Abstract
Purpose:
To identify factors affecting periprocedural morbidity and mortality and long-term survival following hepatic artery embolization (HAE) of hepatic neuroendocrine tumor (NET) metastases.
Materials and Methods:
This single-center, institutional review board–approved retrospective review included 320 consecutive HAEs for NET metastases performed in 137 patients between September 1996 and September 2007. Forty-seven HAEs (15%) were performed urgently to manage refractory symptoms in inpatients (urgent group), and 273 HAEs (85%) were elective (elective group). Overall survival (OS) was estimated by Kaplan–Meier methodology. Complications were categorized per Common Terminology Criteria for Adverse Events, version 4.0. Univariate and multivariate analyses were performed to determine independent predictors for OS, complications, and 30-day mortality. The independent factors were combined to develop clinical risk score groups.
Results:
Urgent HAE (P = .007), greater than 50% liver replacement by tumor (P < .0001), and extrahepatic metastasis (P = .007) were independent predictors for shorter OS. Patients with all three risk factors had decreased OS versus those with none (median, 8.5 vs 86 mo; P < .001). Thirty-day mortality was significantly lower in the elective (1%) versus the urgent group (8.5%; P = .0009). There were eight complications (3%) in the elective group and five (10.6%) in the urgent group (P = .03). Male sex and urgent group were independent factors for higher 30-day mortality rate (P = .023 and P = .016, respectively) and complications (P = .012 and P =.001, respectively).
Conclusions:
Urgent HAE, replacement of more than 50% of liver by tumor, and extrahepatic metastasis are strong independent predictors of shorter OS. Male sex and urgent HAE carry higher 30-day mortality and periprocedural morbidity risks.
Because of their indolent nature, neuroendocrine tumors (NETs) have been described as “cancers in slow motion” (1). Nevertheless, most patients will die from this disease, predominantly as a result of liver metastases (1). Approximately 50% of NETs secrete hormones that may lead to clinical symptoms (2). Arterially directed therapies including transcatheter arterial chemoembolization (3–6), radioembolization (7), and hepatic artery embolization (HAE) (8–10) have been used for the management of NET hepatic metastases. Common indications for arterially directed therapies for NET hepatic metastases include management of hormonal symptoms refractory to somatostatin analogues, radiologic evidence of disease progression, and large tumors causing pain or other bulk symptoms (11). Many reports have shown that HAE and chemoembolization are beneficial to patients with metastatic NETs by reducing hormone levels and tumor size (1,3,10,12–18). However, the literature that focuses on patient selection, procedural circumstances, and clinical factors that may influence the immediate and long-term outcomes of HAE in this patient population is fairly scarce.
The purpose of the present study was to identify factors affecting long-term overall patient survival as well as periprocedural morbidity and mortality related to HAE in patients with NET hepatic metastases.
MATERIALS AND METHODS
Patients treated with HAE for NET hepatic metastases and with at least 5 years of follow-up were eligible for the present review. Patients were included in this study at the time of their very first embolization session. All patients who underwent previous hepatic embolization were excluded. A total of 137 patients who underwent a total of 320 consecutive HAEs between August 1996 and September 2007 comprise this cohort. All patients signed informed consent for HAE. Institutional review board waiver was obtained for this Health Insurance Portability and Accountability Act–compliant retrospective chart review study.
Patients Demographics
Table 1 depicts patient and tumor characteristics. Patients underwent HAE within a median time of 13.3 months (range, 0–201 mo) from the initial diagnosis of liver metastasis. Chart review was performed to identify the referral pattern for HAE. Two distinct referral patterns were identified:
Table 1.
Patient Characteristics
| Characteristic | Carcinoid (n = 78) |
Islet Cell (n = 59) |
|---|---|---|
| Sex | ||
| Male | 26 (33) | 36 (61) |
| Female | 52 (67) | 23 (39) |
| Age (y) | ||
| Median | 60 | 55 |
| Range | 32–85 | 28–83 |
| Race | ||
| White | 67 (86) | 54 (92) |
| Black | 7 (9) | 2 (3) |
| Asian | 1 (1) | 1 (2) |
| Other/Unknown | 3 (4) | 2 (3) |
| Primary origin/tumor type | ||
| Foregut | 23 (30) | – |
| Midgut | 31 (40) | – |
| Hindgut | 7 (9) | – |
| Unknown | 17 (21) | – |
| Insulinoma | – | 2 (3) |
| Glucagonoma | – | 4 (7) |
| Vipoma | – | 4 (7) |
| Gastrinoma | – | 4 (7) |
| Somatostatinoma | – | 1 (2) |
| Islet cell multiple | – | 13 (22) |
| Islet cell nonfunctional | – | 31 (52) |
| Primary histologic grade | ||
| High | 8 (10) | 2 (3) |
| Intermediate | 7 (9) | 6 (10) |
| Low | 33 (42) | 31 (53) |
| Unknown | 30 (39) | 20 (34) |
| Previous octreotide treatment | 55 (71) | 47 (80) |
| Previous liver surgery | 27 (35) | 12 (20) |
| Primary resected | 43 (55) | 28 (47) |
| Extrahepatic metastasis* | 32 (41) | 25 (42) |
| Metastatic liver involvement* | ||
| < 50% | 45 (58) | 30 (51) |
| > 50% | 32 (41) | 26 (44) |
| Unknown | 1 (1) | 3 (5) |
| No. of embolizations | 162 | 156 |
| Median per patient | 2 | 2 |
| Range per patient | 1–10 | 1–9 |
Values in parentheses are percentages.
At presentation.
