Abstract
Coronavirus disease 2019 (COVID-19) management has been challenging for patients with comorbidities. Patients with diabetes and COVID-19, in particular, have shown severe symptoms and rapid progression of the disease. They also have a high mortality rate compared to the non-diabetic population. The high mortality rate is caused in people with diabetes who are in a pro-inflammatory condition; this could worsen COVID-19. In addition, people with diabetes have circulatory issues and COVID-19 infection can lead to further clotting problems. It is critical to understand the mechanisms underlying the adverse clinical outcomes in patients with diabetes and COVID-19. This review discusses various disease conditions contributing to poor prognosis in diabetic COVID-19 patients such as hyperglycemia, insulin resistance, impaired pancreatic function, and production of advanced glycation end products.
Keywords: COVID-19, SARS-CoV-2, diabetics, hyperglycemia, insulin resistance
Impact Statement
Patients with diabetes and COVID-19 are prone to severe infections due to the hyperglycemia-driven increase in virulence of microorganisms and immune dysfunction. As a result, increased hospital admissions, thrombotic complications, and mortality have been observed in patients. Pro-inflammatory states linked with COVID-19 also play a key role in hyperglycemia, thereby worsening COVID-19 prognosis. In this review, we have outlined key factors connected with COVID-19 progression in an effort to understand its complex, bidirectional relationship with diabetes.
Background
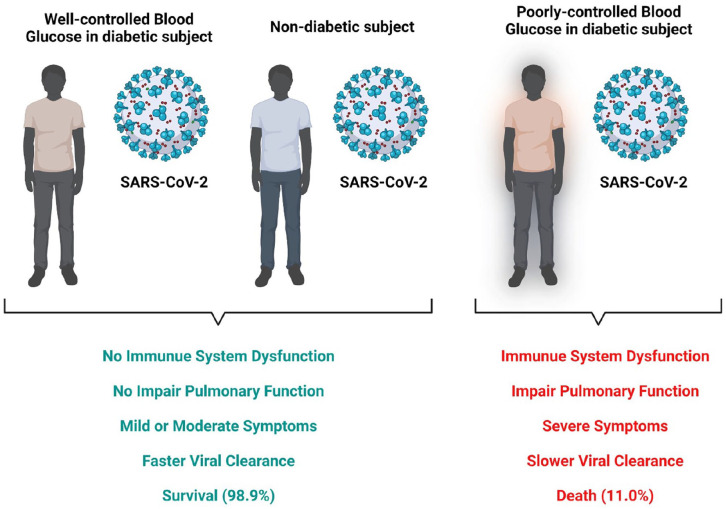
COVID-19 is caused by SARS-CoV-2 (severe acute respiratory syndrome coronavirus 2). The respiratory syndrome has traumatized the world by infecting and taking the lives of larger population. It emerged in December 2019 in Wuhan, China.1 –3 COVID-19 has been linked to the utmost severe and fatal occurrences in people with pre-existing illness like cancer, hypertension, and diabetes. 4 One of the most prevalent comorbidity conditions in critical COVID-19 patients is diabetes mellitus. 5 Diabetes mellitus is an interlinked metabolic disorder affecting 422 million people worldwide 6 and is recognized by significantly elevated blood glucose levels that destructs the blood vessels and nerves. It also causes long-term injury to various parts of the body such as, eyes, brain, immune system, kidney, and heart. 7 COVID-19 and diabetes are linked to chronic and acute inflammation, respectively. COVID-19 can influence clinical progression and outcome of diabetes and vice versa. 8 Patients with diabetes and COVID-19 have a higher mortality rate (7.3%) compared to non-diabetic subjects with a mortality rate of 2.3%. 9
Hyperglycemia, pancreatic function, insulin resistance, advanced glycation end products in relationship with COVID-19
Hyperglycemia, or constantly elevated blood sugar levels, is considered to be the most general metabolic modification connected with type 2 diabetes (T2D). 10 Hyperglycemia and immune responses are intricately intertwined and have a direct impact on the severity of SARS-CoV-2 infection. 11 Raised sugar levels proportionally elevate SARS-CoV-2 replication in human monocytes, and glycolysis withstands SARS-CoV-2 replication through the generation of mitochondrial reactive oxygen species (ROS) and the stimulation of hypoxia-inducible factor. In addition, when SARS-CoV-2 enters the pulmonary system, hypoxia occurs, which stabilizes HIF-α (hypoxia-inducible factor 1-alpha), resulting in overexpression of VEGF (vascular endothelial growth factor) and integrins, which causes vascular permeability and release of cytokines. Another hypothesis states that an increase in dead cells generates ROS, which in turn elevates HIF-α, leading glycolysis to be upregulated. 12 This also causes metabolic derangement, which leads to glycol toxicity. In case of glucotoxicity, the illness is exacerbated indirectly as it assaults in a variety of ways, starting with disrupting glucose homeostasis pathways such as polyol, hexosamine, and sorbitol. Upregulation of proteins involved in cell damage, such as pro-apoptotic, Toll-like receptors (TLR), and others, encouraging apoptosis and fibrosis. During the initial period of SARS-CoV-2 infection, diabetic patients acquired persistent hyperglycemia that worsens on a regular basis. Hyperglycemia may promote viral entry and proliferation. 12 In diabetes mellitus patients, infection causes incretin-like glucagon-like peptide and other insulin-secreting protein dysfunction, resulting in an increase in insulin demand. Infection also boosts the release of glucocorticoids and catecholamines, which worsens the glycemic condition and results in the end products of glycation. 13 High glucose levels have been shown to stimulate several immune cell types, resulting in increased generation of pro-inflammatory cytokines (tumor necrosis factor α (TNF-α), interleukin 1 beta (IL-1β), and IL-6). 14
