Abstract
Background:
Postnatal exposures, including breastfeeding, may influence asthma development.
Objective:
To investigate the association between breastfeeding duration and child asthma. Methods: We studied 2021 mother-child dyads in the ECHO PATHWAYS consortium of prospective pregnancy cohorts (GAPPS, CANDLE, TIDES). Women reported the duration of any and exclusive breastfeeding and child asthma outcomes during follow-up at child age 4 to 6 years. Outcomes included current wheeze (previous 12 months), ever asthma, current asthma (having ≥2 of current wheeze, ever asthma, medication use in past 12–24 months), and strict current asthma (ever asthma with either or both current wheeze and medication use in past 12–24 months). We used multivariable logistic regression to assess associations (odds ratios and 95% confidence intervals) between breastfeeding and asthma outcomes adjusting for potential confounders. We assessed effect modification by mode of delivery, infant sex, and maternal asthma.
Results:
Among women, 33%, 13%, 9% and 45% reported 0<2, 2–4, 5–6, and >6 months of any breastfeeding, respectively. The duration of any breastfeeding had a protective linear trend with ever asthma but no other outcomes. There was a duration-dependent protective association of exclusive breastfeeding and child asthma outcomes (e.g., current asthma adjusted OR [95% CI]: 0.64 [0.41, 1.02], 0.61 [0.38, 0.98], and 0.52 [0.31, 0.87] for durations of 2–4, 5–6, and >6-months respectively, compared to <2 months). For exclusive breastfeeding, protective associations were stronger in dyads with children born by vaginal versus cesarean delivery although interactions were not significant.
Conclusion:
Longer duration of exclusive breastfeeding had a protective association with child asthma.
Keywords: child, asthma, breastfeeding, wheeze
Introduction
Asthma is a chronic disease of the airways, characterized by episodes of bronchial hyperresponsiveness, airway obstruction, and inflammation.1, 2 Because most children develop symptoms before age six, examining early life exposures may inform investigations aimed at elucidating underlying pathophysiology and efforts to prevent or modify disease severity.3 Prenatal and early life exposures, such as infant diet, may influence the subsequent development of asthma through effects on the developing immune and pulmonary systems.4, 5 For example, human milk contains maternal microbiota, oligosaccharides, immune factors, nutrients, hormones, and growth factors that together influence lung and immune system development including shaping of the infant microbiome which includes the signaling of multiple molecular and epigenetic pathways.6–9 Thus, breastfeeding, including duration and dose, is an important potentially modifiable factor in asthma development.
Although the association between breastfeeding and asthma is an active area of research, there have been some inconsistencies in findings and gaps where further investigation is warranted. For example, some studies have found that breastfeeding is protective against asthma in children,10–13 while others have found increased14 or no association with asthma.15 These studies varied in study population, exposure assessment, asthma characterization and age at assessment. Moreover, given that associations may be modified by biological variables (e.g., maternal asthma status and child sex) or environmental factors (e.g., mode of delivery), it is important to systematically assess these associations for effect modification to delineate potential underlying mechanisms and guide practice recommendations.16–19 Additionally, examining both breastfeeding duration and exclusiveness can provide insight into potential duration-response relationships.11 Lastly, broader inclusion of diverse populations in studies of breastfeeding and asthma is important as racial/ethnic disparities in both breastfeeding rates and early childhood asthma have been reported.1, 6, 20, 21 Using harmonized data from three, racially and geographically diverse US pregnancy cohorts, we examined whether both breastfeeding duration and exclusiveness is inversely associated with child asthma. We further assessed for effect modification by maternal asthma, child sex, and mode of delivery.
Methods
Study Population
This investigation included maternal-child dyads enrolled in the NIH ECHO-PATHWAYS consortium which includes three prospective pregnancy cohorts: the Conditions Affecting Neurocognitive Development and Learning in Early Childhood (CANDLE) study, The Infant Development and Environment Study (TIDES), and the Global Alliance to Prevent Prematurity and Stillbirth (GAPPS). CANDLE and TIDES cohorts have been described previously.22, 23 In brief, CANDLE enrolled women in Shelby County (Memphis), TN who were between 16 and 40 years, able to speak and understand English, and had a low medical risk, singleton pregnancy that were between 16- and 28-weeks gestation.22 Between 2006 and 2011, of 1503 women enrolled in CANDLE, 1455 had live births and were active in the study at delivery, and 1157 dyads participated in the study visit at about child age 4–6 years.22 The TIDES study recruited women ≥18 years who were in their first trimester (<13 weeks), able to read and write English and whose pregnancy was not medically threatened.23, 24 Women were recruited across four sites: University of Minnesota Medical Center (Minneapolis, MN); University of California-San Francisco Medical Center (San Francisco, CA); University of Rochester Medical Center (Rochester, NY); and University of Washington (Seattle, WA).23, 24 Overall, 969 women enrolled in TIDES between 2011 and 2013. Of the women active in TIDES after enrollment, 749 had live births and 544 dyads participated in follow-up at child age 4–6 years.23, 24 Lastly, the original GAPPS study enrolled healthy pregnant women. It conducted visits throughout pregnancy and, in a subset, visits in the immediate postpartum period, after which the participants were not engaged in further research activities and infants were not recruited into the GAPPS study. The ECHO PATHWAYS study recontacted women who previously participated in GAPPS based on eligibility criteria (child age, recruitment site, consent to future contact, and biospecimen availability), and enrolled children into the age 4–6 year visit which was the first PATHWAYS study visit for GAPPS25 and included 423 dyads. In total, 2124 dyads from the three cohort studies participated in follow-up. Children older than 7.5 years at follow-up were excluded from the current analysis (N=19). Of the 2,105 dyads that completed study follow-up ≤ 7.5 years, 2,021 had breastfeeding data (4% missing, n=84) and were included in this study.
All cohorts collected psychosocial and demographic characteristics, medical history, and biospecimens prenatally. Postnatally each cohort conducted in-person clinic visits and collected demographic, psychosocial, and medical history data that included assessment of child wheeze and asthma outcomes. The women provided informed consent for themselves and their children. This current study was approved by the University of Washington (UW) IRB.