The urgent group includes patients who were urgently admitted with symptoms attributed to NET hepatic metastases and received inpatient medical care (somatostatin infusion, intravenous hydration and electrolyte management, patient-controlled analgesic pump) for at least 24 hours without symptom improvement. This urgent group included 47 HAEs performed in 30 patients. In these cases, HAE was undertaken as a salvage therapy to control symptoms refractory to medical management. Symptoms at presentation in the urgent group included severe fluid and electrolyte disorders, hypoglycemia with or without seizure activity, and acute abdominal pain.
The elective group included 273 of 320 HAEs (85%) performed electively in 107 patients at a previously scheduled date. These patients came to the hospital only for the embolization and did not require any earlier admission for symptom control. Other than the need for hospitalization for refractory symptomatology, there was no significant difference in baseline characteristic between the elective and the urgent groups (Table 2).
Table 2.
Patient Characteristics in Elective and Urgent Groups at Initial Presentation for Embolization
| Characteristic | Elective | Urgent | P Value |
|---|---|---|---|
| Sex | .74 | ||
| Male | 50 (47) | 13 (43) | |
| Female | 57 (53) | 17 (57) | |
| Age (y) | 58 ± 12 | 56 ± 12 | .34 |
| Pathology | .39 | ||
| Carcinoid | 63 (59) | 15 (50) | |
| Pancreatic NET | 44 (41) | 15 (50) | |
| Liver involvement with metastatic disease | .16 | ||
| ≤ 50% | 63 (59) | 12 (40) | |
| > 50% | 43 (40) | 15 (50) | |
| Unknown | 1 (1) | 3 (10) | |
| Extrahepatic metastasis | .84 | ||
| Yes | 45 (42) | 12 (40) | |
| No | 62 (58) | 18 (60) | |
| Indication for embolization | .23 | ||
| Primary treatment | 31 (29) | 11 (37) | |
| Recurrence after surgery | 21 (20) | 2 (6) | |
| Progression despite medical treatment | 55 (51) | 17 (57) | |
| Mean (95% CI) diagnosis–HAE interval (mo) | 28 (20–36) | 21 (21) | .32 |
Values in parentheses are percentages. Values presented as mean ± standard deviation where applicable.
CI = confidence interval, HAE = hepatic artery embolization, NET = neuroendocrine tumor.
Preprocedure Evaluation
All patients were determined to have unresectable disease by a multidisciplinary team including a hepatobiliary surgeon and a gastrointestinal and hepatobiliary oncologist. Patients with liver-dominant (ie, limited extrahepatic metastases) or liver-only (ie, no extrahepatic metastases) disease were eligible for HAE. Patients were considered to have extrahepatic metastasis if there was radiographic evidence (computed tomography [CT], magnetic resonance imaging, nuclear bone scan) of metastases outside the liver or pathologic confirmation with biopsies from those sites. Fifty-seven patients (42%) presented with extrahepatic metastases at sites including bone (n = 12), lung (n = 8), lymph nodes (n = 37), adrenal gland (n = 2), kidney (n = 1), and spleen (n = 1). In five cases, pathologic confirmation was obtained.
Preprocedure assessment included a dynamic CT scan (most CT studies were triphasic) to demonstrate the size and location of, and arterial blood supply to, the hepatic tumor(s). One gram of cefazolin (GlaxoSmithKline, Philadelphia, Pennsylvania) was administered intravenously before HAE and every 8 hours for 24 hours thereafter. Patients allergic to cephalosporins received coverage of identical duration with clindamycin (Sandoz, East Hanover, New Jersey) or gentamicin (APP, Grand Island, New York). Patients with previous biliary intervention or bilioenteric anastomosis received piperacillin/tazobactam (Pfizer, New York, New York) or cefotetan (APP) intravenously for the period of hospitalization (19). They continued to receive oral ciprofloxacin and metronidazole on discharge for an additional 5 days. Any patient receiving maintenance octreotide (Sandostatin; Sandoz) received their usual morning dose on the day of HAE. In addition, all patients received 250 μg of octreotide subcutaneously on-call for their procedure, and an additional 500 μg mixed in 50 mL of normal saline solution was administered during the procedure for hemodynamic management if needed.
HAE Technique
In patients with bilobar disease, only one lobe was embolized at each session; complete treatment typically consisted of two embolization procedures to treat the right and left hemilivers. The second session was performed 4–8 weeks after the initial HAE. In patients with a large amount of liver volume replaced (50%–75%) by tumor, we performed sublobar rather than lobar treatments at each session. In this subgroup, more than two sessions (range, three to four) were required for treatment completion.
In all instances, stasis in the target vessel was the desired endpoint of HAE. Embolization was initiated with the use of the smallest particles available in an effort to achieve complete filling of the tumor capillary bed and cause terminal vascular blockade. The embolic material used was 50-μm polyvinyl alcohol particles (Cook, Bloomington, Indiana) in 82 embolizations (26%) until March 2001. From March 2001 to September 2007, 40–120-μm Embosphere particles (Merit Medical, South Jordan, Utah) were used in 231 embolizations (72%); 100–300-μm Bead Block particles (Biocompatibles, Oxford, Connecticut) were used in seven embolizations (2%) after March 2006. In a single vessel, the smallest particles were administered until stasis was achieved or a maximum of 10 mL had been administered. If stasis was not achieved, particles of the next larger size were used until stasis was achieved or a maximum of 10 mL had been administered.
Statistical Analysis
The endpoints of the study were patient overall survival (OS), periprocedural (30–d) morbidity, and mortality. OS was measured from the time of first embolization to death from any cause. Kaplan–Meier methodology was used to assess OS. Patients alive at the time of analysis were censored at their last follow-up.
