Abstract
Stroke, including ischemic stroke and hemorrhagic stroke can cause massive neuronal death and disruption of brain structure, which is followed by secondary inflammatory injury initiated by pro-inflammatory molecules and cellular debris. Phagocytic clearance of cellular debris by microglia, the brain’s scavenger cells, is pivotal for neuroinflammation resolution and neurorestoration. However, microglia can also exacerbate neuronal loss by phagocytosing stressed-but-viable neurons in the penumbra, thereby expanding the injury area and hindering neurofunctional recovery. Microglia constantly patrol the central nervous system using their processes to scour the cellular environment and start or cease the phagocytosis progress depending on the “eat me” or “don’t eat me’’ signals on cellular surface. An optimal immune response requires a delicate balance between different phenotypic states to regulate neuro-inflammation and facilitate reconstruction after stroke. Here, we examine the literature and discuss the molecular mechanisms and cellular pathways regulating microglial phagocytosis, their resulting effects in brain injury and neural regeneration, as well as the potential therapeutic targets that might modulate microglial phagocytic activity to improve neurological function after stroke.
Keywords: Brain ischemia, inflammation, metabolism, microglia, stroke
Introduction
Stroke, especially ischemic stroke, are leading causes of disability and mortality worldwide. 1 Dead brain cells after ischemic stroke, and blood cells or toxic cellular debris leaked from ruptured cerebral blood vessels in hemorrhagic stroke triggers profound inflammatory responses in the brain. 2 Persistent presence of these dead or dying cells/cell debris contributes to the inflammatory response, which together with infiltrating peripheral immune cells, 3 leads to the expansion of the resultant damaged area and impairs long-term neurofunctional recovery. 4 Effective clearance of dead cells and cell debris in the injury area is crucial to resolve neuroinflammation, facilitate brain tissue regeneration, and restore cerebral immunological homeostasis after stroke.
Microglia are the professional phagocytes of the central nervous system (CNS). 5 Together with recruited macrophages, they play a pivotal role in orchestrating the immune response, engulfing potentially harmful dead cells and cellular components, and restoring cerebral homeostasis after stroke. 4,6,7 Microglial phagocytosis is generally thought to be beneficial because it clears necrotic brain tissue and toxic cellular debris, and counteracts pro-inflammatory factors. 8 However, recent studies suggest that overactivation of microglial phagocytosis can be detrimental. For instance, stressed but viable brain cells after ischemic or hemorrhagic stroke, which are mostly neurons, can be phagocytosed by microglia/macrophage, exacerbating neuronal loss. 9,10 This excessive phagocytosis of viable neurons can also contribute to delayed brain atrophy and neurodegenerative changes. 6 These viable neuronal cells, when exposed to harmful stimuli, over-express crucial “eat me” signals on the cell surface, including phospholipid phosphatidylserine and calreticulin, which are normally kept at the inner leaflet of the plasma membrane and endoplasmic reticulum in intact neurons. 11,12 Meanwhile, a series of anti-phagocytic signals (e.g. CD47 and other polysialylated proteins) that inhibit phagocytosis by microglia and macrophages, are simultaneously expressed on the cellular surface of neurons. 13 These “don’t eat me” signals are essential to counterbalance the effects of the “eat me” signals and protect healthy neurons from being engulfed by microglia. 13 Infiltrating peripheral immune cells are thought to be a major contributor to the pathogenesis of stroke. 14 Therefore, it is also notable that microglia can also engulf infiltrating immune cells as well as their cellular components, 15 thus they exert further control over the fate of cells after stroke by limiting the accumulation of peripheral infiltrating immune cells in the injured brain. 15
In this review, we examine the up-to-date literature on microglial phagocytosis and the regulatory mechanisms that occur after stroke. We also examine recent advances in potential therapeutics targeting microglial phagocytosis.
Phagocytic signaling after stroke
As the resident phagocytes in the CNS, microglia constantly survey the brain parenchyma with their extensive processes to detect pathological changes in the brain microenvironment. 16 When ischemic stroke related damage occurs, several substances are released by injured or stressed brain cells that act as “find me” signals, including adenosine 5′ triphosphates (ATPs), sphingosine-1-phosphate, and chemokines, 17 which attract neighboring microglia to migrate to the injury site. 18 Microglial phagocytosis involves both the activation of microglia and the presence of specific “eat me” signals on the dead/dying cells. There are also various “eat me” and “don’t eat me” signals that regulate the phagocytosis of dead cells and cellular debris by microglia and other phagocytes as discussed below. (Figure 1).
Figure 1.
Overview of “find me”, “eat me”, “don't eat me” signals that regulate microglia phagocytosis of neurons. (a) “Find me” signals are chemotactic signals, such as ATP and chemokines released from neurons and cell debris, resulting in chemotaxis of microglia to these neurons. (b) “Eat me” signals, such as PS, calreticulin and C3b, are released by stressed or dying neurons and induce phagocytosis of themselves. (c) “Don't eat me” signals including fractalkine and CX3CR1 and CD47 inhibit phagocytosis. All these signals regulate microglial phagocytosis. ATP, adenosine triphosphate; PS, phosphatidylserine; TREM2, Triggering Receptor Expressed on Myeloid Cells 2; LRP, lipoprotein receptor-related protein; CR3, complement receptor 3; CX3CR1, C-X3-C motif chemokine receptor 1; CD47, Cluster of Differentiation 47; SIRPα, signal-regulatory protein α.
“Eat me” signals in microglia: phosphatidylserine, calreticulin, and complement components
The phospholipid phosphatidylserine is an essential “eat me” signal that presents on the cell surface of both dead and stressed-but-viable neurons. 19 It’s notable that the phospholipid phosphatidylserine (PS) also exists in healthy cells, and it can reversibly translocate to the cell surface, releasing damage signals to the surrounding microglia. 12 Toxic signals released from damaged cells lead to oxidative stress, intracellular calcium buildup, ATP depletion, and activation of caspases from apoptotic cells that can cause irreversible translocation of phosphatidylserine presentation to the cell surface. 8 The presence of PS on the cell surface induces microglial phagocytosis to eliminate the dead/dying cells or the cellular debris directly through receptors like the PS receptor or indirectly through adapter proteins such as the product of the growth arrest-specific 6 (Gas6) gene with the TAM family proteins (Tyro3, Axl, c-mer tyrosine kinase (MerTK)). 12,19,20 On the other hand, reversible phosphatidylserine exposure on the cell surface of neurons can occur in response to non-toxic or sublethal cellular stimuli. Under these circumstances, microglia can detect the phagocytic signals but can’t distinguish the type of cellular damage. 6,21 The phagocytosis of these stressed-but-viable neurons might be responsible for delayed neuronal death, brain atrophy, and cognitive decline after ischemic stroke, 6 and blockage of any step involved in PS exposure might prevent endangered neurons from being engulfed and prevent long-term neurodegeneration. 8 Indeed, blocking transmembrane protein 16 F (TMEM16F), a calcium-activated phospholipid scramblase shown to aggravate neuron loss by mediating microglial phagocytosis of neurons after cerebral ischemia, reduced the infarct volume and improved functional recovery after ischemic stroke. 19
Regulation of other “eat me” signals, such as calreticulin or the complement components C1q and C3b, which initiate or promote microglial phagocytosis, may also prove efficacious against unwarranted engulfment of viable neurons. Calreticulin primarily exists in the endoplasmic reticulum but can be translocated to both neuronal and non-neuronal cell surfaces once endoplasmic reticulum stress or apoptosis occurs after ischemic brain injury. 12,22 Calreticulin, once presents on the targeted cell surface, binds to microglial low-density lipoprotein receptor-related protein (LRP) and promotes phagocytosis when microglia are stimulated with lipopolysaccharide (LPS). 11,12 Alterations in C1q and C3b signaling may also abate unwarranted phagocytosis of stressed but viable neurons as they enhance phagocytic signals through distinct signaling pathways: C1q potentiates the signal recognition by binding to cell surface PS, calreticulin, or a desialylated cell surface, and promotes microglial phagocytosis through the production of C3b. 23 In addition, C1q can also directly bind to the functional phagocytic receptor complement receptor 3 (CR3) on the cell surface, and induce phagocytosis by microglia. 12,23,24 Cleavage of C3 generates both C3a and C3b, and C3a apparently activates microglia via C3a receptors (C3aR), which acutely stimulates phagocytosis. Therefore, C3aR antagonists or knock-out were beneficial in mouse models of ischemia/reperfusion injury. 25 Targeted complement inhibition could salvage stressed neurons and inhibit neuroinflammation after ischemic stroke as shown by administration of B4Crry, a complement inhibitor, which prevented microglial activation and microglial phagocytosis of stressed but salvageable neurons guided by the complement opsonins. 26
“Don't eat me” signals: CD47 and sialic acid
Neuronal cells in the ischemic brain can also express “don’t eat me” signals to counterbalance the “eat me” signals, and mitigate potential phagocytic neuronal injury after stroke. For example, CD47 expressed on the target cell surface prevents phagocytosis by modulating the inhibitory receptor, signal-regulatory protein (SIRPα) on microglia. 27 Ligation of SIRPα by CD47 induces phosphorylation of highly conserved tyrosine residues of the cytoplasmic region, providing docking sites for inhibitory phosphatases SH2 domain-containing phosphatase (SHP)-1 to negatively regulate phagocytosis. 27 In addition, desialylated glycoprotein on the neuronal surface enhances the “eat me” signal and promotes phagocytosis, while the sialylation of the cell surface blocks phagocytosis by interacting with microglial sialic acid-binding immunoglobulin-like lectins (SIGLECs) on the cell surface which contains an immunoreceptor tyrosine-based inhibition motif (ITIM) domain. 22 Upon binding with each other, the ITIM domain is phosphorylated and recruits and activates protein tyrosine phosphatases, such as SHP-1 and reverse the tyrosine phosphorylation of signaling proteins, such as Syk, induced by activating receptors to inhibit phagocytosis. 25 Evidence suggests that SIRP/CD47 signaling may play a protective role after ischemic stroke as neurological deficits and neuronal apoptosis after focal cerebral ischemia were all attenuated in SIRPα deficient mice. 28 However, whether this protection is mediated by a reduction in phagocytosis by don't eat me signals warrants further investigation.
