Summary
Human papillomavirus (HPV) is considered to be an etiologic agent of cervical cancer and, recently its relation to oral and oropharyngeal cancer has also been investigated. Oral squamous cell carcinoma (SCC) represents 90% of all malignant tumors that affect the oral cavity. The prevalence of HPV in patients with SCC ranges from 0 to 100%. The most known viral cytopathic effect is koilocytosis, considered to be a major characteristic of HPV infection. Aim: The aim of this study was to verify the prevalence of some peculiar characteristics of HPV - koilocytosis - in oral and oropharyngeal SCC. Study design: transversal cohort. Material and method: Twenty slides with oral and/or oropharyngeal SCC were examined under microscopy. Results: in 15 of them, koilocytosis was found, amounting to 75%. Although we know that polymerase chain reaction (PCR) is the method with the best sensitivity for HPV detection, we began this research looking for koilocytosis, which is highly suggestive of HPV infection. Conclusion: This study is a trial project and we will continue this research with PCR measures to confirm this high prevalence of HPV infection in oral and oropharyngeal SCC.
Key words: carcinoma, papillomavirus, mouth, oropharynx
INTRODUCTION
The Human papillomavirus virus (HPV) is a small sized virus of intranuclear replication with diameter of approximately 50 nm 1. It may infect mucosa and skin surfaces in almost all vertebrate species2. Seventy types of HPV 3 have been identified so far and were classified according to its distribution on nucleic acids in the viral genome. The subtypes found in the oral cavity were 1, 2, 4, 6, 11, 13, 16, 18, 30, 31, 32 and 574.
Comprehensive research studies related some subtypes of HPV to malignant and pre-malignant lesions of cervix, vulva, penis, conjunctive and upper digestive tract and airways5. HPV is universally accepted as an etiologic agent of cervical cancer, and its relation to oral and oropharynx cancer has recently been investigated. The most popular viral cytopathic effect of HPV is koilocytosis and is considered a “major criterion” in HPV infection from the pathophysiological point of view. Koilocytotic atypia consists in nuclear atypia and perinuclear vacuolization. 6
Today, there are molecular biology methods to study HPV such as in situ hybridization (ISH), hybrid capture and PCR that provide not only increased sensitivity in diagnosis, but also the typing of HPV, but costs of such exams are higher if compared against the detection of koilocytosis hematoxylin-eosin stained slides.
HPV and Oral Spinocellular Carcinoma (OSC)
Oral squamous cell carcinoma is also known as epidermoid or oral spinocellular carcinoma (OSC) and accounts for 90% of all malignant tumors that affect the oral cavity7. The most common factors associated with oral cancer development are smoking, alcohol, syphilis, nutritional deficiencies, sunray exposure, trauma, poor hygiene and irritation of the teeth or dental prosthetics8. In addition to those factors, the viruses have been investigated as likely carcinogenic agents. Sÿrjanen et al. 9 suggested HPV may play a role in oral cancer if associated with cell abnormalities found in malignant and pre-malignant lesions of the mouth similar to those that occurred in cervical cancer. The HPV infection is identified as the most common cause of 95% of cervical carcinomas and a large rate of other genital carcinomas10.
Tobacco and alcohol abuse are well established risk factors for oral cancer, but a small rate (15-20%) of the patients do not have any past history of smoking or alcohol addiction suggesting the presence of other risk factors 11 such as HPV, but its role has not been clearly defined yet. An intriguing finding is the rate of approximately 40% reduction in death risk in patients with positive HPV tumors.
HPV 16 is the most common type of virus associated with oral and cervical cancer 12, 13 whereas types 6 and 11 are more commonly found in benign and pre-malignant lesions and rarely found in neoplastic head and neck lesions14.
The importance of HPV infection in oral carcinogenesis is supported by the ability of high risk HPV to immortalize oral keratinocytesin vitro 15.
OBJECTIVE
The objective of this study is to determine the prevalence of suggestive findings of HPV, koilocytosis in OSC of the oral cavity and oropharynx through histological analysis of such lesions.
