Abstract
The outcomes for patients with high-risk DLBCL are suboptimal, especially in Low-middle income countries in comparison to published data from the western world. Most newer therapies aimed at improving outcomes are either unavailable or out of reach for the majority of patients in low-middle income countries. Cyclophosphamide is an easily available and accessible drug that forms the backbone for therapy for DLBCL. We conducted a single-center, open-label randomized pilot study comparing standard RCHOP to RCHOP with fractionated cyclophosphamide (RfCHOP) in patients with newly diagnosed, high-risk DLBCL. Fifty-five patients were randomized- 28 to RfCHOP and 27 to the RCHOP arm. RfCHOP was associated with a higher complete response rate than RCHOP at the end of 6 cycles of therapy (81.2% vs. 59.3%; p-0.062). Grade III/IV adverse events were comparable in both arms with the use of prophylactic GCSF in the RfCHOP arm. At a median follow-up of 22 months, the Median EFS and OS was not reached in either arm. RfCHOP may represent a therapeutic option for patients with newly-diagnosed, high-risk DLBCL, especially in Low-middle income countries. Larger studies are required to confirm these findings.
Keywords: DLBCL, Fractionated cyclophosphamide, Global oncology, RCHOP, Lymphoma
Introduction
Diffuse Large B-Cell Lymphoma (DLBCL) represents the most common Non-Hodgkin Lymphoma in most series from India, accounting for nearly 30–40% of all lymphoma cases[1, 2].
However, nearly 40% of patients with DLBCL either relapse or have refractory disease[3]. In the real-world outcome data from various centers in India, it is reported that patients with high-risk features have a CR rate in the range of 60–65% [2, 4]. The similar value from published studies from developed countries ranges from 75 to 80% [5].
In the past, multiple attempts have failed to improve outcomes in patients with high-risk untreated DLBCL by utilizing more aggressive chemotherapeutic regimens[6–8], alternate rituximab dosing schedules[9–12], alternate anti-CD-20 monoclonal antibodies[13], and addition of multiple novel agents to the standard RCHOP backbone including Bortezomib, Lenalidomide and Ibrutinib[14–16]. The recently published POLARIX trial showed significantly better progression-free survival (PFS) over standard RCHOP in patients with untreated DLBCL[17]. However, at present, the major limitations for the utilization of polatuzumab vedotin are its unavailability in most of the low-middle income countries (LMICs) and the prohibitively high cost of therapy. A cost-effective modification of RCHOP, which improves outcomes, remains elusive.
Cyclophosphamide is an alkylating agent and forms the backbone of RCHOP chemotherapy. High doses of cyclophosphamide have been used in fractionated doses to treat Acute Lymphoid Leukemia and aggressive lymphomas in combination with other chemotherapeutic drugs[18, 19]. Pharmaco-kinetic data from studies reveal that fractionation of cyclophosphamide leads to increased formation of the active metabolite compared to an equivalent single dose. This is accompanied by decreased renal excretion of cyclophosphamide on Day 2, implying that more of the drug is available for metabolism[20, 21]. In-vitro data has demonstrated that cytochrome P450 enzymes are induced by the administration of cyclophosphamide, leading to auto-induction and increased formation of the active metabolite[22]. Fractionated Cyclophosphamide in combination with high-dose methotrexate and cytarabine has been utilized to treat patients with newly diagnosed DLBCL. While this regimen is associated with reasonable response rates, it is accompanied by increased toxicity and high treatment-related mortality, especially in patients with advanced age[23, 24].
In this study, we report the results of a pilot study utilizing fractionated cyclophosphamide in addition to standard RCHOP chemotherapy for patients with newly diagnosed DLBCL with high-risk features. The strategy was chosen to maximize the potential benefits obtained by fractionating cyclophosphamide while mitigating the adverse events associated with more toxic regimens and higher dosing. Further, cyclophosphamide is readily available and accessible worldwide, including LMICs, which is not the case with other drugs used to improve outcomes in this setting. Our primary objective was to study the response rates and the adverse event profile of this regimen compared to standard RCHOP chemotherapy.
Methods
The study was an open-label, randomized, pilot study conducted in a tertiary care referral center in North India between 2019 and 2021. The study was performed in accordance with the Consolidated Standards of Reporting Trials and the Declaration of Helsinki. All patients provided written informed consent before enrollment, and the Institutional Ethics Committee approved the study before starting recruitment. The study was registered at the Clinical Trials Registry of India (CTRI Reg. no CTRI/2019/07/020236).