Periprocedural morbidity was defined as any complication attributable to the HAE procedure that occurred within 30 days from the time of HAE. Thirty-day morbidity was assessed by using the Common Terminology Criteria for Adverse Events, version 4.0. Similarly, any death within 30 days from the time of HAE was considered to represent periprocedural mortality.
Tumor grade, classified in the pathology report on a three-tier system (low, intermediate, and high grade) was available for 77 of 137 patients (56%). For this subset of patients, grade was evaluated in relation to OS. Because of the relatively small numbers in the intermediate-grade (n = 12) and high-grade (n = 5) groups, the analysis used the low-grade group (n = 60) as the reference group to be compared versus the rest of the patients (n = 17) in the intermediate/high grade group. Cox proportional-hazards models were used for univariate and multivariate analyses for OS, and logistic regression was used for complications and 30-day mortality. Variables with a P value of less than .10 in the univariate analyses were included in the multivariate analysis, and nonsignificant variables were removed from the multivariate analysis model one by one until only significant variables remained (ie, backward selection). The resulting model included only independent predictors.
The resulting independent predictors for OS were combined into a survival risk score by giving one point for each factor. Differences in OS between risk score groups were assessed by using Kaplan–Meier methodology. Similarly, the resulting predictors for complications were each given one point, and each patient was assigned to a complication risk score group corresponding to the number of significant factors included. Analysis of morbidity proceeded in the same fashion, except the univariate analysis was performed by using Fisher exact tests and multivariate modelling used logistic regression.
RESULTS
Overall Survival
The median OS for the entire group was 43.1 months, and 1-, 3-, and 5-year OS rates were 82%, 57%, and 35%, respectively. The median OS for carcinoid and pancreatic NET metastasis groups were 43.2 and 43.1 months, respectively. The 1-, 3-, and 5-year OS rates were 88%, 59%, and 37% for the carcinoid group and 72%, 53%, and 33% for the pancreatic NET group, respectively (P = .19).
Patients who underwent elective HAE only (n = 107) had significantly longer median OS than patients treated urgently (urgent group, n = 30) for any of their HAE sessions (median OS, 49 vs 23 mo; P = .019). The 1-, 3-, and 5-year OS rates were 87%, 61%, and 38% for patients treated electively and 63%, 40%, and 27% for those treated urgently, respectively.
The median OS for patients with low-grade tumors (n = 60) was 54.5 months, significantly longer than the 24-month median OS for the 17 patients with intermediate-/high-grade NETs (P = .04). The five patients with high-grade pathology had a very short median OS of 3.33 months.
Table 3 displays univariate analysis of patient and tumor characteristics as variables that may affect OS. Sex, pattern of referral, number of embolizations, hepatic involvement with metastasis, primary tumor resection, previous liver resection, and presence of extrahepatic metastasis were further analyzed in a multivariate model. The final independent factors are displayed in Table 4.
Table 3.
Univariate Analysis of Risk Factors for Overall Survival, Complications, and 30-Day Mortality
| Overall Survival |
Complications |
30-d Mortality |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Factor | HR | 95% CI | P Value | OR | 95% CI | P Value | OR | 95% CI | P Value |
| Sex: male vs female | 1.58 | 1.06–2.37 | .026 | 1.15 | 1.02–1.29 | .020 | 1.09 | 1.01–1.17 | .030 |
| Age: > 65 y vs ≤ 65 y | 1.26 | 0.82–1.93 | .290 | 1.06 | 0.93–1.21 | .387 | 1.04 | 0.96–1.13 | .336 |
| Tumor: carcinoid vs pancreatic NET | 0.84 | 0.57–1.26 | .405 | 1.02 | 0.90–1.15 | .767 | 1.00 | 0.93–1.08 | .991 |
| Grade: low vs intermediate/high* | 1.98 | 1.01–3.82 | .047 | – | – | ||||
| Referral pattern: urgent vs elective | 1.69 | 1.09–2.62 | .020 | 1.27 | 1.11–1.46 | .0008 | 1.11 | 1.02–1.21 | .021 |
| No. of embolizations | 0.88 | 0.78–0.99 | .040 | 0.99 | 0.95–1.02 | .506 | 1.00 | 0.98–1.02 | .883 |
| Hepatic involvement with metastasis: > 50% vs ≤ 50% | 3.10 | 2.04–4.71 | < .001 | 1.10 | 0.98–1.24 | .109 | 1.06 | 0.98–1.15 | .129 |
| Primary tumor resected: yes vs no | 0.67 | 0.45–0.99 | .049 | 0.96 | 0.85–1.08 | .512 | 0.95 | 0.89–1.03 | .209 |
| Previous hepatic resection: yes vs no | 0.53 | 0.34–0.84 | .007 | 0.87 | 0.77–0.99 | .035 | 0.93 | 0.86–1.01 | .077 |
| Extrahepatic disease at first HAE: yes vs no | 1.78 | 1.18–2.67 | .006 | 1.08 | 0.96–1.22 | .191 | 1.03 | 0.96–1.11 | .396 |
CI = confidence interval, HAE = hepatic artery embolization, HR = hazard ratio, NET = neuroendocrine tumor, OR = odds ratio.
Tumor grade was available in only 77 of 137 patients (56%).
Table 4.
Multivariate Analysis of Risk Factors for Overall Survival, Complications, and 30-Day Mortality: Final Models after Backward Selection
| Overall Survival |
Complications |
30-d Mortality |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Independent Factor | HR | 95% CI | P Value | OR | 95% CI | P Value | OR | 95% CI | P Value |
| Hepatic involvement with metastasis: > 50% vs ≤ 50% | 3.27 | 2.13–5.01 | < .0001 | – | – | ||||
| Extrahepatic disease at first HAE: yes vs no | 1.89 | 1.25–2.88 | .003 | – | – | ||||
| Referral pattern: urgent vs elective | 2.08 | 1.30–3.33 | .002 | 1.28 | 1.12–1.46 | 0.001 | 1.11 | 1.02–1.22 | .016 |
| Sex: male vs female | – | 1.16 | 1.04–1.30 | 0.012 | 1.09 | 1.01–1.17 | .023 | ||
CI = confidence interval, HAE = hepatic artery embolization, HR = hazard ratio, OR = odds ratio.
