Abstract
The UspA1 and UspA2 proteins of Moraxella catarrhalis are structurally related, are exposed on the bacterial cell surface, and migrate as very high-molecular-weight complexes in sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Previous analysis of uspA1 and uspA2 mutants of M. catarrhalis strain 035E indicated that UspA1 was involved in adherence of this organism to Chang conjunctival epithelial cells in vitro and that expression of UspA2 was essential for resistance of this strain to killing by normal human serum (C. Aebi, E. R. Lafontaine, L. D. Cope, J. L. Latimer, S. R. Lumbley, G. H. McCracken, Jr., and E. J. Hansen, Infect. Immun. 66:3113–3119, 1998). In the present study, isogenic uspA1, uspA2, and uspA1 uspA2 mutations were constructed in three additional M. catarrhalis strains: 012E, TTA37, and 046E. The uspA1 mutant of strain 012E had a decreased ability to attach to Chang cells. However, inactivation of the uspA1 gene in both strain TTA37 and strain 046E did not cause a significant decrease in attachment ability. Inactivation of the uspA2 gene of strain TTA37 did result in a loss of attachment ability. Nucleotide sequence analysis revealed that the predicted protein encoded by the uspA2 genes of both strains TTA37 and 046E had a N-terminal half that resembled the N-terminal half of UspA1 proteins, whereas the C-terminal half of this protein was nearly identical to those of previously characterized UspA2 proteins. The gene encoding this “hybrid” protein was designated uspA2H. PCR-based analysis revealed that approximately 20% of M. catarrhalis strains apparently possess a uspA2H gene instead of a uspA2 gene. The M. catarrhalis uspA1, uspA2, and uspA2H genes were cloned and expressed in Haemophilus influenzae cells, which were used to prove that both the UspA1 and UspA2H proteins can function as adhesins in vitro.
Moraxella catarrhalis, an unencapsulated, gram-negative bacterium, can cause disease in both the upper and lower respiratory tracts (32). It has been estimated that approximately 20% of cases of acute bacterial otitis media in infants and young children are caused by this organism (6). M. catarrhalis is also associated with nearly one-third of infectious exacerbations of chronic obstructive pulmonary disease in adults (16). The ability of this organism to cause significant morbidity has resulted in increased efforts to develop an efficacious M. catarrhalis vaccine (35).
Outer membrane proteins have received the most attention as possible M. catarrhalis vaccine candidates (9, 19, 20, 31, 33, 43), and even M. catarrhalis lipooligosaccharide may contain potential vaccine components (15). A few of these outer membrane proteins, especially CopB (OMP B2) (4, 38), OMP CD (24), TbpA and TbpB (28), LbpA and LbpB (12), and UspA (ubiquitous surface protein A or HMW-OMP) (20, 26), which consists of two related proteins, UspA1 and UspA2 (2, 3), have been characterized in some detail. Furthermore, changes in expression of M. catarrhalis outer membrane proteins have been shown to affect the ability of this organism to resist clearance from the lungs of animals (27).
The UspA1 and UspA2 surface proteins of M. catarrhalis are structurally related but appear to mediate different biological functions. The amino acid sequences of UspA1 and UspA2 from M. catarrhalis strain 035E are approximately 43% identical, but each possesses an internal segment of 135 amino acids with 93% identity; this region contains an epitope which binds the monoclonal antibody (MAb) 17C7 and is present in all disease isolates of M. catarrhalis tested to date (20). However, these two proteins appear to have different biological functions, with UspA1 having been shown to be essential for attachment of M. catarrhalis strain 035E to Chang conjunctival cells in vitro, whereas UspA2 is involved directly or indirectly in serum resistance of this strain (2). Interestingly, after solubilization of M. catarrhalis cells at 37°C, both UspA1 and UspA2 apparently are present as oligomers or aggregates, each of which migrates in sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) with an apparent molecular weight of greater than 250,000 even though their molecular masses are 88 and 62 kDa, respectively (3).
In the present study, isogenic uspA1, uspA2, and uspA1 uspA2 double mutants were constructed in three additional strains of M. catarrhalis. Analysis of the ability of these mutants to adhere to Chang cells in vitro led to the discovery of a second type of UspA2 protein that is comprised of amino acid segments from both UspA1 and UspA2. Direct evidence that both UspA1 and this second type of UspA2 protein have functional activity as adhesins was obtained by expressing both of these M. catarrhalis proteins as recombinant molecules in Haemophilus influenzae.
MATERIALS AND METHODS
Bacterial strains, plasmids, and culture conditions.
Most of the bacterial strains and plasmids used in this study are listed in Table 1. M. catarrhalis (3), Escherichia coli (11), and H. influenzae (18) strains were routinely cultured as described previously. Antimicrobial supplementation for M. catarrhalis mutants involved kanamycin (15 μg/ml), spectinomycin (15 μg/ml), or chloramphenicol (0.6 μg/ml). For bacterial adherence and serum bactericidal assays, M. catarrhalis strains were grown in broth without antibiotics for two to three generations. Recombinant strains of E. coli were selected with kanamycin (50 μg/ml), spectinomycin (150 μg/ml), or ampicillin (100 μg/ml). H. influenzae recombinant strains were cultured in the presence of chloramphenicol (2 μg/ml). For adherence assays, H. influenzae strains were grown in broth without antibiotics for two to three generations.
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Genotype or description | Reference or Source |
|---|---|---|
| M. catarrhalis | ||
| 035E | Wild-type disease isolate, attaches to Chang cells, serum resistant | 2 |
| 035E.1 | uspA1 mutant of 035E, attachment deficient, serum resistant | 2 |
| 035E.2 | uspA2 mutant of 035E, attaches to Chang cells, serum sensitive | 2 |
| 035E.12 | uspA1 uspA2 mutant of 035E, attachment deficient, serum sensitive | 2 |
| 012E | Wild-type disease isolate, attaches to Chang cells, serum resistant | 1 |
| 012E.1 | uspA1 mutant of 012E, attachment deficient, serum resistant | This study |
| 012E.2 | uspA2 mutant of 012E, attaches to Chang cells, serum sensitive | This study |
| 012E.12 | uspA1 uspA2 mutant of 012E, attachment deficient, serum sensitive | This study |
| TTA37 | Wild-type disease isolate, attaches to Chang cells, serum sensitive | Steven Berk |
| TTA37.1 | uspA1 mutant of TTA37, attaches to Chang cells, serum sensitive | This study |
| TTA37.2 | uspA2H mutant of TTA37, attachment deficient, serum sensitive | This study |
| TTA37.12 | uspA1 uspA2H mutant of TTA37, attachment deficient, serum sensitive | This study |
| 046E | Wild-type disease isolate, attaches to Chang cells, serum resistant | John Nelson |
| 046E.1 | uspA1 mutant of 046E, attaches to Chang cells, serum resistant | This study |
| 046E.2 | uspA2H mutant of 046E, attaches to Chang cells, serum sensitive | This study |
| 046E.12 | uspA1 uspA2H mutant of 046E, attachment deficient, serum sensitive | This study |
| TTA24 | Wild-type disease isolate | 10 |
| P44 | Wild-type disease isolate | 25 |
| ATCC 25240 | Wild-type disease isolate | American Type Culture Collection |
| E22 | Wild-type disease isolate | 5 |
| V1166 | Wild-type isolate from nasopharynx of a healthy child | F. Henderson |
| V1171 | Wild-type isolate from nasopharynx of a healthy child | 10 |
| E. coli DH5α | Host strain for cloning experiments | 37 |
| H. influenzae DB117 | Host strain for cloning experiments | 39 |
| Plasmids | ||
| pBS KS(+) | Cloning vector, Ampr | Stratagene |
| pUSPA1KAN | pBS containing a truncated uspA1 gene from M. catarrhalis strain 035E into which a kanamycin resistance cartridge was inserted | 3 |
| pSPECr | Source of the spectinomycin resistance cartridge | 42 |
| pELU2P44SPEC | pBS containing an incomplete uspA2 gene from M. catarrhalis P44 into which a spectinomycin resistance cartridge was inserted | This study |
| pACYC184 | Cloning vector | New England Biolabs |
| pELU112 | pACYC184 containing the M. catarrhalis 012E uspA1 gene | This study |
| pELU212 | pACYC184 containing the M. catarrhalis 012E uspA2 gene | This study |
| pELU135 | pACYC184 containing the M. catarrhalis 035E uspA1 gene | This study |
| pELU235 | pACYC184 containing the M. catarrhalis 035E uspA2 gene | This study |
| pELU146 | pACYC184 containing the M. catarrhalis 046E uspA1 gene | This study |
| pELU246 | pACYC184 containing the M. catarrhalis 046E uspA2H gene | This study |
| pELU137 | pACYC184 containing the M. catarrhalis TTA37 uspA1 gene | This study |
| pELU237 | pACYC184 containing the M. catarrhalis TTA37 uspA2H gene | This study |
| pELU171 | pACYC184 containing the M. catarrhalis V1171 uspA1 gene | This study |
| pELU271 | pACYC184 containing the M. catarrhalis V1171 uspA2 gene | This study |
| pELU266 | pACYC184 containing the M. catarrhalis V1166 uspA2H gene | This study |
Recombinant DNA methods.
