Abstract
Background
Myostatin is a negative regulator of skeletal muscle mass. On the other hand, growth differentiation factor (GDF)-15 is associated with lower muscle strength and muscle mass. We investigated the relationship between serum GDF-15, myostatin, and sarcopenia in patients receiving cardiovascular surgery through a ROC curve and a multivariate regression analysis.
Methods
Skeletal muscle mass index (SMI) by bioelectrical impedance analysis, hand-grip strength, knee extension strength, and walking speed were measured. Preoperative serum GDF-15 and myostatin levels were determined by ELISA. The sarcopenia index could be expressed as: −0.0042 × [myostatin] + 0.0007 × [GDF-15] + 0.0890 × age + 1.4030 × sex − 0.2679 × body mass index (BMI) − 2.1186. A ROC curve was plotted to identify the optimal cutoff level of the sarcopenia index to detect sarcopenia.
Results
120 patients receiving cardiovascular surgery were included in the study. SMI, hand-grip strength, knee extension strength, and walking speed inversely correlated with GDF-15, but positively correlated with myostatin. In the multivariate stepwise regression analysis, SMI was a determinant of myostatin, and both GDF-15 and myostatin were determinants of SMI and muscle thickness, even after adjustment for age, sex, and BMI. A ROC curve showed that the sarcopenia index was a determinant of sarcopenia (cutoff value −1.0634, area under the curve 0.901, sensitivity 96.9%, specificity 70.9%).
Conclusion
GDF-15 and myostatin are associated with skeletal muscle volume in patients receiving cardiovascular surgery, but these associations are different. The sarcopenia index calculated from GDF-15 and myostatin levels may be a biomarker of sarcopenia.
Keywords: Myostatin, Growth differentiation factor-15, Sarcopenia, Biomarker
1. Introduction
The progressive age-related loss of skeletal muscle mass, strength, and muscle function, termed sarcopenia, is a major threat to self-sufficiency and quality of life [1]. Sarcopenia is also associated with frequent complications and increased mortality in patients with cardiovascular disease [2], [3], [4]. Sarcopenia is diagnosed by the decrease of skeletal muscle mass index (SMI) and decreased grip strength or walking speed. However, measuring SMI is difficult for the general internist because it requires special equipment for bioelectrical impedance testing or dual-energy X-ray absorptiometry.
Growth differentiation factor (GDF)-15 is an independent determinant of prognosis in healthy subjects [5], [6] and heart failure patients [7]. Myostatin, also called GDF-8, is a different member of the GDF family. Myostatin is a robust regulator of muscle development and postnatal growth [1], and maintains skeletal muscle mass and strength in patients with heart failure [8], obesity [9] and renal dysfunction [10]. A multivariate analysis showed that serum myostatin levels were independently associated with muscle wasting in heart failure patients [8]. The study also supported the role of myostatin on role in maintaining skeletal muscle mass and strength in heart failure.
In a study that evaluated the association between serum adiponectin and myostatin in obese patients and used body composition and metabolic indices to identify independent factors, serum adiponectin levels were associated with lower muscle strength and serum myostatin with higher appendicular lean mass [9]. In other research, non-dialysis-dependent renal disease patients were randomly assigned to either strength exercise or balance exercise in addition to endurance training. Regardless of age or comorbidities, plasma myostatin levels increased significantly in both groups, with a significant difference in favor of the strength group [10]. The study also showed that plasma myostatin was significantly positively associated with muscle mass and physical activity before training. These recent reports indicate that myostatin is a positive regulator, in contrast to the conventional view of myostatin as a negative regulator of the maintenance of muscle function. Furthermore, there are reports that GDF-15 and myostatin may be biomarkers of skeletal muscle mass or sarcopenia [11].
Recently, a sarcopenia index calculated from five factors including adiponectin was reported to be highly accurate for the diagnosis of sarcopenia in patients with cardiovascular disease [12]. However, no study has examined a sarcopenia index including GDF-15 and myostatin. Thus, there is a need to explore a novel, simple diagnostic method of sarcopenia assessment that includes GDF-15 and myostatin in preoperative cardiovascular patients. The purpose of this study was to determine the relationship between GDF-15, myostatin and sarcopenia in patients receiving cardiovascular surgery through a ROC curve and a multivariate regression analysis.
2. Methods
2.1. Patients
One hundred twenty patients receiving cardiovascular surgery (72 men [60 %]) at Dokkyo Medical Hospital from October 2015 to April 2018 were included in this study. The patient characteristics are summarized in Table 1. The Regional Ethics Committee of Dokkyo Medical University approved the study protocol (approval number: 27077), which was conducted according to the Declaration of Helsinki. Each patient provided written consent.
Table 1.
Characteristics of patients.
| Number | 120 |
|---|---|
| Male / Female | 72 (60 %) / 48 (40 %) |
| Age, years | 72.0 [66.0–78.8] |
| BMI, kg/m2 | 23.9 ± 4.0 |
| Atrial fibrillation | 6 (5 %) |
| NYHA | 2.0 [1.0–3.0] |
| Risk factors, number | |
| Hypertension | 87 (73 %) |
| Diabetes | 41 (34 %) |
| Dyslipidemia | 59 (49 %) |
| Chronic kidney disease | 63 (53 %) |
| Cardiovascular surgery, number | |
| CABG | 29 (24 %) |
| AS (SAVR or TAVR) | 24 (20 %) |
| AR (SAVR) | 5 (4 %) |
| MR (MVR, MVP) | 16 (13 %) |
| MS (MVR) | 1 (1 %) |
| CABG combined with a valve procedure (SAVR, MVR, MVP) | 13 (11 %) |
| SAVR (AS, AR) with MVR or MVP (MR) | 7 (6 %) |
| Aortic disease (AAR, TAR, HAR, etc.) | 9 (8 %) |
| Others | 16 (13 %) |
| Drugs, number | |
| β-blockers | 63 (53 %) |
| Ca-blockers | 48 (40 %) |
| ACEI/ARB | 68 (57 %) |
| Statin | 59 (49 %) |
| Anti-diabetic drugs | 32 (27 %) |
| eGFR, mL/min/1.73 m2 | 57.1 ± 27.3 |
| BNP, pg/mL | 160.6 [66.4–452.2] |
| HbA1c, % | 5.9 [5.6–6.4] |
| hsCRP, mg/L | 0.15 [0.05–0.71] |
The data are shown as mean ± standard deviation, or, where indicated, median and interquartile range. Categorical variables were expressed as number and percentages. Number, number of patients with a given characteristic. BMI, body mass index; NYHA, New York Heart Association; CABG, coronary artery bypass grafting; AS, aortic stenosis; SAVR, surgical aortic valve replacement; TAVR, transcatheter aortic valve replacement; AR, aortic regurgitation; MR, mitral regurgitation; MVR, mitral valve replacement; MVP, mitral valve plasty; MS, mitral stenosis; AAR, ascending aorta replacement; TAR, total arch replacement; HAR, hemiarch replacement; ACEI, angiotensin converting enzyme inhibitor; ARB, angiotensin II receptor blocker; anti-diabetic drugs (i.e., α-glucosidase inhibitor, sulfonylurea, biguanide, dipeptidyl peptidase-4 inhibitor, sodium glucose cotransporter 2 inhibitor); eGFR, estimated glomerular filtration rate; BNP, brain natriuretic peptide; HbA1c, hemoglobin A1c; hsCRP, high-sensitivity C-reactive protein.