Giving one point to each independent factor affecting OS (urgent embolization, hepatic involvement > 50%, and extrahepatic metastasis), a 0–3-point OS risk score was assigned to each patient. Five patients had a survival risk score of 3 (extrahepatic metastasis, > 50% liver involvement by tumor, and requirement of urgent HAE), 38 had a score of 2 (any two of the three risk factors), 50 had a score of 1 (any of the three risk factors), and 40 had a survival risk score of 0 (no extrahepatic metastasis, liver involvement by tumor less than 50%, underwent HAE electively). In four patients, we were unable to calculate the accurate degree of liver replacement by tumor on outside preprocedural scans and therefore could not assign them into a risk score group. OS by survival risk score groups is depicted in the Figure. Median OS times were 86.3 months (95% confidence interval [CI], 53.97 mo to NR) for risk score 0, 45 months (95% CI, 36.47–63.0 mo) for risk score 1, 20.7 months (95% CI, 12.77–38.7 mo) for risk score 2, and 8.5 months (95% CI, 5.33 mo to NR) for risk score 3, and were significantly different (P < .001).
Figure.
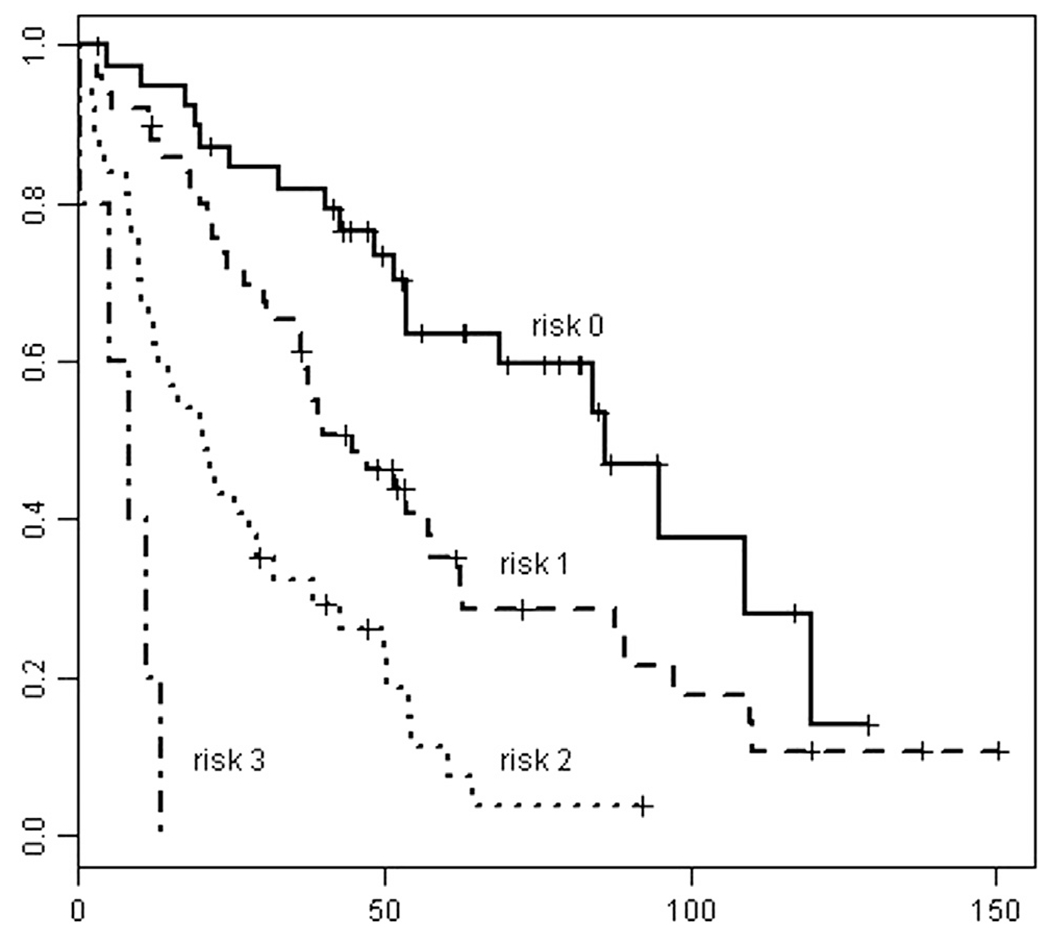
OS by risk score group.
In the subgroup of patients who had a tumor grade available, factors that reached significance on univariate analysis were tumor grade (P = .047), pattern of referral (P = .077), hepatic involvement by metastases of more than 50% (P < .001), and previous liver surgery (P = .009). After including these factors in a multivariate analysis model, grade (P = .003), pattern of referral (P = .018), and hepatic involvement by metastases (P < .001) were independent predictors for OS.
