Abstract
Hypoxia preconditioning is neuroprotective, but the therapeutic effects of intermittent hypoxia were not fully considered. The present study investigated the neuroprotective effect and mechanism of intermittent hypoxia on motor function after cerebral ischemia and explored alternative clinical treatment options. In total, 36 8-week-old male Sprague-Dawley rats were subjected to 60 min of transient middle cerebral artery occlusion (tMCAO) and then randomly divided into a sham-operated group (SHAM), tMCAO-sedentary group (SED), and tMCAO-intermittent hypoxia group (IH). The intervention was performed 1 week after tMCAO and lasted 4 weeks. Rats in the IH group were placed in an animal hypoxic chamber (altitude 5000 m and oxygen concentration of 13%) for 4 h/day and 7 days/week, and rats in the SED group were placed in a normoxic environment for 4 weeks. Body weights, neurological deficit scores, cerebral infarction volume ratios, gait analyses, mitochondrial structure, adenosine triphosphate (ATP) content and AMO-activated protein kinase (AMPK), peroxisome proliferator-activated receptor γ co-activator-1α (PGC-1α), and silencing regulatory protein 3 (Sirt3) expression in the peri-ischemic region brain tissues were detected during the intervention. Compared with the SED group, the body weight of the IH group gradually recovered, and the neurological deficit scores were significantly reduced (P < 0.05). The gait analysis results showed that the pressure of the affected paw and the maximum content area, swing speed, stride length, and other parameters were significantly restored (P < 0.05). The cerebral infarction volume ratio was significantly reduced (P < 0.01). Mitochondrial morphological structure damage in the peri-ischemic region brain tissues recovered, the number was significantly increased (P < 0.05), and the expression of AMPK, PGC-1α, and Sirt3 proteins (P < 0.05), and ATP content were significantly increased (P < 0.05). Intermittent hypoxia may activate the AMPK–PGC-1α–Sirt3 signaling pathway, promote mitochondrial biogenesis, repair mitochondrial ultrastructural damage, and improve mitochondrial function to reduce brain damage and promote motor function recovery in rats with cerebral ischemia.
Keywords: Intermittent hypoxia, cerebral ischemia, mitochondria, motor function
Impact Statement
Early treatment and early recovery significantly improve the prognosis of patients with ischemic stroke. Some stroke patients have limited aerobic exercise capacity due to primary or concomitant diseases, and being sedentary for a long time is not conducive to recovery and increases the risk of recurrence. Intermittent hypoxia training was widely studied in a variety of age-related diseases in recent years, including ischemic stroke. Hypoxia preconditioning is neuroprotective, but the therapeutic effects of intermittent hypoxia were not fully considered. The present study investigated the neuroprotective effects and mechanism of intermittent hypoxia on motor function after cerebral ischemia to investigate whether intermittent hypoxia protected against cerebral ischemia via the regulation of mitochondrial energy metabolism to improve motor function and provide an experimental basis for the clinical application of intermittent hypoxia.
Introduction
Stroke has a high disability rate, high fatality rate, and high recurrence rate. There are 250 million new stroke cases in China annually, and this number is growing, which creates a substantial burden for families and society. 1 Ischemic stroke accounts for 87% of stroke cases. 2 Early diagnosis, early treatment, and early recovery are highly significant to improve the prognosis of patients with ischemic stroke.
Some stroke patients have limited aerobic exercise capacity due to primary or concomitant diseases, and being sedentary for a long time is not conducive to recovery and increases the risk of recurrence. 3 Intermittent hypoxia training was widely studied in a variety of age-related diseases in recent years, including ischemic stroke. Therapeutic intermittent hypoxia training uses alternate exposure to a moderately hypoxic environment, with an oxygen content of approximately 10%, and a normoxic environment, with an oxygen content of 21%, to induce a protective mechanism by enhancing energy metabolism and antioxidant effects.4,5
Neuronal hypoxia after stroke damages mitochondria, interferes with the phosphorylation process and adenosine triphosphate (ATP) supply, and disrupts brain metabolism and homeostasis. 6 ATP provides an impetus for neural plasticity. 7 The intracellular metabolic sensor 5’ adenosine monophosphate-activated protein kinase (AMPK) is activated when ATP production decreases and the adenosine monophosphate (AMP)/ATP ratio increases and improves mitochondrial dysfunction by inducing mitochondrial biogenesis and energy production. 8 Peroxisome proliferator-activated receptor γ co-activator-1α (PGC-1α) is the “initiator” and “master regulator” of mitochondrial occurrence. PGC-1α activation or overexpression compensate for mitochondrial deletion and regulate mitochondrial dysfunction and oxidative damage. 9 Silencing regulatory protein 3 (Sirt3) is a member of the silencing information regulator protein family that is primarily located in the mitochondrial matrix, and increased Sirt3 expression helps alleviate cerebral ischemia. 10 Sirt3 is a downstream target of PGC-1α that is directly regulated by AMP-activated protein kinase (AMPK), and it plays an important role in promoting mitochondrial biogenesis. 11 Our previous studies confirmed that intermittent hypoxia improved cardiac function and exercise tolerance of rats with myocardial infarction via activation of AMPK in the myocardium. 12 Therefore, we hypothesized that intermittent hypoxia would induce mitochondrial biogenesis via the AMPK–PGC-1α–Sirt3 signaling pathway and improve motor function after cerebral ischemia.
The present study used a rat transient middle cerebral artery occlusion (tMCAO) model and detected changes in cerebral infarction volume, gait analysis, mitochondrial morphology, and specific protein levels after intermittent hypoxia intervention. The present study investigated whether intermittent hypoxia protected against cerebral ischemia by regulating mitochondrial energy metabolism to improve motor function, which provides an experimental basis for the clinical application of intermittent hypoxia.
Materials and methods
Experimental animals and groups
Adult male Sprague-Dawley rats (n = 36, 8 weeks old, 280–300 g) were obtained from Beijing Huafukang Biotechnology Co., Ltd. (Beijing, China) and randomly divided into a sham-operated group (SHAM, n = 12), tMCAO-sedentary group (SED, n = 12), and tMCAO-intermittent hypoxia group (IH, n = 12). All animals were housed in a standard laboratory environment (20–25°C, 50–60% relative humidity, 12 h/12 h light/dark cycle) and were given free access to food and water. The experimental procedure was performed according to the National Institutes of Health (NIH) Laboratory Care and Use Guidelines to minimize the number of animals used and animal suffering during the experiment, and the Ethics Committee of Tianjin Medical University approved the study (TMUaMEC 2018037).
Focal cerebral ischemia/reperfusion model
The focal cerebral ischemia/reperfusion model was established as previously described. 13 Under anesthesia (sodium pentobarbital, 40 mg/kg, i.p.), the rats were fixed in the supine position and opened along the median carotid line. Subcutaneous tissue and anterior carotid muscles were passively separated, the left common carotid artery was exposed, and the internal and external carotid arteries were separated from the common carotid artery bifurcation to the distal end. The proximal ends of the left common carotid artery and the external carotid artery were completely ligated, and a fine silicon-coated surgical nylon monofilament (2838-A4, 0.38 ± 0.02 mm, Beijing Xinong Technology Co., Ltd., China) was inserted into the left internal carotid artery to block the middle cerebral artery (up to approximately 18.0–20.0 mm distal to the bifurcation of the carotid artery) for 60 min. The filament was removed for reperfusion. The rectal temperature was maintained at 37 ± 0.5°C during the surgery. The SHAM group underwent the same procedure without filament insertion. After the rats recovered, a Longa score of 1–3 points was used as the standard for successful modeling.