Fasting blood samples were obtained in tubes containing sodium EDTA and in polystyrene tubes without an anticoagulant. Plasma was immediately separated by centrifugation at 3000 rpm at 4 °C for 10 min, and serum was collected by centrifugation at 1000 rpm at room temperature for 10 min. Brain natriuretic peptide (BNP), estimated glomerular filtration rate (eGFR), albumin (Alb), hemoglobin (Hb), high-density lipoprotein cholesterol (HDL-C), and triglycerides (TG) were measured before the operations. The biochemical data were analyzed using routine chemical methods in the Dokkyo Medical University Hospital clinical laboratory. Levels of the inflammatory marker high-sensitivity C-reactive protein (hsCRP) were measured by a latex-enhanced nephelometric immunoassay (N Latex CRP Ⅱ, Dade Behring ltd., Tokyo, Japan).
To measure GDF-15 and myostatin levels, blood samples were drawn into pyrogen-free tubes without EDTA on the morning of cardiovascular surgery. The serum was stored in aliquots at −80 °C for all enzyme-linked immunosorbent assays (ELISAs).
2.2. Enzyme-Linked immunosorbent assay (ELISA)
Serum GDF-15 levels were measured by the Human GDF-15 Quantikine ELISA Kit (DGD150, R&D Systems, Inc., Minneapolis, MN, USA) as previously described [13]. The detection threshold of GDF-15 was 2.0 pg/mL. The serum concentrations of myostatin were measured using the GDF-8/Myostatin Quantikine ELISA kit (DGDF80, R&D Systems, Inc., Minneapolis, MN, USA), and the detection threshold was 2.25 pg/mL.
2.3. Bioelectrical impedance analyzer (BIA) measurements
Body composition was measured with a multi-frequency bioelectrical impedance analyzer (BIA; InBody S10 Biospace, Biospace Co. Ltd., Korea; Model JMW 140) while the patient was in a supine position within days before cardiovascular surgery, as previously described [13], [14]. Body fat volume, body fat percentage, skeletal muscle volume, and SMI were measured. Hand-grip strength for the right hand and knee extension strength for the right leg were measured twice, and the higher value was adopted. Walking speed was measured as the time needed to walk 4 m. The evaluation of sarcopenia was based on the Asian Working Group for Sarcopenia criteria (hand-grip < 26 kgf or walking speed ≤ 0.8 m/s, and SMI < 7.0 kg/m2 for men; hand-grip < 18 kgf or walking speed ≤ 0.8 m/s, and SMI < 5.7 kg/m2 for women).
2.4. Muscle size
Muscle thickness of thigh (MTH) was measured at the midpoint of the thigh length with a real-time linear electronic scanner using a 10 MHz scanning head and ultrasound probe (L4-12 t-RS Probe, GE Healthcare, Tokyo, Japan) and ultrasound (LOGIQ e, GE Healthcare, Tokyo, Japan) within days before surgery, as previously described [13]. The perpendicular distance from the adipose tissue-muscle interface to the muscle-bone interface was considered to represent MTH, measured in the supine position. The measurement was performed twice on the right thigh and the average of the two values was used in the analysis.
2.5. Statistical analysis
All data are presented as the mean ± standard deviation, median and interquartile range, or proportion depending on their distributions. Data normality was evaluated using the Kolmogorov-Smirnov test. Patient characteristics and baseline data were compared between groups using an independent t-test or Mann-Whitney U test. Associations between GDF-15 and myostatin and clinical data were evaluated with Spearman correlation coefficients. Multivariate stepwise regression analysis was used for determining independent predictors of GDF-15, myostatin, SMI, and MTH; the independent variables were factors that had a significant correlation with GDF-15, myostatin, SMI, and MTH, along with the adjustment factors. The sarcopenia index was calculated from logistic regression with the presence of sarcopenia as the dependent factor, and GDF-15, myostatin, age, sex, and BMI as independent factors. A receiver operating characteristic (ROC) curve was plotted to identify an optimal cutoff level of this sarcopenia index to detect sarcopenia. All statistical analyses were performed with SPSS version 28 for Windows (IBM Corp., New York, U.S.A.). A p value of < 0.05 was regarded as significant.
3. Results
The mean age was 72.0 [66.0–78.8] years, and the mean body mass index (BMI) was 23.9 ± 4.0 kg/m2. Most of the patients had expected risk factors such as hypertension, diabetes, dyslipidemia and chronic kidney disease. Patients underwent coronary artery bypass grafting (CABG, n = 29 [24 %]), conventional surgical aortic valve replacement (SAVR, n = 19), transcatheter aortic valve replacement (TAVR, n = 10 [8 %]), mitral valve plasty (MVP, n = 13 [11 %]), mitral valve replacement (MVR, n = 4 [3 %]), CABG combined with a valve procedure (AVR, MVP, or MVR, n = 13 [11 %]), SAVR with MVR or MVP (n = 7 [6 %]), aortic disease surgery (n = 9 [8 %]), or other procedures (n = 16 [13 %]). All patients received medical treatment including β-blockers (53 %), calcium-channel blockers (40 %), angiotensin Ⅱreceptor blockers (ARB) / angiotensin converting enzyme inhibitors (ACEI) (57 %), statins (49 %), and anti-diabetic drugs (27 %) (Table 1).
Table 2 shows a comparison of various indices between male and female patients. The mean age was lower in men than in women (68.5 [64.0–76.0] vs 74.0 [69.0–80.0] years, p = 0.005). The mean body weight was higher in men than in women (65.9 ± 13.2 vs 52.0 ± 10.6 kg, p < 0.001). The mean hand-grip strength (26.7 ± 8.4 vs 16.5 ± 5.1 kgf, p < 0.001), walking speed (0.99 ± 0.32 vs 0.83 ± 0.28 m/s, p = 0.019), knee extension (24.2 ± 10.1 vs 16.6 ± 7.4 kgf, p < 0.001), skeletal muscle mass (24.7 ± 4.1 vs 16.8 ± 3.0 kg, p < 0.001) and SMI (7.15 ± 1.21 vs 5.43 ± 0.92 kg/m2, p < 0.001) were higher in men than in women. The body fat percentage was lower in men than in women (27.4 ± 7.5 vs 37.3 ± 7.9 %, p < 0.001). The mean GDF-15 concentration was not significantly different between the sexes (1361 [948–3395] vs 1188 [703–1763] pg/mL, p = 0.092), but the myostatin concentration was higher in men than in women (544 ± 259 vs 289 [247–389] pg/mL, p < 0.001).
Table 2.
