Abstract
Adult-type Granulosa Cell Tumor of the Ovary (AGCT) is a relatively rare subtype of ovarian cancer, accounting for 2-4% of all ovarian cancer. AGCT originates from proliferating normal preovulatory granulosa cells (GCs) and retains several features of those GCs. The hormonal features of AGCT explain the clinical manifestations and provide reliable markers for early diagnosis and recurrence prediction of the disease. Most AGCT patients are diagnosed at an early stage and usually demonstrate a better prognosis than patients with other types of ovarian cancer. Surgery is crucial for both initial and post-relapse treatments, whereas adjuvant therapy is still in the exploratory stage. In 2009, a population-based screening makes an exciting step, about 97% of AGCT has somatic missense mutations in the transcription factor FOXL2 gene and the FOXL2 mutation is considered to be a molecular characteristic of AGCT. Unfortunately, the FOXL2 mutation does not fully explain the development of AGCT. Ongoing research is focusing on signalling pathways in the molecular pathogenesis of AGCT to identify the possible pathogenetic factors and signal transduction pathways and provide a theoretical basis for targeted treatment. Postoperative recurrence of ovarian AGCT is common and is associated with a high mortality rate, which necessitates regular follow-up. The life management of postoperative patients is also crucial, which requires multidisciplinary experts to design recurrence treatment from the perspective of patients and implement meaningful treatment measures.
Keywords: Adult-type granulosa cell tumor of the ovary, surgery, adjuvant therapy, FOXL2, management
Introduction
Granulosa Cell Tumor of the Ovary (GCT) is a especial kind of rare ovarian cancer, accounting for about 5% malignant ovarian cancer [1-4]. Based on the histological characteristics and clinical symptoms, GCT can be divided into two separate subsets, namely, adult-type GCT (AGCT) and juvenile GCT (JGCT) [5,6]. In contrast to JGCT, AGCT is more common and accounts for approximately 95% of all GCTs [6-8].
AGCT is associated with prominent clinical endocrine manifestations, which is an important reason why most patients are diagnosed at stage Ia (78%-91%) [1,9,10]. Long-term exposure to endogenous oestrogen may lead to endometrial hyperplasia or carcinoma [1,11,12]. AGCT may occur in women at any age; however, patients are usually diagnosed between 50 and 54 years of age during their perimenopausal or early postmenopausal stages [2]. Surgery is the primary treatment for ovarian AGCT. Patients with advanced and recurrent disease usually receive adjuvant therapy, including radiotherapy, chemotherapy, hormonotherapy and targeted therapy. A study conducted in 2009 reported that approximately 97% of the cases of AGCT had somatic missense mutations in the transcription factor FOXL2 (c.402C→G; p.C134W) [13]. Subsequent studies proved that the mutation does not exist in other cancers, including JGCTs [14-17]. The FOXL2 mutation provides theoretical support for the clinical diagnosis, exploration of the pathogenesis and treatment of AGCT. Nonetheless, the pathogenesis of AGCT remains unclear currently despite extensive exploration.
Here, we comprehensively reviewed the clinical manifestations, pathological and imaging features, and the clinical treatment status of AGCT. In addition, we have summarised the latest research on AGCT and discussed the future management and treatment of patients.
Epidemiology and risk factors
AGCT is a relatively uncommon gynaecologic malignancy, with an incidence of less than 3.7 per 100,000 persons [18,19]. A series of retrospective analyses showed that tumor staging is the only prognostic factor for survival in patients with AGCT. The 5-year relative survival rate of patients with early disease was greater than 90%, while that of patients with stage II and stage III or IV disease was 55-75% and 22-50%, respectively [20]. The aetiology of AGCT is obscure. Some cytogenetic studies have reported a unique chromosome aberration pattern in AGCT, including trisomy of chromosome 12 or 14 and monosomy of chromosome 22 [21,22]. However, the aberrations appear neither random nor necessary for the tumorigenesis in this disease. Unlike other types of ovarian cancer, AGCT has a stable karyotype. Studies have shown that aneuploidy and karyotype do not affect the risk. Peutz-Jeghers syndrome (PJS), an autosomal dominant inherited syndrome, has been reported to increase the risk of AGCT [23-27]. Debate continues over whether infertility or drug stimulation in the treatment of infertility is present in the AGCT [28-30]. Although genetic germline mutations in the breast cancer susceptibility genes BRCA1 and BRCA2 have been reported to be associated with a higher risk of epithelial ovarian cancer, AGCT does not appear to have any genetic susceptibility [6]. The incidence of AGCT is relatively high among non-white and obese women, which may be considered important risk factors.
Diagnosis
AGCT is an endocrine ovarian cancer, which shows similar hormone characteristics to GCs, especially abnormal estrogen secretion [20]. Patients often have an abnormal endometrium due to long-term exposure to endogenous, abnormal estrogen. Approximately 26-38% of the patients present with endometrial hyperplasia and approximately 10% of the patients are diagnosed with concurrent endometrial cancer. The abnormal endometrium causes vaginal bleeding, which is the most common symptom of AGCT [3,18,19,31,32]. Premenopausal patients initially exhibit irregular vaginal bleeding, amenorrhea, and rarely infertility due to the abnormal secretion of inhibin [32-34]. Abnormal vaginal bleeding and a unilateral ovarian mass are the most prominent clinical manifestations in postmenopausal patients. A realistic or cystic mass may be observed on ultrasound. Larger masses may cause abdominal distention or pain [12,33]. Ascites is not a significant symptom of AGCT and only 18.6%-21% of the patients present with this symptom at initial diagnosis. It is important to note that symptoms such as abdominal distention and pain are not specific clinical manifestations of AGCT and approximately 20% of the patients are asymptomatic at the time of initial diagnosis.
Pathology
In grossly, AGCT shows significant variations, ranging from small invisible lesions to huge masses, with an average diameter of >10 cm. Ovarian AGCT usually shows solidity with cystic lesions and yellow/grey sections because the mass contains a variable number of tumor cells and fibrous sheath tumor stroma.
AGCT exhibits specific histomorphological patterns, including high-grade and low-grade differentiated patterns (Figure 1). The high-grade differentiated histological pattern usually presents in several forms, including microvesicles, trabecular, island, tubular and hollow tubular forms, although these can be observed simultaneously in AGCT. Call-Exner bodies, the most typical histological pattern of microbubbles, play an important role in histological diagnosis. The low-grade differentiated pattern usually shows a watered-silk or gyriform pattern, which refers to a diffuse distribution and is known as the sarcomatoid type. The well-differentiated and poorly differentiated patterns contain round to oval, pale cells, and the nucleus is typical coffee bean groove. Considering the unique appearance of the nucleus, there is no issue of misdiagnosis between AGCT, undifferentiated carcinoma, adenocarcinoma, and carcinoid tumors. Occasionally, lutealization is obvious, and luteal AGCT may contain more atypical nuclei than the normal.
Figure 1.
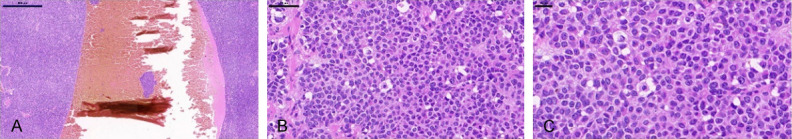
H&E staining of a representative AGCT. A. The tumor contains solid masses and hemorrhage cystic cavity composition. Scale bar = 500 μm. B. Call-Exner bodies. Scale bar = 100 μm. C. The cells are knowns “coffee bean” nuclei, and classic nuclear grooves. Scale bar = 20 μm.
Radiographic findings
An obvious abdominal or pelvic mass is common in patients with AGCT. Therefore, ultrasound evaluation is an important tool to confirm the results of physical examination and obtain qualitative information regarding the mass. Based on the ultrasound and CT scanning results, Ko et al. categorised AGCT into five morphological types, including multilocular cystic, thick-walled single locular cystic, thin-walled single locular cystic, homogeneous solid and heterogeneous solid types [35]. Subsequently, Kim et al. simplified the classification based on the two most commonly observed types as polyseptal cystic lesions and non-lobulated solid lesions of internal cystic lesions [36]. Intratumoural haemorrhage, necrosis and fibrosis can increase the heterogeneity of the imaging findings [37].
Compared with EOC, the initially diagnosed AGCT had no intracystic papillary processes, and the tendency of peritoneal dissemination was small. And the tumor was limited to the ovary. However, recurrent AGCTs are often multifocal. PET/CT scan is not usually used for the diagnosis and follow-up, considering the low activity of 18-F-fluorodeoxyglucose (FDG) in AGCT [38-41].
Screening
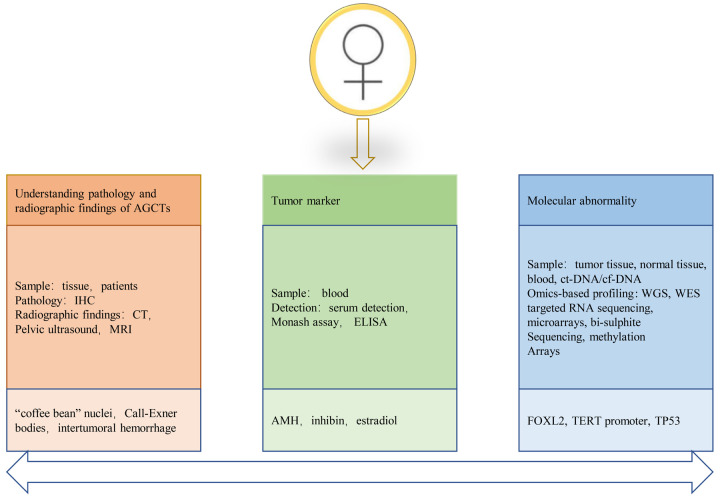
Since AGCT are rare and their histological morphology do not show obvious persuasion, the false positive rate of AGCT is as high as 36% in previous records [32,42]. Sustained efforts have been made to screen the population for accurate early diagnosis and postoperative follow-up of ovarian AGCT (Figure 2). The serum markers inhibin, anti-müllerian hormone (AMH) and estradiol can be used for the early diagnosis and postoperative follow-up of patients with AGCT. However, studies have reported biases; therefore, the detection of serum markers alone is not adequate to diagnose AGCT. Significant progress has been made in the genome screening of AGCT, and FOXL2 mutation has been exclusively identified in this condition. Subsequently, the specificity of FOXL2 mutation was verified in several cohorts of AGCT. Thus, FOXL2 can be considered a molecular marker of AGCT. Recent studies have shown that TERT promoter mutations are common in patients with recurrence, which may become one of the indicators for future follow-up. Currently, the screening of molecular markers through genome and proteome analysis is in progress.
Figure 2.
