Abstract
A prototype family of seven genes encoding the variable surface lipoproteins (Vlps) of Mycoplasma hyorhinis is characterized in the pathogenic SK76 strain, using long-range PCR to amplify and analyze the single chromosomal region containing expressed genes vlpA to -G, each of which is subject to phase and size variation. Smaller families of vlp genes in subclones of SK76 or in another strain of M. hyorhinis, GDL, can be attributed to deletions of specific vlp genes from the prototype array described here. Two genes, vlpA and the newly revealed vlpG, contain repeat motifs in their 3′ coding regions that differ from the short tandem repeats in other vlp genes yet retain structural features common to all vlp gene products. SK76 and GDL vlp gene families are similarly organized and show sequence similarity between corresponding individual vlp genes. In light of the extensive potential for diversity within the vlp gene system, such conservation provides a provisional basis to hypothesize that vlp genes may exist in specific arrays that endow selected functions while retaining common structural features required during phase-variable expression of this set of gene products.
Several species of the wall-less eubacteria Mycoplasma spp. contain in their small genomes sets of related genes encoding alternative surface lipoproteins (recently reviewed in reference 9). Studies of some mycoplasma gene families have revealed that these genes are subject to high-frequency, reversible mutations that generate alternate or composite expression patterns of corresponding gene products, as well as structural variation of the products reflecting changes in intragenic repetitive regions. Whereas specific functions associated with mycoplasmal systems of surface variation are not clear, conservation of such multigenic systems per se suggests that these variable arrays are pivotal elements in the adaptive strategies of mycoplasmas as chronic infectious pathogens of various host species. Determining the range of alternative genes in these families, and the degree and nature of structural variation in the corresponding gene products, can provide initial insight into possible functions. This study examines gene families encoding the variable lipoprotein (Vlp) system of Mycoplasma hyorhinis.
M. hyorhinis chronically infects the swine host, where it causes rhinitis as well as severe serositis and arthritis (13, 14). This agent has also been recently linked to the occurrence of otitis media (8). An experimental swine model of arthritis has been developed using the arthritogenic SK76 strain of M. hyorhinis, a prototype pathogenic strain derived as a cloned isolate from a diseased site in a natural infection (11, 12, 14). Subclones of SK76 propagated by passage in broth culture have been used to characterize the rudimentary Vlp system (11, 12, 21). In addition to its natural host, M. hyorhinis occupies an important artificial niche as one of the most prevalent mycoplasmal tissue culture contaminants worldwide (7). Although the origins and initial pathway for transmitting these cell culture-adapted contaminants are not known, one such isolate, type strain GDL (1), has been characterized and shown to contain additional genes of the Vlp family (10, 22).
Genetic mechanisms underlying mycoplasma surface variation were first elucidated during a study of M. hyorhinis membrane lipoproteins of strains SK76 and GDL (3, 12, 20, 21), which contain repertoires of distinct, single-copy genes encoding surface-exposed Vlps. Reversible, high-frequency mutations govern the phase-variable expression of each Vlp product (12, 21). Insertion or deletion of deoxyadenosine residues in a characteristic promoter region [poly(A) tract] linked to each vlp gene results in drastically altered transcription of the individual genes (3, 21). In addition, size variation of Vlp products results from in-frame insertion or deletion of tandemly repeated intragenic sequences within the 3′ region of individual vlp genes, thereby contracting or expanding the surface-exposed C-terminal region of the corresponding Vlp (12, 21). This structural variation affects the abundance (3) and functional consequences (2) of Vlps on the mycoplasma surface, but the full consequences of this variation are yet to be fully understood. The two independent forms of reversible mutation affect expression and size of the product from each vlp gene. In a propagating population, a single organism carrying multiple vlp genes has the capacity to generate a large number of variants expressing antigenically distinct vlp gene products, either alone or in combinatorial mosaics on the cell surface.
The functions of the Vlp system are likely to be complex. Structural variation in vlp genes of M. hyorhinis was recently shown to govern susceptibility to growth inhibition by host antibody (Ab) produced during infection of swine (2). Nevertheless, this property may represent only one aspect of a system which might mediate diverse additional surface interactions. Knowing the size and extent of diversity embodied in the combinatorial repertoire of vlp genes, particularly in a known pathogenic isolate, will be highly informative in this regard. Defining new or consistent features of the Vlp system, including additional vlp genes, their comparative organization in families, and their common structural features, is an essential step in predicting the functions of the Vlp system in adaptive variation. Comparison of cloned isolates of M. hyorhinis strains SK76 and GDL showed that the vlp repertoires in these two strains comprised three and six vlp genes, respectively (21, 22). However, evidence raising the possibility of additional vlp genes in other isolates of strain SK76 has been reported (22).
A long-range PCR (LR-PCR) procedure capable of amplifying and analyzing complete clusters of contiguous vlp genes was therefore developed and used to characterize the vlp family in strain SK76. This report (i) identifies an expanded prototype vlp gene family in the pathogenic SK76 strain that includes a new vlp gene with an atypical intragenic repeat structure, (ii) shows one vlp gene to have a repeat structure subject to complex spontaneous intragenic variation, and (iii) provides a mechanistic basis for differences observed in the size of vlp gene families in cultured organisms through deletions of vlp genes or gene blocks from a larger prototype vlp family.
MATERIALS AND METHODS
Bacterial strains, plasmids, and culture conditions.
M. hyorhinis SK76 was provided by R. F. Ross, Iowa State University, Ames, as a filter-cloned culture. Isogenic clonal variants derived from M. hyorhinis SK76 and GDL have been previously described (3, 12, 21) and were propagated at 37°C in modified Hayflick broth or agar medium (19). Cloning procedures were performed using the vector pGEM-7Z (Promega Corporation, Madison, Wis.) or the pT7Blue T-Vector (Novagen, Madison, Wis.). Competent Escherichia coli DH5α MCR or DH10B (Life Technologies, Inc., Gaithersburg, Md.) was used as a host to clone recombinant products and grown at 37°C in Luria-Bertani broth supplemented with 100 μg of ampicillin per ml for plasmid preparation.
DNA manipulations and sequencing.
