Abstract
The relationships among cerebral blood flow (CBF), functional connectivity (FC) and suicidal ideation (SI) in major depressive disorder (MDD) patients have remained elusive. In this study, we characterized the changes in CBF and FC among 175 individuals including 47 MDD without SI (MDDNSI), 59 MDD with SI (MDDSI), and 69 healthy control (HC) who underwent arterial spin labeling and resting-state functional MRI scans. Then the voxel-wise CBF, seed-based FC and partial correlation analyses were measured. Mediation analysis was carried out to reveal the effects of FC on the association between CBF and behavioral performances in both subgroups. Results showed that CBF was higher in MDDSI patients in the bilateral precuneus compared to HC and MDDNSI participants. MDDSI patients exhibited enhanced FC in the prefrontal-limbic system and decreased FC in the sensorimotor cortex (SMC) relative to MDDNSI patients. CBF and FC were significantly correlated with clinical variables. More importantly, exploratory mediation analyses identified that abnormal FC can mediate the association between regional CBF and behavioral performances. These results highlight the potential role of precuneus gyrus, prefrontal-limbic system as well as SMC in the process of suicide and provide new insights into the neural mechanism underlying suicide in MDD patients.
Keywords: Depression, suicidal ideation, arterial spin labeling, cerebral blood flow, functional connectivity, mediation analysis
Introduction
Major depressive disorder (MDD) has emerged as a serious global public health problem and it is projected to be the leading economic disease burden by 2030.1,2 The incidence of MDD has increased by 18.4% in the last decade alone. 3 About two-thirds of the MDD patients experience suicidal ideation (SI), 25% exhibit suicidal behaviors, and 15% of them die by suicide.4–6 Moreover, suicide has significantly increased the rates of treatment-resistant depression and delayed the long-term positive outcome in MDD patients. As a core symptom for major depression, SI is defined as considering, thinking about, or planning for suicide without practical action 7 and is highly common in the occurrence and development of suicide.8,9 The degree of SI positively correlates with the likelihood of attempting suicide in the future. 10 Thus, clarifying the mechanisms of SI and reducing the risk of suicide are urgent to be settled currently.
Resting-state functional magnetic resonance imaging (fMRI) assesses changes in brain function, which reflects the spontaneous neural activity of the human brain. 11 Therefore, this method can be used in detecting MDD using specific neurobiological markers and a wealth of neuroimaging studies have been prompted. Existing evidence has linked suicidal behavior to structural and functional changes in the prefrontal-temporal-limbic system. 12 Recent neuroimaging studies have demonstrated a reduction in gray matter volume (GMV), impairment of white matter (WM) integrity, and decrease in amygdala functional connectivity (FC) in the prefrontal-limbic system of patients with bipolar disorder exhibiting SI. 13 MDD patients with SI displayed widespread altered WM, 14 low orbitofrontal-thalamic FC, indicative of impaired decision-making and information integration ability. 15 Recently, accumulating evidence has identified the intricate relationship between cerebral blood flow (CBF) and suicide in individuals with depression. Fluctuation of CBF in the core functional network areas encompassing default-mode network (DMN) is a common phenomenon and CBF-based findings may aid in uncovering MDD biosignatures. 16 Hypoperfusion of the subgenual cingulate cortex increases the risk of suicide in MDD patients with SI and a blunted CBF increase in the prefrontal cortex is thought to generate an opposite effect. 17 However, the potential relationships among the CBF, FC and SI in MDD patients are not well understood.
BOLD fMRI can indirectly reflect neuronal activity, which is a complex interplay involving CBF, cerebral blood volume, and blood oxygen levels. Regional CBF based on arterial spin labeling (ASL) can measure CBF indicators and reflect the energy demand more directly and effectively than fMRI and structural MRI indicators.18–19 Thus, the FC based on CBF difference can better reflect the atypical brain connectivity than directly comparing FC differences. In this paper, we explored the relationships between CBF and FC alterations in MDD patients with or without SI. First, patterns of CBF alteration and the correlations between CBF and behavioral performances in MDD patients with or without SI were illustrated. Second, the FC changes based on those abnormal CBF regions and their behavioral significance were explored. Finally, mediation analyses to explore the effect of altered FCs on the relationship between CBF and behavioral performances in the two subgroup patients were also performed.
Material and methods
Initially, this study incorporated 200 participants. 11 participants could not meet the inclusion criteria. 14 participants were excluded due to poor map qualities. At last, 175 participants in total, including 47 MDD patients with non-suicidal ideation (MDDNSI), 59 MDD patients with suicidal ideation (MDDSI) and 69 healthy control (HC) volunteers, were eventually enlisted into this project. Neuropsychological tests were evaluated and all participants completed the resting-state fMRI scans. HC participants were recruited by community posting and media advertising, and MDD patients were sought in medical centers’ inpatient units and outpatient clinics in the Henan Provincial Mental Hospital Affiliated with Xinxiang Medical University. All participants were Chinese Han descent and were right-handed. In accordance with the guidelines of the Helsinki Declaration of 1975 as revised in 1983, the study was approved by the institutional research ethics committee of Henan Provincial Mental Hospital Affiliated with Xinxiang Medical University (approval ID: 2017-08), and an informed consent was signed prior to the study from all participants.
Inclusion and exclusion criteria
To be included in this study, the MDD patients had to have fulfilled the following: (1) diagnosed with MDD by two independent psychiatrists according to the DSM-IV guidelines; (2) with Hamilton Depression Rating Scale (HAMD)-17 scores >17; (3) between 18 and 59 years, with episodic age <55 years; (4) with no conflicting MRI findings. Those with (1) other underlying major mental illnesses or neural degeneration diseases history; (2) history of drug abuse, craniocerebral trauma, unconsciousness; (3) heart and lung disease; and (4) MRI scan contraindications were excluded from the study.
HC participants must have satisfied the following: a Mini-Mental State Examination (MMSE) score ≥26, and HAMD-17 score ≤7. The exclusion criteria were similar to those of the MDD group.
Subgroup classification of MDD
SI was measured on a scale of 0 to 3 using item 3 of the HAMD-17 scale. A score of 0 was considered the absence of SI as previously described.20–23 As a result, MDD patients were subdivided into MDDNSI and MDDSI groups.