Screening for the early diagnosis and postoperative follow-up of AGCT. Based on the understanding of AGCT biology and evolution, early and recurrent AGCT patients were screened by existing techniques. IHC = Immunohistochemistry. CT = Computed tomography. MRI = Magnetic resonance imaging. ELISA = Enzyme-linked immunosorbent assay. ct-DNA = circulating tumor DNA. cf-DNA = circulating free DNA. WGS = whole genome sequencing. WES = whole exome sequencing.
Tumor markers
Specific tumor markers are crucial for accurate early diagnosis and postoperative follow-up of patients with ovarian AGCT, which can improve the survival rate of patients to a certain extent. The hormonal activity of ovarian AGCT suggests that the hormones synthesized might play a role as tumor markers for the early prediction and postoperative follow-up.
Inhibin
Inhibin is produced by normal ovarian GCs and consists of a dimer of two partially homologous subunits, one of which is covalently linked to the α or β subunit to form inhibin A and inhibin B, respectively [43-46]. Inhibin, a glycoprotein hormone, is involved in the regulation of follicle formation, steroid production, and follicular estrogen secretion [47,48]. In addition, inhibin regulates the secretion of FSH through negative feedback, which leads to a negative correlation between inhibin and FSH in serum [49]. The secretion of FSH usually occurs during the follicular phase in premenopausal women; therefore, inhibin is almost undetectable after menopause [50,51]. Inhibin generation in GCTs was first reported in 1989, along with the correlation between serum inhibin and tumor size [52]. Enzyme-linked immunosorbent assay (ELISA) revealed that inhibin B was more specific than inhibin A in detecting GCTs [53-55]. This claim has been controversial due to the variable specificity of ELISA in detecting certain subunits compared with Monash assays. However, recent studies have confirmed that serum inhibin does not demonstrate specificity for the diagnosis of GCTs. Immunohistochemical analysis confirmed that not all patients with GCT had an increase in inhibin levels, and patients with EOC were also found to have elevated serum inhibin levels [56]. Therefore, it appears contradictory to consider postmenopausal serum inhibin elevation as a tumor marker for GCT.
AMH
AMH, a member of the TGF-β superfamily, is considered a possible tumor marker for GCT [2]. AMH is produced by GCs in the ovary of normal women during reproduction, and the levels vary periodically. AMH plays an important role in the formation of the primary follicle by participating in follicular recruitment by FSH. In a study by Anttonen et al., the expression of AMH correlated negatively with the tumor size in patients with GCTs and decreased AMH level was found in 87% of the tumors larger than 10 cm [57-59]. A cohort of 16 women with AGCT, 58 healthy women, and 75 women with benign and malignant ovarian cancer and non-ovarian cancer underwent AMH testing. The results revealed that AMH was almost undetectable in healthy women after menopause. AMH was elevated in eight of nine patients with progressive GCT and undetectable in 10 of 11 patients in clinical remission. The AMH levels were normal in 93% of other gynaecologic and non-gynaecologic cancers [60]. These results indicate that serum AMH can be considered an effective marker for the diagnosis of ovarian AGCT; however, its application remains limited to clinical studies.
Estradiol
Unlike inhibin and AMH, estradiol is not a reliable marker of tumor cell proliferation. Although its abnormal secretion has been strongly associated with the clinical appearance of GCTs, several studies have shown that GCTs do not necessarily occur with elevated oestradiol levels [61]. Estradiol is not detected in approximately 30% of the patients with GCTs, which may be attributed to the loss of follicular membrane cells [6]. Rey et al. reported a minimal correlation between estradiol levels and the progression of GCTs [60].
Molecular abnormality
The initiation and development of tumors require genetic changes that drive normal cells to transform into highly malignant derivatives [62]. Molecular diagnosis is considered critical to distinguish AGCT from other, often more malignant, ovarian tumors with significantly poor prognosis [63].
FOXL2
FOXL2, a transcription factor of the forkhead family, is found mainly in the follicular granulosa cells of the ovaries and developing eyelids [64-67]. FOXL2 expression has barely been detected in other human tissues, such as the testes and heart [66]. FOXL2 is a sexually dimorphic marker of ovarian differentiation and determines optimal primary follicle formation [68,69]. Mouse knockout studies have emphasized the key role of FOXL2 in follicle development. In addition, GCs do not undergo squamous to cuboidal transition in FOXL2-deficient ovaries, resulting in immature follicle depletion and the absence of mature follicles [70,71]. A study demonstrated that FOXL2 prevents adult ovarian GCs from transforming into functional Sertoli cells, which produce only androgens without estrogen [72]. Garcia-Ortiz et al. reported similar findings signifying the need for FOXL2 to maintain the adult ovarian phenotype and normal function of GCs [68]. In addition, FOXL2 mutation may lead to multiple ovarian dysfunctions, such as the autosomal dominant disease blepharophimosis-ptosis-epicanthus inversus syndrome (BPES) [73]. Premature ovarian failure usually occurs with BPES [74]. Furthermore, FOXL2 mutation may be associated with the pathogenesis of AGCT.
A landmark study on ovarian AGCT, based on whole-transcriptome paired-end RNA sequencing on four different AGCT samples, identified a somatic missense mutation in the FOXL2 gene (c.402→G; p.C134W) [13]. The results revealed that approximately 97% of ovarian AGCTs had the FOXL2 mutation. Furthermore, it was noteworthy that the mutation was not observed in other sex cord/stromal cancers and any unrelated ovarian or breast cancers [14,15,75-77]. A recent survey of a large cohort provided further evidence supporting the finding that FOXL2 is pathognomonic for ovarian AGCT [63]. Reduced FOXL2 has been reported in aggressive JGCT [78]. The mechanism of FOXL2 in AGCT has been studied widely, and two studies have shown that phosphorylated FOXL2 contributes to the growth of the tumor [79,80]. However, an increasing number of studies have focused on the effect of FOXL2 mutation on the development of AGCT. The transcription target of FOXL2 mutation was explored in AGCT, and some researchers hypothesise that the mutation participates in the development of the malignancy by changing its interaction with other factors, including the transcription factor SAMD, BMP family and effectors of TGF-β [81]. A recent study reported that FOXL2C134W transcription-induced mutations play an important role in EMT and cancer resistance [82]. Although wild-type FOXL2 has a positive effect on regulating the proliferation and apoptosis of normal GCs, mutant FOXL2 not only downregulates the death receptors TNFR1 and Fas but also fails to elicit caspase-8-dependent apoptosis signalling [83]. These data reveal that FOXL2 is involved in the pathogenesis of AGCT through multiple pathways. Increasing evidence suggests that FOXL2 mutation may be a major driving force of AGCT; therefore, FOXL2 targeted therapy may offer unprecedented potential to eliminate oncogenes and prevent resistance to conventional chemotherapy.
Telomerase reverse transcriptase promoter
The telomerase reverse transcriptase (TERT) gene encodes a key catalytic subunit of telomerase, playing a role in controlling telomerase activity [84]. Generally, the TERT gene is strictly regulated in most differentiated cells, and the loss of TERT expression leads to a decrease in telomere activity, resulting in shortening during cell division [85]. However, telomere shortening leads to permanent cell growth arrest [86]. Tumor cells can maintain telomere length and telomerase activity by activating TERT to achieve immortality, which is a key step in tumorigenesis [84,86,87]. Telomerase activity is proportional to the expression of TERT in primary GCs, wherein the expression of TERT reduces significantly as the GCs differentiate into luteinized cells and the follicles develop into the corpus luteum [88,89]. Mutation of the TERT promoter is one of the factors involved in telomerase activation that mainly occurs in two hot spots of chromosome 5 (C228T and C250T), which was first identified in malignant melanoma [90,91]. Subsequent studies showed that TERT promoter mutation occurred in various cancers and has been considered for the diagnosis/monitoring of hepatocellular carcinoma, bladder cancer and glioblastoma [92-94].
A study conducted in 2021, using whole-genome sequencing, revealed a novel TERT promoter mutation at C228T in five of 10 AGCT samples [95]. In addition, the study demonstrated a higher frequency of TERT C228T promoter mutation in recurrent AGCTs compared with primary tumors. The findings were confirmed by multiple independent research groups, and a TERT promoter mutation at C250T was identified in a small number of patients [85,96,97].
Previous studies have found that the classic carcinogenic signalling pathways, including c-Myc, NFκB and Wnt/β-catenin may be involved in the transcriptional inhibition of TERT [98]. Notably, activation of the NFκB signalling pathway was observed in KGN cell lines, and p65 nuclear localization was observed in GCT [99,100]. Although studies have not identified mutations in these pathways currently, the findings provide a theoretical possibility that TERT promoter mutation participates in the pathogenesis of ovarian AGCT. Considering the rarity of ovarian AGCT and the limited sample size in previous studies, the involvement of TERT promoter mutation in the development of the disease needs to be verified further.
Treatment
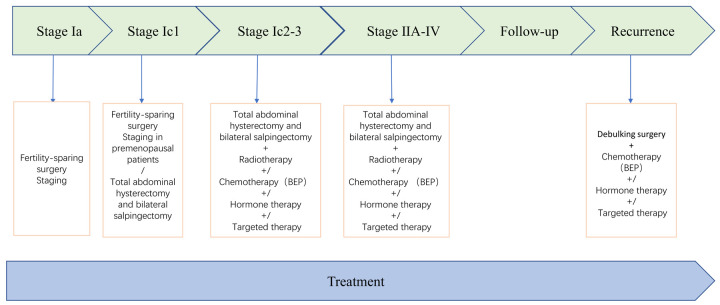
The evidence-based management of AGCT is limited considering the rarity of the condition. Currently, there are no standard international guidelines for the treatment of AGCT, and the International Federation of Gynaecology and Obstetrics (FIGO) staging system is applied routinely. Surgery remains the mainstay of treatment for primary and recurrent AGCT, and there is no other effective treatment. Approximately 80% of the patients with invasive or recurrent AGCT succumb to the disease, indicating the need for more effective treatments [83]. Treatments such as radiation and chemotherapy are not a routine option as for other tumors, and their effectiveness is currently being researched in randomised trials. Radiation, chemotherapy, hormone therapy, and targeted therapy may mitigate the disease and prolong the life of patients with advanced disease and those who cannot undergo surgery after relapse. The most commonly performed treatments are described in Figure 3.
Figure 3.
Treatment plan for AGCT patients.
Surgery
Total abdominal hysterectomy and bilateral salpingectomy are the standard methods for the treatment of primary ovarian AGCT [101]. The stage during primary surgery is crucial to the prognosis of the patients [102]. The majority of patients with ovarian AGCT present with stage IA disease, with tumors commonly confined to the ovary without metastasis [6].