Mycoplasma cells were harvested and subsequently lysed in a buffer containing 1% sodium dodecyl sulfate (SDS), 45 mM Tris (pH 8.0), and 9 mM EDTA for 10 min at 50°C and then for 10 min at 37°C. Genomic DNA was extracted by standard methods (15) or by commercial kits as described below. Agarose gel electrophoresis, restriction endonuclease digestion, ligation, chemical transformation, and electroporation were performed as described elsewhere (15). 3′-Digoxigenin-11-ddUTP (DIG)-labeled oligonucleotides and Southern hybridization were prepared according to the Genius system user's guide for membrane hybridization, version 3.0 (Roche Molecular Biochemicals). For the partial ClaI restriction digestion, 150 ng of the purified (Qiaex II gel extraction kit; Qiagen, Inc., Valencia, Calif.) LR-PCR product of SK76[7] was incubated with 1 U of ClaI (Promega) for 30 or 60 min, and the reaction was stopped by heat inactivation at 65°C for 20 min.
DNA sequencing was performed at the University of Missouri DNA Core Facility, using Taq DyeDeoxy termination and a model 373A or 377 automated sequencer (Applied Biosystems Inc., Foster City, Calif.). Nucleotide sequences were analyzed using the Genetics Computer Group sequence analysis software package (5), version 9.1. Oligonucleotides used in this study were also synthesized by the University of Missouri DNA Core Facility, using a model 394 DNA/RNA synthesizer or model 3948 nucleic acid synthesizer (Applied Biosystems).
Cloning and DNA sequence analysis.
Genomic DNA from M. hyorhinis SK76[7] clonal variant was digested to completion with XbaI. The resulting fragments were inserted into XbaI-restricted, dephosphorylated pGEM-7Z vector. The ligation mixture was used to transform competent E. coli DH5α cells by electroporation (Life Technologies). Recombinant clones carrying vlp genes were detected by hybridization on colonies, using the DIG-labeled oligonucleotide Sig (Table 1). One recombinant plasmid (PT1) contained a 4.6-kbp XbaI insert. This was subcloned by additional ClaI restriction and fully sequenced, revealing a new vlp gene, vlpG, located upstream of the vlpA gene and downstream of an IS1221 element. To identify other vlp genes, the LR-PCR product generated from the SK76[7] genomic template was digested by ClaI, and fragments were cloned into pGEM-7Z. Internal ClaI fragments shown in Fig. 1A (SK76[7]), except the fragment bearing vlpG, were analyzed by this approach.
TABLE 1.
Oligonucleotide probe and primer sequences
| Oligonucleotide designation | Nucleotide sequence (5′ to 3′) | Localization and description |
|---|---|---|
| A | TGAACCTGGAGCTTCACTTTGTTGTGT | Complementary to vlpA repeat motif DNA coding sequence |
| B | CTCCAGAATCTTGTGAATCTGATCCTGTTC | Complementary to vlpB repeat motif DNA coding sequence |
| C | GTGATCCTGATTCTGGAGATTTAGGAGTTG | Complementary to vlpC repeat motif DNA coding sequence |
| D | AAGGATCTTCAGATTCAACTTCAACAAGTAAAGAAC | Complementary to vlpD repeat motif DNA coding sequence |
| E | CAACAACATCAGATGGACAACATTCAAATC | Complementary to vlpE repeat motif DNA coding sequence |
| F | CCAGAGCAAGGAAATAACCAAGGTGGATCAACTCCAACTCCAGAGCAAGG | Complementary to vlpF repeat motif DNA coding sequence |
| G | CCAGTTTCAGTTGTAGAACTTGTTCCTGA | Complementary to vlpG repeat motif DNA coding sequence |
| Sig | AGTTAGTTTTGGTTCGCTTGTGGC | Located in the signal coding region 3′ of the ClaI restriction site; common to all vlps |
| LR-F, N3-F | CCTCTCTTGGTTGAACAATGCTTCC | Located in the 5′ boundary of vlp families in SK76[3], GDL[6], and SK76[7] |
| LR-R, N1-R, N2-R | TTGGCTGTGGCAGTAGCCATAGCT | Located in the 3′ boundary of SK76[3], SK76[7], and GDL[6] vlp families |
| N1-F | TGTGGACAAACAGATAATAATTCATC | Located immediately downstream of the signal peptide sequence of vlpA |
| N3-R, N4-R | AATAGGAACTAAGATGTGTGTTGTGTG | Complementary to the 3′ end of the vlpA DNA coding sequence including part of the tip structure |
| N4-F, N5-F, N6-F | TGTGGTCAAACTAATACAGATCAA | Located immediately downstream of the signal peptide sequence of vlpD |
| N2-F | CACAAGATTCTGGAAATGGATCAAC | Located at the 3′ end of vlpB |
| N5-R | AATAGTTGTTATTTTGAGACTTGGCTATT | Complementary to the 3′ end of vlpF |
| N6-R | TTATTTATTTTGATTTTGTCCATCTGATG | Complementary to the 3′ end of vlpE |
FIG. 1.
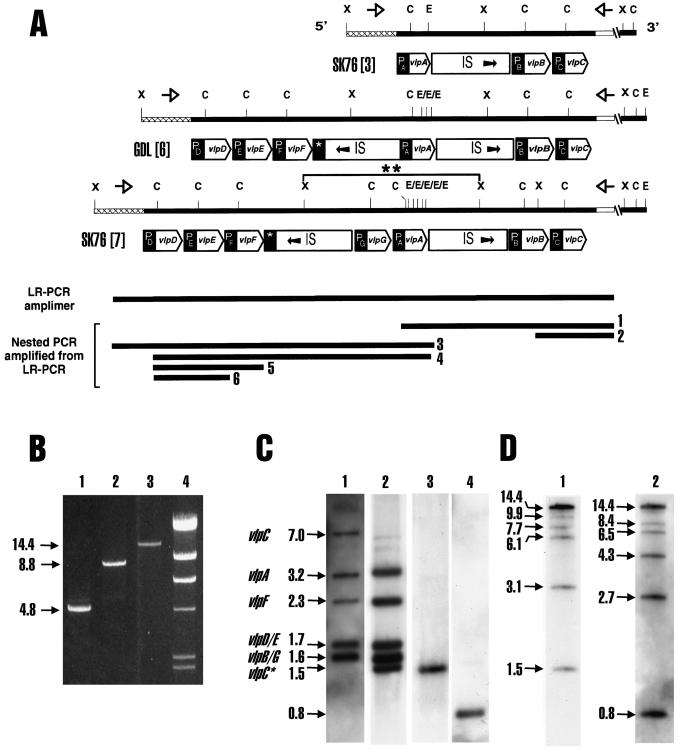
