Abstract
Background
Neutrophil serine proteases are involved in the pathogenesis of COVID-19 and increased serine protease activity has been reported in severe and fatal infection. We investigated whether brensocatib, an inhibitor of dipeptidyl peptidase-1 (DPP-1; an enzyme responsible for the activation of neutrophil serine proteases), would improve outcomes in patients hospitalised with COVID-19.
Methods
In a multicentre, double-blind, randomised, parallel-group, placebo-controlled trial, across 14 hospitals in the UK, patients aged 16 years and older who were hospitalised with COVID-19 and had at least one risk factor for severe disease were randomly assigned 1:1, within 96 h of hospital admission, to once-daily brensocatib 25 mg or placebo orally for 28 days. Patients were randomly assigned via a central web-based randomisation system (TruST). Randomisation was stratified by site and age (65 years or ≥65 years), and within each stratum, blocks were of random sizes of two, four, or six patients. Participants in both groups continued to receive other therapies required to manage their condition. Participants, study staff, and investigators were masked to the study assignment. The primary outcome was the 7-point WHO ordinal scale for clinical status at day 29 after random assignment. The intention-to-treat population included all patients who were randomly assigned and met the enrolment criteria. The safety population included all participants who received at least one dose of study medication. This study was registered with the ISRCTN registry, ISRCTN30564012.
Findings
Between June 5, 2020, and Jan 25, 2021, 406 patients were randomly assigned to brensocatib or placebo; 192 (47·3%) to the brensocatib group and 214 (52·7%) to the placebo group. Two participants were excluded after being randomly assigned in the brensocatib group (214 patients included in the placebo group and 190 included in the brensocatib group in the intention-to-treat population). Primary outcome data was unavailable for six patients (three in the brensocatib group and three in the placebo group). Patients in the brensocatib group had worse clinical status at day 29 after being randomly assigned than those in the placebo group (adjusted odds ratio 0·72 [95% CI 0·57–0·92]). Prespecified subgroup analyses of the primary outcome supported the primary results. 185 participants reported at least one adverse event; 99 (46%) in the placebo group and 86 (45%) in the brensocatib group. The most common adverse events were gastrointestinal disorders and infections. One death in the placebo group was judged as possibly related to study drug.
Interpretation
Brensocatib treatment did not improve clinical status at day 29 in patients hospitalised with COVID-19.
Funding
Sponsored by the University of Dundee and supported through an Investigator Initiated Research award from Insmed, Bridgewater, NJ; STOP-COVID19 trial.
Introduction
COVID-19 is a respiratory disease caused by SARS-CoV-2. In severe cases, patients develop pneumonia that can lead to acute respiratory distress syndrome (ARDS). Respiratory failure is the primary cause of death in people with severe COVID-19.1, 2
Severe COVID-19 is reportedly associated with a dysregulated immune response and hyperinflammation. This response is characterised by the antiviral interferon response, increased concentration of systemic cytokines (eg, interleukin [IL]-6), and an influx of inflammatory cells into the lungs. However, the extent of inflammatory response, in terms of the concentration of IL-6 and other proinflammatory cytokines, is reported to be less than in non-COVID-19 ARDS and sepsis.3 Neutrophils have been less well studied than other immune cell types involved in COVID-19, but as the understanding of the pathophysiology of COVID-19 has evolved, evidence supports an important role for neutrophils in severe disease.4 Increased neutrophil counts are associated with disease severity, and neutrophils have been implicated in amplifying inflammation, coagulopathy, organ damage, and immunothrombosis in people with COVID-19.5, 6 Increased concentrations of neutrophil serine proteases have been identified in the blood and lungs of people with COVID-19, and are associated with disease severity and mortality.7 Although anti-inflammatory treatments (eg, dexamethasone and anti-IL-6 receptor monoclonal antibodies) have been shown to reduce the risk of death in patients with COVID-19, there is an urgent need for additional therapies.8, 9, 10
Research in context.
Evidence before this study
We searched PubMed from Dec 1, 2019, until March 31, 2022, for articles related to COVID-19 and neutrophils, using the search terms COVID and Neutrophil. We did not restrict searches by language and we filtered findings for relevance based on abstract alone. We identified 1238 publications. Extensive evidence implicated neutrophils, neutrophil proteases, and neutrophil extracellular traps in the pathophysiology of COVID-19. Markers of neutrophilic inflammation, such as neutrophil elastase have increased concentration or activity in the blood and bronchoalveolar lavage from patients with COVID-19, and these markers predict poor outcome in patients who are hospitalised. No therapies directly targeting neutrophilic inflammation have been investigated in large-scale clinical trials. Brensocatib is an investigational dipeptidyl peptidase-1 (DPP-1) inhibitor, which has been shown to reduce neutrophilic inflammation in a phase 2 trial in bronchiectasis. We hypothesised that DPP-1 inhibition would improve outcomes in patients in hospital with COVID-19 by reducing neutrophil serine protease activity.
Added value of this study
To our knowledge, this is the first trial to test the clinical efficacy and safety of DPP-1 inhibition with brensocatib in patients hospitalised with COVID-19 who had at least one risk factor for severe disease. Brensocatib treatment did not improve the primary outcome of clinical status at day 29 after being randomly assigned. Similarly, there was no difference between the brensocatib group and placebo group in time to clinical improvement and time to discharge from hospital. Mortality over 28 days was 11% in the placebo group and 15% in the brensocatib group. Prespecified subgroup analyses, based on age, sex, baseline severity, co-medications, and duration of symptoms supported the primary results. Adverse events were reported in 46% of patients given placebo and 45% of patients given brensocatib. Active blood neutrophil elastase levels were reduced over 29 days in the brensocatib group versus in the placebo group.
Implications of all the available evidence
Although multiple studies suggest high amounts of neutrophilic inflammation are associated with worse outcomes in COVID-19, our study does not support targeting neutrophilic inflammation with DPP-1 inhibition as a therapeutic strategy in patients hospitalised with COVID-19. The worse clinical status in the brensocatib group suggests the need for caution in targeting DPP-1 or DPP-1-dependent proteases in patients hospitalised with COVID-19.
Dipeptidyl peptidase-1 (DPP-1) activates neutrophil proteases, such as neutrophil elastase, proteinase 3, and cathepsin G, during maturation in the bone marrow and activates proteases in other immune cells types, such as chymases in mast cells.11, 12 Brensocatib (INS1007, formerly AZD7986) is an oral, selective, competitive, and reversible inhibitor of DPP-1. Brensocatib has been shown to inhibit neutrophil serine protease activity in blood, in animal models, and in healthy people.11 In a phase 2 trial of brensocatib in patients with bronchiectasis, treatment with brensocatib reduced lung inflammation and was associated with prolonged time to first exacerbation.12
To examine whether brensocatib could improve clinical outcomes in people with COVID-19, we conducted a randomised placebo-controlled trial in patients admitted to hospital with COVID-19 in the UK. We hypothesised that brensocatib, by blocking the activity of damaging neutrophil proteases, would improve clinical outcomes over 28 days in patients hospitalised with COVID-19.
Methods
Study design and patients
This multicentre, double-blind, randomised, parallel-group, placebo-controlled trial was done at 14 hospitals in the UK (appendix pp 2–4). Eligible patients were 16 years of age or older with confirmed (confirmed by RT-PCR from combined oropharyngeal and nasopharyngeal swabs) or clinically suspected SARS-CoV-2 infection. Patients were required to have at least one risk factor for severe COVID-19, which were defined as: radiographic infiltrates by imaging, evidence of rales or crackles during physical examination, peripheral capillary oxygen saturation of 94% or less on room air, requiring supplemental oxygen, or a lymphocyte count of less than 1 × 109 cells per L before being randomly assigned.