Generally, pelvic and para-aortic lymph node resection is not recommended, and only large or suspected lymph nodes are removed during the surgery [103]. Surgery can be performed by either laparotomy or laparoscopy, and studies have reported that laparoscopic surgery has high safety and a low recurrence rate [104]. However, more conservative fertility-preserving surgery and unilateral salpingo-ovarian resection are recommended for patients with fertility needs when diagnosed at stage Ia. Several studies have shown that incomplete surgery is not a risk factor for recurrence or AGCT-related death [20,105]. It is worth noting that endometrial biopsy should be performed in patients who undergo conservative surgery to prevent the occurrence of endometrial cancer [106]. There is no clear conclusion about whether patients who choose fertility reservation surgery are undergoing radical surgery after childbirth.
Debulking surgery, when feasible, remains the most effective treatment for recurrent ovarian AGCT [101]. A recent study reported that the common sites of recurrence were the pelvis, liver and intestine, and most cases showed metastasis outside the pelvis [40]. Therefore, patients with recurrence may need multiple organ surgery to achieve optimal volume reduction [104,107]. Patients with a relapse who achieve optimal debulking might benefit from surgery [108].
Radiotherapy
Radiotherapy has been proposed and tested for recurrent disease, postoperative residual disease and palliative treatment [18,109-112]. Previous studies have reported a correlation between radiotherapy and prolonged disease-free survival in patients with advanced or recurrent AGCT. Postoperative radiotherapy has been reported to improve the survival of patients. However, there is limited data from prospective randomised studies regarding the role of radiotherapy in AGCT. Importantly, in the current reports due to the lack of sectional imaging, AGCT patients had significantly different response rates to chemotherapy. In addition, there are many controversies regarding the application of radiotherapy for the treatment of AGCT, including the dose used and the need for total abdominal radiotherapy.
Chemotherapy
In general, the prognosis of patients with stage Ia ovarian AGCT is favourable, and doctors do not recommend adjuvant treatment. Chemotherapy is an option to delay the progression of the disease in patients with advanced, metastatic disease and postoperative recurrence who have been unable to undergo surgery and radiotherapy. AGCT demonstrates a potential response to a single drug as well as combined chemotherapy regimens; however, the reaction rate varies. Chemotherapy does not appear to improve the patient’s condition, and the drugs are associated with toxicity. Considering the rarity of AGCT, the chemotherapy regimen is mainly based on that of EOC, which is a platinum-based combination therapy. The overall rate of effectiveness of the treatment has been reported as 63%-80% [113]. Currently, bleomycin/etoposide/cisplatin (BEP) is used in conventional chemotherapy for AGCT. However, it must be considered that the majority of the current reports rely on a retrospective study of unverified AGCT cohorts, which could be a potential confounding factor. A randomised phase II trial is underway, which compares BEP with paclitaxel/carboplatin in newly diagnosed and recurrent metastatic AGCT.
Hormone therapy and targeted therapy
Noting that AGCT not only express steroid hormone receptors but also have the hormone synthesis function of normal GCs, it is reasonable to treat unresectable AGCT patients with hormone-based methods. In addition, hormone therapy is recommended for patients who do not respond or develop resistance to conventional chemotherapy. Treatment options that have been explored include the use of GnRH agonists, aromatase inhibitors (AIs) and tamoxifen (selective estrogen receptor modulator). A study conducted on 31 patients receiving hormone therapy revealed that 25.8% of the patients achieved complete remission, and nearly half of the patients achieved partial remission [114]. Recently, the multicentre phase II PARAGON trial (ANZGOG-0903) reported that the aromatase inhibitors anastrozole has long-lasting clinical benefits and acceptable toxicity in some asymptomatic patients with ovarian cancer. Randomised trials related to hormone therapy have not been conducted, and published literature includes relatively few retrospective studies and case reports. It is noteworthy that the studies have not been conducted in FOXL2-defined cohorts, and the benefits of the treatments might be associated with a potential bias in patient selection and response assessment. Therefore, the clinical applicability of hormone therapy for ovarian AGCT needs further exploration. Hormone therapy can become an option for the treatment of patients with ovarian AGCT only based on sufficient experimental theory and data from randomised clinical trials.
The current chemotherapy and radiotherapy regimens have not yielded satisfactory results in the treatment of ovarian AGCT. Therefore, it is necessary to develop novel alternative therapies, especially when patients have been unable to pass surgical treatment. More importantly, targeted therapy is more selective and less toxic than chemotherapy. AGCTs have a high blood vessel system; therefore, antiangiogenic drugs have been used in cases of recurrent disease [115,116]. A study conducted at the MD Anderson Cancer Center highlighted the potential role of bevacizumab; however, the results must be interpreted with caution owing to the small sample size (n = 36 patients) [116]. An international randomised clinical trial, conducted by the Groupe d’Investigateurs Nationaux pour les Etudes des Cancers del’Ovaire (GINECO), confirmed that patients who could not be treated surgically did not show clinically significant outcomes following weekly paclitaxel plus bevacizumab treatment [117]. The high-frequency FOXL2 mutation suggests that it could be a potential therapeutic target for AGCT, and some FOX proteins, such as FOX3A, are now considered therapeutic targets [118-120]. Nonetheless, the biological treatment strategy of directly targeting FOX proteins is in its infancy and the high sequence homology between FOX transcription factor family members. The therapeutic drugs targeting FOXL2 might inadvertently affect the regulation of other important FOX transcription factors, thus complicating the theoretical hypothesis of weakening or enhancing a single FOX protein.
Recurrence and follow-up
Research has elucidated that approximately one-third of AGCT patients experience relapse, and more than 50% of these patients succumb to their disease [63,106]. Therefore, close monitoring after surgery and regular follow-up is required to detect relapse at an early stage, which allows specialists to discuss treatment options and maximize potential survival in patients eligible for secondary cytopenia. The progression of AGCT is slow, resulting in an irregular incubation period for recurrence, with some patients experiencing relapse 30 to 40 years after surgery [121]. Therefore, regular follow-up is necessary. Although CA125 is considered the primary indicator of EOC recurrence, its relevance as a marker for AGCT follow-up is controversial [41,122]. Routine follow-up of patients with AGCT includes standard gynaecological examination and the detection of serum markers. The most common site of recurrence is the pelvis, thereby necessitating a standard gynaecological examination. However, more than half of the patients tend to have extrapelvic spread; therefore, the detection of serum markers is an important aspect of the follow-up regimen for patients with ovarian AGCT. Recent studies have suggested that inhibin can be used as the chief indicator of ovarian AGCT during follow-up [52,123]. In addition, a study reported a significant increase in the serum AMH level in patients demonstrating AGCT recurrence; thus, AMH can be used as an indicator during follow-up [124]. The recommendations for follow-up after initial surgery are presented in Table 1.
Table 1.
Recommendations for the follow-up of patients with ovarian AGCT
| Follow-up of patients with AGCT | |
|---|---|
| Physical examination | Standard gynecological examination |
| Radiographic inspection | Computed tomography scan, Chest X-ray |
| Detection of serum markers | AMH, inhibin, estradiol |
| Molecular diagnosis | FOXL2, TERT promoter, TP53 |
| Cycle (>15 years) | First year: every 3 months |
| Second to fifth year: every 4 months | |
| After fifth year: every 6 months | |
Quality of life and symptom management
Given the slow progression of AGCT, patients may undergo a variety of treatments and experience side effects, which can significantly affect their quality of life. Efforts are being made to integrate the patients’ perspectives in clinical therapies that aim not only to prolong but also improve their quality of life. Implementing this idea will mean that multiple experts balance the safety and risks of treatment, especially when adjuvant therapy is not reliable at present. High post-recurrence mortality is also a common phenomenon in patients with ovarian AGCT, which is the most destructive event after the first treatment. More than half of the patients who experience relapse once are likely to have repeated recurrence, indicating a pattern similar to EOC. With each recurrence, the median time to subsequent recurrence is shortened by half, which significantly affects the quality of life of patients [40]. However, improving the quality of life of patients remains a challenge as existing treatments have failed to improve the situation, thus emphasising the need for early initiation of palliative care [125].
Exploring and future
The origin of AGCT has long been confirmed; however, there is no reasonable explanation regarding the evolution of normal GCs into AGCT. The development of a tumor requires the participation of multiple signalling pathways. A series of in vivo and in vitro experiments have been conducted focusing on the hormonal activity, FOXL2 mutation and the common carcinogenic pathways to understand better the pathogenesis of AGCT and identify key therapeutic targets. These experiments provide biological insights to clarify the pathogenesis of AGCT (Table 2). Nonetheless, the outcomes have not been confirmed via clinical trials. Given the rarity of AGCT, successful clinical trials cannot be carried out without international cooperation. Moreover, clinical trials tend to focus on the main carcinogenic signalling pathways, including the PI3K/AKT and MAPK signalling pathways.
Table 2.
Exploring the pathogenesis of ovarian AGCT
| Pathway/Factor | In vitro | In vivo | Ref. |
|---|---|---|---|
| Notch signalling pathway | KGN cell line | [126,127] | |
| BMP signalling pathway | BMPr1a-/- mice, BMPr1b-/- mice | [128,129] | |
| SMAD 4 | SMAD4loxp/loxp AMHR2 Cre mice | [130] | |
| FOXL2-SMAD4 | SMAD4-/-FOXL2-/- mice, FSHD-/- mice | [131] | |
| NFKB signalling pathway | KGN cell line | [132] | |
| PI3K/AKT signalling pathway | FOXO1/3-/- mice | [133] | |
| PI3K/AKT signalling pathway and Wnt signalling pathway | Ptenflox/flox, AMHRcre/+ mice | [133] | |
| GATA4 | KGN cell line | [58,134] | |
| Inhibin | Inhα-/- mice | [135] | |
| Estrogen/FOXL2 signalling pathway | FOXL2-/- KGN cell line; KGN cell line; COV434 cell line | [80] | |
| GATA4-Bcl-2 | KK-1 cell line; COS-7 cell line; KGN cell line | [136] | |
| Activin A/PI3K signalling pathway | Cre+ mice | [137] | |
| Activin/SMAD2/3 signalling pathway | SMAD3-/- mice | [138] |
Determining the pathogenesis of AGCT is beneficial for the development of individualized drugs and the management of patients, especially those with postoperative recurrence. Individualized drugs may determine the key therapeutic targets for AGCT, allowing clinicians to provide more appropriate treatment options for individual patients rather than treating multiple patients with different responses with the same drug. Personalised medicine considers the heterogeneity of tumor response to drugs, thereby permitting the selection and treatment of patients with a good response to a certain drug.
