Abstract
Under anoxic conditions Pseudomonas sp. strain JLR11 can use 2,4,6-trinitrotoluene (TNT) as the sole N source, releasing nitrite from the aromatic ring and subsequently reducing it to ammonium and incorporating it into C skeletons. This study shows that TNT can also be used as a terminal electron acceptor in respiratory chains under anoxic conditions by Pseudomonas sp. strain JLR11. TNT-dependent proton translocation coupled to the reduction of TNT to aminonitrotoluenes has been observed in TNT-grown cells. This extrusion did not occur in nitrate-grown cells or in anaerobic TNT-grown cells treated with cyanide, a respiratory chain inhibitor. We have shown that in a membrane fraction prepared from Pseudomonas sp. strain JLR11 grown on TNT under anaerobic conditions, the synthesis of ATP was coupled to the oxidation of molecular hydrogen and to the reduction of TNT. This phosphorylation was uncoupled by gramicidin. Respiration by Pseudomonas sp. strain JLR11 is potentially useful for the biotreatment of TNT in polluted waters and soils, particularly in phytorhizoremediation, in which bacterial cells are transported to the deepest root zones, which are poor in oxygen.
2,4,6-Trinitrotoluene (TNT) is a major contaminant in many military sites, where manufacturing and decommissioning operations generate large quantities of this explosive as a waste product. Much of this waste is deposited in the soil and in unlined lagoons, from which it often reaches groundwaters through leaching (16, 21). TNT is toxic for many prokaryotes and eukaryotes, and it is mutagenic in Salmonella enterica serovar Typhimurium (23–25, 27). This effect arises from the electrophilic nature of the substituent on the aromatic ring. In fact, TNT oxidizes biological reductants, causing toxicity both directly and through the formation of other reactive products, such as nitroarene radicals (14). Remediation is therefore urgently needed to clean up contaminated sites.
A number of studies have found that mineralization of TNT under aerobic conditions is limited (2, 5, 7, 8, 10, 20, 26). In addition, many aerobic microbes reduce the nitro groups on the aromatic ring to nitroso and hydroxylamino groups, which have a high propensity to react with each other to produce azoxynitrotoluenes in the presence of oxygen (9). These azoxynitrotoluenes are recalcitrant to bioremediation. Degradation of TNT under anaerobic conditions has been explored as an alternative approach to remediation (3, 4, 6, 11, 12, 13, 18, 22). This process has the potential advantages of rapid reduction at a low redox potential and of diminished polymerization reactions due to the absence of oxygen (9, 12, 18).
Pseudomonas sp. strain JLR11, isolated from a wastewater treatment plant, is able to use nitrate, nitrite, and TNT as the N source under anoxic conditions (6). Mass balances with TNT have revealed that about 85% of the total N-TNT content was incorporated as cell biomass (6).
Analyses of culture supernatants detected plausible pathway intermediates, such as 2,4,6-trinitrobenzaldehyde, 2-nitro-4-hydroxybenzoic acid, 4-hydroxybenzaldehyde, and 4-hydroxybenzoic acid, in the productive removal of nitro groups from TNT (6). Strain JLR11 reduced a small fraction of the total TNT to monoaminodinitrotoluenes and diaminomononitrotoluenes, but these products accumulated with time and were not used by the strain as an N source (6). We have determined that the reduced forms of TNT are produced by Pseudomonas sp. strain JLR11 because TNT acts as a final electron acceptor in respiratory chains under anoxic conditions.
MATERIALS AND METHODS
Organism, culture medium, and growth conditions.
Pseudomonas sp. strain JLR11 was grown on M9 minimal medium with glucose (0.1 to 0.5%, wt/vol) or acetate (10 mM) as a C source (6). This strain grows on minimal medium in the presence of 50 μg of kanamycin per ml. When TNT was used as the sole N source, it was supplied at 100 mg/liter and ammonium was omitted from M9 medium. In some experiments nitrate and nitrite were used as a nitrogen source at concentrations of 10 and 2 mM, respectively.