Standard molecular biology techniques were performed as described previously (37) using E. coli strain DH5α or H. influenzae strain DB117 (39) as the host for recombinant DNA manipulations. The procedure for electroporating M. catarrhalis has been described in detail elsewhere (21) and was also used to electroporate H. influenzae.
PCR.
Amplicons used for cloning of the uspA1, uspA2, and uspA2H genes were generated with Pfu DNA polymerase (Stratagene, La Jolla, Calif.). Amplification of other DNA fragments was performed either with the Gene Amp XL PCR kit (Perkin-Elmer Biosystems, Branchburg, N.J.) or with Taq DNA polymerase (Promega, Madison, Wis.) by the manufacturer's procedure.
Construction of isogenic mutants.
The plasmid pUSPA1KAN was used to construct uspA1 mutants of M. catarrhalis strains 012E and TTA37 as described previously (2). The oligonucleotide primers P1 (5′-CGGGATCCCTTCTCCCCCTAAAAATCGCTG-3′) and P2 (5′-AGGGATCCCGCTGTATGCCGCTACTCGCAGCT-3′) (BamHI restriction sites are underlined) were used in PCR to amplify a 3-kb DNA fragment containing an incomplete uspA2 gene with a kanamycin cartridge insertion from the M. catarrhalis uspA2 mutant 035E.2 (2). This PCR product was used to electroporate M. catarrhalis 012E and TTA37; kanamycin-resistant transformants were screened by PCR to identify potential uspA2 mutants of strain 012E and uspA2H mutants of strain TTA37.
To construct isogenic mutants defective in expression of both UspA1 and UspA2, an incomplete uspA2 open reading frame (ORF) was amplified from M. catarrhalis strain P44 by PCR using the oligonucleotide primers P1 and P2. After digestion with BamHI, this 2.2-kb DNA fragment was ligated into pBS KS(+). A 0.4-kb BglII fragment was removed from the middle of this partial uspA2 ORF and was replaced with a cartridge encoding resistance to spectinomycin (42), yielding the plasmid pELU2P44SPEC. EcoRI-digested pELU2P44SPEC was used to electroporate the M. catarrhalis uspA1 mutants 012E.1 and TTA37.1. Spectinomycin-resistant transformants were screened in colony blot radioimmunoassays with the UspA1- and UspA2-reactive MAb 17C7 to identify transformants that had lost the ability to express both proteins. Linearized pELU2P44SPEC was used to electroporate M. catarrhalis 046E to construct an isogenic uspA2H mutant of this strain.
The oligonucleotide primers P3 (5′-AGGGATCCAACGACGGTCCAAGATGG-3′) and P4 (5′-AGGGATCCCCTGCCACCTAAAGCCTTG-3′) (underlined residues are BamHI sites) were used in PCR to amplify a 3.6-kb DNA fragment from M. catarrhalis strain 035E.12 (2) consisting of the entire uspA1 ORF with a chloramphenicol resistance cartridge insertion. This amplicon was introduced into M. catarrhalis strains 046E and 046E.2 by electroporation. Chloramphenicol-resistant colonies were screened by PCR to identify potential uspA1 mutants.
Cloning of M. catarrhalis genes in H. influenzae.
Amplicons of approximately 3 kb containing the uspA1 genes from M. catarrhalis strains 035E, TTA37, 012E, 046E, and V1171 were generated by using the oligonucleotide primers P3 and P4. These PCR products were digested with BamHI and ligated into the vector pACYC184 (New England Biolabs, Inc., Beverly, Mass.). These ligation mixtures were introduced into H. influenzae strain DB117 by electroporation. Chloramphenicol-resistant colonies were screened in colony blot radioimmunoassays with MAb 17C7 to identify recombinants expressing M. catarrhalis UspA1 proteins.
PCR products of approximately 3 kb containing the M. catarrhalis 046E, TTA37, and V1166 uspA2H genes were amplified with the oligonucleotide primers P5 (5′-AGGCATGCGGTTATAGCAATCCCTTG-3′) and P6 (5′-GCGCATGCGCCAGCTTTATTTTATGCAGGG-3′) (the underlining indicates an SphI site). After digestion with SphI, these amplicons were ligated into pACYC184 and used to electroporate H. influenzae; MAb 17C7 was used to identify transformants expressing the UspA2H protein.
The oligonucleotide primers P7 (5′-AGGCATGCTGTCCGCTGATGCTTTCTG-3′) and P8 (5′-AGGCATGCGCTTTTATCCATCACTCAC-3′) (the underlining indicates an SphI site) were used to generate 2.0- to 2.5-kb PCR products containing the uspA2 genes from M. catarrhalis 012E, 035E, and V1171. The SphI-digested amplicons were ligated into pACYC184 and used to electroporate H. influenzae. Chloramphenicol-resistant colonies were screened as described above for expression of UspA2. Both strands of all of the cloned PCR products were sequenced.
Nucleotide sequence analysis.
Nucleotide sequence analysis of recombinant plasmids and PCR amplicons was performed as described previously (10).
Characterization of protein antigens.
SDS-PAGE of proteins present in whole cell lysates and Western blot analysis were performed as described previously (10). The UspA1- and UspA2-reactive MAb 17C7 and the UspA1-specific MAb 24B5 have been described previously (2, 10). Colony blot radioimmunoassays were performed as described previously (2). The indirect antibody accessibility assay was performed as described previously (2) using MAb 17C7 to detect recombinant UspA1, UspA2, and UspA2H proteins on the surface of H. influenzae cells and MAb 24B5 to detect native UspA1 protein on the surface of wild-type and mutant strains of M. catarrhalis.
Adherence and serum resistance assays.
Bacterial adherence assays were performed with Chang conjunctival epithelial cells as previously described (2). Killing of M. catarrhalis strains by 10% (vol/vol) normal human serum was assessed as previously described (2).
Nucleotide sequence accession numbers.
The nucleotide sequences of the genes sequenced in this study have been deposited in GenBank under the following accession numbers: AF181072, M. catarrhalis 012E uspA1; AF181073, M. catarrhalis 012E uspA2; AF181076, M. catarrhalis TTA37 uspA1; AF181075, M. catarrhalis TTA37 uspA2H; U61725, M. catarrhalis 046E uspA1; and AF181074, M. catarrhalis 046E uspA2H.
RESULTS
Construction of isogenic uspA1 and uspA2 mutants of M. catarrhalis 012E, TTA37, and 046E.
The uspA1 and uspA2 gene products of M. catarrhalis 035E were previously shown to be involved in adherence to human conjunctival epithelial cells and in resistance to killing by normal human serum, respectively (2). To determine whether the roles of these surface proteins are conserved among strains of M. catarrhalis, we constructed isogenic uspA1, uspA2, and uspA1 uspA2 double mutants of M. catarrhalis strains 012E, TTA37, and 046E. (For reasons that will be explained below, the uspA2 genes of both strains TTA37 and 046E will be designated uspA2H from this point forward.) PCR-based and Southern blot analyses confirmed that each mutant had the appropriate antibiotic resistance cartridge inserted into the intended target gene (data not shown).
Characterization of selected proteins expressed by wild-type and mutant M. catarrhalis strains.