Complications/Periprocedural Morbidity
A total of 13 major complications (4%), five in the urgent group (10.6%) and eight in the elective group (2.9%; P = .0285), were catalogued per Common Terminology Criteria for Adverse Events as follows: one grade 2, three grade 3, and one grade 4 in the urgent group; and one grade 2 and seven grade 3 in the elective group. These 13 complications of grade 2–4 were successfully managed as follows: hepatic abscesses (n = 4) were treated with drainage and antibiotics, cholecystitis (n = 1) was treated by percutaneous cholecystostomy, acute pulmonary embolism (n = 1) was treated with anticoagulation, renal failure (n = 2) was managed conservatively, sepsis (n = 1) was treated with long courses of antibiotic therapy, common femoral artery dissection (n = 1) was treated with arterial reconstruction, pulmonary edema (n = 1) was managed with diuretics and bilevel positive airway pressure, and seizures (n = 2) were managed with phenytoin.
Patient and tumor characteristics were analyzed for their effect on postembolization complication rates. The results of the univariate analysis are displayed in Table 3. Backward selection was used in the multivariate analysis in the same manner as described earlier, and the final model was revealed, with sex (P = .01) and pattern of referral (P = .001) as independent predictors for complications (Table 4).
The complication risk score groups that resulted after multivariate analysis for complications included 57 patients with a complication risk score of 0 (female; treated electively), 67 patients with a complication risk score of 1 (male or urgent HAE), and 13 patients with a complication risk score of 2 (male patient undergoing urgent HAE). A patient with a risk score of 0 (female; elective HAE) had a 3.5% chance (ie, two in 57) of a complication after HAE, a patient with a risk score of 1 (male or urgent HAE) had an 18% chance (12 in 67) of a complication, and a patient with a score of 2 (male and urgent HAE) had a 46% chance (six in 13) of a complication.
Periprocedural (30-Day) Mortality
There were seven deaths within 30 days of HAE (2.2%). Four (13.3%) occurred in the group of 30 patients admitted to the hospital with exacerbated symptomatology in the setting of refractory disease (ie, urgent group). This urgent group received medical therapies for more than 1 day before urgent HAE in an effort to control the symptoms and stabilize the patient’s condition. There were 47 urgently performed HAEs in 30 inpatients, with a 30-day mortality rate of 8.5%. The 30-day mortality rate in the electively treated patients was 1%: three deaths among 273 elective HAEs (P = .0009).
The causes of death included sepsis (n = 2), cardiopulmonary arrest (n = 1), aspiration pneumonia (n = 1), cerebellar infarct (n = 1), renal failure (n = 1), and cachexia secondary to end-stage disease (n = 1).
The same patient and tumor characteristics were analyzed for effect on 30-day mortality. The results of the univariate analysis are displayed in Table 3. The multivariate analysis final model revealed male sex (P = .023) and urgent referral (P = .016) as independent factors for higher 30-day mortality as outlined in Table 4. There was an insufficient number of events (ie deaths) to reliably create a risk score for periprocedural mortality.
DISCUSSION
The treatment of hepatic metastases from NET is challenging. Although surgical resection of NET hepatic metastases is desirable, only 10%–20% of patients are surgical candidates at presentation (20). OS after hepatic resection varies from 46% to 76% at 5 years and from 35% to 79% at 10 years (21). Variations in surgical survival are most likely related to patient selection. Even in the best surgical series, however, hepatic recurrence rates range from 59% to 97% within 5 years after surgery (22,23). Somatostatin analogues, such as octreotide, have long played a key role in controlling hormonal symptoms related to NET hepatic metastases. More recently, it has been demonstrated that they have a cytostatic effect as well (24). Pasireotide is a somatostatin analogue with a broader spectrum of binding activity than octreotide that is currently being investigated for octreotide-refractory disease (25). Novel therapies such as vascular endothelial growth factor and mammalian target of rapamycin inhibitors are also under study. Most recently, two novel agents (everolimus and sunitinib) were approved for use in advanced pancreatic NET on the basis of positive results of two international large randomized phase III trials (26,27).
Several nonsurgical alternatives are available and successful in the treatment of patients with NET hepatic metastases (28,29). Among those, transcatheter arterial chemoembolization with different combinations of chemotherapeutic agent mixed with particles and lipiodol and HAE with particles only may achieve long-term symptom and tumor control in cases of unresectable disease (4). It is extremely difficult, if not impossible, to make any comparisons between the small number of available series reporting outcomes after arterially directed therapies for NET hepatic metastases. Tumor biology, degree of proliferation, grade, percentage of liver parenchyma replacement by tumor, and previous therapies are some of the important factors that affect outcomes, and these have not been analyzed in most of the available reports. To date, we are aware of no prospective randomized controlled trials comparing HAE and transcatheter arterial chemoembolization or radioembolization (7) in the treatment of NET hepatic metastases. Many centers have chosen transcatheter arterial chemoembolization over HAE (30) for the management of NET hepatic metastases, probably because of early reports demonstrating greater and more durable regression rates in a subgroup of patients who underwent systemic chemotherapy after HAE compared with those who received HAE alone (1).
A published series by Gupta et al (3) suggested that HAE may be more effective than transcatheter arterial chemoembolization for carcinoid NET hepatic metastases, whereas others (30) have indicated that transcatheter arterial chemoembolization may be better. To date, there are contradictory data regarding the added toxicity of chemotherapy in patients treated with transcatheter arterial chemoembolization. Some investigators did not experience higher degrees of toxicity (30), whereas others reported severe complications following chemoembolization (31,32) in the management NET hepatic metastases. One recent report (32) used doxorubicin-eluding bead chemoembolization—these beads have a much better pharmacologic profile than transcatheter arterial chemoembolization, with slow release of chemotherapeutic agent within the tumor—and reported a very high rate of biliary complications compared with traditional chemoembolization. This study (32) indicates that the addition of chemotherapy to spherical particles (ie, transcatheter arterial chemoembolization with doxorubicin-eluding beads), which can cause terminal capillary blockade and locally deliver high doses of doxorubicin, may carry increased risk compared with traditional chemoembolization or HAE with particles only.