Bacterial cells were cultured in batch in a 2-liter bioreactor (Biostat B; Braun Biotech, Madrid, Spain) at a constant temperature (30°C) and pH (7.0 ± 0.1) and with constant stirring (200 ± 2 rpm). The bioreactor was periodically flushed with N2 to maintain anaerobiosis during the assay.
Isolation of Pseudomonas sp. strain JLR11 mutants unable to use TNT as the sole N source.
Mini-Tn5-tellurite was used to generate mutants of Pseudomonas sp. strain JLR11 upon mating of this strain with Escherichia coli CC118λpir (pUT-miniTn5-Tel) as described by Sánchez-Romero et al. (19). Tellurite-resistant transconjugants of strain JLR11 were selected on M9 minimal medium with glucose (0.5%, wt/vol) as the sole C source and 25 μg of kanamycin per ml and 30 μg of potassium tellurite per ml. Among 10,000 transconjugants a clone unable to grow on TNT as the sole N source was found, and it was selected for further studies. This clone was called Pseudomonas sp. strain JLR11-P12E2.
Measurement of proton translocation.
Changes in pH in the extracellular medium of intact cells were measured with a combination electrode (Ingold type) connected to a pH meter (type PHM84 radiometer). All assays were performed at 30°C in an N2 atmosphere.
Analytical methods.
Products which accumulated in culture supernatants were analyzed by high-performance liquid chromatography on a Hewlett-Packard model 1050 chromatograph equipped with a diode array detector and a 5-μm C18RP column (UltraCarb C30 Phenomenex; 15 cm by 4.6 mm). The column was first washed with a mixture of acetonitrile and a solution of 1% (vol/vol) acetic acid in water (2:8 [vol/vol]) for 2 min. Then a linear gradient was applied to reach 100% (vol/vol) acetonitrile over 18 min. The flow was kept constant at 1 ml/min, and the detector was set at 230 and 254 nm to detect aromatic compounds. Gas chromatography-mass spectrometry (GC-MS) analyses were done with an HP6890 GC-MS apparatus. The GC was equipped with a capillary 5% phenylmethyl silicone column (30 m by 0.025 mm).
Preparation of membranes.
Pseudomonas sp. strain JLR11 cells were cultured at 30°C in a 2-liter bioreactor (Biostat B; Braun Biotech) in an N2 atmosphere in minimal medium containing 10 mM acetate, 5 mM ammonium chloride, and 0.5 mM TNT as an electron acceptor. As indicated for preparation of cell membranes, bacteria were also grown on minimal medium with 10 mM acetate and 20 mM nitrate or 2 mM nitrite. Bacteria were harvested at the late exponential growth phase and washed with anoxic buffer (20 mM Tris-HCl [pH 7.3]). Cells were disrupted by passing the suspension 10 times through a French press. Unbroken cells were removed by centrifugation at 5,000 × g for 20 min in a Sorvall 5CR centrifuge. The crude extract was then centrifuged at 90,000 × g for 60 min, and the membrane fraction was resuspended in 1 ml of the above-mentioned anoxic buffer and kept at 4°C in a nitrogen atmosphere in a sealed vial.
Measurement of oxidative phosphorylation.
Anaerobic energy coupling was assessed with cell membranes by determining the amount of ATP synthesized (15). The complete reaction mixture (4 ml) consisted of 15 mM MgCl2, 0.5 mM ADP, 5 mM KPO4H2, 0.5 mM TNT, and 50 mM Tris-HCl (pH 7.3). Assays were run at 30°C in an N2 atmosphere, and the incubation time was 40 min. Esterification of phosphate in the oxidative phosphorylation experiments was determined spectrophotometrically at 340 nm as NADPH formation by using a mixture of 2 U of hexokinase, 1 U of glucose-6-phosphate dehydrogenase, 10 mM glucose, and 0.5 mM NADP+.