Key exclusion criteria were participant (or a legally authorised representative) unable to give informed consent; alanine aminotransferase (ALT) or aspartate aminotransferase (AST) concentration of five times the upper limit of normal within 72 h of being randomly assigned; history of severe liver disease; stage 4 chronic kidney disease or requiring dialysis; absolute neutrophil count less than 1·0 × 109 cells per L within 72 h of being randomly assigned; current treatment with potent Cyp3A4 (cytochrome P450 3A4) inducers or inhibitors (eg, itraconazole, ketoconazole, diltiazem, verapamil, phenytoin, or rifampicin); and current treatment with HIV protease or integrase inhibitors or non-nucleoside reverse transcriptase inhibitors. The complete eligibility criteria are provided in the appendix (pp 5–6).
The trial was done in accordance with the ethical principles of the declaration of Helsinki, International Conference on Harmonisation Good Clinical Practice guidelines, and applicable regulatory requirements. The study was approved by the Scotland A Research Ethics Committee (20/SS/0057). All patients or legal representatives provided written informed consent. An independent, external data monitoring committee (comprising physicians with pulmonary expertise and a statistician experienced in the evaluation of clinical trials) reviewed all adverse events.
Randomisation and masking
Patients were screened for eligibility up to 24 h before random assignment and patients meeting the eligibility criteria were randomly assigned within 96 h of admission to hospital for COVID-19. Patients were randomly assigned via a central web-based randomisation system (TruST). Participants, study staff, and investigators were masked to the study assignment. Brensocatib and placebo tablets were identical and packaged into identical individual high-density polyethylene bottles. Randomisation was stratified by site and age (<65 years or ≥65 years). Within each stratum, blocks were of random sizes of two, four, or six patients. Eligibility for participation and enrollment in the trial was confirmed by the principal investigator or medically qualified delegate.
Procedures
Patients were assigned to either brensocatib (INS1007, formerly AZD7986) 25 mg once daily or placebo once daily orally for 28 days. Patients could be co-enrolled into the RECOVERY trial, but could not receive lopinavir–ritonavir due to potential drug–drug interactions.8 Patients’ clinical status and safety were evaluated daily while they were in hospital and, in patients discharged from hospital, safety assessments were done by telephone on days 3, 5, 11, 15, and 29 after being randomly assigned. Patients in both groups continued to receive other therapies required to manage their condition. Use of other therapies were recorded. Patients continued to receive the intervention after being discharged from hospital until the end of the treatment period (28 days after being randomly assigned).
The 7-point WHO ordinal scale for clinical status (WHO 7-point ordinal scale) was used to assess clinical status at each timepoint (baseline, days 3, 5, 11, 15, and 29 after random assignment; not hospitalised and no limitations on activities [1]; not hospitalised and limitations on activities [2]; hospitalised and not requiring supplemental oxygen [3]; hospitalised and requiring supplemental oxygen [4]; hospitalised and on non-invasive ventilation or high-flow oxygen devices [5]; hospitalised and on invasive mechanical ventilation or extracorporeal membrane oxygenation [6]; and death [7]). The highest score for each day was recorded. The UK National Early Warning Score (NEWS) was used as an efficacy measure and was collected throughout a participant's hospital stay (the number of NEWS measurements were determined by the managing clinicians). Quality of life was assessed using the EQ-5D-5L quality-of-life tool in patients in-person (hospitalised) or over the telephone (discharged). Neutrophil elastase activity was measured as previously described.11 Briefly, whole blood samples were treated with either 10 mg/mL zymosan or with Hanks’ balanced salt solution at 37°C for 30 min. Following incubation, samples were centrifuged, and blood plasma was frozen at –80°C for analysis. Neutrophil elastase activity was measured by cleavage of the specific fluorogenic substrate MeOSuc-AAPV-AMC (Sigma Aldrich, Poole, UK; M9771). The stimulated elastase activity was calculated by subtracting the plasma elastase activity after incubation with buffer alone from the plasma elastase activity following incubation with zymosan stimulation.
Outcomes
The primary objective of the study was the comparison of participant clinical status between treatment groups, assessed with the WHO 7-point ordinal scale. The secondary outcome measures were: time to improvement in one category on the WHO 7-point ordinal scale; clinical status at days 3–15 after first dose; mean change in the 7-point ordinal scale over 29 days from randomisation; time to discharge from hospital or time to a NEWS of 2 or less and maintained for 24 h, whichever occurred first; number of days not requiring oxygen therapy; incidence and duration of new oxygen therapy use during the trial; number of mechanical ventilator-free days; incidence and durations of new mechanical ventilation use during the trial; duration of hospital stay; and 28-day mortality.
Safety assessments were: the cumulative incidence of adverse events and serious adverse events (SAEs) during the trial; discontinuation or temporary suspension of treatment; and adverse events of special interest (hyperkeratosis, infections, and dental complications). Adverse events were reported by the participant, site principal investigators, or delegated staff responsible for detecting, documenting, and recording events that met the definition of an adverse event. Participants discharged from hospital before the end of the trial were given a diary to record adverse events up to day 28. Site principal investigators were responsible for assigning seriousness and causality of adverse events. Additional details are described in the appendix (pp 6–7).
As an exploratory endpoint, we also did a substudy at two centres (Dundee and Sheffield), in which blood was obtained at day 8, 15, and 29 after randomisation for measurement of neutrophil elastase activity.
Statistical analyses
The sample size calculation used the WHO master protocol for COVID-19 trials in March, 2020. We required outcome data on a total of 272 participants to detect an odds ratio (OR) of 2·0 for 5% two-sided α and 85% power, which was inflated to 300 for a potential 9% loss to follow-up. The sample size calculation assumed the following proportions of patients in each WHO class at day 29: not hospitalised and no limitations on activities (42%), not hospitalised and limitations on activities (38%), hospitalised and not requiring supplemental oxygen (8%), hospitalised and requiring supplemental oxygen (7%), hospitalised and on non-invasive ventilation or high flow oxygen devices (2%), hospitalised and on mechanical ventilation or extracorporeal membrane oxygenation (1%), and death (2%). A prespecified blinded sample size re-evaluation by an independent statistician after 100 participants had completed 28 days treatment resulted in an increase in the sample size to 400 patients for 85% power for an OR of 1·75. Efficacy analyses were based on the intention-to-treat population, comprising all randomly assigned patients. The primary efficacy endpoint of the WHO 7-point ordinal scale at day 29 was analysed using ordinal logistic regression, assuming proportional odds, adjusted for the stratifying factors of age as a fixed effect and site, using robust SEs to account for clustering. Results are presented as OR (95% CI), in which an OR greater than 1 indicates better clinical status at day 29 in the brensocatib group. Secondary outcomes based on time-to-event (time to clinical improvement, time to hospital discharge, and 28-day mortality) were analysed by Cox proportional hazards regression, adjusted for age and site (using robust SE). Proportional hazards were assessed visually with log (–log[(survival]) versus log (analysis time) plots. There was some evidence of proportional hazard assumption violation early in follow-up in the mortality analysis and, therefore, we also used a post-hoc analysis of restricted mean survival time to analyse 28 day mortality. We analysed number of days free from oxygen, new oxygen use, days free from ventilation, and new ventilation using negative binomial regression. The EQ-5D-5L quality of life assessment tool was analysed using linear regression adjusted for age and site (using robust SEs). We analysed neutrophil elastase activity in blood during the treatment period using mixed model repeated measures approach. The model included a fixed effect for treatment group and nominal time (1, 8, 15, and 29 days after random assignment), and treatment-by-time interaction. Patient was included in the model as a random intercept and an unstructured covariance structure assumed. We analysed adverse events between brensocatib and placebo groups using negative binomial regression, ordinal regression, or Fisher's exact test.
