Abstract
Cognitive development is marked by age-related improvements across a number of domains, as young children perform worse than their older counterparts on most tasks. However, there are cases in which young children, and even infants, outperform older children and adults. So when, and why, does being young sometimes confer an advantage? This article provides a comprehensive examination of the peculiar cases in which younger children perform better. First, we outline the specific instances in which younger is better across domains, including mastering language, using probabilistic information, detecting causal relations, remembering certain information, and even solving problems. We then examine how children’s reduced cognitive abilities, ongoing brain development, more limited prior knowledge, and heightened tendency to explore benefits their learning, reasoning, perception, and memory from a mechanistic perspective. We hold that considering all of these factors together is essential for understanding the ways in which children’s learning is unique and that science has much to learn from a careful consideration of childhood.
Keywords: cognitive development, learning, memory, reasoning, brain development, trade-offs
Cognitive development is defined by improvement in performance: With age, older children and adults outperform younger children on a variety of tasks across domains. However, there are times when young children, and even infants, have an advantage over older children and adults. Although children take longer to master a new language (Snow & Hoefnagel-Höhle, 1978), they eventually fare much better than adults (Birdsong, 1999; Brown, 1973). In addition, children more accurately detect causal patterns in their environments (Lucas et al., 2014; Seiver et al., 2013; Walker et al., 2016), make more informed decisions based on probabilistic information (Gualtieri & Denison, 2018; Weir, 1964), and are quicker at solving problems that involve using objects in an unconventional manner (Defeyter & German, 2003; German & Defeyter, 2000). Moreover, in some cases, younger children even appear to have more accurate memories than older children and adults (Brainerd et al., 2018; Deng & Sloutsky, 2016). In this article, we offer a unifying perspective on cases in which younger children demonstrate better performance. Although these instances vary widely across domains, we believe that children’s developing cognitive abilities, more limited prior experience, and greater exploration, coupled with periods of neural plasticity and ongoing brain development, explain these advantages in childhood. We first examine the cases in which young children outperform their older counterparts and then outline how the very features that define childhood lead to these advantages.
When Are Younger Children Better Than Older Children and Adults?
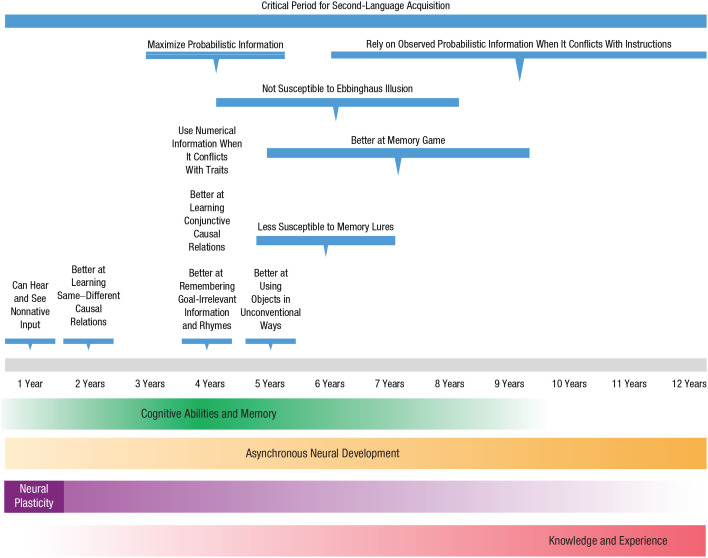
When exactly do children perform better than adults? Figure 1 depicts an approximate timeline of the documented advantages we found in the literature. Children’s advantages appear to cluster around the preschool and early school years, though there are cases of benefits in infancy and later childhood. In this section, we review these instances thematically across childhood.
Fig. 1.
Timeline of advantages and developments in childhood. Information above the timeline, marked by blue lines, depicts children’s advantages across various cognitive tasks. Infants can see and hear information that adults cannot. Around 4 years of age, children show numerous advantages in reasoning, decision-making, and memory over older learners. They also experience some perceptual and memory benefits between the ages of 5 and 8. Notably, the critical period for language acquisition tends to end around the age of 12. Information below the timeline depicts ongoing developments in childhood. Children’s cognitive abilities, depicted by the green line, undergo rapid development before 8 years of age. The yellow line represents asynchronous neural development in childhood: the procedural system develops faster and the declarative system more slowly. The purple line represents neural plasticity, which is especially pronounced during infancy and decreases with age. Finally, children acquire much knowledge throughout childhood, as depicted by the pink line.
When children hear and learn what adults cannot: the case of language
Learning language is one of the greatest feats of childhood. This is a particularly difficult task because learners have to make sense of the imperfect input they are given, which contains large amounts of data and noise. Regardless, infants quickly tune into the sounds used in their native language after only a few months of postnatal listening experience (Polka & Werker, 1994; Werker & Tees, 1984), and by their 4th birthday, children have an extensive vocabulary, produce complex sentences, and understand the rules that govern their native language (Brown, 1973). But how do our youngest, most novice learners overcome this immense learning feat, achieving better attainment than their adult-language-learning counterparts (Newport, 1990)?
Before infants can start learning words, they need to tune into the sounds used in their language. Remarkably, human infants are born seeing and hearing linguistic information that older children and adults miss, although they lose this ability with more experience in their environments. Specifically, infants can discriminate speech sounds and tones used in all of the world’s languages, making them open to all input, regardless of the linguistic environment they are born into (Eimas et al., 1971). Around 8 to 10 months after birth, infants lose the ability to discriminate between nonnative speech sounds and tones (Mattock & Burnham, 2006; Polka & Werker, 1994; Werker & Tees, 1984). This process, referred to as perceptual narrowing, is also observed in the visual domain for signed languages. Indeed, at 4 months of age, infants who are not exposed to any sign language are sensitive to the hand-sign distinctions used in American Sign Language (ASL) but lose this ability by 14 months (Palmer et al., 2012). Perceptual narrowing is not specific to linguistic information either: Six-month-old infants from Western cultures can detect violations in metrical structure from both Western and non-Western music, although they lose the ability to discriminate among non-Western meter by 12 months (Hannon & Treehub, 2005a, 2005b). Thus, while infants are attuning to information in their environments, they simultaneously lose the ability to hear and see things that they could before.
These early commitments to linguistic information matter because they open the door for learning a language’s grammar—the rules that govern its structure. Indeed, children who lose the ability to discriminate nonnative sounds earlier also make progress in learning their native language earlier (Kuhl et al., 2005). In turn, aspects of grammar seem to have their own critical periods. Regardless of modality, the age at which children are exposed to language predicts their acquisition of grammar (Johnson & Newport, 1989). In terms of signed language, an earlier age of exposure leads to better grammatical outcomes. Deaf children who were exposed to signed language after their 12th birthday did not achieve native-like performance on a test of ASL morphology despite having used ASL as their primary language for a minimum of 30 years (Newport, 1990). Similar to first-language acquisition, second-language acquisition also has a critical period for grammar (Johnson & Newport, 1989). Indeed, learning a second language in adulthood generally results in worse learning outcomes, whereas exposure before the age of 12 can result in native-like proficiency (Hartshorne et al., 2018).
Why is this early experience critical for learning grammar? And, in terms of second-language acquisition, why can children achieve higher levels of proficiency than adults, who have more experience processing input and using grammatical rules? Children’s advantage may lie in how they treat the input they are given because, compared with adult learners, they tend to create systematic structure and amplify the structure that is present in their input (Hudson Kam & Newport, 2005; Senghas & Coppola, 2001; Singleton & Newport, 2004). Evidence for children creating structure comes from observations of the creation of Nicaraguan Sign Language (NSL), which was developed by students attending a school for Deaf education. Although children were primarily instructed in spoken Spanish and lipreading, they developed their own system of gestures to communicate with each other. This system of gestures evolved into a signed language with its own grammar through two cohorts of learners, each of which included a wide age range of children. Note that although NSL originated in the gestures of the first cohort of learners, it was systematized by the second cohort of learners, particularly the younger children who were first exposed to the language before the age of 10. This second cohort created a grammar that had structural regularity, as evidenced by their consistent use of spatial modulations to convey specific meanings (Senghas & Coppola, 2001).
Moreover, evidence for children’s ability to amplify structure comes from the case study of Simon, a Deaf child whose late-learning parents were his only source of ASL input (Singleton & Newport, 2004). Because his parents were signers who learned late, they did not provide consistent input and frequently omitted or used incorrect morphemes. Simon, however, amplified the structure he was given and boosted the forms that his parents used relatively consistently, using them to an even greater extent than his parents (Singleton & Newport, 2004). Children’s tendency to impose structure is also evident in artificial grammar-learning experiments. Although children boost the most consistent forms, adults produce variability that is present in the input (Hudson Kam & Newport, 2005). Thus, children create a more consistent, structured grammar, and their tendency to boost consistent forms aids their learning outcomes.
When children see what adults cannot
As illustrated in the previous section, infants’ perceptual systems become specialized because they are exposed to acoustic and linguistic information, something that also occurs in the visual system. Indeed, infants’ visual systems undergo perceptual narrowing for faces of various species: Six-month-old infants can discriminate among monkey faces, whereas 9-month-old infants cannot (Pascalis et al., 2002). And 4- to 6-month-olds, but not 9-month-olds, can discriminate among sheep faces (Simpson et al., 2011). Perceptual narrowing also occurs for infants’ own-race faces. At 3 months, White infants can discriminate between faces from various races, but by 9 months of age, White infants can distinguish between White faces only (Kelly et al., 2007). Together, these findings show that infants are concurrently tuning into linguistic, acoustic, and visual information in their environments, even before their first birthday. But, for the first few months after birth, young infants see and hear things that older infants, children, and adults do not.
