Abstract
Four phase III clinical trials of oral direct factor Xa or thrombin inhibitors demonstrated significantly lower intracranial hemorrhage compared to warfarin in patients with nonvalvular-atrial fibrillation. This is counter-intuitive to the principle that inhibiting thrombosis should increase hemorrhagic risk. We tested the novel hypothesis that anti-thrombin activity decreases the risk of intracerebral hemorrhage by directly inhibiting thrombin-mediated degradation of cerebral microvessel basal lamina matrix, responsible for preventing hemorrhage. Collagen IV, laminin, and perlecan each contain one or more copies of the unique α-thrombin cleavage site consensus sequence. In blinded controlled experiments, α-thrombin significantly degraded each matrix protein in vitro and in vivo in a concentration-dependent fashion. In vivo stereotaxic injection of α-thrombin significantly increased permeability, local IgG extravasation, and hemoglobin (Hgb) deposition together with microvessel matrix degradation in a mouse model. In all formats the direct anti-thrombin dabigatran completely inhibited matrix degradation by α-thrombin. Fourteen-day oral exposure to dabigatran etexilate-containing chow completely inhibited matrix degradation, the permeability to large molecules, and cerebral hemorrhage associated with α-thrombin. These experiments demonstrate that thrombin can degrade microvessel matrix, leading to hemorrhage, and that inhibition of microvessel matrix degradation by α-thrombin decreases cerebral hemorrhage. Implications for focal ischemia and other conditions are discussed.
Keywords: Cerebral microvessels, dabigatran etexilate, extracellular matrix, hemorrhage, thrombin
Introduction
Four randomized phase III clinical studies of oral anticoagulants that directly inhibit factor Xa or thrombin (factor IIa), in the setting of nonvalvular-atrial fibrillation (NVAF), all produced a significantly lower incidence of intracerebral hemorrhage than the standard dose of warfarin.1–5 The RE-LY trial, comparing standard warfarin with the anti-thrombin dabigatran for primary ischemic stroke prevention for NVAF, demonstrated a significantly decreased incidence of intracranial hemorrhage or hemorrhagic stroke, while showing superiority to warfarin for efficacy. 1 A very similar decrease in hemorrhage was observed with all oral anti-factor Xa inhibitors tested so far against warfarin for NVAF.2–5 Explanations for the generally observed relative reduction in intracranial hemorrhage with direct coagulation factor inhibitors in NVAF compared to warfarin have included i) fewer targets for inhibition in the coagulation system compared to warfarin, and therefore better anticoagulation control, ii) relative inhibition of cardiac source thromboembolism compared to warfarin, iii) a relative increase in cerebrovascular thrombosis to limit hemorrhage, and iv) some as yet unknown change in the permeability barrier. However, the common assertion that the risk reduction seen with these agents in NVAF is a consequence of a higher hemorrhagic risk with warfarin management does not acknowledge the excellent warfarin management within all of the direct oral anticoagulant trials. The time in therapeutic range (TTR) was 64.0–68.4% (55.0% in ROCKET-AF where the CHAD2 score was higher 3 ) in these clinical trial settings which is consistent with acceptable clinical practice with warfarin.
Both acute and chronic brain injury target cerebral microvessels, which are unique in structure and function. Acutely, within hours following the onset of focal cerebral ischemia increased permeability of downstream microvessels occurs.6,7 Hemorrhagic transformation of the tissue can also occur, that complicates ischemic stroke.8,9 We have shown that this form of hemorrhage is associated with degradation of microvessel basal lamina matrix in ischemic cerebral regions.9,10 Heo and colleagues further demonstrated the loss of electron density of the microvessel basal lamina within 6 hours of MCA occlusion in the rat. 11 Importantly, in this acute time frame the microvessel basal lamina matrix, which is the barrier to hemorrhage, begins to degrade.9–11 Whether altered microvascular hemostasis in the central nervous system (CNS) is also responsible is still not well understood. 12
While, generally, inhibition of coagulation decreases thrombus formation and thereby increases hemorrhagic risk, in NVAF the oral direct coagulation inhibitors decrease thromboembolic risk, but also appear to decrease the incidence of intracerebral hemorrhage. This unique observation is counterintuitive to our common understanding of hemostasis: with increased anticoagulation the reduction in thrombosis (or thromboembolism, as in NVAF) should increase the risk of hemorrhage. Rosenberg and Aird suggested that hemostasis is organ-specific, and implied that the features of cerebral vascular hemostasis are not fully understood. 13 The recent observations of reduced hemorrhagic transformation in NVAF patients also suggest that there is some link between vascular hemostasis and vessel integrity and that vascular hemostasis might be managed in a unique fashion in the CNS in the setting of focal injury. Here, the direct reversible thrombin inhibitor dabigatran etexilate, in reducing thrombotic risk, could provide a “wedge” into understanding CNS hemorrhage.
Unique to the CNS, particularly in cortical and striatal gray matter, the procoagulant cofactor tissue factor (TF) is expressed by astrocyte end-feet in cerebral microvessels.14–17 del Zoppo and colleagues demonstrated the appearance of fibrin within the walls of striatal microvessels in the ischemic territory by transmission electron microscopy within four (4) hours following middle cerebral artery (MCA) occlusion in the awake non-human primate (see Figure 2 12 ).6,7 Okada and colleagues, using the high-quality murine anti-human fibrin monoclonal antibody MH-1, further demonstrated fibrin deposition in the lumen, wall, and perivascular compartments of microvessels within the ischemic territory within one hour after reperfusion after three hours MCA occlusion. 7 Both observations indicate that thrombin activity can be generated within the microvessel wall, presumably following increased microvessel permeability in consequence of focal ischemia.
Figure 2.
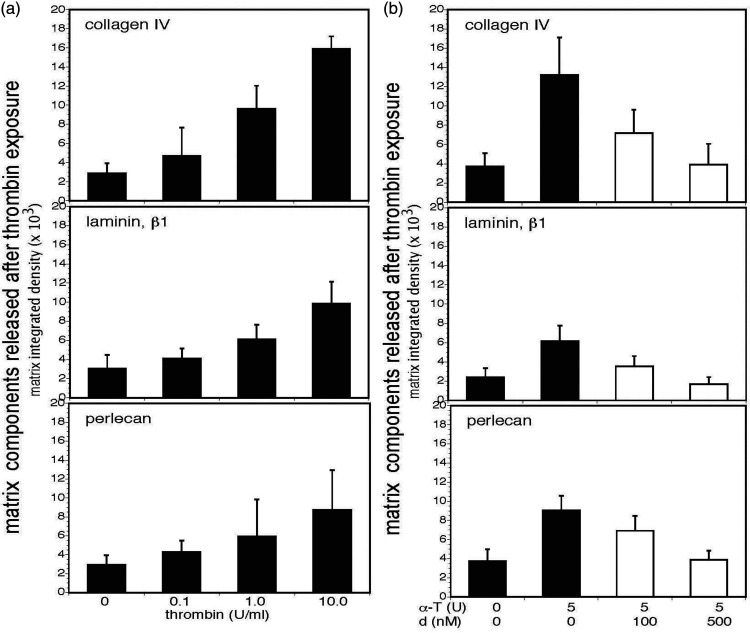
α-thrombin releases matrix products from homogenates of cerebral cortex and dabigatran etexilate prevents their release. Panel a. Concentration-dependent release of products of collagen IV (α1-chain), laminin (β1-chain), and perlecan was detected by immunoblot when homogenates of freshly perfused cortical tissues from naïve adult mice were incubated with α-thrombin (0–10 U/ml) (black bars). Significant differences in the integrated density levels of each purified matrix protein, relative to α-thrombin concentration, were observed: collagen IV, p < 1 × 10−10; laminin, p < 1 × 10−8; and, perlecan, p = 0.002. With each matrix protein there was a significant trend toward increased degradation of the matrix proteins as the concentration of α-thrombin increased. For select individual comparisons, α-thrombin 0U/ml vs 1.0 U/ml: collagen IV, p < 0.0001; laminin, p < 0.0001; and, perlecan, p = 0.0157. Each bar represents n = 9 independent observations and Panel b. Dabigatran etexilate inhibited matrix collagen IV, laminin, and perlecan degradation in homogenates of murine cerebral cortex by 5U murine α-thrombin in a concentration-dependent manner (white bars). Integrated density levels of each matrix protein were significantly different: collagen IV, p < 1 × 10−7; laminin, p < 1 × 10−8; and, perlecan, p < 1 × 10−9. The multiple comparison pattern of perlecan was identical to that of laminin: all pairwise comparisons among the four thrombin combinations were significant, with the exception of the 0/0 vs 5/500 comparison (Bonferroni, α = 0.05). For select individual comparisons, at α-thrombin (α-T) 5 U/ml, dabigatran (d) 0 nM vs100 nM: collagen IV, p = 0.0019; laminin, p = 0.0004; and, perlecan, p = 0.0071. Each bar represents n = 9 independent observations.
Hemorrhagic transformation in the territory-at-risk occurs in up to 65% of ischemic stroke patients and reflects vessel wall injury resulting in blood leakage into the brain, although the exact sequence of molecular events is unclear. 18 Where hemorrhage has taken place, changes in the basal lamina matrix must have occurred. It has also been observed that the risk of intracerebral hemorrhage is greatest with thromboembolism from cardiac sources, which can be made significantly worse by the vitamin K antagonist warfarin. 18 Those observations suggest that hemorrhagic events could result when thromboemboli (from NVAF or atherosclerosis) cause local cerebral ischemia that initiates microvessel matrix degradation. The leakage of plasma coagulation factors could lead to local intra-luminal and intra-mural thrombin generation.