Prespecified subgroups were aged 65 years or older versus younger than 65 years; sex; baseline clinical status; co-enrolment in the RECOVERY trial; and duration of symptoms less than 10 days versus 10 days or more. The analysis included a treatment-by-subgroup interaction.
All estimates are presented with 95% CIs. No imputation of missing data was done. No adjustment was made for multiplicity of comparisons, and p values were displayed only for the primary outcome and safety results, and not displayed for secondary outcomes.
A post-hoc analysis for the primary outcome was adjusted for baseline demographic variables with at least a 5% difference between the groups. The study was prospectively registered with ISRCTN registry, ISRCTN30564012.
Role of the funding source
The funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the article.
Results
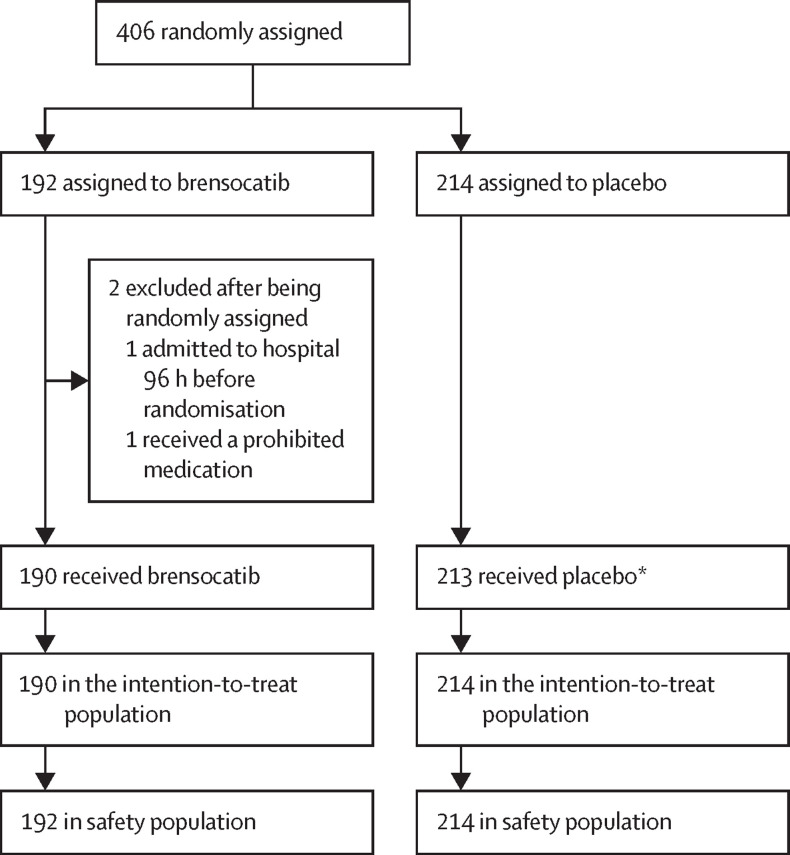
Between June 5, 2020, and Jan 25, 2021, 406 participants were randomly assigned to the placebo group (n=214 patients) or to the brensocatib group (n=192; figure 1 ). There were two post-randomisation exclusions due to ineligibility in the brensocatib group (one patient was admitted to hospital as an inpatient more than 96 h before randomisation and another was receiving a prohibited medication). One patient was randomly assigned but did not receive placebo as they withdrew from study treatment before the first dose. We included these excluded patients in the safety analysis. 404 participants (190 patients in the brensocatib group and 214 in the placebo group) were included in the intention-to-treat analysis.
Figure 1.
Trial profile
*One patient was randomly assigned but did not receive placebo as they withdrew from study treatment before the first dose.
There was a slightly higher proportion of male participants, with chronic obstructive pulmonary disease (COPD), and with crackles on examination, and a lower proportion with hypertension in the brensocatib group than in the placebo group (table 1 ). Clinical status at baseline was balanced. During hospitalisation, 155 (81%) of 192 patients in the brensocatib group and 171 (80%) of 214 in the placebo received low-dose dexamethasone; 47 (25%) in the brensocatib group and 51 (24%) in the placebo received remdesivir; and seven (4% in the brensocatib group and 3% in the placebo group) participants in each group received tocilizumab (co-medications are shown in the appendix p 8).
Table 1.
Baseline characteristics by treatment group
| Placebo (n=214) | Brensocatib (n=190) | ||
|---|---|---|---|
| Mean age, years | 62·0 (14·9) | 62·3 (12·5) | |
| Sex | |||
| Male | 127 (59%) | 125 (66%) | |
| Female | 87 (41%) | 65 (34%) | |
| Ethnicity | |||
| White British | 189 (88%) | 167 (88%) | |
| Irish | 1 (0%) | 2 (1%) | |
| Any other White background | 5 (2%) | 6 (3%) | |
| White and Black Caribbean | 1 (0%) | 0 | |
| White and Black African | 1 (0%) | 0 | |
| Any other mixed or multiple ethnic background | 1 (0%) | 0 | |
| Indian | 5 (2%) | 1 (1%) | |
| Pakistan | 3 (1%) | 4 (2%) | |
| Bangladeshi | 1 (0%) | 0 | |
| Any other Asian background | 2 (1%) | 4 (2%) | |
| African | 0 | 1 (1%) | |
| Any other Black, African, or Caribbean background | 1 (0%) | 0 | |
| Arab | 1 (0%) | 1 (1%) | |
| Any other ethnic group | 3 (1%) | 2 (1%) | |
| Unknown | 0 | 2 (1%) | |
| Smoking status | |||
| Current Smoker | 12 (6%) | 9 (5%) | |
| Never smoked | 98 (46%) | 93 (49%) | |
| Former smoker | 72 (34%) | 67 (35%) | |
| Unknown | 32 (15%) | 21 (11%) | |
| Comorbidities | |||
| Chronic cardiac disease, including congenital heart disease | 37 (17%) | 34 (18%) | |
| Hypertension | 90 (42%) | 70 (37%) | |
| Chronic obstructive pulmonary disease | 22 (10%) | 29 (15%) | |
| Asthma (physician diagnosed) | 38 (18%) | 34 (18%) | |
| Chronic kidney disease (estimated glomerular filtration rate <44 mL/min on dialysis or previous transplant) | 9 (4%) | 7 (4%) | |
| Obesity | 48 (22%) | 41 (22%) | |
| Diabetes with complications | 14 (7%) | 5 (3%) | |
| Diabetes without complications | 33 (15%) | 29 (15%) | |
| Rheumatological disorder | 18 (8%) | 19 (10%) | |
| Median duration of symptoms, days | 8·0 (6·0–11 ·0) | 9·0 (6·0–12·0) | |
| Disease severity | |||
| Required supplemental oxygen | 160 (75%) | 148 (78%) | |
| Peripheral capillary oxygen saturation ≤94% on room air before randomisation | 120 (56%) | 112 (59%) | |
| Radiographic infiltrates by imaging (eg, chest x-ray or CT scan) | 146 (68%) | 138 (73%) | |
| Evidence of rales or crackles on physical examination | 99 (46%) | 101 (53%) | |
| Lymphocyte count less than 1 × 109 cells per L | 95 (44%) | 88 (46%) | |
| Clinical status at randomisation | |||
| Hospitalised and not requiring supplemental oxygen | 50 (23%) | 42 (22%) | |
| Hospitalised and requiring supplemental oxygen | 140 (65%) | 128 (67%) | |
| Hospitalised and on non-invasive ventilation or high flow oxygen devices | 24 (11%) | 20 (11%) | |
| Median 7-point WHO ordinal scale for clinical status | 4 (4–4) | 4 (4–4) | |
| SARS-CoV-2 PCR status | |||
| Confirmed positive SARS-CoV-2 PCR test | 204 (95%) | 186 (98%) | |
| Clinically suspected without confirmed SARS-CoV-2 PCR test | 10 (5%) | 4 (2%) | |
Data are n (%), mean (SD), or median (IQR).
