.97). A similar meta-analysis conducted by Abkar et al. illustrated the relation between hyponatremia and poor outcomes in general among COVID-19 patients, however, no data on the rate of mortality in hyponatremic COVID-19 patients were deduced.[17]
A study done by Chawla et al. stated that hyponatremia is the most common electrolyte disturbance that can lead to more mortality.[84] This association can be justified by the relationship of hyponatremia with the different neurological complications such as encephalopathy, brain edema, and convulsions all of which can lead to severe outcomes including death.[14] Furthermore, hyponatremia may contribute to death indirectly through the rapid correction of low sodium, which can lead to central pontine myelinolysis.[41] In general, hyponatremia is associated with an increased risk of death regardless of the disease with which it is associated.[85]
Our study found that 29.5% of hyponatremic COVID-19 patients were admitted to ICU, and 41.4% needed assisted ventilation. Admission to the ICU and/or the need for mechanical ventilation indicates severe disease.
In a meta-analysis by Lippi et al., the pooled analysis indicated that COVID-19 severity is associated with lower serum sodium.[86] Atila et al. found hyponatremia to be associated with severity parameters measured by national early warning score.[15] In another study by Berni et al., a direct relationship between hyponatremia and low PaO2/FiO2 ratio was drawn, being an important index to assess respiratory performance, with the presence of hypoxia usually necessitating ventilatory support.[11]
A HOPE-COVID-19 registry study found that hyponatremia was associated with hypoxia and receiving intensive therapy, and that the combination of hypoxia and hyponatremia did not increase the risk of mortality compared to hypoxia alone.[28] This implies that hyponatremia might be influencing other factors contributing to ICU admission, rather than oxygen saturation. Interestingly, Martino et al. demonstrated that in-hospital sodium level fluctuations were associated with 20 days earlier need for ICU assistance in hyponatremic patients compared to normonatremic ones.[43]
Our analysis of 10 studies found that hyponatremic COVID-19 patients have 1.9-fold odds to be admitted to the ICU when compared to non-hyponatremic patients, when adjusted for confounding factors, the odds increased to nearly 3-fold. Similar findings were shown by a large retrospective cohort study in CAP, with an increased proportion of hyponatremic patients requiring ICU admission and mechanical ventilation compared to the normonatremic group.[87]
Furthermore, serum sodium levels are well established to be inversely related to IL-6 levels.[11,14] IL-6 is one of the major cytokines that mediates cytokine storms and worsens the prognosis of COVID-19.[88] Thus, low serum sodium might be an indicator of progression to severe disease.[29]
The proximity in results of the prevalence of hyponatremia and the need for intensive care/assisted ventilation between the hyponatremic patients of COVID-19 infection and hyponatremic patients of CAP of other infectious agents might be explained by the similarities of the underlying pathophysiology of hyponatremia in these two groups of patients, with SIADH playing a major role, as well as the release of IL-6 and other mediators, among other factors.[89,90]
Several studies have demonstrated the relationship of multiple factors in COVID-19 patients and the risk of prolonged hospital stay, one of these independent factors is electrolyte disturbance, including hyponatremia. In our study, we have found that hyponatremic COVID-19 patients had a longer duration of hospital stay compared to normonatremic (5.08 h). This is possibly because patients with electrolyte disturbances require more time to correct the abnormalities, that is, why an important component in the management of COVID-19 patients is close monitoring of electrolytes and proper correction of their disturbances.[32] In addition to that, hyponatremic patients may also have other problems and complications that make them stay more in the hospital for appropriate management. Moreover, after adjustment for confounders including age and comorbidities, Atila et al. found that dysnatremia was associated with longer hospitalization, with an odds ratio of 2.7, 95% CI: 2.59–2.93, P < 0.001.[15] However, one study done by Tzoulis et al. did not find any association between hyponatremia and an increase in the LOS, this might be due to the small number of moderate-severe hyponatremic patients in this study.[35]
Habib et al. suggested that hyponatremia on admission could be used as an indicator of COVID-19 infection.[48] However, this might not be accurate, as mentioned previously hyponatremia is not specific to COVID-19 pneumonia alone, and it appears to be of similar prevalence in patients with pneumonia due to other causes.
Our findings are consistent with a recent meta-analysis, which indicated increased odds of severe disease and poor outcome among COVID-19 patients with hyponatremia.[17] However, as for mortality, this association does not necessarily mean causality, since participants in different studies were enrolled with distinct baseline COVID-19 severities, and some might have been admitted directly into ICU due to severe systemic involvement of the disease, with electrolyte imbalance being merely a marker and a manifestation of the physiologic derangement caused by the underlying disease. Although, Frontera et al. in their study found that the severity of low sodium was related to an increase in the odds of mortality by 43%, that remained significant after adjustment for potential confounding factors.[10] Ruiz-Sanchez et al. also concluded that hyponatremia was an independent risk factor that can lead to more deaths (OR 1.5, 95% CI 1.08–2.09; P = 0.016).[16] The same meta-analysis[17] found that hyponatremia has high specificity in predicting poor outcomes in hyponatremic COVID-19 patients, however, with a low sensitivity and poor area under the curve in predicting poor outcomes when measured alone.