Breastfeeding Exposures
The duration of any and exclusive breastfeeding were assessed via questionnaire at approximately 4–6 years (mean age 4.5 years, interquartile range 4.1–5 years). The first exposure was duration of any breastfeeding. We captured the duration of any breastfeeding as none to <2 months, 2–4 months, 5–6 months and >6 months based on responses to the questions “Was your child ever breast fed?” and “How long was your child breastfed?” For women who reported breastfeeding, we defined duration of exclusive breastfeeding, as <2 months, 2–4 months, 5–6 months and >6 months based on responses to the categorical question “How long was your child breastfed without adding formula, juices or other foods?” Exclusive breastfeeding for about 6 months is recommended by the American Academy of Pediatrics and the World Health Organization and data was captured in these categories based off these recommendations.26, 27
Child respiratory outcomes
Child wheeze and asthma outcomes were ascertained using the International Study of Asthma and Allergies in Childhood (ISAAC) questionnaire and additional questionnaires that assessed asthma-specific medication use and diagnosis during the 4 to 6-year visit.28–30 Four wheeze/asthma outcomes were considered in this analysis.28 Current wheeze was defined as affirmative responses to the questions: (1)“Has your child ever had wheezing or whistling in the chest at any time in the past” and (2) “Has your child ever had wheezing or whistling in the chest in the last 12 months?” Ever asthma was defined as an affirmative response to the question: “Has your child ever had asthma?” Current asthma was defined as reporting two out of the following three: 1) current wheeze, 2) ever asthma, or 3) asthma-specific medication use (defined as asthma-specific medication use in the previous 12 months for CANDLE and GAPPS [yes/no] and free-text reporting of asthma or asthma symptoms and medication use for this condition in the previous 24 months for TIDES).31
We defined strict current asthma to identify children who were diagnosed with asthma and had current symptoms as measured by reported prevalent symptoms or medication use. The definition included children who responded affirmatively to the question, “Has your child ever had asthma?” (i.e., ever asthma) and those who had current wheeze and/or asthma-specific medication use.
Covariates
Information obtained at enrollment included maternal age (years), race (White, Black and Other), education level (<high school, high school or General Educational Development (GED), college or technical school and some graduate work or graduate/professional degree), smoking during pregnancy (yes, no), and parity (0, ≥1). Data collected at birth included delivery type (vaginal, cesarean), sex (male, female) and estimated gestational age (EGA) (days). Maternal history of asthma (yes, no), as one measure of a familial predisposition to develop asthma, was assessed at the GAPPS 4–6-year visit, the CANDLE 4-year visit and the TIDES 6-year visit.
Statistical Analysis
Cohort characteristics were summarized by descriptive statistics including frequencies and proportions for categorical data and median and interquartile ranges [IQR:25th, 75th] for continuous variables. Child and maternal characteristics were compared by breastfeeding category using chi-square and Kruskal-Wallis rank sum test, as appropriate. We used multivariable logistic regression to calculate adjusted odds ratios (aOR) and 95% confidence intervals (95% CI) for child wheeze and asthma outcomes at age 4–6 years using separate regressions for duration of any and exclusive breastfeeding.32, 33 The covariates in the main model were selected a priori and included site, maternal age, race, education, prenatal smoking, asthma history, parity, and mode of delivery as well as child EGA and sex. We also performed a mixed effects logistic regression with random effects for site.
A priori selected effect modifiers were maternal asthma, mode of delivery and child sex. We performed interaction analyses with cross-product terms to examine if associations between breastfeeding exposures and childhood wheeze/asthma outcomes were modified by these factors. The p interaction reflects the test of the cross-product term, formally testing the interaction between breast feeding and factor (e.g.: maternal asthma). Its value was considered significant at the level of 0.05. To assess the stability of the pooled analysis to the influence of any one cohort, we completed a series of sensitivity analyses in which, in turn, each of the three cohorts was excluded. Additionally, we performed a linear test for trend across exposure groups using the estimated midpoint (months) of each breastfeeding category (any: 1 month, 3 months, 5.5 months, and 9 months; exclusive: 1 month, 3 months, 5.5 months, and 6.5 months) as linear predictors in logistic regression models. R version 3.6.3 was used for all analysis and statistical tests were considered significant at the level of 0.05.
Results
Maternal and Child Characteristics
Overall, 2,021 dyads with breastfeeding exposure and child follow-up at about 4–6 years were included (Table 1). For the pooled cohort, 38% of women identified as Black, 52% as White, and 6% as Hispanic/Latina. Forty-four percent of women reported a high school education or less. The CANDLE cohort included 63% Black women as compared to GAPPS with 2% and TIDES with 11%. Additionally, in the CANDLE cohort 58% of women had high school as the highest level of educational attainment, while that proportion was 31% in GAPPS and 32% in TIDES. Overall, few women reported smoking during pregnancy (CANDLE 9%, GAPPS 3% and TIDES 5%).
Table 1.