RESULTS AND DISCUSSION
Pseudomonas sp. strain JLR11 uses TNT as the sole N source under anaerobic growth conditions with glucose as the C source (6). The utilization of TNT as an N source involves the removal of the nitro groups and the concomitant reduction of the released nitrite to ammonium ions, which are incorporated into C skeletons. The growth yield under these conditions was higher than would have been expected if the energy had been obtained only through phosphorylation at the substrate level (6). Based on these results we hypothesize that this bacterium uses TNT as the final electron acceptor, so that proton translocation is coupled to the reduction of TNT when the organism is grown anaerobically. Further evidence to support this hypothesis was obtained in the following two sets of assays. We first found that Pseudomonas sp. strain JLR11 grew on minimal medium with acetate as the C source and TNT regardless of the presence of ammonium ions in the culture medium (Table 1). The oxidation of acetate under anoxic conditions required an electron acceptor, a role that only TNT could play in this series of assays. Further support for the role of TNT, other than as an N source for Pseudomonas sp. strain JLR11, came from similar assays but with Pseudomonas sp. strain JLR11-P12E2. This mutant was selected after mini-Tn5-tellurite mutagenesis as unable to grow on TNT as the sole N source. The mutant was blocked in the reduction of the released nitrite to ammonium (A. Esteve-Núñez, A. Caballero, and J. L. Ramos, unpublished results). The results in Table 1 show that the mutant strain was still able to grow, under anaerobic conditions, with acetate as the sole N source and ammonium ions as an N source, but only if TNT was present in the culture medium (Table 1). Analysis of culture supernatants by GC-MS as described in Materials and Methods provided further evidence for the in vivo reduction of TNT by the mutant cells, since we found that about 10% of the total TNT was reduced to 4-amino-2,6-dinitrotoluene. In these assays trace amounts of 2,4-diamino-6-nitrotoluene were also detected. These results were similar to those reported before by Esteve-Núñez and Ramos (6) regarding the reduction of TNT by cultures of the wild-type strain using this xenobiotic as the sole N source under anoxic conditions.
TABLE 1.
Growth of Pseudomonas sp. strain JLR11 and its mutant derivative JLR11-P12E2 on minimal medium under anoxic conditionsa
| Strain | Addition
|
Turbidity | |
|---|---|---|---|
| Ammonium | TNT | ||
| Wild type | − | + | 0.32 |
| + | − | 0.02 | |
| + | + | 0.45 | |
| JLR11-P12E2 | − | + | 0.02 |
| + | − | 0.02 | |
| + | + | 0.30 | |
Cultures were pregrown overnight on M9 minimal medium with glucose as the sole C source under aerobic conditions. Cells were harvested, washed in minimal medium without a C source, and diluted 1:20 in M9 without a nitrogen source and with acetate (10 mM) as the C source. The cultures were supplemented or not with 5 mM ammonium, with or without TNT (100 mg/liter). Cultures were then flushed thoroughly with N2 to achieve anoxic conditions. After 79 h of incubation at 30°C, turbidity was determined in a Perkin-Elmer spectrophotometer at 660 nm (initial turbidity was 0.02).
Proton translocation coupled to the reduction of TNT by Pseudomonas sp. strain JLR11 under anoxic conditions.
