Abstract
Radiotherapy is a localized treatment commonly used in various types of cancer. However, major limitation of radiotherapy is the development of resistance of tumor cells to radiosensitivity. Cordycepin, a predominant functional component of the Cordyceps sinensis, is considered to use in treating tumor cells. In the present study, we investigated the anticancer effect of the combination of radiation and cordycepin in the treatment of Leydig tumor cells. Results showed that the combination treatment has a synergistic effect significantly suppress cell viability and enhance the radiosensitivity in MA-10 mouse Leydig tumor cells. The combination treatment induced MA-10 cell apoptosis through increasing levels of cleaved caspase-3/-8/-9, poly ADP-ribose polymerase (PARP), and cytochrome c and decreasing levels of B-cell lymphoma 2 (Bcl-2). In addition, prolonged sub-G1 and G2/M arrest accompany with cell cycle-related protein regulation was observed in cells that received the combination treatment. The endoplasmic reticulum (ER) stress-related protein expressions were regulated after MA-10 cells treating with a combination of 100 μM cordycepin and 4 Gy radiation. Furthermore, the combination treatment also decreased the Leydig tumor mass by increasing cell apoptosis in tumor-bearing mice. In conclusion, cordycepin enhances radiosensitivity to induce mouse Leydig tumor cells toward apoptosis in vitro and in vivo. This study will provide a scientific basis for the development of therapeutic regimen of testicular cancer.
Keywords: Cordycepin, radiation, Leydig tumor cell, MA-10 cell, apoptosis, caspase, cell cycle, ER stress
Introduction
Testicular cancer is one of the most common cancers in men between the ages of 15 and 35, with approximately 8,000 cases annually in the United States [1]. Testicular cancer is curable with current medical technology, with a 5-year relative survival rate more than 90% [2]. Most testicular cancers originate in germ cells and are divided into 2 histological groups: seminoma and nonseminoma [3]. Approximately 95% of testicular tumors are germ cell origin, and the other 5% are Sertoli or Leydig cell origin [4]. Tumors on the testis can lead to deterioration of spermatogenesis [5]. In addition, 10% of tumors in adults are malignant and metastatic [6]. Since this type of cancer is more common in young men, the effects on fertility should be considered when treating.
The focus of testicular cancer treatment strategies is to avoid long-term toxicity. Radiation therapy is a localized treatment in the clinical routine that uses high-energy radiation to kill cancer cells and induce single- and double-strand DNA breaks of genome [7]. Previous report has also shown that X-rays and gamma rays can cause indirect damage to biological macromolecules through the production of reactive oxygen species (ROS) [8]. The molecular mechanism of radiation-induced cellular damage depends on many factors, including radiation dose, cell type, and the transformed status of the cells [9]. Exposure of mammalian cells to X-rays results in prolongation of the cell cycle, including delay or arrest in G1, S, and G2 phases. In addition, radiation can cause cells permanent arrest in G2/M phase and lead to apoptosis [10]. Numerous studies have found that radiation-induced DNA damage could activate multiple signaling pathways that lead to cell death and accelerated aging [11]. Exposure of cells to radiation may induce multiple cell death mechanisms including necrosis, apoptosis and autophagy [12]. Many cancer cells including lung, prostate and colon cancer cells lead to apoptosis when exposed to radiation doses of 20 Gy [13]. However, radiation doses more than 4 Gy can affect male fertility, resulting in azoospermia in most men [14]. Therefore, the treatment of testicular cancer still needs to consider other more ideal strategies.
Cordycepin, a 3-deoxyadenosine, is the main functional component of Cordyceps sinensis [15]. Previous studies have shown that cordycepin has anticancer activity without significant toxicity to non-cancer cells [16]. The anticancer effects of cordycepin have been studied intensively in a variety of cancers including glioma, oral cancer, breast cancer, lung cancer, hepatocellular carcinoma, bladder cancer, colorectal cancer, testicular cancer, prostate cancer, melanoma and blood cell cancer [15]. Furthermore, cordycepin can inhibit the growth of cancer cells through cell cycle arrest and apoptosis induction [17]. Since cordycepin and adenosine have similar chemical structures, some findings suggest that cordycepin may have anticancer effects through stimulation of adenosine A3 receptors, subsequent activation of glycogen synthase kinase (GSK)-3β, and inhibition of cyclin D1 [18]. We have previously found that cordycepin could induce MA-10 mouse Leydig tumor cell apoptosis by regulating p38 A mitogen-activated protein kinases (MAPKs), phosphoinositide 3-kinases (PI3K)/protein kinase B (PKB or AKT) and unfolded protein response (UPR) signaling pathways [4,19,20]. Recent studies have shown that cordycepin can increase the radiosensitivity of cervical cancer cells and promote apoptosis [21]. Moreover, cordycepin has also been reported to increase radiosensitivity by inhibiting the repair of potentially lethal DNA damage [22] and by inducing cell cycle arrest in G2/M phase [23]. However, whether cordycepin can enhance the sensitivity of testicular Leydig tumor cells to radiotherapy and the mechanisms behind it have not been investigated.
The cell cycle is a series of events that take place in a cell for DNA replication and division to produce two daughter cells. The link between the cell cycle and cancer is obvious because the cell cycle machinery controls cell proliferation. Cancer, on the other hand, is a disease of inappropriate cellular proliferation [24]. However, the development of cancer is uncontrolled cell division, often associated with a series of changes in the activity of cell cycle regulators. As cells progress from G1 to mitosis, a series of cyclin-dependent kinase (CDK) subunits (CDK4, CDK2, and CDK1) are expressed along with a series of cyclins (D, E, A, and B) [25]. CDK4 (in complex with cyclin D) may respond to growth factors early in G1 phase. CDK2 (maybe in complex with cyclin E or cyclin A or both) is essential for DNA replication in S phase. CDK1 (in complex with cyclins A and B) is required for G2/M mitosis. Efforts to alter the regulation of the cell cycle have the potential to be a strategy for cancer treatment.
There are two major signaling pathways involved in apoptosis, including the death receptor (extrinsic) pathway and the mitochondrial (intrinsic) pathway [26]. The extrinsic pathway is initiated by the binding of death receptors to death ligands. Activation of death receptors results in the formation of Fas-associated death domain (FADD) and receptor-associated complexes of caspase-8 and -10, triggering activation of caspase-8/-10 followed by cleavage of caspase-3 and -7 for apoptosis start [27]. Intrinsic pathway signaling cascades can be triggered by a combination of DNA damage, hypoxia, growth factor deprivation, and endoplasmic reticulum (ER) stress [28]. The death signal can activate downstream mediators and cause mitochondria to release the apoptotic compound, cytochrome c. Released cytochrome c binds to apoptotic protease activating factor 1 (APAF-1) to form apoptotic bodies that activate the caspase 9 precursor [29]. Caspase 9 activates effector caspases 3, 6 or 7 and ultimately leads to apoptosis [30]. Both extrinsic and intrinsic pathways can induce caspase 3 cleavage, which in turn cleave poly ADP-ribose polymerase (PARP), rendering it incapable of repairing DNA [31].
ER is the site of protein synthesis and modification and plays an important role in the correct regulation of protein folding in cells. Any disturbance that affects the normal biological function of the ER may lead to the accumulation of misfiled proteins and cause ER stress. When the ER stress exceeds the threshold, the signaling network of the UPR will be activated within the ER to relieve this stress and promote cell survival [32]. Conversely, some studies suggest that UPR may promote apoptosis [33]. The UPR is initiated by the action of three signaling proteins including inositol-1α (IRE1α), protein kinase RNA (PKR)-like ER kinase (PERK) and activating transcription factor-6 (ATF6) [34]. Normally, PERK, ATF6 and IRE1 associate with the chaperone glucose-regulated protein-78 (GRP78)/immunoglobulin heavy chain binding protein (BiP), but as unfolded proteins accumulate and GRP78/BiP dissociates, thereby activating PERK, ATF6 and IRE1 [35]. Activation of PERK may lead to phosphorylation of eukaryotic translation initiation factor 2 (eIF2), which reduces translation and inhibits protein synthesis [36]. P90ATF6 will be transported to the Golgi and cleaved by site 1 and 2 proteases to generate the active transcription factor, P50ATF6 [37]. However, activation of the PERK-eIF2 pathway also induces the transcription factor ATF4 [38]. ATF4 then induces the expression of pro-apoptotic CCAAT/enhancer-binding protein-homologous protein (CHOP) by activating the amino acid response element (AARE) and ATF6 pathways [39], while the IRE1 pathway may also induce the expression of CHOP leading to apoptosis [40]. In addition, IRE1α stimulates downstream apoptosis-signaling kinase 1 (ASK1) and then promotes activation of the kinase Jun-N-terminal kinase (JNK), which can also promote apoptosis [41].
In the present study, we investigated the anticancer effect of the combination treatment of radiation and cordycepin on mouse Leydig tumor cells in vitro and in vivo. We hypothesized that the combination treatment of cordycepin and radiation could cause synergistic cytotoxic effects, that cordycepin could increase the radiosensitivity to promote cell death in mouse Leydig tumor cells. The type of cell death and the underlying mechanisms were examined.
Materials and methods
Cell culture
The MA-10 mouse Leydig tumor cell line was a gift from Dr. Mario Ascoli (University of Iowa, Iowa City, IA, USA). The cells were cultured in the Waymouth’s MB 752/1 medium supplemented with 20 mM HEPES, 1.12 g/L NaHCO3 and 10% fetal bovine serum (FBS) at 37°C in a humidified environment containing 5% CO2 for all the following experiments.
Chemicals
Cordycepin was purchased from Sigma-Aldrich (St. Louis, MO, USA). Dimethyl sulfoxide (DMSO) was purchased from Merck (Darmstadt, Germany). To make a cordycepin solution, DMSO was used to dissolve the cordycepin in final concentration (80 mM) for stock. The stock solution is then further diluted with cell culture medium to working concentrations for experimental use.
Irradiation treatment
Irradiation was performed with 6 MV X-rays using a linear accelerator, Digital M Mevatron Accelerator (Siemens Medical Systems, Malvern, PA, USA), at a dose rate of 5 Gy/min. An additional 2 cm of a tissue-equivalent bolus was placed on the top of the plastic tissue-culture flask or experimental mouse to ensure electronic equilibrium, and 10 cm of tissue-equivalent material was placed under the flask or mouse to obtain full backscatter.