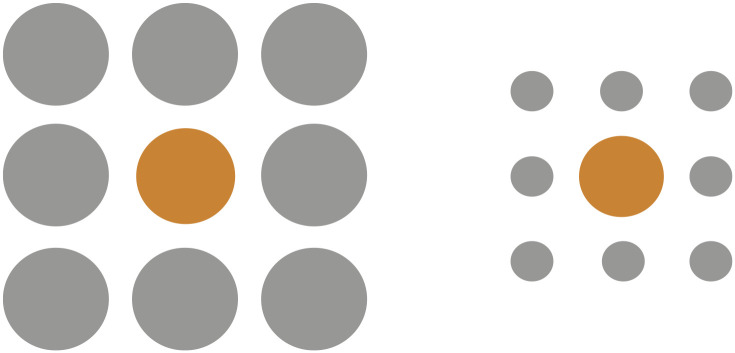
In some cases, children also more accurately see what is really there, making them less susceptible to certain optical illusions. In particular, young children do not experience the Ebbinghaus illusion (Doherty et al., 2010). In this illusion, participants are presented with two circles that are each surrounded by either larger or smaller circles (Fig. 2). When the surrounding circles are smaller than the central circle, they make the central circle appear larger, and when the surrounding circles are larger, they make the central circle look smaller. When asked which central circle is larger, most adults indicate that the central circle surrounded by smaller circles is larger, even when this central circle is actually smaller. Remarkably, children between the ages of 4 and 7 years are not susceptible to this illusion. Moreover, although 8- to 10-year-old children are more susceptible, their experience of the illusion is not as pronounced as adults’ (Doherty et al., 2010). These findings illustrate that context does not affect size perception in early childhood, and although there is gradual development toward adult-like visual processing, younger children can more accurately perceive what is really there.
Fig. 2.
Example of the Ebbinghaus illusion. In the Ebbinghaus illusion, the size of the surrounding gray circles affects our perception of the inner orange circle. That is, when the surrounding gray circles are larger than the inner orange circle, they make the orange circle appear smaller (as on the left), and when the gray circles are smaller than the orange circle, they make the orange circle appear larger (as on the right). In this figure, the orange circle on the left is larger than the orange circle on the right.
When children make better predictions from probabilistic data
Younger children not only see and hear things differently, but they also sometimes make better use of the data they are given. That is, there are cases in which younger children use probabilistic information in their decisions more than older children and adults (Decker et al., 2015; Gualtieri & Denison, 2018; Weir, 1964). Initial evidence of children’s better use of probabilistic information emerged in classic probability-learning studies. In these paradigms, participants were presented with multiple lights and were told that they could select one of the lights by pressing its corresponding button. On each trial, participants could press only one of the buttons, and they were told they would win a small prize if the light they selected lit up. Unbeknownst to participants, the lights activated according to fixed likelihoods (e.g., 75% left light, 25% middle light, 0% right light) that became apparent to participants over multiple trials (Derks & Paclisanu, 1967; Weir, 1964). To increase their chance of success, participants should maximize—that is, almost exclusively choose the option with the highest probability (e.g., always choosing the left light). Instead, adults often match the probabilities in their responses (e.g., choosing the left light 75% of the time and the middle light 25%). However, 3- to 5-year-old children are more likely to maximize than older children and adults (Derks & Paclisanu, 1967; Weir, 1964). Intriguingly, in a probability-learning task in which participants were told that the lower-probability option would yield the highest rewards, children and adolescents abandoned the incorrect instruction as they learned the true likelihoods over the course of the experiment, whereas adults were more biased by this incorrect cue (Decker et al., 2015).
Child-friendly variants of classic judgment and decision-making tasks also highlight 4-year-old children’s ability to use probabilistic data more rationally than both older children and adults. It is well documented that adults frequently discredit base rates: If they are given a personality description (e.g., Jamie enjoys math puzzles and carpentry) taken from a population (e.g., a base rate of 70 lawyers and 30 engineers), adults will overweigh the personality description and neglect the base rate when classifying the individual’s occupation. Adults even neglect base rates in problems in which the personality description is not diagnostic of either occupation, demonstrating a very strong tendency to overrely on the description in lieu of the probabilities (Kahneman & Tversky, 1973). Notably, evidence suggests that 4-year-old children do not neglect this important information. When given conflicting base-rate and personality information, 4-year-olds provided responses more aligned with the base rate and classified an unknown individual as belonging to the majority group, although 6-year-olds and adults relied on the personality description (Gualtieri & Denison, 2018). Thus, younger children were more inclined to rely on probabilistic data in the presence of a conflicting cue, producing more rational responses very early in development.
When children more accurately detect causal patterns
Young children sometimes design better interventions and provide explanations that are more aligned with observed data than older children and adults (Lucas et al., 2014; Seiver et al., 2013; Walker et al., 2016). In one set of experiments, 4- and 5-year-olds more accurately learned a causal rule than adults (Lucas et al., 2014). Participants were presented with evidence that suggested a device was activated disjunctively (i.e., each cause produces an independent effect) or conjunctively (i.e., multiple causes produce the effect together). Although both children and adults inferred the disjunctive principle from the disjunctive activation pattern, they performed differently when presented with the conjunctive activation pattern: Children readily inferred the conjunctive principle, whereas adults inferred a less accurate disjunctive rule. Highlighting an advantage for even younger children, a second set of findings showed that toddlers learned an abstract relation that preschoolers struggled with (Walker et al., 2016). In particular, children observed evidence that suggested a device was activated by two blocks that were identical in color and shape, or two blocks that differed along those dimensions. Whereas 18- to 30-month-olds learned this relation, 30- to 48-month-olds did not. Together, these findings show that very young children sometimes represent the data more accurately for what they are, allowing children to learn rules that are less commonly encountered in our daily lives.
A similar developmental pattern has emerged in work on children’s causal explanations of others’ behavior. In Western cultures, adults often favor explanations that focus on person-specific causes of behavior over those that consider situational factors, which is known as the fundamental attribution error (Jones & Harris, 1967). In a child-friendly variant of this problem, 4-year-olds inferred causes from the data and provided explanations that were aligned with the observed patterns of behavior. That is, if a character acted consistently across activities, 4-year-olds provided causal explanations that focused on the individual, although if both characters acted consistently during the same activity, they provided explanations that highlighted the situation. Conversely, 6-year-old children provided person-specific causal attributions regardless of the pattern of data they observed, much like adults (Seiver et al., 2013). Taken together, these data show that young children learn causal relations that adults miss.
When children have better (or more accurate) memory
Despite ongoing development of children’s memory abilities (Gathercole, 1998), there are some cases in which younger children remember information more accurately than older children and adults (Baker-Ward & Ornstein, 1988; Brainerd et al., 2018; Deng & Sloutsky, 2016; Király et al., 2017). First, preschoolers have better verbatim memory for rhymes than adults (Király et al., 2017). After listening to a verse every night for 10 days, 4-year-olds recalled more words and made fewer errors than adults when reciting the verse. Second, children often win against adults when playing the memory game known as Concentration (Baker-Ward & Ornstein, 1988). In this memory game, participants flip two cards over on each turn, with the goal of uncovering matching pairs of pictures. Although there were no age differences in the number of trials it took participants to uncover all of the pairs, 5- to 9-year-old children were more efficient: They were more likely to find the pair as soon as they knew the location of both pictures (Baker-Ward & Ornstein, 1988; cf., Krøjgaard et al., 2019; Schumann-Hengsteler, 1996). Five-year-olds also made fewer spatial errors (i.e., selecting an incorrect card that is adjacent to the correct card) than older children and adults (Schumann-Hengsteler, 1996).
There are also cases in which children objectively have better memory than adults. First, 6- and 7-year-old children were less likely to inaccurately report that semantically related lures were on a list of words they were tasked with remembering; that is, older children and adults will report that a word was present on a list even though it was not presented (Brainerd et al., 2018; Holliday et al., 2011). Second, when asked to think about a category-level feature (i.e., that cats have beta cells) during initial encoding, 5-year-old children have shown better memory for previously studied items (a particular cat or squirrel) compared with adults (Sloutsky & Fisher, 2004). And third, both 4- and 5-year-old children remembered goal-irrelevant information better than older children and adults (Deng & Sloutsky, 2016; Plebanek & Sloutsky, 2017). That is, when tasked with predicting category membership, older children and adults remembered the diagnostic information better and struggled remembering information that was not initially goal relevant. Conversely, 4- and 5-year-old children remembered the goal-irrelevant information better.
When children are better at solving problems
One final advantage for younger children lies in their ability to solve problems that require unconventional solutions. In particular, young children’s more creative, divergent thinking allows them to take a different approach to solving problems than older children and adults, which can sometimes lead to more effective solutions. Adults find it difficult to generate alternative functions for common objects, known as functional fixedness (i.e., using a box as a candleholder; Adamson, 1952). In a child-friendly version of the task, 5- to 7-year-old children needed to stack a box on top of a tower of blocks to reach a shelf, thus using the box in an unconventional manner (German & Defeyter, 2000). By the age of 6, children performed similarly to adults and showed signs of functional fixedness because it took them longer to come up with the correct solution when it conflicted with the box’s typical function. However, 5-year-olds readily used the objects unconventionally and were faster at solving the problem (Defeyter & German, 2003; German & Defeyter, 2000).
Why Are Younger Children Sometimes Better?