A 2008 publication (33) showed comparable OS and morbidity and mortality rates between patients with NET hepatic metastases treated with transcatheter arterial chemoembolization or HAE. Although any comparison attempts are risky, it appears that the population and results of the present study are comparable to those in previous series from the United States (34) and Germany (35) that used HAE or transcatheter arterial chemoembolization. Older age (36,37), male sex (36,38), extended hepatic metastatic burden (38,39), development of bone or extrahepatic metastases (39,40), poorly differentiated NET (36), and pancreatic origin of the primary NET (36) have been associated with decreased survival in patients with NET hepatic metastases, whereas surgery for primary or liver metastasis resulted in a longer survival (37,38). Two previous studies (3,41) analyzed the variables affecting survival in patients with NET hepatic metastases treated with HAE or transcatheter arterial chemoembolization. In the study of Gupta et al (3), unresected primary tumor, liver involvement with metastasis of greater than 75%, and presence of extrahepatic metastasis were predictors of poorer outcome in patients with pancreatic NET hepatic metastases. In patients who had carcinoid tumors, only male sex was predictive of decreased survival. In addition, pancreatic primary was associated with a shorter OS (3). The more recent study by Hur et al (41) included patients treated with only transcatheter arterial chemoembolization and demonstrated that enterobiliary communication, hepatic tumor burden, and extrahepatic metastasis before the first chemoembolization were associated with poor OS.
OS rates in carcinoid and pancreatic NET groups were identical in the present study. Liver involvement by metastatic disease greater than 50% and presence of extrahepatic metastasis were independent predictors of shorter survival. The risk of death was more than triple for patients with more than 50% metastatic burden and double for patients with extrahepatic disease at the time of first embolization.
At our institution, the decision to perform HAE in patients with NET hepatic metastases is made through a multidisciplinary disease management team, and the patients undergo HAE electively. Less commonly, patients with refractory disease admitted to the hospital for management of exacerbated symptoms are referred for urgent HAE. In the present cohort, the subset of patients who underwent urgent HAE were twice as likely to die than those who received HAE electively. It is intuitive that patients who required admission had poorer performance status than those who received HAE electively, and indeed the urgent group, which comprised patients with refractory symptoms, may represent a surrogate for a relatively compromised performance status. Unfortunately, accurate classification of the performance status by Eastern Cooperative Oncology Group or Karnofsky systems was not available in a significant portion of this cohort, making analysis by performance status impossible. Performance status has been previously shown to be an independent predictor for survival following chemoembolization (42) and radioembolization of primary liver cancer (43,44) and NET hepatic metastases (7).
The constellation of the three identified independent risk factors affecting patient survival (liver involvement, type of referral, and extrahepatic metastasis) was found to greatly impact OS in the group of patients affected. Patients with no risk factors had a significantly longer median OS (86 mo) than patients with one (45 mo), two (21 mo), or all three risk factors (8 mo). This survival risk score described for the first time for patients with NET hepatic metastases may be valuable in making the decision whether HAE or hepatic arterially directed therapy in general is an appropriate therapy for an individual patient with NET hepatic metastases, and also when counseling patients. In patients with higher risk scores, the greatly increased risks should be discussed with referring physicians and patients and their families.
Urgent referral was an independent predictor of increased complications and higher 30-day mortality rate after HAE. Although HAE may be considered an option in advanced disease scenarios, when all other treatments have failed, the higher complication and mortality rates in the urgently treated group show that HAE performed urgently as a salvage treatment is accompanied by worse outcomes. As such, HAE should be offered only after a clear discussion and understanding of the potential risks and benefits. As in many other situations in oncology, patient and family expectations are extremely important and should therefore be addressed before HAE or any hepatic arterial therapy is performed.
There are several limitations to the present study, including its retrospective nature, the relatively small number of subjects, and the lack of direct comparisons of HAE versus other therapies or technologies, including transcatheter arterial chemoembolization, drug-eluting bead chemoembolization, and radioembolization. Another limitation was that tumor grade at the time of primary tumor diagnosis was available for only approximately half of the patients. The presence of refractory symptomatology even after inpatient medical treatment may represent a surrogate of poor performance status and clearly was associated with very poor outcomes after HAE; however, an analysis strictly based on patient performance status was not possible. We recognize the importance of external validation for unbiased evaluation of risk scores; however, we were unable to access a second data set of similar size in this patient population.
Despite its limitations, we believe the present study achieved its main aim of identifying factors affecting periprocedural morbidity and mortality as well as long-term patient survival after HAE for NET hepatic metastases. The present study introduced the concept of clinical risk scores for OS and periprocedural morbidity in patients with hepatic NET metastases, which can help screen patients for HAE or similar hepatic arterially directed therapies and identify those at high risk.
ABBREVIATIONS
- CI
confidence interval
- HAE
hepatic artery embolization
- NET
neuroendocrine tumor
- OS
overall survival
Footnotes
C.T.S. is a paid consultant for SIRTeX Medical (Woburn, Massachusetts). S.B.S. is a paid consultant for Covidien (Mansfield, Massachusetts) and Johnson and Johnson (New Brunswick, New Jersey). None of the other authors have identified a conflict of interest.
