Abstract
Background
Peroxisomes are single membrane-bound organelles named for their role in hydrogen peroxide production and catabolism. However, their cellular functions extend well beyond reactive oxygen species (ROS) metabolism and include fatty acid oxidation of unique substrates that cannot be catabolized in mitochondria, and synthesis of ether lipids and bile acids. Metabolic functions of peroxisomes involve crosstalk with other organelles, including mitochondria, endoplasmic reticulum, lipid droplets and lysosomes. Emerging studies suggest that peroxisomes are important regulators of energy homeostasis and that disruption of peroxisomal functions influences the risk for obesity and the associated metabolic disorders, including type 2 diabetes and hepatic steatosis.
Scope of review
Here, we focus on the role of peroxisomes in ether lipid synthesis, β-oxidation and ROS metabolism, given that these functions have been most widely studied and have physiologically relevant implications in systemic metabolism and obesity. Efforts are made to mechanistically link these cellular and systemic processes.
Major conclusions
Circulating plasmalogens, a form of ether lipids, have been identified as inversely correlated biomarkers of obesity. Ether lipids influence metabolic homeostasis through multiple mechanisms, including regulation of mitochondrial morphology and respiration affecting brown fat-mediated thermogenesis, and through regulation of adipose tissue development. Peroxisomal β-oxidation also affects metabolic homeostasis through generation of signaling molecules, such as acetyl-CoA and ROS that inhibit hydrolysis of stored lipids, contributing to development of hepatic steatosis. Oxidative stress resulting from increased peroxisomal β-oxidation-generated ROS in the context of obesity mediates β-cell lipotoxicity. A better understanding of the roles peroxisomes play in regulating and responding to obesity and its complications will provide new opportunities for their treatment.
Keyword: Peroxisomes, Diabetes, Obesity, Fatty liver, Plasmalogen, Lipid metabolism
1. Introduction
Obesity increases the risk for many diseases and disorders including type 2 diabetes, non-alcoholic fatty liver disease (NAFLD), cardiovascular disease, hypertension, and certain cancers. The prevalence of obesity continues to rise, suggesting a need for new treatment options. Difficulties in treating obesity are directly related to the complex causes, which are biomedical, environmental, and socio-cultural in nature [1]. Similarly, the biomedical bases for the links between obesity and its complications are nuanced and multifactorial. For example, the well-established relationship between obesity and diabetes is mediated in part by pancreatic β-cell physiology, adipose tissue and liver dysfunction, and systemic signaling through various hormones and adipokines [2]. Improving our biochemical understanding of obesity pathogenesis could help improve obesity treatment and aid in prevention of associated diseases.
Recent work implicates peroxisomes in obesity. These single membrane-bound organelles are closely associated with mitochondria. As with mitochondria, peroxisomal number is regulated via variable biogenesis and degradation of the organelle [3]. Unlike mitochondria, however, peroxisomes have no genome. Peroxisomal proteins are encoded in the nucleus, translated on free ribosomes in the cytoplasm, and transported to the peroxisomal matrix by peroxisomal import receptors (Pex5 and Pex7), which recognize their peroxisome targeting sequences (PTS1 and PTS2, respectively) [4].
Peroxisomes are dynamic organelles which coordinate with other metabolic organelles, such as mitochondria and endoplasmic reticulum (ER), to play key roles in ROS and lipid metabolism [5,6]. As essential organelles, peroxisomes are critical for maintaining normal physiology. Mutations in genes involved in de novo peroxisome formation result in peroxisomal biogenesis disorders in the Zellweger spectrum, devastating conditions marked by multiple abnormalities, predominated by neurological impairments and hepatic dysfunction [7,8]. Emerging studies suggest that peroxisomes are also important for regulating energy homeostasis and disruption of peroxisomal functions impacts risk for obesity and its complications (Figure 1).
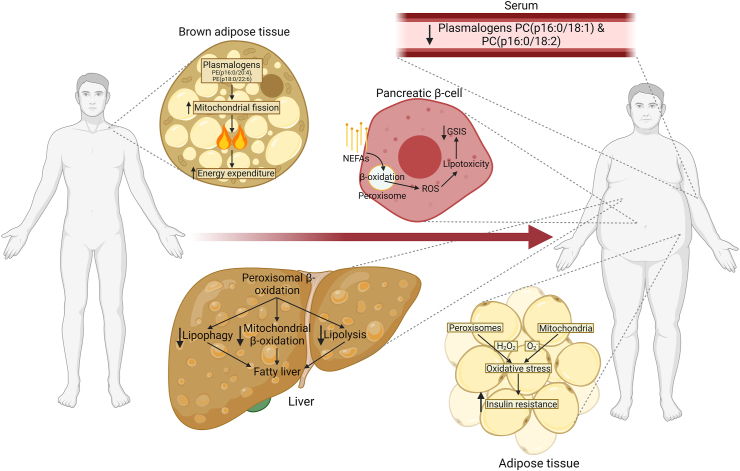
Figure 1.
Role of peroxisomes in various organs across lean and obese states. In brown adipose tissue, peroxisome-derived ether lipids promote mitochondrial fission, which increases thermogenesis and energy expenditure. Certain species of plasmalogen, a form of ether lipid, are decreased in serum of obese individuals. Peroxisomal β-oxidation promotes fatty liver through multiple distinct mechanisms and contributes to non-esterified fatty acid (NEFA)-induced lipotoxicity in pancreatic β-cells, likely contributing to impaired glucose-stimulated insulin secretion (GSIS). Reactive oxygen species (ROS) from both peroxisomal and mitochondrial β-oxidation induce oxidative stress in adipose tissue of obese individuals, causing systemic insulin resistance.
Studies in humans and animals have further suggested that peroxisomes play critical roles in obesity, dyslipidemia, and glucose homeostasis [8,9]. Peroxisomes affect systemic metabolism predominantly through their functions as lipid metabolic organelles, synthesizing ether lipids and oxidizing various fatty acid species, and by coordinated H2O2 synthesis and degradation. Recent work has revealed that peroxisomal β-oxidation negatively regulates mitochondrial β-oxidation, lipolysis, and lipophagy, promoting fatty liver [[10], [11], [12], [13]], while peroxisome-derived ether lipids promote mitochondrial fission and regulate mitochondrial respiration and adipose tissue development [[14], [15], [16]]. This review aims to synthesize recent findings related to the role of peroxisomes in metabolism and metabolic dysregulation in order to better understand the roles peroxisomes play in obesity and obesity-related complications, such as type 2 diabetes mellitus and NAFLD.
2. Major metabolic functions of peroxisomes
Peroxisomes are single membrane-bound organelles which play indispensable roles in cellular lipid metabolism and derive their name from their role in production and catabolism of hydrogen peroxide (H2O2) [17]. However, these versatile organelles are involved in a variety of metabolic processes, including oxidation of very long-chain fatty acids (VLCFA), branched-chain fatty acids, d-amino acids, and polyamines, processes which each generate the reactive oxygen species (ROS) H2O2 [18,19]. Peroxisomes possess dedicated ROS metabolic enzymes such as catalase, which degrades H2O2 to water and oxygen [20], and inducible nitric oxide synthase (NOS2), which produces nitric oxide, a reactive nitrogen species (RNS) [21]. Peroxisomes also contain enzymes involved in the biosynthesis of ether lipids, bile acids, and cholesterol, though these metabolic functions require coordination with ER, mitochondria, or cytosolic enzymes [19,22]. In this review we focus on the peroxisomal role in ether lipid synthesis, fatty acid oxidation and ROS metabolism, given that these functions have been most widely studied and have physiologically relevant implications in systemic metabolism and obesity [[10], [11], [12], [13], [14], [15], [16]].
2.1. Synthesis of ether-linked phospholipids
Peroxisomes are indispensable for synthesis of ether lipids, phospholipids with an ether bond-linked alkyl chain at the sn-1 position of their glycerol backbone, as opposed to an ester bond-linked fatty acyl chain in conventional phospholipids. The head group of ether-linked phospholipids is usually choline or ethanolamine or occasionally serine or inositol. Synthesis of ether lipids, such as plasmalogens and alkylether phospholipids, uses the glycolysis intermediate dihydroxyacetone phosphate (DHAP) as a precursor and involves the DHAP pathway, which is located in peroxisomes and produces 1-O-Alkyl-glycerol-3-phosphate (AGP) as the end product (Figure 2). AGP is the ether lipid analog of lysophosphatidic acid (i.e., 1-acyl-glycerol-3-phosphate, LPA) [23,24]. The peroxisomal protein PexRAP (Peroxisomal Reductase Activating PPARγ), which is named for its proposed role in adipocytes [25], possesses acyl/alkyl-DHAP reductase activity and can synthesize AGP and LPA. Thus, the acyl-DHAP pathway is also a salvage pathway for the synthesis of diacylphospholipids. The subsequent steps for synthesis of ether lipids and peroxisome-derived diacylphospholipids, including the addition of the sn-2 substituent (generally an ester-linked acyl chain) and the completion of phospholipid synthesis, occur in the ER [26]. Plasmalogens are the most common form of ether lipids and have a cis-double bond adjacent to the ether linkage.
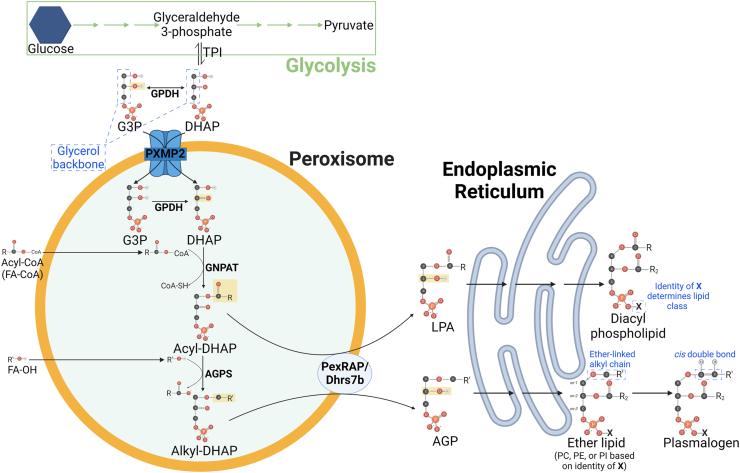
Figure 2.
Peroxisomal lipid synthetic pathway. Glyceraldehyde 3-phosphate, a glycolysis intermediate, can be converted to dihydroxyacetone phosphate (DHAP) by triose phosphate isomerase (TPI). Glycerol-3-phosphate dehydrogenase (GPDH) catalyzes the conversion of DHAP to glycerol 3-phosphate (G3P), though both DHAP and G3P are believed to be transported to the peroxisomal matrix by peroxisomal membrane protein 2 (PXMP2). In peroxisomes GPDH may convert G3P back to DHAP, which subsequently has an acyl chain added to its sn-1 carbon by glyceronephosphate O-acyltransferase (GNPAT), yielding acyl-DHAP. Acyl-DHAP may have its sn-2 carbonyl reduced by PexRAP (Peroxisomal Reductase activating PPARγ; encoded by Dhrs7b) to yield lysophosphatidic acid (LPA) or it may have its sn-1 acyl chain replaced by a new sn-1 alkyl chain to yield alkyl-DHAP, a reaction catalyzed by alkylglycerone phosphate synthase (AGPS). Alkyl-DHAP is then reduced by PexRAP to form 1-O-Alkyl-G3P (AGP). LPA and AGP are shuttled to the endoplasmic reticulum for formation of diacyl phospholipids and ether-linked phospholipids, respectively. Ether lipids may be processed further to generate plasmalogens, a subclass of ether lipids with a cis-double bond.