In light of this, hyponatremia can be used alone, or more preferably with other factors such IL-6 and C-reactive protein (CRP) in predicting the prognosis in this group of patients. It can also be used as a tool in triaging and allocating patients to critical beds and ventilators, especially in resource-constrained settings. Moreover, serum sodium might be used alone in rural areas where testing for other markers such as IL-6 and CRP is not feasible. Berni et al. hypothesized that IL-6 and serum sodium levels, rather than the PaO2/FiO2 ratio, are more reliable in predicting in-hospital mortality.[29]
Hyponatremia can also be used as a biomarker for pulmonary involvement in COVID-19 patients. Martino et al. followed up the trends of sodium level during 21 days of observation. At the end of the observation period, the prevalence of hyponatremia declined to 6%, and the ascending trend of serum sodium levels throughout the hospitalization period reflected the recovery of the patients from pneumonia.[43]
The underlying mechanism that leads to hyponatremia in patients with COVID-19 is multifactorial and varies according to presentation.[91] COVID-19 virus requires ACE-2 receptor to gain access to the cell and inflict harm.[92] These receptors are expressed not only in the lungs but also in the intestine, kidneys, adrenal glands, and heart, resulting in the variation in clinical presentation.[91,93] Our review demonstrated that the most common mechanisms of hyponatremia reported among SARS-COV-2 patients are SIADH, followed by adrenal causes, then hypovolemia. When exploring these mechanisms, they are found to be interrelated. Gastrointestinal losses such as diarrhea can result directly in sodium loss in stool, in addition to a state of hypovolemia that triggers a baroreceptor response. This response, in turn, activates the renin-angiotensin system, resulting in a non-osmotic ADH secretion, that is further augmented by the inflammatory state during the infection through inflammatory cytokines, such as IL-6.[94] Similarly, other factors such as stress and pain can stimulate the hypothalamus to produce ADH, which is used as a biomarker of stress along with cortisol and other hormones.[95,96] In addition, pneumonia, being one of the clinical manifestations of COVID-19, has a major role as well. Although the exact mechanism by which pneumonia is associated with SIADH-induced hyponatremia is still unclear, one way to explain this is by the resultant compensatory hypoxic pulmonary vasoconstriction, which occurs due to ventilation perfusion mismatch. This, in turn, allows stretch receptors in the left atrium to signal the reduced blood volume, resulting in an increased ADH release.[49]
Hashim et al. reported a case of COVID-19-related hyponatremia that was not plausible to be explained by the episodes of vomiting and dehydration, and rather adrenal causes where put first.[68] COVID-19 virus has been reported in literature to affect adrenal glands in direct and indirect ways, where a direct cytopathic effect is presumed to occur when the virus infects these cells, resulting in alteration in cortisol dynamics. Similar to influenza virus, one of the immunoinvasive strategies used by COVID-19 is to suppress the host’s cortisol stress response, as well as express certain amino acids that resemble the host’s ACTH. On that account, the body responds with antibody production that not only attaches to viral amino acids but also to host’s ACTH. Moreover, COVID-19 may affect the hypothalamic-pituitary-adrenal axis, resulting in hyponatremia through, central hypocortisolism, and less often hypothyroidism.[93]
In a case report by De La Flor et al., the severe hyponatremia was unlikely to be due to the presentation of vomiting or use of hydrochlorothiazide. Despite the fact that all the criteria for SIADH were met, the presence of hormonal abnormalities in thyroid, adrenal, and pituitary function alerted to the occurrence of a hypothalamic-pituitary disorder. After a thorough workup, a pituitary macroadenoma was incidentally found on imaging, and the diagnosis of secondary adrenal insufficiency was given.[66] Whereas, Sever et al. reported a case of COVID-19-related hyponatremia presenting with pneumonia, attributing the cause to the use of certain medications, including thiazide diuretic and angiotensin-type1-receptor blocker, that are well established to cause hyponatremia.[74] The diagnosis of COVID-19-related hyponatremia requires meticulous work up, to formulate few differential diagnoses of the most probable contributing causes, while bearing in mind the effect of other factors including medication use.
To the best of our knowledge, this is the first systematic review and meta-analysis to include this large number of papers in COVID-19-related hyponatremia. We have also systematically reviewed case reports and other studies that discussed the pathophysiology of hyponatremia in COVID-19 patients, and to the best of our knowledge, there is no published systematic review covering this subject.
