Abstract
An Acinetobacter sp. genetic screen was used to probe structure-function relationships in vanillate demethylase, a two-component monooxygenase. Mutants with null, leaky, and heat-sensitive phenotypes were isolated. Missense mutations tended to be clustered in specific regions, most of which make known contributions to catalytic activity. The vanillate analogs m-anisate, m-toluate, and 4-hydroxy-3,5-dimethylbenzoate are substrates of the enzyme and weakly inhibit the metabolism of vanillate by wild-type Acinetobacter bacteria. PCR mutagenesis of vanAB, followed by selection for strains unable to metabolize vanillate, yielded mutant organisms in which vanillate metabolism is more strongly inhibited by the vanillate analogs. Thus, the procedure opens for investigation amino acid residues that may contribute to the binding of either vanillate or its chemical analogs to wild-type and mutant vanillate demethylases. Selection of phenotypic revertants following PCR mutagenesis gave an indication of the extent to which amino acid substitutions can be tolerated at specified positions. In some cases, only true reversion to the original amino acid was observed. In other examples, a range of amino acid substitutions was tolerated. In one instance, phenotypic reversion failed to produce a protein with the original wild-type sequence. In this example, constraints favoring certain nucleotide substitutions appear to be imposed at the DNA level.
Vanillate demethylase is a two-component enzyme classified as a IA oxygenase (25, 28). It comprises a reductase containing both a flavin and a [2Fe-2S] redox center and an oxygenase containing, in addition to a substrate-binding site, an iron-binding site and a Rieske-type [2Fe-2S] cluster. Little is known about how structure influences function in vanillate demethylase. Demethylases involved in the metabolism of p-anisate in Pseudomonas putida (1) and vanillate in P. testosteroni (3, 35) and P. fluorescens (5) are known to be air sensitive and unstable. The vanillate demethylases from P. testosteroni and P. fluorescens are mixed-function oxygenases and have a wide substrate specificity: m-anisate, p-anisate, m-toluate, 3,4,5-trimethoxybenzoate, and 3,4-dimethoxybenzoate were oxidized by vanillate-induced cells (5, 36). As described here, the Acinetobacter vanillate demethylase also possesses a broad substrate range.
Inferences can be drawn about the mechanism of vanillate demethylase from results obtained with the evolutionarily related enzyme phthalate dioxygenase (6). In this enzyme, electrons for hydroxylation flow from NADH to flavin mononucleotide to [2Fe-2S] in the reductase and from the Rieske-type [2Fe-2S] center to the Fe2+ site in the oxygenase, where oxygen binding and hydroxylation occur (9, 10, 33, 40). As recently shown for the naphthalene dioxygenase, another member of this group of aromatic dioxygenases, Fe1 of the Rieske [2Fe-2S] center is coordinated by two cysteinyl residues and Fe2 is coordinated by two histidyl residues (14, 15, 18). The iron atom at the active site is coordinated by two histidyl residues and one aspartyl residue (18). Aspartate 205 in the catalytic domain of this enzyme has been shown to be essential for activity (31). The C-terminal regions of the α subunit of the oxygenase component of 2-nitrotoluene 2,3-dioxygenase (30) and biphenyl dioxygenase (26) were shown to be responsible for substrate specificity.
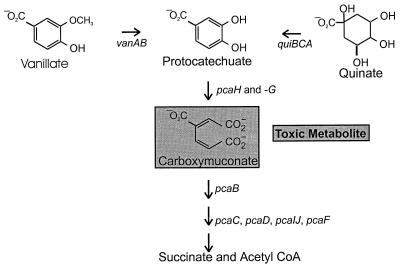
In Acinetobacter strain ADP1 (37) and in different Pseudomonas strains (2, 34, 36), protocatechuate formed by the demethylase undergoes further oxygenative metabolism to carboxymuconate. As shown in Fig. 1, Acinetobacter mutants blocked in carboxymuconate metabolism do not grow in the presence of either vanillate or protocatechuate, thus creating a condition allowing selection of strains carrying secondary mutations blocking expression of either vanillate demethylase (37) or protocatechuate oxygenase (8, 11). Genetic analysis has shown that vanillate demethylase is encoded by contiguous genes, vanA for the terminal oxygenase and vanB for the dioxygenase reductase. Acinetobacter VanA and VanB (37) share amino acid sequence identities of 67 to 77% and 44 to 46% with the respective proteins from Pseudomonas spp. (2, 34).
FIG. 1.
Selection of strains unable to express either vanillate demethylase or protocatechuate oxygenase. A block in pcaB causes accumulation of the toxic metabolite carboxymuconate (12, 37) from vanillate and prevents growth of cells in the presence of this compound. Selection for vanillate-resistant mutants yields strains blocked in either vanAB, structural genes for vanillate demethylase (37), or pcaHG, structural genes for protocatechuate 3,4-dioxygenase (8, 11). The former class of mutants grows in the presence of vanillate but not in the presence of protocatechuate. Protocatechuate itself is somewhat toxic (7), so quinate was used to select vanillate-defective recombinants in which the pcaB mutation has been replaced with wild-type DNA (37). CoA, coenzyme A.
PCR introduces nucleotide substitutions in the amplified DNA segment (4, 19, 22, 39, 41). Resulting amino acid substitutions causing defects in the encoded protein can indicate residues that contribute to protein function. Such analysis is augmented with enzymes like Acinetobacter vanillate demethylase because of the ease with which the organism integrates PCR fragments into its chromosome by natural transformation (8, 19–21). Since it is possible to select directly for strains with defects in vanillate demethylase (37), the combination of PCR mutagenesis and natural transformation offers special advantages for genetic analysis. The consequences of mutation can be observed directly at the phenotype level under conditions in which the mutant enzyme limits the rate of growth. Thus, it is possible to distinguish enzymes with temperature-sensitive or leaky properties from those with null mutations (8, 19–21). This is particularly important for analysis of an enzyme like vanillate demethylase, which is not amenable to analysis in cell extracts.
We present here the results of such an analysis of Acinetobacter vanillate demethylases with defects caused by amino acid substitution. We also describe mutant demethylases with apparent increased affinity for the substrate analogs 3,4-dimethoxybenzoate, m-anisate, m-toluate, and 4-hydroxy-3,5-dimethylbenzoate. The results allow identification of amino acid residues likely to be involved in substrate binding and increase understanding of how structure influences function in the enzyme.
MATERIALS AND METHODS
Organisms and culture conditions.
