Abstract
Nonselected autologous tumor-infiltrating lymphocytes (TILs) may provide advantages over other treatments for solid tumors, including checkpoint inhibitor-refractory melanoma. This retrospective analysis reports a single-center experience of nonselected autologous TILs derived from digested tumors for compassionate use treatment of advanced cutaneous melanoma, including after programmed cell death protein 1 (PD-1) inhibition. Patients with histologically confirmed metastatic cutaneous melanoma and no standard-of-care treatment options underwent tumor resection for TIL product manufacturing. Patients received lymphodepleting chemotherapy with cyclophosphamide for 2 days and fludarabine for 5 days, followed by a single TIL infusion and post-TIL high-dose interleukin (IL)-2. Safety assessments included clinically significant adverse events (AEs). Efficacy assessments included overall response rate (ORR), complete response (CR) rate, disease control rate (DCR), and overall survival. Between October 2011 and August 2019, 21 patients underwent treatment (median follow-up time, 52.2 months from TIL infusion). Among all treated patients, median age was 45 years, median number of disease sites was 4, 100% had M1c or M1d disease, and 90% received prior checkpoint inhibitor. Twelve patients received TILs after prior PD-1 inhibition. The safety profile among all treated patients and the prior PD-1 inhibitor subgroup was generally consistent with lymphodepletion and high-dose IL-2. No treatment-related deaths occurred. Among all patients, the ORR was 67%, CR rate was 19%, and the DCR was 86%, which was consistent with that observed in the prior PD-1 inhibitor subgroup (58%, 8%, and 75%, respectively). Median overall survival in all treated patients and the prior PD-1 inhibitor subgroup was 21.3 months. In total, 5 patients (24%) had durable ongoing responses (>30 months post-TIL infusion) at data cutoff, and all patients who achieved CR remained alive and disease free. To further illustrate how TIL therapy may integrate into established treatment paradigms, several case studies of patients treated in this series were included. Overall, these data demonstrate that manufacturing of nonselected autologous TILs from tumor digests is feasible and resulted in high rates of durable response in poor-risk patient populations, which may address significant unmet medical need.
Keywords: Tumor-infiltrating lymphocytes, melanoma, immunotherapy, immunology, interleukin-2, T cell, checkpoint inhibition, PD-1 inhibitor, compassionate use
Introduction
In the last decade, immunotherapy, including checkpoint inhibition (eg, programmed cell death protein 1 [PD-1] inhibitors), has revolutionized treatment of advanced melanoma and markedly improved patient outcomes [1-4]. Additional advances resulted from identification and targeted inhibition of driver mutations (eg, B-raf proto-oncogene [BRAF]; mitogen-activated protein kinase kinase [MEK]) [5-8]. Despite these advances, patients frequently develop treatment resistance and relapse, with 5-year overall survival (OS) between 34-52%. Those who relapse following PD-1 inhibition and, if appropriate, BRAF and/or MEK inhibition, have limited treatment options [9], highlighting significant unmet medical need for new therapeutic approaches.
The intrinsic antitumor activity and broad T-cell receptor (TCR) repertoire of nonselected tumor-infiltrating lymphocytes (TILs) make them uniquely suited to address the marked clonal heterogeneity characteristic of solid tumors [9-14]. The promise of TIL therapy has been repeatedly demonstrated over many years in the academic setting. Owing to recent advances in manufacturing, TIL production can be scaled up to treat more patients [15,16], as evidenced by several ongoing studies of TILs as monotherapy or in combination [4,17]. In a meta-analysis of 13 studies (n=410), TILs demonstrated durable complete responses (CRs) in patients with advanced melanoma, with an estimated 41% objective response rate [17]. A large phase 2 trial of TIL therapy in patients whose disease had progressed following PD-1 inhibition and, if appropriate, BRAF inhibition, demonstrated clinical utility in this area of high unmet medical need [12]. Toxicity following TIL infusion is largely related to the preparative chemotherapy regimen and post-infusion interleukin (IL)-2 [4,17-19]. Following the resolution of these anticipated and generally manageable adverse events (AEs) that occur immediately before and after the single TIL infusion, side effects related to TIL are rare [4,17-19].
Collectively, these findings provide strong rationale for further clinical development of TIL therapy for advanced melanoma. Here, we present a retrospective analysis of a clinical series of nonselected autologous TILs made from digested tumors for the compassionate use treatment of heavily pretreated patients with advanced cutaneous melanoma, including those with prior PD-1 inhibitor exposure. To supplement these findings, clinical narratives from key patient cases are described.
Patients and methods
Compassionate use program design and conduct
This clinical series reflects a single-center experience of TILs administered at The Christie Hospital, Manchester, United Kingdom. As a compassionate use clinical series, the treatment was approved by institutional review board and National Health Service commissioning; post-treatment blood samples were collected and analyzed under a study approved by South Manchester Research Ethics Committee (Ref: 09/H1003/75). Written informed consent was obtained in accordance with the Declaration of Helsinki.
Patients
Although strict eligibility criteria for treatment were not employed, patient selection guidelines were applied in determining suitability of patients to receive TILs. Patients had advanced, progressive cutaneous melanoma, no standard-of-care treatment options, satisfactory performance status and hematological/biochemical indices, and adequate organ function. Patients should not have had prior allogeneic transplant, extensive skeletal irradiation, symptomatic brain metastasis measuring ≥10 mm in diameter (treated and stable brain metastases were permitted), prior lymphotoxic therapy within 4 weeks of TIL harvest, concurrent serious infection within 28 days prior to treatment, or steroid use ≤3 weeks before treatment, except for physiological replacement doses of steroids. Additional guidelines are provided within Supplementary Methods.
Treatment
Nonselected autologous TILs derived from digested tumor tissue were manufactured under a Medicines and Healthcare Products Regulatory Agency Manufacturing Specials license (Figure S1). Suitable patients underwent resection of ≥1 cm3 of tumor tissue for TIL production. Resected tumor tissue was trimmed to remove necrotic tissue, blood/hemorrhage, fat, and healthy tissue and was subsequently digested enzymatically and mechanically. Digested material was then washed and placed in culture for activation and expansion as previously described [20-22]. Specifically, cells were activated and expanded to a minimum dose of 1×109 T cells using anti-CD3 antibodies, irradiated allogeneic peripheral blood mononuclear cells, and IL-2 [20-22]. Expanded TILs were harvested, washed, and concentrated prior to final formulation and release. Quality control review of the final formulation product was performed using assays to identify T-cell dose, viability, phenotype, and functional activity, as well as screen for microbial contamination. Over the course of this clinical series, tumor procurement was modified to include a controlled-rate cryopreservation step immediately following tumor digestion. Seven days prior to TIL infusion, patients received intravenous lymphodepleting chemotherapy (cyclophosphamide 60 mg/kg/day on Days -7 and -6 and fludarabine 25 mg/m2/day on Days -5 through -1). On Day 0, TILs were infused followed by high-dose IL-2 (600,000-720,000 IU/kg every 8 hours for ≤12 doses). Patients were hospitalized for treatment and discharged when clinically well and following resolution of any AEs and recovery of cell counts to the satisfaction of the care team.
Patients received prophylactic and supportive medications (Table S1). Other concomitant medication could be given as medically indicated. Steroid use was avoided post-TIL infusion but, if necessary, given at the lowest possible dose and shortest duration.
Outcome assessments and analyses
All outcomes were analyzed retrospectively and descriptively. Safety and efficacy data were extracted from primary source documents and placed into a database for quality control and analysis. AEs and changes in laboratory values were collected during hospitalization. Clinically significant AEs were reported using verbatim terms in clinic/hospital notes; similar terms were summarized together per AE lookup table (Table S2). AEs were tabulated by AE terms and defined as those with onset during the lymphodepletion period or post-TIL infusion. Severity and relatedness of AEs are not presented as this was not systematically documented due to the nature of the compassionate use program, precluding definitive attribution of AEs with any particular treatment component. Deaths that occurred post-TIL infusion were tabulated by time windows in relation to the infusion date (i.e., within 30 days, after 30 days and within 3 months, and >3 months). Laboratory results collected during hospitalization and sporadically during outpatient follow-up were summarized through boxplots by timepoints around TIL infusion.
Efficacy outcomes included overall response rate (ORR; combined CR and partial response [PR] rates); disease control rate (DCR; combined CR, PR, and stable disease [SD] rates); OS; change in tumor burden (change from baseline to post-baseline nadir); and time to response (time from TIL infusion to first CR or PR). Disease assessments were conducted per Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1 (v1.1) [23] when possible. Otherwise, responses were assessed per standard clinical disease assessment methods, including positron-emission tomography (PET), computed tomography (CT) without detailed tumor volume measurements, or clinical monitoring (e.g., history and physical examination; laboratory assessments). The standard assessment methods prevented post hoc RECIST v1.1 response determinations. Rigorous confirmation of disease response required by RECIST v1.1 was not applied due to the inconsistent schedule and manner of longitudinal disease assessment utilized in this compassionate use program. Response rates were tabulated, and 95% exact confidence intervals (CIs) were calculated using the Clopper-Pearson method. Tumor burden was measured by the sum of target lesion diameters. Kaplan-Meier (KM) plots, estimates, and 2-sided 95% CIs were generated for OS (derived as the time from TIL infusion date to the date of death from any cause). Patients who were alive at the time of analysis were censored at the last date known to be alive.