MATERIAL AND METHOD
Twenty patients diagnosed with OSC of the oral cavity and oropharynx were examined with optical microscopy to investigate suggestive signs of HPV infection in hematoxylin-eosin stained slides.
Concomitantly, the records of the 20 cases were investigated as shown in Table 1.1.
Table 1.
List of patients with oral or oropharynx OSC.
| Initials | Age | Gender | Race | Smoking | Alcohol abuse | Site | Staging | Lesion | C |
|---|---|---|---|---|---|---|---|---|---|
| F.V.S. | 86 | M | B | ? | ? | JugalMucosa | ? | Ulcer | - |
| A.S. | 69 | M | B | - | - | Hard palate | T4N1Mx | Ulcerated infiltrating | - |
| M.H.M. | 42 | M | P | - | - | Hard palate | T4NoMo | Ulcerated infiltrating | - |
| D.C. | 51 | M | B | + | - | Floor | ? | Infiltrating | - |
| C.A.S. | 61 | M | B | + | - | Soft palate | T1NoMo | Ulcer | - |
| I.D. | 48 | M | B | - | - | Gums | ? | Bone reabsorption | + |
| C.M.F. | 55 | M | B | ? | ? | Soft palate | T4N2cMx | Ulcerated infiltrating | + |
| J.Z.T. | 58 | M | B | + | + | Floor | ? | Ulcerated infiltrating | + |
| R.A.S. | 71 | M | B | ? | ? | Floor | ? | Infiltrating | + |
| A.C.P. | 63 | M | B | + | + | Oropharynx and larynx | T4N2cMx | Infiltrating ulcerated | + |
| G.A.Z. | 47 | M | B | + | + | Soft palate | TxN3Mx | Infiltrating ulcerated | + |
| J.C.G.M | 63 | M | B | + | + | Soft palate | ? | Infiltrating ulcerated | + |
| T.C.M. | 56 | M | B | ? | ? | Oropharynx | T4N2Mo | Infiltrating ulcerated | + |
| S.B.C. | 56 | M | B | ? | ? | Hard palate | ? | Infiltrating ulcerated | + |
| J.T.S. | 80 | M | N | + | + | Tongue | T3N1Mx | Infiltrating ulcerated | + |
| M.E.V. | 55 | F | P | + | - | Tongue | T4N1Mx | Infiltrating ulcerated | + |
| R.A.A. | 64 | M | N | + | - | Oropharynx and base of tongue | T4N2cMx | Vegetating | + |
| R.D.O. | 53 | M | B | + | - | Oropharynx | T4NoMo | ? | + |
| A.G.V. | 53 | M | B | + | + | Floor | T4N2cMo | Exophytic ulcerated | + |
| E.F.F. | 47 | M | P | ? | ? | Tongue and Floor | T4N2cMo | Infiltrating ulcerated | + |
C: koilocytosis
T: tumor
N: lymph node
M: metastasis (TNM classification)
?: non-reported in patient’s records
M: male
F: female
B: white
P: brown
N: black
+: present
-: absent
RESULTS
Seventy-five percent of the cases (15/20) presented koilocytosis, and were identified as positive cases of koilocytosis (C+).
The survey in patients’ records showed that 19 patients were male and 1 patient was female, ages ranging from 42 to 86 years (mean age= 58.9). Fifteen patients were White, three were Brown and two were Black. The group of C+ patients had ages that ranged from 47 to 80 years (mean age= 57.9). The group of C-patients had ages that ranged from 42 to 86 years (mean age= 61.8).
Eleven patients were smokers (55%) and six were alcohol abusers (30%), with 6 patients (30%) - 5 C+ and 1 patient C- that did not have any smoking or alcohol history in their records. The group of C+ patients had 60 % of smokers and 40% of alcoholics, whereas the group C- had 40% smokers and did not have any alcoholic patient as shown in Table 2.
