Abstract
Introduction
Suppurative otitis media is a critical disease causing perforation of the tympanic membrane associated with changes of the mucoperiosteum of the middle ear cleft.
Objective
To isolate causative bacteria from chronic suppurative ear discharges and to ascertain their antibiotic profiles, of patients attending outpatients department in 3 years.
Methods
For isolation of bacteria, samples of ear discharges were grown in suitable media and bacteria were subjected to antibiotic profiling by the Kirby–Bauer's method with presently used antibiotics.
Results
A total of 1043 bacteria were isolated, including Pseudomonas aeruginosa and methicillin resistant Staphylococcus aureus, along with 121 fungal isolates. Among 371 P. aeruginosa isolates, tobramycin 30 had the highest susceptibility rate 93.2%, followed by ceftazidime 30, 91.5% and amikacin 10 μg/disk 64.4%. Of 359 S. aureus isolates, there were 236 coagulase negative S. aureus + methicillin sensitive S. aureus isolates, while 123 isolates were methicillin resistant Staphylococcus aureus with 95.2% isolates susceptible to cloxacillin 15, 83.3% isolates to erythromycin 15 and 78.5% isolates to gentamicin 30 μg/disk. Of 1164, 49 patients presented post aural abscess, 12 patients had intracranial complications, 9 patients had facial palsy and 3 patients had labyrinthitis. More than 90% P. aeruginosa and 90% S. aureus isolates were sensitive to tobramycin 30 and cloxacillin 30 μg/disk, respectively.
Conclusion
Multidrug resistant strains of P. aeruginosa were more prevalent than those of S. aureus in ear discharges. Tobramycin and cloxacillin may be included in the formulatory antibiotic regimen to overcome bacterial infections in chronic suppurative otitis media.
Keywords: Chronic suppurative otitis media, Pseudomonas aeruginosa, MRSA, Intracranial complications
Resumo
Introdução
Otite média supurativa é uma doença importante que causa perfuração da membrana timpânica além de alterações do mucoperiósteo da orelha média.
Objetivo
Isolar as bactérias causadoras a partir da secreção auricular crônica e verificar seus perfis de sensibilidade aos antibióticos em pacientes ambulatoriais durante três anos.
Método
Para o isolamento das bactérias, as amostras de secreções auriculares foram cultivadas em meios adequados e as bactérias foram submetidas à detecção de perfis de sensibilidade aos antibióticos usando o método de Kirby-Bauer para antibióticos usados na atualidade.
Resultados
No total, 1.043 bactérias, incluindo Pseudomonas aeruginosa resistentes à meticilina e Staphylococcus aureus, e 121 fungos isolados foram identificados. Entre 371 isolados de P. aeruginosa, tobramicina 30 μg/disco apresentou a maior taxa de susceptibilidade (93,2%), seguida por ceftazidima 30 μg/disco (91,5%) e amicacina 10 μg/disco (64,4%). De 359 isolados de S. aureus, 236 eram S. aureus coagulase-negativos + S. aureus sensíveis à meticilina (MSSA), enquanto 123 eram MRSA com 95,2% de suscetibilidade à cloxacilina 15 μg/disco, 83,3% sensíveis à eritromicina 15 μg/disco e 78,5% à gentamicina 30 μg/disco. Entre 1.164 pacientes, 49 apresentaram abscesso aural, 12 apresentaram complicações intracranianas, nove apresentaram paralisia facial e três apresentaram labirintite. Mais de 90% das P. aeruginosa isoladas e de 90% de S. aureus eram sensíveis à tobramicina 30 μg/disco e cloxacilina 30 μg/disco, respectivamente.
Conclusão
Cepas multirresistentes de P. aeruginosa foram mais prevalentes que as de S. aureus nas secreções auriculares. Tobramicina e cloxacilina podem ser consideradas na formulação de regime de antibióticos para tratar as infecções bacterianas na OMCS.