Three separate outcome analyses were performed. The first was a safety and efficacy analysis performed on all treated patients. Safety and efficacy analysis was also performed on patients with prior PD-1 inhibitor exposure. Finally, efficacy was assessed on the subgroup of patients with RECIST-evaluable disease. The RECIST-evaluable subgroup comprised all patients with pre- and post-treatment disease assessments collected using CT/magnetic resonance imaging scans, which included quantitative tumor burden measurements in accordance with RECIST version 1.1.
Product assessments
The cell composition, T-cell phenotype, and activation/exhaustion state of the TIL products were assessed. Additional analyses were performed on the final product to profile the CD4/CD8 T-cell ratio and TCR repertoire and clonality. All methods are described within the Supplementary Methods. Assessments were performed using available patient samples for validated analysis; as such, the sample sizes vary by assay type.
Results
All treated patients
Between October 2011 and August 2019, 21 patients with advanced cutaneous melanoma underwent treatment. TILs were manufactured for 4 patients using cryopreserved tumor digests. All patients completed lymphodepleting chemotherapy; the median number of TILs infused was 32×109 cells (range, 8×109-63×109), and the median number of IL-2 doses was 8 (range, 4-11). Patients were hospitalized for a median of 10 days (range, 7-15) following TIL treatment. By the analysis cutoff date (December 31, 2019), patients had a median follow-up time of 52.2 months (range, 4.6-98.8) from the TIL infusion date.
Among all treated patients, 67% had M1c disease, and 33% had M1d disease (Table 1). Median baseline tumor burden was 100 mm (n=20; range, 13-281). Serum lactate dehydrogenase (LDH) levels were elevated above upper limit of normal in 48% of patients and by >2× upper limit of normal in 14%. The median number of prior systemic regimens was 2 (range, 1-9; mean, 3); 90% of patients had prior checkpoint inhibitor therapy (cytotoxic T lymphocyte-associated protein 4 [CTLA-4] inhibitor, 90%; PD-1 inhibitor, 57%; both PD-1 inhibitor and CTLA-4 inhibitor, 57% [38% sequentially and 19% concurrently]). Eleven patients (52%) had tumors with BRAF mutations; of these patients, all had failed prior BRAF inhibitor alone or in combination with a MEK inhibitor.
Table 1.
Demographics and baseline characteristics among all treated patients (N=21) and the prior PD-1 inhibitor subgroup (n=12)
| Prior PD-1 Inhibitor Subgroup | All Treated Patients | |
|---|---|---|
| (n=12) | (N=21) | |
| Age (years), median (range) | 55 (33-64) | 45 (16-68) |
| Male, n (%) | 7 (58) | 15 (71) |
| Stage IV, n (%) | 12 (100) | 21 (100) |
| Time (months) from original diagnosis to TIL treatment, median (range) | 36 (8-177) | 39 (8-177) |
| Disease sites, median (range) | 4 (2-10) | 4 (2-10) |
| M1c disease, n (%) | 9 (75) | 14 (67) |
| M1d disease, n (%) | 3 (25) | 7 (33) |
| History of brain metastasis, n (%) | 4 (33) | 8 (38) |
| Brain metastasis at baseline, n (%) | 3 (25) | 7 (33) |
| Baseline brain metastasis irradiated, n (%) | 3 (25) | 7 (33) |
| Tumor burden (mm)a, median (range) | 123 (51-169) | 100 (13-281) |
| LDH, n (%) | ||
| >ULN to ≤2× ULN | 4 (33) | 7 (33) |
| >2× ULN | 2 (17) | 3 (14) |
| Prior no. of systemic regimens, median (range) | 3 (1-9) | 2 (1-9) |
| Checkpoint inhibitor, n (%) | 12 (100) | 19 (90) |
| PD-1 inhibitor | 12 (100) | 12 (57) |
| CTLA-4 inhibitor | 12 (100) | 19 (90) |
| Dual PD-1/CTLA-4 inhibitor relapsed/refractory | 12 (100) | 12 (57) |
| Cytotoxic therapy, n (%) | 2 (17) | 6 (29) |
| Radiotherapy, n (%) | 6 (50) | 11 (52) |
| Outcome to last prior melanoma therapyb, n (%) | ||
| Refractory | 2 (17) | 4 (19) |
| Relapsed | 3 (25) | 6 (29) |
| Progressed with unknown best response | 6 (50) | 8 (38) |
| Intolerant | 0 | 2 (10) |
| Unknown | 1 (8) | 1 (5) |
| BRAF mutation positive, n (%) | 6 (50) | 11 (52) |
| BRAF inhibitor ± MEK inhibitor | 6 (50) | 11 (52) |
Eleven of 12 and 20 of 21 patients had tumor burden data available at baseline, respectively, as measured by the sum of diameters of all target lesions (local assessment per RECIST v1.1).
Refractory was defined as no response to the prior therapy; relapsed was defined as a response was achieved but the patient’s disease subsequently progressed.
BRAF, B-raf proto-oncogene; CTLA-4, cytotoxic T-lymphocyte-associated protein 4; LDH, lactate dehydrogenase; MEK, mitogen-activated protein kinase kinase; PD-1, programmed cell death protein 1; RECIST, Response Evaluation Criteria in Solid Tumors; TIL, tumor-infiltrating lymphocyte; ULN, upper limit of normal.
The most commonly reported AEs (>1 patient) during the lymphodepletion period were neutropenia (43%) and nausea (19%; Table S3). The most frequently reported AEs (≥25% of patients) post-TIL infusion were thrombocytopenia (62%), pyrexia (57%), rigors (43%), neutropenia (29%), and tachycardia (29%; Table 2), and were primarily lymphodepleting chemotherapy- and IL-2-related toxicities [19]. Two patients experienced vitiligo. Similar AEs were observed among patients with TILs manufactured from cryopreserved tumor digests (Table S4). No unexpected serious AEs were reported. AEs were generally managed supportively and by IL-2 discontinuation.
Table 2.
Adverse events with onset after TIL infusion among all treated patients (N=21)
| Adverse Event Term, n (%) | All Treated Patients |
|---|---|
| (N=21) | |
| Thrombocytopenia | 13 (62) |
| Pyrexia | 12 (57) |
| Rigors | 9 (43) |
| Neutropenia | 6 (29) |
| Tachycardia | 6 (29) |
| Pulmonary edema | 5 (24) |
| Vascular leak | 5 (24) |
| Rash | 4 (19) |
| Atrial fibrillation | 3 (14) |
| Cardiovascular instability | 3 (14) |
| Chest infection | 3 (14) |
| Edema | 3 (14) |
| Confusion | 2 (10) |
| Hypokalemia | 2 (10) |
| Hypotension | 2 (10) |
| Neurological deficit | 2 (10) |
| Renal impairment | 2 (10) |
| Respiratory sepsis | 2 (10) |
| Seizure | 2 (10) |
| Sepsis | 2 (10) |
| Vitiligo | 2 (10) |
| Weight gain | 2 (10) |
| Wheezing | 2 (10) |
| Cough | 1 (5) |
| Diarrhea | 1 (5) |
| Dysphasia | 1 (5) |
| Engraftment syndrome | 1 (5) |
| Hallucinations | 1 (5) |
| Lethargy | 1 (5) |
| PICC line infection | 1 (5) |
| Pleural effusion | 1 (5) |
| Pneumonia | 1 (5) |
| Pneumonitis | 1 (5) |
| Respiratory problems | 1 (5) |
| Tachypnea | 1 (5) |
PICC, peripherally inserted central catheter; TIL, tumor-infiltrating lymphocyte.
Seizures occurred in 2 patients, both with brain metastasis at time of treatment. For 1 patient, seizure occurred 16 days post-infusion. A CT scan confirmed the presence of pre-existing brain metastasis and a new finding of increased edema around the metastasis following recent stereotactic radiotherapy to the site. The other patient, Patient 2, a 16-year-old further described below, had a seizure associated with fever 2 days post-TIL infusion. Both cases of seizure were managed supportively with levetiracetam and resolved without sequelae or recurrence.
A trend towards reduced blood counts in all lineages was observed during the lymphodepletion period (Figure S2). Cell counts and hemoglobin levels generally reached nadirs approximately 1-4 days post-TIL infusion, with recovery to baseline levels approximately 7 days post-infusion.
No treatment-related deaths occurred. Ten patients (48%) died prior to data cutoff (1 death on Day 90 shortly after progressive disease and 9 deaths >3 months after infusion). Of these, 4 deaths were attributed to progressive disease and 1 was possibly attributed, in part, to AEs caused by subsequent therapy given for progressive disease. The remaining 5 patients had documented progressive disease prior to death but the specific causes of death were not reported.
Among all treated patients, the ORR was 67% (95% CI, 43-85), including a CR rate of 19% (95% CI, 5-42), and a DCR of 86% (95% CI, 64-97; Table 3). The median time from TIL infusion to first response was 1.7 months (range, 1-12). In total, 6 patients were not part of the RECIST-evaluable subgroup and were instead followed by PET, CT without detailed tumor burden measurements, and clinical monitoring. Responses were also observed in these patients, including best responses of CR and PR in 2 and 3 patients, respectively. Fluorodeoxyglucose-PET imaging pre- and post-treatment is shown for 1 of the complete responders not followed in the RECIST-evaluable subgroup in Figure S3.
Table 3.