Behavior measurements
All study participants underwent extensive and comprehensive evaluations for neurological and mental status. Demographic information was also captured at baseline. Depression was evaluated using the HAMD-17 scale, 24 whereas the Hamilton Anxiety Rating Scale (HAMA) was used for the degree of anxiety. 25 The HAMD-17 scale assesses five sub-items which include HAMD-anxiety/somatization factor (HAMD-a), HAMD-cognitive disturbance factor (HAMD-c), HAMD-retardation factor (HAMD-r), HAMD-sleep disruption factor (HAMD-s) and HAMD weight factor (HAMD-w). 26
Acquisition of neuroimaging data and imaging parameters
Siemens Verio 3.0-T scanner data were obtained from the Second Affiliated Hospital of Xinxiang Medical University. Participants lay still with their eyes closed during the fMRI scan. Earplugs were used to minimize noise. The heads were held in position using stabilizers. High-resolution T1-weighted anatomical images were acquired using a 3D magnetization-prepared rapid gradient-echo sequence under the following parameters: repetition time (TR) = 1900 ms; echo time (TE) = 2.48 ms; flip angle (FA) = 9°; acquisition matrix of 256 × 256; field of view (FOV) = 250 ×250 mm; thickness = 1.0 mm; gap = 0 mm, number of slices = 176; and acquisition time = 258 s. ASL images were obtained by a pulsed ASL (pASL) sequence. The first volume of all the 105 acquisitions was the M0 image. And the acquisition parameters were: TR = 4000 ms; TE = 12 ms; inversion time 1 (TI1) = 600 ms; inversion time 2 (TI2) = 1600 ms; FA = 90°; acquisition matrix of 64 × 64; FOV = 220 × 220 mm; thickness = 4.0 mm; gap = 1 mm; number of slices = 27 slices, and acquisition time of 434 s. BOLD-fMRI, including 240 volumes, were obtained using gradient-recalled echo-planar imaging (GRE-EPI) sequences under the following parameters: TR = 2000 ms; TE = 25 ms; FA = 90°; acquisition matrix = 64 × 64; FOV = 240 × 240 mm; thickness = 4.0 mm; gap = 0 mm; number of slices = 36 slices; and acquisition time = 486 s.
Image preprocessing
Structural image analyses
Given its significance, the GMV covariate was controlled in the subsequent analyses to reduce the dissect variation.27,28 Optimal voxel-based morphometry (VBM) analyses were performed using the VBM8 toolbox, which was then used to calculate GMV for all the study participants. To ensure that there were no linear changes in MRI images, the anterior-posterior commissure was corrected manually, and the brain images were segmented into different tissue classes of gray matter (GM), WM, and cerebrospinal fluid (CSF) according to the prior probabilities of brain tissues. The optimal template for all the participants was then generated using the VBM-DARTEL.29,30 The GM images were warped to the afore-mentioned template and normalized to Montreal Neurological Institute (MNI) space. Finally, the GM images were resampled to a voxel size of 1.5 mm × 1.5 mm × 1.5 mm and smoothed with a Gaussian kernel of 6-mm full-width at half maximum (FWHM) to improve the signal-to-noise ratio. The modulated GM images were generated for each participant.
CBF calculation
Considering the spatiotemporal noise of ASL data, image processing was performed using the SPM12-based (http://www.fil.ion.ucl.ac.uk/spm) package in ASLtbx. 31 To avoid artifacts caused by improper operation, motion corrections were performed using an algorithm in the ASLtbx. High-pass filtering was a key step to keep the higher half frequency band. The pASL images were then co-registered to the T1 images and spatially smoothed with a Gaussian kernel of 6 × 6 ×6 mm3 FWHM, followed by a single-compartment model after the subtraction of the label images from the control images. After removing outlier time points to enhance CBF map quality, the mean CBF was created from the remaining CBF volumes and was registered into the MNI space after transformation. The relative CBF was obtained to detect perfusion differences. Each participant’s relative CBF was the normalization of their absolute CBF to create uniformity of the CBF values across participants. 32
CBF-based abnormal functional connectivity analyses
Data were preprocessed using the SPM12 and Data Processing & Analysis for Brain Imaging (DPABI 5.1). 33 The first 10 volumes of the scanning session were discarded due to the T1 equilibration effects. The remaining 230 images were corrected for slice timing and realigned (participants with head motion >2 mm in any direction, angular motion >2° of were excluded). The resulting images were normalized to the standard MNI template spatially, resampled to 3-mm isotropic voxels, and smoothed with a Gaussian kernel of 6 × 6 × 6 mm3. The effects of confounding factors including the six head motion parameters, global mean signal, white matter signal, and CSF signal were also removed. Finally, a band-pass filter (0.01 and 0.08 Hz) was applied to the time series for each voxel. Of these, the frame-wise displacement (FD) calculation was performed to index volume-to-volume changes in head position. 34 For FC analysis, the CBF-based region-of-interest (ROI) method was adopted. The relationship between the averaged time course of each CBF-based region and the time courses of all brain voxels was analyzed using Pearson correlation. Fisher z transformation analyses of the correlation coefficients (r) were performed to improve the normality of the data. Finally, the z-FC maps were obtained for further analysis.
Statistical analyses
Demographic and behavioral data
The normality of all continuous variables was checked by the Kolmogorov-Smirnov test. Levene's test was utilized to assess the variance homogeneity. The two-sample t-test was used to examine the differences in the disease duration, HAMD-17 and its sub-scores between MDDNSI and MDDSI groups. The difference of gender was analyzed using chi-square test. One-way analysis of variance (ANOVA) was used for comparing the group differences of age, education, GMV and mean FD among three groups. Post hoc analyses with Bonferroni correction were conducted to find out the source of ANOVA difference. A non-parametric Kruskal-Wallis test was adopted when the Kolmogorov-Smirnov test or Levene's test with p < 0.05 was identified. The significant level was set at p < 0.05. Data were analyzed using SPSS software, version 26 (IBM Corp, Armonk, NY, USA).