Palavras-chave: Otite média crônica supurativa, Pseudomonas aeruginosa, MRSA, Complicações intracranianas
Introduction
The generic term, ‘otitis media’ includes widely, cases of ‘acute otitis media’ (AOM) and cases of ‘otitis media with effusion’ (OME); basically these are non-suppurative. Moreover, ‘chronic otitis media’ (COM) is the gathering of pus from suppurations when infections are chronic; eventually, chronic suppurative otitis media (CSOM) are with inflammation and the production of pus.1 Additionally, CSOM may remain inactive with the potential to be active occasionally, leading to a perforation of the tympanic membrane associated with changes of the mucoperiosteum of the middle ear cleft with/without mucoid or mucopurulent otorrhea.1, 2, 3 It takes usually 2 or 3 weeks or more duration, for the disease to be recognized as active. A healed COM may have permanent abnormalities of the pars tensa; but with an intact pars tensa the occurrence of COM is rare.3 CSOM could lead to hearing loss, intermittent otalgia causing psychological trauma. In CSOM, the most causative bacteria are Klebsiella sp., Proteus sp., Pseudomonas aeruginosa and Staphylococcus aureus.4 And other bacteria commonly isolated from patients with AOM are Haemophilus influenzae, Moraxella catarrhalis and Streptococcus pneumonia.5 Moreover, P. aeruginosa had been seen as a notorious pathogen in this hospital too.6 Mainly found in wounds and urinary tract, it finds ways as bloodstream infection (BSI) to innards causing comorbidities.7 Indeed, the ability of these organisms to form biofilm that may contribute to their frequency in CSOM.8 As it is known, the rate of invasion of a pathogenic bacterium directly depends on its level of drug resistance, apart from immune-conditions of patients.9
Particularly, several clonal variants of S. aureus were resistant to the penicillin group of antibiotics, after which methicillin/oxacillin were introduced for the control. Subsequently, methicillin resistant S. aureus (MRSA), causing surgical site infections and wound emerged.10 The most gruesome situation is that MRSA strains have emerged with concomitant/subsequent resistance to most commonly used antibiotics of groups, aminoglycosides, macrolides, fluoroquinolones, chloramphenicol and tetracycline and many more such as, to cephalosporins, cefems and other β-lactams, ampicillin-sulbactam, amoxicillin-clavulanic acid, ticarcillin-clavulanic acid, piperacillin-tazobactam and the carbapenem, imipenem. Thus, MRSA isolates are MDR too.11 Moreover, the most dominating fungal species were of Candida and Aspergillus along with MRSA; and in a surveillance, 50% patients were diagnosed with candidiasis.10 Indeed, Candida albicans was originally a harmless fungus in healthy persons, but its causes superficial to life-threatening uncontrollable systemic infections due to the emergence of antifungal resistance.12
This work describes surveillance of bacterial flora from ear discharges of patients attending the Outpatients Department (OPD) of ENT department of the hospital, in the last 3 years. And the cited two fungi were too isolated along with bacteria. Antibiograms of isolated bacterial taxa were determined to assess the spectrum of CSOM that would help in rescheduling antimicrobial stewardship program of the hospital or the zone of central Odisha.
Methods
A total of 1230 pus discharges from clinically diagnosed CSOM cases were collected, during January 2012 to January 2015 with sterile cotton swab sticks. Pus swabs were cultured on blood and MacConkey agar plates that were incubated at 37 °C overnight for pathogenic bacteria, which were identified according to the standard method used for bacteria and concomitantly for fungi.6, 13, 14 Antibiotic susceptibility tests of isolated bacteria were done according to Clinical Laboratory Standard Institute guidelines, as described.15, 16 Standard antimicrobial disks (HiMedia, Mumbai) used for S. aureus were, oxacillin, cotrimoxazole, penicillin, cloxacillin, gentamicin, chloramphenicol, ciprofloxacin and vancomycin; similar disks used for P. aeruginosa were gentamicin, chloramphenicol, ciprofloxacin, ceftazidime, piperacillin, carbenecillin and tobramycin.