Collectively, PS, calreticulin, and complement components are the three “eat me” signals that have been identified that induce neuronal cells to activate phagocytic microglia after ischemic stroke. Whereas, CD47 and sialic acid are the recently identified “don’t eat me” signals that inhibit microglia phagocytosis after stroke. Targeting these signals may provide novel therapeutic strategies for the development of future stroke treatment.
Receptors that mediate microglia phagocytosis following stroke
The presence of phagocytic signals on target cells only tags the cells as candidates for microglial phagocytosis. However, actual engulfment by microglia requires the recognition of such tags by specific microglial receptors and activation of specific pathways to initiate the phagocytic process. The receptors that mediate microglial phagocytosis can be divided into three major groups: 1) receptors with high affinity binding foreign pathogens, such as Toll like receptors (TLRs), 2) receptors that can recognize apoptotic cellular components, like triggering receptor expressed on myeloid cells 2 (TREM-2), and 3) receptors involved in phagocytosis of various harmful reactions, such as the complement receptors.
It is notable that microglial receptors, their effector cytokines, as well as intracellular phagocytic pathways might be discriminative in the type of brain components they target. For example, neurons undergo toxic changes after stroke and the removal of dead/dying or stressed-but-viable neurons requires microglial release of Milk Fat Globule Factor-E8 (MFG-E8), which recognize PS “eat me” signals on the neurons, and acts as a tether to bind the target cell. 12,29 Phagocytosis of dead/dying or vulnerable neurons may also require vitronectin receptor (VNR) and MER receptor tyrosine kinase (MERTK) upregulation on the microglial surface after stroke that bind to PS on the neuron surface. 29 Microglial phagocytosis of invading neutrophils also depends on VNR, as well as lectins. 30 On the other hand, developmental and neuroplasticity-induced removal or remodeling of synapses and neurites usually requires C1q, C3b, and CR3 as their main phagocytic pathways, whereas the clearance of neuronal precursors involves oxidant associated caspase activators, CR3 and DNAX activation protein of 12 kDa (DAP12). 12,31,32
The receptors and the corresponding signaling partners that mediate microglial phagocytosis are listed in Supplemental Table 1. A thorough understanding of the role of these distinct receptors and phagocytic pathways in stroke is of great importance, especially when considering possible therapeutic targets. The detailed signaling pathways downstream of the phagocytosis receptors are illustrated in Figure 2.
Figure 2.
Signaling pathways that mediate microglial phagocytosis. (a) TREM-2/DAP12/ERK/PKC pathway that mediates phagocytosis of microglia plays a neuroprotective role in stroke. (b) TLR mediated phagocytosis by MyD88-dependent IRAK4/p38/scavenger receptors pathway or MyD88-independent actin-Cdc42/Rac signaling pathway, which affects both neuroinflammation and phagocytosis for dead/dying cell clearance after stroke. (c) The purinergic receptor UTP triggers phagocytosis through P2Y6R/PLC/InsP3 pathway and promotes the engulfment of cell debris. (d) The phosphatidylserine receptor PS on apoptotic cells enhances microglial phagocytosis of apoptotic cells and modulates microglial proinflammatory signaling. (e) CD36 from scavenger receptor mediates engulfing extravasated erythrocytes and helps with hematoma resolution. TREM2, Triggering Receptor Expressed on Myeloid Cells 2; DAP12, DNAX activation protein 12; SYK, spleen-associated tyrosine kinase; ERK, extracellular signal-regulated kinase; TLR, toll-like receptors; MyD88, Myeloid-Differentiation factor-88; IRAK4, interleukin-1 receptor-associated kinase 4; Rac, reactive oxygen species and mitogen-activated protein kinases; PS, phosphatidylserine; PtdSerR, phosphatidylserine-specific receptor; CREB, Akt-cyclic AMP response element-binding protein; IkB, inhibitor of kB; UTP, uridine 5′-triphosphate; P2Y6R, P2Y6 purinergic receptor; PLC, phospholipase C; DAG, diacylgycerol; PIP2, phosphatidylinositol 4,5-bisphosphate; GTP, guanosine triphosphate; InsP3, inositol 1,4,5-trisphosphate.
After being recognized by phagocytic receptors, the plasma membrane extends and surrounds the phagocytic targets in an actin-dependent manner, with particles finally enclosed within a vesicular phagosome. 33 After formation, this nascent phagosome goes through a series of maturation processes. 33 The phagosome becomes increasingly more acidic, through the accumulation of V-ATPases which pump protons inside, and hydrolytically competent, through the acquisition of lysosomal enzymes. 33 This process is followed by transient fusion with multiple intracellular organelles including the recycling endosomal machinery, the synthetic–secretory apparatus such as the endoplasmic reticulum, secretory lysosomes, and multi-vesicular bodies, which may include the major histocompatibility complex (MHC) compartment and the autophagosome. Finally, the phagosome fuses with pre-existing, dense lysosomal bodies and reaches a pH of 4.5–5.0 to become phagolysosome for the eventual destruction of the phagocytosed particles. 33
Regulatory mechanisms of microglial phagocytosis following stroke
After “eat me” signals are recognized by microglial phagocytic receptors, actin polymerization in microglia is triggered and forms a phagocytic cup before final phagocytosis subsequently take places. 12,34 Once the phagocytic cup closes around the target, the engulfing phagosome goes through a process of maturation, whereby they fuse with endosomes and lysosomes before degrading the engulfed target. 35 Successful regulation of such a process through different modalities may represent a promising therapeutic target that could potentially improve stroke outcome.
Intracellular signaling pathways that regulate microglial phagocytosis
Nuclear factor-erythroid 2 p45-related factor 2
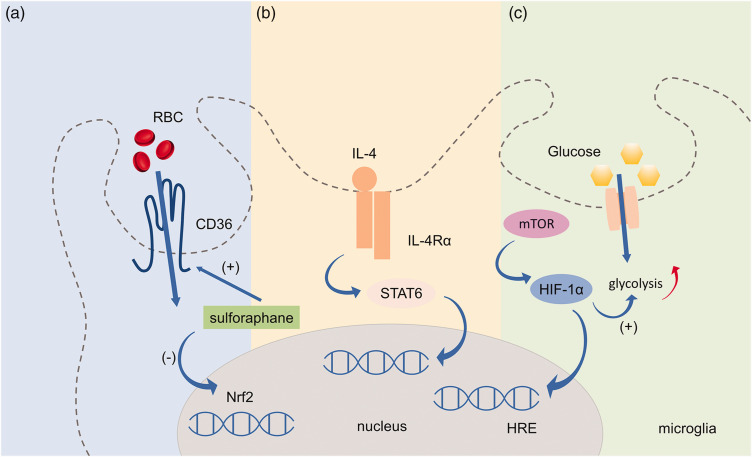
The transcription factor nuclear factor erythroid 2-related factor (Nrf2) is a regulator of cytoprotective antioxidant and anti-inflammatory signaling pathways. 36,37 The Nrf2 agonist, sulforaphane, up-regulated phagocytosis-mediating scavenger receptor CD36 and promoted phagocytosis of red blood cells (RBCs) by microglia after intracerebral hemorrhage (ICH). 38 As microglia represent the primary phagocytic effector cells that mediate hematoma resolution after ICH, means of enhancing clearance of RBCs and their degraded toxins represents a promising therapeutic option. 39 (Figure 3)
Figure 3.
Intracellular signaling pathways that regulate microglial phagocytosis. (a) Nrf2 is a regulator of cytoprotective antioxidant and anti-inflammatory signaling pathway. Nrf2 agonist, sulforaphane, can up-regulate CD36 and promote RBCs phagocytosis in microglia after ICH. (b) IL-4/STAT6 axis is a key enhancer of erythrocyte engulfment and could accelerate hematoma clearance after ICH. (c) HIF-1α facilitates microglial phagocytosis through CD36 and/or MFG-E8, both of which contain HRE and promotes recovery in the acute phase of ischemic stroke. Nrf2, nuclear factor erythroid 2-related factor; RBC, red blood cell; STAT6, signal transducer and activator of transcription 6; IL-4, interleukin-4; IL-4Rα, interleukin-4 receptor α; ICH, intracerebral hemorrhage; HIF-1α, hypoxia-inducible factor-1α; MFG-E8, milk fat globule-epidermal growth factor-factor VIII; mTOR, rapamycin; Aβ, amyloid-beta; AD, Alzheimer's disease.
STAT6 signaling
Signal transducer and activator of transcription 6 (STAT6), a member of the Signal Transducer and Activator of Transcription family of proteins, is principally activated by two cytokines, interleukin (IL)-4 and IL-13. 40 As a key enhancer of erythrocyte engulfment after ICH, STAT6 acting via the IL-4/STAT6 axis promoted long-term recovery in both blood and collagenase injection mouse models of ICH. 39 Thus, intranasal treatment with IL-4 or other STAT6 activators may become a clinically feasible therapy for ICH. 39 Meanwhile, IL-1 receptor-like 1 (ST2) activation might be the crucial downstream step in IL-4/STAT6 induced hematoma clearance. In ischemic stroke models, the STAT6/arginase1 (STAT6/Arg1) pathway mediated the phagocytic clearance of dead/dying cells by both microglia and infiltrating macrophage, and an upregulation of the STAT6/Arg1 signal ameliorated brain injury and enhanced long-term neurofunctional recovery. 4 Hence, the expression of STAT6 and the activity of its related signaling partners are increased after ischemic stroke, which is associated with enhanced microglial/macrophage phagocytosis and better neurofunctional outcome in both ischemic and hemorrhagic stroke. Thus, treatment options targeting STAT6 may improve neurofunctional recovery after stroke by enhancing phagocytosis.
Hypoxia-inducible factor-1α
Hypoxia-inducible factor (HIF) is a heterodimer composed of oxygen-sensitive α (HIF-α) and oxygen-insensitive β subunits. Under hypoxic conditions, instead of being hydroxylated, HIF-α interacts with HIF-1β in the cytoplasm and then translocates into the nucleus where it binds to hypoxia-responsive elements (HREs) of HIF target genes. 41 HIF-1α facilitates microglial phagocytosis through CD36 and/or MFG-E8, both of which contain hypoxia-responsive element (HRE), and promotes recovery in the acute phase of ischemic stroke. 42 HIF-1α was also shown to regulate metabolic control of microglia. 43 HIF increased glycolytic enzymes involved in energy metabolism and balanced between energy demand and supply. 44 The mammalian target of rapamycin (mTOR)-HIF-1α axis has been implicated in amyloid-β-induced metabolic reprogramming of microglia in Alzheimer’s disease (AD), which subsequently led to diminished microglial phagocytic ability. 45 Continued investigation of HIF-1α is warranted in order to assess the beneficial effect of metabolic control of microglia by HIF-1α in stroke models.