In patients with diabetes, the hyperglycemia may impair pulmonary function, which can be aggravated by influenza virus. In animal models of diseases, diabetes is connected to multiple structural alterations to the pulmonary system, including increased vasculature permeability and a collapsed alveolar epithelium.15,16 Uncontrolled hyperglycemia produces aberrant glycated angiotensin-converting enzyme 2 (ACE2) formation not merely in pulmonary system as well as in the nasal pathways, tongue, and oropharynx, potentially increasing viral access sites and disease severity. 17
Elevated amounts of ACE2 in pancreatic islet β-cells can trigger increased injury in islet cells and impaired insulin secretion. 18 With subsequent viral access into β-cells, there is a downregulation of ACE2, resulting in elevated angiotensin levels, which weakens insulin secretion.14,19 Probable mechanisms of pancreatic damage comprise (1) direct cytopathic effect of SARS-CoV-2 replication, (2) systemic response to respiratory malfunction, and (3) damaging immune reaction prompted by SARS-CoV-2 infection. 20 Furthermore, COVID-19 is caused due to continuous acute hypoxia which can impact several body parts, including pancreatic islets, and may directly induce β-cell apoptosis directly. 19 SARS-CoV-2, on the other hand, affects macrophages and neutrophils and suppresses the formation of natural killer cells (NKC). The virus assaults the circulating blood and targets the immune system, destroying lymphocytes CD3, CD4, and CD8 cells, resulting in lymphocytopenia 13 . This is specifically related to SARS-CoV-2 infection as the patients with T2D and COVID-19 have higher serum contents of pro-inflammatory cytokines (TNF-α, IL-1β, IL-6, IL-8, and IFN-γ).14,21 The “cytokine release syndrome, generally called as cytokine storm” is accompanied by other inflammatory indicators like d-dimer, C-reactive protein (CRP), and ferritin in patients with SARS-CoV-2 infection and is connected to intensity of virus. 22 Elevated lactate levels in T2D patients may delay SARS-CoV-2 clearance by blocking the retinoic-acid-inducible RIG-1-like receptor through mitochondrial antiviral signaling protein, resulting in restriction in interferon generation and decreased antiviral response. 23
Insulin resistance is generally associated with obesity, which is a direct pathophysiologic factor of T2D. 24 Over the past few years, there has been a surge of interest in the study of systemic inflammation. Obese people have a chronic systemic pro-inflammatory state that is defined by unusually excessive circulating levels of immune system in blood. 23 ACE2 is a key catalyst connection among insulin resistance and severe COVID-19, as it serves as a pro-hormone and a receptor at primary cellular entry route for SARS-CoV-2. ACE2 produces angiotensin 1 and octapeptide Ang II which cause vasoconstriction and cell proliferation, whereas Ang 1-7 causes vasodilation and inhibits cell growth.14,25 Overexpression of ACE in the pulmonary organ favors ACE2 generation and binding to SARS-CoV-2, and even prompts to downregulate cellular expression of ACE2 contributing to chronic lung damage. ACE2 is increased in mice on an elevated sucrose diet to eliminate surplus angiotensin II and reduce their deleterious effects on insulin sensitivity and glucose carriage through the family of glucose transporter proteins. Patients with T2D cases have higher ACE2 receptor levels, which might aid SARS-CoV-2 in prolong cellular binding, increasing viral load and severity of infection. 26 Insulin-deficient monocytes and macrophages respond poorly to a variety of pathogens. 27 Insulin resistance is also related to surplus blood neutrophil count in patients with T2D, especially important in COVID-19, which is described by neutrophilia and monocytopenia. 28