Chromosomal organization of the vlp gene cluster in M. hyorhinis strains SK76 and GDL. (A) vlp gene families from strain SK76 containing three (SK76[3]) or seven (SK76[7]) genes and from strain GDL containing six genes (GDL[6]). The maps shown are not to scale but reflect the positions and features of all genes. Solid lines show the chromosomal location of ClaI (C), XbaI (X), and EcoRI (E) restriction sites within vlp gene families and the distinct, conserved regions flanking vlp families at the 5′ (stippled) and 3′ (open) boundaries. Corresponding oligonucleotide primers (LR-F and LR-R [Table 1]), used to amplify vlp gene clusters by LR-PCR, are indicated by opposing open arrows above the lines. Large open arrows below each line indicate the location and orientation of ORFs encoding Vlp proteins, and shaded boxes (P) indicate the locations of conserved promoter regions upstream of each vlp gene or in one case a distinctive partial promoter structure downstream of vlpF (*). The positions and orientations of IS1221 elements (IS) are shown as large open boxes. The double asterisk indicates a genomic XbaI fragment from SK76[7] that was cloned and analyzed further (Fig. 2B). The LR-PCR product generated from SK76[7] genomic DNA template is indicated by a line below (LR-PCR amplimer). Additional lines represent nested PCR products generated from the LR-PCR product template by using primers described in Table 1. Nested products 1 through 6 were generated with primer sets N1-F–N1-R, N2-F–N2-R, N3-F–N3-R, N4-F–N4-R, N5-F–N5-R, and N6-F–N6-R, respectively. (B) Ethidium bromide-stained amplimers were generated as described in Materials and Methods from genomic DNA template from clonal isolates SK76[3] (lane 1), GDL[6] (lane 2), and SK76[7] (lane 3) and separated by electrophoresis through 0.9% agarose gels. Sizes of the amplimers, determined using lambda HindIII markers (lane 4), are indicated on the left in kilobase pairs. (C) Southern blot analysis of ClaI restriction fragments from SK76[7] genomic DNA (lane 1) or the LR-PCR product from SK76[7] (lanes 2 through 4). Blots were hybridized with oligonucleotide probes (Table 1) representing a highly conserved signal peptide sequence (Sig) located 3′ of the ClaI site in each vlp gene (lanes 1 and 2), the 3′ flanking primer sequence LR-R (lane 3), or the 5′ flanking sequence, LR-F (lane 4). Chromosomal ClaI fragments bearing specific vlp genes are identified to the left of lane 1, based on hybridization patterns (data not shown) obtained with oligonucleotides specific for vlpA to -G. The sizes of fragments are indicated in kilobase pairs. Comigrating 1.7-kbp fragments bearing vlpD and vlpE, respectively (vlpD/E), and 1.6-kbp fragments bearing vlpB and vlpG, respectively (vlpB/G), were not resolved by the gel system shown. vlpC and vlpC* indicate ClaI fragments from genomic (7.0-kbp) and LR-PCR (1.5-kbp) DNA, respectively, bearing the vlpC gene. (D) ClaI site mapping of the LR-PCR amplimer bearing vlp genes. A partial ClaI digest of the LR-PCR product from SK76[7] was subjected to Southern blot analysis with oligonucleotide probes LR-R (lane 1) and LR-F (lane 2) to identify partially and completely digested fragments bearing these 3′- and 5′-terminal flanking sequences, respectively. Sizes of fragments are indicated to the left of each lane. The terminal 1.5-kbp 3′ fragment and 0.8-kbp 5′ fragment correspond to those in the complete digests shown in panel C, lanes 3 and 4. Additional gels resolving larger fragments are not shown. (This figure was constructed using Adobe Photoshop version 4.0 and 5.0 for Windows NT, Magicscan V4.1, Umax Power Look III scanner, Hewlett-Packard LaserJet 4000N printer, and Dell OptiPlex GXPro or Gateway E-4100 computer.)
Identification and sequence comparison of vlpA genes.
To identify and characterize vlpA genes from assorted clonal isolates, PCR was first used to amplify the vlpA gene from chromosomal DNA. The amplimers were purified and sequenced either directly or after cloning into pT7Blue T-Vector. PCR was performed in a volume of 100 μl with 5 U of AmpliTaq DNA polymerase (PE Corp., Norwalk, Conn.), 0.3 mM each primer, 0.35 mM deoxynucleoside triphosphate mix (10 mM each; Life Technologies), 1.75 mM MgCl2, 1× AmpliTaq DNA polymerase buffer II, and 20 ng of genomic DNA. Thermocycling was performed in a Perkin-Elmer DNA Thermo Cycler 480 with the following conditions: 1 cycle at 94°C for 2 min; 40 cycles at 94°C for 1 min, 57°C for 1 min, 72°C for 2 min; and a final extension cycle of 72°C for 7 min. Ten microliters of the PCR product was analyzed on a 0.8% agarose gel (FMC BioProducts, Rockland, Maine). The resulting PCR products were purified with a QIAquick gel extraction kit (Qiagen), and 375 fmol was sequenced directly. In some instances, indicated below, the purified PCR product was ligated into pT7Blue T-Vector and sequenced using T7 and U19 primers provided by Novagen. Additional internal primers were used to obtain and confirm sequences.
The vlpA gene of the M. hyorhinis strain GDL[6] clonal isolate (see Fig. 3B) was amplified from genomic DNA, as described above, using the forward primer vlpFtip-F 5′-CAAGTCTCAAAATAACAACTATTC-3′, located at the 3′ end of vlpF (Fig. 1A), and the reverse primer p9vlpA-R 5′-TAATAGGAACTAAGATGTGTGTTG-3′, complementary to the 3′ end of vlpA of GDL[6]. This sequence includes a portion of the vlpA tip structure, conserved between SK76[3] and GDL[6]. PCR products containing vlpA were purified and cloned into pT7Blue T-Vector (Novagen). Sequences obtained directly from the PCR product and from the cloned insert were identical.
FIG. 3.