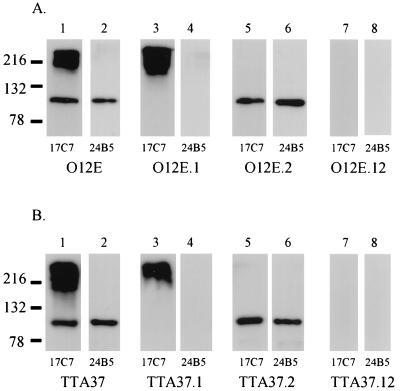
Whole cell lysates were heated at 100°C for 15 min before SDS-PAGE; this treatment allowed distinction of UspA1 and UspA2 proteins probed with a MAb (17C7) reactive with both proteins, as shown previously (10). When these preparations were probed in Western blot analysis with the UspA1-reactive MAb 24B5, it was found that the disruption of the uspA1 ORF in M. catarrhalis 012E.1 (Fig. 1A, lane 4), in 012E.12 (Fig. 1A, lane 8), and in TTA37.1 and TTA37.12 (Fig. 1B, lanes 4 and 8, respectively) abolished expression of the approximately 120-kDa UspA1 protein. The uspA2 mutation in M. catarrhalis 012E.2 (Fig. 1A, lane 5) and the uspA2H mutation in TTA37.2 (Fig. 1B, lane 5) eliminated expression of UspA2, which is present as a very high-molecular-weight complex reactive with MAb 17C7 in these whole cell lysates (Fig. 1A and B, lanes 3). The presence of both uspA1 and uspA2 mutations in strain 012E.12 (Fig. 1A, lanes 7 and 8) and of both uspA1 and uspA2H mutations in strain TTA37.12 (Fig. 1B, lanes 7 and 8) eliminated all MAb 17C7 and MAb 24B5 reactivity from these double mutants. The uspA1, uspA2H, and uspA1 uspA2H mutants of strain 046E had Western blot reactivity patterns identical to those of the 012E and TTA37 mutant sets (data not shown). Taken together, these results indicate that these isogenic mutants lacked expression of the genes that were specifically targeted by our mutagenesis strategy.
FIG. 1.
Western blot analysis of whole cell lysates prepared from M. catarrhalis strains 012E and TTA37 and their corresponding isogenic mutants. (A) Wild-type M. catarrhalis 012E (lanes 1 and 2), the uspA1 mutant 012E.1 (lanes 3 and 4), the uspA2 mutant 012E.2 (lanes 5 and 6), and the uspA1 uspA2 double mutant 012E.12 (lanes 7 and 8). (B) Wild-type M. catarrhalis TTA37 (lanes 1 and 2), the uspA1 mutant TTA37.1 (lanes 3 and 4), the uspA2H mutant TTA37.2 (lanes 5 and 6), and the uspA1 uspA2H double mutant TTA37.12 (lanes 7 and 8). The UspA1- and UspA2-reactive MAb 17C7 was used to probe lanes 1, 3, 5, and 7 in both panels; the UspA1-specific MAb 24B5 was used to probe lanes 2, 4, 6, and 8 in both panels. Molecular mass markers are shown to the left in kilodaltons.
Effect of uspA1 and uspA2 mutations on serum resistance.
Inactivation of the uspA2 gene in M. catarrhalis 035E renders this strain exquisitely sensitive to the bactericidal activity of normal human serum, while the lack of expression of the UspA1 protein does not affect the ability of 035E to resist killing by normal human serum (2). To determine whether the involvement of the uspA2 gene product in serum resistance is a conserved feature of M. catarrhalis strains, we tested the serum-resistant strains 012E and 046E and their isogenic mutants in a serum bactericidal assay. It should be noted that, unlike most disease isolates of M. catarrhalis (41), the wild-type strain TTA37 was completely killed within the first 30 min of incubation with normal human serum (data not shown). Therefore, mutants of M. catarrhalis TTA37 were not tested in the serum bactericidal assay.
The M. catarrhalis uspA2 mutant 012E.2 and the uspA1 uspA2 double mutant 012E.12, both of which lack expression of the UspA2 protein, were completely killed within 30 min of incubation with normal human serum (data not shown). In contrast, lack of expression of the UspA1 protein did not affect the ability of the M. catarrhalis uspA1 mutant 012E.1 to resist killing by normal human serum when compared to the parent strain. The uspA2H mutant and uspA1 uspA2H double mutant of strain 046E were much more sensitive to killing by normal human serum than were the wild-type parent strain and the uspA1 mutant of strain 046E (data not shown).
Effect of uspA1 and uspA2 mutations on the ability of M. catarrhalis strains to adhere to human epithelial cells in vitro.
Lack of expression of the UspA1 protein causes an approximately 60-fold decrease in the ability of M. catarrhalis 035E to adhere to Chang conjunctival epithelial cells in vitro (2). To determine whether the involvement of the uspA1 gene product in the process of adherence to human epithelial cells is a common feature of M. catarrhalis isolates, we tested the ability of the mutants described above to attach to Chang cells.
M. catarrhalis 012E and its uspA2 mutant 012E.2 exhibited similar levels of attachment to Chang cells (Table 2). Lack of expression of the UspA1 protein in both the M. catarrhalis uspA1 mutant 012E.1 and the uspA1 uspA2 double mutant 012E.12 diminished adherence of these mutants to the monolayers by 1 order of magnitude (Table 2). Unexpectedly, however, M. catarrhalis TTA37 and its uspA1 mutant TTA37.1 were found to attach to Chang cells at similar levels (Table 2). Disruption of the uspA2H ORF in the uspA2H mutant TTA37.2 and the uspA1 uspA2H mutant TTA37.12 did result in an approximately 10-fold reduction in adherence of these strains to Chang cells (Table 2). M. catarrhalis 046E, its uspA1 mutant 046E.1, and the uspA2H mutant 046E.2 all exhibited similar levels of attachment to Chang cells (Table 2). When expression of both UspA1 and UspA2H was eliminated in the uspA1 uspA2H mutant 046E.12, this resulted in a 10-fold reduction in adherence (Table 2).
TABLE 2.
Adherence of wild-type and isogenic mutant strains of M. catarrhalis to Chang conjunctival epithelial cells in vitro
| Strain | Genotype | Adherencea |
|---|---|---|
| 012E | Wild type | 123.0 ± 15.57 |
| 012E.1 | uspA1 mutant | 9.3 ± 3.8 (0.0001) |
| 012E.2 | uspA2 mutant | 138.3 ± 19.6 (0.0088) |
| 012E.12 | uspA1 uspA2 mutant | 14.1 ± 3.8 (0.0001) |
| TTA37 | Wild type | 42.4 ± 17.3 |
| TTA37.1 | uspA1 mutant | 45.8 ± 14.7 (0.6593) |
| TTA37.2 | uspA2H mutant | 6.7 ± 2.1 (0.0003) |
| TTA37.12 | uspA1 uspA2H mutant | 4.5 ± 0.5 (0.0002) |
| 046E | Wild type | 52.1 ± 13.1 |
| 046E.1 | uspA1 mutant | 55.2 ± 14.3 (0.6725) |
| 046E.2 | uspA2H mutant | 45.4 ± 18.1 (0.4847) |
| 046E.12 | uspA1 uspA2H mutant | 6.7 ± 2.2 (0.0001) |
Adherence of M. catarrhalis to Chang cells is expressed as the mean (± standard deviation) percentage of the inoculum which adhered to the monolayers. The P value compared to the value for the wild-type parent strain, using a two-tailed t test, is given in parentheses.
Nucleotide sequence analysis of the uspA1 and uspA2 genes of M. catarrhalis strains 012E, TTA37, and 046E.
To investigate a possible correlation between the unexpected biological effects of the uspA1 and uspA2H mutations in strains TTA37 and 046E and the structural features of the relevant proteins, we determined the nucleotide sequences of the uspA1 and uspA2 genes from strain 012E and those of the uspA1 and uspA2H genes from strains TTA37 and 046E. The uspA1 ORFs were predicted to encode proteins ranging in molecular weight from 91,873 to 96,959 (Table 3). The uspA2 gene of M. catarrhalis 012E was predicted to encode a protein of 74,599 Da, whereas the predicted UspA2H proteins from M. catarrhalis 046E and TTA37 were both significantly larger than that of strain 012E (Table 3).
TABLE 3.