CBF and FC analyses
The CBF and FC patterns in each group were obtained using one-sample t test (CBF: corrected with Alphasim, voxel level p < 0.001, cluster level p < 0.01, cluster size >80 × 8 = 640 mm3; FC: corrected with Alphasim, voxel level p < 0.001, cluster level p < 0.01, cluster level >20 × 27 = 540 mm3). The ANOVA was used to compare the group differences of CBF, FC in three groups, after excluding covariates of gender, age, education, GMV and mean FD (CBF: corrected with Alphasim, voxel level p < 0.05, cluster level p < 0.05, cluster size >899 × 8 = 7192 mm3; FC: corrected with Alphasim, voxel level p < 0.05, cluster level p < 0.05, cluster size >211 × 27 = 5697 mm3).
Partial correlation analyses
The relationships between CBF, FC and behavior scores were analyzed using partial correlation analyses, after erasing confounding factors of gender, age, education, GMV and mean FD.
Mediation analyses
First, the voxel-wise correlation analyses between CBF values, FC strength and behavioral scores were carried out separately. Then, the distinctive CBF, FC values were extracted from the overlapping areas. Subsequently, mediation analyses were performed to explore the mediating effect of altered FCs on the relationships between CBF and behavioral performances in the MDDNSI and MDDSI patients, respectively. This analysis was performed using model 4 of PROCESS macro package in SPSS version 26 software. Details of this analysis were provided in the Supplementary Materials.
Results
Demographic information and neuropsychiatric data
Demographic data and behavioral performances of the study participants were shown in Table 1. There was no significant difference in age, gender and mean FD among the three groups. However, both subgroups were less educated than the HC participants. Also, sub-group analysis revealed that MDDSI patients displayed significantly higher HAMD-17, HAMD-c, HAMD-s scores and smaller GMVs when compared with MDDNSI patients (P < 0.05).
Table 1.
Demographic and neuropsychological data across all participants.
| HC (n = 69) | MDD (n = 106) |
P values | ||
|---|---|---|---|---|
| MDDNSI (n = 47) | MDDSI (n = 59) | |||
| Age (years) | 38.55 (12.30) | 36.87 (12.46) | 41.56 (10.63) | 0.115a |
| Gender (F/M) | 38/31 | 19/28 | 36/23 | 0.099b |
| Education levels (years) | 12.57 (3.96) | 10.36 (2.94)c | 10.27 (3.79)d | 0.000a |
| GMV | 637.03 (57.53) | 648.37 (74.08) | 615.88 (46.74)e | 0.017a |
| Mean FD | 0.12 (0.07) | 0.11 (0.06) | 0.12 (0.08) | 0.729a |
| Disease duration (months) | N.A. | 71.53 (80.34) | 75.19 (82.83) | 0.819 |
| HAMD-17 | N.A. | 18.60 (6.04) | 22.85 (4.72)e | 0.000 |
| HAMD-a | N.A. | 4.98 (2.29) | 5.59 (2.41) | 0.185 |
| HAMD-c | N.A | 2.45 (2.09) | 4.29 (2.37)e | 0.000 |
| HAMD-r | N.A. | 6.28 (2.38) | 6.76 (1.84) | 0.239 |
| HAMD-s | N.A. | 3.85 (2.17) | 5.00 (1.87)e | 0.004 |
| HAMD-w | N.A. | 0.45 (0.65) | 0.71 (0.79) | 0.061 |
| HAMA | N.A. | 15.49 (6.65) | 17.68 (5.36) | 0.070 |
Note: ap values were obtained by one-way ANOVA test. When the Kolmogorov-Smirnov test or Levene's test p < 0.05, values were acquired by Kruskal-Wallis test; bp value was obtained by χ2 test; other p values were calculated with two-sample t-test between MDD sub-groups. Unless indicated, data were presented as mean ± standard deviation. Post-hoc analyses were used to reveal the source of ANOVA difference; cstatistical difference was detected between HC group and MDDNSI group; dstatistical difference was detected between HC group and MDDSI group; estatistical difference was detected between MDDNSI group and MDDSI group. F/M: female/male; HC: healthy control; MDD: major depressive disorder; MDDNSI: MDD with non-suicidal ideation; MDDSI: MDD with suicidal ideation; GMV: gray matter volume; FD: frame-wise displacement; HAMD-17: 17-item Hamilton Depression Scale; HAMD-a: Hamilton Depression Scale-anxiety/somatization factor; HAMD-c: Hamilton Depression Scale-cognitive disturbance factor; HAMD-r: Hamilton Depression Scale-retardation factor; HAMD-s: Hamilton Depression Scale-sleep disorder factor; HAMD-w: Hamilton Depression Scale-loss of weight factor; HAMA: Hamilton Anxiety Scale; N.A.: not available.
CBF analyses
The resting-state CBF maps for the three groups were shown in Figure S1. Compared with HCs, the MDDNSI and MDDSI group displayed significantly high CBF in the DMN but low CBF in the bilateral visual cortex and bilateral striatum. The perfusion in the bilateral precuneus in MDDSI group was significantly higher than MDDNSI and HC groups (P < 0.05) (Figure 1(a) and (b), Table S1).
Figure 1.
Group-level differences in CBF and behavioral significance across all participants. (a): Mapping brain regions with significant differences in CBF among three groups. Red color indicates brains regions with elevated CBF and blue color indicates regions with reduced CBF. The color bar represents z scores. (b): Post hoc analysis of the altered CBF in all the groups (Bonferroni correction, p < 0.05). Box plots with each value plotted as dot represent the 25 percentile, the mean (square), the median (line) and the 75 percentile, and whiskers extend to 1.5 times the interquartile range. Significance: *p < 0.05. **p < 0.01, ***p < 0.001. (c): Significantly positive correlations of CBF with HAMD total scores, sub-factor scores, and HAMA in MDDNSI group. (d): Significant correlations of CBF with HAMD total scores, sub-factor scores, and HAMA scores in MDDSI group. CBF: cerebral blood flow; LPCUN: left precuneus; RPCC: right posterior cingulate cortex; LPCC: left posterior cingulate cortex; LSMA: left supplementary motor area; RPCUN: right precuneus; LIns: left insula; LSTG: left superior temporal gyrus; LITG: left inferior temporal gyrus; LVC: left visual cortex; LStr: left striatum; RStr: right striatum; RVC: right visual cortex.