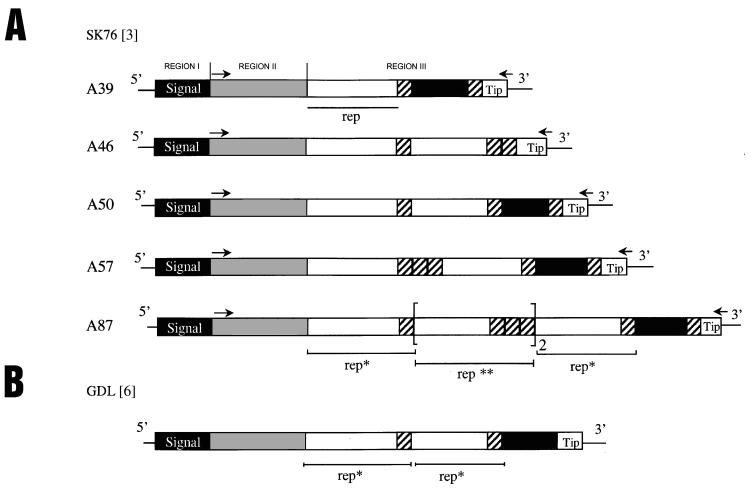
Comparison of vlpA genes among VlpA size variants. (A) Size variants of vlpA (A39, A46, A50, A57, and A87, expressing VlpA protein products of 39, 46, 50, 57, and 87 kDa, respectively, as determined by SDS-PAGE) were derived from a clonal lineage of spontaneous variants from SK76[3] (12, 21). Regions I, II, and III are indicated as in Fig. 2. In region III, a basic repeat unit (rep; underlined) is indicated by an open box. Filled areas in region III represent a modified repeat comprising a 60-bp deletion of the rep unit. Hatched boxes represent identical repeated sequences of 13 amino acids. Larger composite repeated motifs are indicated as rep* and rep**. Brackets depict tandemly repeated rep** sequences. The C-terminal sequence (Tip) is indicated in all variants. Location of the primers used to amplify portions of vlpA genes encoding the mature lipoprotein regions are represented by arrows and are described in Materials and Methods. (B) The structure of vlpA from a previously described (22) cloned isolate of M. hyorhinis strain GDL is depicted, using symbols described for panel A to indicate the repeated structure within region III. (This figure was constructed using Adobe Photoshop version 4.0 and 5.0 for Windows NT, Magicscan V4.1, Umax Power Look III scanner, Hewlett-Packard LaserJet 4000N printer, and Dell OptiPlex GXPro or Gateway E-4100 computer.)
The vlpA genes from spontaneous size variants of SK76[3] were amplified from genomic DNA extracted using a Wizard genomic DNA purification kit (Promega). Primers used for amplification were N1-F (Table 1) and p9vlpA-R. The PCR products were purified and sequenced directly using these same primers. The vlpA amplimer from the clonal variant A87 was also cloned into pT7Blue T-Vector to confirm the sequence. As a result of this analysis, the sequence of the A39 size variant, described in a previous study (21), has been corrected in the GenBank database (accession no. X62936).
LR-PCR and nested PCR.
LR-PCR and nested PCR products were produced using the protocol of the Expand Long Template PCR system (Roche Molecular Biochemicals). The LR-PCR DNA template was generated using primers LR-F and LR-R (Table 1) from genomic DNA. Nested PCR products were generated using 20 ng of the LR-PCR product as template and the nested primer sets listed in Table 1. The annealing temperatures for the primer sets varied and were typically 6 to 9°C below the predicted primer melting temperature. Identification of vlp genes was determined by direct sequencing of corresponding PCR fragments purified with a Qiaex II or QIAquick gel extraction kit (Qiagen) or by sequencing subcloned restriction fragments of the PCR products.
Antibodies to synthetic Vlp peptides and immunoblotting procedures.
The synthetic peptide pepG was prepared on a model 432A peptide synthesizer (Applied Biosystems) by standard 9-fluorenylmethylcarbonyl protection chemistries and purified as previously described (4, 22). The amino acid sequence of pepG, C-SGTSSTTETGSTTESSGQA)DSTSGTSTSI, corresponds to the C-terminal 29 amino acid residues of VlpG deduced from the 3′ region of the vlpG gene sequence determined in this study. The parenthesis indicates the end of a 22-residue repeat unit in the VlpG sequence, and the underlined residues indicate an adjoining partial repeat comprising part of the tip structure. The Cys residue was incorporated at the N terminus in order to couple the peptide to maleimide-activated keyhole limpet hemocyanin, using a hapten carrier conjugate kit (Pierce Chemical Co., Rockford, Ill.). A BALB/c mouse was immunized with three weekly intraperitoneal injections of approximately 15 μg of conjugated peptide emulsified with incomplete Freund adjuvant. The presence of specific anti-pepG polyclonal Abs (PAbs) in the hyperimmune serum was monitored by Western blot analysis of defined clonal variants SK76[3] or GDL[6] that lacked the vlpG gene or of the clonal variant SK76[7] containing the vlpG gene. A monoclonal Ab (MAb) directed to a synthetic peptide representative of the C-terminal repeat and tip structure of VlpA has been described elsewhere (2) and was used as a specific probe for the VlpA product.
Western blotting of SDS-polyacrylamide gel electrophoresis (PAGE)-separated proteins and colony blotting were performed as described elsewhere (12), using PAb to pepG diluted 1:300 or MAb to VlpA (ascites) diluted 1:1,000 as primary Ab, followed by peroxidase-conjugated goat antiserum to mouse immunoglobulin G for detection. Abs were diluted in phosphate-buffered saline containing 10% calf serum.
Nucleotide sequence accession numbers.
The following sequences (with corresponding accession numbers) were deposited in the GenBank sequence database: PCR-derived sequences of A39 (AF193874), A46 (AF193875), A50 (AF193876), A57 (AF193877), and A87 (AF193878), IS1221-vlpG-vlpA-IS1221 from SK76[7] (AF193880), and vlpA from GDL[6] (AF193879).
RESULTS AND DISCUSSION
LR-PCR strategy to amplify and characterize complete vlp gene families in the M. hyorhinis chromosome.
Our previous studies (21, 22) identified the vlp families in two cloned isolates shown in Fig. 1A. One clonal isolate from strain SK76 (SK76[3]) contained three genes, vlpA to -C, whereas a clonal isolate (47.1.2) of type strain GDL contained six genes, including additional genes vlpD to -F (Fig. 1, GDL[6]).
In both strains, all vlp genes occurred in single copy, were clustered as shown in association with IS1221 elements (23), and were flanked by consistent chromosomal boundary sequences. Further analysis of early-passage SK76 revealed a subpopulation that contained more than three vlp genes (10, 22). The presence of this SK76 variant population was compatible with the possibility that the original pathogenic strain (cloned at the time of isolation) contained a more extensive repertoire of vlp genes that might differ from that in the extensively passaged GDL strain derived from tissue culture. To analyze the SK76 population containing the expanded repertoire, a variant (termed SK76[7]; clonal isolate 8II) was selected, and a general method to more directly characterize the composition and organization of vlp gene families was developed. This was needed as an alternative to complement the traditional chromosomal cloning protocols, which were confounded by redundancy and size variation in intergenic and intragenic sequences throughout this region.