Table 2.
Smoking and alcoholism in patients with positive and negative koilocytosis.
| C +(n = 15) | C -(n = 5) | |
|---|---|---|
| Smoking (n = 11) | 60% | 40% |
| Alcoholism (n = 6) | 40% | 0% |
| + Common Site | Palate (27%) and floor (27%) | Palate (60%) |
C+: koilocytosis
The tumor location varied. Most occurred in the palate (35%) - 20% in the soft palate and 15% in the hard palate – followed by the oral cavity floor (25%), oropharynx (20%), tongue (10%), gums (5%) and jugal mucosa (5%). In the C+ group, the most common sites were palate and oral cavity floor, both with 27% rate, whereas in the group C- the most common site involved was the palate region (60%).
In regards to the clinical aspect upon diagnosis, most of the patients had ulcerated infiltrating lesion (60%) and in advanced staging - T4 (50%), in 7 patients (35%) - 5 C+ and 512 2 C- - did not have any report of clinical staging in their records. In the C+ group, 66% of the patients had ulcerated infiltrating lesion and 54% were in advanced staging, whereas in the C- group, 40% of the patients had ulcerated infiltrating lesions and 40% were in advanced staging.
DISCUSSION
In this study, koilocytosis was found in 15 out of the 20 slides investigated, accounting for 75% of the cases.
Koilocytosis first described by Leopoldo Koss et al. in 195616, consists in a picnotic nuclei surrounded by extensive clear halos with a volume generally higher than the cytoplasm if observed in slides under optical microscope, as seen in Figure 1.
Figure 1.
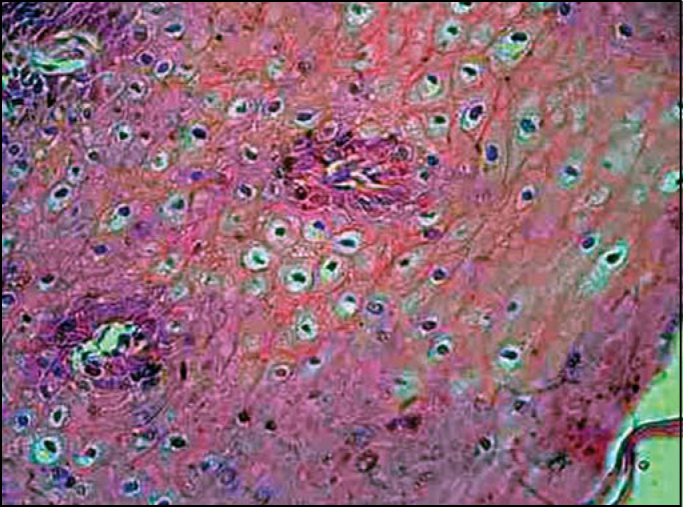
Hematoxylin-eosin stained slides showing koilocytosis: cells with picnotic halo, surrounded by clear outside halos with volume generally higher than that of the cytoplasm.
According to some authors 17, 18, the koilocytosis is a pathognomonic sign of HPV infection serving as a foundation for molecular biology studies.
Some papers in the literature report that HPV frequency in oral carcinoma ranges from 0 to 100%13, 19whereas other investigators report it ranges from 18 to 100% 12, 14. The mean frequency of 25% was estimated by Garlick20 in his review about the topic. As it can be seen, the variability is extreme and may be a consequence of different sample sizes or methods used with variable sensitivity and specificity.
Herrero et al. 21 demonstrated HPV DNA in 3.9% of 766 cancer biopsies of the oral cavity and in 18.3% of the 142 biopsies of oropharynx cancer, through the PCR study. Klussmann et al. 22 found 18% of HPV DNA in cancer biopsies of the oral cavity, 8% in nasopharynx cancer, 25% in hypopharynx cancer and 45% in oropharynx cancer and particularly in tonsil carcinomas (58%). Ritchie et al. 23 detected HPV in 21% of the oral cavity and oropharynx cancers, with 83% of HPV -16. Ha et al. 24 reported 0.98% of HPV DNA in pre-malignant lesions and only 2.9% in malignant lesions of the oral cavity.
