Abstract
Background:
Myelin oligodendrocyte glycoprotein antibody-associated disease (MOGAD) is now recognized as distinct from multiple sclerosis (MS).
Objective:
To evaluate the importance of considering myelin oligodendrocyte glycoprotein (MOG)-immunoglobulin-G (IgG) serology when applying MS diagnostic criteria in children.
Methods:
Within a prospective cohort of children meeting MS criteria (median follow-up = 6 years, interquartile range (IQR) = 4–9), we measured MOG-IgG in serial archived serum obtained from presentation, and compared imaging and clinical features between seropositive and seronegative participants.
Results:
Of 65 children meeting MS criteria (median age = 14.0 years, IQR = 10.9–15.1), 12 (18%) had MOG-IgG at disease onset. Seropositive participants were younger, had brain magnetic resonance imaging (MRI) features atypical for MS, rarely had cerebrospinal fluid (CSF) oligoclonal bands (2/8, 25%), and accumulated fewer T2 lesions over time. On serial samples, 5/12 (42%) were persistently seropositive, 5/12 (42%) became seronegative, and 2/12 (17%) had fluctuating results. All 12 children experienced a disease course different from typical MS.
Conclusion:
While children with MOG-IgG can have clinical, CSF, and MRI features conforming to MS criteria, the presence of MOG-IgG is associated with atypical features and predicts a non-MS disease course. Given MOG-IgG seropositivity can wane over time, testing at first attack is of considerable importance for the diagnosis of MOGAD.
Keywords: All demyelinating diseases (CNS), multiple sclerosis, MRI, autoimmune diseases, all pediatric
Introduction
Anti-myelin oligodendrocyte glycoprotein (MOG) antibodies (MOG-IgG) can be found in children and adults with monophasic or relapsing demyelination, and mounting evidence indicates that the presence of clearly positive MOG-IgG titers defines a diagnosis of Myelin oligodendrocyte glycoprotein antibody-associated disease (MOGAD) and argues against a diagnosis of MS.1–8 MOG-IgG titers often rapidly decline after initial presentation, and relapsing MOG-IgG positive patients may have periods of undetectable antibodies between relapses.3,9,10 As such, the diagnosis of MOGAD cannot be excluded when serostatus was not ascertained proximate to initial clinical presentation, and failure to recognize MOGAD in relapsing patients can lead to a misdiagnosis of MS.
We sought to determine the frequency of MOG-IgG at presentation in a prospective cohort of children who met MS diagnostic criteria and had archived serial serum samples obtained in proximity to clinical presentation, 11 and to evaluate their clinical and magnetic resonance imaging (MRI) features as a function of MOG-IgG serostatus.
Methods
Participants
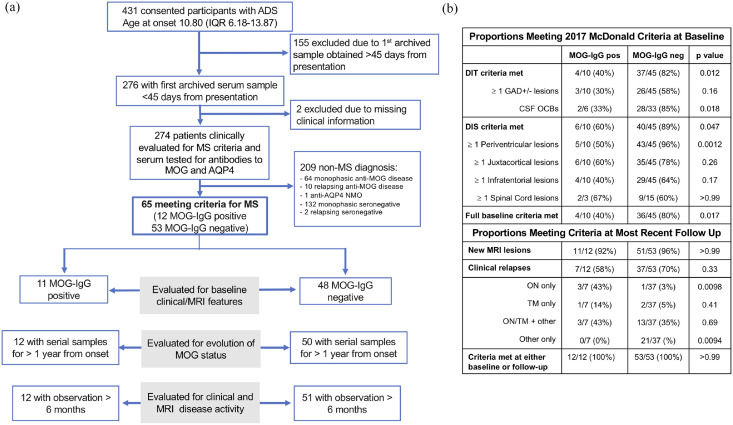
We included 65 children and adolescents meeting MS diagnostic criteria either at onset or over time, recruited between 2004 and 2017 as part of the prospective Canadian Pediatric Demyelinating Disease Study (CPDDS). 12 Most participants were recruited and diagnosed before the clinical availability of MOG-IgG testing, and none of the participants included in this study had clinical anti-MOG testing performed as part of their diagnostic workup. For this study, CPDDS participants were included if they also met the criteria of: (1) first available serum sample obtained within 45 days of initial clinical presentation, (2) complete clinical information available, and (3) a diagnosis of MS based on the 2017 McDonald diagnostic criteria 11 (Figure 1(a)). Data were locked as of May 2018.
Figure 1.
Cohort selection. (a) Flow chart of study design and patient disposition. (b) Proportions of patients meeting 2017 McDonald criteria at baseline and in follow-up. Baseline criteria were evaluated on the baseline MRI (acquired within 45 days from clinical onset), in participants with non-ADEM presentation whose MRI scans were performed with administration of gadolinium-based contrast agent. Fulfillment of the McDonald criteria over time was assessed in all participants including at their most recent follow-up; median (IQR) of 8.14 (6.19–9.80) years in MOG-IgG positive participants and of 6.11 (4.06–9.25) years in MOG-IgG negative participants.
ADS: acquired demyelinating syndrome; ADEM: acute disseminated encephalomyelitis; AQP-4: aquaporin-4; CSF: cerebrospinal fluid; GAD: gadolinium-based contrast agent enhancement; MOG: myelin oligodendrocyte glycoprotein; MS: multiple sclerosis; NMO: neuromyelitis optica; OCB: oligoclonal band; ON: optic neuritis; TM: transverse myelitis.
The clinical phenotype of each attack was determined from case report forms, using our published methods. 13 Children meeting the clinical criteria for acute disseminated encephalomyelitis (ADEM) 14 were not evaluated for a diagnosis of MS at baseline, as stipulated in the 2017 criteria, 11 while they could be evaluated as possible MS if they experienced relapsing non-ADEM events. When clinically indicated, the presence of oligoclonal bands (OCBs) in the cerebrospinal fluid (CSF) was assessed using isoelectric focusing.
The occurrence of new disease activity (new clinical attacks, new MRI lesions or both) was evaluated at all study visits (3, 6, and 12 months, and annually thereafter), and at the time of any off-study clinical visits or admissions. Monofocal and polyfocal presentations were defined as those manifesting signs and symptoms attributable to involvement of a single or multiple location of the central nervous system (CNS), respectively. Monophasic and relapsing outcomes refer to the course of clinical symptoms, with monophasic patients not having new disease activity over time, and relapsing patients experiencing new clinical attacks, more than 30 days from an incident event. For this study, disease activity was assessed among participants with a minimum of 6 months of clinical and MRI observation based on our prior findings in the larger pediatric acquired demyelinating syndrome (ADS) cohort 15 of median time from clinical onset to the first relapse or new lesion of 6.18 months (interquartile range (IQR) = 4.73–11.08). To address the possible confounding effects of different length of follow-up, the annualized relapse rate (ARR) was calculated over the first 2 years of follow-up, restricting the analysis to participants with a minimum observation of 2 years. A standardized clinical examination form was completed at each study visit. Disability outcome was subsequently extrapolated from said form to create an estimated Expanded Disability Status Scale (EDSS) score which was also compared at 2 years from presentation. 16 As a sensitivity analysis, the ARR and EDSS results were also compared at 4 years from presentation. Use of disease-modifying therapies (DMTs) was recorded for all participants. The clinical course was considered atypical for MS based on the presence features identified as different between typical MS and MOGAD courses in our prior studies.9,17 These included as follows: (1) absence of any new lesions after 1 year from presentation, in patients not receiving DMTs or (2) recurrent attacks exclusively in a single CNS location (i.e. optic neuritis). The study was approved by the research ethics boards of all participating institutions. Guardians and participants provided written informed assent/consent.