α-thrombin is a pleiotropic serine protease coagulation factor that has i) hemostatic effects, cleaving fibrinogen to form fibrin in the growing thrombus, and, separately, activating platelets; ii) vascular effects, acting via the PAR-1 receptor to increase endothelial permeability to small molecules; and, iii) proteolytic effects, acting to cleave a variety of cellular proteins. 19 Thrombin proteolysis of cerebral vascular basal lamina matrix and its specific targets, in particular, have received little attention. Liotta and colleagues reported that thrombin can cleave members of several matrix protein families, including laminin, fibronectin, and certain collagens in the setting of cancer.20,21 No other observations in this aspect of vascular integrity in the CNS have been made until now.
The hypothesis tested by our experiments states that thrombin inhibition decreases the risk of intracerebral hemorrhage following a thromboembolic event (that causes focal ischemia) by directly blocking thrombin-mediated degradation of extracellular matrix proteins within the cerebral microvessel basal lamina that are responsible for preventing hemorrhage in the CNS.
While α-thrombin can increase the permeability of microvessel endothelium to 4 kDa molecules in vitro, 22 these experiments address i) the impact of α-thrombin on the basal lamina matrix of cerebral microvessels, and ii) the ability of oral dabigatran etexilate to prevent the substantial matrix protein degradation, increased permeability, and hemorrhage by α-thrombin in an in vivo model of cerebral vascular integrity.
Materials and methods
For exact detailed methods please see the Supplemental Materials and Methods.
Animal materials
All animal protocols and experiments were reviewed and approved by the University of Washington Institutional Animal Care and Use Committee. All mice were male (age 8 weeks) of the C57Bl/6J strain (Jackson Laboratory, CA), and were maintained in a secured pathogen-free facility before and during the studies. Non-human primate samples (male, Papio anubis/cyncephalus) were obtained from archived materials, previously described, from experiments at The Scripps Research Institute (TSRI).6,7,23,24 All experiments conformed to the deliberations of STAIR and the ARRIVE 2.0 Guidelines.25,26
Reagents
Agents
Purified murine α-thrombin was obtained from Haematologic Technologies (Essex Junction, VT) as produced by the methods of Lundblad with the modifications described by Nesheim.22,27,28 Zymography of the purified murine α-thrombin preparation on casein-containing gels demonstrated a single cleavage activity (data not shown).
Dabigatran and dabigatran etexilate were gifts from Boehringer-Ingelheim GmbH; dabigatran was used previously for in vitro permeability studies. 22
Purified collagen IV, laminin, and perlecan were obtained from Sigma-Aldrich (St. Louis, MO).
Immunochemicals
Antibodies used for either immunohistochemistry or immunoblots included: i) for perlecan, the rat anti-mouse monoclonal antibody (MoAb) clone A7L6 MAB 1948 P (Chemicon/Millipore) or the mouse MoAb clone 7E12 (Chemicon); ii) for perlecan domain V, the mouse MoAb clone 268908 (R&D Systems); iii) for laminin, the rabbit polyclonal antibody (PoAb) ab128053 (Abcam), the MoAb LAM 89 (Sigma-Aldrich), and for laminin β1-chain, the rat anti-mouse MoAb LT3 clone ab44941 (Abcam); and, iv) for collagen IV, the rabbit PoAb ab6586 (Abcam), for collagen IV α1-chain, the mouse MoAb clone CIV22 (Sigma-Aldrich) and the rabbit PoAb (Novus Biologicals LLC), and for collagen IV α2-chain, a rabbit PoAb (Sigma-Aldrich). For hemoglobin (Hgb), the rabbit anti-mouse hemoglobin PoAb ab191183 (Abcam) was used. All reagents have been used successfully by this laboratory for the purposes intended here, and in previously published experiments.29,30
Solutions
The standard reagent solution for immunohistochemistry and the stereotaxic injection experiments was PBS modified by the addition of Ca2+ and Mg2+ (DPBS, sterile filtered; nr SH30264.01, HyClone Laboratories, Logan UT).
Chow
Dabigatran etexilate was added to standard dietary chow for rodents (Provimi Kliba AG, Switzerland) and provided as standardized pressed tablets in separate vacuum-sealed pouches by Boehringer Ingelheim Pharma GmbH (Biberach, Germany). Dabigatran etexilate was present in the concentration 10 mg/gm chow. Matched placebo-containing chow was also supplied. The pouches were stored at −20°C prior to thawing and use.
Trans-cardiac perfusion fluid
To clear blood elements from the cerebral microvasculature, isosmotic perfusion fluid containing Ringer’s solution, 18 gm/L bovine serum albumin (BSA), and heparin (2 U/ml) (Fresenius Kabi US, Lake Zurich, IL) was used.6,8,15
Immunoprobe procedures
Immunohistochemistry
Antigens of interest in cerebral microvessels were identified on 10 μm frozen coronal sections of mouse brain, as previously described. 23
Quantitative video-imaging (CVI) microscopy
To quantify immunoreactive signals on the 10 µm frozen sections, randomly chosen non-contiguous full fields (1.80 mm2 images) were scanned in full color (RGB) using a modified Zeiss S100 Invert fluorescence microscope mounted by an AxioCam digital camera, equipped with AxioVision and KS400 software (Zeiss, Oberkochen, Germany), and converted to binary output.29,30 Images were scanned into Image J and the digital data analyzed as previously described.
α-thrombin/matrix degradation assays
The impacts of α-thrombin on the purified matrix proteins collagen IV, laminin, and perlecan were assayed with the purified enzyme and matrix substrates.
Homogenization of cerebral cortex/matrix degradation assays
Matrix substrates from the cerebral microvasculature were obtained by homogenization of cortex dissected from perfused mouse brain at 4 °C with a glass tissue grinder (Dounce; Wheaton Scientific, Millville, NJ). Further details about processing are provided in the Supplemental Materials and Methods.
In vivo permeability assay
The whole brain (in vivo) blood-brain barrier permeability measurements used a pulse-chase method modified from that described by Hawkins et al., and adapted for mice.31,32 Under isoflurane (1%) mice underwent thoracotomy and trans-cardiac pulse perfusion with two fluorescent tracer molecules, fluorescein Na+ (NF) and Evans blue albumin (EBA), in combination in the trans-cardiac perfusion solution. For details of the NF assay and the EBA assay, and the calculations of permeability please see the Supplemental Materials and Methods.
Western immunoblot
Immunoblots of matrix protein degradation products were measured as previously described (Supplemental Materials and Methods).23,29,30,32
Stereotaxic delivery
Under isoflurane anesthesia (1.0–2.5%), mice were placed in a stereotaxic frame (David Kopf Instruments, Los Angeles, CA), the skull opened, and a 33-gauge Hamilton injection needle inserted into the center of the right striatum at bregma −1mm anterior (Y); 2 mm lateral (X); to a depth of −3.5 mm (Z), and 2 µl of test agent was injected over 1 minute. There was 100% survival. The brain tissue was removed following trans-cardiac perfusion with isosmotic buffer containing BSA.
Feeding protocol
For the blinded placebo-controlled dabigatran ingestion experiments mice were distributed into separate cages (4 mice/cage) and randomly assigned to the control (placebo-containing chow) and experimental (dabigatran-containing chow) diet groups. The subjects were fed continuously for 14 days prior to entry into the stereotaxic procedure and for 24 hours thereafter with their respective pellets. Twenty-four hours after α-thrombin or vehicle control injection each subject underwent trans-cardiac perfusion, and cerebral tissue harvest. The assignment of the animals was not known to the investigators performing the assays or performing the statistical analyses until the entire data set was available.
Statistical analysis
Data are expressed as mean ± standard deviation of replicate experiments (with parallel duplicate or triplicate measures) on separate days. The number of measurements is shown in each Figure. Data sets were blinded for analysis using the Latin square assignment method. Group sizes were determined with the goal of ensuring a minimal power of 0.80 to detect moderate effect sizes of 0.2 to 0.25 between controls and other groups at a two-sided α of 0.05. We have utilized general linear models (GLMs) followed by post-hoc multiple comparison procedures if warranted as our standard method of analysis. Since individual sample sizes were typically too small to assess accurately any normality assumption within groups, we chose instead to assess normality on the residuals from the GLM fits. We utilized Lilliefors corrected Kolmogorov-Smirnov tests for normality in these assessments; invariably, p-values were non-significant at the α = 0.05 level. Individual t-tests were performed using Prism®. Significance was set conventionally at p ≤ 0.05. Further details are provided in the Supplemental Materials and Methods.
Data availability statement
Fully anonymized data and detailed protocols will be shared upon request from qualified investigators.
Results
While α-thrombin can increase the permeability of endothelial monolayers, 22 little attention has been paid to α-thrombin’s impact on the vascular matrix components to which endothelial cells adhere. Sequence analyses indicate that the unique α-thrombin cleavage site consensus sequence leu-val-arg-gly-ser (LVRGS) is found in the collagen IV α1-chain (three sites), the laminin β1-chain (one site) and laminin α4-precursor (two sites), and in perlecan (four sites), all constituents of CNS vascular basal lamina matrix (see Supplemental Table 1)
α-thrombin-mediated degradation of CNS vascular matrix proteins
Murine α-thrombin (0-10 U/ml), purified by the method certified for purification of human α-thrombin,22,27,28 degraded isolated matrix collagen IV, laminin, and perlecan in vitro in a concentration-dependent manner (Figure 1(a) and Supplemental Figure 1). Further, when homogenates of freshly perfused unfixed murine cerebral tissue were incubated with α-thrombin for 120 minutes at 37°C immunoreactive products of collagen IV (α1-chain), laminin (β1-chain), and perlecan were released in a concentration-dependent manner over the same α-thrombin concentration range (Figure 2(a) and Supplemental Figure 2).
Figure 1.