Regulations via MicroRNAs
MiRNAs are small non-coding RNAs that play crucial roles in regulating microglial phagocytosis. 46 MiR-155 is a typical multifunctional microRNA involved in immunity and inflammation under various pathological conditions. 47 Inhibition of miR-155 decreased phagocytosis by both microglia and macrophage by downregulating the expression of the phagocytosis marker CD68. 48 It has also been found that stressed neurons might also release extracellular vesicles (EVs) containing miRNA, such as miR-98, as a “help me” signal, which enhanced the communication between neurons and microglia. This enhanced communication aided in preventing microglial phagocytosis of stress-but-viable neurons, and promoted their recovery. 49 Such protective roles of miR-98 might be mediated by inhibiting platelet activating factor receptor (PAFR), one of its target genes. 49 Furthermore, miRNA EVs detected after release into the blood stream, carrying information about the injury might serve as potential biomarkers guiding individualized treatment in stroke patients. Taken together, miRNA-based therapeutic strategies, especially those that target genes involved in microglial phagocytosis, represent a promising approach to promote neurological recovery after stroke.
Regulations by metabolic products
Recently, a large body of studies on immunometabolism has shed new light on the metabolic regulation of microglia phagocytosis. 50 Activated microglia have to undergo significant metabolic changes prior to phagocytosis, and these changes are dictated by their response to various environmental and cellular stresses, resulting in diverse phagocytic functions and phenotypic modifications. 51 Different metabolic states influence microglia phagocytosis as illustrated in Figure 4.
Figure 4.
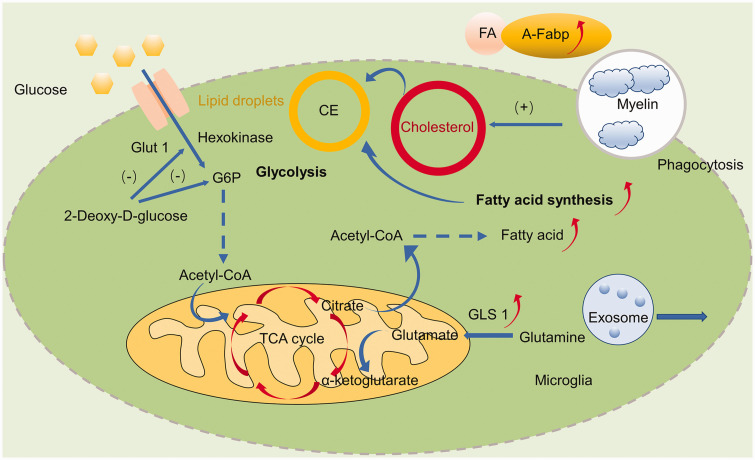
Microglia metabolic reprogramming and related signaling pathways after stroke. Ischemic stroke elicits metabolic profile changes which could affect the phagocytosis of microglia, including increased glycolysis and down-regulated OXPHOS. 2-Deoxy-D-glucose, an inhibitor of hexokinase and G6P could induce microglial phagocytosis and protect against ischemic brain injury. Both circulating and cerebral A-FABP, a lipid chaperone transporting free fatty acids, from microglia was increased. Fatty acid synthesis was increased due to glucose deprivation and phagocytosis of cellular membranes and myelin, leading to the accumulation of cholesterol and lipid droplets. GLS1, an enzyme that catalyzes the hydrolysis of glutamine to produce glutamate, induces pro-inflammatory response and exosome release of microglia. GLUT1, glucose transporter type 1; G6P, glucose 6-phosphate; TCA, tricarboxylic acid cycle; Acetyl-CoA-acetyl, coenzyme A; CE, Cholesteryl esters; FA, Fatty acids; GLS1, glutaminase 1; A-FABP, adipocyte fatty acid-binding protein; OXPHOS, oxidative phosphorylation.
Glucose metabolism of microglia
The survival and functional alteration of microglia in an ever-changing environment have high metabolic demand. 50 Homeostatic microglia generate ATP via glucose metabolism through glycolysis, the tricarboxylic acid cycle (TCA) and oxidative phosphorylation, and undergo distinctly programmed metabolic changes under the pathological circumstances. 52 After stroke, activated microglia mainly rely on glycolysis to meet this energy demand. 51 Whether glycolytic pathways regulate microglial phagocytosis is not definitively known, but it can be speculated that glycolysis may have a significant effect on phagocytosis. 52 Glucose deprivation is a component of the pathophysiology of ischemic stroke and glucose-deprived microglia showed increased phagocytic activity and decreased accumulation of lipids in lipid droplets. 53 Hyperglycolysis, referring to enhanced glucose metabolic rates relative to oxygen, is observed in the penumbra zone, and is characterized by diminished blood supply during ischemic stroke, which is detrimental to tissue survival due to lactate accumulation and reactive oxygen species (ROS) overproduction. Hyperglycolysis and up-regulation of the key glycolytic enzyme hexokinase 2 (HK2) were found to be vital to microglia-mediated neuroinflammation in brain ischemia. However, how hyperglycolysis affects microglial phagocytosis remains to be further determined. It is possible that modulating metabolic processes that drive microglial phagocytosis may provide avenues for future stroke therapies. Indeed, 2-deoxy-d-glucose, an inhibitor of glycolysis via blockage of hexokinase and glucose-6-phosphate isomerase activity, was found to prevent neurodegeneration in vitro by inducing phagocytosis of microglia by other microglia. 54
Pro-inflammatory microglia present increased pentose phosphate pathway (PPP), in which nicotinamide adenine dinucleotide phosphate (NADPH) is produced for ROS generation through NADPH oxidases due to the existence of enzymatic break points in the TCA cycle. 55 ROS are also critical intracellular signaling modulator and responsible for neuronal damage in stroke. 56 During phagocytosis, phagosomes that contain a variety of ROS are derivatives of O2- generated by NADPH oxidase (Nox), actively participating in the modulation of microglial phagocytosis. 57
The low amounts of extracellular ATP present in the homeostasis CNS serves as a neuromodulatory factor, while high amounts of ATP released from damaged neurons and activated glia act as an excitotoxin after acute brain injury. 58 ATP released in large amounts after acute brain injury attenuates microglial phagocytosis through the metabotropic P2Y receptors. 59 Therefore, blockade of this receptor may preserve the phagocytosis of microglia, reverse insufficient clearance of tissue debris by microglia and facilitate CNS tissue repair. 59 AMP-activated protein kinase (AMPK) is a serine/threonine protein kinase playing a key role in the regulation of energy metabolism through promotion of ATP-generating catabolic processes and inhibition of ATP-consuming processes. 60 Using activator of AMPK can also increase the phagocytosis of fluorescent microspheres by microglia and inhibit neuroinflammation in the pathogenesis of stroke. 61
These results suggest the metabolism of glucose plays an important role in the regulation of microglial phagocytosis and future work needs to focus on its therapeutic effect in the model of stroke.
Fatty acid metabolism of microglia
Fatty acid metabolic reprogramming is also involved in the phenotypic shifts in microglia following ischemia. Under conditions of glucose deprivation, as occurs with stroke, microglia retain their ability to phagocytose by utilizing fatty acids as an alternative energy source, which is highlighted by the accumulation of lipid droplets in the glucose-deprived cells. 53 Indeed, detection of increased levels of neutral lipids in brain microglia by magnetic resonance spectroscopy after ischemic stroke suggests continued microglial phagocytosis of cellular membranes. 62 Furthermore, both circulating and cerebral adipocyte fatty acid binding protein (A-FABP) from microglia were found increased during the acute phase of ischemic stroke. 63 By inhibiting microglia transforming growth factor β activated kinase 1 (TAK1), obesity combining with stroke which is related to long term cerebrovascular dysfunction can be reversed, while microglial selective deletion of TAK1 did not improve the outcome of simple ischemic stroke. 64 The interactions between microglia lipid metabolism and its phagocytosis function in stroke are not completely clear. However, it has been investigated in other CNS pathologies. 65 Lipoprotein lipase (LPL), a key enzyme in the lipase family, is highly expressed in microglia and regulates lipid phagocytosis by microglia. 66 LPL facilitates microglial lipid uptake and supports remyelination and repair through the clearance of lipid debris in the model of experimental allergic encephalomyelitis (EAE). 67 As a lipid-carrier regulating lipid homeostasis, apolipoprotein E (ApoE) encodes apolipoprotein E. 68 TREM2 induced APOE signaling and the APOE pathway mediated microglia phenotype switch after phagocytosis of apoptotic neurons in amyotrophic lateral sclerosis (ALS) and AD mouse models. 69 Dong et al. also identified that enhancing microglia-mediated oxidized phosphatidylcholines (OxPCs) clearance via TREM2 could help prevent neurodegeneration in MS. 70 Thus targeting the TREM2-APOE pathway might prevent neuronal loss. 69 Besides, lipid droplets (LDs), the dynamic players in lipid metabolism, also act as immune modulators in myeloid cells. 71 Lipid droplet-accumulating microglia are defective in phagocytosis, contributing to neurodegeneration and its roles in stroke are still unknown. 72
Amino acids and glutamate metabolism of microglia
The metabolism of amino acids plays an important role in microglial functional and phenotypic transformations. 65 Under pro-inflammatory stimuli, microglia undergo glutaminolysis, which supplies metabolites to the TCA cycle generating ATP, 69 which may drive microglia phagocytosis under glucose deprived environments. 53 Glutaminase 1 (GLS1), an enzyme that catalyzes the hydrolysis of glutamine to produce glutamate, was found elevated in microglia after focal cerebral ischemia and promoted microglia polarization into a pro-inflammatory phenotype and exosome release. 73 The metabolism of glutamate may also play a role in the pro-inflammatory shift in microglia as the conversion of glutamate to glutamine in microglia was upregulated in response to LPS 74 and recent studies report that various CNS diseases could be alleviated by inhibiting key enzymes of glutamate metabolism in microglial. 75,76 Little is known, however, about microglial metabolic reprogramming by amino acids under anti-inflammatory conditions. Anti-inflammatory microglia metabolized arginine to proline, which stimulated phagocytic function and enhanced neuroprotection and tissue repair. 77 Interestingly, glutamine showed efficacy at reversing starvation-induced processes in the complete absence of glucose, driven potentially by the ability of glutamine metabolism to maintain phagocytic activity in microglia. 53 Further investigations are needed to clarify how metabolic reprogramming of amino acids in microglia leads to functional alterations in microglial phagocytosis after stroke. It is of note that pre-existing metabolic related risk factors in stroke patients can affect metabolic changes both in the brain and the periphery after stroke. Therefore, metabolic changes in the whole body should also be taken into account in future studies where metabolism altering therapeutics are explored.
