Abstract
Despite the presence and abundance of archaea in low-temperature environments, little information is available regarding their physiological and biochemical properties. In order to investigate the adaptation of archaeal proteins to low temperatures, we purified and characterized the elongation factor 2 (EF-2) protein from the Antarctic methanogen Methanococcoides burtonii, which was expressed in Escherichia coli, and compared it to the recombinant EF-2 protein from a phylogenetically related thermophile, Methanosarcina thermophila. Using differential scanning calorimetry to assess protein stability and enzyme assays for the intrinsic GTPase activity, we identified biochemical and biophysical properties that are characteristic of the cold-adapted protein. This includes a higher activity at low temperatures caused by a decrease of the activation energy necessary for GTP hydrolysis and a decreased activation energy for the irreversible denaturation of the protein, which indicates a less thermostable structure. Comparison of the in vitro properties of the proteins with the temperature-dependent characteristics of growth of the organisms indicates that additional cytoplasmic factors are likely to be important for the complete thermal adaptation of the proteins in vivo. This is the first study to address thermal adaptation of proteins from a free-living, cold-adapted archaeon, and our results indicate that the ability of the Antarctic methanogen to adapt to the cold is likely to involve protein structural changes.
It is now clearly established that archaea are present in low-temperature environments and not restricted to extreme environments such as high-temperature and high-salt habitats (7). In regions of the ocean, archaea have been reported to contribute up to 34% of the procaryotic biomass (8). While this implies that archaea have a significant ecological role, little information is available concerning physiological or biochemical properties of these organisms in these cold habitats. This is largely due to the difficulties in isolating low-temperature-adapted (psychrophilic or psychrotolerant) archaea from the environment and cultivating them in the laboratory. Franzmann and colleagues have, however, successfully isolated and described monocultures of three archaeal organisms from Antarctic lakes (12). One of these, Methanococcoides burtonii, was isolated from the anaerobic, methane-saturated, bottom waters of Ace Lake, where the temperature is a constant 1 to 2°C (13). M. burtonii is a methanogenic archaeon and has a growth temperature range from −2.5 to 28°C, with fastest growth occurring at 23°C.
The mechanisms allowing psychrophilic and psychrotolerant (11, 24, 25) and mesophilic (18, 19, 25, 30) bacteria and eukarya to adapt to low temperatures have been reviewed elsewhere. Organisms growing at low temperatures encounter a number of growth constraints: enzyme reaction rates decrease, the affinity of uptake and transport systems decreases, membranes become less fluid, and nucleic acid structures become more stable. In response, microorganisms have evolved various ways to adapt. For example, increases in membrane fluidity are obtained through a relative increase in polyunsaturated fatty acids, and microorganisms that are restricted to temperature ranges below 15 to 20°C tend to be found in environments that are rich in organic substrates to compensate for their less effective uptake and transport systems. Cold shock and cold acclimation proteins are also synthesized to enable gene expression to continue at low temperatures.
Psychrophilic and psychrotolerant bacteria and eukaryotes appear to compensate for the limitations imposed by reduced thermal energy by producing proteins with a higher specific activity at low temperatures than that of their mesophilic or thermophilic counterparts (15). The increased activity at low temperatures is thought to be due to a higher flexibility of protein structure. As a consequence, cold-adapted proteins are also less thermostable. A number of structural features have been identified as contributing to a less stable or more flexible structure, including the loss of salt bridges, greater solvent interaction with surface structures, and extended loop structures (reviewed in reference 11).
We have recently reported a structural and evolutionary analysis of the archaeal elongation factor 2 (EF-2) proteins from M. burtonii and closely related mesophilic and thermophilic methanogens (31). EF-2 is a GTPase involved in the translocation step of the ribosome during protein synthesis. One of the most significant differences between mesophilic and cold-adapted bacteria is that ribosomes remain active at low temperatures (2, 3, 5, 17, 23, 33). While comparative studies have not been performed with archaea, due to the essential function of protein synthesis, it is likely that ribosomes and associated factors are also thermally adapted. Comparison of the predicted three-dimensional structure of the EF-2 proteins of M. burtonii and a phylogenetically closely related thermophile, Methanosarcina thermophila (fastest growth at 50 to 55°C), has shown that the M. burtonii EF-2 possesses structural features indicative of a more flexible (unstable) protein (31). These features include fewer salt bridges, less-packed hydrophobic cores, and the reduction of proline residues in loop structures. As a result, it is expected that the M. burtonii EF-2 will have lower stability and increased activity at low temperatures.