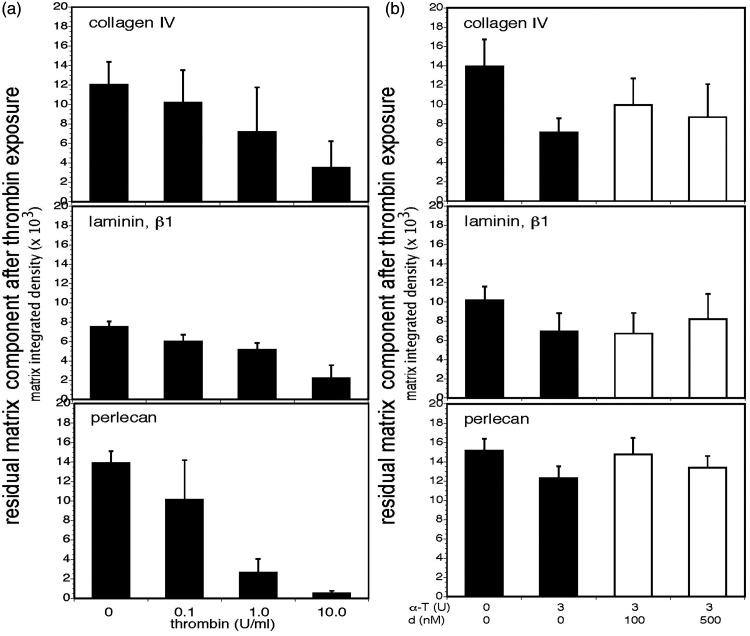
α-thrombin–mediated degradation of purified matrix proteins and inhibition of their degradation. Panel a. Purified murine α-thrombin (0–10 U/ml) degraded three purified matrix proteins in a concentration-dependent manner (black bars). Residual matrix protein content was determined by Western immunoblot with band densities quantified by ImageJ software and reported as “integrated density.” Significant differences in the integrated density levels with each purified matrix protein, relative to thrombin concentrations, were observed: collagen IV, p = 0.00004; laminin, p < 1 × 10−10; and, perlecan, p < 1 × 10−10. For select individual comparisons, α-thrombin 0 U/ml vs 1.0 U/ml: collagen IV, p = 0.020; laminin, p < 0.0001; and, perlecan, p < 0.0001. Each bar represents n = 9 independent observations and Panel b. Dabigatran etexilate inhibited the degradation of purified collagen IV (α1-chain), laminin (β1-chain), and perlecan by 3 U murine α-thrombin in a concentration-dependent manner (white bars). Overall, the integrated density levels of each matrix protein residual were significantly different: collagen IV, p = 0.00006; laminin, p = 0.004; and, perlecan, p = 0.0003. For select individual comparisons, at α-thrombin (α-T) 3 U/ml, dabigatran (d) 0 nM vs100 nM: collagen IV, p = 0.0094; laminin, p = 0.3652; and, perlecan, p = 0.0040. Each bar represents n = 9 independent observations.
Vascular basal lamina matrix degradation by α-thrombin
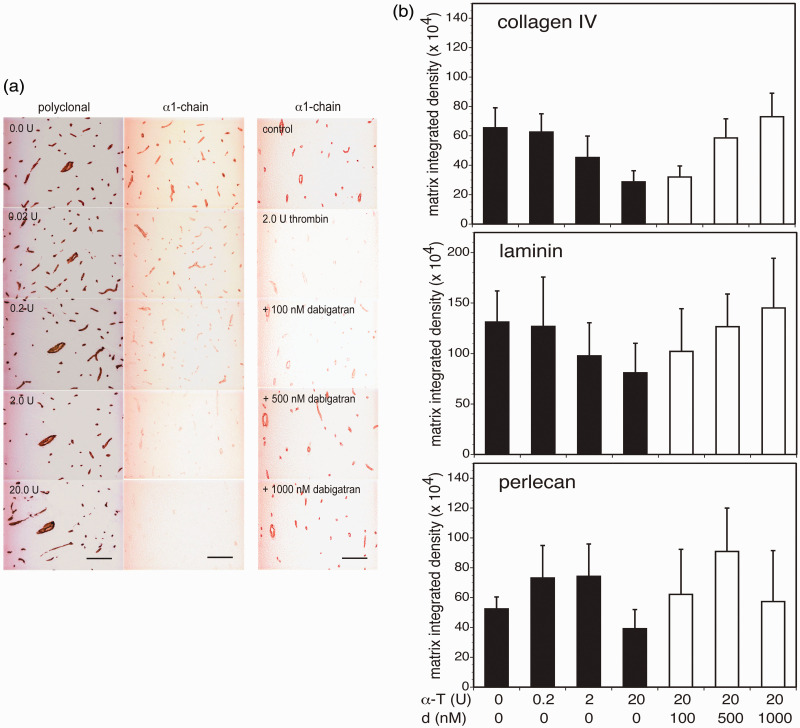
α-thrombin can alter the composition of cerebral microvessel basal lamina matrix, as reported previously for cathepsin L. 23 As demonstrated for collagen IV, incubation of α-thrombin on 10 µm cryosections of adult mouse cortex and of non-human primate striatum decreased α1-chain immunoreactivity, but not that of collagen IV using the PoAb (Figure 3(a) and Supplemental Figure 3, respectively). Simultaneously, in concentration-dependent fashion α-thrombin decreased the microvessel collagen IV (α1-chain), laminin (β1-chain), and perlecan immunoreactivities of adult mouse cortex (Figure 3(b)). Maximum reduction in the three microvessel matrix proteins occurred when 20 U α-thrombin was incubated on mouse tissue (Figure 3(b)). Microvessels of all diameters were affected. Notable was a decrease in mouse tissue collagen IV immunoreactivity (Figure 3(a)), implying an effect of α-thrombin on other (matrix) protein-containing elements of the neuropil.
Figure 3.
α-thrombin and dabigatran etexilate differentially affect microvessel matrix immunoreactivity. Panel a. Immunohistochemistry. Photomicrographs demonstrate decreased collagen IV α1-chain immunoreactivity, but not that of the entire molecule, in cortical microvessel basal lamina by α-thrombin. The concentration-dependent loss in α1-chain immunoreactivity was prevented when dabigatran was co-incubated with 2 U α-thrombin. Magnification bar = 50 µm. NB. The red colorations on the left and right margins of each image are due to refraction of light through the collimator in the low magnification images here. The coloration does not enter into the data quantitation because those images were captured at higher magnification and Panel b. Immunoblots. From general linear model (GLM) analyses with main effects α-thrombin (0, 0.2, 2.0, or 20 U) (black bars) and dabigatran (at 0 (placebo), 100, 500, or 1000 nM) (white bars) on microvessel matrix immunoreactivity, both α-thrombin and dabigatran were significant factors: collagen IV, α-thrombin, p=2 × 10−8, and dabigatran, p = 3 × 10−6; laminin, α-thrombin, p < 0.0001, and dabigatran, p = 2 × 10−5; and, perlecan, α-thrombin, p = 0.028, and dabigatran, p = 0.003. Maximum reduction in microvessel collagen IV (α1-chain), laminin (β1-chain), and perlecan immunoreactivity was observed with 20 U α-thrombin. Co-incubation of 20 U α-thrombin with dabigatran produced a concentration-dependent inhibition of microvessel collagen IV (α1-chain) and laminin (β1-chain) immunoreactivity that was maximal at 500nM dabigatran etexilate. For select individual comparisons, at α-thrombin (α-T) 0U vs 20U: collagen IV, p < 0.0001; laminin, p < 0.0001; and, perlecan, p = 0.0028. At α-thrombin (α-T) 20 U, dabigatran (d) 0 nM vs 500 nM: collagen IV, p < 0.0001; laminin, p = 0.0028; and, perlecan, p = 0.0004. Cohorts consisted of n=9 subjects. NB. Please see the non-human primate data of Supplemental Figure 3.
Preservation of microvessel basal lamina matrix by dabigatran etexilate
In a series of blinded controlled in vitro concentration-finding experiments dabigatran etexilate modestly interfered with α-thrombin-mediated degradation of purified matrix collagen IV (α1-chain), laminin (β1-chain), and perlecan by 3 U/ml α-thrombin (Figure 1(b) and Supplemental Figure 1). Moreover, dabigatran markedly interfered with the degradation of all three matrix proteins in homogenized cortical tissue (Figure 2(b) and Supplemental Figure 2). Maximum inhibition was achieved at dabigatran concentrations of 100 and 500 nM, which translate approximately into 50–250 ng/ml in humans (which are in the clinical range). 33 It seems likely that the cortical microvessel matrix proteins presented conformations more suitable for α-thrombin binding than the purified proteins.
In sectioned cortical tissue, parallel experiments demonstrated that dabigatran co-incubated with 20 U α-thrombin inhibited the loss of both collagen IV and laminin antigens (Figure 3(b)). Interestingly, while the loss of perlecan immunoreactivity could be prevented at 500 nM dabigatran, 1000 nM did not appear to impact perlecan degradation. This may reflect possible conformational specificity of target interactions with α-thrombin and/or the anti-thrombin, or the participation of other tissue-containing proteases in matrix degradation (Figure 3(b)). Similarly, on the non-human primate striatum dabigatran inhibited the effect of 2 U α-thrombin on collagen IV (see Supplemental Figure 3).
Matrix collagen IV degradation by α-thrombin in vivo
The impact of murine α-thrombin (80 mU/2 µl in DPBS) or DPBS alone on cerebral microvessel basal lamina matrix in vivo was examined after stereotaxic injection into the right striatum of adult C57Bl/6 mice by blinded randomized assignment, when whole brain permeability was quantified at 24 hours. Trans-cardiac pulse perfusion with fluorescein Na+ (FN) and Evans blue-albumin (EBA) together detects vascular leakage of substances with molecular masses of ∼332 Da and of 65–70 kDa, respectively.31,32 α-thrombin injection significantly increased whole brain permeability to both FN and EBA 1.53 ± 0.22- and 2.34 ± 0.53-fold, respectively, compared to the DPBS control (p = 0.026 and p = 0.024, respectively; n = 3).