The role of microglial phagocytosis in brain injury and repair after stroke
Microglia play a dual role in the pathogenesis of stroke in both acute and chronic stages. 78 They are the resident macrophages and critical mediators of neuroinflammation in the CNS, which are activated within minutes after stroke. 79 The proliferation of microglia reaches its peak 48-72 h after stroke and can last for several weeks. 80 They are mainly detected in the area of the ischemic core and then extend to the peri-infarct region over time. 81 Activated microglia polarization to either a pro-inflammatory M1 or anti-inflammatory M2 phenotype is an important step to their neuro-immunomodulatory function. 82 Signaling pathways like interferon (IFN)-γ and signal transducer and activator of transcription 1 (STAT1) promote the M1 phenotype and its secretion of pro-inflammatory cytokines such as tumor necrosis factor (TNF)-α, IL-1β and IL-12, culminating in the destruction of intracellular pathogens. 83 IL-4, IL-10, and IL-13 mediate M2 polarization and its secretion of neuroprotective factors like glial cell line derived neurotrophic factor (GDNF), brain derived neurotrophic factor (BDNF), IL-10, and transforming growth factor (TGF)-β to facilitate tissue regeneration after injury. 81,84 Both the M1 and M2 phenotypes express phagocytic receptors, but the M2 phenotype is more efficient at clearing dead neurons than the M1 phenotype. 85 Phagocytosis mediated by the M1 phenotype causes neuronal loss by increasing phagocytosis of viable neurons, 12 whereas phagocytosis mediated by the M2 phenotype may be neuroprotective because of its anti-oxidative function.
In the acute stage of stroke, microglia accelerate damage through pro-inflammatory cytokines while at the same time they help with tissue repair and vessels remodeling. 86 With whole-genome RNA sequencing, macrophages were found to reprogram after ischemic stroke and promote efferocytosis in the mouse brain. 87 Resident microglia phagocytic activity predominates over hematogenous macrophages, and contributes to debris clearance after stroke. 88 They engulf apoptotic cells or damaged myelin debris, which can help to prevent the release of cytotoxic intracellular contents. 89 However, microglia directly engulf endothelial cells and cause the disintegration of blood vessels resulting in the breakdown of the blood brain barrier (BBB), which further accelerates circulating immune cells and their infiltration into the brain parenchyma. 90 After being exposed to ischemia, neurons were shown to reversibly expose PS via the calcium-activated phosphatidylserine scramblase TMEM16F, and microglia phagocytosed stressed-but-still-viable neurons through TMEM16F in the penumbra and caused functional deficits after ischemia–reperfusion. 19 Myeloid cell infiltration, for example neutrophils and their active components, acts as major contributors to pro-inflammatory neuronal damage during the acute phase of stroke, 91 while microglia act to limit this neuronal damage by removing these infiltrating neutrophils by phagocytosis. 3,92 In contrast, plasminogen activator inhibitor type 1 (PAI1) on neutrophils prevents their engulfment by microglia by downregulating VNR-mediated microglial phagocytosis after brain injury. 93 However, whether salvaging stressed-but-viable neurons is good in long term is unknown, since the maintenance of stressed neurons over time may not be beneficial to recovery. As for hemorrhagic stroke, microglia protect the brain tissue by eliminating blood cells and other small molecules. 94 Microglia help eliminate the neurotoxicity of blood products after ICH by phagocytosing and processing extravasated erythrocytes before their lysis and subsequent toxicity occurs. 95
In the chronic stage of stroke, microglia have a dual impact on neurogenesis, angiogenesis, and neuroplasticity. 96 Neurogenesis speeds up after stroke. 97 Microglia in the ipsilateral sub-ventricular zone (SVZ) can promote neurogenesis through up-regulation of TGF-α, 98 while those in the peri-infarct area have the opposite function. As for angiogenesis, perivascular microglia can promote blood vessel disintegration with the upregulation of phagocytic CD68 expression in the ischemic penumbra. 90 In contrast, microglia can release vascular endothelial growth factor (VEGF) to accelerate angiogenesis to help reconstruct blood vessels after stroke. 99 There is also evidence that microglia can modulate neuronal and synaptic functions after ischemic stroke by stimulating proliferation of neural progenitor cells (NPCs). 100 Some synapses in the ischemic areas are turned over after contact with microglia, which suggests that microglia are involved in synaptic pruning after stroke. 100 Microglia also contribute to spontaneous neuronal plasticity after ischemic stroke. Microglial CR3 activation by hypoxia and an inflammatory stimulus can trigger long-term synaptic depression (LTD) in surrounding neurons via NADPH oxidase, one of the main mediators of neurotoxicity in brain trauma and stroke. 101 Furthermore, activity-dependent connections between microglia and synapses are markedly prolonged after ischemic stroke, followed by the disappearance of the presynaptic bouton and synaptic elimination. However, excessive phagocytosis of myelin sheath accelerates demyelination. 100 Microglia selectively phagocytosed myelin sheaths to sculpt myelination in the homeostasis stage 102 and caused myelin sheath damage by excessively engulfing myelin sheath 14 days after ischemic stroke. 10 Berghoff et al. also found that microglia/macrophages internalized (cholesterol-rich) myelin debris, and synthesized sterols to reinforce remyelination by oligodendrocytes in multiple sclerosis (MS). 103 Similar mechanisms might also exist in ischemic stroke and deserve further investigations. As for hemorrhagic stroke, microglia transform into inflammatory cells and the direct phagocytosis and the indirect effects of proinflammatory factors will destroy the vascular endothelium and aggravate the hemorrhagic injury. 25
In conclusion, microglia play a complicated role in both ischemic stroke and hemorrhagic stroke: they engulf dead neurons and neuronal debris to reduce inflammation, but also phagocytose salvageable neurons in the ischemic penumbra. The microglial response to stroke can be divided into an acute, subacute, and long-term response by time, and by the ischemic core or the peri-infarct region by location, which differentially affects their function. Hence, it is inadvisable to simply block or boost microglial phagocytosis. Instead, it is important to maximize the advantages of microglial phagocytosis while avoiding its adverse effects. Therefore, future studies need to consider microglial function in the context of time and location to optimize treatment with therapeutics that modulates phagocytic function. (Figure 5)
Figure 5.
Evolving in vitro and in vivo techniques modeling microglial phagocytosis. In vitro methods either measure the phagocytic activity of individual cells by fluorescent microscopy or a population outcome by flow cytometry through feeding microglia with fluorescently-labeled targets. Phagocytosis in vivo can be inferred by the expression of phagocytic markers, such as CD206 and CD68, or using live cell imaging in the brain slice and transgenic zebrafish models. Aβ, amyloid-beta; EGFP, Enhanced Green Fluorescent Protein.
Evolving techniques modeling microglial phagocytosis
Microglial phagocytosis can be modeled with a wide range of both in vitro and in vivo techniques. Current methods to quantify the phagocytic function of microglia in vitro either measure the phagocytic activity of individual cells (average number of beads or particles/cell) or a population outcome (percent cells that contain phagocytosed material). 33 In most studies, microglial phagocytosis is assessed in vitro by feeding microglia fluorescently-labeled targets, ranging from latex beads to apoptotic cells, cell debris, or Aβ to model phagocytosis under different pathological conditions, 35 and are usually assessed by flow cytometry and fluorescent microscopy. 33 Flow cytometry allows the quantitative assessment of large numbers of cells and the study of different sub-populations, while fluorescent microscopy provides visualized information on the motility and morphology of the cells. 104 Under fluorescent microscopy, microglia are identified as big and round “amoeboid” cells, which represents the “activated morphology”. Clearance of dead neurons is an endogenous function of microglia, which is critical for inflammation resolution after stroke. Assays to measure phagocytosis of dead neurons are also available. Labeling dead neurons with propidium iodide (PI) and exposing them to cultured microglia, the amount of dead neurons in each microglia can be calculated to quantify the phagocytosis efficacy of microglia toward dead neurons. 105,106 Phagocytosis of apoptotic cells can also be assessed. 106 For example, the efficiency of microglial phagocytosis of apoptotic thymocytes is assessed using trypan blue, a non-cell-permeant fluorescein 106 , in concert with flow cytometry and fluorescent microscopy.
High-resolution microscopy incorporates traditional confocal microscopy but offers much higher image quality and details on the microglial phagocytosis process. Lysosomal distribution of microglia can be measured by the gray level co-occurrence matrix (GLCM). Focused ion bean scanning electron microscopy produces a higher 3D resolution image reaching 3–5 nm (x, y, and z), which can distinguish the phagocytosis process from partial to complete engulfment. Moreover, mass spectrometric imaging can visualize spatiotemporal changes in the lipidome after focal cerebral ischemia and bis (monoacylglycerol) phosphate (BMP(22:6/22:6)) can be used as a biomarker for phagocytizing macrophages/microglia cells, as BMP(22:6/22:6) can co-localize with the biomarker CD11b. 107 Traditional phagocytosis assays may not accurately depict the phagocytic process of microglia because they only take a snapshot of their transition status, overlooking the fact that microglia drastically change their morphology and transcriptional profiles under pathological conditions. 108 Indeed, in vivo assays usually recapitulate microglia phagocytosis more accurately. Phagocytosis in vivo can be inferred by the expression of phagocytic markers, such as CD206 and CD68, or live cell imaging in brain tissue. 33 Another approach uses transgenic mice, for example, mouse lines with green fluorescent protein (EGFP) expressed under the control of microglia-specific gene promoters like Iba1. 109 Slice cultures derived from these mice allow live fluorescent imaging of microglial phagocytosis. 110 As for live imaging, PET imaging allows the observance of live changes in microglial phagocytosis with radiotracer [11C]PBR28 or [11C]PK11195 labelled translocator protein (TSPO) on microglial mitochondria. 111
Zebrafish is also a powerful tool to visualize the phagocytic microglia and macrophages. 112 Transgenic line Tg(mpeg1.1:mVenus-CAAX; sox10:mRFP) was created to label microglia membrane with calcium indicator and a threshold was set to distinguish calcium transients from membrane fluctuations. 102 Timelapse imaging of a microglia engulfing molecules could be directly investigated. 102 With this method, we can combine optical, genetic, chemogenetic and behavioral approaches together to detect the mechanism behind microglia phagocytosis. (Figure 5)
Therapeutic strategies targeting microglial phagocytosis after stroke
To date, few effective treatment options are available for ischemic stroke. Tissue plasminogen activator (tPA) is now the only United States Food and Drug Administration (USFDA) approved drug for ischemic stroke treatment. However, the effective window drops within 3–4.5 h after ischemic stroke onset. Microglial based therapies represent a potential therapeutic strategy for stroke since microglia are involved in multiple steps and aspects of the pathogenesis of stroke. 113 As phagocytosis is one of the more critical steps, targeting this process may lead to promising therapeutics.