In this study, we present a comparative biochemical and biophysical characterization of the EF-2 proteins from M. burtonii and M. thermophila. The proteins were overexpressed in Escherichia coli and purified to homogeneity. By using differential scanning calorimetry (DSC), the M. burtonii EF-2 was shown to have lower thermostability than the M. thermophila EF-2. By in vitro GTPase assays, the M. burtonii EF-2 was also shown to possess a higher intrinsic GTPase activity at low temperatures. Moreover, it has been found that the activity and stability profiles of the proteins did not simply correlate with the temperatures at which each methanogen had the highest growth rate. The implications of these findings are discussed.
MATERIALS AND METHODS
Construction of expression vector.
Genomic DNA was extracted and purified from M. burtonii and M. thermophila as described previously (31). The aef-2 genes (accession no. AF003869 and AF022779 for M. burtonii and M. thermophila, respectively) were cloned into the expression vector pCYB2 (New England Biolabs) according to the method of Tillett and Neilan (32), enabling the expression of the genes without additional vector-derived amino acid residues. The recombinant constructs encoded the self-cleavable intein from Saccharomyces cerevisiae and a chitin-binding domain fused to the carboxyl terminus of EF-2. To construct the recombinant plasmids, the genes from each organism were PCR amplified using two different sets of primers. For the first amplification, the primers were EFM (5′-TAGCATATGGGACGAAGGAAGAAAATGGTTGAGCGTGT-3′) and MB3S (5′-CATGCTCATAAAGTCTTCTGC-3′) or MT3S (5′-CATTGAAAGGTAGTCAGAAGC-3′) for M. burtonii and M. thermophila, respectively. For the second amplification, the primers were I5S (5′-AAGAAAATGGTTGAGCGTGT-3′) and MB3L (5′-GGTACCCTTGGCAAAGCACATGCTCATAAAGTCTTCTGC-3′) or MT3L (5′-GGTACCCTTGGCAAAGCACATTGAAAGGTAGTCAGAAGC-3′) for M. burtonii and M. thermophila, respectively. Reactions were carried out in 20-μl volumes with 100 ng of genomic DNA, 10 pmol of each primer, 2.75 mM MgCl2, Taq reaction buffer (Boehringer Mannheim), and 1 U of Taq (Boehringer Mannheim)-PFU (Stratagene) polymerase mix (unit ratio, 10:1) for 25 cycles (95°C, 10 s; 50°C, 20 s; 72°C, 4 min) after initial denaturation for 2 min at 95°C. The vector pCYB2 was also amplified in two PCRs containing the primers V5LM (5′-CCTTCGTCCCATATGCTATGGTCCTTGTTGGTGAAGTG-3′) and V3S (5′-AATGTTTTAATGGCGGATGG-3′), and V5S (5′-TGGTCCTTGTTGGTGAAGTG-3′) and V3L (5′-TGCTTTGCCAAGGGTACCAATGTTTTAATGGCGGATGG-3′). For the vector, reactions were carried out in 20-μl volumes with 1 ng of vector, 10 pmol of each primer, 2.75 mM MgCl2, Taq reaction buffer (Boehringer Mannheim), and 1 U of Taq (Boehringer Mannheim)-PFU (Stratagene) polymerase mix (unit ratio, 10:1) for 20 cycles (95°C, 10 s; 55°C, 20 s; 72°C, 8 min) after initial denaturation for 1 min at 95°C. All amplicons were purified with a Prep-a-gene kit (Bio-Rad) and resuspended in 10 mM Tris-HCl–1 mM EDTA, pH 8. One hundred nanograms of each cleaned PCR product (i.e., two vector products and two aef-2 products) were mixed, adjusted to 100 mM NaCl in a 10-μl volume, and subjected to 3 min at 95°C followed by four cycles with 2 min at 68°C and 15 min at 25°C. The DNA was directly transformed into chemically competent E. coli strain TOP10F′ [F′ {laqIq Tetr}mcrA Δ(mrr-hsdRMS-mcrBC) φ80lacZΔM15 ΔlacX74 deoR recA1 araD139 Δ(ara-leu)7697 galU galK rpsL endA1 nupG] cells. The construction of each recombinant plasmid was verified by restriction digests and complete DNA sequencing of the insert and flanking regions. The constructs used for expression showed no mutations and were termed pMB and pMT for those containing the M. burtonii and M. thermophila aef-2 genes, respectively.
Overexpression and purification.