Laboratory analysis
Analysis was carried out on a total of 363 archived serum samples, acquired within 45 days from clinical onset (baseline), then serially at 3, 6, and 12 months, and yearly thereafter. None of the samples were obtained after treatment with plasma exchange, and only 3/366 samples were obtained within 30 days of exposure to intravenous immunoglobulins (which was not associated with changes in prior or subsequent MOG-IgG results). Archived samples were batch-shipped and analyzed centrally using a live cell-based MOG-IgG1-specific assay 18 and titered using IgG (H + L) as the detecting antibody. The assay cut-off was based on a visual score, ranging from 0 to 4, with scores <1 considered negative, from 1 to <2 low positive, and 2 or greater “high” positive, as per our previously published methods. 9 All samples were also assessed for the presence of anti-aquaporin-4 (AQP4) antibodies. 19
MRI analysis
The research MRI protocol included axial and sagittal T2-weighted, fluid-attenuated inversion recovery (FLAIR)-weighted, and T1-weighted sequences, before and after administration of gadolinium.
MRI scans at presentation (baseline) were analyzed by a team (G.F., R.A.B., B.B., G.L., D.A.C.) trained in the application of the MRI scoring tool 20 and blinded to clinical and serological status. The scoring team independently adjudicated each scan as being “typical” or “atypical” for MS. Based on the information obtained through the scoring tool and evidence from existing literature,9,21 MRI features considered atypical for MS were (1) absence of brain lesions; (2) presence of large ill-defined lesions; (3) diffuse bilateral pattern; or (4) presence of only small/non-specific lesions.
Baseline scans were also scored using the 2017 McDonald criteria. 11 Scans acquired without administration of gadolinium were excluded from the baseline adjudication of criteria, while spine MRI scans acquired as part of the clinical care were considered, whenever available. Cortical lesions were not considered in our scoring, given that our research MRI protocols were not designed for their reliable detection.
Serial brain scans were analyzed for the occurrence of new lesions and lesion resolution. Complete lesion resolution was defined as a normal brain MRI at any timepoint following an abnormal baseline MRI. The most recent research scan was also evaluated for the presence and location of lesions, and for the measurement of T2 and T1 lesion volumes. In particular, T2 lesions were segmented using a Bayesian classifier 22 and then manually corrected by a trained expert, while T1 lesions were automatically segmented as a subset of T2 lesion voxels with T1 intensity less than 87% that of normal appearing white matter.
Statistical analysis
Demographic, clinical, and MRI features were compared between MOG-IgG-positive and negative groups, using chi-square or Fisher’s exact tests for categorical variables and Mann–Whitney U tests for continuous variables. The effect size of these comparisons was computed as standardized difference scores. 23 The analyses were performed using Python (www.python.org, version 3.6.5) and R (www.r-project.org, version 3.1.3). The sensitivity and specificity of the features suggested as red flags were computed using Dag-stat. 24
Results
MOG-IgG antibody results in archived baseline samples
Twelve of the 65 children (18%) diagnosed with MS had MOG-IgG titers above the laboratory cut-off in the samples archived from their initial presentation. Ten of these were high-positive on the IgG1 assay. One had a borderline-positive result in the first sample, followed by a high-positive IgG1 result in the subsequent sample obtained 3 months post-onset. One tested positive exclusively in the H + L assay (titer 200), with titers below the threshold for positivity on the IgG1 assay (Figure 1(a), Table 1). All participants tested negative for anti-AQP4 antibodies.
Table 1.
Baseline clinical, MRI, and laboratory features according to MOG serological status.
| Characteristic | All | MOG-IgG positive | MOG-IgG negative | SD | p-value |
|---|---|---|---|---|---|
| Participants (n) | 65 | 12 | 53 | ||
| Sex (female) (n/%) | 43 (66%) | 8 (67%) | 35 (66%) | 0.013 | >0.99 |
| Age at onset (median (IQR)) | 14.00 (10.93–15.08) | 9.04 (6.50–10.36) | 14.33 (13.01–15.31) | −1.6 | <0.0001 |
| Days from onset to first serum sample collection (median (IQR)) | 14 (6–24) | 22 (6–26) | 13 (7–23) | 0.85 | 0.31 |
| Steroids in the 30 days prior first sample acquisition (n %) | 32 (49%) | 7/12 (58%) | 25/53 (47%) | 0.23 | 0.70 |
| ADEM (n %) | 1 (2%) | 0 (0%) | 1 (2%) | −0.2 | >0.99 |
| Monofocal ON (n %) | 15 (23%) | 5 (42%) | 10 (19%) | 0.51 | 0.19 |
| Polyfocal ON (n %) | 5 (8%) | 1 (8%) | 4 (8%) | 0.029 | >0.99 |
| Monofocal TM (I %) | 6 (9%) | 2 (17%) | 4 (8%) | 0.28 | 0.31 |
| Polyfocal TM (n %) | 4 (6%) | 1 (8%) | 3 (6%) | 0.1 | 0.57 |
| ON + TM (n %) | 1 (2%) | 1 (8%) | 0 (0%) | 0.43 | 0.18 |
| Other than ON/TM/ADEM (n %) | 33 (51%) | 2 (17%) | 31 (58%) | −0.96 | 0.011 |
| Brain MRI typical for MS (n/N %) | 44/59 (75%) | 0/11 (0%) | 44/48 (92%) | −4.70 | <0.0001 |
| Lesions present (n/N %) | 56/59 (95%) | 8/11 (73%) | 48/48 (100%) | −0.87 | 0.0051 |
| Total brain lesions count (median (IQR)) | 12.00 (5.00–16.00) | 4.00 (0.50–11.00) | 14.00 (5.50–16.00) | −0.86 | 0.018 |
| Presence of discrete lesions (n/N %) | 53/56 (95%) | 7/8 (88%) | 46/48 (96%) | −0.3 | 0.38 |
| Presence of only well-defined lesions (n/N %) | 41/56 (73%) | 3/8 (38%) | 38/48 (79%) | −0.93 | 0.026 |
| Diffuse bilateral pattern (n/N %) | 8/56 (14%) | 3/8 (38%) | 5/48 (10%) | 0.67 | 0.078 |
| ⩾1 Cerebellar lesions (n/N %) | 21/56 (38%) | 2/8 (25%) | 19/48 (40%) | −0.32 | 0.7 |
