Abstract
Background
Early identification of patients who will benefit from immune checkpoint inhibitors (ICIs) has recently become a hot issue in cancer immunotherapy. Peripheral cytokines are key regulators in the immune system that can induce the expression of immune checkpoint molecules; however, the association between peripheral cytokines and the efficiency of ICIs remains unclear.
Methods
A systematic review was conducted in several public databases from inception through 3 February 2022 to identify studies investigating the association between peripheral cytokines (i.e., IL-1β, IL-2, IL-2RA, IL-2R, IL-4, IL-5, IL-6, IL-8, IL-10, IL-12, IL-15, IL-17, TNF-α, IFN-γ, and TGF-β) and ICI treatment. Survival data, including overall survival (OS) and/or progression-free survival (PFS), were extracted, and meta-analyses were performed.
Results
Twenty-four studies were included in this analysis. The pooled results demonstrated that the pretreatment peripheral levels of IL-6 (univariate analysis: HR = 2.53, 95% CI = 2.21–2.89, p < 0.00001; multivariate analysis: HR = 2.21, 95% CI = 1.67–2.93, p < 0.00001) and IL-8 (univariate analysis: HR = 2.17, 95% CI = 1.98–2.38, p < 0.00001; multivariate analysis: HR = 1.88, 95% CI= 1.70–2.07, p < 0.00001) were significantly associated with worse OS of cancer patients receiving ICI treatment in both univariate and multivariate analysis. However, high heterogeneity was found for IL-6, which might be attributed to region, cancer type, treatment method, sample source, and detection method.
Conclusion
The peripheral level of IL-8 may be used as a prognostic marker to identify patients with inferior response to ICIs. More high-quality prospective studies are warranted to assess the predictive value of peripheral cytokines for ICI treatment.
Keywords: cytokine, cancer, cancer biomarker, immune checkpoint inhibitors, prognosis, survival analysis
Introduction
Immune escape plays significant roles in occurrence and development of cancer and is one of the most important hallmarks of cancer (1). Immune checkpoints, consisting of co-stimulatory and inhibitory signals, can not only modulate the immune system, but also protect tumor cells from immune killing (1, 2). Reducing the release of negative immunomodulatory factors using immune checkpoint inhibitors (ICIs), either alone or combination, can produce long-lasting antitumor effects, which is one of the standard treatments for numerous tumors (3). There are currently several ICIs approved by the Food and Drug Administration (FDA) in numerous cancer indications, including anti-PD-1, anti-PD-L1, and anti-CTLA-4 antibodies. In addition to the well-investigated molecules, a series of novel immune checkpoint molecules with the potential therapeutic value have been introduced, such as lymphocyte activation gene-3 (LAG-3), T-cell immunoglobulin and mucin-domain containing-3 (TIM-3), and T-cell immunoglobulin and ITIM domain (TIGIT), and monoclonal antibody drugs designed against these novel sites have shown promising curative effects (3, 4).
However, the response rate of ICI treatment varies depending on the cancer type, which is stable at 10%–40%, and most patients progress despite initial response (3, 5). On the other hand, ICI treatment is accompanied by some immune-related adverse events (irAEs), which can be serious or even fatal (6). To avoid ineffective therapies and severe irAEs, early identification of patients who will not respond to ICI treatment has recently become a hot issue in cancer therapy. Diverse predictive biomarkers associated with the response of ICIs include intratumoral expression of PD-L1, tumor mutational burden (TMB), and T-cell infiltration metrics (7). However, it is difficult to establish uniform criteria to quantity these markers, and even more difficult to collect tumor sample before therapy begins; to date, only intratumoral PD-L1 detection has received regulatory companion diagnostic approval for ICI treatment (8). Therefore, it is critical to find new prognostic markers to improve the outcome of cancer patients treated with ICIs.
Cytokines are pleiotropic regulators that can play important roles in controlling cell development, growth, survival, and differentiation, through autocrine or paracrine pathways (9). In the tumor microenvironment (TME), the main types of cytokines involved in intercellular communications include interleukins (ILs), interferons (IFNs), the tumor necrosis factor (TNF) superfamily, chemokines, and growth factors (10). In addition, cytokines can recruit more immune cells into the TME and induce the expression of immune checkpoint proteins, such as PD-1 or TIM-3, to assist tumor cells evading the immune system (11, 12). It has been suggested that serum concentrations of TGF-β and IL-10 are associated with therapeutic effect and higher levels of IL-17 can predict irAEs in melanoma patients treated with ipilimumab (13). In view of its non-invasive and accessible nature, peripheral cytokines may have potential value as prognostic markers of cancer patients treated with ICIs. However, to our knowledge, there is currently no statistical evidence to confirm this hypothesis. Therefore, we performed a comprehensive meta-analysis of relevant clinical trials to investigate the association between peripheral cytokines and the efficacy of ICI treatment in cancer patients.
Methods
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines were utilized to conduct this systematic review and meta-analysis (14).
Information sources and search strategy
A systematic retrieve was conducted in the PubMed, Embase, and Cochrane Library databases from inception through 3 February 2022 to identify articles investigating the relationship between peripheral cytokines and the efficacy of ICI treatment in cancer patients. The search strategy consisted of four major components: (1) “cancer” and synonyms; (2) “prognosis” and synonyms; (3) “immune checkpoint inhibitor” and synonyms; and (4) “cytokine” and synonyms. The search included only studies published in English and human studies. The detailed search strategy for PubMed is shown in Supplementary Table 1 .
Inclusion and exclusion criteria
Studies were screened regarding the following inclusion criteria: (1) patients suffering from any malignant tumors diagnosed by pathologic features or clinical characteristics; (2) treatment option including at least one ICI approved by FDA; (3) any kind of assessment of peripheral cytokines before treatment, mainly including IL-1β, IL-2, IL-2RA, IL-2R, IL-4, IL-5, IL-6, IL-8, IL-10, IL-12, IL-15, IL-17, TNF-α, IFN-γ, and TGF-β (the above types of cytokines were all identified by referring the research of Briukhovetska et al. (15) and all played critical roles in the immune process); and (4) survival analysis contained subgroup comparisons of overall survival (OS) or progression-free survival (PFS) based on concentrations of peripheral cytokine using a hazard ratio (HR). Exclusive criteria include the following: (1) studies involved therapies other than ICIs (e.g., targeted therapy, chemotherapy, radiation therapy, and other immunotherapy); (2) studies reported the serum protein levels that were not related to cytokine; and (3) case report, review, letter, and conference abstract.
Data extraction and quality assessment
Two investigators (MXC and LH) independently extracted HR and 95% confidence interval (CI) from each included studies in addition to methodological and patient characteristics. The Parmar method was used to extract survival data when studies did not provide the original data.
The two investigators (MXC and LH) also independently reviewed the included studies to assess their methodological quality by using Newcastle–Ottawa scale (NOS) score, which ranged from 0 to 9. Studies with scores ≥5 were defined as high-quality studies. Any disagreements were resolved by discussing and receiving a consensus with a third author (LT).
Statistical analysis
Statistical analysis was conducted only when each cytokine contains at least three individual survival data. OS and/or PFS for each cytokine were calculated, providing a pooled HR. The between-study heterogeneity was measured applying I 2 test with the p-value. Significant heterogeneity was suggested if I 2 > 50%, and a random-effects model was used to summary data. Otherwise, we used a fixed-effects model. Publication bias was assessed inspecting funnel plot asymmetry and examined using Egger’s test. A p-value <0.1 was considered the presence of publication bias. Sensitivity analyses were conducted for statistically significant HR by omitting each of the included studies in turn to verify the stability of the pooled results. To further investigate sources of the between-study heterogeneity, subgroup analysis and meta-regression analysis were conducted. The following potential confounders were considered: region, cancer type, sample source, detection time, treatment, and detection method. All analyses were performed using Stata and R software. p-value ≤0.05 was considered statistically significant.