The plasmids pMB and pMT were transformed into E. coli strain BL21 [E. coli B F− dcm lon ompT hsdS(rB− mB−) gal] or BL21 containing the plasmid pUBS520 (4). Cells were grown at 37°C to an optical density at 600 nm of 0.5. Expression of the fusion protein was induced by the addition of 1 mM isopropyl-β-d-thiogalactopyranoside, and cultures were incubated for 16 h at 14°C. Cells were harvested and resuspended in 1/10 culture volume of CBT8 buffer (20 mM Tris-HCl [pH 8], 200 mM NaCl, 5 mM MgCl2, 10 μM GDP, 100 μM phenylmethylsulfonyl fluoride). Cells were lysed in a French pressure cell, and the cell extract was cleared by centrifugation (12,000 × g, 30 min, 4°C). The supernatant was loaded on a chitin bead column (1/100 volume of culture), washed with 10 column volumes of CBT8, quickly flushed with 3 column volumes of CBT8 containing 100 mM dithiothreitol (DTT), and incubated for 16 h at 4°C. Protein fractions were eluted from the column with CBT8 and stored with the addition of 1 volume of glycerol at −20°C. EF-2 protein concentration was determined with the Coomassie Plus protein assay (Pierce) with bovine serum albumin as a standard. Protein purity was determined by visualization by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). The molecular masses of the proteins were determined by matrix-assisted laser desorption ionization–mass spectrometry.
DSC.
Thermal unfolding of the purified EF-2 proteins was investigated by DSC. Freshly purified proteins were concentrated by ultrafiltration to 2 mg/ml and dialyzed against 20 mM morpholinepropanesulfonic acid (MOPS) (pH 7.5) and 1 mM β-mercaptoethanol. The final dialysate was kept as a reference for the DSC. Prior to loading, the samples were filtered (0.2-μm-pore-size filter) and degassed with stirring for 10 min at 4°C. Calorimetry was performed on a MicroCal VP-DSC calorimeter, and data were analyzed with the Origin MicroCal DSC software package.
Intrinsic GTPase activity assay.
The intrinsic GTPase activity of EF-2 was determined essentially as described by Rao and Bodley (22). Cryogenically stored EF-2 proteins were dialyzed against 20 mM MOPS (pH 7.5) and 1 mM DTT. The assay mix (30 μl) contained 5 to 6 μM purified EF-2 protein, 20 mM MOPS (pH 7.5), 1 mM DTT, and various supplements, including the aliphatic alcohols ethylene glycol, ethanol, and 2-propanol and the divalent cations barium, magnesium, and strontium. After 2 min of preincubation at the assay temperature, the reaction was initiated by the addition of 50 μM [α-32P]GTP (0.5 μCi). Aliquots (5 μl) were withdrawn at appropriate time points, the reaction was terminated by the addition of an equal volume of 10% (vol/vol) formic acid, and 2 μl was spotted onto a polyethyleneimine-impregnated cellulose thin-layer chromatography plate. The thin-layer chromatography plate was developed in 0.75 M potassium phosphate (pH 3.4), and the radioactive GDP and GTP were detected and quantified using phosphor screens (Bio-Rad GS425). Image analysis was performed using the Bio-Rad software package Multi-Analyst.
RESULTS AND DISCUSSION
Overexpression and purification of the EF-2 proteins.
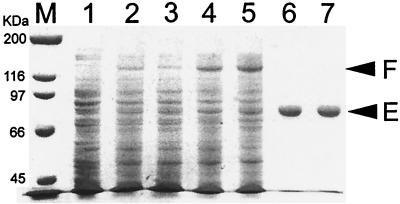
In order to investigate the biochemical and biophysical properties of the EF-2 proteins from M. burtonii and M. thermophila, both proteins were overexpressed in E. coli as fusion proteins with a yeast intein and a chitin-binding domain. This system has been successfully applied to the expression and purification of another archaeal protein (27). In E. coli BL21, expression levels of both fusion proteins were low, with yields estimated at 0.1 to 0.2 mg from pMB and 0.4 to 0.5 mg from pMT for fusion proteins per g of E. coli cell wet weight (cww) (Fig. 1). The M. burtonii and M. thermophila aef-2 genes possess 11/10 (2.9% of a total 730 codons) and 12/18 (4.1%) AGA/AGG codons, respectively. These arginine codons are rarely used in E. coli, and the availability of the cognate tRNA may be limiting expression of the proteins. In order to test this, the plasmid pUBS520, which encodes the E. coli tRNAArgAGA/AGG genes (4), was coexpressed with pMB or pMT. In the presence of pUBS520, yields of the fusion protein were 4 to 20 times higher (∼2 mg per g of cww) (Fig. 1). The purity of the EF-2 proteins expressed from BL21(pUBS520) was estimated by visualization on SDS-PAGE as >95% (Fig. 1), and the overall yield was ∼1 mg per g of cww. The yield from this overexpression and purification procedure is more than 20-fold greater than that for previously described methods for the purification of an elongation factor protein from an archaeal hyperthermophile (9).