Characteristics of dyads (births, 2006–2015)
| CANDLE N=1149 |
GAPPS N=418 |
TIDES N=538 |
Total N=2105 |
|
|---|---|---|---|---|
| Maternal | ||||
| Race/ethnicity, n (%) | ||||
| White | 360 (31) | 331 (83) | 384 (72) | 1075 (52) |
| African-American | 720 (63) | 8 (2) | 57 (11) | 785 (38) |
| Other | 69 (6) | 62 (15) | 90 (16) | 221 (10) |
| Hispanic or Latina | 21 (2) | 59 (14) | 46 (9) | 126 (6) |
| Age (years)* | 26 (22–30) | 31 (27–34) | 31 (28–35) | 28 (24–33) |
| Education, n (%) | ||||
| Less than high school | 130 (11) | 16 (4) | 33 (6) | 179 (9) |
| GED or high school diploma | 536 (47) | 110 (27) | 85 (16) | 731 (35) |
| Technical school/college | 346 (30) | 180 (43) | 165 (31) | 691 (33) |
| ≥Some graduate work | 136 (12) | 109 (26) | 252 (47) | 496 (24) |
| Maternal asthma, yes n (%) | 199 (18) | 75 (18) | 82 (17) | 356 (17) |
| Prenatal smoking, yes n (%) | 104 (9) | 12 (3) | 24 (5) | 140 (7) |
| Child | ||||
| Gestational Age (days)* | 274 (269–279) | 274 (264–281) | 277 (270–284) | 275 (269–281) |
| Age at follow-up (years)* | 4.6 (4.1–4.6) | 5.5 (5.1–6) | 4.5 (4.3–4.8) | 4.5 (4.1–5) |
| Sex, female n (%) | 577 (50) | 204 (49) | 282 (52) | 1063 (50) |
| Firstborn, yes n (%) | 456 (40) | 137 (33) | 286 (54) | 879 (42) |
| Delivery Route, Vaginal n (%) | 718 (63) | 265 (63) | 395 (73) | 1378 (66) |
| Current wheeze†, yes n (%) | 218 (19) | 53 (13) | 52 (10) | 323 (16) |
| Ever asthma, yes n (%) | 168 (15) | 33 (8) | 40 (7) | 241 (12) |
| Current asthma‡, yes n (%) | 182 (16) | 39 (10) | 29 (5) | 250 (12) |
| Strict current asthma§, yes n (%) | 136 (12) | 24 (6) | 29 (6) | 189 (9) |
Median (interquartile range),
Current wheeze: wheezing/whistling in the chest in the past 12 months,
Current asthma: (2 of 3) ever asthma, current wheeze and/or medication use in past 12–24 months,
Strict current asthma: ever asthma and current wheeze, and/or medication use in the past 12–24 months
Among children, 50% were female, 42% were first born and 34% were born by c-section. The median EGA at birth was 39.29 weeks (IQR: 38.43, 40.14). The median age of children at the 4–6-year visit was 4.5 years (IQR: 4.1, 5). Overall, the prevalence of child wheeze/asthma outcomes were 16%, 12%, 12% and 9% for current wheeze, ever asthma, current asthma, and strict current asthma, respectively. By cohort, children in CANDLE had higher prevalence of all outcomes relative to TIDES and GAPPS (Table 1).
Overall, the percentage of women reporting breastfeeding durations of 0<2, 2–4, 5–6, and > 6 months was 33%, 13%, 9%, and 45%, respectively (Figure 1). Most women who breastfed their child >6 months were White (68%) and had more than a high school education (78%). Across cohorts, TIDES had the largest percentage of women who breastfed >6 months (71%), followed by GAPPS (62%) and CANDLE (27%). A larger proportion of women in CANDLE reported never breastfeeding their index child (32%) compared to TIDES (8%) and GAPPS (6%). Among children ever breastfed (N=1553), the percentage of mothers reporting breastfeeding durations of <2, 2–4, 5–6, and > 6 months was 24%, 24%, 28% and 24%, respectively (Figure 1). TIDES had the highest percentage of children (31%) exclusively breastfed for >6 months.
Figure 1.
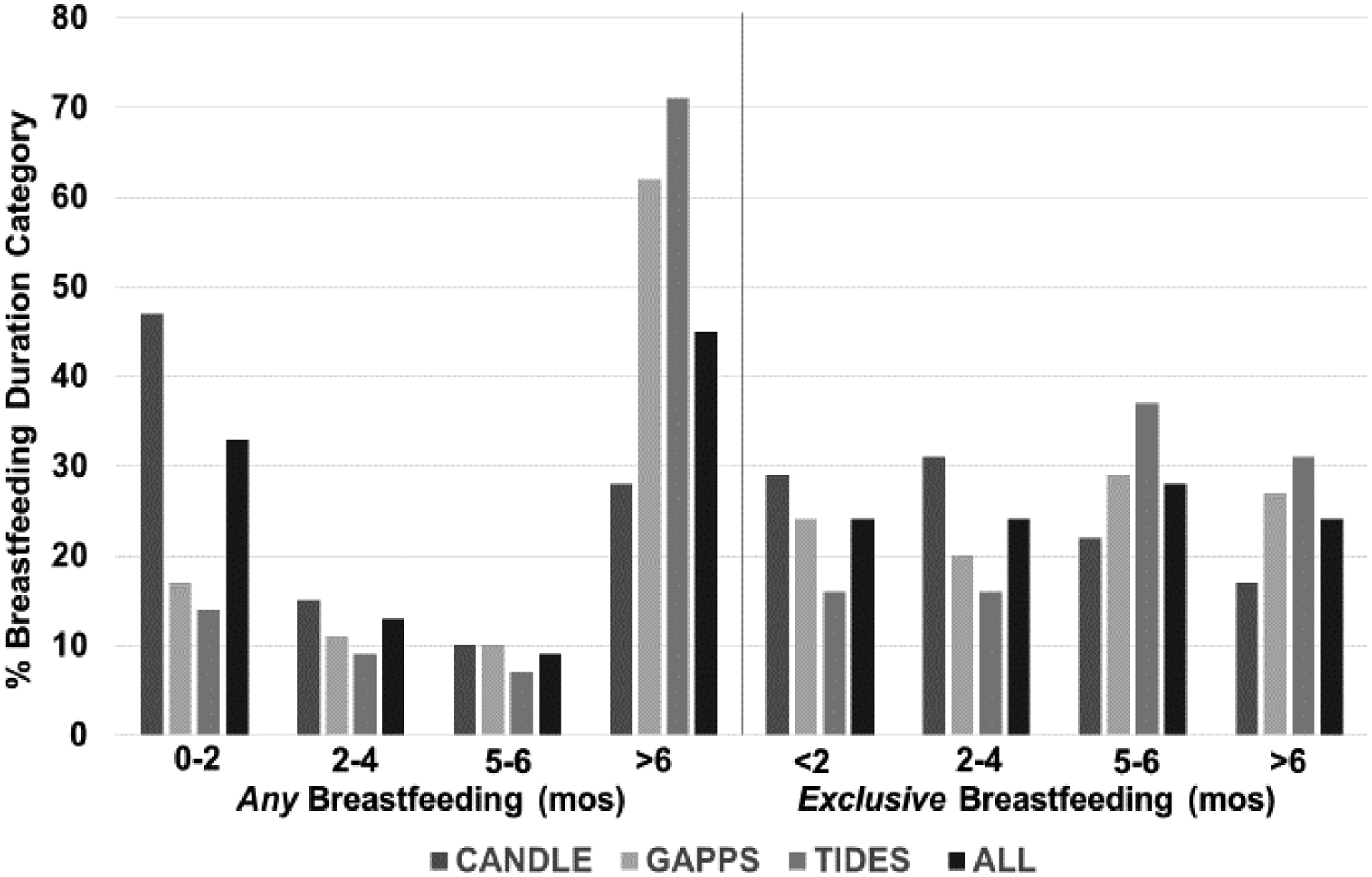
Percentage of any and exclusive breastfeeding in months (mos) by cohort and for total cohort.