The mineral medium described by Juni and Janik (16), supplemented with 10 mM succinate, was routinely used for growth of Acinetobacter strains ADP1 and ADP230 in tubes on a shaker or on plates (solidified with 1.8% [wt/vol] agar) at 37°C. Where indicated, vanillate (3 or 1.5 mM) or quinate (3 mM) was used as the carbon and energy source. The structural analogs were added to medium to a final concentration of 3 mM.
Acinetobacter chromosomal DNA containing vanAB was cloned for overexpression after PCR amplification with Taq polymerase (Quiagen) using primers 5′-ATTGGATCGGTTTCTGGAGCAT-3′ and 5′-GTAGTGAATTCGTAACTCGGAGAG-3′. The latter primer anneals at the end of vanB and introduces an EcoRI site (underlined) into the primer sequence. The resulting PCR fragment was digested with BamHI and EcoRI, gel purified, and ligated into BamHI/EcoRI-digested pUC19. Transformants containing the resulting plasmid (pzR9200) in Escherichia coli JM109 were isolated by selection for ampicillin resistance and screening for expression of vanillate demethylase in the presence of isopropyl-β-d-thiogalactopyranoside (IPTG) induction on plates containing p-toluidine (32).
Induction of vanillate demethylase and measurement of vanillate demethylase activity in whole cells.
Vanillate demethylase was induced in Acinetobacter bacteria by growth of the cells from an overnight inoculum in 10 mM succinate supplemented with either vanillate or one of its chemical analogs at a concentration of 3 mM. After 6 h of incubation, cells were harvested, washed, and resuspended in potassium phosphate buffer (50 mM, pH 7) supplemented with 3 mM vanillate. Samples were taken every 30 min for a total of 3 h, and the remaining vanillate concentration was monitored by high-pressure liquid chromatography.
An overnight Luria-Bertani medium culture of E. coli JM109(pzR9200), which expresses the structural genes of vanillate demethylase, was diluted into fresh Luria-Bertani medium (50 ml), and the cultures were grown for about 2 h at 37°C until they achieved turbidity corresponding to an A600 of 0.5. IPTG was added to a final concentration of 0.5 mM, and the mixture was incubated for 2 h. At a culture turbidity corresponding to an A600 of 1.0, the substrates were added directly to the medium to achieve a final concentration of 5 mM. After 10 to 12 h of incubation, the contents of the flask were centrifuged (10,000 × g at 4°C). The supernatant liquids were adjusted to pH 2 to 4 and extracted with ethyl acetate. The extracted material was dried over anhydrous MgSO4.
Analytical methods.
Chemical conversions by whole cells were monitored by a reverse-phase high-pressure liquid chromatography system using an LC Pump Model 300 from SSI (Scientific Systems, Inc.), a Shimadzu UV Spectrophotometric detector (model SPD-6A), and a Shimadzu Analyzer (Chromatopac C-R 313). Supernatant liquids from cultures were injected directly into a reverse-phase Nova Pak C18 column, eluted with water-methanol (5:1 vol/vol) at a flow rate of 1 ml/min, and monitored at a wavelength of 254 nm. Identification of m-hydroxybenzoate, isovanillate, 3-hydroxy-4,5-dimethoxybenzoate, 3-(hydroxymethy)benzoate,3-(hydroxymethyl)-4-methylbenzoate, and 3-(hydroxymethyl)-4-hydroxy-5-methylbenzoate was achieved with a Hewlett-Packard HP 5890 gas chromatograph and an HP 5971A mass spectrometer equipped with an HP5 column. Samples were derivatized prior to gas chromatography-mass spectroscopy analysis as follows: material was methylated with trimethylsilyldiazomethane (13), and any free hydroxyl groups were further protected with bis(trimethylsilyl)acetamide (29). 1H nuclear magnetic resonance (1H-NMR) spectra of 3-(hydroxymethyl)-4-methylbenzoate and 3-(hydroxymethyl)-4-hydroxy-5-methylbenzoate were recorded on a Bruker 300-MHz spectrometer at 24°C. Samples for 1H-NMR spectroscopy were purified by flash chromatography with ethyl acetate-hexanes (2:3, vol/vol).
Acinetobacter transformation.
Acinetobacter bacteria were transformed as previously described (19). A fresh overnight culture, grown in mineral medium with 10 mM succinate as the carbon and energy source, was diluted 25-fold and grown for 2 h at 37°C. About 600 ng of PCR-amplified DNA was added to 500 μl of the fresh culture, which was incubated for 3 h. Dilutions of the transformation mixture were plated directly onto selective medium or onto nonselective medium for determination of viable counts. For selection of spontaneous mutants and as a control, the same protocol was followed but without the addition of DNA.
PCR for transformation-facilitated mutagenesis.
Taq polymerase (Boehringer Mannheim) was used as indicated by the supplier. Mutagenesis of vanAB was performed using the primers Seq1 and Seq2, which were described in a previous study (37). PCR amplifications were carried out with 10 pmol of each primer, 2.5 nmol of each deoxynucleoside triphosphate, 50 to 100 ng of chromosomal template DNA, and 0.5 U of Taq polymerase in a total volume of 50 μl. The standard protocol had a total of 35 cycles, with a denaturation step at 94°C, primer annealing at 56°C, and elongation at 72°C. The amplified DNA was used without further purification for transformation of Acinetobacter strain ADP230.
Generation and mapping of mutations in vanAB.
Mutations in the van structural genes were selected by the procedure outlined in Fig. 1. After transformation of strain ADP230(ΔpcaBDK1) (12) with PCR-amplified vanAB DNA, mutant strains were selected on mineral agar medium containing 10 mM succinate supplemented with 3 mM vanillate. The ΔpcaBDK1 deletion in these mutants was replaced with wild-type DNA by transformation with linearized plasmid pZR3 (12), followed by selection for growth with quinate. The resulting strains were tested at both 22 and 37°C for the ability to utilize vanillate as the sole carbon source either alone or in the presence of 3,4-dimethoxybenzoate, m-anisate, m-toluate, or 4-hydroxy-3,5-dimethylbenzoate supplied at 3 mM. Mutations in 60 strains were mapped within vanAB with PCR-generated DNA fragments of this region (see Fig. 4) as the donors in transformations (37). For these experiments, cells were grown overnight, diluted 25-fold in fresh medium, and grown for another 2 h and 100 μl was plated on basal-medium plates supplemented with 3 mM vanillate; 500 ng to 1 μg of DNA fragment was added to each plate.