Cell viability test
Cell viability was assessed with Methylthiazoletetrazolium (MTT) assay. MA-10 cells were seeded in 96-well plates containing 1 × 104 cells per well. After 70-80% confluence, cells were treated without or with different concentrations of cordycepin (10, 25, 50 and 100 µM) for 24 and 48 hr, respectively. MTT were following added at different time points with the final concentration of 0.5 mg/ml, and then incubated at 37°C for 4 hr. The medium was removed and DMSO (50 μl) was added into each well to dissolve the crystals by shaking the plate weakly for 20 min in dark. The optical density (O.D.) values in each treatment were then be determined at λ = 570 nm by VersaMax enzyme-linked immunosorbent assay (ELISA) reader (Molecular Devices, LLC, Sunnyvale, CA, USA).
Morphology observation
MA-10 cells were seed at a concentration of 5 × 105 cells/ml and 3 × 105 cells/ml in 25T flask with 4 ml culture medium, and treated without or with different concentrations of cordycepin (10, 25, 50 and 100 µM) and radiation (2, 4, 6 and 8 Gy) for 24 hr. The morphology of cells was examined using Olympus CK40 light microscope (Olympus Corporation, Tokyo, Japan) and images were captured by Olympus DP20 digital camera (Olympus Corporation, Tokyo, Japan).
Trypan blue exclusion test
MA-10 cells were seeded in 25T flask at a density of 5 × 105 cells/flask. The cells were treated without or with different concentrations of cordycepin (0, 10, 25, 50, 100 and 200 μM), different strength of radiation (0, 2, 4, 6 and 8 Gy) and the combination treatments for 24 hr. The treated cells were then suspended by trypsin and mixed with an equal volume of 0.4% trypan blue solution. The stained (dead) and unstained (live) cells were counted on a hemocytometer, and the cell viability was calculated as the percentage of live cells in the sample.
Synergistic interaction analysis
The effect of the combination treatment of cordycepin and radiation was assessed by the combination index (CI) method using CalcuSyn software, which is based on the median effect model as described previously [42]. The experimental data were applied into the CalcuSyn interface and CI values were computed. CI<1, CI = 1, and CI>1 indicate synergism, additive effect, and antagonism, respectively.
Clonogenic assay
MA-10 cells were irradiated under the dosages of 2, 4, 6 or 8 Gy and cordycepin were added to the cells at concentrations of 25 or 50 μM. The cells were then trypsinized and counted. Known numbers of cells were subsequently re-plated in 6-cm culture dishes and returned to the incubator to allow for colony development. After 8 days, colonies (containing ≥50 cells) were stained with 0.5% crystal violet solution. The plating efficiency (PE) is the ratio of the number of colonies to the number of cells seeded in the non-irradiated group. The surviving fraction (SF) was calculated as follows: SF = plating efficiency (PE) of treated cells/PE of control cells. PE (%) was obtained from (colonies counted/cell plated) × 100. Survival curves were then be plotted using the mean surviving fraction values.
Cell cycle analysis
The distribution of cell cycle was determined by propidium iodine (PI) staining through flow cytometric analysis. Briefly, MA-10 cells were treated without or with 25 µM cordycepin for 3, 6, 9, 12 and 24 hr, and 4 Gy radiation for 3, 6, 9, 12 and 24 hr. Cells were then detached by using 1% trypsin and fixed with 70% ethanol at 4°C for 1 day. Fixed cells were mixed with 100 μg/ml RNase and then stained with 40 μg/ml PI solution for 30 min and finally analyzed using FACScan flow cytometer (Becton-Dickinson, Mountain View, CA, USA) with an excitation wavelength of 488 nm and band pass filters of 600 nm. Cells in subG1 phase have less DNA contents on cell cycle distribution, which is considered to be DNA fragmentation and as an outcome of cell apoptosis.
Annexin V/PI double staining assay
MA-10 cells were treated without or with 25 μM cordycepin and/or 4 Gy radiation for 24, 48 and 72 hr, respectively. Cellular apoptosis was detected by Fluorescein Isothiocyanate (FITC) Annexin V Apoptosis Detection Kit (BD Pharmingen™, San Diego, CA, USA) according to manufacturer’s instruction. Samples were analyzed by FACScan flow cytometer (Becton-Dickinson, Mountain View, CA, USA) with an excitation wavelength of 488 nm and band pass filters of 515 and 600 nm for FITC and PI detection, respectively. Data were represented by histogram plots gated into four quadrants containing double-negative (Annexin V-/PI-), PI single positive (Annexin V-/PI+), Annexin V single positive (Annexin V+/PI-) and double positive (Annexin V+/PI+) stained cells, which corresponded to viable, necrotic, early apoptotic and late apoptotic cells, respectively.
Protein extraction and western blotting
Treated cells were lysed with 100 μl lysis buffer (containing 20 mM Tris at pH 7.5, 150 mM NaCl, 1 mM EDTA, 1 mM EGTA, 1% Triton X-100, 2.5 mM sodium pyrophosphate and 1 mM sodium orthovanadate) with proteinase inhibitor for 30 min at room temperature. Lysates were centrifuged at 12,000 rpm for 12 min at 4°C, and supernatants were collected and stored at -20°C until future analysis. Protein concentrations of cell lysates were determined by Bio-Rad protein assay dye reagent concentrate (Hercules, CA, USA). For western blotting, protein samples (25 μg/lane) were separated by 12% sodium dodecyl sulfate-polyacrylamide gel (SDS-PAGE) that performed at 100 V for 2.5 hr with standard running buffer (24 mM Tris-HCl, 0.19 M glycine, 0.5% SDS, pH 8.3) at room temperature and transferred onto polyvinylidene difluoride (PVDF) membrane at 400 mA for 4 hr in transfer buffer (20 mM Tris-HCl, 150 mM glycine, 10% methanol, 0.01% SDS). The membrane was blocked with 5% nonfat milk, washed by tris-buffered saline with 0.1% Tween 20 detergent (TBST) (20 mM Tris base, 137 Mm NaCl, 0.1% Tween 20, pH 7.6), and subsequently incubated with various primary antibodies overnight at 4°C. After washing, the membrane was incubated with the appropriate horseradish peroxidase (HRP)-conjugated secondary antibodies for 1 hr at room temperature. After washing, bands were detected using enhanced chemiluminescence (ECL) substrate and the UVP EC3 BioImaging system (Phoenix, AZ, USA). The optical density of each protein band was quantitated by using ImageJ version 1.50 software (National Institutes of Health, Bethesda, MD, USA). The amount of β-actin in each lane was detected as a control to correct the expression of various proteins. All antibodies used in western blotting were listed in Table 1.
Table 1.
Antibodies used in this study
| Antibody | Manufacturer | Catalogue No. | Species | Applicationa |
|---|---|---|---|---|
| ATF-6β | ThermoFisher | PA5-83845 | Rabbit | WB |
| β-actin | Sigma-Aldrich | A5441 | Mouse | WB |
| Bax | Cell Signaling | #2772 | Rabbit | WB |
| Bcl-2 | Proteintech | 12789-1-AP | Rabbit | WB |
| CD31 | Spring | M3382 | Rabbit | IHC |
| CDK1 | AbCam | ab18 | Mouse | WB |
| CDK2 | Abcam | ab32147 | Rabbit | WB |
| CDK4 | AbCam | ab137675 | Rabbit | WB |
| CHOP | Santa Cruz | AP11955b | Rabbit | WB |
| Cleaved caspase-3 | Cell Signaling | #9661 | Rabbit | WB/IHC |
| Cleaved caspase-8 | Cell Signaling | #9429 | Mouse | WB |
| Cleaved caspase-9 | Cell Signaling | #9509 | Rabbit | WB |
| Cyclin A | Santa Cruz | sc-271682 | Mouse | WB |
| Cyclin B1 | Abcam | ab181593 | Rabbit | WB |
| Cyclin D1 | Abcam | ab16663 | Rabbit | WB |
| Cyclin E1 | Cell Signaling | #20808 | Rabbit | WB |
| Cytochrome c | Cell Signaling | #4272 | Rabbit | WB |
| GRP78 | Santa Cruz | sc-166490 | Mouse | WB |
| PARP | Cell Signaling | #9532 | Rabbit | WB |
| Phosphor-EIF-2α | Cell Signaling | #3398 | Rabbit | WB |
| PERK | Cell Signaling | #3192 | Rabbit | WB |
| Phosphor-IRE1α | Abcam | ab48187 | Rabbit | WB |
| Anti-mouse IgG HRP-conjugated | PerkinElmer | NEF82200-1EA | Goat | WB |
| Anti-rabbit IgG HRP-conjugated | PerkinElmer | NEF81200-1EA | Goat | WB |
WB: Western blotting;
IHC: Immunohistochemistry.
Animal experiments
Male 5- to 7-week-old C57BL/6 mice were purchased from the National Laboratory Animal Breeding and Research Center (Taipei, Taiwan). All animals were housed at the laboratory animal facility (National Cheng-Kung University, Tainan, Taiwan). All mouse experiment protocols were reviewed and approved by the Institutional Animal Care and Use Committee (IACUC) of National Cheng Kung University (IACUC Approval No. 110095). Mice were subcutaneously inoculated with MA-10 cells (7.5 × 105 cells in 0.1 ml of phosphate buffered saline (PBS)) into the right back. 7 days post inoculation, all mice were randomized into 5 groups (n = 5 for each group) as below. In the control group, mice were intraperitoneally (i.p.) inoculated with PBS. In the cordycepin group, mice were administrated with 20 mg/kg cordycepin through i.p. route thrice a week for 2 weeks. In the irradiation (IR) group, mice received a single dose of 4 Gy irradiation. In the Cordycepin + IR group, mice received a single dose of 4 Gy irradiation combined with 20 mg/kg cordycepin through i.p. route thrice a week for 2 weeks. Tumor size and body weight were measured 2-3 days per week. Tumor volume was calculated by the formula: 0.52 × length × width × width. When mice were sacrificed, MA-10 tumor tissues were collected, weighted, and photographed. Tissue samples were then fixed by a 4% paraformaldehyde solution and embedded by paraffin for immunohistochemistry.
Immunohistochemistry
Tumor tissue sections were dewaxed by xylene, dehydrated by ethanol, and blocked endogenous peroxidase activity by incubating with 0.3% H2O2. The sections were infiltrated in sodium citrate buffer (10 mM sodium citrate and 0.05% tween 20, pH 6.0) for 50 min at 120°C autoclave for antigen retrieval. Samples were then blocked with 2% nonfat milk for 1 hr and incubated with primary antibodies at 4°C overnight. Signals were visualized using HRP-conjugated secondary antibody and the chromogenic substrate 3,3-diaminobenzidine (DAB). The sections were then counterstained with hematoxylin and mounted under coverslips. Some tissue sections were stained with hematoxylin and eosin for determining the histopathological changes. All antibodies used in immunohistochemistry were listed in Table 1.