Results
Characteristics of included studies
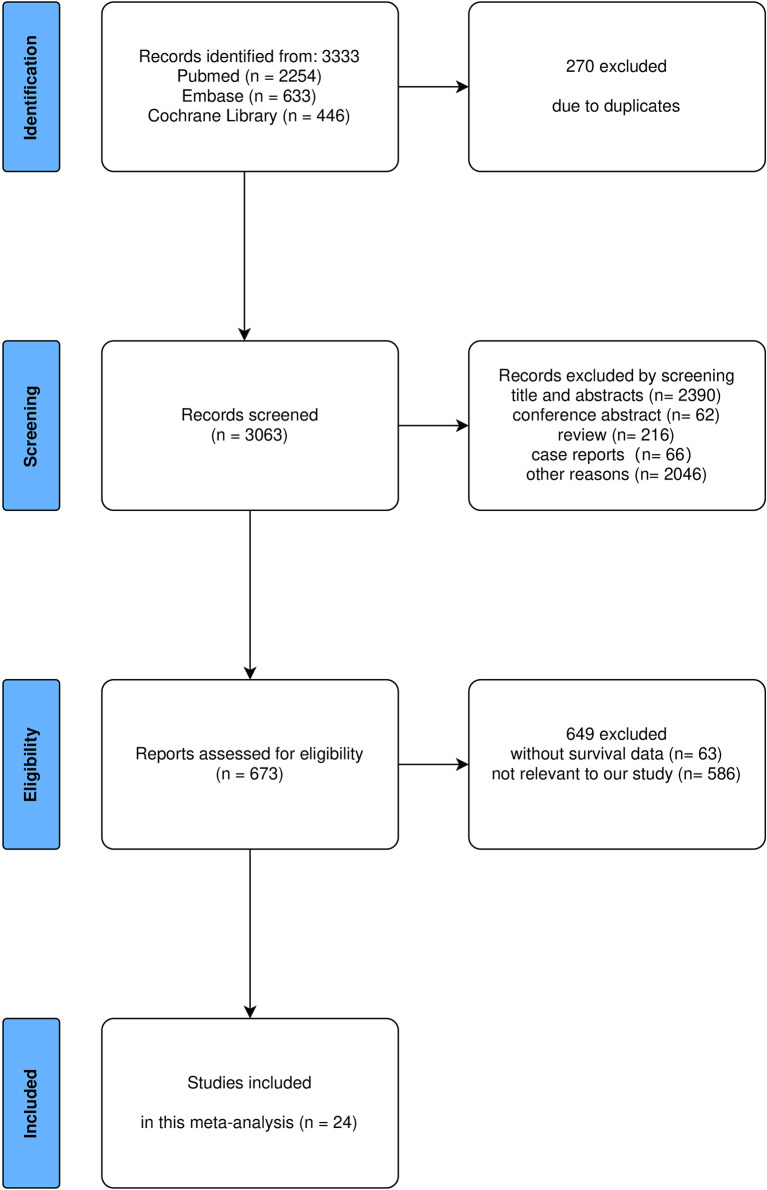
In total, 3,333 records were identified, of which 24 studies met the inclusion criteria and were eligible for this meta-analysis (7, 13, 16–37) ( Figure 1 ). The 24 studies utilized 44 datasets to produce their analyses (4 studies utilized 2 datasets, 1 study utilized 5 datasets, 1 study utilized 6 datasets, and 1 study utilized 7 datasets). The characteristics of the 24 included studies are summarized in Table 1 . These studies were conducted in 11 countries. Nine studies analyzed patients with melanoma, eight studies analyzed patients with lung cancer, two studies analyzed patients with esophageal cancer, one study analyzed patients with prostate cancer, one study analyzed patients with head and neck cancer, and of the rest, three studies included multiple cancer types. A total of 23 studies adopted ICI monotherapy, and 1 study adopted combination ICI plus chemotherapy strategy. The number of patients included in these studies ranged from 16 to 1,642. The NOS scores among included studies ranged from 6 to 10 points.
Figure 1.
Flowchart of study selection.
Table 1.
Study characteristics and quality assessment.
| No. | Study | Sample size (n) | Male (%) | Treatment | Cancer type | Markers measured | Quality rating |
|---|---|---|---|---|---|---|---|
| 1 | Zhou et al., 2021, (16) China | 156 | 89.1 | Anti-PD-1; Anti-PD-L1 | Lung cancer | IL-8 | 6 |
| 2 | Zhao et al., 2021, (17) China | 40 | 85 | Anti-PD-1 | Lung cancer | IL-5; IFN-γ | 8 |
| 3 | Zhang et al., 2021, (18) China | 20 | 90 | Camrelizumab | Esophageal cancer | IL-15 | 6 |
| 4 | Wang et al., 2021, (19) America | 462 | 62 | Immune checkpoint blockade | Melanoma | IL-6 | 9 |
| 5 | Shi et al., 2021, (20) China | 60 | 70 | Anti-PD-1-based chemotherapy | Lung cancer | IL-1β; IL-2; IL-4; IL-5; IL-6; IL-8; IL-10; IFN-γ; TNF-α | 7 |
| 6 | Shenderov et al., 2021, (21) America | 30 | NR | Nivolumab + ipilimumab | Prostate cancer | IL-1β; IL-5; IL-6; IL-8; IL-15; TNF-α | 8 |
| 7 | Klerk et al., 2021, (22) America | 49 | 79.6 | Pembrolizumab | Esophageal cancer | IL-6 | 9 |
| 8 | Arends et al., 2021, (23) America | 367 | 80.9 | Durvalumab | Head and neck cancer | IL-6; IL-8 | 7 |
| 9 | Yuen et al., 2020, (24) America | 1,020 | 78.8 | Atezolizumab | Urothelial carcinoma Renal cell carcinoma |
IL-8 | 10 |
| 10 | Schalper et al., 2020 (7), America | 1,642 | 66.7 | Nivolumab Ipilimumab Nivolumab + Ipilimumab |
Melanoma Renal cell carcinoma Lung cancer |
IL-8 | 9 |
| 11 | Pedersen et al., 2020, (25) Denmark | 16 | 69 | Pembrolizumab Nivolumab + ipilimumab |
Melanoma | IL-6; IL-8; IL-10; IFN-γ; TNF-α | 8 |
| 12 | Laino et al., 2020 (26), America | 1,256 | NR | Nivolumab Ipilimumab Nivolumab + Ipilimumab |
Melanoma | IL-6 | 9 |
| 13 | Koh et al., 2020 (27), Korea | 132 | 79.5 | Pembrolizumab Nivolumab |
Lung cancer | IL-10 | 6 |
| 14 | Keegan et al., 2020 (28), America | 47 | 34 | Pembrolizumab Nivolumab Atezolizumab |
Lung cancer | IL-6 | 8 |
| 15 | Kang et al., 2020 (29), Korea | 125 | 79.2 | Nivolumab Pembrolizumab Atezolizumab |
Lung cancer | IL-6 | 7 |
| 16 | Babačić et al., 2020 (30), Sweden | 24 | 54.2 | Anti-PD-1 | Melanoma | IL-6; IL-12 | 6 |
| 17 | Agulló-Ortuño, et al., 2020 (31), Spain | 27 | 78 | Nivolumab | Lung cancer | IL-8 | 7 |
| 18 | Lim et al., 2019 (13), Australia | 38 | 74 | Anti-PD-1 Anti-CTLA-4 + anti-PD-1 |
Melanoma | IL-2; IL-8; TNF-α | 7 |
| 19 | Hardy-Werbin et al., 2019 (32), Spain | 37 | 64.9 | Ipilimumab | Lung cancer | IL-1β; IL-2; IL-4; IL-5; IL-6; IL-8; IL-10; IFN-γ; TNF-α | 9 |
| 20 | Valpione et al., 2018 (33), England | 140 | 61.4 | Ipilimumab | Melanoma | IL-6 | 8 |
| 21 | Tallerico et al., 2017 (34), Italy | 116 | NR | Ipilimumab | Melanoma | IL-15 | 7 |
| 22 | Sanmamed et al., 2017 (35), America | 48 | 60.4 | Nivolumab Pembrolizumab |
Melanoma Lung cancer |
IL-8 | 7 |
| 23 | Damuzzo et al., 2016 (36), Italy | 44 | 65.9 | Ipilimumab | Melanoma | IL-6 | 8 |
| 24 | Bjoern et al., 2016 (37), Denmark | 40 | 40 | Ipilimumab | Melanoma | IL-6 | 9 |
IL, interleukin; IFN, interferon; TNF, tumor necrosis factor.