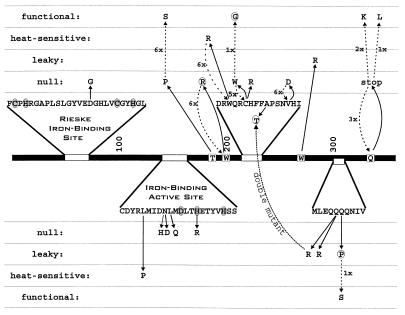
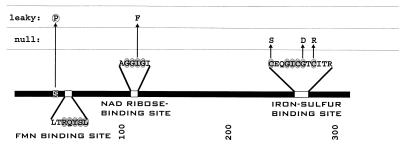
FIG. 4.
PCR-induced mutations altering the activity of VanA. The primary structure of VanA is indicated as a dark bar with relevant portions of the amino acid sequence expanded. Shaded amino acid residues in the primary sequences indicate amino acids conserved in the Rieske iron-binding site and the iron-binding active site. Dark arrows indicate amino acid substitutions caused by PCR mutagenesis. As indicated by the vertical positioning of amino acid substitutions, most caused a null phenotype, as judged by the ability of mutant cells to grow with vanillate. Some of the sequenced mutations caused a heat-sensitive phenotype with respect to growth with vanillate, and some of the mutants were classified as leaky because they allowed slow growth with vanillate. Among the mutants with detectable enzyme function, three fail to grow with vanillate in the presence of vanillate analogs. Substituted amino acids causing this phenotype are surrounded by dotted circles. One of the three mutants had undergone a double mutation, and this is marked by a dotted line connecting the mutant amino acid residues. Mutations restoring van function are indicated by dashed arrows.
Sequence analysis of mutations.
The vanAB region was amplified by PCR with Taq polymerase via the standard procedure for sequence analysis with chromosomal DNA from the mutant strains as the template DNA. PCR-amplified DNA was purified with GeneClean Glassmilk as described by the supplier (Bio 101, Inc.); 200 to 300 ng of the PCR DNA was used as template DNA in cycle sequence reactions with the ABI PRISM dye terminator cycle sequencing kit with Amplitaq DNA polymerase (−FS) as recommended by the supplier (Perkin-Elmer). Cycle sequence products were precipitated with ethanol and sodium acetate (pH 4.8) at −70°C and pelleted in a microcentrifuge at maximum speed. Pellets were washed once with 200 μl of ice-cold 70% (vol/vol) ethanol, air dried for 15 min, and resuspended in a 5:1 (vol/vol) mixture of deionized formamide and 10 mM EDTA (pH 8.0) buffer. DNA fragments were denaturated at 95°C for 2 min prior to electrophoresis on a denaturating 6% polyacrylamide gel in an ABI 373 automated sequencer (Perkin-Elmer ABI) linked to an Apple PowerMac. Sequences were analyzed with the DNA analysis program package DNASTAR (Lasergene).
RESULTS
Activity of VanAB with substrate analogs.
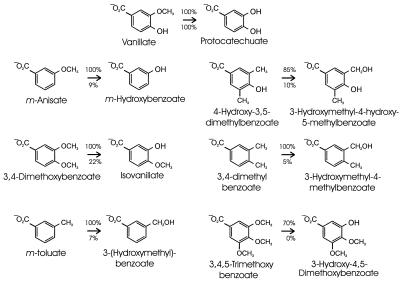
The ability of Acinetobacter vanillate demethylase to transform different substrate analogs was examined with vanillate-grown Acinetobacter cultures. Since such cells might contain enzymes with specificities overlapping that of vanillate demethylase, the survey was repeated with E. coli cells in which cloned Acinetobacter vanAB had been expressed from the lac promoter. Neither the Acinetobacter nor the E. coli cells revealed detectable activity with iso-vanillate, 2,3,4-trimethoxybenzoate, p-anisate, p-toluate, syringate, 3-methoxy-4-nitrobenzoate, 3-methoxyanisole, m-dimethoxybenzene, 3-dimethylaminobenzoate, or p-vinylbenzoate. Activities were observed with vanillate and the six substrate analogs depicted in Fig. 2.
FIG. 2.
Metabolic transformations carried out by Acinetobacter vanillate demethylase with substrate analogs. None of the analogs supported cell growth. Vanillate demethylase was induced by growing wild-type Acinetobacter bacteria with vanillate. The cloned genes for vanillate demethylase were expressed from the lac promoter in E. coli. Washed cell suspensions at an A600 of 1.0 were incubated with vanillate and the depicted chemical analogs. After 10 h, the cells were removed by centrifugation and the relative amounts of the depicted conversions were determined by measurement of the substrate and product concentrations. The respective amounts with vanillate-grown Acinetobacter and E. coli cells containing vanillate demethylase are shown below and above the arrows. The product of vanillate demethylation in Acinetobacter cells was not detected presumably because these cells metabolize the compound completely.
Products produced by demethylation of vanillate analogs were identified by mass spectroscopy after methylation and protection of free hydroxyl groups with bis(trimethylsilyl)acetamide. The following values were observed for the m/z of the fragmentation ion (percentage of base peak and, where mentioned, molecular peak [M+]): m-hydroxybenzoate, 224 (89%, M+), 209 (100%), 177 (87%), 149 (48%), and 135 (19%); iso-vanillate, 254 (27%, M+), 239 (40%), 224 (100%), 193 (52%), and 165 (13%); 3-hydroxy-4,5-dimethoxybenzoate, 284 (56%, M+), 269 (82%), 207 (18%), 195 (100%), and 151 (35%); 3-(hydroxymethyl)benzoate, 238 (11%, M+), 223 (43%), 207 (89%), 177 (40%), 149 (100%), and 133 (21%); 3-(hydroxymethyl)-4-methylbenzoate, 252 (2%, M+), 237 (19%), 221 (43%), 191 (18%), 163 (77%), 162 (100%), and 131 (50%); 3-(hydroxymethyl)-4-hydroxy-5-methylbenzoate, 340 (9%, M+), 325 (13%), 309 (11%), 251 (10%), 221 (100%), 207 (10%), and 178 (75%). NMR spectra revealed the following chemical shifts (s indicates singlet, and d indicates doublet) with reference to tetramethylsilane: for 3-(hydroxymethyl)-4-methylbenzoate, 2.4 ppm (3H, s), 4.65 (2H, s), 7.25 (1H, d, J = 6 Hz), 7.8 (1H, d, J = 6 Hz, 8.05 (1H, s); for 3-(hydroxymethyl)-4-hydroxy-5-methylbenzoate, 2.25 ppm (3H, s), 4.75 (2H, s), 7.7 (1H, s), 7.75 (1H, s).