Intermittent hypoxia training
Intermittent hypoxia was induced by placing tMCAO rats in an animal hypoxic chamber with a simulated oxygen concentration of 13.0% and atmospheric pressure of 54.0 kPa (equivalent to an altitude of 5000 m). The hypoxia program was developed based on a study of the time window of intermittent hypoxia intervention described by Tsai et al. 14 One week after surgery, the following hypoxia intervention was implemented: hypoxia (FiO2 = 13%) for 1 h followed by normoxia (FiO2 = 21%) for 10 min, for four cycles, 4 h/day (10:00 to 14:00), 7 days/week for 4 weeks. Rats in the SHAM and SED groups were placed in the same hypoxic chamber for the same times, but the door was opened to expose the rats to a normoxic environment (FiO2 = 21%).
Neurological function assessment
The modified neurological severity score (mNSS) and beam-walking test were used to evaluate the neurological function of the rats during the intervention. The mNSS is a widely used comprehensive score that includes movement, sensation, balance, and reflex. The total score was between 0 and 18 points, and lower scores indicate better recovery of nerve injury (0 indicates no neurological deficit, and 18 indicates the most serious neurological deficit). 15 The beam-walking test primarily reflects changes in balance, coordination functions, and proprioception. The rats quickly traverse a balance beam (100 cm × 2 cm × 2 cm) that was 1 m from the ground. 16 The scale is presented in Table 1.
Table 1.
Specific behavioral scoring criteria for the beam-walking test.
| Behavior | Score |
|---|---|
| Paws on top of the beam with steady posture | 1 |
| Grasps sides of beam and/or movement instability | 2 |
| One or more paw(s) slip off beam | 3 |
| Attempts to balance on beam but falls off | 4 |
| Drapes over beam and/or hangs on beam and falls off | 5 |
| Falls off beam with no attempt to balance or hang on | 6 |
Gait analysis
A gait analysis was performed using the CatWalk Analysis System (Noldus Information Technology, Wageningen, The Netherlands) after 4 weeks of intervention. Each rat was evaluated at least 3 times. Each time, a set length glass plate was crossed in a specified time (within 10 s), and the entire experimental process was completed in a dark and silent environment. The CatWalk system automatically generated a series of gait parameters, including average speed, run duration, maximum intensity, print area, stride length, base of support (BOS), swing speed, swing, and paw coordination parameters, when identifying and marking each footprint using CatWalk XT 10.6 software for data analyses. 17
Infarct volume assessment
Magnetic resonance imaging (MRI) was performed before and 4 weeks after the intervention using a 3.0 T animal MRI scanner (MagnetomVerio, SIEMENS, Germany) to measure the cerebral infarct volume. Before the scan, the animals were anesthetized with sodium pentobarbital for 5 min to maintain anesthesia during the entire MRI scan. The rats were placed in a stent compatible with the MRI acquisition system and fixed in the supine position, and the body temperature was maintained at 37 ± 0.5°C. T2-weighted imaging (T2WI) was performed to detect the infarct volume from bregma + 1.7 to −6.3 mm using a T2-SPACE sequence with the following parameters: repetition time (TR) = 1000 ms, time to echo (TE) = 155 ms, flip angle (FA) = 120°, field of view (FOV) = 60 × 60 mm2, matrix = 192 × 192, and 72 slices of 0.4 mm thickness without a gap. After the T2WI scan of the brain structure, the areas where the T2 signal value of the ipsilateral hemisphere was abnormally higher than the contralateral hemisphere were selected, and the cerebral infarction volume ratio was calculated as follows: (infarct area of each layer of ipsilateral hemisphere × slice thickness)/(the area of contralateral hemisphere of the same plane × slice thickness) × 100% = the cerebral infarction volume of ipsilateral hemisphere/the cerebral infarction volume of contralateral hemisphere × 100%. 18
Transmission electron microscope assessment of mitochondrial ultrastructure
After 4 weeks of intervention, six rats in each group were randomly selected for deep anesthesia. Anesthetized rats underwent cardiac perfusion with 0.9% normal saline and 4% paraformaldehyde followed by decapitation and brain removal. The ischemic penumbra was separated on ice. The samples were cut into small pieces of 1 mm3 and immediately fixed in a 6% glutaraldehyde solution. Sections with a thickness of 50 nm were prepared according to a conventional electron microscope sample preparation procedure. Six sections of each rat were selected, and the spacing between sections was 100 μm. Six penumbral areas in every section were randomly selected before being photographed at 5000× magnification by transmission electron microscopy (Hitachi HT7700, Japan) to observe the morphological structure and number of brain neuron mitochondria. We first identified the neurons at 1000× magnification. Neuronal membranes are generally surrounded by two dark lines separated by a narrow light-colored gap. The nuclear membrane was identified at 2500× magnification, and the mitochondria in the cytoplasm were analyzed at 5000× magnification. Mitochondria are generally round, oval, or rod-shaped and show a double-layer membrane structure with an intimal crest.
Western blot analysis
Six rats in each group were randomly selected for Western blot analysis. Brain tissues from the peri-ischemic region were dissected from rat brains after 4 weeks of intervention. The protein concentration was quantified using a BCA kit (Solarbio, Beijing, China), and total protein was separated and transferred to polyvinylidene difluoride membranes (Millipore, USA). Membranes were blocked in Tris-buffered saline (TBS) for 2 h at room temperature. The primary antibodies were anti-AMPK (Abcam, UK, 1:1000), anti-PGC-1α (Abcam, UK, 1:1000), anti-Sirt3 (Abcam, UK, 1:1000), and anti-GAPDH (Cell Signaling, USA, 1:1000). Nonspecific sites were blocked, and the membranes were incubated overnight at 4°C. The membranes were washed with TBS-T and incubated with horseradish peroxidase-conjugated secondary antibodies (Cell Signaling, USA, 1:2000) for 1 h at room temperature. ImageJ software was used to detect the gray values of the bands, and the proteins were quantitatively analyzed.
Measurement of ATP content in mitochondrial cerebral tissue
We measured cellular ATP levels in cerebral tissues using an ATP assay kit (Solarbio, China). A volume of 1 mL pyrolysis solution was added to each 0.1 g tissue, and the ice bath homogenate was centrifuged at 8000 ×g for 10 min at 4°C. The supernatant was transferred to another EP tube. Chloroform (500 μL) was added, mixed well and centrifuged at 10,000 ×g for 3 min at 4°C. The supernatant was collected and placed on ice for testing. The ATP standard solution was diluted with distilled water to an appropriate concentration gradient. The ATP detection working solution was fully mixed with the sample or standard solution for 10 s at 37°C and detected using ultraviolet spectrophotometry. The ATP concentration was calculated according to the standard curve data and expressed as μmol/g protein.
Statistical analysis
Statistical analyses were performed using SPSS 20.0 statistical software (SPSS Inc., Cary, NC), and graphs were made using Prism 6.0 (GraphPad, San Diego, CA). Numerical data conforming to a normal distribution are presented as the means ± SD, and the beam-walking test was analyzed using the Mann–Whitney test. One-way analysis of variance (ANOVA) was used to compare multiple groups, and the LSD-t test was used for multiple comparisons between pairs. P < 0.05 was considered significant.
Results
Effect of intermittent hypoxia on body weight
The body weights of each group were the same at baseline (P = 0.915). The weights of each group decreased to varying degrees due to injury after the surgery, but there was no significant difference between the groups before intervention (P = 0.165). After the intervention, the weights of each group gradually recovered, and the fastest weight gain occurred in the SHAM group. After 1 to 4 weeks of intervention, there was a significant difference between the SED and IH groups and the SHAM group (P < 0.05), but there was no significant difference between the SED and IH groups (Figure 1).
Figure 1.