The study recruited patients with high-risk, newly diagnosed DLBCL aged 18 years or above who had an ECOG performance status of 0–2 with adequate renal, liver and cardiac function tests. Further, high-risk cohort was defined if any of the following criteria was met- (1) Non-GCB phenotype on Immunohistochemistry (IHC) according to Hans Protocol or (2) Stage III/IV disease or (3) International Prognostic Index score between 2 and 5 or (4) Bulky disease at baseline defined as at least one node/mass measuring > 7.5 cm in longest diameter. These parameters were chosen as they were associated with inferior outcomes in newly diagnosed DLBCL in previous studies. Patients with active infection with HIV/Hepatitis B/Hepatitis C, history of another lymphoma in the past, documented evidence of CNS lymphoma, current pregnancy/lactation or a history of hematuria in the past six months were excluded from the study.
All biopsy specimens which were suggestive of lymphoma on morphology underwent IHC for CD45, CD20 and CD3 to confirm the diagnosis of large B-cell Non-Hodgkins lymphoma. Further typing on the basis of Hans protocol using CD10, BCL6 and MUM1 was done to subtype into Non-GCB and GCB subtypes. Additional IHC for Ki67, myc and BCL2 was also done depending on the availability of the immunostains.
The study’s primary objective was to compare the response rates of a RCHOP regimen with fractionated cyclophosphamide regimen (referred to as RfCHOP from here onwards) and standard RCHOP therapy. The secondary objective was to assess the regimen’s safety in comparison to the adverse event profile between the two treatment groups. The primary endpoint was the complete response rate at the end of 6 cycles of therapy. Response evaluation was done according to the Revised International Working Group Criteria[25]. Adverse events were graded according to Common terminology criteria for adverse events, version 5.0. An event was classified as documentation of relapse, progressive disease or death due to any cause. The event-free survival (EFS) was calculated from the date of diagnosis to the date of the event, and the overall survival (OS) was calculated from the date of diagnosis to the date of death due to any cause. The follow-up was censored for all patients without an event on 31/12/2021.
Study Procedure
After written informed consent, patients fulfilling the inclusion criteria were enrolled in the study. The two groups were randomized using a computer-generated table in a 1:1 ratio. Patients having a presentation that necessitated immediate therapy (such as organ dysfunction) were permitted to have received up to one cycle of pre-phase chemotherapy up to one month before study enrolment, which could include cyclophosphamide, vincristine and steroids. Patients in the RfCHOP group received Rituximab 375 mg/m2, Cyclophosphamide 500 mg/m2 intravenously on Day 1 followed by Cyclophosphamide 250 mg/m2 orally on Day 2 and 3, Doxorubicin 50 mg/m2 and Vincristine 1.4 mg/m2 (capped at 2 mg) intravenously on Day 1 and Prednisolone 60 mg/m2 orally (capped at 100 mg/day) from Day 1–5. Patients in the RCHOP group received standard RCHOP. All patients were to receive 6 cycles at an interval of 21 days. All patients received anti-emetic medication in dexamethasone 8 mg and palonosetron 0.25 mg intravenously on Day 1 of each cycle and were also given allopurinol with the first cycle of therapy. Primary prophylaxis with granulocyte colony-stimulating factor (GCSF) was given to all patients above 60 years. Each patient had a complete blood count checked between Day 10–14 of each cycle to look for cytopenias. A complete blood count was also checked before every cycle, and chemotherapy was administered only if the absolute neutrophil count was above 1000/mm3.
Patients with grade III/IV neutropenia received GCSF support in subsequent cycles. A pre-planned safety analysis done after recruiting 15 patients in each arm revealed an increased incidence of Grade III/IV neutropenia in the RfCHOP arm. All patients in the RfCHOP arm subsequently received primary prophylaxis with GCSF. The response was assessed using a Whole Body FDG-PET. Interim response assessment was done after three cycles, and final response assessment was done 4–6 weeks after the last cycle of chemotherapy. Patients with a Grade III/IV adverse event underwent dose modification in accordance with the study protocol provided in the supplement. Patients who experienced a grade III/IV adverse event despite dose modification were discontinued from the study. Intrathecal methotrexate was given according to physician discretion (usually if CNS IPI ≥ 4, or involvement of high-risk sites such as Testes, Adrenal); however, no patient received high dose methotrexate-based CNS directed therapy.