As summarized in Fig. 2, the E. coli culture containing cloned vanAB exhibited activities substantially higher than those observed with vanillate-grown Acinetobacter bacteria. In five of the seven observed transformations, metabolic conversions of 10% or less with the induced Acinetobacter cells were greatly exceeded by the E. coli cultures, in which the cloned van genes caused corresponding conversions exceeding 70%. E. coli cultures lacking the cloned genes exhibited no evident activity toward the substrates, so the observed differences can be attributed to elevated expression of the cloned van genes. Attempts to determine enzyme activity in cell extracts were not successful, supporting the reports of others about the instability of demethylases (1, 5, 35).
The vanillate analogs that were transformed by vanillate demethylase share the common property of a methoxy or methyl group in a position meta to the carboxyl group (Fig. 2). Analogs with a hydroxyl group or only hydrogen in the same position were not transformed. These findings are consistent with the view that the enzyme requires a substituent in the meta position for nucleophilic attack. Furthermore, a carboxyl group appears to be essential for substrate binding. The enzyme is able to demethylate one methoxy group or monohydroxylate one methyl group in the meta position (Fig. 2).
Three vanillate analogs inhibited the demethylase-catalyzed transformation of vanillate in Acinetobacter strain ADP1, consistent with the notion that poorly transformed substrates can competitively inhibit the transformation of good substrates. The rate of vanillate removal by such cells in the presence of 3 mM vanillate was 2 mM/h/mg (dry weight) of cells. Relative rates of 77.5, 40, and 15% were observed in the presence of 1, 2, and 3 mM, respectively, m-anisate. At a concentration of 3 mM, 4-hydroxy-3,5-dimethylbenzoate and m-toluate produced respective rates of vanillate removal that were 20 and 45% of that observed with vanillate alone. Similar results were obtained with E. coli cells containing vanillate demethylase. Growth on plates of wild-type Acinetobacter cells with vanillate was inhibited only slightly by the presence of any of the three analogs.
Characterization of PCR-generated vanAB mutations.
Selection for mutations allowing strain ADP230(ΔpcaBDK1) to grow with succinate in the presence of vanillate yielded mutant strains with a frequency 6 × 10−5. This high frequency is consistent with the previously observed genetic instability of vanAB. Even so, transformation of ADP230 with Taq-amplified vanAB DNA, followed by selection on plates containing both vanillate and succinate, led to a 20-fold increase in the mutation frequency.
After replacement of ΔpcaBDK1 with wild-type DNA (Fig. 1), the influence of the PCR-generated mutations on growth with vanillate was determined and the mutations were mapped using specified vanAB DNA fragments as donors (37). All of the 60 strains analyzed contained mutations mapping in vanAB, and the mutant genes in these organisms were sequenced. About half of the sequenced genes contained more than one mutation. Since most of these genes allowed multiple interpretations of how the amino acid sequence influences vanillate demethylase function, they were excluded from further analysis, as were genes containing frameshift mutations. The properties and consequences of the remaining mutations are summarized in Table 1.
TABLE 1.
Nucleotide substitutions that alter translation of vanA or vanB
| Strain | Gene designation | Nucleotide substitution | Amino acid substitution or stop codon created | Phenotype |
|---|---|---|---|---|
| ADP9200 | vanA9200 | A917C | Q306P | Leaky, increased affinity for inhibitors |
| ADP9201 | vanA9203 | A914G | Q305R | Leaky, increased affinity for inhibitors |
| vanA9199 | G670A | A224T | ||
| ADP9202 | vanA9202 | T808A | W270R | Leaky |
| ADP9203 | vanA9203 | A914G | Q305R | Leaky |
| ADP9204a | vanA9204 | T649A | W217R | Heat sensitive, increased affinity for inhibitors |
| ADP9206 | vanA9206 | T437C | L146P | Heat sensitive |
| vanA9198 | T510C | Silent | ||
| ADP9207 | vanA9207 | T660G | C220W | Null |
| ADP9208 | vanA9208 | A182T | D61G | Null |
| ADP9209 | vanA9209 | A448C | N150H | Null |
| ADP9210 | vanA9210 | T683A | V228D | Null |
| ADP9211 | vanA9211 | T595C | W199R | Null |
| ADP9212 | vanA9212 | T452A | L151Q | Null |
| ADP9213 | vanA9213 | A556C | T186P | Null |
| ADP9214 | vanA9214 | T658C | C220R | Null |
| ADP9215 | vanA9215 | A448G | N150D | Null |
| ADP9216 | vanA9216 | A467G | H156R | Null |
| ADP9221 | vanA9221 | C485A | S162-TAG stop | Null |
| ADP9222 | vanA9222 | C982T | Q328-TAA stop | Null |
| ADP9223 | vanA9223 | T161A | L54-TAG stop | Leaky |
| ADP9224 | vanA9224 | A502T | K168-TAA stop | Null |
| ADP9226 | vanA9226 | T288G | Y96-TAG stop | Leaky |
| ADP9227 | vanA9227 | G393A | W131-TGA stop | Null |
| ADP9228 | vanA9228 | A740T | K246-TAA stop | Null |
| ADP9229 | vanA9229 | T521A | L174-TAG stop | Null |
| ADP9231 | vanA9231 | G809A | W270-TAG stop | Null |
| ADP9237 | vanB9237 | T103C | S35P | Leaky, increased affinity for inhibitors |
| ADP9238 | vanB9238 | A337T | I116F | Leaky |
| vanA9197 | T981C | Silent | ||
| ADP9239 | VanB9239 | T820C | C274R | Null |
| ADP9240 | vanB9240 | T796A | C266S | Null |
| ADP9247 | vanB9247 | A312T | K105-TAA stop | Null |
| ADP9248 | vanB9248 | T171A | C57-TGA stop | Null |
| ADP9249 | vanB9249 | A28T | K10-TAA stop | Null |
| ADP9250 | vanB9250 | C676T | Q226-TAA stop | Leaky |
| vanB9251 | T381C | Silent | ||
| ADP9253 | vanB9253 | T174A | C58-TGA stop | Null |
A genotypically identical strain was isolated after a separate round of mutagenesis.