FIG. 1.
SDS–7.5% PAGE of crude E. coli cell extracts after induction and purified EF-2 protein from M. burtonii and M. thermophila. Lane M, broad-range molecular size markers (Bio-Rad); lane 1, crude cell extract of E. coli BL21 containing plasmid pUBS520; lane 2, E. coli BL21 containing pMB; lane 3, E. coli BL21 containing pMT; lane 4, E. coli BL21 containing pUBS520 and pMB; lane 5, E. coli BL21 containing pUBS520 and pMT; lane 6, purified EF-2 from M. burtonii; lane 7, purified EF-2 from M. thermophila. The upper arrowhead (F) indicates the position of the EF-2 fusion protein; the lower arrowhead (E) indicates the position of the EF-2 protein.
The purified proteins from M. burtonii and M. thermophila had a molecular mass as determined by mass spectrometry of 80,567 and 80,631 (±100) Da, respectively, which correlates well with the theoretical masses of 80,477 and 80,564 Da, respectively, derived from the amino acid sequences (31).
Protein stability and thermal unfolding.
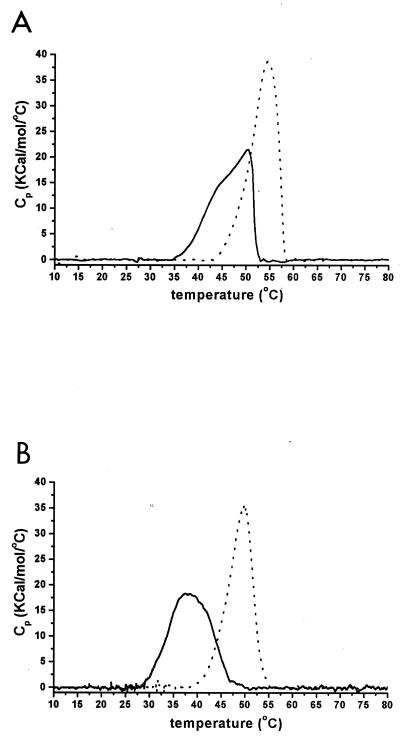
The thermostability of the purified proteins was examined by DSC. At scan rates (v) of 1.5 K/min, the temperature values of the maximum heat capacity (Tm) were 50.5 and 55.6°C for M. burtonii and M. thermophila EF-2, respectively (Fig. 2A). Rescanning of the protein samples after they were heated beyond the transition peak (i.e., 55 to 60°C) and then cooled to 4°C resulted in no further increases in heat capacity (data not shown). This indicates that the unfolding of the EF-2 proteins from both organisms is an irreversible process.
FIG. 2.
Excess heat capacity of the EF-2 proteins from M. burtonii (solid lines) and M. thermophila (dotted lines) versus temperature at scan rates of 1.5 (A) and 0.1 (B) K/min. Measurements were performed in 20 mM MOPS (pH 7.5) and 1 mM β-mercaptoethanol.
When the scan rate was decreased (v = 0.1 K/min), the values for Tm shifted toward lower temperature values with 37.4 and 49.7°C observed for the M. burtonii and M. thermophila EF-2, respectively (Fig. 2B). Significant changes in the shape of the heat capacity curve were also observed. It has previously been shown that the shape of DSC thermograms of irreversible processes is scan rate dependent (26). In order to further analyze the denaturation process, a simple kinetic model for the thermal transition of the EF-2 proteins was used, where the native protein N undergoes an endothermic and irreversible step to a denatured state D with a first-order rate k constant k (N → D). According to this model, the rate constant of the reaction at a given temperature t can be calculated by the formula k = vCp/(Q − Qt) with Cp being the excess heat capacity at t, Qt being the heat evolved at a given t, and Q being the total heat of the process (26).