Characteristics of the uspA1, uspA2, and uspA2H genes and their encoded protein products from selected M. catarrhalis strains
| Strain | Gene | Length of ORF (nt) | Mol wt of predicted protein | No. of amino acids | Predicted start codon |
|---|---|---|---|---|---|
| 012E | uspA1 | 2,766 | 96,959 | 922 | ATG |
| uspA2 | 2,052 | 74,599 | 684 | ATG | |
| TTA37 | uspA1 | 2,619 | 91,873 | 873 | ATG |
| uspA2H | 2,667 | 92,145 | 889 | GTG | |
| 046E | uspA1 | 2,676 | 93,350 | 892 | ATG |
| uspA2H | 2,682 | 94,229 | 894 | GTG |
Approximately 130 to 140 nucleotides (nt) 5′ from the predicted translational start codon of the M. catarrhalis 012E uspA2 ORF were 19 repeats of the tetranucleotide AGAT (data not shown), as previously observed in the 5′ regions of other uspA2 genes (10). The nucleotide sequence upstream of the predicted translational start codon of the three uspA1 ORFs was also found to contain a nucleotide repeat motif. As previously seen with other uspA1 genes (10), poly(G) tracts containing 9 to 11 guanine residues were located 30 nt 5′ from the ATG translational start codon of the uspA1 ORFs in strains 012E, TTA37, and 046E (data not shown). Nucleotide sequence analysis of approximately 1.2 kb of DNA upstream of the proposed translational start codons of the M. catarrhalis 046E and TTA37 uspA2H ORFs did not detect any AGAT repeats or poly(G) tracts but did reveal that these regions were virtually identical in 046E and TTA37 (data not shown). This region did not resemble the upstream regions of either uspA1 or uspA2 genes from previously characterized M. catarrhalis strains. In addition, an ORF encoding a protein product with 26% identity to the InsB protein encoded by IS1 in the Shigella dysenteriae genome (34) was present in this upstream region from strains 046E and TTA37 (data not shown).
Comparison of the predicted amino acid sequences of the UspA1 proteins.
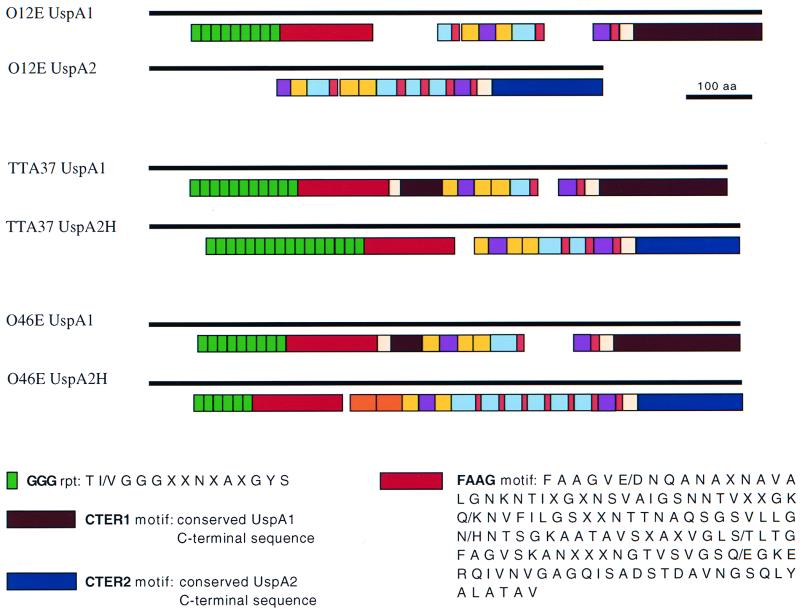
Overall, the UspA1 proteins of strains 012E, TTA37, and 046E were greater than 70% identical (data not shown). As previously observed with the UspA1 proteins from four other M. catarrhalis strains (10), these three UspA1 proteins had virtually identical predicted signal peptides of 49 residues and contained several different amino acid repeats and other motifs (Fig. 2). Of interest are the GGG repeats, which are highly conserved in the N termini of UspA1 proteins (10) and absent from the UspA2 proteins of M. catarrhalis strains 035E, TTA24, ATCC 25238, and V1171 (10) and 012E (Fig. 2). The M. catarrhalis 012E, 046E, and TTA37 UspA1 proteins contained 9, 9, and 11 GGG repeat motifs, respectively (Fig. 2). The GGG repeat is characterized by the consensus sequence T(I/V)GGGXXNXAXGYS where the underlined residues are perfectly conserved. Following the GGG repeats, a region of 135 amino acids (designated FAAG for the first four residues present in this motif) was present in these three UspA1 proteins (Fig. 2) as well as in the four UspA1 proteins previously characterized (10). The C termini of the 012E, TTA37, and 046E UspA1 proteins were also found to contain a stretch of 193 amino acids (designated CTER1 in Fig. 2) that were 97% identical to those observed previously within the C termini of UspA1 proteins (10).
FIG. 2.
Schematic representation of the repetitive amino acid sequences and other motifs present in the UspA1 and UspA2 proteins of M. catarrhalis 012E and in the UspA1 and UspA2H proteins of strains TTA37 and 046E. The solid bars indicates the lengths of the proteins. The colored boxes indicate the positions and relative lengths as well as the numbers of repeats and other motifs. The GGG and FAAG motifs are indicated; the other motifs are described in detail elsewhere (10).
Comparison of the predicted amino acid sequences of the UspA2 and UspA2H proteins.
The UspA2 protein of M. catarrhalis 012E was less than 50% identical to the UspA2H proteins of strains 046E and TTA37, whereas the latter were predicted to be 62% identical. Alignment of the predicted amino acid sequences of these three proteins showed that the signal peptide of 30 residues previously seen in four other UspA2 proteins (10) was present only in the UspA2 protein from M. catarrhalis 012E (data not shown). However, the signal peptide of the UspA2H proteins from strains TTA37 and 046E was nearly identical to that found in the UspA1 proteins of all M. catarrhalis strains characterized to date (10). The C termini of both the UspA2 protein from 012E and the UspA2H proteins from strains TTA37 and 046E were also found to contain a region of 158 residues (designated CTER2 in Fig. 2) that was 87% identical among these three strains.
As before (10), several different amino acid repeats and other motifs were observed in the UspA2 and UspA2H proteins (Fig. 2). Certain amino acid repeats, such as the VEEG, NINNY, LAAY, KASS, and FET motifs, again were found to be shared by both UspA1 and the UspA2 and UspA2H proteins (Fig. 2). Interestingly, the N-terminal halves of the UspA2H proteins of M. catarrhalis TTA37 and 046E contained amino acid sequences that had a high degree of identity with the GGG repeats and FAAG motif present in the UspA1 protein (Fig. 2). These particular motifs were notably absent from the UspA2 proteins of M. catarrhalis 012E (Fig. 2) and strains 035E, TTA24, ATCC 25238, and V1171 (10). In fact, alignment of the predicted amino acid sequences of the N termini of the M. catarrhalis 012E, TTA37, and 046E UspA1 proteins with those of the UspA2H proteins of strains 046E and TTA37 revealed a high degree of homology (data not shown).
Analysis of M. catarrhalis isolates for the presence of a hybrid uspA2 gene.
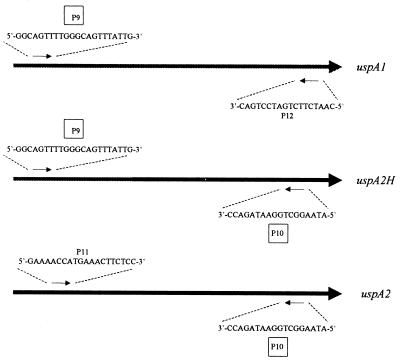
Strains 046E and TTA37 both expressed a UspA2H protein which had an N-terminal half resembling that of a UspA1 protein together with a C-terminal half similar to those of other UspA2 proteins. For the sake of clarity, this type of protein has been designated UspA2H to indicate its apparent “hybrid” nature. To investigate whether other M. catarrhalis isolates might express a UspA2H protein, PCR was used to detect the possible presence of a uspA2H ORF. The oligonucleotide primer P9 (Fig. 3) corresponds to a perfectly conserved region found at the 5′ ends of the uspA1 ORFs of five M. catarrhalis strains (035E, TTA24, 012E, 046E, and TTA37). This same nucleotide sequence is present in the 5′ ends of the uspA2H ORFs in strains 046E and TTA37. The oligonucleotide primer P10 (Fig. 3) was designed to bind a perfectly conserved region at the 3′ ends of both the uspA2 ORFs in strains 035E, TTA24, and 012E and the uspA2H ORFs in strains TTA37 and 046E. For amplification of uspA1 ORFs, the oligonucleotide primers P9 (described above) and P12 (Fig. 3; derived from the 3′ ends of the uspA1 ORFs of strains 035E, 012E, and TTA24) were utilized. To detect the presence of uspA2 ORFs, the oligonucleotide primers P11 (Fig. 3; derived from the 5′ ends of the uspA2 ORFs of strains 035E, 012E, and TTA24) and P10 (described above) were used in PCR.
FIG. 3.
Schematic representation of the placement of the oligonucleotide primers used in PCR to detect the presence of the uspA1, uspA2, and uspA2H genes in 21 M. catarrhalis strains.