Statistical Analysis
The authors used an estimation of 10–15% improvement in CR rate over RCHOP for sample size estimation. Assuming a CR rate of 50% with RCHOP in aggressive DLBCL and a 65% CR rate with RfCHOP, an alpha of 0.05 and power of 80%, the estimated sample size required to prove statistical significance would be 169 patients in each arm. It was decided to conduct a pilot single-center trial and recruit 30 patients to each arm. Due to multiple halts in recruitment due to the Covid-19 pandemic, trial recruitment was stopped after 55 patients had been recruited. For categorical variables, Chi-square and Fischer Exact test were applied. Kaplan Meir curve was created to study the EFS and OS. Log-rank test was used to study the difference in EFS and OS between groups. Significance was set at 0.05 for all tests. The analysis was done using SPSS software (SPSS, Chicago, IL, version 25).
Results
Ninety-three patients were screened and 55 eligible patients were enrolled in the study. Twenty-eight patients were randomized to the RfCHOP arm, and 27 patients were randomized to the RCHOP arm. All patients received at least one cycle of assigned therapy and were included in the intention to treat analysis (Fig. 1). Six patients could not complete the designated six cycles of treatment: 1 in the RfCHOP arm and 5 in the RCHOP arm (Two patients had evidence of progressive disease during chemotherapy and did not complete the designated six cycles, two patients withdrew consent and decided not to get further therapy after 1 and 2 cycles, respectively, and two patients had repeated Grade-III/IV adverse events despite dose modification after which they were removed from the study and given alternative regimens). The median number of chemotherapy cycles administered was 6 in both the study groups.
Fig. 1.
Consort Diagram
Baseline Characteristics
The median age of the patient population was 51 years, with a male: female ratio of 1.63:1 (Males- 61.8%, Females 38.2%). Most patients had stage III-IV disease (69.1%) and 35 patients (63.6%) had B symptoms at presentation. Thirty-seven patients (67.3%) had Non-GCB/ABC subtype of DLBCL on histology. Thirty-seven patients (67.3%) had evidence of extra-nodal disease, with the most common site being the GI tract, with seven patients (12.7%) having primary extra-nodal lymphoma. Eight patients out of 32 patients for whom information was available had double-expressor status on IHC. Both groups were well matched in terms of baseline characteristics (Table 1).
Table 1.
Baseline Characteristics
| Overall (N = 55) | RfCHOP (N = 28) | RCHOP (N = 27) | p value | ||
|---|---|---|---|---|---|
| Age (mean ± SD) | 51.1 ± 14.6 | 49.3 ± 14.3 | 52.8 ± 14.9 | 0.414 | |
| Gender | M- 34; F-21 | Males 20 (71.4%) | Males 14 (51.9%) | 0.135 | |
| Hypertension | 9 (16.4%) | 5 (17.9%) | 4 (14.8%) | 1.000 | |
| Diabetes | 6 (10.9%) | 4 (14.3%) | 2 (7.4%) | 0.669 | |
| Lymphadenopathy | 32 (58.2%) | 16 (57.1%) | 16 (59.3%) | 0.874 | |
| Hepatomegaly | 13 (23.6%) | 7 (25%) | 6 (22.2%) | 0.808 | |
| Splenomegaly | 8 (14.5%) | 5 (17.9%) | 3 (11.1%) | 0.705 | |
| B Symptoms | 35 (63.6%) | 17 (60.7%) | 18 (66.7%) | 0.646 | |
| Stage | I-II | 17 (30.9%) | 10 (35.7%) | 7 (25.9%) | 0.432 |
| III-IV | 38 (69.1%) | 18 (64.3%) | 20 (74.1%) | ||
| Bulky Disease | 25 (45.5%) | 14 (50%) | 11 (40.7%) | 0.491 | |
| Extranodal disease | 37 (67.3%) | 19 (67.9%) | 18 (66.7%) | 0.925 | |
| Elevated LDH | 41 (74.5%) | 21 (75%) | 20 (74.1%) | 0.937 | |
| Bone Marrow involvement | 11 (20%) | 8 (28.6%) | 3 (11.1%) | 0.106 | |