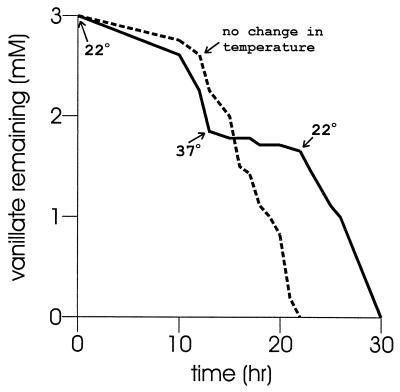
Of 34 mutants with defects in vanAB, 14 contain stop codons (Table 1). Two of the vanA missense mutants are heat sensitive, growing at 22°C but not at 37°C. The growth properties of strain ADP9204 showed that the amino acid substitution W217R rendered the protein unable to support growth at 37°C, but the stability of the protein at the elevated temperature was unknown. This question was addressed by the temperature shift experiment whose results are presented in Fig. 3. When strain ADP9204 was exposed to vanillate at 22°C, the compound was removed after a lag of about 8 h (Fig. 3). A shift in the culture temperature to 37°C stopped the removal of vanillate, and vanillate removal commenced promptly after the culture temperature was restored to 22°C. Thus, the enzyme is not destroyed at the elevated temperature and attains a conformation that allows it to resume activity at the lower temperature.
FIG. 3.
Influence of temperature on vanillate removal by ADP9204(vanA9204). Uninduced cells were incubated with vanillate at 22°C. At 13 h, the time indicated by the arrow labeled 37°C, one of the cultures was shifted to this temperature. The arrow labeled 22°C indicates that the culture was restored to this temperature at 22 h and metabolism of vanillate recommenced.
Many of the amino acid substitutions in the mutant VanA and VanB proteins are in primary-structure segments homologous to regions known to have important functions in other oxygenases. In VanA, D61G presumably disrupts the structure of the Rieske iron-binding site (Fig. 4). Five amino acid substitutions are clustered in the iron-binding active site, although only one of these (H156R) substitutes an amino acid that ligates iron (Fig. 4). The significance of N150 and L151 is highlighted by three amino acid substitutions (N150H, N150D, and L151Q) that cause a null phenotype (Fig. 4). As described above, the amino acid substitution W217R causes a temperature-sensitive phenotype but does not lead to denaturation of the enzyme at the restrictive temperature.
The importance of the VanA primary structure between positions 199 and 228 is indicated by the clustering of five mutations in this protein segment (Fig. 4). Four of these mutations (W199R, C220R, C220W, and V228D) cause a null phenotype, and one mutation (W217R) leads to a temperature-sensitive phenotype. An additional mutation in this region, A224T, alters the phenotype of strains containing Q305R. By itself, the Q305R substitution in VanA produced strain ADP9203 (Table 1), a strain that grows slowly with vanillate. Its rate of growth is unaltered by exposure of cells to the vanillate analogs m-anisate, m-toluate, and 4-hydroxy-3,5-dimethylbenzoate. Growth of strain ADP9201 (which contains both A224T and Q305R in VanA; Fig. 3) is completely inhibited by the analogs. Single amino acid substitutions conferring this phenotype were Q306P in VanA and S35P in VanB (Fig. 5). Growth of the heat-sensitive strain ADP9204 containing the amino acid substitution W217R (Fig. 3 and 4) at the permissive temperature was inhibited by the vanillate analogs.
FIG. 5.
PCR-induced mutations altering the activity of VanB. The primary structure of VanB is indicated as a dark bar with relevant portions of the amino acid sequence expanded. Shaded amino acid residues in the primary sequences indicate conserved amino acid sequences with the depicted functions. Dark arrows indicate amino acid substitutions caused by PCR mutagenesis. The dotted circle indicates an amino acid substitution that appears to increase the sensitivity of vanillate demethylase to competitive inhibition. FMN, flavin mononucleotide.
Four of the five observed single amino acid substitutions in VanB (Fig. 5) occur within peptide segments for which function can be inferred from the study of homologous enzymes. One substitution, I116F, replaces an amino acid in the presumed NAD ribose-binding site of VanB. The substituted isoleucine is conserved in many oxygenases, including toluene monosulfate monooxygenase and chlorobenzoate dioxygenase, yet the I116F substitution results in an enzyme that allows slow growth at both 22 and 37°C. Three of the five amino acid substitutions occur in the highly conserved iron-sulfur-binding site that extends over 12 residues in VanB (Fig. 5).
Nucleotide and amino acid substitutions in phenotypic revertants.
Upon incubation in medium containing vanillate as the sole carbon source, strain ADP9200 gave rise to a secondary mutant strain, ADP9299, that grew rapidly with the compound. The Q306P amino acid substitution in VanA of strain ADP9200 replaced an amino acid that is conserved among vanillate demethylases, so it was anticipated that VanA strain ADP9299 would have undergone a P306Q reversion restoring the conserved amino acid residue. This was not the case. The mutation giving rise to strain ADP9299 causes a P306S substitution in VanA (Fig. 4; Table 2), indicating that there is some latitude in the amino acid substitutions that are tolerated in this position. This finding warranted further investigation of the degree to which amino acid substitution of conserved residues would yield a functional protein.
TABLE 2.