2.2. Peroxisomal fatty acid oxidation
Peroxisomes also play a specialized, indispensable role in fatty acid oxidation. Like mitochondria, peroxisomes are involved in β-oxidation of fatty acids, though the two pathways are different in multiple ways. The enzymes involved in the two pathways are distinct and the pathways thus have unique substrate specificities. While mitochondrial β-oxidation degrades short-, medium-, and most long-chain fatty acids, peroxisomal β-oxidation catabolizes very-long chain fatty acids (VLCFAs; ≥22 Carbons), di- and trihydroxycholestanoic acid (DHCA and THCA), long chain dicarboxylic acids, and certain polyunsaturated fatty acids. In peroxisomal biogenesis disorders, VLCFAs, DHCA, and THCA accumulate, demonstrating the indispensable nature of peroxisomes in oxidizing these substrates [27]. Another key difference results from the fact that since peroxisomes lack an electron transport chain (ETC), peroxisomal β-oxidation requires its products be shuttled to the mitochondria for complete oxidation [28].
In addition to β-oxidation, peroxisomes are involved in alpha-oxidation (α-oxidation), a process by which branched-chain fatty acids undergo oxidative decarboxylation to remove the terminal carboxyl group as CO2. α-oxidation is particularly important in the degradation of multi-methyl branched fatty acids, such as the dietary lipid phytanic acid, in which case it removes a single carbon from the carboxyl end to yield pristanic acid that is subsequently degraded by β-oxidation. α-oxidation takes place exclusively in peroxisomes [29] and is impaired in Refsum disease, a genetic disease associated with multiple symptoms, including retinitis pigmentosa, anosmia, polyneuropathy, and deafness [30]. Although α-oxidation has not yet been implicated in the regulation of systemic metabolism, emerging studies suggest that peroxisomal β-oxidation influences metabolic homeostasis through generation of signaling molecules, as discussed below.
2.3. Role of peroxisomes in ROS metabolism
ROS are radical or non-radical oxygen species which often result from the partial reduction of oxygen during aerobic metabolism and include superoxide (O2−) and hydrogen peroxide (H2O2) [31]. Peroxisomes are a major source of H2O2, which is produced through the actions of multiple FAD-dependent oxidoreductases involved in various peroxisomal metabolic processes, including β-oxidation and bile acid synthesis [32]. In the first dehydrogenation step of both peroxisomal and mitochondrial β-oxidation, acyl-CoA is oxidized to form a double bond between the α- and β-carbon of the acyl chain in a reaction coupled to the reduction of FAD to FADH2 [33]. In mitochondria, the electrons FAD gains in this reaction to become FADH2 are subsequently transferred to the ETC, ultimately reducing molecular oxygen to water [34]. In peroxisomes, which have only one membrane and lack an ETC, these electrons are transported directly from FADH2 to molecular oxygen, generating hydrogen peroxide (H2O2) rather than water [19]. Peroxisomes account for up to 20% of total cellular oxygen consumption and make as much as 35% of total H2O2 in certain tissues [35]. While peroxisomes have antioxidant enzymes such as catalase to reduce H2O2 to water [20], disrupted H2O2 homeostasis has been linked to numerous diseases including diabetes, cancer, obesity, and diabetic renal injury [[36], [37], [38]], demonstrating the significance of this process in systemic metabolism.
3. Interaction of peroxisomes with other organelles in the regulation of lipid metabolism
Peroxisomes work in coordination with other organelles, such as the ER for anabolic functions and mitochondria for catabolic functions [39,40]. Non-autonomy is a nearly ubiquitous feature of the myriad of processes which occur in peroxisomes (reviewed in [5]) and is particularly relevant with respect to peroxisomal lipid metabolism.
Peroxisomes are required for oxidation of VLCFAs, which are primarily synthesized endogenously [27,41]. Peroxisomal β-oxidation relies on mitochondria for oxidation of acetyl-CoA and NADH, two products of each cycle of β-oxidation which must be shuttled from peroxisomes to mitochondria [39]. Not only does peroxisomal β-oxidation prefer longer substrates, but it is unable to degrade the short chain (C6/C8-CoA) fatty acids which remain after multiple rounds of β-oxidation of VLCFAs [42]. These remaining short chain fatty acids then must be shuttled to the mitochondria for complete oxidation [39].
Synthesis of ether lipids requires cooperation between peroxisomes and the ER. ER-peroxisome metabolite exchange may be assisted by the bridge formed between ER membrane VAMP-associated proteins A and B (VAPA and VAPB) and peroxisomal acyl-CoA binding domain containing protein 5 (ACBD5) [43]. Certain enzymes involved in ether lipid biosynthesis, such as PexRAP (encoded by Dhrs7b) are localized to both peroxisomes and ER [25] and have distinct functions in the two locations, further suggesting cooperation between peroxisomes and ER with respect to ether lipid synthesis [44].
Peroxisomes also cooperate with ER and lysosomes to facilitate effective synthesis and transport of cholesterol, which accounts for 30–40 mol% of cellular lipids [45,46]. Cholesterol biosynthesis begins in peroxisomes and is completed in the ER; the rate-limiting enzyme, HMG-CoA reductase, localizes to both peroxisomes and ER [47]. Peroxisome deficiency increases expression of cholesterogenic genes by promoting ER-to-Golgi sterol regulatory-element binding protein (SREBP) trafficking yet has no effect on cholesterol levels [47]. This effect persists even after cholesterol loading, suggesting a role for peroxisomes in cellular cholesterol transport or uptake. Supporting this concept, peroxisomes interact directly with lysosomes through membrane protein synaptotagmin 7 (Syt7) to facilitate cholesterol transport from lysosomes to peroxisomes [48]. Peroxisome biogenesis disorder models show cholesterol accumulation, suggesting that this lysosome–peroxisome interaction is crucial to cellular cholesterol transport and homeostasis.
Lipid droplets are universally conserved storage organelles which contain neutral lipids surrounded by a phospholipid monolayer and play dynamic roles in the storage and mobilization of fatty acids [49]. Literature dating back to the 1980s has suggested spatial association between peroxisomes and lipid droplets, especially in contexts such as adipogenesis, when peroxisomes increase in abundance [[50], [51], [52], [53]]. More recent work has suggested cooperation between peroxisomes and lipid droplets, particularly relating to biogenesis, the rate of which is one mechanism by which both organelles are regulated. Not only do lipid droplet and peroxisome biogenesis occur at the same ER subdomains [54], but both Pex19 and Pex3 are required for insertion of ER- and lipid droplet-resident protein UBXD8 into discrete ER subdomains [55], suggesting a mechanism for coordinated biogenesis of peroxisomes and lipid droplets. Furthermore, fasting induces peroxisome-lipid droplets contact and peroxisome deficiency reduces lipolysis in-vivo, suggesting a close physical and functional cooperation between peroxisomes and lipid droplets in the regulation of adipose tissue lipolysis [13,56].
Peroxisome derived ether lipids constitute 10–20% of neutral lipids in lipid droplets [57], a finding which has been used as argument for bidirectional cooperation between peroxisomes and lipid droplets [58]. However, while peroxisomes are indispensable for the initial steps of ether lipid synthesis, so too are the downstream ER-localized steps [23]. Thus, the high proportion of ether lipids in lipid droplets may reflect a role of ether lipids in lipid droplet formation. In vitro studies have suggested that the peroxisomal acyl-DHAP pathway may account for half of triglycerides synthesized during adipogenesis, though the unaltered adipose tissue morphology of peroxisome-deficient mice suggests that this contribution is dispensable in vivo [24,56].
4. Role of peroxisome-derived lipids in energy-homeostasis and metabolic disorders
Ether lipids are abundant in cell membranes and promote tighter membrane packing due to their lack of an sn-1 carbonyl oxygen, in turn decreasing membrane fluidity and allowing for formation of stronger hydrogen bonds with head groups of neighboring phospholipids [23]. This property may be the mechanism through which peroxisomes regulate membrane dynamics [59,60]. Ether lipids are enriched in lipid raft microdomains, cholesterol-rich membrane regions that facilitate assembly of cell signaling complexes [61]. Depletion of these peroxisome-derived lipids leads to mislocalization of cholesterol [61,62]. Recent studies have implicated ether lipids in regulating ferroptosis susceptibility in mammalian cells and promoting lifespan extension in C. elegans [63,64]. Plasmalogens, the most common form of ether lipids, are thought to function as cellular antioxidants due to the presence of the vinyl ether bond, which makes them highly susceptible to oxidative attacks [65]. Moreover, plasmalogens have been implicated in mediating cell signaling involved in neuroprotection [66].
With the increasing interest in understanding the physiological roles of ether lipids, emerging studies have uncovered a role for these lipids in regulating energy homeostasis and affecting risk for metabolic disorders. Recent work has led to the identification of ether lipids as inversely correlated biomarkers of obese phenotypes, regulators of mitochondrial morphology and function, and mediators of cell signaling related to adipocyte differentiation.
4.1. Ether lipid levels in obesity and diabetes
Lipids account for over half of biological molecules in human plasma. Most free fatty acids in serum associate with albumin, while complex lipids such as phospholipids and triglycerides are transported through serum in plasma lipoproteins [67]. Ethanolamine plasmalogens represent up to 30% of the total phosphatidylethanolamine in lipoproteins [68]. Although liver is a major site for synthesis of ether lipids, including plasmalogens, it has a low intracellular content of these peroxisome-derived lipids. Instead, liver-derived plasmalogens are thought to be transported to other tissues via lipoproteins [68]. However, the plasmalogen content in human atherosclerotic aortas has been reported to be lower than that in normal aortas [69]. This is presumably because plasmalogens, owing to their endogenous antioxidant activity [70], prevent the formation of oxidized low-density lipoprotein (LDL). Oxidized LDL, but not native LDL, is preferentially taken up by macrophages and promotes their conversion to foam cells, a hallmark of atherosclerotic lesions [71]. Accordingly, serum concentrations of plasmalogens are negatively associated with incident myocardial infarction in patients [72] and plasmalogen enrichment through dietary supplementation of an ether lipid precursor has been reported to mitigate atherosclerosis in ApoE-deficient mice [73].
Circulating plasmalogens have also been linked to decreased adiposity. Untargeted metabolomics of serum from healthy women identified choline plasmalogen PC(O-16:0/18:1) as the metabolite most significantly inversely associated with both body-mass index (BMI) and waist circumference. A multivariable analysis revealed plasmalogens PC(O-16:0/18:1) and PC(O-16:0/18:2) as significantly inversely associated with waist circumference independent of BMI, suggesting a specific role for circulating plasmalogens in regulating or responding to body fat distribution [74]. Notably, both PC(O-16:0/18:1) and PC(O-16:0/18:2) are potential products of lysophosphatidylcholine acyltransferase-3 (Lpcat3), which incorporates C18:1 or C18:2 into the sn-2 position of lysophosphatidylcholine to produce phosphatidylcholine (PC) and has been implicated in glucose homeostasis, hepatic steatosis, ferroptosis, and ER stress [[75], [76], [77]], suggesting a possible interplay between peroxisome-derived ether lipids and Lpcat3-mediated phospholipid remodeling.
In contrast to trends seen in serum, untargeted metabolomics of visceral adipose tissue (VAT) from metabolically unhealthy obese, metabolically healthy obese, or nonobese subjects identified significant increases of plasmalogen species and oxidative stress metabolites in tissue from metabolically unhealthy obese patients [78]. The absence of significant increases of plasmalogens or oxidative stress metabolites in VAT of metabolically healthy obese patients may imply that plasmalogens, as potential antioxidants, increase in response to obesity-associated oxidative stress [65,79]. Untargeted lipidomic analysis of adipose tissue in metabolically healthy twin pairs discordant for obesity identified increased ethanolamine plasmalogen levels in adipose tissue of obese twin individuals with a specific enrichment for plasmalogens containing arachidonic acid (C20:4) [80]. It is possible that the use of twins reduced inter-sample variance sufficiently to allow for detection of significant differences in plasmalogen levels between nonobese and metabolically healthy obese subjects which were too small to detect in the context of a larger cohort study such as that used by Candi et al. [78]. If this is indeed the case, then adipose tissue plasmalogens may increase in metabolically healthy obesity and increase further still in metabolically unhealthy obesity, suggesting an inverse relationship between adipose tissue plasmalogen levels and metabolic health.