| Hypoalbuminemia (albumin < 3.5gm/dl) | 13 (23.6%) | 5 (17.9%) | 8 (29.6%) | 0.304 | |
| Elevated CRP (above upper limit of normal) | 41/52 (78.8%) | 22/25 (88%) | 19/27 (70.4%) | 0.177 | |
| Low Vitamin D (below lower limit of normal) | 24/53 (45.3%) | 11/26 (42.3%) | 13/27 (48.1%) | 0.669 | |
| IPI | Low | 12 (21.8%) | 7 (25%) | 5 (18.5%) | 0.560 |
| Intermediate (Low Int + High Int) | 29 (52.8%) | 16 (57.1%) | 13 (48.1%) | ||
| High | 14 (25.4%) | 5 (17.9%) | 9 (33.4%) | ||
| RIPI | V Good | 4 (7.3%) | 3 (10.7%) | 1 (3.7%) | 0.378 |
| Good | 23 (41.8%) | 13 (46.4%) | 10 (37%) | ||
| Poor | 28 (50.9%) | 12 (42.9%) | 16 (59.3%) | ||
| Histological Subtype | Non GCB/ABC Subtype | 37 (67.3%) | 19 (67.9%) | 18 (66.7%) | 0.996 |
| GCB Subtype | 12 (21.8%) | 6 (21.4%) | 6 (22.2%) | ||
| Other | 6 (10.9%) | 3 (10.7%) | 3 (11.1%) | ||
Response Rates
Thirty-nine patients (70.9%) achieved a complete response (CR) at the end of therapy, while 42 patients (76.4%) had an Overall Response (Complete Response + Partial Response) in the entire study. A higher percentage of patients were able to achieve CR (82.1% vs. 59.3%; p-0.062) and an Overall response (85.7% vs. 66.7%; p-0.121) in the RfCHOP arm in comparison to the RCHOP arm (Table 2). Two patients had evidence of progressive disease on interim PET and were taken off the study. All other patients were continued on the same therapy irrespective of response (i.e. Partial response or complete response) on interim PET. Among the 52 patients who received more than 1 cycle of therapy, 19 patients (36.5%) had a delay during chemotherapy delivery in between cycles. The reasons for the delay were infection, neutropenia and logistical issues related to the Covid-19 pandemic. The patients who had delays had a significantly lower CR rate in comparison to patients who got their therapy at the designated 21-day cycle intervals (87.9% vs. 52.6%; p-0.005). However, this did not translate into a statistically significant EFS or OS difference. On subgroup analysis, patients younger than 60 years of age had a statistically significant higher CR rate with RfCHOP in comparison to RCHOP (86.4% vs. 52.9%; p-0.033). There was no statistically significant difference in CR rates in other subgroups (Stage III/IV, High Intermediate/High IPI, Non-GCB/ABC subtype of DLBCL).
Table 2.
Primary Outcomes
| RfCHOP (N = 28) | RCHOP (N = 27) | p value | |
|---|---|---|---|
| Complete Remission | 23 (82.1%) | 16 (59.3%) | 0.062 |
| Overall Response | 24 (85.7%) | 18 (66.7%) | 0.121 |
| Event | 6 (21.4%) | 10 (37%) | 0.203 |
| Death | 3 (10.7%) | 6 (22.2%) | 0.295 |
Adverse Events
The most common grade III/IV adverse event was neutropenia (31 patients, 56.4%). Thirteen patients (23.6%) had febrile neutropenia during their therapy, of which 5 patients required hospital admission for injectable antibiotics. One patient died due to febrile neutropenia in the RfCHOP arm. The incidence of adverse events was not statistically different in the 2 treatment groups (Table 3). A pre-planned safety analysis was done after recruiting 15 patients in each arm. Prior to the interim analysis, 12/15 patients (80%) in the RfCHOP arm had Grade III or above neutropenia and 6/15 patients (40%) had febrile neutropenia. After the routine use of primary prophylaxis with GCSF, the incidence of Grade III or above neutropenia decreased to 5/13 patients (38.4%) and incidence of febrile neutropenia decreased to 3/13 patients (23.1%). Ten patients (18.2%) had a dose reduction due to grade III/IV toxicity during their treatment- 7 in the RfCHOP arm and 3 in the RCHOP arm (p-value 0.295).
Table 3.