169 (79%) of 214 participants in the placebo group and 140 (74%) of 190 in the brensocatib group were discharged from hospital by day 29 (table 2 ). There were three (1%) participants in the placebo group and three (1%) in the brensocatib group whose status was unknown due to loss to follow-up or declining additional follow-up. The adjusted OR from a proportional odds model for clinical outcomes measured by the WHO 7-point ordinal scale was 0·72 (95% CI 0·57–0·92; p=0·0077), favouring the placebo group. The proportional odds assumption was tested and there was no violation for the treatment effect. In a post-hoc analysis adjusted for baseline demographic variables with at least 5% difference between the groups (sex, hypertension, and COPD) the adjusted OR was 0·75 (0·59–0·96).
Table 2.
Estimates of treatment effect on the primary outcome measured by WHO 7-point ordinal scale on day 29 after randomisation
| Placebo (n=214) | Brensocatib (n=190) | Model | Effect size (95% CI) | p value | |
|---|---|---|---|---|---|
| Not hospitalised and no limitations on activities | 40 (19%) | 28 (15%) | Unadjusted | 0·74 (0 ·50–1 ·09) | .. |
| Not hospitalised and limitations on activities | 129 (60%) | 112 (59%) | Adjusted* | 0·72 (0 ·57–0 ·92) | 0·0077 |
| Hospitalised and not requiring supplemental oxygen | 11 (5%) | 7 (4%) | .. | .. | .. |
| Hospitalised and requiring supplemental oxygen | 1 (0%) | 6 (3%) | .. | .. | .. |
| Hospitalised and on non-invasive ventilation or high flow oxygen devices | 1 (0%) | 0 | .. | .. | .. |
| Hospitalised and on invasive mechanical ventilation or extracorporeal membrane oxygenation | 6 (3%) | 5 (3%) | .. | .. | .. |
| Death | 23 (11%) | 29 (15%) | .. | .. | .. |
| Lost to follow-up | 3 (1%) | 3 (1%) | .. | .. | .. |
Data are n (%) unless otherwise specified.
Adjusted for minimisation variables, age and site (using clustered SEs).
We did a prespecified subgroup analyses in patients aged 65 years or older versus those aged younger than 65 years, based on sex, baseline clinical status, co-enrolment in the RECOVERY trial, and duration of symptoms less than 10 days or 10 days or more. Results of subgroup analyses on the primary outcome are shown in appendix (pp 8–16). Effect estimates were consistent across all subgroups for the primary outcome and there was no evidence of effect moderation in any subgroup analysis.
In total, there were 71 (18%) participants (35 [16%] of 214 in the placebo group and 36 [19%] of 190 in the brensocatib group) who discontinued the trial drug during the study period. The main reasons for discontinuation of treatment were participant choice or due to an adverse event. After excluding these participants, 179 participants in the placebo group and 154 in brensocatib group were included in the per-protocol analysis. The adjusted OR from a proportional odds model was 0·79 (95% CI 0·60–1·06; appendix pp 16–17).
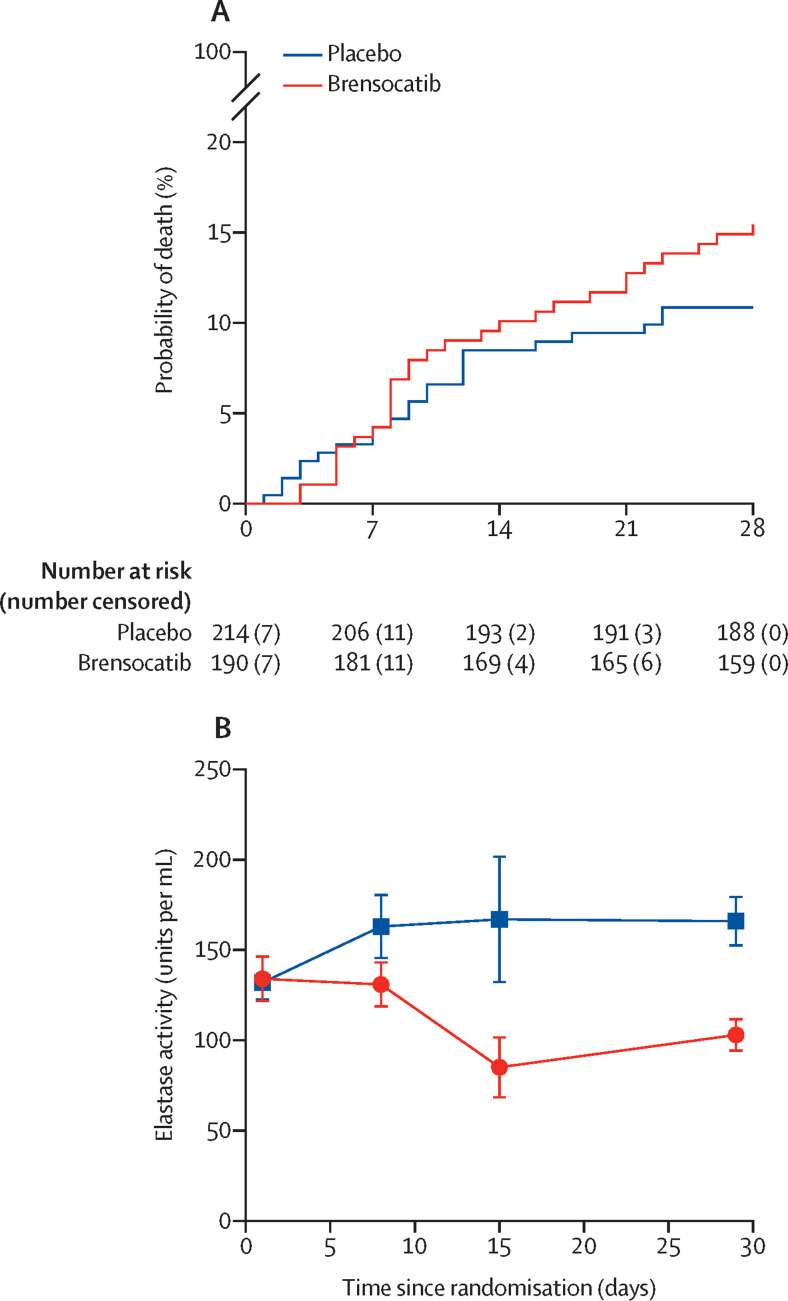
23 (11%) of 214 patients died in the placebo group and 29 (15%) of 190 patients died in the brensocatib group by day 28 after random assignment (table 3 ). The adjusted HR was 1·41 (1·06 to 1·88) for mortality of patients in the brensocatib group versus in the placebo group. The restricted mean survival times were 26·0 (95% CI 25·2 to 26·9) days for placebo and 25·6 (24·7 to 26·5) days for brensocatib (figure 2A ). The difference in restricted mean survival time was –0·43 (95% CI –1·66 to 0·80). With adjustment, the difference was –0·37 (–1·51 to 0·77).