Statistical analysis
Data were expressed as mean ± standard error of the mean (SEM) from three independent experiments. One-way or Two-way ANOVA analysis of variance with the least significant difference test was used to assess the difference among groups or between control and treatments. Statistical analysis was performed using GraphPad Prism version 9 (GraphPad Software, Inc., La Jolla, CA, USA). The significant difference was considered as P<0.05 in all experiments.
Results
Cordycepin reduced MA-10 cell growth ability in time- and dose-dependent manners
The effect of cordycepin on Leydig tumor cell growth was first analyzed with the MTT assay. MA-10 cells were treated with 0, 10, 25, 50, 100 and 200 μM cordycepin for 24 hr (Figure 1A) and 48 hr (Figure 1B). Compared with the untreated control group, the cell viability of MA-10 cells decreased sequentially as the drug concentration increased. At 24 hr post cordycepin treatment, cell viability decreased sequentially from approximately 90% (10 μM cordycepin treatment group) to approximately 50% (≥100 μM cordycepin treatment groups) (Figure 1A). At 48 hr post cordycepin treatment, the cell growth inhibitory effect was more violent than that at 24 hours, and the cell viability decreased sequentially from approximately 70% (10 μM cordycepin treatment group) to approximately 30% (≥50 μM, cordycepin treatment groups) (Figure 1B). These results indicated that the cell growth ability significantly reduced by cordycepin in time- and dose-dependent manners in MA-10 cells.
Figure 1.
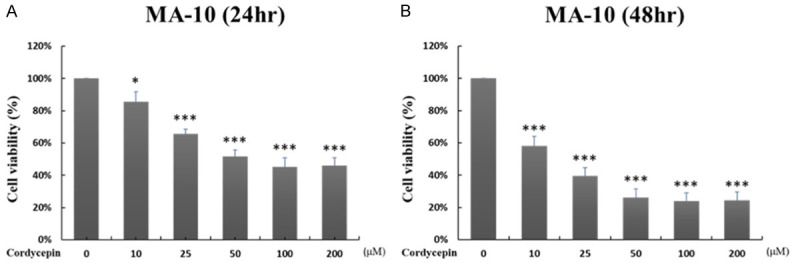
Cordycepin reduced MA-10 growth ability in time- and dose-dependent manners. MA-10 cells were treated with 10, 25, 50, 100 and 200 μM cordycepin for 24 hr (A), and for 48 hr (B), respectively. Cell viabilities were examined by MTT viability test. Results were presented as percentages of cell growth of each group relative to control group. *P<0.05, and ***P<0.001 indicate significant statistical difference compared to the control group.
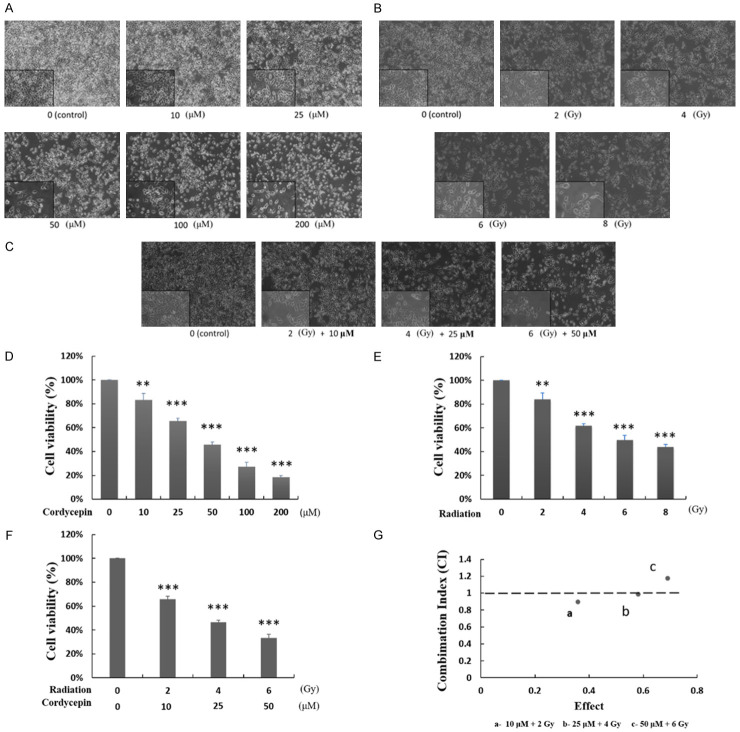
Cordycepin, radiation, and the combination treatment caused morphological changes related to cell death upon MA-10 cells
To determine the cell toxicity of cordycepin, radiation, and the combination effect of cordycepin and radiation, MA-10 cells were treated without or with cordycepin alone (10-200 μM), radiation alone (2-8 Gy), and in combination settings for 24 hr, respectively. The morphology of cells was examined using light microscope and images were captured by digital camera (Figure 2A-C). Untreated MA-10 cells were firmly attached and exhibited the commonly anticipated polygonal-shaped morphology on the cell culture flask. Conversely, cells treated with cordycepin alone (Figure 2A) or radiation alone (Figure 2B) became gradually rounded and more detached as the dose increased. Furthermore, in the combination treatment groups, viable cell density reduction, and cell membrane blebbing and shrinkage were more obvious than in the single treatment groups (Figure 2C). Thus, both single treatments and combination treatments can cause cytotoxicity to MA-10 cells, and the effect of combination treatment is stronger than that of single treatment suggesting that combining two treatments may have an additive or synergistic effect.
Figure 2.
Cordycepin, radiation, and the combination treatment decreased MA-10 cell viability in dose-dependent manners. MA-10 cells were treated with 0, 10, 25, 50, 100 and 200 μM cordycepin for 24 hr (A, D), with 0, 2, 4, 6 and 8 Gy radiation for 24 hr (B, E), and with the combination treatments of cordycepin and radiation for 24 hr (C, F), respectively. The morphology of cells was examined using light microscope and images were captured by digital camera (A-C). Cell viabilities were examined by Trypan blue exclusion test (D-F). Results were presented as percentages of cell growth relative to control groups. CalcuSyn software analysis was used to determine the synergistic effects of combination treatment of cordycepin and radiation on MA-10 cells (G). **P<0.01, and ***P<0.001 indicate significant statistical difference compared to the control group. The data points at “a” and “b” indicate the synergies.
The combination treatment of cordycepin and radiation induced synergistic effect on reducing cell viability
To further confirm the cell death inducing ability of cordycepin alone, radiation alone and the combination treatment in mouse Leydig tumor cells, the Trypan blue exclusion assay was used to analyze cell viability. The results showed that increasing doses of cordycepin (10-200 μM) and radiation (2-8 Gy) for 24 hr significantly decreased MA-10 cell viability in a dose-dependent manner (Figure 2D, 2E) (P<0.01). Moreover, the combination treatment of cordycepin and radiation at 10 μM + 2 Gy, 25 μM + 4 Gy, and 50 μM + 6 Gy conditions significantly reduced cell viability to 67%, 45%, and 36% at 24 hr, respectively (Figure 2F) (P<0.001). The cytotoxicity effect of the combination treatments was more intense compared to each single treatment. To further determine whether the combination treatment of cordycepin and radiation has a synergistic effect, the CaluSyn2.0 program was used. The results demonstrated that the combination index (CI) values at 10 μM + 2 Gy, 25 μM + 4 Gy, and 50 μM + 6 Gy conditions were 0.898 (point a), 0.985 (point b) and 1.174 (point c), respectively (Figure 2G). The CI values at point a and b were less than 1 indicating that the combination treatments at 10 μM + 2 Gy and 25 μM + 4 Gy conditions have a synergistic effect on reducing cell viability. Thus, 25 μM cordycepin plus 4 Gy radiation treatment condition was selected for further mechanism investigation in this study.
Cordycepin enhanced radiosensitivity in MA-10 cells
To verify whether cordycepin could enhance radiosensitivity in MA-10 cells, radiation dose-response survival curves were examined using clonogenic assay (Figure 3). The survival curves of MA-10 cells treated with cordycepin plus radiation were compared with those of cells treated with radiation alone. Data showed that the combination treatment of cordycepin and radiation resulted in significantly decreased survival fractions compared to cells treated with radiation alone (P<0.05) (Figure 3B). Thus, these results suggested that cordycepin could enhance radiosensitivity in MA-10 mouse Leydig tumor cells.
Figure 3.
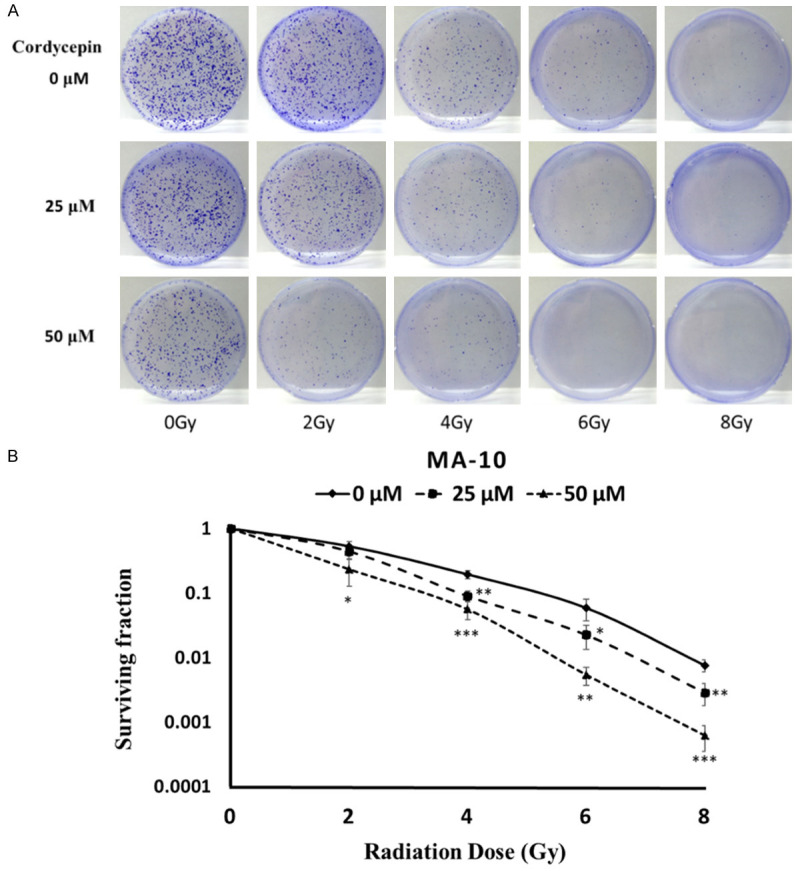
Cordycepin enhanced radiosensitivity in MA-10 cells. MA-10 cells were treated with radiation (0, 2, 4, 6 and 8 Gy) combined with different concentrations of cordycepin (0, 25 and 50 μM). Cells were plated in 6 cm dishes for 8 days. Dishes were stained with crystal violet (A). Colonies containing >50 cells were scored as positive and then the radiation dose-response survival curves were determined (B). The data represent the mean ± S.D. *P<0.05, **P<0.01, and ***P<0.001 indicate significant statistical difference compared to the control group.