| ⩾1 Cerebellar peduncle lesions (n/N %) | 15/56 (27%) | 1/8 (12%) | 14/48 (29%) | −0.42 | 0.43 |
| ⩾1 Brainstem lesions | 33/56 (59%) | 4/8 (50%) | 29/48 (60%) | −0.21 | 0.7 |
| ⩾1 Peri fourth ventricle lesions (n/N %) | 8/56 (14%) | 1/8 (12%) | 7/48 (15%) | −0.061 | >0.99 |
| ⩾1 Periventricular lesions (n/N %) | 51/56 (91%) | 6/8 (75%) | 45/48 (94%) | −0.53 | 0.14 |
| ⩾3 Periventricular lesions (n/N %) | 31/56 (55%) | 1/8 (12%) | 30/48 (62%) | −1.2 | 0.017 |
| ⩾1 Lesion perpendicular to the major axis of the corpus callosum (n/N %) | 37/56 (66%) | 0/8 (0%) | 37/48 (77%) | −2.6 | <0.0001 |
| ⩾1 Basal ganglia lesions (n/N %) | 4/56 (7%) | 0/8 (0%) | 4/48 (8%) | −0.43 | >0.99 |
| ⩾1 Thalamic lesions (n/N %) | 11/56 (20%) | 1/8 (12%) | 10/48 (21%) | −0.23 | >0.99 |
| ⩾1 Juxtacortical lesions (n/N %) | 45/5 (80%) | 7/8 (88%) | 38/48 (79%) | 0.23 | >0.99 |
| ⩾1 T1 hypointense lesions (n/N %) | 51/56 (91%) | 7/8 (88%) | 44/48 (92%) | −0.14 | 0.55 |
| ⩾1 Lesion enhancement (n/N %) a | 36/53 (68%) | 3/7 (43%) | 33/46 (72%) | −0.61 | 0.19 |
| ⩾1 Spinal lesions (n/N %) | 16/19 (84%) | 2/3 (67%) | 14/16 (88%) | −0.51 | 0.42 |
| ⩾1 LETM | 3/16 (19%) | 2/2 (100%) | 1/14 (7%) | 5.09 | 0.11 |
| Total T2 lesion volume (median (IQR)) b | 2.99 (1.17–11.07) | 2.35 (0.42–4.98) | 3.03 (1.33–11.58) | −0.31 | 0.51 |
| Total T1 lesion volume (median (IQR)) b | 0.52 (0.11–2.70) | 0.11 (0.01–0.92) | 0.57 (0.19–2.88) | −0.16 | 0.37 |
| OCBs (n/N %) | 32/44 (73%) | 2/8 (25%) | 30/36 (83%) | −1.4 | 0.0027 |
ADEM: acute disseminated encephalomyelitis; EDSS: Expanded Disability Status Scale; FSS: Functional System Score; LETM: Longitudinally Extensive Transverse Myelitis; OCBs: oligoclonal bands; ON: optic neuritis; SD: standardized difference; TM: transverse myelitis.
Analyses of lesion enhancement were restricted to scans with administration of gadolinium-based contrast agent.
Lesion volume at baseline was available for 6/8 MOG-IgG positive and 33/48 MOG-IgG negative participants with brain lesions at presentation.
Criteria for conferring MS diagnosis (baseline and follow-up)
At presentation, 55 of the 65 participants were evaluable using the 2017 McDonald criteria (i.e. after exclusion of one participant with ADEM presentation and nine with baseline MRI performed without administration of gadolinium-based contrast agent), with a diagnosis of MS conferred at that time in 40 (73%) of them. The remaining 25 met MS diagnostic criteria in follow-up, based on development of clinical relapses, MRI lesions, or both, which were identified a median of 4.1 months from onset (range = 2.3–48.7 months, Figure 1(b)).
Of the 12 MOG-IgG positive participants, the McDonald 2017 criteria could be applied at baseline to 10, of whom only 4 (40%) met both dissemination in space (DIS) and time (DIT) criteria (Figure 1(b)). In contrast, of the 53 MOG-IgG negative participants, the criteria could be applied at baseline to 45, of whom 36 (80%) met both DIS and DIT criteria for MS (p = 0.017).
Over time, all 12 MOG-IgG positive participants demonstrated evidence of new disease activity, 7 (54%) of whom experienced new clinical episodes, and 5 (42%) developed new MRI lesions but no additional clinical attacks (Figure 1(b)).
Of the 53 MOG-IgG negative participants, 37 (70%) experienced further clinical relapses, 15 (28%) developed new MRI lesions but no clinical attacks, and one met the diagnostic criteria for MS at onset though did not develop new signs of disease activity during the available follow-up of 2 years.
Comparison of baseline clinical and MRI features between MOG-IgG positive and negative participants
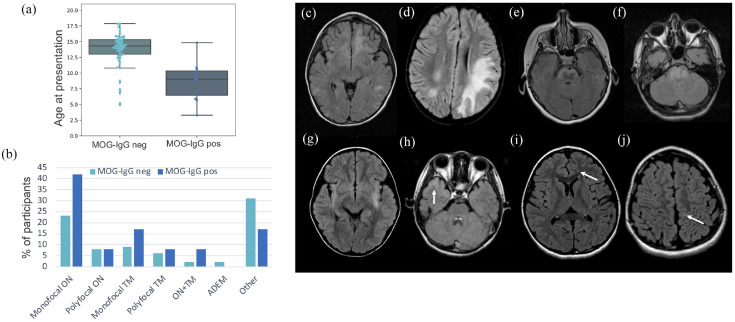
The subgroups of participants compared in each analysis are outlined in Figure 1(a). MOG-IgG positive participants were younger than the seronegative group (Figure 2(a) and Table 1) and 11/12 (92%) were younger than 11 years at onset. MOG-positive participants presented with optic neuritis (ON) and/or transverse myelitis (TM) nearly twice as often as MOG-negative participants (Figure 2(b) and Table 1). ADEM was the presenting phenotype of one seronegative participant.
Figure 2.
Baseline clinical and MRI features. Age at clinical presentation (a) and presenting phenotype (b) in MOG-IgG positive and MOG-IgG negative participants. (c)–(h) Brain MRIs of MOG-IgG positive participants demonstrating atypical features: large confluent lesions (c) and (d), extensive pontine lesions (e) and (f), bilateral ill-defined lesions (g) and ill-defined involvement of the temporal cortex (h), and small, non-specific white matter lesions (i) and (j).
ADEM: acute disseminated encephalomyelitis; MOG: myelin oligodendrocyte glycoprotein; ON: optic neuritis; TM: transverse myelitis.
Of all 12 patients found to be MOG-IgG positive at baseline, 11 had baseline MRI available, all of which were adjudicated as atypical for MS. This was due to the absence of brain lesions in three participants (all presenting with ON), the presence of large, confluent and ill-defined lesions in three participants, the presence of an ill-defined lesion with prominent cortical involvement in one participant, the presence of a large ill-defined pontine lesion in two participants, and the presence of exclusively small, non-specific lesions in two participants (Figure 2(c)–(j)). In contrast, 44 of the 48 (92%) MOG-IgG negative participants had typical MS features on their presenting MRI (p < 0.0001). The four MOG-IgG negative patients with atypical baseline MRI included three children with at least one large, atypical lesion, but in the presence of multiple typical MS lesions. One child had bilateral ill-defined cerebellar peduncle lesions, bilateral T2 hyperintensities of the corticospinal tracts, and scattered smaller ill-defined supra-tentorial lesions.