The pathogenesis of T2D is defined by the non-enzymatic covalent connection of glucose to biomolecules resulting in the development of advanced glycation end products (AGEs) in chronic hyperglycemia of patients with diabetics. 29 AGEs have been recently reported as a contributor to the risk of severe illness from COVID-19 in patients with diabetes.30,31 AGEs have been demonstrated to bind to a variety of surface receptors, including CD36, scavenger receptors type I and II, and galectin-3. 32 AGEs induce the discharge of pro-inflammatory cytokines in monocytes, lymphocytes, and macrophages upon receptor engagement, promoting vascular inflammation and endothelial dysfunction. 10 AGEs generated in diabetic individuals are shown to stimulate the classical complement pathway by detecting C1q, inactivating CD59 and increasing vascular damage in patients with T2D blood vessels. 33 In line with the theory, membrane attack complex deposits found in pulmonary tissue from severe COVID-19-infected patients have demonstrated complement-mediated injury, which subsequently causes vascular inflammation and leads to long-term lung damage. 34
COVID-19 is a worldwide pandemic that has ravaged our human race recently. SARS-COV-2 is an extremely contagious virus with a rapid transmission and mutation. 35 Patients with COVID-19 with previous diseases like cardiovascular disease (7%), diabetes (11%), and hypertension (21%), according to current data, have a greater complication risk and require intensive care.6,36 COVID-19 is more common among diabetics, and the majority of cases are more severe than the non-diabetic population.37 –39
People with diabetes are susceptible to respiratory diseases as they are prone to multidrug-resistant gram-negative bacteria due to compromised cellular and humoral innate immune response.25,36 As a result, chances of bacterial infection are high, leading to increased mortality due to uncontrolled pneumonia.25,40 Moreover, people with diabetes more easily progress to severe symptom, leading to a hospital admission or ICU (intensive care unit) admission more frequently. If the patients show chronic infection, the incidence of nosocomial infection increases and leads to mortality of T2DM which might be increased by decreased immunity of T2DM. 41
COVID-19 has the highest impact on the first-line immune defense in people with diabetes compared to the non-diabetic population.25,42 People with diabetes are in pro-inflammatory state which triggers the cytokine response, leading to an increase in IL-6 and CRP levels in the blood. 43 Evidence indicates that increased IL-6 and CRP levels are physiological indicators of subclinical systemic inflammation, hyperglycemia, insulin resistance, and overt T2D.43,44 These molecules are also linked to cardiovascular diseases, thereby enhancing the risk of cardiovascular complications in patients with diabetes suffering from SARS-CoV-2. 28 CRP has a major role in the acute phase as a downstream mediator. 43 Clinic evidence revealed that 24 patients with COVID-19 and diabetes had high levels of IL-6 and CRP when compared to 26 non-diabetic COVID-19 patients. 40 Increased d-dimer levels have been observed in patients with COVID-19 and diabetes. d-dimers are the smallest fibrinolysis degradation products, players of the hemostatic system of the body, generated as a result of the breakdown of blood clots. It increases the risk of thromboembolism.39,45
ACE2 present in the lungs is known to exhibit anti-inflammatory and antioxidant properties in various disease conditions. Unfortunately, ACE2 is also a receptor for SARS-CoV-2, allowing the entry of virus in the host.14,25 In addition, serum ferritin levels in patients with diabetes and COVID-19 are nearly twofold greater than in non-diabetic patients. 40 Ferritin is important in the generation of monocytes and macrophages; both play key roles in hypertension. As a result, individuals with diabetes and COVID-19 are more likely to develop hypertension. As macrophages are more abundant when hypertension occurs, diabetic patients can acquire hypertension during corona.46,47 Similarly, in diabetic COVID-19 patients, erythrocyte sedimentation rate (ESR), an inflammation-related biomarker, is higher than in non-diabetic patients 40 (Figure 1).