Conclusion
AGCT is a subtype of ovarian cancer with low incidence and a favourable prognosis. The high recurrence rate is the key factor leading to the death of patients. Surgery is the most important treatment for AGCT, and the effectiveness of adjuvant therapy is not ideal. Therefore, there is an urgent need to explore new treatment strategies. Considering the rarity of AGCT, clinical trials conducted with international cooperation are essential to identify novel treatments. Currently, the pathogenesis of AGCT remains unclear, although several studies have shown that FOXL2 mutation and some carcinogenic signalling pathways and factors may be involved in the development of the disease (Figure 4). Exploratory research is being carried out to hypothesis-driven experiments and transformation research. Ovarian AGCT progresses slowly and has a long incubation period, which poses a significant challenge in managing the quality of life of patients. Establishing a strong multidisciplinary network will combine research with clinical practice, which would be beneficial to treat patients and help them manage their daily living activities. The significance of cancer treatment depends on the improvement in clinical symptoms and quality of life of patients. Future explorations should integrate the perspectives of the patients for holistic outcomes.
Figure 4.
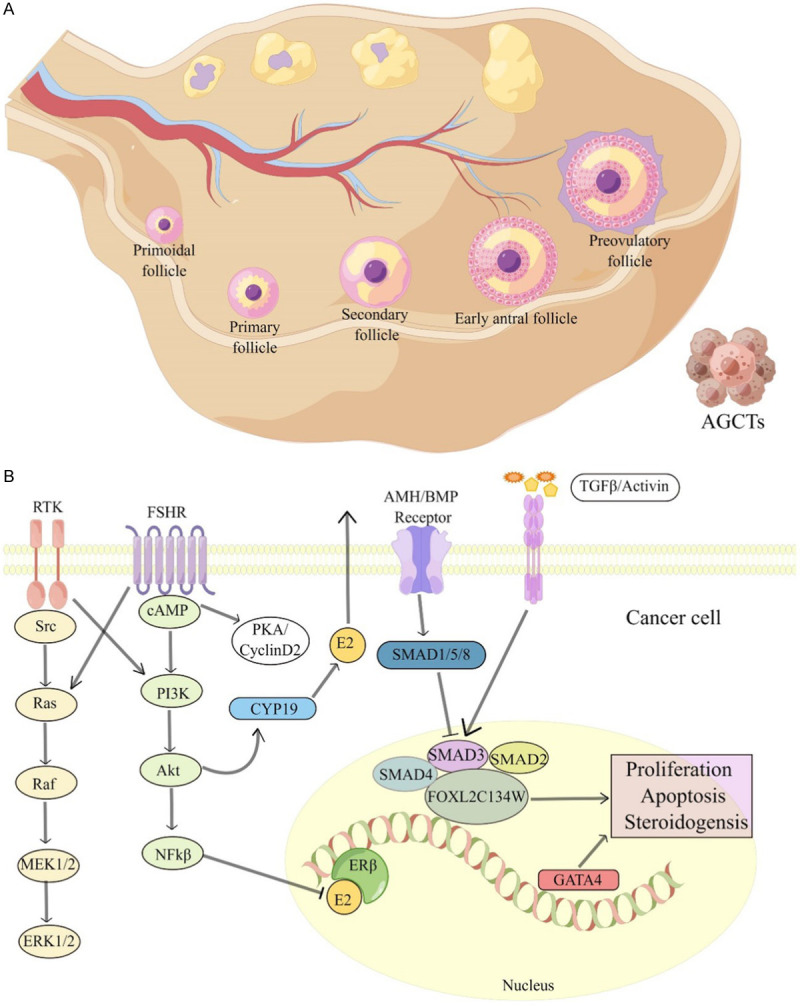
The evolution and pathogenesis of AGCT. A. Schematic representation of AGCT. The occurrence of AGCT needs to undergo a cyclical process of follicular formation, and ultimately originates from the proliferation of follicular granulosa cells before ovulation. B. Various pathways of granulosa cells and granulosa cells involved in proliferation may be involved in AGCT tumorigenesis.
Acknowledgements
This work was supported by grants from the National Natural Science Foundation of China [Grant number: 81802616]. The authors would like to thank Bullet Edits Limited (http://bulletedits.cn) for its linguistic assistance during the preparation of this manuscript.
Disclosure of conflict of interest
None.
References
- 1.Evans AT 3rd, Gaffey TA, Malkasian GD Jr, Annegers JF. Clinicopathologic review of 118 granulosa and 82 theca cell tumors. Obstet Gynecol. 1980;55:231–238. [PubMed] [Google Scholar]
- 2.Pectasides D, Pectasides E, Psyrri A. Granulosa cell tumor of the ovary. Cancer Treat Rev. 2008;34:1–12. doi: 10.1016/j.ctrv.2007.08.007. [DOI] [PubMed] [Google Scholar]
- 3.Fox H, Agrawal K, Langley FA. A clinicopathologic study of 92 cases of granulosa cell tumor of the ovary with special reference to the factors influencing prognosis. Cancer. 1975;35:231–241. doi: 10.1002/1097-0142(197501)35:1<231::aid-cncr2820350128>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 4.Unkila-Kallio L, Tiitinen A, Wahlstrom T, Lehtovirta P, Leminen A. Reproductive features in women developing ovarian granulosa cell tumour at a fertile age. Hum Reprod. 2000;15:589–593. doi: 10.1093/humrep/15.3.589. [DOI] [PubMed] [Google Scholar]
- 5.Jamieson S, Fuller PJ. Molecular pathogenesis of granulosa cell tumors of the ovary. Endocr Rev. 2012;33:109–144. doi: 10.1210/er.2011-0014. [DOI] [PubMed] [Google Scholar]
- 6.Schumer ST, Cannistra SA. Granulosa cell tumor of the ovary. J. Clin. Oncol. 2003;21:1180–1189. doi: 10.1200/JCO.2003.10.019. [DOI] [PubMed] [Google Scholar]
- 7.Scully RE. Juvenile granulosa cell tumor. Pediatr Pathol. 1988;8:423–427. doi: 10.3109/15513818809041579. [DOI] [PubMed] [Google Scholar]
- 8.Young RH, Dickersin GR, Scully RE. Juvenile granulosa cell tumor of the ovary. A clinicopathological analysis of 125 cases. Am J Surg Pathol. 1984;8:575–596. doi: 10.1097/00000478-198408000-00002. [DOI] [PubMed] [Google Scholar]
- 9.Savage P, Constenla D, Fisher C, Shepherd JH, Barton DP, Blake P, Gore ME. Granulosa cell tumours of the ovary: demographics, survival and the management of advanced disease. Clin Oncol (R Coll Radiol) 1998;10:242–245. doi: 10.1016/s0936-6555(98)80008-3. [DOI] [PubMed] [Google Scholar]
- 10.Schwartz PE, Smith JP. Treatment of ovarian stromal tumors. Am J Obstet Gynecol. 1976;125:402–411. doi: 10.1016/0002-9378(76)90577-9. [DOI] [PubMed] [Google Scholar]
- 11.Gusberg SB, Kardon P. Proliferative endometrial response to theca-granulosa cell tumors. Am J Obstet Gynecol. 1971;111:633–643. doi: 10.1016/0002-9378(71)90965-3. [DOI] [PubMed] [Google Scholar]
- 12.Case records of the Massachusetts General Hospital. Weekly clinicopathological exercises. Case 10-1995. A 56-year-old woman with abdominal pain, anemia, and a pelvic mass. N Engl J Med. 1995;332:876–881. doi: 10.1056/NEJM199503303321308. [DOI] [PubMed] [Google Scholar]
- 13.Shah SP, Kobel M, Senz J, Morin RD, Clarke BA, Wiegand KC, Leung G, Zayed A, Mehl E, Kalloger SE, Sun M, Giuliany R, Yorida E, Jones S, Varhol R, Swenerton KD, Miller D, Clement PB, Crane C, Madore J, Provencher D, Leung P, DeFazio A, Khattra J, Turashvili G, Zhao Y, Zeng T, Glover JN, Vanderhyden B, Zhao C, Parkinson CA, Jimenez-Linan M, Bowtell DD, Mes-Masson AM, Brenton JD, Aparicio SA, Boyd N, Hirst M, Gilks CB, Marra M, Huntsman DG. Mutation of FOXL2 in granulosa-cell tumors of the ovary. N Engl J Med. 2009;360:2719–2729. doi: 10.1056/NEJMoa0902542. [DOI] [PubMed] [Google Scholar]
- 14.Jamieson S, Butzow R, Andersson N, Alexiadis M, Unkila-Kallio L, Heikinheimo M, Fuller PJ, Anttonen M. The FOXL2 C134W mutation is characteristic of adult granulosa cell tumors of the ovary. Mod Pathol. 2010;23:1477–1485. doi: 10.1038/modpathol.2010.145. [DOI] [PubMed] [Google Scholar]
- 15.Kim MS, Hur SY, Yoo NJ, Lee SH. Mutational analysis of FOXL2 codon 134 in granulosa cell tumour of ovary and other human cancers. J Pathol. 2010;221:147–152. doi: 10.1002/path.2688. [DOI] [PubMed] [Google Scholar]
- 16.Al-Agha OM, Huwait HF, Chow C, Yang W, Senz J, Kalloger SE, Huntsman DG, Young RH, Gilks CB. FOXL2 is a sensitive and specific marker for sex cord-stromal tumors of the ovary. Am J Surg Pathol. 2011;35:484–494. doi: 10.1097/PAS.0b013e31820a406c. [DOI] [PubMed] [Google Scholar]
- 17.Gershon R, Aviel-Ronen S, Korach J, Daniel-Carmi V, Avivi C, Bar-Ilan D, Barshack I, Meirow D, Ben-Baruch G, Cohen Y. FOXL2 C402G mutation detection using MALDI-TOF-MS in DNA extracted from Israeli granulosa cell tumors. Gynecol Oncol. 2011;122:580–584. doi: 10.1016/j.ygyno.2011.05.008. [DOI] [PubMed] [Google Scholar]
- 18.Malmstrom H, Hogberg T, Risberg B, Simonsen E. Granulosa cell tumors of the ovary: prognostic factors and outcome. Gynecol Oncol. 1994;52:50–55. doi: 10.1006/gyno.1994.1010. [DOI] [PubMed] [Google Scholar]
- 19.Ohel G, Kaneti H, Schenker JG. Granulosa cell tumors in Israel: a study of 172 cases. Gynecol Oncol. 1983;15:278–286. doi: 10.1016/0090-8258(83)90083-5. [DOI] [PubMed] [Google Scholar]
- 20.Colombo N, Parma G, Zanagnolo V, Insinga A. Management of ovarian stromal cell tumors. J. Clin. Oncol. 2007;25:2944–2951. doi: 10.1200/JCO.2007.11.1005. [DOI] [PubMed] [Google Scholar]
- 21.Mayr D, Kaltz-Wittmer C, Arbogast S, Amann G, Aust DE, Diebold J. Characteristic pattern of genetic aberrations in ovarian granulosa cell tumors. Mod Pathol. 2002;15:951–957. doi: 10.1097/01.MP.0000024290.55261.14. [DOI] [PubMed] [Google Scholar]
- 22.Lin YS, Eng HL, Jan YJ, Lee HS, Ho WL, Liou CP, Lee WY, Tzeng CC. Molecular cytogenetics of ovarian granulosa cell tumors by comparative genomic hybridization. Gynecol Oncol. 2005;97:68–73. doi: 10.1016/j.ygyno.2004.12.014. [DOI] [PubMed] [Google Scholar]
- 23.Hemminki A, Tomlinson I, Markie D, Jarvinen H, Sistonen P, Bjorkqvist AM, Knuutila S, Salovaara R, Bodmer W, Shibata D, de la Chapelle A, Aaltonen LA. Localization of a susceptibility locus for Peutz-Jeghers syndrome to 19p using comparative genomic hybridization and targeted linkage analysis. Nat Genet. 1997;15:87–90. doi: 10.1038/ng0197-87. [DOI] [PubMed] [Google Scholar]
- 24.Hemminki A, Markie D, Tomlinson I, Avizienyte E, Roth S, Loukola A, Bignell G, Warren W, Aminoff M, Hoglund P, Jarvinen H, Kristo P, Pelin K, Ridanpaa M, Salovaara R, Toro T, Bodmer W, Olschwang S, Olsen AS, Stratton MR, de la Chapelle A, Aaltonen LA. A serine/threonine kinase gene defective in Peutz-Jeghers syndrome. Nature. 1998;391:184–187. doi: 10.1038/34432. [DOI] [PubMed] [Google Scholar]