Best response rates among all treated patients (N=21) and the prior PD-1 inhibitor subgroup (n=12)
| Prior PD-1 Inhibitor Subgroup | All Treated Patientsa | |||
|---|---|---|---|---|
| (n=12) | (N=21) | |||
|
|
|
|||
| n (%) | 95% CI | n (%) | 95% CI | |
| Overall response rate (CR+PR) | 7 (58) | 28-85 | 14 (67) | 43-85 |
| CR | 1 (8) | 0-38 | 4 (19) | 5-42 |
| PR | 6 (50) | 21-79 | 10 (48) | 26-70 |
| SD | 2 (17) | 2-48 | 4 (19) | 5-42 |
| PD | 3 (25) | 5-57 | 3 (14) | 3-36 |
| Disease control rate (CR+PR+SD) | 9 (75) | 43-95 | 18 (86) | 64-97 |
Includes 2 patients counted as responders who had dabrafenib and MEK inhibitor-refractory disease whose disease was unequivocally progressing on combination therapy prior to TIL therapy and who received post-infusion dabrafenib to prevent tumor flare.
CR, complete response; MEK, mitogen-activated protein kinase kinase; PD, progressive disease; PD-1, programmed cell death protein 1; PR, partial response; SD, stable disease.
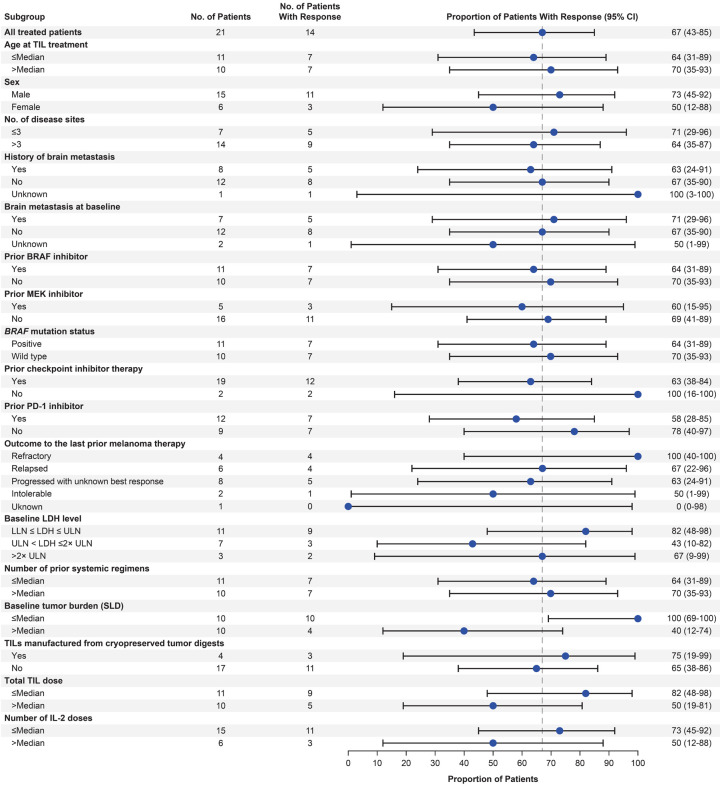
Notwithstanding small sample sizes for certain subgroups, responses were generally consistent across covariates, including age, number of disease sites, number of prior lines of therapy, prior BRAF, MEK, or PD-1 inhibitor, baseline brain metastasis, and baseline tumor burden (Figure 1). Patients with baseline brain metastases (n=7) and those with TILs manufactured from cryopreserved tumor digests (n=4) achieved ORRs of 71% (29% CR) and 75% (25% CR), respectively, which were generally consistent with those observed in all treated patients (Figure 1).
Figure 1.
Response rates by subgroup in all treated patients (N=21). Data include 2 patients counted as responders who had dabrafenib and MEK inhibitor-refractory disease whose disease was unequivocally progressing on combination therapy prior to TIL therapy and who received post-infusion dabrafenib to prevent tumor flare. BRAF, B-raf proto-oncogene; IL-2, interleukin-2; LDH, lactate dehydrogenase; LLN, lower limit of normal; MEK, mitogen-activated protein kinase kinase; ORR, overall response rate; PD-1, programmed cell death protein 1; SLD, sum of target lesion diameters; TIL, tumor-infiltrating lymphocyte; ULN, upper limit of normal.
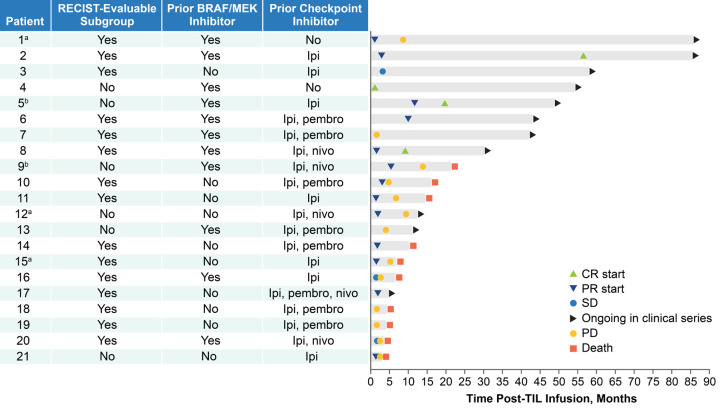
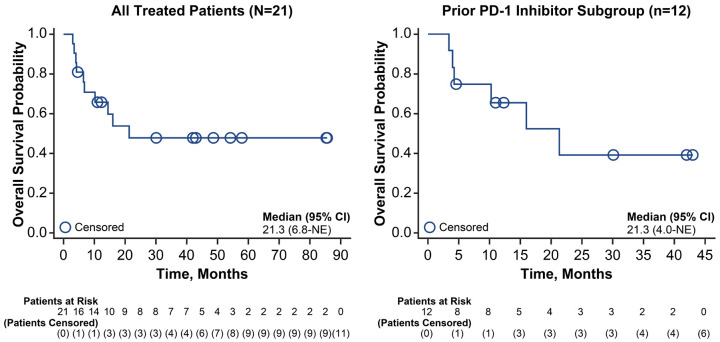
As of the data cutoff date, 5 of 21 patients (24%) had durable ongoing responses of >30 months post-TIL infusion. All 4 patients who achieved a CR remained alive and disease free (range of 30-85 months of follow-up; Figure 2). Median OS for all treated patients was 21.3 months (95% CI, 6.8-not estimable) and the KM estimate of the 24-month OS rate was 48% (95% CI, 24-68; Figure 3).
Figure 2.
Time to response and survival status by patient. aPatient 1 received a checkpoint inhibitor at the time of disease progression; patients 15 and 12 received high-dose IL-2 and checkpoint inhibitor, respectively, prior to documented disease progression. bPatients 5 and 9 had unequivocally BRAF+MEK-refractory melanoma immediately prior to TIL treatment but were continued on dabrafenib, with brief interruptions for tumor harvest and TIL infusion, to prevent tumor flare upon discontinuation. Patient 5 was treated with dabrafenib for 3 months following TIL infusion, at which point the dabrafenib was stopped. Patient 9 achieved a PR that lasted approximately 14 months from TIL infusion during which time dabrafenib was continued. BRAF, B-raf proto-oncogene; CR, complete response; IL-2, interleukin-2; ipi, ipilimumab; MEK, mitogen-activated protein kinase kinase; nivo, nivolumab; PD, progressive disease; pembro, pembrolizumab; PR, partial response; TIL, tumor-infiltrating lymphocyte.
Figure 3.
Overall survival among all treated patients (N=21) and the prior PD-1 inhibitor subgroup (n=12). NE, not estimable; OS, overall survival; PD-1, programmed cell death protein 1.
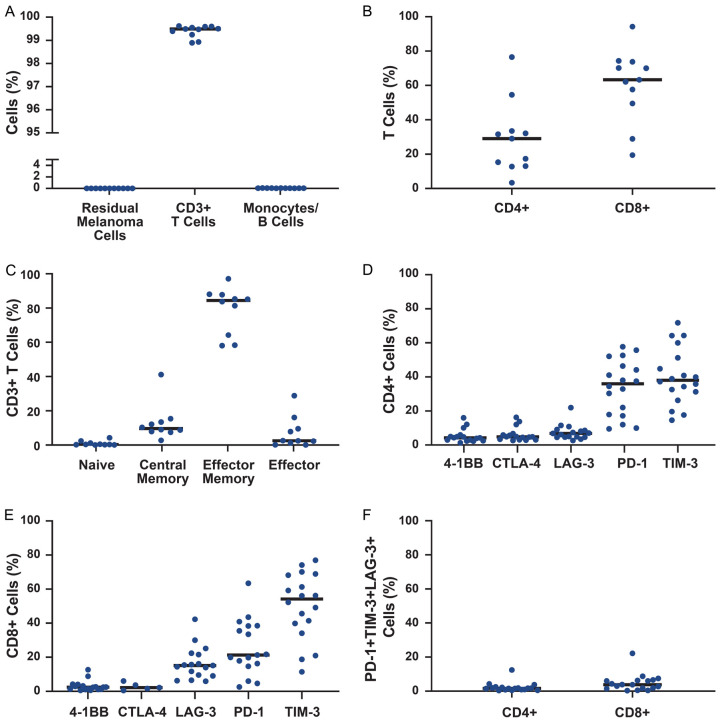
TIL products were primarily composed of T cells with undetectable quantities of monocytes, B cells, or residual melanoma cells (Figure 4A). Products tended to comprise predominantly CD8+ T cells with a primarily effector memory phenotype (Figure 4B, 4C). The differential expression of cell surface activation and exhaustion markers on both CD4+ and CD8+ cells supported an activated rather than an exhausted T-cell phenotype [24,25], with very few cells exhibiting coexpression of PD-1, TIM-3, and LAG-3 (Figure 4D-F). Single-cell RNA sequencing on a limited number of patient samples revealed product-associated clones that were recoverable at up to 4% frequency from peripheral circulation 7 days following infusion (Figure S4). In 1 complete responder, several product-associated clones were persistent in circulation 6 months post-infusion.