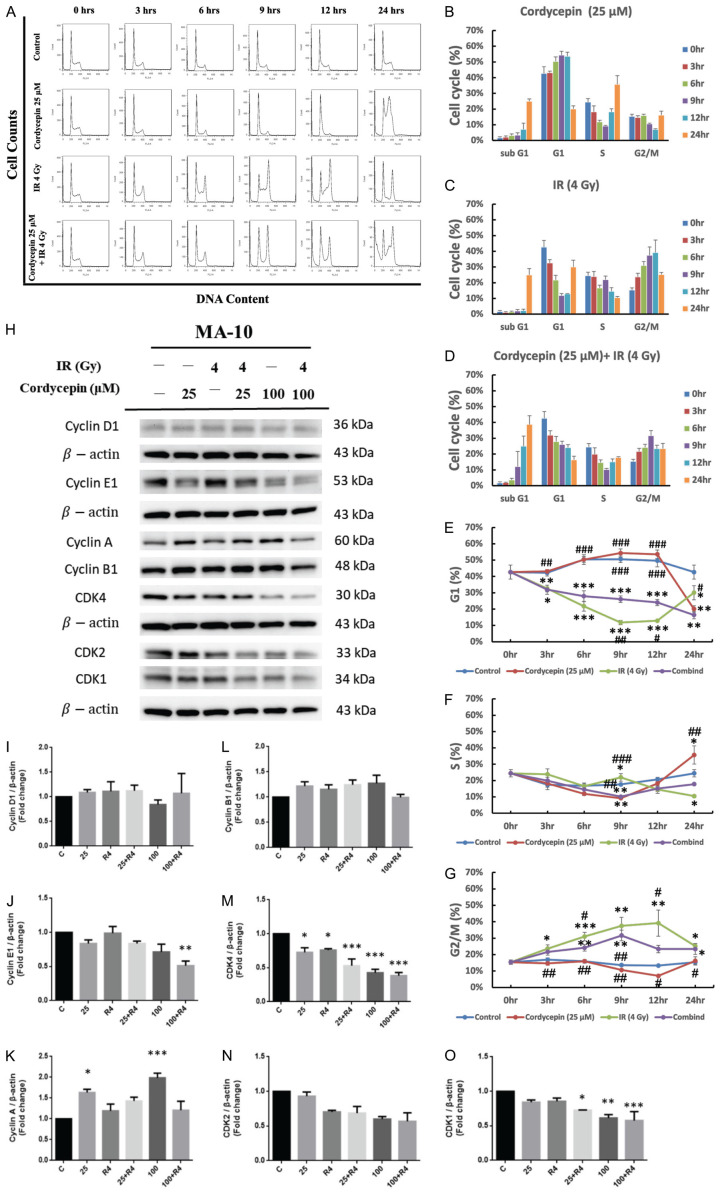
The combination treatment of cordycepin and radiation regulated cell cycle distribution in MA-10 cells
Since radiation combined with cordycepin can reduce MA-10 cell viability, we further determined whether the combination treatments induce cell death through cell cycle arrest. MA-10 cells were treated with 4 Gy radiation alone, 50 μM cordycepin alone, or the combination of both, and the percentages of each cell cycle fraction were observed at different time points (0, 3, 6, 9, 12, and 24 hr) after treatment. The time kinetic curves of the cell cycle were shown in Figure 4A. The populations of MA-10 cells were increased in sub-G1 phase and S phase but decreased in G1 phase at 24 hr post cordycepin alone treatment (Figure 4B). After treating with radiation alone, the populations of MA-10 cells were increased in sub-G1 phase at 24 hr, and G2/M phase from 3 to 12 hr, but decreased in G1 phase from 3 to 12 hr (Figure 4C). As shown in Figure 4D, there was a significant amount of cell accumulation at sub-G1 and G2/M phase in MA-10 cells treated with 4 Gy irradiation plus 50 μM cordycepin. Figure 4E-G shows the time kinetic curves of the G1, S, and G2/M phase of 4 Gy radiation alone, 50 μM cordycepin alone, and the combination treatment on MA-10 cells. The percentage of MA-10 cells in G1 phase significantly decreased after treating with cordycepin at 24 hr, radiation from 3 to 24 hr and the combination treatment from 3 to 24 hr, respectively (Figure 4E). The percentage of MA-10 cells in S phase significantly decreased after treating with cordycepin at 9 hr, radiation at 24 hr and the combination treatment at 9 hr, but significantly increased after treating with cordycepin at 24 hr, respectively (Figure 4F). In addition, G2/M phase arrest could be observed in radiation alone and combination treatment groups which maximized at 12 hr and 9 hr, respectively (Figure 4G). Taken together, the combination treatment of radiation and cordycepin significantly increased subG1 and G2/M phase cell numbers but decrease S and G1 phase cell numbers in MA-10 cells, and prolonged sub-G1 arrest was observed in cells that received the combination treatment compared to those that received cordycepin alone or irradiation alone.
Figure 4.
Cordycepin, radiation, and the combination treatment regulated cell cycle distribution in MA-10 cells. MA-10 cells were treated without or with 25 μM cordycepin and 4 Gy radiation for 3, 6, 9, 12 and 24 hr, respectively. Cells were fixed and then stained with propidium iodide (PI), and cell cycle was measured by flow cytometry. The distribution and percentage of cells in sub G1, G1, S and G2/M phase of the cell cycle are illustrated (A-G). MA-10 cells were treated without or with different concentrations of cordycepin (25 and 100 µM) and radiation (4 Gy) for 24 hr, respectively. Cyclin D1, Cyclin E1, Cyclin A, Cyclin B1, CDK4, CDK2 and CDK1 were detected by western blotting (H-O). *P<0.05, **P<0.01, and ***P<0.001 indicate significant statistical difference compared to the control group. #P<0.05, ##P<0.01 and ###P<0.001 indicate significant statistical difference compared to the combination treatment group.
The combination treatment of cordycepin and radiation regulated cell cycle related protein expression to modulate cell cycle distribution in MA-10 cells
A succession of kinase subunits (CDK4, CDK2, and CDK1) is expressed along with a succession of cyclins (D, E, A, and B) as the cells progress from G1 to mitosis. To further determine whether cordycepin, radiation and the combination treatment could affect cell cycle by regulating cell cycle related proteins, western blotting was used to analyze the expression of Cyclin D1, Cyclin E1, Cyclin A, Cyclin B1, CDK4, CDK2 and CDK1 (Figure 4H). Cyclin B and CDK1 play an important role in G2/M phase transition. Although no significant changes on cyclin B1 expression in all groups, but the expression of CDK1 significantly decreased in 100 μM cordycepin treatment group and combination treatment groups suggesting the G2/M arrest phenomenon which could suppress tumor growth by preventing proper mitosis (Figure 4H, 4L, 4O). Cyclin A and CDK2 are two key regulators of S phase, the results demonstrated that the expressions of cyclin A were significantly increased after treating with cordycepin alone (25 μM and 50 μM), but CDK2 levels showed no significantly changes in all groups (Figure 4H, 4K, 4N). This regulation may cause S to G2 phase transition. Cyclin D, Cyclin E, CDK2, and CDK4 are crucial for maintaining G1 phase. Although no significant changes on cyclin B1 and CDK2 expression in all groups, but cyclin E1 and CDK4 expressions were significantly decreased after combined treating with cordycepin and radiation (P<0.05) suggesting the decrease of percentages of MA-10 cell numbers in G1 phase (Figure 4H, 4J, 4M, 4N). Therefore, cordycepin, radiation, and the combination treatment could affect cell cycle by regulating cell cycle related protein expressions to induce MA-10 cell death.
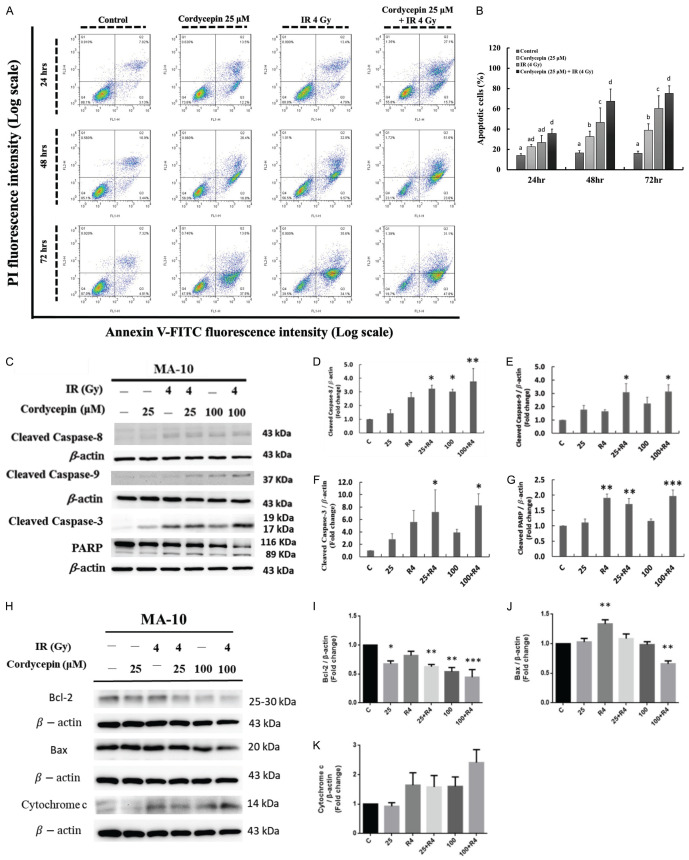
The combination treatment of cordycepin and radiation induced apoptosis in MA-10 cells
To determine whether the apoptotic pathway was involved in cordycepin-, radiation- and the combination treatment-induced cytotoxicity effects in MA-10 cells, annexin V/PI double staining assay was used and analyzed through flow cytometry assay. It is well established that percentages of double-negative cells (viable), annexin V single positive cells (early apoptotic), PI single positive cells (necrotic), and double positive cells (late apoptotic) could be illustrated in four quadrants by this double staining assay to investigate apoptosis [43]. MA-10 cells were treated with 25 μM cordycepin, 4 Gy radiation or radiation plus cordycepin (4 Gy + 25 μM) for 24, 48 and 72 hr, respectively, and the apoptosis effects were analyzed. The results showed that both single treatments and combination treatment significantly induced apoptosis (early plus late apoptosis) of MA-10 cells compared to the control untreated group (P<0.05) (Figure 5A, 5B). Furthermore, the combination treatment of 4 Gy radiation and 25 μM cordycepin for 48 and 72 hr significantly induced more MA-10 cells undergoing apoptosis (70 and 75%, respectively) compared to radiation (47 and 63%, respectively) or cordycepin (37 and 41%, respectively) single treatment (P<0.05) (Figure 5A, 5B). These results illustrate that cordycepin enhanced MA-10 mouse Leydig tumor cell radiosensitivity finally causing apoptosis.