No product was detected when primers P9 and P10 were used in PCR with chromosomal DNA from strain 035E, 012E, or TTA24; these three strains each express a UspA2 protein that has no UspA1-like sequences in its N-terminal half. In contrast, the use of these same two primers in PCR with chromosomal DNAs from strains TTA37 and 046E yielded a 2.5-kb product in both instances. When these same oligonucleotide primers (P9 and P10) were used in PCR with chromosomal DNAs from 15 additional M. catarrhalis strains whose uspA2 genes had not been sequenced, amplicons of 2.3 to 2.5 kb were obtained from several strains, including ATCC 25240, E22, and V1166. A total of 11 disease isolates (035E, 012E, 046E, TTA37, TTA24, P44, P48, ATCC 25238, ATCC 25240, E22, and TTA1) and 10 normal carriage isolates (V1111, V1156, V1171, V1169, V1166, V1153, V1129, V1126, V1118, and V1121) were used in these PCRs. All 21 strains yielded a PCR product when the uspA1-specific primer pair P9 and P12 was used (data not shown). PCR products were obtained with the uspA2-specific primer pair P11 and P10 only from the 16 strains that did not possess a uspA2H gene (the strains other than 046E, TTA37, ATCC 25240, E22, and V1166).
Nucleotide sequence analysis of approximately 700 nt at the 3′ ends of the PCR products amplified with primers P9 and P10 from M. catarrhalis E22, ATCC 25240, and V1166 revealed that the encoded amino acid sequence had a high level of identity with the amino acid sequence typically found in the C-terminal regions of UspA2 proteins (data not shown). The deduced amino acid sequence derived from approximately 700 nt at the 5′ ends of these same amplicons, however, contained the GGG repeats and the FAAG region, which were typically present in the N termini of UspA1 proteins (data not shown).
Expression of the M. catarrhalis UspA1, UspA2, and UspA2H proteins in H. influenzae.
To conclusively determine whether UspA1 and UspA2H proteins functioned as adhesins, the uspA1, uspA2, and uspA2H genes from various M. catarrhalis strains were cloned and expressed in H. influenzae DB117, which has been previously shown to adhere poorly to Chang cells in vitro (40).
Western blot analysis revealed that the UspA1- and UspA2-reactive MAb 17C7 bound a 120-kDa antigen in recombinant H. influenzae cells expressing the UspA1 protein of M. catarrhalis 012E, 035E, V1171, and TTA37 (data not shown). The recombinant UspA1 protein from strain 046E was not detectable in Western blot analysis despite the fact that it could be detected in colony blot radioimmunoassay (data not shown). MAb 17C7-reactive antigens with apparent molecular masses in excess of 200 kDa were detected in H. influenzae strains expressing the UspA2 proteins of strain 012E, 035E, and V1171. Very high-molecular-weight antigens were also detected with MAb 17C7 in whole cell lysates from H. influenzae cells expressing the UspA2H proteins from M. catarrhalis TTA37, 046E, and V1166. MAb 17C7 was used in the indirect antibody accessibility assay to detect recombinant UspA1, UspA2, and UspA2H proteins on the surface of H. influenzae. With the exception of the UspA1 proteins from strains TTA37 and 046E, all of these M. catarrhalis proteins were readily detectable on the surface of the recombinant strains (Table 4).
TABLE 4.
Adherence of recombinant H. influenzae cells to Chang conjunctival epithelial cells in vitro and detection of recombinant proteins on the bacterial cell surface
| Recombinant plasmid | M. catarrhalis proteina | Adherenceb | Detection of protein on cell surfacec |
|---|---|---|---|
| pACYC184 (vector) | 0.2 ± 0.1 | 918 ± 276 | |
| pELU112 | 012E-UspA1 | 35.1 ± 8.7 | 13,870 ± 2,132 |
| pELU212 | 012E-UspA2 | 0.4 ± 0.2 | 5,557 ± 1,546 |
| pELU135 | 035E-UspA1 | 17.4 ± 7.3 | 5,050 ± 1,184 |
| pELU235 | 035E-UspA2 | 0.2 ± 0.1 | 7,175 ± 3,201 |
| pELU171 | V1171-UspA1 | 12.3 ± 7.2 | 6,425 ± 1,049 |
| pELU271 | V1171-UspA2 | 0.4 ± 0.2 | 4,111 ± 1,333 |
| pELU137 | TTA37-UspA1 | 0.9 ± 0.4 | 1,286 ± 206 |
| pELU237 | TTA37-UspA2H | 16.9 ± 6.3 | 11,630 ± 1,351 |
| pELU146 | 046E-UspA1 | 0.8 ± 0.2 | 703 ± 221 |
| pELU246 | 046E-UspA2H | 52.6 ± 15.17 | 28,980 ± 1,703 |
| pELU266 | V1166-UspA2H | 62.3 ± 16.6 | 5,632 ± 199 |
The M. catarrhalis protein expressed by the H. influenzae DB117 clone containing this recombinant plasmid.
Adherence of M. catarrhalis organisms to Chang cells is expressed as the mean (± standard deviation) percentage of the inoculum which adhered to the monolayers.
Counts per minute (mean ± standard deviation) of radioiodinated goat anti-mouse immunoglobulin bound to MAb 17C7 on the surface of recombinant cells. The indirect antibody-accessibility assay was performed as described previously (2).
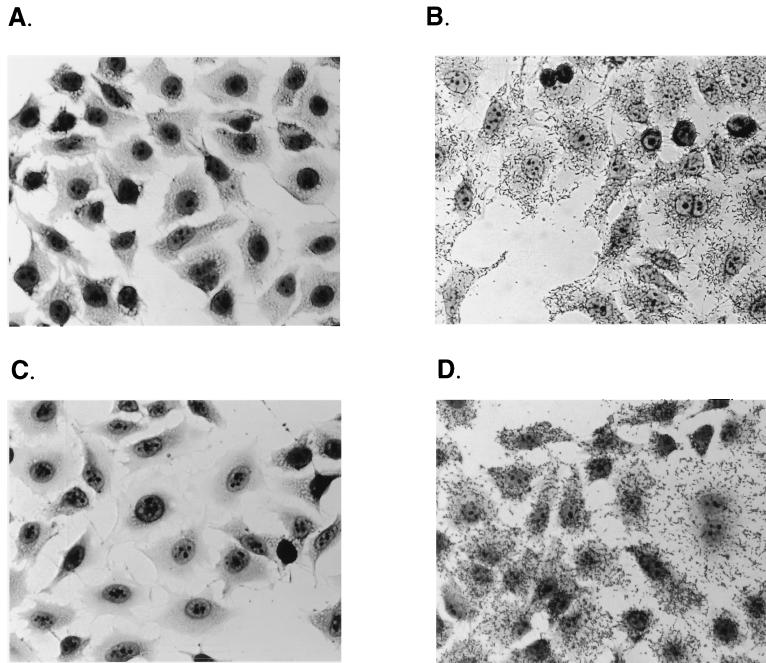
Expression of the M. catarrhalis 012E, 035E, or V1171 UspA1 protein increased the ability of H. influenzae DB117 to adhere to Chang cells by 2 orders of magnitude (Table 4; Fig. 4B). Similarly, expression of the M. catarrhalis TTA37, 046E, or V1166 UspA2H protein increased adherence by a factor of 100-fold (Table 4; Fig. 4D). In contrast, expression of the M. catarrhalis 012E, 035E, or V1171 UspA2 protein had little effect on the adherence of H. influenzae to Chang cells (Table 4; Fig. 4C). Similarly, the recombinant forms of the M. catarrhalis TTA37 and 046E UspA1 proteins had at best a very slight (i.e., fourfold) effect on attachment of H. influenzae to Chang cells (Table 4).
FIG. 4.
Light micrographs of recombinant H. influenzae DB117 clones incubated with Chang conjunctival epithelial cells. Adherence assays were performed as described in Materials and Methods with the following modification: after the last wash to remove nonadherent bacterial cells, the monolayers were fixed for 10 min with methanol and subsequently stained with Giemsa stain. (A) DB117 carrying the cloning vector pACYC184; (B) DB117 carrying pELU112 (expressing 012E UspA1); (C) DB117 carrying pELU212 (expressing 012E UspA2); (D) DB117 carrying pELU246 (expressing 046E UspA2H).