Figure 4.
Final TIL product characteristics. (A) Analysis of cell composition in final product (n=11). Cell subsets were defined as follows: monocytes, CD14+; B cells, CD19+; residual melanoma cells, melanoma markers (CD146, MCAM, MCSP, CD228). (B) Distribution of CD4+ and CD8+ T cells in final product (n=11). (C) Phenotype of T cells in final product (n=10). Cell subsets were defined as follows: naive, CD62L+CD45RO-; central memory, CD62L+CD45RO+; effector memory, CD62L-CD45RO+; and effector, CD62L-CD45RO-. (D) Expression of activation and exhaustion markers on final product (n=18) CD4+ T cells and (E) CD8+ T cells. (F) Exhausted T-cell phenotype, defined as PD-1+TIM-3+LAG-3+ cells. CTLA-4, cytotoxic T-lymphocyte-associated protein 4; LAG-3, lymphocyte activation gene 3; MCAM, melanoma cell adhesion molecule; MSCP, melanoma-associated chondroitin sulfate proteoglycan; PD-1, programmed cell death protein 1; TIM-3, T-cell immunoglobulin and mucin-domain containing protein 3; TIL, tumor-infiltrating lymphocyte.
Prior PD-1 inhibitor subgroup
Twelve patients received TILs after prior PD-1 inhibition, all of whom had disease that was also relapsed after or primarily refractory to CTLA-4 inhibition either in combination or in sequence with PD-1 blockade. The median number of TILs infused was 32×109 cells (range, 8×109-53×109), and the median number of IL-2 doses was 8 (range, 6-9). Median follow-up time was 45.5 months (range, 4.6-59.3) from the TIL infusion date for this subgroup. Patients had higher baseline tumor burden (median, 123 [range, 51-169]) and were more heavily pretreated (Table 1).
Generally, the safety profile of the prior PD-1 inhibitor subgroup was consistent with that of the all-treated population. Neutropenia was the most commonly reported AE during the lymphodepletion period (5 [42%]), though nausea, peripherally inserted central catheter infection, and sepsis were also observed (each in 1 patient [8%]). The most frequently reported AEs (≥25% of patients) post-TIL infusion were thrombocytopenia (75%), pyrexia (50%), rigors (50%), vascular leak (33%), chest infection (25%), and neutropenia (25%; Table S5). AEs were managed supportively and were mostly toxicities related to lymphodepleting chemotherapy and IL-2 [19].
Among those who received prior PD-1 inhibitor, the ORR was 58% (95% CI, 28-85), including an 8% CR rate (95% CI, 0-38), and the DCR was 75% (95% CI, 43-95; Table 3). The median time from TIL infusion to first response was 1.8 months (range, 1.5-10.0). Nine of the patients who received prior PD-1 inhibitor had RECIST-based response assessments, and their outcomes were similar to the overall prior PD-1 inhibitor subgroup (ORR, 56% [95% CI, 21-86]; CR rate, 11% [95% CI, 0-48]). At data cutoff, 2 of 12 patients (17%) had durable ongoing responses (>30 months after TIL infusion; Figure 2). Median OS in the prior PD-1 inhibitor subgroup was 21.3 months (95% CI, 4.0-not estimable; Figure 3), and the 24-month OS rate was 39% (95% CI, 10-68).
RECIST-evaluable subgroup
The RECIST-evaluable subgroup comprised 15 patients who underwent quantitative disease assessment pre- and post-treatment, which included detailed tumor volume measurements, per RECIST v1.1. Patient characteristics for the RECIST-evaluable subgroup were similar to those observed in all treated patients (Table S6).
Among the RECIST-evaluable subgroup, the ORR was 53% (95% CI, 27-79), including a CR rate of 13% (95% CI, 2-40); the DCR was 73% (95% CI, 45-92; Figure S5A). Of 14 patients with detailed post-treatment tumor measurements, 8 demonstrated marked tumor regression (Figure S5B). The median time from TIL infusion to first response was 1.7 months (range, 1-10). Median OS was 16.0 months (95% CI, 4.2-not estimable), and the 24-month OS rate was 44% (95% CI, 18-67; Figure S6A). Median OS was not reached among patients in the RECIST-evaluable subgroup who achieved a response (n=8; 95% CI, 10.2-not estimable) and was 6.5 months among nonresponders (n=7; 95% CI, 3.4-not estimable; Figure S6B).
TIL therapy in context of standard clinical management: hypothesis-generating case studies
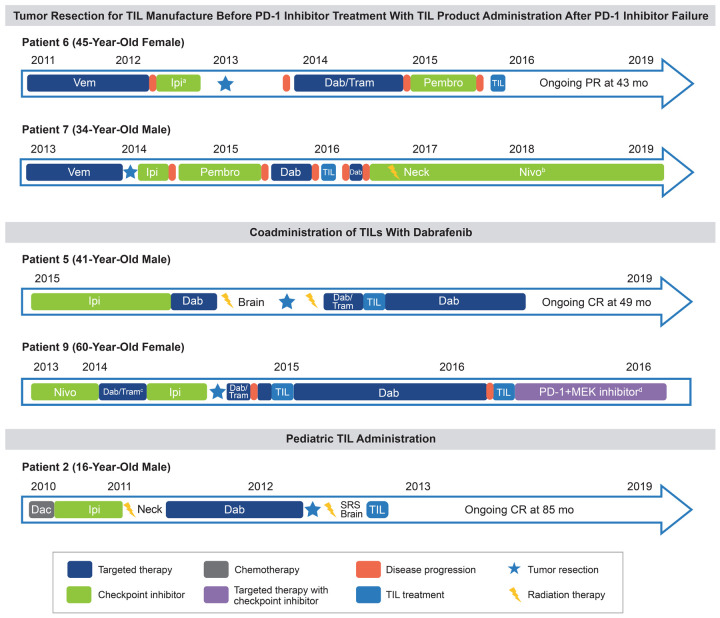
Tumor resection for TIL manufacture before PD-1 inhibitor treatment with TIL product administration after PD-1 inhibitor failure (n=2)
Two patients (Patients 6 and 7) had tumors resected for TIL harvest prior to receiving PD-1 inhibitor therapy (Figure 5). Following resection, TIL manufacturing was initiated, and cells were washed and frozen after outgrowth (Figure S1) as the patient underwent treatment with PD-1 inhibitor. Upon disease progression, the rapid expansion process was initiated, product manufacture was completed, and the product was infused.
Figure 5.
Hypothesis-generating patient cases. The starting event for each patient’s timeline is the initiation of first-line therapy. aPatient presented with hypophysitis while on ipilimumab, requiring hormone replacement with glucocorticoids; no pembrolizumab-associated toxicity was observed. bPatient presented with colitis/pneumonitis while on nivolumab, which was managed by reducing nivolumab frequency (given every 6 weeks). cCombination dabrafenib/trametinib required 50% dose reduction after 3 weeks due to fever and cholecystitis. dPatient went on to receive PD-1 inhibitor with MEK inhibitor. The patient died 21 months after initial TIL infusion, with a combination of progressive disease and possible pneumonitis caused by PD-1 and MEK inhibitors. Dac, dacarbazine; Dab, dabrafenib; Ipi, ipilimumab; MEK, mitogen-activated protein kinase kinase; Nivo, nivolumab; PD-1, programmed cell death protein 1; Pembro, pembrolizumab; SRS, stereotactic radiosurgery; TIL, tumor-infiltrating lymphocyte; Tram, trametinib; Vem, vemurafenib.
Patient 6, a 45-year-old female with BRAF-mutated melanoma, received vemurafenib followed by ipilimumab. While on ipilimumab, the patient presented with hypophysitis, requiring hormone replacement with glucocorticoids. After no response to ipilimumab, the patient had tumor resection for potential TIL manufacture and treatment. Following tumor harvest, the patient went on to receive dabrafenib with trametinib, and later pembrolizumab. Approximately 34 months after tumor harvest and cryopreservation of the TIL manufacturing intermediate, the cells were thawed, rapid expansion was performed, and the patient received the TIL infusion. During the TIL treatment period, the patient experienced short-term cytopenias, confusion, and pneumonia, and recovered with supportive care. Despite tumor harvest for product manufacture occurring prior to PD-1 administration, the product retained antitumor activity. The patient achieved a durable PR to the TIL product administered and remained in an ongoing PR for 42 months at the time of the data cutoff, with no further therapy.
Patient 7, a 34-year-old male, received vemurafenib and achieved a best response of stable disease. The patient then underwent resection for TIL manufacture followed by sequential treatment with ipilimumab, pembrolizumab, and dabrafenib, with disease progression following each therapy. Approximately 22 months after tumor harvest and cryopreservation of the manufacturing intermediate, rapid expansion was performed and the patient received the TIL infusion that was accompanied by low-grade, short-term pneumonia. After no clinical response to TILs, the patient received dabrafenib, followed by nivolumab, then radiation therapy of the neck while continuing nivolumab. Notably, the patient presented with colitis and pneumonitis while on nivolumab, which was managed by reducing dosing frequency to every 6 weeks. At the time of data cutoff, the patient continued to receive nivolumab every 6 weeks and had experienced neither disease progression nor recurrence of colitis or pneumonitis.