Pharmacotherapy targeting microglia phagocytosis for stroke treatment
Numerous established drugs used to treat other ailments, notably minocycline and statins, have recently been shown to modulate microglial functions. Minocycline, a second-generation tetracycline commonly used to treat a vast array of infections, has been shown to reduce tissue infarction after brain focal ischemia. 114 Early post-stroke treatment with minocycline leads to a 57% decrease in the microglia subpopulation that expresses CD68, which is a marker for phagocytic microglia, 115 and this decrease influences neuronal plasticity and contributes to improved recovery. 115 Statins are one of the most widely prescribed drugs for lowering blood cholesterol levels, which may mediate their ability to reduce the risk of ischemic stroke. 116 However, in addition to lowering cholesterol, statins have also been reported to have anti-oxidant, anti-inflammatory, and immunomodulatory effects, including inhibiting microglia phagocytosis in a cholesterol-independent manner. 117 These pleiotropic effects may play a role in the protective effects of statins after ischemic stroke observed clinically 118 and in rodent models of ischemia. 117
Modulation of the phagocytic behavior of microglia has also been shown to be effective after ICH, which tends to be more severe with poorer long-term prognosis compared to ischemic stroke. 119 Indeed, after ICH, bexarotene, a retinoid X receptor agonist, reduced the toxic effect of erythrocyte metabolites and promoted brain recovery by enhancing microglia/macrophage erythrophagocytosis via upregulation of the phagocytic receptors Axl and CD36. 120 The traditional Chinese medicine, wogonin, a major flavonoid compound isolated from scutellaria radix, facilitated hematoma clearance and neurobehavioral recovery after ICH, by stimulating microglial phagocytosis via activating peroxisome proliferater-activated receptor-γ (PPAR-γ) in vitro. 121
Modulation of the phagocytic behavior of microglia by traditional Chinese medicine is also efficacious against ischemic stroke. Salidroside (SLDS), a phenylpropanoid glycoside extracted from the root of Rhodiola rosea L, is known to regulate inflammation. 122 Salidroside reduced cerebral infarction and improved neurological function after cerebral ischemia by modulating microglial polarization and enhancing microglial phagocytosis. 122 Additionally, modulation of complement receptor 3 (CR3, CD11b/CD18), a member of the β2 integrin family involved in the phagocytosis of myelin debris, with Pseudoginsenoside-F11 (PF11), an ocotillol-type saponin, was found to promote phagocytosis of myelin debris, and contributed to the neuroprotection observed after ischemic stroke. 123
Stem cell therapy targeting microglia phagocytosis for stroke treatment
Stem cell therapy has emerged as an experimental treatment to abrogate stroke-induced inflammation in recent years, 124 and microglia are their main targets. Mesenchymal stem cells (MSCs), otherwise known as mesenchymal stromal cells, exhibit neuroprotective and inflammatory effects in models of ischemic injury. 122 EVs derived from MSCs inhibit microglia phagocytosis of viable neurons after hypoxia-ischemic injury by preventing NF-κB-mediated osteopontin (OPN) expression. 125 Adipose stem cells (ASCs) also have potent immunomodulatory capabilities. ASCs from obese donors (ObASCs) promoted a pro-inflammatory phenotype in microglial cells with impaired phagocytosis, 126 and may possess therapeutic efficacy as a biological immunomodulator in models of inflammatory disease like stroke. Importantly, several clinical studies have shown the high efficiency and safety of these stem cell therapies, and therefore they may represent a novel therapeutic strategy for stroke in the future. 127
Dietary therapy targeting microglia phagocytosis for stroke treatment
Numerous epidemiological studies and large-scale clinical trials have shown that the neuroprotective effects of dietary fatty acids after stroke may be related to their anti-inflammatory properties. 128 However, recent data also show that bioactive dietary fatty acids, such polyunsaturated fatty acids (PUFAs) or saturated fatty acids (SFAs), can cross the BBB to regulate microglia function, including phagocytosis. 124 –126 Several in vitro studies in other CNS diseases have assessed the effect of PUFAs of the n-3 series on microglia phagocytosis and found that docosahexaenoic acid (DHA, 22:6n-3) and eicosapentaenoic acid (EPA, 20:5n-3) increased peptide ingestion by microglia in AD 129 and up-regulated gene expression of biomarkers associated with phagocytosis under myelin pathology. 130 In contrast to in vitro studies, in vivo studies with n-3 PUFA deficient mice displayed increased phagocytosis of apoptotic neurons in neuronal hyperactivity, 131 which might be related to a more complicated in vivo microenvironment. Moreover, offspring of mothers fed an n-3 PUFA deficient diet displayed enhanced phagocytosis of synapses in the hippocampus resulting in excessive synaptic pruning and memory deficits. 132 Thus, it is conceivable that n-3 PUFA might also alter microglial phagocytosis after stroke. However, further investigations are warranted to assess whether this altered phagocytic activity proves to be beneficial after stroke. As for SFAs, not much is currently known regarding how they might modulate microglial phagocytosis. Exposure to the saturated free fatty acid palmitate has been shown to switch microglial activity to phagocytosis. 133 Taken together, immunomodulation of microglia by dietary fatty acids may serve as a potential therapy for stroke and other disorders in the CNS, 134 if subsequent research studies continue to report beneficial results.
Knowledge gaps and future perspectives
In this review, we summarize that microglial phagocytosis is a double-edged sword, playing both beneficial and detrimental roles in stroke. We also introduced current therapeutic strategies targeting microglial phagocytosis after stroke. Here we outline some of the key issues that need to be resolved to have a clearer picture of its complicated functions in the pathophysiology of stroke.
Whether microglial phagocytosis is good or detrimental in stroke is still unknown. Different disease conditions, such as ischemic stroke and hemorrhagic stroke, might have distinct effects on microglial phagocytosis. Inflammation and phagocytosis are co-existing pathological mechanisms after stroke, but they usually each dominate during different stages of the disease. Studies have shown decreased microglial phagocytosis during the chronic phase after stroke, specifically in remote areas, such as the hippocampus, which may contribute to inflammatory and maladaptive processes, potentially resulting in cognitive impairment. Thus, from a long-term perspective, microglial phagocytosis might be of great importance for neuroplastic recovery following stroke.135 However, to fine-tune this approach, the spatiotemporal profile of microglial phagocytosis needs to be further investigated.
Most studies have focused on rodent animal models. However, human brain microglia and animal brain microglia are different. For example, they have different neuroinflammatory mediators, such as TGF-β1, which is important in mice but was proven to be less crucial to adult human microglia. In addition, mouse microglia exhibit robust activation upon LPS stimulation via TLR4, and exert both inflammatory and phagocytic functions, whereas in human brain, TLR4 activation doesn’t stimulate the inflammatory response to the same extent as it does in mouse microglia.136 As for rodent models, most studies and publications use young males,137 however the phagocytic ability of microglia is sex-dependent and phagocytosis of neural debris increases with aging in male and female microglia. In clinical settings, age and sex are critical factors in ischemic stroke pathology,138 and future studies should include more female and aged rodent models. More studies are therefore needed to ascertain whether beneficial preclinical observations regarding microglial phagocytosis in stroke can be translated to the human clinical environment.
Invading macrophage associated phagocytosis is found to be dominant in ICH,39 however such data is not quite evident in ischemic stroke. The ability to distinguish between these two cell populations might be needed since the manipulation and modulation of these two cell populations could be different. More attention should also be paid to the comparison of microglial phagocytosis and non-microglial phagocytosis in stroke and their interactions. For example, microglia and astrocyte both have phagocytic properties and secrete similar cytokines. Astrocytes can regulate microglial migration and phagocytosis in an autocrine or paracrine manner.106 In ischemic and hemorrhagic stroke, microglia/macrophages and astrocytes are differentially involved in phagocytosis.139 Inhibiting phagocytosis of microglia or astrocytes in ischemic stroke attenuated brain damage while in hemorrhagic stroke inhibiting phagocytosis of microglia but not astrocytes improved neurobehavioral outcomes.139 Whether they act in collaboration to clear debris is unknown, thus the crosstalk between the two glia cell types needs further investigation.
In conclusion, understanding the alterations in microglial phagocytosis caused by stroke may prove to be a critical step in uncovering new therapies for this disease. Whether phagocytosis after stroke is beneficial or damaging appears to be context dependent, thus any treatment must ultimately balance these effects to establish an immune milieu that favors tissue repair, which may be a significant challenge. Further research is therefore warranted to expand our understanding of microglial phagocytosis after stroke in the pursuit of novel therapeutic ideas for future stroke treatment.