Table 3.
Estimates of treatment effects on secondary endpoints
| Placebo (n=214) | Brensocatib (n=190) | Unadjusted effect estimate (95% CI) | Adjusted*effect estimate (95% CI) | ||
|---|---|---|---|---|---|
| 28 day mortality†, n (%) | 23 (11%) | 29 (15%) | 1·44 (0·83–2·48) | 1·41 (1·06–1·88) | |
| Clinical improvement | |||||
| Patients improved, n (%) | 186 (87%) | 159 (84%) | .. | .. | |
| Time to clinical improvement† | .. | .. | 0·87 (0·70–1·07) | 0·87 (0·76–1·00) | |
| Discharge or UK National Early Warning Score <2† | |||||
| Patients discharged or with UK National Early Warning Score ≤2, n (%) | 195 (91%) | 172 (91%) | .. | .. | |
| Time to discharge or UK National Early Warning Score <2† | .. | .. | 0·98 (0·79–1·21) | 0·98 (0·84–1·13) | |
| Oxygen use and ventilation | |||||
| Oxygen-free days‡ | 24·5 (17·0–27·0) | 24·0 (11·0–27·0) | 0·93 (0·78–1·12), | 0·93 (0·87–0·99) | |
| Duration of new oxygen use‡ | 0·0 (0·0–1·0) | 0·0 (0·0–2·0) | 1·15 (0·39–3·38) | 1·13 (0·73–1·74) | |
| Ventilation-free days§ | 28·0 (26·0–28·0) | 28·0 (22·0–28·0) | 0·85 (0·55–1·30) | 0·84 (0·69–1·04) | |
| Duration of new ventilation use‡ | 0·0 (0·0–0·0) | 0·0 (0·0–0·0) | 1·64 (0·69–3·92) | 1·68 (1·09–2·58) | |
| Mechanical ventilation-free days§ | 28·0 (28·0–28·0) | 28·0 (28·0–28·0) | 0·77 (0·46–1·29) | 0·77 (0·59–1·01) | |
| Duration of new mechanical ventilation use‡ | 0·0 (0·0–0·0) | 0·0 (0·0–0·0) | 1·22 (0·30–4·86) | 1·32 (0·68–2·56) | |
| Duration of hospitalisation‡ | 5·0 (3·0–11·0) | 6·0 (3·0–10·5) | 1·03 (0·84–1·26) | 1·03 (0·92–1·15) | |
Data are median number of days (IQR), unless otherwise specified. Duration of ventilation use includes non-invasive and invasive mechanical ventilation.
Adjusted for minimisation variables; age and site as a random effect.
Hazard ratio from Cox proportional hazards model.
Incidence rate ratio from negative binomial regression.
Odds ratio from ordinal logistic regression model.
Figure 2.
Kaplan-Meier survival curve (A) and neutrophil elastase activity in blood (B)
Data for neutrophil elastase activity in blood are shown as mean (SE) at each timepoint (day 0, 8, 15, and 29 after first randomisation).
Time to clinical improvement was calculated as the number of days from randomisation to the first follow-up day in which there was at least a 1 point improvement in the WHO 7-point ordinal scale. Overall, 186 (87%) patients in the placebo group and 159 (84%) patients in the brensocatib group had a clinical improvement during the study period (adjusted HR 0·87 [0·76–1·00]). The clinical status at timepoints before day 29 (days 3, 5, 8, 11, and 15 after random assignment) are shown in the appendix (pp 17–18). Time to discharge or to a NEWS of 2 or less was not different between the groups (adjusted HR 0·98 [95% CI 0·84–1·13]). The mean change from baseline to day 29 after randomisation in clinical status using the WHO 7-point ordinal scale was a 1·0 point improvement in the brensocatib group (SD 2·0) and a 1·3 point improvement in the placebo group (SD 1·7).
We compared the number of days free from oxygen support between treatment groups using negative binomial regression. The median number of oxygen-free days was 24·5 (IQR 17·0–27·0) days in the placebo group and 24·0 (11·0–27·0) days in the brensocatib group (adjusted incidence rate ratio [IRR] 0·93 [95% CI 0·87–0·99]). For the analysis of duration of new oxygen use, only participants who were hospitalised but did not require supplemental oxygen at baseline were included. In total 81 patients, 42 (52%) patients in the placebo group and 39 (48%) in the brensocatib group, were included in the analysis. There was no association between treatment with brensocatib and new oxygen use versus placebo (IRR 1·13 [0·73–1·74]). For the duration of new ventilation use analysis, only participants who were not requiring ventilatory support at baseline were included. Brensocatib treatment was associated with an increased risk of new ventilation use (adjusted IRR 1·68 [1·09–2·58]) versus the placebo. Ventilation-free days were not significantly different between patients given brensocatib compared with those given placebo. We noted no differences in the duration of new mechanical ventilation, mechanical ventilation-free days, or duration of hospitalisation between the groups (table 3).
The exploratory endpoint of neutrophil elastase activity was done in 152 patients enrolled at two sites (University of Dundee and University of Sheffield; 75 in the brensocatib group and 77 in the placebo group). There was no significant difference in neutrophil elastase activity between the two groups at baseline (134 units per mL [95% CI 108 to 160] in the brensocatib group and 132 units per mL [113 to 152] in the placebo group). Neutrophil elastase activity data were available for patients who were still in hospital at day 8 (n=57) and day 15 (n=29) after random assignment and participants returned for follow-up visits for neutrophil elastase measurement at day 29 (n=98; 50 in the brensocatib group and 48 in the placebo group). Neutrophil elastase activity was reduced from the first measurement at day 8 to day 29 after random assignment in participants given brensocatib. The mean reduction in neutrophil elastase activity at day 29 was –67 units per mL (95% CI –102 to –31) (figure 2B). Additional information is provided in the appendix (p 19).
For safety analysis, 185 (46%) participants, 99 (46%) of 214 in placebo group and 86 (45%) of 192 in the brensocatib group, reported at least one adverse event (OR 0·94 (95% CI 0·64–1·39); p=0·77; table 4 ) and there was no difference in the frequency of adverse events between groups (IRR 0·97 [95% CI 0·73–1·29]; p=0·83). There were 296 events in total, 164 in the placebo group and 132 in the brensocatib group. The most common adverse events were gastrointestinal disorders and infections in both groups. 75 (18%) of all 406 participants had at least one SAE, 35 (16%) participants in placebo group and 40 (21%) in brensocatib group (IRR 1·27 [95% CI 0·81–2·01]; p=0·30). There were 81 SAEs in total; 39 in the placebo group and 42 in brensocatib group. There were 23 (11%) SAEs related to infections and infestations in the placebo group and 26 (14%) in the brensocatib group. Only one death in the placebo group was judged as possibly related to study drug. Detailed safety data are shown in the appendix (pp 19–26).
Table 4.