Phenotypic revertants of vanA mutants
| Parental strain | Phenotypic revertant | Gene designation | Nucleotide substitution | Amino acid substitution | No. of isolates | Phenotype |
|---|---|---|---|---|---|---|
| ADP9200 | vanA9200 | A917C | Q306P | |||
| ADP9299 | vanA9299 | C916T | P306S | 1 | Wild type | |
| ADP9204 | vanA9204 | T649A | W217R | |||
| Wild type | A649T | R217W | 6 | Wild type | ||
| ADP9207 | vanA9207 | T660G | C220W | |||
| Wild type | G660T | W220C | 5 | Wild type | ||
| ADP9207 | vanA9207 | T660G | C220W | |||
| ADP9307 | vanA9307 | T658G | W220G | 1 | Increased affinity for inhibitors | |
| ADP9210 | vanA9210 | T683A | V228D | |||
| Wild type | A683T | D228V | 6 | Wild type | ||
| ADP9210 | vanA9210 | T683A | V228D | |||
| ADP9310 | vanA9210 | A394T | S132C | 1 | Wild type | |
| ADP9211 | vanA9211 | T595C | W199R | |||
| Wild type | C595T | R199W | 6 | Wild type | ||
| ADP9213 | vanA9213 | A556C | T186P | |||
| ADP9313 | vanA9313 | C556T | P186S | 6 | Wild type | |
| ADP9222 | vanA9222 | C982T | Q328stop | |||
| Wild type | T982C | Stop328Q | 3 | Wild type | ||
| ADP9222 | vanA9222 | C982T | Q328stop | |||
| ADP9322 | vanA9322 | T982A | Stop328K | 2 | Wild type | |
| ADP9222 | vanA9222 | C982T | Q328stop | |||
| ADP9323 | vanA9323 | A983T | Stop328L | 1 | Wild type |
The initial target of this study was the stop codon substituting the wild-type codon for Q328 in VanA of strain ADP9222 (Fig. 4; Table 2). The amino acid residue was of interest because it is conserved among oxygenases as distant as Comamonas testosteroni toluene monosulfate oxygenases (17), which has an amino acid sequence identity of only 33% with the Acinetobacter enzyme. DNA containing vanA from strain ADP9222 was mutagenized by PCR amplification, and mutations that restored the ability to grow with vanillate were selected after strain ADP9222 had been transformed with this DNA. The nucleotide sequences of vanA from six of these phenotypic revertants were determined, and three of the strains had undergone true stop328Q reversion. However, as shown in Fig. 4 and Table 2, in one of the phenotypic reverants (stop328L), the original amide residue was replaced with a hydrophobic residue and two of the revertants (stop328K) contained a basic residue at this position. It thus appears that conservation of the glutamine in the widely divergent proteins is not the consequence of direct selective pressure for its function. Toleration of limited variation was demonstrated by consistent substitution of one alcohol side chain for another, as phenotypic reversion of T186P was achieved by P186S.
Three mutated residues in VanA were invariably restored to the wild type after six separate treatments with PCR-mutagenized DNA. The respective R199W, R217W, and D228V reversions indicate that the wild-type residues are under some selective constraints (Fig. 4; Table 2). True reversion of the C220W mutation was observed in five cases, but one phenotypic revertant contained a glycyl residue rather than the wild-type cysteinyl residue, which is conserved in all known vanillate demethylase sequences (Fig. 4; Table 2). Growth of strain ADP9307 containing the glycyl substitution was inhibited by vanillate analogs, suggesting that this mutation alters the affinity of the enzyme for its substrate.
DISCUSSION
The substrate range of the vanillate demethylase shows that this enzyme, like p-toluenesulfonate monooxygenase (23), catalyzes demethylation or monohydroxylation with a variety of different aromatic substrates. Catalysis appears to depend upon the presence of a carboxyl group and a methyl or methoxy substituent in the meta position, allowing a nucleophilic attack (Fig. 2); three analogs (m-anisate, m-toluate, and 4-hydroxy-3,5-dimethylbenzoate) inhibited the activity of the demethylase. The activity of the enzymes involved in the degradation of protocatechuate was not affected by any of these compounds.
The distribution of PCR-generated mutations creating nonsense codons is, as expected, fairly random throughout vanA and vanB (Table 1). The null phenotype caused by termination at residue 328 demonstrates that the carboxy-terminal 31 amino acid residues of this protein are required for activity, and termination codons at earlier sites in the sequence presumably disrupt vanillate demethylase activity by giving rise to even smaller protein products. Of particular interest are three stop codons, two in vanA and one in vanB, that result in a leaky phenotype (Table 1). Evidently, the translational context (24, 27, 38) of these codons allows readthrough sufficient to permit slow utilization of vanillate.
The selected missense codons either replace essential amino acid residues or perturb the protein's structure so that it loses function. An example of the former is H156R, substituting an iron-binding ligand in VanA, and an example of the latter is the W217R substitution, creating a protein that is functional at 22°C but not at 37°C (Fig. 3). Clustering of missense mutations within specified segments of the primary structures of VanA and VanB is significant because it highlights contributions of these segments to enzyme function. The five missense mutations recovered in VanB occur either within or near portions of the primary structure that can be assigned functions on the basis of sequence comparison with related proteins (Fig. 5). Similarly, most of the recovered missense mutations are clustered in four primary-structure regions in VanA. Two of these have well-defined functions in the binding of iron, and the functions of the other two regions are less clear (Fig. 4). Some mutations in the latter two regions appear to increase the affinity of the enzyme for inhibitors (Tables 1 and 2; Fig. 4), suggesting that these regions contribute to substrate binding, a function known to be associated with VanA. The finding that cells containing S35P in VanB appeared to be sensitive to competitive inhibition by vanillate analogs raises the possibility that VanB makes a contribution, possibly indirect, to substrate binding.
The fact that relatively few of the selected missense mutations occur outside of clusters where function can be inferred suggests that vanillate demethylase can accumulate amino acid substitutions at many sites without losing function. A direct approach to understanding amino acid residues that can be tolerated at a specified position is the use of PCR mutagenesis to determine amino acid substitutions that allow phenotypic reversion of a known mutation (19). In the present study, flexibility was indicated by phenotypic reversion of a stop codon resulting in functional proteins with overall Q328K and Q328L substitutions (Table 2; Fig. 4). Therefore, the demonstrated conservation of glutamine at this position in widely divergent oxygenases cannot be taken as evidence of severe functional constraints. The importance of W199, W217, and V228 was underlined by the fact that activity was restored to mutants with substitutions of these residues only by direct reversion to the wild type (Table 2; Fig. 4). The same general pattern was observed with reversion of C220W, but in a single instance, a functional protein was formed by substituting a glycyl residue for the original cysteinyl residue at this position. Intriguingly, the protein containing this amino acid substitution was relatively sensitive to competitive inhibitors of vanillate demethylase, reinforcing the interpretation that this portion of the protein may contribute to substrate binding.
In one case, strain ADP9310 (Table 2), the consequence of a null mutation (V228D in VanA) overcame a mutation altering an amino acid (S132C in VanA) that is distant in the primary sequence. This finding raises the possibility that the different amino acid residues affected by mutation is within physical proximity in the folded protein. Observation of the second-site mutation was nearly masked by the predominance of six strains in which the V228 mutation had reverted to the wild type (Table 2). Therefore, additional evidence gained by probing such second-site mutations would be sharpened by seeking revertants created by PCR mutagenesis with primers excluding the primary mutation.