The body weights of rats during and after tMCAO surgery (x ± s, n = 6).
tMCAO: transient middle cerebral artery occlusion; SHAM: sham-operated group; SED: tMCAO-sedentary group; IH: tMCAO-intermittent hypoxia group.
*P < 0.05; **P < 0.01 versus SHAM; #P < 0.05; ##P < 0.01; ###P < 0.001 versus SHAM.
Effect of intermittent hypoxia on neurological function
The mNSS score of the SHAM group was 0, and the beam-walking test score was 1. There were no significant differences in the mNSS or beam-walking test scores between the SED and IH groups before the intervention. After 1 to 4 weeks of intervention, the mNSS score of the IH group was always significantly lower than the SED group (P1w = 0.013, P2w = 0.005, P3w = 0.005, P4w = 0.001). After 2 to 4 weeks of intervention, the beam-walking test score of the IH group was significantly lower than the SED group (P2w = 0.006, P3w = 0.005, P4w = 0.018; Figure 2(a) and (b)). The within-group comparison showed that the SED and IH groups had the same tendency to recover neurological function, and the recovery in the IH group was more significant in the first 2 weeks of intervention (PmNSS-IH < 0.001, PmNSS-SED < 0.01; PBeam-walking-IH < 0.01, PBeam-walking-SED < 0.05; Figure 2(c) and (d)).
Figure 2.
(a, c) mNSS scores and (b, d) beam-walking test scores of the tMCAO rats during each week of intervention.
mNSS: modified neurological severity score; tMCAO: transient middle cerebral artery occlusion; SHAM: sham-operated group; SED: tMCAO-sedentary group; IH: tMCAO-intermittent hypoxia group.
(a, b): Comparison of the results between the SHAM, SED, and IH groups.
*P < 0.05; **P < 0.01 versus IH.
(c, d): Comparison of the results at different time points within the SED and IH groups.
*P < 0.05; **P < 0.01; ***P < 0.001.
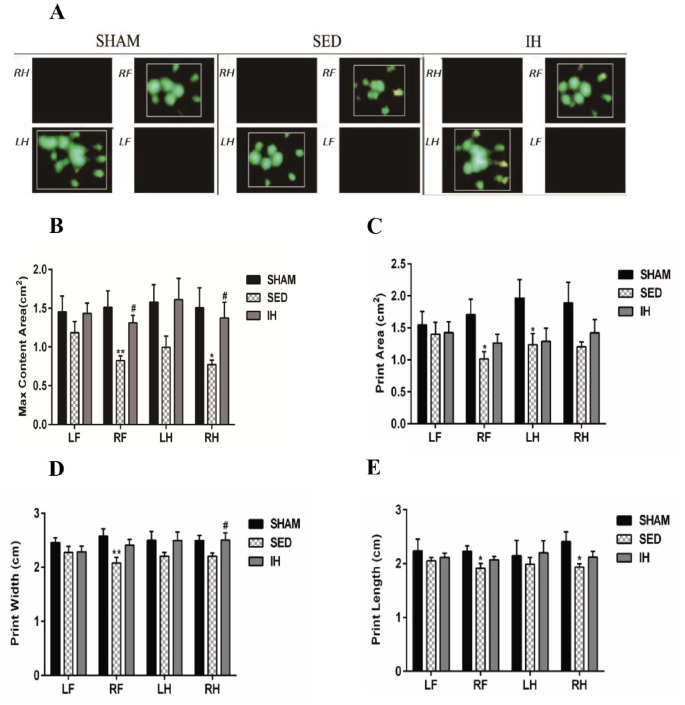
Effect of intermittent hypoxia on gait analysis
After 4 weeks of intervention, the rats in each group were analyzed using a CatWalk XT 10.6 gait analysis system, and the parameters were classified into four categories: basic motion, static, dynamic, and balance coordination parameters.
Basic motion parameters
The run duration was the duration of the animal’s passage through the prescribed route. The average speed was the average speed of the animal’s body in the recorded run. The results showed that the run duration of rats in the SED group was significantly longer than the SHAM group (P = 0.001), and the average speed was significantly slower (P = 0.001). Compared with the SED group, the IH group had a significantly shortened run duration (P = 0.027) and an increased average speed (P = 0.014). There was no significant difference between the IH and SHAM groups (Figure 3).
Figure 3.
(a) Run duration and (b) average speed ( ± s, n = 6) of rats in each group after 4 weeks of intervention.
tMCAO: transient middle cerebral artery occlusion; SHAM: sham-operated group; SED: tMCAO-sedentary group; IH: tMCAO-intermittent hypoxia group.
**P < 0.01 versus SHAM; #P < 0.05 versus SED.
Static parameters
The maximum intensity was the maximum pressure of a complete paw. The maximum content area was the maximum area of a paw that came into contact with the glass plate. The print area was the surface area of the complete print, including all parts during standing. The print width was the width (vertical direction) of the complete paw print. The print length was the length (horizontal direction) of the complete paw print. The results showed that the maximum intensity (PRF = 0.004, PLH = 0.031, PRH = 0.007), maximum content area (PRF = 0.003, PRH = 0.017), print area (PRF = 0.012, PLH = 0.038), print width (PRF = 0.008), and print length (PRF = 0.021, PRH = 0.016) of the SED group were significantly reduced compared with the SHAM group. The maximum intensity (PRF = 0.045, PRH = 0.005), maximum content area (PRF = 0.026, PRH = 0.042), and print width (PRH = 0.048) of the IH group were significantly improved compared with the SED group (Table 2, Figures 4 and 5).
Table 2.
Comparison of CatWalk static parameters of rats in each group.
| Parameters | SHAM | SED | IH |
|---|---|---|---|
| Max intensity (a.u.) | |||
| LF | 218.888 ± 0.806 | 207.852 ± 3.839 | 215.070 ± 7.032 |
| RF | 220.555 ± 2.560 | 201.968 ± 4.357** | 213.917 ± 4.406 † |
| LH | 220.828 ± 1.683 | 199.013 ± 7.534* | 216.570 ± 8.174 |
| RH | 219.862 ± 1.879 | 200.167 ± 5.617** | 220.778 ± 4.910 †† |
| Max content area (cm2) | |||
| LF | 1.453 ± 0.204 | 1.185 ± 0.143 | 1.435 ± 0.130 |
| RF | 1.512 ± 0.212 | 0.825 ± 0.060** | 1.312 ± 0.096 † |
| LH | 1.580 ± 0.224 | 0.997 ± 0.143 | 1.610 ± 0.275 |
| RH | 1.505 ± 0.258 | 0.773 ± 0.058* | 1.375 ± 0.201 † |
| Print area (cm2) | |||
| LF | 1.544 ± 0.210 | 1.402 ± 0.186 | 1.424 ± 0.171 |
| RF | 1.708 ± 0.238 | 1.011 ± 0.118* | 1.264 ± 0.137 |
| LH | 1.966 ± 0.288 | 1.234 ± 0.173* | 1.288 ± 0.205 |
| RH | 1.887 ± 0.327 | 1.203 ± 0.076 | 1.422 ± 0.205 |
| Print width (cm) | |||
| LF | 2.456 ± 0.089 | 2.275 ± 0.110 | 2.289 ± 0.104 |
| RF | 2.574 ± 0.137 | 2.077 ± 0.104** | 2.409 ± 0.105 |
| LH | 2.499 ± 0.166 | 2.207 ± 0.066 | 2.497 ± 0.156 |
| RH | 2.496 ± 0.093 | 2.203 ± 0.058 | 2.504 ± 0.132 † |
| Print length (cm) | |||
| LF | 2.234 ± 0.220 | 2.052 ± 0.060 | 2.116 ± 0.078 |
| RF | 2.230 ± 0.100 | 1.913 ± 0.090* | 2.070 ± 0.067 |
| LH | 2.147 ± 0.285 | 1.987 ± 0.122 | 2.199 ± 0.227 |
| RH | 2.412 ± 0.180 | 1.935 ± 0.059* | 2.119 ± 0.104 |
SHAM: sham-operated group; tMCAO: transient middle cerebral artery occlusion; SED: tMCAO-sedentary group; IH: tMCAO-intermittent hypoxia group; LF: left forepaw; RF: right forepaw; LH: left hind paw; RH: right hind paw (R is the affected side).