Based on the premise (confirmed below) that the vlp family in SK76[7] would be flanked in the chromosome by the distinctive sequences at each boundary of the two known families, we designed primers (shown as opposing arrows in Fig. 1A) and developed conditions for LR-PCR amplification of complete vlp gene clusters from chromosomal DNA templates derived from mycoplasmal clonal isolates. Examples of the products obtained with these flanking primers are shown in Fig. 1B. LR-PCR amplimers from the three-gene SK76[3] isolate and six-gene GDL[6] isolate showed the expected 4.8- and 8.8-kbp sizes, respectively, as predicted from previous cloning, restriction analysis, and sequencing of these vlp gene families (21, 22). The authenticity of LR-PCR amplimers from these vlp gene families was confirmed by diagnostic Southern hybridization of ClaI and XbaI fragments as previously described (21, 22). Amplification products were reproducibly obtained and, based on subsequent sequencing of various regions and comparison to known vlp gene sequences, appeared to be free of PCR-derived errors in amplification (data not shown).
The vlp family of the SK76[7] clonal subpopulation was analyzed using LR-PCR in conjunction with conventional mapping techniques. First, an LR-PCR amplimer of approximately 14.4 kbp was obtained (Fig. 1B, lane 3). This was larger than known vlp families and could reflect highly extended repeat structures in individual vlp genes, additional IS1221 elements, additional vlp genes, or unknown sequences. Southern hybridization with probes specific for each of the known vlp genes was used to further characterize the LR-PCR amplimers. These probes represented the distinctive repeat regions of each of the genes vlpA to -F as described previously (3, 21, 22). Because vlp genes contain a highly conserved DNA sequence encoding the prolipoprotein signal peptide common to all Vlp products, and because this sequence contains a distinctive ClaI restriction site accounting for all ClaI sites in the known vlp gene clusters (Fig. 1A), ClaI restriction fragments could be used to segregate individual vlp genes (21, 22). ClaI fragments bearing vlp genes could be identified by their hybridization with a conserved signal peptide probe (Sig) immediately downstream of the ClaI site and further distinguished using specific probes for individual vlp genes (Table 1).
ClaI restriction of chromosomal DNA from the SK76[7] isolate, or of the LR-PCR amplimer generated from this isolate, enabled assignment of known vlp genes to ClaI fragments and a comparison of the genomic organization with that found in the LR-PCR product. The pattern of ClaI restriction fragments hybridizing with vlp gene probes was completely concordant between chromosomal DNA and the LR-PCR product, with the anticipated exception of a 1.5-kbp ClaI fragment at the 3′ end of the LR-PCR product bearing vlpC. Figure 1C (lanes 1 and 2) compares these patterns, using the conserved vlp signal peptide probe to elucidate all ClaI fragments bearing vlp genes. Probes specific to known vlp genes (and later to a new gene, vlpG, described below) further assigned specific genes to these fragments and additionally confirmed that known vlpA to -F gene sequences were present in SK76[7]. Higher-resolution gels revealed two pairs of nearly comigrating bands (data not shown), indicated in Fig. 1C as vlpD/E and vlpB/G. To complete the restriction analysis of the LR-PCR product, the distal ClaI fragments at the 5′ and 3′ ends of the amplimer were identified with probes representing the chromosomal boundary primers used to generate the LR-PCR product. The 1.5-kbp 3′ ClaI fragment is shown in lane 3 (this fragment also bears vlpC), and the 0.8-kbp 5′ ClaI fragment is shown in lane 4 (this fragment bears no target for vlp probes). XbaI fragments of the LR-PCR amplimer were also subjected to hybridization with vlp-specific probes, in order to map specific genes onto these larger fragments. The distribution of individual vlp genes on ClaI and XbaI fragments is summarized in Table 2. With the exception of an unusually large XbaI fragment bearing vlpA, the pattern was generally comparable to that for the vlp family previously identified (22) in GDL[6]. As in earlier studies, no evidence for vlp-related sequences outside this cluster was found in strain SK76[7].
TABLE 2.
Localization of individual vlp genes in M. hyorhinis SK76[7]
| Probea | Size (kbp) of restriction fragmentb
|
Nested PCRc
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Genomic DNA
|
LR-PCR
|
|||||||||
| ClaI | XbaI | ClaI | XbaI | 1 | 2 | 3 | 4 | 5 | 6 | |
| A (vlpA) | 3.2 | 4.6 | 3.2 | 4.6 | + | − | + | + | − | − |
| B (vlpB) | 1.6 | 2.7 | 1.6 | 2.7 | + | − | − | − | − | − |
| C (vlpC) | 7.0 | >12 | 1.5 | 2.7 | + | + | − | − | − | − |
| D (vlpD) | 1.7 | 5.5 | 1.7 | 5.5 | − | − | + | + | + | + |
| E (vlpE) | 1.7 | 5.5 | 1.7 | 5.5 | − | − | + | + | + | + |
| F (vlpF) | 2.3 | 5.5 | 2.3 | 5.5 | − | − | + | + | + | − |
| G (vlpG) | 1.6 | 4.6 | 1.6 | 4.6 | − | − | + | + | − | − |
vlp-specific oligonucleotide probes are described in Table 1 and correspond to region III repeat sequences specific for individual vlp genes.
Genomic DNA extracted from SK76[7] or the LR-PCR product amplified from SK76[7] genomic DNA template was digested to completion with restriction enzyme ClaI or XbaI. Replica Southern blots were hybridized with probes to individual vlp genes. Numbers represent sizes of the DNA fragments recognized by individual vlp-specific probes. Two fragments not hybridizing with the vlp-specific probes are omitted from these data: the 5′-terminal ClaI fragment (0.8 kbp) of the LR-PCR product, and an XbaI fragment (1.3 kbp) encompassing a portion of vlpB but excluding most of the region III sequence recognized by the vlpB probe. vlpD, vlpE, and vlpF probes hybridized to the same 5.5-kbp XbaI fragment; vlpA and vlpG probes hybridized to the same 4.6-kbp XbaI fragment.
Nested PCR products 1 through 6 (Fig. 1A) were generated as described in Materials and Methods and hybridized in a dot blot format with vlp-specific oligonucleotide probes. Presence (+) or absence (−) of a hybridization signal is indicated. Hybridization data are not shown.
Due to similarity in the sizes of some ClaI fragments bearing vlp genes, an independent method was used to determine the ordered positions of ClaI sites in the LR-PCR product. This was accomplished by partial ClaI restriction of the amplimer followed by Southern hybridization of the resulting fragments with the 3′ or 5′ primers used to generate the LR-PCR amplimer (Fig. 1D, lanes 1 and 2, respectively). As seen in the examples shown, the size interval between fragments could be used to calculate the distance between successive ClaI sites, from the 3′ or 5′ end. Results from ClaI site mapping, along with mapping of vlp genes to limit fragments shown in Table 2, are consistent with the map shown in Fig. 1A (SK76[7]).