The strength of our study also comes from the stratification of the poor outcome measures into separate strata, namely: Mortality, need for intensive care, ventilatory support, and LOS, rather than pooling the outcomes together into one mean effect. This gives comprehensive information regarding the prognosis and helps more in dealing with real-life situations where not all the patients have the same prognosis.
Finally, our meta-analysis did not include papers with high risk of bias, adding to the strength and reliability of the study.
Limitations
First, we were not able to undergo subgroup analysis to explore the heterogeneity. It might be explained by the variability in the responses of different healthcare systems around the globe to the pandemic, the different management plans that might impact the prognosis, as well as the resources availability in each country.
Second, meta-regression analysis to explore the impact of other factors – such as chronic illness or use of antihypertensive – on the prognosis was not possible to conduct, due to scarcity of data available in the included studies.
In addition, causality could not be ascertained as some patients might have been admitted with very poor health initially, which introduces bias. Moreover, it was not possible to determine the state of electrolyte level in the included patients before diagnosis and admission, which introduces another source of bias. Finally, patients were admitted with variable background characteristics, such as using diuretics or having established chronic kidney disease, which might affect serum sodium levels.
Conclusion
Hyponatremia is found in a significant proportion of COVID-19 patients, carrying around twice the risk for mortality, ICU admission, and assisted ventilation, with longer duration of hospitalization than normonatremic COVID-19 patients. SIADH is found to be the most common mechanism involved in COVID-19 hyponatremia. Health-care providers need to be aware of the clinically important association between hyponatremia and adverse clinical outcomes to ensure prompt and effective management.
Ethics Approval and Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Availability of Data and Materials
The datasets used and analyzed during the present study are available from the corresponding author on reasonable request.
Competing Interests
The authors declare that they have no competing interests.
Funding
Not applicable.
Authors’ Contributions
RK undertook research design; BI and SM undertook data analysis; RK, BI, MA, RH, AF, and RA participated in searching databases; RK, BI, MA, RH, AF, and RA undertook articles screening; and RK, BI, MA, RH, and RA undertook data extraction. All authors interpreted the results and participated in drafting the manuscript. All authors revised and approved the final manuscript. Reem jamal yousif khidir1, Basil abubakr yagoub ibrahim1 1Contributed equally to this work.
Acknowledgment
Not applicable.
References
- 1.Guo C, Bo Y, Lin C, Li HB, Zeng Y, Zhang Y, et al. Meteorological factors and COVID-19 incidence in 190 countries:An observational study. Sci Total Environ. 2021;757:143783. doi: 10.1016/j.scitotenv.2020.143783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Colavita F, Lapa D, Carletti F, Lalle E, Bordi L, Marsella P, et al. SARS-CoV-2 isolation from ocular secretions of a patient with COVID-19 in Italy with prolonged viral RNA detection. Ann Intern Med. 2020;173:242–3. doi: 10.7326/M20-1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323:1061–9. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention. Post-COVID Conditions. Atlanta, Georgia: Centers for Disease Control and Prevention; [Last accessed on 2022 Jan 03]. Available from: https://www.cdc.gov/coronavirus/2019-ncov/long-term-effects/index.html . [Google Scholar]
- 6.Sadeghi S, Keivany E, Naderi Z, Purajam S, Nasri E. Electrolyte imbalance in patients with COVID-19 pneumonia. J Renal Inj Prev. 2022:11. [Google Scholar]
- 7.SeyedAlinaghi SA, Afsahi AM, MohsseniPour M, Behnezhad F, Salehi MA, Barzegary A, et al. Late complications of COVID-19;a systematic review of current evidence. Arch Acad Emerg Med. 2020;9:1–15. doi: 10.22037/aaem.v9i1.1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sterns RH. Diagnostic Evaluation of Adults with Hyponatremia. [Last accessed on 2022 Jan 03]. Available from: https://www.uptodate.com/contents/diagnostic-evaluation-of-adults-with-hyponatremia .