Comparison of clinical data between male and female patients.
| Male, n = 72 | Female, n = 48 | p value | |
|---|---|---|---|
| Age (years) | 68.5 [64.0–76.0] | 74.0 [69.0–80.0] | 0.005** |
| Body weight (kg) | 65.9 ± 13.2 | 52.0 ± 10.6 | <0.001*** |
| BMI (kg/m2) | 23.4 [21.7–26.4] | 23.5 ± 3.9 | 0.528 |
| Hand-grip strength (kgf) | 26.7 ± 8.4 | 16.5 ± 5.1 | <0.001*** |
| Walking speed (m/s) | 0.99 ± 0.32 | 0.83 ± 0.28 | 0.019* |
| Knee extension strength (kgf) | 24.2 ± 10.1 | 16.6 ± 7.4 | <0.001*** |
| MTH (cm) | 2.36 ± 0.80 | 2.15 ± 0.65 | 0.211 |
| Skeletal muscle mass (kg) | 24.7 ± 4.1 | 16.8 ± 3.0 | <0.001*** |
| SMI (kg/m2) | 7.15 ± 1.21 | 5.43 ± 0.92 | <0.001*** |
| Body fat mass (kg) | 17.5 [12.7–21.8] | 20.4 ± 6.2 | 0.068 |
| Body fat percentage (%) | 27.4 ± 7.5 | 37.3 ± 7.9 | <0.001*** |
| GDF-15 (pg/mL) | 1361 [948–3395] | 1188 [703–1763] | 0.092 |
| Myostatin (pg/mL) | 544 ± 259 | 289 [247–389] | <0.001*** |
| BNP (pg/mL) | 163.8 [64.2–488.4] | 160.6 [70.7–350.9] | 0.991 |
| hsCRP (mg/L) | 0.15 [0.05–0.76] | 0.15 [0.03–0.67] | 0.287 |
| eGFR (mL/min/1.73 m2) | 55.4 ± 30.1 | 60.7 ± 22.9 | 0.280 |
| Alb (g/dL) | 3.9 [3.3–4.3] | 3.9 ± 0.6 | 0.169 |
| Hb (g/dL) | 12.4 ± 2.1 | 11.9 [10.5–13.2] | 0.273 |
Data are shown as mean ± standard deviation, or, where indicated, median and interquartile range. *, p < 0.05, **, p < 0.01, ***, p < 0.001. BMI, body mass index; MTH, muscle thickness; SMI, skeletal muscle mass index; GDF, growth differentiation factor; BNP, brain natriuretic peptide, hsCRP, high-sensitivity C-reactive protein; eGFR, estimated glomerular filtration rate; Alb, albumin; Hb, hemoglobin.
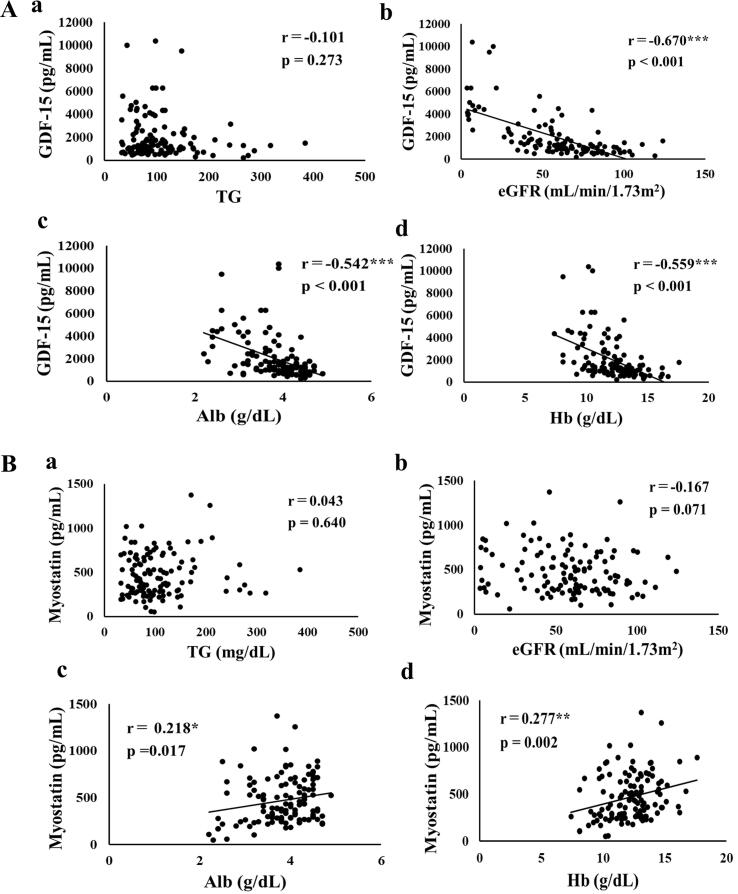
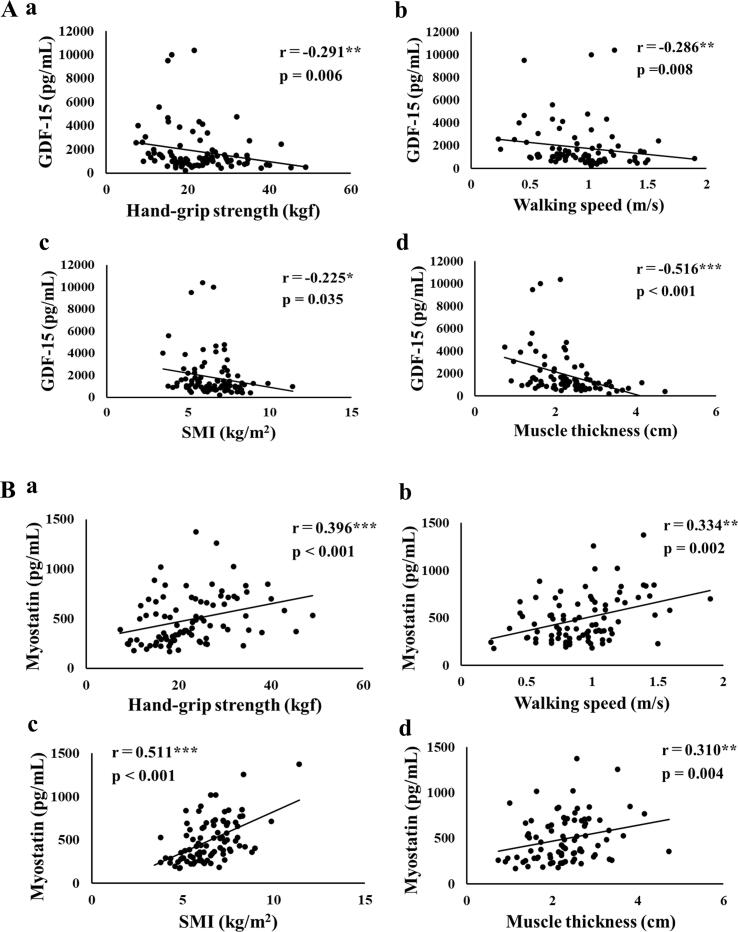
Fig. 1 and Fig. 2 show the correlation coefficients and p values of serum GDF-15 and myostatin with clinical data. GDF-15 was significantly negatively correlated with eGFR (r = -0.670, p < 0.001, Fig. 1Ab), Alb (r = -0.542, p < 0.001, Fig. 1Ac), Hb (r = -0.559, p < 0.001, Fig. 1Ad), hand-grip strength (r = -0.291, p = 0.006, Fig. 2Aa), walking speed (r = -0.286, p = 0.008, Fig. 2Ab), SMI (r = -0.225, p = 0.035, Fig. 2Ac), and MTH (r = -0.516, p < 0.001, Fig. 2Ad).