Peripheral cytokine levels and its association with OS
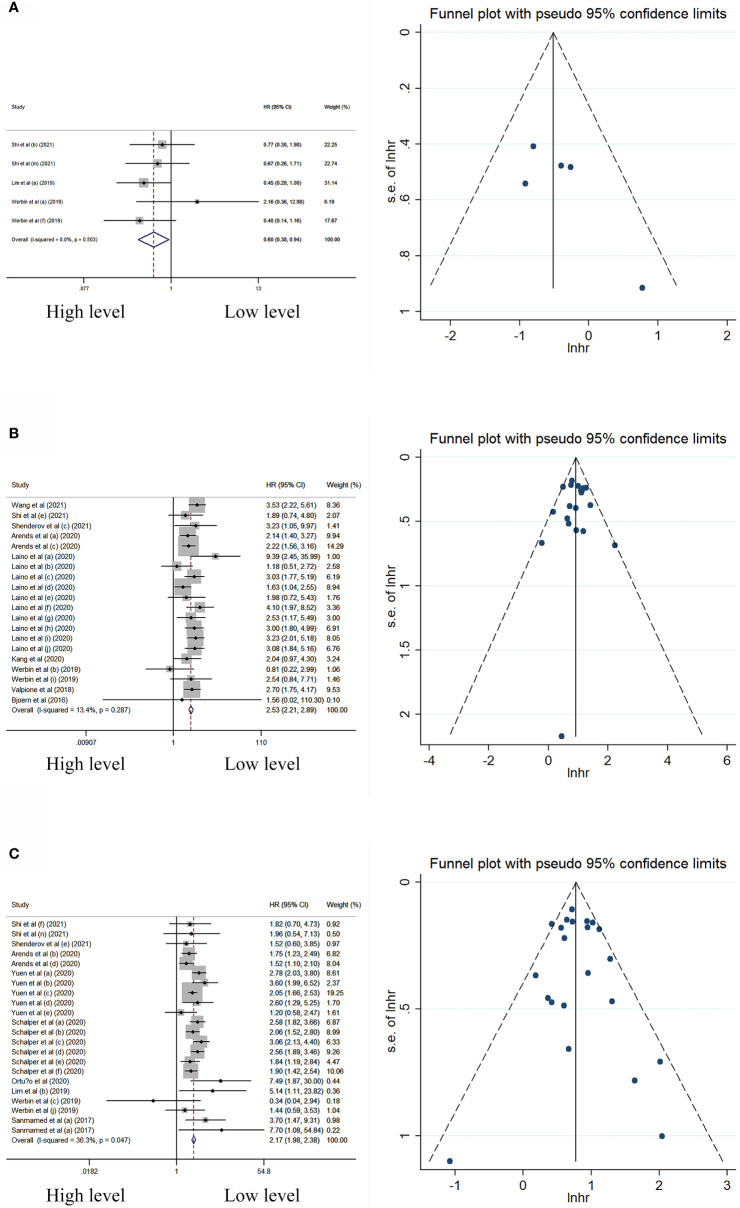
In univariate analysis, peripheral IL-2 level was significantly associated with better OS, while the levels of IL-6 and IL-8 were significantly associated with worse OS ( Table 2 ; Figure 2 ). Neither significant heterogeneity nor publication bias was detected in these meta-analyses.
Table 2.
Meta-analysis investigated the association between cytokines and OS.
| Cytokines | Studies (n) | HR (95% CI) | p-value | I 2 (%) | p-value Egger’s test |
|---|---|---|---|---|---|
| Univariate Analysis | |||||
| IL-1β | 3 | 0.60 (0.33, 1.09) | 0.09 | 0 | 0.11 |
| IL-2 | 3 | 0.60 (0.38, 0.94) | 0.02 | 0 | 0.14 |
| IL-4 | 2 | 0.96 (0.49, 1.88) | 0.90 | 49 | 0.12 |
| IL-5 | 3 | 0.77 (0.44, 1.34) | 0.36 | 0 | 0.17 |
| IL-6 | 9 | 2.53 (2.21, 2.89) | <0.00001 | 13 | 0.89 |
| IL-8 | 9 | 2.17 (1.98, 2.38) | <0.00001 | 36 | 0.61 |
| IL-10 | 3 | 1.61 (0.91, 2.83) | 0.10 | 0 | 0.32 |
| TNF-α | 4 | 1.44 (0.66, 3.14) | 0.35 | 60 | 0.66 |
| Multivariable Analysis | |||||
| IL-6 | 8 | 2.21 (1.67, 2.93) | <0.00001 | 85 | 0.00 |
| IL-8 | 2 | 1.88 (1.70, 2.07) | <0.00001 | 0 | 0.68 |
| IL-15 | 2 | 1.77 (1.08, 1.32) | 0.0006 | 30 | 0.64 |
IL, interleukin; TNF, tumor necrosis factor.
Figure 2.
Forest plot and funnel plot of studies investigating levels of peripheral (A) IL-2, (B) IL-6, and (C) IL-8 in univariate analysis of OS.
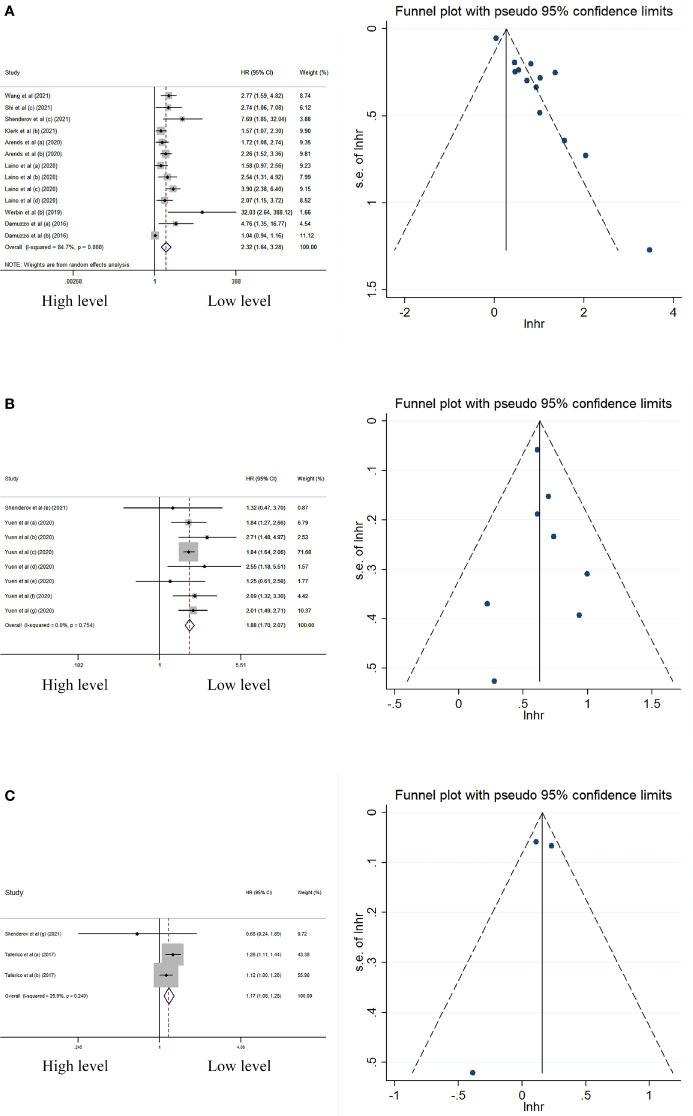
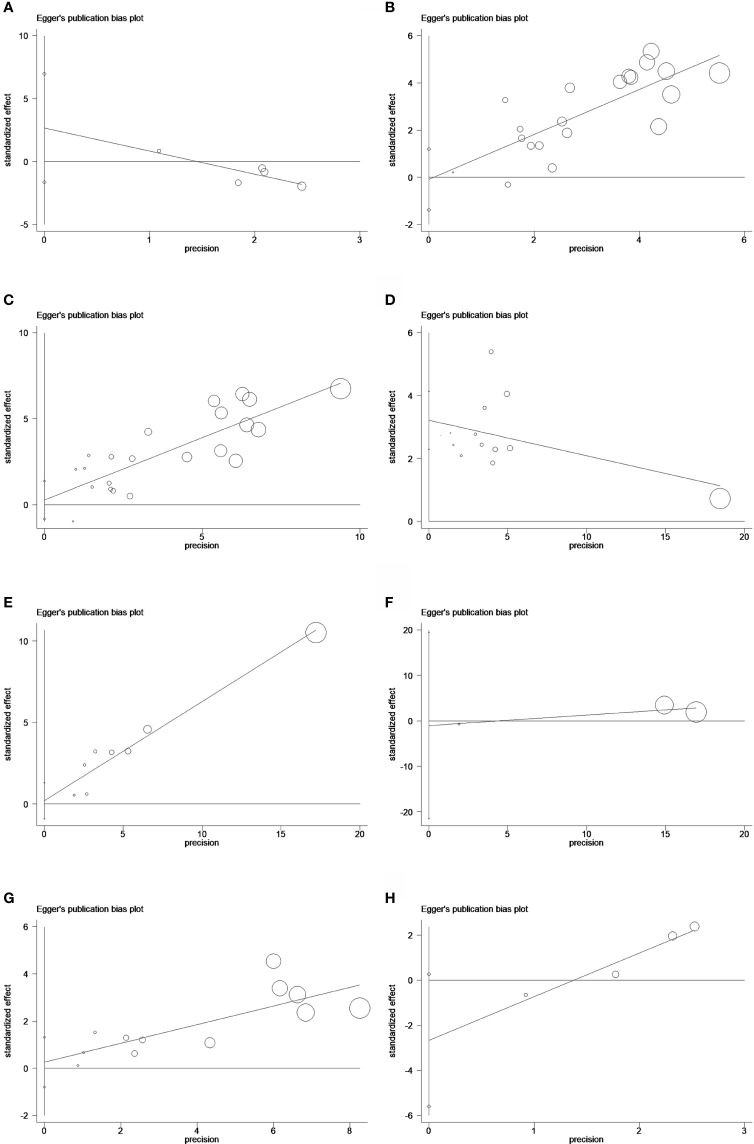
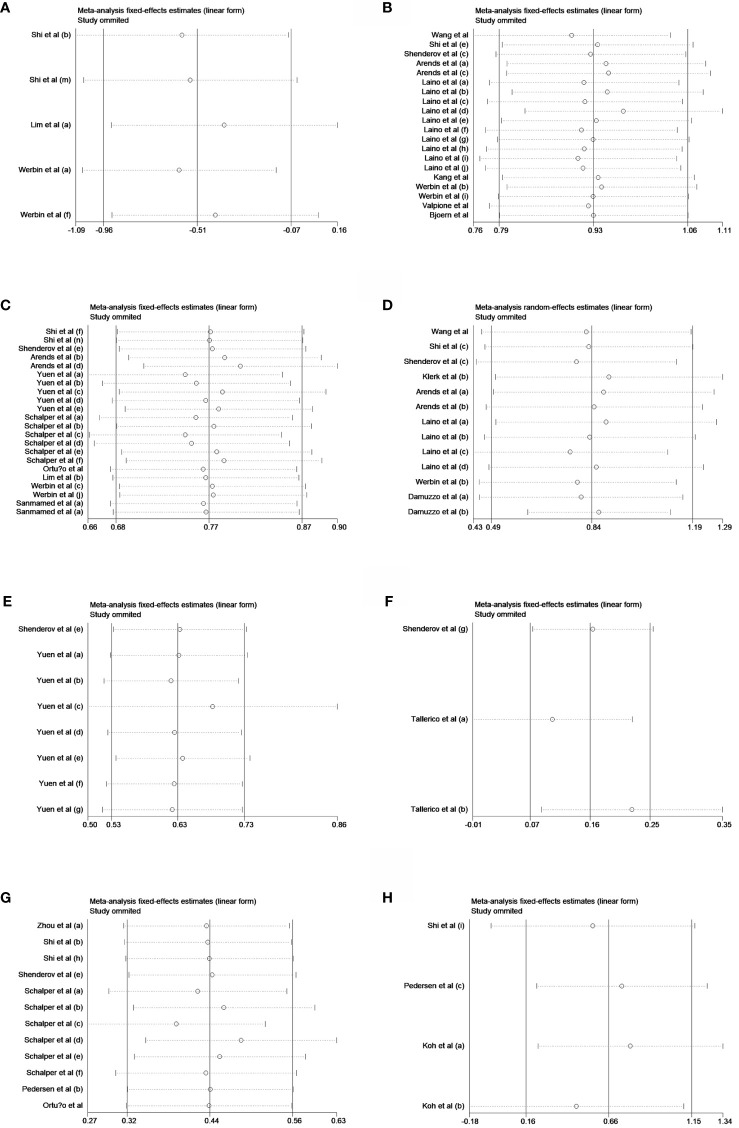