Now that we have outlined the instances in which younger children hold an advantage, we can examine why, exactly, younger is better in these circumstances. Although these cases are varied across domains, it seems that they are due in part to limits in children’s cognitive abilities, patterns of brain development, periods of neural plasticity, weaker prior knowledge, and a heightened tendency to explore. We posit that these factors, at times with varying degrees of influence, lead to young children’s superior outcomes.
Cognitive abilities
Compared with their older counterparts, young children have more limited cognitive abilities. Specifically, their long-term memory, working memory, cognitive control, and attentional capacities undergo great change throughout early childhood. Turning to children’s memories first, much work has shown that both long-term and working memory abilities develop greatly throughout childhood (despite the idiosyncratic instances reviewed above). Children’s long-term memory (i.e., their ability to remember information over time) improves significantly throughout childhood (Gathercole, 1998; Ghetti & Angelini, 2008), but especially during earlier childhood (roughly before the age of 7 years). Moreover, children’s working memory (i.e., their ability to hold and manipulate information in their mind’s eye or ear) improves rapidly in the early school years and continues to develop into later childhood (Gathercole et al., 2004).
Relatedly, children’s cognitive control—the process by which goals influence behavior (including attention and working memory)—changes greatly with age, especially during the preschool years. Indeed, it has been suggested that children shift from a more reactive, control-free mindset through 4 years of age to a more proactive, control-based mindset in which they can proactively set goals, anticipate events, and generate predictions (Munakata et al., 2011). Inhibition (the ability to withhold a prepotent response) is central to this and also increases significantly through early childhood (Davidson et al., 2006; Williams et al., 1999). Along these lines, young children’s ability to selectively attend to an item (among distractors) changes greatly through early childhood (Plude et al., 1994), reaching mature levels for simple search tasks by 6 years of age (Hommel et al., 2004).
Although these developments generally aid children’s learning outcomes as they get older, limits in these cognitive abilities seem to help younger children in quite a few circumstances. Indeed, a number of theories have put forth a link between limits in children’s cognitive abilities and their superior learning outcomes, particularly when it comes to language. The “less-is-more” hypothesis suggests that children’s limited working memory capacity underlies their language-learning prowess: Children’s better analysis of linguistic input is a virtue of their reduced working memory because they store less information in smaller forms (Newport, 1990). Relatedly, children’s limited working memory has been said to help in the detection of strong relations among the input because children work with smaller samples of data that reflect more extreme relationships (Kareev, 1995). Providing support for this proposal, empirical work has shown that adults with more limited working memory capacity detected stronger correlations within the data they were given (Kareev et al., 1997).
Turning to limits in cognitive control, an additional set of theories has suggested that reduced control (and increased perseveration) aids children’s language (Ramscar & Gitcho, 2007) and other learning outcomes (Thompson-Schill et al., 2009). These limits in children’s cognitive control are thought to underlie their tendency to regularize linguistic input. That is, reduced control leads to greater perseveration and engagement with input or responses that are more frequent, thus boosting the more frequent response. This could help children acquire grammar and explain why we see periods of overregularization (e.g., producing the plural for foot as “foots”) when children are learning irregular plurals.
A related set of thinking has focused on children’s poor long-term memories and the fact that they are more likely to forget, arguing that although forgetting information impedes learning something new, it also prioritizes highly relevant information for learning via greater repetition (Vlach, 2014). Children forget information more often because of their poor long-term memory, leading to more attempts at reactivation. It has been argued that these additional reactivation attempts support memory for relevant information that is present at repeated learning events and concurrently deters memory for irrelevant information.
We posit that these limits in cognitive abilities can also explain why children confer advantages in domains other than language. Recall that young preschoolers tend to maximize the most likely outcome, whereas older children and adults probability match. Preschoolers’ limited cognitive resources may lead them to maximize the probabilities (Thompson-Schill et al., 2009). Although maximizing is more rational, it only requires that participants track the most frequent option and select it somewhat habitually. Younger children may have an approximate representation of the values, which includes an understanding of which option is highly frequent. They may perseverate on the high-probability option because their limited working memory makes it difficult for them to track and reproduce the lower-probability options. Older learners, aided by better developed cognitive abilities, can inhibit this more prepotent response and track the likelihoods associated with all options. From this, they produce patterns that match the likelihoods (i.e., probability match), generate and test additional hypotheses, and flexibly switch between different strategies on the basis of the evidence they have observed.
Children’s limited cognitive abilities may also lead them to make better use of probabilistic information in decision-making tasks. Given their limited working memory and cognitive flexibility, 4-year-old children may have trouble considering all the information at hand. That is, 4-year-olds’ tendency to provide the more rational base-rate response seems to be due to difficulty with weighing more than one piece of information. Six-year-olds’ better developed working memory and cognitive flexibility could help them weigh both pieces of information and consider different potential responses (Gualtieri & Denison, 2018).
Moreover, younger children’s more limited cognitive abilities aid their performance in causal-reasoning tasks. Recall that younger children sometimes pick up on causal-activation patterns that older children and adults miss. Because young children have less cognitive control, their exploration is also less controlled, allowing them to entertain hypotheses that older learners might not consider, such as those based on limited evidence or those that are less similar to other hypotheses considered (Gopnik et al., 2015). Furthermore, children’s cognitive abilities likely affect how they weigh information when making inferences. Because of more limited working memory and reduced integration abilities in memory (Edgin et al., 2014), children may have trouble integrating evidence with their own prior beliefs and thus stick with the data they have observed in these tasks. Although older children and adults are better equipped to handle all available data, they seem to overweigh their prior beliefs (Lucas et al., 2014; Seiver et al., 2013), thus sometimes leading to more biased inferences when their prior beliefs conflict with the data at hand (Decker et al., 2015).
Young children’s more limited cognitive abilities can explain their immunity to functional fixedness, also allowing them to explore more unconventional hypotheses (Thompson-Schill et al., 2009). Recall that participants in these paradigms are tasked with devising solutions that involve using objects in an unconventional way. At first glance, this interpretation seems counterintuitive: Older problem-solvers, who have better developed inhibitory control and cognitive flexibility, should be better at inhibiting their knowledge of prepotent conventional uses. In turn, this should allow them to think more flexibly about alternative solutions (German & Defeyter, 2000). However, less cognitive control actually improved adults’ performance on these tasks: Adults whose left prefrontal cortex was inhibited via transcranial direct current stimulation were faster and could generate more novel responses, likely because of reductions in the top-down filtering of bottom-up information (Chrysikou et al., 2013). Thus, 5-year-olds’ more limited cognitive abilities seem to aid their problem-solving when devising more unconventional, creative solutions.
Finally, ongoing cognitive development can also explain age-related changes in children’s ability to remember goal-irrelevant information. Whereas younger children distribute their attention more broadly, older children and adults are better at selectively attending to information (Plude et al., 1994). Because of this, younger children pick up on more features in the stimuli, including those that might not currently be relevant, whereas older children and adults allocate their attention to only goal-relevant information (Deng & Sloutsky, 2016; Plebanek & Sloutsky, 2017). This difference in how younger and older learners allocate attention affects their memory of the information presented.
Asynchronous neurocognitive development
It is noteworthy that the neural structures in the brain that give rise to these changes in cognitive abilities also change greatly with age, and at different rates. Indeed, much of the theory emphasizing the possible benefits of children’s reduced cognitive control and working memory focuses specifically on the especially slow development of the prefrontal cortex (Lenroot & Giedd, 2006; Ramscar & Gitcho, 2007; Thompson-Schill et al., 2009). In fact, much can be learned from looking at children’s unique learning strengths from a neurocognitive perspective and considering the developmental profile of the many brain regions that are involved in learning and memory more broadly construed.
Along these lines, a classic neurocognitive view holds that the brain learns in different ways by using two specialized systems: a procedural or nondeclarative learning system that is thought to depend on the basal ganglia and cerebellum (Shohamy et al., 2004) and a declarative or explicit learning system that depends on the hippocampus and prefrontal and parietal cortices (Gabrieli, 1998). The procedural system is generally thought to learn information slowly (over many experiences) and without awareness on the part of the learner (Knowlton et al., 1994; Shohamy et al., 2009), making it particularly well suited for learning nonverbalizable distributions and rules (Nomura & Reber, 2008; Ullman, 2001). The declarative system, on the other hand, can learn much more quickly (from even just one experience) and generally produces knowledge that learners can be aware of: They can verbalize or declare it (Squire, 2004). These two learning systems develop at different rates: The procedural learning system matures earlier than the declarative system (Amso & Davidow, 2012; Finn et al., 2016) 1 ; some have even argued that procedural and other implicit systems are developmentally invariant (Hasher & Zacks, 1984; Janacsek et al., 2012; Thomas & Nelson, 2001).
In addition to benefits in learning from having reduced cognitive capacities as discussed in the previous section—which are generally speaking declarative, conscious, and effortful—we propose that there is an additional benefit to learning as a young child by virtue of having a faster developing procedural learning system that is left unchecked by a slower developing declarative system (see also Finn et al., 2014).
Addressing these advantages, it has been proposed that learning a language’s grammar is achieved mostly through the procedural learning system (Ullman, 2001). Indeed, the learning of nonverbalizable distributions is central to understanding a language’s grammar (Maratsos & Chalkley, 1980; Mintz et al., 2002). The fact that the procedural system develops more quickly could explain why children can learn the grammar of both first and second languages better than adults because it could allow for children to focus on nonverbalizable distributions in the data, without interference from the declarative system (Ullman, 2001).