Summary of safety results
| Placebo (n=214) | Brensocatib 25 mg (n=192) | Odds ratio (95% CI) | p value | ||
|---|---|---|---|---|---|
| Any TEAE | 99 (46%) | 86 (45%) | 0·94 (0·64–1·39) | 0·77 | |
| TEAE maximum severity | |||||
| Mild | 44 (21%) | 36 (19%) | .. | .. | |
| Moderate | 24 (11%) | 12 (6%) | .. | .. | |
| Severe | 31 (15%) | 38 (20%) | 1·01 (0·70– 1·47) | 0·94 | |
| TEAEs in ≥5% of patients in any group* | |||||
| Gastrointestinal disorders | 30 (14%) | 18 (9%) | 0·63 (0·34–1·18) | 0·15 | |
| Infections and infestations | 31 (15%) | 31 (16%) | 1·14 (0·66–1·95) | 0·64 | |
| Respiratory, thoracic, and mediastinal disorders | 12 (6%) | 15 (8%) | 1·43 (0·65–3·13) | 0·38 | |
| Nervous system disorders | 11 (5%) | 11 (6%) | 1·12 (0·47–2·65) | 0·79 | |
| Skin and subcutaneous tissue disorders | 14 (7%) | 12 (6%) | 0·95 (0·43–2·11) | 0·91 | |
| TEAE resulting in treatment discontinuation | 7 (3%) | 9 (5%) | 1·45 (0·53–3·98) | 0·47 | |
| Adverse events of special interest | |||||
| Hyperkeratosis | 0 | 0 | .. | .. | |
| Dental complications | 1 (0%) | 0 | .. | 1·00 | |
| Secondary infections | 7 (3%) | 6 (3%) | 0·95 (0·31–2·89) | 0·93 | |
| Serious TEAE | |||||
| Infections and infestations | 23 (11%) | 26 (14%) | 1·30 (0·72–2·37) | 0·39 | |
| Respiratory, thoracic, and mediastinal disorders | 5 (2%) | 6 (3%) | .. | 0·76 | |
| Gastrointestinal disorders | 2 (1%) | 1 (1%) | .. | 1·00 | |
| Relation to drug treatment† | |||||
| Adverse event related to study drug | 37 (17%) | 39 (20%) | 1·22 (0·74–2·01) | 0·44 | |
| Deaths related to study drug | 1 (0%) | 0 | .. | 1·00 | |
Data are n (%), unless otherwise specified. Safety analyses were based on the safety population, which included all patients who received at least one dose of brensocatib or placebo. p values are brensocatib group vs placebo logistic regression, except TEAE maximum severity, which was analysed using ordinal regression. If odds ratios were not provided then p values were determined using Fisher's exact test. TEAE=treatment-emergent adverse event.
Adverse events are reported and classified based on Medical Dictionary for Regulatory Activities terminology.
Judged as possible or probable by the site principal investigator.
Quality-of-life scores using the EQ-5D-5L quality-of-life tool were collected for 151 participants in the placebo group and 131 participants in the brensocatib group. There was no association between treatment and quality-of-life scores with an adjusted mean difference of 0·003 (95% CI –0·060 to 0·066). Detailed analyses are shown in the appendix (p 27).
Discussion
Among patients hospitalised with COVID-19, treatment with brensocatib for 28 days was associated with a worse clinical status on the WHO 7-point ordinal scale at the end of treatment than was placebo. The number of deaths was higher in the brensocatib group than in the placebo group. Participants randomly assigned to brensocatib also had significantly fewer oxygen-free days and an increased duration of new ventilator use, which indicated worse respiratory function during the study period. Several other study outcomes, including time to hospital discharge, time to clinical improvement, and duration of hospitalisation were not significantly different between the groups. Results were consistent among subgroups, and also in the per-protocol and post-hoc analyses, and no results showed a benefit associated with brensocatib treatment. Despite the worse clinical status at day 29 following randomisation according to the 7-point WHO ordinal scale, there were no large differences between the groups in the frequency of adverse events and SAEs. In addition, no increase in infections was seen in the bresocatib treatment group, suggesting that treatment did not increase the risk of bacterial coinfection.
The exploratory endpoint of neutrophil elastase release in the blood suggests that the treatment had the desired effect on inhibition of neutrophil serine proteases, which is consistent with the pharmacology research previously showing inhibition of neutrophil serine protease activity in the blood and the lungs of patients given this dose.11, 12 As expected, reductions in neutrophil elastase were detected at the first timepoint (day 8) due to the release into the circulation of newly formed neutrophils from the bone marrow that were deficient in active neutrophil serine proteases, such as elastase. Therefore, the lack of efficacy observed in these patients who were hospitalised with COVID-19 is unlikely to be explained by an absence of a pharmacological effect.
A large number of observational studies have suggested that neutrophils are important in the pathobiology of severe COVID-19, and neutrophil-related proteins, including serine proteases, are linked with outcomes, including mortality.7, 13, 14, 15 It has been hypothesised that the release of neutrophil extracellular traps and the associated antimicrobial proteins and proteases induce lung damage and promote alveolar oedema, leading to worse outcomes.7, 13 Despite these findings, our results found that an effective serine protease inhibitor did not improve clinical outcomes at this stage of the disease.
In this study, the difference in outcomes observed in patients given brensocatib compared with those given placebo, in terms of worse clinical status and increased requirement for new ventilation use and reduced oxygen-free days, suggests that inhibition of DPP-1 could have impaired recovery from COVID-19. However, our results should be interpreted with caution as the sample size of this study is small compared with much larger platform trials8, 16 that have tested other agents for COVID-19 treatment and because of the inconsistent results observed with other anti-inflammatory or immunomodulatory therapies administered at different stages of the disease. For example, a small randomised open-label trial of the anti-IL-6 receptor monoclonal antibody tocilizumab in 129 patients with COVID-19 in Brazil was terminated early due to a large increase in mortality reported in the tocilizumab group (OR for mortality of 6·42 [95% CI 1·59–43·2]).17 Subsequently, larger open-label trials had shown the effectiveness of tocilizumab in preventing mortality and requirement for mechanical ventilation in patients who have been hospitalised with COVID-19.10, 18, 19 It is therefore possible that our results represent a chance finding. Dexamethasone was found to be effective in patients requiring supplementary oxygen or ventilatory support with a duration of illness greater than 7 days; however, it was ineffective in patients who did not require oxygen and had a duration of illness less than 7 days.8 The concept that the inflammatory phase of COVID-19 occurs late informed the design of our study. We are unable to comment on whether protease inhibition earlier in the course of disease might have been more beneficial than in our study. We observed some baseline imbalances in the study, with a higher proportion of male patients, a higher proportion of patients with COPD, and a longer duration of symptoms in the brensocatib group and more patients in the placebo group. These differences were small and adjusting for baseline imbalances in a post-hoc analysis did not affect the primary conclusions.
Additional research is required to understand the potential mechanisms underlying our results and to characterise the role of neutrophils in COVID-19. In-vitro and mouse models of respiratory viral infections suggest that neutrophils can have both a positive and negative role in host response.20, 21, 22 Neutrophil recruitment and response involve a delicate balance between these protective and deleterious effects. Although capable of inducing tissue damage, neutrophils have been shown to suppress T-cell-mediated immune pathology in influenza infection, dependent on CD11b and CD18 (Mac1).23 There are sparse data available on potential beneficial effects of neutrophil serine proteases but neutrophil elastase has been reported to enhance the release of immunomodulatory prostaglandins and to cleave receptors on T cells, macrophages, and neutrophils to limit hyperinflammation.24, 25, 26 Uptake of neutrophil extracellular traps by macrophages has been shown to promote type I interferon generation via neutrophil elastase-mediated pathways.27 T cell, macrophage, and type I interferon signalling are also central to the immune pathology in COVID-19.28 Although these mechanisms are plausible explanations for our findings, in our study, we did not have direct measures of pulmonary inflammation or effects on pulmonary immunity.29 Although our results might suggest a need for caution in testing inhibitors of neutrophil serine proteases in COVID-19, it is important to note that DPP-1 has functions beyond those in neutrophils. In DPP-1-deficient murine models, serine proteases are inactivated in a range of inflammatory and immune cells, including cytotoxic T lymphocytes, natural killer cells, and mast cells, suggesting that the effects seen in our study are not solely the result of targeting neutrophils.30, 31, 32
The limitations of the study should be acknowledged. Patients could receive additional interventions, either as part of clinical care or through co-enrolment in the RECOVERY trial. Since the majority of patients who were co-enrolled in the RECOVERY trial were given azithromycin, aspirin, colchicine, or convalescent plasma, all of which were found to have no effect on outcomes and the probability of receiving these interventions was equal between the two groups, they are unlikely to have affected the results of the study. Although we measured neutrophil elastase activity in blood as an exploratory endpoint in the study, we were unable to measure neutrophil serine protease activity in bronchoalveolar lavage and so we cannot comment on the extent of any anti-inflammatory effects of brensocatib in the lungs.