- 9.Filatov A, Sharma P, Hindi F, Espinosa PS. Neurological complications of coronavirus disease (COVID-19):Encephalopathy. Cureus. 2020;12:e7352. doi: 10.7759/cureus.7352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Corona G, Giuliani C, Parenti G, Norello D, Verbalis JG, Forti G, et al. Moderate hyponatremia is associated with increased risk of mortality:Evidence from a meta-analysis. PLoS One. 2013;8:e80451. doi: 10.1371/journal.pone.0080451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Berni A, Malandrino D, Parenti G, Maggi M, Poggesi L, Peri A. Hyponatremia, IL-6, and SARS-CoV-2 (COVID-19) infection:May all fit together? J Endocrinol Invest. 2020;43:1137–9. doi: 10.1007/s40618-020-01301-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Upadhyay A, Jaber BL, Madias NE. Incidence and prevalence of hyponatremia. Am J Med. 2006;119(7 Suppl 1):S30–5. doi: 10.1016/j.amjmed.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 13.Cuesta M, Thompson CJ. The syndrome of inappropriate antidiuresis (SIAD) Best Pract Res. 2016;30:175–87. doi: 10.1016/j.beem.2016.02.009. [DOI] [PubMed] [Google Scholar]
- 14.Frontera JA, Valdes E, Huang J, Lewis A, Lord AS, Zhou T, et al. Prevalence and impact of hyponatremia in patients with coronavirus disease 2019 in New York city. Crit Care Med. 2020;48:E1211–7. doi: 10.1097/CCM.0000000000004605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Atila C, Sailer CO, Bassetti S, Tschudin-Sutter S, Bingisser R, Siegemund M, et al. Prevalence and outcome of dysnatremia in patients with COVID-19 compared to controls. Eur J Endocrinol. 2021;184:413–22. doi: 10.1530/EJE-20-1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hirsch JS, Uppal NN, Sharma P, Khanin Y, Shah HH, Malieckal DA, et al. Prevalence and outcomes of hyponatremia and hypernatremia in patients hospitalized with COVID-19. Nephrol Dial Transplant. 2021;36:1135–8. doi: 10.1093/ndt/gfab067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Akbar MR, Pranata R, Wibowo A, Irvan Sihite TA, Martha JW. The prognostic value of hyponatremia for predicting poor outcome in patients with COVID-19:A systematic review and meta-analysis. Front Med. 2021;8:1–7. doi: 10.3389/fmed.2021.666949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions:Explanation and elaboration. J Clin Epidemiol. 2009;62:e1–34. doi: 10.1016/j.jclinepi.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 19.Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A. Rayyan-a web and mobile app for systematic reviews. Syst Rev. 2016;5:1–10. doi: 10.1186/s13643-016-0384-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Joanna Briggs Institute. Critical Appraisal Tools. Adelaide, Australia: Joanna Briggs Institute; 2020. [Last accessed on 2022 Jan 10]. Available from: https://jbi.global/critical-appraisal-tools . [Google Scholar]
- 21.Gruszecki A. In:Textbook of Natural Medicine. Amsterdam, Netherlands: Elsevier; 2020. [Last accessed on 2022 Jan 06]. Mineral status evaluation; pp. 193–207.e6. Available from: https://linkinghub.elsevier.com/retrieve/pii/B9780323430449000236 . [Google Scholar]
- 22.Borenstein M, Hedges LV, Higgins JP, Rothstein HR. A basic introduction to fixed-effect and random-effects models for meta-analysis. Res Synth Methods. 2010;1:97–111. doi: 10.1002/jrsm.12. [DOI] [PubMed] [Google Scholar]
- 23.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088. [PubMed] [Google Scholar]
- 24.Duval S, Tweedie R. Trim and fill:A simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56:455–63. doi: 10.1111/j.0006-341x.2000.00455.x. [DOI] [PubMed] [Google Scholar]
- 25.Comprehensive Meta-Analysis Software (CMA) [Last accessed on 2022 Jan 12]. Available from: https://www.meta-analysis.com .
- 26.Khan F, Khokhar A, Ali Joyo RM, Ahmad N, Shamshad A, Hanif MA. Frequency of electrolyte imbalances (sodium and potassium) in patients with Covid-19 disease. Pak J Med Heal Sci. 2020;14:1190–2. [Google Scholar]