A final interesting link between obesity-associated disease and total ethanolamine plasmalogen levels exists in the nervous system, where plasmalogens constitute roughly 70% of total phosphatidylethanolamine (PE) in myelin sheaths [81]. In obese db/db (leptin receptor-deficient diabetic) mice, loss of PE plasmalogen species has been implicated as an underlying mechanism of diabetic neuropathy, preceding any structural or functional defects in mitochondria, myelin sheaths, or axons [82]. Surprisingly, this loss of plasmalogens is driven not by hyperglycemia but instead by obesity and hyperlipidemia themselves. Not only are these findings useful as potential biomarkers of different subtypes of diabetic neuropathy, but they also provide new insight into the roles of plasmalogens in the central nervous system.
4.2. Regulation of mitochondrial dynamics and respiration by peroxisome-derived lipids
Given the uneven cellular distribution of peroxisome-derived ether lipids, it is important to consider not only their total levels in the context of obesity, but also their cellular functions and the mechanistic nature of their relationships to obesity. Recent work suggests that ether lipids influence energy homeostasis through regulation of mitochondrial dynamics and respiration.
Dynamic regulation of mitochondrial morphology and number is achieved through highly coordinated mitochondrial fission and fusion [83]. In metabolic tissues, these processes can drastically impact systemic energy expenditure [84]. For example, mitochondrial morphology is considered an important regulator of the thermogenic capacity of adipocytes, with brown adipocyte mitochondria exhibiting more fragmented and circular shape that promotes uncoupled respiration, while white adipocyte mitochondria exhibit an elongated shape that is associated with increased ATP synthesis activity [[85], [86], [87]]. Recent studies using mice with adipose-specific knockout of the critical peroxisome-biogenesis factor Pex16 (Pex16-AKO) suggest that peroxisome-derived lipids are required for brown fat-mediated thermogenesis through regulation of cold-induced mitochondrial fission [16]. Pex16-AKO mice had impaired mitochondrial fission in brown adipocytes, causing decreased uncoupled respiration and thermogenesis and increased diet-induced obesity (Figure 3). Knockdown of the peroxisomal ether-lipid synthetic enzyme GNPAT in adipocytes recapitulated the Pex16-AKO phenotype in a cell-autonomous manner, and dietary supplementation with plasmalogen precursors rescued the phenotype of high-fat diet fed Pex16-AKO mice. Together, these data demonstrate that ether lipids play a crucial role in systemic energy expenditure by regulating mitochondrial dynamics in adipose tissue [16].
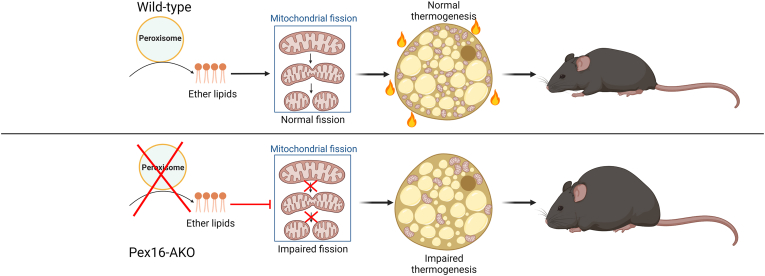
Figure 3.
Adipose-specific peroxisome deficiency impairs mitochondrial fission and increases diet-induced obesity. Mice with adipose-specific knockout of Pex16 (Pex16-AKO) display impaired mitochondrial fission in brown adipose tissue, decreased thermogenesis, and increased diet-induced obesity. This phenotype is rescued by supplementation of ether-lipid synthetic intermediates, implicating ether lipids as the mediator linking peroxisomes with mitochondrial fission and thermogenesis.
Further supporting peroxisome-to-mitochondria functional lipid signaling, newly published studies suggests that peroxisome-derived lipids promote efficient mitochondrial respiration and cell growth by regulating assembly of the respirasome, a mitochondrial supercomplex consisting of complexes I, III and IV [14]. These authors demonstrated that mitochondrial phospholipids are sensitive to cellular levels of the pyrimidine nucleotide cytidine triphosphate (CTP) and coordinate with the de novo pyrimidine synthesis enzyme dihydroorotate dehydrogenase (DHODH). DHODH then signals to peroxisomes to promote ether lipid synthesis and transport to mitochondria for respirasome assembly [14].
Inter-organ communication might also be involved in regulation of mitochondrial function by peroxisome-derived lipids. Recent studies identified decreased serum plasmalogens as biomarkers in Leigh Syndrome French-Canadian variant (LSFC), a monogenic mitochondrial disorder in which a mutation in Leucine-rich PPR-motif-containing protein (Lrpprc), a protein that regulates RNA metabolism in mitochondria and nuclei, causes mitochondrial dysfunction, neurodegeneration, and hepatic lactic acidosis [9,88,89]. These findings were recapitulated in a LSFC mouse model with liver-specific knockout of Lrpprc, implying that perturbed hepatic peroxisomal ether lipid synthesis is responsible for the decreased serum plasmalogens in LSFC subjects and possibly some of the associated defects in other tissues, such as the brain, where plasmalogens account for approximately 20% of phospholipids [40] and are a major component of myelin [90]. It is unclear whether altered peroxisomal lipid metabolism is a direct effect of Lrpprc mutation or a response to mitochondrial dysfunction, though either case suggests a relevant role for circulating plasmalogens in the LSFC phenotype [91].
4.3. Role of peroxisome derived lipids in adipose tissue development and function
Peroxisome-derived lipids are also thought to be partial agonists of PPARγ (peroxisome proliferator-activated receptor gamma), a ligand-activated transcription factor critical for adipogenesis. As the name suggests, PPARγ belongs to a family of nuclear receptors originally identified based on their ability to be activated by agents that promote peroxisome proliferation [92]. PPARγ is necessary and sufficient for adipose tissue development [93]. It regulates the expression of a large number of genes in adipocytes [94,95], including genes involved in peroxisomal biogenesis [3]. Inhibition of de novo peroxisome formation by knockdown of the critical peroxisomal biogenesis factor Pex16 has been shown to block PPARγ activation and adipocyte differentiation. Treatment of cells with alkylglycerol, an ether lipid precursor that enters the synthetic pathway downstream of the peroxisomal steps, restores adipogenesis in peroxisome-deficient adipocytes, suggesting that peroxisome-derived ether lipids are required for PPARγ activation during adipogenesis [15]. Moreover, a role for peroxisome-derived lipids in adipose tissue development was suggested by studies in mice with global knockout of Pex7 [96], a peroxisomal biogenesis factor required for import of peroxisomal matrix proteins, including the ether lipid synthetic enzyme AGPS [97]. These mice lack plasmalogens and have severely decreased body fat content. Remarkably, the decreased adiposity could be rescued by feeding the mice a diet enriched in alkylglycerol [96]. Together, these studies suggests that the increased peroxisomal biogenesis and ether lipid synthesis in response to PPARγ activation may represent a feed-forward mechanism to support the adipogenic program.
PPARγ has a large ligand binding pocket [98] and can interact with and be activated by various lipids, including fatty acids, fatty acyl-CoA species, eicosanoids, and oxidized phospholipids [99]. Multiple ether lipids have also been identified as potential ligands of PPARγ, including azelaoyl phosphatidylcholine (1-O-hexadecyl-2-O-(9-carboxyoctanoyl)-sn-glyceryl-3-phosphocholine) [100]. In addition, both AGP and LPA, direct products of the peroxisomal protein PexRAP, have been shown to be high affinity partial agonists for PPARγ [[101], [102], [103]]. Given that PPARγ can be activated by various lipids, it is possible that its endogenous ligands in general exhibit partial agonism, thus allowing selective recruitment of distinct coactivators to regulate unique subsets of genes. In an effort to identify potential endogenous ligands of PPARγ, we used mass spectrometry to search for lipids bound to tagged PPARγ affinity captured from adipocytes [25]. This search led to the identification of several species of ether lipid analogs of PC that were bound to PPARγ in a rosiglitazone-displaceable manner. Treatment of cells with one of these lipid species, 1-O-octadecenyl-2-palmitoyl-3-glycerophosphocholine (18:1e/16:0-GPC), promoted PPARγ activation and increased adipogenic gene expression [25]. Additional evidence for a pro-adipogenic role of ether lipids was provided by an untargeted metabolomics screen, which showed that endogenous synthesis of monoalkylglycerol ether lipids (MAGE) increases early during 3T3-L1 adipogenesis and that treatment with these lipids promotes adipocyte differentiation, albeit through an undefined mechanism [104].
Alkyglycerols have also been implicated in protection against childhood obesity. These precursors of ether-linked phospholipids, including plasmalogens, were recently shown to be enriched in breast milk. Alkylglycerols from breast milk were found to prevent beige-to-white adipose tissue remodeling in infants through a mechanism involving their conversion by adipose tissue macrophages (ATM) to a bioactive lipid called platelet-activating factor (PAF), which acts in a paracrine manner to promote IL6 release, leading to adipose tissue browning [105].
5. Peroxisomal β-oxidation and the regulation of lipid homeostasis in adipose tissue and liver
As lipid metabolic organelles, peroxisomes play important roles in cellular lipid homeostasis through their direct synthesis and degradation of various lipid species. Peroxisomal β-oxidation is transcriptionally regulated by PPARα to mediate catabolism of VLCFAs [106], but the physiological effects of this process have remained unclear. Emerging studies suggest that peroxisomal β-oxidation is an important regulator of cellular lipid homeostasis that increases the risk for NAFLD via multiple mechanisms, including inhibition of adipose triglyceride lipase (ATGL)-mediated lipolysis [11], autophagic degradation of lipid droplets [12], and mitochondrial β-oxidation substrate availability [10,107]. Through regulation of lipid homeostasis in adipose tissue and liver, peroxisomal β-oxidation has the potential to affect systemic metabolism and obesity.
5.1. Role of peroxisomal fatty acid oxidation in lipolysis
Lipolysis is a cytosolic process through which triglycerides stored in lipid droplets are hydrolyzed, generating free fatty acids and glycerol. It involves induction of β-adrenergic receptor signaling, resulting in the activation of a protein kinase A (PKA)-dependent pathway [108]. Only recently has any regulatory relationship between peroxisomes and lipolysis emerged. Mice lacking functional peroxisomes in adipocytes were discovered to have reduced rates of lipolysis, though the use of the nonspecific ap2/Fabp4 promoter to drive Pex5 knockout called into question the specificity of the observed effects [56,109]. A more recent study clarified the role of peroxisomes in fasting-induced lipolysis using adiponectin-Cre-driven Pex5 knockout in mice and RNAi-mediated Pex5 knockdown in C. elegans [13]. Fasting is known to induce lipolysis in adipocytes by activating PKA, which was found to be associated with a kinesin family member C3 (KIFC3)-dependent movement of peroxisomes to lipid droplets [13]. Furthermore, part of the increase in lipolytic activity in response to fasting is mediated by Pex5 recruitment of ATGL to peroxisome-lipid droplet contact sites. Pex5 depletion does not affect basal lipolysis, implying that Pex5 recruitment of ATGL to lipid droplet occurs primarily as a mechanism to upregulate lipolysis under non-basal conditions [13]. As Pex5 is required for normal peroxisome biogenesis, its role in fasting-induced lipolytic upregulation reveals a novel relationship between peroxisomes and cellular lipid homeostasis.
Insulin is a potent inhibitor of lipolysis in adipocytes, activating the PI3K-AKT pathway which deactivates both hormone sensitive lipase (HSL) and forkhead box O1 (FOXO1) and decreases ATGL expression [108,[110], [111], [112]]. The effect of insulin on hepatic lipolysis in vivo is somewhat less clear [113,114], but insulin also inhibits both mitochondrial and peroxisomal β-oxidation in hepatocytes [115], which is likely through FOXO1 signaling [116]. Thus, both adipose tissue lipolysis and hepatic peroxisomal β-oxidation are negatively regulated by insulin, the predominant extracellular cue of the fed state in vivo. A direct role of peroxisomal β-oxidation in regulation of lipolysis in response to intracellular cues has only recently emerged, however.