Figure 1.
COVID-19 and diabetics.
Source: Created with BioRender.com. (A color version of this figure is available in the online journal.)
Challenges
Hyperglycemia is believed to affect the function of the immune response to injections, which leads to the uncontrolled spread of pathogens (including viruses, e.g., SARS-CoV-2) in diabetic subjects. The condition of hyperglycemia does not increase the vulnerability of patients to be infected with SARS-CoV-2. Still, the disease severity of COVID-19 can be increased by the comorbidity of diabetics.13,48,49 COVID-19 may influence the blood levels of glucose;50,51 in such conditions, clinical guidelines should be strictly managed in subjects with diabetes. Recent several studies have discovered that many mechanisms are believed to be responsible for a highlighted clinical severity of COVID-19 in people with diabetes, 13 so scientists and clinicians required more time and studies to understand further in detail about mechanisms involved in COVID-19 and diabetes. This will enable clinicians to prescribe medicine for subjects with diabetes during infections and recovery from COVID-19. Other challenges include the lockdown of standard outpatient clinics, decreased inpatient capacity, medicine shortage, staff shortage, and very importantly undiagnosed cases/events.
Conclusions
Better scientific knowledge about the molecular mechanism by which immune dysfunctions occur in subjects with diabetes can lead to novel treatments and even preventions for infections, thus improving the outcome of COVID-19 patients with diabetics. Evidence suggests that vaccines are effective against COVID-19, so subjects with diabetes should be vaccinated as soon as available. A better and improved strict guideline for management of subjects with diabetes should be in place. Finally, it is necessary to adhere to strict social distancing protocols to block the chain of infection.
Footnotes
Authors’ Contributions: PG, HP, SHSR, MPJ, CMH, BA, UMR, SG, AK, PR, and KS contributed to the preparation of the initial draft and subsequent revisions. All authors approved the final version of the article.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs: Prakash Gangadaran  https://orcid.org/0000-0002-0658-4604
https://orcid.org/0000-0002-0658-4604
Manasi P Jogalekar  https://orcid.org/0000-0003-1307-4829
https://orcid.org/0000-0003-1307-4829
Prasanna Ramani  https://orcid.org/0000-0002-5236-7141
https://orcid.org/0000-0002-5236-7141
References
- 1. Jogalekar MP, Veerabathini A, Gangadaran P. Novel 2019 coronavirus: genome structure, clinical trials, and outstanding questions. Exp Biol Med (Maywood) 2020;245:964–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Jogalekar MP, Veerabathini A, Gangadaran P. SARS-CoV-2 variants: a double-edged sword. Exp Biol Med (Maywood) 2021;246:1721–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Manivannan M, Jogalekar MP, Kavitha MS, Maran BAV, Gangadaran P. A mini-review on the effects of COVID-19 on younger individuals. Exp Biol Med (Maywood) 2021;246:293–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, Xiang J, Wang Y, Song B, Gu X, Guan L, Wei Y, Li H, Wu X, Xu J, Tu S, Zhang Y, Chen H, Cao B. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 2020; 395:1054–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Li B, Yang J, Zhao F, Zhi L, Wang X, Liu L, Bi Z, Zhao Y. Prevalence and impact of cardiovascular metabolic diseases on COVID-19 in China. Clin Res Cardiol 2020;109:531–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Venkatesh N, Paldus B, Lee MH, MacIsaac RJ, Jenkins AJ, O’Neal DN. COVID-19, type 1 diabetes clinical practice, research, and remote medical care: a view from the land down-under. J Diabetes Sci Technol 2020;14:803–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Knapp S. Diabetes and infection: is there a link? A mini-review. Gerontology 2013;59:99–104 [DOI] [PubMed] [Google Scholar]
- 8. Diabetes, https://www.who.int/health-topics/diabetes#tab=tab_1 (accessed 20 March 2022).