Supplemental Material
Supplemental material, sj-pdf-1-jcb-10.1177_0271678X221098841 for Microglial phagocytosis and regulatory mechanisms after stroke by Weijie Chen, Yueman Zhang, Xiaozhu Zhai, Lv Xie, Yunlu Guo, Chen Chen, Yan Li, Fajun Wang, Ziyu Zhu, Li Zheng, Jieqing Wan and Peiying Li in Journal of Cerebral Blood Flow & Metabolism
Footnotes
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work is supported by National Natural Science Foundation of China (NSFC, 91957111, 81971096, 82061130224 to PL; 82171279 to JW), New Frontier Technology Joint Research Program (SHDC12019102 to PL), Shanghai Municipal Education Commission-Gaofeng Clinical Medical Grant Support (20181805 to PL), Shuguang Program (20SG17 to PL), and Shanghai Outstanding Academic Leaders Program (20XD1422400 to PL), Newton Advanced Fellowship grant provided by the UK Academy of Medical Sciences (NAF\R11\1010 to PL), Renji Innovative Research Program (PYII20-03 to PL). Shanghai Science and Technology Commission (Outstanding Academic Leader Program 20XD1422400 to PL, and DWZX210320 to JW). This work is also supported by the Shanghai Engineering Research Center of Peri-operative Organ Support and Function Preservation (20DZ2254200).
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
ORCID iD: Peiying Li https://orcid.org/0000-0002-5721-9914
References
- 1.Feske SK. Ischemic stroke. Am J Med 2021; 134: 1457–1464. [DOI] [PubMed] [Google Scholar]
- 2.Mastorakos P, Mihelson N, Luby M, et al. Temporally distinct myeloid cell responses mediate damage and repair after cerebrovascular injury. Nat Neurosci 2021; 24: 245–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jones KA, Maltby S, Plank MW, et al. Peripheral immune cells infiltrate into sites of secondary neurodegeneration after ischemic stroke. Brain Behav Immun 2018; 67: 299–307. [DOI] [PubMed] [Google Scholar]
- 4.Cai W, Dai X, Chen J, et al. STAT6/Arg1 promotes microglia/macrophage efferocytosis and inflammation resolution in stroke mice. JCI Insight 2019; 4: e131355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yenari MA. Microglia, the brain's double agent. J Cereb Blood Flow Metab 2020; 40: S3–S5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Neher JJ, Emmrich JV, Fricker M, et al. Phagocytosis executes delayed neuronal death after focal brain ischemia. Proc Natl Acad Sci U S A 2013; 110: E4098–4107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zheng K, Lin L, Jiang W, et al. Single-cell RNA-seq reveals the transcriptional landscape in ischemic stroke. J Cereb Blood Flow Metab 2022; 42: 56–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brown GC. Neuronal loss after stroke due to microglial phagocytosis of stressed neurons. IJMS 2021; 22: 13442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kluge MG, Abdolhoseini M, Zalewska K, et al. Spatiotemporal analysis of impaired microglia process movement at sites of secondary neurodegeneration post-stroke. J Cereb Blood Flow Metab 2019; 39: 2456–2470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang LY, Pan J, Mamtilahun M, et al. Microglia exacerbate white matter injury via complement C3/C3aR pathway after hypoperfusion. Theranostics 2020; 10: 74–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fricker M, Oliva-Martin MJ, Brown GC. Primary phagocytosis of viable neurons by microglia activated with LPS or abeta is dependent on calreticulin/LRP phagocytic signalling. J Neuroinflammation 2012; 9: 196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brown GC, Neher JJ. Microglial phagocytosis of live neurons. Nat Rev Neurosci 2014; 15: 209–216. [DOI] [PubMed] [Google Scholar]
- 13.Lehrman EK, Wilton DK, Litvina EY, et al. CD47 protects synapses from excess microglia-mediated pruning during development. Neuron 2018; 100: 120–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zera KA, Buckwalter MS. The local and peripheral immune responses to stroke: implications for therapeutic development. Neurotherapeutics 2020; 17: 414–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Otxoa-de-Amezaga A, Miró-Mur F, Pedragosa J, et al. Microglial cell loss after ischemic stroke favors brain neutrophil accumulation. Acta Neuropathol 2019; 137: 321–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang K, Li J, Zhang Y, et al. Central nervous system diseases related to pathological microglial phagocytosis. CNS Neurosci Ther 2021; 27: 528–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jiang CT, Wu WF, Deng YH, et al. Modulators of microglia activation and polarization in ischemic stroke (review). Mol Med Rep 2020; 21: 2006–2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Abe N, Nishihara T, Yorozuya T, et al. Microglia and macrophages in the pathological Central and peripheral nervous systems. Cells 2020; 9: 2132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang Y, Li H, Li X, et al. TMEM16F aggravates neuronal loss by mediating microglial phagocytosis of neurons in a rat experimental cerebral ischemia and reperfusion model. Front Immunol 2020; 11: 1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Suzuki J, Fujii T, Imao T, et al. Calcium-dependent phospholipid scramblase activity of TMEM16 protein family members. J Biol Chem 2013; 288: 13305–13316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Neher JJ, Neniskyte U, Brown GC. Primary phagocytosis of neurons by inflamed microglia: potential roles in neurodegeneration. Front Pharmacol 2012; 3: 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vilalta A, Brown GC. Neurophagy, the phagocytosis of live neurons and synapses by glia, contributes to brain development and disease. FEBS J 2018; 285: 3566–3575. [DOI] [PubMed] [Google Scholar]
- 23.Linnartz B, Kopatz J, Tenner AJ, et al. Sialic acid on the neuronal glycocalyx prevents complement C1 binding and complement receptor-3-mediated removal by microglia. J Neurosci 2012; 32: 946–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zengeler KE, Lukens JR. Innate immunity at the crossroads of healthy brain maturation and neurodevelopmental disorders. Nat Rev Immunol 2021; 21: 454–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.He C, Liu R, Fan Z, et al. Microglia in the pathophysiology of hemorrhagic stroke and the relationship between microglia and pain after stroke: a narrative review. Pain Ther 2021; 10: 927–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alawieh A, Langley EF, Tomlinson S. Targeted complement inhibition salvages stressed neurons and inhibits neuroinflammation after stroke in mice. Sci Transl Med 2018; 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gitik M, Liraz-Zaltsman S, Oldenborg PA, et al. Myelin down-regulates myelin phagocytosis by microglia and macrophages through interactions between CD47 on myelin and SIRPα (signal regulatory protein-α) on phagocytes. J Neuroinflammation 2011; 8: 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang L, Lu Y, Deng S, et al. SHPS-1 deficiency induces robust neuroprotection against experimental stroke by attenuating oxidative stress. J Neurochem 2012; 122: 834–843. [DOI] [PubMed] [Google Scholar]
- 29.Liu Y, Yang X, Guo C, et al. Essential role of MFG-E8 for phagocytic properties of microglial cells. PloS One 2013; 8: e55754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Strecker JK, Schmidt A, Schabitz WR, et al. Neutrophil granulocytes in cerebral ischemia – evolution from killers to key players. Neurochem Int 2017; 107: 117–126. [DOI] [PubMed] [Google Scholar]
- 31.Cunningham CL, Martinez-Cerdeno V, Noctor SC. Microglia regulate the number of neural precursor cells in the developing cerebral cortex. J Neurosci 2013; 33: 4216–4233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wakselman S, Bechade C, Roumier A, et al. Developmental neuronal death in hippocampus requires the microglial CD11b integrin and DAP12 immunoreceptor. J Neurosci 2008; 28: 8138–8143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Galloway DA, Phillips AEM, Owen DRJ, et al. Phagocytosis in the brain: homeostasis and disease. Front Immunol 2019; 10: 790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brown GC, Vilalta A. How microglia kill neurons. Brain Res 2015; 1628: 288–297. [DOI] [PubMed] [Google Scholar]
- 35.Sierra A, Abiega O, Shahraz A, et al. Janus-faced microglia: beneficial and detrimental consequences of microglial phagocytosis. Front Cell Neurosci 2013; 7: 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lei X, Li H, Li M, et al. The novel Nrf2 activator CDDO-EA attenuates cerebral ischemic injury by promoting microglia/macrophage polarization toward M2 phenotype in mice. CNS Neurosci Ther 2021; 27: 82–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Imai T, Matsubara H, Hara H. Potential therapeutic effects of Nrf2 activators on intracranial hemorrhage. J Cereb Blood Flow Metab 2021; 41: 1483–1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhao X, Sun G, Ting SM, et al. Cleaning up after ICH: the role of Nrf2 in modulating microglia function and hematoma clearance. J Neurochem 2015; 133: 144–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xu J, Chen Z, Yu F, et al. IL-4/STAT6 signaling facilitates innate hematoma resolution and neurological recovery after hemorrhagic stroke in mice. Proc Natl Acad Sci U S A 2020; 117: 32679–32690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Karpathiou G, Papoudou-Bai A, Ferrand E, et al. STAT6: a review of a signaling pathway implicated in various diseases with a special emphasis in its usefulness in pathology. Pathol Res Pract 2021; 223: 153477. [DOI] [PubMed] [Google Scholar]
- 41.McGettrick AF, O'Neill LAJ. The role of HIF in immunity and inflammation. Cell Metab 2020; 32: 524–536. [DOI] [PubMed] [Google Scholar]
- 42.Bok S, Kim Y-E, Woo Y, et al. Hypoxia-inducible factor-1α regulates microglial functions affecting neuronal survival in the acute phase of ischemic stroke in mice. Oncotarget 2017; 8: 111508–111521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.York EM, Zhang J, Choi HB, et al. Neuroinflammatory inhibition of synaptic long-term potentiation requires immunometabolic reprogramming of microglia. Glia 2021; 69: 567–578. [DOI] [PubMed] [Google Scholar]
- 44.Del Rey MJ, Valin A, Usategui A, et al. Hif-1alpha knockdown reduces glycolytic metabolism and induces cell death of human synovial fibroblasts under normoxic conditions. Sci Rep 2017; 7: 3644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Baik SH, Kang S, Lee W, et al. A breakdown in metabolic reprogramming causes microglia dysfunction in Alzheimer's disease. Cell Metab 2019; 30: 493–507.e496. [DOI] [PubMed] [Google Scholar]