Figure 5.
Cordycepin and radiation induced cell apoptosis in MA-10 cells. MA-10 cells were treated without or with 25 or 50 μM cordycepin and/or 4 Gy radiation for 24, 48 and 72 hr, respectively, and stained with annexin V and propidium iodide (PI). The original density plots of Flow Cytometry analysis were showed (A). Data were analyzed by two-way ANOVA with Tukey’s multiple comparisons test. Different superscripts above each column indicated significant difference among each treatment (P<0.05) (B). Apoptosis related protein were detected by western blotting and normalized with β-actin (43 kDa) in each lane (C-K). *P<0.05, **P<0.01, and ***P<0.001 indicate significant statistical difference compared to the control group.
The combination treatment of cordycepin and radiation induced apoptosis through caspase cascade in MA-10 cells
Caspases, a family of aspartic acid-specific proteases, are the major effectors of apoptosis [44]. To examine whether cordycepin and/or radiation could induce cell apoptosis by activating caspase pathway, MA-10 cells were treated without or with 25 or 100 μM cordycepin and/or 4 Gy radiation for 24 hr, repectively. The cleavages of caspase-8 (death receptor pathway), caspase-9 (mitochondrial pathway), caspase-3 and PARP were determined by western blotting. The results demonstrated that cleaved caspase-8 levels significantly increased after treatment with 100 μM cordycepin and the combination treatments of 25 or 100 μM cordycepin and 4 Gy radiation for 24 hr, respectively (P<0.05) (Figure 5C, 5D). The expression of cleaved caspase-9 (Figure 5C, 5E) and cleaved caspase-3 (Figure 5C, 5F) were also increased significantly following the combination treatments of 25 or 100 μM cordycepin and 4 Gy radiation for 24 hr (P<0.05). In addition, cleaved PARP (89 kDa) significantly increased after treatment with 4 Gy radiation and the combination treatments of 25 or 100 μM cordycepin and 4 Gy radiation for 24 hr, respectively (Figure 5C, 5G) (P<0.05). These results showed that combination treatments significantly increased cleaved caspase-3/-8/-9 and PARP protein expressions in MA-10 cells indicating that the combination treatment-induced cell death is through caspase-dependent apoptosis pathway.
The combination treatment of cordycepin and radiation regulated apoptosis-related protein expressions to induce MA-10 cell apoptosis
The Bcl-2 family protein involved in the regulation of apoptotic cell death consisting of anti-apoptotic and pro-apoptotic members. The anti-apoptotic members of this family such as Bcl-2 could prevent cytochrome c releasing from mitochondria into the cytoplasm to activate caspases and cause apoptosis. In contrast, pro-apoptotic members of this family such as Bax could induce mitochondria to release cytochrome c into the cytoplasm, thereby leading to caspase activation [45]. Thus, apoptosis-related protein expressions including Bcl-2, Bax, and total cytochrome c were also analyzed (Figure 5C-K) to further confirm the apoptosis phenomenon induced by the combination treatments. In MA-10 cells, Bcl-2 levels significantly decreased after treatment with 25 and 100 μM cordycepin and the combination treatments of 25 or 100 μM cordycepin and 4 Gy radiation for 24 hr, respectively (P<0.05) (Figure 5H, 5I).
The results also showed that the levels of total cytochrome c were increased in MA-10 cells following the combination treatment of 100 μM cordycepin and 4 Gy radiation compared to each single treatment group (Figure 5H, 5K). Unexpectedly, the combination treatment of 100 μM cordycepin and 4 Gy radiation significantly decreased, but 4 Gy radiation single treatment significantly increased the expression of Bax, respectively (Figure 5H, 5J) indicating that Bax may not play a pro-apoptotic role in combination treatment to induce MA-10 cell apoptosis. Therefore, the combination treatments of cordycepin and radiation could activate caspase via the decrease on Bcl-2 and increase on total cytochrome c to induce apoptosis.
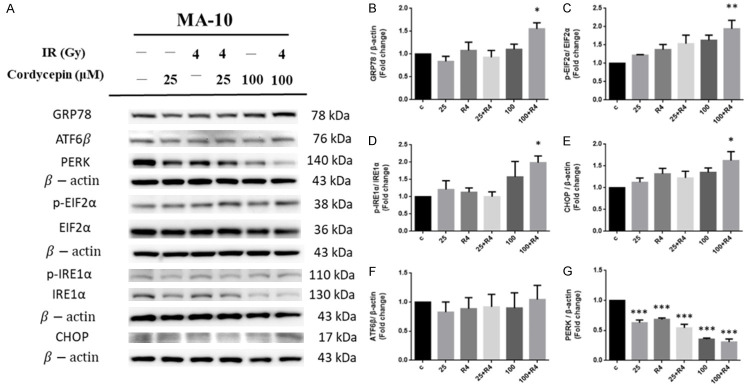
The combination treatment of cordycepin and radiation regulated ER stress pathway to induce MA-10 cell apoptosis
ER is highly sensitive to stresses that perturb cellular energy levels, the redox state or Ca2+ concentration could lead the reducing of the protein folding capacity of the ER, which results in the accumulation and aggregation of unfolded proteins-a condition referred to as ER stress [46]. Studies have shown that a distinct program of pro-apoptotic signals activate the cell death machinery if ER stress cannot be remedied [47]. To further study whether the combination treatment of cordycepin and radiation would regulate ER stress pathways to induce apoptosis in MA-10 cells, ER stress-related proteins including GRP78, PERK, p-EIF2α, EIF2α, ATF6β, p-IRE1α, IRE1α and CHOP in MA-10 cells were analyzed by western blotting. The results demonstrated that the expressions of PERK were significantly decreased by both single treatments and combination treatments compared to the control untreated group (P<0.05). Besides, the combination treatment reduced the expression of PERK compared to the radiation treatment alone (Figure 6A, 6G). However, the expression levels of ATF6β showed no significant change with both single treatments and combination treatments compared to the control untreated group (Figure 6A, 6F). Interestingly, the combination treatment of 100 μM cordycepin and 4 Gy radiation for 24 hr significantly increased the expression of GRP78 (Figure 6A, 6B), p-EIF2α (Figure 6A, 6C), p-IRE1α (Figure 6A, 6D) and CHOP (Figure 6A, 6E), respectively (P<0.05). The CHOP is well known with the function to promote apoptotic cell death as it is activated [46]. Thus, these results indicated that the combination treatment of cordycepin and radiation could regulate the expressions of ER stress-related proteins to induce apoptosis in MA-10 cells.
Figure 6.
The effect of cordycepin and radiation on ER stress pathway in MA-10 cells. MA-10 cells were treated without or with different concentrations of cordycepin (25 and 100 µM) and/or radiation (4 Gy) for 24 hr, respectively. GRP78, p-EIF2α, p-IRE1α, CHOP, ATF6β and PERK were detected by western blotting (A). The integrated optical densities (IOD) of GRP78, p-EIF2α, p-IRE1α, CHOP, ATF6β and PERK were normalized with β-actin (43 kDa) in each lane. *P<0.05, **P<0.01, and ***P<0.001 indicate significant statistical difference compared to the control group (B-G).
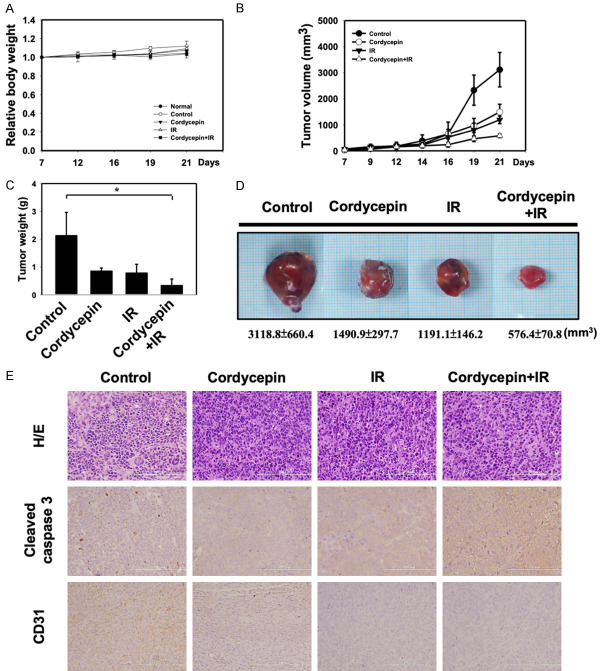
The combination treatment of cordycepin and radiation synergistically inhibited tumorigenesis of MA-10 cells in vivo
We next used a C57BL/6 mouse model to determine the anticancer effect of cordycepin alone, radiation alone, and the combination treatment on MA-10 tumorigenesis in vivo. MA-10 cells were injected subcutaneously into C57BL/6 mice and allowed to grow for 7 days, prior to randomization of the mice into each experimental group. During evaluation of the antitumor activity, no apparent changes in mouse body weight were observed in both the treatment and the control groups compared to the normal mouse group (Figure 7A). Mice treated with cordycepin alone, radiation alone, or in combination resulted in smaller tumor volume (Figure 7B) and lighter tumor weight (Figure 7C) compared to the control untreated group. Dramatically, the combination treatment of cordycepin and radiation shows the most superior anti-tumor effect than each single treatment group. The same pattern can be observed in the size and weight of tumors removed after mice sacrifice (Figure 7D). These data suggested that the combination treatment with radiation and cordycepin synergistically inhibited tumorigenesis of MA-10 cells in C57BL/6 mice. To further investigate the inhibitory effect of cordycepin alone, radiation alone, and combination treatments in vivo, immunohistochemistry (IHC) examinations of cleaved caspase-3 and CD31 were carried out. Results showed that the tumor tissue expressions of cleaved caspase-3 were increased in the combination treatment group, whereas the expressions of CD31, an angiogenic marker, were decreased in tumor tissues obtained from each single treatment and the combination treatment groups (Figure 7E). These data indicating that the combination treatment of radiation and cordycepin decreased the Leydig tumor mass by reducing angiogenesis and increasing cell apoptosis.
Figure 7.