Fig. 1.
A. Correlation between serum concentrations of GDF-15 and (a) TG, (b) eGFR, (c) Alb, and (d) Hb. B. Correlation between serum concentrations of myostatin and (a) TG, (b) eGFR, (c) Alb, and (d) Hb. *, p < 0.05, **, p < 0.01, ***, p < 0.001.
Fig. 2.
Correlation between clinical data and serum concentration of GDF-15 and myostatin. A. Relationships between serum concentration of GDF-15, and (a) handgrip strength, (b) walking speed, (c) SMI, and (d) muscle thickness. B. Relationships between serum concentration of myostatin, and (a) handgrip strength, (b) walking speed, (c) SMI, and (d) muscle thickness. *, p < 0.05, **, p < 0.01, ***, p < 0.001.
On the other hand, myostatin was positively correlated with Alb (r = 0.218, p = 0.017, Fig. 1Bc), Hb (r = 0.277, p = 0.002, Fig. 1Bd), hand-grip strength (r = 0.396, p < 0.001, Fig. 2Ba), walking speed (r = 0.334, p = 0.002, Fig. 2Bb), SMI (r = 0.511, p < 0.001, Fig. 2Bc), and MTH (r = 0.310, p = 0.004, Fig. 2Bd).
Multivariate stepwise regression analysis with serum GDF-15 levels as the dependent variable and clinical data (hsCRP, eGFR, Hb, Alb, and BNP) as independent variables was performed for all patients as shown in Table 3A. Multivariate regression analysis (Table 3A) showed that eGFR (β = -0.482, p < 0.001), Hb (β = -0.302, p < 0.001), and BNP (β = 0.156, p = 0.027) were independent variables to predict serum GDF-15 levels after adjusting for age, sex, and BMI. Multivariate stepwise regression analysis between myostatin and the clinical data (hand-grip strength, knee extension strength, walking speed, MTH, skeletal muscle mass, SMI and body fat percentage) was performed as shown in Table 3B. Multivariate regression analysis showed that SMI (β = 0.457, p = 0.010) was an independent variable to predict myostatin after adjusting for age, sex, and BMI.
Table 3.
Multivariate stepwise analyses to determine serum GDF-15 and myostatin concentration and muscle mass.
| A: Multivariate stepwise analysis of serum GDF-15 concentration and clinical data | ||
|---|---|---|
| Dependent variable: GDF-15 | Adjusted R2: Model 1, 0.536; Model 2, 0.565 |
|
| Model 1 | Model 2 | |
| Independent variable | β value (p) | β value (p) |
| hsCRP | 0.115 (0.093) | 0.096 (0.160) |
| eGFR | −0.494*** (<0.001) | −0.482*** (<0.001) |
| Hb | −0.288*** (<0.001) | −0.302*** (<0.001) |
| Alb | −0.145 (0.093) | −0.070 (0.433) |
| BNP | 0.197** (0.005) | 0.156* (0.027) |
| B: Multivariate stepwise analysis of serum myostatin concentration and clinical data | ||
| Dependent variable: myostatin | Adjusted R2: Model 1, 0.330; Model 2, 0.304 |
|
| Model 1 | Model 2 | |
| Independent variable | β value (p) | β value (p) |
| Hand-grip strength | −0.074 (0.569) | −0.133 (0.361) |
| Knee extension strength | −0.156 (0.190) | −0.169 (0.166) |
| Walking speed | 0.195 (0.087) | 0.218 (0.065) |
| MTH | −0.029 (0.823) | −0.010 (0.948) |
| Skeletal muscle mass | −0.028 (0.902) | −0.221 (0.498) |
| SMI | 0.481*** (<0.001) | 0.457* (0.010) |
| Body fat percentage | −0.255* (0.016) | −0.160 (0.395) |
| C: Multivariate stepwise analysis of (a) SMI and (b) MTH and clinical data (a) | ||
| Dependent variable: SMI | Adjusted R2: Model 1, 0.343; Model 2, 0.704 |
|
| Model 1 | Model 2 | |
| Independent variable | β value (p) | β value (p) |
| GDF-15 | −0.266** (0.004) | −0.227** (0.001) |
| Myostatin (b) |
0.586*** (<0.001) | 0.341*** (<0.001) |
| Dependent variable: MTH Adjusted R2: Model 1, 0.254; Model 2, 0.510 | ||
| Model 1 | Model 2 | |
| Independent variable | β value (p) | β value (p) |
| GDF-15 | −0.433*** (<0.001) | −0.270** (0.002) |
| Myostatin | 0.369*** (<0.001) | 0.234** (0.008) |
*, p < 0.05, **, p < 0.01, ***, p < 0.001. Model 1, unadjusted; Model 2, adjusted by age, sex, and body mass index. SMI, skeletal muscle mass index, hsCRP, high-sensitivity C-reactive protein; eGFR, estimated glomerular filtration rate; Hb, hemoglobin; Alb, albumin; BNP, brain natriuretic peptide; MTH, muscle thickness.
In contrast, multivariate stepwise regression analysis showed that GDF-15 (β = -0.227, p = 0.001) and myostatin (β = 0.341, p < 0.001) were independent determinants of SMI (Table 3Ca), and GDF-15 (β = -0.270, p = 0.002) and myostatin (β = 0.234, p = 0.008) were independent determinants of MTH (Table 3Cb), even adjusting for age, sex, and BMI.
Table 4 shows a comparison of various indicators of patients with and without sarcopenia overall. Compared to no sarcopenia, the mean age was significantly higher in those with sarcopenia (77.0 [72.8–80.0] vs 68.0 [63.0–74.0] years, p < 0.001) and the mean BMI was lower (22.6 ± 3.7 vs 25.8 ± 4.0 kg/m2, p < 0.001). Among patients with sarcopenia, the mean hand-grip strength (16.3 ± 5.1 vs 26.8 ± 8.1 kgf, p < 0.001), walking speed (0.73 ± 0.28 vs 1.02 [0.88–1.15] m/s, p < 0.001), knee extension strength (15.3 ± 6.2 vs 24.8 ± 9.3 kgf, p < 0.001), MTH (1.74 ± 0.50 vs 2.63 ± 0.62 cm, p < 0.001), skeletal muscle mass (17.4 ± 3.5 vs 23.8 ± 4.7 kg, p < 0.001), and SMI (5.27 ± 0.85 vs 7.16 ± 1.14 kg/m2, p < 0.001) were significantly lower.
Table 4.