Niv et al. 25 conducted a study to investigate HPV presence in oral cavity carcinoma and found a positive rate of 17.3%; in all cases the tumor was located in the anterior tonsillar pillar. In other studies, the most common tumor sites where they found HPV were jugal and palate mucosa (40-50%) 26. In this study, the most common locations were palate and oral cavity floor, both with 27% prevalence.
Positive HPV carcinoma seem to be a distinctive entity (involve more basal cells and have lower inflammatory component), with distinct biology (less p 53 mutation), distinct risk factors (less association with tobacco and alcohol and a different clinical outcome - higher survival rate) 11. Conversely, this study did not show lower association with tobacco and alcoholism, but higher percentage of alcoholic and smokers in group C+.
Cruz et al. 27 observed that patients under 60 years of age with oral carcinoma presented eight times higher risk of being infected by HPV than people older than 60 years. In this study, men presented increased HPV positive responses than women, but an association between HPV infection and tumor location, clinical staging in diagnosis or smoking, and alcoholism could not be found. This survey did not show any significant difference of age range between groups C+ and C-.
Klussmann ET al. 28 reported a statistically significant difference between HPV prevalence in oropharynx tumor (45%), especially in palatine tonsils (58%) against the other sites of the oral cavity (7 to 25%).
Some authors 11, 28 believe that positive HPV palatine tonsil carcinomas constitute an independent tumor entity. Klussmann et al. 28 found out that these patients present statistically lower exposure level to known risk factors such as smoking and alcoholism. They also found a statistically significant correlation between HPV and low level of tumor differentiation, contrary to the previous findings the authors mentioned.
Miller and Johnstone29 concluded in their meta-analysis from 1982-1997 that HPV is an independent risk factor and extremely important in OSC, and their results indicated that HPV detection is twice to three times more common in oral pre-malignant lesions and 4 to 7 times more common in OSC if compared against normal oral mucosa.
Brandwein et al. 30 did not find any statistical association between HPV and TNM staging and also between clinical and differentiation aspects of the tumor. In this study, there were no statistically significant differences found between negative and positive HPV regarding its disease-free interval and survival time.
Although we know that PCR is the best current method to detect HPV this study began by investigating koilocytosis in hematoxylin-eosin stained slides, which is highly suggestive of HPV infection. This study is part of a pilot project that will be followed up by PCR tests to be performed in all slides previously examined to confirm the high prevalence of HPV infection in oral OSC. The histological analysis by itself may suggest the presence of HPV and is a screening method of HPV-related lesions. It is quite a useful method in centers in which sophisticated molecular biology survey methods are unavailable.
CONCLUSION
Careful anatomic pathological survey of OSC of oral cavity and oropharynx showed high prevalence of koilocytosis - 75% -, indicating a likely high HPV prevalence in this type of tumors.
Footnotes
Article submited on March 24, 2005.