These cases illustrate that TILs may retain antitumor activity despite being collected early in the course of treatment and before administration of immunomodulatory agents, such as PD-1 and CTLA-4 inhibitors. This opens the possibility of harvesting and banking tumors for patients who have not yet failed standard therapies. Such a strategy would significantly reduce complexity and urgency associated with scheduling and performing tumor harvest, TIL product manufacturing, and administration once the patient’s disease has progressed on all approved drugs. Tumor harvests could even occur at the time of standard-of-care procedures, like at the time of definitive surgical resection for localized disease, thus eliminating the need for an additional surgery only for TIL production.
Coadministration of TILs with dabrafenib (n=2)
Treatment of BRAF-mutated melanoma with targeted BRAF ± MEK inhibitors is a mainstay of melanoma therapy [5,7]. Development of resistance to such therapy, however, is nearly universal and can sometimes be associated with aggressive tumor growth at the time of treatment discontinuation [26]. To prevent such tumor flare, 2 patients in this clinical series who had developed progressive disease while on combination dabrafenib and MEK inhibitor therapy were continued on dabrafenib during TIL treatment, with brief interruptions for tumor harvest for TIL product manufacture and subsequent infusion (Figure 5).
Patient 5, a 41-year-old male, had progressed on 3 prior lines of therapy, including ipilimumab, and was progressing on dabrafenib with metastases to the liver, brain, and lung at the time of TIL treatment. Dabrafenib was interrupted approximately 5 weeks prior to liver resection for TIL harvest. The patient restarted dabrafenib, and trametinib was added after surgery while the product was being manufactured. Both agents were held prior to lymphodepleting chemotherapy and TIL administration. Following TIL infusion, the patient received high-dose IL-2 and experienced increasingly severe fevers (>40°C) and rigors, necessitating cessation after 7 doses. Thereafter, the patient had rapid and sustained lymphocyte engraftment. Dabrafenib monotherapy was restarted approximately 2 weeks after TIL infusion to prevent rapid tumor growth and was continued for 3 months, at which point it was stopped given the patient was responding post TIL therapy. There were no new or unusual safety signals observed during coadministration of TILs with dabrafenib. Off all therapy, the patient achieved a CR at 20 months after TIL infusion, and remained in durable CR at time of data cutoff.
Patient 9, a 60-year-old female, had progressed on nivolumab, combination dabrafenib/trametinib (which required 50% dose reduction due to fever and cholecystitis), and ipilimumab. At the time of TIL administration, she had metastases to lung, pleura, and bone. Dabrafenib and trametinib were stopped for tumor resection and were resumed to prevent tumor flare during TIL manufacture. While awaiting product generation, the patient’s disease continued to progress. Dabrafenib and trametinib were briefly held again for TIL treatment, which was accompanied by fever and rigors and fluid retention requiring diuresis. Despite unequivocal resistance to dabrafenib, it was resumed to mitigate tumor flare. The patient achieved a PR that lasted approximately 14 months from TIL infusion, during which time dabrafenib was continued. Similar to Patient 5, there were no new or unusual safety signals observed during coadministration of TILs with dabrafenib. At 14 months post-TIL infusion, the patient had disease progression of pleural metastasis, prompting discontinuation of dabrafenib and TIL retreatment. Despite an initial response, the patient went on to receive PD-1 inhibitor with MEK inhibitor. The patient died 21 months after initial TIL infusion, with a combination of progressive disease and possible pneumonitis caused by PD-1 and MEK inhibitors.
These 2 cases provide evidence that targeted BRAF/MEK inhibition may continue with brief interruptions for tumor harvest and TIL administration without interfering with TIL activity. As emerging clinical data suggest sequencing targeted therapy after failure of frontline immunotherapy in BRAF-mutated melanoma [27], such patients will likely be considered for TIL therapy while their disease is progressing on BRAF/MEK-targeted agents. Administration of TILs followed by a period of BRAF inhibition did not lead to increased toxicity and may have prevented aggressive tumor growth during the period of TIL engraftment. Discontinuation of treatment with BRAF inhibitors may be possible once tumor flare is no longer a concern.
These findings build on preclinical data that suggest the BRAF inhibitor vemurafenib may induce higher levels of MAPK activation, intratumoral cytokine secretion, and cytotoxic activity of adoptively transferred T cells [28]. They are also supported by work from Sarnaik et al who documented the feasibility of combining vemurafenib with TIL therapy in 12 patients with BRAF-mutated melanoma. Those findings showed improved feasibility of TILs and comparable antitumor activity with continued vemurafenib prior to TIL infusion compared with patients in a previous study that did not allow BRAF inhibition during TIL manufacture in which 35% of patients enrolled were unable to proceed to TIL infusion due to disease progression [29,30].
TIL administration in a pediatric patient (n=1)
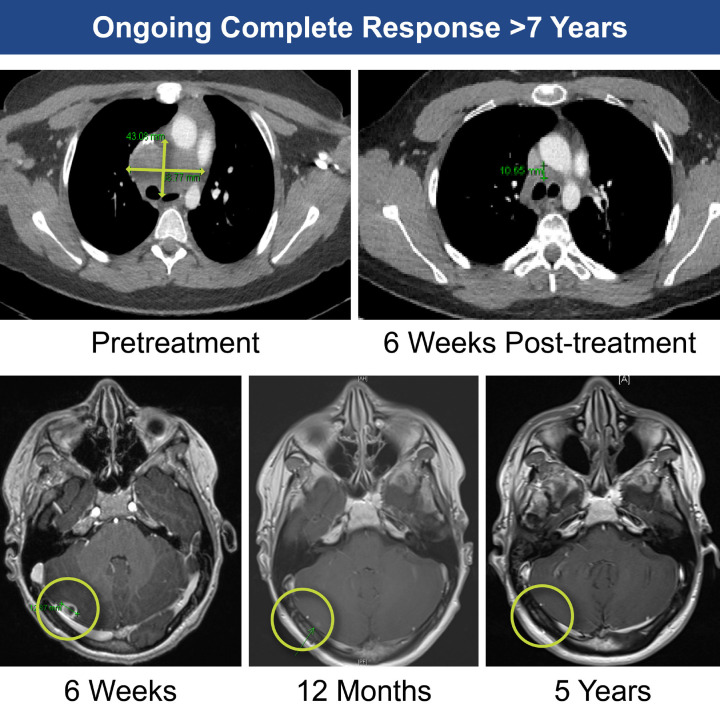
Although rare, melanoma is known to occur in children [31,32]. Patient 2, a 16-year-old male, had BRAF-mutated melanoma, bulky disease (sum of lesion diameters, 103 mm), and extensive mediastinal disease and brain metastases (Figure 5). The patient was relapsed/refractory to 3 prior lines of therapy, including ipilimumab and dabrafenib, before receiving TILs. Following TIL infusion, the patient received 8 doses of IL-2 and experienced expected IL-2-related toxicities, including tachycardia, fever, and cardiovascular instability. After the 6th dose of IL-2, seizure in the setting of high fever and tachycardia/shortness of breath occurred, all of which resolved spontaneously. The seventh dose of IL-2 was delayed and prophylactic levetiracetam was administered; no further seizure events were observed. The patient experienced neutropenia from Days 1-6 without need for platelet support. Rapid reduction in disease burden was achieved at 6 weeks, with onset of a PR at 3 months. By 60 months, the patient’s response deepened to a radiographic CR (Figure 6). At time of data cutoff, the patient remained in ongoing CR for >7 years post-TIL therapy (follow-up time, 85 months), without any further anticancer therapy.
Figure 6.
Successful treatment of brain metastases in a 16-year-old (Patient 2).
Discussion
Melanoma is a markedly immunogenic cancer with high mutational burden and inter- and intratumoral heterogeneity. This immunogenicity has likely enabled its treatment with T-cell-based therapies like immune checkpoint inhibitors and TILs [9,33,34]. Targeted therapy and checkpoint inhibitors have indeed revolutionized treatment of advanced melanoma, but relapse is common and those who experience disease progression after these approaches have poor outcomes and limited treatment options [9,26,35]. This unmet medical need may be addressed by therapeutic approaches that are patient-specific and possess broad antigen specificity. Nonselected autologous TILs represent a highly personalized therapeutic approach that leverages each patient’s unique population of tumor-reactive T cells that encompass a broad panel of TCRs that can target patient-specific tumor-associated antigens, including neoantigens [4,9,13,17,36]. This retrospective compassionate use clinical series confirms that nonselected TIL products can be manufactured from digested tumors and deliver clinical benefit to patients with advanced cutaneous melanoma, including those patients who experience disease progression after checkpoint inhibition and, if applicable, targeted therapy.
The safety profile of the TIL treatment regimen in this clinical series was similar to that in other TIL studies. AEs were primarily attributable to lymphodepleting chemotherapy and/or high-dose IL-2 rather than the TIL product, and product-specific AEs were uncommon [17,19]. With respect to the latter, 2 cases of vitiligo were observed, both in patients who achieved CRs. Generally, AEs were self-limited and managed by supportive care within the hospital setting. Of note, AE reporting in this series was limited to those documented within hospital notes. Accordingly, it is possible that AEs were underreported, particularly any that may have occurred outside the hospitalization window.
