Abstract
Mitochondrial transplantation/transfer has been increasingly recognized as a potential way for cell and tissue revitalization. In a recent study, Gabelein et al. reported a novel method for single cells mitochondria transplantation using “nanosyringe”. This technique combines atomic force microscopy, optical microscopy, and nanofluidics that enable intra- and intercellular organelle micromanipulation and cell-to-cell mitochondria transplantation with up to 95% success rate. The transferred mitochondria fuse to the host mitochondrial network and donor mtDNA incorporate into the recipient mitochondrial genome. The nanosyringe technique provides a novel tool for future mitochondrial research to offer insight into mitochondrial replacement therapy for stroke and fundamental mitochondrial biology.
Keywords: Mitochondria, mitochondrial transfer, mitochondrial transplantation, nanosyringe, revitalization
Life is the interplay between energy and structure through metabolism, in which mitochondria serve as the central hub for both bioenergetics and biosynthesis. Consequently, mitochondrial dysfunction is highly implicated in a wide range of pathological conditions such as diabetes, cancers, neurodegenerative diseases, and stroke. Various therapeutic approaches have been developing for rescue mitochondrial function to improve the prognosis of these diseases. Pioneering studies indicate that mitochondrial transplantation might be a plausible approach to replenish the damaged mitochondria.1–3 A variety of techniques, including microinjection, coincubation, and magnetomitotransfering have been attempted to improve the efficiency of mitochondrial transplantation. However, all currently established technologies have insufficient efficiencies and mitochondrial transplantation between single cells has not been possible to date.
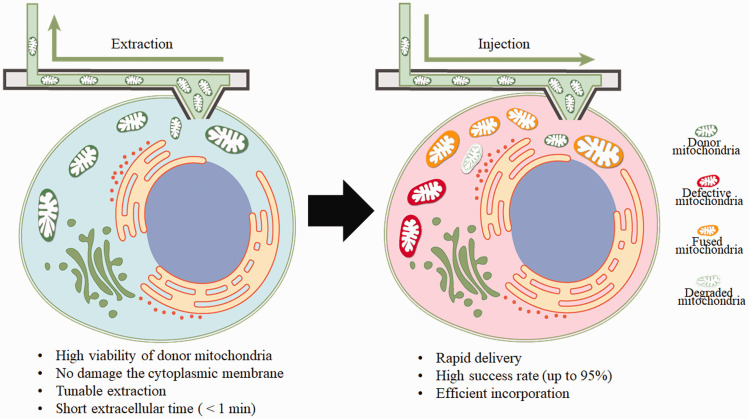
In the March issue of PloS Biology, the team from Zurich, led by Julia A. Vorholt, established a single-cell technology based on fluidic force microscope (FluidFM) for inter- and intracellular micromanipulation of mitochondria. 4 FluidFM combines the high-precision force-regulated approach of an atomic force microscope with the volumetric dispensing of nanoscale pipets under optical inspection, providing the forces and volume control relevant for single-cell manipulation. 5 With this technology, they achieved tunable organelle extraction from single cultured cells without damage cytoplasmic membrane and rapid delivery of the fresh mitochondria to the recipient cells with a success rate up to 95% (Figure 1). Combined with fluorescent labeling, their research demonstrated that the nanosyringe approach preserves mitochondria viability, facilitates their internalization, and improves their incorporation into the mitochondrial network of the recipient cells.
Figure 1.
Therapeutic application of mitochondrial transplantation using the cell-to-cell nanosyringe. Mitochondria of a healthy donor cell are extracted and rapidly injected into a recipient cell. Most of the transplanted mitochondria will fuse with the recipient mitochondria network with a few degrade.
Using this nanosyringe approach, their study offers answers to several critical issues regarding mitochondrial transplantation/transfer therapy. As mitochondria are highly heterogeneous and display distinctive morphology and functional properties, mitochondria from different tissue may not be compatible to each other. 6 The established nanosyringe technique allows real time monitoring of mitochondrial dynamics and tracing mitochondrial fate in the recipient cultured cells. The current study presented that U2OS cells showed little selection for the mitochondria from organelle donor HeLa cells, and the transplanted mitochondria were fused with the mitochondrial network of the recipient cells. Primary human endothelia keratinocytes (HEKa) incorporate a majority of transplanted HeLa cell mitochondria into their network via mitochondrial fusion, and were capable to cope with damaged mitochondria upon transplantation. Interestingly, they found that the fusion or degradation of the transplanted mitochondria was not affected by either amount or state of transplant mitochondria. The host healthy HEKa cells response similar to small or large amount, depolarized or healthy mitochondria. The long-term studies of single cells mitochondrial transplantation over generations of host cells demonstrated the propagation of the transplanted mtDNA within the recipient cell’s mitochondrial genome.