Adverse Events
| RfCHOP arm (N = 28) | RCHOP arm (N = 27) | |||
|---|---|---|---|---|
| Grade I-II | Grade III-V | Grade I-II | Grade III-V | |
| Anemia | 15 (53.5%) | 7 (25%) | 8 (29.6%) | 6 (22.2%) |
| Neutropenia | 7 (25%) | 17 (60.7%) | 8 (29.6%) | 14 (51.9%) |
| Febrile Neutropenia | - | 9 (32.1%) | - | 4 (14.8%) |
| Thrombocytopenia | 10 (35.7%) | 2 (7.1%) | 11 (40.7%) | 0 |
| Diarrhoea | 6 (21.4%) | 1 (3.6%) | 2 (7.4%) | 2 (7.4%) |
| Constipation | 4 (14.3%) | 1 (3.6%) | 7 (25.9%) | 1 (3.7%) |
| Vomiting | 6 (21.4%) | - | 8 (29.6%) | - |
| Neuropathy | 10 (35.7%) | 1 (3.6%) | 8 (29.6%) | - |
Relapse and Survival
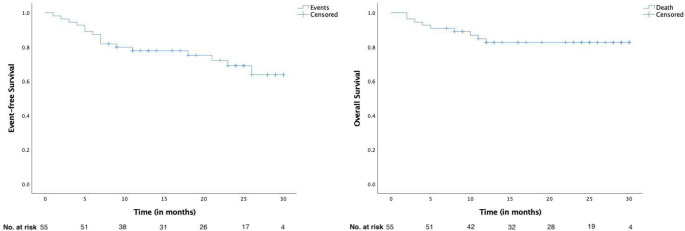
The Median follow-up for all patients was 22 months (Range 2–30 months). Thirteen patients (23.6%) experienced a relapse or had refractory disease during follow-up, including the development of CNS disease in 4 patients. Nine patients (16.4%) died during follow up (Table 2). The most common cause of death was progressive/refractory disease in 8 patients, and one patient died due to febrile neutropenia post her 6th cycle of therapy. More patients in the RCHOP group experienced an event than the RfCHOP group, but this difference was not statistically significant (37% vs. 21%; p-0.203). There was no significant difference in the relapse rate in both arms (29.6% vs. 17.9%; p- 0.304). The Median Event-free survival (EFS) and Overall Survival (OS) was not reached for both groups (Fig. 2). The estimated 2-year EFS was 77.8% (95% CI- 63.2 – 87%) and estimated 2-year OS was 82.6% (95% CI- 72.2 − 93%). There was no difference in the EFS and OS between the 2 study groups (Fig. 3).
Fig. 2.
Event-free survival and Overall Survival for the entire cohort
Fig. 3.
Comparison between RfCHOP and RCHOP for event-free survival and overall survival
Discussion
In our study, we used a higher, fractionated dose of cyclophosphamide in newly diagnosed DLBCL patients with high-risk characteristics. This strategy was based on previous data highlighting a higher response rate when patients were treated with more aggressive regimens containing fractionated cyclophosphamide and pharmaco-kinetic data suggesting increased active metabolite formation when cyclophosphamide was given in a fractionated manner. In our study, patients receiving RfCHOP had a higher CR rate than patients receiving RCHOP. On subgroup analysis, in patients younger than 60 years of age, RfCHOP was associated with a statistically significantly higher CR rate than RCHOP. The number of patients experiencing an event during follow-up was also higher in the RCHOP group. However, this was not statistically significant. The median EFS and OS were not achieved for the entire cohort, and there was no difference between the EFS and OS between the two treatment groups.
Fractionated Cyclophosphamide has been used as a part of the Hyper-CVAD regimen to treat patients with high-risk DLBCL. In the phase II study by Oki et al., patients with high-risk DLBCL were randomized to receive standard R-CHOP or R-HCVAD/R-MA [23]. The R-HCVAD/R-MA arm was associated with higher response rates, but high treatment related mortality in patients > 45 years of age. Similarly, in a retrospective analysis by Mato et al., patients with high-risk DLBCL who had received R-HCVAD/R-MA had a higher 3-year PFS and OS in comparison to historical cohorts [24]. Both these studies have used a much more intensive regimen, with a much higher dose of cyclophosphamide in comparison to our study. RfCHOP is given on an outpatient basis and has an adverse event profile which is comparable to RCHOP rather than the regimens used in the above studies.
The response rate seen with standard RCHOP in our study is lower than described in published clinical trials [17]. There may be multiple reasons for this finding. Our study focused solely on newly diagnosed DLBCL with high-risk features such as Stage III/IV disease, Non-GCB/ABC cell-of-origin subtype on IHC, IPI 2–5 and baseline bulky disease. This may have led to a lower-than-expected response rate with RCHOP therapy. Recently published Indian registry data shows that patients with DLBCL treated with RCHOP or RCHOP like therapy had a CR rate of 67.7% [2]. Other real-world data published from India shows the CR rate to be 75% in patients with newly-diagnosed DLBCL [4]. However, both these sets of data include a heterogenous population of DLBCL patients. For example, in the single center real-world data published from AIIMS, B symptoms were seen in 43% of patients, bulky disease was seen in 35% of patients and 51% of patients had stage III/IV disease [4]. In comparison, 63.6%, 45.5% and 69% of patients had B symptoms, bulky disease and stage III/IV disease in our study respectively. The lower response rates seen in the Indian studies in comparison to western literature may be due to a multitude of factors such as late presentation, poor socio-economic support, lack of adherence to the intensity of regimen and retrospective nature of analysis. Considering the fact that both published real-world data sets are an unselected population of DLBCL patients with a mix of patients with good-risk and high-risk disease, we feel that our CR rate of 60% with RCHOP in patients with high-risk features is in accordance to what is practically seen in India. Table 4 compares our studies characteristics with other published data from India and the west.