- 27.Mallipattu SK, Jawa R, Moffitt R, Hajagos J, Fries B, Nachman S, et al. Geospatial distribution and predictors of mortality in hospitalized patients with COVID-19:A cohort study. Open Forum Infect Dis. 2020;7:1–7. doi: 10.1093/ofid/ofaa436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ruiz-Sánchez JG, Núñez-Gil IJ, Cuesta M, Rubio MA, Maroun-Eid C, Arroyo-Espliguero R, et al. prognostic impact of hyponatremia and hypernatremia in COVID-19 pneumonia. A HOPE-COVID-19 (health outcome predictive evaluation for COVID-19) registry analysis. Front Endocrinol (Lausanne) 2020;11:1–12. doi: 10.3389/fendo.2020.599255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Berni A, Malandrino D, Corona G, Maggi M, Parenti G, Fibbi B, et al. Serum sodium alterations in SARS CoV-2 (COVID-19) infection:Impact on patient outcome. Eur J Endocrinol. 2021;185:137–44. doi: 10.1530/EJE-20-1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Malieckal DA, Uppal NN, Ng JH, Jhaveri KD, Hirsch JS, Abate M, et al. Electrolyte abnormalities in patients hospitalized with COVID-19. Clin Kidney J. 2021;14:1704–7. doi: 10.1093/ckj/sfab060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.De Carvalho H, Richard MC, Chouihed T, Goffinet N, Le Bastard Q, Freund Y, et al. Electrolyte imbalance in COVID-19 patients admitted to the emergency department:A case-control study. Intern Emerg Med. 2021;16:1945–50. doi: 10.1007/s11739-021-02632-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tezcan ME, Gokce GD, Sen N, Kaymak NZ, Ozer RS. Baseline electrolyte abnormalities would be related to poor prognosis in hospitalized coronavirus disease 2019 patients. New Microbes New Infect. 2020;37:100753. doi: 10.1016/j.nmni.2020.100753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Aggarwal S, Garcia-Telles N, Aggarwal G, Lavie C, Lippi G, Henry BM. Clinical features, laboratory characteristics, and outcomes of patients hospitalized with coronavirus disease 2019 (COVID-19):Early report from the United States. Diagnosis. 2020;7:91–6. doi: 10.1515/dx-2020-0046. [DOI] [PubMed] [Google Scholar]
- 34.Hu W, lv X, Li C, Xu Y, Qi Y, Zhang Z, et al. Disorders of sodium balance and its clinical implications in COVID-19 patients:A multicenter retrospective study. Intern Emerg Med. 2021;16:853–62. doi: 10.1007/s11739-020-02515-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tzoulis P, Waung JA, Bagkeris E, Hussein Z, Biddanda A, Cousins J, et al. Dysnatremia is a predictor for morbidity and mortality in hospitalized patients with COVID-19. J Clin Endocrinol Metab. 2021;106:1637–48. doi: 10.1210/clinem/dgab107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sarvazad H, Cahngaripour SH, Roozbahani NE, Izadi B. Evaluation of electrolyte status of sodium, potassium and magnesium, and fasting blood sugar at the initial admission of individuals with COVID-19 without underlying disease in Golestan Hospital, Kermanshah. New Microbes New Infect. 2020;38:100807. doi: 10.1016/j.nmni.2020.100807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang C, Zhou L, Chen J, Yang Y, Huang T, Fu M, et al. The differences of clinical characteristics and outcomes between imported and local patients of COVID-19 in Hunan:A two-center retrospective study. Respir Res. 2020;21:1–10. doi: 10.1186/s12931-020-01551-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wu Y, Hou B, Liu J, Chen Y, Zhong P. Risk factors associated with long-term hospitalization in patients with COVID-19:A single-centered, retrospective study. Front Med. 2020;7:1–10. doi: 10.3389/fmed.2020.00315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Voets PJ, Frölke SC, Vogtländer NP, Kaasjager KA. COVID-19 and dysnatremia:A comparison between COVID-19 and non-COVID-19 respiratory illness. SAGE Open Med. 2021;9:205031212110277. doi: 10.1177/20503121211027778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fernández LS, Iturriaga LA, Yandiola PP, Ocaña RM, Fernández SP, Huget ET, et al. Bacteraemic pneumococcal pneumonia and SARS-CoV-2 pneumonia:Differences and similarities. Int J Infect Dis. 2022;115:39–47. doi: 10.1016/j.ijid.2021.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Emektar E, Karaarslan FN, Koç F, Dağar S, Uzunosmanoğlu H, Çorbacıoğlu ŞK. Evaluation of hyponatremia and predictors of hyponatremia in patients hospitalized with the COVID-19. Eur J Emerg Med. 2021;20:183–9. [Google Scholar]
- 42.Ayus JC, Negri AL, Moritz ML, Lee KM, Caputo D, Borda ME, et al. Hyponatremia, inflammation at admission, and mortality in hospitalized COVID-19 patients:A prospective cohort study. Front Med. 2021;8:1–12. doi: 10.3389/fmed.2021.748364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Martino M, Falcioni P, Giancola G, Ciarloni A, Salvio G, Silvetti F, et al. Sodium alterations impair the prognosis of hospitalized patients with COVID-19 pneumonia. Endocr Connect. 2021;10:1344–51. doi: 10.1530/EC-21-0411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.De Carvalho H, Letellier T, Karakachoff M, Desvaux G, Caillon H, Papuchon E, et al. Hyponatremia is associated with poor outcome in COVID-19. J Nephrol. 2021;34:991–8. doi: 10.1007/s40620-021-01036-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Adrogué HJ, Madias NE. The challenge of hyponatremia. J Am Soc Nephrol. 2012;23:1140–8. doi: 10.1681/ASN.2012020128. [DOI] [PubMed] [Google Scholar]