In this clinical series, patients had been heavily pretreated, had several high-risk features (e.g., advanced disease, high disease burden, and high LDH), and lacked standard-of-care treatment options. The majority (57%) of patients were relapsed/refractory to both PD-1 and CTLA-4 inhibitors. Nevertheless, TIL products generated high ORRs, with similar results among all treated patients and amongst subgroups, including those patients with PD-1 inhibitor-resistant disease (67% and 58%, respectively). The kinetics of response were rapid and durable with 24% of patients in ongoing response >30 months after TIL infusion. The majority of responding patients demonstrated >1 consecutive scan showing ongoing disease response. However, there was not a schedule of assessments that governed the schedule and manner of disease monitoring as is generally used in a clinical trial; thus, confirmation of response per RECIST v1.1 was not possible for all responders.
Notably, approximately 75% of patients with advanced melanoma develop brain metastases [37,38]. Highlighting the unmet need in this population, treatments for brain metastases are limited, and although outcomes have improved with checkpoint inhibitor therapy, treatment is associated with notable morbidity [39,40]. In this series, patients with a history of brain metastases achieved an ORR of 71% (29% CR), supporting existing literature [38,41] and highlighting that TILs may be an effective treatment option for this difficult-to-treat patient population that is frequently excluded from clinical trials. Collectively, these results highlight the successful application of nonselected autologous TILs to address unmet medical need for patients with advanced melanoma, including those refractory to checkpoint inhibitor therapy and targeted therapy.
Unlike off-the-shelf treatments, TIL therapy is a complex multistep and multidisciplinary intervention requiring surgical resection of the tumor, an offsite manufacturing process, and inpatient administration sequenced with lymphodepletion and high-dose IL-2 [19]. Management of rapidly progressing, treatment-refractory melanoma in the midst of the preparation for TILs can be challenging. A number of case studies have been included in this report that illustrate several commonly encountered clinical scenarios and provide evidence that clinical implementation of TIL therapy may be further optimized. First, we detail 2 patients who underwent tumor harvest before PD-1 inhibitor therapy and then went on to receive PD-1 inhibition during TIL manufacturing. One of these patients developed a durable PR to TIL infusion 34 months after tumor resection. The other patient, whose TIL product was administered 22 months after surgical resection, did not have a response to TIL but surprisingly went on to develop durable disease control to repeat exposure to PD-1 blockade despite having previously failed PD-1 inhibition.
These cases suggest that early tumor collection and banking may offer additional options for patients with early or high-risk disease. Notably, digested tumors are freezer-stable long-term, which may enable tumor collection during routine surgery and the potential to manufacture and administer TIL therapy if a patient relapses. Additionally, these results suggest sensitivity to PD-1 inhibitor may be restored by TIL therapy, supporting combination treatment with TILs and PD-1/programmed death-ligand 1 axis blockade.
Tumor flare after BRAF-positive disease progression and discontinuation of BRAF inhibitors often poses a difficult clinical problem [42]. We detail 2 patients in whom TILs were coadministered with dabrafenib. No inherent limitations on efficacy were observed with the combination, and importantly, no added toxicities were seen. Both patients achieved response (1 CR, 1 PR) and subsequently discontinued dabrafenib. These observations are supported by findings observed in a pilot study of TILs in combination with vemurafenib in patients with metastatic melanoma, where no new safety signals emerged, and objective responses were observed [43].
These results of a compassionate use program at a single institution suggest that treatment with nonselected TILs manufactured from both fresh and frozen tumor digests is feasible and may offer significant clinical and logistical benefit to patients with advanced melanoma who otherwise lack standard-of-care treatment options, warranting future prospective clinical trials. Notably, the product herein was manufactured with an earlier version of the ITIL-168 manufacturing process [44]. A global, multicenter, pivotal phase 2 clinical trial of ITIL-168 in adults and pediatric patients aged 12 and above with advanced melanoma who received prior PD-1 inhibitor will build on the observations shared here (DELTA-1; NCT05050006; EudraCT, 2020-003862-37). DELTA-1 and other ongoing and future TIL studies may afford new therapeutic options for patients with significant unmet medical need.
Acknowledgements
The authors would like to thank all the staff within The Christie NHS Foundation Trust and The Christie Clinic (Manchester, United Kingdom) who worked tirelessly to provide high-quality care to all the patients in this report. The authors thank Holly Askew for her support with manufacturing and testing; Catherine Sharpe, PhD, for her support with optimizing methods, updating standard operating procedures and test samples, and contributing to data analysis; Michelle Le Brocq, PhD, for her support with conducting data analyses per standard operating procedures and collating data; Tom Scott, MS, for his support in executing studies to optimize methods and test samples, and contributing to data analyses; Leyuan Bao, PhD, for her contributions with collecting data; and Maggie Chen, PhD, and Paul Robbins, PhD, for their assistance with translational data analysis. Medical writing support was provided by Christopher Waldapfel, PharmD, of Instil Bio, Inc., and Ashley Skorusa, PhD, and Jennifer Yang, PhD, of Nexus Global Group Science with funding from Instil Bio, Inc.
Disclosure of conflict of interest
M. Pillai reports speakers’ bureau participation for Bristol Myers Squibb, Ipsen, Pfizer, and Novartis; and travel support from EUSA Pharma and Bristol Myers Squibb. Y. Jiang, P. Velazquez, D. Chonzi, and Z. J. Roberts report employment with and stock or other ownership in Instil Bio, Inc. P. C. Lorigan reports honoraria from Amgen, Merck, Merck Sharp & Dohme (MSD), NeraCare GmbH, Novartis, Oncology Education, Pierre Fabre, and Roche; consultancy or advisory role for Amgen, Bristol Myers Squibb, MSD, Nektar, Novartis, and Pierre Fabre; speakers’ bureau participation for Bristol Myers Squibb, MSD, Novartis, and Pierre Fabre; research funding from Bristol Myers Squibb; and travel support from Bristol Myers Squibb and MSD. F. C. Thistlethwaite reports consulting or advisory role for Achilles, Bayer, Bristol Myers Squibb, Evelo Therapeutics, GSK, T-knife, and Zelluna Immunotherapy; research funding from Novartis; and serves as the coordinating or local physician investigator for AbbVie, Achilles Ltd, Adaptimmune, Agalimmune, AstraZeneca, AVEO, Bristol Myers Squibb, Chugai Pharmaceutical Co., CytomX, Daiichi Sankyo, GenMab, GSK, Immunocore, Incyte, Janssen, Kymab Ltd, Millennium Pharmaceuticals/Takeda, Novartis, Pfizer, Roche, and Synthon. M. Thomas reports employment with Cellular Therapeutics, Ltd, Immetacyte, Ltd, and Instil Bio, Inc. N. Kirillova reports former employment with and stock or other ownership in Immetacyte, Inc; and stock or other ownership in Instil Bio, Inc. J. S. Bridgeman reports employment with Instil Bio, Inc; stock or other ownership in Immetacyte and Instil Bio, Inc; and patents, royalties, or other intellectual property from Instil Bio, Inc. G. Kueberuwa reports employment with, stock or other ownership in, and patents, royalties or other intellectual property from Instil Bio, Inc. S. Biswas reports employment with Instil Bio, Inc; stock or other ownership in Instil Bio, Inc. and Kite, a Gilead Company; and patents, royalties, or other intellectual property from City of Hope. R. D. Guest reports employment with, stock or other ownership in, and patents, royalties, or other intellectual property from Instil Bio, Inc; and leadership role at Immetacyte, Inc. R. E. Hawkins reports employment with and stock or other ownership in Instil Bio, Inc; consultancy or advisory role for Anaveon AG and NovalGen, Ltd; and nonexecutive director for and stock or other ownership in Bivictrix Plc.