In this regard, recently published studies suggest that peroxisomal β-oxidation acts as a sensor of intracellular fatty acids to inhibit lipolysis. ROS from peroxisomal β-oxidation was shown to act as a novel signaling molecule by stabilizing disulfide bonds within the peroxin Pex2, in turn increasing total Pex2 protein levels. Pex2 then poly-ubiquitinates ATGL for proteasome-targeted degradation, completing a novel signaling pathway by which peroxisomal β-oxidation negatively regulates basal ATGL-mediated lipolysis and thus affects lipid homeostasis (Figure 4) [11]. These findings that peroxisomal β-oxidation inhibits lipolysis in both adipocytes and hepatocytes appear to contradict the relationship which would be predicted based on insulin signaling. Insulin inhibits lipolysis in adipose tissue and peroxisomal β-oxidation in liver, which would suggest that the two processes are regulated in the same direction in response to systemic energy cues, yet these studies show that peroxisomal β-oxidation negatively regulates lipolysis. This discrepancy likely suggests that, in the absence of insulin (basal state), cellular lipolysis is fine-tuned in response to intracellular levels of fatty acids, though insulin-mediated effects likely predominate in the fed state.
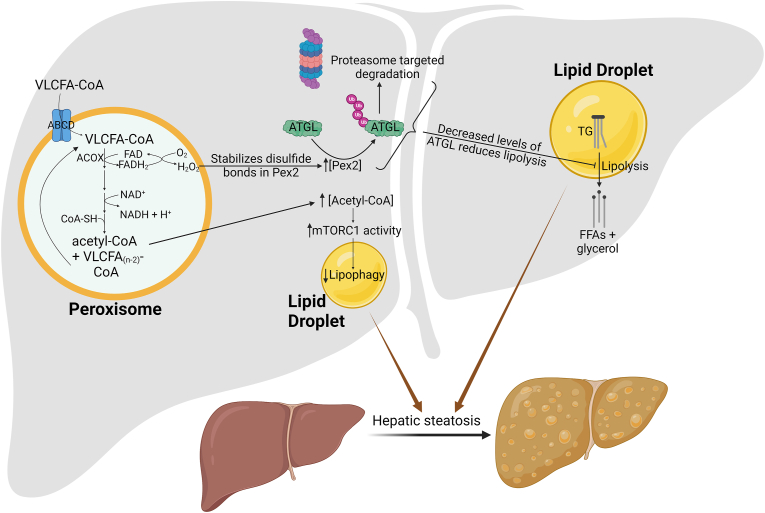
Figure 4.
Peroxisomal β-oxidation promotes nonalcoholic fatty liver disease through multiple unique mechanisms. The first step of peroxisomal β-oxidation reduces FAD to FADH2, which in turn reduces O2 to H2O2. The oxidant stabilizes disulfide bonds in peroxisomal biogenesis factor 2 (Pex2), thus increasing the protein levels of Pex2, which mediates poly-ubiquitination of adipose triglyceride lipase (ATGL). This promotes targeted degradation of ATGL by proteasomes, resulting in impaired lipolysis and increased hepatic steatosis. An alternative mechanism involves the use of acetyl-CoA, a product of peroxisomal β-oxidation, in acetylation of regulatory-associated protein of mTOR (Raptor), part of the mammalian target of rapamycin complex 1 (mTORC1). Acetylation of Raptor activates mTORC1, resulting in impaired lipophagy and aberrant hepatic lipid accumulation.
5.2. Inhibition of lipophagy by peroxisomal β−oxidation-derived acetyl-CoA
Western diets or prolonged fasting both cause NAFLD despite increased hepatic peroxisomal β-oxidation [117]. These contexts reveal a novel additional mechanism by which activation of peroxisomal β-oxidation may contribute to hepatic steatosis. Recent studies from our laboratory suggest that peroxisomal β-oxidation in the liver promotes steatosis by inhibiting lipophagy [12], autophagic degradation of lipid droplets that is distinct from cytosolic lipolysis. Lipophagy is an alternative process for releasing fatty acids from triglycerides and involves encapsulation of lipid droplets within an autophagosome, which fuses with a lysosome, resulting in hydrolysis of triglycerides by lysosomal acid lipase A (LAL) [118]. Acetyl-CoA is an important metabolic regulator of autophagy whose depletion is sufficient to promote autophagic degradation [119]. Our studies indicate that Acox1-mediated peroxisomal β-oxidation is a major source of acetyl-CoA which inhibits lipophagy. Mechanistically, peroxisome-derived acetyl-CoA mediates acetylation of Raptor [12], a component of the mechanistic target of rapamycin kinase (mTOR) complex 1 (mTORC1), a key growth and metabolism regulatory complex that senses nutrients and inhibits autophagy [120]. Thus, inhibiting Acox1, which catalyzes the rate-limiting step of peroxisomal β-oxidation and is required for peroxisomal acetyl-CoA production, ameliorates fasting- or high-fat diet-induced hepatic steatosis. Consistent with these results, selective inhibition of hepatic mTORC1 inhibits NAFLD, reaffirming the physiological significance of the link between mTORC1 activity and hepatic steatosis in vivo [121]. Together, these findings demonstrate that peroxisomal β-oxidation-derived acetyl-CoA activates mTORC1, inhibiting autophagy and lipophagy and contributing to the development of NAFLD in response to fasting or obesogenic diet feeding (Figure 4). Additional work is required to understand how peroxisomal β-oxidation impacts whole-body energy metabolism.
5.3. Regulation of mitochondrial fatty acid oxidation by peroxisomal β-oxidation
Peroxisomal β-oxidation also regulates cellular lipid homeostasis through the SIRT1/AMPK pathway and by modulating levels of malonyl-CoA, which inhibits mitochondrial β-oxidation substrate availability [10,122]. Rapeseed (canola) oil causes hepatic steatosis in animals [123], likely due to its high concentration of erucic acid (C22:1ω9), which is preferentially oxidized in peroxisomes rather than mitochondria. The mechanism by which high-erucic-acid rapeseed oil induces hepatic steatosis was long unclear as the animals had high levels of hepatic PPARα activation and peroxisomal β-oxidation [124], both of which might be expected to decrease fat accumulation. Exploring this paradoxical effect led to the elucidation of a role for peroxisomal β-oxidation in regulating mitochondrial β-oxidation. Peroxisomal β-oxidation of erucic acid generates acetate and elevates the cellular NADH/NAD + ratio, inhibiting the SIRT1-AMPK pathway and leading to increased acetyl-CoA synthetase (ACS) and acetyl-CoA carboxylase (ACC) activities and elevated malonyl-CoA levels. High levels of malonyl-CoA inhibit transfer of long chain fatty acids into mitochondria, thus blocking mitochondrial β-oxidation [107]. These findings reveal a novel regulatory relationship between peroxisomal and mitochondrial β-oxidation wherein erucic acid oxidation by the former regulates substrate availability of the latter by modulating cellular acetate levels and NADH/NAD+ ratio [10]. Notably, regulation of mitochondrial β-oxidation by peroxisomal β-oxidation occurs in contexts beyond merely erucic acid catabolism. Inhibiting Acox1, the rate limiting enzyme in peroxisomal β-oxidation, with 10,12-tricosadiynoic acid increases mitochondrial fatty acid oxidation in high-fat diet fed rats by activating the SIRT1-AMPK pathway, in turn decreasing hepatic steatosis, reducing diet-induced obesity, and reducing serum triglyceride and insulin levels [122]. Together, these studies demonstrate that peroxisomal and mitochondrial β-oxidation activities are inversely related due to peroxisomal β-oxidation inhibiting the SIRT1-AMPK pathway and blocking mitochondrial β-oxidation through malonyl-CoA formation.
5.4. Anabolic functions of peroxisomal β-oxidation
Finally, peroxisomal β-oxidation regulates cellular lipid homeostasis by potentially providing substrate for synthesis of ether lipids and cholesterol. Early studies observed that peroxisomal β-oxidation-derived acetyl-CoA can be incorporated into PE plasmalogens in rat liver [125]. Rats in this experiment received clofibrate treatment to inhibit cholesterol and bile acid biosyntheses and promote peroxisomal β-oxidation and were subsequently fed [1–14C] lignoceric acid, which is exclusively degraded by peroxisomal β-oxidation [125]. While acetyl-CoA from peroxisomal β-oxidation was found to be incorporated into PE plasmalogens, whether this occurs under normal physiological conditions remains unclear. Recent work, however, has further suggested a lipogenic role for products of peroxisomal β-oxidation. In a type-1 diabetic mouse model, excessive hepatic peroxisomal β-oxidation was shown to generate a surplus of free acetate, which in turn stimulates and serves as a precursor for cholesterol biosynthesis without affecting the gene expression and abundance of key cholesterol biosynthetic enzymes. Moreover, inhibiting peroxisomal β-oxidation with the Acox1 inhibitor 10,12-tricosadiynoic acid decreases cholesterol biosynthesis by reducing acetate formation [126]. While the relationship between hepatic peroxisomal β-oxidation and circulating LDL-cholesterol levels was not explored, it is possible that activation of cholesterol biosynthesis by peroxisomal β-oxidation contributes to diabetes-associated hypercholesterolemia since peroxisomal fatty acid oxidation in the liver is strongly induced in the diabetic state [[127], [128], [129]].
6. Role of peroxisomal ROS production in obesity and diabetes
Oxidative stress, the state in which pro-oxidative processes overwhelm cellular antioxidant defenses through disrupted redox signaling, is a well-recognized feature of obesity in numerous tissues [130]. Obese humans have higher levels of oxidative stress in white adipose tissue [131] and oxidative stress correlates with insulin resistance and intra-abdominal obesity [132]. Obesity-associated oxidative stress is generally thought to result from prolonged nutritional excess and hyperinsulinemia, together increasing metabolic demand on the mitochondria and consequently ETC activity, leading to increased ROS production by complexes I and III [133,134]. Increased ROS, in the form of H2O2 or superoxide (O2−), inhibits insulin signaling in adipocytes and thus causes insulin resistance [135]. Oxidative stress-induced inhibition of insulin signaling functions as a last line of defense against ROS-mediated cellular damage, as accumulating ROS indicates to the cell that the presently available antioxidant defenses are insufficient relative to the rate of ROS production and therefore insulin signaling must be inhibited to decrease ETC activity and the ROS it produces. In this way, adipose tissue oxidative stress represents an adaptive response to overnutrition, which has the untoward effects of causally linking obesity to insulin resistance and the myriad of other associated metabolic disorders such as type 2 diabetes [136]. Mitochondria are a major source of cellular ROS, particularly , and have been the focus of many studies on the role of oxidative stress in obesity and diabetes [133]. Peroxisomes are also major sources of ROS, particularly H2O2, and thus also have potential to mediate obesity-induced insulin resistance [137].
Although peroxisomes produce up to 35% of total H2O2 in certain tissues [35], the role of adipose tissue peroxisomal ROS metabolism in obesity and insulin resistance remains relatively unexplored. Levels of peroxisomal markers decrease in brown adipose tissue of ob/ob (leptin-deficient obese) mice relative to lean controls [138] and obesity-resistant mice have enhanced peroxisomal β-oxidation in VAT [139], together suggesting an inverse relationship between ROS-producing peroxisomal enzymes and obesity-associated oxidative stress in adipose tissue. Consistent with these correlations, a recent study found that treating 3T3-L1 adipocytes with H2O2 decreases abundance of many peroxisomal proteins, suggesting that adipocytes might downregulate peroxisomal biogenesis in response to oxidative stress, possibly to decrease peroxisome ROS production [140]. Decreasing adipose tissue oxidative stress in vivo by adipose-specific double overexpression of the antioxidant enzymes superoxide dismutase and catalase, a primarily peroxisomal protein, demonstrates that oxidative stress inhibits the healthy expansion of adipose tissue and confirms that adipose tissue oxidative stress is sufficient to cause insulin resistance [141]. Although this study did not exclusively explore peroxisomal ROS metabolism, the specific contribution of peroxisomes to ROS production in these conditions represents an exciting area of future research, considering the significance of adipose tissue oxidative stress in obesity-associated insulin resistance.