The most remarkable phenotypic revertants were those obtained after mutagenized DNA restored the wild-type phenotype to strains containing the T186P substitution in VanA (Fig. 4; Table 2). In each of six independent isolates, wild-type activity had been restored not by true reversion but by a different substitution at the same nucleotide position causing a P186S amino acid substitution. It seems that the alcohol functions of threonine and serine side chains are both important and interchangeable in VanA; indeed, both residues are found at position 186 in sequenced vanillate demethylases. The remarkable observation is the preferential substitution by secondary mutation of serine for the original threonine, and the basis for this may lie in the nature of the nucleotide substitutions that were available for selection. The initial nucleotide substitution is a relatively unusual transversion, A556C, in strain ADP9213, whereas the nucleotide substitution giving rise to the phenotypic revertant is the relatively frequent transition C556T (Table 2). This finding illustrates that it would be unwise to regard nucleotide substitutions as entirely random when charting the course of amino acid substitutions in evolution.
ACKNOWLEDGMENTS
This research was supported by grants DAAG55-98-1-0232 from the Army Research Office and MCB-9603980 from the National Science Foundation. B.M. was supported by a postdoctoral fellowship from the Deutscher Akademischer Austauschdienst (DAAD). A.S. was supported by a postdoctoral fellowship from the Spanish Ministerio de Educacion y Ciencia.
Footnotes
Publication 23 from the Biological Transformation Center in the Yale Biospherics Intitute.
REFERENCES
- 1.Bernhardt F H P H S H. A 4-methoxybenzoate O-demethylase from Pseudomonas putida: a new type of monooxygenase system. Eur J Biochem. 1975;57:241–256. doi: 10.1111/j.1432-1033.1975.tb02296.x. [DOI] [PubMed] [Google Scholar]
- 2.Brunel F, Davison J. Cloning and sequencing of Pseudomonas genes encoding vanillate demethylase. J Bacteriol. 1988;170:4924–4930. doi: 10.1128/jb.170.10.4924-4930.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Buswell J A, Ribbons D W. Vanillate O-demethylase from Pseudomonas spp. Methods Enzymol. 1988;161:294–301. doi: 10.1016/0076-6879(88)61032-9. [DOI] [PubMed] [Google Scholar]
- 4.Cadwell R C, Joyce G F. Randomization of genes by PCR mutagenesis. Genome Res. 1992;2:28–33. doi: 10.1101/gr.2.1.28. [DOI] [PubMed] [Google Scholar]
- 5.Cartwright N J, Smith A R W. Bacterial attack on phenolic esters: an enzyme demethylating vanillic acid. Biochem J. 1967;102:826–841. doi: 10.1042/bj1020826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Correll C C, Batie C J, Ballou D P, Ludwig M L. Phthalate dioxygenase reductase: a modular structure for electron transfer from pyridine nucleotides to [2Fe-2S] Science. 1992;258:1604–1610. doi: 10.1126/science.1280857. [DOI] [PubMed] [Google Scholar]
- 7.D'Argenio D A, Segura A, Coco W M, Bünz P V, Ornston L N. The physiological contribution of Acinetobacter PcaK, a transport system that acts upon protocatechuate, can be masked by the overlapping specificity of VanK. J Bacteriol. 1999;181:3505–3515. doi: 10.1128/jb.181.11.3505-3515.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.D'Argenio D A, Vetting M W, Ohlendorf D H, Ornston L N. Substitution, insertion, deletion, suppression, and altered substrate specificity in functional protocatechuate 3,4-dioxygenases. J Bacteriol. 1999;181:6478–6487. doi: 10.1128/jb.181.20.6478-6487.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gassner G, Wang L, Batie C, Ballou D P. Reaction of phthalate dioxygenase reductase with NADH and NAD: kinetic and spectral characterization of intermediates. Biochemistry. 1994;33:12184–12193. doi: 10.1021/bi00206a022. [DOI] [PubMed] [Google Scholar]
- 10.Gassner G T, Ludwig M L, Gatti D L, Correll C C, Ballou D P. Structure and mechanism of the iron-sulfur flavoprotein phthalate dioxygenase reductase. FASEB J. 1995;9:1411–1418. doi: 10.1096/fasebj.9.14.7589982. [DOI] [PubMed] [Google Scholar]
- 11.Gerischer U, Ornston L N. Spontaneous mutations in pcaH and -G, structural genes for protocatechuate 3,4-dioxygenase in Acinetobacter calcoaceticus. J Bacteriol. 1995;177:1336–1347. doi: 10.1128/jb.177.5.1336-1347.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hartnett G B, Averhoff B, Ornston L N. Selection of Acinetobacter calcoaceticus mutants deficient in the p-hydroxybenzoate hydroxylase gene (pobA), a member of a supraoperonic cluster. J Bacteriol. 1990;172:6160–6161. doi: 10.1128/jb.172.10.6160-6161.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hashimoto N A T S T. New methods and reagents in organic synthesis 14. A simple efficient preparation of methyl esters with tri methylsilyl diazomethane and its application to gas chromatographic analysis of fatty-acids. Chem Pharm Bull. 1981;29:1475–1478. [Google Scholar]
- 14.Jiang H, Parales R E, Gibson D T. The α subunit of toluene dioxygenase from Pseudomonas putida F1 can accept electrons from reduced FerredoxinTOL but is catalytically inactive in the absence of the β subunit. Appl Environ Microbiol. 1999;65:315–318. doi: 10.1128/aem.65.1.315-318.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jiang H, Parales R E, Lynch N A, Gibson D T. Site-directed mutagenesis of conserved amino acids in the alpha subunit of toluene dioxygenase: potential mononuclear non-heme iron coordination sites. J Bacteriol. 1996;178:3133–3139. doi: 10.1128/jb.178.11.3133-3139.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Juni E, Janik A. Transformation of Acinetobacter calcoaceticus (Bacterium anitratum) J Bacteriol. 1969;98:281–288. doi: 10.1128/jb.98.1.281-288.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Junker F, Kiewitz R, Cook A M. Characterization of the p-toluenesulfonate operon tsaMBCD and tsaR in Comamonas testosteroni T-2. J Bacteriol. 1997;179:919–927. doi: 10.1128/jb.179.3.919-927.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kauppi B, Lee K, Carredano E, Parales R E, Gibson D T, Eklund H, Ramaswamy S. Structure of an aromatic-ring-hydroxylating dioxygenase-naphthalene 1,2-dioxygenase. Structure. 1998;6:571–586. doi: 10.1016/s0969-2126(98)00059-8. [DOI] [PubMed] [Google Scholar]