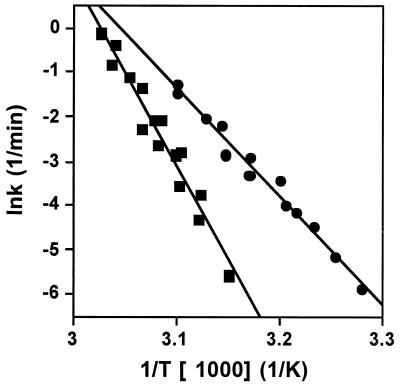
The calculated values of k (as lnk) for the M. burtonii and M. thermophila EF-2 proteins for three different scan rates (v = 0.1, 0.5, and 1.5 K/min) were plotted against the inverse of the absolute temperature (in K) (Fig. 3). According to the Arrhenius equation [k = Ae(−E/RT)], the slope of the straight line corresponds to −E/R, with E being the activation energy of the process and R being the universal gas constant. From this, values for E of 203 and 351 kJ/mol were calculated for M. burtonii and M. thermophila EF-2, respectively. These data show that the activation energy for the unfolding process of EF-2 from M. burtonii is significantly lower than that for M. thermophila and indicate that the M. burtonii EF-2 has a less stable structure.
FIG. 3.
Arrhenius plot for the reaction rate of thermal denaturation (calculated as described in the text) for M. burtonii EF-2 (circles) and M. thermophila EF-2 (squares). Values for three different scan rates (0.1, 0.5, and 1.5 K/min) were used. The straight lines represent linear fits to the data, which were used to calculate the activation energy of the reaction.
It should be noted that the model applied to analyze the thermograms may only represent an approximate description of the EF-2 unfolding and denaturation process. Deconvolution of the thermograms using various models incorporated in the Origin software (see description in Materials and Methods) indicated that the thermal unfolding of both proteins is best described by a model with three or more separate, non-two-phase unfolding events. This is consistent with the known multidomain structure of the bacterial homologue to EF-2, elongation factor G (1, 6), and the predicted structure of EF-2 (31). The model presented above, however, is sufficient to make a qualitative interpretation about the overall thermolability of the EF-2 proteins and to let us reach the conclusion that the M. burtonii protein is less stable.
In vitro activity.
The EF-2 proteins from both organisms showed a low intrinsic activity for the hydrolysis of GTP to GDP. For example, 0.02 mol of GTP was hydrolyzed per mol of M. thermophila EF-2 per min at 40°C. This is comparable to the activity in the absence of stimulating factors described for the EF-2 protein from the archaeal hyperthermophile Sulfolobus solfataricus (0.016 mol of GTP hydrolyzed per mol of S. solfataricus EF-2 per min at 60°C) (20).
The intrinsic activity of S. solfataricus EF-2 has been shown to be stimulated about 300-fold by the presence of aliphatic alcohols and divalent cations. This stimulatory effect was attributed to the increased affinity of the EF-2 for GTP and was proposed to involve a conformational change in a hydrophobic region near the catalytic site (21). The effect of various combinations of aliphatic alcohols and divalent cations was tested with the EF-2 proteins from M. burtonii and M. thermophila. The highest level of stimulation (about eightfold) for both proteins was observed by the addition of 10 mM BaCl2 and 10% (vol/vol) 2-propanol. As a result, these conditions were used for subsequent activity assays. These conditions are similar to those used for the S. solfataricus EF-2 (greatest stimulation by 8 mM BaCl2 and 40% ethylene glycol) and E. coli elongation factor G (16-fold stimulation by 20% 2-propanol) (10).
In the GTPase assays, the amount of GTP hydrolysis was directly proportional to the amount of purified EF-2 added, thereby indicating that the reaction observed is enzymatically catalyzed. When a maltose-binding protein was purified by the same procedures, the GTP hydrolysis observed was equivalent to that observed for spontaneous GTP hydrolysis (data not shown). This demonstrates that the activity in assays containing the EF-2 proteins is not a result of contaminating enzymes.