The percentage of children enrolled in PATHWAYS in each breastfeeding duration category for the any and exclusive breastfeeding exposures.
Breastfeeding duration and child wheeze/asthma outcomes
We first assessed the association between duration of any breastfeeding and child wheeze/asthma outcomes in unadjusted and multivariable analyses (eTable 1). In multivariable analyses, breastfeeding duration was not associated with child wheeze/asthma outcomes (Figure 2). Although the overall adjusted association was not statistically significant for the outcome ever asthma, there was a significant linear trend towards protection (plinear=0.034). In models with ever asthma as the outcome, longer duration of any breastfeeding trended with decreased odds (aOR [95%CI], including 0.79 [0.49, 1.24]; 0.72 [0.42,1.25]; and 0.65 [0.43,0.97] for 2–4 months, 5–6 months, and >6 months, respectively, when compared to children in the none to <2 months category (Figure 2).
Figure 2.
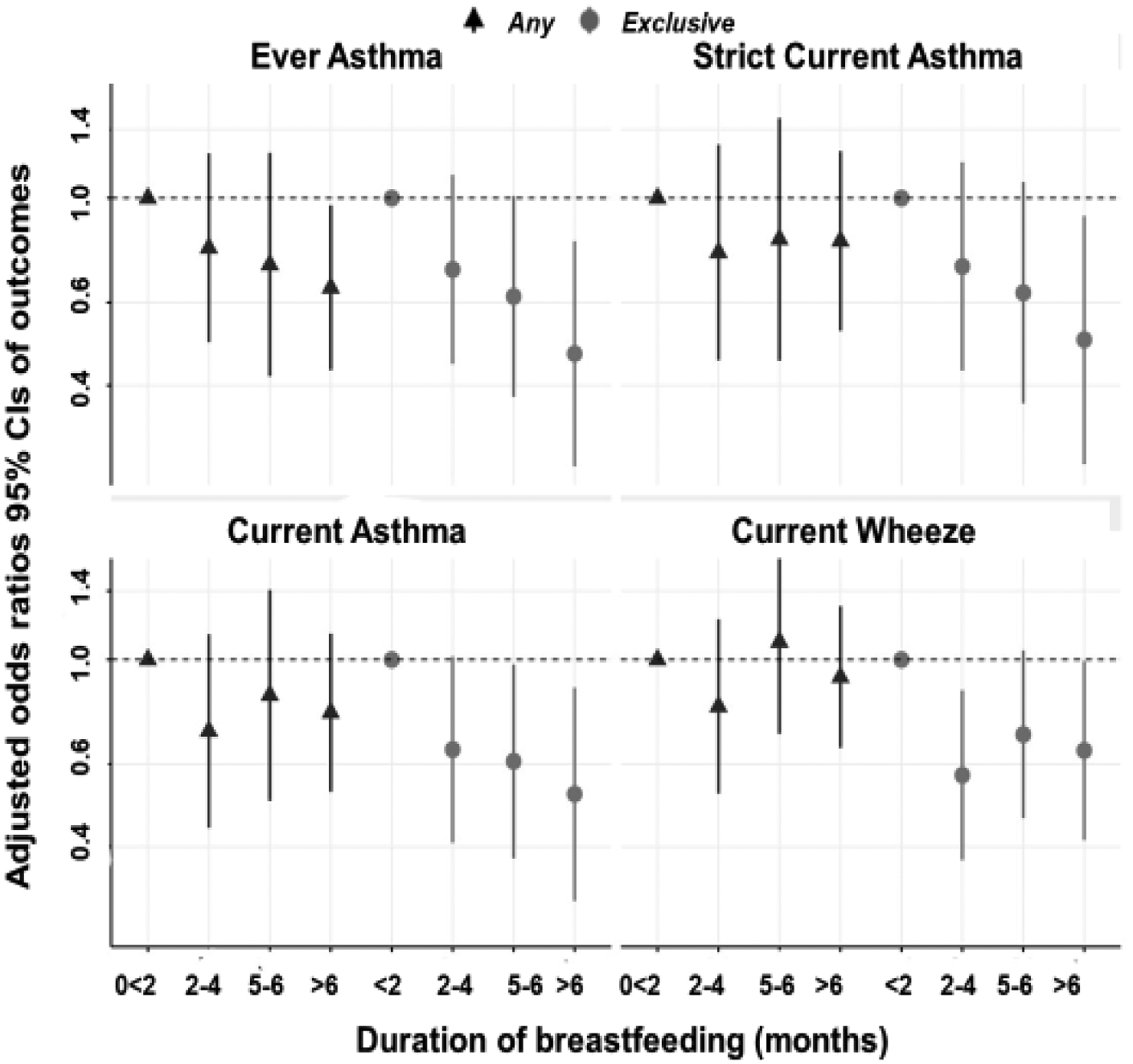
Associations of any and exclusive breastfeeding with asthma outcomes. Adjusted odds ratios and 95% CIs are graphed in separate models (referent group: none to <2 months [any] or <2months [exclusive]). Covariates: site, maternal age, race, education, asthma, parity, smoking during pregnancy, delivery mode, gestational age, and child sex.