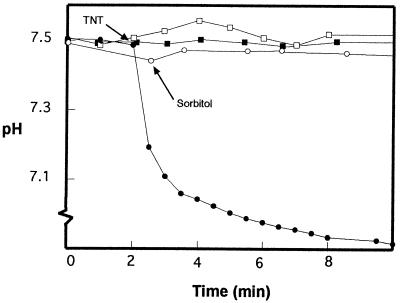
To test whether proton translocation occurred when TNT was added to an anoxic suspension of wild-type cells, we grew Pseudomonas sp. strain JLR11 cells under anoxic conditions on minimal medium with acetate, ammonium, and TNT. Cells were washed and divided into two aliquots; one was boiled and the other was left untreated. Cells were incubated in an isotonic solution of 250 mM sorbitol, and after exhaustive molecular nitrogen bubbling, TNT was added to reach 250 μM. In cultures of living cells we observed a decrease in the pH of the extracellular medium, with maximal acidification after 5 min (Fig. 1). Preincubation of living cells with cyanide in the presence of TNT prevented proton extrusion (data not shown). The pH of the extracellular medium did not change when boiled cells were used instead of living cells.
FIG. 1.
Proton translocation coupled to the reduction of TNT by Pseudomonas sp. strain JLR11 under anoxic conditions. Cells grown anaerobically in the presence of TNT were suspended in 8 ml of an anaerobic 250 mM sorbitol solution. The suspension was divided into four aliquots; two were boiled for 2 min (squares), and the others were left untreated (circles). Cells were incubated at 30°C with stirring in an N2 atmosphere. At the time indicated by the arrow, TNT was added to two of the samples to reach a final concentration of 250 μM (closed symbols), while the other samples were kept as controls (open symbols). The pH of the extracellular medium was measured with a pH electrode as described in Materials and Methods.
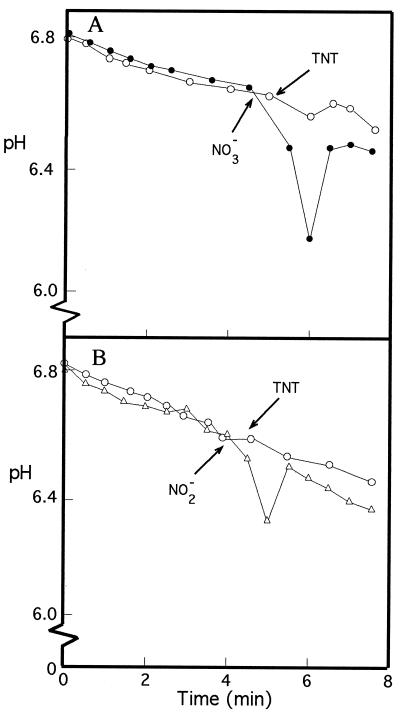
Pseudomonas sp. strain JLR11 can also use nitrate and nitrite as the final electron acceptor under anoxic conditions. When nitrite- or nitrate-grown cells extruded protons in response to the addition of nitrite and nitrate (ΔpH = −0.4 unit), respectively, the external pH remained unchanged after TNT was added (Fig. 2). This suggests that nitrate and nitrite respiration and TNT respiration by Pseudomonas sp. strain JLR11 are, at least in part, independent processes.
FIG. 2.
Proton translocation coupled to reduction of nitrate and nitrite by Pseudomonas sp. strain JLR11 under anoxic conditions. Assay conditions are as described in the legend for Fig. 1, except that the cells were grown on nitrate (A) or on nitrite (B). At the time indicated by the arrow, TNT (500 μM [open circles]) nitrate (150 μM [closed circles]), or nitrite (150 μM [open triangles]) was added.
H2-TNT oxidoreduction in membranes prepared from Pseudomonas sp. strain JLR11 cells.
Membranes from Pseudomonas sp. strain JLR11 cells grown with TNT as the electron acceptor catalyzed the reduction of TNT, a process that was accompanied by the oxidation of hydrogen. Because membranes prepared from nitrate-grown cells did not reduce TNT, we concluded that TNT reduction was specific. In addition, there was no reduction of the compound when the membranes were boiled before TNT was added.