- 46.Naaraayan A, Pant S, Jesmajian S. Severe hyponatremic encephalopathy in a patient with COVID-19. Mayo Clin Proc. 2020;95:2285–6. doi: 10.1016/j.mayocp.2020.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Habib MB, Hamad MK, Kalash T, Ahmed A, Mohamed MF. COVID-19 pneumonia complicated by seizure due to severe hyponatremia. Cureus. 2021;13:e15603. doi: 10.7759/cureus.15603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Habib MB, Sardar S, Sajid J. Acute symptomatic hyponatremia in setting of SIADH as an isolated presentation of COVID-19. IDCases. 2020;21:e00859. doi: 10.1016/j.idcr.2020.e00859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sheikh MM, Ahmad E, Jeelani HM, Riaz A, Muneeb A. COVID-19 pneumonia:An emerging cause of syndrome of inappropriate antidiuretic hormone. Cureus. 2020;12:e8841. doi: 10.7759/cureus.8841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chowdhury MR, Akter KS, Moula MM, Kabir MA, Bhuiyan SI, Das BC. COVID-19 presented with syndrome of inappropriate ADH secretion(SIADH):A case report from Bangladesh. Respir Med Case Rep. 2020;31:101290. doi: 10.1016/j.rmcr.2020.101290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ata F, Almasri H, Sajid J, Yousaf Z. COVID-19 presenting with diarrhoea and hyponatraemia. BMJ Case Rep. 2020;13:e235456. doi: 10.1136/bcr-2020-235456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sherazi A, Bedi P, Udevbulu E, Rubin V, Alasadi L, Spitalewitz S. Hyponatremia and encephalopathy in a 55-year-old woman with syndrome of inappropriate antidiuretic hormone secretion as an isolated presentation of SARS-CoV-2 infection. Am J Case Rep. 2021;22:e930135. doi: 10.12659/AJCR.930135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kleybolte J, Storek B, Hegner B. SARS-CoV-2-induced SIADH:A novel cause of hyponatremia. Z Gerontol Geriatr. 2021;54:301–4. doi: 10.1007/s00391-021-01863-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Miró MF, Arguedas SM, Ruscalleda LF. Syndrome of inappropriate antidiuretic hormone secretion associated with a SARS-CoV-2 pneumonia. Med Clin. 2021;156:195–6. doi: 10.1016/j.medcle.2020.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Saleh AO, Al-Shokri SD, Ahmed A, Musa AE, Mohamed MF. Urinary retention and severe hyponatremia:An unusual presentation of COVID-19. Eur J Case Reports Intern Med. 2020;7:001905. doi: 10.12890/2020_001905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gemcioglu E, Karabuga B, Ercan A, Erden A. A case of inappropriate antidiuretic hormone secretion syndrome associated with COVID-19 pneumonia. Acta Endocrinol. 2020;16:110–1. doi: 10.4183/aeb.2020.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ho KS, Narasimhan B, Kumar A, Flynn E, Salonia J, El-Hachem K, et al. Syndrome of inappropriate antidiuretic hormone as the initial presentation of COVID-19:A novel case report. Nefrologia. 2021;41:218–20. doi: 10.1016/j.nefroe.2021.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cassia MA, Casazza R, Napodano P, Cozzolino M. COVID-19 infection and acute kidney injury:Cause or complication? Blood Purif. 2022;51:288–91. doi: 10.1159/000516336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Amir M, Renata A, Ratana LT. Symptomatic sinus bradycardia due to electrolyte imbalances in syndrome of inappropriate antidiuretic hormone (SIADH) related Covid-19:A case report. BMC Infect Dis. 2021;21:465. doi: 10.1186/s12879-021-06143-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chittal AR, Rao SJ, Lakra P, Zulty ME. Asymptomatic hyponatremia precipitated by COVID-19 pneumonia. J Community Hosp Intern Med Perspect. 2021;11:779–81. doi: 10.1080/20009666.2021.1979738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Saad G, Ben Abdelkrim A, El Abed YH, Tahri S, Gorchene A, Maaroufi A, et al. Disorders of sodium balance in covid-19 patients:Two tunisian patients report. Pan Afr Med J. 2021;39:199. doi: 10.11604/pamj.2021.39.199.27626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fajri M, Essafti M, Aloua R, Mouaffak Y. Severe case of COVID-19 pneumonia complicated by SIADH. Ann Med Surg. 2022;73:103153. doi: 10.1016/j.amsu.2021.103153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Elmahal M, Lohana P, Anvekar P, Ali SR, Allah SA. Syndrome of inappropriate antidiuretic hormone secretion-induced encephalopathy in a patient with COVID-19. Cureus. 