Next, we investigated unadjusted and multivariable associations between duration of exclusive breastfeeding and child wheeze/asthma outcomes (eTable 2) and several of the associations were statistically significant after confounder adjustment (Figure 2). For example, longer duration of exclusive breastfeeding was associated with decreased odds of current asthma (aOR [95%CI]) of 0.64 [0.41, 1.02], 0.61 [0.38, 0.98], and 0.52 [0.31, 0.87] for 2–4 months, 5–6 months, and >6 months, respectively, when compared to children in the exclusively breastfed <2 months (Figure 2). Additionally, protective associations for current wheeze, ever asthma and strict current asthma were found for comparisons between the referent and >6 months of exclusive breastfeeding (Figure 2). Linear trends also were observed with longer duration of exclusive breastfeeding (current wheeze plinear=0.061, ever asthma plinear=0.005, current asthma plinear= 0.009, strict current asthma plinear=0.018).
We next examined effect modification of the association of breastfeeding exposures and child respiratory outcomes. For mode of delivery, there were no statistically significant interactions between the association of duration of any breastfeeding and child wheeze/asthma outcomes (eTable 3). When assessing for effect modification between exclusive breastfeeding duration and mode of delivery on child wheeze/asthma outcomes, protective associations were consistently stronger in dyads with vaginal deliveries compared to cesareans, although interactions were not statistically significant. For example, in the subset of children who were born vaginally, those who were exclusively breastfed either 2–4 months, 5–6 months or >6 months had aORs [95% CI] for current asthma of 0.48 [0.26, 0.86]; 0.46 [0.25, 0.86] and 0.29 [0.14, 0.61], respectively, compared to children exclusively breastfed <2 months. In the subset of children who were born by cesarean, those who were exclusively breasted for either 2–4 months, 5–6 months or >6 months had aORs [95%CI] for current asthma of 0.95 [0.47, 1.92]; 0.84 [0.41, 1.72] and 0.98 [0.47, 2.06], compared to children exclusively breastfed <2 months (pinteraction =0.12).
When assessing for effect modification by history of maternal asthma, any breastfeeding duration was associated with protective associations for current asthma in children whose mothers did not have asthma (e.g., aOR: 0.58 (95% CI: 0.37, 0.92) for >6 months vs. 0–2 months), but not among children whose mothers had asthma (e.g., aOR: 1.49 (95% CI: 0.79, 2.80) for >6 months vs. 0–2 months) (pinteraction =0.013). Odds ratios followed a similar direction for other outcomes; however interactions were not significant (pinteraction = 0.12 – 0.18). There was no evidence of effect modification by maternal asthma history for exclusive breastfeeding duration (all pinteraction > 0.67), wherein we observed inverse (protective) relationships regardless of maternal asthma history. However, estimates for those with maternal asthma (n = 279) lacked precision (e.g., aOR current asthma = 0.76 [95% CI: 0.32, 1.81]). There was no indication of effect modification by infant sex, in the association of any (all pinteraction >0.05) or exclusive (all pinteraction>0.20) breastfeeding duration and child asthma outcomes
Sensitivity analyses
Lastly, we completed sensitivity analyses, leaving one cohort out of the pooled analysis at a time (eFigure 1 and eFigure 2). Relative to the original pooled analysis, results did not appreciably change in models that excluded TIDES or GAPPS. When excluding our largest cohort, CANDLE, associations were generally consistent with the pooled analysis but were notably less precise due to decreased sample size. The largest differences due to CANDLE exclusion were seen for associations between any breastfeeding duration and strict current asthma and current wheeze. Although the exclusive breastfeeding point estimates had wide confidence intervals and included the null, estimates were consistent with the pooled analysis allowing us to assert that results were robust to site exclusion. Additionally, as a sensitivity analysis, we performed a mixed effects logistic regression model with site as a random effect and results were consistent.
Discussion
Prenatal and early life dietary exposures are potential modifiable factors that may influence asthma development in children.7, 8 In this large, socioeconomically, and racial/ethnically diverse cohort of over 2,000 mother-child dyads, we found evidence of protective associations between longer duration of exclusive breastfeeding and child asthma outcomes. These associations were robust even after adjustment for many established confounders. In contrast, duration of any breastfeeding, which included dyads with either partial or exclusive breastfeeding, was not strongly associated with subsequent development of wheeze or asthma, apart from ever asthma.