Another independent approach was used to localize specific vlp genes and determine their order and orientation. This employed specific primers to generate nested PCR products amplified from the LR-PCR product as template. Unique sequences in particular vlp genes were used to design forward and reverse primer pairs that encompass subsets of vlp genes (Table 1). These were used to generate a nested set of amplimers (Fig. 1A, numbered 1 through 6) that could be positioned on the restriction map shown (SK76[7]). The specificities of primer sequences and sizes of discrete nested PCR products confirmed the positions and relative orientations of most vlp genes in this complex. Finally, to further confirm the predicted map shown, specific vlp probes were hybridized to the nested PCR products 1 through 6, in a dot blot matrix summarized in Table 2. All of these approaches confirmed the map shown in Fig. 1A for SK76[7]. The one abnormality in SK76[7] compared to previously reported vlp families was the presence of an exceptionally large (4.6-kbp) XbaI fragment bearing vlpA.
Identification and positional analysis of vlpG, a structurally distinctive and phase-variable vlp gene.
To examine the genomic region adjoining vlpA, chromosomal DNA from isolate SK76[7] was digested to completion with XbaI, and a library was generated by ligating these fragments with XbaI-digested vector pGEM-7Z, as described in Materials and Methods. This vector also places inserts under the inducible T7 promoter for overexpression in E. coli. Clones from this library that hybridized with the Sig probe (Table 1) were collected, and the insert of one of these (PT1) was subcloned and sequenced.
The sequence of the 4.6-kbp XbaI DNA fragment in PT1 revealed two adjacent open reading frames (ORFs) typical of vlp gene structures (Fig. 1A and 2A) described previously (17, 20–22). Comparisons with known vlp gene sequences identified one ORF as vlpA (also confirmed by independent serologic means; see below). The second ORF was not previously known and was designated vlpG. As illustrated in Fig. 1A, vlpG and vlpA are similarly oriented and are flanked by portions of two IS1221 elements, positioned in divergent orientations. A vlpG-specific oligonucleotide probe was synthesized and used to localize vlpG by Southern hybridization (Fig. 1C; Table 2) to ClaI or XbaI restriction fragments generated from genomic DNA and the LR-PCR amplimer from SK76[7]. The orientation of the 4.6-kbp XbaI fragment within the family was further established. First, specific nested PCR products from the LR-PCR amplimer template were generated. As indicated in Fig. 1A, products 3 and 4 were generated using a reverse primer representing the specific 3′ end of vlpA, and product 1 was generated using a forward primer in a specific portion (region II) of vlpA. Second, dot hybridization patterns established the distribution of all seven vlp genes on these nested PCR products (Table 2). Collectively, these data confirmed the positions and orientations of vlpG and vlpA.
FIG. 2.
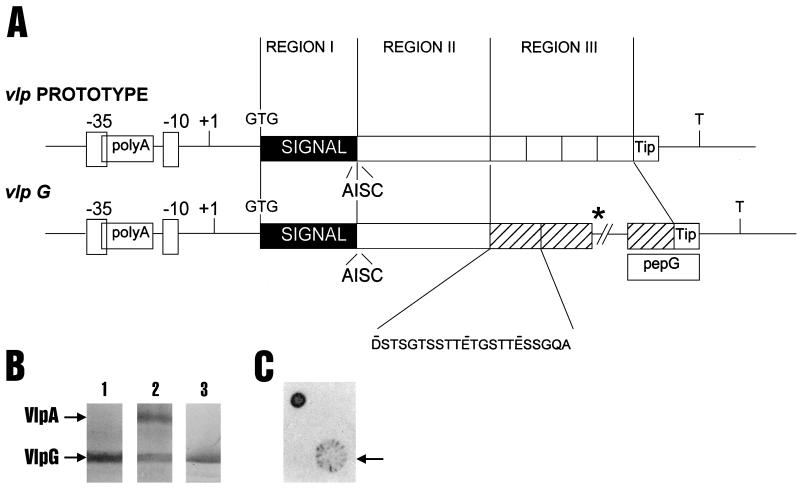
Structural features and phase-variable expression of the vlpG gene. (A) Schematic structure of a prototype vlp gene compared to that of vlpG, which contains a characteristic promoter with a homopolymeric tract of adenine residues (polyA) located between the −35 and −10 sequences and a likely transcription terminator (T) (3, 12, 20). The predicted VlpG protein is initiated with a GTG start codon, followed by a highly conserved signal peptide (region I) with a prolipoprotein signal peptidase recognition site, AISC, characteristic of known Vlp proteins (20, 22). The mature VlpG protein is encoded by region II (divergent among Vlps) and region III (composed of a tandemly repeated sequence, specific for this Vlp). The distal C-terminal portion of VlpG (Tip) is composed of a partial repeat motif. The amino acid sequence of the repeated motif (in single-letter code; hatched) in region III (showing negatively charged residues) and the location of a sequence used to generate synthetic peptide pepG are indicated below the schematic structure of vlpG. Additional repeats in region III (*) are not shown. (B) Expression of Vlp proteins from recombinant vlpG and vlpA genes. The genomic XbaI fragment from SK76[7] bearing genes vlpG and vlpA (** in Fig. 1A) was cloned and expressed in E. coli under the T7 promoter (16) as previously described (22), and Western immunoblots of bacterial lysates (lanes 1 and 2) were stained with PAb to pepG (lane 1) or a combination of this PAb and a MAb (2) to VlpA (lane 2). Lane 3 represents a Western blot of SK76[7] mycoplasmas run on the same (8%) gel and immunostained with PAb to pepG. Relative molecular masses of recombinant VlpA and VlpG were 87 and 82 kDa, respectively. (C) Phase variation of VlpG. A colony immunoblot of SK76[7] stained with PAb to pepG is shown, with a sectored colony indicated by the arrow. (This figure was constructed using Adobe Photoshop version 4.0 and 5.0 for Windows NT, Magicscan V4.1, Umax Power Look III scanner, Hewlett-Packard LaserJet 4000N printer, and Dell OptiPlex GXPro or Gateway E-4100 computer.)