DISCUSSION
Despite advances made in the characterization of the biochemical and immunogenic properties of the M. catarrhalis UspA1 and UspA2 proteins (2, 3, 8–10, 31), information about the structure-function relationships inherent in these two macromolecules remains limited. Analysis of isogenic uspA1 and uspA2 mutants of M. catarrhalis strain 035E (2) provided the first indication that these two proteins, which have in common a 135-amino-acid region that contains the epitope for MAb 17C7, might have different functions. Lack of expression of UspA1 severely impaired the ability of M. catarrhalis 035E to adhere to human epithelial cells in vitro, whereas the uspA2 mutation rendered this strain sensitive to complement-mediated killing by normal human serum (2). In addition, the UspA1 protein purified from M. catarrhalis 035E binds to HEp-2 cells, whereas the UspA2 protein purified from the same strain binds these same cells very weakly (9). Furthermore, antibodies raised against purified UspA1, but not UspA2, were sufficient to significantly abrogate adherence of strain 035E to HEp-2 cells (9). It could be inferred from these findings that UspA1 and UspA2 likely had distinct biological functions and that UspA1 was directly or indirectly involved in the physical interaction of M. catarrhalis 035E with human epithelial cells in vitro.
Analysis of additional uspA1 and uspA2 mutants in the present study revealed that, at least in strain 012E, inactivation of the uspA1 gene could be correlated with loss of adherence ability in vitro (Table 2). In contrast, inactivation of the uspA2H gene of strain TTA37, but not the uspA1 gene, adversely affected the ability of this strain to adhere to Chang cells in vitro. This finding led to the discovery of a second type of UspA2 protein (UspA2H) in strain TTA37 which has a C-terminal half that is virtually identical to the C-terminal half of previously described UspA2 proteins (10) but which has an N-terminal half that is very different from that of other UspA2 proteins. More specifically, this region of the UspA2H protein contains the GGG amino acid repeats and the FAAG region, which have been observed in the N termini of all UspA1 proteins examined to date (10).
A PCR-based survey of a number of M. catarrhalis isolates identified additional strains which likely encoded a UspA2H protein. Determination of the complete nucleotide sequence of the uspA2H gene from strain 046E and partial nucleotide sequence analysis of the PCR products obtained from strains V1166, E22, and ATCC 25240 indicated that the predicted UspA2H proteins from these strains had primary amino acid sequences very similar to that of the UspA2H protein of strain TTA37.
These data derived from mutant analysis still provided only indirect evidence that UspA1 and now UspA2H were involved in the ability of M. catarrhalis strains to attach to Chang cells in vitro. To demonstrate directly that these M. catarrhalis proteins were adhesins, both the uspA1 and uspA2H genes were cloned and expressed in H. influenzae DB117, an organism that does not normally adhere well to Chang conjunctival cells (40). H. influenzae recombinant clones expressing the M. catarrhalis 035E or 012E UspA1 protein exhibited a dramatic increase in adherence to Chang cells (Table 4; Fig. 4). These results clearly demonstrate that the UspA1 proteins from these M. catarrhalis isolates, whose isogenic uspA1 mutants are deficient in attachment ability, function as an adhesin for Chang cells in vitro. Furthermore, recombinant H. influenzae clones expressing M. catarrhalis TTA37, 046E, or V1166 UspA2H protein exhibited a significant increase in adherence ability, a result which confirmed that the UspA2H protein is directly involved in the attachment ability of these M. catarrhalis strains. In contrast, H. influenzae strains expressing the uspA2 genes from strains 035E and 012E did not attach to Chang monolayers (Table 4; Fig. 4). This latter finding can be correlated directly with the lack of effect of uspA2 mutations on the ability of these two M. catarrhalis strains to attach to Chang cells (2) (Table 2). Therefore, UspA2H proteins can function as adhesins in vitro and, as such, are functionally distinct from the UspA2 proteins of previously characterized M. catarrhalis strains, including 035E, TTA24, ATCC 25238, and V1171 (10).
The results in Tables 2 and 4 and Fig. 4 indicate that both the UspA1 and UspA2H proteins mediate a physical interaction between M. catarrhalis and Chang cells in vitro. Both proteins have striking identity in their N-terminal regions (Fig. 2), and it is tempting to speculate that the region of these proteins containing the GGG repeats and FAAG motif is responsible for this interaction, although it is equally likely that another amino acid sequence(s) in the same region could be responsible for attachment to Chang cells. Both the UspA1 and UspA2 proteins have been proposed to be part of the autotransporter family of secreted proteins (22), which includes gene products that form large macromolecular complexes on the surface of bacterial cells and which often function as adherence factors (22). The N termini of some of these autotransporter proteins have been proposed to be exposed on the surface of bacteria (22). The next logical step will be to identify the region(s) of the UspA1 and UspA2H proteins responsible for conferring on M. catarrhalis the ability to adhere to human epithelial cells in vitro. Our ability to express functional, recombinant UspA1 and UspA2H proteins in H. influenzae should allow construction of strains expressing chimeric molecules containing different portions of UspA1 and UspA2.
It is interesting that expression of the UspA1 protein of either strain TTA37 or strain 046E in H. influenzae did not promote attachment to Chang cells (Table 4). Nucleotide sequence analysis of the M. catarrhalis DNA inserts in the respective recombinant plasmids revealed no mutations caused by PCR amplification. However, the use of MAb 17C7 in an indirect antibody accessibility assay (2) indicated that the recombinant forms of these two UspA1 proteins were not expressed on the surface of the H. influenzae cells (data not shown). This finding provides a physical explanation for the lack of attachment ability of the recombinant H. influenzae cells expressing either of these UspA1 proteins, although it is not clear why these two proteins were not translocated to the surface of H. influenzae.
The phenotypes of the isogenic uspA1 and uspA2H mutants of strains TTA37 and 046E must be interpreted carefully. Lack of expression of UspA1 by M. catarrhalis strain 046E.1 did not eliminate the ability of this uspA1 mutant to attach to Chang cells (Table 4), because it was still expressing the UspA2H protein. Similarly, lack of expression of UspA2H by strain 046E.2 did not adversely affect attachment, because this uspA2H mutant still expressed a UspA1 protein. In contrast, the uspA2H mutant of strain TTA37 exhibited a dramatic decrease in its ability to attach to Chang cells (Table 2). UspA1, however, was found to be exposed on the surface of both the wild-type TTA37 strain and its uspA2H mutant in an indirect antibody accessibility assay using the UspA1-specific MAb 24B5 (data not shown). Thus, the UspA1 protein from the wild-type strain TTA37 appears to be defective and unable to confer adherence ability on this strain, because the lack of expression of the UspA2H protein is sufficient to abrogate adherence of this particular isolate to Chang cells (Table 2). Structure-function analysis of the UspA1 protein should ultimately allow determination of the region(s) in the TTA37 UspA1 protein responsible for this apparent dysfunction. Alternatively, at this time we cannot exclude the possibility that lack of expression of UspA2H in the TTA37.2 mutant somehow adversely affected the orientation and thus the function of the UspA1 protein.
Although the UspA1 and UspA2H proteins have now been shown to be adhesins which mediate attachment to Chang cells in vitro, it is likely that M. catarrhalis strains express other adhesins. It has been reported that the CD outer membrane protein of M. catarrhalis bound human mucin purified from the middle ears of children with otitis media (36). Expression of a 200-kDa protein has been found to be exclusively associated with hemagglutinating isolates of M. catarrhalis (13); this proposed hemagglutination factor has been recently associated with the formation of a fibrillar layer surrounding the bacteria which was observed to physically contact erythrocytes (14).
Although the UspA2 and UspA2H proteins are structurally distinct, the results of the mutant analyses indicate that both are involved in the ability of their respective M. catarrhalis strains to survive killing mediated by the complement components present in normal human serum. The lack of expression of UspA2 in strains 035E.2 (2) and 012E.2 and the lack of expression of UspA2H by M. catarrhalis 046E.2 rendered these strains exquisitely sensitive to killing by normal human serum. It is tempting to speculate that the C-terminal halves of both UspA2 and UspA2H may somehow be involved in serum resistance, since this region is common to both proteins (Fig. 2). However, the C-terminal half of the UspA2H protein of the serum-sensitive M. catarrhalis strain TTA37 exhibits a high degree of sequence identity to the C-terminal half of the UspA2H protein of the serum-resistant M. catarrhalis strain 046E. In addition, the mechanism by which many disease isolates of M. catarrhalis resist killing by normal human serum is unclear, although it has been proposed that binding of vitronectin to the surface of serum-resistant M. catarrhalis cells inhibits the formation of the membrane attack complex (41) and it has been shown that purified UspA2 binds vitronectin (9). The construction of isogenic uspA2 mutants of various M. catarrhalis strains as well as the newly realized ability to express the UspA2 and UspA2H proteins in the heterologous background of H. influenzae DB117 may help to elucidate the involvement of these proteins in protecting M. catarrhalis from killing by normal human serum.