In multivariate analysis, the peripheral level of IL-6, IL-8, and IL-15 was significantly associated with worse OS ( Table 2 ; Figure 3 ). No significant heterogeneity and publication bias were observed in summary HRs of IL-8 and IL-15. The sensitivity analyses for IL-8 and IL-15 also displayed relatively robust results ( Figures 4E, F ). However, the heterogeneity in the summary effect for IL-6 was large (I 2 = 85% [75%–91%]). Subgroup analysis showed that European patients, melanoma, ipilimumab monotherapy, blood sample type, on-treatment sample type, and using flow cytometry-based detection method were associated with higher heterogeneity compared with other regions (e.g., America or China), other cancer types (e.g., lung cancer or prostate cancer), other treatment methods (e.g., nivolumab or pembrolizumab), other sample types (serum or plasma), other detection methods (e.g., ELISA or Luminex), and baseline sample type ( Table 3 ). Meta-regression analysis did not show any statistically significant covariates that could explain the heterogeneity ( Supplementary Table 3 ). However, the funnel plot for IL-6 had apparent asymmetry and the p-value of Egger’s test was less than 0.05 (Egger’s test: p-value = 0), indicating the existence of publication bias.
Figure 3.
Forest plot and funnel plot of studies investigating levels of peripheral (A) IL-6, (B) IL-8, and (C) IL-15 in multivariable analysis of OS.
Figure 4.
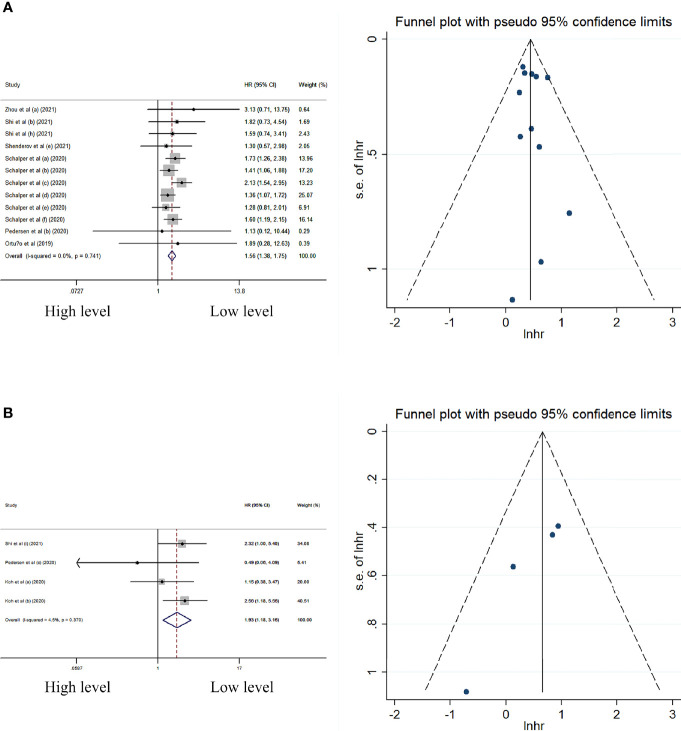
Forest plot and funnel plot of studies investigating levels of peripheral (A) IL-8 and (B) IL-10 in univariate analysis of PFS.
Table 3.