Beyond language, a faster developing procedural system could explain why children can learn from probabilistic data and why they can learn certain causal relationships that adults struggle with. The role of the procedural system in learning about probabilistic relationships has been documented extensively (Gluck et al., 2002; Knowlton et al., 1994) and has been observed in children (Amso & Davidow, 2012; Finn et al., 2016; Janacsek et al., 2012), making this the likely system involved in learning about probabilistic relationships. But the key to children’s possible advantage in probability learning likely has more to do with the slow development of the declarative system. Indeed, the declarative system is thought by some to directly compete with the procedural system (Poldrack et al., 2001), meaning that when it comes on-line it could directly block the operation of the procedural system, making it harder for older children and adults to use this for pattern learning. Even if declarative and procedural systems do not compete for learning, the early development of the procedural system relative to the declarative can help explain why children are likely to learn probabilistic information better because their underdeveloped declarative system could lead them to rely more heavily on procedural processes. Although these theories need to be tested with experimental work, having a procedural system that is relatively more mature than the declarative system could lead children to weigh their slow learning of probabilistic data more heavily, or even rely on this entirely.
This balance of neurocognitive systems could also be helpful for learning causal relationships that are not common (i.e., a conjunctive rule; Lucas et al., 2014). Here again, the procedural system will be helpful in extracting patterns of data from observations. And, in turn, a better developed declarative system aids in the use of prior knowledge (discussed in greater detail later) because it calls on general knowledge and similar experiences from memory. Thus, having a less mature declarative system benefits young children by making them less likely to rely on prior knowledge in new, yet similar, situations (and rely on current observations instead). A similar logic applies for thinking about children’s prowess in finding novel solutions in functional-fixedness tasks—younger children may be less likely to call on their knowledge of an object’s conventional functions and instead rely on their current observations to determine its potential features.
Plasticity
Another aspect of brain development that deserves much careful consideration is neural plasticity: the malleability of the brain—in terms of neuronal structure or connectivity—that is generally thought to be caused by some aspect of the environment. Indeed, increased neural plasticity during infancy is integral to children’s learning across a number of domains and is often cited as fostering children’s superior language-learning outcomes (Lenneberg, 1967). Greater plasticity in infancy has been documented in myriad animal and human models, all of which show the immense capacity for the juvenile brain to reorganize on the basis of experience in many forms, from exposure to bird song in finches (Woolley, 2012), to visual distortions (in the form of prism glasses) in barn owls (Knudsen & Knudsen, 1990), and even recovery after the removal of visual occlusions (cataracts) in humans (Maurer, 2017).
Indeed, work on plasticity in the developing human brain has identified critical periods, in which infants’ brains are particularly plastic and sensitive to environmental input (Hensch, 2004). During a critical period, infants’ experiences change their synaptic networks by strengthening and weakening relevant connections to reflect the information in their environment (Werker & Hensch, 2015). Moreover, the development of neural circuits that use γ-aminobutyric acid (GABA; the brain’s most prevalent inhibitory neurotransmitter) plays an important role in the timing of critical periods (Fagiolini & Hensch, 2000; Hensch, 2004; Werker & Hensch, 2015). Before the maturation of GABA circuits, brain activity is mostly excitatory. As GABA circuits mature, inhibitory processes gradually kick in: Because GABA is an inhibitory neurotransmitter, the maturity of GABA circuits balances the mostly excitatory activity in the infant brain, switching it into a plastic state by creating an optimal excitatory–inhibitory balance for learning and consolidating new information. These windows of plasticity are limited because circuits eventually shift to a stable state and consolidate information learned during plasticity (Werker & Hensch, 2015). In general, increased plasticity means that the brain is more vulnerable and more likely to reorganize on the basis of experience, although these periods of plasticity can hold certain perceptual and learning advantages for infants, who see and hear things that children and adults cannot.
Notably, much research has examined the role of critical periods in language development and has highlighted important maturational timelines associated with plasticity. In particular, perceptual narrowing of phonetic contrasts follows a trajectory that matches an infant’s gestational age and not their chronological age (Peña et al., 2012). Indeed, infants who were born 3 months premature did not lose the ability to discriminate between nonnative contrasts until they caught up in gestational age, although they experienced more high-quality input outside of the uterus than full-term infants of the same gestational age (Peña et al., 2012).
Moreover, these critical periods seem tied to excitatory-inhibitory balances within the brain. Exposure to selective serotonin-reuptake inhibitors (SSRIs) in utero accelerates the onset of postnatal critical periods, closing the plasticity window earlier in infancy (Weikum et al., 2012). Because SSRIs increase levels of GABA in the infant brain, the optimal excitatory-inhibitory balance for plasticity is experienced earlier because of the added inhibitory influence of GABA. Infants whose mothers took SSRIs while pregnant lost the ability to discriminate nonnative contrasts earlier than infants whose mothers did not take SSRIs while pregnant, suggesting that exposure to GABA initiated the critical period for perceptual tuning earlier. Thus, critical periods are based in the brain, and plasticity seems to be driven by maturation and the regulation of excitatory-inhibitory circuits.
Plasticity during infancy also plays a role in perceptual narrowing to faces (Kelly et al., 2007; Pascalis et al., 2002; Simpson et al., 2011) and musical structure (Hannon & Treehub, 2005a, 2005b), although work linking these directly to the excitatory-inhibitory balance in the brain is as yet outstanding. Experience has a pronounced impact on infants’ visual-processing abilities: Six-month-old infants who were regularly presented with picture books containing other-race (Heron-Delaney et al., 2011) and primate faces (Pascalis et al., 2005) did not experience the perceptual narrowing for other-race or primate faces (respectively) that typically occurs at 9 months. Experience can also prolong periods of plasticity for acoustic information, even after a critical period has “closed.” Indeed, with additional exposure to non-Western music, 12-month-olds regained sensitivity to non-Western metrical structure (Hannon & Trehub, 2005b). A similar effect in language learning was found by Yoshida et al. (2010), who showed that 10-month-olds were able to relearn a “lost” phonetic contrast (Hindi distinction between retroflex /Da/ and dental /da/) with sufficient bimodal exposure to the contrast. Thus, across visual and acoustic modalities, there are periods of plasticity in which infants’ brains appear to be more malleable and open to environmental input, making learning and reorganization possible.
The picture regarding the role of plasticity throughout childhood is less clear, although it appears that children’s brains are more open to reorganization compared with adults. Indeed, the developing human brain undergoes a protracted period (lasting even into one’s 20s or 30s) in which cortical and subcortical gray matter is reduced and white matter is increased (Lebel & Beaulieu, 2011; Lenroot & Giedd, 2006; Shaw et al., 2008). Reductions in gray matter are thought to reflect the developmental process of synaptic pruning, in which redundant connections are eliminated and “effective” connections are used and strengthened (Nowakowski & Hayes, 2002). In addition, an increase in white matter is thought to reflect an increase in myelin, the sheath-covering axons that can act as “glue” in the human brain, preventing major reorganization (or plasticity) while stabilizing existing networks (McGee et al., 2005). Throughout childhood, adolescence, and even early adulthood, the human brain is more plastic in these regards.
The ease with which children can rework and overwrite knowledge provides a strong case for prolonged periods of plasticity in development, highlighting the important role of experience throughout childhood. That is, children’s knowledge of linguistic and visual information is more amendable to experience than adults’, particularly before 12 years of age (Hartshorne et al., 2018; McKone et al., 2019). Moreover, compelling evidence for plasticity in childhood comes from adults born in Korea that were adopted into French families as children. Children were exposed only to Korean before adoption but heard only French after adoption. When listening to Korean as adults, they could not discriminate between Korean phonemes (Ventureyra et al., 2004) and produced neural responses that resembled exposure to other unknown languages (Pallier et al., 2003), which is consistent with complete reorganization resulting from the immersive input after adoption. Childhood experience with other-race faces can also lead to significant reorganization. That is, children born in Asian countries adopted by White European families have shown both a reduced (de Heering et al., 2010) and even a completely reversed (Sangrigoli et al., 2005) other-race effect. Note that increased (and even extensive) exposure to other-race faces in adulthood does not appear to shift discrimination abilities, although experience before the age of 12 does lead to better recognition of other-race faces (McKone et al., 2019). Taken together, children’s processing abilities are extremely amendable to experience and can greatly reorganize, and even overwrite, information learned during critical periods in infancy, even years after these critical periods have presumably closed.
Another example of greater plasticity extending into childhood comes from work looking at the age of onset of dense cataracts that block all patterned visual input to the back of the eye. Congenital cataracts produce a host of long-term consequences on vision, especially reductions in visual acuity (Maurer, 2017). If the onset of dense cataracts is later (between 4 months and 15 years), visual acuity is better than in congenital cases but still much worse than control participants. Moreover, acuity was abnormal for the patients who experienced deprivation before 5 years of age but normal for patients who experienced deprivation after 11 years of age (Maurer, 2017). Thus, the visual system is particularly vulnerable early in development because experience shapes visual ability more at younger ages, especially up to 5 years.
A final way of looking at plasticity in childhood is examining the neural representation of skills that were acquired earlier in life. Along these lines, string players had larger motor representations related to the fingers used to play their instrument, and these representations were especially larger in players who picked up their instrument earlier (Elbert et al., 1995). Likewise, the age that a learner begins to learn a second language affects how the language is processed in their brain: If a second language is learned early, it activates overlapping areas associated with native language processing, but if it is learned later, it activates adjacent areas (Kim et al., 1997). This effect matters most for a language’s grammar (Wartenburger et al., 2003), which we know is subject to a critical period.
Taken together, this work indicates that infants, but also children, are much more plastic than their older counterparts, leading to crucial differences in perception that allow them to hear and see things that older children and adults cannot. 2 Further, children’s greater ability to learn from and reorganize on the basis of experience likely contributes to their unique success observed in other domains. In particular, greater plasticity in younger learners means experience holds more weight, has more power to reorganize, and is also more vulnerable to being overwritten on the basis of subsequent experience. Although much work is needed to determine how these variables play out during learning and reasoning in children, plasticity could help explain why children change their behavior in accordance with observed patterns of data more quickly than adults (Decker et al., 2015) and why previous experience (and knowledge) often play a reduced role in their learning and reasoning, an idea that we review more fully in the next section.