- 25.Christian CD, McLoughlin TG, Cathcart ER, Eisenberg MM. Peutz-Jeghers syndrome associated with functioning ovarian tumor. JAMA. 1964;190:935–938. doi: 10.1001/jama.1964.03070230071027. [DOI] [PubMed] [Google Scholar]
- 26.Hart WR, Kumar N, Crissman JD. Ovarian neoplasms resembling sex cord tumors with annular tubules. Cancer. 1980;45:2352–2363. doi: 10.1002/1097-0142(19800501)45:9<2352::aid-cncr2820450920>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 27.Young RH, Welch WR, Dickersin GR, Scully RE. Ovarian sex cord tumor with annular tubules: review of 74 cases including 27 with Peutz-Jeghers syndrome and four with adenoma malignum of the cervix. Cancer. 1982;50:1384–1402. doi: 10.1002/1097-0142(19821001)50:7<1384::aid-cncr2820500726>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 28.Willemsen W, Kruitwagen R, Bastiaans B, Hanselaar T, Rolland R. Ovarian stimulation and granulosa-cell tumour. Lancet. 1993;341:986–988. doi: 10.1016/0140-6736(93)91071-s. [DOI] [PubMed] [Google Scholar]
- 29.Venn A, Healy D, McLachlan R. Cancer risks associated with the diagnosis of infertility. Best Pract Res Clin Obstet Gynaecol. 2003;17:343–367. doi: 10.1016/s1521-6934(02)00128-1. [DOI] [PubMed] [Google Scholar]
- 30.Venn A, Watson L, Bruinsma F, Giles G, Healy D. Risk of cancer after use of fertility drugs with in-vitro fertilisation. Lancet. 1999;354:1586–1590. doi: 10.1016/S0140-6736(99)05203-4. [DOI] [PubMed] [Google Scholar]
- 31.Stenwig JT, Hazekamp JT, Beecham JB. Granulosa cell tumors of the ovary. A clinicopathological study of 118 cases with long-term follow-up. Gynecol Oncol. 1979;7:136–152. doi: 10.1016/0090-8258(79)90090-8. [DOI] [PubMed] [Google Scholar]
- 32.Cronje HS, Niemand I, Bam RH, Woodruff JD. Review of the granulosa-theca cell tumors from the emil Novak ovarian tumor registry. Am J Obstet Gynecol. 1999;180:323–327. doi: 10.1016/s0002-9378(99)70207-3. [DOI] [PubMed] [Google Scholar]
- 33.Lappohn RE, Burger HG, Bouma J, Bangah M, Krans M. Inhibin as a marker for granulosa cell tumor. Acta Obstet Gynecol Scand Suppl. 1992;155:61–65. doi: 10.1111/j.1600-0412.1992.tb00008.x. [DOI] [PubMed] [Google Scholar]
- 34.Segal R, DePetrillo AD, Thomas G. Clinical review of adult granulosa cell tumors of the ovary. Gynecol Oncol. 1995;56:338–344. doi: 10.1006/gyno.1995.1060. [DOI] [PubMed] [Google Scholar]
- 35.Ko SF, Wan YL, Ng SH, Lee TY, Lin JW, Chen WJ, Kung FT, Tsai CC. Adult ovarian granulosa cell tumors: spectrum of sonographic and CT findings with pathologic correlation. AJR Am J Roentgenol. 1999;172:1227–1233. doi: 10.2214/ajr.172.5.10227493. [DOI] [PubMed] [Google Scholar]
- 36.Kim SH, Kim SH. Granulosa cell tumor of the ovary: common findings and unusual appearances on CT and MR. J Comput Assist Tomogr. 2002;26:756–761. doi: 10.1097/00004728-200209000-00016. [DOI] [PubMed] [Google Scholar]
- 37.van der Vorst MJ, Bleeker MC, Boven E. Unusual presentation of granulosa cell tumor of the ovary. J. Clin. Oncol. 2010;28:e554–e556. doi: 10.1200/JCO.2010.29.6905. [DOI] [PubMed] [Google Scholar]
- 38.Huang YT, Lee JC, Kumar AS. Variable F-18 fluorodeoxyglucose avidity of metastatic recurrent adult granulosa cell tumor. Clin Nucl Med. 2009;34:710–712. doi: 10.1097/RLU.0b013e3181b539e4. [DOI] [PubMed] [Google Scholar]
- 39.Raj G, Proietto A, Jaaback K. Positron emission tomography and granulosa cell tumor recurrence: a report of 2 cases. Int J Gynecol Cancer. 2009;19:1542–1544. doi: 10.1111/IGC.0b013e3181a84819. [DOI] [PubMed] [Google Scholar]
- 40.Bryk S, Farkkila A, Butzow R, Leminen A, Tapper J, Heikinheimo M, Unkila-Kallio L, Riska A. Characteristics and outcome of recurrence in molecularly defined adult-type ovarian granulosa cell tumors. Gynecol Oncol. 2016;143:571–577. doi: 10.1016/j.ygyno.2016.10.002. [DOI] [PubMed] [Google Scholar]
- 41.Tak JY, Chong GO, Park JY, Lee SJ, Lee YH, Hong DG. Adult granulosa cell tumor presenting with massive ascites, elevated CA-125 level, and low (18)F-fluorodeoxyglucose uptake on positron emission tomography/computed tomography. Obstet Gynecol Sci. 2015;58:423–426. doi: 10.5468/ogs.2015.58.5.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Miller BE, Barron BA, Wan JY, Delmore JE, Silva EG, Gershenson DM. Prognostic factors in adult granulosa cell tumor of the ovary. Cancer. 1997;79:1951–1955. doi: 10.1002/(sici)1097-0142(19970515)79:10<1951::aid-cncr16>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 43.Burger HG. What do inhibin measurements tell a clinician today? Ann Med. 1992;24:419–421. doi: 10.3109/07853899209166988. [DOI] [PubMed] [Google Scholar]
- 44.Thompson TB, Cook RW, Chapman SC, Jardetzky TS, Woodruff TK. Beta A versus beta B: is it merely a matter of expression? Mol Cell Endocrinol. 2004;225:9–17. doi: 10.1016/j.mce.2004.02.007. [DOI] [PubMed] [Google Scholar]
- 45.Stenvers KL, Findlay JK. Inhibins: from reproductive hormones to tumor suppressors. Trends Endocrinol Metab. 2010;21:174–180. doi: 10.1016/j.tem.2009.11.009. [DOI] [PubMed] [Google Scholar]
- 46.Sehested A, Juul AA, Andersson AM, Petersen JH, Jensen TK, Muller J, Skakkebaek NE. Serum inhibin A and inhibin B in healthy prepubertal, pubertal, and adolescent girls and adult women: relation to age, stage of puberty, menstrual cycle, follicle-stimulating hormone, luteinizing hormone, and estradiol levels. J Clin Endocrinol Metab. 2000;85:1634–1640. doi: 10.1210/jcem.85.4.6512. [DOI] [PubMed] [Google Scholar]
- 47.Hsueh AJ, Dahl KD, Vaughan J, Tucker E, Rivier J, Bardin CW, Vale W. Heterodimers and homodimers of inhibin subunits have different paracrine action in the modulation of luteinizing hormone-stimulated androgen biosynthesis. Proc Natl Acad Sci U S A. 1987;84:5082–5086. doi: 10.1073/pnas.84.14.5082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.McLachlan RI, Robertson DM, De Kretser DM, Burger HG. Advances in the physiology of inhibin and inhibin-related peptides. Clin Endocrinol (Oxf) 1988;29:77–112. doi: 10.1111/j.1365-2265.1988.tb00251.x. [DOI] [PubMed] [Google Scholar]
- 49.Burger HG. Evidence for a negative feedback role of inhibin in follicle stimulating hormone regulation in women. Hum Reprod. 1993;8(Suppl 2):129–132. doi: 10.1093/humrep/8.suppl_2.129. [DOI] [PubMed] [Google Scholar]
- 50.Burger HG, Robertson DM, Cahir N, Mamers P, Healy DL, Jobling T, Groome N. Characterization of inhibin immunoreactivity in post-menopausal women with ovarian tumours. Clin Endocrinol (Oxf) 1996;44:413–418. doi: 10.1046/j.1365-2265.1996.627450.x. [DOI] [PubMed] [Google Scholar]
- 51.Groome NP, Illingworth PJ, O’Brien M, Pai R, Rodger FE, Mather JP, McNeilly AS. Measurement of dimeric inhibin B throughout the human menstrual cycle. J Clin Endocrinol Metab. 1996;81:1401–1405. doi: 10.1210/jcem.81.4.8636341. [DOI] [PubMed] [Google Scholar]
- 52.Lappohn RE, Burger HG, Bouma J, Bangah M, Krans M, de Bruijn HW. Inhibin as a marker for granulosa-cell tumors. N Engl J Med. 1989;321:790–793. doi: 10.1056/NEJM198909213211204. [DOI] [PubMed] [Google Scholar]
- 53.Petraglia F, Luisi S, Pautier P, Sabourin JC, Rey R, Lhomme C, Bidart JM. Inhibin B is the major form of inhibin/activin family secreted by granulosa cell tumors. J Clin Endocrinol Metab. 1998;83:1029–1032. doi: 10.1210/jcem.83.3.4800. [DOI] [PubMed] [Google Scholar]
- 54.Robertson DM, Cahir N, Burger HG, Mamers P, Groome N. Inhibin forms in serum from postmenopausal women with ovarian cancers. Clin Endocrinol (Oxf) 1999;50:381–386. doi: 10.1046/j.1365-2265.1999.00656.x. [DOI] [PubMed] [Google Scholar]
- 55.Mom CH, Engelen MJ, Willemse PH, Gietema JA, ten Hoor KA, de Vries EG, van der Zee AG. Granulosa cell tumors of the ovary: the clinical value of serum inhibin A and B levels in a large single center cohort. Gynecol Oncol. 2007;105:365–372. doi: 10.1016/j.ygyno.2006.12.034. [DOI] [PubMed] [Google Scholar]
- 56.Robertson DM, Pruysers E, Burger HG, Jobling T, McNeilage J, Healy D. Inhibins and ovarian cancer. Mol Cell Endocrinol. 2004;225:65–71. doi: 10.1016/j.mce.2004.02.014. [DOI] [PubMed] [Google Scholar]