REFERENCES
- 1.Sarruf MBJM, Dias EP. Avaliação citopatológica da cavidade bucal em pacientes portadores de infecção genital pelo papilomavírus humano (HPV) J Bras Doenças Sex Trans. 1997;9(2):4–18. [Google Scholar]
- 2.Howley PM, Schlegel R. The human papillomaviruses. An overview. (Review) Am J Med. 1988;85(2A):155–158. [PubMed] [Google Scholar]
- 3.Oliveira LHS. Virologia Humana. Cultura Médica; Rio de Janeiro: 1994. p. 311. [Google Scholar]
- 4.Kellokoski JK, Syrjanen SM, Syrjanem KJY, Kiloski M. Oral mucosal changes in women with genital HPV infection. J Oral Pathol Med. 1990;19:142–148. doi: 10.1111/j.1600-0714.1990.tb00813.x. [DOI] [PubMed] [Google Scholar]
- 5.Pfister H, Fuchs PG, Volcker HE. Human papillomavirus DNA in conjuntival papilloma. Graefes Arch Clin Exp Ophthamol. 1985;223:164–167. doi: 10.1007/BF02148894. [DOI] [PubMed] [Google Scholar]
- 6.Cotran RS, Kumar V, Robbins SL, Schoen FJ. Patologia Estrutural e Funcional. 5º. Guanabara-Koogan; Rio de Janeiro: 1996. p. 937. [Google Scholar]
- 7.Neville BW, Damm DD, Allen CM, Bouquot JE. Patologia Oral e Maxilofacial. 1º. Guanabara-Koogan; Rio de Janeiro: 1998. p. 705. [Google Scholar]
- 8.Shafer WG, Hine MK, Levy BM. Tratado de Patologia Bucal. 2º. Interamericana; Rio de Janeiro: 1985. [Google Scholar]
- 9.Syrjanen K, Gissman L, Koss K. Papillomaviruses and human disease. Springer; Berlin/ Heidelberg/ New York: 1987. pp. 104–137. [Google Scholar]
- 10.Rosenberg SK, Greenberg MD, Reid R. Sexually transmitted papilloma viral infection in men. Obstet Gynecol Clin North Am. 1987;14:495–512. [PubMed] [Google Scholar]
- 11.Gillison ML, Koch WM, Capone RB, et al. Evidence for a causal association between human papillomavirus and a subset of head and neck cancers. JNCI. 2000;92(9):709–720. doi: 10.1093/jnci/92.9.709. [DOI] [PubMed] [Google Scholar]
- 12.Yeudall WA. Human papillomavirus and oral neoplasia. Oral Oncol Eur J Cancer. 1992;28B:61–66. doi: 10.1016/0964-1955(92)90015-s. [DOI] [PubMed] [Google Scholar]
- 13.Woods K, Shillitoe E, Spitz M, Schantz S, Adler-Stortz K. Analysis of human papillomavirus DNA in oral squamous cell carcinomas. J Oral Pathol Med. 1993;22:101–108. doi: 10.1111/j.1600-0714.1993.tb01038.x. [DOI] [PubMed] [Google Scholar]
- 14.Snijders PJ, Scholes AG, Hart CA, Jones AS, Vaughan ED, Woolgar JA, et al. Prevalence of mucosotropic human papillomaviruses in squamous-cell carcinomas of the head and neck. Int J Cancer. 1996;66:464–469. doi: 10.1002/(SICI)1097-0215(19960516)66:4<464::AID-IJC9>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 15.Sugerman PB, Shillitoe EJ. The high risk human papillomaviruses and oral cancer: evidence for and against a causal relationship. Oral Dis. 1997;3:130–147. doi: 10.1111/j.1601-0825.1997.tb00025.x. [DOI] [PubMed] [Google Scholar]
- 16.Koss LG, Durfee GR. Unusual patterns of squamous epithelium of the uterine cervix: cytologic and pathologic study of koilocytic atypia. Ann N Y Acad Sci. 1956;63:1245–1261. doi: 10.1111/j.1749-6632.1956.tb32134.x. [DOI] [PubMed] [Google Scholar]
- 17.Syrjänen K, Syrjänen S, Lamberg M, Pyrhönen S, Nuutinen J. Morphological and immunohistochemical evidence suggesting human papillomavirus (HPV) involvement in oral squamous cell carcinogenesis. Int J Oral Surg. 1983;12:418–424. doi: 10.1016/s0300-9785(83)80033-7. [DOI] [PubMed] [Google Scholar]