Antibiotic sensitivity and detection of MRSA
The standard MTCC number 7443 strain and all the isolated S. aureus strains were subjected to antibiotic sensitivity tests with antibiotics, by the Kirby-Bauer's method (disk diffusion) detailed previously.11 For the detection of MRSA, chromogenic agar media test was used; pure clinical isolates of S. aureus were streaked onto MRSA-agar media as described.11 Muller-Hinton Agar (MHA) plates were incubated at 37 °C for 18 h and inhibition-zone diameters were measured. A value of inhibition-zone diameter less than 22 mm was reported as oxacillin resistant and that more than 21 mm was considered as oxacillin sensitive.11
Identification of fungi
Direct microscopic examination of the cotton swab with samples was carried out by mounting sample lots treated with 1–2 drops of 10–20% KOH for 15–30 min. Each specimen-lot was inoculated on two sets of Sabouraud's dextrose agar slopes, one set with chloramphenicol and the other set with cycloheximide (chloramphenicol – 0.05 mg/mL, cycloheximide – 0.5 mg/mL). Cultures were incubated at room temperature for 4–6 weeks and were observed regularly for possible growth. Fungal isolates were identified on the basis of duration of growth and surface morphology of colonies, as well as pigment production on the reverse and microscopic examination of hyphae in lacto phenol cotton blue preparation.6, 16
Results
From 1230 collected samples, 1164 bacterial and fungal colonies grew as 629 single and 535 mixed colonies on agar plates and no microbial growth was seen with 66 samples. There were 1043 bacterial and 121 fungal isolates in total. The most common causal bacteria isolated were 220 isolates of S. aureus with and 188 isolates of P. aeruginosa; and 19 isolates of S. aureus were MRSA, and 64 isolates were coagulase negative S. aureus (CONS). Bacteria, P. aeruginosa was isolated in 183 of the total 1164 samples that yielded mixed colonies of S. aureus, Klebsiella sp. and Proteus sp., followed by Escherichia coli, given in Table 1. Fungi accounted for 63 isolates of Aspergillus sp. and 68 isolates of Candida sp. as both single and mixed colonies from 1164 growth-yielding samples, given in Table 1.
Table 1.
Growth of bacteria and fungi in cultures of ear discharge samples of OPD patients with CSOM as single colony and mixed colonies.
| Organisms | Single colony isolates n1 = 629 (100) |
Mixed colony isolates n2 = 535 (100) |
Total isolates (n1 + n2) = n = 1164 (100) |
|---|---|---|---|
| Enterobacter sp. | 19 (03.0) | – | 19 (01.6) |
| CONS | 64 (10.1) | – | 64 (05.4) |
| MRSA | 19 (03.0) | 104 (19.7) | 123 (10.5) |
| MSSA | 137 (22.0) | 35 (06.8) | 172 (14.7) |
| E. coli | 47 (07.7) | 51 (09.8) | 98 (08.4) |
| Citrobacter sp. | 34 (05.4) | – | 34 (02.9) |
| Klebsiella sp. | 47 (07.7) | 45 (8.7) | 92 (07.9) |
| Proteus sp. | 19 (03.0) | 41 (7.7) | 60 (05.1) |
| P. aeruginosa | 188 (30.1) | 183 (34.3) | 371 (31.8) |
| Aspergillus sp. | 33 (05.3) | 30 (05.8) | 63 (05.4) |
| Candida sp. | 22 (03.5) | 46 (08.8) | 68 (05.8) |
CONS, coagulase negative Staphylococcus; MRSA, methicillin resistant S. aureus; MSSA, methicillin sensitive S. aureus; OPD, outpatients department; percent values are in parenthesis; n or total colonies = 1164, from the total 1230 samples; the rest 66 samples had no growth; there were 121 (63 + 68) fungal isolates.