- 57.Chang HL, Pahlavan N, Halpern EF, MacLaughlin DT. Serum Mullerian Inhibiting Substance/anti-Mullerian hormone levels in patients with adult granulosa cell tumors directly correlate with aggregate tumor mass as determined by pathology or radiology. Gynecol Oncol. 2009;114:57–60. doi: 10.1016/j.ygyno.2009.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Weenen C, Laven JS, Von Bergh AR, Cranfield M, Groome NP, Visser JA, Kramer P, Fauser BC, Themmen AP. Anti-Mullerian hormone expression pattern in the human ovary: potential implications for initial and cyclic follicle recruitment. Mol Hum Reprod. 2004;10:77–83. doi: 10.1093/molehr/gah015. [DOI] [PubMed] [Google Scholar]
- 59.Anttonen M, Unkila-Kallio L, Leminen A, Butzow R, Heikinheimo M. High GATA-4 expression associates with aggressive behavior, whereas low anti-Mullerian hormone expression associates with growth potential of ovarian granulosa cell tumors. J Clin Endocrinol Metab. 2005;90:6529–6535. doi: 10.1210/jc.2005-0921. [DOI] [PubMed] [Google Scholar]
- 60.Rey RA, Lhomme C, Marcillac I, Lahlou N, Duvillard P, Josso N, Bidart JM. Antimullerian hormone as a serum marker of granulosa cell tumorsof the ovary: comparative study with serum alpha-inhibin and estradiol. Am J Obstet Gynecol. 1996;174:958–965. doi: 10.1016/s0002-9378(96)70333-2. [DOI] [PubMed] [Google Scholar]
- 61.Kaye SB, Davies E. Cyclophosphamide, adriamycin, and cis-platinum for the treatment of advanced granulosa cell tumor, using serum estradiol as a tumor marker. Gynecol Oncol. 1986;24:261–264. doi: 10.1016/0090-8258(86)90035-1. [DOI] [PubMed] [Google Scholar]
- 62.Vogelstein B, Kinzler KW. The path to cancer --three strikes and you’re out. N Engl J Med. 2015;373:1895–1898. doi: 10.1056/NEJMp1508811. [DOI] [PubMed] [Google Scholar]
- 63.McConechy MK, Farkkila A, Horlings HM, Talhouk A, Unkila-Kallio L, van Meurs HS, Yang W, Rozenberg N, Andersson N, Zaby K, Bryk S, Butzow R, Halfwerk JB, Hooijer GK, van de Vijver MJ, Buist MR, Kenter GG, Brucker SY, Kramer B, Staebler A, Bleeker MC, Heikinheimo M, Kommoss S, Blake Gilks C, Anttonen M, Huntsman DG. Molecularly defined adult granulosa cell tumor of the ovary: the clinical phenotype. J Natl Cancer Inst. 2016;108:djw134. doi: 10.1093/jnci/djw134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Li J, Bao R, Peng S, Zhang C. The molecular mechanism of ovarian granulosa cell tumors. J Ovarian Res. 2018;11:13. doi: 10.1186/s13048-018-0384-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cocquet J, De Baere E, Gareil M, Pannetier M, Xia X, Fellous M, Veitia RA. Structure, evolution and expression of the FOXL2 transcription unit. Cytogenet Genome Res. 2003;101:206–211. doi: 10.1159/000074338. [DOI] [PubMed] [Google Scholar]
- 66.Cocquet J, Pailhoux E, Jaubert F, Servel N, Xia X, Pannetier M, De Baere E, Messiaen L, Cotinot C, Fellous M, Veitia RA. Evolution and expression of FOXL2. J Med Genet. 2002;39:916–921. doi: 10.1136/jmg.39.12.916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Crisponi L, Deiana M, Loi A, Chiappe F, Uda M, Amati P, Bisceglia L, Zelante L, Nagaraja R, Porcu S, Ristaldi MS, Marzella R, Rocchi M, Nicolino M, Lienhardt-Roussie A, Nivelon A, Verloes A, Schlessinger D, Gasparini P, Bonneau D, Cao A, Pilia G. The putative forkhead transcription factor FOXL2 is mutated in blepharophimosis/ptosis/epicanthus inversus syndrome. Nat Genet. 2001;27:159–166. doi: 10.1038/84781. [DOI] [PubMed] [Google Scholar]
- 68.Garcia-Ortiz JE, Pelosi E, Omari S, Nedorezov T, Piao Y, Karmazin J, Uda M, Cao A, Cole SW, Forabosco A, Schlessinger D, Ottolenghi C. Foxl2 functions in sex determination and histogenesis throughout mouse ovary development. BMC Dev Biol. 2009;9:36. doi: 10.1186/1471-213X-9-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ottolenghi C, Omari S, Garcia-Ortiz JE, Uda M, Crisponi L, Forabosco A, Pilia G, Schlessinger D. Foxl2 is required for commitment to ovary differentiation. Hum Mol Genet. 2005;14:2053–2062. doi: 10.1093/hmg/ddi210. [DOI] [PubMed] [Google Scholar]
- 70.Schmidt D, Ovitt CE, Anlag K, Fehsenfeld S, Gredsted L, Treier AC, Treier M. The murine winged-helix transcription factor Foxl2 is required for granulosa cell differentiation and ovary maintenance. Development. 2004;131:933–942. doi: 10.1242/dev.00969. [DOI] [PubMed] [Google Scholar]
- 71.Uda M, Ottolenghi C, Crisponi L, Garcia JE, Deiana M, Kimber W, Forabosco A, Cao A, Schlessinger D, Pilia G. Foxl2 disruption causes mouse ovarian failure by pervasive blockage of follicle development. Hum Mol Genet. 2004;13:1171–1181. doi: 10.1093/hmg/ddh124. [DOI] [PubMed] [Google Scholar]
- 72.Uhlenhaut NH, Jakob S, Anlag K, Eisenberger T, Sekido R, Kress J, Treier AC, Klugmann C, Klasen C, Holter NI, Riethmacher D, Schutz G, Cooney AJ, Lovell-Badge R, Treier M. Somatic sex reprogramming of adult ovaries to testes by FOXL2 ablation. Cell. 2009;139:1130–1142. doi: 10.1016/j.cell.2009.11.021. [DOI] [PubMed] [Google Scholar]
- 73.Crisponi L, Uda M, Deiana M, Loi A, Nagaraja R, Chiappe F, Schlessinger D, Cao A, Pilia G. FOXL2 inactivation by a translocation 171 kb away: analysis of 500 kb of chromosome 3 for candidate long-range regulatory sequences. Genomics. 2004;83:757–764. doi: 10.1016/j.ygeno.2003.11.010. [DOI] [PubMed] [Google Scholar]
- 74.Beysen D, Vandesompele J, Messiaen L, De Paepe A, De Baere E. The human FOXL2 mutation database. Hum Mutat. 2004;24:189–193. doi: 10.1002/humu.20079. [DOI] [PubMed] [Google Scholar]
- 75.Rosario R, Wilson M, Cheng WT, Payne K, Cohen PA, Fong P, Shelling AN. Adult granulosa cell tumours (GCT): clinicopathological outcomes including FOXL2 mutational status and expression. Gynecol Oncol. 2013;131:325–329. doi: 10.1016/j.ygyno.2013.08.031. [DOI] [PubMed] [Google Scholar]
- 76.Nolan A, Joseph NM, Sangoi AR, Rabban J, Zaloudek C, Garg K. FOXL2 mutation status in granulosa theca cell tumors of the ovary. Int J Gynecol Pathol. 2017;36:568–574. doi: 10.1097/PGP.0000000000000377. [DOI] [PubMed] [Google Scholar]
- 77.D’Angelo E, Mozos A, Nakayama D, Espinosa I, Catasus L, Munoz J, Prat J. Prognostic significance of FOXL2 mutation and mRNA expression in adult and juvenile granulosa cell tumors of the ovary. Mod Pathol. 2011;24:1360–1367. doi: 10.1038/modpathol.2011.95. [DOI] [PubMed] [Google Scholar]
- 78.Kalfa N, Philibert P, Patte C, Ecochard A, Duvillard P, Baldet P, Jaubert F, Fellous M, Sultan C. Extinction of FOXL2 expression in aggressive ovarian granulosa cell tumors in children. Fertil Steril. 2007;87:896–901. doi: 10.1016/j.fertnstert.2006.11.016. [DOI] [PubMed] [Google Scholar]
- 79.Pisarska MD, Kuo FT, Bentsi-Barnes IK, Khan S, Barlow GM. LATS1 phosphorylates forkhead L2 and regulates its transcriptional activity. Am J Physiol Endocrinol Metab. 2010;299:E101–E109. doi: 10.1152/ajpendo.00534.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wu J, Miao C, Lv X, Zhang Y, Li Y, Wang D. Estrogen regulates forkhead transcription factor 2 to promote apoptosis of human ovarian granulosa-like tumor cells. J Steroid Biochem Mol Biol. 2019;194:105418. doi: 10.1016/j.jsbmb.2019.105418. [DOI] [PubMed] [Google Scholar]
- 81.Kobel M, Gilks CB, Huntsman DG. Adult-type granulosa cell tumors and FOXL2 mutation. Cancer Res. 2009;69:9160–9162. doi: 10.1158/0008-5472.CAN-09-2669. [DOI] [PubMed] [Google Scholar]
- 82.Weis-Banke SE, Lerdrup M, Kleine-Kohlbrecher D, Mohammad F, Sidoli S, Jensen ON, Yanase T, Nakamura T, Iwase A, Stylianou A, Abu-Rustum NR, Aghajanian C, Soslow R, Da Cruz Paula A, Koche RP, Weigelt B, Christensen J, Helin K, Cloos PAC. Mutant FOXL2(C134W) hijacks SMAD4 and SMAD2/3 to drive adult granulosa cell tumors. Cancer Res. 2020;80:3466–3479. doi: 10.1158/0008-5472.CAN-20-0259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Leung DTH, Fuller PJ, Chu S. Impact of FOXL2 mutations on signaling in ovarian granulosa cell tumors. Int J Biochem Cell Biol. 2016;72:51–54. doi: 10.1016/j.biocel.2016.01.003. [DOI] [PubMed] [Google Scholar]
- 84.Shay JW, Wright WE. Telomeres and telomerase: three decades of progress. Nat Rev Genet. 2019;20:299–309. doi: 10.1038/s41576-019-0099-1. [DOI] [PubMed] [Google Scholar]
- 85.Yuan X, Larsson C, Xu D. Mechanisms underlying the activation of TERT transcription and telomerase activity in human cancer: old actors and new players. Oncogene. 2019;38:6172–6183. doi: 10.1038/s41388-019-0872-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 87.Kim NW, Piatyszek MA, Prowse KR, Harley CB, West MD, Ho PL, Coviello GM, Wright WE, Weinrich SL, Shay JW. Specific association of human telomerase activity with immortal cells and cancer. Science. 1994;266:2011–2015. doi: 10.1126/science.7605428. [DOI] [PubMed] [Google Scholar]