Supporting Information
References
- 1.Hamid O, Robert C, Daud A, Hodi FS, Hwu WJ, Kefford R, Wolchok JD, Hersey P, Joseph RW, Weber JS, Dronca R, Gangadhar TC, Patnaik A, Zarour H, Joshua AM, Gergich K, Elassaiss-Schaap J, Algazi A, Mateus C, Boasberg P, Tumeh PC, Chmielowski B, Ebbinghaus SW, Li XN, Kang SP, Ribas A. Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. N Engl J Med. 2013;369:134–144. doi: 10.1056/NEJMoa1305133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel JC, Akerley W, van den Eertwegh AJ, Lutzky J, Lorigan P, Vaubel JM, Linette GP, Hogg D, Ottensmeier CH, Lebbé C, Peschel C, Quirt I, Clark JI, Wolchok JD, Weber JS, Tian J, Yellin MJ, Nichol GM, Hoos A, Urba WJ. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD, Sosman JA, Atkins MB, Leming PD, Spigel DR, Antonia SJ, Horn L, Drake CG, Pardoll DM, Chen L, Sharfman WH, Anders RA, Taube JM, McMiller TL, Xu H, Korman AJ, Jure-Kunkel M, Agrawal S, McDonald D, Kollia GD, Gupta A, Wigginton JM, Sznol M. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rohaan MW, van den Berg JH, Kvistborg P, Haanen J. Adoptive transfer of tumor-infiltrating lymphocytes in melanoma: a viable treatment option. J Immunother Cancer. 2018;6:102. doi: 10.1186/s40425-018-0391-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Flaherty KT, Infante JR, Daud A, Gonzalez R, Kefford RF, Sosman J, Hamid O, Schuchter L, Cebon J, Ibrahim N, Kudchadkar R, Burris HA 3rd, Falchook G, Algazi A, Lewis K, Long GV, Puzanov I, Lebowitz P, Singh A, Little S, Sun P, Allred A, Ouellet D, Kim KB, Patel K, Weber J. Combined BRAF and MEK inhibition in melanoma with BRAF V600 mutations. N Engl J Med. 2012;367:1694–1703. doi: 10.1056/NEJMoa1210093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Long GV, Menzies AM, Nagrial AM, Haydu LE, Hamilton AL, Mann GJ, Hughes TM, Thompson JF, Scolyer RA, Kefford RF. Prognostic and clinicopathologic associations of oncogenic BRAF in metastatic melanoma. J. Clin. Oncol. 2011;29:1239–1246. doi: 10.1200/JCO.2010.32.4327. [DOI] [PubMed] [Google Scholar]
- 7.Long GV, Stroyakovskiy D, Gogas H, Levchenko E, de Braud F, Larkin J, Garbe C, Jouary T, Hauschild A, Grob JJ, Chiarion Sileni V, Lebbe C, Mandalà M, Millward M, Arance A, Bondarenko I, Haanen JB, Hansson J, Utikal J, Ferraresi V, Kovalenko N, Mohr P, Probachai V, Schadendorf D, Nathan P, Robert C, Ribas A, DeMarini DJ, Irani JG, Casey M, Ouellet D, Martin AM, Le N, Patel K, Flaherty K. Combined BRAF and MEK inhibition versus BRAF inhibition alone in melanoma. N Engl J Med. 2014;371:1877–1888. doi: 10.1056/NEJMoa1406037. [DOI] [PubMed] [Google Scholar]
- 8.Chapman PB, Hauschild A, Robert C, Haanen JB, Ascierto P, Larkin J, Dummer R, Garbe C, Testori A, Maio M, Hogg D, Lorigan P, Lebbe C, Jouary T, Schadendorf D, Ribas A, O’Day SJ, Sosman JA, Kirkwood JM, Eggermont AM, Dreno B, Nolop K, Li J, Nelson B, Hou J, Lee RJ, Flaherty KT, McArthur GA BRIM-3 Study Group. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med. 2011;364:2507–2516. doi: 10.1056/NEJMoa1103782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van den Berg JH, Heemskerk B, van Rooij N, Gomez-Eerland R, Michels S, van Zon M, de Boer R, Bakker NAM, Jorritsma-Smit A, van Buuren MM, Kvistborg P, Spits H, Schotte R, Mallo H, Karger M, van der Hage JA, Wouters MWJM, Pronk LM, Geukes Foppen MH, Blank CU, Beijnen JH, Nuijen B, Schumacher TN, Haanen JBAG. Tumor infiltrating lymphocytes (TIL) therapy in metastatic melanoma: boosting of neoantigen-specific T cell reactivity and long-term follow-up. J Immunother Cancer. 2020;8:e000848. doi: 10.1136/jitc-2020-000848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Besser MJ, Shapira-Frommer R, Itzhaki O, Treves AJ, Zippel DB, Levy D, Kubi A, Shoshani N, Zikich D, Ohayon Y, Ohayon D, Shalmon B, Markel G, Yerushalmi R, Apter S, Ben-Nun A, Ben-Ami E, Shimoni A, Nagler A, Schachter J. Adoptive transfer of tumor-infiltrating lymphocytes in patients with metastatic melanoma: intent-to-treat analysis and efficacy after failure to prior immunotherapies. Clin Cancer Res. 2013;19:4792–4800. doi: 10.1158/1078-0432.CCR-13-0380. [DOI] [PubMed] [Google Scholar]
- 11.Andersen R, Borch TH, Draghi A, Gokuldass A, Rana MAH, Pedersen M, Nielsen M, Kongsted P, Kjeldsen JW, Westergaard MCW, Radic HD, Chamberlain CA, Hölmich LR, Hendel HW, Larsen MS, Met Ö, Svane IM, Donia M. T cells isolated from patients with checkpoint inhibitor-resistant melanoma are functional and can mediate tumor regression. Ann Oncol. 2018;29:1575–1581. doi: 10.1093/annonc/mdy139. [DOI] [PubMed] [Google Scholar]
- 12.Sarnaik AA, Hamid O, Khushalani NI, Lewis KD, Medina T, Kluger HM, Thomas SS, Domingo-Musibay E, Pavlick AC, Whitman ED, Martin-Algarra S, Corrie P, Curti BD, Oláh J, Lutzky J, Weber JS, Larkin JMG, Shi W, Takamura T, Jagasia M, Qin H, Wu X, Chartier C, Graf Finckenstein F, Fardis M, Kirkwood JM, Chesney JA. Lifileucel, a tumor-infiltrating lymphocyte therapy, in metastatic melanoma. J. Clin. Oncol. 2021;39:2656–2666. doi: 10.1200/JCO.21.00612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lu YC, Yao X, Crystal JS, Li YF, El-Gamil M, Gross C, Davis L, Dudley ME, Yang JC, Samuels Y, Rosenberg SA, Robbins PF. Efficient identification of mutated cancer antigens recognized by T cells associated with durable tumor regressions. Clin Cancer Res. 2014;20:3401–3410. doi: 10.1158/1078-0432.CCR-14-0433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rosenberg SA, Yang JC, Sherry RM, Kammula US, Hughes MS, Phan GQ, Citrin DE, Restifo NP, Robbins PF, Wunderlich JR, Morton KE, Laurencot CM, Steinberg SM, White DE, Dudley ME. Durable complete responses in heavily pretreated patients with metastatic melanoma using T-cell transfer immunotherapy. Clin Cancer Res. 2011;17:4550–4557. doi: 10.1158/1078-0432.CCR-11-0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rosenberg SA, Packard BS, Aebersold PM, Solomon D, Topalian SL, Toy ST, Simon P, Lotze MT, Yang JC, Seipp CA, et al. Use of tumor-infiltrating lymphocytes and interleukin-2 in the immunotherapy of patients with metastatic melanoma. A preliminary report. N Engl J Med. 1988;319:1676–1680. doi: 10.1056/NEJM198812223192527. [DOI] [PubMed] [Google Scholar]
- 16.Kradin RL, Kurnick JT, Lazarus DS, Preffer FI, Dubinett SM, Pinto CE, Gifford J, Davidson E, Grove B, Callahan RJ, et al. Tumour-infiltrating lymphocytes and interleukin-2 in treatment of advanced cancer. Lancet. 1989;1:577–580. doi: 10.1016/s0140-6736(89)91609-7. [DOI] [PubMed] [Google Scholar]
- 17.Dafni U, Michielin O, Lluesma SM, Tsourti Z, Polydoropoulou V, Karlis D, Besser MJ, Haanen J, Svane IM, Ohashi PS, Kammula US, Orcurto A, Zimmermann S, Trueb L, Klebanoff CA, Lotze MT, Kandalaft LE, Coukos G. Efficacy of adoptive therapy with tumor-infiltrating lymphocytes and recombinant interleukin-2 in advanced cutaneous melanoma: a systematic review and meta-analysis. Ann Oncol. 2019;30:1902–1913. doi: 10.1093/annonc/mdz398. [DOI] [PubMed] [Google Scholar]
- 18.Puzanov I, Diab A, Abdallah K, Bingham CO 3rd, Brogdon C, Dadu R, Hamad L, Kim S, Lacouture ME, LeBoeuf NR, Lenihan D, Onofrei C, Shannon V, Sharma R, Silk AW, Skondra D, Suarez-Almazor ME, Wang Y, Wiley K, Kaufman HL, Ernstoff MS. Managing toxicities associated with immune checkpoint inhibitors: consensus recommendations from the Society for Immunotherapy of Cancer (SITC) toxicity management working group. J Immunother Cancer. 2017;5:95. doi: 10.1186/s40425-017-0300-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wolf B, Zimmermann S, Arber C, Irving M, Trueb L, Coukos G. Safety and tolerability of adoptive cell therapy in cancer. Drug Saf. 2019;42:315–334. doi: 10.1007/s40264-018-0779-3. [DOI] [PubMed] [Google Scholar]
- 20.Besser MJ, Shapira-Frommer R, Treves AJ, Zippel D, Itzhaki O, Schallmach E, Kubi A, Shalmon B, Hardan I, Catane R, Segal E, Markel G, Apter S, Nun AB, Kuchuk I, Shimoni A, Nagler A, Schachter J. Minimally cultured or selected autologous tumor-infiltrating lymphocytes after a lympho-depleting chemotherapy regimen in metastatic melanoma patients. J Immunother. 2009;32:415–423. doi: 10.1097/CJI.0b013e31819c8bda. [DOI] [PubMed] [Google Scholar]