Prior knowledge
As noted, one mechanism that underpins many of children’s superior learning outcomes is their reduced knowledge about the world around them. It may go without saying, but young children generally have less knowledge than older children and adults. With additional experience and knowledge, age-related improvements in crystalized intelligence (i.e., knowledge gained through experience, such as vocabulary and general facts) persist into adulthood (Li et al., 2004). Moreover, children’s semantic networks are reworked over the course of development. They are initially sparse, but they become larger and more structured as they are enhanced and fine-tuned with experience and additional knowledge (Comesaña et al., 2014; Dubossarsky et al., 2017). Indeed, children do have knowledge about the world around them, and prior knowledge does affect what information is learned (Brod & Shing, 2019), although, importantly, the influence of prior knowledge may be attenuated in children (Brod et al., 2013). Although prior knowledge and experience can help us make decisions, learn new information, and solve problems, having less prior knowledge seems to benefit children’s learning outcomes in particular circumstances.
Notably, limits in children’s knowledge lead to advantages in causal-reasoning tasks. Over time, we learn general rules about how causal systems typically work (e.g., that the individual properties of an object tend to produce an effect). Although this can help us pick up other rules that fit with this principle, it constrains our ability to learn from patterns of evidence that suggest conjunctive or relational principles. Indeed, young learners are afforded with advantages in cases in which having less, and weaker, prior knowledge aids in their interpretation of the evidence because the pattern of evidence conflicts with these prior beliefs (Lucas et al., 2014; Seiver et al., 2013; Walker et al., 2016). That is, both children and adults have a similar hypothesis regarding the efficacy of individual objects, although it appears to be weaker and more amendable to additional evidence in children (Lucas et al., 2014). Moreover, children, who have less prior knowledge in general, have more bandwidth to test unique hypotheses that are not constrained by their previous experiences (Gopnik et al., 2015).
This logic also applies to young children’s success on functional-fixedness tasks (Defeyter & German, 2003; German & Defeyter, 2000) because their weaker prior knowledge affords them with an advantage over older children and adults. Younger children seem to view the conventional, intended use of an object as one of many features, which can make them more flexible when generating novel uses. Conversely, because older children and adults have more experience and extensive knowledge of conventional uses, this information may impede their ability to generate solutions that use objects in unconventional ways.
We also acquire knowledge that helps us predict and explain others’ behavior. As children increasingly interact with those around them, they begin to learn how traits and behavior aid their predictions. In turn, a stronger prior for person-specific information may lead children to undervalue other useful pieces of information, such as probabilistic data. By 6 years of age, children in Western cultures have a strong belief about how factors related to the individual are best for understanding behavior: They prefer trait-based explanations of behavior (Seiver et al., 2013) and rely on individuating information in their decisions (Gualtieri & Denison, 2018). Moreover, 4-year-olds seem to have a weaker prior for trait-based information, although they are quite practiced in using probabilistic information to inform their predictions. This may be why 4-year-olds rely on covariation data in their causal explanations (Seiver et al., 2013) and probabilistic information in their decisions (Gualtieri & Denison, 2018).
Prior knowledge also has a significant impact on what information is remembered, typically supporting memory for new information that is aligned with this knowledge (Brod et al., 2013). However, limits in children’s knowledge can also benefit their memories. Recall that younger children are less susceptible to semantic lures compared with older children and adults (Brainerd et al., 2018; Holliday et al., 2011). Young children’s semantic networks appear to be less structured and contain weaker relations between items (Comesaña et al., 2014; Dubossarsky et al., 2017). It is likely that this sparser semantic organization makes children less susceptible to these types of errors because young children experience higher levels of false memories when presented with lists of more age-appropriate information (Carneiro et al., 2007). Because the associations between these items are stronger, children are susceptible to semantic lures when presented with more age-appropriate lists. Taken together, these findings suggest that children’s reduced knowledge can lead to successes across domains, including their reasoning, problem-solving, and memory.
Greater exploration
On a final note, children’s tendency to explore is important for understanding their unique successes in learning (for reviews, see Gopnik, 2020; Nussenbaum & Hartley, 2019). Indeed, when tasked with balancing explore–exploit tensions—exploring to gain more information versus exploiting what is known to gain rewards—children explore more than adults (Blanco & Sloutsky, 2021; Gopnik, 2020; Meder et al., 2021) in ways that are systematic and directed (Blanco & Sloutsky, 2021; Meder et al., 2021; Schulz et al., 2019). Although increased exploration can prevent younger learners from more optimally exploiting rewards (Blanco & Sloutsky, 2021; Meder et al., 2021; Schulz et al., 2019), it can allow them to pick up on information that adults miss. Although work is needed to test these links directly, greater exploration has clear applications for learning the nuanced distribution of information required for learning a language’s grammar. It could also enable greater flexibility in reinforcement-learning tasks, allowing children to adjust from an incorrect instruction (Decker et al., 2015). And finally, children’s greater exploration—of hypotheses in particular—could lead them to make more correct inferences from patterns of causal data (Gopnik et al., 2015; Lucas et al., 2014) and aid in their ability to solve problems with unconventional solutions (Defeyter & German, 2003; German & Defeyter, 2000). Notably, developmental differences in exploration could be the result of ongoing cognitive development (Blanco & Sloutsky, 2021) and weaker prior knowledge (Gopnik, 2020); these changes, together with developmental shifts in neuroplasticity and patterns of brain development, make childhood a particularly unique and successful period for learning.
Conclusion
Childhood is often regarded as a time in development in which children undergo substantial periods of change to catch up with adults’ more sophisticated abilities. This view often neglects the magical aspects of infancy and childhood that are integral in understanding early experiences: Infants and children can sometimes see, hear, learn, remember, and reason in ways that are much better than adults. In this article, we documented these instances of childhood superiority across domains and argued that key aspects of being a child—having immature cognitive abilities, asynchronous periods of brain development, greater brain plasticity, less knowledge, and a stronger tendency to explore—can produce these unique and very special advantages.
Here we note that children’s perception and learning can be thought of in many ways as being more yoked to the actual input in the environment. Indeed, the instances we document regarding their prowess in perception shows that they can see and hear things adults miss. They also produce rational responses that are more aligned with the data, whether probabilistic or causal, and adapt their behavior on the basis of shifts in the environment more. And although they may remember less overall, children’s memories appear to be more grounded in their lived experience because they are less likely to falsely recall semantically related elements and sometimes even more detail of particular items.
Given these advantages, it is possible that all of these aspects of childhood are an adaptive feature of human development and not a bug (see Gopnik, 2020). Thus, infants and children present an opportunity for understanding the roles of cognitive ability, neural structures, plasticity, and knowledge in shaping perception, learning, and reasoning, thus deepening our mechanistic understanding of these factors. In addition, understanding how these factors, together, shape the magical experience that is childhood can help us understand the multifaceted nature of human cognition. In presenting these cases of childhood superiority in perception, learning, and reasoning across these diverse lines of research, we hope to inspire research that similarly traverses domains, whether linguistic or perceptual, to move toward a greater understanding of human cognition across the life span.
Although this developmental pattern is quite clear from behavioral work, there are many open questions about developmental changes in the associated neurobiology. In particular, we know that structural brain changes occur throughout childhood in brain regions that are associated with procedural and implicit learning as well, including the basal ganglia (Ostby et al., 2009) and hippocampus (Gogtay et al., 2006; Lee et al., 2014), through late childhood, leaving pressing questions about how the procedural system is behaviorally quite functional so early in life. Although this is a question for ongoing work, we think the role of sensory cortical regions in slow nondeclarative learning (McClelland et al., 1995) is likely to play a central role for very young learners and infants especially (Gómez, 2017).
It should be noted that, with sufficient and incrementally titrated exposure, there are many instances of plasticity in adulthood (e.g., Knudsen & Knudsen, 1990; for a comprehensive review of plasticity in adulthood, see Lillard & Erisir, 2011). And importantly, adults can even become experts in discriminating among novel and highly complex stimuli with sufficient experience (Gauthier et al., 1998).
Footnotes
ORCID iDs: Samantha Gualtieri  https://orcid.org/0000-0002-5403-0059
https://orcid.org/0000-0002-5403-0059
Amy S. Finn  https://orcid.org/0000-0002-7717-3562
https://orcid.org/0000-0002-7717-3562
Transparency
Action Editor: Laura A. King
Editor: Laura A. King
Declaration of Conflicting Interests: The author(s) declared that there were no conflicts of interest with respect to the authorship or the publication of this article.
Funding: This work was supported in part by Canadian Foundation for Innovation and Ontario Research Fund Grant 34947, Natural Sciences and Engineering Research Council of Canada Discovery Grant RGPIN-2016-05, and Social Sciences and Humanities Research Council Insight Program Grant 435171493 (to A. S. Finn).