- 88.Wang W, Chen H, Li R, Ouyang N, Chen J, Huang L, Mai M, Zhang N, Zhang Q, Yang D. Telomerase activity is more significant for predicting the outcome of IVF treatment than telomere length in granulosa cells. Reproduction. 2014;147:649–657. doi: 10.1530/REP-13-0223. [DOI] [PubMed] [Google Scholar]
- 89.Mordechai A, Wasserman M, Abramov M, Ben-Menahem D, Har-Vardi I, Levitas E, Priel E. Increasing telomerase enhanced steroidogenic genes expression and steroid hormones production in rat and human granulosa cells and in mouse ovary. J Steroid Biochem Mol Biol. 2020;197:105551. doi: 10.1016/j.jsbmb.2019.105551. [DOI] [PubMed] [Google Scholar]
- 90.Huang FW, Hodis E, Xu MJ, Kryukov GV, Chin L, Garraway LA. Highly recurrent TERT promoter mutations in human melanoma. Science. 2013;339:957–959. doi: 10.1126/science.1229259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Horn S, Figl A, Rachakonda PS, Fischer C, Sucker A, Gast A, Kadel S, Moll I, Nagore E, Hemminki K, Schadendorf D, Kumar R. TERT promoter mutations in familial and sporadic melanoma. Science. 2013;339:959–961. doi: 10.1126/science.1230062. [DOI] [PubMed] [Google Scholar]
- 92.Labgaa I, Villacorta-Martin C, D’Avola D, Craig AJ, von Felden J, Martins-Filho SN, Sia D, Stueck A, Ward SC, Fiel MI, Mahajan M, Tabrizian P, Thung SN, Ang C, Friedman SL, Llovet JM, Schwartz M, Villanueva A. A pilot study of ultra-deep targeted sequencing of plasma DNA identifies driver mutations in hepatocellular carcinoma. Oncogene. 2018;37:3740–3752. doi: 10.1038/s41388-018-0206-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hurst CD, Platt FM, Knowles MA. Comprehensive mutation analysis of the TERT promoter in bladder cancer and detection of mutations in voided urine. Eur Urol. 2014;65:367–369. doi: 10.1016/j.eururo.2013.08.057. [DOI] [PubMed] [Google Scholar]
- 94.Wang K, Liu T, Liu C, Meng Y, Yuan X, Liu L, Ge N, Liu J, Wang C, Ren H, Yan K, Hu S, Xu Z, Fan Y, Xu D. TERT promoter mutations and TERT mRNA but not FGFR3 mutations are urinary biomarkers in Han Chinese patients with urothelial bladder cancer. Oncologist. 2015;20:263–269. doi: 10.1634/theoncologist.2014-0391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Groeneweg JW, Roze JF, Peters EDJ, Sereno F, Brink AGJ, Paijens ST, Nijman HW, van Meurs HS, van Lonkhuijzen LRCW, Piek JMJ, Lok CAR, Monroe GR, van Haaften GW, Zweemer RP. FOXL2 and TERT promoter mutation detection in circulating tumor DNA of adult granulosa cell tumors as biomarker for disease monitoring. Gynecol Oncol. 2021;162:413–420. doi: 10.1016/j.ygyno.2021.05.027. [DOI] [PubMed] [Google Scholar]
- 96.Da Cruz Paula A, da Silva EM, Segura SE, Pareja F, Bi R, Selenica P, Kim SH, Ferrando L, Vahdatinia M, Soslow RA, Vidal A, Gatius S, Przybycin CG, Abu-Rustum NR, Matias-Guiu X, Rubin BP, Reis-Filho JS, DeLair DF, Weigelt B. Genomic profiling of primary and recurrent adult granulosa cell tumors of the ovary. Mod Pathol. 2020;33:1606–1617. doi: 10.1038/s41379-020-0514-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Alexiadis M, Rowley SM, Chu S, Leung DTH, Stewart CJR, Amarasinghe KC, Campbell IG, Fuller PJ. Mutational landscape of ovarian adult granulosa cell tumors from whole exome and targeted TERT promoter sequencing. Mol Cancer Res. 2019;17:177–185. doi: 10.1158/1541-7786.MCR-18-0359. [DOI] [PubMed] [Google Scholar]
- 98.Mitchell TJ, Turajlic S, Rowan A, Nicol D, Farmery JHR, O’Brien T, Martincorena I, Tarpey P, Angelopoulos N, Yates LR, Butler AP, Raine K, Stewart GD, Challacombe B, Fernando A, Lopez JI, Hazell S, Chandra A, Chowdhury S, Rudman S, Soultati A, Stamp G, Fotiadis N, Pickering L, Au L, Spain L, Lynch J, Stares M, Teague J, Maura F, Wedge DC, Horswell S, Chambers T, Litchfield K, Xu H, Stewart A, Elaidi R, Oudard S, McGranahan N, Csabai I, Gore M, Futreal PA, Larkin J, Lynch AG, Szallasi Z, Swanton C, Campbell PJ TRACERx Renal Consortium. Timing the landmark events in the evolution of clear cell renal cell cancer: TRACERx renal. Cell. 2018;173:611–623. e617. doi: 10.1016/j.cell.2018.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Jamieson S, Fuller PJ. Characterization of the inhibitor of kappaB kinase (IKK) complex in granulosa cell tumors of the ovary and granulosa cell tumor-derived cell lines. Horm Cancer. 2013;4:277–292. doi: 10.1007/s12672-013-0146-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Chu S, Nishi Y, Yanase T, Nawata H, Fuller PJ. Transrepression of estrogen receptor beta signaling by nuclear factor-kappab in ovarian granulosa cells. Mol Endocrinol. 2004;18:1919–1928. doi: 10.1210/me.2004-0021. [DOI] [PubMed] [Google Scholar]
- 101.Ray-Coquard I, Morice P, Lorusso D, Prat J, Oaknin A, Pautier P, Colombo N ESMO Guidelines Committee. Non-epithelial ovarian cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2018;29(Suppl 4):iv1–iv18. doi: 10.1093/annonc/mdy001. [DOI] [PubMed] [Google Scholar]
- 102.Lee IH, Choi CH, Hong DG, Song JY, Kim YJ, Kim KT, Lee KW, Park IS, Bae DS, Kim TJ. Clinicopathologic characteristics of granulosa cell tumors of the ovary: a multicenter retrospective study. J Gynecol Oncol. 2011;22:188–195. doi: 10.3802/jgo.2011.22.3.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Colombo N, Peiretti M, Garbi A, Carinelli S, Marini C, Sessa C ESMO Guidelines Working Group. Non-epithelial ovarian cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2012;23(Suppl 7):vii20–vii26. doi: 10.1093/annonc/mds223. [DOI] [PubMed] [Google Scholar]
- 104.Fotopoulou C, Savvatis K, Braicu EI, Brink-Spalink V, Darb-Esfahani S, Lichtenegger W, Sehouli J. Adult granulosa cell tumors of the ovary: tumor dissemination pattern at primary and recurrent situation, surgical outcome. Gynecol Oncol. 2010;119:285–290. doi: 10.1016/j.ygyno.2010.06.031. [DOI] [PubMed] [Google Scholar]
- 105.Bryk S, Farkkila A, Butzow R, Leminen A, Heikinheimo M, Anttonen M, Riska A, Unkila-Kallio L. Clinical characteristics and survival of patients with an adult-type ovarian granulosa cell tumor: a 56-year single-center experience. Int J Gynecol Cancer. 2015;25:33–41. doi: 10.1097/IGC.0000000000000304. [DOI] [PubMed] [Google Scholar]
- 106.Farkkila A, Haltia UM, Tapper J, McConechy MK, Huntsman DG, Heikinheimo M. Pathogenesis and treatment of adult-type granulosa cell tumor of the ovary. Ann Med. 2017;49:435–447. doi: 10.1080/07853890.2017.1294760. [DOI] [PubMed] [Google Scholar]
- 107.Ertas IE, Gungorduk K, Taskin S, Akman L, Ozdemir A, Goklu R, Terek MC, Ozsaran A, Dikmen Y, Yildirim Y, Ortac F. Prognostic predictors and spread patterns in adult ovarian granulosa cell tumors: a multicenter long-term follow-up study of 108 patients. Int J Clin Oncol. 2014;19:912–920. doi: 10.1007/s10147-013-0630-x. [DOI] [PubMed] [Google Scholar]
- 108.Mangili G, Ottolina J, Gadducci A, Giorda G, Breda E, Savarese A, Candiani M, Frigerio L, Scarfone G, Pignata S, Rossi R, Marinaccio M, Lorusso D. Long-term follow-up is crucial after treatment for granulosa cell tumours of the ovary. Br J Cancer. 2013;109:29–34. doi: 10.1038/bjc.2013.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.E C, Samant R, Fung MF, Le T, Hopkins L, Senterman M. Palliative radiotherapy for recurrent granulosa cell tumor of the ovary: a report of 3 cases with radiological evidence of response. Gynecol Oncol. 2006;102:406–410. doi: 10.1016/j.ygyno.2006.02.034. [DOI] [PubMed] [Google Scholar]
- 110.Disaia P, Saltz A, Kagan AR, Rich W. A temporary response of recurrent granulosa cell tumor to adriamycin. Obstet Gynecol. 1978;52:355–358. [PubMed] [Google Scholar]
- 111.Lee IW, Levin W, Chapman W, Goldberg RE, Murphy KJ, Milosevic M. Radiotherapy for the treatment of metastatic granulosa cell tumor in the mediastinum: a case report. Gynecol Oncol. 1999;73:455–460. doi: 10.1006/gyno.1998.5338. [DOI] [PubMed] [Google Scholar]
- 112.Hauspy J, Beiner ME, Harley I, Rosen B, Murphy J, Chapman W, Le LW, Fyles A, Levin W. Role of adjuvant radiotherapy in granulosa cell tumors of the ovary. Int J Radiat Oncol Biol Phys. 2011;79:770–774. doi: 10.1016/j.ijrobp.2009.12.005. [DOI] [PubMed] [Google Scholar]