P < 0.05; **P < 0.01 versus SHAM; †P < 0.05; ††P < 0.01 versus SED.
Figure 4.
Foot pressure and maximum intensity of rats in each group after 4 weeks of intervention ( ± s, n = 6). (a) Three-dimensional graph of the footprint (the vertical axis is intensity) and (b) max intensity.
LF: left forepaw; RF: right forepaw; LH: left hind paw; RH: right hind paw (R is the affected side); tMCAO: transient middle cerebral artery occlusion; SHAM: sham-operated group; SED: tMCAO-sedentary group; IH: tMCAO-intermittent hypoxia group.
*P < 0.05; **P < 0.01 versus SHAM; #P < 0.05; ##P < 0.01 versus SED. (A color version of this figure is available in the online journal.)
Figure 5.
CatWalk static parameters of rats in each group after 4 weeks of intervention ( ± s, n = 6). (a) Representative paw prints of each group; (b) maximum content area; (c) print area; (d) print width; (e) print length.
LF: left forepaw; RF: right forepaw; LH: left hind paw; RH: right hind paw (R is the affected side); tMCAO: transient middle cerebral artery occlusion; SHAM: sham-operated group; SED: tMCAO-sedentary group; IH: tMCAO-intermittent hypoxia group.
*P < 0.05; **P < 0.01 versus SHAM; #P < 0.05 versus SED. (A color version of this figure is available in the online journal.)
Dynamic parameters
The stand was the duration in seconds of contact of a paw with the glass plate. The swing was the duration in seconds of no contact of a paw with the glass plate. The swing speed was the speed (distance unit/second) of the paw during swing. The stride length was the distance (in distance units) between successive placements of the same paw. The BOS was the base of support, which represented the average width of front paw or hind paw. The results showed that the stand (PLF = 0.037), swing (PLF = 0.014, PRF = 0.030, PRH = 0.012), swing speed (PLF = 0.020, PRF = 0.001, PLH = 0.017, PRH = 0.008), stride length (PLF = 0.001, PRF = 0.004, PLH = 0.021, PRH = 0.003), and BOS (PHP = 0.010) of the SED group were significantly worse than the SHAM group. The swing (PRF = 0.040, PRH = 0.012), swing speed (PLF = 0.035, PRF = 0.029, PRH = 0.039), stride length (PLF = 0.010, PRF = 0.048, PRH = 0.024), and BOS (PHP = 0.024) of the IH group were significantly improved compared with the SED group (Table 3 and Figure 6).
Table 3.
Comparison of CatWalk dynamic parameters of rats in each group.
| Parameters | SHAM | SED | IH |
|---|---|---|---|
| Stand (s) | |||
| LF | 0.271 ± 0.025 | 0.376 ± 0.046* | 0.300 ± 0.022 |
| RF | 0.282 ± 0.025 | 0.403 ± 0.072 | 0.338 ± 0.046 |
| LH | 0.308 ± 0.020 | 0.418 ± 0.067 | 0.338 ± 0.035 |
| RH | 0.336 ± 0.024 | 0.404 ± 0.059 | 0.328 ± 0.037 |
| Swing (s) | |||
| LF | 0.141 ± 0.004 | 0.204 ± 0.024* | 0.187 ± 0.014 |
| RF | 0.170 ± 0.009 | 0.237 ± 0.032* | 0.174 ± 0.008 † |
| LH | 0.131 ± 0.005 | 0.141 ± 0.007 | 0.135 ± 0.005 |
| RH | 0.143 ± 0.010 | 0.233 ± 0.036* | 0.143 ± 0.009 † |
| Swing speed (cm/s) | |||
| LF | 85.627 ± 8.407 | 64.292 ± 3.397* | 83.366 ± 4.418 † |
| RF | 85.080 ± 6.630 | 59.093 ± 2.958** | 74.805 ± 3.278 † |
| LH | 100.033 ± 5.379 | 81.319 ± 2.708* | 95.561 ± 6.111 |
| RH | 96.140 ± 8.674 | 71.594 ± 2.999** | 89.794 ± 3.553 † |
| Stride length (cm) | |||
| LF | 14.862 ± 0.388 | 12.289 ± 0.545** | 14.040 ± 0.292 † |
| RF | 14.940 ± 0.522 | 12.657 ± 0.614** | 14.107 ± 0.184 † |
| LH | 14.755 ± 0.477 | 12.493 ± 0.921* | 14.285 ± 0.275 |
| RH | 15.028 ± 0.562 | 12.137 ± 0.761** | 14.169 ± 0.287 † |
| BOS (cm) | |||
| Front paws | 2.793 ± 0.262 | 2.494 ± 0.206 | 2.643 ± 0.204 |
| Hind paws | 2.973 ± 0.128 | 2.476 ± 0.127* | 2.900 ± 0.101 † |
SHAM: sham-operated group; tMCAO: transient middle cerebral artery occlusion; SED: tMCAO-sedentary group; IH: tMCAO-intermittent hypoxia group; LF: left forepaw; RF: right forepaw; LH: left hind paw; RH: right hind paw (R is the affected side); BOS: base of support.
P < 0.05; **P < 0.01 versus SHAM; †P < 0.05 versus SED.
Figure 6.
CatWalk dynamic parameters of rats in each group after 4 weeks of intervention ( ± s, n = 6). (a) Plain view of the gait of each group; (b) stand; (c) swing; (d) swing speed; (e) representative paw prints of each group; (f) stride length; (g) base of support (BOS).
tMCAO: transient middle cerebral artery occlusion; SHAM: sham-operated group; SED: tMCAO-sedentary group; IH: tMCAO-intermittent hypoxia group; LF: left forepaw; RF: right forepaw; LH: left hind paw; RH: right hind paw (R is the affected side).
*P < 0.05; **P < 0.01 versus SHAM; #P < 0.05 versus SED. (A color version of this figure is available in the online journal.)
Balance coordination parameters
The step cycle was the time in seconds between two consecutive initial contacts of the same paw, which was equal to the sum of the stand and swing. The step sequence lists the order in which the paws were placed on the glass plate. The support formula was the number of paws that were simultaneously on the glass plate in a section. The most commonly used support formula in rats is three-paw simultaneous support, followed by two-paw diagonal support or four-paw support. The regularity index expresses the number of normal step sequence patterns relative to the total number of paw placements. The results showed that the step cycle of the four paws in the SED group was significantly longer than the SHAM group (P < 0.05), and the IH group was significantly better than the SED group (P < 0.05; Figure 7(a)). For the step sequence, the proportion of AB was the largest, followed by CA and CB, with no significant difference. The three-step sequences of RA, RB, and AA did not appear in the three groups (Figure 7(b)). The support formula showed that the proportion of three-paw support was significantly reduced compared with the SHAM group (P = 0.036), and the proportion of four-paw support was increased in the SED group. Compared with the SED group, the proportion of three-paw support was significantly increased (P = 0.029), and the proportion of four-paw support decreased significantly (P = 0.042) in the IH group (Figure 7(c)). There was no significant difference between the IH and SHAM groups. There was no significant difference in the regularity index between the three groups (P = 0.166; Figure 7(d)).
Figure 7.