This work builds upon previous investigations of infant diet and asthma. Studies that examined duration of breastfeeding have reported protective associations. Specifically, a cohort study of ~880 Australian and Swedish children with a family history of asthma found that infants breastfed ≥ 3 months had lower relative risk of asthma at 5 years and 8 years.34 In addition, a Dutch study of over 5,000 dyads, found that shorter duration of breastfeeding (<2 months) was associated with current asthma and early wheezing (≤3 years old) and less exclusive breastfeeding (≤4 months) was associated with early wheezing; however this study did not evaluate for potential modification by factors such as maternal asthma.11 In contrast, in a Belarus follow-up study of a cluster-randomized controlled trial focused on breastfeeding promotion where the intervention was associated with longer duration of breastfeeding at three months, there was no associated decrease in asthma or wheeze14. Of note, the prevalence of atopic outcomes was several-fold lower than those seen in most U.S. populations, so it is unclear how generalizable these results are to our study population.14 Despite these divergent findings, recent meta-analyses support the protective effect of more versus less breastfeeding on the development of asthma.35, 36 Specifically, the meta-analyses showed that the protective association is strongest in children 0–2 years old and only modest after age 7.35
In addition to the primary analyses, we assessed for effect modification in the associations between duration of any and exclusive breastfeeding and childhood asthma/wheeze outcomes by maternal asthma history, infant sex or delivery type. For maternal asthma history as a potential effect modifier, we observed protective associations for any breastfeeding duration among dyads without maternal asthma but not among dyads with maternal asthma, although this interaction reached statistical significance only for the current asthma outcome. We did not observe evidence of effect modification by maternal asthma history for exclusive breastfeeding. Taken together, future studies should consider how the dose of breastfeeding, presumably higher in exclusive breastfeeding, influences asthma risk in children with a familial predisposition to develop asthma. When considering effect modification by child sex, although there was not statistically significant interaction, prospective studies with larger samples across the different breastfeeding duration strata may provide insights as studies have had mixed results16, 17, 37
We also investigated the interaction of mode of delivery which has a known influence on establishing the gut microbiome postnatally5 and growing evidence suggests that the microbiome perturbation may be associated with asthma risk.38–40 Although the p-values for interaction did not reach statistical significance, there is the suggestion of a stronger protective association between duration of exclusive breastfeeding and asthma in children born vaginally compared to those born by c-section. While exclusive breastfeeding for approximately the first 6 months is recommended for infants except in rare cases, these novel findings highlight the potential additional benefit of vaginal delivery and exclusive breastfeeding in child respiratory health. Interestingly, an investigation of 673 dyads suggested that mode of delivery and exclusive breastfeeding modified the association between maternal atopy and allergic outcomes in young children.41 Thus, our investigation of exposures that modify the association of breastfeeding and child asthma suggests a potential mechanistic area of exploration as well as supports future investigations with larger sample sizes and that assess the role of antibiotic prophylaxis during caesareans.42
Literature suggests that human breast milk plays a key role in disease prevention and the subsequent development of chronic conditions like necrotizing enterocolitis, diabetes and asthma5. Previous studies have suggested that breastmilk components such as oligosaccharides influence the developing immune system by promoting a healthier gut microbiome composition and the immune system in turn modulates lung development through specific cytokines, immunoglobins and hormones5,8, 9 Consequently, the changing gut microbiome through multiple molecular pathways and epigenetic modifications affect the development of allergic disease.38 While the mechanisms are not fully delineated, previous work in the field and the trend toward a dose response in many of the analyses in this current study, suggest a prolonged and high dose of breast milk exposure is beneficial and pivotal for child asthma prevention.
There are strengths and limitations to consider in this study. Strengths include the well-characterized, diverse study population that resulted from pooling three prospective pregnancy cohorts. By including Black children who historically have experienced lower rates of breastfeeding, our work can potentially inform targeted interventions that address the breastfeeding disparity in the United States.6 Moreover, this study assessed duration of breastfeeding and potential duration-dependent dose response, controlled for key confounders, and tested for potential effect modification by maternal asthma, child sex and mode of delivery.
Limitations include ascertaining maternal report of breastfeeding at child age 4 to 6 which may result in misclassification. However, previous research studies have largely supported validity and reliability of maternal recall of infant feeding practices.43–45 For example, maternal recollection of breastfeeding was accurate in two studies that examined recall six years after delivery and in a Norwegian study that examined recall after 20 years.43 van Zykl and co-authors found accurate recall of exclusive breastfeeding duration after 10 years (r = 0.70, p<0.05).45 An additional limitation included the inability to differentiate between breastmilk from direct breastfeeding and breastmilk fed through a bottle.46, 47 Although the outcomes are based on parent-report, a validated questionnaire was used and is widely used to identify asthma and wheeze.30 In addition, there may be unmeasured confounding to consider for further cautious interpretation.
Lastly, this study strengthens current breastfeeding recommendations which reflect recent analysis that show lower risk of asthma with more versus less breastfeeding.35, 48 Although guidelines recommend not delaying the introduction of solid foods to prevent atopic disease, the strongest evidence applies to peanut allergy, where in a randomized trial (RCT) including high-risk infants between 4–11 months, infants randomized to consume peanuts in the first 60 months of life had reduced risk of peanut allergy.49 A follow-up study to the RCT did not find that breastfeeding duration was impacted by early introduction of peanut containing foods.50
In summary, in this racially diverse cohort study, we found that longer duration of exclusive breastfeeding was associated with decreased odds of child asthma. Our study extends the understanding of the association between breastfeeding duration and child respiratory health and informs future investigations of asthma and lung function in older children and how exposures may modify associations.
Supplementary Material
Acknowledgements
We are grateful for the participation of families enrolled in the CANDLE, GAPPS and TIDES cohorts, as well as the dedication of the respective research staff and investigators. The ECHO PATHWAYS Data Center staff harmonized data across cohorts and compiled the analytic dataset used in this study. This research was conducted using data collected on behalf of the GAPPS Repository.
Funding:
ECHO PATHWAYS is funded by NIH (1UG3OD023271,4UH3OD023271).
The Conditions Affecting Neurocognitive Development and Learning in Early Childhood (CANDLE) study was funded by the Urban Child Institute and NIH (R01 HL109977) and Dr. Carroll was also supported by K24 HL150312 and R01HL132338.
The Infant Development and the Environment Study (TIDES) was funded by NIH (R01ES016863,1R01ES25169, P30 ES005022 and UH3OD023305-05).
Select surveys were administered in REDCap, which was developed with NIH funds (UL1 TR002319, KL2 TR002317, and TL1 TR002318).