Compared to lesions in MOG-IgG negative participants, lesions in MOG-IgG positive participants were less likely to have exclusively well-defined margins, less likely to be oriented perpendicularly to the major axis of the corpus callosum, and less likely to enhance following gadolinium administration (Table 1).
The presence of CSF-OCBs was assessed in 42/66 participants; they were positive in only 2/8 (25%) MOG-IgG positive participants, in contrast to most MOG-IgG negative participants (30/36, 86%, p = 0.0027).
Evolution of MOG-IgG status
Serial serum samples were obtained in 62 participants over a median of 4.98 years (IQR = 2.03–7.44, Figure 3). None of the initially seronegative participants converted to seropositive in follow-up samples. Among the 11 of these 62 who were MOG-IgG positive at presentation, 5 persisted positive in all follow-up samples (median follow-up = 6.08 years, IQR = 2.66–8.19), 2 fluctuated between MOG-IgG positive and negative status (median follow-up = 6.97 and 7.44 years), and 4 converted to seronegative (median time to conversion = 1.6 years (IQR = 0.9–2.9), median follow-up = 6.18 years (IQR = 4.76–7.33)). The patient who had an initial borderline MOG-IgG result, then found to be MOG-IgG positive at 3 months, converted to MOG-IgG negative at 1 year, and remained seronegative for the subsequent 5 years of follow-up. While three of the MOG-IgG positive participants were treated with DMT (glatiramer acetate, interferon-beta, and cyclophosphamide) at some point during follow-up, in all cases, DMT was initiated subsequently to collection of the first seronegative sample.
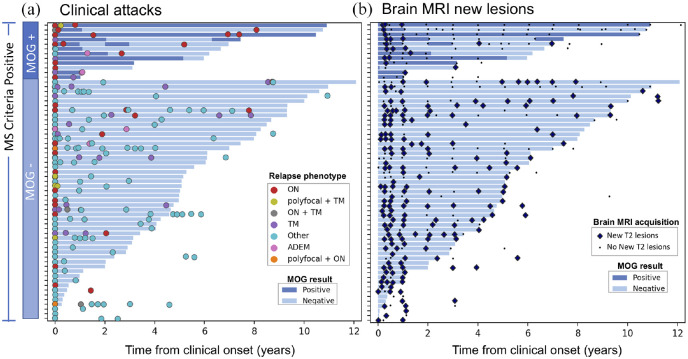
Figure 3.
Clinical and imaging disease course. Each bar indicates the serological follow-up of an individual participant, with dark and light blue colors indicating seropositive and seronegative status, respectively. (a) Colored circles correspond to clinical relapses. Participants initially MOG-IgG positive show a preponderance of episodes of monofocal ON. (b) Black dots indicate the time of acquisition of follow-up brain MRI scans with no new T2 lesions, while the large blue diamonds indicate the follow-up brain MRI with detection of new T2 lesions. New lesions were detected across both seropositive and negative participants, with most MOG-IgG positive participants showing new lesions at the earliest follow-up timepoints, and relatively few subsequently.
Clinical course according to MOG-IgG status
Among the 63 participants with at least 6 months of clinical and MRI follow-up, the median time to the second clinical attack and the ARR in the first 2 years did not differ between MOG-IgG positive (n = 12) and negative participants (n = 51) (Table 2). The two groups differed in relapse phenotype, with MOG-IgG positive participants showing a clear preponderance of episodes of isolated ON, and less frequently mono/polyfocal TM or ADEM. Conversely, most attacks in MOG-IgG negative participants had phenotypes distinct from ON, TM or ADEM (Figure 3(a)).
Table 2.
Clinical and MRI disease activity in participants with at least 6 months of clinical and MRI follow-up.
| MOG-IgG positive (n = 12) | MOG-IgG negative (n = 51) | SD | p-value | |
|---|---|---|---|---|
| Years of clinical follow-up (median (IQR)) | 8.14 (6.19 to 9.80) | 6.11 (4.06 to 9.25) | 0.43 | 0.096 |
| Ever treated with DMT (n/N %) a | 3/12 (25%) | 42/51 (82%) | −0.89 | 0.00028 |
| Clinical relapses (n/N %) | 7/12 (58%) | 36/51 (71%) | −0.26 | 0.58 |
| Years from onset to second clinical attack (when occurred) (median (IQR)) | 0.74 (0.29 to 1.20) | 0.90 (0.43 to 1.97) | −0.18 | 0.41 |
| ARR in the first 2 years (median (IQR)) b | 1.00 (0.50 to 1.00) | 1.00 (0.50 to 1.25) | −0.079 | 0.18 |
| EDSS at 2 years (median (IQR)) c | 2 (1 to 3) | 1 (0 to 1.50) | 0.25 | 0.024 |
| Years from previous relapse to 2 years EDSS assessment | 1.25 (0.82 to 1.88) | 0.84 (0.37 to 1.43) | 0.47 | 0.044 |
| Visual FSS at 2 years (median (IQR)) | 2 (0.25 to 3) | 0 (0 to 1) | 0.38 | 0.0034 |
| New brain T2 lesions (n/N %) | 11/12 (92%) | 50/51 (98%) | −0.29 | 0.35 |
| Years from onset to first new T2 lesion (when occurred) (median (IQR)) | 0.34 (0.29 to 1.14) | 0.31 (0.26 to 0.58) | 0.21 | 0.51 |
| Complete lesion resolution (n/N %) d | 1/8 (13%) | 0/46 (0%) | 0.53 | 0.15 |
| Years from first to last MRI scan (median (IQR)) | 5.22 (3.78 to 9.34) | 5.02 (1.10 to 6.36) | 0.44 | 0.11 |
| Most recent MRI features | ||||
| Lesions present (n/N %) | 9/12 (75%) | 51/51 (100%) | −0.82 | 0.0055 |
| Total brain lesions count (median (IQR)) e | 4.5 (4 to 12) | >15 (11 to >15) | −1.2 | 0.00092 |
| ⩾ 1 Periventricular lesions (n/N %) e | 5/9 (56%) | 50/51 (98%) | −1.2 | 0.0012 |
| ⩾ 1 Lesion perpendicular to the major axis of the corpus callosum (n/N %) e | 3/9 (33%) | 43/51 (84%) | −1.2 | 0.0033 |
| ⩾ 1 Infratentorial lesions (n/N %) e | 5/9 (56%) | 41/51 (80%) | −0.55 | 0.19 |
| ⩾ 1 Juxtacortical lesions (n/N %) e | 7/9 (78%) | 43/51 (84%) | −0.17 | 0.64 |
| ⩾ 1 T1 hypointense lesions (n/N %) e | 4/9 (44%) | 46/51 (90%) | −1.2 | 0.0042 |
| Total T2 lesion volume at last MRI scan (cc) (median (IQR)) e | 0.40 (0.06 to 1.18) | 4.31 (2.64 to 10.26) | −0.84 | 0.00044 |
| Total T1 lesion volume at last MRI scan (cc) (median (IQR)) e | 0.018 (0.011 to 0.15) | 1.35 (0.67 to 4.70) | −0.79 | 0.00012 |
| T2 lesion volume change from baseline to last follow-up (cc) (median (IQR)) f | −0.14 (−3.04 to −0.020) | 0.33 (−0.72 to 2.18) | −0.54 | 0.21 |
ARR: annualized relapse rate; DMT: disease-modifying therapy; EDSS: Expanded Disability Status Scale; FSS: Functional System Score; SD: standardized difference; IQR: interquartile range.