- 9. Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA 2020;323:1239–42 [DOI] [PubMed] [Google Scholar]
- 10. Fehrenbach H, Kasper M, Tschernig T, Shearman MS, Schuh D, Müller M. Receptor for advanced glycation endproducts (RAGE) exhibits highly differential cellular and subcellular localisation in rat and human lung. Cell Mol Biol (Noisy-le-grand) 1998;44:1147–57 [PubMed] [Google Scholar]
- 11. Blair M. Diabetes mellitus review. Urol Nurs 2016;36:27–36 [PubMed] [Google Scholar]
- 12. Codo AC, Davanzo GG, Monteiro L, de B, de Souza GF, Muraro SP, Virgilio da-Silva-JV, Prodonoff JS, Carregari VC, de Biagi Junior CAO, Crunfli F, Jimenez Restrepo JL, Vendramini PH, Reis-de-Oliveira G, Bispo Dos Santos K, Toledo-Teixeira DA, Parise PL, Martini MC, Marques RE, Carmo HR, Borin A, Coimbra LD, Boldrini VO, Brunetti NS, Vieira AS, Mansour E, Ulaf RG, Bernardes AF, Nunes TA, Ribeiro LC, Palma AC, Agrela MV, Moretti ML, Sposito AC, Pereira FB, Velloso LA, Vinolo MAR, Damasio A, Proença-Módena JL, Carvalho RF, Mori MA, Martins-de-Souza D, Nakaya HI, Farias AS, Moraes-Vieira PM. Elevated glucose levels favor SARS-CoV-2 infection and monocyte response through a HIF-1α/glycolysis-dependent axis. Cell Metab 2020;32:437–4465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lim S, Bae JH, Kwon HS, Nauck MA. COVID-19 and diabetes mellitus: from pathophysiology to clinical management. Nat Rev Endocrinol 2021;17:11–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Muniyappa R, Gubbi S. COVID-19 pandemic, coronaviruses, and diabetes mellitus. Am J Physiol Endocrinol Metab 2020;318:E736–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Philips BJ, Meguer JX, Redman J, Baker EH. Factors determining the appearance of glucose in upper and lower respiratory tract secretions. Intensive Care Med 2003;29:2204–10 [DOI] [PubMed] [Google Scholar]
- 16. Schofield J, Leelarathna L, Thabit H. COVID-19: impact of and on diabetes. Diabetes Ther 2020;11:1429–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Brufsky A. Hyperglycemia, hydroxychloroquine, and the COVID-19 pandemic. J Med Virol 2020;92:770–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Batlle D, Jose Soler M, Ye M. ACE2 and diabetes: ACE of ACEs. Diabetes 2010;59:2994–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zheng X, Zheng X, Wang X, Ma Z, Gupta Sunkari V, Botusan I, Takeda T, Björklund A, Inoue M, Catrina S-B, Brismar K, Poellinger L, Pereira TS. Acute hypoxia induces apoptosis of pancreatic β-cell by activation of the unfolded protein response and upregulation of CHOP. Cell Death Dis 2012;3:e322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ugwueze CV, Ezeokpo BC, Nnolim BI, Agim EA, Anikpo NC, Onyekachi KE. COVID-19 and diabetes mellitus: the link and clinical implications. Dubai Diabetes Endocrinol J 2020;26:69–77 [Google Scholar]
- 21. Ye Q, Wang B, Mao J. The pathogenesis and treatment of the “cytokine storm” in COVID-19. J Infect 2020;80:607–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wang J, Jiang M, Chen X, Montaner LJ. Cytokine storm and leukocyte changes in mild versus severe SARS-CoV-2 infection: review of 3939 COVID-19 patients in China and emerging pathogenesis and therapy concepts. J Leukoc Biol 2020;108:17–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zhang W, Wang G, Xu Z-G, Tu H, Hu F, Dai J, Chang Y, Chen Y, Lu Y, Zeng H, Cai Z, Han F, Xu C, Jin G, Sun L, Pan B-S, Lai S-W, Hsu C-C, Xu J, Chen Z-Z, Li H-Y, Seth P, Hu J, Zhang X, Li H, Lin H-K. Lactate is a natural suppressor of RLR signaling by targeting MAVS. Cell 2019;178:176–189.e15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wondmkun YT. Obesity, insulin resistance, and type 2 diabetes: associations and therapeutic implications. Diabetes Metab Syndr Obes 2020;13:3611–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Barber TM. COVID-19 and diabetes mellitus: implications for prognosis and clinical management. Expert Rev Endocrinol Metab 2020;15:227–36 [DOI] [PubMed] [Google Scholar]