Subgroup analysis of IL-6 multivariable OS.
| Subgroup | N studies | Hazard ratio (95% CI) | I 2 (%) | p-value for heterogeneity |
|---|---|---|---|---|
| Region | ||||
| Europe | 2 | 3.82 (0.69,21.16) | 84 | 0.002 |
| America/China | 6 | 2.24 (1.79, 2.81) | 41 | 0.08 |
| Cancer type | ||||
| Melanoma | 3 | 2.24 (1.34, 3.74) | 89 | <0.00001 |
| Other | 5 | 2.25 (1.52, 3.35) | 55 | 0.05 |
| Drug | ||||
| Ipilimumab | 5 | 3.16 (1.58, 6.33) | 90 | <0.00001 |
| Other | 3 | 1.87 (1.54, 2.28) | 0 | 0.58 |
| Sample source | ||||
| Blood | 1 | 1.95 (0.45, 8.43) | 82 | 0.02 |
| Plasma/serum | 7 | 2.33 (1.81, 2.99) | 49 | 0.03 |
| Detection time | ||||
| On-treatment | 2 | 1.50 (0.60, 3.78) | 75 | 0.04 |
| Baseline | 7 | 2.38 (1.83, 3.09) | 52 | 0.02 |
| Detection method | ||||
| Flow cytometry | 1 | 1.95 (0.45, 8.43) | 82 | 0.02 |
| ELISA/Luminex | 7 | 2.33 (1.81, 2.99) | 49 | 0.03 |
No statistical significance was observed in the univariate analysis of IL-1β, IL-4, IL-5, IL-10, and TNF-α. The forest plots for these cytokines are shown in Supplementary Figure 1 .
Peripheral cytokine levels and its association with PFS
In univariate analysis, the peripheral levels of IL-8 and IL-10 were associated with better PFS without significant heterogeneity and publication bias in these meta-analyses ( Table 4 ; Figure 4 ).
Table 4.
Meta-analysis investigated the association between cytokines and PFS.
| Cytokines | Studies (n) | HR (95%CI) | p-value | I 2(%) | p-value Egger’s test |
|---|---|---|---|---|---|
| Univariate Analysis | |||||
| IL−5 | 3 | 1.15 (0.72, 1.85) | 0.56 | 45 | 0.64 |
| IL−6 | 5 | 1.61 (0.86, 3.02) | 0.1 | 53 | 0.90 |
| IL-8 | 6 | 1.56 (1.38, 1.75) | <0.00001 | 0 | 0.59 |
| IL-10 | 3 | 1.93 (1.18, 3.16) | 0.001 | 5 | 0.06 |
| TNF-α | 3 | 0.71 (0.14, 3.57) | 0.73 | 74 | 0.06 |
| IFN-γ | 3 | 1.11 (0.40, 3.07) | 0.85 | 65 | 0.87 |
| Multivariable Analysis | |||||
| IL-6 | 3 | 1.75 (0.43, 7.08) | 0.45 | 88 | 0.29 |
IL, interleukin; IFN, interferon; TNF, tumor necrosis factor.
Figure 6.
Egger’s publication bias plots of peripheral (A) IL-2, (B) IL-6, and (C) IL-8 in univariate analysis of OS and (D) IL-6, (E) IL-8, and (F) IL-15 in multivariable analysis of OS and (G) IL-8 and (H) IL-10 in univariate analysis of PFS.
No statistical significance was observed in the univariate analysis of IL-5, IL-6, TNF-α, and IFN-γ, and in the multivariable analysis of IL-6. The forest plots for these cytokines are shown in Supplementary Figures 2, 3 .
Sensitivity analyses and Egger's publication bias plots
Sensitivity analyses showed that all pooled results were relatively robust ( Figures 5A–H ). Egger's publication bias plots were shown in Figures 6A–H .
Figure 5.
Sensitivity analyses of peripheral (A) IL-2, (B) IL-6, and (C) IL-8 in univariate analysis of OS and (D) IL-6, (E) IL-8, and (F) IL-15 in multivariable analysis of OS and (G) IL-8 and (H) IL-10 in univariate analysis of PFS.
Discussion
This meta-analysis investigated the association between the peripheral cytokine levels and the outcome of ICIs in cancers. Low levels of IL-6, IL-8, and IL-15 were found on multivariable analysis to be associated with better OS of cancer patients treated with ICIs. Although the levels of IL-2, IL-6, IL-8, and IL-10 could also affect PFS or OS of patients in univariate analysis, the statistical evidence of univariate analysis was weak and further studies were needed to confirm these findings.
To the best of our knowledge, this is the first meta-analysis to investigate how peripheral cytokines affect the efficacy of ICI treatment in cancer patients. In the univariate analysis of OS, patients treated with ICIs whose IL-2 increased had a better OS. IL-2 plays a significant role in modulating the immune system, in both innate and adaptive immunity (10). IL-2 is not only an essential mediator that promotes T-cell clonal expansion, survival, and differentiation after antigen exposure, but also a growth factor and enhancer for CD4+T cells as well as natural killer (NK) cells (10, 32). Because modulating the activity of T cells through the interaction between immune checkpoint molecules may lead to decreased IL-2 secretion, the release of this inhibition with ICIs could increase IL-2 secretion (38), which, in turn, enhances the immune response. However, multiple latest studies have shown that IL-2 induces immunosuppressive regulatory T (Treg) cell activity through binding with a high-affinity trimeric receptor composed of α, β, γ subunits on the surface of Treg cell and thereby inhibit antitumor response (10, 39). Given the possible double effect of IL-2 in tumor immune environment and the relatively few studies that have been published, the relationship between IL-2 and ICIs needs further study.
In the univariate analysis of PFS, high level of IL-10 was associated with worse PFS in cancer patients. IL-10 is usually considered as an immunosuppressive and anti‐inflammatory cytokine regulating the growth and differentiation of various immune cells (40). The inhibitory effect of IFN-γ secretion mediated by IL-10 was achieved via interacting with APC function of macrophages and inhibiting cytokines produced by activated dendritic cells (41). An additional property of IL-10 is its anti-inflammatory trait to inhibit the antigen-presenting capacity of monocytes (42). All the above were aligned with the results of our meta-analysis. However, it has been recently shown that IL-10 may stimulate the immune response on specific cell types and contexts (41). High level of IL-10 was able to promote activation and proliferation of CD8+ T cells in chronic inflammation and cancer (43). The efficacy and safety of high-dose IL-10 combined with ICIs in cancer treatment have been verified in clinical trials, and the clinical results were safe and tolerated (NCT02009449). In light of recent studies, we have to revisit our research data. While the heterogeneity was small, the survival data from two of the four studies were not statistically significant, and more data may be needed to demonstrate the prognostic significance of IL-10 in ICI treatment.
IL-15, a member of the four-α-helix bundle family of cytokines, could stimulate the proliferation of immune cells including T cell, B cell, and NK cell through JAK1/3 STAT3/5 signaling molecules (39). Combination therapy of IL-15 and ICIs was able to facilitate clearance of immune checkpoints and to achieve prolonged survival in animal experiments (44, 45). In addition, an antitumor combination strategy, IL-15 in combination with monoclonal antibody, was gradually emerging, and clinical trials for leukemia and lymphoma have been carried out (46). Taking all the above results, IL-15 seems to act as an anti-cancer factor, which is contrary to our conclusion. Given that only three survival data included in the statistical analysis of IL-15, and one of them was not statistically significant, more data were still needed to further verify this finding.
Until now, there were relatively numerous studies discussing the association with IL-6, IL-8, and ICI treatment. The statistical analysis of IL-6 and IL-8 was robust and adequate in our meta-analysis, and the result showed that high levels of IL-6 and IL-8 were significantly associated with the prognosis of cancer patients treated with ICIs, for both OS and PFS. IL-6 is emerging as a regulator of immune system through multiple mechanisms, especially in patients treated with ICIs, from promoting expression of the T helper-associated cytokine IL-4 (47) and suppressing IFN-γ expression (48), to inducing the expression of angiogenic factors, such as vascular endothelial growth factor (VEGF), and downregulating expression of CD80, CD86, and IL-12 (49). In addition to its immunosuppressive function, IL-6 also has multiple pro-tumorigenic activities. IL-6 contributes to malignancy formation and proliferation by inducing expression of STAT3 and its downstream target genes, which encode proteins that drive tumorigenesis, such as cyclin D1 or BCL2- like protein 1 (BCL-xL) (26). STAT3, in turn, promotes IL-6 gene expression, which forms a positive feedback loop to accelerate tumor formation. There have been several studies suggesting that inhibiting IL‐6 activity can increase the response rate of cancer patients to ICIs, and ultimately improve outcomes for patients (23). Combined blockade of IL-6 and PD-1/PD-L1 abolishes mutual regulation of their immunosuppressive effects and lead to synergistic antitumor effects. Recently, clinical trials of ICIs combined with anti-IL-6 or anti-IL-6 receptor for cancer patients have started or are being initiated (NCT03999749, NCT04258150, and NCT03821246) (23).