- 19.Kok R G, D'Argenio D A, Ornston L N. Combining localized PCR mutagenesis and natural transformation in direct genetic analysis of a transcriptional regulator gene, pobR. J Bacteriol. 1997;179:4270–4276. doi: 10.1128/jb.179.13.4270-4276.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kok R G, D'Argenio D A, Ornston L N. Mutation analysis of PobR and PcaU, closely related transcriptional activators in Acinetobacter. J Bacteriol. 1998;180:5058–5069. doi: 10.1128/jb.180.19.5058-5069.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kok R G, Young D M, Ornston L N. Phenotypic expression of PCR-Generated random mutations in a Pseudomonas putida gene after its introduction into an Acinetobacter chromosome by natural transformation. Appl Environ Microbiol. 1999;65:1675–1680. doi: 10.1128/aem.65.4.1675-1680.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leung D W, Chen E, Goeddel D V. A method for random mutagenesis of a defined DNA segment using a modified polymerase chain reaction. Technique. 1989;1:11–15. [Google Scholar]
- 23.Locher H H, Leisinger T, Cook A M. 4-Toluene sulfonate methyl-monooxygenase from Comamonas testosteroni T-2: purification and some properties of the oxygenase component. J Bacteriol. 1991;173:3741–3748. doi: 10.1128/jb.173.12.3741-3748.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Makrides S C. Strategies for achieving high-level expression of genes in Escherichia coli. Microbiol Rev. 1996;60:512–538. doi: 10.1128/mr.60.3.512-538.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mason J R, Cammack R. The electron-transport proteins of hydroxylating bacterial dioxygenases. Annu Rev Microbiol. 1992;46:277–305. doi: 10.1146/annurev.mi.46.100192.001425. [DOI] [PubMed] [Google Scholar]
- 26.Mondello F J, Turcich M P, Lobos J H, Erickson B D. Identification and modification of biphenyl dioxygenase sequences that determine the specificity of polychlorinated biphenyl degradation. Appl Environ Microbiol. 1997;63:3096–3103. doi: 10.1128/aem.63.8.3096-3103.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mottagui-Tabar S, Isaksson L A. Only the last amino acids in the nascent peptide influence translation termination in Escherichia coli genes. FEBS Lett. 1997;414:165–170. doi: 10.1016/s0014-5793(97)00978-2. [DOI] [PubMed] [Google Scholar]
- 28.Nakatsu C H, Straus N A, Wyndham R C. The nucleotide sequence of the Tn5271 3-chlorobenzoate 3,4-dioxygenase genes (cbaAB) unites the class IA oxygenases in a single lineage. Microbiology. 1995;141:485–495. doi: 10.1099/13500872-141-2-485. [DOI] [PubMed] [Google Scholar]
- 29.Ohlmeyer M H, Swanson R N, Dillard L W, Reader J C, Asouline G, Kobayashi R, Wigler M, Still W C. Complex synthetic chemical libraries indexed with molecular tags. Proc Natl Acad Sci USA. 1993;90:10922–10926. doi: 10.1073/pnas.90.23.10922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Parales J V, Parales R E, Resnick S M, Gibson D T. Enzyme specificity of 2-nitrotoluene 2,3-dioxygenase from Pseudomonas sp. strain JS42 is determined by the C-terminal region of the α subunit of the oxygenase component. J Bacteriol. 1998;180:1194–1199. doi: 10.1128/jb.180.5.1194-1199.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Parales R E, Parales J V, Gibson D T. Aspartate 205 in the catalytic domain of naphthalene dioxygenase is essential for activity. J Bacteriol. 1999;181:1831–1837. doi: 10.1128/jb.181.6.1831-1837.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Parke D. Application of p-toluidine in chromogenic detection of catechol and protocatechuate, diphenolic intermediates in catabolism of aromatic compounds. Appl Environ Microbiol. 1992;58:2694–2697. doi: 10.1128/aem.58.8.2694-2697.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pavel E G, Martins L J, Ellis W R, Jr, Solomon E I. Magnetic circular dichroism studies of exogenous ligand and substrate binding to the non-heme ferrous active site in phthalate dioxygenase. Chem Biol. 1994;1:173–183. doi: 10.1016/1074-5521(94)90007-8. [DOI] [PubMed] [Google Scholar]
- 34.Priefert H, Rabenhorst J, Steinbuchel A. Molecular characterization of genes of Pseudomonas sp. strain HR199 involved in bioconversion of vanillin to protocatechuate. J Bacteriol. 1997;179:2595–2607. doi: 10.1128/jb.179.8.2595-2607.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ribbons D W. Requirement of two protein fractions for O-demethylase activity in Pseudomonas testosteroni. FEBS Lett. 1971;12:161–165. doi: 10.1016/0014-5793(71)80058-3. [DOI] [PubMed] [Google Scholar]
- 36.Ribbons D W. Stoichiometry of O-demethylase activity in Pseudomonas aeruginosa. FEBS Lett. 1970;8:101–104. doi: 10.1016/0014-5793(70)80235-6. [DOI] [PubMed] [Google Scholar]
- 37.Segura A, Bünz P V, D'Argenio D A, Ornston L N. Genetic analysis of a chromosomal region containing vanA and vanB, genes required for conversion of either ferulate or vanillate to protocatechuate in Acinetobacter. J Bacteriol. 1999;181:3494–3504. doi: 10.1128/jb.181.11.3494-3504.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tate W P, Poole E S, Mannering S A. Hidden infidelities of the translational stop signal. Prog Nuclic Acid Res Mol Biol. 1996;52:293–335. doi: 10.1016/s0079-6603(08)60970-8. [DOI] [PubMed] [Google Scholar]
- 39.Tindall K R, Kunkel T A. Fidelity of DNA synthesis by the Thermus aquaticus DNA polymerase. Biochemistry. 1988;27:6008–6013. doi: 10.1021/bi00416a027. [DOI] [PubMed] [Google Scholar]
- 40.Tsang H T, Batie C J, Ballou D P, Penner-Hahn J E. X-ray absorption spectroscopy of the [2Fe-2S] Rieske cluster in Pseudomonas cepacia phthalate dioxygenase. Determination of core dimensions and iron ligation. Biochemistry. 1989;28:7233–7240. doi: 10.1021/bi00444a015. [DOI] [PubMed] [Google Scholar]
- 41.Zhou Y, Zhang X, Ebright R H. Random mutagenesis of gene-sized DNA molecules by use of PCR with Taq DNA polymerase. Nucleic Acids Res. 1991;19:6052. doi: 10.1093/nar/19.21.6052. [DOI] [PMC free article] [PubMed] [Google Scholar]