In addition to MS-specific DMTs, cyclophosphamide was used in one MOG-positive and three MOG-negative participants. One MOG-negative participant was on therapy with minocycline for 1 year during the study period.
The ARR in the first 2 years was assessed in the 57 participants (9 MOG-IgG positive and 48 MOG-IgG negative) with at least 2 years of clinical follow-up.
EDSS at 2 years was available for 52 participants (10 MOG-IgG positive and 42 MOG-IgG negative), and visual FSS in 50 participants (10 MOG-IgG positive and 40 MOG-IgG negative).
Lesion resolution was evaluated among participants with brain lesions in the baseline scans. Five of the 52 MOG-IgG negative with observation >6 months did not have an MRI scan within 45 days from onset and could not be evaluated for resolution of the baseline lesions.
The number of lesions, their location, and total lesion volume were computed only among subjects with brain lesions in the last MRI scan. Measurement of T2 lesion volume at last follow-up was available for 9 MOG-IgG positive and 47 MOG-IgG negative, while for T1, lesion volume was available for 6 MOG-IgG positive and 33 MOG-IgG negative.
The analysis of T2 lesion volume change from baseline to last follow-up was computed with the inclusion of participants with and without lesions at baseline and last FU scans, but restricted to those with lesion volume measurement available at both baseline and last follow-up. These included 6 MOG-IgG positive and 28 MOG-IgG negative.
New lesions on brain MRI were detected in nearly all participants regardless of MOG-IgG status (Figure 3(b)). New lesions in MOG-IgG positive participants were commonly detected within a few months from the presenting episode. Of the participants with MRI follow-up greater than 1 year, only 3/13 (27%) MOG-IgG positive showed new T2 lesions beyond the first year from presentation, versus 30/34 (88%) of the MOG-IgG negative ones (p = 0.00028).
Complete resolution of all brain lesions was uncommon, with lesions present at the most recent brain MRI (acquired a median (IQR) of 4.99 (1.11–7.00) years from presentation) in 9/12 (75%) MOG-IgG positive and in all 51 MOG-IgG negative participants (Table 2). Nonetheless, the most recent MRI scans of the nine MOG-IgG positive participants with persistent brain T2 lesions showed fewer lesions compared to MOG-IgG negative participants, contributing to a smaller total T2 lesion volume (Table 2).
T1 hypointense lesions at most recent MRI were detected in fewer MOG-IgG positive participants (4/9; 44%) compared to 46/51 (90%) MOG-IgG negative (p = 0.0042), associated with a reduced total T1 lesion volume. Seropositive participants also exhibited less frequent lesions periventricular or perpendicular to the major axis of the corpus callosum (Table 2). Of the four MOG-IgG negative participants with presenting imaging features atypical for MS, three developed typical new T2 lesions and evolved into a more classical MS imaging profile over time, while one continued to appear atypical at his most recent MRI evaluation (performed 8 years from presentation).
At 2 years from presentation, all participants had low EDSS scores, although EDSS scores were greater in the MOG-IgG positive group (Table 2), mainly driven by deficits in visual function: 7/11 (63%) MOG-IgG positive participants had visual deficits, which were moderate (functional system score (FSS) = 3) in four cases and less severe (FSS = 1 or 2) in three cases. The visual function at 2 years was not recorded in one participant. The four participants without visual impairment had either no neurological deficits (n = 2), pyramidal (n = 1, FSS = 1) or bladder dysfunction (n = 1, FSS = 1). In contrast, visual deficits were present in 15/41 (37%) MOG-IgG negative participants evaluated for visual function at 2 years, reaching moderate severity (FSS = 3) in only one case. The sensitivity analysis at 4 years from presentation showed results similar to those at 2 years for ARR (median (IQR) = 0.5 (0.25–0.5) in MOG-positive (n = 10) vs 0.5 (0.5–0.75) in MOG-negative (n = 39)) and EDSS (median (IQR) = 2.00 (1.50–3.00) in MOG-positive (n = 10) vs 1.50 (0.00–1.50) in MOG-negative (n = 28)).
Discussion
We retrospectively ascertained the presence of MOG-IgG in archived serum samples from children meeting MS diagnostic criteria 11 identified as part of a prospective cohort of patients with CNS demyelination, well before MOGAD was recognized. We found MOG-IgG at presentation in 18% of these participants, a frequency substantially higher than reported in adult MS cohorts, where MOG seropositivity is typically below 1% and does not exceed 5%, and the vast majority of such patients have low or borderline titres.6,7,25–27 Our finding likely reflect, at least in part, our study design with assessment of MOG-IgG results from samples obtained at the time of presentation, therefore including initially MOG-positive children who subsequently converted to seronegative. In addition, it could also reflect the relatively high pre-test probability of MOG-IgG among children with acquired CNS demyelination, compared to adults, 27 and highlights the importance of a critical evaluation of young patients whose MS diagnosis was conferred before recognition of MOGAD.
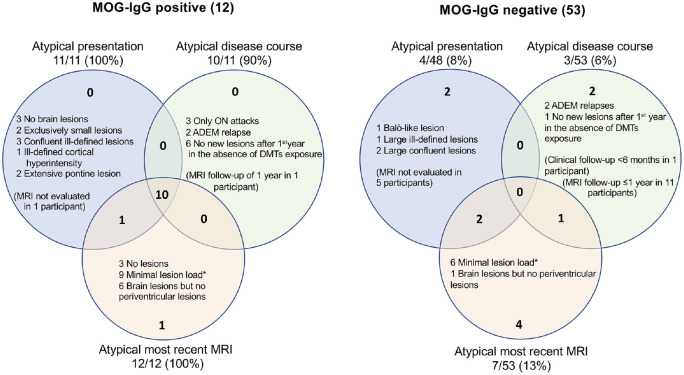
All the MOG-IgG positive children meeting the 2017 McDonald MS diagnostic criteria in our cohort presented features atypical of MS (Figure 4), which could serve as “red flags” prompting consideration of MOGAD (Table 3). In particular, MOG-IgG positive patients tended not to demonstrate the classic ovoid brain MRI lesions of MS, and their initial lesions tended, over time, to dramatically reduce in size, yielding a very low cumulative lesion volume. While substantial decrease in lesion volume has been previously described as a common manifestation in particularly young MS patients (i.e. those presenting before the age of 11 years), 28 our findings suggest such prior reports may have overestimated this association due to inclusion of children with MOGAD masquerading as MS. Similarly, while prior studies suggested lower frequency of CSF-OCBs in younger children with MS, 29 OCBs were detected in 85% of MOG-IgG negative children in our study, a proportion similar to adult-onset MS. 30 Most MOG-IgG positive participants in our study were younger than 11 years at presentation, reinforcing the caution recommended when applying the 2017 McDonald criteria to this age group. 11 The majority of features of MOG-IgG positive children observed in this study are aligned with what expected in typical pediatric MOGAD. 9 Exceptions are a low proportion of children presenting with ADEM, and an overall higher frequency of clinical relapses and of silent new brain MRI lesions,17,31 attributable to the selection of children meeting MS diagnostic criteria for the inclusion to this study.