At this point, it is appropriate to address issues concerning the structures of the uspA1, uspA2, and uspA2H genes. The data in the present study, together with those from two previous reports (3, 10), include the nucleotide sequences of a total of eight uspA1 genes, six uspA2 genes, and two uspA2H genes. Examination of these sequences raises two important questions: what mechanism(s) is responsible for the variability in certain regions of the uspA1 and uspA2 genes, and how did the uspA2H genes originate?
Most of the interstrain variability in the uspA1 genes and in the uspA2 genes is localized to the 5′ half of each ORF (data not shown). In contrast, the 3′ end of each uspA1 and uspA2 gene is highly conserved, as is the 5′ end of each gene, including the beginning of the ORF. This clustering of sequence polymorphisms amidst two highly conserved regions is reminiscent of the mosaic structure described for ORFs that encode surface-exposed proteins of other bacterial pathogens, including Neisseria meningitidis (23, 29), E. coli (30), and Neisseria gonorrhoeae (17). In these examples, horizontal genetic exchange has been proposed as the major mechanism by which mosaic genes are generated. Similar to several of the aforementioned pathogens, M. catarrhalis can be transformed in vitro (7); therefore, horizontal transfer of genetic material via transformation followed by homologous recombination is a possible mechanism for generating mosaicism in the uspA1 and uspA2 ORFs. In addition, the presence of both a uspA1 gene and a uspA2 (or uspA2H) gene in each M. catarrhalis strain studied to date indicates that intrachromosomal recombination between uspA1 and uspA2 loci could also be involved.
The origin of the uspA2H genes remains open to speculation. However, it is apparent that the uspA2H gene was not just the result of a simple double-crossover event between a uspA1 gene and a uspA2 gene. The 3′ end of uspA2H is virtually identical to that of uspA2, and the 5′ end of the uspA2H ORF is clearly derived from a uspA1 gene. However, the 1.2-kb region upstream of the translational start codon in the uspA2H ORFs in strains TTA37 and 046E was not derived from either uspA1 or uspA2, thus posing an interesting question about the source of this nucleotide sequence. The presence in this upstream DNA of an ORF encoding a predicted protein similar to that expressed by an insertion element (i.e., IS1) (34) raises the possibility that several different genetic events were involved in the development of uspA2H genes.
In summary, we have identified two distinct yet closely related adhesins of M. catarrhalis. Because bacterial adherence is an important first step in the colonization of the upper respiratory tract by M. catarrhalis, the findings reported in this study have implications for both vaccine development and the design of therapeutic approaches which could interfere with the adherence properties conferred by UspA1 or UspA2H. It may be feasible to prevent establishment of M. catarrhalis in the nasopharynx, thus precluding subsequent development of otitis media caused by this organism.
ACKNOWLEDGMENTS
This study was supported by Public Health Service grant AI36344 and by Texas Advanced Technology Program Award 003660-087 to E.J.H. C.A. was supported by a research grant for young investigators from Novartis AG, Basel, Switzerland.
We thank Steven Berk, John Nelson, and Frederick Henderson for providing isolates of M. catarrhalis.
REFERENCES
- 1.Aebi C, Cope L D, Latimer J L, Thomas S E, Slaughter C A, McCracken G H, Jr, Hansen E J. Mapping of a protective epitope of the CopB outer membrane protein of Moraxella catarrhalis. Infect Immun. 1998;66:540–548. doi: 10.1128/iai.66.2.540-548.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aebi C, Lafontaine E R, Cope L D, Latimer J L, Lumbley S R, McCracken G H, Jr, Hansen E J. Phenotypic effect of isogenic uspA1 and uspA2 mutations on Moraxella catarrhalis 035E. Infect Immun. 1998;66:3113–3119. doi: 10.1128/iai.66.7.3113-3119.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aebi C, Maciver I, Latimer J L, Cope L D, Stevens M K, Thomas S E, McCracken G H, Jr, Hansen E J. A protective epitope of Moraxella catarrhalis is encoded by two different genes. Infect Immun. 1997;65:4367–4377. doi: 10.1128/iai.65.11.4367-4377.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aebi C, Stone B, Beucher M, Cope L D, Maciver I, Thomas S E, McCracken G H, Jr, Sparling P F, Hansen E J. Expression of the CopB outer membrane protein by Moraxella catarrhalis is regulated by iron and affects iron acquisition from transferrin and lactoferrin. Infect Immun. 1996;64:2024–2030. doi: 10.1128/iai.64.6.2024-2030.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beaulieu D, Ouellette M, Bergeron M G, Roy P H. Characterization of a plasmid isolated from Branhamella catarrhalis and detection of plasmid sequences within the genome of a B. catarrhalis strain. Plasmid. 1988;20:158–162. doi: 10.1016/0147-619x(88)90020-0. [DOI] [PubMed] [Google Scholar]
- 6.Bluestone C D, Stephenson J S, Martin L M. Ten-year review of otitis media pathogens. Pediatr Infect Dis J. 1992;11:S7–S11. doi: 10.1097/00006454-199208001-00002. [DOI] [PubMed] [Google Scholar]
- 7.Catlin B W, Cunningham L S. Genetic transformation of Neisseria catarrhalis by deoxyribonucleate preparations having different average base compositions. J Gen Microbiol. 1964;37:341–352. doi: 10.1099/00221287-37-3-341. [DOI] [PubMed] [Google Scholar]
- 8.Chen D, Barniak V, VanDerMeid K R, McMichael J C. The levels and bactericidal capacity of antibodies directed against the UspA1 and UspA2 outer membrane proteins of Moraxella (Branhamella) catarrhalis in adults and children. Infect Immun. 1999;67:1310–1316. doi: 10.1128/iai.67.3.1310-1316.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen D, McMichael J C, van der Meid K R, Hahn D, Mininni T, Cowell J, Eldridge J. Evaluation of purified UspA from Moraxella catarrhalis as a vaccine in a murine model after active immunization. Infect Immun. 1996;64:1900–1905. doi: 10.1128/iai.64.6.1900-1905.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cope L D, Lafontaine E R, Slaughter C A, Hasemann C A, Jr, Aebi C, Henderson F W, McCracken G H., Jr Characterization of the Moraxella catarrhalis uspA1 and uspA2 genes and their encoded products. J Bacteriol. 1999;181:4026–4034. doi: 10.1128/jb.181.13.4026-4034.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cope L D, Lumbley S R, Latimer J L, Klesney-Tait J, Stevens M K, Johnson L S, Purven M, Munson R S, Jr, Lagergard T, Radolf J D, Hansen E J. A diffusible cytotoxin of Haemophilus ducreyi. Proc Natl Acad Sci USA. 1997;94:4056–4061. doi: 10.1073/pnas.94.8.4056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Du R P, Wang Q J, Yang Y-P, Schryvers A B, Chong P, Klein M H, Loosmore S M. Cloning and expression of the Moraxella catarrhalis lactoferrin receptor genes. Infect Immun. 1998;66:3656–3665. doi: 10.1128/iai.66.8.3656-3665.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fitzgerald M, Mulcahy R, Murphy S, Keane C, Coakley D, Scott T. A 200 kDa protein is associated with haemagglutinating isolates of Moraxella (Branhaemella) catarrhalis. FEMS Immunol Med Microbiol. 1997;18:209–216. doi: 10.1111/j.1574-695X.1997.tb01047.x. [DOI] [PubMed] [Google Scholar]
- 14.Fitzgerald M, Mulcahy R, Murphy S, Keane C, Coakley D, Scott T. Transmission electron microscopy studies of Moraxella (Branhamella) catarrhalis. FEMS Immunol Med Microbiol. 1999;23:57–66. doi: 10.1111/j.1574-695X.1999.tb01717.x. [DOI] [PubMed] [Google Scholar]