- 46.Bernstein DL, Zuluaga-Ramirez V, Gajghate S, et al. miR-98 reduces endothelial dysfunction by protecting blood-brain barrier (BBB) and improves neurological outcomes in mouse ischemia/reperfusion stroke model. J Cereb Blood Flow Metab 2020; 40: 1953–1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zingale VD, Gugliandolo A, Mazzon E. MiR-155: an important regulator of neuroinflammation. IJMS 2021; 23: 90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pena-Philippides JC, Caballero-Garrido E, Lordkipanidze T, et al. In vivo inhibition of miR-155 significantly alters post-stroke inflammatory response. J Neuroinflammation 2016; 13: 287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yang J, Cao LL, Wang XP, et al. Neuronal extracellular vesicle derived miR-98 prevents salvageable neurons from microglial phagocytosis in acute ischemic stroke. Cell Death Dis 2021; 12: 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Devanney NA, Stewart AN, Gensel JC. Microglia and macrophage metabolism in CNS injury and disease: the role of immunometabolism in neurodegeneration and neurotrauma. Exp Neurol 2020; 329: 113310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mehta MM, Weinberg SE, Chandel NS. Mitochondrial control of immunity: beyond ATP. Nat Rev Immunol 2017; 17: 608–620. [DOI] [PubMed] [Google Scholar]
- 52.Lynch MA. Can the emerging field of immunometabolism provide insights into neuroinflammation? Prog Neurobiol 2020; 184: 101719. [DOI] [PubMed] [Google Scholar]
- 53.Churchward MA, Tchir DR, Todd KG. Microglial function during glucose deprivation: inflammatory and neuropsychiatric implications. Mol Neurobiol 2018; 55: 1477–1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vilalta A, Brown GC. Deoxyglucose prevents neurodegeneration in culture by eliminating microglia. J Neuroinflammation 2014; 11: 58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Peruzzotti-Jametti L, Pluchino S. Targeting mitochondrial metabolism in neuroinflammation: towards a therapy for progressive multiple sclerosis. Trends Mol Med 2018; 24: 838–855. [DOI] [PubMed] [Google Scholar]
- 56.Orellana-Urzúa S, Rojas I, Líbano L, et al. Pathophysiology of ischemic stroke: role of oxidative stress. Curr Pharm Des 2020; 26: 4246–4260. [DOI] [PubMed] [Google Scholar]
- 57.Sun HN, Kim SU, Lee MS, et al. Nicotinamide adenine dinucleotide phosphate (NADPH) oxidase-dependent activation of phosphoinositide 3-kinase and p38 mitogen-activated protein kinase signal pathways is required for lipopolysaccharide-induced microglial phagocytosis. Biol Pharm Bull 2008; 31: 1711–1715. [DOI] [PubMed] [Google Scholar]
- 58.Di Virgilio F, Ceruti S, Bramanti P, et al. Purinergic signalling in inflammation of the central nervous system. Trends Neurosci 2009; 32: 79–87. [DOI] [PubMed] [Google Scholar]
- 59.Fang KM, Yang CS, Sun SH, et al. Microglial phagocytosis attenuated by short-term exposure to exogenous ATP through P2X receptor action. J Neurochem 2009; 111: 1225–1237. [DOI] [PubMed] [Google Scholar]
- 60.Jiang S, Li T, Ji T, et al. AMPK: potential therapeutic target for ischemic stroke. Theranostics 2018; 8: 4535–4551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Labuzek K, Liber S, Gabryel B, et al. Metformin increases phagocytosis and acidifies lysosomal/endosomal compartments in AMPK-dependent manner in rat primary microglia. Naunyn Schmiedebergs Arch Pharmacol 2010; 381: 171–186. [DOI] [PubMed] [Google Scholar]
- 62.Gasparovic C, Rosenberg GA, Wallace JA, et al. Magnetic resonance lipid signals in rat brain after experimental stroke correlate with neutral lipid accumulation. Neurosci Lett 2001; 301: 87–90. [DOI] [PubMed] [Google Scholar]
- 63.Liao B, Geng L, Zhang F, et al. Adipocyte fatty acid-binding protein exacerbates cerebral ischaemia injury by disrupting the blood-brain barrier. Eur Heart J 2020; 41: 3169–3180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Shen Q, Chen Z, Zhao F, et al. Reversal of prolonged obesity-associated cerebrovascular dysfunction by inhibiting microglial Tak1. Nat Neurosci 2020; 23: 832–841. [DOI] [PubMed] [Google Scholar]
- 65.Yang S, Qin C, Hu ZW, et al. Microglia reprogram metabolic profiles for phenotype and function changes in central nervous system. Neurobiol Dis 2021; 152: 105290. [DOI] [PubMed] [Google Scholar]
- 66.Zhang Y, Chen K, Sloan SA, et al. An RNA-sequencing transcriptome and splicing database of glia, neurons, and vascular cells of the cerebral cortex. J Neurosci 2014; 34: 11929–11947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bruce KD, Gorkhali S, Given K, et al. Lipoprotein lipase is a feature of Alternatively-Activated microglia and may facilitate lipid uptake in the CNS during demyelination. Front Mol Neurosci 2018; 11: 57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Liu CC, Liu CC, Kanekiyo T, et al. Apolipoprotein E and Alzheimer disease: risk, mechanisms and therapy. Nat Rev Neurol 2013; 9: 106–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Krasemann S, Madore C, Cialic R, et al. The TREM2-APOE pathway drives the transcriptional phenotype of dysfunctional microglia in neurodegenerative diseases. Immunity 2017; 47: 566–581.e569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dong Y, D'Mello C, Pinsky W, et al. Oxidized phosphatidylcholines found in multiple sclerosis lesions mediate neurodegeneration and are neutralized by microglia. Nat Neurosci 2021; 24: 489–503. [DOI] [PubMed] [Google Scholar]
- 71.den Brok MH, Raaijmakers TK, Collado-Camps E, et al. Lipid droplets as immune modulators in myeloid cells. Trends Immunol 2018; 39: 380–392. [DOI] [PubMed] [Google Scholar]
- 72.Marschallinger J, Iram T, Zardeneta M, et al. Lipid-droplet-accumulating microglia represent a dysfunctional and proinflammatory state in the aging brain. Nat Neurosci 2020; 23: 194–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gao G, Li C, Zhu J, et al. Glutaminase 1 regulates neuroinflammation after cerebral ischemia through enhancing microglial activation and Pro-Inflammatory exosome release. Front Immunol 2020; 11: 161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Nakajima K, Kanamatsu T, Takezawa Y, et al. Up-regulation of glutamine synthesis in microglia activated with endotoxin. Neurosci Lett 2015; 591: 99–104. [DOI] [PubMed] [Google Scholar]
- 75.Khoury ES, Sharma A, Ramireddy RR, et al. Dendrimer-conjugated glutaminase inhibitor selectively targets microglial glutaminase in a mouse model of Rett syndrome. Theranostics 2020; 10: 5736–5748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhu X, Nedelcovych MT, Thomas AG, et al. JHU-083 selectively blocks glutaminase activity in brain CD11b(+) cells and prevents depression-associated behaviors induced by chronic social defeat stress. Neuropsychopharmacology 2019; 44: 683–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mantovani A, Biswas SK, Galdiero MR, et al. Macrophage plasticity and polarization in tissue repair and remodelling. J Pathol 2013; 229: 176–185. [DOI] [PubMed] [Google Scholar]
- 78.Lyu J, Xie D, Bhatia TN, et al. Microglial/macrophage polarization and function in brain injury and repair after stroke. CNS Neurosci Ther 2021; 27: 515–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hu X, Liou AK, Leak RK, et al. Neurobiology of microglial action in CNS injuries: receptor-mediated signaling mechanisms and functional roles. Prog Neurobiol 2014; 119-120: 60–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Denes A, Vidyasagar R, Feng J, et al. Proliferating resident microglia after focal cerebral ischaemia in mice. J Cereb Blood Flow Metab 2007; 27: 1941–1953. [DOI] [PubMed] [Google Scholar]
- 81.Hu X, Li P, Guo Y, et al. Microglia/macrophage polarization dynamics reveal novel mechanism of injury expansion after focal cerebral ischemia. Stroke 2012; 43: 3063–3070. [DOI] [PubMed] [Google Scholar]
- 82.Ronaldson PT, Davis TP. Regulation of blood-brain barrier integrity by microglia in health and disease: a therapeutic opportunity. J Cereb Blood Flow Metab 2020; 40: S6–S24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ting SM, Zhao X, Zheng X, et al. Excitatory pathway engaging glutamate, calcineurin, and NFAT upregulates IL-4 in ischemic neurons to polarize microglia. J Cereb Blood Flow Metab 2020; 40: 513–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Dai X, Chen J, Xu F, et al. TGFα preserves oligodendrocyte lineage cells and improves white matter integrity after cerebral ischemia. J Cereb Blood Flow Metab 2020; 40: 639–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.McWhorter FY, Wang T, Nguyen P, et al. Modulation of macrophage phenotype by cell shape. Proc Natl Acad Sci U S A 2013; 110: 17253–17258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Shi C, Pamer EG. Monocyte recruitment during infection and inflammation. Nat Rev Immunol 2011; 11: 762–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zhang W, Zhao J, Wang R, et al. Macrophages reprogram after ischemic stroke and promote efferocytosis and inflammation resolution in the mouse brain. CNS Neurosci Ther 2019; 25: 1329–1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Schilling M, Besselmann M, Müller M, et al. Predominant phagocytic activity of resident microglia over hematogenous macrophages following transient focal cerebral ischemia: an investigation using green fluorescent protein transgenic bone marrow chimeric mice. Exp Neurol 2005; 196: 290–297. [DOI] [PubMed] [Google Scholar]
- 89.Fukumoto Y, Tanaka KF, Parajuli B, et al. Neuroprotective effects of microglial P2Y(1) receptors against ischemic neuronal injury. J Cereb Blood Flow Metab 2019; 39: 2144–2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Jolivel V, Bicker F, Binamé F, et al. Perivascular microglia promote blood vessel disintegration in the ischemic penumbra. Acta Neuropathol 2015; 129: 279–295. [DOI] [PubMed] [Google Scholar]