Oxidative stress is also believed to be involved in pancreatic β-cell lipotoxicity, which often occurs as a result of prolonged elevations of circulating nonesterified fatty acids (NEFA). β-cell lipotoxicity impairs glucose-stimulated insulin secretion and contributes to the loss of β-cells in type 2 diabetes [142]. Certain lipotoxic fatty acids, such as palmitic acid, are oxidized by both peroxisomes and mitochondria [143], processes which could contribute to lipotoxicity-associated oxidative stress since both mitochondrial and peroxisomal β-oxidation produce ROS [144]. Catalase, the peroxisomal H2O2 antioxidant enzyme, is thought to be a “forbidden gene” whose expression must be suppressed for normal β-cell functioning, in theory leaving β-cells particularly susceptible to peroxisome-derived ROS [145]. β-cell-specific peroxisome deficiency compromises β-cell integrity and promotes cell death in vivo, leading to glucose intolerance and impaired insulin secretion in both normal chow- or high fat diet-fed mice [146]. While these findings suggest an indispensable role for peroxisomes in β-cell function and viability, they cannot distinguish which of the many peroxisomal functions is responsible for this phenotype.
A specific role for peroxisomal fatty acid oxidation in β-cell lipotoxicity is suggested by the fact that peroxisome-localized overexpression of catalase in β-cells decreases NEFA-induced lipotoxicity [147]. These results suggest that ROS, primarily H2O2, derived from peroxisomal β-oxidation of NEFA is the key mediator of lipotoxicity in pancreatic β-cells. An indispensable signaling role for peroxisomal ROS is consistent with the notion that catalase, which would eliminate most peroxisomal ROS, is not expressed in β-cells. Why β-cells do not express catalase, leaving them poorly defended against both H2O2 formed during peroxisomal β-oxidation and the resultant lipotoxicity, remains unanswered. Recent findings have demonstrated that, while high doses of ER stress are deleterious and promote β-cell death, low doses of ER stress are adaptive and promote β-cell proliferation under hyperglycemic conditions [148]. One theory for the lack of catalase expression in β-cells could be that peroxisomal oxidative stress functions analogously to ER stress and is beneficial at low levels yet harmful at high levels. Such a mechanism, which is analogous to a previously discovered one in mitochondria [149], would provide a rationale for the lack of catalase expression in β-cells, as the presence of catalase would greatly reduce the sensitivity of such a signaling pathway.
7. Summary and future directions
Peroxisomes are important metabolic organelles which coordinate with ER, lysosomes, and mitochondria to play key roles in lipid and ROS metabolism. In metabolic tissues such as adipose tissue, liver, and pancreas, peroxisomes are emerging as cellular regulators of various processes, with close connections to systemic metabolism and obesity. With the increasing interest in the role of peroxisomes in metabolic health and disease, a number of key questions remain.
It is unknown why there are multiple mechanisms through which peroxisomes regulate fatty acid mobilization and catabolism; an improved understanding of these processes could help elucidate therapeutic targets for decreasing lipid storage and promoting lipid oxidation as an obesity treatment. Numerous studies have associated decreased circulating ether lipids with obesity and BMI, although it is unclear if this is a cause or a response to disease. The mechanistic nature of this relationship also remains unclear. Suggesting a potential protective role against obesity, these peroxisome-derived lipids are a component of mitochondrial membranes and may affect mitochondrial respiration and energy expenditure by regulating assembly of the respirasome and increasing thermogenic capacity of brown adipocytes by mediating cold-induced mitochondrial fission [14,16]. Further supporting a causative role for ether lipids in decreasing obesity, breast milk-derived alkylglycerols have been reported to promote adipocyte browning [105].
Adipose tissue ROS is believed to causally link obesity with insulin resistance, yet the specific contribution of peroxisome-derived ROS in this process has not been explored, possibly since mitochondrial ROS is increased in obesity by hyperinsulinemia and thus a seemingly more obvious mediator. Oxidative stress correlates in proportion with the amount of ROS produced relative to the antioxidative defenses present, however. Thus, even if the absolute ROS production by peroxisomal β-oxidation is less than that from mitochondrial β-oxidation, a better understanding of the former might help uncover new therapeutic targets to reduce or prevent obesity-associated insulin resistance and metabolic syndrome by reducing cellular ROS. Finally, as a potential mediator of lipotoxicity in pancreatic β-cells, peroxisome derived ROS are a possible therapeutic target to prevent glucose intolerance in obesity and β-cell loss in type 2 diabetes. The notable lack of catalase expression in these cells, however, is unlikely an accident, and rather represents an evolutionarily selected feature which requires further study if therapeutic treatment is to be progressed. Further investigation into the role of peroxisomal ROS production and other metabolic processes regulated by peroxisomes might uncover novel mechanisms through which obesity and diabetes can be treated or diagnosed clinically.
Author contributions
Both authors contributed to this work by writing and editing the manuscript and preparing figures.
Acknowledgments
The work in the Lodhi lab is supported by NIH grants R01DK115867, R01DK118333, R01DK132239, and P30DK020579. We apologize to those colleagues whose work could not be cited here due to space constraints.
Conflict of interest
No conflict of interest exists.
Data availability
No data was used for the research described in the article.
References
- 1.Blüher M. Obesity: global epidemiology and pathogenesis. Nature Reviews Endocrinology. 2019;15:288–298. doi: 10.1038/s41574-019-0176-8. [DOI] [PubMed] [Google Scholar]
- 2.Klein S., Gastaldelli A., Yki-Järvinen H., Scherer P.E. Why does obesity cause diabetes? Cell Metabolism. 2022;34:11–20. doi: 10.1016/j.cmet.2021.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lodhi I.J., Semenkovich C.F. Peroxisomes: a nexus for lipid metabolism and cellular signaling. Cell Metabolism. 2014;19:380–392. doi: 10.1016/j.cmet.2014.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hasan S., Platta H.W., Erdmann R. Import of proteins into the peroxisomal matrix. Frontiers in Physiology. 2013;4:261. doi: 10.3389/fphys.2013.00261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wanders R.J.A., Waterham H.R., Ferdinandusse S. Peroxisomes and their central role in metabolic interaction networks in humans. Subcellular Biochemistry. 2018;89:345–365. doi: 10.1007/978-981-13-2233-4_15. [DOI] [PubMed] [Google Scholar]
- 6.Sugiura A., Mattie S., Prudent J., McBride H.M. Newly born peroxisomes are a hybrid of mitochondrial and ER-derived pre-peroxisomes. Nature. 2017;542:251–254. doi: 10.1038/nature21375. [DOI] [PubMed] [Google Scholar]
- 7.Steinberg S.J., Dodt G., Raymond G.V., Braverman N.E., Moser A.B., Moser H.W. Peroxisome biogenesis disorders. Biochimica et Biophysica Acta. 2006;1763:1733–1748. doi: 10.1016/j.bbamcr.2006.09.010. [DOI] [PubMed] [Google Scholar]
- 8.Baes M., Van Veldhoven P.P. Hepatic dysfunction in peroxisomal disorders. Biochimica et Biophysica Acta (BBA) - Molecular Cell Research. 2016;1863:956–970. doi: 10.1016/j.bbamcr.2015.09.035. [DOI] [PubMed] [Google Scholar]
- 9.Ruiz M., Cuillerier A., Daneault C., Deschênes S., Frayne I.R., Bouchard B., et al. Lipidomics unveils lipid dyshomeostasis and low circulating plasmalogens as biomarkers in a monogenic mitochondrial disorder. JCI Insight. 2019;4 doi: 10.1172/jci.insight.123231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen X., Shang L., Deng S., Li P., Chen K., Gao T., et al. Peroxisomal oxidation of erucic acid suppresses mitochondrial fatty acid oxidation by stimulating malonyl-CoA formation in the rat liver. Journal of Biological Chemistry. 2020;295:10168–10179. doi: 10.1074/jbc.RA120.013583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ding L., Sun W., Balaz M., He A., Klug M., Wieland S., et al. Peroxisomal β-oxidation acts as a sensor for intracellular fatty acids and regulates lipolysis. Nature Metabolism. 2021;3:1648–1661. doi: 10.1038/s42255-021-00489-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.He A., Chen X., Tan M., Chen Y., Lu D., Zhang X., et al. Acetyl-CoA derived from hepatic peroxisomal β-oxidation inhibits autophagy and promotes steatosis via mTORC1 activation. Molecular Cell. 2020;79:30–42.e34. doi: 10.1016/j.molcel.2020.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kong J., Ji Y., Jeon Y.G., Han J.S., Han K.H., Lee J.H., et al. Spatiotemporal contact between peroxisomes and lipid droplets regulates fasting-induced lipolysis via PEX5. Nature Communications. 2020;11 doi: 10.1038/s41467-019-14176-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bennett C.F., O'Malley K.E., Perry E.A., Balsa E., Latorre-Muro P., Riley C.L., et al. Peroxisomal-derived ether phospholipids link nucleotides to respirasome assembly. Nature Chemical Biology. 2021;17:703–710. doi: 10.1038/s41589-021-00772-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hofer D.C., Pessentheiner A.R., Pelzmann H.J., Schlager S., Madreiter-Sokolowski C.T., Kolb D., et al. Critical role of the peroxisomal protein PEX16 in white adipocyte development and lipid homeostasis. Biochimica et Biophysica Acta (BBA) - Molecular and Cell Biology of Lipids. 2017;1862:358–368. doi: 10.1016/j.bbalip.2016.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Park H., He A., Tan M., Johnson J.M., Dean J.M., Pietka T.A., et al. Peroxisome-derived lipids regulate adipose thermogenesis by mediating cold-induced mitochondrial fission. Journal of Clinical Investigation. 2019;129:694–711. doi: 10.1172/JCI120606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.De Duve C., Baudhuin P. Peroxisomes (microbodies and related particles) Physiological Reviews. 1966;46:323–357. doi: 10.1152/physrev.1966.46.2.323. [DOI] [PubMed] [Google Scholar]
- 18.Schrader M., Fahimi H.D. Mammalian peroxisomes and reactive oxygen species. Histochemistry and Cell Biology. 2004;122:383–393. doi: 10.1007/s00418-004-0673-1. [DOI] [PubMed] [Google Scholar]
- 19.Waterham H.R., Ferdinandusse S., Wanders R.J. Human disorders of peroxisome metabolism and biogenesis. Biochimica et Biophysica Acta. 2016;1863:922–933. doi: 10.1016/j.bbamcr.2015.11.015. [DOI] [PubMed] [Google Scholar]
- 20.Antonenkov V.D., Grunau S., Ohlmeier S., Hiltunen J.K. Peroxisomes are oxidative organelles. Antioxidants and Redox Signaling. 2010;13:525–537. doi: 10.1089/ars.2009.2996. [DOI] [PubMed] [Google Scholar]