Partial correlation analyses between CBF and behavioral scores
In MDDNSI patients, we found positive correlations between the CBF in the left visual cortex (LVC) and behavior scores, including HAMD-17 (R2 = 0.205, P = 0.002), HAMD-a (R2 = 0.100, P = 0.039), HAMD-r (R2 = 0.124, P = 0.020) and HAMD-w (R2 = 0.141, P = 0.013) scores. A positive relationship was also observed between the CBF in the right visual cortex and HAMD-c (R2 = 0.176, P = 0.005) scores. In MDDSI patients, a positive correlation between the CBF in left posterior cingulate cortex (PCC) and HAMD-c (R2 = 0.071, P = 0.049) as well as HAMD-r (R2 = 0.076, P = 0.042) scores was detected. Likewise, a positive correlation between the CBF in left supplementary motor area (LSMA) and HAMD-r (R2 = 0.095, P = 0.022) scores was also found. The CBF in right PCC displayed a positive correlation with HAMD-c (R2 = 0.126, P = 0.008) scores and a negative correlation with HAMD-w scores (R2 = 0.098, P = 0.020) (Figure 1(c) and (d)).
FC analyses among the three groups
Resting-state CBF-based FC maps across the three groups were illustrated in Figure S2. Each network comprised a positive and negative network. Compared with the HC group, FCs were significantly low in the MDD subgroups in most regions. Compared with the MDDNSI, the FCs of the left precuneus-left middle temporal gyrus (MTG), LSMA-right orbitofrontal cortex (OFC), right striatum-bilateral medial prefrontal cortex (mPFC) were relatively high in MDDSI patients, while the FCs were similar to the HC group in these areas. Compared with the MDDNSI and HC groups, the FCs of the LVC-bilateral sensorimotor cortex (SMC) were also significantly lower in the MDDSI patients (P < 0.05) (Figure 2, Table S2).
Figure 2.
Group-level differences of seed-based FC networks in all participants. (a1) to (j1): Brain regions with significant differences in FC among the three groups shown in a node map. (a2) to (j2): Post hoc analysis of the altered FCs in all the groups (Bonferroni correction, p < 0.05). Box plots with each value plotted as dot represent the 25 percentile, the mean (square), the median (line) and the 75 percentile, and whiskers extend to 1.5 times the interquartile range. Significance: *p < 0.05. **p < 0.01, ***p < 0.001. FC: functional connectivity; LPCUN: left precuneus; RPCUN: right precuneus; LPCC: left posterior cingulate cortex; RPCC: right posterior cingulate cortex; LVC: left visual cortex; RVC: right visual cortex; LStr: left striatum; RStr: right striatum; LSMA: left supplementary motor area; LIns: left insula; RdmPFC: right dorsomedial prefrontal cortex; LMTG: left middle temporal gyrus; RMTG: right middle temporal gyrus; RrACC: right rostral anterior cingulate cortex; RITG: right inferior temporal gyrus; RMTG: right middle temporal gyrus; RFFA: right fusiform area; RSMC: right sensorimotor cortex; LSMC: left sensorimotor cortex; RdlPFC: right dorsolateral prefrontal cortex; RPUT: right putamen; BmPFC: bilateral medial prefrontal cortex; ROFC: right orbitofrontal cortex; RSMA: right supplementary motor area; LTPJ: left temporal parietal junction.
Partial correlation between FC and behavioral scores
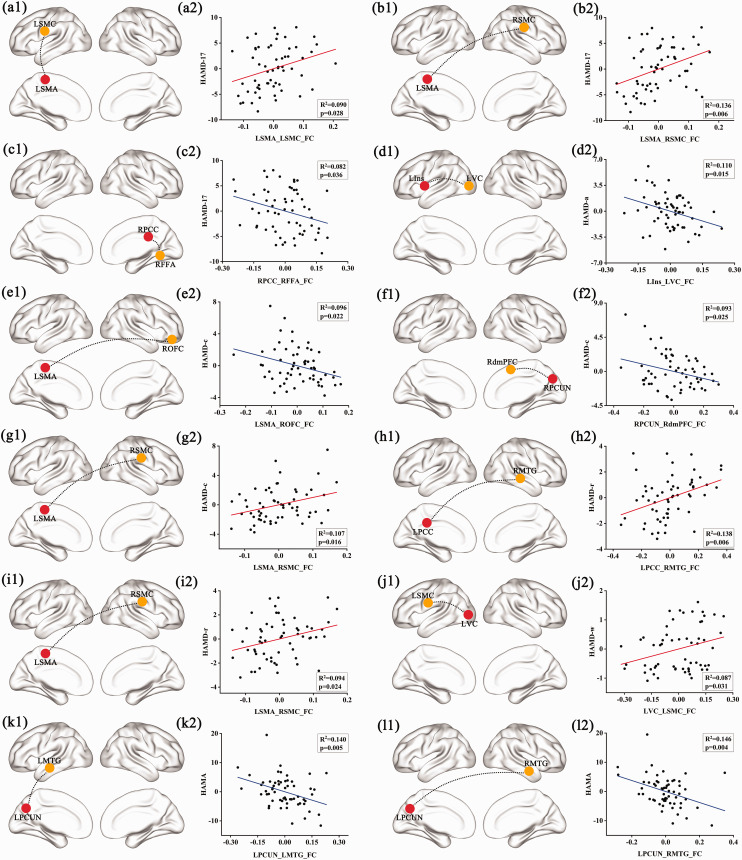
As highlighted in Figure 3, there was no significant correlation between altered FCs and behavioral performances in the MDDNSI group, but the correlations were more widespread in the MDDSI group. More specifically, we observed a positive correlation between the FCs of LSMA-left SMC (R2 = 0.090, P = 0.028) as well as LSMA-right SMC (R2 = 0.136, P = 0.006) and HAMD-17 scores, while a negative correlation was found between the FCs of the right PCC-right fusiform area and HAMD-17 scores (R2 = 0.082, P = 0.036). A negative correlation was observed between the FCs of the left insula (LIns)-LVC and HAMD-a scores (R2 = 0.110, P = 0.015). The FCs of LSMA-right OFC (R2 = 0.096, P = 0.022) as well as the right precuneus-right dorsomedial prefrontal cortex (R2 = 0.093, P = 0.025) displayed a negative association with HAMD-c scores whereas an opposite relationship was observed between the FCs of LSMA-right SMC and HAMD-c scores (R2 = 0.107, P = 0.016). There was a positive correlation between the FCs of left PCC-right MTG (R2 = 0.138, P = 0.006) as well as LSMA-right SMC (R2 = 0.094, P = 0.024) and HAMD-r scores. The FCs of LVC-left SMC positively correlated with HAMD-w scores (R2 = 0.087, P = 0.031). A negative correlation was found between the FCs of left precuneus-left MTG (R2 = 0.140, P = 0.005) as well as the left precuneus-right MTG (R2 = 0.146, P = 0.004) and HAMA scores.