IL-8, a pro-inflammatory chemokine and overexpressed in many cancers, was shown to enhance the growth and invasion abilities of tumor cells in multiple mechanisms, including angiogenesis, epithelial-to-mesenchymal transition (EMT), formation of neutrophil extracellular traps (NETs), and infiltration of immunosuppressive and pro-tumorigenic myeloid inflammatory cells (50–52). Multiple studies have shown that the NF-kB signaling pathway plays a significant role in maintaining the mesenchymal phenotype of tumor cells and inducing cytoskeletal rearrangement to increasing chemotaxis and invasion (53, 54). On the process shown above, IL-8 binds to CXCR1 on tumor cells in an autocrine manner to trigger a downstream cascade reaction of the signaling pathway resulting in increased NF-kB activity (54). IL-8 can alter TME in indirect ways, such as increasing angiogenesis within the tumor or recruiting higher numbers of infiltrating immune suppressive cells (55). In addition, high serum level of IL-8 is associated with tumor burden and resistance to ICIs, and blocking the IL-8/IL-8R receptor axis can augment the efficacy of ICIs (7, 55, 56). The efficacy and safety of HuMaxIL8 (an anti-IL-8 monoclonal antibody) in multiple clinical trials are being evaluated, mostly in combination with nivolumab (NCT03689699, NCT04050462, and NCT03400332). Meanwhile, IL-8 receptor antagonists, including the dual CXCR1/2 inhibitor SX682 and the CXCR2 antagonist AZD5069, are also being assessed in combination with multiple anti-PD-1 or anti-PD-L1 mAbs (55).
It is noteworthy that in addition to the above-discussed cytokines, there are several other cytokines directly involved in the activation of an effective antitumor response, such as IL-12 and TGF-β (15). Disappointingly, there were not enough studies to conduct meta-analyses for IL-12 and TGF-β after conducting systematic retrieve. There were four articles investigating the association of IL-12 and the efficacy of ICIs. Only the study of Babačić et al. (30) contained the relevant survival data, and the remaining three studies (57–59) only indicated that the higher level of IL-12 at baseline was associated with clinical benefit from ICIs; however, no relevant survival data were provided. For TGF-β, only four studies roughly met our inclusion criteria after screening title and abstracts. Two studies (60, 61) investigated gene signatures of TGF-β rather than peripheral TGF-β concentration. One study (62) measured the peripheral level of TGF-β 1 week after anti-PD-1 immunotherapy, which did not meet our requirement. The work of Feun et al. (63) provided KM curves for OS and PFS based on the baseline plasma level of TGF-β in hepatocellular carcinoma (HCC) patients treated with pembrolizumab. Relevant survival data can extract from curves, but the accuracy is poor. Therefore, more high-quality clinical studies are warranted to assess the predictive value of IL-12 and TGF-β for ICI treatment.
There are several limitations in our meta-analysis. First, this study is only based on the data of the three commonly used public databases (PubMed, Embase, and Cochrane of library), and thus relevant papers may be omitted. Second, on some occasions, we had to calculate HR and 95% CI based on the data extracted from the Kaplan–Meier survival curve, which may not provide the most accurate survival data. However, this practice did not significantly disrupt the stability of the result. Third, significant heterogeneity was observed in the multivariable analysis of evaluating the prognostic role of IL-6 for OS, which might be attributed to cancer type, treatment method, sample source, detection time, and detection method according to the results of subgroup analysis. In addition, we also perform a meta-regression analysis to discuss the source of heterogeneity; however, no statistically significant covariates were observed.
Conclusion
Our study is the first comprehensive meta-analysis investigating the association between peripheral cytokine levels and the outcome of ICI treatment in cancer patients to date. The peripheral level of IL-8 may be used as a prognostic marker to identify patients with inferior response to ICIs. More high-quality prospective studies are warranted to assess the predictive value of peripheral cytokines for ICI treatment.
Data availability statement
The original contributions presented in the study are included in the article/ Supplementary Material . Further inquiries can be directed to the corresponding author.
Author contributions
X-CM and TL designed the study. C-CY, Y-FY, and L-JY performed the systematic search. X-CM and HL selected eligible articles and conducted the quality assessment. X-CM, Z-ND, Y-CY, Z-RD, and D-XW analyzed and interpreted the data, and drafted the manuscript. TL revised the manuscript. All authors have read and approved the final version of the manuscript.
Funding
This work was supported by grants from the Taishan Scholars Program for Young Expert of Shandong Province (Grant No. tsqn20161064), National Natural Science Foundation of China (Grant Nos. 82073200 and 81874178), Funds for Independent Cultivation of Innovative Team from Universities in Jinan (Grant No. 2020GXRC023), and Major Basic Research of Shandong Provincial Natural Science Foundation (Grant No. ZR202105070027).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2022.884592/full#supplementary-material
Abbreviations
ICIs, immune checkpoint inhibitors; FDA, Food and Drug Administration; LAG-3, lymphocyte activation gene-3; TIM-3, T-cell immunoglobulin and mucin-domain containing-3; TIGIT, T-cell immunoglobulin and ITIM domain; IrAEs, immune-related adverse events; TMB, tumor mutational burden; TME, tumor microenvironment; TNF, tumor necrosis factor; OS, overall survival; PFS, progression-free survival; HR, hazard ratio; CI, confidence interval; NOS, Newcastle–Ottawa scale; NK, natural killer; Treg, regulatory T; IFN, interferon; VEGF, vascular endothelial growth factor; BCL-xL, BCL2-like protein 1; EMT, epithelial-to-mesenchymal transition; NETs, neutrophil extracellular traps; HCC, hepatocellular carcinoma.