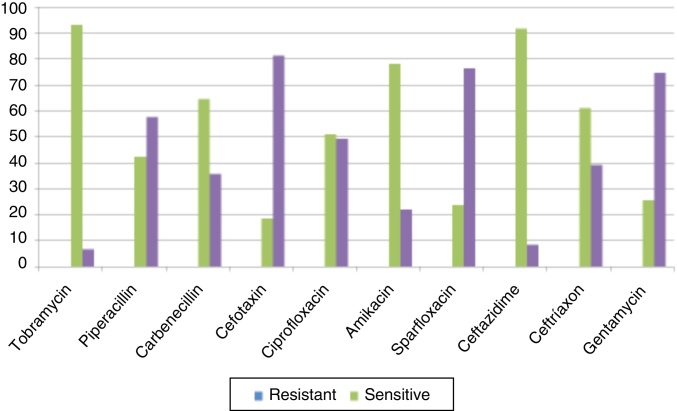
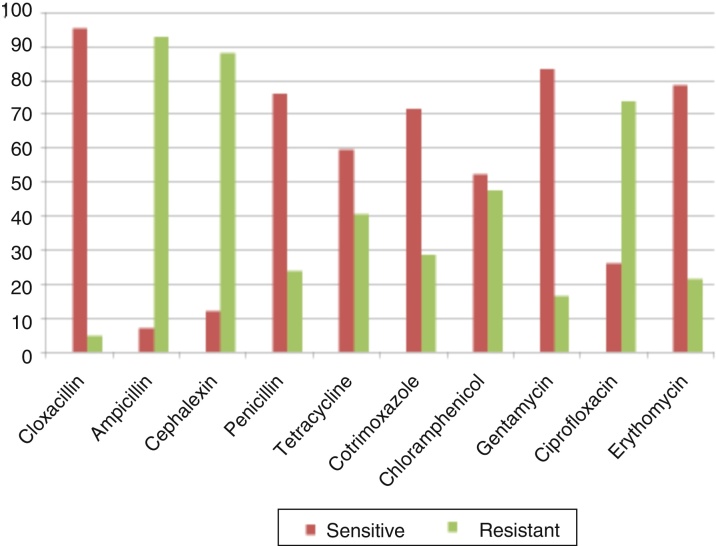
Antibiograms of the most common bacteria, P. aeruginosa and S. aureus (other than MRSA) are depicted in Fig. 1. Among P. aeruginosa, tobramycin 30 μg/disk had the highest susceptibility rate as 93.2%, followed by ceftazidime 30 μg/disk 91.5% and amikacin 10 μg/disk 64.4%, given in Fig. 2. And 95.2% S. aureus isolates were susceptible to cloxacillin 15 μg/disk, followed by 83.3% isolates to erythromycin 15 μg/disk and 78.5% isolates to gentamicin 30 μg/disk, given in Fig. 3. All MRSA isolates were MDR; however, none of those isolates were resistant to vancomycin 30 μg/disk.
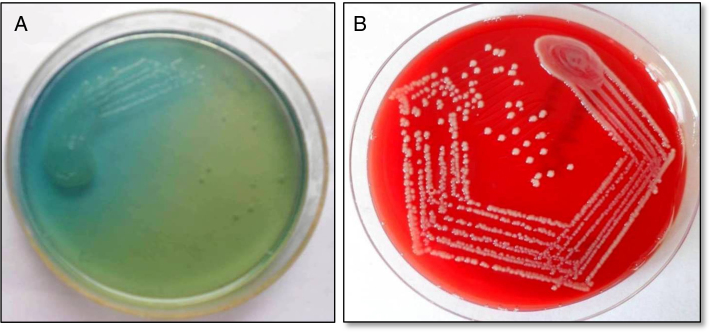
Figure 1.
(A) Confluent growth and isolated green colonies of P. aeruginosa on nutrient agar plate; and (B) methicillin resistant colorless colonies of S. aureus (MRSA) on blood agar plate.
Figure 2.
Antibiogram of P. aeruginosa.