Figure 3.
Correlations of seed-based FC with behavioral scores in MDDSI group but not in MDDNSI group. (a1) to (l1): Seed regions presented in red nodes and target regions with significant differences in FC described in yellow nodes. (a2) to (l2): Significant correlation of FC with HAMD total scores, sub-factor scores, and HAMA. Red line indicates positive correlation while blue line indicates negative correlation. LSMA: left supplementary motor area; LSMC: left sensorimotor cortex; RSMC: right sensorimotor cortex; RPCC: right posterior cingulate cortex; RFFA: right fusiform area; LIns: left insula; LVC: left visual cortex; ROFC: right orbitofrontal cortex; RPCUN: right precuneus; RdmPFC: right dorsomedial prefrontal cortex; LPCC: left posterior cingulate cortex; RMTG: right middle temporal gyrus; LPCUN: left precuneus; LMTG: left middle temporal gyrus; RMTG: right middle temporal gyrus.
Mediation analyses
We found that abnormal FC could mediate the association between CBF and behavioral performances (Figure 4). Specifically, the left precuneus, LIns network connectivity mediated the association between abnormal CBF in the brain regions with right MTG, right OFC and HAMD-c as well as HAMA scores in MDDNSI participants. The LSMA, the left inferior temporal gyrus network connectivity significantly mediated the association between abnormal CBF in the brain regions with bilateral PCC and retardation traits in MDDSI patients.
Figure 4.
Mediation effects of seed-based FC on the association between abnormal CBF and behavioral scores in MDDNSI and MDDSI groups. (a): The LPCUN, LIns network connectivity mediating the association between abnormal CBF in the brain regions with RMTG, ROFC, and HAMD-c scores as well as HAMA scores in MDDNSI participants. (b): The LSMA, LITG network connectivity mediating the association between abnormal CBF in the brain regions with BPCC and retardation traits in MDDSI patients. CBF: cerebral blood flow; FC: functional connectivity; HAMD-c: Hamilton Depression Scale-cognitive disturbance factor; HAMD-r: Hamilton Depression Scale-Retardation factor; HAMA: Hamilton Anxiety Scale; LPCUN: left precuneus; LIns: left insula; RMTG: right middle temporal gyrus; ROFC: right orbital frontal cortex; LSMA: left supplementary motor area; LITG: left inferior temporal gyrus; BPCC: bilateral posterior cingulate cortex.
Discussion
To the best of our knowledge, this cross-sectional study is the first to integrate the CBF and FC approaches to reveal the potential mechanism underlying SI in MDD patients. First, abnormally high CBF perfusion was found in bilateral precuneus of MDDSI patients. Second, the brain lesions related to SI were characterized by disrupted FC in the prefrontal-limbic system and SMC. Third, the CBF-based abnormal FC could mediate the association between altered CBF and behavioral performances in MDDSI patients.
First, ASL-derived CBF may be useful in understanding brain metabolism and function, 35 which can uncover a more specific neural activity. 36 The DMN, which involves the precuneus/PCC, mPFC as well as parietal cortex is widely distributed across brain regions. 37 Functional brain hubs at rest were confirmed primarily in the DMN, insula lobe, visual areas, and shared a striking overlap with the distribution of blood flow supply. 38 That is to say, the abnormal CBF in MDD tended to occur in hub-like regions. For example, the visual cortex center was the major region of receiving and processing visual information. The MDD is also a kind of visual perceptive disorder. 39 The decreased CBF in visual cortex could affect the mood, cognitive function and other neuropsychological activities, which was similar to other research. 40 However, given the significant CBF deficits between participants with or without SI, the abnormal brain metabolic activity was the differentiating factor between the two groups of patients. Altered activities of the regions involved in DMN are linked to the suicide risk in mood disorders. 41 The precuneus is the core hub within DMN, which may be an important region for emotional processing, episodic memory, and self-reflection.42–44 As such, the left precuneus is metabolically overactive in patients with refractory depression. 45 A history of self-injury has a positive correlation with the FC between the right precuneus and putamen. 46 Thus, abnormally high perfusion in the precuneus may be closely related to suicidal thoughts in depressed patients.
Second, according to the neurovascular coupling hypothesis, abnormal CBF may be tightly related to FC changes in the brain. 47 Hence, analysis of the FC network based on abnormal CBF may deepen our understanding of such a mechanism. However, abnormal CBF was observed in the two temporal lobes of MDD patients, but the FC remained normal. This was an early indicator for the development of abnormal CBF in MDD patients, rather than FC. Our findings are generally consistent with previous reports in patients with SI. This implies that integrative dysfunctions of the prefrontal-limbic system may contribute to suicide-related thoughts in MDD patients. Further network analyses revealed that the absolute values of FC in left precuneus-left MTG, LSMA-right OFC, right striatum-bilateral mPFC among three groups presented a U-shape trajectory. In other words, the FC strength trended to decrease from HCs to MDDNSI and increase from MDDNSI to MDDSI with progress in the stages of the disease. The nonlinear alterations indicated that the brain activities were inhibited in the early stages. Our correlation analysis between the altered ROI-FC values and behavioral scores showed that there was no significant correlation between the altered FCs and behavioral performances in the MDDNSI group, while there were wide correlations in MDDSI group, which also reflected the above problems to some extent. And a compensatory increase would happen at an advanced stage. This FC enhancement may link to either compensatory redistribution or dedifferentiation. 48 Thus, the reason why FC appears to “normalize” maybe be that the compensatory brain function may also account for the observations of increased FCs in MDDSI patients. Intriguingly, we observed a reduced FC between the LVC and SMC of MDDSI patients, relative to those of MDDNSI and HC groups. Numerous studies have shown that depression induces varied executive dysfunction such as impaired response and impulse control disorder. This precentral gyrus mediates the executive function of response inhibition, 49 underlying the important relationship among the pathogenic factors of suicide. 50 Put differently, impaired inhibitory control disrupts negative emotional response and increases the risk of suicide. 51 Overt SI is inextricably linked to functional and cortical abnormalities in precentral gyrus in MDD patients.52–53 In addition, the emotional response participates in the brain feedback on the physical movement of SI patients. 54 MDD adolescents with higher SI displayed slow response in the postcentral gyrus (sensory perception) and the precentral gyrus (motor activity), 55 consistent with our findings. Jointly, these findings provide cues and information about SI neural circuits and support our hypotheses that the abnormal FCs in prefrontal-limbic system and SMC may play crucial parts in the vulnerability for SI patients.