References
- 1. Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell (2011) 144(5):646–74. doi: 10.1016/j.cell.2011.02.013 [DOI] [PubMed] [Google Scholar]
- 2. Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer (2012) 12(4):252–64. doi: 10.1038/nrc3239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ribas A, Wolchok JD. Cancer immunotherapy using checkpoint blockade. Science (2018) 359(6382):1350–5. doi: 10.1126/science.aar4060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Morad G, Helmink BA, Sharmaand P, Wargo JA. Hallmarks of response, resistance, and toxicity to immune checkpoint blockade. Cell (2021) 184(21):5309–37. doi: 10.1016/j.cell.2021.09.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. O'Donnell JS, Teng MWL, Smyth MJ. Cancer immunoediting and resistance to T cell-based immunotherapy. Nat Rev Clin Oncol (2019) 16(3):151–67. doi: 10.1038/s41571-018-0142-8 [DOI] [PubMed] [Google Scholar]
- 6. Dolladille C, Ederhy S, Sassier M, Cautela J, Thuny F, Cohen AA, et al. Immune checkpoint inhibitor rechallenge after immune-related adverse events in patients with cancer. JAMA Oncol (2020) 6(6):865–71. doi: 10.1001/jamaoncol.2020.0726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Schalper KA, Carleton M, Zhou M, Chen T, Feng Y, Huang SP, et al. Elevated serum interleukin-8 is associated with enhanced intratumor neutrophils and reduced clinical benefit of immune-checkpoint inhibitors. Nat Med (2020) 26(5):688–92. doi: 10.1038/s41591-020-0856-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Havel JJ, Chowell D, Chan TA. The evolving landscape of biomarkers for checkpoint inhibitor immunotherapy. Nat Rev Cancer (2019) 19(3):133–50. doi: 10.1038/s41568-019-0116-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lin JX, Leonard WJ. Fine-tuning cytokine signals. Annu Rev Immunol (2019) 37:295–324. doi: 10.1146/annurev-immunol-042718-041447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Propper DJ, Balkwill FR. Harnessing cytokines and chemokines for cancer therapy. Nat Rev Clin Oncol (2022) 19(4):237–53. doi: 10.1038/s41571-021-00588-9 [DOI] [PubMed] [Google Scholar]
- 11. Engelhardt B, Ransohoff RM. Capture, crawl, cross: the T cell code to breach the blood-brain barriers. Trends Immunol (2012) 33(12):579–89. doi: 10.1016/j.it.2012.07.004 [DOI] [PubMed] [Google Scholar]
- 12. Zhang ZN, Zhu ML, Chen YH, Fu YJ, Zhang TW, Jiang YJ, et al. Elevation of Tim-3 and PD-1 expression on T cells appears early in HIV infection, and differential Tim-3 and PD-1 expression patterns can be induced by common γ -chain cytokines. BioMed Res Int (2015) 2015:916936. doi: 10.1155/2015/916936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lim SY, Lee JH, Gide TN, Menzies AM, Guminski A, Carlino MS, et al. Circulating cytokines predict immune-related toxicity in melanoma patients receiving anti-PD-1-Based immunotherapy. Clin Cancer Res (2019) 25(5):1557–63. doi: 10.1158/1078-0432.CCR-18-2795 [DOI] [PubMed] [Google Scholar]
- 14. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PloS Med (2009) 6(7):e1000100. doi: 10.1371/journal.pmed.1000100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Briukhovetska D, Dörr J, Endres S, Libby P, Dinarelloand CA, Kobold S. Interleukins in cancer: from biology to therapy. Nat Rev Cancer (2021) 21(8):481–99. doi: 10.1038/s41568-021-00363-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zhou J, Chao Y, Yao D, Ding N, Li J, Gao L, et al. Impact of chronic obstructive pulmonary disease on immune checkpoint inhibitor efficacy in advanced lung cancer and the potential prognostic factors. Transl Lung Cancer Res (2021) 10(5):e000842. doi: 10.21037/tlcr-21-214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zhao Q, Bi Y, Sun H, Xiao M. Serum IL-5 and IFN-γ are novel predictive biomarkers for anti-PD-1 treatment in NSCLC and GC patients. Dis Markers (2021) 2021:5526885. doi: 10.1155/2021/5526885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zhang W, Yan C, Zhang T, Chen X, Dong J, Zhao J, et al. Addition of camrelizumab to docetaxel, cisplatin, and radiation therapy in patients with locally advanced esophageal squamous cell carcinoma: a phase 1b study. Oncoimmunology (2021) 10(1):e000204. doi: 10.1080/2162402x.2021.1971418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wang Y, Ramachandran V, Sui D, Xu K, Haydu LE, Fang S, et al. Evaluation of plasma interleukin-6 in melanoma patients as a prognostic and checkpoint immunotherapy predictive biomarker. J Invest Dermatol (2021) 142(7):2046–2049.e3. doi: 10.1016/j.jid.2021.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Shi Y, Liu X, Du J, Zhang D, Liu J, Chen M, et al. Circulating cytokines associated with clinical outcomes in advanced non-small cell lung cancer patients who received chemoimmunotherapy. Thorac Cancer (2021) 13(2):219–227. doi: 10.1111/1759-7714.14248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Shenderov E, Boudadi K, Fu W, Wang H, Sullivan R, Jordan A, et al. Nivolumab plus ipilimumab, with or without enzalutamide, in AR-V7-expressing metastatic castration-resistant prostate cancer: a phase-2 nonrandomized clinical trial. Prostate (2021) 81(6):a028472. doi: 10.1002/pros.24110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. de Klerk LK, Patel AK, Derks S, Pectasides E, Augustin J, Uduman M, et al. Phase II study of pembrolizumab in refractory esophageal cancer with correlates of response and survival. J Immunother Cancer (2021) 9(9):e002472. doi: 10.1136/jitc-2021-002472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Arends R, Guo X, Baverel PG, González-García I, Xie J, Morsli N, et al. Association of circulating protein biomarkers with clinical outcomes of durvalumab in head and neck squamous cell carcinoma. Oncoimmunology (2021) 10(1):1898104. doi: 10.1080/2162402x.2021.1898104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Yuen KC, Liu LF, Gupta V, Madireddi S, Keerthivasan S, Li C, et al. High systemic and tumor-associated IL-8 correlates with reduced clinical benefit of PD-L1 blockade. Nat Med (2020) 26(5):693–698. doi: 10.1038/s41591-020-0860-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Pedersen JG, Madsen AT, Gammelgaard KR, Aggerholm-Pedersen N, Sørensen BS, Øllegaard TH, et al. Inflammatory cytokines and ctDNA are biomarkers for progression in advanced-stage melanoma patients receiving checkpoint inhibitors. Cancers (Basel) (2020) 12(6):1414. doi: 10.3390/cancers12061414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Laino AS, Woods D, Vassallo M, Qian X, Tang H, Wind-Rotolo M, et al. Serum interleukin-6 and C-reactive protein are associated with survival in melanoma patients receiving immune checkpoint inhibition. J Immunother Cancer (2020) 8(1):e000842. doi: 10.1136/jitc-2020-000842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Koh J, Kim Y, Lee KY, Hur JY, Kim MS, Kim B, et al. MDSC subtypes and CD39 expression on CD8(+) T cells predict the efficacy of anti-PD-1 immunotherapy in patients with advanced NSCLC. Eur J Immunol (2020) 50(11):1810–1819. doi: 10.1002/eji.202048534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Keegan A, Ricciuti B, Garden P, Cohen L, Nishihara R, Adeni A, et al. Plasma IL-6 changes correlate to PD-1 inhibitor responses in NSCLC. J Immunother Cancer (2020) 8(2):e000678. doi: 10.1136/jitc-2020-000678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kang DH, Park CK, Chung C, Oh IJ, Kim YC, Park D, et al. Baseline serum interleukin-6 levels predict the response of patients with advanced non-small cell lung cancer to pd-1/pd-l1 inhibitors. Immune Network (2020) 20(3):1–11. doi: 10.4110/in.2020.20.e27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Babačić H, Lehtiö J, Pico de Coaña Y, Pernemalmand M, Eriksson H. In-depth plasma proteomics reveals increase in circulating PD-1 during anti-PD-1 immunotherapy in patients with metastatic cutaneous melanoma. J Immunother Cancer (2020) 8(1):e000204. doi: 10.1136/jitc-2019-000204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Agulló-Ortuño MT, Gómez-Martín O, Ponce S, Iglesias L, Ojeda L, Ferrer I, et al. Blood predictive biomarkers for patients with non-small-cell lung cancer associated with clinical response to nivolumab. Clin Lung Cancer (2020) 21(1):75–85. doi: 10.1016/j.cllc.2019.08.006 [DOI] [PubMed] [Google Scholar]