REFERENCES
- 1.Moertel CG, Johnson CM, McKusick MA, et al. The management of patients with advanced carcinoid tumors and islet cell carcinomas. Ann Intern Med 1994; 120:302–309. [DOI] [PubMed] [Google Scholar]
- 2.Metz DC, Jensen RT. Gastrointestinal neuroendocrine tumors: pancreatic endocrine tumors. Gastroenterology 2008; 135:1469–1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gupta S, Johnson MM, Murthy R, et al. Hepatic arterial embolization and chemoembolization for the treatment of patients with metastatic neuroendocrine tumors: variables affecting response rates and survival. Cancer 2005; 104:1590–1602. [DOI] [PubMed] [Google Scholar]
- 4.Roche A, Girish BV, de Baere T, et al. Trans-catheter arterial chemoembolization as first-line treatment for hepatic metastases from endocrine tumors. Eur Radiol 2003; 13:136–140. [DOI] [PubMed] [Google Scholar]
- 5.Ruszniewski P, Rougier P, Roche A, et al. Hepatic arterial chemoembolization in patients with liver metastases of endocrine tumors. A prospective phase II study in 24 patients. Cancer 1993; 71:2624–2630. [DOI] [PubMed] [Google Scholar]
- 6.Therasse E, Breittmayer F, Roche A, et al. Transcatheter chemoembolization of progressive carcinoid liver metastasis. Radiology 1993; 189:541–547. [DOI] [PubMed] [Google Scholar]
- 7.Memon K, Lewandowski RJ, Mulcahy MF, et al. Radioembolization for neuroendocrine liver metastases: safety, imaging, and long-term outcomes. Int J Radiat Onco Biol Phys 2012; 83:887–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chamberlain RS, Canes D, Brown KT, et al. Hepatic neuroendocrine metastases: does intervention alter outcomes? J Am Coll Surg 2000; 190:432–445. [DOI] [PubMed] [Google Scholar]
- 9.Sward C, Johanson V, Nieveen van Dijkum E, et al. Prolonged survival after hepatic artery embolization in patients with midgut carcinoid syndrome. Br J Surg 2009; 96:517–521. [DOI] [PubMed] [Google Scholar]
- 10.Brown KT, Koh BY, Brody LA, et al. Particle embolization of hepatic neuroendocrine metastases for control of pain and hormonal symptoms. J Vasc Interv Radiol 1999; 10:397–403. [DOI] [PubMed] [Google Scholar]
- 11.Madoff DC, Gupta S, Ahrar K, Murthy R, Yao JC. Update on the management of neuroendocrine hepatic metastases. J Vasc Interv Radiol 2006; 17:1235–1249. [DOI] [PubMed] [Google Scholar]
- 12.Ajani JA, Carrasco CH, Charnsangavej C, Samaan NA, Levin B, Wallace S. Islet cell tumors metastatic to the liver: effective palliation by sequential hepatic artery embolization. Ann Intern Med 1988; 108:340–344. [DOI] [PubMed] [Google Scholar]
- 13.Drougas JG, Anthony LB, Blair TK, et al. Hepatic artery chemoembolization for management of patients with advanced metastatic carcinoid tumors. Am J Surg 1998; 175:408–412. [DOI] [PubMed] [Google Scholar]
- 14.Eriksson BK, Larsson EG, Skogseid BM, Lofberg AM, Lorelius LE, Oberg KE. Liver embolizations of patients with malignant neuroendocrine gastrointestinal tumors. Cancer 1998; 83:2293–2301. [PubMed] [Google Scholar]
- 15.Hajarizadeh H, Ivancev K, Mueller CR, Fletcher WS, Woltering EA. Effective palliative treatment of metastatic carcinoid tumors with intraarterial chemotherapy/chemoembolization combined with octreotide acetate. Am J Surg 1992; 163:479–483. [DOI] [PubMed] [Google Scholar]
- 16.Kress O, Wagner HJ, Wied M, Klose KJ, Arnold R, Alfke H. Transarterial chemoembolization of advanced liver metastases of neuroendocrine tumors—a retrospective single-center analysis. Digestion 2003; 68:94–101. [DOI] [PubMed] [Google Scholar]
- 17.Loewe C, Schindl M, Cejna M, Niederle B, Lammer J, Thurnher S. Permanent transarterial embolization of neuroendocrine metastases of the liver using cyanoacrylate and lipiodol: assessment of mid- and long-term results. AJR Am J Roentgenol 2003; 180:1379–1384. [DOI] [PubMed] [Google Scholar]
- 18.Perry LJ, Stuart K, Stokes KR, Clouse ME. Hepatic arterial chemoembolization for metastatic neuroendocrine tumors. Surgery 1994; 116:1111–1116. [PubMed] [Google Scholar]
- 19.Kim W, Clark TW, Baum RA, Soulen MC. Risk factors for liver abscess formation after hepatic chemoembolization. J Vasc Interv Radiol 2001; 12:965–968. [DOI] [PubMed] [Google Scholar]
- 20.Dejong CH, Parks RW, Currie E, Piris J, Redhead DN, Garden OJ. Treatment of hepatic metastases of neuroendocrine malignancies: a 10-year experience. J R Coll Surg Edinb 2002; 47:495–499. [PubMed] [Google Scholar]
- 21.Frilling A, Li J, Malamutmann E, Schmid KW, Bockisch A, Broelsch CE. Treatment of liver metastases from neuroendocrine tumours in relation to the extent of hepatic disease. Br J Surg 2009; 96:175–184. [DOI] [PubMed] [Google Scholar]
- 22.Sarmiento JM, Que FG. Hepatic surgery for metastases from neuroendocrine tumors. Surg Oncol Clin N Am 2003; 12:231–242. [DOI] [PubMed] [Google Scholar]
- 23.Musunuru S, Chen H, Rajpal S, et al. Metastatic neuroendocrine hepatic tumors: resection improves survival. Arch Surg 2006; 141:1000–1004. [DOI] [PubMed] [Google Scholar]