Third, the mediation analyses suggested that CBF-based FC could be inferred as the bridge between CBF and behavioral scores. MDD patients often present with cognitive dysfunction. Abnormal FCs between temporal lobe and precuneus which serve as the areas that contributed to cognitive deterioration, are considered to play an important role in MDD pathophysiology. 56 Our result indicates that the right MTG perfusion changes affect behavioral performances at the circuits level in MDDNSI patients. Moreover, patients with anxiety disorders represented hyperactivity of the insular cortex, suggesting that the insula is an important brain region within the emotional circuit with anxiety.57–58 And a growing body of literature recognizes that OFC also engages the process of emotional regulation including anxiety. 59 In addition, decreased activation in the left prefrontal cortex and anxiety-specific increased activation in the right prefrontal cortex were proposed in MDD patients. 60 Our results highlight that CBF alterations cause anxious dysfunction via the disrupted FC between left insula and right OFC in MDDNSI patients. The PCC is also a metabolically active brain region. Psychomotor retardation is a key symptom of MDD. PCC is involved in emotional regulation, episodic memory and cognitive functions. Abnormalities in this region may affect emotional problems including rumination, brooding, and suicidal ideation.61–62 It exerts neural effects in psychomotor retardation and delay in MDDSI individuals, which was different from the results of MDDNSI group, suggesting that PCC is more closely involved in process of SI. The findings of this study have expanded our understanding of the relationship between CBF, FC and behavioral performances. However, little is known about this complex relationship and further investigation is needed.
This study has several limitations and drawbacks as well. First, being the maiden cross-section study, follow-up researches are required to validate the generalizability of the current result. Second, the potential impacts of treatment and the period between onset of illness and time of assessment were not evaluated. Treatment naïve individuals who recently developed MDD and exhibited more analog clinical characteristics are mainly preferred. Third, SI was measured using item 3 of the HAMD-17 scale. Two more professional and comprehensive scales, Columbia suicide severity rating scale and Beck suicide ideation scale, should be added to improve relevant studies in the subsequent data collection. Fourth, white matter hyperintensity (WMH) is remarkably common among the elderly and associated with suicidality in the late-life depression. But we did not find the potential association between WMH and suicide severity. Future work is needed to probe this relationship further.
Taken together, the most prominent representation is the hyperperfusion in the bilateral precuneus, increased FC in the prefrontal-limbic system and decreased FC in LVC-SMC in MDDSI patients. Importantly, as expected, the CBF-based abnormal FC could mediate the association between altered CBF and behavioral performances. These findings reveal the potential mechanism underlying the SI in the MDD patients at the cerebral blood perfusion and neural network level, suggesting that the combination use of ASL and FC approaches maybe an effective approach to help explore the neuroimaging characteristics of MDD patients with SI, as well as offer new insight for suicidal prevention and treatment intervention.
Supplemental Material
Supplemental material, sj-pdf-1-jcb-10.1177_0271678X221090998 for Altered resting-state cerebral blood flow and functional connectivity mediate suicidal ideation in major depressive disorder by Dandan Fan, Cancan He, Xinyi Liu, Feifei Zang, Yao Zhu, Haisan Zhang, Hongxing Zhang, Zhijun Zhang and Chunming Xie in Journal of Cerebral Blood Flow & Metabolism
Acknowledgements
We would like to thank all participants, without whom this research would not have been accomplished.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was funded by the National Key Projects for Research and Development Program of China (2016YFC1305800, 2016YFC1305802), the National Natural Science Foundation of China (82071204, 81671256, 81871069), the Key Project for Research and Development Program of Jiangsu Province (BE2018741), the Key Projects of Jiangsu Commission of Health (ZDB2020008), Jiangsu Innovation & Entrepreneurship Team Program and Zhongyuan One Thousand Talents Program (204200510020).
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Authors’ contributions: CMX conceived the study and provided funding. DDF, CCH, XYL, FFZ and YZ collected clinical and fMRI data. DDF performed data analysis and drafted the manuscript. CMX, HSZ, HXZ, ZJZ modified and proofread the manuscript. All authors reviewed and approved the final version of the manuscript.
Supplemental material: Supplemental material for this article is available online.