CatWalk balance coordination parameters of rats in each group after 4 weeks of intervention ( ± s, n = 6). (a) Step cycle; (b) step sequence; (c) support; (d) regularity index.
Step sequence: CA, right forepaw → left forepaw → right hind paw → left hind paw; CB, left forepaw → right forepaw → left hind paw → right hind paw; RA, right forepaw → left forepaw → left hind paw → right hind paw; RB, left forepaw → right forepaw → right hind paw → left hind paw; AA, right forepaw → right hind paw → left forepaw → left hind paw; AB, left forepaw → right hind paw → right forepaw → left hind paw. Support: single, one paw supported; diagonal, two diagonal paws supported; girdle, two forepaws or hind paws supported; lateral, two paws on the same side supported; three, three paws supported; four, four paws supported. tMCAO: transient middle cerebral artery occlusion; SHAM: sham-operated group; SED: tMCAO-sedentary group; IH: tMCAO-intermittent hypoxia group; LF: left forepaw; RF: right forepaw; LH: left hind paw; RH: right hind paw (R is the affected side).
*P < 0.05; **P < 0.01 versus SHAM; #P < 0.05 versus SED.
Effects of intermittent hypoxia on the cerebral infarction volume
Before and after 4 weeks of intervention, MRI scans were performed to detect the cerebral infarct volume, and the infarct volume ratio was used to reduce the influence of cerebral edema. The infarct volume ratio in the SHAM group was always 0. There was no significant difference in the cerebral infarction volume ratio between the SED and IH groups before intervention (P = 0.631). After 4 weeks of intervention, the cerebral infarction volumes of the SED and IH groups significantly reduced compared to before intervention (P < 0.001). The cerebral infarction volume of the IH group was significantly reduced compared with the SED group (P = 0.001; Figure 8).
Figure 8.
The cerebral infarction volume ratio of rats in each group before and 4 weeks after intervention ( ± s, n = 6). (a) Representative coronal T2 images of each group. Abnormal T2 high signals are observed in the left hemisphere. (b) Quantitative analysis of the cerebral infarction volume ratio detected by MRI.
MRI: magnetic resonance imaging; tMCAO: transient middle cerebral artery occlusion; SHAM: sham-operated group; SED: tMCAO-sedentary group; IH: tMCAO-intermittent hypoxia group.
**P < 0.01; ***P < 0.001.
Effect of intermittent hypoxia on the mitochondrial ultrastructure
After 4 weeks of intervention, the number and ultrastructure of mitochondria in the cortex ischemic penumbra area were observed using transmission electron microscopy (TEM). The number and morphology of mitochondria in the SHAM group were normal, circular, or oval-shaped, with integrated double membranes and identifiable cristae. The number of mitochondria in the SED group decreased significantly compared with the SHAM group (P = 0.000), and the mitochondria showed obvious swelling and vacuolation. The inner and outer mitochondrial membranes were ruptured, with fractured cristae. The number of mitochondria in the IH group significantly increased (P = 0.027), the mitochondria were evenly distributed, the membrane structure was intact, the cristae were identifiable, and swelling and vacuolization significantly improved (Figure 9).
Figure 9.
The ultrastructure and number of mitochondria in the peri-infarct subfield of the cortex of rats in each group after 4 weeks of intervention ( ± s, n = 6). (a) Mitochondrial ultrastructure image. N represents neuronal nuclei, and the arrow points to mitochondria (bar = 1.0 μm). (b) Quantification of the number of mitochondria in each field of transmission electron microscopy.
tMCAO: transient middle cerebral artery occlusion; SHAM: sham-operated group; SED: tMCAO-sedentary group; IH: tMCAO-intermittent hypoxia group.
***P < 0.001 versus SHAM; #P < 0.05 versus SED. (A color version of this figure is available in the online journal.)
Effects of intermittent hypoxia on the expression of mitochondrial biogenesis proteins
After 4 weeks of intervention, the expression levels of AMPK and Sirt3 in the SED group were significantly downregulated compared with the SHAM group (PAMPK = 0.044, PSirt3 = 0.005). The expression levels of AMPK, PGC-1α, and Sirt3 in the IH group were significantly increased compared with the SED group (PAMPK = 0.034, PPGC-1α = 0.01, PSirt3 = 0.003; Figure 10).
Figure 10.

The expression levels of mitochondrial biogenesis proteins in the peri-infarct subfield of the cortex of rats in each group after 4 weeks of intervention ( ± s, n = 6). (a, c, e) Representative Western blot bands of AMPK, PGC-1α, and Sirt3 proteins, respectively. (b, d, f) Quantitative analysis corresponding to AMPK, PGC-1α, and Sirt3 proteins, respectively.
tMCAO: transient middle cerebral artery occlusion; SHAM: sham-operated group; SED: tMCAO-sedentary group; IH: tMCAO-intermittent hypoxia group.
*P < 0.05; **P < 0.01 versus SHAM; #P < 0.05; ##P < 0.01 versus SED.
Effect of intermittent hypoxia on mitochondrial function
The ATP content was measured to evaluate the protective effect of intermittent hypoxia on mitochondrial function. After 4 weeks of intervention, the ATP level of the SED group was significantly lower than the SHAM group (P = 0.001), and the ATP level of the IH group was significantly higher than the SED group (P = 0.04; Figure 11).
Figure 11.

Mitochondrial ATP levels of rats in each group after 4 weeks of intervention ( ± s, n = 6).
ATP: adenosine triphosphate; tMCAO: transient middle cerebral artery occlusion; SHAM: sham-operated group; SED: tMCAO-sedentary group; IH: tMCAO-intermittent hypoxia group.
**P < 0.01 versus SHAM; #P < 0.05 versus SED.
Discussion
Intermittent hypoxia was widely used in the research of various diseases in recent years. The present study found that intermittent hypoxia significantly improved the neurological deficits and gait of tMCAO rats, reduced the cerebral infarction volume, and promoted improvements of mitochondrial ultrastructure and function in the cortex ischemic penumbra area, which may be related to the upregulation of AMPK, PGC-1α, and Sirt3 protein expression levels.
Intermittent hypoxia has a certain degree of safety. Relevant studies showed that weight loss after stroke was an adverse event 19 that negatively affected prognosis and functional recovery. Severe weight loss increases mortality, which is closely related to fat loss, skeletal muscle atrophy, decreased anabolism, increased catabolism, eating disorders, pain, and infection. 20 Although the weight of the rats in the tMCAO groups decreased, the weight of the rats in each group gradually recovered as the intervention progressed. The weight of the IH group was relatively light, but there was no significant difference from the SED group. This change may be related to the increase in the resting metabolic rate and the decrease in physical activity levels in the hypoxic environment. 21 None of the animals died or had increased cerebral infarction volume during intermittent hypoxia intervention, which indicated that intermittent hypoxia intervention promoted weight recovery and reduced the risk of stroke recurrence in cerebral ischemic rats. Manukhina et al. 22 found that intermittent hypoxia improved behavior and adrenal dysfunction in post-traumatic stress rats. This result shows that intermittent hypoxia intervention has an obvious anti-stress effect. Zhu et al. 23 found that IH intervention promoted neurogenesis in the subventricular region and dentate gyrus of the hippocampus in normal adult rats, and neurogenesis in the subventricular region and dentate gyrus of the hippocampus was beneficial to functional recovery after ischemic brain injury. Therefore, IH intervention has a positive effect in normal and cerebral ischemic rats.