Figure 3.
Antibiogram of S. aureus.
Of samples of 1164 patients, 73 had complicated and 1091 patients had uncomplicated CSOM, as detailed: only 48 cases had as single bacterium isolated as a single colony, while the remaining 198 cases had two or more bacteria isolates as mixed colonies, given in Table 2. Of the total 1230 patients, 49 had post aural abscess, 12 patients had intracranial complications, 9 patients presented with facial palsy and 3 patient presented with labyrinthitis, given in Table 3. Furthermore, it was seen that the trend of intracranial complications was gradually decreasing while intracranial complications were in an increasing trend, although there was no significant change in overall incidences of CSOM. From 359 S. aureus samples, a total of 123 MRSA strainsand 236 strains of ‘CONS + (MSSA)’ (methicillin sensitive S. aureus) strains, as both single and mixed colonies. The minimum inhibitory concentration (MIC) range against oxacillin was 16–512 μg/mL, the MIC range was 1–4 μg/mL, for MRSA and ‘CONS + MSSA’. These MIC values confirmed the presence of MRSA strains, as the break point for being resistant to oxacillin was ≥4 μg/mL, given in Table 4.
Table 2.
Numbers of growing organisms from cultures of ear discharge samples in patients with complicated and uncomplicated CSOM.
| Types of organisms | Complicated CSOM | Uncomplicated CSOM | Total |
|---|---|---|---|
| As single colony | 48 (0.05) | 581 (0.49) | n1 = 629 (0.54) |
| As mixed colonies | 25 (0.02) | 510 (0.43) | n2 = 535 (0.45) |
| Total | 73 (0.06) | 1091 (0.94) | n = 1164 (100) |
See note of Table 1.
Table 3.
Numbers of patients with complications as comorbidities causing CSOM in 3 years.
| Year | Facial palsy | Intracranial complication | Post-aural abscess | Labyrinthitis | Total |
|---|---|---|---|---|---|
| 2012 | 3 | 3 | 16 | 2 | 24 |
| 2013 | 2 | 4 | 18 | 0 | 24 |
| 2014 | 4 | 5 | 15 | 1 | 25 |
| Total | 9 (12.9) | 12 (16.6) | 49 (67.1) | 3 (3.3) | 73 (100) |
Percent values are in parenthesis.
Table 4.
Detection of MRSA and ‘CONS + MSSA’ isolates based on MIC values due to oxacillin in a 12 × 8 micro-titer plate.
| Well | Oxacillin (μg/mL) | Number of isolates |
|
|---|---|---|---|
| MRSA = 123 | CONS + MSSA = 236 | ||
| 1 | 0 | 123 | 236 |
| 2 | ≤0.25 | – | – |
| 3 | 0.5 | – | – |
| 4 | 1 | – | 83 |
| 5 | 2 | – | 75 |
| 6 | 4 | – | 78 |
| 7 | 8 | – | – |
| 8 | 16 | 23 | – |
| 9 | 32 | 26 | – |
| 10 | 64 | 27 | – |
| 11 | 128 | 29 | – |
| 12 | ≥256 | 28 | – |
The oxacillin stock solution of 512 μg/mL was serially diluted at each successive well, from the 12th well for final concentration of 0.25 μg/mL oxacillin at the 2nd well; –, no growth; total Staphylococcus sp. = MRSA with 123 + (CONS + MSSA) with 236 = 359 colonies. Results of the second repeated experiment are presented.