- 32. Hardy-Werbin M, Rocha P, Arpi O, Taus Á, Nonell L, Durán X, et al. Serum cytokine levels as predictive biomarkers of benefit from ipilimumab in small cell lung cancer. Oncoimmunology (2019) 8(6):e1593810. doi: 10.1080/2162402X.2019.1593810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Valpione S, Pasquali S, Campana LG, Piccin L, Mocellin S, Pigozzo J, et al. Sex and interleukin-6 are prognostic factors for autoimmune toxicity following treatment with anti-CTLA4 blockade. J Transl Med (2018) 16(1):94. doi: 10.1186/s12967-018-1467-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Tallerico R, Cristiani CM, Staaf E, Garofalo C, Sottile R, Capone M, et al. IL-15, TIM-3 and NK cells subsets predict responsiveness to anti-CTLA-4 treatment in melanoma patients. Oncoimmunology (2017) 6(2):e1261242. doi: 10.1080/2162402x.2016.1261242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sanmamed MF, Perez-Gracia JL, Schalper KA, Fusco JP, Gonzalez A, Rodriguez-Ruiz ME, et al. Changes in serum interleukin-8 (IL-8) levels reflect and predict response to anti-PD-1 treatment in melanoma and non-small-cell lung cancer patients. Ann Oncol (2017) 28(8):1988–1995. doi: 10.1093/annonc/mdx190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Damuzzo V, Solito S, Pinton L, Carrozzo E, Valpione S, Pigozzo J, et al. Clinical implication of tumor-associated and immunological parameters in melanoma patients treated with ipilimumab. Oncoimmunology (2016) 5(12):e1249559. doi: 10.1080/2162402x.2016.1249559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Bjoern J, Juul Nitschke N, Zeeberg Iversen T, Schmidt H, FodeandI K, Svane M. Immunological correlates of treatment and response in stage IV malignant melanoma patients treated with Ipilimumab. Oncoimmunology (2016) 5(4):e1100788. doi: 10.1080/2162402x.2015.1100788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Krummel MF, Allison JP. CTLA-4 engagement inhibits IL-2 accumulation and cell cycle progression upon activation of resting T cells. J Exp Med (1996) 183(6):2533–40. doi: 10.1084/jem.183.6.2533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Waldmann TA. Cytokines in cancer immunotherapy. Cold Spring Harb Perspect Biol (2018) 10(12). doi: 10.1101/cshperspect.a028472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Asadullah K, Sterry W, Volk HD. Interleukin-10 therapy–review of a new approach. Pharmacol Rev (2003) 55(2):241–69. doi: 10.1124/pr.55.2.4 [DOI] [PubMed] [Google Scholar]
- 41. Saraiva M, Vieira P, O'Garra A. Biology and therapeutic potential of interleukin-10. J Exp Med (2020) 217(1):e20190418. doi: 10.1084/jem.20190418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. de Waal Malefyt R, Haanen J, Spits H, Roncarolo MG, te Velde A, Figdor C, et al. Interleukin 10 (IL-10) and viral IL-10 strongly reduce antigen-specific human T cell proliferation by diminishing the antigen-presenting capacity of monocytes via downregulation of class II major histocompatibility complex expression. J Exp Med (1991) 174(4):915–24. doi: 10.1084/jem.174.4.915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Fioravanti J, Di Lucia P, Magini D, Moalli F, Boni C, Benechet AP, et al. Effector CD8(+) T cell-derived interleukin-10 enhances acute liver immunopathology. J Hepatol (2017) 67(3):543–8. doi: 10.1016/j.jhep.2017.04.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Yu P, Steel JC, Zhang M, Morrisand JC, Waldmann TA. Simultaneous blockade of multiple immune system inhibitory checkpoints enhances antitumor activity mediated by interleukin-15 in a murine metastatic colon carcinoma model. Clin Cancer Res (2010) 16(24):6019–28. doi: 10.1158/1078-0432.CCR-10-1966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Yu P, Steel JC, Zhang M, Morris JC, Waitz R, Fasso M, et al. Simultaneous inhibition of two regulatory T-cell subsets enhanced interleukin-15 efficacy in a prostate tumor model. Proc Natl Acad Sci U S A (2012) 109(16):6187–92. doi: 10.1073/pnas.1203479109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Vincent M, Teppaz G, Lajoie L, Solé V, Bessard A, Maillasson M, et al. Highly potent anti-CD20-RLI immunocytokine targeting established human b lymphoma in SCID mouse. MAbs (2014) 6(4):1026–37. doi: 10.4161/mabs.28699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Rincón M, Anguita J, Nakamura T, Fikrigand E, Flavell RA. Interleukin (IL)-6 directs the differentiation of IL-4-producing CD4+ T cells. J Exp Med (1997) 185(3):461–9. doi: 10.1084/jem.185.3.461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Diehl S, Anguita J, Hoffmeyer A, Zapton T, Ihle JN, Fikrig E, et al. Inhibition of Th1 differentiation by IL-6 is mediated by SOCS1. Immunity (2000) 13(6):805–15. doi: 10.1016/S1074-7613(00)00078-9 [DOI] [PubMed] [Google Scholar]
- 49. Tsukamoto H, Fujieda K, Senju S, Ikeda T, Oshiumiand H, Nishimura Y. Immune-suppressive effects of interleukin-6 on T-cell-mediated anti-tumor immunity. Cancer Sci (2018) 109(3):523–30. doi: 10.1111/cas.13433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Baggiolini M, Walz A, Kunkel SL. Neutrophil-activating peptide-1/interleukin 8, a novel cytokine that activates neutrophils. J Clin Invest (1989) 84(4):1045–9. doi: 10.1172/JCI114265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Fernando RI, Castillo MD, Litzinger M, Hamiltonand DH, Palena C. IL-8 signaling plays a critical role in the epithelial-mesenchymal transition of human carcinoma cells. Cancer Res (2011) 71(15):5296–306. doi: 10.1158/0008-5472.CAN-11-0156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. David JM, Dominguez C, Hamiltonand DH, Palena C. The IL-8/IL-8R axis: A double agent in tumor immune resistance. Vaccines (Basel) (2016) 4(3):22. doi: 10.3390/vaccines4030022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Geng R, Tan X, Wu J, Pan Z, Yi M, Shi W, et al. RNF183 promotes proliferation and metastasis of colorectal cancer cells via activation of NF-κB-IL-8 axis. Cell Death Dis (2017) 8(8):e2994. doi: 10.1038/cddis.2017.400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Zhang B, Shi L, Lu S, Sun X, Liu Y, Li H, et al. Autocrine IL-8 promotes f-actin polymerization and mediate mesenchymal transition via ELMO1-NF-κB-Snail signaling in glioma. Cancer Biol Ther (2015) 16(6):898–911. doi: 10.1080/15384047.2015.1028702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Fousek K, Horn LA, Palena C. Interleukin-8: A chemokine at the intersection of cancer plasticity, angiogenesis, and immune suppression. Pharmacol Ther (2021) 219:107692. doi: 10.1016/j.pharmthera.2020.107692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Alfaro C, Sanmamed MF, Rodríguez-Ruiz ME, Teijeira Á, Oñate C, González Á, et al. Interleukin-8 in cancer pathogenesis, treatment and follow-up. Cancer Treat Rev (2017) 60:24–31. doi: 10.1016/j.ctrv.2017.08.004 [DOI] [PubMed] [Google Scholar]
- 57. Chehrazi-Raffle A, Meza L, Alcantara M, Dizman N, Bergerot P, Salgia N, et al. Circulating cytokines associated with clinical response to systemic therapy in metastatic renal cell carcinoma. J Immunother Cancer (2021) 9(3):e002009. doi: 10.1136/jitc-2020-002009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Costantini A, Takam Kamga P, Julie C, Corjon A, Dumenil C, Dumoulin J, et al. Plasma biomarkers screening by multiplex ELISA assay in patients with advanced non-small cell lung cancer treated with immune checkpoint inhibitors. Cancers (Basel) (2020) 13(1):97. doi: 10.3390/cancers13010097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Boutsikou E, Domvri K, Hardavella G, Tsiouda D, Zarogoulidisand K, Kontakiotis T. Tumour necrosis factor, interferon-gamma and interleukins as predictive markers of antiprogrammed cell-death protein-1 treatment in advanced non-small cell lung cancer: a pragmatic approach in clinical practice. Ther Adv Med Oncol (2018) 10:1758835918768238. doi: 10.1177/1758835918768238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Shah MA, Cunningham D, Metges JP, Van Cutsem E, Wainberg Z, Elboudwarej E, et al. Randomized, open-label, phase 2 study of andecaliximab plus nivolumab versus nivolumab alone in advanced gastric cancer identifies biomarkers associated with survival. J Immunother Cancer (2021) 9(12):e003580. doi: 10.1136/jitc-2021-003580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Ni Y, Soliman A, Joehlin-Price A, Rose PG, Vlad A, Edwards RP, et al. High TGF-β signature predicts immunotherapy resistance in gynecologic cancer patients treated with immune checkpoint inhibition. NPJ Precis Oncol (2021) 5(1):101. doi: 10.1038/s41698-021-00242-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Koh J, Hur JY, Lee KY, Kim MS, Heo JY, Ku BM, et al. Regulatory (FoxP3(+) T cells and TGF-β predict the response to anti-PD-1 immunotherapy in patients with non-small cell lung cancer. Sci Rep (2020) 10(1):18994. doi: 10.1038/s41598-020-76130-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Feun LG, Li YY, Wu C, Wangpaichitr M, Jones PD, Richman SP, et al. Phase 2 study of pembrolizumab and circulating biomarkers to predict anticancer response in advanced, unresectable hepatocellular carcinoma. Cancer (2019) 125(20):3603–14. doi: 10.1002/cncr.32339 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The original contributions presented in the study are included in the article/ Supplementary Material . Further inquiries can be directed to the corresponding author.