Intermittent hypoxia improves neurological deficits in rats with cerebral ischemia. Cerebral ischemia generally causes motor dysfunction. 24 The present study found that neurological function recovered spontaneously to a certain extent after tMCAO. Intermittent hypoxia significantly reduced the mNSS and beam-walking scores, accelerated neurological recovery, and improved functional outcomes. The balance function recovered later. A number of studies showed that early motor balance and coordination training affected the plasticity of the subcortical region of the ischemic hemisphere after stroke, which was accompanied with the recovery of sensorimotor function.25,26 Adaptive training induced by intermittent hypoxia provides a certain degree of protection or improves motor performance for some diseases, 27 and our previous study demonstrated that 4 weeks of intermittent hypoxia improved exercise endurance in rats with myocardial infarction. 12 Patterns of intermittent hypoxia exposure to short-term (minutes to hours) and relatively mild (>12% FiO2) conditions protect specific cells, tissues, or organs from severe hypoxia and ischemic damage. The hypoxia parameters (4 h/d, FiO2 = 13%) used in the present study effectively rescued neuronal cells after cerebral ischemia 28 and improved neurological dysfunction.
Intermittent hypoxia improved gait parameters in rats with cerebral ischemia. Ischemic stroke causes temporary or permanent gait disturbance. Gait ability is an important predictor of the health and quality of life of stroke patients, and it is one criterion for measuring the effectiveness of stroke treatments. 29 The present study used the CatWalk Analysis System for evaluation, 30 and the results showed that intermittent hypoxia increased the movement speed of rats and improved the maximum strength, maximum content area, print area, print width, and print length parameters of the affected paw (right side), especially the improvement of the right hind paw of the main bearing. During exercise, the stand of the left forepaw and the swing of the right (affected) paws were prolonged, the swing speed and stride length of all paws were significantly reduced, and the BOS of the hind paw was significantly shortened in the SED group. These changes may be due to the bias of the center of gravity to the left, and the left forepaw plays an important compensatory support role in the walking process, 31 which is similar to the gait of stroke patients with clinically limited hip flexion and knee flexion. 32 Intermittent hypoxia intervention significantly shortened the swing of the right (affected) paws, increased the swing speed and stride length of the right (affected) paws, improved the BOS of the hind paw, and improved the stability of motion. From the perspective of balance coordination parameters, the motor function of the paws was impaired, and the step cycle was prolonged after cerebral ischemia. The step cycle was significantly shortened and tended toward a normal state after intermittent hypoxia. The support formula of healthy rats was primarily three-paw support. The proportion of four-paw support increased significantly after cerebral ischemia, and the proportion of three-paw support and diagonal two-paw support increased significantly after intermittent hypoxia. The balance and coordination ability recovered significantly. The AB pattern (left forepaw → right hind paw → right forepaw → left hind paw) was the most common step sequence in healthy rats, which accounted for 80–95%, and remained the highest in animals with neurological damage. 33 Our study also demonstrated this result. The regularity index was not significantly different between the three groups because only one extra or missing paw placement by one or multiple paws was defined as “irregular” steps. As long as four paws were used in a single step cycle, it was almost certainly normal in most cases, regardless of the step sequence or paws placement. 33 These results demonstrated that intermittent hypoxia improved gait parameters and promoted the recovery of motor function and the overall balance and coordination of rats with cerebral ischemia.
Intermittent hypoxia reduced the cerebral infarction volume. Damage to brain tissue in cerebral infarction is a dynamic evolutionary process. The interruption of cerebral blood flow leads to complex changes in perfusion, electrolysis, metabolism, and neurotransmitters. 34 Measurement of the cerebral infarction volume is helpful for the early and timely diagnosis and treatment, and it is important for predicting later functional recovery. Li et al. 18 used MRI detection and found that EGb 761 significantly reduced the cerebral infarction volume of MCAO rats, reduced gray matter and white matter damage, and contributed to behavior recovery and endogenous neurogenesis. Our results showed that intermittent hypoxia intervention significantly reduced the cerebral infarction volume of tMCAO rats, which was consistent with the conclusions of Tsai et al. 14 Therefore, for severe patients with large cerebral infarction, intermittent hypoxia treatment at an early stage helped reduce brain damage and promoted the recovery of neurological function.
Intermittent hypoxia helps repair mitochondrial morphological structure and function after cerebral ischemia and promotes mitochondrial biogenesis. Cerebral ischemia and hypoxia lead to cell necrosis and apoptosis, which result in the mitochondrial dysfunction and destruction of mitochondrial gene expression 35 that lead to difficulty in ATP synthesis, increased reactive oxygen species (ROS) production and Ca2+ accumulation, and the exacerbation of mitochondrial damage and apoptosis. 36 Intermittent hypoxia maintains mitochondrial membrane potential and improves the tolerance of mitochondria to high calcium levels by increasing adenosine diphosphate (ADP) and AMP in mitochondria. 37 The present study found that the mitochondria in the SED group showed obvious swelling and vacuolization, rupture of the inner and outer membranes, fractured cristae, significantly reduced numbers of mitochondria, and decreased ATP levels. After intermittent hypoxia intervention, the mitochondria were evenly distributed, the membrane structure was intact, the cristae structure was identifiable, the swelling and vacuolation were significantly improved, the number was significantly increased, and the ATP level was increased. The repair of mitochondrial structure and dysfunction contributes to the regulation of neuroplasticity. Mitochondrial biogenesis may counteract the harmful effects of oxidative stress by increasing the number of mitochondria and provide sufficient energy for the process of neuroplasticity. 38 These studies suggest that intermittent hypoxia has a neuroprotective effect by repairing damaged mitochondria and promoting mitochondrial biogenesis.
Intermittent hypoxia may regulate mitochondria by activating the AMPK–PGC-1α–Sirt3 signaling pathway. AMPK is activated under energy stress to maintain metabolic homeostasis and alleviate mitochondrial dysfunction under severe energy crisis by initiating the biogenesis of new mitochondria. 39
PGC-1α is a central regulator of mitochondrial biogenesis, and it translates many physiological stimuli into specific metabolic programs and coordinates the activity of various transcription factors involved in mitochondrial proliferation. 40 Ashabi et al. 41 demonstrated that AMPK induced neuronal mitochondrial biogenesis in rats with cerebral ischemia via the PGC-1α signaling pathway. The present study found that the expression levels of PGC-1α and AMPK were downregulated after cerebral ischemia, and intermittent hypoxia intervention upregulated their expression. PGC-1α is a key downstream molecule of AMPK, 42 and we inferred that AMPK regulated mitochondrial biogenesis by reducing its expression or phosphorylation.
Sirt3 is a necessary downstream target for PGC-1α-induced mitochondrial-related gene expression and mitochondrial biogenesis, 43 and PGC-1α–Sirt3 signaling is essential for regulating mitochondrial oxidative stress and biogenesis. 44 The overexpression of Sirt3 via lentiviral transfection protected cortical neurons from oxidative stress by regulating mitochondrial Ca2+ and mitochondrial biogenesis. 45 Xie et al. 46 found that the activation of Sirt3 was a key mechanism of LanCL1 reduction of oxidative stress and protection of mitochondrial function.
Increasing evidence shows that AMPK phosphorylation and Sirt3 deacetylation are necessary for activating PGC-1α. Therefore, the AMPK–PGC-1α–Sirt3 axis is a key signaling pathway in mitochondrial biogenesis and the regulation of mitochondrial homeostasis. Previous studies of various diseases, such as spinal cord injury, 47 myofascial pain syndrome, 48 and Type 2 diabetes skeletal muscle insulin resistance, 11 showed that the AMPK–PGC-1α–Sirt3 pathway improved mitochondrial dysfunction, reduced mitochondrial damage, and played a protective role in mitochondria. The AMPK–PGC-1α signals in the SED group in the present study were significantly downregulated and resulted in reduced Sirt3 expression. Intermittent hypoxia intervention activated AMPK–PGC-1α signaling and increased Sirt3 expression. Therefore, we speculated that intermittent hypoxia reduced mitochondrial damage by activating the AMPK–PGC-1α–Sirt3 axis, increasing mitochondrial biogenesis and improving mitochondrial function.