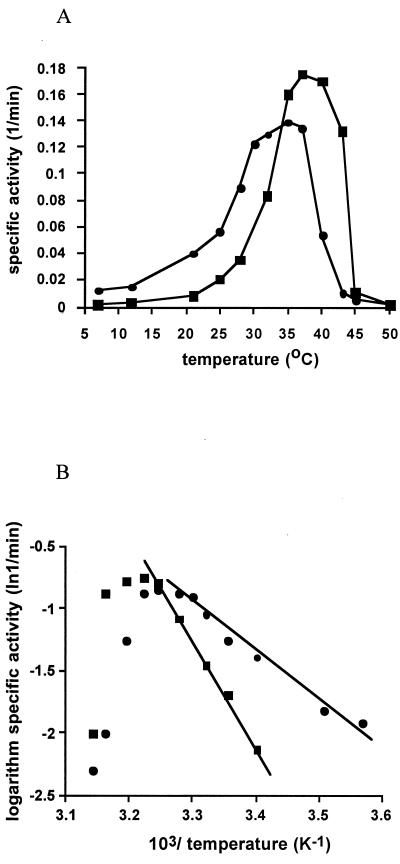
Initial rates were used to determine the temperature-dependent, specific activity of both elongation factors. Marginal differences in temperature optima for activity (34 and 36°C) and maximum reaction rates (0.14 to 0.18 mol of GTP hydrolyzed per mol of EF-2 per min) were observed for EF-2 proteins from M. burtonii and M. thermophila, respectively (Fig. 4A). While the activity profiles for the proteins are similar, the loss of activity occurs at a lower temperature for the M. burtonii EF-2. In addition, the M. burtonii EF-2 has measurable activity at 7°C, whereas the lowest temperature for which activity was observed for the M. thermophila EF-2 was 21°C.
FIG. 4.
(A) Specific activity of the GTP hydrolysis for M. burtonii EF-2 (circles) and M. thermophila EF-2 (squares) versus temperature. (B) Arrhenius plot showing the logarithm of GTP hydrolysis for M. burtonii EF-2 (circles) and M. thermophila EF-2 (squares) versus the reciprocal of the absolute temperature. Lines represent the regression of the linear range used to determine the activation energy of catalysis (for values, see the text). Activity was measured in the presence of 10% propanol and 10 mM BaCl2.
From the linear range of the Arrhenius plot, the activation energy for GTP hydrolysis was determined to be 35.5 and 70.7 kJ/mol for M. burtonii and M. thermophila EF-2, respectively (Fig. 4B). The activation energy for GTP hydrolysis of EF-2 from the moderate hyperthermophile S. solfataricus (fastest growth at 70 to 80°C), is 85 kJ/mol (20). These data for activation energy are consistent with the thermal energy of the environments in which the archaea grow and clearly demonstrate that the EF-2 proteins have undergone adaptations to enable catalytic activity under different thermal constraints.
Correlation of in vitro stability and activity with cellular physiology.
The in vitro activity and stability profiles of the proteins were examined with respect to the upper temperature limits of growth of the organism and the temperature at which the organism has the highest growth rate. The M. thermophila EF-2 shows no activity and a partial unfolding of the protein at the temperature at which the organism has maximal growth rate (50 to 55°C). This may indicate that the in vitro assay conditions do not reflect physiological conditions in vivo and that intracellular factors may be important for stabilizing the protein. M. thermophila is known to produce and accumulate small, highly water-soluble molecules called compatible solutes in response to hyperosmotic conditions (29). Compatible solutes have been shown to stabilize proteins against heat stress (14, 16, 28) and may therefore play a role in thermal stabilization of the M. thermophila EF-2 protein in vivo.
The M. burtonii EF-2 shows its maximal in vitro activity (34°C) above the maximal growth temperature of the organism (28°C). Similar patterns of activity in comparison to temperature ranges of low-temperature-adapted microorganisms have frequently been observed (11). It is possible that the cytoplasm of the cold-adapted methanogen (and possibly of bacteria) contains factors that increase flexibility of the protein, thereby augmenting activity at low temperatures. Furthermore, EF-2 binds in vivo to rRNA and ribosomal proteins, and these interactions might modulate the temperature-dependent activity profiles of the EF-2 proteins.
These studies, by comparing the in vitro activity and stability properties of the EF-2 proteins from M. burtonii and M. thermophila, show that the M. burtonii protein possesses characteristics of a low-temperature-adapted protein. The in vitro characteristics, however, do not simply correlate with the maximal growth rates. In future studies, we will focus on the effects of intracellular components on in vitro activity and stability in order to gain a more complete understanding of the mechanisms of physiological adaptation to the cold.
ACKNOWLEDGMENTS
We thank Anne Poljak, Charles Gerday, Thierry Lohienne, Paul March, Ralf Mattes, and Daniel Tillett for help and advice and Paul Curmi and Staffan Kjelleberg for critical review of the manuscript.
This work was supported by the Australian Research Council, Large Grants scheme.