Table 4.
| Tilly et al. | Mytelka et al. | Nair et al. | Gogia et al. | Present study | |
|---|---|---|---|---|---|
| No. of patients | 879 | 257 | 1961 | 417 | 55 |
| Regimen |
Pola-R-CHP- 440 RCHOP- 439 |
RCHOP (80%) | RCHOP (68%) | RCHOP |
RfCHOP- 28 RCHOP- 27 |
| B symptoms | - | 43% | 43% | 64% | |
| High Intermediate/ High IPI | 62% | 100% | 30% | 19% (High risk) | 51% |
| Bulky Disease | 43.8% | 32% | 46% | ||
| Non-GCB Subtype | 33% | - | 54% | 47% | 67% |
| Stage III/IV | 88.7% | 89% | 57% | 51% | 69% |
| CR |
Pola-RCHP- 78% RCHOP-74% |
77% | 67% | 75% |
RfCHOP- 82% RCHOP- 59% |
The adverse events observed in both the treatment arms were similar. The overall incidence of febrile neutropenia and grade III/IV neutropenia in the RCHOP arm were similar to reported in other trials [14, 16]. Ten patients had dose reductions due to grade III/IV toxicity, with seven in the RfCHOP arm. The RfCHOP arm also had a higher incidence of febrile neutropenia than the RCHOP arm, although this was not statistically significant. However, after the interim analysis and institution of primary prophylaxis with GCSF, the incidence of febrile neutropenia and neutropenia decreased in the RfCHOP arm. Overall, with the use of primary GCSF prophylaxis, RfCHOP was well tolerated by the patients with no patient developing Grade-III/IV mucositis or cystitis.
Our study has some limitations. It was a single-center study and the observers were not blinded for the intervention. FISH for detecting double-hit and triple-hit lymphoma was not done in any of the patients. A significant number of screened patients could not be enrolled in the study, most commonly due to having an ECOG performance status > 2. This limits the applicability of the regimen in the real-world setting. Also, since this was a pilot study, the numbers involved were small, and the study was not powered to look at a significant difference between the 2 treatment groups.
Conclusions
Overall, our study suggests that RfCHOP may be associated with better response rates than RCHOP in patients with newly diagnosed, high-risk DLBCL. A larger, multi-centre study is required to confirm our findings. Most newer regimens being tried to improve the outcomes for DLBCL focus on newer, targeted therapies (such as Polatuzumab) which often take years before they are accessible in LMICs. Due to the low cost and easy availability all over the world, cyclophosphamide fractionation is easily doable without any major financial burden. Hence, this regimen is especially attractive for patients being treated in LMICs.
Therefore, if larger studies do confirm improved response rates and efficacy of RfCHOP over RCHOP, the regimen will have a valuable impact on the treatment of DLBCL.
Acknowledgements
We acknowledge Ms. Chandni for help in data-keeping and follow up of the study patients.
Funding Information
The authors did not receive any funding for this trial.
Data Availability
The data is available on request to the corresponding author.
Declaration
Disclosure Statement
The authors have no conflicts of interest to report.
Ethical Approval
The study was approved by the institute ethics committee and the trial was registered with CTRI (CTRI Reg. no CTRI/2019/07/020236).