- 21.Tran KQ, Zhou J, Durflinger KH, Langhan MM, Shelton TE, Wunderlich JR, Robbins PF, Rosenberg SA, Dudley ME. Minimally cultured tumor-infiltrating lymphocytes display optimal characteristics for adoptive cell therapy. J Immunother. 2008;31:742–751. doi: 10.1097/CJI.0b013e31818403d5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dudley ME, Gross CA, Somerville RP, Hong Y, Schaub NP, Rosati SF, White DE, Nathan D, Restifo NP, Steinberg SM, Wunderlich JR, Kammula US, Sherry RM, Yang JC, Phan GQ, Hughes MS, Laurencot CM, Rosenberg SA. Randomized selection design trial evaluating CD8+-enriched versus unselected tumor-infiltrating lymphocytes for adoptive cell therapy for patients with melanoma. J. Clin. Oncol. 2013;31:2152–2159. doi: 10.1200/JCO.2012.46.6441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, Rubinstein L, Shankar L, Dodd L, Kaplan R, Lacombe D, Verweij J. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 24.Speiser DE, Utzschneider DT, Oberle SG, Münz C, Romero P, Zehn D. T cell differentiation in chronic infection and cancer: functional adaptation or exhaustion? Nat Rev Immunol. 2014;14:768–774. doi: 10.1038/nri3740. [DOI] [PubMed] [Google Scholar]
- 25.Gattinoni L, Speiser DE, Lichterfeld M, Bonini C. T memory stem cells in health and disease. Nat Med. 2017;23:18–27. doi: 10.1038/nm.4241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schadendorf D, van Akkooi ACJ, Berking C, Griewank KG, Gutzmer R, Hauschild A, Stang A, Roesch A, Ugurel S. Melanoma. Lancet. 2018;392:971–984. doi: 10.1016/S0140-6736(18)31559-9. [DOI] [PubMed] [Google Scholar]
- 27.Atkins MB, Lee SJ, Chmielowski B, Ribas A, Tarhini AA, Truong TG, Davar D, O’Rourke MA, Curti BD, Brell JM, Kendra KL, Ikeguchi A, Wolchok JD, Kirkwood JM. DREAMseq (doublet, randomized evaluation in advanced melanoma sequencing): a phase III trial-ECOG-ACRIN EA6134. J. Clin. Oncol. 2021;39:356154–356154. [Google Scholar]
- 28.Koya RC, Mok S, Otte N, Blacketor KJ, Comin-Anduix B, Tumeh PC, Minasyan A, Graham NA, Graeber TG, Chodon T, Ribas A. BRAF inhibitor vemurafenib improves the antitumor activity of adoptive cell immunotherapy. Cancer Res. 2012;72:3928–3937. doi: 10.1158/0008-5472.CAN-11-2837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pilon-Thomas S, Kuhn L, Ellwanger S, Janssen W, Royster E, Marzban S, Kudchadkar R, Zager J, Gibney G, Sondak VK, Weber J, Mulé JJ, Sarnaik AA. Efficacy of adoptive cell transfer of tumor-infiltrating lymphocytes after lymphopenia induction for metastatic melanoma. J Immunother. 2012;35:615–620. doi: 10.1097/CJI.0b013e31826e8f5f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sarnaik A, Hall M, Mullinax J, Royster E, Richards A, Crago G, Zager J, Vernon S, Weber J, Pilon-Thomas S. Clinical results of combined vemurafenib and tumor-infiltrating lymphocyte therapy for metastatic melanoma. J Immunother Cancer. 2015;3:P49. [Google Scholar]
- 31.Dean PH, Bucevska M, Strahlendorf C, Verchere C. Pediatric melanoma: a 35-year population-based review. Plast Reconstr Surg Glob Open. 2017;5:e1252. doi: 10.1097/GOX.0000000000001252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ferrari A, Bono A, Baldi M, Collini P, Casanova M, Pennacchioli E, Terenziani M, Marcon I, Santinami M, Bartoli C. Does melanoma behave differently in younger children than in adults? A retrospective study of 33 cases of childhood melanoma from a single institution. Pediatrics. 2005;115:649–654. doi: 10.1542/peds.2004-0471. [DOI] [PubMed] [Google Scholar]
- 33.Arozarena I, Wellbrock C. Phenotype plasticity as enabler of melanoma progression and therapy resistance. Nat Rev Cancer. 2019;19:377–391. doi: 10.1038/s41568-019-0154-4. [DOI] [PubMed] [Google Scholar]
- 34.Alexandrov LB, Nik-Zainal S, Wedge DC, Aparicio SA, Behjati S, Biankin AV, Bignell GR, Bolli N, Borg A, Børresen-Dale AL, Boyault S, Burkhardt B, Butler AP, Caldas C, Davies HR, Desmedt C, Eils R, Eyfjörd JE, Foekens JA, Greaves M, Hosoda F, Hutter B, Ilicic T, Imbeaud S, Imielinski M, Jäger N, Jones DT, Jones D, Knappskog S, Kool M, Lakhani SR, López-Otín C, Martin S, Munshi NC, Nakamura H, Northcott PA, Pajic M, Papaemmanuil E, Paradiso A, Pearson JV, Puente XS, Raine K, Ramakrishna M, Richardson AL, Richter J, Rosenstiel P, Schlesner M, Schumacher TN, Span PN, Teague JW, Totoki Y, Tutt AN, Valdés-Mas R, van Buuren MM, van’t Veer L, Vincent-Salomon A, Waddell N, Yates LR, Zucman-Rossi J, Futreal PA, McDermott U, Lichter P, Meyerson M, Grimmond SM, Siebert R, Campo E, Shibata T, Pfister SM, Campbell PJ, Stratton MR. Signatures of mutational processes in human cancer. Nature. 2013;500:415–421. doi: 10.1038/nature12477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Weichenthal M, Ugurel S, Leiter UM, Satzger I, Kähler KC, Welzel J, Pföhler C, Feldmann-Böddeker I, Meier FE, Terheyden P, Haferkamp S, Herbst R, Ulrich J, Utikal J, Kreuter A, Gutzmer R, Schadendorf D, Mohr P. Salvage therapy after failure from anti-PD-1 single agent treatment: a study by the German ADOReg melanoma registry. J. Clin. Oncol. 2019;37:9505–9505. [Google Scholar]
- 36.Shilyansky J, Nishimura MI, Yannelli JR, Kawakami Y, Jacknin LS, Charmley P, Rosenberg SA. T-cell receptor usage by melanoma-specific clonal and highly oligoclonal tumor-infiltrating lymphocyte lines. Proc Natl Acad Sci U S A. 1994;91:2829–2833. doi: 10.1073/pnas.91.7.2829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Harrison BE, Johnson JL, Clough RW, Halperin EC. Selection of patients with melanoma brain metastases for aggressive treatment. Am J Clin Oncol. 2003;26:354–357. doi: 10.1097/01.COC.0000020963.71379.FE. [DOI] [PubMed] [Google Scholar]
- 38.Hong JJ, Rosenberg SA, Dudley ME, Yang JC, White DE, Butman JA, Sherry RM. Successful treatment of melanoma brain metastases with adoptive cell therapy. Clin Cancer Res. 2010;16:4892–4898. doi: 10.1158/1078-0432.CCR-10-1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tawbi HA, Forsyth PA, Algazi A, Hamid O, Hodi FS, Moschos SJ, Khushalani NI, Lewis K, Lao CD, Postow MA, Atkins MB, Ernstoff MS, Reardon DA, Puzanov I, Kudchadkar RR, Thomas RP, Tarhini A, Pavlick AC, Jiang J, Avila A, Demelo S, Margolin K. Combined nivolumab and ipilimumab in melanoma metastatic to the brain. N Engl J Med. 2018;379:722–730. doi: 10.1056/NEJMoa1805453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kluger HM, Chiang V, Mahajan A, Zito CR, Sznol M, Tran T, Weiss SA, Cohen JV, Yu J, Hegde U, Perrotti E, Anderson G, Ralabate A, Kluger Y, Wei W, Goldberg SB, Jilaveanu LB. Long-term survival of patients with melanoma with active brain metastases treated with pembrolizumab on a phase II trial. J. Clin. Oncol. 2019;37:52–60. doi: 10.1200/JCO.18.00204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mehta GU, Malekzadeh P, Shelton T, White DE, Butman JA, Yang JC, Kammula US, Goff SL, Rosenberg SA, Sherry RM. Outcomes of adoptive cell transfer with tumor-infiltrating lymphocytes for metastatic melanoma patients with and without brain metastases. J Immunother. 2018;41:241–247. doi: 10.1097/CJI.0000000000000223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Keilholz U, Ascierto PA, Dummer R, Robert C, Lorigan P, van Akkooi A, Arance A, Blank CU, Chiarion Sileni V, Donia M, Faries MB, Gaudy-Marqueste C, Gogas H, Grob JJ, Guckenberger M, Haanen J, Hayes AJ, Hoeller C, Lebbé C, Lugowska I, Mandalà M, Márquez-Rodas I, Nathan P, Neyns B, Olofsson Bagge R, Puig S, Rutkowski P, Schilling B, Sondak VK, Tawbi H, Testori A, Michielin O. ESMO consensus conference recommendations on the management of metastatic melanoma: under the auspices of the ESMO Guidelines Committee. Ann Oncol. 2020;31:1435–1448. doi: 10.1016/j.annonc.2020.07.004. [DOI] [PubMed] [Google Scholar]
- 43.Deniger DC, Kwong ML, Pasetto A, Dudley ME, Wunderlich JR, Langhan MM, Lee CR, Rosenberg SA. A pilot trial of the combination of vemurafenib with adoptive cell therapy in patients with metastatic melanoma. Clin Cancer Res. 2017;23:351–362. doi: 10.1158/1078-0432.CCR-16-0906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gastman B, Hamid O, Corrie P, Gibney G, Daniels G, Chmielowski B, Thomas S, Domingo-Musibay E, Lawrence D, Jiang Y, Kennedy A, Aycock J, Alvarez-Rodriguez R, Robbins P, Le Gall J, Roberts Z, Hawkins R, Sarnaik A. DELTA-1: a global, multicenter phase 2 study of ITIL-168, an unrestricted autologous tumor-infiltrating lymphocyte (TIL) cell therapy, in adult patients with advanced cutaneous melanoma. J Immunother Cancer. 2021;9:A573. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.