- 21.Fransen M., Nordgren M., Wang B., Apanasets O. Role of peroxisomes in ROS/RNS-metabolism: implications for human disease. Biochimica et Biophysica Acta. 2012;1822:1363–1373. doi: 10.1016/j.bbadis.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 22.Chiang J.Y. Bile acid metabolism and signaling. Comprehensive Physiology. 2013;3:1191–1212. doi: 10.1002/cphy.c120023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dean J.M., Lodhi I.J. Structural and functional roles of ether lipids. Protein Cell. 2018;9:196–206. doi: 10.1007/s13238-017-0423-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hajra A.K., Larkins L.K., Das A.K., Hemati N., Erickson R.L., MacDougald O.A. Induction of the peroxisomal glycerolipid-synthesizing enzymes during differentiation of 3T3-L1 adipocytes. Role in triacylglycerol synthesis. Journal of Biological Chemistry. 2000;275:9441–9446. doi: 10.1074/jbc.275.13.9441. [DOI] [PubMed] [Google Scholar]
- 25.Lodhi I.J., Yin L., Jensen-Urstad A.P., Funai K., Coleman T., Baird J.H., et al. Inhibiting adipose tissue lipogenesis reprograms thermogenesis and PPARγ activation to decrease diet-induced obesity. Cell Metabolism. 2012;16:189–201. doi: 10.1016/j.cmet.2012.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gibellini F., Smith T.K. The Kennedy pathway--De novo synthesis of phosphatidylethanolamine and phosphatidylcholine. IUBMB Life. 2010;62:414–428. doi: 10.1002/iub.337. [DOI] [PubMed] [Google Scholar]
- 27.Wanders R.J.A., Waterham H.R. Biochemistry of mammalian peroxisomes revisited. Annual Review of Biochemistry. 2006;75:295–332. doi: 10.1146/annurev.biochem.74.082803.133329. [DOI] [PubMed] [Google Scholar]
- 28.Wanders R.J., van Grunsven E.G., Jansen G.A. Lipid metabolism in peroxisomes: enzymology, functions and dysfunctions of the fatty acid alpha- and beta-oxidation systems in humans. Biochemical Society Transactions. 2000;28:141–149. doi: 10.1042/bst0280141. [DOI] [PubMed] [Google Scholar]
- 29.Jansen G.A., Mihalik S.J., Watkins P.A., Moser H.W., Jakobs C., Denis S., et al. Phytanoyl-CoA hydroxylase is present in human liver, located in peroxisomes, and deficient in Zellweger syndrome: direct, unequivocal evidence for the new, revised pathway of phytanic acid alpha-oxidation in humans. Biochemical and Biophysical Research Communications. 1996;229:205–210. doi: 10.1006/bbrc.1996.1781. [DOI] [PubMed] [Google Scholar]
- 30.Wierzbicki A.S. Peroxisomal disorders affecting phytanic acid alpha-oxidation: a review. Biochemical Society Transactions. 2007;35:881–886. doi: 10.1042/BST0350881. [DOI] [PubMed] [Google Scholar]
- 31.Ray P.D., Huang B.W., Tsuji Y. Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cellular Signalling. 2012;24:981–990. doi: 10.1016/j.cellsig.2012.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fransen M., Lismont C. Redox signaling from and to peroxisomes: progress, challenges, and prospects. Antioxidants and Redox Signaling. 2019;30:95–112. doi: 10.1089/ars.2018.7515. [DOI] [PubMed] [Google Scholar]
- 33.Schrader M., Godinho L.F., Costello J.L., Islinger M. The different facets of organelle interplay-an overview of organelle interactions. Frontiers in Cell and Developmental Biology. 2015;3:56. doi: 10.3389/fcell.2015.00056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Massart J., Begriche K., Buron N., Porceddu M., Borgne-Sanchez A., Fromenty B. Drug-induced inhibition of mitochondrial fatty acid oxidation and steatosis. Current Pathobiology Reports. 2013;1:147–157. [Google Scholar]
- 35.Boveris A., Oshino N., Chance B. The cellular production of hydrogen peroxide. Biochemical Journal. 1972;128:617–630. doi: 10.1042/bj1280617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cipolla C.M., Lodhi I.J. Peroxisomal dysfunction in age-related diseases. Trends in Endocrinology and Metabolism. 2017;28:297–308. doi: 10.1016/j.tem.2016.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shin S.K., Cho H.W., Song S.E., Im S.S., Bae J.H., Song D.K. Oxidative stress resulting from the removal of endogenous catalase induces obesity by promoting hyperplasia and hypertrophy of white adipocytes. Redox Biology. 2020;37 doi: 10.1016/j.redox.2020.101749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hwang I., Lee J., Huh J.Y., Park J., Lee H.B., Ho Y.S., et al. Catalase deficiency accelerates diabetic renal injury through peroxisomal dysfunction. Diabetes. 2012;61:728–738. doi: 10.2337/db11-0584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Westin M.A.K., Hunt M.C., Alexson S.E.H. Short- and medium-chain carnitine acyltransferases and acyl-CoA thioesterases in mouse provide complementary systems for transport of β-oxidation products out of peroxisomes. Cellular and Molecular Life Sciences. 2008;65:982–990. doi: 10.1007/s00018-008-7576-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Braverman N.E., Moser A.B. Functions of plasmalogen lipids in health and disease. Biochimica et Biophysica Acta. 2012;1822:1442–1452. doi: 10.1016/j.bbadis.2012.05.008. [DOI] [PubMed] [Google Scholar]
- 41.Kishimoto Y., Moser H.W., Kawamura N., Platt M., Pallante S.L., Fenselau C. Adrenoleukodystrophy: evidence that abnormal very long chain fatty acids of brain cholesterol esters are of exogenous origin. Biochemical and Biophysical Research Communications. 1980;96:69–76. doi: 10.1016/0006-291x(80)91182-1. [DOI] [PubMed] [Google Scholar]
- 42.Lazarow P.B. Rat liver peroxisomes catalyze the beta oxidation of fatty acids. Journal of Biological Chemistry. 1978;253:1522–1528. [PubMed] [Google Scholar]
- 43.Kors S., Hacker C., Bolton C., Maier R., Reimann L., Kitchener E.J.A., et al. Regulating peroxisome–ER contacts via the ACBD5-VAPB tether by FFAT motif phosphorylation and GSK3β. Journal of Cell Biology. 2022;221 doi: 10.1083/jcb.202003143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Honsho M., Tanaka M., Zoeller R.A., Fujiki Y. Distinct functions of acyl/alkyl dihydroxyacetonephosphate reductase in peroxisomes and endoplasmic reticulum. Frontiers in Cell and Developmental Biology. 2020;8:855. doi: 10.3389/fcell.2020.00855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ikonen E., Zhou X. Cholesterol transport between cellular membranes: a balancing act between interconnected lipid fluxes. Developmental Cell. 2021;56:1430–1436. doi: 10.1016/j.devcel.2021.04.025. [DOI] [PubMed] [Google Scholar]
- 46.Lange Y., Ye J., Rigney M., Steck T.L. Regulation of endoplasmic reticulum cholesterol by plasma membrane cholesterol. The Journal of Lipid Research. 1999;40:2264–2270. [PubMed] [Google Scholar]
- 47.Charles K.N., Shackelford J.E., Faust P.L., Fliesler S.J., Stangl H., Kovacs W.J. Functional peroxisomes are essential for efficient cholesterol sensing and synthesis. Frontiers in Cell and Developmental Biology. 2020;8 doi: 10.3389/fcell.2020.560266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chu B.-B., Liao Y.-C., Qi W., Xie C., Du X., Wang J., et al. Cholesterol transport through lysosome-peroxisome membrane contacts. Cell. 2015;161:291–306. doi: 10.1016/j.cell.2015.02.019. [DOI] [PubMed] [Google Scholar]
- 49.Barbosa A.D., Savage D.B., Siniossoglou S. Lipid droplet-organelle interactions: emerging roles in lipid metabolism. Current Opinion in Cell Biology. 2015;35:91–97. doi: 10.1016/j.ceb.2015.04.017. [DOI] [PubMed] [Google Scholar]
- 50.Novikoff A.B., Novikoff P.M., Rosen O.M., Rubin C.S. Organelle relationships in cultured 3T3-L1 preadipocytes. The Journal of Cell Biology. 1980;87:180–196. doi: 10.1083/jcb.87.1.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Novikoff A.B., Novikoff P.M. Microperoxisomes and peroxisomes in relation to lipid metabolism. Annals of the New York Academy of Sciences. 1982;386:138–152. doi: 10.1111/j.1749-6632.1982.tb21412.x. [DOI] [PubMed] [Google Scholar]
- 52.Blanchette-Mackie E.J., Dwyer N.K., Barber T., Coxey R.A., Takeda T., Rondinone C.M., et al. Perilipin is located on the surface layer of intracellular lipid droplets in adipocytes. The Journal of Lipid Research. 1995;36:1211–1226. [PubMed] [Google Scholar]
- 53.Liu J., Lu W., Shi B., Klein S., Su X. Peroxisomal regulation of redox homeostasis and adipocyte metabolism. Redox Biology. 2019;24 doi: 10.1016/j.redox.2019.101167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Joshi A.S., Nebenfuehr B., Choudhary V., Satpute-Krishnan P., Levine T.P., Golden A., et al. Lipid droplet and peroxisome biogenesis occur at the same ER subdomains. Nature Communications. 2018;9:2940. doi: 10.1038/s41467-018-05277-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schrul B., Kopito R.R. Peroxin-dependent targeting of a lipid-droplet-destined membrane protein to ER subdomains. Nature Cell Biology. 2016;18:740–751. doi: 10.1038/ncb3373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Martens K., Bottelbergs A., Peeters A., Jacobs F., Espeel M., Carmeliet P., et al. Peroxisome deficient aP2-Pex5 knockout mice display impaired white adipocyte and muscle function concomitant with reduced adrenergic tone. Molecular Genetics and Metabolism. 2012;107:735–747. doi: 10.1016/j.ymgme.2012.10.015. [DOI] [PubMed] [Google Scholar]
- 57.Bartz R., Li W.H., Venables B., Zehmer J.K., Roth M.R., Welti R., et al. Lipidomics reveals that adiposomes store ether lipids and mediate phospholipid traffic. The Journal of Lipid Research. 2007;48:837–847. doi: 10.1194/jlr.M600413-JLR200. [DOI] [PubMed] [Google Scholar]
- 58.Schrul B., Schliebs W. Intracellular communication between lipid droplets and peroxisomes: the Janus face of PEX19. Biological Chemistry. 2018;399:741–749. doi: 10.1515/hsz-2018-0125. [DOI] [PubMed] [Google Scholar]
- 59.Han X.L., Gross R.W. Plasmenylcholine and phosphatidylcholine membrane bilayers possess distinct conformational motifs. Biochemistry. 1990;29:4992–4996. doi: 10.1021/bi00472a032. [DOI] [PubMed] [Google Scholar]
- 60.Lohner K. Is the high propensity of ethanolamine plasmalogens to form non-lamellar lipid structures manifested in the properties of biomembranes? Chemistry and Physics of Lipids. 1996;81:167–184. doi: 10.1016/0009-3084(96)02580-7. [DOI] [PubMed] [Google Scholar]
- 61.Rodemer C., Thai T.P., Brugger B., Kaercher T., Werner H., Nave K.A., et al. Inactivation of ether lipid biosynthesis causes male infertility, defects in eye development and optic nerve hypoplasia in mice. Human Molecular Genetics. 2003;12:1881–1895. doi: 10.1093/hmg/ddg191. [DOI] [PubMed] [Google Scholar]
- 62.Almsherqi Z.A. Potential role of plasmalogens in the modulation of biomembrane morphology. Frontiers in Cell and Developmental Biology. 2021;9 doi: 10.3389/fcell.2021.673917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zou Y., Henry W.S., Ricq E.L., Graham E.T., Phadnis V.V., Maretich P., et al. Plasticity of ether lipids promotes ferroptosis susceptibility and evasion. Nature. 2020;585:603–608. doi: 10.1038/s41586-020-2732-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cedillo L., Li S., Ahsan F.M., Emans S., Adedoja A., Dao K., et al. Cold Spring Harbor Laboratory; 2021. Ether lipid biosynthesis promotes lifespan extension and enables diverse prolongevity paradigms. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Engelmann B. Plasmalogens: targets for oxidants and major lipophilic antioxidants. Biochemical Society Transactions. 2004;32:147–150. doi: 10.1042/bst0320147. [DOI] [PubMed] [Google Scholar]
- 66.Hossain M.S., Mawatari S., Fujino T. Biological functions of plasmalogens. Advances in Experimental Medicine & Biology. 2020;1299:171–193. doi: 10.1007/978-3-030-60204-8_13. [DOI] [PubMed] [Google Scholar]