REFERENCES
- 1.Ævarrson A, Brazhinikov E, Grabber M, Zheltonosova J, Chirgadze Y, Al-Karadaghi S, Svensson L A, Liljas A. Three-dimensional structure of the ribosomal translocase: elongation factor G from Thermus thermophilus. EMBO J. 1994;13:3669–3677. doi: 10.1002/j.1460-2075.1994.tb06676.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Araki T. Changes in rates of synthesis of individual proteins in a psychrophilic bacterium after a shift in temperature. Can J Microbiol. 1991;37:840–847. doi: 10.1139/m91-145. [DOI] [PubMed] [Google Scholar]
- 3.Berger F, Morellet N, Menu F, Potier P. Cold shock and cold acclimation proteins in the psychrotrophic bacterium Arthrobacter globiformis SI 55. J Bacteriol. 1996;178:2999–3007. doi: 10.1128/jb.178.11.2999-3007.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brinkmann U, Mattes R E, Buckel P. High-level expression of recombinant genes in Escherichia coli is dependent on the availability of the dnaY gene product. Gene. 1989;85:109–114. doi: 10.1016/0378-1119(89)90470-8. [DOI] [PubMed] [Google Scholar]
- 5.Broeze R J, Solomon C J, Pope D H. Effects of low temperature on in vivo and in vitro protein synthesis in Escherichia coli and Pseudomonas fluorescens. J Bacteriol. 1978;134:861–874. doi: 10.1128/jb.134.3.861-874.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Czworkowski J, Wang J, Seitz T A, Moore P R. The crystal structure of elongation factor G complexed with GDP, at 2.7Å resolution. EMBO J. 1994;13:3661–3668. doi: 10.1002/j.1460-2075.1994.tb06675.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.DeLong E F. Archaeal means and extremes. Science. 1998;280:542–543. doi: 10.1126/science.280.5363.542. [DOI] [PubMed] [Google Scholar]
- 8.DeLong E F, Wu K Y, Prezelin B B, Jovine R V M. High abundance of archaea in Antarctic marine plankton. Nature. 1994;371:695–697. doi: 10.1038/371695a0. [DOI] [PubMed] [Google Scholar]
- 9.deVendittis E, Amatruda M R, Raimo G, Bocchini V. Heterologous expression in Escherichia coli of the gene encoding an archaeal thermoacidophilic elongation factor 2. Properties of the recombinant protein. Biochimie. 1997;79:303–308. doi: 10.1016/s0300-9084(97)83518-3. [DOI] [PubMed] [Google Scholar]
- 10.deVendittis E, Masullo M, Bocchini V. The elongation factor G carries a catalytic site for GTP-hydrolysis which is revealed by using 2-propanol in the absence of ribosomes. J Biol Chem. 1986;261:4445–4450. [PubMed] [Google Scholar]
- 11.Feller G, Narinx E, Arpigny J L, Aittaleb M, Baise E, Genicot S, Gerday C. Enzymes from psychrophilic organisms. FEMS Microbiol Rev. 1996;18:189–202. [Google Scholar]
- 12.Franzmann P D. Examination of Antarctic procaryotic diversity through molecular comparison. Biodivers Conserv. 1996;5:1295–1305. [Google Scholar]
- 13.Franzmann P D, Springer N, Ludwig W, De M E C, Rohde M. A methanogenic archaeon from Ace Lake, Antarctica: Methanococcoides burtonii sp. nov. Syst Appl Microbiol. 1992;15:573–581. [Google Scholar]
- 14.Galinski E A. Compatible solutes of halophilic eubacteria: molecular principles, water-solute interaction, stress protection. Experientia. 1993;49:487–496. [Google Scholar]
- 15.Gerday C, Aittaleb M, Arpigny J L, Baise E, Chessa J P, Garsoux G, Feller G. Psychrophilic enzymes: a thermodynamic challenge. Biochim Biophys Acta. 1997;1342:119–131. doi: 10.1016/s0167-4838(97)00093-9. [DOI] [PubMed] [Google Scholar]
- 16.Hensel R, Koenig H. Thermoadaptation of methanogenic bacteria by intracellular ion concentration. FEMS Microbiol Lett. 1988;49:75–79. [Google Scholar]