We observed synthesis of ATP coupled to H2-TNT oxidation-reduction in membranes prepared from Pseudomonas sp. strain JLR11 cells. The rate of ATP synthesis was 450 nmol per mg of protein (Table 2). No ATP synthesis was observed when the membrane preparation was incubated with gramicidin before the addition of TNT (Table 2). As expected, in the absence of ADP no ATP synthesis occurred. In the absence of TNT the rate of ATP synthesis was about 15% of the rate found in the presence of the nitroarene. When nitrate and nitrite replaced TNT as the final electron acceptor, the rates of ATP synthesis were in the order of 10 and 36%, respectively, of the rate in the presence of TNT (Table 2).
TABLE 2.
Phosphorylation coupled to hydrogen oxidation and TNT reductiona
| Reaction mixture | Amt of ATP formed (nmol/mg of membrane protein) |
|---|---|
| Complete | 450 |
| Complete plus gramicidin | 1 |
| Without ADP | 0 |
| Without H2 | 15 |
| Without TNT | 30 |
| Without TNT plus nitrate | 45 |
| Without TNT plus nitrite | 100 |
The complete reaction mixture and the reaction conditions are described in Materials and Methods. When indicated, the reaction mixture was supplemented with 0.5 mM nitrate or nitrite. ATP formation was determined as described in Materials and Methods.
It should be noted that the anaerobic oxidative phosphorylation observed in the membrane system was coupled to the oxidation of hydrogen. This should not be interpreted as indicating that only H2 oxidation can be coupled to phosphorylation; instead, our in vivo results indicate that it is thermodinamically possible that the oxidation of acetate coupled to TNT reduction may also be coupled to ATP synthesis.
Conclusions.
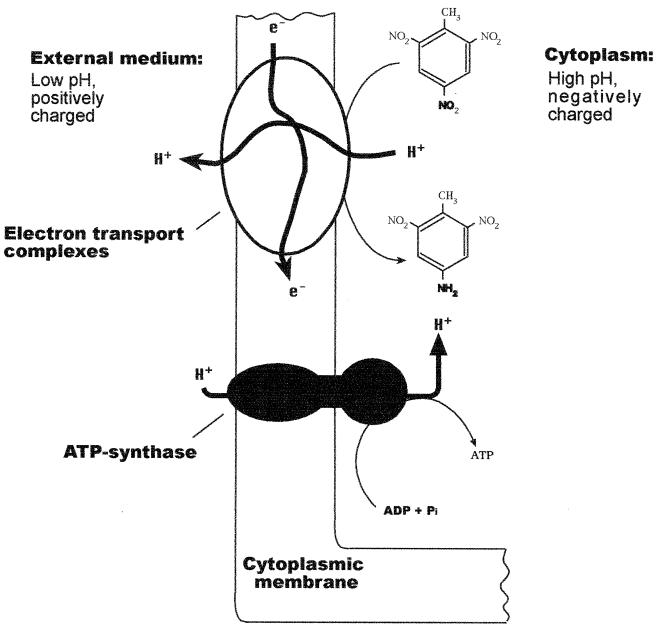
Our results indicate that the reduction of TNT in Pseudomonas sp. strain JLR11 is linked to proton extrusion, which may contribute to a transmembrane electrochemical proton gradient of sufficient magnitude to drive ATP synthesis (Fig. 3). The role of TNT as an electron acceptor was suggested before by Boopathy and Kulpa (1), although this study is the first to demonstrate experimentally that the reduction of TNT to the corresponding aminonitrotoluenes is of physiological importance as an energy conservation system under anoxic conditions. In terms of energy coupling, this system is similar to the energy coupling in nitrate reduction during denitrification, although the finding that vesicles of TNT-grown cells did not reduce NO3− indicates that different terminal reductases are involved in these processes. The physiological role of TNT respiration in Pseudomonas sp. strain JLR11 raises the possibility of interesting environmental applications in anaerobic environments polluted with TNT, in which the pollutant can be used not only as an N source but also as a terminal electron acceptor.
FIG. 3.
Scheme showing the coupling of electron donor compounds, TNT oxidoreduction, and ATP synthesis.