- 67.Quehenberger O., Dennis E.A. The human plasma lipidome. New England Journal of Medicine. 2011;365:1812–1823. doi: 10.1056/NEJMra1104901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Vance J.E. Lipoproteins secreted by cultured rat hepatocytes contain the antioxidant 1-alk-1-enyl-2-acylglycerophosphoethanolamine. Biochimica et Biophysica Acta. 1990;1045:128–134. doi: 10.1016/0005-2760(90)90141-j. [DOI] [PubMed] [Google Scholar]
- 69.Buddecke E., Andresen G. [Studies on the chemistry of the arterial wall. IV. Quantitative determination of the acetalphosphatides (plasmalogens) in human aorta with consideration to arteriosclerosis] Hoppe-Seyler's Zeitschrift fur physiologische Chemie. 1959;314:38–45. [PubMed] [Google Scholar]
- 70.Zoeller R.A., Morand O.H., Raetz C.R. A possible role for plasmalogens in protecting animal cells against photosensitized killing. Journal of Biological Chemistry. 1988;263:11590–11596. [PubMed] [Google Scholar]
- 71.Steinbrecher U.P., Parthasarathy S., Leake D.S., Witztum J.L., Steinberg D. Modification of low density lipoprotein by endothelial cells involves lipid peroxidation and degradation of low density lipoprotein phospholipids. Proceedings of the National Academy of Sciences of the United States of America. 1984;81:3883–3887. doi: 10.1073/pnas.81.12.3883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Moxon J.V., Jones R.E., Wong G., Weir J.M., Mellett N.A., Kingwell B.A., et al. Baseline serum phosphatidylcholine plasmalogen concentrations are inversely associated with incident myocardial infarction in patients with mixed peripheral artery disease presentations. Atherosclerosis. 2017;263:301–308. doi: 10.1016/j.atherosclerosis.2017.06.925. [DOI] [PubMed] [Google Scholar]
- 73.Rasmiena A.A., Barlow C.K., Stefanovic N., Huynh K., Tan R., Sharma A., et al. Plasmalogen modulation attenuates atherosclerosis in ApoE- and ApoE/GPx1-deficient mice. Atherosclerosis. 2015;243:598–608. doi: 10.1016/j.atherosclerosis.2015.10.096. [DOI] [PubMed] [Google Scholar]
- 74.Stevens V.L., Carter B.D., McCullough M.L., Campbell P.T., Wang Y. Metabolomic profiles associated with BMI, waist circumference, and diabetes and inflammation biomarkers in women. Obesity (Silver Spring) 2020;28:187–196. doi: 10.1002/oby.22670. [DOI] [PubMed] [Google Scholar]
- 75.Rong X., Albert C.J., Hong C., Duerr M.A., Chamberlain B.T., Tarling E.J., et al. LXRs regulate ER stress and inflammation through dynamic modulation of membrane phospholipid composition. Cell Metabolism. 2013;18:685–697. doi: 10.1016/j.cmet.2013.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lagrost L., Masson D. The expanding role of lyso-phosphatidylcholine acyltransferase-3 (LPCAT3), a phospholipid-remodeling enzyme, in health and disease. Current Opinion in Lipidology. 2022;33(3):193–198. doi: 10.1097/MOL.0000000000000820. [DOI] [PubMed] [Google Scholar]
- 77.Zhang Q., Yao D., Rao B., Jian L., Chen Y., Hu K., et al. The structural basis for the phospholipid remodeling by lysophosphatidylcholine acyltransferase 3. Nature Communications. 2021;12 doi: 10.1038/s41467-021-27244-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Candi E., Tesauro M., Cardillo C., Lena A.M., Schinzari F., Rodia G., et al. Metabolic profiling of visceral adipose tissue from obese subjects with or without metabolic syndrome. Biochemical Journal. 2018;475:1019–1035. doi: 10.1042/BCJ20170604. [DOI] [PubMed] [Google Scholar]
- 79.Chattopadhyay M., Khemka V.K., Chatterjee G., Ganguly A., Mukhopadhyay S., Chakrabarti S. Enhanced ROS production and oxidative damage in subcutaneous white adipose tissue mitochondria in obese and type 2 diabetes subjects. Molecular and Cellular Biochemistry. 2015;399:95–103. doi: 10.1007/s11010-014-2236-7. [DOI] [PubMed] [Google Scholar]
- 80.Pietiläinen K.H., Róg T., Seppänen-Laakso T., Virtue S., Gopalacharyulu P., Tang J., et al. Association of lipidome remodeling in the adipocyte membrane with acquired obesity in humans. PLoS Biology. 2011;9 doi: 10.1371/journal.pbio.1000623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Farooqui A.A., Horrocks L.A. Plasmalogens: workhorse lipids of membranes in normal and injured neurons and glia. The Neuroscientist. 2001;7:232–245. doi: 10.1177/107385840100700308. [DOI] [PubMed] [Google Scholar]
- 82.Palavicini J.P., Chen J., Wang C., Wang J., Qin C., Baeuerle E., et al. Early disruption of nerve mitochondrial and myelin lipid homeostasis in obesity-induced diabetes. JCI Insight. 2020;5 doi: 10.1172/jci.insight.137286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mishra P., Chan D.C. Mitochondrial dynamics and inheritance during cell division, development and disease. Nature Reviews Molecular Cell Biology. 2014;15:634–646. doi: 10.1038/nrm3877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Liesa M., Shirihai O.S. Mitochondrial dynamics in the regulation of nutrient utilization and energy expenditure. Cell Metabolism. 2013;17:491–506. doi: 10.1016/j.cmet.2013.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Cinti S. Transdifferentiation properties of adipocytes in the adipose organ. American Journal of Physiology. Endocrinology and Metabolism. 2009;297:E977–E986. doi: 10.1152/ajpendo.00183.2009. [DOI] [PubMed] [Google Scholar]
- 86.Pisani D.F., Barquissau V., Chambard J.C., Beuzelin D., Ghandour R.A., Giroud M., et al. Mitochondrial fission is associated with UCP1 activity in human brite/beige adipocytes. Molecular Metabolism. 2018;7:35–44. doi: 10.1016/j.molmet.2017.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wikstrom J.D., Mahdaviani K., Liesa M., Sereda S.B., Si Y., Las G., et al. Hormone-induced mitochondrial fission is utilized by brown adipocytes as an amplification pathway for energy expenditure. EMBO Journal. 2014;33:418–436. doi: 10.1002/embj.201385014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bchetnia M., Tardif J., Morin C., Laprise C. Expression signature of the Leigh syndrome French-Canadian type. Molecular Genetics and Metabolism Reports. 2022;30 doi: 10.1016/j.ymgmr.2022.100847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Cooper M.P., Qu L., Rohas L.M., Lin J., Yang W., Erdjument-Bromage H., et al. Defects in energy homeostasis in Leigh syndrome French Canadian variant through PGC-1alpha/LRP130 complex. Genes & Development. 2006;20:2996–3009. doi: 10.1101/gad.1483906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Berger J., Dorninger F., Forss-Petter S., Kunze M. Peroxisomes in brain development and function. Biochimica et Biophysica Acta. 2016;1863:934–955. doi: 10.1016/j.bbamcr.2015.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ruiz M., Cuillerier A., Dupuis F., Morue P., Bouchard B., Robillard-Frayne I., et al. Unravelling a role of LRPPRC in peroxisomal lipid metabolism through lipidomic investigations in human and mouse. The FASEB Journal. 2017;31:782–783. [Google Scholar]
- 92.Issemann I., Green S. Activation of a member of the steroid hormone receptor superfamily by peroxisome proliferators. Nature. 1990;347:645–650. doi: 10.1038/347645a0. [DOI] [PubMed] [Google Scholar]
- 93.Rosen E.D., Spiegelman B.M. What we talk about when we talk about fat. Cell. 2014;156:20–44. doi: 10.1016/j.cell.2013.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lefterova M.I., Zhang Y., Steger D.J., Schupp M., Schug J., Cristancho A., et al. PPARgamma and C/EBP factors orchestrate adipocyte biology via adjacent binding on a genome-wide scale. Genes & Development. 2008;22:2941–2952. doi: 10.1101/gad.1709008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Mikkelsen T.S., Xu Z., Zhang X., Wang L., Gimble J.M., Lander E.S., et al. Comparative epigenomic analysis of murine and human adipogenesis. Cell. 2010;143:156–169. doi: 10.1016/j.cell.2010.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Brites P., Ferreira A.S., da Silva T.F., Sousa V.F., Malheiro A.R., Duran M., et al. Alkyl-glycerol rescues plasmalogen levels and pathology of ether-phospholipid deficient mice. PLoS One. 2011;6 doi: 10.1371/journal.pone.0028539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Itzkovitz B., Jiralerspong S., Nimmo G., Loscalzo M., Horovitz D.D., Snowden A., et al. Functional characterization of novel mutations in GNPAT and AGPS, causing rhizomelic chondrodysplasia punctata (RCDP) types 2 and 3. Human Mutation. 2012;33:189–197. doi: 10.1002/humu.21623. [DOI] [PubMed] [Google Scholar]
- 98.Xu H.E., Li Y. Ligand-dependent and -independent regulation of PPAR gamma and orphan nuclear receptors. Science Signaling. 2008;1:pe52. doi: 10.1126/scisignal.148pe52. [DOI] [PubMed] [Google Scholar]
- 99.Lodhi I.J., Wei X., Semenkovich C.F. Lipoexpediency: de novo lipogenesis as a metabolic signal transmitter. Trends in Endocrinology and Metabolism. 2011;22:1–8. doi: 10.1016/j.tem.2010.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Davies S.S., Pontsler A.V., Marathe G.K., Harrison K.A., Murphy R.C., Hinshaw J.C., et al. Oxidized alkyl phospholipids are specific, high affinity peroxisome proliferator-activated receptor gamma ligands and agonists. Journal of Biological Chemistry. 2001;276:16015–16023. doi: 10.1074/jbc.M100878200. [DOI] [PubMed] [Google Scholar]
- 101.McIntyre T.M., Pontsler A.V., Silva A.R., St Hilaire A., Xu Y., Hinshaw J.C., et al. Identification of an intracellular receptor for lysophosphatidic acid (LPA): LPA is a transcellular PPARgamma agonist. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:131–136. doi: 10.1073/pnas.0135855100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Tsukahara T., Tsukahara R., Yasuda S., Makarova N., Valentine W.J., Allison P., et al. Different residues mediate recognition of 1-O-oleyllysophosphatidic acid and rosiglitazone in the ligand binding domain of peroxisome proliferator-activated receptor gamma. Journal of Biological Chemistry. 2006;281:3398–3407. doi: 10.1074/jbc.M510843200. [DOI] [PubMed] [Google Scholar]
- 103.Zhang C., Baker D.L., Yasuda S., Makarova N., Balazs L., Johnson L.R., et al. Lysophosphatidic acid induces neointima formation through PPARgamma activation. Journal of Experimental Medicine. 2004;199:763–774. doi: 10.1084/jem.20031619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Homan E.A., Kim Y.G., Cardia J.P., Saghatelian A. Monoalkylglycerol ether lipids promote adipogenesis. Journal of the American Chemical Society. 2011;133:5178–5181. doi: 10.1021/ja111173c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Yu H., Dilbaz S., Coßmann J., Hoang A.C., Diedrich V., Herwig A., et al. Breast milk alkylglycerols sustain beige adipocytes through adipose tissue macrophages. Journal of Clinical Investigation. 2019;129:2485–2499. doi: 10.1172/JCI125646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Dreyer C., Keller H., Mahfoudi A., Laudet V., Krey G., Wahli W. Positive regulation of the peroxisomal beta-oxidation pathway by fatty acids through activation of peroxisome proliferator-activated receptors (PPAR) Biology of the Cell. 1993;77:67–76. doi: 10.1016/s0248-4900(05)80176-5. [DOI] [PubMed] [Google Scholar]