Figure 4.
Summary of features considered atypical for MS.
Features considered atypical for MS, in both MOG-IgG positive and MOG-IgG negative participants meeting MS diagnostic criteria. For each serologically defined subgroup, the atypical features are shown at presentation, during the disease course, and at time of their most recent brain MRI scan. As shown in the intersections of the two Venn diagrams, the majority (10/12) of MOG-IgG positive participants exhibited atypical features from initial presentation and throughout their disease course including their most recent MRI, while none (0/53) of the MOG-IgG negative MS patients exhibited any of these atypical features throughout their course.
*Minimal lesion load at last MRI evaluation was defined as total T2 lesion volume >0 and <1cc.
Table 3.
Red flags prompting evaluation of MOG-IgG antibody status in children meeting MS diagnostic criteria.
| Sensitivity (95% CI) | Specificity (95% CI) | |
|---|---|---|
| Presenting features | ||
| Age at onset <11 years | 0.92 (0.62–1.0) | 0.89 (0.77–0.96) |
| Normal brain MRI or exclusively small non-specific lesions | 0.45 (0.17–0.77) | 1.00 |
| Large brain lesions with ill-defined borders | 0.55 (0.23–0.83) | 0.92 (0.80–0.98) |
| Diffuse confluent bilateral lesion pattern | 0.27 (0.6–0.61) | 1.00 |
| Disease course | ||
| Relapses restricted to the optic nerve | 0.25 (0.05–0.57) | 0.98 (0.90–1.00) |
| Complete or near complete resolution of initial MRI T2 lesions a | 0.75 (0.35–0.97) | 0.89 (0.77–0.96) |
| Absence of new T2 lesions after 1 year from presentation (in patients not treated with high efficacy DMTs) | 0.73 (0.39–0.94) | 0.88 (0.73–0.97) |
Defined as normal brain MRI or total T2 lesion volume >0 and <1cc at last MRI evaluation.
Only 3 MOG-IgG positive participants fulfilled 2017 MS criteria at baseline (two cases meeting DIT thanks to presence of CSF-OCBs and one due to simultaneous enhancing and non-enhancing lesions), while all of them were diagnosed with MS by meeting criteria over follow-up. Notably, in one-third of MOG-IgG positive participants, development of new asymptomatic brain lesions was the only evidence of further disease activity, although they were typically detected within the first months from presentation and contributed to minimal increase of total T2 lesion volume over time. Since only 25% MOG-IgG positive patients were treated with MS DMTs at any point (compared to 82% MOG-IgG negative patients), the relatively limited cumulative lesion burden in seropositive patients is unlikely be attributed solely to therapeutic disease suppression.
In our prior publication on a larger MOGAD cohort, we observed an overall lower chance for future relapses in patients who converted to seronegative, although seroconversion did not entirely exclude the possibility of further attacks. 9 Given the small number of MOG-IgG positive participants, we did not investigate the relationship between MOG-IgG titers at presentation, or their evolution over time, on the probability to experience further disease activity in this study.
Our imaging findings generally support prior studies suggesting that atypical MRI features at presentation can provide a reasonable index of suspicion for MOGAD.32,33 They also strongly support the diagnosis of MOGAD in all our MOG-IgG seropositive participants, including in the only patient with initial borderline-positive results, where particular caution should be applied to rule out false-positivity. 27 In adult MS cohorts, low or borderline MOG-IgG titers are frequently considered as “false positives,” while higher titers are thought to be more convincingly indicative of MOGAD. 27 Our findings agree with the concept of “true positives,” given that our seropositive patients followed a disease course consistent with MOGAD rather than MS.
The observations that MOG-IgG titers often decline rapidly after clinical onset,3,9,10,34 and that MOGAD patients may have prolonged periods of undetectable antibodies between relapses3,9,10 confound the ability to distinguish between relapsing MOGAD and MS when testing is performed months after onset. Hence, in children who do not have available testing at onset, atypical brain MRI features should prompt consideration of possible “missed” MOGAD. The exposure to steroid treatment could potentially contribute to a faster reduction of antibody levels in the weeks following treatment initiation, possibly increasing the yield of false negative results over time. Although we cannot entirely exclude this occurrence in our study cohort, the acquisition of samples in close proximity to clinical presentation, and the negative history for steroid exposure prior sample acquisition in over half of the seronegative participants, limit the extent in which this event could have affected our results.
A strength of our study is the comprehensive prospective clinical and MRI characterization of our cohort, and our ability to evaluate the presence of MOG-IgG antibodies in samples obtained in proximity to clinical presentation. Limitations include relatively small sample size and the acquisition of spine MRI and CSF analysis only in a subset of participants, according to clinical indication. While our research protocol did not direct the timing or selection of DMTs, we did not feel that therapy affected our ability to identify the atypical clinical features of MOG-IgG positive patients, as 9/12 never received DMT. Other features, such as serology evidence of prior Epstein–Barr virus (EBV) infection, 35 could contribute in differentiating pediatric MS from MOGAD, 36 and should be object of future studies.
In conclusion, a clinically meaningful minority of young children meeting MS diagnostic criteria have MOGAD. While high-positive MOG-IgG at baseline supports a MOGAD diagnosis, the waning of MOG-IgG titers over time can lead to a missed diagnostic opportunity for MOGAD. When serologic testing is not obtained at baseline, clinical and MRI features atypical for MS should still raise suspicion of MOGAD.
Acknowledgments
The authors acknowledge the invaluable assistance of Julien Sirois, MSc (Montreal Neurological Institute, McGill University, Montreal, Quebec, Canada), and the Experimental Therapeutics Program team members at the Montreal Neurological Institute for judicious handling of patient and control samples.