Comparison of clinical data between patients with and without sarcopenia.
| Sarcopenia (+), n = 34 | Sarcopenia (-), n = 55 | p value | |
|---|---|---|---|
| Age (years) | 77.0 [72.8–80.0] | 68.0 [63.0–74.0] | <0.001*** |
| BMI (kg/m2) | 22.6 ± 3.7 | 25.8 ± 4.0 | <0.001*** |
| Hand-grip strength (kgf) | 16.3 ± 5.1 | 26.8 ± 8.1 | <0.001*** |
| Walking speed (m/s) | 0.73 ± 0.28 | 1.02 [0.88–1.15] | <0.001*** |
| Knee extension strength (kgf) | 15.3 ± 6.2 | 24.8 ± 9.3 | <0.001*** |
| MTH (cm) | 1.74 ± 0.50 | 2.63 ± 0.62 | <0.001*** |
| Skeletal muscle mass (kg) | 17.4 ± 3.5 | 23.8 ± 4.7 | <0.001*** |
| SMI (kg/m2) | 5.27 ± 0.85 | 7.16 ± 1.14 | <0.001*** |
| Body fat mass (kg) | 17.5 ± 5.9 | 20.1 [15.5–23.6] | 0.107 |
| Body fat percentage (%) | 32.8 ± 10.7 | 31.1 ± 7.9 | 0.420 |
| Myostatin (pg/mL) | 316 [245–540] | 488 [359–702] | 0.008** |
| GDF-15 (pg/mL) | 1630 [1034–3946] | 948 [655–1444] | <0.001*** |
| BNP (pg/mL) | 320.1 [172.7–770.4] | 92.0 [46.3–302.6] | <0.001*** |
| HDL-C (mg/dL) | 56.7 ± 19.8 | 51.6 ± 13.3 | 0.147 |
| TG (mg/dL) | 89.8 ± 44.6 | 102.0 [70.0–137.0] | 0.050* |
| hsCRP (mg/L) | 0.25 [0.05–0.75] | 0.12 [0.05–0.52] | 0.355 |
| eGFR (mL/min/1.73 m2) | 51.3 ± 26.9 | 62.3 ± 23.1 | 0.044* |
| Alb (g/dL) | 3.6 ± 0.6 | 4.0 ± 0.5 | 0.001** |
| Hb (g/dL) | 11.3 ± 1.6 | 13.0 ± 1.5 | <0.001*** |
Data are shown as mean ± standard deviation, or, where indicated, median and interquartile range. *, p < 0.05, **, p < 0.01, ***, p < 0.001. BMI, body mass index; MTH, muscle thickness; SMI, skeletal muscle mass index; GDF, growth differentiation factor; BNP, brain natriuretic peptide; HDL-C, high-density lipoprotein cholesterol; TG, triglyceride; hsCRP, high-sensitivity C-reactive protein; eGFR, estimated glomerular filtration rate; Alb, albumin; Hb, hemoglobin.
The mean serum GDF-15 concentration was significantly higher in patients with sarcopenia (1630 [1034–3946] vs 948 [655–1444] pg/mL, p < 0.001), while the mean myostatin concentration was lower (316 [245–540] vs 488 [359–702] pg/mL, p = 0.008). BNP (320.1 [172.7–770.4] vs 92.0 [46.3–302.6] pg/mL, p < 0.001) was higher in patients with sarcopenia, but TG (89.8 ± 44.6 vs 102.0 [70.0–137.0] mg/dL, p = 0.050), eGFR (51.3 ± 26.9 vs 62.3 ± 23.1 mL/min/1.73 m2 (p = 0.008), Alb (3.6 ± 0.6 vs 4.0 ± 0.5 g/dL, p = 0.001), and Hb (11.3 ± 1.6 vs 13.0 ± 1.5 g/dL, p < 0.001) were lower.
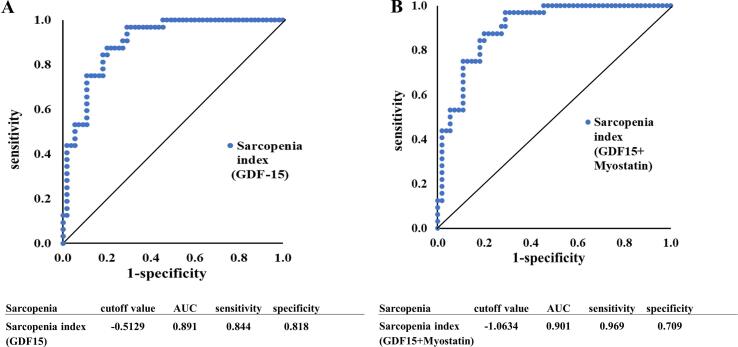
Fig. 3 shows ROC curves for biomarkers defining sarcopenia. Logistic regression analysis with the presence of sarcopenia as the dependent factor and GDF-15, age, sex, and BMI as independent factors showed that GDF-15, age, sex, and BMI were all significant to identify sarcopenia, and an expression for the sarcopenia index of 0.0006 × [GDF-15] + 0.0970 × age + 1.7889 × sex − 0.2522 × BMI - 5.2761 was obtained. The ROC curve for the presence of sarcopenia using this sarcopenia index expression showed a cutoff value of −0.5129, area under the curve (AUC) 0.891, sensitivity 84.4 %, and specificity 81.8 %.
Fig. 3.
A ROC curve to identify the optimal sarcopenia index cutoff level for detecting sarcopenia in cardiac surgery patients. A. The sarcopenia index was calculated using GDF-15, age, sex, and BMI. B. The sarcopenia index was calculated using GDF-15, myostatin, age, sex, and BMI.
Logistic regression analysis with GDF-15, myostatin, age, sex, and BMI as independent factors, with the presence of sarcopenia as the dependent factor, showed that GDF-15, myostatin, age, sex, and BMI were all significant to predict sarcopenia, and the sarcopenia index could be expressed as: −0.0042 × [myostatin] + 0.0007 × [GDF-15] + 0.0890 × age + 1.4030 × sex − 0.2679 × BMI − 2.1186. The ROC curve for the presence of sarcopenia using this sarcopenia index showed a cutoff value of −1.0634, AUC 0.901, sensitivity 96.9 %, and specificity 70.9 %.
4. Discussion
In this study, SMI, hand-grip strength, knee extension strength, and walking speed were inversely correlated with GDF-15 concentration and positively correlated with myostatin concentration in patients receiving cardiovascular surgery. Serum albumin and hemoglobin were inversely correlated with GDF-15 and positively correlated with myostatin. Patients with sarcopenia had higher levels of GDF-15 and lower levels of myostatin. In multivariate regression analysis, SMI was a determinant of myostatin, and both GDF-15 and myostatin were determinants of SMI and MTH, even after adjustment for age, sex, and BMI. The sarcopenia index, which was calculated using GDF-15, myostatin, age, sex, and BMI, was a determinant of sarcopenia (AUC 0.901, sensitivity 96.9 %, specificity 70.9 %).