ACKNOWLEDGMENTS
This work was supported by a grant from the European Commission (BIO4-CT97-2040). The work of Abraham Esteve-Núñez in Konstanz, Germany, was supported by the GPoll program of the European Science Foundation.
REFERENCES
- 1.Boopathy R, Kulpa C F. Trinitrotoluene as a sole nitrogen source for a sulfate-reducing bacterium Desulfovibrio sp. (B. strain) isolated from an anaerobic digester. Curr Microbiol. 1992;25:235–241. doi: 10.1007/BF01570724. [DOI] [PubMed] [Google Scholar]
- 2.Boopathy R, Manning J, Kulpa C F. A laboratory study of the bioremediation of 2,4,6-trinitrotoluene-contaminated soil using aerobic/anoxic soil slurry reactor. Water Environ Res. 1998;70:80–86. [Google Scholar]
- 3.Boopathy R, Wilson M, Kulpa C F. Anaerobic removal of 2,4,6-trinitrotoluene (TNT) under different electron accepting conditions: laboratory study. Water Environ Res. 1993;65:271–275. [Google Scholar]
- 4.Drzyzga D, Bruns-Nagel D, Goroutzy T, Bloterogel K-H, Gemsa D, van Löw E. Mass balance studies with 14C-labeled 2,4,6-trinitrotoluene (TNT) mediated by an anaerobic Desulfovibrio species and an aerobic Serratia species. Curr Microbiol. 1998;37:380–386. doi: 10.1007/s002849900397. [DOI] [PubMed] [Google Scholar]
- 5.Duque E, Haïdour A, Godoy F, Ramos J L. Construction of a Pseudomonas hybrid strain that mineralizes 2,4,6-trinitrotoluene. J Bacteriol. 1993;175:2278–2283. doi: 10.1128/jb.175.8.2278-2283.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Esteve-Núñez A, Ramos J L. Metabolism of 2,4,6-trinitrotoluene by Pseudomonas sp. JLR11. Environ Sci Technol. 1998;32:3802–3808. [Google Scholar]
- 7.Fernando T J, Bumpus J A, Aust S D. Biodegradation of TNT (2,4,6-trinitrotoluene) by Phanerochaete chrysosporium. Appl Environ Microbiol. 1990;56:1666–1671. doi: 10.1128/aem.56.6.1666-1671.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Funck S B, Pasti-Grisby M B, Feliciano E C, Crawford D L. Bioremediation of recalcitrant organics. Columbus, Ohio: Battelle; 1995. pp. 329–350. [Google Scholar]
- 9.Haïdour A, Ramos J L. Identification of products resulting from the biological reduction of 2,4,6-trinitrotoluene, 2,4-dinitrotoluene, and 2,6-dinitrotoluene by Pseudomonas sp. Environ Sci Technol. 1996;30:2365–2370. [Google Scholar]
- 10.Herre A, Michels J, Scheibner K, Fritsche W. Fourth International In Situ and On Site Bioremediation Symposium. Vol. 2. Columbus, Ohio: Battelle Press; 1997. pp. 493–498. [Google Scholar]
- 11.Hughes J B, Wong C H Y, Zhang C H. Anaerobic biotransformation of 2,4-dinitrotoluene and 2,6-dinitrotoluene by Clostridium acetobutylicum: a pathway through dihydroxylamino intermediates. Environ Sci Technol. 1999;33:1065–1070. [Google Scholar]
- 12.Lenke H, Warrelmann J, Dann G, Hund, Sieglen V, Walter U, Knackmuss H J. Biological treatment of TNT-contaminated soil. 2. Biological induced immobilization of the contaminants and full-scale application. Environ Sci Technol. 1998;32:1964–1971. [Google Scholar]
- 13.Lewis T A, Ederer M M, Crawford R L, Crawford D L. Microbial transformation of 2,4,6-trinitrotoluene. J Ind Microbiol Biotechnol. 1997;18:89–96. doi: 10.1038/sj.jim.2900257. [DOI] [PubMed] [Google Scholar]