References
- Adamson R. E. (1952). Functional fixedness as related to problem solving: A repetition of three experiments. Journal of Experimental Psychology, 44(4), 288–291. 10.1037/h0062487 [DOI] [PubMed] [Google Scholar]
- Amso D., Davidow J. (2012). The development of implicit learning from infancy to adulthood: Item frequencies, relations, and cognitive flexibility. Developmental Psychobiology, 54(6), 664–673. 10.1002/dev.20587 [DOI] [PubMed] [Google Scholar]
- Baker-Ward L., Ornstein P. A. (1988). Age differences in visual-spatial memory performance: Do children really out-perform adults when playing Concentration? Bulletin of the Psychonomic Society, 26(4), 331–332. 10.3758/BF03337672 [DOI] [Google Scholar]
- Birdsong D. (1999). Second language acquisition and the critical period hypothesis. Routledge. [Google Scholar]
- Blanco N. J., Sloutsky V. M. (2021). Systematic exploration and uncertainty dominate young children’s choices. Developmental Science, 24(2), Article e13026. 10.1111/desc.13026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brainerd C. J., Reyna V. F., Holliday R. E. (2018). Developmental reversals in false memory: Development is complementary, not compensatory. Developmental Psychology, 54(9), 1773–1784. 10.1037/dev0000554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brod G., Shing Y. L. (2019). A boon and a bane: Comparing the effects of prior knowledge on memory across the lifespan. Developmental Psychology, 55(6), 1326–1337. 10.1037/dev0000712 [DOI] [PubMed] [Google Scholar]
- Brod G., Werkle-Bergner M., Shing Y. L. (2013). The influence of prior knowledge on memory: A developmental cognitive neuroscience perspective. Frontiers in Behavioral Neuroscience, 7, Article 139. 10.3389/fnbeh.2013.00139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown R. (1973). A first language: The early stages. Harvard University Press. [Google Scholar]
- Carneiro P., Albuquerque P., Fernandez A., Esteves F. (2007). Analyzing false memories in children with associative lists specific for their age. Child Development, 78(4), 1171–1185. 10.1111/j.1467-8624.2007.01059.x [DOI] [PubMed] [Google Scholar]
- Chrysikou E. G., Hamilton R. H., Coslett H. B., Datta A., Bikson M., Thompson-Schill S. L. (2013). Noninvasive transcranial direct current stimulation over the left prefrontal cortex facilitates cognitive flexibility in tool use. Cognitive Neuroscience, 4(2), 81–89. 10.1080/17588928.2013.768221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comesaña M., Fraga I., Moreira A. J., Frade C. S., Soares A. P. (2014). Free associate norms for 139 European Portuguese words for children from different age groups. Behavior Research Methods, 46(2), 564–574. 10.3758/s13428-013-0388-0 [DOI] [PubMed] [Google Scholar]
- Davidson M. C., Amso D., Anderson L. C., Diamond A. (2006). Development of cognitive control and executive functions from 4 to 13 years: Evidence from manipulations of memory, inhibition, and task switching. Neuropsychologia, 44(11), 2037–2078. 10.1016/j.neuropsychologia.2006.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decker J. H., Lourenco F. S., Doll B. B., Hartley C. A. (2015). Experiential reward learning outweighs instruction prior to adulthood. Cognitive, Affective, & Behavioral Neuroscience, 15(2), 310–320. 10.3758/s13415-014-0332-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Defeyter M. A., German T. P. (2003). Acquiring an understanding of design: Evidence from children’s insight problem solving. Cognition, 89(2), 133–155. 10.1016/S0010-0277(03)00098-2 [DOI] [PubMed] [Google Scholar]
- de Heering A., de Liedekerke C., Deboni M., Rossion B. (2010). The role of experience during childhood in shaping the other-race effect. Developmental Science, 13(1), 181–187. 10.1111/j.1467-7687.2009.00876.x [DOI] [PubMed] [Google Scholar]
- Deng W. S., Sloutsky V. M. (2016). Selective attention, diffused attention, and the development of categorization. Cognitive Psychology, 91, 24–62. https://10.1016/j.cogpsych.2016.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derks P. L., Paclisanu M. I. (1967). Simple strategies in binary prediction by children and adults. Journal of Experimental Psychology, 73(2), 278–285. 10.1037/h0024137 [DOI] [Google Scholar]
- Doherty M. J., Campbell N. M., Tsuji H., Phillips W. A. (2010). The Ebbinghaus illusion deceives adults but not young children. Developmental Science, 13(5), 714–721. 10.1111/j.1467-7687.2009.00931.x [DOI] [PubMed] [Google Scholar]
- Dubossarsky H., De Deyne S., Hills T. T. (2017). Quantifying the structure of free association networks across the life span. Developmental Psychology, 53(8), 1560–1570. 10.1037/dev0000347 [DOI] [PubMed] [Google Scholar]
- Edgin J. O., Spanò G., Kawa K., Nadel L. (2014). Remembering things without context: Development matters. Child Development, 85(4), 1491–1502. 10.1111/cdev.12232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eimas P. D., Siqueland E. R., Jusczyk P., Vigorito J. (1971). Speech perception in infants. Science, 171(3968), 303–306. [DOI] [PubMed] [Google Scholar]
- Elbert T., Pantev C., Wienbruch C., Rockstroh B., Taub E. (1995). Increased cortical representation of the fingers of the left hand in string players. Science, 270(5234), 305–307. 10.1126/science.270.5234.305 [DOI] [PubMed] [Google Scholar]
- Fagiolini M., Hensch T. K. (2000). Inhibitory threshold for critical-period activation in primary visual cortex. Nature, 404(6774), 183–186. 10.1038/35004582 [DOI] [PubMed] [Google Scholar]
- Finn A. S., Kalra P. B., Goetz C., Leonard J. A., Sheridan M. A., Gabrieli J. D. (2016). Developmental dissociation between the maturation of procedural memory and declarative memory. Journal of Experimental Child Psychology, 142, 212–220. 10.1016/j.jecp.2015.09.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finn A. S., Lee T., Kraus A., Kam C. L. H. (2014). When it hurts (and helps) to try: The role of effort in language learning. PLOS ONE, 9(7), Article e101806. 10.1371/journal.pone.0101806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabrieli J. D. (1998). Cognitive neuroscience of human memory. Annual Review of Psychology, 49(1), 87–115. 10.1146/annurev.psych.49.1.87 [DOI] [PubMed] [Google Scholar]
- Gathercole S. E. (1998). The development of memory. Journal of Child Psychology and Psychiatry, 39(1), 3–27. 10.1111/1469-7610.00301 [DOI] [PubMed] [Google Scholar]
- Gathercole S. E., Pickering S. J., Ambridge B., Wearing H. (2004). The structure of working memory from 4 to 15 years of age. Developmental Psychology, 40(2), 177–190. 10.1037/0012-1649.40.2.177 [DOI] [PubMed] [Google Scholar]
- Gauthier I., Williams P., Tarr M. J., Tanaka J. (1998). Training ‘Greeble’ experts: A framework for studying expert object recognition processes. Vision Research, 38(15–16), 2401–2428. 10.1016/S0042-6989(97)00442-2 [DOI] [PubMed] [Google Scholar]
- German T. P., Defeyter M. A. (2000). Immunity to functional fixedness in young children. Psychonomic Bulletin & Review, 7(4), 707–712. 10.3758/BF03213010 [DOI] [PubMed] [Google Scholar]
- Ghetti S., Angelini L. (2008). The development of recollection and familiarity in childhood and adolescence: Evidence from the dual-process signal detection model. Child Development, 79(2), 339–358. 10.1111/j.1467-8624.2007.01129.x [DOI] [PubMed] [Google Scholar]
- Gluck M. A., Shohamy D., Myers C. (2002). How do people solve the weather prediction task? Individual variability in strategies for probabilistic category learning. Learning & Memory, 9(6), 408–418. 10.1101/lm.45202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogtay N., Nugent T. F., III., Herman D. H., Ordonez A., Greenstein D., Hayashi K. M., Clasen L., Toga A. W., Giedd J. N., Rapoport J. L., Thompson P. M. (2006). Dynamic mapping of normal human hippocampal development. Hippocampus, 16(8), 664–672. 10.1002/hipo.20193 [DOI] [PubMed] [Google Scholar]
- Gómez R. L. (2017). Do infants retain the statistics of a statistical learning experience? Insights from a developmental cognitive neuroscience perspective. Philosophical Transactions of the Royal Society B: Biological Sciences, 372(1711), Article 20160054. 10.1098/rstb.2016.0054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gopnik A. (2020). Childhood as a solution to explore–exploit tensions. Philosophical Transactions of the Royal Society B: Biological Sciences, 375(1803), Article 20190502. 10.1098/rstb.2019.0502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gopnik A., Griffiths T. L., Lucas C. G. (2015). When younger learners can be better (or at least more open-minded) than older ones. Current Directions in Psychological Science, 24(2), 87–92. 10.1177/0963721414556653 [DOI] [Google Scholar]
- Gualtieri S., Denison S. (2018). The development of the representativeness heuristic in young children. Journal of Experimental Child Psychology, 174, 60–76. 10.1016/j.jecp.2018.05.006 [DOI] [PubMed] [Google Scholar]
- Hannon E. E., Trehub S. E. (2005. a). Metrical categories in infancy and adulthood. Psychological Science, 16(1), 48–55. 10.1111/j.0956-7976.2005.00779.x [DOI] [PubMed] [Google Scholar]