- 18.Premoli-de-Percoco G, Galindo I, Ramirez JL, Perrone M, Rivera H. Detection of human papillomavirus-related oral verruca vulgaris among Venezuelans. J Oral Pathol Med. 1993;22:113–116. doi: 10.1111/j.1600-0714.1993.tb01040.x. [DOI] [PubMed] [Google Scholar]
- 19.Dekmezian RH, Batsakis JG, Goepfert H. In situ hybridization of papillomavirus DNA in head and neck squamous cell carcinomas. Arch. Otolaryngol. Head Neck Surg. 1987;113:819–821. doi: 10.1001/archotol.1987.01860080025008. [DOI] [PubMed] [Google Scholar]
- 20.Garlick JA, Taichman IB. Human papillomavirus infection of the oral mucosa. Am J Dermatopathol. 1991;13:386–395. doi: 10.1097/00000372-199108000-00010. [DOI] [PubMed] [Google Scholar]
- 21.Herrero R, et al. Human papillomavirus and oral cancer: the International Agency for Research on Cancer multicenter study. J Natl Cancer Inst. 2003;95(23):1772–1783. doi: 10.1093/jnci/djg107. [DOI] [PubMed] [Google Scholar]
- 22.Klussmann JP, Weissenborn SJ, Wieland U, Dries V, Eckel HE, Pfister HJ, Fuchs PG. Human pappilomavirus-positive tonsillar carcinomas: a different tumor entity? Med Microbiol Immunol. 2003 (Berl);192(3):129–132. doi: 10.1007/s00430-002-0126-1. [DOI] [PubMed] [Google Scholar]
- 23.Ritchie JM, Smith EM, Summersgill KF, Hoffman HT, Wang D, Klussmann JP, Turek LP, Haugen TH. Human papillomavirus infection as a prognostic factor in carcinomas of the oral cavity and oropharynx. Int J Cancer. 2003;104(3):336–344. doi: 10.1002/ijc.10960. [DOI] [PubMed] [Google Scholar]
- 24.Ha PK, Pai SI, Westra WH, Gillison ML, Tong BC, Sidransky D, Califano JA. Real-time quantitative PCR demonstrates low prevalence of human papillomavirustype 16 in premalignant and malignant lesions of the oral cavity. Clin Cancer Res. 2002;8(5):1203–1209. [PubMed] [Google Scholar]
- 25.Niv A, Sion-Vardi N, Gatot A, Nash M, Fliss DM. Identification and typing of human papillomavirus (HPV) in squamous cell carcinoma of the oral cavity and oropharynx. J Laryngol Otol. 2000;114:41–46. doi: 10.1258/0022215001903870. [DOI] [PubMed] [Google Scholar]
- 26.Shindoh M, Chiba I, Yasuda M, et al. Detection of human papillomavirus DNA sequences in oral squamous cell carcinomas and their relation to p54 and proliferating cell nuclear antigen expression. Cancer. 1995;76:1513–1521. doi: 10.1002/1097-0142(19951101)76:9<1513::aid-cncr2820760903>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 27.Cruz IBF, Snijders PJF, Steenbergen RDM, et al. Age- dependence of human papillomavirus DNA presence in oral squamous cell carcinomas. Oral Oncol Eur J Cancer. 1996;32B:55–62. doi: 10.1016/0964-1955(95)00060-7. [DOI] [PubMed] [Google Scholar]
- 28.Klussmann JP, Weissenborn SJ, Weiland U, Dries V, Kolligs J, Jungehuelsing M, et al. Prevalence, distribution, and viral load of human papillomavirus 16 DNA in tonsillar carcinomas. Cancer. 2001;92:2875–2884. doi: 10.1002/1097-0142(20011201)92:11<2875::aid-cncr10130>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 29.Miller CS, Johnstone BM. Human papillomavirus as a risk factor for oral squamous cell carcinoma: a meta-analysis, 1982-1997. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2001;91:622–635. doi: 10.1067/moe.2001.115392. [DOI] [PubMed] [Google Scholar]
- 30.Brandwein M, Zeitlin J, Nuovo GJ, et al. HPV detection using “hot start” polymerase chain reaction in patients with oral cancer: a clinicopathological study of 64 patients. (Review) Mod Pathol. 1994;7:720–727. [PubMed] [Google Scholar]