In summary, intermittent hypoxia may have a neuroprotective effect by activating the AMPK–PGC-1α–Sirt3 signaling pathway, promoting mitochondrial biogenesis, repairing mitochondrial ultrastructural injury, and improving mitochondrial dysfunction to reduce cerebral infarction volume and promote the recovery of motor function in rats with cerebral ischemia. However, mitochondrial biogenesis is a complex process, and the effects of intermittent hypoxia on downstream-related transcription factors and the specific mechanisms need further study. Mitochondrial biogenesis may be one of the neuroprotective mechanisms of intermittent hypoxia. We will explore the aspects of mitochondrial autophagy, oxidative stress, and apoptosis in the future. The present study broadens the scope of the application of intermittent hypoxia, provides a new treatment idea for severe patients who cannot engage in active exercises in the early stage of clinical practice, and provides an experimental basis for the early clinical application of intermittent hypoxia.
Footnotes
Authors’ Contributions: All authors participated in the design, interpretation of the studies and analysis of the data, and review of the manuscript. CL, CH, and CK performed the experiments, and YS and CK wrote the manuscript.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the National Key R&D Program of China (grant numbers 2017YFC1308504 and 2017YFC1104004) and the Tianjin Key Medical Discipline (Specialty) Construction Project.
ORCID iD: Chunxiao Wan  https://orcid.org/0000-0002-0934-9300
https://orcid.org/0000-0002-0934-9300
References
- 1. Liu L, Chen W, Zhou H, Duan W, Li S, Huo X, Xu W, Huang L, Zheng H, Liu J, Liu H, Wei Y, Xu J, Wang Y, Chinese Stroke Association Stroke Council Guideline Writing Committee. Chinese Stroke Association guidelines for clinical management of cerebrovascular disorders: executive summary and 2019 update of clinical management of ischaemic cerebrovascular diseases. Stroke Vasc Neurol 2020;5:159–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Expert Panel on Neurologic I, Salmela MB, Mortazavi S, Jagadeesan BD, Broderick DF, Burns J, Deshmukh TK, Harvey HB, Hoang J, Hunt CH, Kennedy TA, Khalessi AA, Mack W, Patel ND, Perlmutter JS, Policeni B, Schroeder JW, Setzen G, Whitehead MT, Cornelius RS, Corey AS. ACR appropriateness criteria((R)) cerebrovascular disease. J Am Coll Radiol 2017;14:S34–61 [DOI] [PubMed] [Google Scholar]
- 3. MacKay-Lyons M, Billinger SA, Eng JJ, Dromerick A, Giacomantonio N, Hafer-Macko C, Macko R, Nguyen E, Prior P, Suskin N, Tang A, Thornton M, Unsworth K. Aerobic exercise recommendations to optimize best practices in care after stroke: AEROBICS 2019 update. Phys Ther 2020;100:149–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Manukhina EB, Downey HF, Shi X, Mallet RT. Intermittent hypoxia training protects cerebrovascular function in Alzheimer’s disease. Exp Biol Med 2016;241:1351–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Tantingco G, Ryou MG. Normobaric intermittent hypoxic training regulates microglia phenotype and enhances phagocytic activity. Exp Biol Med 2020;245:740–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gao J, Qian T, Wang W. CTRP3 Activates the AMPK/SIRT1-PGC-1alpha pathway to protect mitochondrial biogenesis and functions in cerebral ischemic stroke. Neurochem Res 2020;45:3045–58 [DOI] [PubMed] [Google Scholar]
- 7. Yu K, Kuang S, Wang C, Wang Y, Liu G, Xie H, Jiang C, Wu J, Wang N, Wu Y. Changes in mitochondria-associated protein expression and mitochondrial function in response to 2 weeks of enriched environment training after cerebral ischaemia-reperfusion injury. J Mol Neurosci 2020;70:413–21 [DOI] [PubMed] [Google Scholar]
- 8. Jackson CW, Escobar I, Xu J, Perez-Pinzon MA. Effects of ischemic preconditioning on mitochondrial and metabolic neuroprotection: 5’ adenosine monophosphate-activated protein kinase and sirtuins. Brain Circ 2018;4:54–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chen SD, Yang DI, Lin TK, Shaw FZ, Liou CW, Chuang YC. Roles of oxidative stress, apoptosis, PGC-1alpha and mitochondrial biogenesis in cerebral ischemia. Int J Mol Sci 2011;12:7199–215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Yang X, Zhang Y, Geng K, Yang K, Shao J, Xia W. Sirt3 protects against ischemic stroke injury by regulating HIF-1alpha/VEGF signaling and blood-brain barrier integrity. Cell Mol Neurobiol 2021;41:1203–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Shi L, Zhang T, Zhou Y, Zeng X, Ran L, Zhang Q, Zhu J, Mi M. Dihydromyricetin improves skeletal muscle insulin sensitivity by inducing autophagy via the AMPK-PGC-1alpha-Sirt3 signaling pathway. Endocrine 2015;50:378–89 [DOI] [PubMed] [Google Scholar]
- 12. Xu K, Meng X, Huang C, Wan C. Intermittent hypoxia intervention improves cardiac function and exercise tolerance in rats with myocardial infarction. Journal of Tianjin Medical University 2019;25:459–62 [Google Scholar]
- 13. Longa EZ, Weinstein PR, Carlson S, Cummins R. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke 1989;20: 84–91 [DOI] [PubMed] [Google Scholar]
- 14. Tsai YW, Yang YR, Chen GH, Chang HC, Wang RY. The time window of intermittent hypoxia intervention after middle cerebral artery occlusion. Chin J Physiol 2008;51:324–8 [PubMed] [Google Scholar]
- 15. Chen J, Li Y, Wang L, Zhang Z, Lu D, Lu M, Chopp M. Therapeutic benefit of intravenous administration of bone marrow stromal cells after cerebral ischemia in rats. Stroke 2001;32:1005–11 [DOI] [PubMed] [Google Scholar]
- 16. Clifton GL, Jiang JY, Lyeth BG, Jenkins LW, Hamm RJ, Hayes RL. Marked protection by moderate hypothermia after experimental traumatic brain injury. J Cereb Blood Flow Metab 1991;11:114–21 [DOI] [PubMed] [Google Scholar]
- 17. Fan QY, Liu JJ, Zhang GL, Wu HQ, Zhang R, Zhan SQ, Liu N. Inhibition of SNK-SPAR signaling pathway promotes the restoration of motor function in a rat model of ischemic stroke. J Cell Biochem 2018; 119:1093–110 [DOI] [PubMed] [Google Scholar]
- 18. Li MZ, Zhang Y, Zou HY, Ouyang JY, Zhan Y, Yang L, Cheng BC, Wang L, Zhang QX, Lei JF, Zhao YY, Zhao H. Investigation of Ginkgo biloba extract (EGb 761) promotes neurovascular restoration and axonal remodeling after embolic stroke in rat using magnetic resonance imaging and histopathological analysis. Biomed Pharmacother 2018;103:989–1001 [DOI] [PubMed] [Google Scholar]