- 24.Rinke A, Muller HH, Schade-Brittinger C, et al. Placebo-controlled, double-blind, prospective, randomized study on the effect of octreotide LAR in the control of tumor growth in patients with metastatic neuroendocrine midgut tumors: a report from the PROMID Study Group. J Clin Oncol 2009; 27:4656–4663. [DOI] [PubMed] [Google Scholar]
- 25.Kvols LK, Oberg KE, O’Dorisio TM, et al. Pasireotide (SOM230) shows efficacy and tolerability in the treatment of patients with advanced neuroendocrine tumors refractory or resistant to octreotide LAR: results from a phase II study. Endocr Relat Cancer 2012; 19:657–666. [DOI] [PubMed] [Google Scholar]
- 26.Yao JC, Shah MH, Ito T, et al. Everolimus for advanced pancreatic neuroendocrine tumors. N Engl J Med 2011; 364:514–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Raymond E, Dahan L, Raoul JL, et al. Sunitinib malate for the treatment of pancreatic neuroendocrine tumors. N Engl J Med 2011; 364:501–513. [DOI] [PubMed] [Google Scholar]
- 28.Oberg K, Norheim I, Lundqvist G, Wide L. Cytotoxic treatment in patients with malignant carcinoid tumors. Response to streptozocin—alone or in combination with 5-FU. Acta Oncol 1987; 26:429–432. [DOI] [PubMed] [Google Scholar]
- 29.Auernhammer CJ, Goke B. Therapeutic strategies for advanced neuroendocrine carcinomas of jejunum/ileum and pancreatic origin. Gut 2011; 60:1009–1021. [DOI] [PubMed] [Google Scholar]
- 30.Ruutiainen AT, Soulen MC, Tuite CM, et al. Chemoembolization and bland embolization of neuroendocrine tumor metastases to the liver. J Vasc Interv Radiol 2007; 18:847–855. [DOI] [PubMed] [Google Scholar]
- 31.Winkelbauer FW, Niederle B, Pietschmann F, et al. Hepatic artery embolotherapy of hepatic metastases from carcinoid tumors: value of using a mixture of cyanoacrylate and ethiodized oil. AJR Am J Roentgenol 1995; 165:323–327. [DOI] [PubMed] [Google Scholar]
- 32.Guiu B, Deschamps F, Aho S, et al. Liver/biliary injuries following chemoembolisation of endocrine tumours and hepatocellular carcinoma: lipiodol vs. drug-eluting beads. J Hepatol 2012; 56:609–617. [DOI] [PubMed] [Google Scholar]
- 33.Pitt SC, Knuth J, Keily JM, et al. Hepatic neuroendocrine metastases: chemo- or bland embolization? J Gastrointest Surg 2008; 12:1951–1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gupta S, Yao JC, Ahrar K, et al. Hepatic artery embolization and chemoembolization for treatment of patients with metastatic carcinoid tumors: the M.D. Anderson experience. Cancer J 2003; 9:261–267. [DOI] [PubMed] [Google Scholar]
- 35.Vogl TJ, Gruber T, Naguib NN, Hammerstingl R, Nour-Eldin NE. Liver metastases of neuroendocrine tumors: treatment with hepatic transarterial chemotherapy using two therapeutic protocols. AJR Am J Roentgenol 2009; 193:941–947. [DOI] [PubMed] [Google Scholar]
- 36.Lepage C, Rachet B, Coleman MP. Survival from malignant digestive endocrine tumors in England and Wales: a population-based study. Gastroenterology 2007; 132:899–904. [DOI] [PubMed] [Google Scholar]
- 37.Durante C, Boukheris H, Dromain C, et al. Prognostic factors influencing survival from metastatic (stage IV) gastroenteropancreatic well-differentiated endocrine carcinoma. Endocr Relat Cancer 2009; 16:585–597. [DOI] [PubMed] [Google Scholar]
- 38.Solorzano CC, Lee JE, Pisters PW, et al. Nonfunctioning islet cell carcinoma of the pancreas: survival results in a contemporary series of 163 patients. Surgery 2001; 130:1078–1085. [DOI] [PubMed] [Google Scholar]
- 39.Yu F, Venzon DJ, Serrano J, et al. Prospective study of the clinical course, prognostic factors, causes of death, and survival in patients with long-standing Zollinger-Ellison syndrome. J Clin Oncol 1999; 17:615–630. [DOI] [PubMed] [Google Scholar]
- 40.Panzuto F, Nasoni S, Falconi M, et al. Prognostic factors and survival in endocrine tumor patients: comparison between gastrointestinal and pancreatic localization. Endocr Relat Cancer 2005; 12:1083–1092. [DOI] [PubMed] [Google Scholar]
- 41.Hur S, Chung JW, Kim HC, et al. Survival outcomes and prognostic factors of transcatheter arterial chemoembolization for hepatic neuroendocrine metastases. J Vasc Interv Radiol 2013; 24:947–956. [DOI] [PubMed] [Google Scholar]
- 42.Dhanasekaran R, Kooby DA, Staley CA, Kauh JS, Khanna V, Kim HS. Prognostic factors for survival in patients with unresectable hepatocellular carcinoma undergoing chemoembolization with doxorubicin drug-eluting beads: a preliminary study. HPB 2010; 12:174–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dunfee BL, Riaz A, Lewandowski RJ, et al. Yttrium-90 radioembolization for liver malignancies: prognostic factors associated with survival. J Vasc Interv Radiol 2010; 21:90–95. [DOI] [PubMed] [Google Scholar]
- 44.Sangro B, Carpanese L, Cianni R, et al. Survival after yttrium-90 resin microsphere radioembolization of hepatocellular carcinoma across Barcelona clinic liver cancer stages: a European evaluation. Hepatology 2011; 54:868–878. [DOI] [PubMed] [Google Scholar]