This nanosyringe-mediated cell-to-cell mitochondrial transfer provides a novel and efficient approach to treat neurological diseases, including stroke. Mitochondrial damage is one of the hall markers of stroke due to the insufficient supply of oxygen and glucose, and has been considered as a potential therapeutic target. 8 With the advantage of rapid replenishment of healthy mitochondria, this highly efficient and mini-invasive cell-to-cell mitochondrial transfer shows profound potential for the treatment of stroke. In addition, this technique can be used in conjunction with stem cells or induced pluripotent stem cells to generate healthy cells and reintroduce the cells into patients to improve stroke-induced neurological deficits.8,9 However, there are additional issues need to be addressed before its wide application. Mitochondrial DNA are maternally inherited and closely interact with nuclear DNA. The introduction of allogenic mtDNA may interfere with the nuclear-mtDNA communication of the recipient cells, ultimately affect genomic expression and phenotype. 7 Future research using cell-to-cell mitochondria transplantation with single-cell sequencing technology may enable the identification of the metabolic and genetic factors that impact nuclear mitochondrial crosstalk. The transplanted mitochondria may induce immune response for their bacterial origin or mitochondrial damage-associated molecular patterns (DAMPs). Several studies have reported the increase of proinflammatory chemokines and cytokines after mitochondrial transplantation, and the underlying mechanisms still remains unknown. 10 It is plausible that degradation of the transplanted mitochondria in large amount upon mitochondria transplantation may elicit immune response through DAMPs. The cell-to-cell mitochondria transplantation presented in this paper provide a method that might fine-tune the condition of donor and recipient cells as well as amount of mitochondria for transferring in long-term studies. Thus, this technique will facilitate future research and bring new perspective for mitochondria replacement therapy.
Footnotes
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: National Institutes of Health grants R01NS109583 (SY) and National Natural Science Foundation of China 82071283 (QH).
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Lippert T, Borlongan CV. Prophylactic treatment of hyperbaric oxygen treatment mitigates inflammatory response via mitochondria transfer. CNS Neurosci Ther 2019; 25: 815–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen W, Huang J, Hu Y, et al. Mitochondrial transfer as a therapeutic strategy against ischemic stroke. Transl Stroke Res 2020; 11: 1214–1228. [DOI] [PubMed] [Google Scholar]
- 3.Park JH, Nakamura Y, Li W, et al. Effects of O-GlcNAcylation on functional mitochondrial transfer from astrocytes. J Cereb Blood Flow Metab 2021; 41: 1523–1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gabelein CG, Feng Q, Sarajlic E, et al. Mitochondria transplantation between living cells. PLoS Biol 2022; 20: e3001576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guillaume-Gentil O, Potthoff E, Ossola D, et al. Force-controlled manipulation of single cells: from AFM to FluidFM. Trends Biotechnol 2014; 32: 381–388. [DOI] [PubMed] [Google Scholar]
- 6.Johnson DT, Harris RA, French S, et al. Tissue heterogeneity of the mammalian mitochondrial proteome. Am J Physiol Cell Physiol 2007; 292: C689–697. [DOI] [PubMed] [Google Scholar]
- 7.An H, Zhou B, Ji X. Mitochondrial quality control in acute ischemic stroke. J Cereb Blood Flow Metab 2021; 41: 3157–3170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tseng N, Lambie SC, Huynh CQ, et al. Mitochondrial transfer from mesenchymal stem cells improves neuronal metabolism after oxidant injury in vitro: the role of miro1. J Cereb Blood Flow Metab 2021; 41: 761–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cozene BM, Russo E, Anzalone R, et al. Mitochondrial activity of human umbilical cord mesenchymal stem cells. Brain Circ 2021; 7: 33–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ali Pour P, Hosseinian S, Kheradvar A. Mitochondrial transplantation in cardiomyocytes: Foundation, methods, and outcomes. Am J Physiol Cell Physiol 2021; 321: C489–C503. [DOI] [PMC free article] [PubMed] [Google Scholar]