- 19. Yang J, Kim E, Beltran C, Cho S. Corticosterone-mediated body weight loss is an important catabolic process for poststroke immunity and survival. Stroke 2019;50:2539–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Scherbakov N, Pietrock C, Sandek A, Ebner N, Valentova M, Springer J, Schefold JC, von Haehling S, Anker SD, Norman K, Haeusler KG, Doehner W. Body weight changes and incidence of cachexia after stroke. J Cachexia Sarcopenia Muscle 2019;10:611–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kayser B, Verges S. Hypoxia, energy balance and obesity: from pathophysiological mechanisms to new treatment strategies. Obes Rev 2013; 14:579–92 [DOI] [PubMed] [Google Scholar]
- 22. Manukhina EB, Tseilikman VE, Tseilikman OB, Komelkova MV, Kondashevskaya MV, Goryacheva AV, Lapshin MS, Platkovskii PO, Alliluev AV, Downey HF. Intermittent hypoxia improves behavioral and adrenal gland dysfunction induced by posttraumatic stress disorder in rats. J Appl Physiol 2018;125:931–7 [DOI] [PubMed] [Google Scholar]
- 23. Zhu LL, Zhao T, Li HS, Zhao H, Wu LY, Ding AS, Fan WH, Fan M. Neurogenesis in the adult rat brain after intermittent hypoxia. Brain Res 2005;1055:16. [DOI] [PubMed] [Google Scholar]
- 24. Frechou M, Margaill I, Marchand-Leroux C, Beray-Berthat V. Behavioral tests that reveal long-term deficits after permanent focal cerebral ischemia in mouse. Behav Brain Res 2019;360:69–80 [DOI] [PubMed] [Google Scholar]
- 25. Seo HG, Kim DY, Park HW, Lee SU, Park SH. Early motor balance and coordination training increased synaptophysin in subcortical regions of the ischemic rat brain. J Korean Med Sci 2010;25:1638–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Omiyale O, Crowell CR, Madhavan S. Effect of Wii-based balance training on corticomotor excitability post stroke. J Mot Behav 2015;47: 190–200 [DOI] [PubMed] [Google Scholar]
- 27. Almendros I, Wang Y, Gozal D. The polymorphic and contradictory aspects of intermittent hypoxia. Am J Physiol Lung Cell Mol Physiol 2014; 307:L129–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Qiao Y, Liu Z, Yan X, Luo C. Effect of intermittent hypoxia on neuro-functional recovery post brain ischemia in mice. J Mol Neurosci 2015;55: 923–30 [DOI] [PubMed] [Google Scholar]
- 29. Ghai S, Ghai I. Effects of (music-based) rhythmic auditory cueing training on gait and posture post-stroke: a systematic review & dose-response meta-analysis. Sci Rep 2019;9:2183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Orgah JO, Ren J, Liu X, Orgah EA, Gao XM, Zhu Y. Danhong injection facilitates recovery of post-stroke motion deficit via Parkin-enhanced mitochondrial function. Restor Neurol Neurosci 2019;37: 375–95 [DOI] [PubMed] [Google Scholar]
- 31. Cao Y, Sun N, Yang JW, Zheng Y, Zhu W, Zhang ZH, Wang XR, Shi GX, Liu CZ. Does acupuncture ameliorate motor impairment after stroke? An assessment using the CatWalk gait system. Neurochem Int 2017;107:198–203 [DOI] [PubMed] [Google Scholar]
- 32. Milot MH, Nadeau S, Gravel D, Requiao LF. Bilateral level of effort of the plantar flexors, hip flexors, and extensors during gait in hemiparetic and healthy individuals. Stroke 2006;37:2070–5 [DOI] [PubMed] [Google Scholar]
- 33. Wang Y, Bontempi B, Hong SM, Mehta K, Weinstein PR, Abrams GM, Liu J. A comprehensive analysis of gait impairment after experimental stroke and the therapeutic effect of environmental enrichment in rats. J Cereb Blood Flow Metab 2008;28:1936–50 [DOI] [PubMed] [Google Scholar]
- 34. Barone FC, Feuerstein GZ. Inflammatory mediators and stroke: new opportunities for novel therapeutics. J Cereb Blood Flow Metab 1999;19:819–34 [DOI] [PubMed] [Google Scholar]
- 35. Ji F, Zhao C, Wang B, Tang Y, Miao Z, Wang Y. The role of 5-hydroxymethylcytosine in mitochondria after ischemic stroke. J Neurosci Res 2018;96:1717–26 [DOI] [PubMed] [Google Scholar]
- 36. Bakthavachalam P, Shanmugam PST. Mitochondrial dysfunction: silent killer in cerebral ischemia. J Neurol Sci 2017;375:417–23 [DOI] [PubMed] [Google Scholar]
- 37. Chen J, Liao W, Gao W, Huang J, Gao Y. Intermittent hypoxia protects cerebral mitochondrial function from calcium overload. Acta Neurol Belg 2013;113:507–13 [DOI] [PubMed] [Google Scholar]
- 38. Habash T, Saleh A, Roy Chowdhury SK, Smith DR, Fernyhough P. The proinflammatory cytokine, interleukin-17A, augments mitochondrial function and neurite outgrowth of cultured adult sensory neurons derived from normal and diabetic rats. Exp Neurol 2015;273:177–89 [DOI] [PubMed] [Google Scholar]
- 39. Toyama EQ, Herzig S, Courchet J, Lewis TL, Jr., Loson OC, Hellberg K, Young NP, Chen H, Polleux F, Chan DC, Shaw RJ. Metabolism amp-activated protein kinase mediates mitochondrial fission in response to energy stress. Science 2016;351:275–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Palikaras K, Tavernarakis N. Mitochondrial homeostasis: the interplay between mitophagy and mitochondrial biogenesis. Exp Gerontol 2014; 56:182–8 [DOI] [PubMed] [Google Scholar]
- 41. Ashabi G, Khodagholi F, Khalaj L, Goudarzvand M, Nasiri M. Activation of AMP-activated protein kinase by metformin protects against global cerebral ischemia in male rats: interference of AMPK/PGC-1alpha pathway. Metab Brain Dis 2014;29:47–58 [DOI] [PubMed] [Google Scholar]
- 42. Canto C, Gerhart-Hines Z, Feige JN, Lagouge M, Noriega L, Milne JC, Elliott PJ, Puigserver P, Auwerx J. AMPK regulates energy expenditure by modulating NAD+ metabolism and SIRT1 activity. Nature 2009;458:1056–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Guan Y, Cui ZJ, Sun B, Han LP, Li CJ, Chen LM. Celastrol attenuates oxidative stress in the skeletal muscle of diabetic rats by regulating the AMPK-PGC1alpha-SIRT3 signaling pathway. Int J Mol Med 2016;37: 1229–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Matsushima S, Sadoshima J. The role of sirtuins in cardiac disease. Am J Physiol Heart Circ Physiol 2015;309:H1375–189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Dai SH, Chen T, Wang YH, Zhu J, Luo P, Rao W, Yang YF, Fei Z, Jiang XF. Sirt3 protects cortical neurons against oxidative stress via regulating mitochondrial Ca2+ and mitochondrial biogenesis. Int J Mol Sci 2014;15:14591–609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Xie Z, Cao BQ, Wang T, Lei Q, Kang T, Ge CY, Gao WJ, Hui H. LanCL1 attenuates ischemia-induced oxidative stress by Sirt3-mediated preservation of mitochondrial function. Brain Res Bull 2018;142:216–23 [DOI] [PubMed] [Google Scholar]
- 47. Liu SG, Wang YM, Zhang YJ, He XJ, Ma T, Song W, Zhang YM. ZL006 protects spinal cord neurons against ischemia-induced oxidative stress through AMPK-PGC-1alpha-Sirt3 pathway. Neurochem Int 2017;108:230–7 [DOI] [PubMed] [Google Scholar]
- 48. Ye L, Li M, Wang Z, Yang Z, Zhang J, Fang H, He Z, Wang X. Depression of mitochondrial function in the rat skeletal muscle model of myofascial pain syndrome is through down-regulation of the AMPK-PGC-1alpha-SIRT3 axis. J Pain Res 2020;13:1747–56 [DOI] [PMC free article] [PubMed] [Google Scholar]