Abbreviations:
- CANDLE
Conditions Affecting Neurocognitive Development and Learning in Early Childhood
- TIDES
The Infant Development and Environment Study
- GAPPS
Global Alliance to Prevent Prematurity and Stillbirth
- ISAAC
International Study of Asthma and Allergies in Childhood
Footnotes
Conflict of Interest: The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References:
- 1.Keet CA, McCormack MC, Pollack CE, Peng RD, McGowan E, Matsui EC. Neighborhood poverty, urban residence, race/ethnicity, and asthma: Rethinking the inner-city asthma epidemic. J Allergy Clin Immunol. 2015;135(3):655–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Veeranki SP, Gebretsadik T, Mitchel EF, Tylavsky FA, Hartert TV, Cooper WO, et al. Maternal Folic Acid Supplementation During Pregnancy and Early Childhood Asthma. Epidemiology. 2015;26(6):934–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Just J, Bourgoin-Heck M, Amat F. Clinical phenotypes in asthma during childhood. Clin Exp Allergy. 2017;47(7):848–855. [DOI] [PubMed] [Google Scholar]
- 4.Kozyrskyj AL, Bahreinian S, Azad MB. Early life exposures: impact on asthma and allergic disease. Curr Opin Allergy Clin Immunol. 2011;11(5):400–406. [DOI] [PubMed] [Google Scholar]
- 5.Indrio F, Martini S, Francavilla R, Corvaglia L, Cristofori F, Mastrolia SA, et al. Epigenetic Matters: The Link between Early Nutrition, Microbiome, and Long-term Health Development. Front Pediatr. 2017;5:178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Anstey EH, Chen J, Elam-Evans LD, Perrine CG. Racial and Geographic Differences in Breastfeeding - United States, 2011–2015. MMWR Morb Mortal Wkly Rep. 2017;66(27):723–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beasley R, Semprini A, Mitchell EA. Risk factors for asthma: is prevention possible? Lancet. 2015;386(9998):1075–1085. [DOI] [PubMed] [Google Scholar]
- 8.Miliku K, Azad MB. Breastfeeding and the Developmental Origins of Asthma: Current Evidence, Possible Mechanisms, and Future Research Priorities. Nutrients. 2018;10(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Penders J, Stobberingh EE, van den Brandt PA, Thijs C. The role of the intestinal microbiota in the development of atopic disorders. Allergy. 2007;62(11):1223–1236. [DOI] [PubMed] [Google Scholar]
- 10.Ahmadizar F, Vijverberg SJH, Arets HGM, de Boer A, Garssen J, Kraneveld AD, et al. Breastfeeding is associated with a decreased risk of childhood asthma exacerbations later in life. Pediatr Allergy Immunol. 2017;28(7):649–654. [DOI] [PubMed] [Google Scholar]
- 11.den Dekker HT, Sonnenschein-van der Voort AM, Jaddoe VW, Reiss IK, de Jongste JC, Duijts L. Breastfeeding and asthma outcomes at the age of 6 years: The Generation R Study. Pediatr Allergy Immunol. 2016;27(5):486–492. [DOI] [PubMed] [Google Scholar]
- 12.Kim A, Lim G, Oh I, Kim Y, Lee T, Lee J. Perinatal factors and the development of childhood asthma. Ann Allergy Asthma Immunol. 2018;120(3):292–299. [DOI] [PubMed] [Google Scholar]
- 13.Klopp A, Vehling L, Becker AB, Subbarao P, Mandhane PJ, Turvey SE, et al. Modes of Infant Feeding and the Risk of Childhood Asthma: A Prospective Birth Cohort Study. J Pediatr. 2017;190:192–199.e192. [DOI] [PubMed] [Google Scholar]
- 14.Kramer MS, Matush L, Vanilovich I, Platt R, Bogdanovich N, Sevkovskaya Z, et al. Effect of prolonged and exclusive breast feeding on risk of allergy and asthma: cluster randomised trial. Bmj. 2007;335(7624):815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sears MR, Greene JM, Willan AR, Taylor DR, Flannery EM, Cowan JO, et al. Long-term relation between breastfeeding and development of atopy and asthma in children and young adults: a longitudinal study. Lancet. 2002;360(9337):901–907. [DOI] [PubMed] [Google Scholar]
- 16.Elliott L, Henderson J, Northstone K, Chiu GY, Dunson D, London SJ. Prospective study of breast-feeding in relation to wheeze, atopy, and bronchial hyperresponsiveness in the Avon Longitudinal Study of Parents and Children (ALSPAC). J Allergy Clin Immunol. 2008;122(1):49–54, 54 e41–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Scholtens S, Wijga AH, Brunekreef B, Kerkhof M, Hoekstra MO, Gerritsen J, et al. Breast feeding, parental allergy and asthma in children followed for 8 years. The PIAMA birth cohort study. Thorax. 2009;64(7):604–609. [DOI] [PubMed] [Google Scholar]
- 18.Lossius AK, Magnus MC, Lunde J, Stordal K. Prospective Cohort Study of Breastfeeding and the Risk of Childhood Asthma. J Pediatr. 2018;195:182–189.e182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Klein SL, Flanagan KL. Sex differences in immune responses. Nat Rev Immunol. 2016;16(10):626–638. [DOI] [PubMed] [Google Scholar]
- 20.Johnson CC, Havstad SL, Ownby DR, Joseph CLM, Sitarik AR, Biagini Myers J, et al. Pediatric asthma incidence rates in the United States from 1980 to 2017. J Allergy Clin Immunol. 2021;148(5):1270–1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kirksey K A social history of racial disparities in breastfeeding in the United States. Soc Sci Med. 2021;289:114365. [DOI] [PubMed] [Google Scholar]
- 22.Tylavsky FA, Kocak M, Murphy LE, Graff JC, Palmer FB, Volgyi E, et al. Gestational Vitamin 25(OH)D Status as a Risk Factor for Receptive Language Development: A 24-Month, Longitudinal, Observational Study. Nutrients. 2015;7(12):9918–9930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barrett ES, Sathyanarayana S, Janssen S, Redmon JB, Nguyen RH, Kobrosly R, et al. Environmental health attitudes and behaviors: findings from a large pregnancy cohort study. Eur J Obstet Gynecol Reprod Biol. 2014;176:119–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bornehag CG, Lindh C, Reichenberg A, Wikstrom S, Unenge Hallerback M, Evans SF, et al. Association of Prenatal Phthalate Exposure With Language Development in Early Childhood. JAMA Pediatr. 2018;172(12):1169–1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Global Alliance to Prevent Prematurity and Stillbirth. Bioservices Projects-ECHO 2020. [Available from: https://www.gapps.org/] [Google Scholar]