Preoperative malnutrition is frequently observed in cardiac surgery patients and improvement of malnutrition should be considered prior to cardiac surgery [15], [16]. The results of this study showed that GDF-15 was inversely correlated with muscle mass, muscle function, and nutritional indices, while myostatin was positively correlated with these indices. The maintenance of normal muscle mass and function depends on the dynamic equilibrium between positive and negative regulators of skeletal muscle [17]. In a previous study, serum levels of GDF-15 were found to be elevated in patients with quadriceps atrophy following cardiac surgery [18]. Elevated GDF-15 expression was also shown in frail patients at the intensive care unit, in whom increased GDF-15 was associated with decreased expression of several muscle microRNAs involving skeletal muscle growth [19]. These results for GDF-15 are consistent with the findings of the present study.
Myostatin is a robust regulator of muscle development and postnatal growth, and its activity is controlled by many complex posttranslational events. It is not clear whether myostatin abundance or activity is affected by age and whether myostatin plays a causal role in sarcopenia [1]. Also, although myostatin, along with GDF-15, is considered a negative regulator of skeletal muscle [17], the present study suggested that myostatin, unlike GDF-15, may be a positive regulator of skeletal muscle maintenance in patients receiving cardiac surgery.
Skladany et al. [11] evaluated the association between myostatin and muscle mass, its association with inflammation, and the added value of myostatin to predict survival in hospitalized patients with advanced chronic liver disease. In male patients, myostatin was positively correlated with C-reactive protein (CRP), hand-grip strength, central forearm muscle circumference, and transverse psoas muscle index; in female patients, myostatin was positively correlated with CRP and hand-grip strength. Mortality was higher in male patients with myostatin levels lower than 1600 pg/mL. The model for end-stage liver disease score (MELD) and myostatin cutoff were independent predictors of mortality in males, but not in females. Thus, in males with chronic liver disease, myostatin levels directly reflected muscle mass, and low levels independently predicted prognosis. In females, on the other hand, myostatin was not associated with muscle mass or prognosis. Nishikawa et al. [20] also examined myostatin and psoas muscle index (PMI) by computed tomography in patients with liver cirrhosis. In multivariate analysis, older age and low PMI were significant determinants of poor survival, while high myostatin levels tended to be a significant determinant (p = 0.0855). PMI, albumin, and branched-chain amino acid to tyrosine ratio were inversely correlated with myostatin in both males and females. Thus, even in patients with chronic liver disease, the association of myostatin with sarcopenia and mortality remains unclear. Further studies on the association between myostatin and sarcopenia and even mortality in patients before cardiac surgery are needed.
In the present study, myostatin was low in patients with sarcopenia, and there are several reports on the mechanism of this phenomenon [9], [10], [21]. Myostatin suppresses muscle satellite cell differentiation, so when myostatin levels decrease after exercise, the decrease in myostatin promotes skeletal muscle anabolism. However, myostatin secretion also increases when the skeletal muscle mass is high, and increased myostatin acts to control muscle mass [9]. A strong positive correlation between muscle mass index and myostatin was also found in advanced stages of liver cirrhosis, which was consistent with the fact that as liver disease worsens, the secretion of other muscle regulators such as testosterone [22] and insulin-like growth factor-1 (IGF-1) [23] decreases, and the role of myostatin as a muscle synthesis promoter becomes more important [21]. On the other hand, there are reports that myostatin and IGF-1 act as counter-regulatory molecules against muscle hypertrophy [10], [24]. In vitro experiments showed that myostatin expression is increased in cardiomyocytes when stretched, and that its expression was dependent on IGF-1 [25]. These findings suggested that training leads to muscle growth, in part by increasing IGF-1 levels, which in turn increases the “braking” function of myostatin. Hence, we examined the association between myostatin and IGF-1 and did not find a significant association (r = 0.158, p = 0.185), suggesting that myostatin may be a muscle synthesis promoter rather than an inhibitor. Furthermore, in patients with heart failure (HF), neurohumoral activation is accompanied by increased serum levels of inflammatory cytokines (tumor necrosis factor (TNF) α, interleukin (IL)-1β, and IL-6) [26], and systemic markers of inflammatory cytokines contribute to skeletal muscle atrophy in HF patients [27], [28]. In the present study, myostatin was not significantly associated with TNFα (r = -0.092, p = 0.317) or hsCRP in patients receiving cardiovascular surgery, suggesting that inflammatory cytokines are not involved in the mechanism of low myostatin levels.
Based on multivariate analysis, in the present study, skeletal muscle mass and muscle strength were determinants of myostatin in patients before cardiac surgery, and both GDF-15 and myostatin were determinants of muscle mass. In a previous study, GDF-15 was an independent determinant of all-cause mortality in healthy subjects [6]. GDF-15 was also a strong determinant of all-cause, cardiovascular, and non-cardiovascular mortality in community-dwelling older adults, adding incremental value to the traditional risk factors amino-terminal pro-BNP (NT-proBNP) and CRP [5]. In a study exploring the prognostic utility of GDF-15 in heart failure patients, GDF-15 was still an independent determinant of mortality after correction for clinical data and established biomarkers of adverse prognosis, including NT-proBNP, renal dysfunction, anemia, and hyperuricemia [7]. The negative regulation of skeletal muscle maintenance by GDF-15 seen in this study may be involved in the prognostic utility of GDF-15. However, further studies are needed on GDF-15 and myostatin, including in HF patients.
In the present study, the sarcopenia index calculated from GDF-15 and myostatin was a strong regulator of sarcopenia in ROC curve analysis. Recently, some reports showed that myostatin was a biomarker to predict sarcopenia in patients [21], [29], [30]. In a previous study that performed ROC curve analysis for myostatin levels, the (log10 myostatin) / creatine phosphokinase ratio and albumin / myostatin ratio were found to have acceptable diagnostic accuracy in ruling out sarcopenia in all patients with liver cirrhosis [21]. However, the best diagnostic performance was demonstrated in patients with MELD scores not lower than 15 (AUC 0.829 and 0.801, respectively). Also, myostatin concentrations were low in patients with sarcopenia, and the ROC curve showed that myostatin was the only variable capable of identifying sarcopenia (cutoff value < 2.5 ng/mL, AUC 0.78, sensitivity 0.93, specificity 0.66) [29]. Furthermore, in patients after ST-elevation myocardial infarction, serum myostatin concentrations were positively correlated with muscle mass and muscle strength, and low myostatin was associated with in-hospital mortality, with a cutoff value of<2.20 ng/mL [30]. In that study, multivariate logistic regression showed that high myostatin was associated with lower in-hospital mortality when adjusted for beta blocker use (OR, 0.228; 95 % CI, 0.054–0.974; p = 0.046).
As for a sarcopenia index, a previous study showed that stepwise multivariate logistic regression analysis revealed that adiponectin, sialic acid, age, sex, and BMI were independent factors for sarcopenia detection in patients with cardiovascular disease [12]. The sarcopenia index, which was derived from a diagnostic regression equation for sarcopenia detection that included the above five independent factors, showed high accuracy in ROC curve analysis (sensitivity 94.9 %, specificity 69.9 %). However, no study has examined whether a sarcopenia index calculated using indices including GDF-15 and myostatin predicts sarcopenia. The present study suggests that the sarcopenia index including GDF-15 and myostatin may be a novel and simple diagnostic method of sarcopenia assessment in patients before cardiovascular surgery.