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Prakash G, Sharma A, Raina V, Kumar L, Sharma MC, Mohanti BK. B cell non-Hodgkin’s lymphoma: Experience from a tertiary care cancer center. Ann Hematol. 2012;91:1603–1611. doi: 10.1007/s00277-012-1491-5. [DOI] [PubMed] [Google Scholar]
- 2.Nair R, Bhurani D, Rajappa S, Kapadia A, Reddy Boya R, Sundaram S, et al. Diffuse Large B-Cell Lymphoma: Clinical Presentation and Treatment Outcomes From the OncoCollect Lymphoma Registry. Article. 2022;11:1. doi: 10.3389/fonc.2021.796962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sehn LH, Salles G, Diffuse Large B-Cell, Lymphoma N Engl J Med. 2021;384:842–858. doi: 10.1056/NEJMRA2027612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gogia A, Nair S, Arora S, Kumar L, Sharma A, Gupta R, et al. Impact of Cell-of-Origin on Outcome of Patients With Diffuse Large B-Cell Lymphoma Treated With Uniform R-CHOP Protocol: A Single-Center Retrospective Analysis From North India. Front Oncol. 2021;11:5043. doi: 10.3389/FONC.2021.770747/BIBTEX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mytelka DS, Li L, Stafkey-Mailey D, Liepa AM, Hess LM, Farrelly E, et al. Treatment patterns and outcomes among patients with high-intermediate/high-risk diffuse large B-cell lymphoma in the USA. Hematology. 2015;20:442–448. doi: 10.1179/1607845414Y.0000000228. [DOI] [PubMed] [Google Scholar]
- 6.Récher C, Coiffier B, Haioun C, Molina TJ, Fermé C, Casasnovas O, et al. Intensified chemotherapy with ACVBP plus rituximab versus standard CHOP plus rituximab for the treatment of diffuse large B-cell lymphoma (LNH03-2B): An open-label randomised phase 3 trial. The Lancet. 2011;378:1858–1867. doi: 10.1016/S0140-6736(11)61040-4. [DOI] [PubMed] [Google Scholar]
- 7.Bartlett NL, Wilson WH, Jung S-H, Hsi ED, Maurer MJ, Pederson LD et al (2019) Dose-Adjusted EPOCH-R Compared With R-CHOP as Frontline Therapy for Diffuse Large B-Cell Lymphoma: Clinical Outcomes of the Phase III Intergroup Trial Alliance/CALGB 50303. J Clin Oncol JCO1801994. 10.1200/JCO.18.01994 [DOI] [PMC free article] [PubMed]
- 8.Stiff PJ, Unger JM, Cook JR, Constine LS, Couban S, Stewart DA, et al. Autologous transplantation as consolidation for aggressive non-Hodgkin’s lymphoma. N Engl J Med. 2013;369:1681–1690. doi: 10.1056/NEJMOA1301077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cunningham D, Hawkes EA, Jack A, Qian W, Smith P, Mouncey P, et al. Rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisolone in patients with newly diagnosed diffuse large B-cell non-Hodgkin lymphoma: A phase 3 comparison of dose intensification with 14-day versus 21-day cycles. The Lancet. 2013;381:1817–1826. doi: 10.1016/S0140-6736(13)60313-X. [DOI] [PubMed] [Google Scholar]
- 10.Hüttmann A, Rekowski J, Müller SP, Hertenstein B, Franzius C, Mesters R et al Six versus eight doses of rituximab in patients with aggressive B cell lymphoma receiving six cycles of CHOP: results from the “Positron Emission Tomography-Guided Therapy of Aggressive Non-Hodgkin Lymphomas” (PETAL) trial. Annals of Hematology 2019. 10.1007/s00277-018-3578-0 [DOI] [PubMed]
- 11.Lugtenburg PJ, de Nully Brown P, van der Holt B, D’Amore F, Koene HR, Berenschot HW, et al. Randomized phase III study on the effect of early intensification of rituximab in combination with 2-weekly CHOP chemotherapy followed by rituximab or no maintenance in patients with diffuse large B-cell lymphoma: Results from a HOVON-Nordic Lymphoma Grou. J Clin Oncol. 2018;34:7504–7504. doi: 10.1200/jco.2016.34.15_suppl.7504. [DOI] [Google Scholar]
- 12.Murawski N, Pfreundschuh M, Zeynalova S, Poeschel V, Hänel M, Held G, et al. Optimization of rituximab for the treatment of DLBCL (I): Dose-dense rituximab in the DENSE-R-CHOP-14 trial of the DSHNHL. Ann Oncol. 2014;25:1800–1806. doi: 10.1093/annonc/mdu208. [DOI] [PubMed] [Google Scholar]