- 107.McGarry J.D., Mannaerts G.P., Foster D.W. A possible role for malonyl-CoA in the regulation of hepatic fatty acid oxidation and ketogenesis. Journal of Clinical Investigation. 1977;60:265–270. doi: 10.1172/JCI108764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Grabner G.F., Xie H., Schweiger M., Zechner R. Lipolysis: cellular mechanisms for lipid mobilization from fat stores. Nature Metabolism. 2021;3:1445–1465. doi: 10.1038/s42255-021-00493-6. [DOI] [PubMed] [Google Scholar]
- 109.Lee K.Y., Russell S.J., Ussar S., Boucher J., Vernochet C., Mori M.A., et al. Lessons on conditional gene targeting in mouse adipose tissue. Diabetes. 2013;62:864–874. doi: 10.2337/db12-1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Chakrabarti P., Kandror K.V. FoxO1 controls insulin-dependent adipose triglyceride lipase (ATGL) expression and lipolysis in adipocytes. Journal of Biological Chemistry. 2009;284:13296–13300. doi: 10.1074/jbc.C800241200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.I O.S., Zhang W., Wasserman D.H., Liew C.W., Liu J., Paik J., et al. FoxO1 integrates direct and indirect effects of insulin on hepatic glucose production and glucose utilization. Nature Communications. 2015;6:7079. doi: 10.1038/ncomms8079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Zhao J., Wu Y., Rong X., Zheng C., Guo J. Anti-lipolysis induced by insulin in diverse pathophysiologic conditions of adipose tissue. Diabetes, Metabolic Syndrome and Obesity: Targets and Therapy. 2020;13:1575–1585. doi: 10.2147/DMSO.S250699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Edgerton D.S., Kraft G., Smith M., Farmer B., Williams P.E., Coate K.C., et al. Insulin's direct hepatic effect explains the inhibition of glucose production caused by insulin secretion. JCI Insight. 2017;2 doi: 10.1172/jci.insight.91863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Perry R.J., Camporez J.G., Kursawe R., Titchenell P.M., Zhang D., Perry C.J., et al. Hepatic acetyl CoA links adipose tissue inflammation to hepatic insulin resistance and type 2 diabetes. Cell. 2015;160:745–758. doi: 10.1016/j.cell.2015.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Hamel F.G., Bennett R.G., Upward J.L., Duckworth W.C. Insulin inhibits peroxisomal fatty acid oxidation in isolated rat hepatocytes. Endocrinology. 2001;142:2702–2706. doi: 10.1210/endo.142.6.8178. [DOI] [PubMed] [Google Scholar]
- 116.Ling A.V., Gearing M.E., Semova I., Shin D.J., Clements R., Lai Z.W., et al. FoxO1 is required for most of the metabolic and hormonal perturbations produced by hepatic insulin receptor deletion in male mice. Endocrinology. 2018;159:1253–1263. doi: 10.1210/en.2017-00870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ipsen D.H., Lykkesfeldt J., Tveden-Nyborg P. Molecular mechanisms of hepatic lipid accumulation in non-alcoholic fatty liver disease. Cellular and Molecular Life Sciences. 2018;75:3313–3327. doi: 10.1007/s00018-018-2860-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Schulze R.J., Sathyanarayan A., Mashek D.G. Breaking fat: the regulation and mechanisms of lipophagy. Biochimica et Biophysica Acta (BBA) - Molecular and Cell Biology of Lipids. 2017;1862:1178–1187. doi: 10.1016/j.bbalip.2017.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Marino G., Pietrocola F., Eisenberg T., Kong Y., Malik S.A., Andryushkova A., et al. Regulation of autophagy by cytosolic acetyl-coenzyme A. Molecular Cell. 2014;53:710–725. doi: 10.1016/j.molcel.2014.01.016. [DOI] [PubMed] [Google Scholar]
- 120.Simcox J., Lamming D.W. The central moTOR of metabolism. Developmental Cell. 2022;57:691–706. doi: 10.1016/j.devcel.2022.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Gosis B.S., Wada S., Thorsheim C., Li K., Jung S., Rhoades J.H., et al. Inhibition of nonalcoholic fatty liver disease in mice by selective inhibition of mTORC1. Science. 2022;376 doi: 10.1126/science.abf8271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Zeng J., Deng S., Wang Y., Li P., Tang L., Pang Y. Specific inhibition of acyl-CoA oxidase-1 by an acetylenic acid improves hepatic lipid and reactive oxygen species (ROS) metabolism in rats fed a high fat diet. Journal of Biological Chemistry. 2017;292:3800–3809. doi: 10.1074/jbc.M116.763532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Norseth J., Christophersen B.O. Chain shortening of erucic acid in isolated liver cells. FEBS Letters. 1978;88:353–357. doi: 10.1016/0014-5793(78)80210-5. [DOI] [PubMed] [Google Scholar]
- 124.Ellinghaus P., Wolfrum C., Assmann G., Spener F., Seedorf U. Phytanic acid activates the peroxisome proliferator-activated receptor alpha (PPARalpha) in sterol carrier protein 2-/sterol carrier protein x-deficient mice. Journal of Biological Chemistry. 1999;274:2766–2772. doi: 10.1074/jbc.274.5.2766. [DOI] [PubMed] [Google Scholar]
- 125.Hayashi H., Oohashi M. Incorporation of acetyl-CoA generated from peroxisomal beta-oxidation into ethanolamine plasmalogen of rat liver. Biochimica et Biophysica Acta. 1995;1254:319–325. doi: 10.1016/0005-2760(94)00194-4. [DOI] [PubMed] [Google Scholar]
- 126.Zhang X., Wang Y., Yao H., Deng S., Gao T., Shang L., et al. Peroxisomal β-oxidation stimulates cholesterol biosynthesis in the liver in diabetic mice. Journal of Biological Chemistry. 2022;298 doi: 10.1016/j.jbc.2022.101572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Asayama K., Sandhir R., Sheikh F.G., Hayashibe H., Nakane T., Singh I. Increased peroxisomal fatty acid beta-oxidation and enhanced expression of peroxisome proliferator-activated receptor-alpha in diabetic rat liver. Molecular and Cellular Biochemistry. 1999;194:227–234. doi: 10.1023/a:1006930513476. [DOI] [PubMed] [Google Scholar]
- 128.Cassader M., Ruiu G., Gambino R., Alemanno N., Veglia F., Pagano G. Hypercholesterolemia in non-insulin-dependent diabetes mellitus: different effect of simvastatin on VLDL and LDL cholesterol levels. Atherosclerosis. 1993;99:47–53. doi: 10.1016/0021-9150(93)90049-z. [DOI] [PubMed] [Google Scholar]
- 129.Horie S., Ishii H., Suga T. Changes in peroxisomal fatty acid oxidation in the diabetic rat liver. Journal of Biochemistry. 1981;90:1691–1696. doi: 10.1093/oxfordjournals.jbchem.a133645. [DOI] [PubMed] [Google Scholar]
- 130.Ji L.L., Yeo D. Oxidative stress: an evolving definition. Faculty Reviews. 2021;10:13. doi: 10.12703/r/10-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Furukawa S., Fujita T., Shimabukuro M., Iwaki M., Yamada Y., Nakajima Y., et al. Increased oxidative stress in obesity and its impact on metabolic syndrome. Journal of Clinical Investigation. 2004;114:1752–1761. doi: 10.1172/JCI21625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Frohnert B.I., Sinaiko A.R., Serrot F.J., Foncea R.E., Moran A., Ikramuddin S., et al. Increased adipose protein carbonylation in human obesity. Obesity (Silver Spring) 2011;19:1735–1741. doi: 10.1038/oby.2011.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Masschelin P.M., Cox A.R., Chernis N., Hartig S.M. The impact of oxidative stress on adipose tissue energy balance. Frontiers in Physiology. 2019;10:1638. doi: 10.3389/fphys.2019.01638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.May J.M., de Haën C. Insulin-stimulated intracellular hydrogen peroxide production in rat epididymal fat cells. Journal of Biological Chemistry. 1979;254:2214–2220. [PubMed] [Google Scholar]
- 135.Rudich A., Tirosh A., Potashnik R., Hemi R., Kanety H., Bashan N. Prolonged oxidative stress impairs insulin-induced GLUT4 translocation in 3T3-L1 adipocytes. Diabetes. 1998;47:1562–1569. doi: 10.2337/diabetes.47.10.1562. [DOI] [PubMed] [Google Scholar]
- 136.Jankovic A., Korac A., Buzadzic B., Otasevic V., Stancic A., Daiber A., et al. Redox implications in adipose tissue (dys)function--A new look at old acquaintances. Redox Biology. 2015;6:19–32. doi: 10.1016/j.redox.2015.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Newsholme P., Cruzat V.F., Keane K.N., Carlessi R., de Bittencourt P.I., Jr. Molecular mechanisms of ROS production and oxidative stress in diabetes. Biochemical Journal. 2016;473:4527–4550. doi: 10.1042/BCJ20160503C. [DOI] [PubMed] [Google Scholar]
- 138.Zhou L., Yu M., Arshad M., Wang W., Lu Y., Gong J., et al. Coordination among lipid droplets, peroxisomes, and mitochondria regulates energy expenditure through the CIDE-ATGL-PPARα pathway in adipocytes. Diabetes. 2018;67:1935–1948. doi: 10.2337/db17-1452. [DOI] [PubMed] [Google Scholar]
- 139.Xie W.D., Wang H., Zhang J.F., Li J.N., Can Y., Qing L., et al. Enhanced peroxisomal β-oxidation metabolism in visceral adipose tissues of high-fat diet-fed obesity-resistant C57BL/6 mice. Experimental and Therapeutic Medicine. 2011;2:309–315. doi: 10.3892/etm.2011.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Piao L., Dorotea D., Jiang S., Koh E.H., Oh G.T., Ha H. Impaired peroxisomal fitness in obese mice, a vicious cycle exacerbating adipocyte dysfunction via oxidative stress. Antioxidants and Redox Signaling. 2019;31:1339–1351. doi: 10.1089/ars.2018.7614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Okuno Y., Fukuhara A., Hashimoto E., Kobayashi H., Kobayashi S., Otsuki M., et al. Oxidative stress inhibits healthy adipose expansion through suppression of SREBF1-mediated lipogenic pathway. Diabetes. 2018;67:1113–1127. doi: 10.2337/db17-1032. [DOI] [PubMed] [Google Scholar]
- 142.Carpentier A., Mittelman S.D., Lamarche B., Bergman R.N., Giacca A., Lewis G.F. Acute enhancement of insulin secretion by FFA in humans is lost with prolonged FFA elevation. American Journal of Physiology. 1999;276:E1055–E1066. doi: 10.1152/ajpendo.1999.276.6.E1055. [DOI] [PubMed] [Google Scholar]
- 143.Violante S., Achetib N., van Roermund C.W.T., Hagen J., Dodatko T., Vaz F.M., et al. Peroxisomes can oxidize medium- and long-chain fatty acids through a pathway involving ABCD3 and HSD17B4. The FASEB Journal. 2019;33:4355–4364. doi: 10.1096/fj.201801498R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Kunau W.H., Dommes V., Schulz H. beta-oxidation of fatty acids in mitochondria, peroxisomes, and bacteria: a century of continued progress. Progress in Lipid Research. 1995;34:267–342. doi: 10.1016/0163-7827(95)00011-9. [DOI] [PubMed] [Google Scholar]
- 145.Pullen T.J., Rutter G.A. When less is more: the forbidden fruits of gene repression in the adult β-cell. Diabetes, Obesity and Metabolism. 2013;15:503–512. doi: 10.1111/dom.12029. [DOI] [PubMed] [Google Scholar]
- 146.Baboota R.K., Shinde A.B., Lemaire K., Fransen M., Vinckier S., Van Veldhoven P.P., et al. Functional peroxisomes are required for β-cell integrity in mice. Molecular Metabolism. 2019;22:71–83. doi: 10.1016/j.molmet.2019.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Elsner M., Gehrmann W., Lenzen S. Peroxisome-generated hydrogen peroxide as important mediator of lipotoxicity in insulin-producing cells. Diabetes. 2011;60:200–208. doi: 10.2337/db09-1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Stamateris R.E., Sharma R.B., Kong Y., Ebrahimpour P., Panday D., Ranganath P., et al. Glucose induces mouse β-cell proliferation via IRS2, MTOR, and cyclin D2 but not the insulin receptor. Diabetes. 2016;65:981–995. doi: 10.2337/db15-0529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Hamanaka R.B., Chandel N.S. Mitochondrial reactive oxygen species regulate cellular signaling and dictate biological outcomes. Trends in Biochemical Sciences. 2010;35:505–513. doi: 10.1016/j.tibs.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.