The present study has several limitations. First, the study included a small number patients who underwent different types of cardiovascular surgery. Therefore, our findings may not necessarily be applicable to the general population of patients undergoing cardiovascular surgery. Secondly, considering that the blood tests were done on the day of surgery, anxiety and fasting might have effects on the results of GDF-15 and myostatin. Thus, further studies using a large number of patients and detailed analysis are required to clarify whether a sarcopenia index calculated using preoperative GDF-15 and myostatin levels can be a biomarker for sarcopenia.
In conclusion, GDF-15 and myostatin were associated with skeletal muscle mass in patients undergoing cardiovascular surgery, but the association differed between them. The sarcopenia index calculated using GDF-15 and myostatin may be a potential biomarker for sarcopenia.
Funding
This study was supported in part by JSPS KAKENHI Grant No 19H03981 and 22H03457 (to T.N.) and 20K11259 (to T.F.). The funding sources for this study had no roles in study design, data collection, analysis, or interpretation. The opinions expressed herein are those of the authors. There was no additional external funding received for this study.
6. Institutional Review Board Statement
This study was conducted according to the Declaration of Helsinki and approved by the Ethics Committee of Dokkyo Medical University with approval number 27077 on 13 October 2015.
CRediT authorship contribution statement
Riichi Nishikawa: Formal analysis, Resources. Taira Fukuda: Conceptualization, Formal analysis, Funding acquisition. Akiko Haruyama: Resources. Ikuko Shibasaki: Resources. Suomi Yamaguchi: Resources. Takuo Arikawa: Resources. Syotaro Obi: Resources. Hirohisa Amano: Resources. Hiroshi Yagi: Resources. Masashi Sakuma: Resources. Shichiro Abe: Resources. Hirotsugu Fukuda: Supervision. Shigeru Toyoda: Supervision. Toshiaki Nakajima: Conceptualization, Data curation, Funding acquisition.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
We would like to thank all of the cardiovascular doctors at Dokkyo Heart Center and staff members for helping us with this study.
References
- 1.White T.A., LeBrasseur N.K. Myostatin and sarcopenia: opportunities and challenges - a mini-review. Gerontology. 2014;60(4):289–293. doi: 10.1159/000356740. [DOI] [PubMed] [Google Scholar]
- 2.Fülster S., Tacke M., Sandek A., Ebner N., Tschöpe C., Doehner W., Anker S.D., von Haehling S. Muscle wasting in patients with chronic heart failure: results from the studies investigating co-morbidities aggravating heart failure (SICA-HF) Eur. Heart J. 2013;34(7):512–519. doi: 10.1093/eurheartj/ehs381. [DOI] [PubMed] [Google Scholar]
- 3.Barbalho S.M., Flato U.A.P., Tofano R.J., Goulart R.d.A., Guiguer E.L., Detregiachi C.R.P., Buchaim D.V., Araújo A.C., Buchaim R.L., Reina F.T.R., Biteli P., Reina D.O.B.R., Bechara M.D. Physical exercise and myokines: relationships with sarcopenia and cardiovascular complications. Int. J. Mol. Sci. 2020;21(10):3607. doi: 10.3390/ijms21103607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shimura T., Yamamoto M., Kano S., Kagase A., Kodama A., Koyama Y., Tsuchikane E., Suzuki T., Otsuka T., Kohsaka S., Tada N., Yamanaka F., Naganuma T., Araki M., Shirai S., Watanabe Y., Hayashida K. Impact of the clinical frailty scale on outcomes after transcatheter aortic valve replacement. Circulation. 2017;135(21):2013–2024. doi: 10.1161/CIRCULATIONAHA.116.025630. [DOI] [PubMed] [Google Scholar]
- 5.Daniels L.B., Clopton P., Laughlin G.A., Maisel A.S., Barrett-Connor E. Growth-differentiation factor-15 is a robust, independent predictor of 11-year mortality risk in community-dwelling older adults: the Rancho Bernardo Study. Circulation. 2011;123(19):2101–2110. doi: 10.1161/CIRCULATIONAHA.110.979740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Doerstling S., Hedberg P., Öhrvik J., Leppert J., Henriksen E. Growth differentiation factor 15 in a community-based sample: age-dependent reference limits and prognostic impact. Upsala J. Med. Sci. 2018;123(2):86–93. doi: 10.1080/03009734.2018.1460427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kempf T., von Haehling S., Peter T., Allhoff T., Cicoira M., Doehner W., Ponikowski P., Filippatos G.S., Rozentryt P., Drexler H., Anker S.D., Wollert K.C. Prognostic utility of growth differentiation factor-15 in patients with chronic heart failure. J. Am. Coll. Cardiol. 2007;50(11):1054–1060. doi: 10.1016/j.jacc.2007.04.091. [DOI] [PubMed] [Google Scholar]
- 8.Furihata T., Kinugawa S., Fukushima A., Takada S., Homma T., Masaki Y., Abe T., Yokota T., Oba K., Okita K., Tsutsui H. Serum myostatin levels are independently associated with skeletal muscle wasting in patients with heart failure. Int. J. Cardiol. 2016;220:483–487. doi: 10.1016/j.ijcard.2016.06.231. [DOI] [PubMed] [Google Scholar]
- 9.Kurose S., Onishi K., Takao N., Miyauchi T., Takahashi K., Kimura Y., Sanada K. Association of serum adiponectin and myostatin levels with skeletal muscle in patients with obesity: A cross-sectional study. PLoS ONE. 2021;16(1):e0245678. doi: 10.1371/journal.pone.0245678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhou Y., Hellberg M., Hellmark T., Höglund P., Clyne N. Muscle mass and plasma myostatin after exercise training: a substudy of Renal Exercise (RENEXC)-a randomized controlled trial. Nephrol. Dial. Transplant.: Official Publ. Eur. Dialy. Transplant. Assoc. – Eur. Renal Assoc. 2021;36(1):95–103. doi: 10.1093/ndt/gfz210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Skladany L., Koller T., Molcan P., Vnencakova J., Zilincan M., Jancekova D., Kukla M. Prognostic usefulness of serum myostatin in advanced chronic liver disease: its relation to gender and correlation with inflammatory status. J. Physiol. Pharmacol.: Official J. Polish Physiol. Soc. 2019;70(3) doi: 10.26402/jpp.2019.3.03. [DOI] [PubMed] [Google Scholar]