Footnotes
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: G.F., M.W., J.O.M., D.A.C., and G.L. report no disclosures. P.W. and the University of Oxford hold patents for antibody assays and receive royalties. He has received speaker honoraria from Alexion, Roche, and UBC. He is co-director of Oxford Autoimmune Diagnostic Laboratory where MOG-IgG testing is performed. R.A.B. is the founder and president of ShadowLab Research Inc. and has provided advisory services or received personal compensation for consulting from NeuroRx Research, Biogen Idec, and the Population Council. D.L.A. reports personal fees for consulting from Acorda, Biogen, Celgene, F. Hoffmann-La Roche, Frequency Therapeutics, GeNeuro, MedImmune, Merck-Serono, Novartis, and Sanofi-Aventis; grants from Biogen, Immunotec, and Novartis; and an equity interest in NeuroRx Research. R.A.M. receives research funding from: CIHR, Research Manitoba, Multiple Sclerosis Society of Canada, Multiple Sclerosis Scientific Foundation, Crohn’s and Colitis Canada, National Multiple Sclerosis Society, CMSC, and US Department of Defense. She is supported by the Waugh Family Chair in Multiple Sclerosis. She is a co-investigator on studies funded by Biogen Idec and Roche. E.A.Y. reports personal fees for consulting from Biogen, F. Hoffmann-La Roche, and Alexion; grants from Biogen, Ontario Institute for Regenerative Medicine, Stem Cell Network, Centre for Brain and Mental Health, The Peterson Foundation, National MS Society, MS Scientific Foundation, National Institutes of Health, Canadian Institutes of Health Research, and Consortium of MS Centers. B.B. receives funding from the Multiple Sclerosis Scientific Foundation, and the National Multiple Sclerosis Society. She has received consultancy fees from Novartis, UCB pharmaceuticals, and Medscape. She serves as a non-remunerated advisor on clinical trial design for Novartis, Biogen, Teva Neuroscience, and Sanofi-Aventis. A.B.-O. participated as a speaker in meetings sponsored by and received consulting fees and/or grant support from Janssen/Actelion, Atara Biotherapeutics, Biogen Idec, Celgene/Receptos, Roche/Genentech, MedImmune, Merck/EMD Serono, Novartis, and Sanofi-Genzyme.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was funded by the Multiple Sclerosis Scientific Research Foundation (Drs E.A.Y., R.A.M., D.L.A., B.B., and A.B.-O.). Dr G.F. was funded by the Clinical Research Training Scholarship in Multiple Sclerosis from the American Academy of Neurology. Dr.s P.W. and M.W. are supported by NHS National Specialised Commissioning Group for Neuromyelitis Optica, UK. Dr R.A.M. is supported by the Waugh Family Chair in Multiple Sclerosis. Dr B.B. is funded by the National MS Society, NIH, and the Multiple Sclerosis Society of Canada. Dr. A.B.-O. is funded by the NIH, Immune Tolerance Network (ITN), National MS Society, and the Multiple Sclerosis Society of Canada.
ORCID iDs: Giulia Fadda  https://orcid.org/0000-0001-9658-815X
https://orcid.org/0000-0001-9658-815X
E Ann Yeh  https://orcid.org/0000-0002-5393-7417
https://orcid.org/0000-0002-5393-7417
Ruth Ann Marrie  https://orcid.org/0000-0002-1855-5595
https://orcid.org/0000-0002-1855-5595
Douglas L Arnold  https://orcid.org/0000-0003-4266-0106
https://orcid.org/0000-0003-4266-0106
Contributor Information
Giulia Fadda, Center for Neuroinflammation and Neurotherapeutics, and Multiple Sclerosis Division, Department of Neurology, Perelman School of Medicine, University of Pennsylvania, Philadelphia, PA, USA/Montreal Neurological Institute, McGill University, Montreal, QC, Canada.
Patrick Waters, Oxford Autoimmune Neurology Group, Nuffield Department of Clinical Neurosciences, University of Oxford, Oxford, UK.
Mark Woodhall, Oxford Autoimmune Neurology Group, Nuffield Department of Clinical Neurosciences, University of Oxford, Oxford, UK.
Robert A Brown, ShadowLab Research Inc., Toronto, ON, Canada.
Julia O’Mahony, Institute of Health Policy, Management and Evaluation, University of Toronto and The Hospital for Sick Children, Toronto, ON, Canada.
Denise A Castro, Department of Diagnostic Imaging, Neurosciences and Mental Health, SickKids Research Institute, Toronto, ON, Canada/Department of Diagnostic Radiology, Queen’s University, Kingston, ON, Canada.
Giulia Longoni, Department of Pediatrics (Neurology), The Hospital for Sick Children, Division of Neuroscience and Mental Health, SickKids Research Institute, University of Toronto, Toronto, ON, Canada.
E Ann Yeh, Department of Pediatrics (Neurology), The Hospital for Sick Children, Division of Neuroscience and Mental Health, SickKids Research Institute, University of Toronto, Toronto, ON, Canada.
Ruth Ann Marrie, Departments of Internal Medicine and Community Health Sciences, Max Rady College of Medicine, Rady Faculty of Health Sciences, University of Manitoba, Winnipeg, MB, Canada.
Douglas L Arnold, Montreal Neurological Institute, McGill University, Montreal, QC, Canada.
Brenda Banwell, Division of Child Neurology, Department of Neurology, The Children’s Hospital of Philadelphia, Perelman School of Medicine, University of Pennsylvania, Philadelphia, PA, USA.
Amit Bar-Or, Center for Neuroinflammation and Neurotherapeutics, and Multiple Sclerosis Division, Department of Neurology, Perelman School of Medicine, University of Pennsylvania, Philadelphia, PA, USA.