- 14.Mason R P, Josephy P D. Toxicity of nitroaromatic compounds. New York, N.Y: Hemisphere; 1985. pp. 121–140. [Google Scholar]
- 15.Peck H D., Jr Evidence for oxidative phosphorylation during reduction of sulfate with hydrogen by Desulfovibrio desulfuricans. J Biol Chem. 1969;235:2734–2738. [PubMed] [Google Scholar]
- 16.Pennington J C, Patrick W H., Jr Adsorption and desorption of 2,4,6-trinitrotoluene by soils. J Environ Qual. 1990;195:559–567. [Google Scholar]
- 17.Preuss A, Fimpel J, Dieckert G. Anaerobic transformation of 2,4,6-trinitrotoluene (TNT) Arch Microbiol. 1993;159:345–353. doi: 10.1007/BF00290917. [DOI] [PubMed] [Google Scholar]
- 18.Rieger P, Knackmuss H J. Basic knowledge and perspectives on biodegradation of 2,4,6-trinitrotoluene and related nitroaromatic compounds in contaminated soil. In: Spain J C, editor. Biodegradation of nitroaromatic compounds. New York, N.Y: Plenum Publishing Co.; 1995. pp. 1–18. [Google Scholar]
- 19.Sánchez-Romero J M, Díaz-Oreja R, de Lorenzo V. Resistance to tellurite as a selection marker for genetic manipulations of Pseudomonas strains. Appl Environ Microbiol. 1998;64:4040–4046. doi: 10.1128/aem.64.10.4040-4046.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Scheibner K, Hofrichter M, Herre A, Michels J, Fritsche W. Screening for fungi intensively mineralizing 2,4,6-trinitrotoluene. Appl Microbiol Biotechnol. 1997;47:452–457. doi: 10.1007/s002530050955. [DOI] [PubMed] [Google Scholar]
- 21.Selim H M, Xue S K, Iskandar I K. Transport of 2,4,6-trinitrotoluene and hexahydro-1,3,5-trinitro-1,3,5-triazine in soils. Soil Sci. 1995;160:328–339. [Google Scholar]
- 22.Sembries S, Crawford R L. Production of Clostridium bifermentans spores as inoculum for bioremediation of nitroaromatic contaminants. Appl Environ Microbiol. 1997;63:2100–2104. doi: 10.1128/aem.63.5.2100-2104.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Spanggord R J, Mortelmans K E, Griffin A F, Simmon V E. Mutagenicity in Salmonella typhimurium and structure activity relationships of waste water components emanating from the manufacture of trinitrotoluene. Environ Mutagen. 1982;4:163–179. doi: 10.1002/em.2860040207. [DOI] [PubMed] [Google Scholar]
- 24.Styles J A, Cross M F. Activity of 2,4,6-TNT in an in vitro mammalian gene mutation assay. Cancer Lett. 1983;20:103–108. doi: 10.1016/0304-3835(83)90194-5. [DOI] [PubMed] [Google Scholar]
- 25.Tan E L, Ho C H, Griest W H, Tyndall R L. Mutagenicity of trinitrotoluene and its metabolites formed during composting. J Toxicol Environ Health. 1992;36:165–175. doi: 10.1080/15287399209531632. [DOI] [PubMed] [Google Scholar]
- 26.Widrig D L, Boopathy R, Manning J F. Bioremediation of TNT-contaminated soil: a laboratory study. Environ Toxicol Chem. 1997;16:1141–1148. [Google Scholar]
- 27.Won W D, Disalvo L H N J. Toxicity and mutagenicity of 2,4,6-trinitrotoluene and its microbial metabolites. Appl Environ Microbiol. 1976;31:576–580. doi: 10.1128/aem.31.4.576-580.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]