References
- 1.Huang CH, Lin MC, Hsieh CL. Acupuncture treatment reduces incidence of Parkinson's disease in patients with depression: a population-based retrospective cohort study in Taiwan. Front Aging Neurosci 2020; 12: 591640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gong L, He C, Yin Y, et al. Mediating role of the reward network in the relationship between the dopamine multilocus genetic profile and depression. Front Mol Neurosci 2017; 10: 292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kanamori S, Takamiya T, Inoue S, et al. Frequency and pattern of exercise and depression after two years in older Japanese adults: the JAGES longitudinal study. Sci Rep 2018; 8: 11224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hay SI, Jayaraman SP, Truelsen T, et al. Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990–2015: a systematic analysis for the global burden of disease study 2015. Lancet 2016; 388: 1545–1602. [DOI] [PMC free article] [PubMed]
- 5.Oh HM, Lee JS, Kim SW, et al. A standardized herbal drug, exerts an anti-depressive effect in a social isolation stress-induced mouse model. Front Pharmacol 2019; 10: 1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sokero TP, Melartin TK, Rytsala HJ, et al. Suicidal ideation and attempts among psychiatric patients with major depressive disorder. J Clin Psychiatry 2003; 64: 1094–1100. [DOI] [PubMed] [Google Scholar]
- 7.Klonsky ED, May AM, Saffer BY. Suicide, suicide attempts, and suicidal ideation. Annu Rev Clin Psychol 2016; 12: 307–330. [DOI] [PubMed] [Google Scholar]
- 8.Brunoni AR, Nunes MA, Lotufo PA, et al. Acute suicidal ideation in middle-aged adults from Brazil. Results from the baseline data of the Brazilian longitudinal study of adult health (ELSA-Brasil). Psychiatry Res 2015; 225: 556–562. [DOI] [PubMed] [Google Scholar]
- 9.Liu X, He C, Fan D, et al. Alterations of core structural network connectome associated with suicidal ideation in major depressive disorder patients. Transl Psychiatry 2021; 11: 243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ordaz SJ, Goyer MS, Ho TC, et al. Network basis of suicidal ideation in depressed adolescents. J Affect Disord 2018; 226: 92–99. [DOI] [PubMed] [Google Scholar]
- 11.Logothetis NK. What we can do and what we cannot do with fMRI. Nature 2008; 453: 869–878. [DOI] [PubMed] [Google Scholar]
- 12.Renteria ME, Schmaal L, Hibar DP, et al. Subcortical brain structure and suicidal behaviour in major depressive disorder: a meta-analysis from the ENIGMA-MDD working group. Transl Psychiatry 2017; 7: e1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Johnston J, Wang F, Liu J, et al. Multimodal neuroimaging of frontolimbic structure and function associated with suicide attempts in adolescents and young adults with bipolar disorder. Am J Psychiatry 2017; 174: 667–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Taylor WD, Boyd B, McQuoid DR, et al. Widespread white matter but focal gray matter alterations in depressed individuals with thoughts of death. Prog Neuropsychopharmacol Biol Psychiatry 2015; 62: 22–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim K, Kim SW, Myung W, et al. Reduced orbitofrontal-thalamic functional connectivity related to suicidal ideation in patients with major depressive disorder. Sci Rep 2017; 7: 15772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cooper CM, Chin FC, Liu P, et al. Discovery and replication of cerebral blood flow differences in major depressive disorder. Mol Psychiatry 2020; 25: 1500–1510. [DOI] [PubMed] [Google Scholar]
- 17.Audenaert K, Goethals I, Van Laere K, et al. SPECT neuropsychological activation procedure with the verbal fluency test in attempted suicide patients. Nucl Med Commun 2002; 23: 907–916. [DOI] [PubMed] [Google Scholar]
- 18.Galiano A, Mengual E, Garcia DER, et al. Coupling of cerebral blood flow and functional connectivity is decreased in healthy aging. Brain Imaging Behav 2020; 14: 436–450. [DOI] [PubMed] [Google Scholar]
- 19.Zhang N, Qin J, Yan J, et al. Increased ASL-CBF in the right amygdala predicts the first onset of depression in healthy young first-degree relatives of patients with major depression. J Cereb Blood Flow Metab 2020; 40: 54–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pu S, Nakagome K, Yamada T, et al. Suicidal ideation is associated with reduced prefrontal activation during a verbal fluency task in patients with major depressive disorder. J Affect Disord 2015; 181: 9–17. [DOI] [PubMed] [Google Scholar]
- 21.Ballard ED, Lally N, Nugent AC, et al. Neural correlates of suicidal ideation and its reduction in depression. Int J Neuropsychopharmacol 2015; 18: 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Park YM, Lee BH, Lee SH. The association between serum lipid levels, suicide ideation, and central serotonergic activity in patients with major depressive disorder. J Affect Disord 2014; 159: 62–65. [DOI] [PubMed] [Google Scholar]
- 23.Pu S, Setoyama S, Noda T. Association between cognitive deficits and suicidal ideation in patients with major depressive disorder. Sci Rep 2017; 7: 11637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry 1960; 23: 56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang T, Zhu J, Xu L, et al. Add-on rTMS for the acute treatment of depressive symptoms is probably more effective in adolescents than in adults: evidence from real-world clinical practice. Brain Stimul 2019; 12: 103–109. [DOI] [PubMed] [Google Scholar]
- 26.Cleary P. GW. Factor analysis of the Hamilton depression scale. Drugs Exp Clin Res 1977; 1: 115–120. [Google Scholar]
- 27.Xie C, Bai F, Yuan B, et al. Joint effects of gray matter atrophy and altered functional connectivity on cognitive deficits in amnestic mild cognitive impairment patients. Psychol Med 2015; 45: 1799–1810. [DOI] [PubMed] [Google Scholar]
- 28.Xie C, Shao Y, Fu L, et al. Identification of hyperactive intrinsic amygdala network connectivity associated with impulsivity in abstinent heroin addicts. Behav Brain Res 2011; 216: 639–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ashburner J. A fast diffeomorphic image registration algorithm. Neuroimage 2007; 38: 95–113. [DOI] [PubMed] [Google Scholar]
- 30.D’Ambrosio E, Jauhar S, Kim S, et al. The relationship between grey matter volume and striatal dopamine function in psychosis: a multimodal (18)F-DOPA PET and voxel-based morphometry study. Mol Psychiatry 2021; 26: 1332–1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang Z, Aguirre GK, Rao H, et al. Empirical optimization of ASL data analysis using an ASL data processing toolbox: ASLtbx. Magn Reson Imaging 2008; 26: 261–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zheng Q, Martin-Saavedra JS, Saade-Lemus S, et al. Cerebral pulsed arterial spin labeling perfusion weighted imaging predicts language and motor outcomes in neonatal hypoxic-ischemic encephalopathy. Front Pediatr 2020; 8: 576489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yan CG, Wang XD, Zuo XN, et al. DPABI: data processing & analysis for (resting-state) brain imaging. Neuroinformatics 2016; 14: 339–351. [DOI] [PubMed] [Google Scholar]