Combination treatment synergistically inhibits tumorigenesis of MA-10 cells in tumor-bearing C57BL/6 mice. C57BL/6 mice were treated with IR (4 Gy) or cordycepin (20 mg/kg) alone or in combination. A. Measurement of body weight in mice 2-3 days per week. B. MA-10 transplanted tumor growth curves in mice. Data are presented as the relative tumor volume normalized to the initial tumor volume measured on Day 7 as a function of time after start of treatment. C. Measurement of tumor weight of transplanted MA-10 in mice. D. Direct observation of mice with tumors from the control and cordycepin, IR alone or in combination groups. E. H&E staining and immunohistochemical staining for analysis of Cleaved caspase 3-positive and CD31-positive cells (brown), Microscopic view with 200X. *P<0.05 indicates significant statistical difference compared to the control group.
Discussion
Clinically, treatment of testicular cancer is mainly through surgical operation and combined with chemotherapy or radiation therapy in some serious cases [48]. Although radiation therapy can effectively kill cancer cells, it also harms the viability of normal cells [49]. The clinical drug used in testicular cancer chemotherapy can lead to side effects in patients, may induce drug resistance, and even reduce male fertility [50]. Leydig tumors mainly effect on teenager, long-term toxicity should be avoided in treatment to reduce the impact on normal reproductive function. Therefore, the development of novel therapeutic strategies to reduce toxicity and side effects remains the focus of current medical research efforts. Cordyceps sinensis, also called the “winter-worm, summer-plant” in Chinese, has long been used as a tonic supplement for sexual and reproductive dysfunctions in oriental society. Cordycepin is a constituent isolated from the mycelia of Cordyceps sinensis [51]. It has been demonstrated that cordycepin has anti-tumor effect on various tumors such as melanoma, neuroblastoma, lung carcinoma, and oral squamous cancer [52-55] and could increase radiosensitivity in cervical cancer cells to promote apoptotic cell death [21]. Moreover, cordycepin can also stimulate normal Leydig cell steroidogenesis [56,57]. In the present study, we examined synergistic effect of the combination of radiation and cordycepin in mouse Leydig tumor cells. Our data demonstrated that the combination treatment of cordycepin and radiation resulted in significantly decreased survival fractions compared to cells treated with radiation alone suggesting that cordycepin could enhance radiosensitivity in MA-10 mouse Leydig tumor cells. Stunningly, the IC50 doses of MA-10 cells treated with cordycepin alone and radiation alone are 50 μM and 6 Gy, respectively, but only 25 μM cordycepin plus 4 Gy radiation are needed in the combination treatment to achieve IC50 level (Figure 8). These results reveal that the future use of cordycepin combined with radiotherapy in testicular cancer treatment could through reducing the therapeutic dose achieve the same therapeutic effect to avoid long-term toxicity and reduce the impact on testicular function. Whether this combination therapy strategy can be applied to other types of cancer treatment needs to be further explored.
Figure 8.
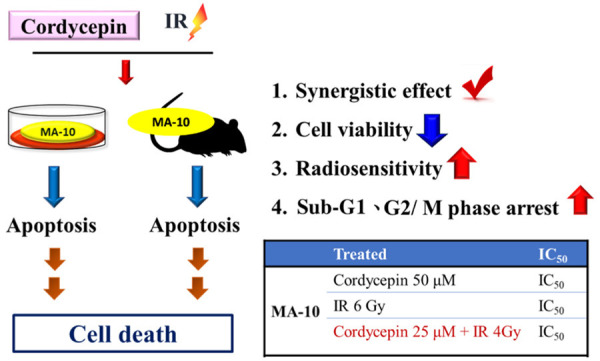
The effect of the combination treatment of cordycepin and radiation in mouse Leydig tumor cells. The combination treatment of cordycepin and radiation has a synergistic effect could significantly suppress cell viability and enhance the radiosensitivity in MA-10 cells. In addition, cordycepin and radiation could significantly induce sub-G1 and G2/M phase arrest in MA-10 cells. Moreover, the combination treatment of cordycepin and radiation induces MA-10 cells to progress toward apoptosis in vitro and in vivo.
Radiotherapy and chemotherapy induce cancer cell death mainly through three pathways including apoptosis, autophagy, and necrosis. In the present study, radiation and/or cordycepin treatment induced apoptosis in MA-10 cells, while combination treatment induced a higher percentage of cells towards apoptosis as well as more obvious morphological changes related to apoptosis. Caspases, the major effectors of apoptosis, playing essential roles in programmed cell death, and the activity of caspases is delicately regulated by a variety of proteins [58]. Previous reports demonstrated that cordycepin could induce the activation of cleavage caspase-8/-9/-3 and cleavage of PARP in cancer cells [59]. Moreover, the radiation treatment could induce the expression of cleaved caspase-3 in MCF-7 breast cancer cells [60]. These results support our current observation in MA-10 cells as that cordycepin and/or radiation induced cleavage caspase proteins to induce apoptosis through both intrinsic and extrinsic pathways in MA-10 cells. Bcl-2 family proteins play a pivotal role in deciding whether a cell will live or die, the intrinsic apoptotic pathway is regulated by these proteins [61]. Study has shown that cordycepin can reduce the expression level of the anti-apoptotic protein Bcl-2, while the expression level of the pro-apoptotic protein Bax remains unchanged, eventually causing apoptosis in human lung cancer cells [62]. These results are similar to our current observations that both the single treatments and the combination treatment decreased Bcl-2 expression but not related to Bax expression in MA-10 cells. Furthermore, the reduction of Bcl-2 protein level can cause apoptosis by increasing the expression level of cytochrome c in non-small cell lung cancer cells as parallel our results in this study [63].
Cell cycle progression is regulated through the four different cell cycle phases: G1, S, G2 and M. Failure of these steps can lead to abnormal growth or apoptosis [64]. G2/M arrest is a common phenomenon following DNA damage after radiation treatment [65]. Studies have indicated that radiation induces G2/M arrest in A549 lung cancer cells and in meningioma cells [10,66]. Rapidly dying and radiosensitive cells undergo apoptosis at different time points in the cell cycle, whereas slowly dying cells display a variety of cell cycle arrest patterns, starting apoptosis only after accumulation of cells in the G2/M fraction [67]. In this study, the 4 Gy radiation single treatment induced remarkable G2/M arrest and the combination treatment significantly prolonged G2/M fraction arrest which consequently induced cell undergoing apoptosis. In addition, prolonged sub-G1 arrest was observed in cells that received the combination treatment compared to those that received cordycepin alone or radiation alone indicating that cordycepin enhances radiosensitivity to induce apoptosis through cell cycle arrest in MA-10 cells. The progression of cell cycle is generally accomplished under the interaction of cyclins and CDKs [68]. Previous reports have illustrated that CDK inhibitors could activate apoptotic cell death by inducing cell cycle arrest at G1/S and G2/M phases in cancer cells [69]. In addition, these compounds are referred as strong apoptotic inducers in breast, colon and lung cancer cells by blocking specifically CDK1, 2, 4, 7 and 9 [70]. Our results showed that the combination treatments could also decrease the expression of CDK1, CDK2 and CDK4 and then induce G2/M arrest in MA-10 cells. Cyclin D, Cyclin E, CDK2, and CDK4 are crucial for maintaining G1 phase. Cyclin E1 and CDK4 expressions were significantly decreased after combined treating with cordycepin and radiation suggesting the decrease of percentages of MA-10 cell numbers in G1 phase caused by sub-G1 arrest. Our current observations are not exceptional to other studies.
ER stress could induce cancer cell apoptosis. CHOP, a member of the C/EBP transcription factor family, is induced by ER stress and thus causes apoptosis [71]. Recent studies have shown that cordycepin activated the PERK/eIF2α/ATF3/CHOP and the IRE1/XBP1 UPR pathways and finally kill testicular cancer cells [48]. In addition, ER stress has been demonstrated to sensitize cancer cells to radiation [72]. In our observations, the combination treatment of 100 μM cordycepin and 4 Gy radiation for 24 hr did increase the expression of GRP78, p-eIF2α, p-IRE1α and CHOP illustrating that the combination treatment could stimulate ER stress to induce MA-10 cell toward apoptosis. Thus, our observations are not unprecedented. It has been shown that ROS generation is associated with ER stress activation. Enhanced ROS generation or oxidative stress might be linked to subsequent ER stress and ER stress-dependent cancer cell apoptosis [73]. Indeed, we have found that cordycepin alone, radiation alone, and the combination treatments did induce ROS production in MA-10 cells, and the effect of the combination treatment is stronger than that of single treatment (unshown data). Therefore, the production of ROS may be the reason that induces ER stress and eventually leads to apoptosis of MA-10 cells.
Our in vivo experimental data showed that cordycepin effectively enhanced the radiosensitivity of MA-10 cells, resulting in a decrease in the weight and volume of the transplanted tumor and an increase in the expression of cleaved caspase-3 in the tumor tissue compared to each single treatment and no treatment control groups. This result confirms our in vitro experimental conclusion in the present study. However, it has been reported that cordycepin not only has the function of inducing apoptosis of cancer cells, but also has biological effects on the inflammatory response, many signal transduction pathways and cell migration [74]. Furthermore, except directly killing cancer cells by damaging DNA, radiotherapy can also indirectly cause cancer cell death by inducing ROS [7,8]. Whether cordycepin treatment and/or radiation treatment can affect the viability of MA-10 Leydig tumor cells through other mechanisms in vivo, such as interaction with the ROS pathway, is still a mystery. Therefore, the detailed molecular mechanism of the antitumor effect induced by cordycepin and/or radiation treatment in vivo still needs to be further studied in the future. Tumor tissue must obtain sufficient supply of nutrients and gases through angiogenesis for survive in vivo. Interestingly, the expression of CD31, a marker of angiogenesis, was significantly lower in tumor tissue from mice treated with cordycepin alone, radiation alone, and in combination than that in the untreated group. How cordycepin treatment and/or radiation treatment can affect angiogenesis, thereby inducing antitumor activity, is also a direction that future research can explore.
In summary, cordycepin enhanced radiosensitivity to induce apoptosis in MA-10 mouse Leydig tumor cells by activating both extrinsic and intrinsic caspase pathways, cell cycle arrest, and ER stress, which is involved in the regulation of caspases, cyclins/CDKs, pro-apoptotic/anti-apoptotic proteins, and ER stress-related proteins. The combination treatment of cordycepin and radiation decreased the Leydig tumor mass by increasing cell apoptosis in vivo (Figure 8). These results illustrate helpful evidence for designing more effective chemotherapy agent using cordycepin with proper concentration and suitable radiation dosage to treat testicular cancers with less adverse effect after testicular cancer therapy.
Acknowledgements
This study was supported by the Ministry of Science and Technology, Taiwan (MOST 110-2320-B-006-025-MY3 to Bu-Miin Huang; MOST 110-2811-B-006-569 to Yi-Ping Lee). The authors are grateful for the support from the Core Research Laboratory, College of Medicine, National Cheng Kung University.