Discussion
CSOM is a disease associated with the structural change in middle ear; and permanent abnormality of pars tensa or pars flaccid, mostly occur as sequelae of long standing middle ear effusion, inadequately treated AOM, eustachian tube dysfunction or even from a negative middle ear pressure. In the developing countries, poverty, ignorance, dearth of specialists and limited access to medical care amongst others conspire to worsen the occurrence and complications of CSOM17; poor living conditions, poor access to medical care, inadequate medical treatment, recurrent upper respiratory tract infections and nasal diseases have been recognized as risk factors for CSOM.18 Atticoantral disease most commonly is involved with the pars flaccida and posterior superior quadrant of pars tensa. It is characterized by the formation of a retraction pocket in which, keratin and desquamated epithelial debris accumulate to produce cholesteatoma; eventually it is considered to be a dangerous form of the disease because of the development of several intracranial and extracranial complications.18 Moreover, staphylococci are a part of the normal flora, but those remain invasive causing a variety of body infections. S. aureus is the most notorious nosocomial pathogen and in community too.11
Although the clinical relevance of CONS is still controversial, patients at risk of CONS infections include neonates, those with intravascular catheters, prosthetic devices and surgical wounds in immune-compromised individuals. The remarkable ability of S. aureus and CONS to acquire antibiotic resistance limits therapeutic options, attended with high rates of morbidity and mortality, including costs of hospitalization.19 Particularly, several clonal variants of S. aureus and MRSA were reported resistant to the penicillin group of antibiotics, methicillin/oxacillin. Moreover, in a German study, it was reported that a majority of MRSA strains were from wound infections (56.9%), with pneumonia cases being the second most common (21.0%), followed by BSI (15.1%).11
Conclusion
MDR strains of P. aeruginosa and MRSA were most prevalent is ear discharges of patients with CSOM. Of the total 1164, 49 patients presented post aural abscess, 12 patients had intracranial complications, 9 patients had facial palsy and 3 patients had labyrinthitis. This study revealed ciprofloxacin as less effective in the treatment of active CSOM, and tobramycin and cloxacillin could preferably be used to treat CSOM.
Funding
This work was supported by the major research project n° BT/PR8214/PBD/17/863/2013 on bacterial infections, from Department of Biotechnology (DBT), Govt. of India, New Delhi, awarded to RN Padhy. This work is a part of PhD thesis of SN Rath, a JRF in the DBT project, in Biotechnology of S‘O’A University, Bhubaneswar. We are grateful to Prof. Rankanidhi Samal, for critical appreciation and thankful to Prof. Gangadhara Sahoo, Dean, IMS and Sum Hospital, Bhubaneswar, for extended facilities.
Conflicts of interest
The authors declare no conflicts of interest.
Footnotes
Please cite this article as: Rath S, Das SR, Padhy RN. Surveillance of bacteria Pseudomonas aeruginosa and MRSA associated with chronic suppurative otitis media (CSOM). Braz J Otorhinolaryngol. 2017;83:201–6.
Peer Review under the responsibility of Associação Brasileira de Otorrinolaringologia e Cirurgia Cérvico-Facial.
References
- 1.Berman S. Otitis media in children. N Engl J Med. 1995;23:1560–1565. doi: 10.1056/NEJM199506083322307. [DOI] [PubMed] [Google Scholar]
- 2.Browning G.G., Merchant S.N., Kelly G., Swan I.R., Canter R., McKerrow S.W. vol. 19. Edward Arnold Ltd.; 2008. Chronic otitis media; pp. 3406–3410. (Scott-Brown's otorhinolaryngology head and neck). [Google Scholar]