- 26.BREASTFEEDING SO, Eidelman AI, Schanler RJ, Johnston M, Landers S, Noble L, et al. Breastfeeding and the Use of Human Milk. Pediatrics. 2012;129(3):e827–e841. [DOI] [PubMed] [Google Scholar]
- 27.World Health Organization. Breastfeeding: World Health Organization; 2021. [Available from: https://www.who.int/health-topics/breastfeeding#tab=tab_2.] [Google Scholar]
- 28.Rosa MJ, Hartman TJ, Adgent M, Gardner K, Gebretsadik T, Moore PE, et al. Prenatal polyunsaturated fatty acids and child asthma: Effect modification by maternal asthma and child sex. J Allergy Clin Immunol. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Roy A, Kocak M, Hartman TJ, Vereen S, Adgent M, Piyathilake C, et al. Association of prenatal folate status with early childhood wheeze and atopic dermatitis. Pediatr Allergy Immunol. 2018;29(2):144–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Asher MI, Keil U, Anderson HR, Beasley R, Crane J, Martinez F, et al. International Study of Asthma and Allergies in Childhood (ISAAC): rationale and methods. Eur Respir J. 1995;8(3):483–491. [DOI] [PubMed] [Google Scholar]
- 31.Adgent MA, Carroll KN, Hazlehurst MF, Loftus CT, Szpiro AA, Karr CJ, et al. A combined cohort analysis of prenatal exposure to phthalate mixtures and childhood asthma. Environ Int. 2020;143:105970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Doi SA, Furuya-Kanamori L, Xu C, Lin L, Chivese T, Thalib L. Questionable utility of the relative risk in clinical research: a call for change to practice. J Clin Epidemiol. 2020. [DOI] [PubMed] [Google Scholar]
- 33.Cook TD. Advanced statistics: up with odds ratios! A case for odds ratios when outcomes are common. Acad Emerg Med. 2002;9(12):1430–1434. [DOI] [PubMed] [Google Scholar]
- 34.Brew BK, Kull I, Garden F, Almqvist C, Bergstrom A, Lind T, et al. Breastfeeding, asthma, and allergy: a tale of two cities. Pediatr Allergy Immunol. 2012;23(1):75–82. [DOI] [PubMed] [Google Scholar]
- 35.Lodge CJ, Tan DJ, Lau MX, Dai X, Tham R, Lowe AJ, et al. Breastfeeding and asthma and allergies: a systematic review and meta-analysis. Acta Paediatr. 2015;104(467):38–53. [DOI] [PubMed] [Google Scholar]
- 36.Dogaru CM, Nyffenegger D, Pescatore AM, Spycher BD, Kuehni CE. Breastfeeding and childhood asthma: systematic review and meta-analysis. Am J Epidemiol. 2014;179(10):1153–1167. [DOI] [PubMed] [Google Scholar]
- 37.Liptzin DR, Landau LI, Taussig LM. Sex and the lung: Observations, hypotheses, and future directions. Pediatr Pulmonol. 2015;50(12):1159–1169. [DOI] [PubMed] [Google Scholar]
- 38.McLoughlin RM, Mills KH. Influence of gastrointestinal commensal bacteria on the immune responses that mediate allergy and asthma. J Allergy Clin Immunol. 2011;127(5):1097–1107; quiz 1108–1099. [DOI] [PubMed] [Google Scholar]
- 39.Stokholm J, Blaser MJ, Thorsen J, Rasmussen MA, Waage J, Vinding RK, et al. Maturation of the gut microbiome and risk of asthma in childhood. Nat Commun. 2018;9(1):141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stokholm J, Thorsen J, Chawes BL, Schjorring S, Krogfelt KA, Bonnelykke K, et al. Cesarean section changes neonatal gut colonization. J Allergy Clin Immunol. 2016;138(3):881–889 e882. [DOI] [PubMed] [Google Scholar]
- 41.Sitarik AR, Kasmikha NS, Kim H, Wegienka G, Havstad S, Ownby D, et al. Breast-feeding and delivery mode modify the association between maternal atopy and childhood allergic outcomes. J Allergy Clin Immunol. 2018;142(6):2002–2004 e2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bollig C, Nothacker M, Lehane C, Motschall E, Lang B, Meerpohl JJ, et al. Prophylactic antibiotics before cord clamping in cesarean delivery: a systematic review. Acta Obstet Gynecol Scand. 2018;97(5):521–535. [DOI] [PubMed] [Google Scholar]
- 43.Li R, Ingol TT, Smith K, Oza-Frank R, Keim SA. Reliability of Maternal Recall of Feeding at the Breast and Breast Milk Expression 6 Years After Delivery. Breastfeed Med. 2020;15(4):224–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Amissah EA, Kancherla V, Ko YA, Li R. Validation Study of Maternal Recall on Breastfeeding Duration 6 Years After Childbirth. J Hum Lact. 2017;33(2):390–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.van Zyl Z, Maslin K, Dean T, Blaauw R, Venter C. The accuracy of dietary recall of infant feeding and food allergen data. J Hum Nutr Diet. 2016;29(6):777–785. [DOI] [PubMed] [Google Scholar]
- 46.Ogbuanu IU, Karmaus W, Arshad SH, Kurukulaaratchy RJ, Ewart S. Effect of breastfeeding duration on lung function at age 10 years: a prospective birth cohort study. Thorax. 2009;64(1):62–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dethlefsen L, McFall-Ngai M, Relman DA. An ecological and evolutionary perspective on human-microbe mutualism and disease. Nature. 2007;449(7164):811–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Greer FR, Sicherer SH, Burks AW, Committee On N, Section On A, Immunology. The Effects of Early Nutritional Interventions on the Development of Atopic Disease in Infants and Children: The Role of Maternal Dietary Restriction, Breastfeeding, Hydrolyzed Formulas, and Timing of Introduction of Allergenic Complementary Foods. Pediatrics. 2019;143(4). [DOI] [PubMed] [Google Scholar]
- 49.Du Toit G, Roberts G, Sayre PH, Bahnson HT, Radulovic S, Santos AF, et al. Randomized trial of peanut consumption in infants at risk for peanut allergy. N Engl J Med. 2015;372(9):803–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Feeney M, Du Toit G, Roberts G, Sayre PH, Lawson K, Bahnson HT, et al. Impact of peanut consumption in the LEAP Study: Feasibility, growth, and nutrition. J Allergy Clin Immunol. 2016;138(4):1108–1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