Disclosure of conflict of interest
None.
References
- 1.Milose JC, Filson CP, Weizer AZ, Hafez KS, Montgomery JS. Role of biochemical markers in testicular cancer: diagnosis, staging, and surveillance. Open Access J Urol. 2011;4:1–8. doi: 10.2147/OAJU.S15063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Papworth DG, Lloyd RA. Cancer survival in the USA, 1973-1990: a statistical analysis. Br J Cancer. 1998;78:1514–1515. doi: 10.1038/bjc.1998.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Richiardi L, Scelo G, Boffetta P, Hemminki K, Pukkala E, Olsen JH, Weiderpass E, Tracey E, Brewster DH, McBride ML, Kliewer EV, Tonita JM, Pompe-Kirn V, Kee-Seng C, Jonasson JG, Martos C, Brennan P. Second malignancies among survivors of germ-cell testicular cancer: a pooled analysis between 13 cancer registries. Int J Cancer. 2007;120:623–631. doi: 10.1002/ijc.22345. [DOI] [PubMed] [Google Scholar]
- 4.Pan BS, Wang YK, Lai MS, Mu YF, Huang BM. Cordycepin induced MA-10 mouse Leydig tumor cell apoptosis by regulating p38 MAPKs and PI3K/AKT signaling pathways. Sci Rep. 2015;5:13372. doi: 10.1038/srep13372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Petersen PM, Skakkebaek NE, Rorth M, Giwercman A. Semen quality and reproductive hormones before and after orchiectomy in men with testicular cancer. J Urol. 1999;161:822–826. [PubMed] [Google Scholar]
- 6.Bertram KA, Bratloff B, Hodges GF, Davidson H. Treatment of malignant Leydig cell tumor. Cancer. 1991;68:2324–2329. doi: 10.1002/1097-0142(19911115)68:10<2324::aid-cncr2820681036>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 7.Lomax ME, Folkes LK, O’Neill P. Biological consequences of radiation-induced DNA damage: relevance to radiotherapy. Clin Oncol (R Coll Radiol) 2013;25:578–585. doi: 10.1016/j.clon.2013.06.007. [DOI] [PubMed] [Google Scholar]
- 8.Borrego-Soto G, Ortiz-Lopez R, Rojas-Martinez A. Ionizing radiation-induced DNA injury and damage detection in patients with breast cancer. Genet Mol Biol. 2015;38:420–432. doi: 10.1590/S1415-475738420150019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jonathan EC, Bernhard EJ, McKenna WG. How does radiation kill cells? Curr Opin Chem Biol. 1999;3:77–83. doi: 10.1016/s1367-5931(99)80014-3. [DOI] [PubMed] [Google Scholar]
- 10.Gogineni VR, Nalla AK, Gupta R, Dinh DH, Klopfenstein JD, Rao JS. Chk2-mediated G2/M cell cycle arrest maintains radiation resistance in malignant meningioma cells. Cancer Lett. 2011;313:64–75. doi: 10.1016/j.canlet.2011.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Panganiban RA, Snow AL, Day RM. Mechanisms of radiation toxicity in transformed and non-transformed cells. Int J Mol Sci. 2013;14:15931–15958. doi: 10.3390/ijms140815931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Verheij M, Bartelink H. Radiation-induced apoptosis. Cell Tissue Res. 2000;301:133–142. doi: 10.1007/s004410000188. [DOI] [PubMed] [Google Scholar]
- 13.Han Y, Wang Y, Xu HT, Yang LH, Wei Q, Liu Y, Zhang Y, Zhao Y, Dai SD, Miao Y, Yu JH, Zhang JY, Li G, Yuan XM, Wang EH. X-radiation induces non-small-cell lung cancer apoptosis by upregulation of Axin expression. Int J Radiat Oncol Biol Phys. 2009;75:518–526. doi: 10.1016/j.ijrobp.2009.05.040. [DOI] [PubMed] [Google Scholar]
- 14.Rowley MJ, Leach DR, Warner GA, Heller CG. Effect of graded doses of ionizing radiation on the human testis. Radiat Res. 1974;59:665–678. [PubMed] [Google Scholar]
- 15.Tuli HS, Sharma AK, Sandhu SS, Kashyap D. Cordycepin: a bioactive metabolite with therapeutic potential. Life Sci. 2013;93:863–869. doi: 10.1016/j.lfs.2013.09.030. [DOI] [PubMed] [Google Scholar]
- 16.Aramwit P, Porasuphatana S, Srichana T, Nakpheng T. Toxicity evaluation of cordycepin and its delivery system for sustained in vitro anti-lung cancer activity. Nanoscale Res Lett. 2015;10:152. doi: 10.1186/s11671-015-0851-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liao Y, Ling J, Zhang G, Liu F, Tao S, Han Z, Chen S, Chen Z, Le H. Cordycepin induces cell cycle arrest and apoptosis by inducing DNA damage and up-regulation of p53 in Leukemia cells. Cell Cycle. 2015;14:761–771. doi: 10.1080/15384101.2014.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nakamura K, Shinozuka K, Yoshikawa N. Anticancer and antimetastatic effects of cordycepin, an active component of cordyceps sinensis. J Pharmacol Sci. 2015;127:53–56. doi: 10.1016/j.jphs.2014.09.001. [DOI] [PubMed] [Google Scholar]
- 19.Chang MM, Pan BS, Wang CY, Huang BM. Cordycepin-induced unfolded protein response-dependent cell death, and AKT/MAPK-mediated drug resistance in mouse testicular tumor cells. Cancer Med. 2019;8:3949–3964. doi: 10.1002/cam4.2285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chang MM, Hong SY, Yang SH, Wu CC, Wang CY, Huang BM. Anti-cancer effect of cordycepin on FGF9-induced testicular tumorigenesis. Int J Mol Sci. 2020;21:8336. doi: 10.3390/ijms21218336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Seong da B, Hong S, Muthusami S, Kim WD, Yu JR, Park WY. Cordycepin increases radiosensitivity in cervical cancer cells by overriding or prolonging radiation-induced G2/M arrest. Eur J Pharmacol. 2016;771:77–83. doi: 10.1016/j.ejphar.2015.12.022. [DOI] [PubMed] [Google Scholar]
- 22.Hiraoka W, Tanabe K, Kuwabara M, Sato F, Matsuda A, Ueda T. Sensitization of X-irradiated Chinese hamster V79 cells by derivatives of pyrimidine nucleosides. J Radiat Res. 1988;29:246–254. doi: 10.1269/jrr.29.246. [DOI] [PubMed] [Google Scholar]
- 23.Lee SJ, Kim SK, Choi WS, Kim WJ, Moon SK. Cordycepin causes p21WAF1-mediated G2/M cell-cycle arrest by regulating c-Jun N-terminal kinase activation in human bladder cancer cells. Arch Biochem Biophys. 2009;490:103–109. doi: 10.1016/j.abb.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 24.Collins K, Jacks T, Pavletich NP. The cell cycle and cancer. Proc Natl Acad Sci U S A. 1997;94:2776–2778. doi: 10.1073/pnas.94.7.2776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sherr CJ. Mammalian G1 cyclins. Cell. 1993;73:1059–1065. doi: 10.1016/0092-8674(93)90636-5. [DOI] [PubMed] [Google Scholar]
- 26.Gupta S. Molecular steps of cell suicide: an insight into immune senescence. J Clin Immunol. 2000;20:229–239. doi: 10.1023/a:1006653917314. [DOI] [PubMed] [Google Scholar]
- 27.Ashkenazi A. Targeting the extrinsic apoptosis pathway in cancer. Cytokine Growth Factor Rev. 2008;19:325–331. doi: 10.1016/j.cytogfr.2008.04.001. [DOI] [PubMed] [Google Scholar]
- 28.Sarvothaman S, Undi RB, Pasupuleti SR, Gutti U, Gutti RK. Apoptosis: role in myeloid cell development. Blood Res. 2015;50:73–79. doi: 10.5045/br.2015.50.2.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hill MM, Adrain C, Duriez PJ, Creagh EM, Martin SJ. Analysis of the composition, assembly kinetics and activity of native Apaf-1 apoptosomes. EMBO J. 2004;23:2134–2145. doi: 10.1038/sj.emboj.7600210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shawgo ME, Shelton SN, Robertson JD. Caspase-mediated Bak activation and cytochrome c release during intrinsic apoptotic cell death in Jurkat cells. J Biol Chem. 2008;283:35532–35538. doi: 10.1074/jbc.M807656200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Boulares AH, Yakovlev AG, Ivanova V, Stoica BA, Wang G, Iyer S, Smulson M. Role of poly (ADP-ribose) polymerase (PARP) cleavage in apoptosis. Caspase 3-resistant PARP mutant increases rates of apoptosis in transfected cells. J Biol Chem. 1999;274:22932–22940. doi: 10.1074/jbc.274.33.22932. [DOI] [PubMed] [Google Scholar]
- 32.Rodvold JJ, Chiu KT, Hiramatsu N, Nussbacher JK, Galimberti V, Mahadevan NR, Willert K, Lin JH, Zanetti M. Intercellular transmission of the unfolded protein response promotes survival and drug resistance in cancer cells. Sci Signal. 2017;10:eaah7177. doi: 10.1126/scisignal.aah7177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Oslowski CM, Urano F. Measuring ER stress and the unfolded protein response using mammalian tissue culture system. Methods Enzymol. 2011;490:71–92. doi: 10.1016/B978-0-12-385114-7.00004-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sano R, Reed JC. ER stress-induced cell death mechanisms. Biochim Biophys Acta. 2013;1833:3460–3470. doi: 10.1016/j.bbamcr.2013.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Willis MS, Townley-Tilson WH, Kang EY, Homeister JW, Patterson C. Sent to destroy: the ubiquitin proteasome system regulates cell signaling and protein quality control in cardiovascular development and disease. Circ Res. 2010;106:463–478. doi: 10.1161/CIRCRESAHA.109.208801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pavio N, Romano PR, Graczyk TM, Feinstone SM, Taylor DR. Protein synthesis and endoplasmic reticulum stress can be modulated by the hepatitis C virus envelope protein E2 through the eukaryotic initiation factor 2alpha kinase PERK. J Virol. 2003;77:3578–3585. doi: 10.1128/JVI.77.6.3578-3585.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schindler AJ, Schekman R. In vitro reconstitution of ER-stress induced ATF6 transport in COPII vesicles. Proc Natl Acad Sci U S A. 2009;106:17775–17780. doi: 10.1073/pnas.0910342106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rozpedek W, Pytel D, Mucha B, Leszczynska H, Diehl JA, Majsterek I. The role of the PERK/eIF2alpha/ATF4/CHOP signaling pathway in tumor progression during endoplasmic reticulum stress. Curr Mol Med. 2016;16:533–544. doi: 10.2174/1566524016666160523143937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Logue SE, Cleary P, Saveljeva S, Samali A. New directions in ER stress-induced cell death. Apoptosis. 2013;18:537–546. doi: 10.1007/s10495-013-0818-6. [DOI] [PubMed] [Google Scholar]