- Hannon E. E., Trehub S. E. (2005. b). Tuning in to musical rhythms: Infants learn more readily than adults. Proceedings of the National Academy of Sciences, USA, 102(35), 12639–12643. 10.1073/pnas.0504254102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartshorne J. K., Tenenbaum J. B., Pinker S. (2018). A critical period for second language acquisition: Evidence from 2/3 million English speakers. Cognition, 177, 263–277. 10.1016/j.cognition.2018.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasher L., Zacks R. (1984). Automatic processing of fundamental information: The case of frequency of occurrence. American Psychologist, 39(12), 1372–1388. 10.1037/0003-066X.39.12.1372 [DOI] [PubMed] [Google Scholar]
- Hensch T. K. (2004). Critical period regulation. Annual Review of Neuroscience, 27, 549–579. 10.1146/annurev.neuro.27.070203.144327 [DOI] [PubMed] [Google Scholar]
- Heron-Delaney M., Anzures G., Herbert J. S., Quinn P. C., Slater A. M., Tanaka J. W., Lee K., Pascalis O. (2011). Perceptual training prevents the emergence of the other race effect during infancy. PLOS ONE, 6(5), Article e19858. 10.1371/journal.pone.0019858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holliday R. E., Brainerd C. J., Reyna V. F. (2011). Developmental reversals in false memory: Now you see them, now you don’t! Developmental Psychology, 47(2), 442–449. [DOI] [PubMed] [Google Scholar]
- Hommel B., Li K. Z. H., Li S. -C. (2004). Visual search across the life span. Developmental Psychology, 40(4), 545–558. 10.1037/0012-1649.40.4.545 [DOI] [PubMed] [Google Scholar]
- Hudson Kam C. L., Newport E. L. (2005). Regularizing unpredictable variation: The roles of adult and child learners in language formation and change. Language Learning and Development, 1(2), 151–195. 10.1080/15475441.2005.9684215 [DOI] [Google Scholar]
- Janacsek K., Fiser J., Nemeth D. (2012). The best time to acquire new skills: Age-related differences in implicit sequence learning across the human lifespan. Developmental Science, 15(4), 496–505. 10.1111/j.1467-7687.2012.01150.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson J. S., Newport E. L. (1989). Critical period effects in second language learning: The influence of maturational state on the acquisition of English as a second language. Cognitive Psychology, 21(1), 60–99. https://10.1016/0010-0285(89)90003-0 [DOI] [PubMed] [Google Scholar]
- Jones E. E., Harris V. A. (1967). The attribution of attitudes. Journal of Experimental Social Psychology, 3(1), 1–24. https://10.1016/0022-1031(67)90034-0 [Google Scholar]
- Kahneman D., Tversky A. (1973). On the psychology of prediction. Psychological Review, 80(4), 237–251. 10.1037/h0034747 [DOI] [Google Scholar]
- Kareev Y. (1995). Through a narrow window: Working memory capacity and the detection of covariation. Cognition, 56(3), 263–269. 10.1016/0010-0277(95)92814-G [DOI] [PubMed] [Google Scholar]
- Kareev Y., Lieberman I., Lev M. (1997). Through a narrow window: Sample size and the perception of correlation. Journal of Experimental Psychology: General, 126(3), 278–287. 10.1037/0096-3445.126.3.278 [DOI] [Google Scholar]
- Kelly D. J., Quinn P. C., Slater A. M., Lee K., Ge L., Pascalis O. (2007). The other-race effect develops during infancy: Evidence of perceptual narrowing. Psychological Science, 18(12), 1084–1089. 10.1111/j.1467-9280.2007.02029.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K. H., Relkin N. R., Lee K. M., Hirsch J. (1997). Distinct cortical areas associated with native and second languages. Nature, 388(6638), 171–174. 10.1038/40623 [DOI] [PubMed] [Google Scholar]
- Király I., Takacs S., Kaldy Z., Blaser E. (2017). Preschoolers have better long-term memory for rhyming text than adults. Developmental Science, 20(3), Article e12398. 10.1111/desc.12398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowlton B. J., Squire L. R., Gluck M. A. (1994). Probabilistic classification learning in amnesia. Learning & Memory, 1(2), 106–120. [PubMed] [Google Scholar]
- Knudsen E. I., Knudsen P. F. (1990). Sensitive and critical periods for visual calibration of sound localization by barn owls. Journal of Neuroscience, 10(1), 222–232. 10.1523/JNEUROSCI.10-01-00222.1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krøjgaard P., Sonne T., Lerebourg M., Lambek R., Kingo O. S. (2019). Eight-year-olds, but not six-year-olds, perform just as well as adults when playing Concentration: Resolving the enigma? Consciousness and Cognition, 69, 81–94. 10.1016/j.concog.2019.01.015 [DOI] [PubMed] [Google Scholar]
- Kuhl P. K., Conboy B. T., Padden D., Nelson T., Pruitt J. (2005). Early speech perception and later language development: Implications for the “critical period.” Language Learning and Development, 1(3–4), 237–264. 10.1080/15475441.2005.9671948 [DOI] [Google Scholar]
- Lebel C., Beaulieu C. (2011). Longitudinal development of human brain wiring continues from childhood into adulthood. Journal of Neuroscience, 31(30), 10937–10947. 10.1523/JNEUROSCI.5302-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J. K., Ekstrom A. D., Ghetti S. (2014). Volume of hippocampal subfields and episodic memory in childhood and adolescence. NeuroImage, 94, 162–171. 10.1016/j.neuroimage.2014.03.019 [DOI] [PubMed] [Google Scholar]
- Lenneberg E. H. (1967). Biological foundations of language. John Wiley & Sons. [Google Scholar]
- Lenroot R. K., Giedd J. N. (2006). Brain development in children and adolescents: Insights from anatomical magnetic resonance imaging. Neuroscience & Biobehavioral Reviews, 30(6), 718–729. 10.1016/j.neubiorev.2006.06.001 [DOI] [PubMed] [Google Scholar]
- Li S. C., Lindenberger U., Hommel B., Aschersleben G., Prinz W., Baltes P. B. (2004). Transformations in the couplings among intellectual abilities and constituent cognitive processes across the life span. Psychological Science, 15(3), 155–163. [DOI] [PubMed] [Google Scholar]
- Lillard A. S., Erisir A. (2011). Old dogs learning new tricks: Neuroplasticity beyond the juvenile period. Developmental Review, 31(4), 207–239. 10.1016/j.dr.2011.07.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas C. G., Bridgers S., Griffiths T. L., Gopnik A. (2014). When children are better (or at least more open-minded) learners than adults: Developmental differences in learning the forms of causal relationships. Cognition, 131(2), 284–299. https://10.1016/j.cognition.2013.12.010 [DOI] [PubMed] [Google Scholar]
- Maratsos M. P., Chalkley M. A. (1980). The internal language of children’s syntax: The nature and ontogenesis of syntactic categories. In Nelson K. E. (Ed.), Children’s language (Vol. 2). Gardner Press. [Google Scholar]
- Mattock K., Burnham D. (2006). Chinese and English infants’ tone perception: Evidence for perceptual reorganization. Infancy, 10(3), 241–265. https://10.1207/s15327078in1003_3 [Google Scholar]
- Maurer D. (2017). Critical periods re-examined: Evidence from children treated for dense cataracts. Cognitive Development, 42, 27–36. 10.1016/j.cogdev.2017.02.006 [DOI] [Google Scholar]
- McClelland J. L., McNaughton B. L., O’Reilly R. C. (1995). Why there are complementary learning systems in the hippocampus and neocortex: Insights from the successes and failures of connectionist models of learning and memory. Psychological Review, 102(3), 419–457. 10.1037/0033-295X.102.3.419 [DOI] [PubMed] [Google Scholar]
- McGee A. W., Yang Y., Fischer Q. S., Daw N. W., Strittmatter S. M. (2005). Experience-driven plasticity of visual cortex limited by myelin and Nogo receptor. Science, 309(5744), 2222–2226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKone E., Wan L., Pidcock M., Crookes K., Reynolds K., Dawel A., Kidd E., Fiorentini C. (2019). A critical period for faces: Other-race face recognition is improved by childhood but not adult social contact. Scientific Reports, 9, Article 12820. 10.1038/s41598-019-49202-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meder B., Wu C. M., Schulz E., Ruggeri A. (2021). Development of directed and random exploration in children. Developmental Science, 24(4), Article e13095. 10.1111/desc.13095 [DOI] [PubMed] [Google Scholar]
- Mintz T. H., Newport E. L., Bever T. G. (2002). The distributional structure of grammatical categories in speech to young children. Cognitive Science, 26(4), 393–424. 10.1207/s15516709cog2604_1 [DOI] [Google Scholar]
- Munakata Y., Herd S. A., Chatham C. H., Depue B. E., Banich M. T., O’Reilly R. C. (2011). A unified framework for inhibitory control. Trends in Cognitive Sciences, 15(10), 453–459. 10.1016/j.tics.2011.07.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newport E. L. (1990). Maturational constraints on language learning. Cognitive Science, 14(1), 11–28. 10.1207/s15516709cog1401_2 [DOI] [Google Scholar]
- Nomura E. M., Reber P. J. (2008). A review of medial temporal lobe and caudate contributions to visual category learning. Neuroscience & Biobehavioral Reviews, 32(2), 279–291. 10.1016/j.neubiorev.2007.07.006 [DOI] [PubMed] [Google Scholar]
- Nowakowski R. S., Hayes N. L. (2002). General principles of CNS development. In Johnson M. H., Munakata Y., Gilmore R. O. (Eds.), Brain development and cognition: A reader (2nd ed., pp. 57–82). Blackwell Publishing. [Google Scholar]