- 113.Ray-Coquard I, Brown J, Harter P, Provencher DM, Fong PC, Maenpaa J, Ledermann JA, Emons G, Rigaud DB, Glasspool RM, Mezzanzanica D, Colombo N. Gynecologic Cancer InterGroup (GCIG) consensus review for ovarian sex cord stromal tumors. Int J Gynecol Cancer. 2014;24:S42–S47. doi: 10.1097/IGC.0000000000000249. [DOI] [PubMed] [Google Scholar]
- 114.van Meurs HS, van Lonkhuijzen LR, Limpens J, van der Velden J, Buist MR. Hormone therapy in ovarian granulosa cell tumors: a systematic review. Gynecol Oncol. 2014;134:196–205. doi: 10.1016/j.ygyno.2014.03.573. [DOI] [PubMed] [Google Scholar]
- 115.Perren TJ, Swart AM, Pfisterer J, Ledermann JA, Pujade-Lauraine E, Kristensen G, Carey MS, Beale P, Cervantes A, Kurzeder C, du Bois A, Sehouli J, Kimmig R, Stahle A, Collinson F, Essapen S, Gourley C, Lortholary A, Selle F, Mirza MR, Leminen A, Plante M, Stark D, Qian W, Parmar MK, Oza AM ICON7 Investigators. A phase 3 trial of bevacizumab in ovarian cancer. N Engl J Med. 2011;365:2484–2496. doi: 10.1056/NEJMoa1103799. [DOI] [PubMed] [Google Scholar]
- 116.Brown J, Brady WE, Schink J, Van Le L, Leitao M, Yamada SD, de Geest K, Gershenson DM. Efficacy and safety of bevacizumab in recurrent sex cord-stromal ovarian tumors: results of a phase 2 trial of the Gynecologic Oncology Group. Cancer. 2014;120:344–351. doi: 10.1002/cncr.28421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ray-Coquard I, Harter P, Lorusso D, Dalban C, Vergote I, Fujiwara K, Gladieff L, Luck HJ, Floquet A, Chevalier-Place A, Schnelzer A, Pignata S, Selle F, Sehouli J, Brocard F, Mangili G, Pautier P, De Giorgi U, Provansal M, Heudel PE. Effect of weekly paclitaxel with or without bevacizumab on progression-free rate among patients with relapsed ovarian sex cord-stromal tumors: the ALIENOR/ENGOT-ov7 randomized clinical trial. JAMA Oncol. 2020;6:1923–1930. doi: 10.1001/jamaoncol.2020.4574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Sunters A, Madureira PA, Pomeranz KM, Aubert M, Brosens JJ, Cook SJ, Burgering BM, Coombes RC, Lam EW. Paclitaxel-induced nuclear translocation of FOXO3a in breast cancer cells is mediated by c-Jun NH2-terminal kinase and Akt. Cancer Res. 2006;66:212–220. doi: 10.1158/0008-5472.CAN-05-1997. [DOI] [PubMed] [Google Scholar]
- 119.Zeng Z, Samudio IJ, Zhang W, Estrov Z, Pelicano H, Harris D, Frolova O, Hail N Jr, Chen W, Kornblau SM, Huang P, Lu Y, Mills GB, Andreeff M, Konopleva M. Simultaneous inhibition of PDK1/AKT and Fms-like tyrosine kinase 3 signaling by a small-molecule KP372-1 induces mitochondrial dysfunction and apoptosis in acute myelogenous leukemia. Cancer Res. 2006;66:3737–3746. doi: 10.1158/0008-5472.CAN-05-1278. [DOI] [PubMed] [Google Scholar]
- 120.Essafi A, Fernandez de Mattos S, Hassen YA, Soeiro I, Mufti GJ, Thomas NS, Medema RH, Lam EW. Direct transcriptional regulation of Bim by FoxO3a mediates STI571-induced apoptosis in Bcr-Abl-expressing cells. Oncogene. 2005;24:2317–2329. doi: 10.1038/sj.onc.1208421. [DOI] [PubMed] [Google Scholar]
- 121.Hines JF, Khalifa MA, Moore JL, Fine KP, Lage JM, Barnes WA. Recurrent granulosa cell tumor of the ovary 37 years after initial diagnosis: a case report and review of the literature. Gynecol Oncol. 1996;60:484–488. doi: 10.1006/gyno.1996.0078. [DOI] [PubMed] [Google Scholar]
- 122.Timmerman D, Moerman P, Vergote I. Meigs’ syndrome with elevated serum CA 125 levels: two case reports and review of the literature. Gynecol Oncol. 1995;59:405–408. doi: 10.1006/gyno.1995.9952. [DOI] [PubMed] [Google Scholar]
- 123.Farkkila A, Koskela S, Bryk S, Alfthan H, Butzow R, Leminen A, Puistola U, Tapanainen JS, Heikinheimo M, Anttonen M, Unkila-Kallio L. The clinical utility of serum anti-Mullerian hormone in the follow-up of ovarian adult-type granulosa cell tumors--A comparative study with inhibin B. Int J Cancer. 2015;137:1661–1671. doi: 10.1002/ijc.29532. [DOI] [PubMed] [Google Scholar]
- 124.Geerts I, Vergote I, Neven P, Billen J. The role of inhibins B and antimullerian hormone for diagnosis and follow-up of granulosa cell tumors. Int J Gynecol Cancer. 2009;19:847–855. doi: 10.1111/IGC.0b013e3181a702d1. [DOI] [PubMed] [Google Scholar]
- 125.Basch E, Deal AM, Kris MG, Scher HI, Hudis CA, Sabbatini P, Rogak L, Bennett AV, Dueck AC, Atkinson TM, Chou JF, Dulko D, Sit L, Barz A, Novotny P, Fruscione M, Sloan JA, Schrag D. Symptom monitoring with patient-reported outcomes during routine cancer treatment: a randomized controlled trial. J. Clin. Oncol. 2016;34:557–565. doi: 10.1200/JCO.2015.63.0830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Irusta G, Pazos MC, Abramovich D, De Zuniga I, Parborell F, Tesone M. Effects of an inhibitor of the gamma-secretase complex on proliferation and apoptotic parameters in a FOXL2-mutated granulosa tumor cell line (KGN) Biol Reprod. 2013;89:9. doi: 10.1095/biolreprod.113.108100. [DOI] [PubMed] [Google Scholar]
- 127.Terauchi KJ, Shigeta Y, Iguchi T, Sato T. Role of Notch signaling in granulosa cell proliferation and polyovular follicle induction during folliculogenesis in mouse ovary. Cell Tissue Res. 2016;365:197–208. doi: 10.1007/s00441-016-2371-4. [DOI] [PubMed] [Google Scholar]
- 128.Tripurani SK, Cook RW, Eldin KW, Pangas SA. BMP-specific SMADs function as novel repressors of PDGFA and modulate its expression in ovarian granulosa cells and tumors. Oncogene. 2013;32:3877–3885. doi: 10.1038/onc.2012.392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Van Nieuwenhuysen E, Lambrechts S, Lambrechts D, Leunen K, Amant F, Vergote I. Genetic changes in nonepithelial ovarian cancer. Expert Rev Anticancer Ther. 2013;13:871–882. doi: 10.1586/14737140.2013.811174. [DOI] [PubMed] [Google Scholar]
- 130.Hahn SA, Schutte M, Hoque AT, Moskaluk CA, da Costa LT, Rozenblum E, Weinstein CL, Fischer A, Yeo CJ, Hruban RH, Kern SE. DPC4, a candidate tumor suppressor gene at human chromosome 18q21.1. Science. 1996;271:350–353. doi: 10.1126/science.271.5247.350. [DOI] [PubMed] [Google Scholar]
- 131.Fortin J, Boehm U, Deng CX, Treier M, Bernard DJ. Follicle-stimulating hormone synthesis and fertility depend on SMAD4 and FOXL2. FASEB J. 2014;28:3396–3410. doi: 10.1096/fj.14-249532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Bilandzic M, Chu S, Wang Y, Tan HL, Fuller PJ, Findlay JK, Stenvers KL. Betaglycan alters NFkappaB-TGFbeta2 cross talk to reduce survival of human granulosa tumor cells. Mol Endocrinol. 2013;27:466–479. doi: 10.1210/me.2012-1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Liu Z, Ren YA, Pangas SA, Adams J, Zhou W, Castrillon DH, Wilhelm D, Richards JS. FOXO1/3 and PTEN depletion in granulosa cells promotes ovarian granulosa cell tumor development. Mol Endocrinol. 2015;29:1006–1024. doi: 10.1210/me.2015-1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Kyronlahti A, Kauppinen M, Lind E, Unkila-Kallio L, Butzow R, Klefstrom J, Wilson DB, Anttonen M, Heikinheimo M. GATA4 protects granulosa cell tumors from TRAIL-induced apoptosis. Endocr Relat Cancer. 2010;17:709–717. doi: 10.1677/ERC-10-0041. [DOI] [PubMed] [Google Scholar]
- 135.Matzuk MM, Finegold MJ, Su JG, Hsueh AJ, Bradley A. Alpha-inhibin is a tumour-suppressor gene with gonadal specificity in mice. Nature. 1992;360:313–319. doi: 10.1038/360313a0. [DOI] [PubMed] [Google Scholar]
- 136.Kyronlahti A, Ramo M, Tamminen M, Unkila-Kallio L, Butzow R, Leminen A, Nemer M, Rahman N, Huhtaniemi I, Heikinheimo M, Anttonen M. GATA-4 regulates Bcl-2 expression in ovarian granulosa cell tumors. Endocrinology. 2008;149:5635–5642. doi: 10.1210/en.2008-0148. [DOI] [PubMed] [Google Scholar]
- 137.Kim SY, Ebbert K, Cordeiro MH, Romero MM, Whelan KA, Suarez AA, Woodruff TK, Kurita T. Constitutive activation of PI3K in oocyte induces ovarian granulosa cell tumors. Cancer Res. 2016;76:3851–3861. doi: 10.1158/0008-5472.CAN-15-3358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Coutts SM, Childs AJ, Fulton N, Collins C, Bayne RA, McNeilly AS, Anderson RA. Activin signals via SMAD2/3 between germ and somatic cells in the human fetal ovary and regulates kit ligand expression. Dev Biol. 2008;314:189–199. doi: 10.1016/j.ydbio.2007.11.026. [DOI] [PubMed] [Google Scholar]