- 40.Ma Y, Brewer JW, Diehl JA, Hendershot LM. Two distinct stress signaling pathways converge upon the CHOP promoter during the mammalian unfolded protein response. J Mol Biol. 2002;318:1351–1365. doi: 10.1016/s0022-2836(02)00234-6. [DOI] [PubMed] [Google Scholar]
- 41.Ron D, Hubbard SR. How IRE1 reacts to ER stress. Cell. 2008;132:24–26. doi: 10.1016/j.cell.2007.12.017. [DOI] [PubMed] [Google Scholar]
- 42.Chou TC, Talalay P. Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Adv Enzyme Regul. 1984;22:27–55. doi: 10.1016/0065-2571(84)90007-4. [DOI] [PubMed] [Google Scholar]
- 43.Rieger AM, Nelson KL, Konowalchuk JD, Barreda DR. Modified annexin V/propidium iodide apoptosis assay for accurate assessment of cell death. J Vis Exp. 2011:2597. doi: 10.3791/2597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Logue SE, Martin SJ. Caspase activation cascades in apoptosis. Biochem Soc Trans. 2008;36:1–9. doi: 10.1042/BST0360001. [DOI] [PubMed] [Google Scholar]
- 45.Donovan M, Cotter TG. Control of mitochondrial integrity by Bcl-2 family members and caspase-independent cell death. Biochim Biophys Acta. 2004;1644:133–147. doi: 10.1016/j.bbamcr.2003.08.011. [DOI] [PubMed] [Google Scholar]
- 46.Szegezdi E, Logue SE, Gorman AM, Samali A. Mediators of endoplasmic reticulum stress-induced apoptosis. EMBO Rep. 2006;7:880–885. doi: 10.1038/sj.embor.7400779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tabas I, Ron D. Integrating the mechanisms of apoptosis induced by endoplasmic reticulum stress. Nat Cell Biol. 2011;13:184–190. doi: 10.1038/ncb0311-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Huang MM, Cheaib JG, Su ZT, Biles MJ, Sharma R, Zhang A, Singla N, Bass EB, Pierorazio PM. Assessing quality of care in the diagnosis and treatment of early-stage testicular cancer: a critical review and summary. Urol Oncol. 2021;39:400–408. doi: 10.1016/j.urolonc.2021.02.001. [DOI] [PubMed] [Google Scholar]
- 49.Baskar R, Lee KA, Yeo R, Yeoh KW. Cancer and radiation therapy: current advances and future directions. Int J Med Sci. 2012;9:193–199. doi: 10.7150/ijms.3635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stephenson WT, Poirier SM, Rubin L, Einhorn LH. Evaluation of reproductive capacity in germ cell tumor patients following treatment with cisplatin, etoposide, and bleomycin. J. Clin. Oncol. 1995;13:2278–2280. doi: 10.1200/JCO.1995.13.9.2278. [DOI] [PubMed] [Google Scholar]
- 51.Chen YC, Chen YH, Pan BS, Chang MM, Huang BM. Functional study of cordyceps sinensis and cordycepin in male reproduction: a review. J Food Drug Anal. 2017;25:197–205. doi: 10.1016/j.jfda.2016.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nakamura K, Yoshikawa N, Yamaguchi Y, Kagota S, Shinozuka K, Kunitomo M. Antitumor effect of cordycepin (3’-deoxyadenosine) on mouse melanoma and lung carcinoma cells involves adenosine A3 receptor stimulation. Anticancer Res. 2006;26:43–47. [PubMed] [Google Scholar]
- 53.Baik JS, Kwon HY, Kim KS, Jeong YK, Cho YS, Lee YC. Cordycepin induces apoptosis in human neuroblastoma SK-N-BE(2)-C and melanoma SK-MEL-2 cells. Indian J Biochem Biophys. 2012;49:86–91. [PubMed] [Google Scholar]
- 54.Chen YH, Hao LJ, Hung CP, Chen JW, Leu SF, Huang BM. Apoptotic effect of cisplatin and cordycepin on OC3 human oral cancer cells. Chin J Integr Med. 2014;20:624–632. doi: 10.1007/s11655-013-1453-3. [DOI] [PubMed] [Google Scholar]
- 55.Wu WC, Hsiao JR, Lian YY, Lin CY, Huang BM. The apoptotic effect of cordycepin on human OEC-M1 oral cancer cell line. Cancer Chemother Pharmacol. 2007;60:103–111. doi: 10.1007/s00280-006-0354-y. [DOI] [PubMed] [Google Scholar]
- 56.Leu SF, Poon SL, Pao HY, Huang BM. The in vivo and in vitro stimulatory effects of cordycepin on mouse leydig cell steroidogenesis. Biosci Biotechnol Biochem. 2011;75:723–731. doi: 10.1271/bbb.100853. [DOI] [PubMed] [Google Scholar]
- 57.Pao HY, Pan BS, Leu SF, Huang BM. Cordycepin stimulated steroidogenesis in MA-10 mouse Leydig tumor cells through the protein kinase C pathway. J Agric Food Chem. 2012;60:4905–4913. doi: 10.1021/jf205091b. [DOI] [PubMed] [Google Scholar]
- 58.Engidawork E, Gulesserian T, Yoo BC, Cairns N, Lubec G. Alteration of caspases and apoptosis-related proteins in brains of patients with Alzheimer’s disease. Biochem Biophys Res Commun. 2001;281:84–93. doi: 10.1006/bbrc.2001.4306. [DOI] [PubMed] [Google Scholar]
- 59.Jeong JW, Jin CY, Park C, Hong SH, Kim GY, Jeong YK, Lee JD, Yoo YH, Choi YH. Induction of apoptosis by cordycepin via reactive oxygen species generation in human leukemia cells. Toxicol In Vitro. 2011;25:817–824. doi: 10.1016/j.tiv.2011.02.001. [DOI] [PubMed] [Google Scholar]
- 60.Yang XH, Edgerton S, Thor AD. Reconstitution of caspase-3 sensitizes MCF-7 breast cancer cells to radiation therapy. Int J Oncol. 2005;26:1675–1680. [PubMed] [Google Scholar]
- 61.Skommer J, Brittain T, Raychaudhuri S. Bcl-2 inhibits apoptosis by increasing the time-to-death and intrinsic cell-to-cell variations in the mitochondrial pathway of cell death. Apoptosis. 2010;15:1223–1233. doi: 10.1007/s10495-010-0515-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hwang JH, Park SJ, Ko WG, Kang SM, Lee DB, Bang J, Park BJ, Wee CB, Kim DJ, Jang IS, Ko JH. Cordycepin induces human lung cancer cell apoptosis by inhibiting nitric oxide mediated ERK/Slug signaling pathway. Am J Cancer Res. 2017;7:417–432. [PMC free article] [PubMed] [Google Scholar]
- 63.Miyake N, Chikumi H, Takata M, Nakamoto M, Igishi T, Shimizu E. Rapamycin induces p53-independent apoptosis through the mitochondrial pathway in non-small cell lung cancer cells. Oncol Rep. 2012;28:848–854. doi: 10.3892/or.2012.1855. [DOI] [PubMed] [Google Scholar]
- 64.Williams GH, Stoeber K. The cell cycle and cancer. J Pathol. 2012;226:352–364. doi: 10.1002/path.3022. [DOI] [PubMed] [Google Scholar]
- 65.Ho SY, Chen WC, Chiu HW, Lai CS, Guo HR, Wang YJ. Combination treatment with arsenic trioxide and irradiation enhances apoptotic effects in U937 cells through increased mitotic arrest and ROS generation. Chem Biol Interact. 2009;179:304–313. doi: 10.1016/j.cbi.2008.12.015. [DOI] [PubMed] [Google Scholar]
- 66.Chang HY, Shih MH, Huang HC, Tsai SR, Juan HF, Lee SC. Middle infrared radiation induces G2/M cell cycle arrest in A549 lung cancer cells. PLoS One. 2013;8:e54117. doi: 10.1371/journal.pone.0054117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Aldridge DR, Radford IR. Explaining differences in sensitivity to killing by ionizing radiation between human lymphoid cell lines. Cancer Res. 1998;58:2817–2824. [PubMed] [Google Scholar]
- 68.Wesierska-Gadek J, Borza A, Komina O, Maurer M. Impact of roscovitine, a selective CDK inhibitor, on cancer cells: bi-functionality increases its therapeutic potential. Acta Biochim Pol. 2009;56:495–501. [PubMed] [Google Scholar]
- 69.Arisan ED, Obakan P, Coker-Gurkan A, Calcabrini A, Agostinelli E, Unsal NP. CDK inhibitors induce mitochondria-mediated apoptosis through the activation of polyamine catabolic pathway in LNCaP, DU145 and PC3 prostate cancer cells. Curr Pharm Des. 2014;20:180–188. doi: 10.2174/13816128113199990029. [DOI] [PubMed] [Google Scholar]
- 70.Sutherland RL, Musgrove EA. CDK inhibitors as potential breast cancer therapeutics: new evidence for enhanced efficacy in ER+ disease. Breast Cancer Res. 2009;11:112. doi: 10.1186/bcr2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Endo M, Mori M, Akira S, Gotoh T. C/EBP homologous protein (CHOP) is crucial for the induction of caspase-11 and the pathogenesis of lipopolysaccharide-induced inflammation. J Immunol. 2006;176:6245–6253. doi: 10.4049/jimmunol.176.10.6245. [DOI] [PubMed] [Google Scholar]
- 72.Yoshino H, Kumai Y, Kashiwakura I. Effects of endoplasmic reticulum stress on apoptosis induction in radioresistant macrophages. Mol Med Rep. 2017;15:2867–2872. doi: 10.3892/mmr.2017.6298. [DOI] [PubMed] [Google Scholar]
- 73.Verfaillie T, Garg AD, Agostinis P. Targeting ER stress induced apoptosis and inflammation in cancer. Cancer Lett. 2013;332:249–264. doi: 10.1016/j.canlet.2010.07.016. [DOI] [PubMed] [Google Scholar]
- 74.Radhi M, Ashraf S, Lawrence S, Tranholm AA, Wellham PAD, Hafeez A, Khamis AS, Thomas R, McWilliams D, de Moor CH. A systematic review of the biological effects of cordycepin. Molecules. 2021;26:5886. doi: 10.3390/molecules26195886. [DOI] [PMC free article] [PubMed] [Google Scholar]