References
- 1. Ketelslegers IA, Van Pelt DE, Bryde S, et al. Anti-MOG antibodies plead against MS diagnosis in an acquired demyelinating syndromes cohort. Mult Scler 2015; 21(12): 1513–1520. [DOI] [PubMed] [Google Scholar]
- 2. Hacohen Y, Absoud M, Deiva K, et al. Myelin oligodendrocyte glycoprotein antibodies are associated with a non-MS course in children. Neurol Neuroimmunol Neuroinflamm 2015; 2(2): e81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hennes EM, Baumann M, Schanda K, et al. Prognostic relevance of MOG antibodies in children with an acquired demyelinating syndrome. Neurology 2017; 89: 900–908. [DOI] [PubMed] [Google Scholar]
- 4. Jurynczyk M, Geraldes R, Probert F, et al. Distinct brain imaging characteristics of autoantibody-mediated CNS conditions and multiple sclerosis. Brain 2017; 140: 617–627. [DOI] [PubMed] [Google Scholar]
- 5. Hacohen Y, Wong YY, Lechner C, et al. Disease course and treatment responses in children with relapsing myelin oligodendrocyte glycoprotein antibody-associated disease. JAMA Neurol 2018; 75: 478–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cobo-Calvo A, d’Indy H, Ruiz A, et al. Frequency of myelin oligodendrocyte glycoprotein antibody in multiple sclerosis: A multicenter cross-sectional study. Neurol Neuroimmunol Neuroinflamm 2020; 7(2): 649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mariotto S, Ferrari S, Monaco S, et al. Clinical spectrum and IgG subclass analysis of anti-myelin oligodendrocyte glycoprotein antibody-associated syndromes: A multicenter study. J Neurol 2017; 264(12): 2420–2430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fadda G, Armangue T, Hacohen Y, et al. Paediatric multiple sclerosis and antibody-associated demyelination: Clinical, imaging, and biological considerations for diagnosis and care. Lancet Neurol 2021; 20(2): 136–149. [DOI] [PubMed] [Google Scholar]
- 9. Waters P, Fadda G, Woodhall M, et al. Serial anti-myelin oligodendrocyte glycoprotein antibody analyses and outcomes in children with demyelinating syndromes. JAMA Neurol 2019; 77: 82–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jarius S, Ruprecht K, Kleiter I, et al. MOG-IgG in NMO and related disorders: A multicenter study of 50 patients—Part 1: Frequency, syndrome specificity, influence of disease activity, long-term course, association with AQP4-IgG, and origin. J Neuroinflammation 2016; 13: 279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Thompson AJ, Banwell BL, Barkhof F, et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol 2018; 17: 162–173. [DOI] [PubMed] [Google Scholar]
- 12. Banwell B, Bar-Or A, Arnold DL, et al. Clinical, environmental, and genetic determinants of multiple sclerosis in children with acute demyelination: A prospective national cohort study. Lancet Neurol 2011; 10(5): 436–445. [DOI] [PubMed] [Google Scholar]
- 13. Banwell B, Krupp L, Kennedy J, et al. Clinical features and viral serologies in children with multiple sclerosis: A multinational observational study. Lancet Neurol 2007; 6(9): 773–781. [DOI] [PubMed] [Google Scholar]
- 14. Krupp LB, Banwell B, Tenembaum S, et al. Consensus definitions proposed for pediatric multiple sclerosis and related disorders. Neurology 2007; 68: S7–S12. [DOI] [PubMed] [Google Scholar]
- 15. Verhey LH, Branson HM, Shroff MM, et al. MRI parameters for prediction of multiple sclerosis diagnosis in children with acute CNS demyelination: A prospective national cohort study. Lancet Neurol 2011; 10(12): 1065–1073. [DOI] [PubMed] [Google Scholar]
- 16. O’Mahony J, Marrie RA, Laporte A, et al. Recovery from central nervous system acute demyelination in children. Pediatrics 2015; 136(1): e115–e123. [DOI] [PubMed] [Google Scholar]
- 17. Fadda G, Banwell B, Waters P, et al. Silent new brain MRI lesions in children with MOG-antibody associated disease. Ann Neurol 2021; 89(2): 408–413. [DOI] [PubMed] [Google Scholar]
- 18. Waters P, Woodhall M, O’Connor KC, et al. MOG cell-based assay detects non-MS patients with inflammatory neurologic disease. Neurol Neuroimmunol Neuroinflamm 2015; 2(3): e89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Waters P, Reindl M, Saiz A, et al. Multicentre comparison of a diagnostic assay: Aquaporin-4 antibodies in neuromyelitis optica. J Neurol Neurosurg Psychiatry 2016; 87(9): 1005–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fadda G, Brown RA, Longoni G, et al. MRI and laboratory features and the performance of international criteria in the diagnosis of multiple sclerosis in children and adolescents: A prospective cohort study. Lancet Child Adolesc Health 2018; 2(3): 191–204. [DOI] [PubMed] [Google Scholar]
- 21. Filippi M, Preziosa P, Banwell BL, et al. Assessment of lesions on magnetic resonance imaging in multiple sclerosis: Practical guidelines. Brain 2019; 142: 1858–1875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Francis SJ. Automatic lesion identification in MRI of multiple sclerosis patients. PhD Thesis, McGill University, Montreal, QC, Canada, 2004. [Google Scholar]
- 23. Yang D, Dalton JE. A unified approach to measuring the effect size between two groups using SAS®. SAS Global Forum 2012, http://citeseerx.ist.psu.edu/viewdoc/summary?doi=10.1.1.456.9301
- 24. Mackinnon A. A spreadsheet for the calculation of comprehensive statistics for the assessment of diagnostic tests and inter-rater agreement. Comput Biol Med 2000; 30(3): 127–134. [DOI] [PubMed] [Google Scholar]
- 25. Spadaro M, Gerdes LA, Krumbholz M, et al. Autoantibodies to MOG in a distinct subgroup of adult multiple sclerosis. Neurol Neuroimmunol Neuroinflamm 2016; 3(5): e257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Waters PJ, Komorowski L, Woodhall M, et al. A multicenter comparison of MOG-IgG cell-based assays. Neurology 2019; 92: e1250–e1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sechi E, Buciuc M, Pittock SJ, et al. Positive predictive value of myelin oligodendrocyte glycoprotein autoantibody testing. JAMA Neurol 2021; 78: 741–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Chabas D, Castillo-Trivino T, Mowry EM, et al. Vanishing MS T2-bright lesions before puberty: A distinct MRI phenotype? Neurology 2008; 71: 1090–1093. [DOI] [PubMed] [Google Scholar]
- 29. Chabas D, Ness J, Belman A, et al. Younger children with MS have a distinct CSF inflammatory profile at disease onset. Neurology 2010; 74: 399–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Dobson R, Ramagopalan S, Davis A, et al. Cerebrospinal fluid oligoclonal bands in multiple sclerosis and clinically isolated syndromes: A meta-analysis of prevalence, prognosis and effect of latitude. J Neurol Neurosurg Psychiatry 2013; 84(8): 909–914. [DOI] [PubMed] [Google Scholar]
- 31. Camera V, Holm-Mercer L, Ali AAH, et al. Frequency of new silent MRI lesions in myelin oligodendrocyte glycoprotein antibody disease and aquaporin-4 antibody neuromyelitis optica spectrum disorder. JAMA Netw Open 2021; 4: e2137833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hacohen Y, Mankad K, Chong WK, et al. Diagnostic algorithm for relapsing acquired demyelinating syndromes in children. Neurology 2017; 89: 269–278. [DOI] [PubMed] [Google Scholar]
- 33. Baumann M, Grams A, Djurdjevic T, et al. MRI of the first event in pediatric acquired demyelinating syndromes with antibodies to myelin oligodendrocyte glycoprotein. J Neurol 2018; 265(4): 845–855. [DOI] [PubMed] [Google Scholar]
- 34. Hyun JW, Woodhall MR, Kim SH, et al. Longitudinal analysis of myelin oligodendrocyte glycoprotein antibodies in CNS inflammatory diseases. J Neurol Neurosurg Psychiatry 2017; 88(10): 811–817. [DOI] [PubMed] [Google Scholar]
- 35. Bjornevik K, Cortese M, Healy BC, et al. Longitudinal analysis reveals high prevalence of Epstein–Barr virus associated with multiple sclerosis. Science 2022; 375: 296–301. [DOI] [PubMed] [Google Scholar]
- 36. Nourbakhsh B, Cordano C, Asteggiano C, et al. Multiple sclerosis is rare in Epstein–Barr virus-seronegative children with central nervous system inflammatory demyelination. Ann Neurol 2021; 89(6): 1234–1239. [DOI] [PMC free article] [PubMed] [Google Scholar]