- 12.Harada H., Kai H., Shibata R., Niiyama H., Nishiyama Y., Murohara T., Yoshida N., Katoh A., Ikeda H., Mogi M. New diagnostic index for sarcopenia in patients with cardiovascular diseases. PLoS ONE. 2017;12(5):e0178123. doi: 10.1371/journal.pone.0178123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nakajima T., Shibasaki I., Sawaguchi T., Haruyama A., Kaneda H., Nakajima T., Hasegawa T., Arikawa T., Obi S., Sakuma M., Ogawa H., Toyoda S., Nakamura F., Abe S., Fukuda H., Inoue T. Growth differentiation factor-15 (GDF-15) is a biomarker of muscle wasting and renal dysfunction in preoperative cardiovascular surgery patients. J. Clin. Med. 2019;8(10) doi: 10.3390/jcm8101576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yazawa H., Fukuda T., Kaneda H., Waku R., Sakuma M., Matsumoto A., Toyoda S., Abe S., Nakamura F., Inoue T., Nakajima T. Association of serum growth differentiation factor-15 with eGFR and hemoglobin in healthy older females. Int. J. Cardiol. Heart Vasculature. 2020;31 doi: 10.1016/j.ijcha.2020.100651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yu P.-J., Cassiere H.A., Dellis S.L., Manetta F., Kohn N., Hartman A.R. Impact of preoperative prealbumin on outcomes after cardiac surgery. JPEN J. Parenteral Enteral Nutr. 2015;39(7):870–874. doi: 10.1177/0148607114536735. [DOI] [PubMed] [Google Scholar]
- 16.Goldfarb M., Lauck S., Webb J.G., Asgar A.W., Perrault L.P., Piazza N., Martucci G., Lachapelle K., Noiseux N., Kim D.H., Popma J.J., Lefèvre T., Labinaz M., Lamy A., Peterson M.D., Arora R.C., Morais J.A., Morin J.-F., Rudski L.G., Afilalo J. Malnutrition and mortality in frail and non-frail older adults undergoing aortic valve replacement. Circulation. 2018;138(20):2202–2211. doi: 10.1161/CIRCULATIONAHA.118.033887. [DOI] [PubMed] [Google Scholar]
- 17.Kalinkovich A., Livshits G. Sarcopenia-the search for emerging biomarkers. Ageing Res. Rev. 2015;22:58–71. doi: 10.1016/j.arr.2015.05.001. [DOI] [PubMed] [Google Scholar]
- 18.Bloch S.A.A., Lee J.Y., Wort S.J., Polkey M.I., Kemp P.R., Griffiths M.J.D. Sustained elevation of circulating growth and differentiation factor-15 and a dynamic imbalance in mediators of muscle homeostasis are associated with the development of acute muscle wasting following cardiac surgery. Crit. Care Med. 2013;41(4):982–989. doi: 10.1097/CCM.0b013e318274671b. [DOI] [PubMed] [Google Scholar]
- 19.Bloch S.A.A., Lee J.Y., Syburra T., Rosendahl U., Griffiths M.J.D., Kemp P.R., Polkey M.I. Increased expression of GDF-15 may mediate ICU-acquired weakness by down-regulating muscle microRNAs. Thorax. 2015;70(3):219–228. doi: 10.1136/thoraxjnl-2014-206225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nishikawa H., Enomoto H., Ishii A., Iwata Y., Miyamoto Y., Ishii N., Yuri Y., Hasegawa K., Nakano C., Nishimura T., Yoh K., Aizawa N., Sakai Y., Ikeda N., Takashima T., Takata R., Iijima H., Nishiguchi S. Elevated serum myostatin level is associated with worse survival in patients with liver cirrhosis. J. Cachexia Sarcopenia Muscle. 2017;8(6):915–925. doi: 10.1002/jcsm.12212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alexopoulos T., Vasilieva L., Kontogianni M.D., Tenta R., Georgiou A., Stroumpouli E., Mani I., Alexopoulou A. Myostatin in combination with creatine phosphokinase or albumin may differentiate patients with cirrhosis and sarcopeniaAm. J. Physiol. Gastrointest. Liver Physiol. 2021;321(5):G543–G551. doi: 10.1152/ajpgi.00184.2021. [DOI] [PubMed] [Google Scholar]
- 22.Sinclair M., Grossmann M., Gow P.J., Angus P.W. Testosterone in men with advanced liver disease: abnormalities and implications. J. Gastroenterol. Hepatol. 2015;30(2):244–251. doi: 10.1111/jgh.12695. [DOI] [PubMed] [Google Scholar]
- 23.Adamek A., Kasprzak A. Insulin-like growth factor (IGF) system in liver diseases. Int. J. Mol. Sci. 2018;19(5):1308. doi: 10.3390/ijms19051308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mak R.H., Rotwein P. Myostatin and insulin-like growth factors in uremic sarcopenia: the yin and yang in muscle mass regulation. Kidney Int. 2006;70(3):410–412. doi: 10.1038/sj.ki.5001622. [DOI] [PubMed] [Google Scholar]
- 25.Shyu K., Ko W., Yang W., Wang B., Kuan P. Insulin-like growth factor-1 mediates stretch-induced upregulation of myostatin expression in neonatal rat cardiomyocytes. Cardiovasc. Res. 2005;68(3):405–414. doi: 10.1016/j.cardiores.2005.06.028. [DOI] [PubMed] [Google Scholar]
- 26.Torre-Amione G., Kapadia S., Lee J., Durand J.B., Bies R.D., Young J.B., Mann D.L. Tumor necrosis factor-alpha and tumor necrosis factor receptors in the failing human heart. Circulation. 1996;93(4):704–711. doi: 10.1161/01.cir.93.4.704. [DOI] [PubMed] [Google Scholar]
- 27.Anker S.D., Chua T.P., Ponikowski P., Harrington D., Swan J.W., Kox W.J., Poole-Wilson P.A., Coats A.J.S. Hormonal changes and catabolic/anabolic imbalance in chronic heart failure and their importance for cardiac cachexia. Circulation. 1997;96(2):526–534. doi: 10.1161/01.cir.96.2.526. [DOI] [PubMed] [Google Scholar]
- 28.Hambrecht R., Schulze P.C., Gielen S., Linke A., Möbius-Winkler S., Yu J., Kratzsch J.J., Baldauf G., Busse M.W., Schubert A., Adams V., Schuler G. Reduction of insulin-like growth factor-I expression in the skeletal muscle of noncachectic patients with chronic heart failure. J. Am. Coll. Cardiol. 2002;39(7):1175–1181. doi: 10.1016/s0735-1097(02)01736-9. [DOI] [PubMed] [Google Scholar]
- 29.Echeverria I., Besga A., Sanz B., Amasene M., Hervás G., Barroso J., Rodriguez‐Larrad A., Irazusta J. Identification of frailty and sarcopenia in hospitalised older people. Eur. J. Clin. Invest. 2021;51(4) doi: 10.1111/eci.v51.410.1111/eci.13420. [DOI] [PubMed] [Google Scholar]
- 30.Oliveira P.G.S., Schwed J.F., Chiuso-Minicucci F., Duarte S.R.S., Nascimento L.M., Dorna M.S., Costa N.A., Okoshi K., Okoshi M.P., Azevedo P.S., Polegato B.F., Paiva S.A.R., Zornoff L.A.M., Minicucci M.F. Association between serum myostatin levels, hospital mortality, and muscle mass and strength following ST-elevation myocardial infarction. Heart Lung Circulation. 2022;31(3):365–371. doi: 10.1016/j.hlc.2021.08.018. [DOI] [PubMed] [Google Scholar]