Next, preliminary experiments with stereotaxic injections of either 2 µl [DPBS], [α-thrombin + DPBS], or [α-thrombin+dabigatran] into the mouse striatum in blinded randomized fashion evaluated dabigatran inhibition of α-thrombin activity. Based on the pulse perfusion permeability study, injection of 40mU murine α-thrombin significantly decreased microvessel collagen IV (α1-chain) immunoreactivity compared with DPBS alone, and 500 nM dabigatran substantially prevented the reduction in collagen IV (α1-chain) immunoreactivity (Supplemental Figure 4). The microvessel α1-chain immunoreactivity in the [α-thrombin+dabigatran] cohort was not different from DPBS alone. This experiment set the conditions and power for the subsequent extended in vivo experiments.
Inhibition of α-thrombin-mediated microvessel matrix degradation in vivo
Experiments were then designed to test the impact of oral dabigatran etexilate vs placebo, in separate formulated chows ingested daily over 14 days, on the ability of α-thrombin to alter cerebral microvessel matrix structure and permeability in naïve mice. At the end of 14 days of each diet, the subjects received an intra-striatal injection of DPBS or 40 mU α-thrombin in a randomized blinded four-arm study. At 24 hours post-injection, following trans-cardiac perfusion, cerebral tissues were retrieved and the microvessel matrix component immunoreactivities and IgG extravasation were quantified from serial 10 µm frozen sections. Also, two separate parallel studies with identical treatment cohorts were performed for pulse perfusion whole brain permeability and for hemorrhage.
Cerebral vascular permeability
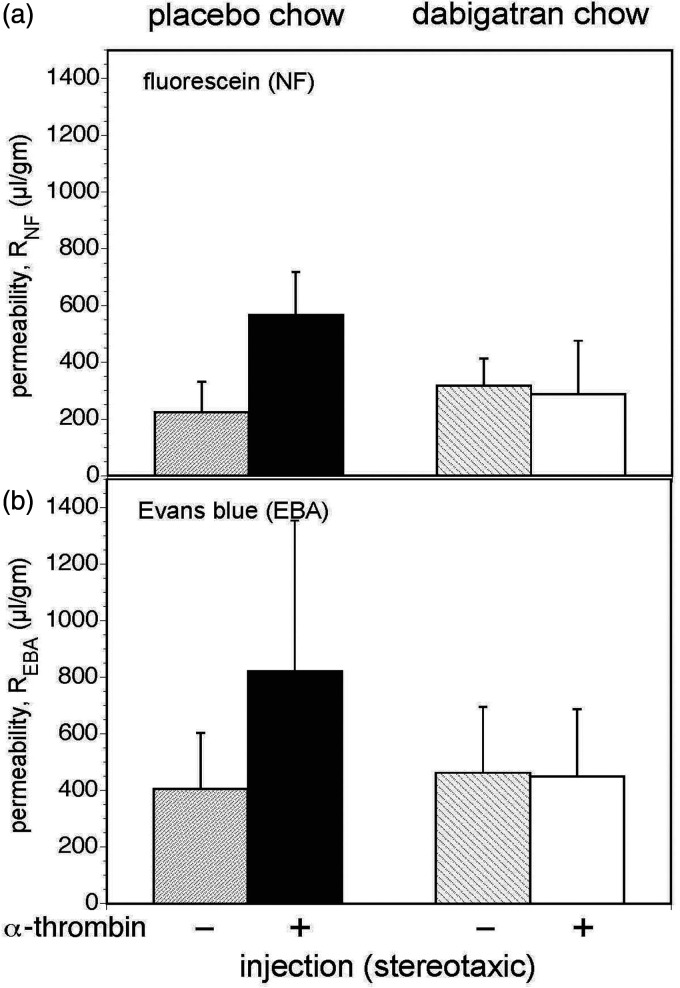
In pulse-chase permeability experiments, injection of 40 mU α-thrombin significantly increased both fluorescein flux and EBA flux compared to DPBS injection in the cohort exposed to placebo-containing chow (Figure 4), indicating significant extravasation of molecules up to 65–70 kDa mass. Ingestion of the dabigatran-containing chow prevented any significant extravasation of either marker (Figure 4).
Figure 4.
Ingestion of dabigatran inhibits increased whole brain permeability following stereotaxic α-thrombin injection. In a balanced factorial design, 24 animals received placebo-containing chow or dabigatran-containing chow for 14 days, and then received intra-striatal injections of either placebo or α-thrombin. Whole (global) brain permeability was measured at 24 hours. Panel a. The permeability to fluorescein Na (RNF) was assessed with 3 replicate readings per animal. A GLM analysis of permeability found a significant α-thrombin effect (p = 1.2 × 10−5), a significant dabigatran effect (p = 0.007), and a large α-thrombin × dabigatran interaction (p = 4 × 10−7). Subsequent pair-wise comparisons found that dabigatran chow lowered permeability compared to placebo chow, and injection of α-thrombin was associated with increased permeability. The significant interaction owes to the significantly higher permeability of the placebo chow–α-thrombin group (black bars) than any of the other three groups (Bonferroni, α = 0.05) and Panel b. The permeability to albumin (REBA) was also assessed with 3 replicate readings per animal. A GLM analysis of permeability found a significant α-thrombin effect (p = 0.011), a significant dabigatran effect (p = 0.045), and a large α-thrombin × dabigatran interaction (p = 0.007). Subsequent pair-wise comparisons yielded results qualitatively identical to those in Panel A (Bonferroni, α = 0.05).
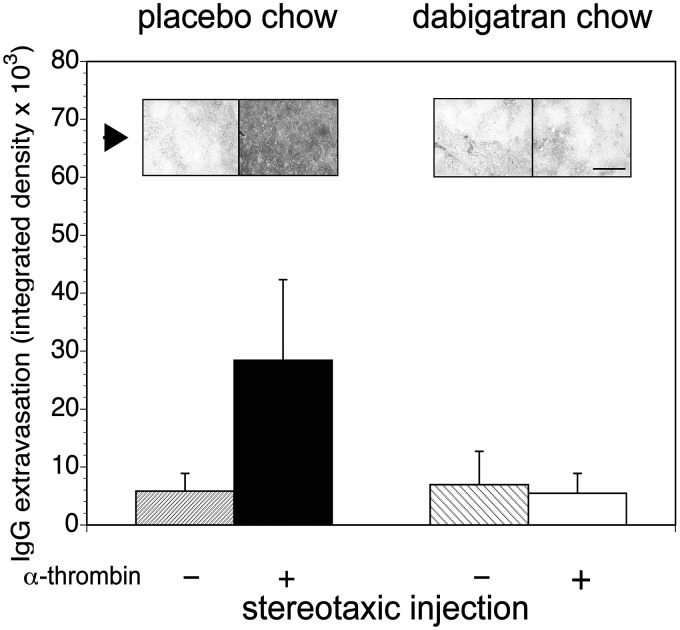
Permeability by IgG extravasation
In a second set of experiments, IgG extravasation increased significantly in the α-thrombin (40 mU) injection group compared to the DPBS injection group receiving placebo-containing chow (Figure 5), indicating permeability to ∼150 kDa molecules. In the group that ingested dabigatran chow and after stereotaxic α-thrombin injection, no increase in IgG extravasation above the animals receiving placebo chow or dabigatran chow and no α-thrombin injection was observed. In subjects receiving dabigatran-containing chow and local α-thrombin, IgG was not detected in the neuropil (Figure 5).
Figure 5.
Dabigatran prevents α-thrombin-induced increase in microvessel permeability to IgG. In a factorial design, stereotaxic injection of either placebo (–) or α-thrombin (+) into the mouse striatum significantly increased IgG extravasation into the neuropil from the microvasculature in animals receiving α-thrombin and placebo chow (see inset (arrow) photomicrographs of immunoreactive IgG in matched fields). Dabigatran-containing chow significantly reduced IgG extravasation (white bar) compared to the placebo chow (black bar). A GLM analysis found a significant α-thrombin effect (p = 0.001), a significant dabigatran effect (p < 0.001), and a large α-thrombin × dabigatran interaction (p = 0.0002). A total of 31 animals received placebo-containing chow (n = 15) or dabigatran-containing chow (n = 16). Magnification bar=100 µm.