In summary, this multicentre, double-blind, randomised, parallel-group, placebo-controlled trial done during the first and second waves of the COVID-19 pandemic in the UK showed no significant benefit of brensocatib compared with placebo in patients who were hospitalised with COVID-19.
Data sharing
No data are available for sharing.
Declaration of interests
JDC reports grants and personal fees from AstraZeneca, Boehringer-Ingelheim, Chiesi, GSK, Gilead Sciences, Grifols, Insmed, Janssen, Novartis, and Zambon. CB reports grants from the UK National Institute for Health and Care Research Biomedical Research Centre during the conduct of the study; grants and personal fees from GSK, AstraZeneca, Chiesi, Boehringer-Ingelheim, Genentech, Roche, Sanofi, Regeneron, Merck, TEVA, Mologic, 4DPharma, and Novartis. AART reports grants and personal fees from British Heart Foundation and Actelion Pharmaceuticals. JU reports personal fees from Gilead Sciences and ViiV Healthcare and from Celltrion; and is supported by the UK Medical Research Council (MR/T023791/1). DPSD reports grants and personal fees from GSK, Vir Biotechnology, AstraZeneca, and Boehringer-Ingelheim. ASm has received non-financial support for clinical trial work from AstraZeneca, GSK, Chiesi, and Oncimmune; and has done consultancy work with AstraZeneca and GSK. MP reports non-financial support for clinical trial work from AstraZeneca, GSK, Chiesi, and Oncimmune and consultancy work with AstraZeneca and GSK. All other authors report no competing interests.
Acknowledgments
Acknowledgments
This study was funded by an investigator-initiated research grant from Insmed (Bridgewater, NJ, USA). The authors acknowledge Stephen Senn (Edinburgh, UK) for independent statistical advice and Alex McConnachie (University of Glasgow, Glasgow, UK), Aran Singanayagam (Imperial College, London, UK), Oriol Sibila (Hospital Clínic Barcelona, Barcelona, Spain), and Petra Rauchhaus (University of Dundee, Dundee, UK) for serving as the independent data monitoring committee. The STOP-COVID19 study was designated an urgent public health priority study by the UK National Institute for Health and Care Research. The authors acknowledge the funding and logistical support from the UK National Institute for Health and Care Research.
Contributors
This study was conceived and designed by HRK, MBL, and JDC. Trial management and conduct were done by HRK, MBL, MB, FM-N, SA, EL, JG, AMC, and JDC. Patient enrolment and data collection were done by HA-L, BJMN, DC, JC, JS, LDi, PR, JU, AH, DPSD, BS, DD, MS, AART, CB, ASm, MP, AART, and JDC. Laboratory analysis was done by HRK, MBL, YHG, TP, RCH, LD, CH, RD, HT, HR, DC, AMC, ASh, and JDC. Statistical analysis was done by TV, GM, and JDC. HRK, TV, GM, and JDC had directly accessed and verified the data. The manuscript was drafted by HRK, MBL, and JDC. All authors reviewed, contributed to, and approved the final version of the article.
Contributor Information
STOP-COVID19 Investigators:
James Chalmers, Hani Abo-Leyah, Benjamin JM New, Christine Almaden-Boyle, David Connell, Jennifer Taylor, Jodie Strachan, Heather Loftus, Lesley Young, Angela Strachan, Margaret Band, Fiona McLaren-Neil, Kristina Pilvinyte, Simon Adamson, Eva Lahnsteiner, Petra Rauchhaus, Fiona Hogarth, Jacob George, Tricia Burns, Elizabeth Coote, Marney Keiller, Manish Patel, Andrew Smith, Elizabeth Sage, Jamie Cooper, David Miller, Davinder Dosanjh, Benjamin Sutton, Jonathan Underwood, Sharon Frayling, Matthew Haynes, Lauren Broad, Laura Jones, Karen Rahilly, Catherine Oliver, Terriann Evans, Andrea Balan, Rhys Davies, Donal Forde, Clemency Nye, Dr Haboubi, Zoe Hilton, Jennie Williams, Alison McQueen, Mark Spears, Ian Edmond, Dario Salutous, Laura McGenily, Rhona Scott, Eilidh Henderson, Andrea Collins, Devesh Dhasmana, Patrick Liu, Ana Morrow, Mandy Couser, Fleur Davey, Alexander Hicks, Laura Wiffen, Lauren Fox, Mohamed Abdelrahim, Alexander Darbyshire, Elena Cowen, Megan Rowley, Benjamin Giles, Yingjia Yang, Tom Brown, Hitasha Rupani, Elizabeth Hawes, Debi Barnes, Fiona Brogan, Roneleeh Bungue-Tuble, Serena Howe, Charlotte Turner, Sonia Baryschpolec, Bev Longhurst, Maria Moon, Lynn Watkins, Michelle Baker-Moffat, Lisa Murray, Yasmin Harrington-Davies, Kate Burrows, Chrissie Minnis, Mary Wands, Adefunke Bamgboye, Charlotte Wong, Christopher Brightling, Sarah Diver, Richard Russell, Hamish McAuley, Omer Elneima, Ahmed Yousuf, Paula McCourt, Beverley Hargadon, Sarah Parker, Michelle Bourne, Jay Suntharalingam, Tom Hartley, Vidan Masan, Sharon Sturney, Rob MacKenzie, Clare Marchand, Rebecca Mason, Katie White, Alison Kirby, Manjula Meda, Lavanya Diwakar, Peter Russell, Joanne Finn, Sophie Harris, Carol Muir, Gemma Cook, Nikki Staines, Chris Cook, AA Roger Thompson, Alison Condliffe, Rebecca Hull, Rebecca Dowey, Helena Turton, Paul Collini, Zoé Gabriel, Simon Hardman, Helen Newell, Janet Middle, Phillip Simpson, Hayley Colton, Joann Barker, Katie Birchall, Kate Harrington, Kay Housley, Rebecca Lenagh, Jayne Wilson, Joan Wesonga, Rachel Whitham, Sarah Bird, Yvonne Jackson, Angeline Mbuyisa, Samantha Anderson, Anna Wilson, Faith Kibutu, Sara Walker, Kay Cawthron, Irene Macharia, Lynne Smart, Anna Emery, Alice Howell, Elizabeth Hurditch, Amber Ford, Kim Turner, Lisa Watson, Helen Bowler, Tracy Jackson, Carol Jaques, Nichole Dyer, Shelley Ducker, Vicky Goodall, and Emily Udale
Supplementary Material
References
- 1.Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Torres Acosta MA, Singer BD. Pathogenesis of COVID-19-induced ARDS: implications for an ageing population. Eur Respir J. 2020;56 doi: 10.1183/13993003.02049-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Leisman DE, Ronner L, Pinotti R, et al. Cytokine elevation in severe and critical COVID-19: a rapid systematic review, meta-analysis, and comparison with other inflammatory syndromes. Lancet Respir Med. 2020;8:1233–1244. doi: 10.1016/S2213-2600(20)30404-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sinha S, Rosin NL, Arora R, et al. Dexamethasone modulates immature neutrophils and interferon programming in severe COVID-19. Nat Med. 2022;28:201–211. doi: 10.1038/s41591-021-01576-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ackermann M, Anders HJ, Bilyy R, et al. Patients with COVID-19: in the dark-NETs of neutrophils. Cell Death Differ. 2021;28:3125–3139. doi: 10.1038/s41418-021-00805-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schurink B, Roos E, Radonic T, et al. Viral presence and immunopathology in patients with lethal COVID-19: a prospective autopsy cohort study. Lancet Microbe. 2020;1:e290–e299. doi: 10.1016/S2666-5247(20)30144-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Seren S, Derian L, Keleş I, et al. Proteinase release from activated neutrophils in mechanically ventilated patients with non-COVID-19 and COVID-19 pneumonia. Eur Respir J. 2021;57 doi: 10.1183/13993003.03755-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Horby P, Lim WS, Emberson JR, et al. Dexamethasone in hospitalized patients with COVID-19. N Engl J Med. 2021;384:693–704. doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brown MJ, Alazawi W, Kanoni S. Interleukin-6 receptor antagonists in critically ill patients with COVID-19. N Engl J Med. 2021;385:1147–1149. doi: 10.1056/NEJMc2108482. [DOI] [PubMed] [Google Scholar]