- 34.Power JD, Barnes KA, Snyder AZ, et al. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage 2012; 59: 2142–2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.de la Torre JC. Cerebral hemodynamics and vascular risk factors: setting the stage for Alzheimer's disease. J Alzheimers Dis 2012; 32: 553–567. [DOI] [PubMed] [Google Scholar]
- 36.Liu TT, Brown GG. Measurement of cerebral perfusion with arterial spin labeling: Part 1. Methods. J Int Neuropsychol Soc 2007; 13: 517–525. [DOI] [PubMed] [Google Scholar]
- 37.van Wingen GA, Tendolkar I, Urner M, et al. Short-term antidepressant administration reduces default mode and task-positive network connectivity in healthy individuals during rest. Neuroimage 2014; 88: 47–53. [DOI] [PubMed] [Google Scholar]
- 38.Liang X, Zou Q, He Y, et al. Coupling of functional connectivity and regional cerebral blood flow reveals a physiological basis for network hubs of the human brain. Proc Natl Acad Sci U S A 2013; 110: 1929–1934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Spies M, Klobl M, Hoflich A, et al. Association between dynamic resting-state functional connectivity and ketamine plasma levels in visual processing networks. Sci Rep 2019; 9: 11484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nagafusa Y, Okamoto N, Sakamoto K, et al. Assessment of cerebral blood flow findings using 99mTc-ECD single-photon emission computed tomography in patients diagnosed with major depressive disorder. J Affect Disord 2012; 140: 296–299. [DOI] [PubMed] [Google Scholar]
- 41.Sobczak AM, Bohaterewicz B, Marek T, et al. Altered functional connectivity differences in salience network as a neuromarker of suicide risk in euthymic bipolar disorder patients. Front Hum Neurosci 2020; 14: 585766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cheng X, Chen J, Zhang X, et al. Alterations in resting-state global brain connectivity in bipolar I disorder patients with prior suicide attempt. Bipolar Disord 2021; 23: 474–486. [DOI] [PubMed] [Google Scholar]
- 43.Amft M, Bzdok D, Laird AR, et al. Definition and characterization of an extended social-affective default network. Brain Struct Funct 2015; 220: 1031–1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cavanna AE, Trimble MR. The precuneus: a review of its functional anatomy and behavioural correlates. Brain 2006; 129: 564–583. [DOI] [PubMed] [Google Scholar]
- 45.Leech R, Sharp DJ. The role of the posterior cingulate cortex in cognition and disease. Brain 2014; 137: 12–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cao J, Chen JM, Kuang L, et al. Abnormal regional homogeneity in young adult suicide attempters with no diagnosable psychiatric disorder: a resting state functional magnetic imaging study. Psychiatry Res 2015; 231: 95–102. [DOI] [PubMed] [Google Scholar]
- 47.Wei Y, Wu L, Wang Y, et al. Disrupted regional cerebral blood flow and functional connectivity in pontine infarction: a longitudinal MRI study. Front Aging Neurosci 2020; 12: 577899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang Y, Wang J, Jia Y, et al. Shared and specific intrinsic functional connectivity patterns in unmedicated bipolar disorder and major depressive disorder. Sci Rep 2017; 7: 3570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tsujii N, Mikawa W, Tsujimoto E, et al. Reduced left precentral regional responses in patients with major depressive disorder and history of suicide attempts. Plos One 2017; 12: e175249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bari A, Robbins TW. Inhibition and impulsivity: behavioral and neural basis of response control. Prog Neurobiol 2013; 108: 44–79. [DOI] [PubMed] [Google Scholar]
- 51.Mann JJ. Neurobiology of suicidal behaviour. Nat Rev Neurosci 2003; 4: 819–828. [DOI] [PubMed] [Google Scholar]
- 52.Hwang JP, Lee TW, Tsai SJ, et al. Cortical and subcortical abnormalities in late-onset depression with history of suicide attempts investigated with MRI and voxel-based morphometry. J Geriatr Psychiatry Neurol 2010; 23: 171–184. [DOI] [PubMed] [Google Scholar]
- 53.Sublette ME, Milak MS, Galfalvy HC, et al. Regional brain glucose uptake distinguishes suicide attempters from non-attempters in major depression. Arch Suicide Res 2013; 17: 434–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kragel PA, LaBar KS. Somatosensory representations link the perception of emotional expressions and sensory experience. eNeuro 2016; 3: 169–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nebel MB, Joel SE, Muschelli J, et al. Disruption of functional organization within the primary motor cortex in children with autism. Hum Brain Mapp 2014; 35: 567–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Finotelli P, Dipasquale O, Costantini I, et al. Exploring resting-state functional connectivity invariants across the lifespan in healthy people by means of a recently proposed graph theoretical model. Plos One 2018; 13: e206567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ju A, Fernandez-Arroyo B, Wu Y, et al. Expression of serotonin 1A and 2A receptors in molecular- and projection-defined neurons of the mouse insular cortex. Mol Brain 2020; 13: 99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Liu CH, Liu CZ, Zhu XQ, et al. Increased posterior insula-sensorimotor connectivity is associated with cognitive function in healthy participants with sleep complaints. Front Hum Neurosci 2018; 12: 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Clarke HF, Horst NK, Roberts AC. Regional inactivations of primate ventral prefrontal cortex reveal two distinct mechanisms underlying negative bias in decision making. Proc Natl Acad Sci U S A 2015; 112: 4176–4181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ironside M, Browning M, Ansari TL, et al. Effect of prefrontal cortex stimulation on regulation of amygdala response to threat in individuals with trait anxiety: a randomized clinical trial. JAMA Psychiatry 2019; 76: 71–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fransson P, Marrelec G. The precuneus/posterior cingulate cortex plays a pivotal role in the default mode network: evidence from a partial correlation network analysis. Neuroimage 2008; 42: 1178–1184. [DOI] [PubMed] [Google Scholar]
- 62.Chase HW, Segreti AM, Keller TA, et al. Alterations of functional connectivity and intrinsic activity within the cingulate cortex of suicidal ideators. J Affect Disord 2017; 212: 78–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-jcb-10.1177_0271678X221090998 for Altered resting-state cerebral blood flow and functional connectivity mediate suicidal ideation in major depressive disorder by Dandan Fan, Cancan He, Xinyi Liu, Feifei Zang, Yao Zhu, Haisan Zhang, Hongxing Zhang, Zhijun Zhang and Chunming Xie in Journal of Cerebral Blood Flow & Metabolism