Matrix content
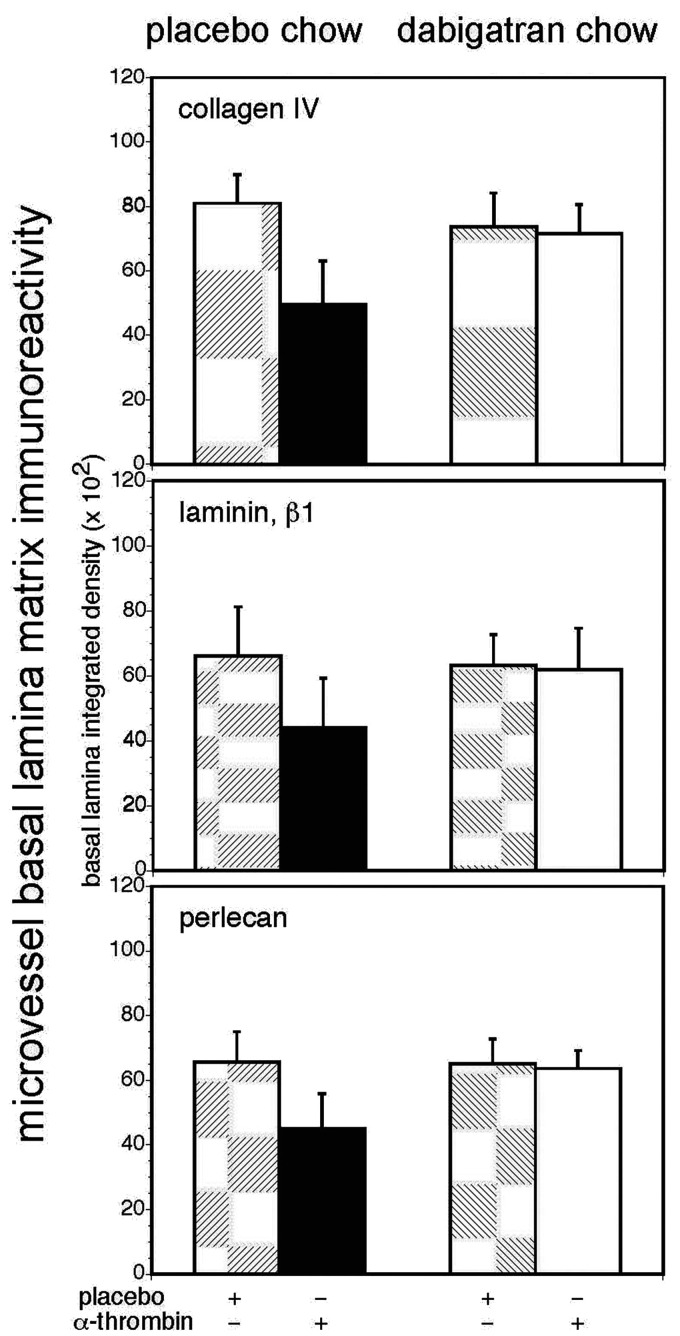
In serial sections from the same subjects, injection of α-thrombin (40 mU) alone significantly decreased collagen IV (α1-chain), laminin (β1-chain), and perlecan immunoreactivity in striatal microvessels compared to DPBS in the cohort receiving placebo-containing chow, whereas no significant change in cerebral microvessel matrix immunoreactivity was observed in subjects ingesting the dabigatran-containing chow after the α-thrombin injection compared to animals receiving placebo chow or dabigatran chow and no α-thrombin injection (Figure 6).
Figure 6.
Dabigatran prevents α-thrombin-induced loss of microvessel basal lamina matrix proteins in vivo. In a balanced factorial design, employing 32 animals, stereotaxic striatal injection of 40 mU α-thrombin significantly decreased microvessel basal lamina collagen IV (α1-chain), laminin (β1-chain), and perlecan immunoreactivity (black bars) compared to placebo in subjects fed placebo-containing chow, while dabigatran ingestion prevented this decrease (white bars). Upper graph. Collagen IV. A GLM analysis of subsequent microvessel collagen IV (α1-chain) immunoreactivity with main effects α-thrombin and dabigatran, found a significant α-thrombin effect (p = 0.001), a borderline significant dabigatran effect (p = 0.063), and a large α-thrombin × dabigatran interaction (p = 0.001), owing to the significantly lower level of collagen IV immunoreactivity (placebo chow–α-thrombin group). Middle graph. Laminin. Similarly, for the laminin β1-chain a significant α-thrombin effect (p = 0.019), a non-significant dabigatran effect (p = 0.125), and a significant α-thrombin × dabigatran interaction (p = 0.036) were found, owing to the significantly lower level of laminin immunoreactivity (placebo chow–α-thrombin group). Lower graph. Perlecan.Continued.For microvessel perlecan immunoreactivity significant main effects for α-thrombin (p = 0.002) and for dabigatran (p = 0.008) and a significant α-thrombin × dabigatran interaction (p = 0.005) were found. The significant interaction owes to the significantly decreased perlecan immunoreactivity in the placebo chow–α-thrombin group relative to the other groups (Bonferroni, α = 0.05).
Hemorrhage
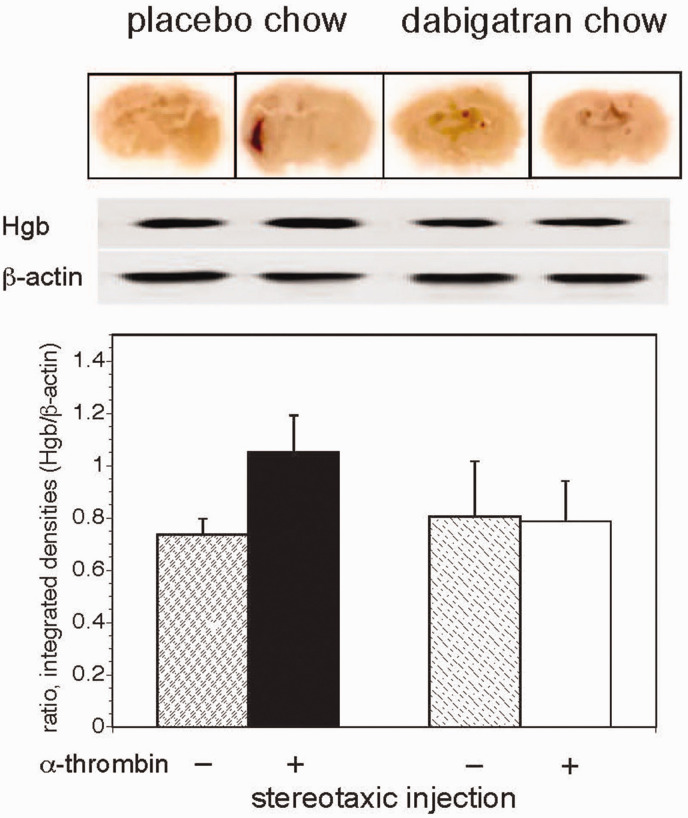
A third set of stereotaxic injection experiments patterned on those above examined the impact of murine α-thrombin on cerebral hemorrhage 24 hours after stereotaxic injection. Preliminary studies indicated that immunoblots of homogenized tissue for murine hemoglobin (Hgb) provided the most sensitive assay for evidence of hemorrhage under these conditions (data not shown). The immunoblots demonstrated a significant increase in cerebral Hgb deposition in the subjects ingesting placebo chow that received intra-striatal α-thrombin (80mU) compared with those receiving placebo injections (Figure 7). Furthermore, in the two groups that received dabigatran there was no difference in Hgb deposition detected, but rather a significant reduction in Hgb deposition after α-thrombin injection compared to the placebo chow/placebo injection group. Notably, in the dabigatran/placebo group two animals displayed small hemorrhages in the striatum along the needle track that probably accounted for the larger standard deviation in this group.
Figure 7.
Dabigatran prevents α-thrombin-induced cerebral hemorrhage in vivo. Stereotaxic striatal α-thrombin injection in subjects receiving placebo-containing chow produced an increase in hemoglobin deposition over those receiving dabigatran-containing chow (n = 9 subjects in each condition, in a balanced factorial design of 36 animals receiving respective chows for 14 days each). Upper image. Coronal sections demonstrate hemoglobin deposition at 24 hours along the injection track in the placebo chow–α-thrombin group only following α-thrombin injection. All sections are scanned wet-mounts of immunohistochemistry preparations for hemoglobin at no magnification (normal image size), which explains the apparent lack of expected detail. Middle image. Evidence of increased hemoglobin immunoreactivity on Western immunoblot in the placebo chow–α-thrombin group compared to the other groups. Lower graph. Immunoreactive hemoglobin relative to β-actin from immunoblots in the middle panel. A GLM analysis of the hemoglobin disposition found a significant α-thrombin effect (p = 0.006), a dabigatran effect of borderline significance (p = 0.062), and a large α-thrombin × dabigatran interaction (p = 0.002). Subsequent pair-wise comparisons demonstrated an increased hemoglobin deposition in the placebo chow–α-thrombin group compared to the other three groups, which were not different among themselves (Bonferroni, α = 0.05).
Discussion
A largely inaccessible aspect of brain vascular response is how intravascular hemostasis interacts with the microvessel wall during injury. In the early hours following the onset of focal cerebral ischemia, in the ischemic core, thrombin is acutely generated within the cerebral microvessel wall. We first demonstrated these microvessel wall events in the non-human primate focal ischemia model of MCA occlusion/reperfusion.6,7 By 4 hours after MCA occlusion fibrin was evident within the microvessel wall, consistent with local thrombin generation. Thrombin is generated when prothrombin (factor II) in plasma interacts with TF in the microvessel astrocyte end-feet.14–17 The further meaning of those observations was not apparent until clinical trials of the direct oral anticoagulants, the anti-thrombin dabigatran and the anti-factor Xa inhibitors, in NVAF all demonstrated a significant reduction in intracerebral hemorrhagic risk compared with warfarin.1–5 That consistent observation could not be explained simply by peripheral inhibition of hemostasis, which might be expected to increase cerebral hemorrhagic risk (even if thromboembolic risk were reduced).
The hypothesis tested here states that pharmacologic anti-thrombin activity could decrease the risk of intracerebral hemorrhage following a thromboembolic event (that causes focal ischemia) by directly inhibiting in situ thrombin-mediated degradation of the cerebral microvessel basal lamina that is responsible for preventing hemorrhage. Together, based on the unique thrombin cleavage sequence, these experiments demonstrate that i) α-thrombin can directly degrade the matrix proteins collagen IV, laminin, and perlecan in vitro and in brain-derived samples of both non-human primate and mouse, and in vivo in cerebral microvessels of the mouse; ii) their degradation is compatible with the thrombin cleavage sequences in these major cerebral vascular matrix components; iii) matrix degradation both in vitro and in vivo can be blocked by dabigatran, directly and after oral ingestion, at clinically-relevant concentrations; and, iv) increased microvessel permeability and hemoglobin deposition in vivo can be prevented by this oral anti-thrombin. These events provide a link between intravascular hemostasis and cerebral microvessel basal lamina matrix integrity, as might occur under conditions of cerebral injury, where the microvessel permeability barrier is breached.