2021;13:e16671. doi: 10.7759/cureus.16671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Anand U, Kumar R, Priyadarshi RN, John AG. Hyponatremia in COVID-19 infection:One should think beyond SIADH. Med J Armed Forces India. 2021;77:S522–4. doi: 10.1016/j.mjafi.2021.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tantisattamo E, Reddy UG, Duong DK, Ferrey AJ, Ichii H, Dafoe DC, et al. Hyponatremia:A possible immuno-neuroendocrine interface with COVID-19 in a kidney transplant recipient. Transpl Infect Dis. 2020;22:e13355. doi: 10.1111/tid.13355. [DOI] [PubMed] [Google Scholar]
- 66.De La Flor Merino JC, Reyes LM, Gravalos TL, Conde AR, del Pozo MR. An unusual case of severe acute hyponatremia in patient with COVID-19 infection. Nefrologia. 2020;40:356–8. doi: 10.1016/j.nefro.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Álvarez-Troncoso J, Larrauri MZ, Vega MD, Vallano RG, Peláez EP, Rojas-Marcos PM, et al. Case report:COVID-19 with bilateral adrenal hemorrhage. Am J Trop Med Hyg. 2020;103:1156–7. doi: 10.4269/ajtmh.20-0722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hashim M, Athar S, Gaba WH. New onset adrenal insufficiency in a patient with COVID-19. BMJ Case Rep. 2021;14:e237690. doi: 10.1136/bcr-2020-237690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Machado IF, Menezes IQ, Figueiredo SR, Coelho FM, Terrabuio DR, Ramos DV, et al. Primary adrenal insufficiency due to bilateral adrenal infarction in COVID-19. J Clin Endocrinol Metab. 2022;107:e394–400. doi: 10.1210/clinem/dgab557. [DOI] [PubMed] [Google Scholar]
- 70.Sánchez J, Cohen M, Zapater JL, Eisenberg Y. Primary adrenal insufficiency after COVID-19 infection. AACE Clin Case Rep. 2021;8:51–3. doi: 10.1016/j.aace.2021.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tee LY, Yap B, Sidhu GK, Goh KS, Rosario BH. Atypical presentation of COVID-19 in an older adult:Lethargy and vomiting from severe hypovolemic hyponatremia. Geriatr Gerontol Int. 2020;20:839–40. doi: 10.1111/ggi.13990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Khan AA, Ata F, Munir W, Yousaf Z. Fluid replacement versus fluid restriction in COVID-19 associated hyponatremia. Cureus. 2020;12:e9059. doi: 10.7759/cureus.9059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mansoor E, Khoudari G, Abou Saleh M, Elfadawy N, Cooper GS, Katz J, et al. The many faces of COVID-19:Atypical presentation of COVID-19 in a patient with Crohn's disease with acute diarrhea leading to severe hypovolemic hyponatremia a case report. Am J Gastroenterol. 2020;115:1730–1. doi: 10.14309/ajg.0000000000000814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sever MY, Uzun N, Ceritli S, Mutlu H, Bayramoğlu A. Hyponatremia in COVID-19 patient using angiotensin Type 1 receptor (AT1R) blocker and diuretic:A case report. J Res Clin Med. 2020;8:22. [Google Scholar]
- 75.Akyil FT, Akyil M, Ağca MC, Güngör A, Ozantürk E, Söğüt G, et al. Hyponatremia prolongs hospital stay and hypernatremia better predicts mortality than hyponatremia in hospitalized patients with community-acquired pneumonia. Tuberk Toraks. 2019;67:239–47. doi: 10.5578/tt.68779. [DOI] [PubMed] [Google Scholar]
- 76.Krüger S, Ewig S, Giersdorf S, Hartmann O, Frechen D, Rohde G, et al. Dysnatremia, vasopressin, atrial natriuretic peptide and mortality in patients with community-acquired pneumonia:Results from the german competence network CAPNETZ. Respir Med. 2014;108:1696–705. doi: 10.1016/j.rmed.2014.09.014. [DOI] [PubMed] [Google Scholar]
- 77.Ravioli S, Gygli R, Funk GC, Exadaktylos A, Lindner G. Prevalence and impact on outcome of sodium and potassium disorders in patients with community-acquired pneumonia:A retrospective analysis. Eur J Intern Med. 2021;85:63–7. doi: 10.1016/j.ejim.2020.12.003. [DOI] [PubMed] [Google Scholar]
- 78.Nair V, Niederman MS, Masani N, Fishbane S. Hyponatremia in community-acquired pneumonia. Am J Nephrol. 2007;27:184–90. doi: 10.1159/000100866. [DOI] [PubMed] [Google Scholar]