- 13.Vitolo U, Trněný M, Belada D, Burke JM, Carella AM, Chua N, et al. Obinutuzumab or Rituximab Plus Cyclophosphamide, Doxorubicin, Vincristine, and Prednisone in Previously Untreated Diffuse Large B-Cell Lymphoma. J Clin Oncol. 2017;35:3529–3537. doi: 10.1200/JCO.2017.73.3402. [DOI] [PubMed] [Google Scholar]
- 14.Davies A, Cummin TE, Barrans S, Maishman T, Mamot C, Novak U, et al. Gene-expression profiling of bortezomib added to standard chemoimmunotherapy for diffuse large B-cell lymphoma (REMoDL-B): an open-label, randomised, phase 3 trial. Lancet Oncol. 2019;20:649–662. doi: 10.1016/S1470-2045(18)30935-5/ATTACHMENT/8D119871-79B9-4998-9398-135C8574A3F0/MMC1.PDF. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Younes A, Sehn LH, Johnson P, Zinzani PL, Hong X, Zhu J, et al. Randomized Phase III Trial of Ibrutinib and Rituximab Plus Cyclophosphamide, Doxorubicin, Vincristine, and Prednisone in Non–Germinal Center B-Cell Diffuse Large B-Cell Lymphoma. J Clin Oncol. 2019 doi: 10.1200/JCO.18.02403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nowakowski GS, Chiappella A, Gascoyne RD, Scott DW, Zhang Q, Jurczak W, et al. ROBUST: A Phase III Study of Lenalidomide Plus R-CHOP Versus Placebo Plus R-CHOP in Previously Untreated Patients With ABC-Type Diffuse Large B-Cell Lymphoma. J Clin Oncol. 2021;39:1317–1328. doi: 10.1200/JCO.20.01366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tilly H, Morschhauser F, Sehn LH, Friedberg JW, Trněný M, Sharman JP, et al. Polatuzumab Vedotin in Previously Untreated Diffuse Large B-Cell Lymphoma. N Engl J Med. 2021 doi: 10.1056/NEJMOA2115304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Widmer F, Balabanov S, Soldini D, Samaras P, Gerber B, Manz MG, et al. R-hyper-CVAD versus R-CHOP/cytarabine with high-dose therapy and autologous haematopoietic stem cell support in fit patients with mantle cell lymphoma: 20 years of single-center experience. Ann Hematol. 2018;97:277–287. doi: 10.1007/s00277-017-3180-x. [DOI] [PubMed] [Google Scholar]
- 19.Thomas DA, O’brien S, Cortes J, Giles FJ, Faderl S, Verstovsek S et al Outcome with the hyper-CVAD regimens in lymphoblastic lymphoma 2004; 104:1624–30. 10.1182/blood-2003-12-4428 [DOI] [PubMed]
- 20.Busse D, Busch FW, Bohnenstengel F, Eichelbaum M, Fischer P, Opalinska J, et al. Dose escalation of cyclophosphamide in patients with breast cancer: consequences for pharmacokinetics and metabolism. J Clin Oncol. 1997;15:1885–1896. doi: 10.1200/JCO.1997.15.5.1885. [DOI] [PubMed] [Google Scholar]
- 21.Mantadakis BE, Herrera L, Leavey PJ, Bash RO, Winick NJ, Kamen BA. Cyclophosphamide and Etoposide for Children With Advanced or Refractory Solid Tumors: A Phase II. Window Study. 2010;18:2576–2581. doi: 10.1200/JCO.2000.18.13.2576. [DOI] [PubMed] [Google Scholar]
- 22.Chang TK, Yu L, Maurel P, Waxman DJ. Enhanced cyclophosphamide and ifosfamide activation in primary human hepatocyte cultures: response to cytochrome P-450 inducers and autoinduction by oxazaphosphorines. Cancer Res. 1997;57:1946–1954. [PubMed] [Google Scholar]
- 23.Oki Y, Westin JR, Vega F, Chuang H, Fowler N, Neelapu S, et al. Prospective phase II study of rituximab with alternating cycles of hyper-CVAD and high-dose methotrexate with cytarabine for young patients with high-risk diffuse large B-cell lymphoma. Br J Haematol. 2013;163:611–620. doi: 10.1111/bjh.12585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mato A, Feldman T, Zielonka T, Singavi A, Gadaletta G, Waksmundzki K, et al. Rituximab, cyclophosphamide-fractionated, vincristine, doxorubicin and dexamethasone alternating with rituximab, methotrexate and cytarabine overcomes risk features associated with inferior outcomes in treatment of newly diagnosed, high-risk diffuse large. Leuk Lymphoma. 2013;54:2606–2612. doi: 10.3109/10428194.2013.783909. [DOI] [PubMed] [Google Scholar]
- 25.Cheson BD, Pfistner B, Juweid ME, Gascoyne RD, Specht L, Horning SJ, et al. Revised response criteria for malignant lymphoma. J Clin Oncol. 2007;25:579–586. doi: 10.1200/JCO.2006.09.2403. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data is available on request to the corresponding author.