The cerebral microvessel wall includes the endothelium, basal lamina matrix, astrocyte foot processes, and pericytes and histiocytes embedded in the matrix. Within this unique structure the intact matrix is central to limiting the risk of CNS hemorrhage.9,10,24,34 The observation that α-thrombin can increase the permeability of primary cerebral endothelial cell monolayers to small molecules in a capillary model, that is preventable by dabigatran etexilate, 28 is alone insufficient to explain the reduction in hemorrhagic risk seen clinically in NVAF. The very early appearance of thrombin activity in the microvessel wall coincides with the first wave of striatal edema and increased permeability following MCA occlusion,6,35,36 the acute loss of endothelial cell matrix β1-integrin immunoreactivity, 35 and the acute and early loss of matrix constituents.9,10,23,34
Endogenous factors in limiting thrombin action in the CNS
During focal ischemia fibrin is formed when plasma leaks through the microvessel wall encountering TF in astrocyte end-feet (the glia limitans),16,17 where TF:factor VIIa is formed.6,7,29 Functional inhibition of TF:factor VIIa prevents fibrin formation, and reduces intra-microvessel obstruction following focal ischemia. 7 In and beyond the vessel wall, fibrin forms where thrombin is generated. Because hemorrhage is associated with the loss of microvessel matrix in ischemic cerebral regions of primate and rodent focal ischemia models,9,10,24,34 and the microvessel basal lamina is degraded early during focal ischemia,9,10,23,37 where hemorrhage has taken place, degradation of the basal lamina matrix must have occurred.9,10 Could local thrombin generated during focal ischemia cause basal lamina degradation?
The microvessel wall and procoagulant activity
Clinically, it is assumed that manipulation of hemostasis/coagulation peripherally will have a comparable effect within the brain vasculature. This view underlies the anti-thrombotic approaches to reduce cerebral thromboembolic risk. The experiments here support a new premise that hemorrhagic risk during NVAF relates to i) silent thromboembolic focal ischemic events that produce localized microvessel structural injury, ii) increased barrier permeability with plasma leakage, iii) the local generation of thrombin when the leaked plasma interacts with perivascular TF, iv) local degradation of microvessel basal lamina matrix by thrombin, and v) consequent focal hemorrhage. This formulation accords with observations from recent clinical trials that hemorrhagic risk compared to warfarin is reduced by decreasing thrombin activity directly or at the level of the prothrombinase complex.2–5
An immediate limitation of the proof of this premise is the absence of small animal models that mimic both ischemic and hemorrhagic events in atrial fibrillation (AF), although a few models of dysrhythmia have been developed. Hence, currently, neither dabigatran nor the factor Xa inhibitors can be tested for their impact on hemostasis and vascular wall interactions in AF experimentally. Also, while a comparison with warfarin exposure would be instructive, warfarin dosing in rodents to obtain an equipotent anti-thrombin effect is sufficiently problematic that a direct comparison is not possible.38,39 As a result, these experiments focus on steps iii), iv), and v) of the premise above – how thrombin can alter microvessel structure and promote hemorrhage during cerebral injury.
Reports that α-thrombin can cleave laminin, fibronectin, and certain collagens in cancer environments20,21 underscore the lack of data on direct thrombin effects on CNS vascular integrity/infrastructure. Although cleavage is highly specific, thrombin can hydrolyse other proteins, which becomes more general in the denatured state. 19 The CNS limits thrombin activity through interactions with circulating anti-thrombin (AT), tissue factor pathway inhibitor (TFPI), and perivascular protease nexin (PN)-1.40–42 Hence, fibrin formation within the microvessel wall and glia limitans implies that any effects of unopposed thrombin within the microvessel wall must involve high concentrations locally. These events require initial microvessel injury.
Anti-thrombin effects
Dabigatran is a reversible direct highly specific thrombin inhibitor that can penetrate organ systems and has a wide volume of distribution of the aqueous extracellular compartment (1 L/kg). 33 In contrast, the direct anti-thrombin argatroban, that has been used to modulate focal ischemic injury and hemorrhage in model systems,43,44 has a much lower volume of distribution, and also inhibits activation of factors V, VIII, and XIII, protein C activation, fibrin formation, and platelet activation. It also does not penetrate the brain and is therefore unsuitable for these studies.
Matrix degradation
Based on the presence of the unique thrombin cleavage site sequence, in vitro experiments demonstrated that α-thrombin can cause concentration-dependent degradation of the three basal lamina matrix constituents collagen IV, laminin, and perlecan, purified or in cerebral tissue. Dabigatran inhibits matrix substrate degradation, demonstrating the specificity of the α-thrombin action. Important to these studies is the α-thrombin purity.
Ex vivo, on 10 µm cryosections of normal cortex, α-thrombin decreased microvessel basal lamina matrix protein immunoreactivity on the same microvessels in a concentration-dependent manner. These changes were prevented by co-incubation with dabigatran etexilate at human therapeutic concentrations (∼90–185 ng/ml, trough-peak). 45 The incubation studies also imply that α-thrombin can cleave non-vascular matrix and other proteins of the neuropil (Figure 3). This may occur either directly or through release of other matrix proteases held latent in cerebral tissue compartments. A further aspect is the potential stimulation of matrix protease release by thrombin during ischemia. For instance, thrombin and prothrombin in the neuropil can activate microglia,46–49 as can plasma fibronectin and vitronectin, 24 the latter releasing (pro-)MMP-9. Initiation of matrix degradation could lead to further release of inactive proteases stored within the basal lamina matrix.
With these background experiments, the impact of α-thrombin to alter microvessel matrix structure and to increase microvessel permeability measured locally by IgG and globally in the CNS were confirmed in vivo. The significance of these findings is emphasized by the ability of dabigatran exposure for two weeks, ingested to known clinically relevant steady state levels, 45 to prevent both α-thrombin-mediated microvessel matrix degradation and increased microvessel permeability to tracers and larger 150 kDa plasma molecules.
Notable was the variable regional increase in IgG leakage suggesting variability of the increased permeability following direct α-thrombin injection (Figure 5). Subject-to-subject variability was also observed with the whole brain permeability determinations (NF vs EBA) (Figure 4), which are in accord with the variability of thrombin/fibrin deposition seen in the non-human primate following focal cerebral ischemia.7,16
Hemorrhage
Typically, thrombin exposure is associated with intravascular thrombosis, however here hemoglobin deposition was observed in the regions of α-thrombin injection, which appeared extravascular by 24 hours. To avoid behavioral alterations, the stereotaxic settings were arranged such that tissue injury was minimized with the small volume of modest α-thrombin concentration and an incubation time limited to 24 hours. Hemolysis or erythrolysis of an intracerebral hemorrhage can occur within 24 hours in small animal models, 50 so that tissue Hgb deposition can be detectable in the absence of overt hemorrhage. It is possible that higher local α-thrombin levels with longer incubation times could have produced more significant hemorrhage, as hemorrhagic infarction is associated with cerebral microvessel basal lamina degradation.9,10 This scenario is consistent with the concept that hemorrhagic transformation during NVAF would require a microvessel-injuring (e.g., ischemic) event that generates thrombin locally to initiate degradation of the basal lamina.
The possibility that dabigatran etexilate itself could induce hemorrhage at clinically relevant doses/concentrations must be considered. In healthy volunteers receiving dabigatran etexilate no hemorrhagic adverse events were observed, except when higher doses were employed, which were more frequently associated with hematomas at venipuncture sites. 51 Furthermore, in the mice ingesting dabigatran-containing chow in these studies, in experimental studies of arteriosclerosis, and in a recent report of murine models of ageing and of cerebral amyloid angiography, no obvious intracerebral or peripheral hemorrhage was described.52–55 In the experiments here, modest hemorrhage was observed in two mice that received dabigatran. A relevant feature of the anti-thrombin dabigatran is that the volume of distribution is large (1 L/kg in humans), 33 such that in the CNS the perivascular or parenchymal concentration of dabigatran is likely sufficient to inhibit α-thrombin activity generated in the microvessel basal lamina when/if an embolic event occurs, thereby reducing the apparent hemorrhagic risk.
Limitations
Both the cell culture models and in vivo studies are based on successful basic and clinically-relevant studies in the non-human primate.6,7,29,30 However, because there are no appropriate AF models that mimic the conditions of human NVAF, elements of the hypothesis must be studied separately, and some of the in vivo conditions indirectly. This is particularly true of the α-thrombin concentrations in the ischemic microvasculature generated post-ischemia (as shown in the non-human primate 7 ). Hence, concentration-escalation studies were performed with the assumption that if fibrin formation occurs in the microvessel wall or beyond, thrombin activity would have been unopposed by endogenous inhibitors. It cannot be ruled out that sectioning of the cerebral tissues ex vivo exposed basal lamina matrix structures to other proteases in the non-vascular tissue, potentially increasing their sensitivity to α-thrombin action. Differences seen in matrix protein responses among the assays most likely also represent differences in matrix protein accessibility and conformation, in addition to the location of cleavage sequences. Isolation methods are likely to have already altered their conformation from the normal vascular state in vivo. In the in vivo experiments there was no evidence of microvessel thrombosis, suggesting that the thrombin effects here were mostly extravascular, consistent with the stereotaxic delivery. The possibility to examine the effects of α-thrombin directly with dabigatran was an advantage to these studies. This is particularly true for the impact of other matrix proteases generated by focal ischemia, and i) their effects on the generation of thrombin, and ii) the degradation of matrix. Together these observations suggest a contribution of the vascular wall, which cannot be reproduced in cell culture studies. 22
Generalization of the observations
The novel hypothesis was generated by clinical observations in recent NVAF trials, and the studies here highlight the effect of thrombin activity on microvessel matrix structure to explain hemorrhagic events caused by thrombin. It is suggested that in other CNS disorders active thrombin might play a role. 56 These disorders include, but are not limited to, focal cerebral ischemia (ischemic stroke), intracerebral hemorrhage (ICH), 57 arteriovenous malformations (AVMs), 58 multiple sclerosis (MS), 59 germinal matrix hemorrhage, 60 intracerebral hemorrhage due to hypertension,61,62 traumatic brain injury, 63 and CNS neoplasms. 64 To our knowledge at this time the ability of thrombin to degrade cerebral vascular components has not been studied in those disorders or their model systems.