- 91.Jickling GC, Liu D, Ander BP, et al. Targeting neutrophils in ischemic stroke: translational insights from experimental studies. J Cereb Blood Flow Metab 2015; 35: 888–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Neumann J, Sauerzweig S, Ronicke R, et al. Microglia cells protect neurons by direct engulfment of invading neutrophil granulocytes: a new mechanism of CNS immune privilege. J Neurosci 2008; 28: 5965–5975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Jeon H, Kim JH, Kim JH, et al. Plasminogen activator inhibitor type 1 regulates microglial motility and phagocytic activity. J Neuroinflammation 2012; 9: 149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ren H, Han R, Chen X, et al. Potential therapeutic targets for intracerebral hemorrhage-associated inflammation: an update. J Cereb Blood Flow Metab 2020; 40: 1752–1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zheng Y, Tan X, Cao S. The critical role of erythrolysis and microglia/macrophages in clot resolution after intracerebral hemorrhage: a review of the mechanisms and potential therapeutic targets. Cell Mol Neurobiol 2022. doi: 10.1007/s10571-021-01175-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Qin C, Zhou LQ, Ma XT, et al. Dual functions of microglia in ischemic stroke. Neurosci Bull 2019; 35: 921–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Tsai YW, Yang YR, Wang PS, et al. Intermittent hypoxia after transient focal ischemia induces hippocampal neurogenesis and c-Fos expression and reverses spatial memory deficits in rats. PloS One 2011; 6: e24001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Choi JY, Kim JY, Kim JY, et al. M2 phenotype microglia-derived cytokine stimulates proliferation and neuronal differentiation of endogenous stem cells in ischemic brain. Exp Neurobiol 2017; 26: 33–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Moon S, Chang MS, Koh SH, et al. Repair mechanisms of the neurovascular unit after ischemic stroke with a focus on VEGF. IJMS 2021; 22: 8543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Sandvig I, Augestad IL, Håberg AK, et al. Neuroplasticity in stroke recovery. The role of microglia in engaging and modifying synapses and networks. Eur J Neurosci 2018; 47: 1414–1428. [DOI] [PubMed] [Google Scholar]
- 101.Zhang J, Malik A, Choi HB, et al. Microglial CR3 activation triggers long-term synaptic depression in the hippocampus via NADPH oxidase. Neuron 2014; 82: 195–207. [DOI] [PubMed] [Google Scholar]
- 102.Hughes AN, Appel B. Microglia phagocytose myelin sheaths to modify developmental myelination. Nat Neurosci 2020; 23: 1055–1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Berghoff SA, Spieth L, Sun T, et al. Microglia facilitate repair of demyelinated lesions via post-squalene sterol synthesis. Nat Neurosci 2021; 24: 47–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ramesha S, Rayaprolu S, Rangaraju S. Flow cytometry approach to characterize phagocytic properties of Acutely-Isolated adult microglia and brain macrophages in vitro. J Vis Exp 2020; 160. doi: 10.3791/61467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Zhao X, Zhang L, Ting SM, et al. Phagocytosis assay of microglia for dead neurons in primary rat brain cell cultures. Bio Protoc 2016; 6: e1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Jeon H, Lee S, Lee WH, et al. Analysis of glial secretome: the long pentraxin PTX3 modulates phagocytic activity of microglia. J Neuroimmunol 2010; 229: 63–72. [DOI] [PubMed] [Google Scholar]
- 107.Nielsen MM, Lambertsen KL, Clausen BH, et al. Mass spectrometry imaging of biomarker lipids for phagocytosis and signalling during focal cerebral ischaemia. Sci Rep 2016; 6: 39571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Gosselin D, Skola D, Coufal NG, et al. An environment-dependent transcriptional network specifies human microglia identity. Science 2017; 356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Hirasawa T, Ohsawa K, Imai Y, et al. Visualization of microglia in living tissues using Iba1-EGFP transgenic mice. J Neurosci Res 2005; 81: 357–362. [DOI] [PubMed] [Google Scholar]
- 110.Dailey ME, Eyo U, Fuller L, et al. Imaging microglia in brain slices and slice cultures. Cold Spring Harb Protoc 2013; 2013: 1142–1148. [DOI] [PubMed] [Google Scholar]
- 111.Carrier M, Robert M, González Ibáñez F, et al. Imaging the neuroimmune dynamics across space and time. Front Neurosci 2020; 14: 903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Eichhoff G, Brawek B, Garaschuk O. Microglial calcium signal acts as a rapid sensor of single neuron damage in vivo. Biochim Biophys Acta 2011; 1813: 1014–1024. [DOI] [PubMed] [Google Scholar]
- 113.Li LZ, Huang YY, Yang ZH, et al. Potential microglia-based interventions for stroke. CNS Neurosci Ther 2020; 26: 288–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Lu Y, Zhou M, Li Y, et al. Minocycline promotes functional recovery in ischemic stroke by modulating microglia polarization through STAT1/STAT6 pathways. Biochem Pharmacol 2021; 186: 114464. [DOI] [PubMed] [Google Scholar]
- 115.Yew WP, Djukic ND, Jayaseelan JSP, et al. Early treatment with minocycline following stroke in rats improves functional recovery and differentially modifies responses of peri-infarct microglia and astrocytes. J Neuroinflammation 2019; 16: 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Zhao W, Xiao ZJ, Zhao SP. The benefits and risks of statin therapy in ischemic stroke: a review of the literature. Neurol India 2019; 67: 983–992. [DOI] [PubMed] [Google Scholar]
- 117.Zhang P, Zhang X, Huang Y, et al. Atorvastatin alleviates microglia-mediated neuroinflammation via modulating the microbial composition and the intestinal barrier function in ischemic stroke mice. Free Radic Biol Med 2021; 162: 104–117. [DOI] [PubMed] [Google Scholar]
- 118.Christophe B, Karatela M, Sanchez J, et al. Statin therapy in ischemic stroke models: a meta-analysis. Transl Stroke Res 2020; 11: 590–600. [DOI] [PubMed] [Google Scholar]
- 119.Zhao X, Kruzel M, Ting SM, et al. Optimized lactoferrin as a highly promising treatment for intracerebral hemorrhage: pre-clinical experience. J Cereb Blood Flow Metab 2021; 41: 53–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Chang CF, Massey J, Osherov A, et al. Bexarotene enhances macrophage erythrophagocytosis and hematoma clearance in experimental intracerebral hemorrhage. Stroke 2020; 51: 612–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Zhuang J, Peng Y, Gu C, et al. Wogonin accelerates hematoma clearance and improves neurological outcome via the PPAR-γ pathway after intracerebral hemorrhage. Transl Stroke Res 2021; 12: 660–675. [DOI] [PubMed] [Google Scholar]
- 122.Liu X, Wen S, Yan F, et al. Salidroside provides neuroprotection by modulating microglial polarization after cerebral ischemia. J Neuroinflammation 2018; 15: 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Liu Y, Wu C, Hou Z, et al. Pseudoginsenoside-F11 accelerates microglial phagocytosis of myelin debris and attenuates cerebral ischemic injury through complement receptor 3. Neuroscience 2020; 426: 33–49. [DOI] [PubMed] [Google Scholar]
- 124.Stonesifer C, Corey S, Ghanekar S, et al. Stem cell therapy for abrogating stroke-induced neuroinflammation and relevant secondary cell death mechanisms. Prog Neurobiol 2017; 158: 94–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Xin D, Li T, Chu X, et al. MSCs-extracellular vesicles attenuated neuroinflammation, synapse damage and microglial phagocytosis after hypoxia-ischemia injury by preventing osteopontin expression. Pharmacol Res 2021; 164: 105322. [DOI] [PubMed] [Google Scholar]
- 126.Harrison MAA, Wise RM, Benjamin BP, et al. Adipose-derived stem cells from obese donors polarize macrophages and microglia toward a Pro-Inflammatory phenotype. Cells 2020; 10: 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Zhou G, Wang Y, Gao S, et al. Potential mechanisms and perspectives in ischemic stroke treatment using stem cell therapies. Front Cell Dev Biol 2021; 9: 646927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Belayev L, Hong SH, Freitas RS, et al. DHA modulates MANF and TREM2 abundance, enhances neurogenesis, reduces infarct size, and improves neurological function after experimental ischemic stroke. CNS Neurosci Ther 2020; 26: 1155–1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Hjorth E, Zhu M, Toro VC, et al. Omega-3 fatty acids enhance phagocytosis of Alzheimer's disease-related amyloid-β42 by human microglia and decrease inflammatory markers. J Alzheimers Dis 2013; 35: 697–713. [DOI] [PubMed] [Google Scholar]
- 130.Chen S, Zhang H, Pu H, et al. n-3 PUFA supplementation benefits microglial responses to myelin pathology. Sci Rep 2014; 4: 7458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Abiega O, Beccari S, Diaz-Aparicio I, et al. Neuronal hyperactivity disturbs ATP microgradients, impairs microglial motility, and reduces phagocytic receptor expression triggering apoptosis/microglial phagocytosis uncoupling. PLoS Biol 2016; 14: e1002466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Madore C, Leyrolle Q, Morel L, et al. Essential omega-3 fatty acids tune microglial phagocytosis of synaptic elements in the mouse developing brain. Nat Commun 2020; 11: 6133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Tu TH, Kim H, Yang S, et al. Linoleic acid rescues microglia inflammation triggered by saturated fatty acid. Biochem Biophys Res Commun 2019; 513: 201–206. [DOI] [PubMed] [Google Scholar]
- 134.Fourrier C, Remus-Borel J, Greenhalgh AD, et al. Docosahexaenoic acid-containing choline phospholipid modulates LPS-induced neuroinflammation in vivo and in microglia in vitro. J Neuroinflammation 2017; 14: 170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Rudolph M, Schmeer CW, Gunther M, et al. Microglia-mediated phagocytosis of apoptotic nuclei is impaired in the adult murine hippocampus after stroke. Glia 2021; 69: 2006–2022. [DOI] [PubMed] [Google Scholar]
- 136.Masuda T, Sankowski R, Staszewski O, et al. Spatial and temporal heterogeneity of mouse and human microglia at single-cell resolution. Nature 2019; 566: 388–392. [DOI] [PubMed] [Google Scholar]
- 137.Shi L, Rocha M, Leak RK, et al. A new era for stroke therapy: integrating neurovascular protection with optimal reperfusion. J Cereb Blood Flow Metab 2018; 38: 2073–2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Roy-O'Reilly M, McCullough LD. Age and sex are critical factors in ischemic stroke pathology. Endocrinology 2018; 159: 3120–3131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Shi X, Luo L, Wang J, et al. Stroke subtype-dependent synapse elimination by reactive gliosis in mice. Nat Commun 2021; 12: 6943. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-jcb-10.1177_0271678X221098841 for Microglial phagocytosis and regulatory mechanisms after stroke by Weijie Chen, Yueman Zhang, Xiaozhu Zhai, Lv Xie, Yunlu Guo, Chen Chen, Yan Li, Fajun Wang, Ziyu Zhu, Li Zheng, Jieqing Wan and Peiying Li in Journal of Cerebral Blood Flow & Metabolism