- 26. Rao S, Lau A, So HC. Exploring diseases/traits and blood proteins causally related to expression of ACE2, the putative receptor of SARS-CoV-2: a Mendelian randomization analysis highlights tentative relevance of diabetes-related traits. Diabetes Care 2020;43:1416–26 [DOI] [PubMed] [Google Scholar]
- 27. Ieronymaki E, Daskalaki MG, Lyroni K, Tsatsanis C. Insulin signaling and insulin resistance facilitate trained immunity in macrophages through metabolic and epigenetic changes. Front Immunol 2019;10:1330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rizo-Téllez SA, Méndez-García LA, Flores-Rebollo C, Alba-Flores F, Alcántara-Suárez R, Manjarrez -Reyna AN, Baltazar-López N, Hernández-Guzmán VA, León-Pedroza JI, Zapata-Arenas R, González-Chávez A, Hernández-Ruíz J, Carrillo-Ruíz JD, Serrano-Loyola R, Guerrero-Avendaño GML, Escobedo G. The neutrophil-to-monocyte ratio and lymphocyte-to-neutrophil ratio at admission predict in-hospital mortality in Mexican patients with severe SARS-CoV-2 infection (Covid-19). Microorganisms 2020;8:1560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Maessen DE, Stehouwer CD, Schalkwijk CG. The role of methylglyoxal and the glyoxalase system in diabetes and other age-related diseases. Clin Sci (Lond) 2015;128:839–61 [DOI] [PubMed] [Google Scholar]
- 30. Sellegounder D, Zafari P, Rajabinejad M, Taghadosi M, Kapahi P. Advanced glycation end products (AGEs) and its receptor, RAGE, modulate age-dependent COVID-19 morbidity and mortality. A review and hypothesis. Int Immunopharmacol 2021;98:107806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Rojas A, Lindner C, Gonzàlez I, Morales MA. Advanced-glycation end-products axis: a contributor to the risk of severe illness from COVID-19 in diabetes patients. World J Diabetes 2021;12:590–602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mulrennan S, Baltic S, Aggarwal S, Wood J, Miranda A, Frost F, Kaye J, Thompson PJ. The role of receptor for advanced glycation end products in airway inflammation in CF and CF related diabetes. Sci Rep 2015;5:8931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Chikazawa M, Shibata T, Hatasa Y, Hirose S, Otaki N, Nakashima F, Ito M, Machida S, Maruyama S, Uchida K. Identification of C1q as a binding protein for advanced glycation end products. Biochemistry 2016;55:435–46 [DOI] [PubMed] [Google Scholar]
- 34. Manzo G. COVID-19 as an immune complex hypersensitivity in antigen excess conditions: theoretical pathogenetic process and suggestions for potential therapeutic interventions. Front Immunol 2020;11:566000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Spanakis EK. Diabetes and technology in the covid-19 pandemic crisis. J Diabetes Sci Technol 2021;15:377–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Singh AK, Gupta R, Ghosh A, Misra A. Diabetes in COVID-19: prevalence, pathophysiology, prognosis and practical considerations. Diabetes Metab Syndr 2020;14:303–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. CDCMMWR. Preliminary estimates of the prevalence of selected underlying health conditions among patients with coronavirus disease 2019—United States, February 12–March 28, 2020. MMWR Morb Mortal Wkly Rep 2020;69:382–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Liu J, Liu Y, Xiang P, Pu L, Xiong H, Li C, Zhang M, Tan J, Xu Y, Song R, Song M, Wang L, Zhang W, Han B, Yang L, Wang X, Zhou G, Zhang T, Li B, Wang Y, Chen Z, Wang X. Neutrophil-to-lymphocyte ratio predicts critical illness patients with 2019 coronavirus disease in the early stage. J Transl Med 2020;18:206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wu C, Chen X, Cai Y, Xia J, Zhou X, Xu S, Huang H, Zhang L, Zhou X, Du C, Zhang Y, Song J, Wang S, Chao Y, Yang Z, Xu J, Zhou X, Chen D, Xiong W, Xu L, Zhou F, Jiang J, Bai C, Zheng J, Song Y. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med 2020;180:934–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Guo W, Li M, Dong Y, Zhou H, Zhang Z, Tian C, Qin R, Wang H, Shen Y, Du K, Zhao L, Fan H, Luo S, Hu D. Diabetes is a risk factor for the progression and prognosis of COVID-19. Diabetes Metab Res Rev 2020;36:e3319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Nikniaz Z, Somi MH, Dinevari MF, Taghizadieh A, Mokhtari L. Diabesity associates with poor COVID-19 outcomes among hospitalized patients. J Obes Metab Syndr 2021;30:149–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Zhang J, Kong W, Xia P, Xu Y, Li L, Li Q, Yang L, Wei Q, Wang H, Li H, Zheng J, Sun H, Xia W, Liu G, Zhong X, Qiu K, Li Y, Wang H, Wang Y, Song X, Liu H, Xiong S, Liu Y, Cui Z, Hu Y, Chen L, Pan A, Zeng T. Impaired fasting glucose and diabetes are related to higher risks of complications and mortality among patients with coronavirus disease 2019. Front Endocrinol (Lausanne) 2020;11:525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Pal R, Bhadada SK. COVID-19 and diabetes mellitus: an unholy interaction of two pandemics. Diabetes Metab Syndr 2020;14:513–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Pradhan AD, Manson JE, Rifai N, Buring JE, Ridker PM. C-reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. JAMA 2001;286:327–34 [DOI] [PubMed] [Google Scholar]
- 45. Adam SS, Key NS, Greenberg CS. D-dimer antigen: current concepts and future prospects. Blood 2009;113:2878–87 [DOI] [PubMed] [Google Scholar]
- 46. Kloc M, Ghobrial RM, Lewicki S, Kubiak JZ. Macrophages in diabetes mellitus (DM) and COVID-19: do they trigger DM? J Diabetes Metab Disord 2020;19:2045–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Chen G, Li X, Gong Z, Xia H, Wang Y, Wang X, Huang Y, Barajas-Martinez H, Hu D. Hypertension as a sequela in patients of SARS-CoV-2 infection. PLoS ONE 2021;16:e0250815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Erener S. Diabetes, infection risk and COVID-19. Mol Metab 2020;39: 101044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Zhu L, She Z-G, Cheng X, Qin J-J, Zhang X-J, Cai J, Lei F, Wang H, Xie J, Wang W, Li H, Zhang P, Song X, Chen X, Xiang M, Zhang C, Bai L, Xiang D, Chen M-M, Liu Y, Yan Y, Liu M, Mao W, Zou J, Liu L, Chen G, Luo P, Xiao B, Zhang C, Zhang Z, Lu Z, Wang J, Lu H, Xia X, Wang D, Liao X, Peng G, Ye P, Yang J, Yuan Y, Huang X, Guo J, Zhang B-H, Li H. Association of blood glucose control and outcomes in patients with COVID-19 and pre-existing type 2 diabetes. Cell Metab 2020;31:1068–10773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Lee MS, Lee R, Ko CW, Moon JE. Increase in blood glucose level and incidence of diabetic ketoacidosis in children with type 1 diabetes mellitus in the Daegu-Gyeongbuk area during the coronavirus disease 2019 (COVID-19) pandemic: a retrospective cross-sectional study. J Yeungnam Med Sci 2022;39:46–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. COVID-19 may trigger hyperglycemia and worsen disease by harming fat cells. WCM Newsroom, https://news.weill.cornell.edu/news/2021/10/covid-19-may-trigger-hyperglycemia-and-worsen-disease-by-harming-fat-cells (accessed 21 March 2022).