Implications
These findings imply that: 1) a dabigatran-inhibitable extravascular thrombin activity can contribute to increased microvessel permeability observed in cerebral ischemia, 2) matrix degradation by this activity is the principal alteration that is associated with structural changes of the permeability barrier that allows hemorrhage, and 3) preservation of brain microvessel integrity may underlie the novel reduction in hemorrhagic risk by dabigatran (and other indirect inhibitors of thrombin activity) in CNS ischemia associated with AF compared to warfarin. This is because hemorrhage is associated with significant decreases in microvessel basal lamina matrix in the ischemic territories.9–11,23
These considerations are consistent with the clinical situation in which a focal ischemic event during anticoagulant-treated NVAF could generate thrombin in the microvessel wall, thereby leading to hemorrhage. Because any oral anticoagulant, including warfarin, does not completely eliminate the risk of thromboembolism or hemorrhage during NVAF, matrix degradation could take place over considerable time from a silent thromboembolic event. Concentrations and durations of α-thrombin exposure could be determined that would support this scenario.
Conclusions
While the use of anticoagulants is central to the prophylaxis against potential thromboembolic stroke in patients with NVAF, the risk of intracerebral hemorrhage is a major concern. Species-compatible α-thrombin can mediate cleavage of the matrix proteins collagen IV (α1-chain), laminin (β1-chain), and perlecan found in the basal lamina of cerebral microvessels, while increasing cerebral microvessel permeability. The anti-thrombin dabigatran at clinically-relevant dosing completely prevents the degradation of cerebral microvessel basal lamina matrix by extravascular α-thrombin, also preventing associated increased microvessel permeability caused by α-thrombin, and tissue hemoglobin deposition as hemorrhage. These observations have broader implications for changes in microvessel matrix structure by thrombin generated during other disorders of brain injury where hemorrhage is evident. Experiments with focal ischemia and other models (to determine hemorrhagic outcomes), that are complex technically, will require these studies as a basis and will be much more difficult to interpret without them.
Supplemental Material
Supplemental material, sj-pdf-1-jcb-10.1177_0271678X221099092 for Intracerebral hemorrhage and thrombin-induced alterations in cerebral microvessel matrix by Yu-Huan Gu, Brian T Hawkins, Yoshikane Izawa, Yoji Yoshikawa, James A Koziol and Gregory J del Zoppo in Journal of Cerebral Blood Flow & Metabolism
Acknowledgements
We wish to thank Dr. Joanne van Ryn for discussions on this topic. We thank Ms. G. I. Berg for her superb expert assistance in the development of this manuscript.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by research grants R01 NS 053716 and R01 NS 038710 from the National Institutes of Health (GJ del Zoppo) and a grant award to Dr. del Zoppo from the Boehringer Ingelheim GmbH. Support from the Uehara Memorial Foundation (YI) is gratefully acknowledged. The laboratory also received funds from Mr. and Mrs. A. Gonsalves to whom it is warmly grateful.
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Authors’ contributions: Y-H. Gu was responsible for experiment design, performed both in vitro and in vivo experiments (blinded), acquired data, made direct contributions to the manuscript, and reviewed and edited the manuscript. B. T. Hawkins and Y. Izawa contributed to experiment design, performed preliminary in vitro experiments (blinded), made direct contributions to the manuscript, and reviewed and edited the manuscript. Y. Yoshikawa contributed to the further theoretical considerations involving microvessel alterations in NVAF and added his expertise in small animal modeling, the translation to the clinical settings, and data analysis. J. A. Koziol contributed to the experiment design, performed power analyses based on preliminary data and statistical analyses (on the blinded data sets), made direct contributions to the manuscript, and reviewed and edited the manuscript. G. J. del Zoppo was responsible for project design, experiment design, and data acquisition, and has made direct contributions to the manuscript, and has reviewed and edited the manuscript. All co-authors have read and approve this manuscript.
ORCID iD: Brian T Hawkins https://orcid.org/0000-0001-6719-5402
Supplemental material: Supplemental material for this article is available online.
References
- 1.Connolly SJ, Ezekowitz MD, Yusuf S, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med 2009; 361: 1139–1151. [DOI] [PubMed] [Google Scholar]
- 2.Connolly SJ, Eikelboom J, Joyner C, et al. Apixaban in patients with atrial fibrillation. N Engl J Med 2011; 364: 806–817. [DOI] [PubMed] [Google Scholar]
- 3.Patel M, Mahaffey KW, Garg J, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med 2011; 365: 883–891. [DOI] [PubMed] [Google Scholar]
- 4.Granger CB, Alexander JH, McMurray JJ, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med 2011; 365: 981–992. [DOI] [PubMed] [Google Scholar]
- 5.Giugliano RP, Ruff CT, Braunwald E, et al. Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med 2013; 369: 2093–2104. [DOI] [PubMed] [Google Scholar]
- 6.del Zoppo GJ, Schmid-Schönbein GW, Mori E, et al. Polymorphonuclear leukocytes occlude capillaries following middle cerebral artery occlusion and reperfusion in baboons. Stroke 1991; 22: 1276–1283. [DOI] [PubMed] [Google Scholar]
- 7.Okada Y, Copeland BR, Fitridge R, et al. Fibrin contributes to microvascular obstructions and parenchymal changes during early focal cerebral ischemia and reperfusion. Stroke 1994; 25: 1847–1853. [DOI] [PubMed] [Google Scholar]
- 8.del Zoppo GJ, Copeland BR, Harker LA, et al. Experimental acute thrombotic stroke in baboons. Stroke 1986; 17: 1254–1265. [DOI] [PubMed] [Google Scholar]
- 9.Hamann GF, Okada Y, del Zoppo GJ. Hemorrhagic transformation and microvascular integrity during focal cerebral ischemia/reperfusion. J Cereb Blood Flow Metab 1996; 16: 1373–1378. [DOI] [PubMed] [Google Scholar]
- 10.Hamann GF, Okada Y, Fitridge R, et al. Microvascular basal lamina antigens disappear during cerebral ischemia and reperfusion. Stroke 1995; 26: 2120–2126. [DOI] [PubMed] [Google Scholar]
- 11.Kwon I, Kim EH, del Zoppo GJ, et al. Ultrastructural and temporal changes of the microvascular basement membrane and astrocyte interface following focal cerebral ischemia. J Neurosci Res 2009; 87: 668–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.del Zoppo GJ, Izawa Y, Hawkins BT. Hemostasis and alterations of the central nervous system. Semin Thromb Hemost 2013; 39: 856–875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rosenberg RD, Aird WC. Vascular bed-specific hemostasis and hypercoagulable states. N Engl J Med 1999; 340: 1555–1564. [DOI] [PubMed] [Google Scholar]
- 14.Drake TA, Morrissey JH, Edgington TS. Selective cellular expression of tissue factor in human tissues: implications for disorders of hemostasis and thrombosis. Am J Pathol 1989; 134: 1087–1097. [PMC free article] [PubMed] [Google Scholar]
- 15.del Zoppo GJ, Yu JQ, Copeland BR, et al. Tissue factor localization in non-human primate cerebral tissue. Thromb Haemost 1992; 68: 642–647. [PubMed] [Google Scholar]
- 16.Thomas WS, Mori E, Copeland BR, et al. Tissue factor contributes to microvascular defects following cerebral ischemia. Stroke 1993; 24: 847–853. [DOI] [PubMed] [Google Scholar]
- 17.Mackman N, Sawdey MS, Keeton MR, et al. Murine tissue factor gene expression in vivo. Tissue and cell specificity and regulation by lipopolysaccharide. Am J Pathol 1993; 143: 76–84. [PMC free article] [PubMed] [Google Scholar]
- 18.Okada Y, Yamaguchi T, Minematsu K, et al. Hemorrhagic transformation in cerebral embolism. Stroke 1989; 20: 598–603. [DOI] [PubMed] [Google Scholar]
- 19.Walz DA, Seegers WH, Reuterby J, et al. Proteolytic specificity of thrombin. Thromb Res 1974; 4: 713–717. [DOI] [PubMed] [Google Scholar]
- 20.Liotta LA, Goldfarb RH, Terranova VP. Cleavage of laminin by thrombin and plasmin: alpha-thrombin selectively cleaves the beta chain of laminin. Thromb Res 1981; 21: 663–673. [DOI] [PubMed] [Google Scholar]
- 21.Liotta LA, Goldfarb RH, Brundage R, et al. Effect of plasminogen activator (urokinase), plasmin, and thrombin on glycoprotein and collagenous components of basement membrane. Cancer Res 1981; 41: 4629–4636. [PubMed] [Google Scholar]
- 22.Hawkins BT, Gu Y-H, Izawa Y, et al. Dabigatran abrogates brain endothelial cell permeability in response to thrombin. J Cereb Blood Flow Metab 2015; 35: 985–992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fukuda S, Fini CA, Mabuchi T, et al. Focal cerebral ischemia induces active proteases that degrade microvascular matrix. Stroke 2004; 35: 998–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.del Zoppo GJ, Frankowski H, Gu Y-H, et al. Microglial cell activation is a source of metalloproteinase generation during hemorrhagic transformation. J Cereb Blood Flow Metab 2012; 32: 919–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stroke Therapy Academic Industry Roundtable (STAIR). Recommendations for standards regarding preclinical neuroprotective and restorative drug development. Stroke 1999; 30: 2752–2758. [DOI] [PubMed] [Google Scholar]