- 10.Chalmers JD, Crichton ML, Goeminne PC, et al. Management of hospitalised adults with coronavirus disease 2019 (COVID-19): a European Respiratory Society living guideline. Eur Respir J. 2021;57 doi: 10.1183/13993003.00048-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Palmér R, Mäenpää J, Jauhiainen A, et al. Dipeptidyl peptidase 1 inhibitor AZD7986 induces a sustained, exposure-dependent reduction in neutrophil elastase activity in healthy subjects. Clin Pharmacol Ther. 2018;104:1155–1164. doi: 10.1002/cpt.1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chalmers JD, Haworth CS, Metersky ML, et al. Phase 2 trial of the DPP-1 inhibitor brensocatib in bronchiectasis. N Engl J Med. 2020;383:2127–2137. doi: 10.1056/NEJMoa2021713. [DOI] [PubMed] [Google Scholar]
- 13.Veras FP, Pontelli MC, Silva CM, et al. SARS-CoV-2-triggered neutrophil extracellular traps mediate COVID-19 pathology. J Exp Med. 2020;217 doi: 10.1084/jem.20201129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zuo Y, Yalavarthi S, Shi H, et al. Neutrophil extracellular traps in COVID-19. JCI Insight. 2020;5 doi: 10.1172/jci.insight.138999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McElvaney OJ, McEvoy NL, McElvaney OF, et al. Characterization of the inflammatory response to severe COVID-19 illness. Am J Respir Crit Care Med. 2020;202:812–821. doi: 10.1164/rccm.202005-1583OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Horby P, Mafham M, Linsell L, et al. Effect of hydroxychloroquine in hospitalized patients with COVID-19. N Engl J Med. 2020;383:2030–2040. doi: 10.1056/NEJMoa2022926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Veiga VC, Prats JAGG, Farias DLC, et al. Effect of tocilizumab on clinical outcomes at 15 days in patients with severe or critical coronavirus disease 2019: randomised controlled trial. BMJ. 2021;372:n84. doi: 10.1136/bmj.n84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.RECOVERY Collaborative Group Tocilizumab in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. Lancet. 2021;397:1637–1645. doi: 10.1016/S0140-6736(21)00676-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.REMAP-CAP Investigators. Gordon AC, Mouncey PR, et al. Interleukin-6 receptor antagonists in critically ill patients with COVID-19—preliminary report. medRxiv. 2021 doi: 10.1101/2021.01.07.21249390. published online Jan 7. (preprint). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Geerdink RJ, Pillay J, Meyaard L, Bont L. Neutrophils in respiratory syncytial virus infection: a target for asthma prevention. J Allergy Clin Immunol. 2015;136:838–847. doi: 10.1016/j.jaci.2015.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Akk A, Springer LE, Pham CT. Neutrophil extracellular traps enhance early inflammatory response in sendai virus-induced asthma phenotype. Front Immunol. 2016;7:325. doi: 10.3389/fimmu.2016.00325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Porto BN, Stein RT. Neutrophil extracellular traps in pulmonary diseases: too much of a good thing? Front Immunol. 2016;7:311. doi: 10.3389/fimmu.2016.00311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tak T, Rygiel TP, Karnam G, et al. Neutrophil-mediated suppression of influenza-induced pathology requires CD11b/CD18 (MAC-1) Am J Respir Cell Mol Biol. 2018;58:492–499. doi: 10.1165/rcmb.2017-0021OC. [DOI] [PubMed] [Google Scholar]
- 24.Domon H, Maekawa T, Isono T, Furuta K, Kaito C, Terao Y. Proteolytic cleavage of HLA class II by human neutrophil elastase in pneumococcal pneumonia. Sci Rep. 2021;11 doi: 10.1038/s41598-021-82212-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van den Berg CW, Tambourgi DV, Clark HW, Hoong SJ, Spiller OB, McGreal EP. Mechanism of neutrophil dysfunction: neutrophil serine proteases cleave and inactivate the C5a receptor. J Immunol. 2014;192:1787–1795. doi: 10.4049/jimmunol.1301920. [DOI] [PubMed] [Google Scholar]
- 26.Perng DW, Wu YC, Tsai MC, et al. Neutrophil elastase stimulates human airway epithelial cells to produce PGE2 through activation of p44/42 MAPK and upregulation of cyclooxygenase-2. Am J Physiol Lung Cell Mol Physiol. 2003;285:L925–L930. doi: 10.1152/ajplung.00182.2002. [DOI] [PubMed] [Google Scholar]
- 27.Apel F, Andreeva L, Knackstedt LS, et al. The cytosolic DNA sensor cGAS recognizes neutrophil extracellular traps. Sci Signal. 2021;14 doi: 10.1126/scisignal.aax7942. [DOI] [PubMed] [Google Scholar]
- 28.Stephenson E, Reynolds G, Botting RA, et al. Single-cell multi-omics analysis of the immune response in COVID-19. Nat Med. 2021;27:904–916. doi: 10.1038/s41591-021-01329-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tate MD, Deng YM, Jones JE, Anderson GP, Brooks AG, Reading PC. Neutrophils ameliorate lung injury and the development of severe disease during influenza infection. J Immunol. 2009;183:7441–7450. doi: 10.4049/jimmunol.0902497. [DOI] [PubMed] [Google Scholar]
- 30.Turk D, Janjić V, Stern I, et al. Structure of human dipeptidyl peptidase I (cathepsin C): exclusion domain added to an endopeptidase framework creates the machine for activation of granular serine proteases. EMBO J. 2001;20:6570–6582. doi: 10.1093/emboj/20.23.6570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pham CT, Ley TJ. Dipeptidyl peptidase I is required for the processing and activation of granzymes A and B in vivo. Proc Natl Acad Sci USA. 1999;96:8627–8632. doi: 10.1073/pnas.96.15.8627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wolters PJ, Pham CT, Muilenburg DJ, Ley TJ, Caughey GH. Dipeptidyl peptidase I is essential for activation of mast cell chymases, but not tryptases, in mice. J Biol Chem. 2001;276:18551–18556. doi: 10.1074/jbc.M100223200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No data are available for sharing.