- 17.Michel V, Lehoux I, Depret G, Anglade P, Labadie J, Hebraud M. The cold shock response of the psychrotrophic bacterium Pseudomonas fragi involves four low-molecular-mass nucleic acid-binding proteins. J Bacteriol. 1997;179:7331–7342. doi: 10.1128/jb.179.23.7331-7342.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Panoff J-M, Thammavongs B, Guéguen M, Boutibonnes P. Cold stress response in mesophilic bacteria. Cryobiology. 1998;36:75–83. doi: 10.1006/cryo.1997.2069. [DOI] [PubMed] [Google Scholar]
- 19.Phadtare S, Alsins J, Inouye M. Cold-shock response and cold-shock proteins. Curr Opin Microbiol. 1999;2:175–180. doi: 10.1016/S1369-5274(99)80031-9. [DOI] [PubMed] [Google Scholar]
- 20.Raimo G, Masullo M, Bocchini V. Studies of the polypeptide elongation factor 2 from Sulfolobus solfataricus. J Biol Chem. 1995;270:21082–21085. doi: 10.1074/jbc.270.36.21082. [DOI] [PubMed] [Google Scholar]
- 21.Raimo G, Masullo M, Scarano G, Bocchini V. The site for GTP hydrolysis on the archaeal elongation factor 2 is unmasked by aliphatic alcohols. Biochimie. 1996;78:832–837. doi: 10.1016/s0300-9084(97)84335-0. [DOI] [PubMed] [Google Scholar]
- 22.Rao S, Bodley J W. Expression, purification and characterization of the G domain of Saccharomyces cerevisiae elongation factor 2. Protein Expr Purif. 1996;8:91–96. doi: 10.1006/prep.1996.0078. [DOI] [PubMed] [Google Scholar]
- 23.Roberts M E, Inniss W E. The synthesis of cold shock proteins and cold acclimation proteins in the psychrophilic bacterium Aquaspirillum arcticum. Curr Microbiol. 1992;25:275–278. [Google Scholar]
- 24.Russell N. Cold adaptation in microorganism. Phil Trans R Soc Lond Ser B. 1990;326:595–611. doi: 10.1098/rstb.1990.0034. [DOI] [PubMed] [Google Scholar]
- 25.Russell N J, Hamamoto T. Psychrophiles. In: Horikoshi K, Grant W D, editors. Extremophiles: microbial life in extreme environments. New York, N.Y: Wiley-Liss, Inc.; 1998. pp. 25–45. [Google Scholar]
- 26.Sanchez-Ruiz J M, Lopez-Lacomba J L, Cortijo M, Mateo P L. Differential scanning calorimetry of the irreversible thermal denaturation of thermolysin. Biochemistry. 1988;27:1648–1652. doi: 10.1021/bi00405a039. [DOI] [PubMed] [Google Scholar]
- 27.Schleper C, Swanson R V, Mathur E R, DeLong E F. Characterization of a DNA-polymerase from the uncultivated psychrophilic archaeon Cenarchaeum symbiosum. J Bacteriol. 1997;179:7803–7811. doi: 10.1128/jb.179.24.7803-7811.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shima S, Herault D A, Berkessel A, Thauer R K. Activation and thermostabilization effects of cyclic 2,3-diphosphoglycerate on enzymes from the hyperthermophile Methanopyrus kandleri. Arch Microbiol. 1998;170:469–472. doi: 10.1007/s002030050669. [DOI] [PubMed] [Google Scholar]
- 29.Sowers K R, Robertson D E, Noll D, Gunsalus R P, Roberts M F. Nɛ-Acetyl-β-lysine: an osmolyte synthesized by methanogenic archaeabacteria. Proc Natl Acad Sci USA. 1990;87:9083–9087. doi: 10.1073/pnas.87.23.9083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thieringer H A, Jones P G, Inouye M. Cold shock and adaptation. Bioessays. 1998;20:49–57. doi: 10.1002/(SICI)1521-1878(199801)20:1<49::AID-BIES8>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 31.Thomas T, Cavicchioli R. Archaeal cold-adapted proteins: structural and evolutionary analysis of the elongation factor 2 proteins from psychrophilic, mesophilic and thermophilic methanogens. FEBS Lett. 1998;439:281–286. doi: 10.1016/s0014-5793(98)01375-1. [DOI] [PubMed] [Google Scholar]
- 32.Tillett D, Neilan B A. Enzyme-free cloning: a rapid method to clone PCR-products independent of vector restriction enzyme sites. Nucleic Acids Res. 1999;27:e26. doi: 10.1093/nar/27.19.e26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Whyte L G, Inniss W E. Cold shock proteins and cold acclimation proteins in a psychrotrophic bacterium. Can J Microbiol. 1992;38:1281–1285. doi: 10.1139/m96-100. [DOI] [PubMed] [Google Scholar]