- Nussenbaum K., Hartley C. A. (2019). Reinforcement learning across development: What insights can we draw from a decade of research? Developmental Cognitive Neuroscience, 40, Article 100733. 10.1016/j.dcn.2019.100733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostby Y., Tamnes C. K., Fjell A. M., Westlye L. T., Due-Tonnessen P., Walhovd K. B. (2009). Heterogeneity in subcortical brain development: A structural magnetic resonance imaging study of brain maturation from 8 to 30 years. The Journal of Neuroscience, 29(38), 11772–11782. 10.1523/JNEUROSCI.1242-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pallier C., Dehaene S., Poline J. B., LeBihan D., Argenti A. M., Dupoux E., Mehler J. (2003). Brain imaging of language plasticity in adopted adults: Can a second language replace the first? Cerebral Cortex, 13(2), 155–161. 10.1093/cercor/13.2.155 [DOI] [PubMed] [Google Scholar]
- Palmer S. B., Fais L., Golinkoff R. M., Werker J. F. (2012). Perceptual narrowing of linguistic sign occurs in the 1st year of life. Child Development, 83(2), 543–553. https://10.1111/j.1467-8624.2011.01715.x [DOI] [PubMed] [Google Scholar]
- Pascalis O., de Haan M., Nelson C. A. (2002). Is face processing species-specific during the first year of life? Science, 296(5571), 1321–1323. 10.1126/science.1070223 [DOI] [PubMed] [Google Scholar]
- Pascalis O., Scott L. S., Kelly D. J., Shannon R. W., Nicholson E., Coleman M., Nelson C. A. (2005). Plasticity of face processing in infancy. Proceedings of the National Academy of Sciences, USA, 102(14), 5297–5300. 10.1073/pnas.0406627102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peña M., Werker J. F., Dehaene-Lambertz G. (2012). Earlier speech exposure does not accelerate speech acquisition. Journal of Neuroscience, 32(33), 11159–11163. 10.1523/JNEUROSCI.6516-11.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plebanek D. J., Sloutsky V. M. (2017). Costs of selective attention: When children notice what adults miss. Psychological Science, 28(6), 723–732. 10.1177/0956797617693005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plude D. J., Enns J. T., Brodeur D. (1994). The development of selective attention: A life-span overview. Acta Psychologica, 86, 227–272. [DOI] [PubMed] [Google Scholar]
- Poldrack R. A., Clark J., Paré-Blagoev E. A., Shohamy D., Moyano J. C., Myers C., Gluck M. A. (2001). Interactive memory systems in the human brain. Nature, 414(6863), 546–550. 10.1038/35107080 [DOI] [PubMed] [Google Scholar]
- Polka L., Werker J. F. (1994). Developmental changes in perception of nonnative vowel contrasts. Journal of Experimental Psychology: Human Perception and Performance, 20(2), 421–435. 10.1037/0096-1523.20.2.421 [DOI] [PubMed] [Google Scholar]
- Ramscar M., Gitcho N. (2007). Developmental change and the nature of learning in childhood. Trends in Cognitive Sciences, 11(7), 274–279. 10.1016/j.tics.2007.05.007 [DOI] [PubMed] [Google Scholar]
- Sangrigoli S., Pallier C., Argenti A. M., Ventureyra V. A. G., de Schonen S. (2005). Reversibility of the other-race effect in face recognition during childhood. Psychological Science, 16(6), 440–444. 10.1111/j.0956-7976.2005.01554.x [DOI] [PubMed] [Google Scholar]
- Schulz E., Wu C. M., Ruggeri A., Meder B. (2019). Searching for rewards like a child means less generalization and more directed exploration. Psychological Science, 30(11), 1561–1572. 10.1177/0956797619863663 [DOI] [PubMed] [Google Scholar]
- Schumann-Hengsteler R. (1996). Children’s and adults’ visuospatial memory: The game concentration. The Journal of Genetic Psychology, 157(1), 77–92. [DOI] [PubMed] [Google Scholar]
- Seiver E., Gopnik A., Goodman N. D. (2013). Did she jump because she was the big sister or because the trampoline was safe? Causal inference and the development of social attribution. Child Development, 84(2), 443–454. https://10.1111/j.1467-8624.2012.01865.x [DOI] [PubMed] [Google Scholar]
- Senghas A., Coppola M. (2001). Children creating language: How Nicaraguan sign language acquired a spatial grammar. Psychological Science, 12(4), 323–328. 10.1111/1467-9280.00359 [DOI] [PubMed] [Google Scholar]
- Shaw P., Kabani N. J., Lerch J. P., Eckstrand K., Lenroot R., Gogtay N., Greenstein D., Clasen L., Evans A., Rapoport J. L., Giedd J. N., Wise S. P. (2008). Neurodevelopmental trajectories of the human cerebral cortex. Journal of Neuroscience, 28(14), 3586–3594. 10.1523/JNEUROSCI.5309-07.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shohamy D., Myers C. E., Grossman S., Sage J., Gluck M. A., Poldrack R. A. (2004). Cortico-striatal contributions to feedback-based learning: Converging data from neuroimaging and neuropsychology. Brain, 127(4), 851–859. 10.1093/brain/awh100 [DOI] [PubMed] [Google Scholar]
- Shohamy D., Myers C. E., Hopkins R. O., Sage J., Gluck M. A. (2009). Distinct hippocampal and basal ganglia contributions to probabilistic learning and reversal. Journal of Cognitive Neuroscience, 21(9), 1820–1832. 10.1162/jocn.2009.21138 [DOI] [PubMed] [Google Scholar]
- Simpson E. A., Varga K., Frick J. E., Fragaszy D. (2011). Infants experience perceptual narrowing for nonprimate faces. Infancy, 16(3), 318–328. 10.1111/j.1532-7078.2010.00052.x [DOI] [PubMed] [Google Scholar]
- Singleton J. L., Newport E. L. (2004). When learners surpass their models: The acquisition of American sign language from inconsistent input. Cognitive Psychology, 49(4), 370–407. 10.1016/j.cogpsych.2004.05.001 [DOI] [PubMed] [Google Scholar]
- Sloutsky V. M., Fisher A. V. (2004). When development and learning decrease memory: Evidence against category-based induction in children. Psychological Science, 15(8), 553–558. 10.1111/j.0956-7976.2004.00718.x [DOI] [PubMed] [Google Scholar]
- Snow C. E., Hoefnagel-Höhle M. (1978). The critical period for language acquisition: Evidence from second language learning. Child Development, 49, 1114–1128. [Google Scholar]
- Squire L. R. (2004). Memory systems of the brain: A brief history and current perspective. Neurobiology of Learning and Memory, 82(3), 171–177. 10.1016/j.nlm.2004.06.005 [DOI] [PubMed] [Google Scholar]
- Thomas K. M., Nelson C. A. (2001). Serial reaction time learning in preschool- and school-age children. Journal of Experimental Child Psychology, 79(4), 364–387. 10.1006/jecp.2000.2613 [DOI] [PubMed] [Google Scholar]
- Thompson-Schill S. L., Ramscar M., Chrysikou E. G. (2009). Cognition without control: When a little frontal lobe goes a long way. Current Directions in Psychological Science, 18(5), 259–263. https://10.1111/j.1467-8721.2009.01648.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullman M. T. (2001). A neurocognitive perspective on language: The declarative/procedural model. Nature Reviews Neuroscience, 2(10), 717–726. 10.1038/35094573 [DOI] [PubMed] [Google Scholar]
- Ventureyra V. A., Pallier C., Yoo H. Y. (2004). The loss of first language phonetic perception in adopted Koreans. Journal of Neurolinguistics, 17(1), 79–91. 10.1016/S0911-6044(03)00053-8 [DOI] [Google Scholar]
- Vlach H. A. (2014). The spacing effect in children’s generalization of knowledge: Allowing children time to forget promotes their ability to learn. Child Development Perspectives, 8(3), 163–168. 10.1111/cdep.12079 [DOI] [Google Scholar]
- Walker C. M., Bridgers S., Gopnik A. (2016). The early emergence and puzzling decline of relational reasoning: Effects of knowledge and search on inferring abstract concepts. Cognition, 156, 30–40. https://10.1016/j.cognition.2016.07.008 [DOI] [PubMed] [Google Scholar]
- Wartenburger I., Heekeren H. R., Abutalebi J., Cappa S. F., Villringer A., Perani D. (2003). Early setting of grammatical processing in the bilingual brain. Neuron, 37(1), 159–170. 10.1016/S0896-6273(02)01150-9 [DOI] [PubMed] [Google Scholar]
- Weikum W. M., Oberlander T. F., Hensch T. K., Werker J. F. (2012). Prenatal exposure to antidepressants and depressed maternal mood alter trajectory of infant speech perception. Proceedings of the National Academy of Sciences, USA, 109(Suppl. 2), 17221–17227. 10.1073/pnas.1121263109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weir M. W. (1964). Developmental changes in problem-solving strategies. Psychological Review, 71(6), 473–490. 10.1037/h0041785 [DOI] [PubMed] [Google Scholar]
- Werker J. F., Hensch T. K. (2015). Critical periods in speech perception: New directions. Annual Review of Psychology, 66, 173–196. 10.1146/annurev-psych-010814-015104 [DOI] [PubMed] [Google Scholar]
- Werker J. F., Tees R. C. (1984). Cross-language speech perception: Evidence for perceptual reorganization during the first year of life. Infant Behavior and Development, 7(1), 49–63. 10.1016/S0163-6383(84)80022-3 [DOI] [Google Scholar]
- Williams B. R., Ponesse J. S., Schachar R. J., Logan G. D., Tannock R. (1999). Development of inhibitory control across the life span. Developmental Psychology, 35(1), 205–213. 10.1037/0012-1649.35.1.205 [DOI] [PubMed] [Google Scholar]
- Woolley S. M. N. (2012). Early experience shapes vocal neural coding and perception in songbirds. Developmental Psychobiology, 54(6), 612–631. 10.1002/dev.21014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida K. A., Pons F., Maye J., Werker J. F. (2010). Distributional phonetic learning at 10 months of age. Infancy, 15(4), 420–433. 10.1111/j.1532-7078.2009.00024.x [DOI] [PubMed] [Google Scholar]