- 3.Afolabi O.A., Fadare J.O., Omokanye H.K., Olatoke F., Odi T.O., Saka M.J., et al. Socioeconomic challenges of chronic suppurative otitis media management in state tertiary health facility in Nigeria. Egypt J Ear Nose Throat Allied Sci. 2014;15:17–22. [Google Scholar]
- 4.Couzos S., Lea T., Mullar R. Effectiveness of ototopical antibiotics for CSOM in Aboriginal children, a community based multicenter double blind randomized controlled trial. Med J Aust. 2003;4:185–190. doi: 10.5694/j.1326-5377.2003.tb05496.x. [DOI] [PubMed] [Google Scholar]
- 5.Bluestone C.D., Klein J.O. In: Otitis media in infants and children. Microbiology. 3rd ed. Bluestone C.D., Klein J.O., editors. WB Saunders; Philadelphia, PA: 2001. pp. 79–101. [Google Scholar]
- 6.Sahu M.C., Padhy R.N. Bayesian evaluation of two conventional diagnostic methods for pathogenic fungal infections. J Acute Med. 2014;4:109–119. [Google Scholar]
- 7.Askarian M., Hossseini R., Kheirandish P. Incidence and outcome of nosocomial infections in female burn patients in Shiraz, Iran. Am J Infect Control. 2004;32:23–26. doi: 10.1016/j.ajic.2003.03.004. [DOI] [PubMed] [Google Scholar]
- 8.Ahmed B., Hydri A.S., Ejaz A., Farooq S., Zaidi S.K., Afridi A.A. Microbiology of ear discharge in Quetta. J Coll Physician Surg Pak. 2005;15:583–584. [PubMed] [Google Scholar]
- 9.Giamarellos-Bourboulis E.J., Papadimitriou E., Galanakis N., Antonopoulou A., Tsaganos T., Kanellakopoulou K. Multidrug resistance to antimicrobials as a predominant factor influencing patient survival. Int J Antimicrob Agent. 2006;27:476–481. doi: 10.1016/j.ijantimicag.2005.12.013. [DOI] [PubMed] [Google Scholar]
- 10.Wisplinghoff H., Bischoff T., Tallent S.M., Seifert H., Wenzel R.P., Edmond M.B. Nosocomial bloodstream infections in US hospitals: analysis of 24,179 cases from a prospective nationwide surveillance study. Clin Infect Dis. 2004;39:309–317. doi: 10.1086/421946. [DOI] [PubMed] [Google Scholar]
- 11.Dubey D., Rath S., Sahu M.C., Patnaik L., Debata N.K., Padhy R.N. Surveillance of infection status of drug resistant Staphylococcus aureus in an Indian teaching hospital. Asian Pac J Trop Dis. 2013;3:133–142. [Google Scholar]
- 12.Richardson M., LasseFlorl C. Changing epidemiology of systemic fungal infections. Clin Microbiol Infect. 2008;14:5–24. doi: 10.1111/j.1469-0691.2008.01978.x. [DOI] [PubMed] [Google Scholar]
- 13.Rath S.N., Padhy R.N. Surveillance of acute community acquired urinary tract bacterial infections. J Acute Dis. 2015;3:186–195. [Google Scholar]
- 14.Prajna L., Vijayakumar A. Jaypee Broth Med Publishers; New Delhi: 2008. Atlas of fungal corneal ulcers clinical features and laboratory identification methods. [Google Scholar]
- 15.Clinical and Laboratory Standards Institute . Wayne; PA: 2011. Performance standards for antimicrobial susceptibility testing. [PubMed] [Google Scholar]
- 16.Rath S.N., Panda M., Sahu M.C., Padhy R.N. Bayesian analysis of two diagnostic methods for paediatric ringworm infections in a teaching hospital. J Mycol Méd. 2015;25:191–199. doi: 10.1016/j.mycmed.2015.06.065. [DOI] [PubMed] [Google Scholar]
- 17.Vikram B.K., Khaja N., Udayashankar S.G. Clinico-epidemiological study of complicated and uncomplicated chronic suppurative otitis media. J Laryngol Otol. 2008;122:226–242. doi: 10.1017/S0022215107000278. [DOI] [PubMed] [Google Scholar]
- 18.Chowdhury M.A., Alauddin A. Comparative study between tubotympanic and atticoantral type of chronic suppurative otitis media. Bangladesh Med Res Counc Bull. 2002;1:36–44. [PubMed] [Google Scholar]
- 19.Zong Z., Peng C., Lu X. Diversity of SCCmec elements in methicillin resistant coagulase-negative staphylococci clinical isolates. PLoS ONE. 2011;5:191–200. doi: 10.1371/journal.pone.0020191. [DOI] [PMC free article] [PubMed] [Google Scholar]