vlpG contained the typical promoter and poly(A) tract characteristic of vlp genes and a stem-loop structure (ΔG = −10.5 kcal) 3′ of the translation stop (3, 20, 21). The VlpG ORF shown in Fig. 2A extends from a GTG start codon (typical of Vlp proteins) through structural motifs consistent with Vlp prolipoprotein processing and repeated regions. Like ORFs encoding other Vlps, VlpG contains no UGA (Trp) codons and is overlapped on both strands by additional ORFs (17, 20–22). To directly evaluate the presence and expression features of a putative vlpG gene translation product, a specific PAb to a synthetic peptide (pepG) comprising the predicted C-terminal tip and repeat structure of VlpG was prepared and used to detect the gene product in two ways. First, as shown in Fig. 2B, T7 induction of the recombinant PT1 plasmid, containing the XbaI insert bearing vlpG and vlpA, resulted in overexpression in E. coli of two products identified, respectively, by antibody to pepG and a previously described MAb to VlpA (2). The PAb to VlpG recognized a product of similar size in Western blots of mycoplasmal proteins (Fig. 2B, lane 3) prepared from isolate SK76[7]. This result confirmed the translation and the reading frame (defined by the Ab) of the VlpG product. Because the Ab to VlpG was determined not to immunostain other known Vlp products (A to F), using an array of variants of SK76[3] and GDL which lack the vlpG gene (data not shown), it was further used in colony immunoblotting (18) to determine whether the VlpG product expressed in SK76[7] was subject to phase variation. As indicated in Fig. 2C, the marked sectoring of single colonies revealed by Ab to VlpG was consistent with the notion that this gene product shared with other Vlps the feature of phase variation. This was very likely due to mutations in the poly(A) tract of vlpG (Fig. 2A), analogous to other vlp genes, that would affect transcription (3). Finally, the accessibility of the VlpG repeat region III to the Ab directed to pepG was consistent with the predicted orientations and surface locations of all other known products of vlp genes (11, 12).
A noteworthy feature of the VlpG structure is the distinctive (22-residue) length of the tandem protein repeats in region III of the product (Fig. 2A). Despite this longer unit, compared to 12 or 13 residues for analogous units in VlpA-F (21, 22), VlpG shares the charge distribution and composition found in repeat units of all Vlps, representing a net negative charge and high content of Gly, Ser, and Thr (17, 20, 22). An unusual feature of VlpG, like all Vlps, is the discordance between the mass of the protein predicted from the deduced sequence and the predicted migration of the product in SDS-PAGE. This feature has recently been attributed to properties of region III repeats in these proteins (6). The entire sequence of the vlpG gene could not be precisely determined due to its extensive repetitive region. However, sequence data clearly revealed 13 full repeats, all of which were identical. This number was also consistent with the number of repeats determined by probing fragments generated from a recombinant insert bearing vlpG, after partial restriction with XmnI, which recognizes a site in each repeat unit of vlpG (data not shown). Consequently, the calculated mass of the mature VlpG protein from the SK76[7] strain with 13 full repeats was 30.3 kDa, predicting a much faster migration in SDS-PAGE than the 82-kDa relative molecular mass observed for both the recombinant and authentic mycoplasmal VlpG products shown in Fig. 2B.
Overall, the vlpG gene found in SK76[7] encodes a unique repetitive structure within a product otherwise typical of the Vlp family of lipoproteins. Moreover, the seven-gene repertoire in this isolate is likely to represent the complete vlp gene family in the original pathogenic SK76 strain (11, 12, 22).
VlpA contains a complex repetitive structure.
While VlpB to -G contain a region III comprised of nearly identical, tandemly repeated units of 12, 13, or (in VlpG) 22 amino acids, comparative analysis of vlpA genes from clonal variants of SK76[3], SK76[7], and GDL[6] available from this or previous studies revealed a more complex repeat structure. The repeat structure of the shortest known VlpA variant, A39 from SK76[3] (Fig. 3A), contains all known repeat elements of region III in the VlpA product. These include a sequence of 39 amino acids (rep), a version of rep with a deletion of 60 bp, a small repeat of 13 amino acids, and a conserved distal C-terminal sequence (Tip). A39 is the smallest of four spontaneous deletion size variants emerging in broth culture from a clonal population of SK76[3] expressing the largest known VlpA, A87 (Fig. 3A). The vlpA sequence from this A87 clonal variant revealed a much more complex repeat structure in region III, containing composites of the same basic elements arranged in alternative configurations as shown in Fig. 3A (including, for example, rep* and rep**). From the A87 variant of SK76[3], three other spontaneous deletion variants, in addition to A39, have been obtained by screening for colony opacity variants that were known to be correlated with altered length of the VlpA products expressed (12). Sequences from these variants (A46, A50, and A57), like that of A39, showed a variety of in-frame deletions within the vlpA gene. These deletions could be explained by the elimination of various internal repeated elements from the A87 configuration through intragenic deletion mechanisms (Fig. 3A).
As the vlpA gene of the SK76[7] isolate was sequenced, we were able to confirm that it too contained the specific A87 configuration. This might be expected since the SK76[7] and SK76[3] isolates had the same origin prior to culture passage. Finally, sequencing of vlpA from the long-term tissue culture passaged GDL strain showed an intermediate size VlpA, with a repeat structure having the same basic elements but in yet another unique configuration (Fig. 3B). Interestingly, this structure could also have been generated in principle by intragenic deletions from a configuration such as that in SK76 A87. In light of analysis of these deletion variants and our earlier demonstration that the vlpA gene is also subject to expansion in length from small and intermediate forms (21), these results indicate that vlpA has a more complex and distinctive repeat structure than other vlp genes and that diverse variants can occur spontaneously during propagation in culture conditions.
The natural vlp repertoire.
We propose that the enlarged vlp gene family in the pathogenic SK76 strain, containing seven distinct vlp genes, may be a prototype of vlp families in the natural host environment. Simple deletions from this configuration could yield the SK76[3] family, derived from the same clonal stock of SK76 after passage in broth medium, or the six-gene family in the tissue culture contaminant GDL, isolated on a different continent over an interval spanning several years (22). Further examination of field and pathogenic isolates should allow us to test the hypothesis that the natural vlp family contains seven functional vlp genes. Horizontal sampling may also reveal the conservation of the genomic context of these arrays, as well as the stability of genetic signatures linked to IS1221 elements within vlp families (Fig. 1). Finally, such studies will determine whether or not the entire family resides on a larger element, or can be located at diverse chromosomal locations.
The range and nature of functions associated with a possibly fixed array of structurally diverse vlp genes remains an intriguing enigma. Considering the nearly limitless diversity (17, 20) embodied in the Vlp system (e.g., through intergenic recombination, frameshift mutations in overlapping reading frames, or specific composites created by multiple reiterated sequences in region III of vlpA or the extended repeat unit in region III of vlpG), selection and maintenance of a specific array of Vlp products will be a critical point to establish, since it would argue strongly for specific functions associated with each product, presumably selected in the natural host niche. This study establishes the hypothesis and tools to address these questions and identifies additional forms of diversity in two Vlp products that may be useful in understanding Vlp structure and function.