- 15.Gu X-X, Chen J, Barenkamp S J, Robbins J B, Tsai C-M, Lim D J, Battey J. Synthesis and characterization of lipooligosaccharide-based conjugates as vaccine candidates for Moraxella (Branhamella) catarrhalis. Infect Immun. 1998;66:1891–1897. doi: 10.1128/iai.66.5.1891-1897.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hager H, Verghese A, Alvarez S, Berk S L. Branhamella catarrhalis respiratory infections. Rev Infect Dis. 1987;9:1140–1149. doi: 10.1093/clinids/9.6.1140. [DOI] [PubMed] [Google Scholar]
- 17.Halter R, Pohlner J, Meyer T F. Mosaic-like organization of IgA protease genes in Neisseria gonorrhoeae generated by horizontal genetic exchange in vivo. EMBO J. 1989;8:2737–3744. doi: 10.1002/j.1460-2075.1989.tb08415.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hansen E J, Gonzales F R, Chamberlain N R, Norgard M V, Miller E E, Cope L D, Pelzel S E, Gaddy B, Clausell A. Cloning of the gene encoding the major outer membrane protein of Haemophilus influenzae type b. Infect Immun. 1988;56:2709–2716. doi: 10.1128/iai.56.10.2709-2716.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Helminen M E, Maciver I, Latimer J L, Cope L D, McCracken G H, Jr, Hansen E J. A major outer membrane protein of Moraxella catarrhalis is a target for antibodies that enhance pulmonary clearance of the pathogen in an animal model. Infect Immun. 1993;61:2003–2010. doi: 10.1128/iai.61.5.2003-2010.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Helminen M E, Maciver I, Latimer J L, Klesney-Tait J, Cope L D, Paris M M, McCracken G H, Jr, Hansen E J. A large, antigenically conserved protein on the surface of Moraxella catarrhalis is a target for protective antibodies. J Infect Dis. 1994;170:867–872. doi: 10.1093/infdis/170.4.867. [DOI] [PubMed] [Google Scholar]
- 21.Helminen M E, Maciver I, Latimer J L, Lumbley S R, Cope L D, McCracken G H, Jr, Hansen E J. A mutation affecting expression of a major outer membrane protein of Moraxella catarrhalis alters serum resistance and survival of this organism in vivo. J Infect Dis. 1993;168:1194–1201. doi: 10.1093/infdis/168.5.1194. [DOI] [PubMed] [Google Scholar]
- 22.Henderson I R, Navarro-Garcia F, Nataro J P. The great escape: structure and function of the autotransporter proteins. Trends Microbiol. 1998;6:370–378. doi: 10.1016/s0966-842x(98)01318-3. [DOI] [PubMed] [Google Scholar]
- 23.Hobbs M M, Seiler A, Achtman M, Cannon J G. Microevolution within a clonal population of pathogenic bacteria: recombination, gene duplication and horizontal genetic exchange in the opa gene family of Neisseria meningitidis. Mol Microbiol. 1994;12:171–180. doi: 10.1111/j.1365-2958.1994.tb01006.x. [DOI] [PubMed] [Google Scholar]
- 24.Hsiao C B, Sethi S, Murphy T F. Outer membrane protein CD of Branhamella catarrhalis: sequence conservation in strains recovered from the human respiratory tract. Microb Pathog. 1995;19:215–225. doi: 10.1016/s0882-4010(95)90272-4. [DOI] [PubMed] [Google Scholar]
- 25.Jordan K L, Berk S H, Berk S L. A comparison of serum bactericidal activity and phenotypic characteristics of bacteremic, pneumonia-causing strains, and colonizing strains of Branhamella catarrhalis. Am J Med. 1990;88(5A):28S–32S. doi: 10.1016/0002-9343(90)90258-f. [DOI] [PubMed] [Google Scholar]
- 26.Klingman K L, Murphy T F. Purification and characterization of a high-molecular-weight outer membrane protein of Moraxella (Branhamella) catarrhalis. Infect Immun. 1994;62:1150–1155. doi: 10.1128/iai.62.4.1150-1155.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kyd J M, Cripps A W, Murphy T F. Outer-membrane antigen expression by Moraxella (Branhamella) catarrhalis influences pulmonary clearance. J Med Microbiol. 1998;47:159–168. doi: 10.1099/00222615-47-2-159. [DOI] [PubMed] [Google Scholar]
- 28.Luke N R, Russo T A, Luther N, Campagnari A A. Use of an isogenic mutant constructed in Moraxella catarrhalis to identify a protective epitope of outer membrane B1 defined by monoclonal antibody 11C6. Infect Immun. 1999;67:681–687. doi: 10.1128/iai.67.2.681-687.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maiden M C J. Population genetics of a transformable bacterium: the influence of horizontal genetic exchange on the biology of Neisseria meningitidis. FEMS Microbiol Lett. 1993;112:243–250. doi: 10.1111/j.1574-6968.1993.tb06457.x. [DOI] [PubMed] [Google Scholar]
- 30.McGraw E A, Li J, Selander R K, Whittman T S. Molecular evolution and mosaic structure of alpha, beta and gamma intimins of pathogenic Escherichia coli. Mol Biol Evol. 1999;16:12–22. doi: 10.1093/oxfordjournals.molbev.a026032. [DOI] [PubMed] [Google Scholar]
- 31.McMichael J C, Fiske M J, Fredenburg R A, Chakravarti D N, VanDerMeid K R, Barniak V, Caplan J, Bortell E, Baker S, Arumugham R, Chen D. Isolation and characterization of two proteins from Moraxella catarrhalis that bear a common epitope. Infect Immun. 1998;66:4374–4381. doi: 10.1128/iai.66.9.4374-4381.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Murphy T F. Branhamella catarrhalis: epidemiology, surface antigenic structure, and immune response. Microbiol Rev. 1996;60:267. doi: 10.1128/mr.60.2.267-279.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Murphy T F, Kyd J M, John A, Kirkham C, Cripps A W. Enhancement of pulmonary clearance of Moraxella (Branhamella) catarrhalis following immunization with outer membrane protein CD in a mouse model. J Infect Dis. 1998;178:1667–1675. doi: 10.1086/314501. [DOI] [PubMed] [Google Scholar]
- 34.Ohtsubo H, Nyman K, Doroszkiewicz W, Othsubo E. Multiple copies of iso-insertion sequence of IS1 in Shigella dysenteriae chromosome. Nature. 1981;292:640–643. doi: 10.1038/292640a0. [DOI] [PubMed] [Google Scholar]
- 35.Pelton S I, Klein J O. The promise of immunoprophylaxis for prevention of acute otitis media. Pediatr Infect Dis J. 1999;18:926–935. doi: 10.1097/00006454-199910000-00018. [DOI] [PubMed] [Google Scholar]
- 36.Reddy M S, Murphy T F, Faden H S, Bernstein J M. Middle ear mucin glycoprotein: purification and interaction with nontypeable Haemophilus influenzae and Moraxella catarrhalis. Otolaryngol Head Neck Surg. 1997;116:175–180. doi: 10.1016/S0194-59989770321-8. [DOI] [PubMed] [Google Scholar]
- 37.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 38.Sethi S, Surface J M, Murphy T F. Antigenic heterogeneity and molecular analysis of CopB of Moraxella (Branhamella) catarrhalis. Infect Immun. 1997;65:3666–3671. doi: 10.1128/iai.65.9.3666-3671.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Setlow J K, Brown D C, Boling M E, Mattingly A, Gordon M P. Repair of deoxyribonucleic acid in Haemophilus influenzae. J Bacteriol. 1968;95:546–558. doi: 10.1128/jb.95.2.546-558.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.St. Geme J W, III, de la Morena M L, Falkow S. A Haemophilus influenzae IgA protease-like protein promotes intimate interaction with human epithelial cells. Mol Microbiol. 1994;14:217–233. doi: 10.1111/j.1365-2958.1994.tb01283.x. [DOI] [PubMed] [Google Scholar]
- 41.Verduin C M, Jansze M, Hol C, Mollnes T E, Verhoef J, van Dijk H. Differences in complement activation between complement-resistant and complement-sensitive Moraxella (Branhamella) catarrhalis strains occur at the level of membrane attack complex formation. Infect Immun. 1994;62:589–595. doi: 10.1128/iai.62.2.589-595.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Whitby P W, Morton D J, Stull T L. Construction of antibiotic resistance cassettes with multiple paired restriction sites for insertional mutagenesis of Haemophilus influenzae. FEMS Microbiol Lett. 1998;158:57–60. doi: 10.1111/j.1574-6968.1998.tb12800.x. [DOI] [PubMed] [Google Scholar]
- 43.Yang Y-P, Myers L E, Mcguinness U, Chong P, Kwok Y, Klein M H, Harkness R E. The major outer membrane protein, CD, extracted from Moraxella (Branhamella) catarrhalis is a potential vaccine antigen that induces bactericidal antibodies. FEMS Immunol Med Microbiol. 1997;17:187–199. doi: 10.1111/j.1574-695X.1997.tb01012.x. [DOI] [PubMed] [Google Scholar]