- 26.Percie Du Sert N, Hurst V, Ahluwalia A, et al. The ARRIVE guidelines 2.0: updated guidelines for reporting animal research. J Cereb Blood Flow Metab 2020; 40: 1769–1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fenton JW, Fasco MJ, Stackrow AB. Human thrombins. Production, evaluation, and properties of alpha-thrombin. J Biol Chem 1977; 252: 3587–3598. [PubMed] [Google Scholar]
- 28.Lundblad RL, Kingdon HS, Mann KG. Thrombin. Methods Enzymol 1976; 45: 156–176. [DOI] [PubMed] [Google Scholar]
- 29.Osada T, Gu Y-H, Kanazawa M, et al. M Interendothelial claudin-5 expression depends upon cerebral endothelial cell-matrix adhesion by b1-integrins. J Cereb Blood Flow Metab 2011; 31: 1972–1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Izawa Y, Gu Y-H, Osada T, et al. beta1-integrin-matrix interactions modulate cerebral microvessel endothelial cell tight junction expression and permeability. J Cereb Blood Flow Metab 2018; 38: 641–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hawkins BT, Egleton RD. Fluorescence imaging of blood-brain barrier disruption. J Neurosci Methods 2006; 151: 262–267. [DOI] [PubMed] [Google Scholar]
- 32.Hawkins BT, Ocheltree SM, Norwood KM, et al. Decreased blood-brain barrier permeability to fluorescein in streptozotocin-treated rats. Neurosci Lett 2007; 411: 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stangier J, Clemens A. Pharmacology, pharmacokinetics, and pharmacodynamics of dabigatran etexilate, an oral direct thrombin inhibitor. Clin Appl Thromb Hemost 2009; 15 Suppl 1: 9S–16S. [DOI] [PubMed] [Google Scholar]
- 34.Hamann GF, Liebetrau M, Martens H, et al. Microvascular basal lamina injury after experimental focal cerebral ischemia and reperfusion in the rat. J Cereb Blood Flow Metab 2002; 22: 526–533. [DOI] [PubMed] [Google Scholar]
- 35.Tagaya M, Haring H-P, Stuiver I, et al. Rapid loss of microvascular integrin expression during focal brain ischemia reflects neuron injury. J Cereb Blood Flow Metab 2001; 21: 835–846. [DOI] [PubMed] [Google Scholar]
- 36.Belayev L, Busto R, Zhao W, et al. Quantitative evaluation of blood-brain barrier permeability following middle cerebral artery occlusion in rats. Brain Res 1996; 739: 88–96. [DOI] [PubMed] [Google Scholar]
- 37.Gu Y-H, Kanazawa M, Hung SY, et al. Cathepsin L acutely alters microvessel integrity within the neurovascular unit during focal cerebral ischemia. J Cereb Blood Flow Metab 2015; 35: 1888–1900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lefebvre S, Hascoet C, Damin-Pernik M, et al. Monitoring of antivitamin K-dependent anticoagulation in rodents – towards an evolution of the methodology to detect resistance in rodents. Pestic Biochem Physiol 2017; 138: 29–36. [DOI] [PubMed] [Google Scholar]
- 39.Lund M. Comparative effect of the three rodenticides warfarin, difenacoum and brodifacoum on eight rodent species in short feeding periods. J Hyg (Lond) 1981; 87: 101–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hollister RD, Kisiel W, Hyman BT. Immunohistochemical localization of tissue factor pathway inhibitor-1 (TFPI-1), a Kunitz proteinase inhibitor, in Alzheimer's disease. Brain Res 1996; 728: 13–19. [PubMed] [Google Scholar]
- 41.Wagner SL, Van Nostrand WE, Lau AL, et al. Co-distribution of protease nexin-1 and protease nexin-2 in brains of non-human primates. Brain Res 1993; 626: 90–98. [DOI] [PubMed] [Google Scholar]
- 42.Niclou SP, Suidan HS, Pavlik A, et al. Changes in the expression of protease-activated receptor 1 and protease nexin-1 mRNA during rat nervous system development and after nerve lesion. Eur J Neurosci 1998; 10: 1590–1607. [DOI] [PubMed] [Google Scholar]
- 43.Sugawara T, Jadhav V, Ayer R, et al. Thrombin inhibition by argatroban ameliorates early brain injury and improves neurological outcomes after experimental subarachnoid hemorrhage in rats. Stroke 2009; 40: 1530–1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nagatsuna T, Nomura S, Suehiro E, et al. Systemic administration of argatroban reduces secondary brain damage in a rat model of intracerebral hemorrhage: histopathological assessment. Cerebrovasc Dis 2005; 19: 192–200. [DOI] [PubMed] [Google Scholar]
- 45.Van Ryn J, Stangier J, Haertter S, et al. Dabigatran etexilate – a novel, reversible, oral direct thrombin inhibitor: interpretation of coagulation assays and reversal of anticoagulant activity. Thromb Haemost 2010; 103: 1116–1127. [DOI] [PubMed] [Google Scholar]
- 46.Choi SH, Joe EH, Kim SU, et al. Thrombin-induced microglial activation produces degeneration of nigral dopaminergic neurons in vivo. J Neurosci 2003; 23: 5877–5886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Suo Z, Wu M, Citron BA, et al. Persistent protease-activated receptor 4 signaling mediates thrombin-induced microglial activation. J Biol Chem 2003; 278: 31177–31183. [DOI] [PubMed] [Google Scholar]
- 48.Suo Z, Wu M, Ameenuddin S, et al. Participation of protease-activated receptor-1 in thrombin-induced microglial activation. J Neurochem 2002; 80: 655–666. [DOI] [PubMed] [Google Scholar]
- 49.Ryu J, Min KJ, Rhim TY, et al. Prothrombin kringle-2 activates cultured rat brain microglia. J Immunol 2002; 168: 5805–5810. [DOI] [PubMed] [Google Scholar]
- 50.Dang G, Yang Y, Wu G, et al. Early erythrolysis in the hematoma after experimental intracerebral hemorrhage. Transl Stroke Res 2017; 8: 174–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Stangier J, Rathgen K, Stahle H, et al. The pharmacokinetics, pharmacodynamics and tolerability of dabigatran etexilate, a new oral direct thrombin inhibitor, in healthy male subjects. Br J Clin Pharmacol 2007; 64: 292–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lee IO, Kratz MT, Schirmer SH, et al. The effects of direct thrombin inhibition with dabigatran on plaque formation and endothelial function in apolipoprotein E-deficient mice. J Pharmacol Exp Ther 2012; 343: 253–257. [DOI] [PubMed] [Google Scholar]
- 53.Kadoglou NP, Moustardas P, Katsimpoulas M, et al. The beneficial effects of a direct thrombin inhibitor, dabigatran etexilate, on the development and stability of atherosclerotic lesions in apolipoprotein E-deficient mice: dabigatran etexilate and atherosclerosis. Cardiovasc Drugs Ther 2012; 26: 367–374. [DOI] [PubMed] [Google Scholar]
- 54.Pingel S, Tiyerili V, Mueller J, et al. Thrombin inhibition by dabigatran attenuates atherosclerosis in ApoE deficient mice. aoms 2014; 1: 154–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Michael N, Grigoryan MM, Kilday K, et al. Effects of dabigatran in mouse models of aging and cerebral amyloid angiopathy. Front Neurol 2019; 10: 966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shlobin NA, Har-Even M, Itsekson-Hayosh Z, et al. Role of thrombin in central nervous system injury and disease. Biomolecules 2021; 11: 562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Aronowski J, Zhao X. Molecular pathophysiology of cerebral hemorrhage: secondary brain injury. Stroke 2011; 42: 1781–1786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Keep RF, Zhou N, Xiang J, et al. Vascular disruption and blood-brain barrier dysfunction in intracerebral hemorrhage. Fluids Barriers CNS 2014; 11: 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Davalos D, Baeten KM, Whitney MA, et al. Early detection of thrombin activity in neuroinflammatory disease. Ann Neurol 2014; 75: 303–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gao F, Liu F, Chen Z, et al. Hydrocephalus after intraventricular hemorrhage: the role of thrombin. J Cereb Blood Flow Metab 2014; 34: 489–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yang Y, Zhang M, Kang X, et al. Thrombin-induced microglial activation impairs hippocampal neurogenesis and spatial memory ability in mice. Behav Brain Funct 2015; 11: 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Xue M, Del Bigio MR. Injections of blood, thrombin, and plasminogen more severely damage neonatal mouse brain than mature mouse brain. Brain Pathol 2005; 15: 273–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Xi G, Reiser G, Keep RF. The role of thrombin and thrombin receptors in ischemic, hemorrhagic and traumatic brain injury: deleterious or protective? J Neurochem 2003; 84: 3–9. [DOI] [PubMed] [Google Scholar]
- 64.Krenzlin H, Lorenz V, Alessandri B. The involvement of thrombin in the pathogenesis of glioblastoma. J Neurosci Res 2017; 95: 2080–2085. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-jcb-10.1177_0271678X221099092 for Intracerebral hemorrhage and thrombin-induced alterations in cerebral microvessel matrix by Yu-Huan Gu, Brian T Hawkins, Yoshikane Izawa, Yoji Yoshikawa, James A Koziol and Gregory J del Zoppo in Journal of Cerebral Blood Flow & Metabolism
Data Availability Statement
Fully anonymized data and detailed protocols will be shared upon request from qualified investigators.