- 79.Akirov A, Diker-Cohen T, Steinmetz T, Amitai O, Shimon I. Sodium levels on admission are associated with mortality risk in hospitalized patients. Eur J Intern Med. 2017;46:25–9. doi: 10.1016/j.ejim.2017.07.017. [DOI] [PubMed] [Google Scholar]
- 80.Conti P, Younes A. Coronavirus COV-19/SARS-CoV-2 affects women less than men:Clinical response to viral infection. J Biol Regul Homeost Agents. 2020;34:339–43. doi: 10.23812/Editorial-Conti-3. [DOI] [PubMed] [Google Scholar]
- 81.Gemmati D, Bramanti B, Serino ML, Secchiero P, Zauli G, Tisato V. COVID-19 and individual genetic susceptibility/receptivity:Role of ACE1/ACE2 genes, immunity, inflammation and coagulation. Might the double X-chromosome in females be protective against SARS-CoV-2 compared to the single X-chromosome in males?Int J Mol Sci. 2020;21:3474. doi: 10.3390/ijms21103474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Rastrelli G, Di Stasi V, Inglese F, Beccaria M, Garuti M, Di Costanzo D, et al. Low testosterone levels predict clinical adverse outcomes in SARS-CoV-2 pneumonia patients. Andrology. 2021;9:88–98. doi: 10.1111/andr.12821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Çayan S, Uğuz M, Saylam B, Akbay E. Effect of serum total testosterone and its relationship with other laboratory parameters on the prognosis of coronavirus disease 2019 (COVID-19) in SARS-CoV-2 infected male patients:A cohort study. Aging Male. 2020;23:1493–503. doi: 10.1080/13685538.2020.1807930. [DOI] [PubMed] [Google Scholar]
- 84.Chawla A, Sterns RH, Nigwekar SU, Cappuccio JD. Mortality and serum sodium:Do patients die from or with hyponatremia? Clin J Am Soc Nephrol. 2011;6:960–5. doi: 10.2215/CJN.10101110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Tierney WM, Martin DK, Greenlee MC, Zerbe RL, McDonald CJ. The prognosis of hyponatremia at hospital admission. J Gen Intern Med. 1986;1:380–5. doi: 10.1007/BF02596422. [DOI] [PubMed] [Google Scholar]
- 86.Lippi G, South AM, Henry BM. Electrolyte imbalances in patients with severe coronavirus disease 2019 (COVID-19) Ann Clin Biochem. 2020;57:262–5. doi: 10.1177/0004563220922255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zilberberg MD, Exuzides A, Spalding J, Foreman A, Jones AG, Colby C, et al. Hyponatremia and hospital outcomes among patients with pneumonia:A retrospective cohort study. BMC Pulm Med. 2008;8:16. doi: 10.1186/1471-2466-8-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zhang C, Wu Z, Li JW, Zhao H, Wang GQ. Cytokine release syndrome in severe COVID-19:Interleukin-6 receptor antagonist tocilizumab may be the key to reduce mortality. Int J Antimicrob Agents. 2020;55:105954. doi: 10.1016/j.ijantimicag.2020.105954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Gheorghe G, Ilie M, Bungau S, Stoian AM, Bacalbasa N, Diaconu CC. Is there a relationship between covid-19 and hyponatremia? Medicina (Lithuania) 2021;57:1–7. doi: 10.3390/medicina57010055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Edmonds ZV. Hyponatremia in pneumonia. J Hosp Med. 2012;7(Suppl 4):S11–3. doi: 10.1002/jhm.1933. [DOI] [PubMed] [Google Scholar]
- 91.De La FLor JC, Marschall A, Biscotti B, del Pozo MR. Hyponatremia in COVID-19 infection should only think about SIADH? J Clin Nephrol Ren Care. 2020;6:57. [Google Scholar]
- 92.Behl T, Kaur I, Bungau S, Kumar A, Uddin S, Kumar C, et al. The dual impact of ACE2 in COVID-19 and ironical actions in geriatrics and pediatrics with possible therapeutic solutions. Life Sci. 2020;257:118075. doi: 10.1016/j.lfs.2020.118075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Pal R. COVID-19, hypothalamo-pituitary-adrenal axis and clinical implications. Endocrine. 2020;68:251–2. doi: 10.1007/s12020-020-02325-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Yousaf Z, Al-Shokri SD, Al-Soub H, Mohamed MF. COVID-19-associated SIADH:A clue in the times of pandemic! Am J Physiol Endocrinol Metab. 2020;318:E882–5. doi: 10.1152/ajpendo.00178.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Jezova D, Skultetyova I, Tokarev DI, Bakos P, Vigas M. Vasopressin and oxytocin in stress. Ann N Y Acad Sci. 1995;771:192–203. doi: 10.1111/j.1749-6632.1995.tb44681.x. [DOI] [PubMed] [Google Scholar]
- 96.Noushad S, Ahmed S, Ansari B, Mustafa UH, Saleem Y, Hazrat H. Physiological biomarkers of chronic stress:A systematic review. Int J Health Sci (Qassim) 2021;15:46–59. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and analyzed during the present study are available from the corresponding author on reasonable request.