ACKNOWLEDGMENTS
We thank Mary F. Kim and Jeong Im for preparation and analysis of key peptide and serologic reagents used in this study and Matthew Mouw for assistance in chromosomal analyses.
This work was supported in part by USPHS grant AI31656 from the National Institute of Allergy and Infectious Diseases and by a University of Missouri—Columbia Molecular Biology Program fellowship (C.C.).
REFERENCES
- 1.Butler M, Leach R H. A mycoplasma which induces acidity and cytopathic effect in tissue culture. J Gen Microbiol. 1964;34:285–294. doi: 10.1099/00221287-34-2-285. [DOI] [PubMed] [Google Scholar]
- 2.Citti C, Kim M F, Wise K S. Elongated versions of Vlp surface lipoproteins protect Mycoplasma hyorhinis escape variants from growth-inhibiting host antibodies. Infect Immun. 1997;65:1773–1785. doi: 10.1128/iai.65.5.1773-1785.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Citti C, Wise K S. Mycoplasma hyorhinis vlp gene transcription: critical role in phase variation and expression of surface lipoproteins. Mol Microbiol. 1995;18:649–660. doi: 10.1111/j.1365-2958.1995.mmi_18040649.x. [DOI] [PubMed] [Google Scholar]
- 4.Cleavinger C M, Kim M F, Im J H, Wise K S. Identification of mycoplasma membrane proteins by systematic TnphoA mutagenesis of a recombinant library. Mol Microbiol. 1995;18:283–293. doi: 10.1111/j.1365-2958.1995.mmi_18020283.x. [DOI] [PubMed] [Google Scholar]
- 5.Devereux J, Haeberli P, Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984;12:387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Leigh S, Citti C, Im J, Wise K. Aberrant structural features and instability of internal sequences in Vlp lipoproteins defined by analysis of recombinant Vlp fusion proteins. IOM Lett. 1996;4:386–387. [Google Scholar]
- 7.McGarrity G J, Kotani H, Butler G H. Mycoplasmas and tissue culture cells. In: Maniloff J, McElhaney R N, Finch L R, Baseman J B, editors. Mycoplasmas: molecular biology and pathogenesis. Washington, D.C.: American Society for Microbiology; 1992. pp. 445–454. [Google Scholar]
- 8.Morita T, Fukuda H, Awakura T, Shimada A, Umemura T, Kazama S, Yagihashi T. Demonstration of Mycoplasma hyorhinis as a possible primary pathogen for porcine otitis media. Vet Pathol. 1995;32:107–111. doi: 10.1177/030098589503200202. [DOI] [PubMed] [Google Scholar]
- 9.Razin S, Yogev D, Naot Y. Molecular biology and pathogenicity of mycoplasmas. Microbiol Mol Biol Rev. 1998;62:1094–1156. doi: 10.1128/mmbr.62.4.1094-1156.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rosengarten R, Theiss P M, Yogev D, Wise K S. Antigenic variation in Mycoplasma hyorhinis: increased repertoire of variable lipoproteins expanding surface diversity and structural complexity. Infect Immun. 1993;61:2224–2228. doi: 10.1128/iai.61.5.2224-2228.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rosengarten R, Wise K S. Phenotypic switching in mycoplasmas: phase variation of diverse surface lipoproteins. Science. 1990;247:315–318. doi: 10.1126/science.1688663. [DOI] [PubMed] [Google Scholar]
- 12.Rosengarten R, Wise K S. The Vlp system of Mycoplasma hyorhinis: combinatorial expression of distinct size variant lipoproteins generating high-frequency surface antigenic variation. J Bacteriol. 1991;173:4782–4793. doi: 10.1128/jb.173.15.4782-4793.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ross R F. Mycoplasmal pneumonia of swine. In: Leman A D, Straw B E, Mengeling W L, D'Allaire S, Taylor D J, editors. Diseases of swine. Ames, Iowa: Iowa State University Press; 1990. pp. 537–543. [Google Scholar]
- 14.Ross R F, Dale S E, Duncan J R. Experimentally induced Mycoplasma hyorhinis arthritis in swine: immune response to 26th postinoculation week. Am J Vet Res. 1973;34:367–372. [PubMed] [Google Scholar]
- 15.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- 16.Tabor S, Richardson C C. A bacteriophage T7 RNA polymerase/promoter system for controlled exclusive expression of specific genes. Proc Natl Acad Sci USA. 1985;82:1074–1078. doi: 10.1073/pnas.82.4.1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wise K S. Adaptive surface variation in mycoplasmas. Trends Microbiol. 1993;1:59–63. doi: 10.1016/0966-842X(93)90034-O. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wise K S, Kim M F, Watson-McKown R. Variant membrane proteins. In: Razin S, Tully J G, editors. Molecular and diagnostic procedures in mycoplasmology. New York, N.Y: Academic Press; 1995. pp. 227–241. [Google Scholar]
- 19.Wise K S, Watson R K. Mycoplasma hyorhinis GDL surface protein antigen p120 defined by monoclonal antibody. Infect Immun. 1983;41:1332–1339. doi: 10.1128/iai.41.3.1332-1339.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wise K S, Yogev D, Rosengarten R. Antigenic variation. In: Maniloff J, McElhaney R N, Finch L R, Baseman J B, editors. Mycoplasmas: molecular biology and pathogenesis. Washington, D.C.: American Society for Microbiology; 1992. pp. 473–490. [Google Scholar]
- 21.Yogev D, Rosengarten R, Watson-McKown R, Wise K S. Molecular basis of Mycoplasma surface antigenic variation: a novel set of divergent genes undergo spontaneous mutation of periodic coding regions and 5′ regulatory sequences. EMBO J. 1991;10:4069–4079. doi: 10.1002/j.1460-2075.1991.tb04983.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yogev D, Watson-McKown R, Rosengarten R, Im J H, Wise K S. Increased structural and combinatorial diversity in an extended family of genes encoding Vlp surface proteins of Mycoplasma hyorhinis. J Bacteriol. 1995;177:5636–5643. doi: 10.1128/jb.177.19.5636-5643.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zheng J, McIntosh M A. Characterization of IS1221 from Mycoplasma hyorhinis: expression of its putative transposase in Escherichia coli incorporates a ribosomal frameshift mechanism. Mol Microbiol. 1995;16:669–685. doi: 10.1111/j.1365-2958.1995.tb02429.x. [DOI] [PubMed] [Google Scholar]